Abstract
Peptide receptor radionuclide therapy (PRRT) is a highly effective anti-cancer treatment modality for patients with non-resectable, metastasized neuroendocrine tumors (NETs). During PRRT, specific receptors that are overexpressed on the cancer cells are targeted with a peptide labeled with a DNA-damaging radionuclide. Even though PRRT is a powerful treatment for metastasized NET patients, the majority still cannot be cured at this stage of the disease. Hence, many investigators focus on improving the therapeutic efficacy of this therapy. Improving PRRT can, for example, be achieved by using other radionuclides with different physical properties, by combining PRRT with radiosensitizing agents or by radiolabeling peptides with different characteristics. However, due to lack of extensive knowledge of radiobiological responses of cancer cells to PRRT, biological parameters that influence absorbed dose or that might even elicit insensitivity to therapy remain elusive and the context in which these improvements will be successful warrants further investigation. In this review, we will discuss the development of PRRT, its clinical merits in current treatment and future perspectives. We will highlight different radionuclides and their benefits and pitfalls, as well as different peptide-conjugates that hold these radionuclides. We will zoom in on the latest developments regarding combinatorial treatments and how investigators from different disciplines such as dosimetry and radiobiology are now joining forces to improve PRRT for NETs.
Keywords: Peptide receptor radionuclide therapy (PRRT), Combination therapy, Neuroendocrine tumors (NET), Radiopharmaceuticals, Radiobiology, Therapeutic effects
1. INTRODUCTION
Resection can be an effective strategy for patients with localized cancer; however, its feasibility becomes limited in patients with metastasized disease. A large group of patients with neuroendocrine tumors (NETs) will present with metastases at the time of diagnosis, making NETs – although classified as relatively rare – a very lethal type of cancer [1]. NETs are a heterogeneous group of cancers that can arise from different organs, with different phenotypes in terms of differentiation status, pathological features and proliferative index [2, 3]. Despite the large variations between NETs, there are also features that are commonly found among these tumors, which are even described as reliable biomarkers, such as high protein expression levels of neural cell adhesion molecule (N-CAM) [4], chromogranin A (CgA) [5], synaptophysin [6] and the membrane-protein somatostatin receptor subtype 2 (SST2) [7]. SST2 is an exploitable target for anti-NET treatments with e.g. the somatostatin analogues (SSAs) octreotide or lanreotide, which bind the receptors with high affinity and specificity [8]. Labeling of SSAs with radionuclides such as indium-111 or later on with gallium-68 provided a powerful method for the detection of NETs [9, 10]. By labeling these SSAs with radionuclides that damage the DNA of the recipient cells, the imaging modality was transformed into a therapy: peptide receptor radionuclide therapy (PRRT). This is a marvelous example of ‘theranostics’, depicting the combination of therapy and diagnostics. Here, coupling a radionuclide that can be used for both imaging and therapy to a peptide enables the visualization and treatment of tumors simultaneously and allows tracking of the PRRT (Fig. 1) [11].
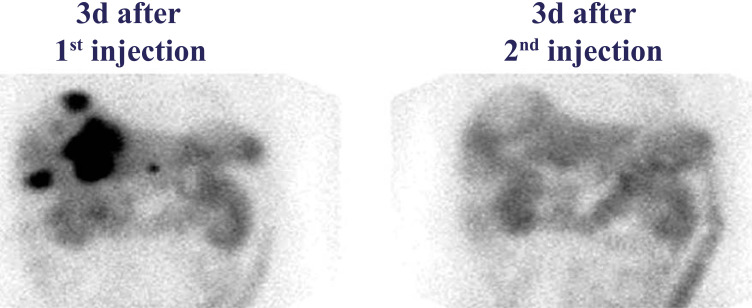
Fig. (1).
SPECT images of liver metastases in a patient 3 days post injection of [177Lu]Lu-DOTA-TATE. Black signal depicts the SST2-positive tumor lesions 3 days after the first injection (left panel), but not after the second injection (right panel). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
A successful imaging method that was developed for the detection of NETs is called OctreoScan, which uses diethylenetriaminepentaacetic acid-d-phenylalanine (DTPA)-octreotide radiolabeled with indium-111 (indium-111-DTPA-D-Phe1-octreotide) for single-photon emission computed tomography (SPECT) of NETs [10]. Although, originally used for diagnostics, investigators discovered that high doses of indium-111-DTPA-D-Phe1-octreotide could also be used therapeutically and on administering, symptom relief and disease stabilization were shown in some patients [12-14]. Although PRRT with indium-111-DTPA-D-Phe1-octreotide had a positive effect on the quality of life, overall a relatively modest response was seen in certain patients, urging the improvement of PRRT [15].
PRRT has undergone many improvements over the years due to the availability of different chelators, peptides, and radionuclides. PRRT using [177Lu]Lu-1,4,7,10-tetraazacyclo-dodecane-1,4,7,10-tetraacetic acid (DOTA),[Tyr3]-octreotate ([177Lu]Lu-DOTA-TATE) was successfully tested in the phase 3 multi-center trial NETTER-1 [16]. In this trial, a significantly higher response rate and longer progression-free survival were shown, along with mitigating adverse effects, compared to the standard of care long-acting repeatable (LAR) octreotide administrations.
In late 2017 and the beginning of 2018, [177Lu]Lu-DOTA-TATE under the name of ‘Lutathera’ was approved by the European Medicines Agency (EMA) and Food and Drug Administration (FDA) respectively, for the treatment of SST2-positive gastroenteropancreatic (GEP) NETs in adults. Even though [177Lu]Lu-DOTA-TATE is successful in the treatment of GEP-NETs in terms of survival benefits, the majority of the patients will ultimately relapse, showing there is still much to gain in terms of improvement of PRRT efficacy. Therefore, the possibilities in the field of PRRT for NETs are under widespread and active investigation.
2. IMPROVING PEPTIDE RECEPTOR RADIONUCLIDE THERAPY
2.1. Radionuclides
For therapy of NETs, different types of radionuclides that can induce DNA damage during radioactive decay and which lead to cancer cell death can be used. These radionuclides have specific physical properties and can, therefore, induce different therapeutic effects (Table 1). There is a large variation in the range between the different radiation qualities: 0.05-12 mm for β--particles, 40-100 µm for α-particles, Auger electrons have a short range of <0.5 µm, while γ-irradiation can easily pass through the human body [17]. The short range of α-particles and Auger electrons results in high linear energy transfer (LET) of 50-230 keV/µm and 4-26 keV/µm, respectively, while β--particles have a LET of 0.1-1.0 keV/µm [18, 19].
Table 1.
Different radionuclides used in the imaging or therapy of NETs and their basic characteristics.
| Radionuclide | Decay | Half-life | Energy (max) |
Range
(max) |
Application |
|---|---|---|---|---|---|
| 111In | γ, Auger-electron |
2.8 days | 245 KeV 19 KeV |
500 µm | Imaging/Therapy |
| 177Lu | β-, γ | 6.7 days | 498 KeV | 1.7 mm | Imaging/Therapy |
| 90Y | β-, γ | 64.2 hours | 2.284 MeV | 11 mm | Therapy |
| 213Bi | α/β- | 46 minutes | 6-8 MeV | 84 µm | Therapy |
| 225Ac | α/β- | 9.9 days | 6-8 MeV | 61 µm | Therapy |
Among therapeutic radionuclides for PRRT in the treatment of NETs, the most prominent and the only clinically FDA and EMA approved is lutetium-177 in [177Lu]Lu-DOTA-TATE. Lutetium-177 has a half-life of 6.7 days and during its decay, β--particles with a maximum energy of 498 keV and low-energy γ photons (208 keV and 113 keV) are emitted [20, 21]. The β--particles can penetrate soft tissue up to 1.7 mm and thus induce DNA damage locally upon binding at the tumor site [22]. Due to the physical properties of lutetium-177, patients can be treated while the tumor response can be monitored simultaneously by SPECT.
For application in the treatment of NETs, also other radionuclides have shown therapeutic potential. Comparative (pre)clinical studies have presented promising results for different β--emitters, especially yttrium-90 [23-26]. Researchers have shown that yttrium-90 coupled to DOTA-TATE also elicits a significant clinical and radiological response in NET patients [27]. Yttrium-90 has a half-life of 64.2 hours and emits β--particles with a maximum energy of 2.27 MeV, which can penetrate soft tissue to a depth of around 11 mm [28, 29]. Its particles have a longer range compared to lutetium-177, and therefore it has been hypothesized that PRRT using [90Y]Y-[DOTA-Tyr3]octreotide (DOTA-TOC) or [90Y]Y-DOTA-TATE is more suited for bigger lesions, while [177Lu]Lu-DOTA-TATE treatment is preferred for smaller lesions [30]. However, it has also been shown that yttrium-90-labeled peptides had a higher chance of inducing high-grade renal toxicity compared to lutetium-177-labeled peptides, especially in patients with risk factors relevant for creatinine clearance loss [31, 32]. Nevertheless, to limit renal toxicity, a cocktail of lysine and arginine can be administered to patients after which potential toxicity can be monitored, increasing the safety of the radiopeptide [33-34]. So far, maybe more promising is the combination of yttrium-90 and lutetium-177 for the treatment of NETs. There is compelling evidence that administering [177Lu]Lu- and [90Y]Y-DOTA-TATE either in one cycle or sequentially has benefits for overall survival compared to monotherapies of the two [35, 36]. Moreover, no severe additional adverse effects have been shown, rendering such combination therapies a feasible option [37, 38]. However, the efficacy will have to be determined using randomized studies.
Next to β--emitters also radionuclides that emit α-particles are gaining interest for the treatment of NETs [19, 39]. Principal examples of α-emitters that have been (pre)clinically tested for NET treatment are actinium-225 and bismuth-213. Bismuth-213 is a short-lived radiometal with a half-life of 45.6 minutes, mean energy of 8.32 MeV and a range of 84 µm [19]. The therapeutic efficacy of bismuth-213 coupled to DOTA-TATE was found to be superior compared to [177Lu]Lu-DOTA-TATE in different cancer cell lines [40, 41]. In SST2-positive cancer in vivo models, it was shown that PRRT using bismuth-213 labeled DOTA-TATE had a clear anti-tumor benefit, although this was not directly compared to lutetium-177 [42]. However, in practice, it can be logistically difficult to treat patients with bismuth-213 labeled compounds because there is limited time from radiolabeling to administration due to its relatively short half-life. Therefore, other α-particle emitting radionuclides are being explored for potential applicability in PRRT. Promising preclinical results have been observed for actinium-225, which has a half-life of 9.9 days, mean energy of 6.83 MeV and an average range of 61 µm in soft-tissues [39, 43, 44]. Actinium-225 emits multiple subsequent α-particles, one of which is bismuth-213, therefore inducing a high level of localized damage. In both in vitro and in vivo experiments, it was shown that actinium-225 labeled DOTA-TOC can more effectively kill neuroendocrine cancer cells compared to lutetium-177 [45]. Overall, α-emitting radionuclides show great promise when it comes to improving therapeutic outcomes for patients. This is corroborated by preliminary data from a clinical study where DOTA-TATE was labeled with actinium-225, which led to a strong anti-tumor response in NET patients [46]. Also, clinical studies focusing on other malignancies have reported the effectiveness of targeted alpha therapy, as with certain doses, no severe toxicity was reported in these studies with strong anti-cancer effectiveness [47-49]. In a report of a clinical study where PRRT with lead-212, whose daughter nuclide is an α-emitter, in NETs was used and a favorable safety profile was also observed [50]. In contrast, other studies have shown severe side effects depending on the dose and target molecule of treatment, as was reported for the salivary glands in the prostate-specific membrane antigen (PSMA)-targeted treatment for prostate cancer [51, 52]. Moreover, due to recoil energy during α-particle, decay radionuclides can be released from the chelator freeing daughter atoms which can accumulate in organs and might translate in increased radiotoxicity [53]. Therefore caution in the use of α-emitters is important, as of yet no long-term follow up studies have been performed to assess safety and toxicity of PRRT using α-emitters.
For the treatment of NET patients, the use of α-emitters or combinations with different radionuclides, although feasible and promising, still remains to be proven effective and safe. What PRRT regimen of radionuclides and in what order or combination this will be most efficacious for NET treatment warrants further investigation.
2.2. Different Chelators and Peptides
A SST2-targeting peptide consists of a SST2 binding domain, a linker, and a radionuclide labeling domain, named a chelator. For different radionuclides, certain chelators are required to ensure the stability of the PRRT compound, because if the compound is unstable, delivering radioactivity to the target will be less efficient. On this subject, much has been described already and more information about matching chelators to radionuclides can be found in a published review article [54]. In the case of lutetium-177 or yttrium-90, the isotope is most often bound in a bifunctional DOTA chelator, which in turn will link the radiometal to a targeting peptide such as Tyr3-octreotate [55]. DOTA complexes are currently the chelator of choice for many radionuclides, as DOTA-based chelators have a high thermostability and biological inertness. However, for some radionuclides such as copper-64 or lead-212, which have shown promising possibilities for imaging and therapy of NETs respectively, DOTA for radiolabeling has sub-optimal characteristics. Here, the chelator of choice would be a derivative of DOTA such as 1-oxa-4,7,10-triazacyclododecane-S-5-(4-nitrobenzyl)-4,7,10-triaceticacid (p-NO2-Bn-Oxo) for copper-64 [56] or 1,4,7,10-tetrakis(carbamoylmethyl)-l,4,7,10-tetraazacyclododecane (TCMC) for lead-212 [54]. Finding the optimal chelator can result in better stability and thus more efficient targeting and less adverse effects.
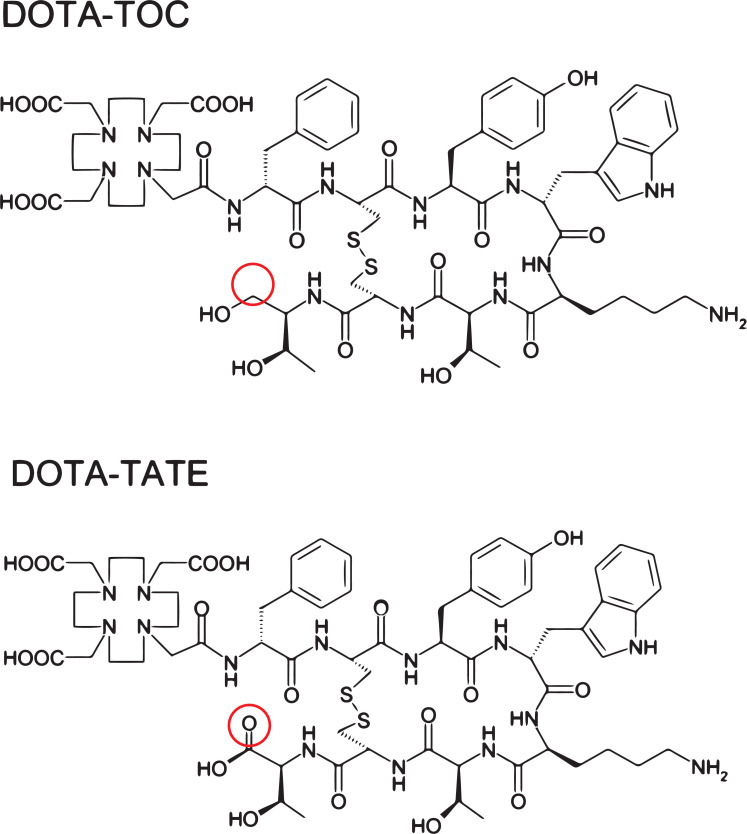
Next to different chelators, the peptides that are used for targeted radionuclide therapy can also vary. In the treatment of NETs, two pivotal somatostatin analogues are used for transporting lutetium-177 to the cancer cells: Tyr3-octreotate (i.e. TATE) or Tyr3-octreotide (i.e. TOC). These are agonistic compounds that get internalized upon binding their target. Researchers observed that despite the relatively small difference in chemical structure between octreotide and octreotate (Fig. 2), the biological effects can differ evidently. A clinical study has reported that the residence time of [177Lu]Lu-DOTA-TATE in NET patients was significantly higher compared to the same patients that received [177Lu]Lu-DOTA-TOC [57]. The longer retention time was also observed in the kidneys and spleen, probably due to the stability of the compound; however, the authors concluded that ultimately the higher tumor dose of [177Lu]Lu-DOTA-TATE would be beneficial and superior over using [177Lu]Lu-DOTA-TOC. It was shown in vitro that coupling TATE or TOC to [111In]In-DTPA alters the binding affinities of the radiopeptide to SST2 [58]. Also, in the same study using SST2-positive tumor xenografted rats, a higher in vivo uptake was measured for [111In]In-DTPA-TATE. Another group also presented biological differences in terms of binding affinity between the two peptides [59]. Here, it was observed that [90Y]Y-DOTA-TOC had a lower affinity to SST2 compared to [90Y]Y-DOTA-TATE. Moreover, in these analyses, it was shown that the change in the binding affinities was dependent on the radiometal, used for labeling. This shows that subtle chemical changes may have pronounced effects on the biodistribution and that these should always be evaluated and characterized preclinically.
Fig. (2).
Chemical structures of DOTA-TOC (upper panel) and DOTA-TATE (lower panel). The structural difference entails a reduction of the C-terminal threonine for octreotide/DOTA-TOC (encircled in red). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Peptides can also be adapted to alter the mechanism of action of a PRRT compound. The somatostatin analogue DOTA-Cpa-c[D-Cys-Aph(Hor)-D-Aph(Cbm)-Lys-Thr-Cys]- D-Tyr-NH2 (DOTA-JR11) binds with high affinity to SST2 and is, in contrast to octreotate and octreotide, a receptor antagonist [60]. Radiolabeled DOTA-JR11 is not internalized upon binding to a cancer cell. It was reported that uptake or binding of [177Lu]Lu-DOTA-JR11 was higher in SST2-positive cells and xenografts compared to [177Lu]Lu-DOTA-TATE and that this corresponded to higher levels of induced DNA damage [61]. Moreover, the higher uptake also led to an improved therapeutic outcome in the mice. In line with this, a clinical pilot study, where tumor and organ doses of [177Lu]Lu-DOTA-JR11 and [177Lu]Lu-DOTA-TATE were compared in the same patients, also showed a higher uptake and tumor retention time for the receptor antagonist compared to the agonist, leading to a higher tumor radiation dose for [177Lu]Lu-DOTA-JR11 [62]. Unfortunately, also a higher uptake level was observed in the normal organs for [177Lu]Lu-DOTA-JR11, yet the tumor-to-background ratio was favorable. However, how efficacious antagonists such as [177Lu]Lu-DOTA-JR11 are will have to be directly compared to [177Lu]Lu-DOTA-TATE in (pre)clinical studies.
Ultimately, the target affinity, peptide-radionuclide stability and thus the total absorbed dose to the tumor and normal organs will greatly depend on PRRT compound design. Whether a more optimal design will be enough to increase therapeutic outcomes for patients remains to be seen, but is indisputably important for reliable patient treatment.
2.3. Radiosensitization
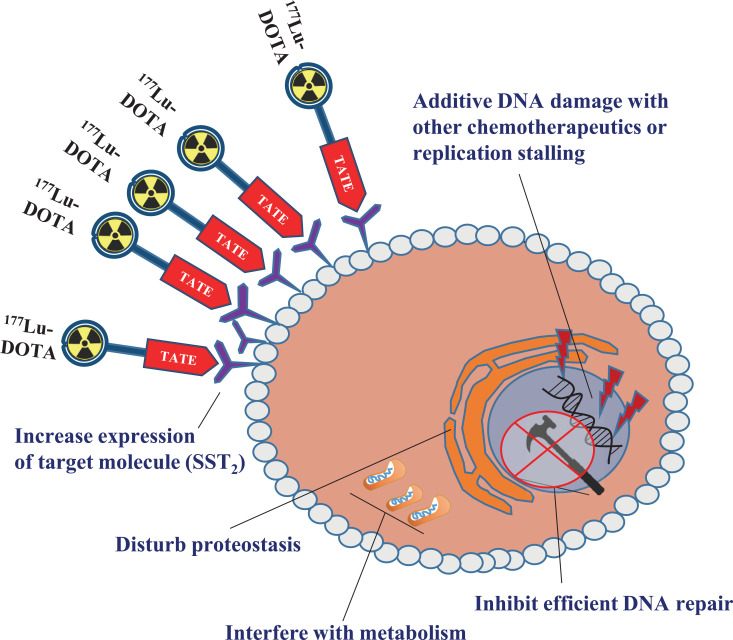
To improve PRRT efficacy, various strategies can be applied, such as a combination of PRRT with other therapies. Currently, more and more studies are showing the potential for different combinatorial regimes [63]. For example, the combination of PRRT with agents that can increase the radiosensitivity of NETs by affecting the DNA damage response (DDR), that can increase target receptor expression via epigenetic regulation, or agents that can inhibit or alter essential cellular processes such as protein folding or metabolism (Fig. 3).
Fig. (3).
Possible modes of radiosensitization in the context of PRRT in NETs. Strategies involving induction of replication stress or direct DNA damage, inhibition of DNA repair, increase of target expression, interference with proteostasis or cellular metabolism are schematically depicted. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
The DDR is activated upon PRRT and consists of various pathways that either drive a cell towards survival or death. DNA damage repair pathway activation is essential for cellular survival and will, therefore, counteract PRRT efficacy. This suggests that inhibition of DNA repair can lead to improvement of PRRT efficacy without increasing the administered radioactive dose. Inhibition of efficient repair of [177Lu]Lu-DOTA-TATE induced DNA single-strand breaks will increase the amount of cytotoxic DNA double-strand breaks (DSBs) when a cell replicates. This concept was proven in patient NET-slices and SST2-positive cancer cells, which showed a synergistic increase in induced DNA damage levels and subsequent cell death when [177Lu]Lu-DOTA-TATE was combined with the Poly (ADP Ribose) Polymerase (PARP) inhibitor olaparib [64]. This was further supported by a second study where different PARP inhibitors showed effective inhibition of PARylation and subsequent synergistic anti-cancer efficacy in two NET cell lines [65]. As other DDR inhibitors have already shown promise in the field of External Beam Radiation Therapy (EBRT), more potential radiosensitizers, such as ataxia telangiectasia mutated- or DNA-dependent protein kinase inhibitors might also show potential in improving PRRT efficacy [66].
Next to enhancing DNA damage by blocking efficient DNA repair, it is also feasible to increase PRRT efficacy by directly increasing the level of DNA damage or by reducing efficient DNA replication via a combination of PRRT with frequently used chemotherapeutics. This might enhance replications stress and induce catastrophic failure in these cells. One study showed that combining [177Lu]Lu-DOTA-TATE with temozolomide, a DNA alkylating agent, increased tumor control in SST2-positive tumor xenografted mice [67]. Furthermore, it was shown that tumor perfusion and thus, the uptake of [177Lu]Lu-DOTA-TATE in temozolomide treated mice was increased compared to control. This indicates that temozolomide might increase PRRT efficacy via two mechanisms: higher uptake of radioactivity and additional DNA damage induction beyond PRRT. Another study using the same xenograft model showed an increase in tumor control and subsequent survival when PRRT was combined with carboplatin, an inducer of intra- and interstrand crosslinks, and etoposide, an inducer of replication stress [68]. The clinical feasibility of adding approaches that induce additional DNA damage or restrict DNA replication was shown in a phase 1-2 study where the combination of [177Lu]Lu-DOTA-TATE with temozolomide and capecitabine, an inhibitor of DNA synthesis, was not only well-tolerated but also seemed to improve the response rate of reported [177Lu]Lu-DOTA-TATE alone, albeit preliminary [69]. Another study from the same group showed that the combination of [177Lu]Lu-DOTA-TATE and capecitabine resulted in tumor control in 94% of the patients without increased radiotoxicity [70]. In contrast, another study reported no superior anti-cancer effects of the combination of capecitabine with [177Lu]Lu-DOTA-TATE [71], however in this study, a smaller group of patients with a specific type of NETs was enrolled, making it difficult to directly compare these two studies.
A different potential strategy to increase the radiosensitivity of PRRT is the disturbance of proper protein folding. This might impair the ability of the cell to employ proteins that are essential for survival after PRRT. For example, it was shown that inhibition of heat shock protein 90 (HSP90), a folding chaperone protein, with ganetespib increased radiosensitivity of NET cells to PRRT both in vitro and in vivo in xenografted mice [72]. This was further substantiated by another study where the HSP90 inhibitor onalespib was used to radiosensitize NET cells both in 2D and 3D cultures [73]. Here, the investigators also showed an increase in DNA damage signaling and subsequent apoptotic factors, but not in all examined NET cell lines. A target group that has not been exploited extensively yet in the context of PRRT is cellular metabolism. Targeting components of cellular metabolism to increase radiosensitivity has been shown to be feasible [74], however, the exact mode of action often remains veiled due to a plethora of possible cellular consequences, such as increased oxidative stress or altered activity of important enzymes such as sirtuins, ADP-ribosyl cyclases or PARPs. One study showed complete remission in a cohort of mice with NET xenografts that were pretreated with an inhibitor of nicotineamide phosphoribosyltransferase, an enzyme crucial in nicotineamide-adenine-dinucleotide metabolism, before [177Lu]Lu-DOTA-TATE injection [75]. To our knowledge, no other studies have combined direct targeting of cellular metabolism with [177Lu]Lu-DOTA-TATE yet.
Enhanced sensitivity of NETs to PRRT can also be achieved via upregulation of the therapy target. One study showed an increase in SST2 transcript- and protein levels after the use of epigenetic drugs, such as the histon-deacetylase inhibitor (HDACi) valproic acid (VPA). Moreover, increased uptake of [68Ga]Ga-DOTA-TATE was observed in many of these cells, demonstrating the feasibility of upregulation of PRRT key molecules [76]. Another group also tested the uptake of [125I]I-[Tyr3]octreotide, where a strong increase in uptake was observed which correlated with strong upregulation of SST2 after treatment with 5-aza-dC, VPA or the combination of these two [77]. Although promising, the effects of epigenetic drugs on the expression of SST2 in healthy tissues and potential subsequent elevated toxicity remain largely unknown.
Not all possible combination therapies will increase PRRT efficacy and some might even counteract therapeutic efficacy. This was for example shown in a preclinical study where RAD001, an inhibitor of mammalian target of rapamycin (mTOR), was administered to SST2-positive tumor bearing rats together with PRRT, and no increase of therapeutic efficacy was observed. Contrasting the expectations of the investigators, in the rats that were treated with RAD001 metastases were formed, but not in the PRRT monotherapy group [78, 79]. Despite these negative preclinical results, a phase I clinical study was performed, which showed that the combination of the mTOR inhibitor everolimus and PRRT was well tolerated in terms of toxicity [80]. Within the 6 month time-frame of treatment and follow-up, 44% of treated patients had a partial response. However, the number of patients in the study was insufficient to provide statistical analyses to compare the response rate to PRRT alone. The feasibility of mTOR inhibitors in combination with PRRT will thus warrant more (pre)clinical investigation.
Whether some compounds radiosensitize NETs with specific pathological grading or NETs with specific mutations remains to be elucidated and underlying mechanisms will have to be better understood in order to enhance the clinical success rate. Also, how the described combinations will weigh against [177Lu]Lu-DOTA-TATE alone or how well tolerated such combination therapies are in the long-term will have to be shown in the coming years with more (pre)clinical data.
3. NEED FOR RADIOBIOLOGY
Since the discovery of X-rays by Roentgen in 1895, research on the effects of EBRT on healthy and cancer tissues has been extensive. These investigations describe the effects of ionizing radiation on DNA damage induction and repair, metabolism, proteostasis, acquisition of therapy resistance, and many more [81]. However, due to differences in dose-rate, duration and quality of radiation exposure, the biological effects of the two radiation types might vary tremendously, and thus prevent simple extrapolation of EBRT knowledge- to PRRT. In addition, the complexity of the intra- and interpatient heterogeneity of NETs might also contribute to PRRT response and prognosis [82]. Since biological parameters contribute to uncertainties in predicting PRRT responses, we and others published a ‘Call to arms’, pleading for more fundamental and translational research in the field of radiobiology of molecular radiotherapies (MRT) [83]. Here, we emphasized that in order to improve therapeutic outcomes for MRTs such as PRRT, knowledge has to be gained concerning biomarkers predicting therapy response, MRT effects on subcellular structures and also the role of microenvironmental factors on therapeutic efficacy.
Different studies on the applicability of biomarkers for prognostic purposes and evaluation of therapy response were published. In one preclinical study, investigators observed that certain parameters measured using magnetic resonance imaging (MRI) potentially revealed the therapeutic response of NETs to [177Lu]Lu-DOTA-TATE [84]. The feasibility of biomarkers for prognostic purposes in a clinical setting was also shown in another study, where the authors compared blood circulating NET-specific transcripts to CgA for their predictive value [85]. Here, this set of transcripts, coined the ‘NETest’, was shown to be more sensitive than CgA in the diagnosis of NETs, but also in the prediction of PRRT response. Biomarkers predicting response could potentially be implemented to adapt the treatment planning of individual patients.
As induction of DNA damage is the main anti-cancer mode of action of PRRT, it is essential to understand the effects of different radionuclides on the DNA. However, not much is known yet about the type of DNA lesions that are induced by PRRT. One study has reported that in vitro surviving cell fractions after equivalent doses of internal β-- and γ-irradiation, were lower using β--irradiation, indicating a higher tumor cell killing efficacy [86]. Moreover, when analyzing gene expression, the authors found differences in the magnitude and kinetics of DNA repair genes. Another study investigated gene expression profiles by RNA sequencing of proton- and γ whole-body irradiated mice [87]. Here, the investigators showed that γ-radiation responsive genes only partly overlapped with proton-responsive genes. This indicates different cellular responses to certain radiation qualities. More subtle differences between different radiation qualities were shown in a study where the number and kinetics of induced DSBs were compared between internal β-- and external γ-radiation [88]. Here it was observed that the initial fraction of all DNA break repair was faster for γ- than for β--irradiation. Differences in repair kinetics might be even more relevant in the context of PRRT, where the radioactive decay in/on the cell induces DNA damage at different rates and with different effects on the cell compared to EBRT.
Next to the activation of the DNA damage response, there are many cellular and microenvironmental processes that might be differentially impacted by different radiation types. The microenvironment itself can also impact radiation therapy. It is well described that hypoxia can counteract radiation efficacy [89]. How this exactly impacts PRRT and its dosimetry is not well known, but it is hypothesized that hypoxia will especially impede low-LET irradiation, such as therapies with β--emitters, and will interfere less with high-LET α-emitters [39]. Next to hypoxia, more factors that can elicit therapy resistance have been described, such as the presence of cancer-associated fibroblasts, the composition of extracellular matrix, etc. [90]. In the coming years, the impact of these factors in the context of PRRT will have to be investigated.
Since radiobiology and dosimetry are intricately linked, questions of how subcellular distribution of radionuclides will influence micro-dosimetry and downstream radiation effects will have to be answered. Researchers that modeled cellular dosimetry for multiple radionuclides observed that dose-deposition was greatly dependent on the size of the cell, but also that specific radionuclides exert a higher dose-deposition when located near the nucleus instead of on the cell surface [91]. This indicates the need for subcellular dosimetry. That subcellular targeting is feasible has been reported in a study where copper-64 or lutetium-177 were coupled to a nanoparticle that targets mitochondria [92]. Here, a high tumor uptake was observed in vivo and specific mitochondrial uptake in vitro. Since radiolabeled DOTA-TATE is internalized into NET cells, it would be beneficial to investigate its subcellular distribution, especially in the context of short-range α-particle- or auger-electron emitters. Modeling of the effects and influences of different subcellular localizations and cellular shapes on micro dose-distributions is under active investigation [93], but if and how dosimetry should subsequently be adapted is still under debate.
The field of radiobiology still has a lot of ground to cover as many radiation effects of PRRT remain elusive. How timing and mode of administration, dose-rate and thus absorbed dose will impact both therapeutic efficacy and potential adverse effects warrants further investigation. In the past, the importance of administration routes by comparing intra-arterial with intra-venous PRRT injections was already reported [94]. Here, it was observed that when injecting [111In]In-DTPA-octreotide intra-arterially, the radioactive uptake in GEP-NETs was two times higher compared to intra-venous injections. Another study also reported higher tumor uptake of [177Lu]Lu-DOTA-3-iodo-Tyr3-octreotate after intra-arterial injection compared to intra-venous injections, emphasizing the importance of the mode of administration [95].
Ultimately, what cellular and microenvironmental differences might be induced due to different radiation qualities and dose-rates, and by what factors absorbed dose or its biological effectiveness might be influenced remains largely unknown and will be the subject of investigation in the coming years.
CONCLUSION
Many exciting lines of PRRT research are conducted on the intersection of biophysics, radiochemistry, radio and cellular biology and dosimetry. It is the combination of all these disciplines that enables the proper design of combinatorial treatment strategies, the incorporation of different radionuclides for different radio-peptides, the discovery of new target molecules, and mapping the dose- and biological effects of PRRT in order to predict the therapeutic outcome or adapt treatment planning. However, many challenges remain. Technical differences between studies from research groups, such as the injected dose, the xenograft model or timing- or modes of administration can complicate comparisons and translation of results towards the clinic. As the absorbed dose is important to the anti-tumor effect of PRRT [96, 97], more standardized treatment regimes in terms of specific radioactivity would be beneficial for putting enhanced efficacy into context, paving an easier way towards clinical research.
PRRT is a powerful anti-cancer therapy and many different angles of research are being actively investigated to improve the therapy. Also, as the field of nuclear medicine is rapidly growing, the multidisciplinary approaches grow with it. This increasing body of interdisciplinary collaborations will pave the way towards a radiant future for PRRT and the patients receiving it.
Acknowledgements
Declared none.
LIST OF ABBREVIATIONS
- CgA
Chromogranin A
- DDR
DNA Damage Response
- DOTA
1,4,7,10-tetraazacyclo-dodecane-1,4,7,10-tetraacetic Acid
- DTPA
Diethylenetriaminepentaacetic Acid-d-Phenylalanine
- JR11
Cpa-c[D-Cys-Aph(Hor)-D-Aph(Cbm)-Lys-Thr-Cys]-D-Tyr-NH2
- GEP
Gastoenteropancreatic
- HDAC
Histon-deacetylase
- MRI
Magnetic Resonance Imaging
- N-CAM
Neural Cell Adhesion Molecule
- NET
Neuroendocrine Tumor
- PARP
Poly(ADP-ribose)polymerase
- p-NO2-Bn-Oxo
1-oxa-4,7,10-triazacyclododecane-S-5-(4-nitrobenzyl)-4,7,10-triaceticacid
- PRRT
Peptide Receptor Radionuclide Therapy
- PSMA
Prostate-specific Membrane Antigen
- SPECT
Single-photon Emission Computed Tomography
- SSA
Somatostatin Analogue
- SST2
Somatostatin Receptor Subtype 2
- TATE
Tyr3-octreotate
- TCMC
1,4,7,10-tetrakis(carbamoylmethyl)-l,4,7,10-tetraazacyclododecane
- TOC
Tyr3-octreotide
- VPA
Valproic Acid
Consent for Publication
Not applicable.
Funding
This work was supported by funding from Daniel den Hoed Foundation, Netherlands. The funders had no role in the design, writing, and decision to submit this article.
conflict of interest
MdJ and JN have an investigator-initiated project contract with Advanced Accelerator Applications, a Novartis Company. This had no role in the preparation or submission of this article.
REFERENCES
- 1.Riihimäki M., Hemminki A., Sundquist K., Sundquist J., Hemminki K. The epidemiology of metastases in neuroendocrine tumors. Int. J. Cancer. 2016;139(12):2679–2686. doi: 10.1002/ijc.30400. [DOI] [PubMed] [Google Scholar]
- 2.Kim J.Y., Hong S.M., Ro J.Y. Recent updates on grading and classification of neuroendocrine tumors. Ann. Diagn. Pathol. 2017;29:11–16. doi: 10.1016/j.anndiagpath.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Barakat M.T., Meeran K., Bloom S.R. Neuroendocrine tumours. Endocr. Relat. Cancer. 2004;11(1):1–18. doi: 10.1677/erc.0.0110001. [DOI] [PubMed] [Google Scholar]
- 4.Hofving T., Arvidsson Y., Almobarak B., Inge L., Pfragner R., Persson M., Stenman G., Kristiansson E., Johanson V., Nilsson O. The neuroendocrine phenotype, genomic profile and therapeutic sensitivity of GEPNET cell lines. Endocr. Relat. Cancer. 2018;25(3):367–380. doi: 10.1530/ERC-17-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Modlin I.M., Gustafsson B.I., Moss S.F., Pavel M., Tsolakis A.V., Kidd M., Chromogranin A. Chromogranin A--biological function and clinical utility in neuro endocrine tumor disease. Ann. Surg. Oncol. 2010;17(9):2427–2443. doi: 10.1245/s10434-010-1006-3. [DOI] [PubMed] [Google Scholar]
- 6.Gould V.E., Lee I., Wiedenmann B., Moll R., Chejfec G., Franke W.W. Synaptophysin: a novel marker for neurons, certain neuroendocrine cells, and their neoplasms. Hum. Pathol. 1986;17(10):979–983. doi: 10.1016/S0046-8177(86)80080-6. [DOI] [PubMed] [Google Scholar]
- 7.Kulaksiz H., Eissele R., Rössler D., Schulz S., Höllt V., Cetin Y., Arnold R. Identification of somatostatin receptor subtypes 1, 2A, 3, and 5 in neuroendocrine tumours with subtype specific antibodies. Gut. 2002;50(1):52–60. doi: 10.1136/gut.50.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolin E.M. The expanding role of somatostatin analogs in the management of neuroendocrine tumors. Gastrointest. Cancer Res. 2012;5(5):161–168. [PMC free article] [PubMed] [Google Scholar]
- 9.Krenning E.P., Bakker W.H., Breeman W.A., Koper J.W., Kooij P.P., Ausema L., Lameris J.S., Reubi J.C., Lamberts S.W. Localisation of endocrine-related tumours with radioiodinated analogue of somatostatin. Lancet. 1989;1(8632):242–244. doi: 10.1016/S0140-6736(89)91258-0. [DOI] [PubMed] [Google Scholar]
- 10.Krenning E.P., Kwekkeboom D.J., Bakker W.H., Breeman W.A., Kooij P.P., Oei H.Y., van Hagen M., Postema P.T., de Jong M., Reubi J.C. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur. J. Nucl. Med. 1993;20(8):716–731. doi: 10.1007/BF00181765. [DOI] [PubMed] [Google Scholar]
- 11.Baum R.P., Kulkarni H.R. THERANOSTICS: From molecular imaging using Ga-68 labeled tracers and PET/CT to personalized radionuclide therapy - The bad berka experience. Theranostics. 2012;2(5):437–447. doi: 10.7150/thno.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krenning E.P., Kooij P.P., Bakker W.H., Breeman W.A., Postema P.T., Kwekkeboom D.J., Oei H.Y., de Jong M., Visser T.J., Reijs A.E. Radiotherapy with a radiolabeled somatostatin analogue, [111In-DTPA-D-Phe1]-octreotide. A case history. Ann. N. Y. Acad. Sci. 1994;733:496–506. doi: 10.1111/j.1749-6632.1994.tb17300.x. [DOI] [PubMed] [Google Scholar]
- 13.Fjälling M., Andersson P., Forssell-Aronsson E., Grétarsdóttir J., Johansson V., Tisell L.E., Wängberg B., Nilsson O., Berg G., Michanek A., Lindstedt G., Ahlman H. Systemic radionuclide therapy using indium-111-DTPA-D-Phe1-octreotide in midgut carcinoid syndrome. J. Nucl. Med. 1996;37(9):1519–1521. [PubMed] [Google Scholar]
- 14.Bomanji J. B., Papathanasiou N. D. 2012.
- 15.Levine R., Krenning E.P. Clinical history of the theranostic radionuclide approach to neuroendocrine tumors and other types of cancer: Historical review based on an interview of Eric P. Krenning by Rachel Levine. J. Nucl. Med. 2017;58(Suppl. 2):3S–9S. doi: 10.2967/jnumed.116.186502. [DOI] [PubMed] [Google Scholar]
- 16.Strosberg J., El-Haddad G., Wolin E., Hendifar A., Yao J., Chasen B., Mittra E., Kunz P.L., Kulke M.H., Jacene H., Bushnell D., O’Dorisio T.M., Baum R.P., Kulkarni H.R., Caplin M., Lebtahi R., Hobday T., Delpassand E., Van Cutsem E., Benson A., Srirajaskanthan R., Pavel M., Mora J., Berlin J., Grande E., Reed N., Seregni E., Öberg K., Lopera Sierra M., Santoro P., Thevenet T., Erion J.L., Ruszniewski P., Kwekkeboom D., Krenning E. Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N. Engl. J. Med. 2017;376(2):125–135. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choppin G.R., Liljenzin J-O., Rydberg J.A.N. 2002. [Google Scholar]
- 18.Kassis A.I. The amazing world of auger electrons. Int. J. Radiat. Biol. 2004;80(11-12):789–803. doi: 10.1080/09553000400017663. [DOI] [PubMed] [Google Scholar]
- 19.Kassis A.I. Therapeutic radionuclides: biophysical and radiobiologic principles. Semin. Nucl. Med. 2008;38(5):358–366. doi: 10.1053/j.semnuclmed.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kondev F.G. Nuclear Data Sheets for A = 177. Nucl. Data Sheets (N.Y. N.Y.) 2003;98(4):801–1095. doi: 10.1006/ndsh.2003.0006. [DOI] [Google Scholar]
- 21.Schötzig U., Schrader H., Schönfeld E., Günther E., Klein R. Standardisation and decay data of 177Lu and 188Re. Appl. Radiat. Isot. 2001;55(1):89–96. doi: 10.1016/S0969-8043(00)00362-6. [DOI] [PubMed] [Google Scholar]
- 22.Bodei L., Mueller-Brand J., Baum R.P., Pavel M.E., Hörsch D., O’Dorisio M.S., O’Dorisio T.M., Howe J.R., Cremonesi M., Kwekkeboom D.J., Zaknun J.J. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging. 2013;40(5):800–816. doi: 10.1007/s00259-012-2330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valkema R., Pauwels S., Kvols L.K., Barone R., Jamar F., Bakker W.H., Kwekkeboom D.J., Bouterfa H., Krenning E.P. Survival and response after peptide receptor radionuclide therapy with [90Y-DOTA0,Tyr3]octreotide in patients with advanced gastroenteropancreatic neuroendocrine tumors. Semin. Nucl. Med. 2006;36(2):147–156. doi: 10.1053/j.semnuclmed.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Miller W.H., Hartmann-Siantar C., Fisher D., Descalle M.A., Daly T., Lehmann J., Lewis M.R., Hoffman T., Smith J., Situ P.D., Volkert W.A. Evaluation of beta-absorbed fractions in a mouse model for 90Y, 188Re, 166Ho, 149Pm, 64Cu, and 177Lu radionuclides. Cancer Biother. Radiopharm. 2005;20(4):436–449. doi: 10.1089/cbr.2005.20.436. [DOI] [PubMed] [Google Scholar]
- 25.Gudkov S.V., Shilyagina N.Y., Vodeneev V.A., Zvyagin A.V. Targeted radionuclide therapy of human tumors. Int. J. Mol. Sci. 2015;17(1):33. doi: 10.3390/ijms17010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capello A., Krenning E.P., Breeman W.A., Bernard B.F., Konijnenberg M.W., de Jong M. Tyr3-octreotide and Tyr3-octreotate radiolabeled with 177Lu or 90Y: peptide receptor radionuclide therapy results in vitro. Cancer Biother. Radiopharm. 2003;18(5):761–768. doi: 10.1089/108497803770418300. [DOI] [PubMed] [Google Scholar]
- 27.Vinjamuri S., Gilbert T.M., Banks M., McKane G., Maltby P., Poston G., Weissman H., Palmer D.H., Vora J., Pritchard D.M., Cuthbertson D.J. Peptide receptor radionuclide therapy with (90)Y-DOTATATE/(90)Y-DOTATOC in patients with progressive metastatic neuroendocrine tumours: assessment of response, survival and toxicity. Br. J. Cancer. 2013;108(7):1440–1448. doi: 10.1038/bjc.2013.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu T-J., Chiu H-Y., Yu J., Cautela M.P., Sarmento B. In: Nanotechnologies in Preventive and Regenerative Medicine. Uskoković V., Uskoković D.P., editors. Amsterdam: Elsevier; 2018. Nanotechnologies for early diagnosis, in situ disease monitoring, and prevention. pp. 1–92. [Google Scholar]
- 29.Chinol M., Franceschini R., Paganelli G., Pecorale A., Paiano A. Simple production of Yttrium-90 in a chemical form suitable to clinical grade radioconjugates.; Proceedings of the 22nd International Badgastein Symposium; 1997. pp. 327–332. [Google Scholar]
- 30.Kunikowska J., Królicki L., Hubalewska-Dydejczyk A., Mikołajczak R., Sowa-Staszczak A., Pawlak D. Clinical results of radionuclide therapy of neuroendocrine tumours with 90Y-DOTATATE and tandem 90Y/177Lu-DOTATATE: which is a better therapy option? Eur. J. Nucl. Med. Mol. Imaging. 2011;38(10):1788–1797. doi: 10.1007/s00259-011-1833-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valkema R., Pauwels S.A., Kvols L.K., Kwekkeboom D.J., Jamar F., de Jong M., Barone R., Walrand S., Kooij P.P., Bakker W.H., Lasher J., Krenning E.P. Long-term follow-up of renal function after peptide receptor radiation therapy with (90)Y-DOTA(0),Tyr(3)-octreotide and (177)Lu-DOTA(0), Tyr(3)-octreotate. J. Nucl. Med. 2005;46(Suppl. 1):83S–91S. [PubMed] [Google Scholar]
- 32.Cremonesi M., Ferrari M.E., Bodei L., Chiesa C., Sarnelli A., Garibaldi C., Pacilio M., Strigari L., Summers P.E., Orecchia R., Grana C.M., Botta F. Correlation of dose with toxicity and tumour response to 90Y- and 177Lu-PRRT provides the basis for optimization through individualized treatment planning. Eur. J. Nucl. Med. Mol. Imaging. 2018;45(13):2426–2441. doi: 10.1007/s00259-018-4044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rolleman E.J., Valkema R., de Jong M., Kooij P.P., Krenning E.P. Safe and effective inhibition of renal uptake of radiolabelled octreotide by a combination of lysine and arginine. Eur. J. Nucl. Med. Mol. Imaging. 2003;30(1):9–15. doi: 10.1007/s00259-002-0982-3. [DOI] [PubMed] [Google Scholar]
- 34.Lenain R., Hamroun A., Lion G., Chamley P., Bui L., Lionet A., Hazzan M., Provôt F. Description of a transient proximal tubulopathy induced by amino acids perfusion in peptide receptor radionuclide therapy: A case report. Medicine (Baltimore) 2019;98(52):e18478. doi: 10.1097/MD.0000000000018478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Todorović-Tirnanić M., Kaemmerer D., Prasad V., Hommann M., Baum R.P. Intraoperative somatostatin receptor detection after peptide receptor radionuclide therapy with (177)Lu- and (90)Y-DOTATOC (tandem PRRNT) in a patient with a metastatic neuroendocrine tumor. Recent Results Cancer Res. 2013;194:487–496. doi: 10.1007/978-3-642-27994-2_28. [DOI] [PubMed] [Google Scholar]
- 36.Baum R.P., Kulkarni H.R., Singh A., Kaemmerer D., Mueller D., Prasad V., Hommann M., Robiller F.C., Niepsch K., Franz H., Jochems A., Lambin P., Hörsch D. Results and adverse events of personalized peptide receptor radionuclide therapy with 90Yttrium and 177Lutetium in 1048 patients with neuroendocrine neoplasms. Oncotarget. 2018;9(24):16932–16950. doi: 10.18632/oncotarget.24524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seregni E., Maccauro M., Chiesa C., Mariani L., Pascali C., Mazzaferro V., De Braud F., Buzzoni R., Milione M., Lorenzoni A., Bogni A., Coliva A., Lo Vullo S., Bombardieri E. Treatment with tandem [90Y]DOTA-TATE and [177Lu]DOTA-TATE of neuroendocrine tumours refractory to conventional therapy. Eur. J. Nucl. Med. Mol. Imaging. 2014;41(2):223–230. doi: 10.1007/s00259-013-2578-5. [DOI] [PubMed] [Google Scholar]
- 38.Hörsch D., Ezziddin S., Haug A., Gratz K.F., Dunkelmann S., Miederer M., Schreckenberger M., Krause B.J., Bengel F.M., Bartenstein P., Biersack H.J., Pöpperl G., Baum R.P. Effectiveness and side-effects of peptide receptor radionuclide therapy for neuroendocrine neoplasms in Germany: A multi-institutional registry study with prospective follow-up. Eur. J. Cancer. 2016;58:41–51. doi: 10.1016/j.ejca.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Navalkissoor S., Grossman A. Targeted alpha particle therapy for neuroendocrine tumours: The next generation of peptide receptor radionuclide therapy. Neuroendocrinology. 2019;108(3):256–264. doi: 10.1159/000494760. [DOI] [PubMed] [Google Scholar]
- 40.Chan H.S., de Blois E., Morgenstern A., Bruchertseifer F., de Jong M., Breeman W., Konijnenberg M. In Vitro comparison of 213Bi- and 177Lu-radiation for peptide receptor radionuclide therapy. PLoS One. 2017;12(7):e0181473. doi: 10.1371/journal.pone.0181473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nayak T.K., Norenberg J.P., Anderson T.L., Prossnitz E.R., Stabin M.G., Atcher R.W. Somatostatin-receptor-targeted alpha-emitting 213Bi is therapeutically more effective than beta(-)-emitting 177Lu in human pancreatic adenocarcinoma cells. Nucl. Med. Biol. 2007;34(2):185–193. doi: 10.1016/j.nucmedbio.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Chan H.S., Konijnenberg M.W., de Blois E., Koelewijn S., Baum R.P., Morgenstern A., Bruchertseifer F., Breeman W.A., de Jong M. Influence of tumour size on the efficacy of targeted alpha therapy with (213)Bi-[DOTA(0),Tyr(3)]-octreotate. EJNMMI Res. 2016;6(1):6. doi: 10.1186/s13550-016-0162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miederer M., Scheinberg D.A., McDevitt M.R. Realizing the potential of the Actinium-225 radionuclide generator in targeted alpha particle therapy applications. Adv. Drug Deliv. Rev. 2008;60(12):1371–1382. doi: 10.1016/j.addr.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scheinberg D.A., McDevitt M.R. Actinium-225 in targeted alpha-particle therapeutic applications. Curr. Radiopharm. 2011;4(4):306–320. doi: 10.2174/1874471011104040306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miederer M., Henriksen G., Alke A., Mossbrugger I., Quintanilla-Martinez L., Senekowitsch-Schmidtke R., Essler M. Preclinical evaluation of the alpha-particle generator nuclide 225Ac for somatostatin receptor radiotherapy of neuroendocrine tumors. Clin. Cancer Res. 2008;14(11):3555–3561. doi: 10.1158/1078-0432.CCR-07-4647. [DOI] [PubMed] [Google Scholar]
- 46.Ballal S., Yadav M., Bal C., Tripathi M., Sahoo R. Early results of 225Ac-DOTATATE targeted alpha therapy in metastatic gastroenteropancreatic neuroendocrine tumors: First clinical experience on safety and efficacy. J. Nucl. Med. 2019;•••:60. [Google Scholar]
- 47.Kratochwil C., Giesel F.L., Bruchertseifer F., Mier W., Apostolidis C., Boll R., Murphy K., Haberkorn U., Morgenstern A. (2)(1)(3)Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: a first-in-human experience. Eur. J. Nucl. Med. Mol. Imaging. 2014;41(11):2106–2119. doi: 10.1007/s00259-014-2857-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sathekge M., Bruchertseifer F., Knoesen O., Reyneke F., Lawal I., Lengana T., Davis C., Mahapane J., Corbett C., Vorster M., Morgenstern A. 225Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: a pilot study. Eur. J. Nucl. Med. Mol. Imaging. 2019;46(1):129–138. doi: 10.1007/s00259-018-4167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jurcic J.G. Clinical studies with bismuth-213 and actinium-225 for hematologic malignancies. Curr. Radiopharm. 2018;11(3):192–199. doi: 10.2174/1874471011666180525102814. [DOI] [PubMed] [Google Scholar]
- 50.Delpassand E., Tworowska I., Shanoon F., Nunez R., Flores Ii L., Muzammil A., Stallons T., Saidi A., Torgue J. First clinical experience using targeted alpha-emitter therapy with 212Pb-DOTAMTATE (AlphaMedixTM) in patients with SSTR(+) neuroendocrine tumors. J. Nucl. Med. 2019;60(Suppl. 1):559–559. [Google Scholar]
- 51.Langbein T., Chaussé G., Baum R.P. Salivary gland toxicity of PSMA radioligand therapy: Relevance and preventive strategies. J. Nucl. Med. 2018;59(8):1172–1173. doi: 10.2967/jnumed.118.214379. [DOI] [PubMed] [Google Scholar]
- 52.Kratochwil C., Bruchertseifer F., Rathke H., Bronzel M., Apostolidis C., Weichert W., Haberkorn U., Giesel F.L., Morgenstern A. Targeted α-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: Dosimetry estimate and empiric dose finding. J. Nucl. Med. 2017;58(10):1624–1631. doi: 10.2967/jnumed.117.191395. [DOI] [PubMed] [Google Scholar]
- 53.Kruijff R.M., Raavé R., Kip A., Molkenboer-Kuenen J., Morgenstern A., Bruchertseifer F., Heskamp S., Denkova A.G. The in vivo fate of 225Ac daughter nuclides using polymersomes as a model carrier. Sci. Rep. 2019;9(1):11671. doi: 10.1038/s41598-019-48298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Price E.W., Orvig C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2014;43(1):260–290. doi: 10.1039/C3CS60304K. [DOI] [PubMed] [Google Scholar]
- 55.De León-Rodríguez L.M., Kovacs Z. The synthesis and chelation chemistry of DOTA-peptide conjugates. Bioconjug. Chem. 2008;19(2):391–402. doi: 10.1021/bc700328s. [DOI] [PubMed] [Google Scholar]
- 56.Ferreira C.L., Yapp D.T., Lamsa E., Gleave M., Bensimon C., Jurek P., Kiefer G.E. Evaluation of novel bifunctional chelates for the development of Cu-64-based radiopharmaceuticals. Nucl. Med. Biol. 2008;35(8):875–882. doi: 10.1016/j.nucmedbio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 57.Esser J.P., Krenning E.P., Teunissen J.J., Kooij P.P., van Gameren A.L., Bakker W.H., Kwekkeboom D.J. Comparison of [(177)Lu-DOTA(0),Tyr(3)]octreotate and [(177)Lu-DOTA(0), Tyr(3)]octreotide: which peptide is preferable for PRRT? Eur. J. Nucl. Med. Mol. Imaging. 2006;33(11):1346–1351. doi: 10.1007/s00259-006-0172-9. [DOI] [PubMed] [Google Scholar]
- 58.de Jong M., Breeman W.A., Bakker W.H., Kooij P.P., Bernard B.F., Hofland L.J., Visser T.J., Srinivasan A., Schmidt M.A., Erion J.L., Bugaj J.E., Mäcke H.R., Krenning E.P. Comparison of (111)In-labeled somatostatin analogues for tumor scintigraphy and radionuclide therapy. Cancer Res. 1998;58(3):437–441. [PubMed] [Google Scholar]
- 59.Reubi J.C., Schär J.C., Waser B., Wenger S., Heppeler A., Schmitt J.S., Mäcke H.R. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur. J. Nucl. Med. 2000;27(3):273–282. doi: 10.1007/s002590050034. [DOI] [PubMed] [Google Scholar]
- 60.Fani M., Braun F., Waser B., Beetschen K., Cescato R., Erchegyi J., Rivier J.E., Weber W.A., Maecke H.R., Reubi J.C. Unexpected sensitivity of sst2 antagonists to N-terminal radiometal modifications. J. Nucl. Med. 2012;53(9):1481–1489. doi: 10.2967/jnumed.112.102764. [DOI] [PubMed] [Google Scholar]
- 61.Dalm S.U., Nonnekens J., Doeswijk G.N., de Blois E., van Gent D.C., Konijnenberg M.W., de Jong M. Comparison of the therapeutic response to treatment with a 177Lu-labeled somatostatin receptor agonist and antagonist in preclinical models. J. Nucl. Med. 2016;57(2):260–265. doi: 10.2967/jnumed.115.167007. [DOI] [PubMed] [Google Scholar]
- 62.Wild D., Fani M., Fischer R., Del Pozzo L., Kaul F., Krebs S., Fischer R., Rivier J.E., Reubi J.C., Maecke H.R., Weber W.A. Comparison of somatostatin receptor agonist and antagonist for peptide receptor radionuclide therapy: a pilot study. J. Nucl. Med. 2014;55(8):1248–1252. doi: 10.2967/jnumed.114.138834. [DOI] [PubMed] [Google Scholar]
- 63.Adant S., Shah G.M., Beauregard J.M. Combination treatments to enhance peptide receptor radionuclide therapy of neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging. 2019;41:907–921. doi: 10.1007/s00259-019-04499-x. [DOI] [PubMed] [Google Scholar]
- 64.Nonnekens J., van Kranenburg M., Beerens C.E., Suker M., Doukas M., van Eijck C.H., de Jong M., van Gent D.C. Potentiation of peptide receptor radionuclide therapy by the PARP inhibitor olaparib. Theranostics. 2016;6(11):1821–1832. doi: 10.7150/thno.15311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Purohit N.K., Shah R.G., Adant S., Hoepfner M., Shah G.M., Beauregard J.M. Potentiation of 177Lu-octreotate peptide receptor radionuclide therapy of human neuroendocrine tumor cells by PARP inhibitor. Oncotarget. 2018;9(37):24693–24706. doi: 10.18632/oncotarget.25266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nickoloff J.A., Boss M.K., Allen C.P., LaRue S.M. Translational research in radiation-induced DNA damage signaling and repair. Transl. Cancer Res. 2017;6(Suppl. 5):S875–S891. doi: 10.21037/tcr.2017.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bison S.M., Haeck J.C., Bol K., Koelewijn S.J., Groen H.C., Melis M., Veenland J.F., Bernsen M.R., de Jong M. Optimization of combined temozolomide and peptide receptor radionuclide therapy (PRRT) in mice after multimodality molecular imaging studies. EJNMMI Res. 2015;5(1):62. doi: 10.1186/s13550-015-0142-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lewin J., Cullinane C., Akhurst T., Waldeck K., Watkins D.N., Rao A., Eu P., Mileshkin L., Hicks R.J. Peptide receptor chemoradionuclide therapy in small cell carcinoma: from bench to bedside. Eur. J. Nucl. Med. Mol. Imaging. 2015;42(1):25–32. doi: 10.1007/s00259-014-2888-2. [DOI] [PubMed] [Google Scholar]
- 69.Claringbold P.G., Price R.A., Turner J.H. Phase I-II study of radiopeptide 177Lu-octreotate in combination with capecitabine and temozolomide in advanced low-grade neuroendocrine tumors. Cancer Biother. Radiopharm. 2012;27(9):561–569. doi: 10.1089/cbr.2012.1276. [DOI] [PubMed] [Google Scholar]
- 70.Claringbold P.G., Brayshaw P.A., Price R.A., Turner J.H. Phase II study of radiopeptide 177Lu-octreotate and capecitabine therapy of progressive disseminated neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging. 2011;38(2):302–311. doi: 10.1007/s00259-010-1631-x. [DOI] [PubMed] [Google Scholar]
- 71.Yadav M.P., Ballal S., Bal C. Concomitant 177Lu-DOTATATE and capecitabine therapy in malignant paragangliomas. EJNMMI Res. 2019;9(1):13. doi: 10.1186/s13550-019-0484-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hofving T., Sandblom V., Arvidsson Y., Shubbar E., Altiparmak G., Swanpalmer J., Almobarak B., Elf A.K., Johanson V., Elias E., Kristiansson E., Forssell-Aronsson E., Nilsson O. 177Lu-octreotate therapy for neuroendocrine tumours is enhanced by Hsp90 inhibition. Endocr. Relat. Cancer. 2019;26(4):437–449. doi: 10.1530/ERC-18-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lundsten S., Spiegelberg D., Stenerlöw B., Nestor M. The HSP90 inhibitor onalespib potentiates 177Lu-DOTATATE therapy in neuroendocrine tumor cells. Int. J. Oncol. 2019;55(6):1287–1295. doi: 10.3892/ijo.2019.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao Y., Butler E.B., Tan M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 2013;4(3):e532. doi: 10.1038/cddis.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Elf A.K., Bernhardt P., Hofving T., Arvidsson Y., Forssell-Aronsson E., Wängberg B., Nilsson O., Johanson V. NAMPT inhibitor GMX1778 enhances the efficacy of 177Lu-DOTATATE treatment of neuroendocrine tumors. J. Nucl. Med. 2017;58(2):288–292. doi: 10.2967/jnumed.116.177584. [DOI] [PubMed] [Google Scholar]
- 76.Taelman V.F., Radojewski P., Marincek N., Ben-Shlomo A., Grotzky A., Olariu C.I., Perren A., Stettler C., Krause T., Meier L.P., Cescato R., Walter M.A. Upregulation of key molecules for targeted imaging and therapy. J. Nucl. Med. 2016;57(11):1805–1810. doi: 10.2967/jnumed.115.165092. [DOI] [PubMed] [Google Scholar]
- 77.Veenstra M.J., van Koetsveld P.M., Dogan F., Farrell W.E., Feelders R.A., Lamberts S.W.J., de Herder W.W., Vitale G., Hofland L.J. Epidrug-induced upregulation of functional somatostatin type 2 receptors in human pancreatic neuroendocrine tumor cells. Oncotarget. 2016;9(19):14791–14802. doi: 10.18632/oncotarget.9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pool S.E., Bison S., Koelewijn S.J., van der Graaf L.M., Melis M., Krenning E.P., de Jong M. mTOR inhibitor RAD001 promotes metastasis in a rat model of pancreatic neuroendocrine cancer. Cancer Res. 2013;73(1):12–18. doi: 10.1158/0008-5472.CAN-11-2089. [DOI] [PubMed] [Google Scholar]
- 79.Bison S.M., Pool S.E., Koelewijn S.J., van der Graaf L.M., Groen H.C., Melis M., de Jong M. Peptide receptor radionuclide therapy (PRRT) with [(177)Lu-DOTA(0),Tyr(3)]octreotate in combination with RAD001 treatment: further investigations on tumor metastasis and response in the rat pancreatic CA20948 tumor model. EJNMMI Res. 2014;4:21. doi: 10.1186/s13550-014-0021-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Claringbold P.G., Turner J.H. NeuroEndocrine tumor therapy with lutetium-177-octreotate and everolimus (NETTLE): A phase I study. Cancer Biother. Radiopharm. 2015;30(6):261–269. doi: 10.1089/cbr.2015.1876. [DOI] [PubMed] [Google Scholar]
- 81.Baskar R., Dai J., Wenlong N., Yeo R., Yeoh K.W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014;1:24. doi: 10.3389/fmolb.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nuñez-Valdovinos B., Carmona-Bayonas A., Jimenez-Fonseca P., Capdevila J., Castaño-Pascual Á., Benavent M., Pi Barrio J.J., Teule A., Alonso V., Custodio A., Marazuela M., Segura Á., Beguiristain A., Llanos M., Martinez Del Prado M.P., Diaz-Perez J.A., Castellano D., Sevilla I., Lopez C., Alonso T., Garcia-Carbonero R. Neuroendocrine tumor heterogeneity adds uncertainty to the world health organization 2010 classification: Real-World data from the spanish tumor registry (R-GETNE). Oncologist. 2018;23(4):422–432. doi: 10.1634/theoncologist.2017-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Terry S.Y.A., Nonnekens J., Aerts A., Baatout S., de Jong M., Cornelissen B., Pouget J.P. Call to arms: need for radiobiology in molecular radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging. 2019;46(8):1588–1590. doi: 10.1007/s00259-019-04334-3. [DOI] [PubMed] [Google Scholar]
- 84.Montelius M., Spetz J., Jalnefjord O., Berger E., Nilsson O., Ljungberg M., Forssell-Aronsson E. Identification of potential MR-derived biomarkers for tumor tissue response to 177Lu-octreotate therapy in an animal model of small intestine neuroendocrine tumor. Transl. Oncol. 2018;11(2):193–204. doi: 10.1016/j.tranon.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bodei L., Kidd M., Modlin I.M., Severi S., Drozdov I., Nicolini S., Kwekkeboom D.J., Krenning E.P., Baum R.P., Paganelli G. Measurement of circulating transcripts and gene cluster analysis predicts and defines therapeutic efficacy of peptide receptor radionuclide therapy (PRRT) in neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging. 2016;43(5):839–851. doi: 10.1007/s00259-015-3250-z. [DOI] [PubMed] [Google Scholar]
- 86.Kumar C., Jayakumar S., Pandey B.N., Samuel G., Venkatesh M. Cellular and molecular effects of beta radiation from I-131 on human tumor cells: a comparison with gamma radiation. Curr. Radiopharm. 2014;7(2):138–143. doi: 10.2174/1874471007666140716115938. [DOI] [PubMed] [Google Scholar]
- 87.Ricciotti E., Sarantopoulou D., Grant G.R., Sanzari J.K., Krigsfeld G.S., Kiliti A.J., Kennedy A.R., Grosser T. Distinct vascular genomic response of proton and gamma radiation-A pilot investigation. PLoS One. 2019;14(2):e0207503. doi: 10.1371/journal.pone.0207503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dikomey E., Franzke J. DNA repair kinetics after exposure to X-irradiation and to internal beta-rays in CHO cells. Radiat. Environ. Biophys. 1986;25(3):189–194. doi: 10.1007/BF01221225. [DOI] [PubMed] [Google Scholar]
- 89.Murland S.L. Tumour oxygenation: The importance of hypoxia, anemia, and angiogenesis in radiation therapy. Can. J. Med. Radiat. Technol. 2005;36(1):21–33. doi: 10.1016/S0820-5930(09)60054-2. [DOI] [Google Scholar]
- 90.Sun Y. Tumor microenvironment and cancer therapy resistance. Cancer Lett. 2016;380(1):205–215. doi: 10.1016/j.canlet.2015.07.044. [DOI] [PubMed] [Google Scholar]
- 91.Lee D., Li M., Bednarz B., Schultz M.K. Modeling cell and tumor-metastasis dosimetry with the particle and heavy ion transport code system (PHITS) software for targeted alpha-particle radionuclide therapy. Radiat. Res. 2018;190(3):236–247. doi: 10.1667/RR15081.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu B., Wei H., He Q., Ferreira C.A., Kutyreff C.J., Ni D., Rosenkrans Z.T., Cheng L., Yu F., Engle J.W., Lan X., Cai W. Efficient uptake of 177 Lu-Porphyrin-PEG nanocomplexes by tumor mitochondria for multimodal-imaging-guided combination therapy. Angew. Chem. Int. Ed. Engl. 2018;57(1):218–222. doi: 10.1002/anie.201710232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tamborino G. Cellular dosimetry of [177Lu]Lu-DOTATATE radionuclide therapy: the impact of modelling assumptions on the correlations with in vitro cytotoxicity. EJNMMI Phys. 2019;7(1) doi: 10.1186/s40658-020-0276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pool S.E., Kam B.L., Koning G.A., Konijnenberg M., Ten Hagen T.L., Breeman W.A., Krenning E.P., de Jong M., van Eijck C.H. [(111)In-DTPA]octreotide tumor uptake in GEPNET liver metastases after intra-arterial administration: an overview of preclinical and clinical observations and implications for tumor radiation dose after peptide radionuclide therapy. Cancer Biother. Radiopharm. 2014;29(4):179–187. doi: 10.1089/cbr.2013.1552. [DOI] [PubMed] [Google Scholar]
- 95.Braat A.J.A.T., Snijders T.J., Seute T., Vonken E.P.A. Will 177Lu-DOTATATE treatment become more effective in salvage meningioma patients, when boosting somatostatin receptor saturation? A promising case on intra-arterial administration. Cardiovasc. Intervent. Radiol. 2019;42(11):1649–1652. doi: 10.1007/s00270-019-02262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ilan E., Sandström M., Wassberg C., Sundin A., Garske-Román U., Eriksson B., Granberg D., Lubberink M. Dose response of pancreatic neuroendocrine tumors treated with peptide receptor radionuclide therapy using 177Lu-DOTATATE. J. Nucl. Med. 2015;56(2):177–182. doi: 10.2967/jnumed.114.148437. [DOI] [PubMed] [Google Scholar]
- 97.Pauwels S., Barone R., Walrand S., Borson-Chazot F., Valkema R., Kvols L.K., Krenning E.P., Jamar F. Practical dosimetry of peptide receptor radionuclide therapy with (90)Y-labeled somatostatin analogs. J. Nucl. Med. 2005;46(Suppl. 1):92S–98S. [PubMed] [Google Scholar]