Abstract
Background and Purpose
Platelet-to-neutrophil ratio (PNR) was suggested to be an independent protective predictor for 90-days outcomes in acute ischemic stroke (AIS) patients in previous studies. This study aims to investigate the association between PNR and outcomes of AIS in intravenous thrombolysis (IVT) group.
Methods
Data on acute ischemic stroke patients who received intravenous thrombolysis from April 2015 to March 2019 were collected. We defined the PNR value at admission as pre-IVT PNR and after IVT within 24 h was defined as post-IVT PNR. Clinical outcome indicators included early neurological deterioration (END), hemorrhagic transformation (HT), delayed neurological deterioration (DND), and poor 3-month outcome (3m-mRS >2).
Results
A total of 581 patients were enrolled in the final analysis. The age was 61(53-69) years, and 423(72.8%) were males. Post-IVT PNR was independently associated with hemorrhagic transformation (OR = 0.974; 95%CI = 0.956-0.992; P=0.006), early neurological deterioration (OR = 0.939; 95%CI = 0.913-0.966; P = 0.01), delayed neurological deterioration (OR = 0.949; 95%CI = 0.912-0.988; P = 0.011), and poor outcome (OR = 0.962; 95%CI = 0.948-0.976; P<0.001). PNR level was identified as high (at the cut-off value or above) or low (below the cut-off value) according to receiver operating curve (ROC) analyses on each endpoint. Comparison of early neurological deterioration, hemorrhagic transformation, delayed neurological deterioration, and poor 3-month outcome (3m-mRS >2) between patients at high and low levels for platelet-to-neutrophil ratio (PNR) showed statistical differences (p<0.001).
Conclusion
Post-IVT PNR was independently associated with early neurological deterioration, hemorrhagic transformation, delayed neurological deterioration, and poor 3-month outcome. Lower PNR can predict a worse outcome.
Keywords: Acute ischemic stroke, intravenous thrombolysis, early neurological deterioration, hemorrhagic transformation, delayed neurological deterioration
1. Introduction
Stroke is the second leading cause of death worldwide and also the leading cause of long-term disability [1]. Ischemic stroke is the most common type of stroke [2]. An occurrence of acute ischemic stroke (AIS) always leads to the death of brain tissues and focal neurological deficits. Recombinant tissue plasminogen activator (rtPA) is the only thrombolytic agent approved by the FDA for ischemic stroke therapy. But, owing to the limitation of the narrow therapeutic time window (4.5 h from the onset of symptoms of ischemic stroke) and the potential risk of hemorrhagic transformation (HT), only partial patients can benefit from intravenous thrombolysis (IVT) [3].
According to recent studies, the key role of the inflammatory process has been increasingly recognized in thrombosis. It is well known that platelets adhere to the damaged vessel wall at the site of injury and release types of granules containing enzymes after activation [4-6]. Previous studies demonstrated evidences that leukocytes were recruited by thrombi and invoked thrombo-inflammatory response correlating with the degree of organ injury and clinical outcome [7, 8]. Neutrophils rapidly respond to the ischemic site [9] and release reactive oxygen species (ROS), proteases and cytokines, resulting in a series of brain tissue damage including disruption of the blood-brain barrier (BBB) and cerebral edema [10, 11]. Platelet-to-neutrophil ratio (PNR) is a new biomarker that combines platelets and neutrophil counts. Compared with single platelet counts and neutrophil counts, PNR reflects the severity of both thrombosis and inflammation, revealing the connection between the two processes. One previous study investigated the potential association of PNR with different histological types of ovarian epithelial carcinomas [12]. In the stroke field, a recent study suggested that the level of PNR on admission is associated with the prognosis of AIS patients [13]. No existing studies have reported whether there is a similar association for the AIS patients treated with IVT.
For the first time, we focused on the clinical value of PNR in predicting the outcome in AIS patients treated with IVT. In consideration of IVT, treatment might change the blood cell counts and ratio, and we involved PNR both before and after IVT into the analysis.
2. Materials and methods
2.1. Study Population
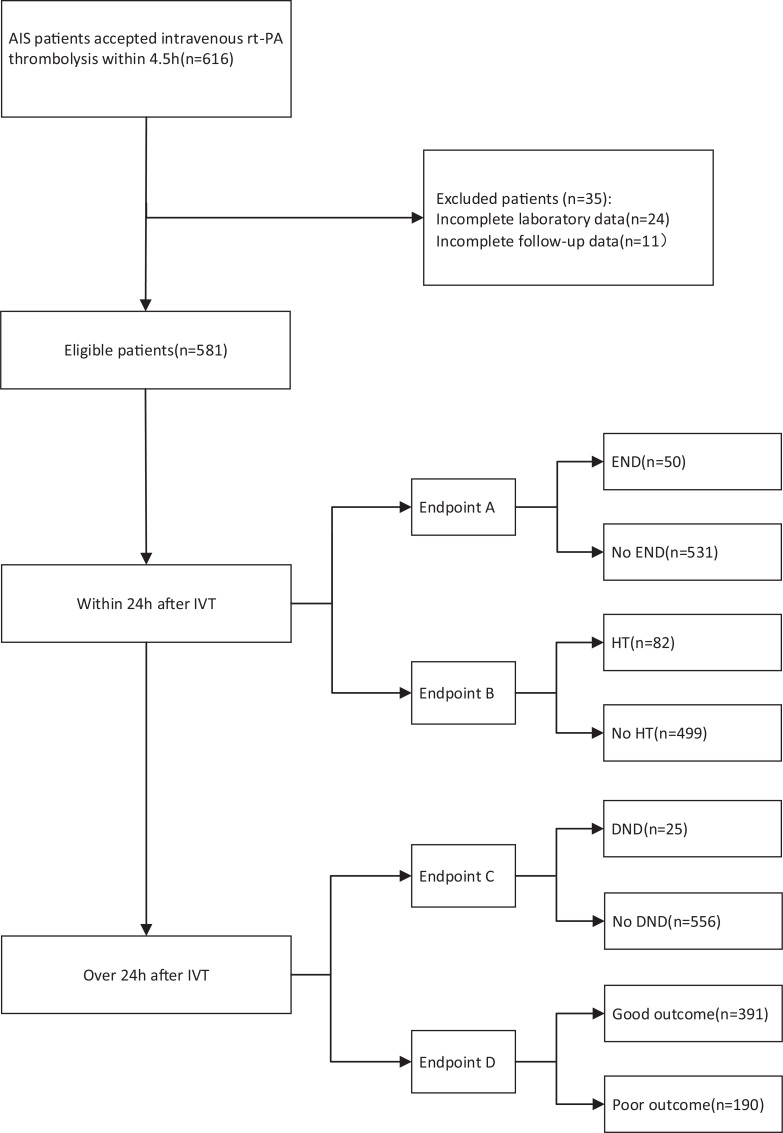
Data from this retrospective study were collected at the First Hospital of Jilin University. Acute ischemic stroke patients who received intravenous thrombolysis treatment from April 2015 to March 2019 were included. A total of 616 patients diagnosed with acute ischemic stroke (AIS) accepted intravenous thrombolysis (IVT) rt-PA treatment within 4.5 h of stroke onset. Patients who lost laboratory data (24) and follow-up data (11) were excluded from this study. Finally, 581 patients were included in the study (Fig. 1).
Fig. (1).
The flow chart of the study.
Abbreviations: AIS, acute ischemic stroke; END, early neurologic deterioration; HT, hemorrhagic transformation; DND, delayed neurological deterioration; IVT, intravenous thrombolysis.
A total of 616 patients diagnosed with acute ischemic stroke (AIS) accepted intravenous thrombolysis (IVT) rt-PA treatment within 4.5 h of stroke onset. Patients who lost laboratory data (24) and follow-up data (11) were excluded from this study. Finally, 581 patients were included in the study.
2.2. Data Collection
Based on the clinical manifestations and signs, an ex- perienced clinician determined whether the patient met the clinical case description of acute stroke, and stroke severity was assessed on admission using the National Institute of Health Stroke Scale (NIHSS) score. All patients underwent emergent computerized tomography (CT) scan before IVT to rule out the possibility of hemorrhagic stroke. We recorded baseline characteristics including demographics (age and sex), vascular risk factors(current smoking, current drinking, atrial fibrillation, coronary artery disease, diabetes, hypertension, and prior stroke), past medical history (antihypertensive therapy, antiplatelet therapy, and hypoglycemic therapy) and baseline parameters (including admission blood glucose, admission systolic and diastolic blood pressure levels, and onset-to-treatment time for IV rt-PA). Venous blood samples were collected both at admission and within 24 h after IVT. The PNR value was calculated according to platelet and neutrophil counts both at admission and after thrombolysis therapy. We defined the PNR value at admission as pre-IVT PNR. Similarly, the PNR value after IVT within 24 h was defined as post-IVT PNR. The procedures of this study were in accord with ethical standards of the responsible institutional or regional committee on human experimentation.
2.3. Outcomes
Four clinical outcome indicators included early neurolo-gical deterioration, hemorrhagic transformation, delayed neurological deterioration, and poor 3-month outcome. Hemorrhagic transformation was defined as any visible hemorrhage on brain CT within 24 h after thrombolysis. Early neurological deterioration was defined as ≥4-point increase in scores on the NIHSS or dead within 24 h after intravenous thrombolysis. Delayed neurological deterioration was similarly defined as early neurological deterioration but for the period 24 h to 7 d. 3-months clinical outcome was measured using the modified Rankin Scale (mRS). Poor 3-month outcome was defined as an mRS score of 3-6, and good outcome was defined as an mRS score of 0-2.
2.4. Statistical Analysis
Data were analyzed using the Statistical Program for Social Sciences version 22.0 (SPSS, IBM, West Grove, PA, USA). The difference between the 2 groups was tested using the Mann–Whitney U-test for nonparametrically distributed variables. The differences between categorical variables were determined using the χ2 test. Median with IQR and percentage were used to describe the distribution of continuous and categorical variables, respectively. The receiver opera-ting characteristic (ROC) curve was used to evaluate the prognosis effect of PNR. Multivariate logistic regression analysis was used to examine risk factors for each endpoint, classified as 1 when present and 0 when absent. P<0.05 was used to establish statistical significance in all comparisons between groups.
3. Results
3.1. Patients’ Characteristics
Of the 581 patients enrolled in our study, the age was 61(53-69) years, and 423(72.8%) were males. The baseline NIHSS score was 9(5-13). The time from stroke onset to IVT infusion was 180(141-230) min. We accessed outcome events of patients who accepted IVT at different time points: 24 h, 24 h-7 d, and 3 months after thrombolysis. Among 581 eligible patients, 50 (8.6%) presented with early neurological deterioration and 82(14.1%) presented with the hemorrhagic transformation (determined by 24 h-head CT) in the first 24 h after IVT. 25(4.3%) developed delayed neurological deterioration in 24 h-7d after IVT. After 3 months, 190(32.7%) patients had poor outcome (3m-mRS>2). Baseline clinical characteristics and outcomes are summarized in Table 1.
Table 1.
Clinical characteristics of the study population.
| Variables | Total (n = 581) |
|---|---|
| Demographic data | - |
| Age, years, median (IQR) | 61 (53-69) |
| Sex (male), n (%) | 423 (72.8) |
| Risk factors | - |
| Current smoking, n (%) | 326 (56.1) |
| Current drinking, n (%) | 253 (43.5) |
| Atrial fibrillation, n (%) | 27 (4.6) |
| Coronary artery disease, n (%) | 116 (20) |
| Diabetes, n (%) | 189 (32.5) |
| Hypertension, n (%) | 302 (52) |
| Time from stroke onset to IVT infusion (min), median (IQR) | 180 (141-230) |
| Prior stroke, n (%) | 88 (15.1) |
| Laboratory tests | - |
| Systolic blood pressure, mmHg, median (IQR) | 154 (138-165) |
| Diastolic blood pressure, mmHg, median (IQR) | 89 (80-98) |
| Baseline blood glucose, mmol/L, median (IQR) | 6.92 (6.13-8.65) |
| Baseline NIHSS score, median (IQR) | 9 (5-13) |
| Baseline neutrophil(109/L), median (IQR) | 5.41 (3.98-7.44) |
| Baseline PLT (109/L), median (IQR) | 200 (170-231) |
| Baseline PNR, median (IQR) | 36.71 (26.04-51.29) |
| Post-IVT neutrophil (109/L), median (IQR) | 5.39 (4.23-7.26) |
| Medications | - |
| Antihypertensive therapy, n (%) | 200 (34.4) |
| Antiplatelet therapy, n (%) | 73 (12.6) |
| Hypoglycemic therapy, n (%) | 102 (17.6) |
| Post-IVT PLT (109/L), median (IQR) | 205 (173-237) |
| Post-IVT PNR, median (IQR) | 37.76 (27.16-49.33) |
| Outcome events | - |
| END, n (%) | 50 (8.6) |
| HT, n (%) | 82 (14.1) |
| DND, n (%) | 25 (4.3) |
| Poor 3-month outcome, n (%) | 190 (32.7) |
Abbreviations: NIHSS, National Institute of Health Stroke Scale; PLT, Platelet; PNR, Platelet-To-Neutrophil Ratio; IVT, intravenous Thrombolysis; END, Early Neurologic Deterioration; HT, Hemorrhagic Transformation; DND, Delayed Neurological Deterioration.
3.2. The Association of PNR Levels with END, HT, DND, and Poor 3-month Outcome
We divided all eligible patients into groups according to the presence or absence of each clinical outcome indicators. The post-IVT PNR value was lower in patients who deve-loped any of the 4 clinical outcome indicators [(29.92 vs. 38.06, p<0.01) in early neurological deterioration, (30.73 vs. 38.64, p<0.01) hemorrhagic transformation, (17.18 vs. 37.76, p<0.01) delayed neurological deterioration, (28.92 vs. 41.67, p<0.01) and poor 3-month outcome]. However, lower Pre-PNR value (32.47 vs. 37.19, p = 0.011) was associated with hemorrhagic transformation, but not with the other endpoints. The comparisons of other main characteristics and laboratory results according to the presence/absence of each endpoint are presented in Tables 2 and 3.
Table 2.
Clinical characteristics of patients according to the presence/absence of END and HT after IVT treatment.
| Variables |
Total
(n = 581) |
No END
(n = 531) |
END (n = 50) | P |
No HT
(n = 499) |
HT
(n = 82) |
P |
|---|---|---|---|---|---|---|---|
| Age, years, median (IQR) | 61 (53-69) | 62 (53-69) | 59.5 (53.5-67.5) | 0.448 | 62 (54-70) | 57 (50.75-66) | 0.006 |
| Sex (male), n (%) | 423 (72.8) | 385 (72.5) | 38 (76) | 0.595 | 358 (71.7) | 65 (79.3) | 0.156 |
| Current smoking, n (%) | 326 (56.1) | 300 (56.5) | 26 (52) | 0.54 | 277 (55.5) | 49 (59.8) | 0.473 |
| Current drinking, n (%) | 253 (43.5) | 235 (44.3) | 18 (36) | 0.26 | 212 (42.5) | 41 (50) | 0.203 |
| Atrial fibrillation, n (%) | 27 (4.6) | 27 (5.1) | 0 | 0.102 | 16 (3.2) | 11 (13.4) | <0.01 |
| Coronary artery disease, n (%) | 116 (20) | 107 (20.2) | 9 (18) | 0.716 | 103 (20.6) | 13 (15.9) | 0.315 |
| Diabetes, n (%) | 189 (32.5) | 167 (31.5) | 22 (44) | 0.07 | 153 (30.7) | 36 (43.9) | 0.018 |
| Hypertension, n (%) | 302 (52) | 271 (51) | 31 (62) | 0.138 | 260 (52.1) | 42 (51.2) | 0.882 |
| Time from stroke onset to IVT infusion (min), median (IQR) | 180 (141-230) | 180 (141-230) | 177.5 (150-225) | 0.992 | 179 (138-227) | 203 (153.75-236.25) | 0.109 |
| Prior stroke, n (%) | 88 (15.1) | 81 (15.3) | 7 (14) | 0.813 | 75 (15) | 13 (15.9) | 0.847 |
| Systolic blood pressure, mmHg, median (IQR) | 154 (138-165) | 154 (138-165) | 156.5 (144.25-167.25) | 0.271 | 154 (138-165) | 154 (139.5-165) | 0.910 |
| Diastolic blood pressure, mmHg, median (IQR) | 89 (80-98) | 89 (80-98) | 89.5 (80-98) | 0.988 | 89 (80-98) | 89 (82-98) | 0.390 |
| Antihypertensive therapy, n (%) | 200 (34.4) | 183 (34.5) | 17 (34) | 0.947 | 172 (34.5) | 28 (34.1) | 0.955 |
| Antiplatelet therapy, n (%) | 73 (12.6) | 62 (11.7) | 11 (22) | 0.035 | 56 (11.2) | 17 (20.7) | 0.016 |
| Hypoglycemic therapy, n (%) | 102 (17.6) | 89 (16.8) | 13 (26) | 0.101 | 83 (16.6) | 19 (23.2) | 0.149 |
| Baseline blood glucose, mmol/L, median (IQR) | 6.92 (6.13-8.65) | 6.92 (6.14-8.65) | 7.03 (5.97-9.06) | 0.815 | 6.92 (6.09-8.62) | 6.91 (6.18-9.56) | 0.444 |
| Baseline NIHSS score, median (IQR) | 9 (5-13) | 9 (5-13) | 6 (3-10) | <0.01 | 8 (5-12) | 12 (8-15) | <0.01 |
| Baseline neutrophil (109/L), median (IQR) | 5.41 (3.98-7.44) | 5.38 (3.95-7.38) | 5.65 (4.33-7.74) | 0.194 | 5.38 (3.94-7.17) | 6.17 (4.22-8.39) | 0.038 |
| Baseline PLT (109/L), median (IQR) | 200 (170-231) | 198 (168-230) | 213 (191.25-243.25) | 0.025 | 201 (170-231) | 192.5 (169.5-234) | 0.532 |
| Baseline PNR, median (IQR) | 36.71 (26.04-51.29) | 36.7 (26.09-51.46) | 37.73 (24.41-51.33) | 0.949 | 37.19 (26.59-52.61) | 32.47 (23.80-42.32) | 0.011 |
| Post-IVT neutrophil (109/L), median (IQR) | 5.39 (4.23-7.26) | 5.29 (4.16-7.07) | 7.17 (5.20-9.84) | <0.01 | 5.27 (4.13-7.06) | 6.3 (4.84-8.90) | <0.01 |
| Post-IVT PLT (109/L), median (IQR) | 205 (173-237) | 204 (172-237) | 219.5 (191-249.25) | 0.068 | 205 (173-238) | 204.5 (174.5-237.25) | 0.86 |
| Post-IVT PNR, median (IQR) | 37.76 (27.16-49.33) | 38.06 (27.88-50.11) | 29.92 (22.14-38.13) | 0.001 | 38.64 (28.42-50.93) | 30.73 (23.14-41.57) | <0.01 |
Abbreviations: END, Early Neurologic Deterioration; HT, Hemorrhagic Transformation; NIHSS, National Institute of Health Stroke Scale; PLT, Platelet; PNR, Platelet-to-Neutrophil Ratio; IVT, Intravenous Thrombolysis.
Table 3.
Clinical characteristics of patients according to the presence/absence of DND and 3 months outcome after IVT treatment.
| Variables |
Total
(n = 581) |
No DND
(n = 556) |
DND
(n = 25) |
P | Good 3-month outcome (n = 391) | Poor 3-month outcome (n = 190) | P |
|---|---|---|---|---|---|---|---|
| Age, years, median (IQR) | 61 (53-69) | 61(53-69) | 65 (52-72.5) | 0.349 | 60 (52-68) | 63 (54-71) | 0.007 |
| Sex (male), n (%) | 423 (72.8) | 406 (73) | 17 (68) | 0.581 | 283 (72.4) | 140 (73.7) | 0.74 |
| Current smoking, n (%) | 326 (56.1) | 313 (56.3) | 13 (52) | 0.672 | 226 (57.8) | 100 (52.6) | 0.239 |
| Current drinking, n (%) | 253 (43.5) | 245 (44.1) | 8 (32) | 0.234 | 171 (43.7) | 82 (43.2) | 0.895 |
| Atrial fibrillation, n (%) | 27 (4.6) | 24 (4.3) | 3 (12) | 0.074 | 20 (5.1) | 7 (3.7) | 0.442 |
| Coronary artery disease, n (%) |
116 (20) | 110 (19.8) | 6 (24) | 0.606 | 75 (19.2) | 41 (21.6) | 0.498 |
| Diabetes, n (%) | 189 (32.5) | 175 (31.5) | 14 (56) | 0.01 | 117 (29.9) | 72 (37.9) | 0.054 |
| Hypertension, n (%) | 302 (52) | 283 (50.9) | 19 (76) | 0.014 | 190 (48.6) | 112 (58.9) | 0.019 |
| Time from stroke onset to IVT infusion (min), median (IQR) | 180 (141-230) | 180 (141-228.75) | 175 (146.5-239) | 0.674 | 182 (144-231) | 175 (134.75-230) | 0.138 |
| Prior stroke, n (%) | 88 (15.1) | 84 (15.1) | 4 (16) | 0.903 | 52 (13.3) | 36 (18.9) | 0.075 |
| Systolic blood pressure, mmHg, median (IQR) | 154 (138-165) | 154 (138-165) | 160 (144-167) | 0.589 | 152 (138-164) | 157 (142-169) | 0.009 |
| Diastolic blood pressure, mmHg, median (IQR) | 89 (80-98) | 89 (80-98) | 92 (82.5-99.5) | 0.649 | 89 (80-98) | 89 (81-98) | 0.599 |
| Antihypertensive therapy, n (%) |
200 (34.4) | 188 (33.8) | 12 (48) | 0.144 | 124 (31.7) | 76 (40) | 0.049 |
| Antiplatelet therapy, n (%) | 73 (12.6) | 68 (12.2) | 5 (20) | 0.252 | 45 (11.5) | 28 (14.7) | 0.271 |
| Hypoglycemic therapy, n (%) | 102 (17.6) | 95 (17.1) | 7 (28) | 0.161 | 65 (16.6) | 37 (19.5) | 0.397 |
| Baseline blood glucose, mmol/L, median (IQR) | 6.92 (6.13-8.65) | 6.88 (6.08-8.64) | 7.27 (6.6-9.37) | 0.166 | 6.8 (6.02-8.62) | 7.12 (6.28-8.72) | 0.154 |
| Baseline NIHSS score, median (IQR) |
9 (5-13) | 9 (5-12) | 14 (9-19) | 0.002 | 8 (4-11) | 12 (8-15) | <0.01 |
| Baseline neutrophil (109/L), median (IQR) | 5.41 (3.98-7.44) | 5.38 (3.96-7.27) | 8.09 (4.10-10.76) | 0.056 | 5.25 (3.79-7.15) | 5.67 (4.27-8.04) | 0.016 |
| Baseline PLT (109/L), median (IQR) |
200 (170-231) | 200.5 (170-231) | 195 (168-232.5) | 0.968 | 197 (170-230) | 203 (170-236) | 0.260 |
| Baseline PNR, median (IQR) | 36.71 (26.04-51.29) | 36.82 (26.29-51.37) | 30.77 (17.68-51.04) | 0.118 | 37 (26.57-52.49) | 36.33 (23.96-48.28) | 0.169 |
| Post-IVT neutrophil (109/L), median (IQR) | 5.39 (4.23-7.26) | 5.31 (4.20-7.12) | 9.86 (5.60-14.38) | <0.01 | 4.88 (3.87-6.16) | 6.83 (5.29-9.55) | <0.01 |
| Post-IVT PLT (109/L), median (IQR) |
205 (173-237) | 205 (173.25-238) | 204 (164.5-228.5) | 0.701 | 204 (172-237) | 208.5 (179-238.75) | 0.249 |
| Post-IVT PNR, median (IQR) | 37.76 (27.16-49.33) | 37.81 (28.01-49.51) | 17.18 (13.33-38.58200) | <0.01 | 41.67 (30.97-52.14) | 28.92 (20.89-40.64) | <0.01 |
Abbreviations: DND, Delayed Neurological Deterioration; NIHSS, National Institute of Health Stroke Scale; PLT, Platelet; PNR, Platelet-to-Neutrophil Ratio; IVT, Intravenous Thrombolysis.
3.3. Logistic Regression Analyses
Logistic regression analyses were used for exploring the association between PNR before/after thrombolysis and at 4 different endpoints. All risk factors were introduced into the multivariate logistic regression analysis. The results of the multivariate logistic regression analyses are detailed in Table 4. The value of post-IVT PNR can predict hemorrhagic transformation (OR = 0.974; 95%CI = 0.956-0.992; P = 0.006), early neurological deterioration (OR = 0.939; 95%CI = 0.913-0.966; P = 0.01), delayed neurological deterioration (OR = 0.949; 95%CI = 0.912-0.988; P = 0.011), and poor 3-month outcome (OR = 0.962; 95%CI = 0.948-0.976; P<0.01). However, baseline PNR value had no association with any of the 4 endpoints.
Table 4.
Multivariate logistic regression analysis of the relationship between PNR and four outcome events.
| Dependent Variables | Baseline Data | Post-IVT Data | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | Pa | OR | 95% CI | Pa | |
| END | 0.999 | 0.990-1.008 | 0.775 | 0.939 | 0.913-0.966 | <0.01 |
| HT | 1.000 | 1.000-1.000 | 0.367 | 0.974 | 0.956-0.992 | 0.006 |
| DND | 0.993 | 0.966-1.022 | 0.647 | 0.949 | 0.912-0.988 | 0.011 |
| Poor 3-month outcome | 1.000 | 1.000-1.000 | 0.797 | 0.962 | 0.949-0.976 | <0.01 |
aAdjusted for age, sex, current smoking, current drinking, hypertension, diabetes, atrial fibrillation, prior stroke, time from stroke onset to r-tPA infusion, coronary artery disease, systolic blood pressure, diastolic blood pressure, antihypertensive therapy, antiplatelet therapy, antiplatelet therapy, hypoglycemic therapy, baseline blood glucose, and NIHSS score at baseline.
3.4. Receiver Operating Curve Analyses
Receiver operating curve (ROC) analysis results are detailed in Table 5. According to the results, post-IVT PNR has a higher accuracy for prognosis compared with baseline PNR (all p>0.05). Area under curve (AUC) for early neurological deterioration, hemorrhagic transformation, delayed neurological deterioration, and poor 3-month outcome were 0.647 (95% CI = 0.572-0.723, p<0.01), 0.647 (95% CI = 0.584-0.710, p = 0.01), 0.762 (95% CI = 0.637-0.887, p<0.01), and 0.705 (95% CI = 0.660-0.751, p<0.01), respectively.
Table 5.
Diagnostic values of the PNR for four outcome events.
| - | Outcome Events | Threshold | AUC | 95% CI | Sensitivity, % | Specificity, % | P |
|---|---|---|---|---|---|---|---|
| Baseline PNR | END | 33.1 | 0.503 | 0.421-0.584 | 72.0% | 42.7% | 0.949 |
| HT | 46.4 | 0.587 | 0.525-0.649 | 34.1% | 86.6% | 0.011 | |
| DND | 22.2 | 0.592 | 0.461-0.724 | 86.0% | 60.0% | 0.118 | |
| Poor 3-month outcome | 49.6 | 0.535 | 0.485-0.585 | 29.9% | 78.4% | 0.169 | |
| Post-IVT PNR | END | 37.9 | 0.647 | 0.572-0.723 | 50.8% | 24.0% | 0.010 |
| HT | 35.6 | 0.647 | 0.584-0.710 | 59.7% | 30.5% | <0.001 | |
| DND | 26.8 | 0.762 | 0.637-0.887 | 79.3% | 72.0% | <0.001 | |
| Poor 3-month outcome | 30.8 | 0.705 | 0.660-0.751 | 76.0% | 55.8% | <0.001 |
Abbreviations: PNR, Platelet-to-Neutrophil Ratio; IVT, Intravenous Thrombolysis; END, Early Neurologic Deterioration; HT, Hemorrhagic Transformation; DND, Delayed Neurological Deterioration.
According to the ROC curves, we calculated the best cut-off values of post-IVT PNR as 37.9(sensitivity 50 8%, specificity 24%) for early neurological deterioration, 35.6 (sensitivity 59.7%, specificity 30.5%) for hemorrhagic transformation, 26.8 (sensitivity 79.3%, specificity 72%) for delayed neurological deterioration, and 30.8(sensitivity 76%, specificity 55.8%) for poor 3-month outcome. For each endpoint, the post-IVT PNR level was identified as high (at the cut-off value or above) or low (below the cut-off value), and the patients were divided into 2 groups by each cut-off value of post-IVT PNR, respectively. The comparison of endpoints between patients at high and low levels for platelet-to-neutrophil ratio (PNR) is presented in Fig. (2). The results showed that patients of lower post-IVT PNR level tended to have higher percentage of worse outcomes.
Fig. (2).
Comparison of endpoints between patients at high and low levels for platelet-to-neutrophil ratio (PNR).
Abbreviations: END, early neurologic deterioration; HT, hemorrhagic transformation; DND, delayed neurological deterioration. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
PNR level was identified as high (at the cut-off value or above) or low (below the cut-off value) according to receiver operating curve (ROC) analyses on each endpoint. The results showed that patients of lower post-IVT PNR level tended to have higher percentage of worse outcomes.
4. Discussion
In this study, we found that 24-h post IVT PNR was associated with early neurological deterioration, hemorrhagic transformation, delayed neurological deterioration, and poor 3-month outcome. Lower post-IVT PNR level could predict worse outcomes. Nevertheless, pre-PNR before IVT was not independently associated with any of the 4 outcome measures. Our results indicated that the post-IVT PNR might become a new predictor of the prognosis of AIS patients who accepted IVT treatment.
As a new parameter put forward recently, the research of PNR in the stroke field is still rare. The study of Jin et al. [13] indicated that PNR might be an autocephaly protective predictor for 90-days outcome in AIS patients. They also suggested that higher PNR value might predict better outcome. In addition, several studies found PNR was correlated with thrombosis [14]. For example, Long et al. [15] proposed that PNR might be an indicator of blood hyperco-agulable state, and an increased PNR level may induce a gastric cancer-related ischemic stroke. However, the relationship between PNR and the prognosis of IVT patients has not been explored.
As is well-known, platelets are the primary cells in the process of thrombosis. When stroke happens, platelets are activated and adhered to the injured site of vessel wall Inflammation cells can also be recruited by platelet granules and interact with activated platelets, leading to vascular inflammation and further resulting in brain tissue damage [7, 8, 10, 11]. Neutrophils are the first responders among all the inflammation cells to injury site in initial few minutes to hours [16]. During the process of migrating across cerebral endothelium, neutrophils also released kinds of proteases and ROS, which contribute to the BBB disruption after ischemic stroke [10]. To date, numerous researches have revealed that the interaction between platelet and neutrocyte amplifies the inflammatory response of thrombosis. Intravenous thrombolysis (IVT) treatment would cause blood cells changed in count and percentage, resulting in platelet-to-neutrophil ratio (PNR) value changing.
Platelet-to-neutrophil ratio (PNR) is an index, which reflects a balance of platelet and neutrophil, connecting the process of thrombosis and inflammation. Patients of severe acute ischemic stroke may present a state of peripheral blood platelet count decrease because of platelet persistent activation and finally exhaustion [17]. A lower circulating platelet count may represent a more severe infarction and the occurrence of hemorrhagic transformation or other dangerous events. Both the decrease in platelet resulting from thrombosis and the rising of neutrophil due to inflammation can lead to a decrease in PNR. In addition, intravenous thrombolysis (IVT) treatment can also influence PNR value by changing platelets or neutrophils. Some researchers found that rtPA not only converts plasminogen to plasmin, which degrades fibrin in thrombus and forms soluble fibrin degradation products, but also aggravates platelet activation and aggregation [18, 19]. According to Gensicke et al. [20], decreasing platelet counts are associated with the occurrence of hemorrhagic transformation in IVT-treated stroke patients. Moreover, according to Maestrini et al. [21] higher neutrophil counts after IVT therapy are associated with worse outcomes. And they assessed the potential mechanism for explaining the relationship between poor outcome and neutrophil is the disruption of the blood-brain barrier by releasing MMP-9 and increased reactive oxygen and nitrogen species [21]. All the above studies suggested that post-IVT PNR, rather than the value before thrombolysis treatment, could be a potential predictor for the prognosis of patients.
According to our study, the PNR value after rtPA thrombolysis, but not that before rtPA infusion, was prospectively associated with both short-term outcome and long-term outcome, which suggested the underlying effect of rtPA treatment on platelet and neutrophil.
Compared with other studies, our research has the following strengths: All blood samples were taken both before and after thrombolysis in 24 h. Our data reflected a dynamic change of PNR value in IVT patients. And based on a large sample size, our results became more reliable and convin-cing. This study also, for the first time indicated the value of PNR in predicting the prognosis of IVT. PNR may become a predictive factor in future studies. There are also several limitations to this study. First, the study had all of the common drawbacks of retrospective studies, and a further confounder may exist; Second, all data were collected only in one hospital, and it might lead to selection bias. In addition, infection and other diseases that may affect inflammation that occurred during treatment were not taken into consideration.
Conclusion
We found that PNR after thrombolysis therapy was independently associated with early neurological deterioration, hemorrhagic transformation, delayed neurological deterioration, and poor 3-month outcome (mRS>2). In addition, lower PNR could predict a worse outcome. This finding could help neurologists predict and improve stroke outcomes after rtPA treatment in clinical settings. The exact mechanisms need further study.
Acknowledgements
The authors thank the patients and their families and appreciate the study participants for their assistance in this study.
Funding Statement
This work was supported by the National Natural Science Foundation of China (Grant no. 81771243), the Program for JLUSTIRT (Grant no. 2017TD-12), and the Jilin Provincial Key Laboratory (Grant no. 20190901005JC) to Yi Yang.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study protocol has been approved by the Ethics Committee of the First Hospital of Jilin University, China.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
Availability of Data and Materials
The authors confirm that the data supporting the findings of this study are available within the article.
FUNDING
This work was supported by the National Natural Science Foundation of China (Grant no. 81771243), the Program for JLUSTIRT (Grant no. 2017TD-12), and the Jilin Provincial Key Laboratory (Grant no. 20190901005JC) to Yi Yang.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Donnan G.A., Fisher M., Macleod M., Davis S.M. Stroke. Lancet. 2008;371(9624):1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 2.Meschia J.F., Brott T. Ischaemic stroke. Eur. J. Neurol. 2018;25(1):35–40. doi: 10.1111/ene.13409. [DOI] [PubMed] [Google Scholar]
- 3.Ho W.M., Reis C., Akyol O., et al. Pharmacological management options to prevent and reduce ischemic hemorrhagic transformation. Curr. Drug Targets. 2017;18(12):1441–1459. doi: 10.2174/1389450117666160818115850. [DOI] [PubMed] [Google Scholar]
- 4.Andrews R.K., López J.A., Berndt M.C. Molecular mechanisms of platelet adhesion and activation. Int. J. Biochem. Cell Biol. 1997;29(1):91–105. doi: 10.1016/S1357-2725(96)00122-7. [DOI] [PubMed] [Google Scholar]
- 5.Gremmel T., Frelinger A.L., III, Michelson A.D. Platelet physiology. Semin. Thromb. Hemost. 2016;42(3):191–204. doi: 10.1055/s-0035-1564835. [DOI] [PubMed] [Google Scholar]
- 6.Holinstat M. Normal platelet function. Cancer Metastasis Rev. 2017;36(2):195–198. doi: 10.1007/s10555-017-9677-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diacovo T.G., Roth S.J., Buccola J.M., Bainton D.F., Springer T.A. Neutrophil rolling, arrest, and transmigration across activated, surface-adherent platelets via sequential action of P-selectin and the beta 2-integrin CD11b/CD18. Blood. 1996;88(1):146–157. doi: 10.1182/blood.V88.1.146.146. [DOI] [PubMed] [Google Scholar]
- 8.Hagberg I.A., Roald H.E., Lyberg T. Adhesion of leukocytes to growing arterial thrombi. Thromb. Haemost. 1998;80(5):852–858. [PubMed] [Google Scholar]
- 9.Segel G.B., Halterman M.W., Lichtman M.A. The paradox of the neutrophil’s role in tissue injury. J. Leukoc. Biol. 2011;89(3):359–372. doi: 10.1189/jlb.0910538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolaczkowska E., Kubes P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013;13(3):159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 11.Jickling G.C., Liu D., Ander B.P., Stamova B., Zhan X., Sharp F.R. Targeting neutrophils in ischemic stroke: Translational insights from experimental studies. J. Cereb. Blood Flow Metab. 2015;35(6):888–901. doi: 10.1038/jcbfm.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bednarska K., Król E., Głowacka E., et al. Analysis of preoperative blood platelet parameters in terms of diversity of epithelial ovarian cancer. Medicine (Baltimore) 2018;97(12):e0180–e80. doi: 10.1097/MD.0000000000010180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin P., Li X., Chen J., et al. Platelet-to-neutrophil ratio is a prognostic marker for 90-days outcome in acute ischemic stroke. J. Clin. Neurosci. 2019;63:110–115. doi: 10.1016/j.jocn.2019.01.028. [DOI] [PubMed] [Google Scholar]
- 14.Ren B., Duan M., Liu Z., et al. fibrinogen, neutrophil-to-lymphocyte rate and platelet-to-neutrophil rate as novel acute phase indicators in patients with thromboangiitis obliterans. Ann. Vasc. Surg. 2019;65:137–144. doi: 10.1016/j.avsg.2019.11.020. [DOI] [PubMed] [Google Scholar]
- 15.Long H., Qin K., Chen J., et al. Biomarkers of gastric cancer-related ischemic stroke and its underlying pathogenesis. Medicine (Baltimore) 2018;97(17):e0493–e93. doi: 10.1097/MD.0000000000010493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perez-de-Puig I., Miró-Mur F., Ferrer-Ferrer M., et al. Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol. 2015;129(2):239–257. doi: 10.1007/s00401-014-1381-0. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y., Xiao Y., Lin Z., et al. the role of circulating platelets microparticles and platelet parameters in acute ischemic stroke patients. J. Stroke Cerebrovasc. Dis. 2015;24(10):2313–2320. doi: 10.1016/j.jstrokecerebrovasdis.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubenstein M.H., Finn A.V., Leinbach R.C., et al. Short-term intravenous eptifibatide infusion combined with reduced dose recombinant tissue plasminogen activator inhibits platelet recruitment at sites of coronary artery injury. J. Am. Coll. Cardiol. 2004;43(2):287–294. doi: 10.1016/j.jacc.2003.08.039. [DOI] [PubMed] [Google Scholar]
- 19.Matosevic B., Knoflach M., Werner P., et al. Fibrinogen degradation coagulopathy and bleeding complications after stroke thrombolysis. Neurology. 2013;80(13):1216–1224. doi: 10.1212/WNL.0b013e3182897015. [DOI] [PubMed] [Google Scholar]
- 20.Gensicke H., Al Sultan A.S., Strbian D., et al. Thrombolysis in Stroke Patients (TRISP) Collaborators Intravenous thrombolysis and platelet count. Neurology. 2018;90(8):e690–e697. doi: 10.1212/WNL.0000000000004982. [DOI] [PubMed] [Google Scholar]
- 21.Maestrini I., Strbian D., Gautier S., et al. Higher neutrophil counts before thrombolysis for cerebral ischemia predict worse outcomes. Neurology. 2015;85(16):1408–1416. doi: 10.1212/WNL.0000000000002029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.