Abstract
Background
Metabolic information provided by 18F-FDG PET/CT are useful for initial staging, therapy planning, response evaluation, and to a lesser extent for the follow-up of non-small cell lung cancer (NSCLC). To date, there are no established clinical guidelines in treatment response and early detection of recurrence.
Objective
To provide an overview of 18F-FDG PET/CT in NSCLC and in particular, to discuss its utility in treatment response evaluation and restaging of lung cancer.
Methods
A comprehensive search was used based on PubMed results. From all studies published in English those that explored the role of 18F-FDG PET/CT in the treatment response scenario were selected.
Results
Several studies have demonstrated that modifications in metabolic activity, expressed by changes in SUV both in the primary tumor as well as in regional lymph nodes, are associated with tumor response and survival. Beside SUV, other metabolic parameters (i.e. MTV, TLG, and percentage changes) are emerging to be helpful for predicting clinical outcomes.
Conclusion
18F-FDG parameters appear to be promising factors for evaluating treatment response and for detecting recurrences, although larger prospective trials are needed to confirm these evidences and to determine optimal cut-off values.
Keywords: Lung cancer, NSCLC, FDG PET, response evaluation, restaging, PET/CT
1. INTRODUCTION
Lung cancer is the leading cause of cancer-related death worldwide. Its management needs a multidisciplinary approach where imaging techniques play an essential role. 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography (18F-FDG PET/CT) has become a powerful tool in lung cancer management due to its ability to assess regional lymph node spread more precisely than CT scan and to detect metastatic lesions that would have been missed or equivocal at conventional imaging. ESMO guidelines for non-small-cell lung cancer (NSCLC) consider 18F-FDG PET/CT mandatory for staging at diagnosis and recommend its use when recurrence is suspected based on CT scan [1]. 18F-FDG PET/CT is also considered the most sensitive modality in detecting bone metastasis with higher sensitivity and specificity than bone scintigraphy. Nevertheless, follow-up with 18F-FDG PET/CT is not routinely recommended, due to its high sensitivity and relatively low specificity [2]. NCCN guidelines recommend 18F-FDG PET/CT as an initial assessment at the diagnosis of NSCLC and for restaging after induction therapy [3]. The aim of this monograph is to discuss the utility of 18F-FDG PET/CT in treatment response evaluation and the restaging of lung cancer.
2. CLINICAL CONTEXT
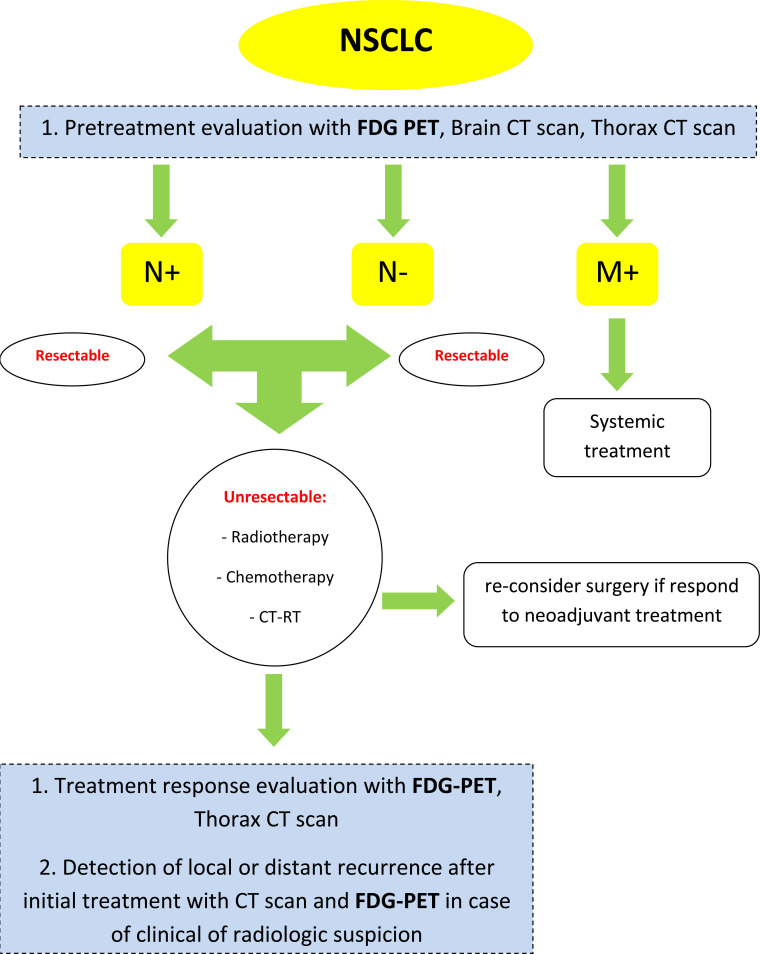
The use of 18F-FDG PET/CT is considered in a different clinical setting (Fig. 1).
Fig. (1).
Flow diagram; use of 18F-FDG PET/CT in different setting. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
2.1. Differential Diagnosis of Solitary Pulmonary Nodules
A solitary pulmonary nodule is defined as a discrete, well-marginated, rounded opacity less than or equal to 3 cm in diameter that is completely surrounded by lung parenchyma, does not touch the hilum or mediastinum, and is not associated with adenopathy, atelectasis, or pleural effusion. Lesions larger than 3 cm are considered malignant until proven otherwise [4]. 18F-FDG PET/CT is accurate in differentiating benign from malignant lesions as small as 1 cm; observation of the metabolic activity of the nodules, measured by standardized uptake value (SUV), has proven to help in the differential diagnosis. An overall sensitivity of 96% (range, 83%-100%), specificity of 79% (range, 52%-100%), and accuracy of 91% (range, 86%-100%) can be expected [5, 6]. False-negative results can occur in lesions smaller than 1 cm and in tumors with a low metabolism, like carcinoid tumors and bronchioloalveolar cell carcinomas; false-positive results are expected in inflammatory conditions.
2.2. Staging at Diagnosis
Around 18-36% of patients with NSCLC have distant metastases at diagnosis. Detection of metastasis at initial staging plays a key role in the most appropriate management of disease, with a direct impact on prognosis; 18F-FDG PET/CT is not able to detect brain metastases, due to the high glucose uptake of normal brain parenchyma [7]. When the absence of metastasis is proved, the most important decision is between those patients that can immediately undergo surgery and those who are unresectable but can benefit from neoadjuvant chemotherapy, radiotherapy alone, or a combined approach of chemotherapy and radiotherapy. In case of positive ipsilateral mediastinal lymph nodes (N2 disease), neoadjuvant platinum-based chemotherapy can be considered; in case of contralateral mediastinal lymph nodes or supraclavicular nodes (N3 disease), surgery is usually excluded and a combination of concomitant or sequential chemo-radiation therapy is considered the gold standard [8]. The good negative predictive value of 18F-FDG PET/CT in lymph node assessment is its most important characteristic, thus permitting a correct staging avoiding invasive procedures. In addition, in the case of pleural effusion, a negative PET/CT scan can reduce the number of thoracenteses or thoracoscopic biopsies. Currently, 18F-FDG PET/CT and brain/thorax CT-scan are used for correct initial staging of newly diagnosed lung cancers.
2.3. Treatment Response Evaluation
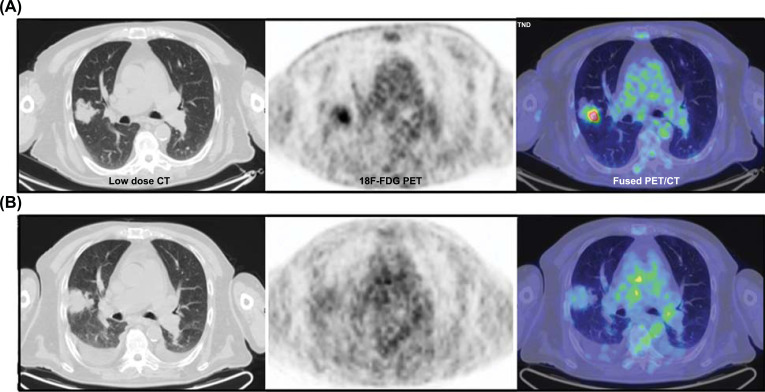
Traditionally tumor response to treatments is evaluated by CT scan taking into account the morphologic changes and using Response Evaluation Criteria In Solid Tumors (RECIST) [9]. However, 18F-FDG PET/CT provides functional information and detects metabolic changes earlier than morphologic changes. In patients with locally advanced NSCLC who undergo induction chemotherapy (Fig. 2), a correct restaging is decisive: CT scan has some limitations because small-sized lymph nodes can still harbor metastatic disease, whereas large lymph nodes can be inflammatory. This is noteworthy for an accurate restaging after induction treatment, where the involvement of ipsilateral and subcarinal mediastinal lymph nodes (N2 disease) as well as for contralateral hilar, mediastinal, and ipsilateral and contralateral supraclavicular involvement (N3 disease) is crucial for subsequent decisions and its intrinsic prognostic value. In a meta-analysis including 13 studies and 414 patients with NSCLC, the predictive value of 18F-FDG PET/CT in NSCLC patients with a pathological response after neoadjuvant therapy was significantly higher than that CT scan (P < 0.05) [10].
Fig. (2).
Response evaluation in neo-adjuvant setting; the clinical example illustrated in the figure represents an Adenocarcinoma (T2a pN2) of the right upper lobe treated with induction chemotherapy prior to surgery; after 3 cycles of therapy the patient presented with a stable disease on contrast enhanced CT, while there was a clear metabolic response on PET as visualized on axial views before (A) and after the treatment (B). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
2.4. Detection of Local or Distant Recurrence After Initial Treatment (Surgery, Chemoradiotherapy, or Radiotherapy)
Using the new staging system, 5-year survival rates were: stage IA1 92%, IA2 83%, IA3 77%; stage IB, 68%; stage IIA 60%; stage IIB 53%; stage IIIA 36%, IIIB 36%, IIIC 13%; stage IVA 10%, IVB 0%. Several clinic-pathological factors are known to be associated with recurrence in early-stage NSCLC, such as tumor size (T-stage), nodal involvement (N-stage), and smoking history. In the early stages, a regular follow-up program includes conventional imaging techniques like CT scan or X-Rays. A meta-analysis including 1035 patients and 13 articles compared the diagnostic value of FDG-PET with conventional imaging techniques for the detection of lung cancer recurrence. In this report, FDG-PET was found to be a superior modality for the diagnosis of local or distant recurrence [11]. Nevertheless, 18F-FDG PET/CT seems not a valid tool after stereotactic body radiation therapy in patients with inoperable stage I NSCLC because of a persistent 18F-FDG uptake that could be related to inflammation and fibrosis [12]. As a matter of fact, PET/TC is currently used only in patients with clinical or radiologic evidence of recurrence.
3. POSTTREATMENT EVALUATION WITH 18F-FDG PET/CT
Based on the clinical context, metabolic information provided by 18F-FDG PET/CT can be useful for the steps of lung cancer workup [13-15]. However, despite recent evidences on the role of 18F-FDG PET/CT in treatment response and early detection of recurrence, there are no established clinical guidelines in this scenario and data from literature are not univocal due to variations in methods, patient characteristics, time of imaging and response definition. Moreover, most of the to-date-available data refer to patients treated with cytotoxic agents [16, 17].
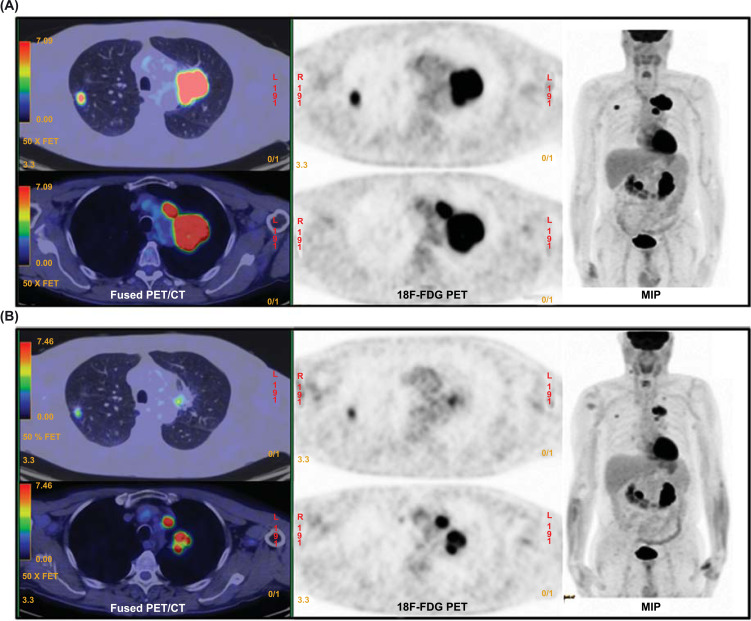
Several studies have demonstrated that modifications in metabolic activity, expressed by changes in SUV during induction therapy or at interim evaluation, are associated with tumor response and survival [18-21]. Kim et al. [19] retrospectively analyzed the prognostic value of 18F-FDG PET/CT in 42 patients with locally advanced NSCLC (stage IIIA-N2). Patients with reduction of SUVmax below 2.5 after 2 or 4 cycles of preoperative chemotherapy (Fig. 3), with or without radiation therapy, were considered responders and showed a median time to recurrence significantly longer than those with incomplete response (28.3 vs 9.1 months, respectively), whereas there was no significant difference in median overall survival rate. Similarly, Barnett et al. [20] evaluated the predictive value of SUVmax in 545 NSCLC patients treated with neoadjuvant therapy (chemotherapy, radiation therapy, or chemoradiotherapy) followed by surgery. On multivariate analysis, an increase versus a stable or reduced SUVmax after induction therapy was associated with worse survival.
Fig. (3).
Response evaluation during the course of chemotherapy; in this figure are illustrated the MIP (maximal intensity projection) and axial views of 18F-FDG PET/CT scans at baseline (A) and after 3 cycles of chemotherapy (B) of a patient with bilateral adenocarcinoma (cT1: right upper lobe; cT3N2: left upper lobe). Both primary tumor lesions and secondary adenopathies show a partial metabolic and morphological response to therapy. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
In a recent meta-analysis, the impact of SUVmax in the primary tumor as a predictor of local recurrence and increased risk of death was also highlighted for NSCLC patients receiving radiotherapy, both before and after the procedure [22]. Similar results were confirmed in other papers regardless of the type of radiation treatment, either SBRT (Stereotactic Body Radiation Therapy) or conventional radiation [20, 23-25].
Along with glucose uptake of the primary tumor, verification of the involvement of regional lymph nodes after induction therapy is fundamental for treatment planning in order to perform the best patients’ selection for surgery. As a matter of fact, differentiating between N2 or N3 status is crucial for subsequent decisions and its intrinsic prognostic value [26-29]. Kremer et al. [26] examined the value of 18F-FDG PET/CT in assessing the response to induction therapy at mediastinal lymph nodes among a cohort of 45 NSCLC patients with N2 status. They found posttreatment median SUVmax of N2 nodes significantly lower in responding than non-responding patients and suggested mediastinoscopy as not mandatory in patients with a negative PET/CT after neoadjuvant therapy. Nevertheless, an invasive procedure is still recommended to confirm lymph node involvement, although some studies have shown the utility of 18F-FDG PET/CT in the restaging of locally advanced NSCLC [28].
As previously discussed, SUVmax is the semi-quantitative PET/CT parameter widely used in clinical routine and the preferred parameter for evaluating the therapeutic response. However, tumors of large dimensions with central necrosis show highly heterogeneous 18F-FDG uptake and SUVmax may not represent the exact distribution of metabolic status. Therefore, in this case, it may be helpful to adopt metabolic parameters that incorporate both tumor volume and the intensity of uptake [14]. Metabolic tumor volume (MTV) and total lesion glycolysis (TLG) are two such parameters, although in the literature, they are limited mostly to the pretreatment setting in retrospective studies [30-32]. In the posttreatment scenario, Soussan et al. [33] analyzed the prognostic role of volume 18F-FDG PET/CT parameters in 32 patients with stage III NSCLC after induction chemotherapy followed by surgery. In a multivariate analysis, authors found that both tumor volume and TLG, along with SUVmax, SUVmean, and SUVpeak, were an independent prognostic factor for event-free survival. In particular, patients with MTV <22 ml or TLG <66 ml after induction therapy had better event-free survival than those with higher values. Nevertheless, none of the investigated indices was prognostic for OS. On the other hand, Kahraman et al. [34] assessed the response prediction and prognostic value of TLG in 30 patients with stage IV NSCLC treated with erlotinib. They found that MTV and TLG absolute values both at early (1 week) and late (6 week) assessment in the course of erlotinib constituted strong predictor factors for PFS, while the prognostic impact of the same parameters at baseline was limited. As can be observed, they used metabolic parameters as absolute values rather than cutoff values adopted in the previous study abovementioned. In the literature, different methods for tumor volume and TLG calculation, highlighting the lack and, thus, the need of a standardized process can be observed. Interestingly, some studies have demonstrated prognostic superiority for volume-based parameters (MTV and TLG) than SUVmax, although they are all based on pretreatment evaluation [35, 36]. Thus, larger prospective trials are needed to support the accurate use of MTV and TLG.
3.1. Criteria for Response Assessment
To date, a largely accepted consensus on the best imaging modality for posttreatment response evaluation has not been obtained [37, 38]. World Health Organization (WHO) criteria, RECIST, and RECIST 1.1 are the first criteria widely adopted in clinical routine and clinical trials for determining treatment response in solid tumors [17, 39]. However, these criteria are based on morphologic characteristics and they are not free from errors. First of all, inter- and intra-reader variability related to tumor lesion measurements tend to classify wrongly between 30-40% of tumor progression. Secondly, and probably the most important, they cannot detect any change in the metabolic activity of tumor cells, which usually occurs earlier than changes in size. This aspect is relevant especially in the last years where new anticancer therapies that stabilize the disease are increasing.
On the other hand, response assessment based on PET/CT parameters, such as European Organization for Research and Treatment of Cancer (EORTC) and PET Response Criteria in Solid Tumors (PERCIST) can detect a change in tumor metabolic activity earlier than the reduction in tumor size. In fact, the rationale is that the decrease of 18F-FDG uptake would be associated with a loss of viable cancer cells, while an increase in glucose metabolism and physically growing of cells in progressive tumors. Some studies have demonstrated the superiority of PERCIST compared to RECIST criteria in the assessment of tumor response and survival, both in NSCLC and SCLC (small-cell lung cancer) [40-43]. EORTC and PERCIST criteria propose using quantitative changes in SUV as an index of response: the first suggests a reduction of a minimum 25% in SUVmax, whereas the second recommends a reduction of 30% in SUVpeak corrected for lean body mass or an absolute drop of 0.8 SUVpeak units.
More recently, Hopkins criteria have been proposed as new PET-based criteria of response for lung cancer [44]. These criteria, which reproduce in some way Deauville score from lymphoma, are essentially qualitative considering mediastinal blood pool and liver 18F-FDG uptake as the reference standards. In detail, score 1 is a 18F-FDG uptake less than or equal to the mediastinal pool, score 2 if greater than mediastinal pool but less than liver, score 3 identifies likely inflammatory changes when 18F-FDG uptake is greater than liver but without focal aspect, while score 4 and 5 (focal and intense activity greater than liver) were referred to residual tumor.
As can be observed in the abovementioned criteria, volumetric or glycolytic changes in 18F-FDG uptake are not part of the existing response criteria, though they have shown a prognostic capability in NSCLC [30, 45]. However, there are limited data in the literature, and mostly retrospective in nature, comparing MTV and TLG versus available therapy response criteria, hence future studies are needed to confirm the findings.
3.2. Other Metabolic Parameters
Along with the absolute value of metabolic parameters at a single time-point, either at pre or posttreatment evaluation, other papers have investigated the role of the percentage change in 18F-FDG activity as a potential predictor of survival in patients with lung cancer. Zhang et al. [46] found ΔSUVmax of 50% as a significant parameter of response and survival at 1-year and 2-years, whilst in the study of Huang et al. [47], ΔMTV was the only independent prognostic factor for OS. Similarly, Usmanij et al. [45] assessed the role of early 18F-FDG PET/CT in a cohort of 28 NSCL patients treated chemoradiotherapy. ΔTLG was found to be associated with PFS, with a corresponding range of decrease comprises between 38-52%.
Furthermore, recent studies have shown that background activity-based PET metrics (background subtracted lesion activity (BSL) and background subtracted volume (BSV) are promising new prognostic NSCLC markers [48, 49]. For example, Burger et al. [49] showed that BSL and BSV were significantly better correlated with tumor response than MTV and TLG.
3.3. Limitations
Even if the studies above reported demonstrate 18F-FDG PET/CT parameters to be important and accurate in the management of NSCLC, other studies have failed to find a correlation between SUVmax and survival or response assessment in NSCLC patients. For example, Roy et al. [50] evaluated the prognostic role of PET/CT in 21 patients with locally advanced NSCLC. Although a significant decrease in post-treatment SUVmax was observed in metabolic responders, the authors did not find a significant difference in terms of PFS and OS between the responders and non-responders. One of the main limitations of SUVmax in the response evaluation is that it does not consider the spatial distribution of metabolic activity, as it quantifies 18-FDG uptake from a single hot pixel within the tumor mass. Additionally, it has been observed that SUVmax cutoff value, chosen to discriminate between favorable and unfavorable prognosis, is quite wide among studies ranging from 2.4 to 20 [22, 51]. This wide range could be related to different factors, such as uptake time, patient obesity, blood glucose level, image noise, technical issues (methods of attenuation correction, and reconstruction), or variation of the cohort studied. Similarly to SUVmax, the use of MTV and TLG has been characterized by arbitrary cutoff values and lack of a consensus uniformly accepted. In a recent meta-analysis by Im H-J et al. [52], MTV ranged between 0.3 and 68.3, while TLG between 9.6 and 525. Moreover, other issues related to volume-based PET/CT calculations are represented by the need for dedicated software/workstation and which threshold of SUV to be used. Different approaches have been described, from an absolute threshold value of 2.5 to a relative threshold mostly of 42%, but also 40% or 50% of SUVmax [48]. For instance, an adenocarcinoma tumor mass with an SUVmax of 2.4 is unmeasurable on MTV or TLG with an absolute threshold of SUV 2.5. In contrast, a lesion with high and uniform FDG activity is likely to be overestimated if a relative threshold of 50% of SUVmax is used.
Studies of the diagnostic performance of 18F-FDG PET/CT in the posttreatment assessment of lung cancer are summarized in Tables 1, 2 and 3.
4. ROLE OF PET/CT IN THE ERA OF IMMUNE CHECKPOINT INHIBITORS
In the last few years, immunotherapy with checkpoint inhibitors is offering new hope to cancer patients, comprising NSCLC [53]. Recently, three PD-1/PD-L1 blocking agents have been approved in either untreated (pembrolizumab) or pretreated patients (nivolumab, pembrolizumab and atezolizumab). Despite the improvement in survival outcomes showed in pivotal trials, not all patients benefit from this new approach as well as response evaluation to these new drugs is still challenging. In fact, at early evaluation, morphologic (RECIST, irRC, irRECIST) and metabolic criteria (EORTC and PERCIST) have shown a low inter-criteria agreement (k-values = 0.48-0.7) in a recent paper by Cho and colleagues [54], though in melanoma patients, suggesting that the reactivation of the immune system might determine an early increase of 18F-FDG uptake in the responder patients. As a consequence, the same group has tried to combine RECIST and PERCIST criteria to predict response to treatment with checkpoint inhibitors. They named these criteria PET/CT Criteria for early prediction of response to Immune-checkpoint inhibitor Therapy and showed that after 3-4 weeks, tumors classified as a stable disease by RECIST and with a change over 15.5% in SULpeak by 18F-FDG PET/CT had a good response at the end of treatment. One of the main problems with checkpoint inhibitors is related to the inflammatory reactions that recall neutrophils, macrophages and activate T cells on the tumor site. Consequently, 18F-FDG cannot be considered as a specific marker. In this scenario, a recent imaging technique based on monoclonal antibody specific combined with radioactive elements, so-called Immuno-PET might assist for response assessment [55]. Most of these new radiotracers are still in a preclinical setting, but they are tracing the path to better evaluate response in the course of checkpoint inhibitors.
CONCLUSION
Metabolic parameters extracted by 18F-FDG PET/CT, ranging from the historical SUVmax to the most recent MTV and TLG, seem to be valuable prognostic and predictive factors in NSCLC patients. The inclusion of quantitative 18F-FDG indexes, beyond SUVmax, to assess tumor response after each treatment represents the next future and the way for better patients’ selection, and cost-effectiveness. However, further large prospective clinical studies are necessary to confirm the prognostic role of 18F-FDG PET/CT and to determine the optimal cut-off values.
Table 1.
Posttreatment absolute 18F-FDG PET/CT parameters values.
| Author | Study | No Patients, Stage | Treatment | PET/CT Parameters Evaluation | Conclusions |
|---|---|---|---|---|---|
| Decoster [18] | Prospective | 31 NSCLC, inoperable III | Chemotherapy (paclitaxel, carboplatin and gemcitabine) + radiotherapy | SUVmax | Post-SUVmax <2.5 was associated with better time-to-progression and OS rates |
| Kim [19] | Retrospective | 42 NSCLC, IIIA N2 | Chemotherapy ± radiotherapy + surgery | SUVmax | Post-SUVmax <2.5 was associated with longer median time to recurrence, no significant difference in median OS time |
| Barnett [20] | Retrospective | 545 NSCLC, I-IV | Induction therapy (chemotherapy, radiotherapy, or chemoradiotherpy) + surgery | SUVmax | Increase in post-SUVmax for OS: HR= 2.04 (95% CI, 1.32 to 3.16) |
| Na [22] | Meta-analysis | 1518 NSCLC, I-IV | Chemoradiotherapy, SBRT | SUVmax | Post-SUVmax correlated with: LC: HR = 2.01 (95% CI, 1.16-3.46) OS: HR = 2.47 (95% CI, 0.58-13.03) |
| Clarke [24] | Retrospective | 82 NSCLC, T1-T2 | SBRT | SUVmax | Post-SUVmax <2 significantly associated with a lower risk of distant metastases |
| Bollineni [25] | Retrospective | 132 NSCLC, I | SBRT | SUVmax | Post-SUVmax >5 significantly associated with failure of LC: HR = 9.5 (95% CI, 1.8-49.0) |
| Kremer [26] | 42 NSCLC, III N2 | Chemotherapy, chemoradiotherapy | SUVmax of N2 | Post-SUVmax values after therapy significantly different between responding and non-responding N2 nodes | |
| Soussan [33] | Prospective | 32 NSCLC, III | Chemotherapy + surgery | SUVmax SUVmean SUVpeak MTV TLG |
All metabolic parameters were prognostic factor for event-free survival, but not for OS |
Abbreviations: HR, hazard ratio; LC, local control; NSCLC, non-small cell lung carcinoma; OS, overall survival; SUV, standardized uptake value.
Table 2.
Criteria for response assessment with 18F-FDG PET/CT.
| Author | Study | No Patients, Stage | Treatment | PET/CT Parameters Evaluation | Conclusions |
|---|---|---|---|---|---|
| Fledelius, [30] | Retrospective | 21 NSCLC, IIB-III | Cisplatin, vinorelbine | PERCIST | Responders vs non-responders: Median OS: 8.6 vs 2.6 mo, p < 0.001 Median PFS: 8.4 vs 2.7 mo, p < 0.001 |
| Ding [40] | Retrospective | 44 NSCLC II, III, IV | Chemotherapy | PERCIST, RECIST |
PERCIST was a significant factor for predicting DFS: HR= 3.20 (95% CI: 1.85-5.54). |
| Usmanij, 2017 [41] | Prospective | 25 NSCLC, inoperable IIIB-IV | Paclitaxel, carboplatin, bevacizumab ± erlotinib | PERCIST | Responders vs non-responders: Median OS: 9.1 vs 4.4 mo, p < 0.001 Median PFS: 22.8 vs 1.7 mo, p < 0.001 |
| Ziai [42] | Retrospective | 29 SCLC | Chemotherapy ± radiotherapy | EORTC PERCIST |
Perfect concordance was achieved between the EORTC and PERCIST criteria |
| Shang [43] | Prospective | 35 NSCLC, III-IV | Chemotherapy | RECIST EORTC PERCIST |
EORTC criteria and PERCIST were more sensitive and accurate than RECIST for the detection of an early therapeutic response |
| Sheikhbahaei [44] | Retrospective | 201 lung cancer, I-IV | All | Hopkins criteria | OS: HR = 2.12, p < 0.001 |
Abbreviations: EORTC, European organisation for research and treatment of cancer; HR, hazard ratio; LC, local control; NSCLC, non-small cell lung carcinoma; PERCIST, positron emission tomography response criteria in solid tumors; OS, overall survival; RECIST, response evaluation criteria in solid tumors.
Table 3.
Other posttreatment PET/CT parameters.
| Author | Study | No Patients, Stage | Treatment | PET/CT Parameters Evaluation | Conclusions |
|---|---|---|---|---|---|
| Barnett [20] | Retrospective | 78 NSCLC, I-IV | Chemotherapy, radiotherapy, or chemoradiotherpy + surgery | ΔSUVmax of N2 | % Reduction in N2 SUV >0% HR= 0.42 (95% CI 0.18-0.98) p=0.044 % Reduction in N2 >60% HR= 0.53 (0.30-0.93) p=0.028 |
| Wang [23] | Prospective | 44 NSCLC, inoperable I-III | Radiotherapy | ΔSUV | % Change of metabolic activity was shown to be predictive of survival. OS: HR = 0.97 (95% CI, 0.95-0.99); PFS: HR = 0.97 (95% CI, 0.96-0.99) |
| Kahraman [34] | Prospective | 30 NSCLC, IV | Erlotinib | TLG, ΔTLG |
Post-TLG and ΔTLG levels measured at early (1 week) or late (6 weeks) were predictive factors for PFS. |
| Usmanij, 2013 [45] | Retrospective | 28 NSCLC, III | Chemoradiotherapy | ΔTLG | ΔTLG associate with PFS: HR = 1.17 (95% CI, 1.01-1.35) |
| Zhang [46] | Prospective | 46 NSCLC, III | Chemoradiotherapy | ΔSUVmax | ΔSUVmax >50% predicted early therapy response and PFS |
| Huang [47] | Prospective | 53 NSCLC, | Chemoradiotherapy | ΔMTV | ΔMTV independent prognostic factor for OS |
| Burger [48] | Retrospective | 44 NSCLC, II-IV | Neoadjuvant chemotherapy | BSV BSL |
Post-BSV and post-BSL correlated with histopathologic tumor regression score |
| Roy [50] | Prospective | 21 NSCLC, III | Chemoradiotherapy | ΔSUVmax | ΔSUVmax >50% t did not provide any prognostic significance. |
Abbreviations: BSL, background subtracted lesion activity; BSV, background subtracted volume; Δ, percentage change; HR, hazard ratio; MTV, metabolic tumor volume; NSCLC, non-small cell lung carcinoma; OS, overall survival; SUV, standardized uptake value; TLG, total lesion glycolysis.
Acknowledgements
Declared none.
List of abbreviations
- 18F-FDG
18F-Fluorodeoxyglucose
- BSL
Background Subtracted Lesion Activity
- BSV
Background Subtracted Volume
- EORTC
Organization for Research and Treatment of Cancer
- irRC
Immune-Related Response Criteria
- irRECIST
RECIST for Immune Based Therapeutics
- MTV
Metabolic Tumor Volume
- NSCLC
Non-Small Cell Lung Carcinoma
- PERCIST
PET Response Criteria in Solid Tumors
- PET/CT
Positron Emission Tomography/Computed Tomography
- RECIST
Response Evaluation Criteria In Solid Tumors
- SBRT
Stereotactic Body Radiation Therapy
- SUV
Standardized Uptake Value
- TLG
Total Lesion Glycolysis
- WHO
World Health Organization
Funding Statement
The authors thank the AIRC (Associazione Italiana per la Ricerca sul Cancro) for the support on research with the Ggrant No. 18,923.
Consent for Publication
Not applicable.
Funding
The authors thank the AIRC (Associazione Italiana per la Ricerca sul Cancro) for the support on research with the Ggrant No. 18,923.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Postmus P.E., Kerr K.M., Oudkerk M. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017;284:iv1–iv21. doi: 10.1093/annonc/mdx222. [DOI] [PubMed] [Google Scholar]
- 2.Novello S., Barlesi F., Califano R., Cufer T., Ekman S., Levra M.G., Kerr K., Popat S., Reck M., Senan S., Simo G.V., Vansteenkiste J., Peters S. ESMO guidelines committee. metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016;27(Suppl. 5):v1–v27. doi: 10.1093/annonc/mdw326. [DOI] [PubMed] [Google Scholar]
- 3.Ettinger D.S., Aisner D.L., Wood D.E., Akerley W., Bauman J., Chang J.Y., Chirieac L.R., D’Amico T.A., Dilling T.J., Dobelbower M., Govindan R., Gubens M.A., Hennon M., Horn L., Lackner R.P., Lanuti M., Leal T.A., Lilenbaum R., Lin J., Loo B.W., Martins R., Otterson G.A., Patel S.P., Reckamp K., Riely G.J., Schild S.E., Shapiro T.A., Stevenson J., Swanson S.J., Tauer K., Yang S.C., Gregory K., Hughes M. NCCN guidelines insights: non-small cell lung cancer, version 5.2018. J. Natl. Compr. Canc. Netw. 2018;16(7):807–821. doi: 10.6004/jnccn.2018.0062. [DOI] [PubMed] [Google Scholar]
- 4.Austin J.H., Müller N.L., Friedman P.J., Hansell D.M., Naidich D.P., Remy-Jardin M., Webb W.R., Zerhouni E.A. Glossary of terms for CT of the lungs: recommendations of the Nomenclature Committee of the Fleischner Society. Radiology. 1996;200(2):327–331. doi: 10.1148/radiology.200.2.8685321. [DOI] [PubMed] [Google Scholar]
- 5.Gould M.K., Maclean C.C., Kuschner W.G., Rydzak C.E., Owens D.K. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA. 2001;285(7):914–924. doi: 10.1001/jama.285.7.914. [DOI] [PubMed] [Google Scholar]
- 6.Zhuang H., Pourdehnad M., Lambright E.S., Yamamoto A.J., Lanuti M., Li P., Mozley P.D., Rossman M.D., Albelda S.M., Alavi A. Dual time point 18F-FDG PET imaging for differentiating malignant from inflammatory processes. J. Nucl. Med. 2001;42(9):1412–1417. [PubMed] [Google Scholar]
- 7.Quint L.E. Staging non-small cell lung cancer. Cancer Imaging. 2007;7:148–159. doi: 10.1102/1470-7330.2007.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martini N., Kris M.G., Ginsberg R.J. The role of multimodality therapy in locoregional non-small cell lung cancer. Surg. Oncol. Clin. N. Am. 1997;6(4):769–791. doi: 10.1016/S1055-3207(18)30303-X. [DOI] [PubMed] [Google Scholar]
- 9.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., Dancey J., Arbuck S., Gwyther S., Mooney M., Rubinstein L., Shankar L., Dodd L., Kaplan R., Lacombe D., Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Zhang C., Liu J., Tong J., Sun X., Song S., Huang G. 18F-FDG-PET evaluation of pathological tumour response to neoadjuvant therapy in patients with NSCLC. Nucl. Med. Commun. 2013;34(1):71–77. doi: 10.1097/MNM.0b013e3283599999. [DOI] [PubMed] [Google Scholar]
- 11.He Y.Q., Gong H.L., Deng Y.F., Li W.M. He YQ1. Diagnostic efficacy of PET and PET/CT for recurrent lung cancer: a meta-analysis. Acta Radiol. 2014;55(3):309–317. doi: 10.1177/0284185113498536. [DOI] [PubMed] [Google Scholar]
- 12.Cuaron J., Dunphy M., Rimner A. Role of FDG-PET scans in staging, response assessment, and follow-up care for non-small cell lung cancer. Front. Oncol. 2013;2:208. doi: 10.3389/fonc.2012.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ung Y.C., Maziak D.E., Vanderveen J.A., Smith C.A., Gulenchyn K., Lacchetti C., Evans W.K. Lung Cancer Disease Site Group of Cancer Care Ontario’s Program in Evidence-Based Care. 18Fluorodeoxyglucose positron emission tomography in the diagnosis and staging of lung cancer: a systematic review. J. Natl. Cancer Inst. 2007;99(23):1753–1767. doi: 10.1093/jnci/djm232. [DOI] [PubMed] [Google Scholar]
- 14.Hicks R.J. Role of 18F-FDG PET in assessment of response in non-small cell lung cancer. J. Nucl. Med. 2009;50(Suppl. 1):31S–42S. doi: 10.2967/jnumed.108.057216. [DOI] [PubMed] [Google Scholar]
- 15.Sheikhbahaei S., Mena E., Yanamadala A., Reddy S., Solnes L.B., Wachsmann J., Subramaniam R.M. The value of FDG PET/CT in treatment response assessment, follow-up, and surveillance of lung cancer. AJR Am. J. Roentgenol. 2017;208(2):420–433. doi: 10.2214/AJR.16.16532. [DOI] [PubMed] [Google Scholar]
- 16.Cheng G., Huang H. Prognostic value of 18F-fluorodeoxyglucose PET/computed tomography in non-small-cell lung Cancer. PET Clin. 2018;13(1):59–72. doi: 10.1016/j.cpet.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Nishino M., Hatabu H., Johnson B.E., McLoud T.C. State of the art: Response assessment in lung cancer in the era of genomic medicine. Radiology. 2014;271(1):6–27. doi: 10.1148/radiol.14122524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Decoster L., Schallier D., Everaert H., Nieboer K., Meysman M., Neyns B., De Mey J., De Grève J. Complete metabolic tumour response, assessed by 18-fluorodeoxyglucose positron emission tomography (18FDG-PET), after induction chemotherapy predicts a favourable outcome in patients with locally advanced non-small cell lung cancer (NSCLC). Lung Cancer. 2008;62(1):55–61. doi: 10.1016/j.lungcan.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Kim S.H., Lee J.H., Lee G.J., Jeong S., Kwak Y.K., Kim H.K., Cho D.G., Park Y.H., Yu M., Yoon S.C. Interpretation and prognostic value of positron emission tomographycomputed tomography after induction chemotherapy with or without radiation in IIIA-N2 non-small cell lung cancer patients who receive curative surgery. Medicine (Baltimore) 2015;94(24):e955. doi: 10.1097/MD.0000000000000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnett S.A., Downey R.J., Zheng J., Plourde G., Shen R., Chaft J., Akhurst T., Park B.J., Rusch V.W. Utility of routine PET imaging to predict response and survival after induction therapy for non-small cell lung cancer. Ann. Thorac. Surg. 2016;101(3):1052–1059. doi: 10.1016/j.athoracsur.2015.09.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skoura E., Datseris I.E., Platis I., Oikonomopoulos G., Syrigos K.N. Role of positron emission tomography in the early prediction of response to chemotherapy in patients with non--small-cell lung cancer. Clin. Lung Cancer. 2012;13(3):181–187. doi: 10.1016/j.cllc.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Na F., Wang J., Li C., Deng L., Xue J., Lu Y. Primary tumor standardized uptake value measured on F18-Fluorodeoxyglucose positron emission tomography is of prediction value for survival and local control in non-small-cell lung cancer receiving radiotherapy: meta-analysis. J. Thorac. Oncol. 2014;9(6):834–842. doi: 10.1097/JTO.0000000000000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J., Wong K.K., Piert M., Stanton P., Frey K.A., Kong F.S. Metabolic response assessment with 18F-FDG PET/CT: inter-method comparison and prognostic significance for patients with non-small cell lung cancer. J. Radiat. Oncol. 2015;4(3):249–256. doi: 10.1007/s13566-015-0184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clarke K., Taremi M., Dahele M., Freeman M., Fung S., Franks K., Bezjak A., Brade A., Cho J., Hope A., Sun A. Stereotactic body radiotherapy (SBRT) for non-small cell lung cancer (NSCLC): is FDG-PET a predictor of outcome? Radiother. Oncol. 2012;104(1):62–66. doi: 10.1016/j.radonc.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 25.Bollineni V.R., Widder J., Pruim J., Langendijk J.A., Wiegman E.M. Residual 18F-FDG-PET uptake 12 weeks after stereotactic ablative radiotherapy for stage I non-small-cell lung cancer predicts local control. Int. J. Radiat. Oncol. Biol. Phys. 2012;83(4):e551–e555. doi: 10.1016/j.ijrobp.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Kremer R., Peysakhovich Y., Dan L.F., Guralnik L., Kagna O., Nir R.R., Bar-Shalom R. FDG PET/CT for assessing the resectability of NSCLC patients with N2 disease after neoadjuvant therapy. Ann. Nucl. Med. 2016;30(2):114–121. doi: 10.1007/s12149-015-1038-7. [DOI] [PubMed] [Google Scholar]
- 27.De Leyn P., Stroobants S., De Wever W., Lerut T., Coosemans W., Decker G., Nafteux P., Van Raemdonck D., Mortelmans L., Nackaerts K., Vansteenkiste J. Prospective comparative study of integrated positron emission tomography-computed tomography scan compared with remediastinoscopy in the assessment of residual mediastinal lymph node disease after induction chemotherapy for mediastinoscopy-proven stage IIIA-N2 Non-small-cell lung cancer: a Leuven Lung Cancer Group Study. J. Clin. Oncol. 2006;24(21):3333–3339. doi: 10.1200/JCO.2006.05.6341. [DOI] [PubMed] [Google Scholar]
- 28.Stamatis G. Staging of lung cancer: the role of noninvasive, minimally invasive and invasive techniques. Eur. Respir. J. 2015;46(2):521–531. doi: 10.1183/09031936.00126714. [DOI] [PubMed] [Google Scholar]
- 29.Kamel M.K., Rahouma M., Ghaly G., Nasar A., Port J.L., Stiles B.M., Nguyen A.B., Altorki N.K., Lee P.C. Clinical predictors of persistent mediastinal nodal disease after induction therapy for stage IIIA N2 non-small cell lung cancer. Ann. Thorac. Surg. 2017;103(1):281–286. doi: 10.1016/j.athoracsur.2016.06.061. [DOI] [PubMed] [Google Scholar]
- 30.Fledelius J., Khalil A.A., Hjorthaug K., Frøkiaer J. Using positron emission tomography (PET) response criteria in solid tumours (PERCIST) 1.0 for evaluation of 2′-deoxy-2′-[18F] fluoro-D-glucose-PET/CT scans to predict survival early during treatment of locally advanced non-small cell lung cancer (NSCLC). J. Med. Imaging Radiat. Oncol. 2016;60(2):231–238. doi: 10.1111/1754-9485.12427. [DOI] [PubMed] [Google Scholar]
- 31.Winther-Larsen A., Fledelius J., Sorensen B.S., Meldgaard P. Metabolic tumor burden as marker of outcome in advanced EGFR wild-type NSCLC patients treated with erlotinib. Lung Cancer. 2016;94:81–87. doi: 10.1016/j.lungcan.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 32.Castello A, Toschi L, Rossi S. Predictive and prognostic role of metabolic response in patients with stage III NSCLC Treated with neoadjuvant chemotherapy. 2019. [DOI] [PubMed]
- 33.Soussan M., Chouahnia K., Maisonobe J-A., Boubaya M., Eder V., Morère J.F., Buvat I. Prognostic implications of volume-based measurements on FDG PET/CT in stage III non-small-cell lung cancer after induction chemotherapy. Eur. J. Nucl. Med. Mol. Imaging. 2013;40(5):668–676. doi: 10.1007/s00259-012-2321-7. [DOI] [PubMed] [Google Scholar]
- 34.Kahraman D., Holstein A., Scheffler M., Zander T., Nogova L., Lammertsma A.A., Boellaard R., Neumaier B., Dietlein M., Wolf J., Kobe C. Tumor lesion glycolysis and tumor lesion proliferation for response prediction and prognostic differentiation in patients with advanced non-small cell lung cancer treated with erlotinib. Clin. Nucl. Med. 2012;37(11):1058–1064. doi: 10.1097/RLU.0b013e3182639747. [DOI] [PubMed] [Google Scholar]
- 35.Zaizen Y., Azuma K., Kurata S., Sadashima E., Hattori S., Sasada T., Imamura Y., Kaida H., Kawahara A., Kinoshita T., Ishibashi M., Hoshino T. Prognostic significance of total lesion glycolysis in patients with advanced non-small cell lung cancer receiving chemotherapy. Eur. J. Radiol. 2012;81(12):4179–4184. doi: 10.1016/j.ejrad.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Hyun S.H., Ahn H.K., Ahn M-J., Ahn Y.C., Kim J., Shim Y.M., Choi J.Y. Volume-based assessment with 18F-FDG PET/CT improves outcome prediction for patients with stage IIIA-N2 non-small cell lung cancer. AJR Am. J. Roentgenol. 2015;205(3):623–628. doi: 10.2214/AJR.14.13847. [DOI] [PubMed] [Google Scholar]
- 37.Colt H.G., Murgu S.D., Korst R.J., Slatore C.G., Unger M., Quadrelli S. RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J. Nucl. Med. 2013;50:1122S–1150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crabtree T.D., Puri V., Chen S.B., Gierada D.S., Bell J.M., Broderick S., Krupnick A.S., Kreisel D., Patterson G.A., Meyers B.F. Does the method of radiologic surveillance affect survival after resection of stage I non-small cell lung cancer? 2015. [DOI] [PMC free article] [PubMed]
- 39.Wahl R.L., Jacene H., Kasamon Y., Lodge M.A. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009;50(Suppl. 1):122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding Q., Cheng X., Yang L., Zhang Q., Chen J., Li T., Shi H. PET/CT evaluation of response to chemotherapy in non-small cell lung cancer: PET response criteria in solid tumors (PERCIST) versus response evaluation criteria in solid tumors (RECIST). J. Thorac. Dis. 2014;6(6):677–683. doi: 10.3978/j.issn.2072-1439.2014.05.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Usmanij E.A., Natroshvili T., Timmer-Bonte J.N.H., Oyen W.J.G., van der Drift M.A., Bussink J., Geus-Oei L.F. The predictive value of early in-treatment 18F-FDG PET/CT response to chemotherapy in combination with bevacizumab in advanced nonsquamous non-small cell lung cancer. J. Nucl. Med. 2017;58(8):1243–1248. doi: 10.2967/jnumed.116.185314. [DOI] [PubMed] [Google Scholar]
- 42.Ziai D., Wagner T., El Badaoui A., Hitzel A., Woillard J.B., Melloni B., Monteil J. Therapy response evaluation with FDG-PET/CT in small cell lung cancer: a prognostic and comparison study of the PERCIST and EORTC criteria. Cancer Imaging. 2013;13:73–80. doi: 10.1102/1470-7330.2013.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shang J., Ling X., Zhang L., Tang Y., Xiao Z., Cheng Y., Guo B., Gong J., Huang L., Xu H. Comparison of RECIST, EORTC criteria and PERCIST for evaluation of early response to chemotherapy in patients with non-small-cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging. 2016;43(11):1945–1953. doi: 10.1007/s00259-016-3420-7. [DOI] [PubMed] [Google Scholar]
- 44.Sheikhbahaei S., Mena E., Marcus C., Wray R., Taghipour M., Subramaniam R.M. 18F-fluorodeoxyglucose PET/CT: therapy response assessment interpretation (Hopkins criteria) and survival outcomes in lung cancer patients. J. Nucl. Med. 2016;57(6):855–860. doi: 10.2967/jnumed.115.165480. [DOI] [PubMed] [Google Scholar]
- 45.Usmanij E.A., de Geus-Oei L.F., Troost E.G., Peters-Bax L., van der Heijden E.H., Kaanders J.H., Oyen W.J., Schuurbiers O.C., Bussink J. 18F-FDG PET early response evaluation of locally advanced non-small cell lung cancer treated with concomitant chemoradiotherapy. J. Nucl. Med. 2013;54(9):1528–1534. doi: 10.2967/jnumed.112.116921. [DOI] [PubMed] [Google Scholar]
- 46.Zhang H.Q., Yu J.M., Meng X., Yue J.B., Feng R., Ma L. Prognostic value of serial [18F]fluorodeoxyglucose PET-CT uptake in stage III patients with non-small cell lung cancer treated by concurrent chemoradiotherapy. Eur. J. Radiol. 2011;77(1):92–96. doi: 10.1016/j.ejrad.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 47.Huang W., Fan M., Liu B., Fu Z., Zhou T., Zhang Z., Gong H., Li B. Value of metabolic tumor volume on repeated 18F-FDG PET/CT for early prediction of survival in locally advanced non-small cell lung cancer treated with concurrent chemoradiotherapy. J. Nucl. Med. 2014;55(10):1584–1590. doi: 10.2967/jnumed.114.142919. [DOI] [PubMed] [Google Scholar]
- 48.Burger I.A., Vargas H.A., Apte A., Beattie B.J., Humm J.L., Gonen M., Larson S.M., Ross Schmidtlein C. PET quantification with a histogram derived total activity metric: superior quantitative consistency compared to total lesion glycolysis with absolute or relative SUV thresholds in phantoms and lung cancer patients. Nucl. Med. Biol. 2014;41(5):410–418. doi: 10.1016/j.nucmedbio.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burger I.A., Casanova R., Steiger S., Husmann L., Stolzmann P., Huellner M.W., Curioni A., Hillinger S., Schmidtlein C.R., Soltermann A. 18F-FDG PET/CT of non-small cell lung carcinoma under neoadjuvant chemotherapy: background-based adaptive-volume metrics outperform TLG and MTV in predicting histopathologic response. J. Nucl. Med. 2016;57(6):849–854. doi: 10.2967/jnumed.115.167684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roy S., Pathy S., Kumar R., Mohanti B.K., Raina V., Jaiswal A., Taywade S., Garg K., Thulkar S., Mohan A., Mathur S., Behera D. Efficacy of 18F-fluorodeoxyglucose positron emission tomography/computed tomography as a predictor of response in locally advanced non-small-cell carcinoma of the lung. Nucl. Med. Commun. 2016;37(2):129–138. doi: 10.1097/MNM.0000000000000422. [DOI] [PubMed] [Google Scholar]
- 51.Liu J., Dong M., Sun X., Li W., Xing L., Yu J. Prognostic value of 18F-FDG PET/CT in surgical non-small cell lung cancer: a meta-analysis. PLoS One. 2016;11(1):e0146195. doi: 10.1371/journal.pone.0146195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Im H-J., Pak K., Cheon G.J., Kang K.W., Kim S.J., Kim I.J., Chung J.K., Kim E.E., Lee D.S. Prognostic value of volumetric parameters of (18)F-FDG PET in non-small-cell lung cancer: a meta-analysis. Eur. J. Nucl. Med. Mol. Imaging. 2015;42(2):241–251. doi: 10.1007/s00259-014-2903-7. [DOI] [PubMed] [Google Scholar]
- 53.Rossi S., Castello A., Toschi L., Lopci E. Immunotherapy in non-small-cell lung cancer: potential predictors of response and new strategies to assess activity. Immunotherapy. 2018;10(9):797–805. doi: 10.2217/imt-2017-0187. [DOI] [PubMed] [Google Scholar]
- 54.Cho S.Y., Lipson E.J. Im, H.J.; Rowe, S.P.; Gonzalez, E.M.; Blackford, A.; Chirindel, A.; Pardoll, D.M.; Topalian, S.L.; Wahl, R.L. Prediction of Response to Immune Checkpoint Inhibitor Therapy Using Early-Time-Point 18F-FDG PET/CT Imaging in Patients with Advanced Melanoma. J. Nucl. Med. 2017;58(9):1421–1428. doi: 10.2967/jnumed.116.188839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Natarajan A., Mayer A.T., Xu L., Reeves R.E., Gano J., Gambhir S.S. Novel radiotracer for ImmunoPET imaging of PD-1 checkpoint expression on tumor infiltrating lymphocytes. Bioconjug. Chem. 2015;26(10):2062–2069. doi: 10.1021/acs.bioconjchem.5b00318. [DOI] [PubMed] [Google Scholar]