Abstract
Background
There is some controversy whether stroke history is an independent risk factor for poor prognosis of stroke or not. This study aimed to investigate the difference of mortality, disability and recurrent rate of ischemic stroke patients without and with stroke history, as well as to explore the effect of stroke history on stroke prognosis.
Methods
We analyzed patients with ischemic stroke enrolled in the China National Stroke Registry which was a nationwide, multicenter, and prospective registry of consecutive patients with acute cerebrovascular events from 2007 to 2008. Multivariable logistic regression was performed to assess the risk of worse prognosis of stroke history in patients with ischemic stroke.
Results
A total of 8181(65.9%) patients without stroke history and 4234(34.1%) patients with stroke history were enrolled in the study. The mortality, recurrence, modified Rankin Scale (mRS) 3-6 rate was 11.4%, 14.7% and 28.5% respectively at 1 year for patients without stroke history, which was significantly lower than that of 17.3%, 23.6%, 42.1% in patients with stroke history, respectively. Multivariable analysis showed that patients with stroke history had higher risk of death [odds ratio (OR) 1.34,95% confidence interval (CI) 1.17-1.54], recurrence (OR 1.47, 95 % CI 1.31-1.65) and mRS 3-6 (OR 1.49,95% CI 1.34-1.66) at 1 year.
Conclusion
After adjusting for the potential confounders, stroke history was still an independent risk factor for poor prognosis of ischemic stroke, which further emphasizes the importance of secondary prevention of ischemic stroke. The specific causes of poor prognosis in patients with history of stroke need to be furtherly investigated.
Keywords: Ischemic stroke, risk factors, prognosis, registry, mortality, disability
1. INTRODUCTION
Stroke is a major cause of death and disability worldwide. Approximately three-quarters of the global burden of stroke deaths (approximately 6.5 million per year) and associated disability-adjusted life years (approximately 113 million) occurred in low- and middle-income countries [1, 2]. In China, stroke is the leading cause of death and disability, accounting for nearly 2 million deaths and more than 3 million new cases in 2013 [3, 4]. About 2 of 3 strokes are first-ever events; while one-third have a history of stroke [5].
Risks of stroke prognosis are attributable to many nonmodifiable and modifiable risk factors, such as age, male gender, hypertension, diabetes mellitus, dyslipidemia, and history of stroke [6]. However, few studies have specifically investigated the impact of stroke history on the prognosis of stroke. Generally, recurrent stroke is considered to be more likely to be fatal or disabling than first-ever stroke [7-9], though there are also studies showing that previous stroke history was not an independent predictor of case fatalities [10]. Otherwise, the study by TIAregistry.org showed that previous stroke or TIA is an independent predictor of stroke recurrence in non-Asians, but not in Asians [11].
In this study, we presented the clinical characteristics and prognosis of patients with and without stroke history from the China National Stroke Registry (CNSR), and aimed to assess the difference of prognosis, including case- fatality, recurrence and functional outcome, between the two kinds of ischemic stroke at 3-month, 6-month, and 1-year; furthermore, we aimed to investigate the effect of stroke history on stroke prognosis.
2. MATERIALS AND METHODS
2.1. Study Population
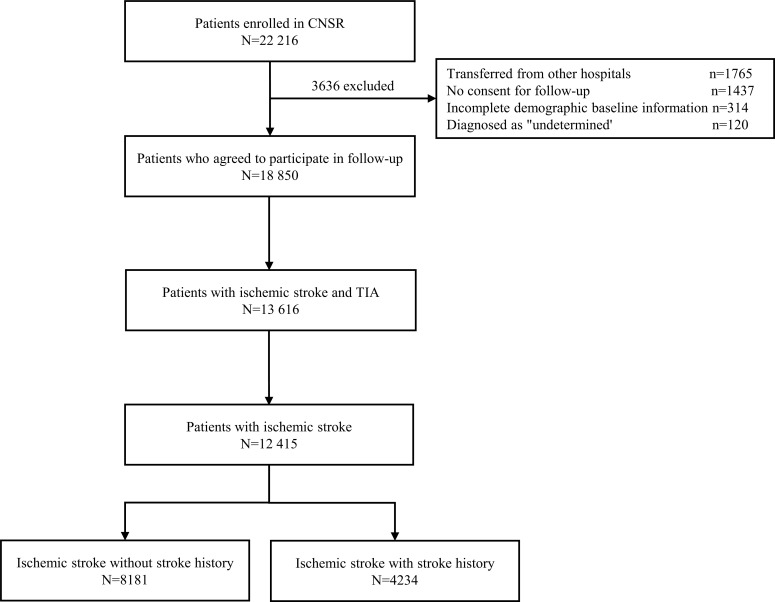
The study population was from the CNSR which was a national hospital-based prospective cohort study designed to evaluate the quality of care in hospitalized patients with acute cerebrovascular disease and measure clinical and functional outcomes at 12 months after disease onset. The design of the CNSR has been described previously [12]. Generally, the registry recruited consecutive patients from 132 centers across China if they were 18 years or older and presented within 14 days of symptom onset of a transient ischemic attack, ischemic stroke, intracranial hemorrhage, or subarachnoid hemorrhage confirmed by computed Tomography (CT) or magnetic resonance imaging (MRI). From September 2007 to August 2008, a total of 22 216 patients were approached to participate in the study. Finally, 12415 patients with ischemic stroke were divided into two groups: those with and without stroke history (Fig. 1).
Fig. (1).
flowchart for ischemic stroke patients with and without stoke history. A total of 22,216 patients were enrolled in CNSR. First, 3636 patients were excluded due to transfers from other hospitals, no consent for follow up, incomplete demographic baseline information, and diagnosis as undetermined. Second, 5234 patients were excluded because of the diagnosis of haemorrhagic cerebrovascular diseases. In addition, 1201 patients were excluded because of the diagnosis of TIA. Finally, 12,415 patients with ischemic stroke, including 8181 without stroke history and 4234 with, were included in this study for analysis. Abbreviations: CNSR, China National Stroke Registry; TIA, Transient Ischemic Attack.
2.2. Data Collection
Trained research coordinators at each institute collected baseline information, including patient demographics, vascular risk factors, stroke severity [National Institutes of Health Stroke Scale (NIHSS) score], medication use, and diagnosis. Vascular risk factors included a history of stroke, hypertension, diabetes mellitus, dyslipidemia, atrial fibrillation, coronary heart disease, smoking, and moderate or heavy alcohol consumption (≥2 alcoholic drinks per day). Hypertension was defined as systolic blood pressure (SBP) ≥140 mm Hg or diastolic blood pressure (DBP) ≥90 mm Hg, and the use of any antihypertensive drug or self-reported history of hypertension. Diabetes was defined as fasting glucose level ≥7.0 mmol/L, non-fasting glucose concentration ≥11.1 mmol/L, the use of any glucose-lowering drugs, or any self-reported history of diabetes. Dyslipidemia was defined as serum triglyceride level ≥150 mg/dL, low-density lipoprotein cholesterol level ≥140 mg/dL, high-density lipoprotein cholesterol level ≥40 mg/dL, the use of any lipid-lowering drugs, or any self-reported history of dyslipidemia. Body mass index (BMI) was calculated by dividing measured weight in kilograms by the square of measured height in meters and was categorized as 25, 25-30, and >30 kg/m2.
2.3. Outcome Assessment
Follow-up was performed by a centralized telephone interview. Data collection was performed by trained research personnel who were blinded to patient baseline clinical status. Patients were asked the standardized follow-up questions at 3, 6, and 12 months after stroke onset. Outcome data collected included all-cause death, stroke recurrence, and modified Rankin Scale (mRS). Recurrent cerebrovascular events included ischemic stroke, intracranial hemorrhage (ICH), and subarachnoid hemorrhage (SAH).
2.4. Statistical Analysis
Patient characteristics were summarized as mean values and standard deviation for continuous parameters, or absolute count and percentage for categorical parameters. The student t-test was used to compare continuous parameters, while χ2-test was used for categorical parameters. Demographic data and clinical manifestations in patients with stroke history were compared with first-ever stroke patients. The number and proportion of missing data for these variables were shown as following: BMI (1220, 9.8%), family history of stroke (805, 6.5%), NIHSS at admission (505, 4.1%), and Glasgow Coma Scale (GCS) (24, 0.2%). The associations between stroke history and death, further recurrence, and mRS 3-6 were analyzed using multivariable logistic regression after adjusting for potential confounders including age, gender, history of disease,smoking, drinking, NIHSS, medicine use before admission and during hospitalization, and complications during hospitalization. All data were analyzed by SAS version 9.4 statistical software (SAS Institute Inc, Cary, NC).
3. RESULTS
Among 12, 415 ischemic stroke patients, 8181 (65.9%) were patients without stroke history, while 4234 (34.1%) were patients with stroke history. There was no significant difference in gender (61.3% vs. 62.4%, P = 0.25) between patients without and with stroke history, but the patients with stroke history were 3.2 years older than first-ever patients on average (67.6 vs. 64.4, P<0.0001). The patients with stroke history had a similar proportion of smoking(39.6% vs. 40.0%, P = 0.72)with patients without stroke history. Comparing patients without stroke history, patients with stroke history had a lower proportion of moderate or heavy alcohol drinking (10.9% vs. 6.7%, P<0.0001) (Table 1).
Table 1.
Baseline characteristics of patients with and without a history of stroke.
| - | Total(n = 12415) | Without History(n = 8181) | With History(n = 4234) | P value |
|---|---|---|---|---|
| Age (year), mean ± SD | 65.5 ± 12.3 | 64.4 ± 12.8 | 67.6 ± 11.1 | <0.0001 |
| Gender (male), n (%) | 7658 (61.7%) | 5017 (61.3%) | 2641 (62.4%) | 0.25 |
| Smoking, n (%) | 4934 (39.7%) | 3242 (39.6%) | 1692 (40.0%) | 0.72 |
| Alcohol, n (%) | 1171 (9.4%) | 888 (10.9%) | 283 (6.7%) | <0.0001 |
| Health insurance, n (%) | 10052 (81%) | 6485 (79.3%) | 3567 (84.2%) | <0.0001 |
| BMI (Kg/m2) *, n (%) | - | - | - | 0.20 |
| >25.0 | 6815 (60.9%) | 4501 (61.1%) | 2314 (60.5%) | - |
| 25.0-29.9 | 3788 (33.8%) | 2463 (33.4%) | 1325 (34.6%) | - |
| ≥30 | 592 (5.3%) | 406 (5.5%) | 186 (4.9%) | - |
| Family history*, n (%) | 1519 (13.1%) | 926 (12.0%) | 593 (15.2%) | <0.0001 |
| Medical history, n (%) | - | - | - | - |
| Hypertension | 9000 (72.5%) | 5616 (68.6%) | 3384 (79.9%) | <0.0001 |
| Diabetes Mellitus | 3216 (25.9%) | 1914 (23.4%) | 1302 (30.8%) | <0.0001 |
| Hyperlipidemia | 1390 (11.2%) | 712 (8.7%) | 678 (16.0%) | <0.0001 |
| Coronary heart diseases | 1792 (14.4%) | 1026 (12.5%) | 766 (18.1%) | <0.0001 |
| Antiplatlet, n (%) | 2067 (16.6%) | 794 (9.7%) | 1273 (30.1%) | <0.0001 |
| Anticogulation, n (%) | 279 (2.2%) | 116 (1.4%) | 163 (3.8%) | <0.0001 |
| Prestroke mRS ≥2*, n (%) | 1165 (9.5%) | 276 (3.4%) | 889 (21.4%) | <0.0001 |
| NIHSS*, n (%) | - | - | - | <0.0001 |
| 0-3 | 4550 (38.2%) | 3183(40.5%) | 1367(33.7%) | - |
| 4-7 | 3512 (29.5%) | 2301(29.3%) | 1211(29.9%) | - |
| 8-14 | 2332 (19.6%) | 1487(18.9%) | 845 (20.8%) | - |
| 15-21 | 904 (7.6%) | 550 (7.0%) | 354 (8.7%) | - |
| ≥22 | 612 (5.1%) | 336 (4.3%) | 276 (6.8%) | - |
| Median (25th-75th) | 4 (2-9) | 4 (2-9) | 5 (2-10) | <0.0001 |
| GCS*, n (%) | - | - | - | <0.0001 |
| 3-5 | 176 (1.4%) | 98 (1.2%) | 78 (1.8%) | - |
| 6-10 | 959 (7.7%) | 557 (6.8%) | 402 (9.5%) | - |
| 11-15 | 11256 (90.8%) | 7507 (92.0%) | 3749 (88.6%) | - |
| Blood pressure (mmHg) | - | - | - | - |
| Mean SBP, mean ± SD | 151.2 (24.13) | 151.33 (24.33) | 150.97 (23.72) | 0.43 |
| Mean DBP, mean ± SD | 87.9 (13.99) | 88.1 (14.01) | 87.4 (13.95) | 0.02 |
*: Denominator may be different due to missing data. Abbreviations: NIHSS, national institutes of health stroke scale; mRS, Modified Rankin Scale.
Before admission, patients with stroke history were more likely to have a history of hypertension, diabetes mellitus, dyslipidemia, coronary heart diseases, taking antithrombosis medicine, and have a worse functional status, but similar for BMI. At admission, NIHSS in patients with stroke history was significantly higher than patients without stroke history (median: 5 vs. 4, P<0.0001). There was no statistical difference in the proportion of patients presenting to the hospital within 3 h of symptoms (21.1% vs. 22.5%, P = 0.10), and in the proportion of patients given thrombolysis medicine (4.6% vs. 3.9%, P = 0.10). The level of diastolic blood pressure at admission was higher in patients with stroke history, but the systolic blood pressure was not significantly different between patients without and with patients (Table 1).
During hospitalization, patients with stroke history had higher mortality (3.6% vs. 2.4%, P = 0.0002) and recurrent risk (5.8% vs. 3.1%, P<0.0001) than patients without stroke history. During follow-up, patients without stroke history had higher mortality (10.5% vs. 7.2%, P<0.0001), mRS 3-6 (30.8% vs. 22.6%, P<0.0001) and recurrent risk(16.1% vs. 10.3%, P<0.0001) than patients with stroke history at 3- month, with further higher mortality (17.3% vs. 11.4%, P<0.0001), mRS 3-6 (42.1% vs. 28.5%, P<0.0001) and recurrent risk(23.6% vs. 14.7%, P<0.0001), respectively at 12- month (Table 2).
Table 2.
Comparison of Outcome in Patients with and without a history of stroke.
| - | Total(n = 12415) | Without History(n = 8181) | With History(n = 4234) | P value |
|---|---|---|---|---|
| In-hospital | - | - | - | - |
| Mortality (%) | 352 (2.8%) | 199 (2.4%) | 153 (3.6%) | 0.0002 |
| Recurrence (%) | 497 (4.0%) | 253 (3.1%) | 244 (5.8%) | <0.0001 |
| 3-month | - | - | - | - |
| Mortality (%) | 1037 (8.4%) | 591 (7.2%) | 446 (10.5%) | <0.0001 |
| mRS 3-6 (%) | 4189 (33.8%) | 1848 (22.6%) | 1304 (30.8%) | <0.0001 |
| Recurrent stroke* (%) | 1494 (12.3%) | 824 (10.3%) | 670 (16.1%) | <0.0001 |
| 6-month | - | - | - | - |
| Mortality (%) | 1324 (10.7%) | 736 (9.0%) | 588 (13.9%) | <0.0001 |
| mRS 3-6 (%)* | 4129 (33.3%) | 2370 (29.0%) | 1759 (41.7%) | <0.0001 |
| Recurrent stroke* (%) | 1848 (15.5%) | 1019 (13.0%) | 829 (20.3%) | <0.0001 |
| 12-month | - | - | - | - |
| Mortality (%) | 1664 (13.4%) | 931 (11.4%) | 733 (17.3%) | <0.0001 |
| mRS 3-6 (%)* | 4101 (33.1%) | 2326 (28.5%) | 1775 (42.1%) | <0.0001 |
| Recurrent stroke (%)* | 2050 (17.7%) | 1115 (14.7%) | 935 (23.6%) | <0.0001 |
*: Denominator may be different due to missing data. Abbreviation: mRS, Modified Rankin Scale.
The unadjusted odds ratio (OR) of stroke history for death, mRS 3-6, and recurrence at 3-month, 6-month, and 12-month are shown in Table 3. After adjusting for potential confounders, multivariable logistic regression analyses showed that stroke history was an independent factor for death, mRS 3-6, and stroke recurrence at 3, 6, and 12- month, respectively, except for borderline statistical significance for death at 3-month (Table 3).
Table 3.
Unadjusted and adjusted Odds Ratios of poor outcomes in ischemic stroke patients with or without stroke history.
| - | Unadjusted OR (95% CI) | Adjusted OR (95% CI)* |
|---|---|---|
| 3-month | - | - |
| Death | 1.51 (1.33-1.72) | 1.18 (0.99-1.39) |
| MRS 3-6 | 1.66 (1.54-1.80) | 1.34 (1.20-1.49) |
| Recurrence | 1.68 (1.50-1.87) | 1.38 (1.21-1.57) |
| 6-month | - | - |
| Death | 1.63 (1.45-1.83) | 1.29 (1.11-1.51) |
| MRS 3-6 | 1.75 (1.62-1.89) | 1.41 (1.27-1.57) |
| Recurrence | 1.71 (1.54-1.89) | 1.40 (1.24-1.57) |
| 12-month | - | - |
| Death | 1.63 (1.47-1.81) | 1.34 (1.16-1.54) |
| MRS 3-6 | 1.82 (1.69-1.97) | 1.49 (1.34-1.66) |
| Recurrence | 1.79 (1.63-1.97) | 1.47 (1.31-1.65) |
*: Adjusting for potential confounders including age, gender, history of hypertension, dyslipidemia, diabetes mellitus, coronary heart disease and atrial fibrillation, family history of stroke, smoking, drinking, modified Rankin scale before stroke onset, NIHSS, medicine use before admission (anticoagulant, antiplatelet, antihypertensive, dyslipidemia, management of diabetes mellitus) and during hospitalization (tissue-type plasminogen activator, anticoagulant, antiplatelet, dyslipidemia, management of diabetes mellitus), and complications during hospitalization (urinary tract infection, pneumonia, gastrointestinal bleeding).
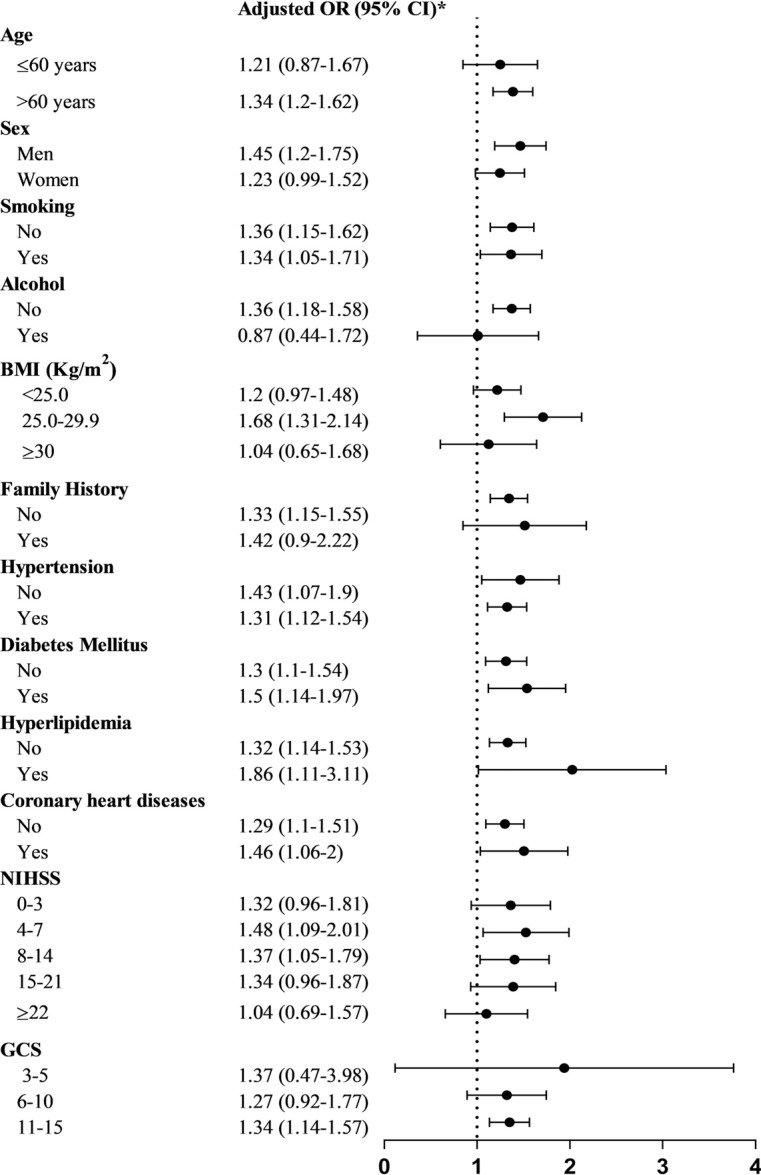
In the subgroup analysis according to age, sex, smoking, alcohol, BMI, family history, hypertension, DM, hyperlipidemia, coronary heart diseases, NIHSS and GCS, stroke history showed significantly higher 12-month mortality in most subgroups; and in some subgroups, only trend instead of statistical difference was found mainly because of limited samples (Fig. 2).
Fig. (2).
Subgroup analysis of 12-month mortality of patients with and without stroke history. *adjusted OR had been adjusted for potential confounders including age, gender, history of hypertension, dyslipidemia, diabetes mellitus, coronary heart disease and atrial fibrillation, family history of stroke, smoking, drinking, modified Rankin scale before stroke onset, NIHSS, medicine use before admission (anticoagulant, antiplatelet, antihypertensive, dyslipidemia, management of diabetes mellitus) and during hospitalization (tissue-type plasminogen activator, anticoagulant, antiplatelet, dyslipidemia, management of diabetes mellitus), and complications during hospitalization (urinary tract infection, pneumonia, gastrointestinal bleeding). Abbreviations: OR, odds ratio; BMI, Body Mass Index; NIHSS, National Institutes of Health Stroke Scale; GCS, Glasgow Coma Scale.
4. DISCUSSION
On the large scale, nationwide stroke registry in China, we found that patients with stroke history had a worse prognosis compared to first-ever patients, and stroke history was an independent risk factor for poor prognosis of short and long term. It provided strong evidence that secondary prevention after stroke should be further emphasized for reducing mortality, disability, and recurrence for stroke patients.
We found that a significantly higher proportion of hypertension and diabetes mellitus, as well as taking antithrombosis and hypoglycemic drugs in patients with stroke history. These findings may be explained by the low awareness of risk factors in first-ever stroke patients, which leads to inadequate control of their personal risk factors for stroke prevention [13].
The case fatality of stroke varies considerably between different ethnic and geographic populations [14-16]. The observed mortality of 13.4% in our study is similar to that reported in previous studies in Chinese stroke populations, but much lower than that reported in the Western countries (23.6%) [17, 18]. The relatively lower mortality in our study compared to the western countries may be associated with the less severity of stroke patients [19, 20]. However, about 18% of patients in our study had a recurrent stroke in the first year, which was higher than that of 11% reported in a recent meta-analysis of 13 studies of 9115 stroke patients mostly in Western populations [21]. The high recurrent risk in China may reflect the inadequate use of those proven secondary preventive treatments in stroke patients.
In this study, the mortality of patients with stroke history was higher than that of patients without stroke history in 6 and 12 months, however, there is an only borderline significant difference in 3-month mortality after adjusting for various risk factors. This may partly be due to the progress of new medical technology and health insurance coverage which mainly targets decreasing short-term mortality [22-24]. Compared to the first-ever stroke, we found that the mortality of patients with stroke history was still significantly higher at the 6 and 12-month follow-up, and the mRS 3-6 was persistently higher at any follow-up point, which indicates that the prognosis of patients with stroke history is more serious than that of the first-ever stroke. The reasons for the higher mortality and disability rate of patients with stroke history remain a mystery to this day. After adjustment of age, medical history, disease severity, etc. stroke history is still significantly associated with a worse prognosis. Thus, it is difficult to explain by the generally known risk factors such as age and severity of the disease. The prognosis of patients with stroke history is worse, which may be related to the poor reactivity to antithrombosis agents, or poor social/ family support after discharge from the hospital. However, specific mechanisms need to be further clarified.
Our study also has some limitations. Firstly, the enrolled patients were restricted to hospitalized patients, and out-patients were not included, which probably influences the re- presentative of the stroke patients. Secondly, the history of stroke did not distinguish between hemorrhagic and ischemic stroke, because many patients were often difficult to clarify their own stroke types. With the consideration that ischemic stroke is more common than intracerebral hemorrhage even in recurrent intracerebral types [25, 26], in this study, some patients were included with a history of intra- cerebral hemorrhage. This study suggests that stroke history significantly exacerbates the prognosis of ischemic stroke even without differentiating stroke types. Thirdly, the follow-up by telephone interview might introduce misjudgment into the functional outcome evaluation because some of the interviewees might not be able to answer the questions accurately. However, evidence from previous studies indicates that structured telephone assessment of the mRS is reliable and comparable with a face-to-face interview with the good agreement [27]. Despite these limitations, our study is based on the CNSR, which is a nationwide, prospective, multicenter, and comprehensive registry designed to observe the characteristics and outcomes of stroke patients. The results strongly suggest that patients with recurrent stroke have a worse prognosis. Intensive secondary prevention could be conducted to partly reduce stroke recurrence, thus improve the prognosis. However, it also notably implies that some stroke patients may respond poorly to the treatment and are prone to recurrence. Further studies should focus on finding potential mechanisms, including genes, medicine meta- bolism, and medicine resistance.
CONCLUSION
In conclusion, the mortality and disability rates of patients with stroke history are significantly higher than that of first-ever stroke patients, which suggests clinical physicians to pay more attention to prevent recurrent stroke. Further studies are needed to investigate the determinants of outcomes in patients with stroke history to provide optimal stroke treatment and management for patients with stroke history.
AUTHORS' CONTRIBUTIONS
Yongjun Wang designed the study. Gaifen Liu conceived the study. Haiqiang Qin drafted the article, with further contributions from Penglian Wang, Miaoxin Yu, and Guitao Zhang. Haiqiang Qin, Runhua Zhang and Gaifen Liu managed and analyzed the data. All authors interpreted data and approved the final version of the article.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- BMI
Body Mass Index
- CI
Confidence Interval
- CT
Computed Tomography
- CNSR
China National Stroke Registry
- DBP
Diastolic Blood Pressure
- GCS
Glasgow Coma Scale
- ICH
Intracranial Hemorrhage
- MRI
Magnetic Resonance Imaging
- mRS
Modified Rankin Scale
- NIHSS
National Institutes of Health Stroke Scale
- OR
Odds Ratio
- SBP
Systolic Blood Pressure
- SAH
Subarachnoid Hemorrhage
Funding Statement
This work was financially supported by National key R & D Plan of Ministry of Science and Technology of China (Grant no. 2016YFC1301604, 2017YFC1307702); National Natural Science Foundation of China (Grant no. 81870907); National key R & D Plan of Ministry of Science and Technology of China (Grant no. 2016YFC0901002, 2018YFC- 1312303), Ministry of Science and Technology and the Ministry of Health of the People’s Republic of China (Grant no. 2006BA101A11, and 2009CB521905), National Science and Technology Major Project (Grant no. 2017ZX0930- 4018), Capital’s Funds for Health Improvement and Research (Grant no. 2020-1-2041).
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Central Ethics Committee at Beijing Tiantan Hospital, Capital Medical University, China (Approval no. 2006BAI01A11).
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was financially supported by National key R & D Plan of Ministry of Science and Technology of China (Grant no. 2016YFC1301604, 2017YFC1307702); National Natural Science Foundation of China (Grant no. 81870907); National key R & D Plan of Ministry of Science and Technology of China (Grant no. 2016YFC0901002, 2018YFC- 1312303), Ministry of Science and Technology and the Ministry of Health of the People’s Republic of China (Grant no. 2006BA101A11, and 2009CB521905), National Science and Technology Major Project (Grant no. 2017ZX0930- 4018), Capital’s Funds for Health Improvement and Research (Grant no. 2020-1-2041).
CONFLICT OF INTEREST
The authors have no conflicts of interest, financial or otherwise.
REFERENCES
- 1.Feigin V.L., Roth G.A., Naghavi M., Parmar P., Krishnamurthi R., Chugh S., Mensah G.A., Norrving B., Shiue I., Ng M., Estep K., Cercy K., Murray C.J.L., Forouzanfar M.H. Global Burden of Diseases, Injuries and Risk Factors Study 2013 and Stroke Experts Writing Group. Global burden of stroke and risk factors in 188 countries, during 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016;15(9):913–924. doi: 10.1016/S1474-4422(16)30073-4. [DOI] [PubMed] [Google Scholar]
- 2.Feigin V.L., Krishnamurthi R.V., Parmar P., Norrving B., Mensah G.A., Bennett D.A., Barker-Collo S., Moran A.E., Sacco R.L., Truelsen T., Davis S., Pandian J.D., Naghavi M., Forouzanfar M.H., Nguyen G., Johnson C.O., Vos T., Meretoja A., Murray C.J., Roth G.A. GBD 2013 Writing Group; GBD 2013 Stroke Panel Experts Group. Update on the global burden of ischemic and hemorrhagic stroke in 1990-2013: The GBD 2013 Study. Neuroepidemiology. 2015;45(3):161–176. doi: 10.1159/000441085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou M., Wang H., Zhu J., Chen W., Wang L., Liu S., Li Y., Wang L., Liu Y., Yin P., Liu J., Yu S., Tan F., Barber R.M., Coates M.M., Dicker D., Fraser M., González-Medina D., Hamavid H., Hao Y., Hu G., Jiang G., Kan H., Lopez A.D., Phillips M.R., She J., Vos T., Wan X., Xu G., Yan L.L., Yu C., Zhao Y., Zheng Y., Zou X., Naghavi M., Wang Y., Murray C.J., Yang G., Liang X. Cause-specific mortality for 240 causes in China during 1990-2013: A systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet. 2016;387(10015):251–272. doi: 10.1016/S0140-6736(15)00551-6. [DOI] [PubMed] [Google Scholar]
- 4.Wang W., Jiang B., Sun H., Ru X., Sun D., Wang L., Wang L., Jiang Y., Li Y., Wang Y., Chen Z., Wu S., Zhang Y., Wang D., Wang Y., Feigin V.L. NESS-China Investigators. Prevalence, Incidence, and Mortality of Stroke in China: Results from a Nationwide Population-Based Survey of 480 687 Adults. Circulation. 2017;135(8):759–771. doi: 10.1161/CIRCULATIONAHA.116.025250. [DOI] [PubMed] [Google Scholar]
- 5.LaBresh K.A., Reeves M.J., Frankel M.R., Albright D., Schwamm L.H. Hospital treatment of patients with ischemic stroke or transient ischemic attack using the “Get With The Guidelines” program. Arch. Intern. Med. 2008;168(4):411–417. doi: 10.1001/archinternmed.2007.101. [DOI] [PubMed] [Google Scholar]
- 6.Hackam D.G., Spence J.D. Combining multiple approaches for the secondary prevention of vascular events after stroke: A quantitative modeling study. Stroke. 2007;38(6):1881–1885. doi: 10.1161/STROKEAHA.106.475525. [DOI] [PubMed] [Google Scholar]
- 7.Coull A.J., Rothwell P.M. Underestimation of the early risk of recurrent stroke: Evidence of the need for a standard definition. Stroke. 2004;35(8):1925–1929. doi: 10.1161/01.STR.0000133129.58126.67. [DOI] [PubMed] [Google Scholar]
- 8.Hankey G.J., Jamrozik K., Broadhurst R.J., Forbes S., Burvill P.W., Anderson C.S., Stewart-Wynne E.G. Long-term risk of first recurrent stroke in the Perth Community Stroke Study. Stroke. 1998;29(12):2491–2500. doi: 10.1161/01.STR.29.12.2491. [DOI] [PubMed] [Google Scholar]
- 9.Lee J.D., Hu Y.H., Lee M., Huang Y.C., Kuo Y.W., Lee T.H. High risk of one-year stroke recurrence in patients with younger age and prior history of ischemic stroke. Curr. Neurovasc. Res. 2019;16(3):250–257. doi: 10.2174/1567202616666190618164528. [DOI] [PubMed] [Google Scholar]
- 10.Lekoubou A., Nkoke C., Dzudie A., Kengne A.P. Stroke admission and case-fatality in an urban medical unit in sub-Saharan Africa: A fourteen year trend study from 1999 to 2012. J. Neurol. Sci. 2015;350(1-2):24–32. doi: 10.1016/j.jns.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Hoshino T., Uchiyama S., Wong L.K.S., Sissani L., Albers G.W., Bornstein N.M., Caplan L.R., Donnan G.A., Ferro J.M., Hennerici M.G., Labreuche J., Lavallée P.C., Molina C., Rothwell P.M., Steg P.G., Touboul P.J., Vicaut É., Amarenco P. TIAregistry.org Investigators. Differences in characteristics and outcomes between asian and non-asian patients in the TIAregistry.org. Stroke. 2017;48(7):1779–1787. doi: 10.1161/STROKEAHA.117.016874. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y., Cui L., Ji X., Dong Q., Zeng J., Wang Y., Zhou Y., Zhao X., Wang C., Liu L., Nguyen-Huynh M.N., Claiborne Johnston S., Wong L., Li H. China National Stroke Registry Investigators. The China National Stroke Registry for patients with acute cerebrovascular events: Design, rationale, and baseline patient characteristics. Int. J. Stroke. 2011;6(4):355–361. doi: 10.1111/j.1747-4949.2011.00584.x. [DOI] [PubMed] [Google Scholar]
- 13.Powers B.J., Oddone E.Z., Grubber J.M., Olsen M.K., Bosworth H.B. Perceived and actual stroke risk among men with hypertension. J. Clin. Hypertens. (Greenwich) 2008;10(4):287–294. doi: 10.1111/j.1751-7176.2008.07797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asplund K., Ashburner S., Cargill K., Hux M., Lees K., Drummond M. GAIN International Investigators. Health care resource use and stroke outcome. Multinational comparisons within the GAIN International trial. Int. J. Technol. Assess. Health Care. 2003;19(2):267–277. doi: 10.1017/S0266462303000242. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y., Wright N., Guo Y., Turnbull I., Kartsonaki C., Yang L., Bian Z., Pei P., Pan D., Zhang Y., Qin H., Wang Y., Lv J., Liu M., Hao Z., Wang Y., Yu C., Peto R., Collins R., Li L., Clarke R., Chen Z. China Kadoorie Biobank Collaborative Group. Mortality and recurrent vascular events after first incident stroke: A 9-year community-based study of 0·5 million Chinese adults. Lancet Glob. Health. 2020;8(4):e580–e590. doi: 10.1016/S2214-109X(20)30069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin H., Chen Y., Liu G., Turnbull I., Zhang R., Li Z., Wang Y., Liu L., Zhao X., Chen Z., Wang Y. Management characteristics and prognosis after stroke in China: Findings from a large nationwide stroke registry. Stroke Vasc Neurol 2020; [Epub ahead of print]. doi: 10.1136/svn-2020-000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saposnik G., Hill M.D., O’Donnell M., Fang J., Hachinski V., Kapral M.K. Registry of the Canadian Stroke Network for the Stroke Outcome Research Canada (SORCan) Working Group. Variables associated with 7-day, 30-day, and 1-year fatality after ischemic stroke. Stroke. 2008;39(8):2318–2324. doi: 10.1161/STROKEAHA.107.510362. [DOI] [PubMed] [Google Scholar]
- 18.Fonarow G.C., Reeves M.J., Zhao X., Olson D.M., Smith E.E., Saver J.L., Schwamm L.H. Get With the Guidelines-Stroke Steering Committee and Investigators. Age-related differences in characteristics, performance measures, treatment trends, and outcomes in patients with ischemic stroke. Circulation. 2010;121(7):879–891. doi: 10.1161/CIRCULATIONAHA.109.892497. [DOI] [PubMed] [Google Scholar]
- 19.Fonarow G.C., Reeves M.J., Smith E.E., Saver J.L., Zhao X., Olson D.W., Hernandez A.F., Peterson E.D., Schwamm L.H. GWTG-Stroke Steering Committee and Investigators. Characteristics, performance measures, and in-hospital outcomes of the first one million stroke and transient ischemic attack admissions in get with the guidelines-stroke. Circ. Cardiovasc. Qual. Outcomes. 2010;3(3):291–302. doi: 10.1161/CIRCOUTCOMES.109.921858. [DOI] [PubMed] [Google Scholar]
- 20.Cumbler E., Wald H., Bhatt D.L., Cox M., Xian Y., Reeves M., Smith E.E., Schwamm L., Fonarow G.C. Quality of care and outcomes for in-hospital ischemic stroke: Findings from the National Get With The Guidelines-Stroke. Stroke. 2014;45(1):231–238. doi: 10.1161/STROKEAHA.113.003617. [DOI] [PubMed] [Google Scholar]
- 21.Mohan K.M., Wolfe C.D., Rudd A.G., Heuschmann P.U., Kolominsky-Rabas P.L., Grieve A.P. Risk and cumulative risk of stroke recurrence: A systematic review and meta-analysis. Stroke. 2011;42(5):1489–1494. doi: 10.1161/STROKEAHA.110.602615. [DOI] [PubMed] [Google Scholar]
- 22.Minnerup J., Wersching H., Unrath M., Berger K. Explaining the decrease of in-hospital mortality from ischemic stroke. PLoS One. 2015;10(7):: e0131473. doi: 10.1371/journal.pone.0131473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nimptsch U., Mansky T. Stroke unit care and trends of in-hospital mortality for stroke in Germany 2005-2010. Int. J. Stroke. 2014;9(3):260–265. doi: 10.1111/ijs.12193. [DOI] [PubMed] [Google Scholar]
- 24.Wang W., Wang D., Liu H., Sun H., Jiang B., Ru X., Sun D., Chen Z., Wang Y. Trend of declining stroke mortality in China: reasons and analysis. Stroke Vasc. Neurol. 2017;2(3):132–139. doi: 10.1136/svn-2017-000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zia E., Engström G., Svensson P.J., Norrving B., Pessah-Rasmussen H. Three-year survival and stroke recurrence rates in patients with primary intracerebral hemorrhage. Stroke. 2009;40(11):3567–3573. doi: 10.1161/STROKEAHA.109.556324. [DOI] [PubMed] [Google Scholar]
- 26.Hill M.D., Silver F.L., Austin P.C., Tu J.V. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke. 2000;31(1):123–127. doi: 10.1161/01.STR.31.1.123. [DOI] [PubMed] [Google Scholar]
- 27.Merino J.G., Lattimore S.U., Warach S. Telephone assessment of stroke outcome is reliable. Stroke. 2005;36(2):232–233. doi: 10.1161/01.STR.0000153055.43138.2f. [DOI] [PubMed] [Google Scholar]