Abstract
Constipation in gestating and lactating sows is common and the inclusion of dietary fiber may help to alleviate this problem. We investigated the effects of inulin (INU) and isomalto-oligosaccharide (IMO), two sources of soluble dietary fiber, on gastrointestinal motility-related hormones, short-chain fatty acids (SCFA), fecal microflora, and reproductive performance in pregnant sows. On day 64 of gestation, 30 sows were randomly divided into three groups and fed as follows: a basal diet, a basal diet with 0.5% INU, and a basal diet with 0.5% IMO. We found that INU and IMO significantly modulated the levels of gastrointestinal motility-related hormones, as evidenced by an increase in substance P (P < 0.05), and a decrease in the vasoactive intestinal peptide concentrations (P < 0.05), indicating the capacity of INU and IMO to alleviate constipation. Furthermore, IMO enhanced the concentrations of acetic, propionic, isobutyric, butyric, isovaleric, and valeric acids in the feces (P < 0.05). High-throughput sequencing showed that IMO and INU increased the fecal microflora α- and β-diversity (P < 0.05). Methanobrevibacter was more abundant (P < 0.05), whereas the richness of Turicibacter was lower in the INU and IMO groups than in the control group (P < 0.05). In addition, IMO significantly increased litter size (P < 0.05). Overall, our findings indicate that INU and IMO can relieve constipation, optimize intestinal flora, and promote reproductive performance in pregnant sows.
Keywords: constipation alleviation, inulin, isomalto-oligosaccharide, reproductive performance, sows
Introduction
Constipation in sows in the late gestation and lactation period is a common problem, particularly in summer. Constipation leads to dystocia or stillbirth, toxins in feces, uteritis, and mastitis and also influences the survival and growth of the piglets (Gu et al., 2019). Furthermore, constipation causes postpartum pain in sows and prolongs the time to litter (Oliviero et al., 2010). In addition, this problem may also increase the release and absorption of bacterial endotoxins, resulting in postpartum dysfunction syndrome in sows (Tabeling et al., 2003). Many factors cause constipation in sows, such as an unsuitable diet structure, the use of limited stalls in modern intensive breeding farms, and restricted feeding of pregnant sows (Oliviero et al., 2010).
Disordered gastrointestinal metabolism and the intestinal nervous system are also major causes of constipation. Gastrointestinal hormones are polypeptides secreted by multiple endocrine cells, which can regulate secretion from digestive glands and gut motility (Ning and Zhang, 2009; Sui and Tian, 2014). Serum substance P (SP), motilin (MTL), calcitonin gene-related peptide (CGRP), and vasoactive intestinal peptide (VIP) are associated with constipation (Zhao and Tong, 2012). They can regulate gut motility and secretions within the digestive tract, which are considered to be indices of constipation (Jia et al., 2020). The increase of MTL and SP can promote intestinal peristalsis to relieve constipation (Deng et al., 2021). In mice with constipation caused by diphenoxylate, the levels of NO and VIP in serum decrease, and the SP and MTL contents increase in mulberry-treated groups (Hu et al., 2019). Compared to the control group, the serum levels of MTL and SP in mice with constipation treated using Lactobacillus fermentum were significantly higher, whereas the level of somatostatin was lower (Zhao et al., 2015).
Inulin (INU) is a soluble dietary fiber that cannot be digested and absorbed by the body (Roberfroid, 2007). The main chain of the inulin fructosan molecule is linked by a β-(2,1) glucoside bond and there is a glucose at the nonreducing end (Srikanth et al., 2015). Many studies have shown that it has a variety of physiological functions (Kietsiriroje et al., 2015). Inulin plays a positive role in mice with diabetes induced by a high-fat diet (Shao et al., 2020), as well as in lowering blood sugar (Gao et al., 2019). It can be used as a bifidogenic factor to stimulate the immune system, reduce pathogenic bacteria, and relieve constipation (Kaur and Gupta, 2002). Paßlack et al. (2015) found that inulin increased the number of Enterococcus cells in the feces of pregnant sows and the cecum of piglets, indicating that inulin can improve the intestinal microbial structure of piglets. Diets supplemented with inulin significantly influence antioxidative capacity in growing pigs and increase litter size (Lepczyński et al., 2021).
Isomalto-oligosaccharide (IMO) is one of the most common commercial oligosaccharides and another soluble dietary fiber (Patel and Goyal, 2011; Sasaki et al., 2020). According to the study of Mizubuchi et al. (2005), IMO activates the immune system and improves sow health. In high-fat diet-induced mice, IMO alters metabolism by preventing intestinal flora dysbiosis (Singh et al., 2017). Isomalto-oligosaccharide sulfate inhibits cell proliferation and metastasis, and induces cell apoptosis (Xiao et al., 2011). In addition, Gu et al. (2019) showed that IMO affects the metabolism and milk quality of sows and improves their lactating performance. As a dietary fiber, IMO is known to improve growth performance, raise levels of jejunum butyrate and isobutyrate, and increase the thymus index in broilers (Zhang et al., 2003).
Although the effects of INU and IMO on nutritional and immune health have been revealed, few studies have investigated their effects on reducing constipation in pregnant sows. Therefore, the objectives of the present study were to assess the effects of INU and IMO on the reproductive performance of sows and to investigate the mechanism by analyzing gastrointestinal motility-related hormones, antioxidant indices, fecal short-chain fatty acids, and flora composition.
Materials and Methods
All procedures involving animal were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Zhejiang A&F University and were approved by the Animal Ethics Committee of Zhejiang A&F University.
Animals and treatments
A total of 30 Landrace sows at 64 d of gestation were used in this experiment. According to the principle of similar body weight and number of births, the sows were randomly divided into three groups (n = 10 per group). The three groups of sows were treated as follows: a negative control group (NCO) was fed a basal diet, an INU group was fed the basal diet supplemented with 0.5% INU, and an IMO group was fed the basal diet with 0.5% IMO. The experiment was run for 40 d. The basal diet was designed to conform to the nutritional requirements of the NRC (2002) and contained no antibiotics (Table 1). The feed was served twice daily, and water was provided ad libitum. The piggery was cleaned daily and disinfected every 2 wk.
Table 1.
Composition and nutrient levels of the basal diet (calculated as 90% dry matter)
| Ingredients | Content, % | Nutrient level | Content |
|---|---|---|---|
| Corn | 59.00 | ME, kcal/kg | 3,300.00 |
| Soybean meal | 16.00 | CP, % | 14.5 |
| bran | 21.00 | Lys, % | 0.45 |
| Vitamin-mineral Premix1 | 4.00 | Met, % | 0.13 |
| Met+Cys, % | 0.32 | ||
| Thr, % | 0.37 | ||
| Ile, % | 0.21 | ||
| Ca,% | 0.49 | ||
| TP, % | 0.41 | ||
| Total | 100 |
1Supplied the following per kg of diet: vitamin A, 125000 IU; vitamin D, 2000 IU; vitamin E, 64.855 mg; pantothenic acid, 16.074 mg; vitamin B6, 1.510 mg; biotin, 0.005 mg; vitamin B12, 3.65 mg; niacin, 0.015g; choline, 1295.153 mg; folic acid, 0.015 mg; vitamin K, 1.1 mg; vitamin B1, 0.577 mg; vitamin B2, 4.963 mg; Fe, 137.71 mg; Cu, 22.25mg; Co, 0.983 mg; Mn, 43.1 mg; Zn, 118.65 mg; I, 1.1 mg and Se, 0.324 mg.
Sample collection
At 104 d of gestation, serum samples extracted from the front cavity vein were collected from the 30 sows and centrifuged at 3,000 g for 10 min at 4 °C. The supernatant was collected in 1.5 mL Eppendorf tubes. All samples were stored at −80 °C for further analysis.
Serum parameter analysis
The gastrointestinal motility-related hormone contents, specifically CGRP, SP, VIP, and MTL as well as the antioxidant capacity parameters (CAT and SOD) were determined using microplate assay kits following the manufacturer’s instructions. The kits were obtained from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Short-chain fatty acid analysis
Fecal short-chain fatty acid (SCFAs), including acetic, propionic, butyric, isobutyric, valeric, and isovaleric acids, were tested using gas chromatography. Briefly, feces (0.5 g) were mixed evenly with precooled sterile water at a ratio of 1:2 and centrifuged at 15,000 g for 15 min at 4 °C. Thereafter, 1 mL of supernatant was blended with 0.2 mL of 25% metaphosphoric acid and thoroughly mixed. After standing for 30 min at 0 °C, the tubes were centrifuged again at 15,000 g for 10 min at 4 °C. The supernatant was filtered into a sample bottle for further analysis.
Feces microflora content determined by 16S rRNA sequencing
The CTAB/SDS method was used to extract genomic DNA from the fecal samples. After purification and determination of the concentration, DNA was diluted to 1 ng/μL with sterile water. The V4 region of the 16S rRNA gene was analyzed using primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTW TCTAAT-3′). The library was constructed using the NEB Next Ultra DNA Library Prep Kit for Illumina (New England Biolabs Inc., Ipswich, MA). Sequencing was performed on an Illumina HiSeq platform (Novogene, Beijing, China).
Reproductive performance
After delivery, the reproductive performance of the 30 sows was recorded, including litter size, litter birth weight, number of weak young, and number of stillbirths.
Statistical analysis
All data were analyzed using the SPSS 22.0 software (SPSS Inc., Chicago, IL). A value of P < 0.05 was considered statistically significant for one-way ANOVA. GraphPad Prism 7 (GraphPad Prism Inc., San Diego, CA) was used to draw all the histograms.
Results
Effects of inulin and isomalto-oligosaccharide on gastrointestinal motility-related hormones
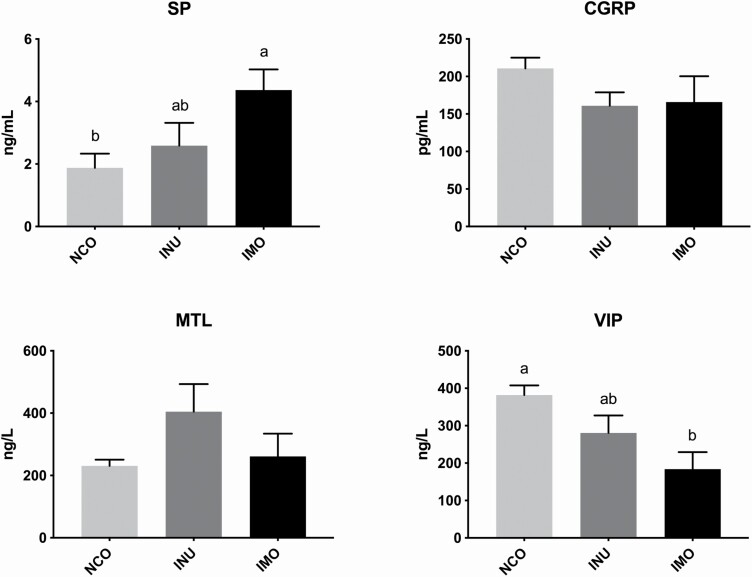
The effects of INU and IMO on gastrointestinal motility-related hormones in the pregnant sows are shown in Figure 1. The sows fed IMO had significantly more SP compared to those in the control group (P < 0.05), whereas there was no significant difference between the INU and NCO groups (P > 0.05). The IMO sows had lower VIP levels when compared with the NCO sows (P < 0.05). No significant effects in the levels of CGRP and MTL were observed.
Figure 1.
Effect of inulin and isomalto-oligosaccharide on neurotransmitter content in sows: the content of SP, VIP, CGRP, and MTL in sow serum, respectively. NCO represents control sows; INU represents sows fed with inulin; IMO represents sows fed with isomalto-oligosaccharide. Different letters indicate a significant difference at P < 0.05.
Effects of inulin and isomalto-oligosaccharide on antioxidation indexes
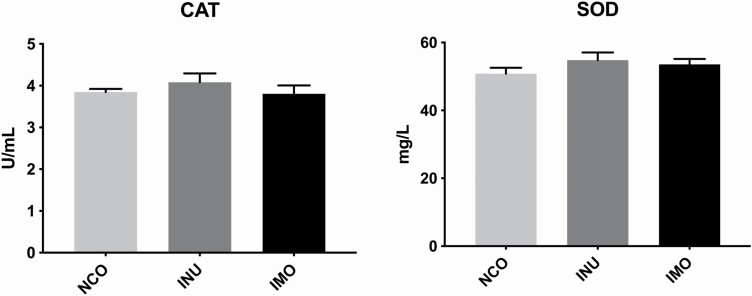
The effects of INU and IMO on the antioxidant capacity of sows are shown in Figure 2. Compared to the control group, no significant effects on the levels of CAT and SOD were noted in sows fed INU and IMO (P > 0.05), indicating that INU and IMO had no significant effect on the antioxidant activity.
Figure 2.
Effect of inulin and isomalto-oligosaccharide on antioxidant capacity in sows: the content of CAT and SOD in sow serum, respectively. NCO represents control sows; INU represents sows fed with inulin; IMO represents sows fed with isomalto-oligosaccharide. Different letters indicate a significant difference at P< 0.05.
Effects of inulin and isomalto-oligosaccharide on concentrations of short-chain fatty acids
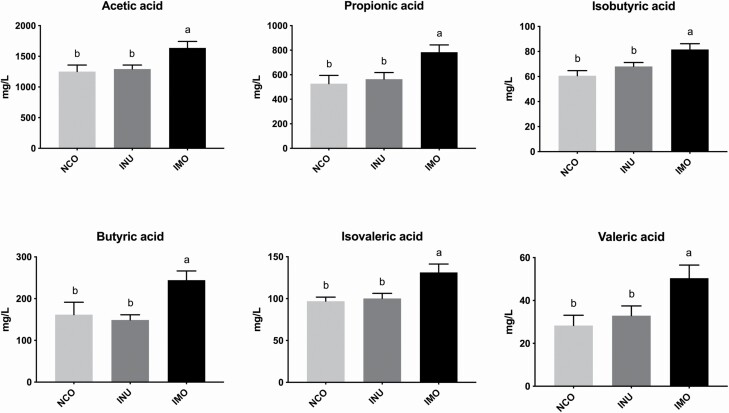
The effects of INU and IMO on the colonic concentrations of SCFAs in sows are shown in Figure 3. The levels of acetic, propionic, isobutyric, butyric, isovaleric, and valeric acids were significantly higher in the IMO group compared to the control group (P < 0.05). Nevertheless, there were no significant differences between the INU and NCO groups (P > 0.05).
Figure 3.
Effect of inulin and isomalto-oligosaccharide on volatile fatty acids in feces content of sows: the content of acetic acid, propionic acid, isobutyric acid, butyric acid, isovaleric acid, and valeric acid in sow feces content, respectively. NCO represents control sows; INU represents sows fed with inulin; IMO represents sows fed with isomalto-oligosaccharide. Different letters indicate a significant difference at P < 0.05.
Effects of inulin and isomalto-oligosaccharide on microflora structure in sow feces
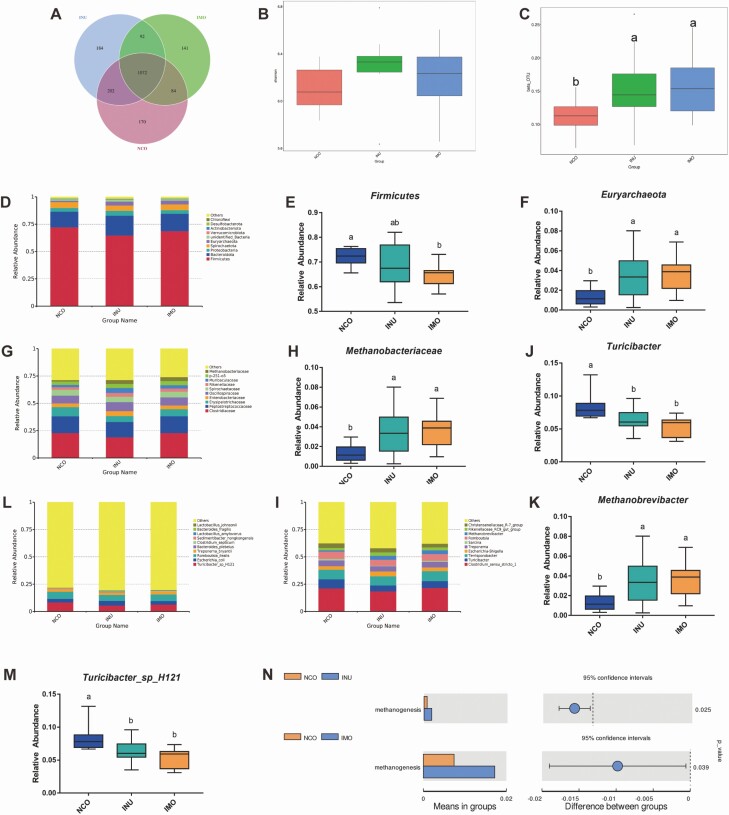
The effects of INU and IMO on the microflora structure of the sow feces are shown in Figure 4. The fecal microbiota composition is shown in the Venn diagram (Figure 4A). A total of 1,945 observed taxonomic units (OTUs) were shared among the three groups. The control, IMO and INU groups had 170, 141, and 184 unique OTU, respectively. Supplementation with IMO and INU significantly increased the β-diversity of the fecal flora (P < 0.05; Figure 4C) but had no significant effect on the Shannon index (P > 0.05; Figure 4B).
Figure 4.
Summary of the microbial community in the feces contents of sows. (A) Venn diagram summarizing the numbers of common and unique operational taxonomic units (OTUs) in the microflora community in the feces contents of sows. (B) Shannon index, reflecting species diversity within and between groups. (C) β-OTU index, reflecting species diversity within and between groups. (D–M) Top 10 taxa with differences in relative abundance between groups (phylum, family, genus, and species levels). (N) Tax4Fun function predictive analysis of inulin and isomalto-oligosaccharide on sow fecal flora.
Firmicutes and Bacteroidetes were the dominant bacterial phyla in the feces (Figure 4D). The addition of IMO significantly decreased Firmicutes (P < 0.05; Figure 4E). At the family level, Clostridiaceae and Peptostreptococcaceae were the dominant strains (Figure 4G). Supplementation with IMO and INU significantly increased the abundance of the Euryarchaeota (P < 0.05; Figure 4F) and Methanobacteriaceae (P < 0.05; Figure 4H). At the genus level, we found that Clostridium sensu stricto 1, Terrisporobacter, and Turicibacter were the dominant genera in all fecal samples (Figure 4I). At the species level, Turicibacter sp. Strain H121 and Romboutsia spp. were the dominant species (Figure 4L). Compared to the control group, the relative abundance of Turicibacter and Turicibacter sp. Strain H121 in the IMO and INU groups was significantly lower (P < 0.05; Figure 4J and M), whereas the relative abundance of Methanobrevibacter was significantly higher (P < 0.05; Figure 4K). Supplementation with INU and IMO significantly increased the abundance of methanogens in the fecal microflora suggesting that these dietary fibers improved the ability of the intestinal flora to produce methane (P < 0.05; Figure 4N).
Effects of inulin and isomalto-oligosaccharide on the reproductive performance of sows
Sows fed IMO had a significantly larger litter size compared to the control group (P < 0.05; Table 2). However, litter birth weight, number of weak young, and number of stillbirths in the IMO and INU groups were similar to those in the control group (P > 0.05).
Table 2.
Effects of inulin and isomalto-oligosaccharide on reproductive performance of sows1
| Item | Treatment | SEM2 | P Value | ||
|---|---|---|---|---|---|
| NCO | INU | IMO | |||
| Litter size | 10.14a | 10.14a | 11.14b | 0.19 | 0.037 |
| Litter birth weight | 15.12 | 15.44 | 16.48 | 0.33 | 0.221 |
| Number of weak young | 0.57 | 0.14 | 0.43 | 0.16 | 0.498 |
| Number of stillbirths | 0.29 | 0.29 | 0.29 | 0.14 | 1.000 |
1NCO, INU, and IMO represents the sows supplemented with basal diet, sows supplemented with the inulin and sows supplemented with isomalto-oligosaccharide, respectively. Sows were regarded as the experimental units.
2Pooled SEM; n = 10 per treatment.
a,b means within the same raw with different superscripts differ significantly (P < 0.05).
Discussion
Studies have shown that fiber reduction in sow causes negative effects, including increased rigid behaviors, gastric ulcers, and constipation (Lee and Close, 1987), indicating that fiber plays a positive role in relieving constipation and improving reproductive performance. High-fiber diets can balance nutrient levels and promote feed intake to improve the reproductive performance of sows (Yu et al., 2020). Oliviero et al. (2009) found that sows fed 7% crude fiber at the end of pregnancy had less constipation, and their intestinal activity recovered more quickly than those fed only 3.8% crude fiber. Tabeling et al. (2003) found that diets supplemented with 40% dried beet pulp had no adverse effects on labor and reproductive performance. According to Lan et al. (2020), IMO and INU significantly alleviated constipation in rats by increasing the number, weight and water content of fecal pellets and shortening the defecation time of the first black stool. We observed that dietary supplementation with IMO reduced constipation symptoms in sows and increased the litter size. It is known that IMO can promote adjustments in the intestinal microecological environment of mice and stimulate defecation (Zonglian, 2001). In particular, IMO has been shown to increase the water content in the feces of constipated mice, thereby reducing intestinal transportation time (Wang et al., 2017).
Dietary fiber may play an important role in changing the concentration of intestinal peristaltic neurotransmitters, particularly SP and MTL, which are excitability neurotransmitters, and VIP and CGRP, which are inhibitory neurotransmitters. These four neurotransmitters play a key role in relation to the occurrence of constipation. In rats with functional constipation, manual acupuncture and moxibustion can improve intestinal motility and decrease the expression of CGRP, PAR-4, and TRPV 1 in colon tissue (Zhang et al., 2017), and it has been reported that acupoint application can increase the immune activity of SP in rats with functional constipation (Meng and Liu, 2019). Bacterial cellulose, a promising dietary fiber to relieve constipation, can increase the secretion of SP and MTL and reduce the level of VIP (Zhai et al., 2018). In constipated mice, Lactobacillus plantarum YS-3 can elevate the serum levels of MTL, and SP (Zhao et al., 2018). In a study by Liu et al. (2019), Konjac mannan oligosaccharides increased the serum levels of MTL, SP and acetylcholinesterase (AchE), and inhibited somatostatin and VIP to effectively improve intestinal function and promote the relief of constipation. Furthermore, Lan et al. (2020) have shown that INU and IMO increased the serum levels of MTL and SP, decreased VIP and CGRP in rats with diphenoxylate-induced constipation. The findings of these studies indicate that an increase in SP and MTL and a decrease in VIP and CGRP could result in the relief of constipation. Similarly, our study showed that INU and IMO could increase the serum levels of MTL and SP and decrease VIP and CGRP in pregnant sows, suggesting that IMO and INU have a positive effect on relieving constipation. Jiang et al. (2020) found Durio zibethinus Murr rind polysaccharide can significantly improve the intestinal transport rate, influence gastrointestinal peristalsis, and increase the level of MTL, SP, and gastrin in constipated rats. These hormonal changes can promote intestinal peristalsis, increase intestinal osmotic pressure, and maintain a water balance suitable for facilitating defecation (Su et al., 2019).
The addition of fiber to feed can increase the dietary SCFAs content and, acetic acid and butyric acid, in particular, can improve intestinal barrier function and protect the host from bacterial invasion (Fukuda et al., 2011). Adding appropriate fiber to the diet of pigs increases microbial richness and promotes SCFAs metabolism (Pu et al., 2020). In a study by Ndou et al. (2019), dietary supplementation with soluble fiber increased the quantity of SCFAs and the contribution rate of SCFAs from fermentation to total tract digestible energy. Piglets fed with 2.5% Astragalus membranaceus stems and leaves tended to have slightly higher concentrations of acetic acid, butyric acid, propionic acid, and total SCFAs (Che et al., 2019). Sugar beet pulp and oat bran increased the total SCFAs concentration in the ileal digesta and feces (Zhao et al., 2020). The addition of dextrin can lead to a change in SCFAs concentration, which indicates that the intestinal microbiota and barrier function may have changed (Chastain et al., 2019). Similarly, we found that the addition of IMO significantly increased the concentrations of acetic, propionic, butyric, isobutyric, valeric, and isovaleric acids, suggesting that IMO is more effective in promoting the content of SCFAs than INU. It has been shown that IMO can effectively improve the growth performance of broilers and increase the content of fatty acids in the cecum (Mookiah et al., 2014). In addition, Wu et al. (2017) found that the ileum villus height and total SCFAs concentration in the cecum were higher in the IMO group in weaned pigs, suggesting that IMO may improve reproductive performance by promoting intestinal health. In other studies, IMO has been found to increase cecal acetic and propionic acids, and colonic acetic and propionic acids as well as total SCFAs concentrations in broilers (Zhang et al., 2003).
Furthermore, dietary fiber is known to alter the composition of microorganisms to maintain or restore the microbial balance. Niu et al. (2019) found Firmicutes and Bacteroidetes to be the most dominant phyla in sow feces. Turicibacter spp. are closely related to immune function and bowel diseases (Allen et al., 2015). In mice, a ketogenic diet increased the relative abundance of Akkermansia muciniphila and Lactobacillus, which are potentially beneficial gut bacteria and reduce the relative abundance of Desulfovibrio and Turicibacter, which may have a pro-inflammatory effect (Ma et al., 2018). Liang et al. (2019) found that indigo naturalis can modulate the intestinal microbiota community to ameliorate DSS-induced colitis in mice. A marine microalga Chlorella pyrenoidosa ethanol extract significantly reduced dyslipidemia and the content of Turicibacter in hyperlipidemic rats fed a high-fat diet (Wan et al., 2018). High fiber mixed with xylanase increased α-diversity and decreased Turicibacter in the cecal contents (Petry et al., 2021). Our results showed that IMO and INU significantly increased the β-diversity of the fecal flora. At the genus level, INU and IMO reduced the abundance of Turicibacter and enriched the level of Methanobrevibacter in sow feces. At the species level, Turicibacter sp. Strain H121 in the IMO and INU groups was significantly lower than that in the control group. Turicibacter is pro-inflammatory bacteria, and Turicibacter abundance increases during enteritis (Bretin et al., 2018). Turicibacter may have negative effects on intestinal health and weight gain (Wan et al., 2018), and may also cause age-related defects in the intestinal barrier, leading to more severe colitis (Liu et al., 2020). Turicibacter content decreased in the intestine of sows treated with aureomycin, indicating that Turicibacter may adversely affect the host (Rettedal et al., 2009).
Diet is an important factor that can result in differences in fecal bacterial abundance and diversity. Tax4Fun was used to predict the intestinal flora function (Aßhauer et al., 2015). Using the Tax4Fun sequencing method, we found that dietary IMO and INU significantly increased the abundance of methanogens, which are thought to have been part of the intestinal flora of pigs for thousands of years and play an important role in maintaining host health (Pike and Forster, 2018). Moreover, methanogens can maintain intestinal health by improving fiber degradation, which is associated with constipation relief (Chaucheyras-Durand et al., 2010). Methanobrevibacter, a methanogen, is an environmentally important microorganism. A natural culture of methanogens isolated from herbivores can bioconvert lignocellulosic materials to methane (Jin et al., 2011) and a co-culture of anaerobic fungi and methanogens has been shown to degrade fibers (Li et al., 2021). Methanogens can not only improve the fiber degradation ability of anaerobic fungi but may also help anaerobic fungi resist harsh environments (Mountfort and Asher, 1985; Stewart and Richardson, 1989).
In summary, supplementation of INU and IMO in the diets of pregnant sows can relieve constipation by increasing the content of excitability neurotransmitters SP and reducing the content of inhibitory neurotransmitters VIP, improve the sow’s reproductive performance and regulate the intestinal flora by significantly reducing the abundance of potentially pathogenic bacteria, such as Turicibacter. In addition, IMO enhanced the concentrations of acetic, propionic, isobutyric, butyric, isovaleric, and valeric acids in the feces.
Acknowledgments
The present research was supported by the Zhejiang Provincial Key Research and Development Program (No. 2019C02051 and No.2020C02032), Zhejiang Provincial Leading Innovation Team Project (No.2020R01015), and the National key research and development program of China (2018YFE0112700).
Glossary
Abbreviations
- AchE
acetylcholinesterase
- CGRP
calcitonin gene-related peptide
- IMO
isomalto-oligosaccharide
- INU
inulin
- MTL
motilin
- SCFAs
short-chain fatty acids
- SP
substance P
- VIP
vasoactive intestinal peptide
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Allen, J. M., Berg Miller M. E., Pence B. D., Whitlock K., Nehra V., Gaskins H. R., White B. A., Fryer J. D., and Woods J. A.. . 2015. Voluntary and forced exercise differentially alters the gut microbiome in C57BL/6J mice. J. Appl. Physiol. (1985). 118: 1059–1066. doi: 10.1152/japplphysiol.01077.2014. [DOI] [PubMed] [Google Scholar]
- Aßhauer, K. P., Wemheuer B., Daniel R., and Meinicke P.. . 2015. Tax4Fun: predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics 31:2882–2884. doi: 10.1093/bioinformatics/btv287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretin, A., Lucas C., Larabi A., Dalmasso G., Billard E., Barnich N., Bonnet R., and Nguyen H. T. T.. . 2018. AIEC infection triggers modification of gut microbiota composition in genetically predisposed mice, contributing to intestinal inflammation. Sci. Rep. 8:12301. doi: 10.1038/s41598-018-30055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastain, C. S., Richert B. T., Schinckel A. P., Johnson T. A., Wickware C. L., Thayer M. T., Mills K. M., Feldpausch J. A., Palencia J. Y., and Richert J. A.. . 2019. 146 Effects of feeding soluble fiber (dextrin) to pigs pre-and post-weaning on growth performance and volatile fatty acid (VFA) production. J. Anim. Sci. 97(Supplement_2):84-84. doi: 10.1093/jas/skz122.153. [DOI] [Google Scholar]
- Chaucheyras-Durand, F., Masséglia S., Fonty G., and Forano E.. . 2010. Influence of the composition of the cellulolytic flora on the development of hydrogenotrophic microorganisms, hydrogen utilization, and methane production in the rumens of gnotobiotically reared lambs. Appl. Environ. Microbiol. 76:7931–7937. doi: 10.1128/AEM.01784-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che, D., Adams S., Wei C., Gui-Xin Q., Atiba E. M., and Hailong J.. . 2019. Effects of Astragalus membranaceus fiber on growth performance, nutrient digestibility, microbial composition, VFA production, gut pH, and immunity of weaned pigs. Microbiologyopen 8:e00712. doi: 10.1002/mbo3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, Z., Fu Z., Yan W., Nie K., Ding L., Ma D., Huang H., Li T., Xie J., and Fu L.. . 2021. The different effects of Chinese Herb Solid Drink and lactulose on gut microbiota in rats with slow transit constipation induced by compound diphenoxylate. Food Res. Int. 143:110273. doi: 10.1016/j.foodres.2021.110273. [DOI] [PubMed] [Google Scholar]
- Fukuda, S., Toh H., Hase K., Oshima K., Nakanishi Y., Yoshimura K., Tobe T., Clarke J. M., Topping D. L., Suzuki T., . et al. 2011. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469:543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- Gao, T., Jiao Y., Liu Y., Li T., Wang Z., and Wang D.. . 2019. Protective effects of konjac and inulin extracts on type 1 and type 2 diabetes. J. Diabetes Res. 2019:3872182. doi: 10.1155/2019/3872182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, X. L., Song Z. H., Li H., Wu S., Wu S. S., Ding Y. N., He X., Yin Y. L., and Fan Z. Y.. . 2019. Effects of dietary isomaltooligosaccharide and Bacillus spp. supplementation during perinatal period on lactational performance, blood metabolites, and milk composition of sows. J. Sci. Food Agric. 99:5646–5653. doi: 10.1002/jsfa.9821. [DOI] [PubMed] [Google Scholar]
- Hu, T. G., Wen P., Fu H. Z., Lin G. Y., Liao S. T., and Zou Y. X.. . 2019. Protective effect of mulberry (Morus atropurpurea) fruit against diphenoxylate-induced constipation in mice through the modulation of gut microbiota. Food Funct. 10:1513–1528. doi: 10.1039/c9fo00132h. [DOI] [PubMed] [Google Scholar]
- Jia, F., Yang S., Ma Y., Gong Z., Cui W., Wang Y., and Wang W.. . 2020. Extraction optimization and constipation-relieving activity of dietary fiber from Auricularia polytricha. Food Biosci. 33:100506. doi: 10.1016/j.fbio.2019.100506. [DOI] [Google Scholar]
- Jiang, H., Dong J., Jiang S., Liang Q., Zhang Y., Liu Z., Ma C., Wang J., and Kang W.. . 2020. Effect of Durio zibethinus rind polysaccharide on functional constipation and intestinal microbiota in rats. Food Res. Int. 136:109316. doi: 10.1016/j.foodres.2020.109316. [DOI] [PubMed] [Google Scholar]
- Jin, W., Cheng Y. F., Mao S. Y., and Zhu W. Y.. . 2011. Isolation of natural cultures of anaerobic fungi and indigenously associated methanogens from herbivores and their bioconversion of lignocellulosic materials to methane. Bioresour. Technol. 102:7925–7931. doi: 10.1016/j.biortech.2011.06.026. [DOI] [PubMed] [Google Scholar]
- Kaur, N., and Gupta A. K.. . 2002. Applications of inulin and oligofructose in health and nutrition. J. Biosci. 27:703–714. doi: 10.1007/BF02708379. [DOI] [PubMed] [Google Scholar]
- Kietsiriroje, N., Kwankaew J., Kitpakornsanti S., and Leelawattana R.. . 2015. Effect of phytosterols and inulin-enriched soymilk on LDL-cholesterol in Thai subjects: a double-blinded randomized controlled trial. Lipids Health Dis. 14:146. doi: 10.1186/s12944-015-0149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan, J., Wang K., Chen G., Cao G., and Yang C.. . 2020. Effects of inulin and isomalto-oligosaccharide on diphenoxylate-induced constipation, gastrointestinal motility-related hormones, short-chain fatty acids, and the intestinal flora in rats. Food Funct. 11:9216–9225. doi: 10.1039/d0fo00865f. [DOI] [PubMed] [Google Scholar]
- Lee, P. A., and Close W. H.. . 1987. Bulky feeds for pigs: A consideration of some non-nutritional aspects. Livest. Prod. Sci. 16:395–405. doi: 10.1016/0301-6226(87)90008-X. [DOI] [Google Scholar]
- Lepczyński, A., Herosimczyk A., Barszcz M., Ożgo M., Michałek K., Grabowska M., Tuśnio A., Szczerbińska D., and Skomiał J.. . 2021. Diet supplemented either with dried chicory root or chicory inulin significantly influence kidney and liver mineral content and antioxidative capacity in growing pigs. Animal 15:100129. doi: 10.1016/j.animal.2020.100129. [DOI] [PubMed] [Google Scholar]
- Li, Y., Meng Z., Xu Y., Shi Q., Ma Y., Aung M., Cheng Y., and Zhu W.. . 2021. Interactions between anaerobic fungi and methanogens in the rumen and their biotechnological potential in biogas production from lignocellulosic materials. Microorganisms. 9:190. doi: 10.3390/microorganisms9010190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, Y. N., Yu J. G., Zhang D. B., Zhang Z., Ren L. L., Li L. H., Wang Z., and Tang Z. S.. . 2019. Indigo naturalis ameliorates dextran sulfate sodium-induced colitis in mice by modulating the intestinal microbiota community. Molecules. 24:4086. doi: 10.3390/molecules24224086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, A., Lv H., Wang H., Yang H., Li Y., and Qian J.. . 2020. Aging increases the severity of colitis and the related changes to the gut barrier and gut microbiota in humans and mice. J. Gerontol. A. Biol. Sci. Med. Sci. 75:1284–1292. doi: 10.1093/gerona/glz263. [DOI] [PubMed] [Google Scholar]
- Liu, X., Chen S., Yan Q., Li Y., and Jiang Z.. . 2019. Effect of Konjac mannan oligosaccharides on diphenoxylate-induced constipation in mice. J. Funct. Foods. 57:399–407. doi: 10.1016/j.jff.2019.04.036 [DOI] [Google Scholar]
- Ma, D., Wang A. C., Parikh I., Green S. J., Hoffman J. D., Chlipala G., Murphy M. P., Sokola B. S., Bauer B., Hartz A. M. S., . et al. 2018. Ketogenic diet enhances neurovascular function with altered gut microbiome in young healthy mice. Sci. Rep. 8:6670. doi: 10.1038/s41598-018-25190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, X. M., and Liu X. T.. . 2019. [Effect of application of herbal medicine paste to “Tianshu” (ST25)on intestinal mobility and expression of vasoactive intestinal peptide and substance P in colonic myenteric plexus in rats with functional constipation]. Zhen Ci Yan Jiu 44:906–910. doi: 10.13702/j.1000-0607.180760. [DOI] [PubMed] [Google Scholar]
- Mizubuchi, H., Yajima T., Aoi N., Tomita T., and Yoshikai Y.. . 2005. Isomalto-oligosaccharides polarize Th1-like responses in intestinal and systemic immunity in mice. J. Nutr. 135:2857–2861. doi: 10.1093/jn/135.12.2857. [DOI] [PubMed] [Google Scholar]
- Mookiah, S., Sieo C. C., Ramasamy K., Abdullah N., and Ho Y. W.. . 2014. Effects of dietary prebiotics, probiotic and synbiotics on performance, caecal bacterial populations and caecal fermentation concentrations of broiler chickens. J. Sci. Food Agric. 94:341–348. doi: 10.1002/jsfa.6365. [DOI] [PubMed] [Google Scholar]
- Mountfort, D. O., and Asher R. A.. . 1985. Production and regulation of cellulase by two strains of the rumen anaerobic fungus Neocallimastix frontalis. Appl. Environ. Microbiol. 49:1314–1322. doi: 10.1128/aem.49.5.1314-1322.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndou, S. P., Kiarie E., and Nyachoti C. M.. . 2019. Flaxseed meal and oat hulls supplementation: impact on predicted production and absorption of volatile fatty acids and energy from hindgut fermentation in growing pigs. J. Anim. Sci. 97:302–314. doi: 10.1093/jas/sky399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning, Y., and W. Zhang. 2009. Relationship between gastrointestinal hormones and functional constipation. Int. J. Intern. Med. 7:399–404. [Google Scholar]
- Niu, Q., Li P., Hao S., Kim S., Du T., Hua J., and Huang R.. . 2019. Characteristics of gut microbiota in sows and their relationship with apparent nutrient digestibility. Int. J. Mol. Sci. 20. doi: 10.3390/ijms20040870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliviero, C., Heinonen M., Valros A., and Peltoniemi O.. . 2010. Environmental and sow-related factors affecting the duration of farrowing. Anim. Reprod. Sci. 119:85–91. doi: 10.1016/j.anireprosci.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Oliviero, C., Kokkonen T., Heinonen M., Sankari S., and Peltoniemi O.. . 2009. Feeding sows with high fibre diet around farrowing and early lactation: impact on intestinal activity, energy balance related parameters and litter performance. Res. Vet. Sci. 86:314–319. doi: 10.1016/j.rvsc.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Paßlack, N., Vahjen W., and Zentek J.. . 2015. Dietary inulin affects the intestinal microbiota in sows and their suckling piglets. BMC Vet. Res. 11:51. doi: 10.1186/s12917-015-0351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, S., and Goyal A.. . 2011. Functional oligosaccharides: production, properties and applications. World J Microbiol Biotechnol. 27:1119–1128. doi: 10.1007/s11274-010-0558-5 [DOI] [Google Scholar]
- Petry, A. L., Patience J. F., Huntley N. F., Koester L. R., Bedford M. R., and Schmitz-Esser S.. . 2021. Xylanase supplementation modulates the microbiota of the large intestine of pigs fed corn-based fiber by means of a stimbiotic mechanism of action. Front. Microbiol. 12:619970. doi: 10.3389/fmicb.2021.619970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike, L. J., and Forster S. C.. . 2018. A new piece in the microbiome puzzle. Nat. Rev. Microbiol. 16:186. doi: 10.1038/nrmicro.2018.24. [DOI] [PubMed] [Google Scholar]
- Pu, G., Li P., Du T., Niu Q., Fan L., Wang H., Liu H., Li K., Niu P., Wu C., . et al. 2020. Adding appropriate fiber in diet increases diversity and metabolic capacity of distal gut microbiota without altering fiber digestibility and growth rate of finishing pig. Front. Microbiol. 11:533. doi: 10.3389/fmicb.2020.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettedal, E., Vilain S., Lindblom S., Lehnert K., Scofield C., George S., Clay S., Kaushik R. S., Rosa A. J., Francis D., . et al. 2009. Alteration of the ileal microbiota of weanling piglets by the growth-promoting antibiotic chlortetracycline. Appl. Environ. Microbiol. 75:5489–5495. doi: 10.1128/AEM.02220-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberfroid, M. 2007. Prebiotics: the concept revisited. J. Nutr. 137(3 Suppl 2):830S–837S. doi: 10.1093/jn/137.3.830S. [DOI] [PubMed] [Google Scholar]
- Sasaki, H., Lyu Y., Nakayama Y., Nakamura F., Watanabe A., Miyakawa H., Nakao Y., and Shibata S.. . 2020. Combinatorial effects of soluble, insoluble, and organic extracts from Jerusalem artichokes on gut microbiota in mice. Microorganisms. 8:954. doi: 10.3390/microorganisms8060954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao, T., Yu Q., Zhu T., Liu A., Gao X., Long X., and Liu Z.. . 2020. Inulin from Jerusalem artichoke tubers alleviates hyperglycaemia in high-fat-diet-induced diabetes mice through the intestinal microflora improvement. Br. J. Nutr. 123:308–318. doi: 10.1017/S0007114519002332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, D. P., Singh J., Boparai R. K., Zhu J., Mantri S., Khare P., Khardori R., Kondepudi K. K., Chopra K., and Bishnoi M.. . 2017. Isomalto-oligosaccharides, a prebiotic, functionally augment green tea effects against high fat diet-induced metabolic alterations via preventing gut dysbacteriosis in mice. Pharmacol. Res. 123:103–113. doi: 10.1016/j.phrs.2017.06.015. [DOI] [PubMed] [Google Scholar]
- Srikanth, R., Reddy C. H., Siddartha G., Ramaiah M. J., and Uppuluri K. B.. . 2015. Review on production, characterization and applications of microbial levan. Carbohydr. Polym. 120:102–114. doi: 10.1016/j.carbpol.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Stewart, C. S., and Richardson A. J.. . 1989. Enhanced resistance of anaerobic rumen fungi to the ionophores monensin and lasalocid in the presence of methanogenic bacteria. J. Appl. Bacteriol. 66:85–93. doi: 10.1111/j.1365-2672.1989.tb02458.x. [DOI] [PubMed] [Google Scholar]
- Su, H., Chen J., Miao S., Deng K., Liu J., Zeng S., Zheng B., and Lu X.. . 2019. Lotus seed oligosaccharides at various dosages with prebiotic activity regulate gut microbiota and relieve constipation in mice. Food Chem. Toxicol. 134:110838. doi: 10.1016/j.fct.2019.110838. [DOI] [PubMed] [Google Scholar]
- Sui, N., and Z. Tian. 2014. Effect of Zhu yang tong bian decoction on 5-HT and VIP and expression in intestinal tissue of mouse slow transit constipation model. J. Liaoning. Univ. Tradit. Chin. Med. 5:32–36. [Google Scholar]
- Tabeling, R., Schwier S., and Kamphues J.. . 2003. Effects of different feeding and housing conditions on dry matter content and consistency of faeces in sows. J. Anim. Physiol. Anim. Nutr. (Berl). 87:116–121. doi: 10.1046/j.1439-0396.2003.00423.x. [DOI] [PubMed] [Google Scholar]
- Wan, X., Li T., Liu D., Chen Y., Liu Y., Liu B., Zhang H., and Zhao C.. . 2018. Effect of marine microalga ethanol extract on lipid metabolism and gut microbiota composition in high-fat diet-fed rats. Mar. Drugs. 16. doi: 10.3390/md16120498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L., Hu L., Yan S., Jiang T., Fang S., Wang G., Zhao J., Zhang H., and Chen W.. . 2017. Effects of different oligosaccharides at various dosages on the composition of gut microbiota and short-chain fatty acids in mice with constipation. Food Funct. 8:1966–1978. doi: 10.1039/c7fo00031f. [DOI] [PubMed] [Google Scholar]
- Wu, Y., Pan L., Shang Q., Ma X., Long S., Xu Y., and Piao X.. . 2017. Effects of isomalto-oligosaccharides as potential prebiotics on performance, immune function and gut microbiota in weaned pigs. Anim. Feed Sci. Tech. 230:126–135. doi: 10.1016/j.anifeedsci.2017.05.013 [DOI] [Google Scholar]
- Xiao, C. L., Tao Z. H., Guo L., Li W. W., Wan J. L., Sun H. C., Wang L., Tang Z. Y., Fan J., and Wu W. Z.. . 2011. Isomalto oligosaccharide sulfate inhibits tumor growth and metastasis of hepatocellular carcinoma in nude mice. BMC Cancer 11:150. doi: 10.1186/1471-2407-11-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, M., Gao T., Liu Z., and Diao X.. . 2020. Effects of dietary supplementation with high fiber (stevia residue) on the fecal flora of pregnant sows. Animals. 10:2247. doi: 10.3390/ani10122247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai, X., Lin D., Zhao Y., and Yang X.. . 2018. Bacterial cellulose relieves diphenoxylate-induced constipation in rats. J. Agric. Food Chem. 66:4106–4117. doi: 10.1021/acs.jafc.8b00385. [DOI] [PubMed] [Google Scholar]
- Zhang, W. F., Li D. F., Lu W. Q., and Yi G. F.. . 2003. Effects of isomalto-oligosaccharides on broiler performance and intestinal microflora. Poult. Sci. 82:657–663. doi: 10.1093/ps/82.4.657. [DOI] [PubMed] [Google Scholar]
- Zhang, X., X. P. Zhang, Y. Z. Du, H. J. Xing, R. Jia, L. J. Pan, J. Xu, and C. S. Jia. 2017. Effect of manual acupuncture, electroacupuncture and moxibustion on intestinal motility and expression of enteric nervous activity related proteins in functional constipation rats. Zhen Ci Yan Jiu. 42(5):407–412. [PubMed] [Google Scholar]
- Zhao, J. S., and W. D. Tong. 2012. Pathophysiology of slow transit constipation. Zhonghua Wei Chang Wai Ke Za Zhi. 15(7):758–760. [PubMed] [Google Scholar]
- Zhao, J., Bai Y., Zhang G., Liu L., and Lai C.. . 2020. Relationship between dietary fiber fermentation and volatile fatty acids’ concentration in growing pigs. Animals. 10:263. doi: 10.3390/ani10020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X., Qian Y., Suo H., Du M., Li G., Liu Z., and Li J.. . 2015. Preventive effect of Lactobacillus fermentum Zhao on activated carbon-induced constipation in mice. J. Nutr. Sci. Vitaminol. (Tokyo). 61:131–137. doi: 10.3177/jnsv.61.131. [DOI] [PubMed] [Google Scholar]
- Zhao, X., Yi R., Qian Y., and Park K. Y.. . 2018. Lactobacillus plantarum YS-3 prevents activated carbon-induced constipation in mice. J. Med. Food 21:575–584. doi: 10.1089/jmf.2017.4109. [DOI] [PubMed] [Google Scholar]