Abstract
To elucidate the pharmacological effects of Rho‐associated coiled‐coil containing protein kinase inhibitors (ROCK‐is), ripasudil (Rip), Y27632, and KD025, on human orbital fatty tissue, the human orbital fibroblasts (HOFs) were three‐dimensional (3D) cultured for 12 days. The effects of ROCK‐is on the physical properties of the 3D‐cultured HOF spheroids, including their sizes and physical stiffness, their adipogenesis by lipid staining, and the mRNA expression of adipogenesis‐related genes, PPARγ and AP2, and extracellular matrix (ECM) including collagen (COL) 1, 4, and 6, and fibronectin were analyzed. A significant increase in the sizes, physical stiffness, lipid staining, and mRNA expression of adipogenesis‐related genes, COL4 and COL6, and a decrease in COL1 expression were observed with adipogenesis (DIF+). In the presence of ROCK‐is, such DIF+‐induced effects were differently modulated as follows: (1) the sizes were not affected or significantly enhanced by Rip, Y27632, or KD025, (2) the physical stiffness was significantly decreased in Rip and Y27632, but was substantially increased in KD025, (3) the lipid staining was further enhanced or significantly suppressed by Rip, Y27632, or KD025, and both PPARγ and AP2 expression were significantly downregulated or upregulated by KD025 or Rip, and (4) Rip upregulated the expression of COL4, Y27632 upregulated the expression of COL1, COL4, and COL6, and KD025 upregulated the expression of COL1 and COL4. This study indicates that ROCK‐is significantly and differently modulate physical properties of the 3D HOF spheroids as well as their adipogenesis.
Keywords: human orbital fibroblasts (HOFs), Rho kinase, ROCK, ROCK inhibitor, three‐dimensional (3D) tissue culture
1. INTRODUCTION
The only evidence‐based therapy for glaucomatous optic neuropathy, the most frequent disease leading to irreversible blindness, 1 , 2 , 3 is to suitably lower the intraocular pressure (IOP) by antiglaucoma medications and/or glaucoma surgeries. 4 Among antiglaucoma medications, prostaglandin analogues (PGs) are recognized as the first‐line medication due to their great hypotensive efficacy in addition to very few systemic side effects. 5 Recently, PGs induced periocular side effects, including deepening of the upper eyelid sulcus (DUES) and other manifestations have been identified among their long‐term users. 6 PG‐induced atrophy of orbital adipose tissues was suspected as a possible mechanism for causing DUES, 7 Since then, antiglaucoma medication‐induced periocular side effects have begun to attract great attentions. To study this periocular effects especially toward orbital fatty tissue, we successfully obtained an in vivo model replicating DUES pathogenesis by using three‐dimensional (3D) cell cultures of 3T3‐L1 cells 8 as well as human orbital fibroblasts (HOFs). 9 Thus, we suggested that our 3D spheroid culture methods will rationally be applicable to study the periocular effects of several antiglaucoma drugs especially on orbital fatty tissues.
Rho‐associated coiled‐coil containing protein kinases (ROCKs), belonging to the serine–threonine protein kinase family, are well known to be involved in the regulation of actin cytoskeleton remodeling. 10 , 11 , 12 , 13 , 14 Two types of ROCKs, ROCK1 (ROKβ) and ROCK2 (ROKα) share their amino acid compositions of the carboxyl termini, the catalytic kinase domain and the Rho‐binding domain except their coiled‐coil region. 15 , 16 Functionally, ROCK1 and ROCK2 play important roles in the regulation of actin cytoskeleton organization, cytokinesis, differentiation, apoptosis, glucose metabolism, cell adhesion/motility, and inflammation. 17 , 18 , 19 The expression of ROCKs are also recognized within the ocular and periocular tissues, including the trabecular meshwork, ciliary muscles, and the retina, 15 , 16 and therefore, these are involved in the ocular pathophysiology in several ocular diseases such as cataracts, retinopathy, and corneal dysfunction. 10 , 11 , 20 , 21 , 22 , 23 These observations, in turn, strongly suggest that ROCKs may alternatively become therapeutic targets for these ocular diseases. In fact, it was revealed that ROCK inhibitors (ROCK‐is) could reduce IOP in several animal models, 24 , 25 and one of the ROCK‐is, ripasudil hydrochloride hydrate (Rip), a nonselective ROCK‐i, is already available as a new type of medications for the treatment of glaucoma and ocular hypertension. 26 , 27 Since it has been revealed that conjunctival hyperemia as one of periocular side effects of Rip, Rip and other ROCK‐is may cause unknown other periocular manifestations. 26 , 27 In addition, since, as described above, ROCKs, ROCK1 and ROCK2, multiply contribute pathophysiology in general, it is of great interest to elucidate periocular effects of ROCK‐is including Rip, especially toward orbital fatty tissues. In fact, our recent pilot study using a 3D spheroid culture of the 3T3‐L1 cells, which is known as the most popular preadipocyte used for adipogenesis‐related study, suggested that ROCK‐is significantly affect physical properties, adipogenesis, and extracellular matrix (ECM) expression of the 3T3‐L1 3D spheroids. 28 Taken together, these observations rationally allowed us to study further the effects of ROCK‐is toward HOFs.
Therefore, in the present study, we studied the effects of several ROCK‐is, including nonselective ROCK‐is, Y27632 and Rip, and a selective ROCK2 inhibitor, KD025, on lipid metabolism, especially on adipocyte volume, and their ECM expression through adipogenesis on our 3D‐cultured HOFs.
2. MATERIALS AND METHODS
This study, which was performed at the Sapporo Medical University Hospital, Japan, was approved by the institutional review board (approved number, 312‐3190) and according to the tenets of the Declaration of Helsinki as well as national laws for the protection of personal data. Informed consent was obtained from all participants in this study.
2.1. Isolation of HOFs and 3D cultures of HOFs
Human orbital fibroblasts were isolated surgically as described previously using orbital fat explants from four patients with orbital fat herniation. Thereafter, 3D cultures of HOFs and induction of their adipogenic differentiation were processed for 12 days as described recently. 29 , 30 For evaluating the efficacy of ROCK‐is, 1 or 10 µM ripasudil (Rip), Y27632, or KD025 were added from Day 1 through Day 12.
2.2. Lipid staining of 3D‐cultured HOFs by BODIPY
BODIPY lipid staining of 3D‐cultured HOF spheroids was performed as described previously. 29 , 31 , 32 Briefly, 4% paraformaldehyde‐fixed 3D spheroids were incubated in a mixture of 0.1% BODIPY (#D3922; Thermo Fisher Scientific), 0.1% DAPI (#D523; Doujin), and 0.1% phalloidin (#20553; Funakoshi) in PBS containing 3% bovine serum albumin for 3 h. The fluorescence intensity of the BODIPY was detected using a Nikon A1 confocal microscope (Tokyo, Japan) and quantified using the Image J software version 2.0.0 (NIH).
2.3. Quantitative PCR
Using total RNA extraction by a RNeasy mini kit (Qiagen), and the reverse transcription by the SuperScript IV kit (Invitrogen) were processed to make cDNA according to the manufacturer's instructions. The real‐time PCR with the Universal Taqman Master mix was performed using a StepOnePlus machine (Applied Biosystems/Thermo Fisher Scientific). cDNA levels expressed as fold‐change relative to the expression of a housekeeping 36B4 (Rplp0) gene was calculated. Sequences of the primers and Taqman probes used are given below:
Human RPLP0
Probe: 5′‐/56‐FAM/CCCTGTCTT/ZEN/CCCTGGGCATCAC/3IABkFQ/‐3′; forward: 5′‐TCGTCTTTAAACCCTGCGTG‐3′; reverse: 5′‐TGTCTGCTCCCACAATGAAAC‐3′.
Human COL1A1
Probe: 5′‐/56‐FAM/TCCAGGGCC/ZEN/AAGACGAAGACATC/3IABkFQ/‐3′, forward: 5′‐GACATGTTCAGCTTTGTGGAC‐3′; reverse: 5′‐TTCTGTACGCAGGTGATTGG‐3′.
Human COL4A1
Probe: 5′‐/56‐FAM/TCATACAGA/ZEN/CTTGGCAGCGGCT/3IABkFQ/‐3′, forward: 5′‐AGAGAGGAGCGAGATGTTCA‐3′; reverse: 5′‐TGAGTCAGGCTTCATTATGTTCT‐3′.
Human COL6A1
Forward: 5′‐CCTCGTGGACAAAGTCAAGT‐3′; reverse: 5′‐GTGAGGCCTTGGATGATCTC‐3′.
Human FN1
Forward: 5′‐CGTCCTAAAGACTCCATGATCTG‐3′; reverse: 5′‐ACCAATCTTGTAGGACTGACC‐3′.
2.4. Microindentation force measurement
icroindentation force of the spheroids was measured using a microsqueezer (CellScale) as described previously. 30 Briefly, a single spheroid placed on a 3 mm × 3 mm plate was compressed to achieve 50% deformation in 20 s using a microcamera. The required strain (μN) was measured and force/displacement (μN/μm) was calculated.
2.5. Statistical analysis
All statistical analyses were performed using Graph Pad Prism 8 (GraphPad Software). To analyze the difference between groups, a group analysis with two‐way ANOVA followed by Tukey's multiple comparison test were performed. Data are presented as arithmetic means ± SEM.
3. RESULTS
To study the pharmacological effects of several ROCK‐is on orbital fatty tissues, physical properties such as sizes and stiffness of 3D HOFs spheroids were investigated in the presence of 1 or 10 μM ripasudil (Rip), Y27632, or KD025. The 3D cultures of HOFs at Day 12 and sizes of each 3D spheroid among the experimental groups were plotted as shown in Figure 1A,B. As described in our previous studies, 29 , 31 uniform round‐shape spheroidal 3D spheroids from 20,000 HOFs cells were obtained (Figure 1C), and upon adipogenic induction (DIF+), these sizes were significantly enlarged. This DIF+‐induced enlargements of 3D HOF spheroids were substantially enhanced in the presence of Y27632 or KD025 and this enhancement was concentration dependent, although such enlargement effects were not observed in the presence of Rip (Figure 1A,B). Similarly, microindentation analysis indicated that the physical stiffness of 3D HOF spheroids were significantly increased by DIF+, and the additive effects of ROCK‐is to DIF+ were also concentration dependent. However, the efficacies among the ROCK‐is forms were significantly different, that is, pan‐ROCK‐is, Rip and Y27632, or ROCK2‐i, KD025, induced significant suppressive or enhancing effects (Figure 2).
FIGURE 1.
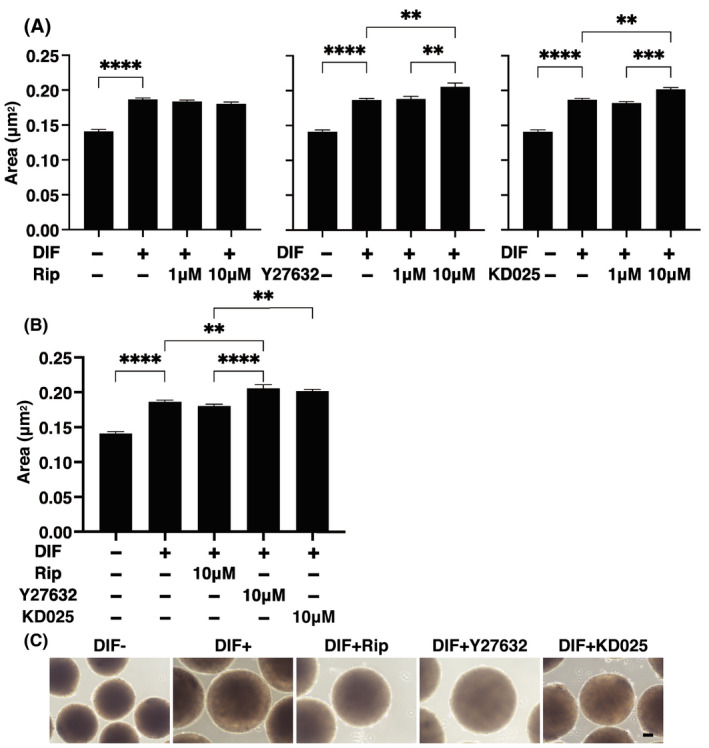
Effects of ROCK‐is on the area sizes of the 3D human orbital fibroblast (HOF) spheroids during adipogenesis. At Day 12, the mean area sizes (μm2) of the 3D spheroids of HOFs preadipocytes (DIF−) and their adipogenic differentiation (DIF+) without or with 1 or 10 µM ripasudil (Rip), Y27632, or KD025 are plotted in panel (A). Among the different ROCK‐is forms, to compare their effects toward HOFs adipogenesis, the mean area sizes (μm2) of 10 µM of each ROCK‐is were replotted in panel (B), and their representative phase contrast microscopic images are shown in panel (C). All experiments were performed in triplicate using fresh preparations, each of which consisted of 16 spheroids. Data are presented as arithmetic means ±standard error of the mean (SEM). **p < 0.01, ***p < 0.005, ****p < 0.001 (ANOVA followed by Tukey's multiple comparison test). ROCK, Rho‐associated coiled‐coil containing protein kinase
FIGURE 2.
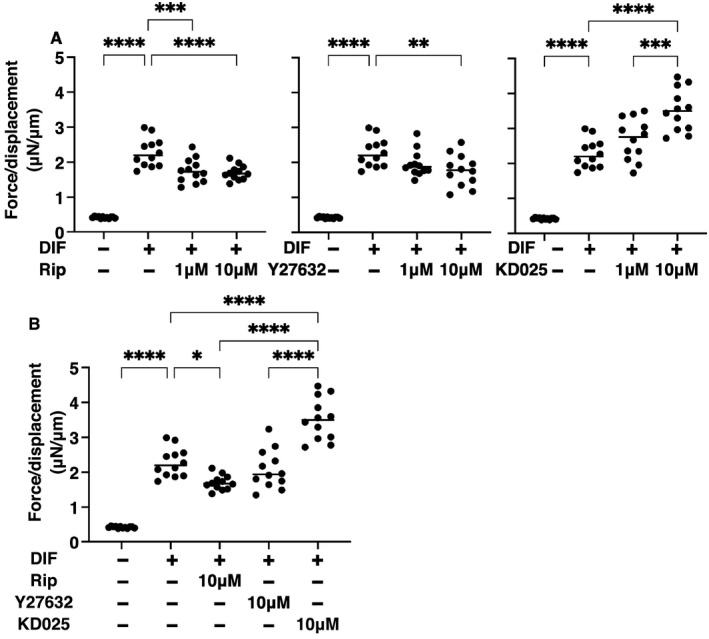
Effects of ROCK‐is on physical stiffness of the 3T3‐L1 3D spheroids. At Day 12, the 3D human orbital fibroblast (HOF) spheroids of preadipocytes (DIF−) and their adipogenic differentiation (DIF+) without or with 1 or 10 µM ripasudil (Rip), Y27632, or KD025 were subjected to a physical solidity analysis using a microsqueezer. The force required to induce deformation until half diameter was reached (μN/μm force/displacement) were measured and the data are potted in panel A. Among the different ROCK‐is forms, to compare their effects toward HOFs adipogenesis, the physical stiffness of 10 µM of each ROCK‐is were replotted in panel B. All experiments were performed using freshly prepared 12–20 spheroids. *p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.001 (ANOVA followed by Tukey's multiple comparison test). ROCK, Rho‐associated coiled‐coil containing protein kinase
To elucidate on the underlying mechanisms of ROCK‐is‐induced effects on the physical properties of 3D HOF spheroid, lipid staining by BODIPY and mRNA expression of adipogenesis‐related genes, PPARγ and AP2, and ECMs including collagen 1 (COL1), COL4, COL6, and fibronectin (FN) were studied in the presence of 10 μM ROCK‐is, which caused more effects on the physical properties as above. As shown in Figure 3, upon DIF+, their staining intensities and gene expression of PPARγ and AP2 were significantly increased as observed in our previous studies. 29 , 31 , 32 In the presence of Rip or Y27632, such DIF+ induced increase in BODIPY staining was further enhanced; although the gene expression of PPARγ was not altered, the gene expression of AP2 was significantly increased by Rip. Surprisingly, KD025 induced significant downregulation of PPARγ and AP2 expression, which is more evident in the latter, and almost no staining by BODIPY. In terms of mRNA expression of ECMs, COL4 and COL6 were downregulated or upregulated upon DIF+ as similarly observed in our previous studies. 29 , 31 , 32 In the presence of ROCK‐is, such DIF+‐induced changes of COL4 were all enhanced. While in contrast, DIF+‐induced expression of COL1 and COL6 were significantly enhanced by Y27632 and KD025, and Y27632, respectively. These diversity in the efficacies among ROCK‐is may be attributed to differences in their preference for ROCK1 or ROCK2 inhibitory activities (Rip; ROCK 1<ROCK 2, Y‐27632; ROCK 1>ROCK 2) 33 , 34 in addition to the paradoxical inhibitory effects of KD025 as described by Diep et al. 35
FIGURE 3.
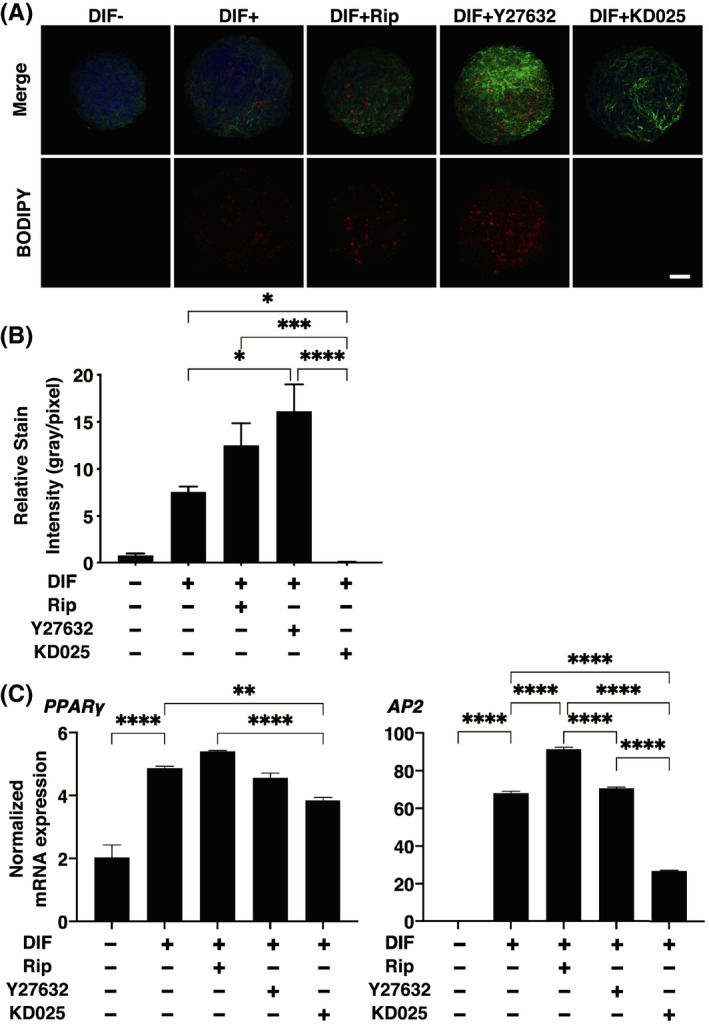
Effects of ROCK‐is on adipogenesis of 3D human orbital fibroblast (HOF) spheroids. The 3D HOF spheroids from Day 12 was prepared under several conditions: preadipocytes (DIF−) and their adipogenic differentiation (DIF+) without or with 10 µM ripasudil (Rip), Y27632, or KD025. These samples were immunostained with DAPI (blue), phalloidin (green), and BODIPY (red) (panel A, scale bar: 100 μm) and their staining intensities were plotted (panel B). The mRNA expressions of adipogenesis‐related genes including Pparγ and Ap2 under above conditions were plotted in panel ©. All experiments were performed in duplicate using fresh preparations, each consisting of 16 spheroids. Data are presented as arithmetic means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.001 (ANOVA followed by Tukey's multiple comparison test). ROCK, Rho‐associated coiled‐coil containing protein kinase
4. DISCUSSION
ROCKs are known to negatively regulate adipocyte differentiation. 36 In the 3T3‐L1 cells, ROCKs are known to inhibit adipogenesis, and in turn, their inhibitors, Y‐27632 and fasudil are identified to stimulate adipocyte differentiation. 36 In the present study, pan‐ROCK inhibitors, Rip and Y27632, also significantly enhanced adipogenesis of the 3D HOF spheroids. As it was shown that ROCK2 but not ROCK1 is responsible for the suppressive effects toward their adipogenesis, 36 we expected higher enhancement effects of adipogenesis by a specific inhibitor of ROCK2, KD025. However, in contrast, KD025 inhibited mRNA expression of PPARγ slightly and AP2 substantially in the 3D HOF spheroids, in which almost no BODIPY lipid staining was observed. Such paradoxical anti‐adipogenesis effects by KD025 in the 3T3‐L1 cells were also reported. 35 Furthermore, this paradoxical phenomenon by KD025 was also observed in the stiffness analysis of the 3D HOF spheroids (Figure 2). In their study, key genes such as PPARγ and C/EBPα were significantly blocked by KD025, whereas the expression of early adipogenic genes was not affected. In addition, the end stage of adipogenesis, KD025 did not affect lipid accumulation, and mitotic clonal expansion. Based on these findings, they suggested that KD025 regulates its targets during the intermediate stage of adipogenesis. To support this suggestion, in our current study, suppressive effects of mRNA expression toward AP2 were much more evident than those of PPARγ.
In terms of the expression of major ECMs in adipocytes or adipose tissues, it has been reported that main types of adipose ECM are the main fibril‐forming COL1 and microfibrillar COL4 and COL6. COL1 is the most abundant ECM, which serve to impart stiffness to tissues and organs. 37 COL4 and six foam network of COLs and are involved in a variety of cellular functions including the regulation of fibril assemblies and organization, integrating cells, and matrix structures and/or the integration of different matrix structures such as basement membranes. 38 , 39 In agreement with these network‐forming ECMs have the capacity to organize the 3D tissue architecture. 40 , 41 ROCK‐is‐induced upregulation of COL1, COL4, or COL6 was observed within the 3D HOF spheroids, in which their sizes were increased in the present study. FN forms a weak molecular conformation and can be changed by the binding of allosteric partners or strain resulting from cell contractile forces. 42 In the present study, FN expression were not significantly affected by ROCK‐is. Therefore, unbalance between unchanged FN and significant upregulation of the network‐forming COLs, COL4, and COL6 by ROCK‐is, may presumably cause substantial decrease in the physical stiffness of the 3D HOF spheroids. This speculation is rationally supported by the fact that the increase in 3D spheroid size was induced in the presence of 10 μM ROCK‐is, but not in the presence of 1 μM ROCK‐is as shown in Figure 1, and in the presence of 10 μM concentrations of ROCK‐is, the gene expression of ECM were modified as shown in Figure 4. Similar to this, the inhibition of ROCKs is also known to reduce the extent of mechanical tension and stiffness in cells, and decrease ECM synthesis and rigidity in various cell types. 43 , 44
FIGURE 4.
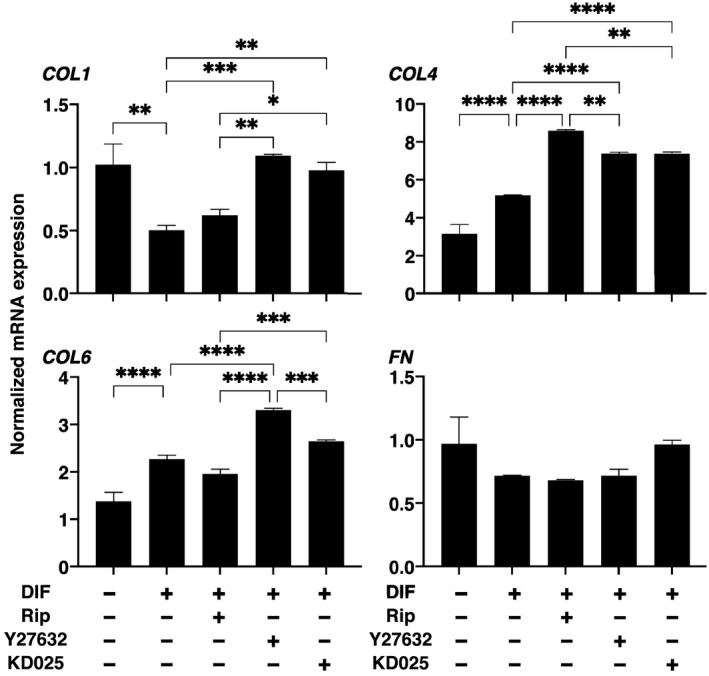
Effects of ROCK‐is on mRNA expressions of mRNA expression of ECMs in 3D human orbital fibroblast (HOF) spheroids. At Day 12, the 3D HOF spheroids of preadipocytes (DIF−) and their adipogenic differentiation (DIF+) without or with 10 µM ripasudil (Rip), Y27632, or KD025 were subjected to a quantitive PCR analysis to estimate the mRNA expression of ECMs. All experiments were performed in duplicate using fresh preparations, each of which consisted of 16 spheroids. Data are presented as arithmetic means ± SEM. COL1, collagen 1; COL4, collagen 4; COL6, collagen 6; ECM, extracellular matrix; FN, fibronectin. *p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.001 (ANOVA followed by Tukey's multiple comparison test). ROCK, Rho‐associated coiled‐coil containing protein kinase
Concerning the periocular effects of ROCK‐is especially on orbital fatty tissues, only limited information is currently available. However, in our previous studies, we demonstrated that pan‐ROCK‐is, Rip and Y27632, induced the formation of significantly larger sized 3D spheroids from DIF+ 3T3‐L1 cells 28 or DIF‐HOFs obtained from patients with Grave's orbitopathy. 45 However, a paradoxical phenomenon of physical stiffness of the 3D 28 spheroid was observed upon adipogenesis, that is, an increase (HOF) 30 , 46 but a decrease (3T3‐L1) 32 was observed in addition to the paradoxical inhibition of the ROCK2 inhibitor, KD025 toward adipogenesis 35 were also observed in our current study. The underlying mechanisms responsible for causing these paradoxical phenomena remain unknown because of study limitations and need to be elucidated.
Our current observations indicated that ROCK‐is significantly altered the physical properties as well as adipogenesis of 3D HOF spheroids. However, in a clinical situation, adipocytes are already differentiated when they are exposed to glaucoma medications, and further study on the effects of ROCK‐is toward undifferentiated preadipocytes in addition to investigations related to the study limitations as above will be required.
AUTHOR CONTRIBUTIONS
Y.I. designed and performed experiments, analyzed data, and wrote the paper. H.I, M.W., and U.A. analyzed the data. H.O. analyzed the data and provided conceptual advice. F.H. designed the experiments, analyzed the data, and wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no conflicts of interest associated with this manuscript.
Hikage F, Ichioka H, Watanabe M, Umetsu A, Ohguro H, Ida Y. ROCK inhibitors modulate the physical properties and adipogenesis of 3D spheroids of human orbital fibroblasts in different manners. FASEB BioAdvances. 2021;3:866–872. 10.1096/fba.2021-00037
REFERENCES
- 1. Weinreb RN, Khaw PT. Primary open‐angle glaucoma. Lancet (London, England). 2004;363:1711‐1720. [DOI] [PubMed] [Google Scholar]
- 2. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Caprioli J, Coleman AL. Blood pressure, perfusion pressure, and glaucoma. Am J Ophthalmol. 2010;149:704‐712. [DOI] [PubMed] [Google Scholar]
- 4. van der Valk R, Webers CA, Schouten JS, Zeegers MP, Hendrikse F, Prins MH. Intraocular pressure‐lowering effects of all commonly used glaucoma drugs: a meta‐analysis of randomized clinical trials. Ophthalmology. 2005;112:1177‐1185. [DOI] [PubMed] [Google Scholar]
- 5. Li T, Lindsley K, Rouse B, et al. Comparative effectiveness of first‐line medications for primary open‐angle glaucoma: a systematic review and network meta‐analysis. Ophthalmology. 2016;123:129‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peplinski LS, Albiani Smith K. Deepening of lid sulcus from topical bimatoprost therapy. Optom Vis Sci. 2004;81:574‐577. [DOI] [PubMed] [Google Scholar]
- 7. Serrero G, Lepak NM. Prostaglandin F2alpha receptor (FP receptor) agonists are potent adipose differentiation inhibitors for primary culture of adipocyte precursors in defined medium. Biochem Biophys Res Commun. 1997;233:200‐202. [DOI] [PubMed] [Google Scholar]
- 8. Ida Y, Hikage F, Itoh K, Ida H, Ohguro H. Prostaglandin F2alpha agonist‐induced suppression of 3T3‐L1 cell adipogenesis affects spatial formation of extra‐cellular matrix. Sci Rep. 2020;10:7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Itoh K, Hikage F, Ida Y, Ohguro H. Prostaglandin F2alpha agonists negatively modulate the size of 3D organoids from primary human orbital fibroblasts. Invest Ophthalmol Vis Sci. 2020;61:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bertrand J, Winton MJ, Rodriguez‐Hernandez N, Campenot RB, McKerracher L. Application of Rho antagonist to neuronal cell bodies promotes neurite growth in compartmented cultures and regeneration of retinal ganglion cell axons in the optic nerve of adult rats. J Neurosci. 2005;25:1113‐1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tan H‐B, Zhong Y‐S, Cheng Y, Shen X. Rho/ROCK pathway and neural regeneration: a potential therapeutic target for central nervous system and optic nerve damage. Int J Ophthalmol. 2011;4:652‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sagawa H, Terasaki H, Nakamura M, et al. A novel ROCK inhibitor, Y‐39983, promotes regeneration of crushed axons of retinal ganglion cells into the optic nerve of adult cats. Exp Neurol. 2007;205:230‐240. [DOI] [PubMed] [Google Scholar]
- 13. Watabe H, Abe S, Yoshitomi T. Effects of Rho‐associated protein kinase inhibitors Y‐27632 and Y‐39983 on isolated rabbit ciliary arteries. Jpn J Ophthalmol. 2011;55:411‐417. [DOI] [PubMed] [Google Scholar]
- 14. Stiles JM, Kurisetty V, Mitchell DC, Bryan BA. Rho kinase proteins regulate global miRNA expression in endothelial cells. Cancer Genomics Proteomics. 2013;10:251‐263. [PubMed] [Google Scholar]
- 15. Nakagawa O, Fujisawa K, Ishizaki T, Saito Y, Nakao K, Narumiya S. ROCK‐I and ROCK‐II, two isoforms of Rho‐associated coiled‐coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996;392:189‐193. [DOI] [PubMed] [Google Scholar]
- 16. Fukiage C, Mizutani K, Kawamoto Y, Azuma M, Shearer TR. Involvement of phosphorylation of myosin phosphatase by ROCK in trabecular meshwork and ciliary muscle contraction. Biochem Biophys Res Comm. 2001;288:296‐300. [DOI] [PubMed] [Google Scholar]
- 17. Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12:722‐734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446‐456. [DOI] [PubMed] [Google Scholar]
- 19. Chun KH, Araki K, Jee Y, et al. Regulation of glucose transport by ROCK1 differs from that of ROCK2 and is controlled by actin polymerization. Endocrinology. 2012;153:1649‐1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yokota T, Utsunomiya K, Taniguchi K, Gojo A, Kurata H, Tajima N. Involvement of the Rho/Rho kinase signaling pathway in platelet‐derived growth factor BB‐induced vascular endothelial growth factor expression in diabetic rat retina. Jpn J Ophthalmol. 2007;51:424‐430. [DOI] [PubMed] [Google Scholar]
- 21. Arita R, Hata Y, Nakao S, et al. Rho kinase inhibition by fasudil ameliorates diabetes‐induced microvascular damage. Diabetes. 2009;58:215‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hollanders K, Van Bergen T, Kindt N, et al. The effect of AMA0428, a novel and potent ROCK inhibitor, in a model of neovascular age‐related macular degeneration. Invest Ophthalmol Vis Sci. 2015;56:1335‐1348. [DOI] [PubMed] [Google Scholar]
- 23. Okumura N, Koizumi N, Ueno M, et al. The new therapeutic concept of using a Rho kinase inhibitor for the treatment of corneal endothelial dysfunction. Cornea. 2011;30:S54‐S59. [DOI] [PubMed] [Google Scholar]
- 24. Kameda T, Inoue T, Inatani M, et al. The effect of Rho‐associated protein kinase inhibitor on monkey Schlemm's canal endothelial cells. Invest Ophthalmol Vis Sci. 2012;53:3092‐3103. [DOI] [PubMed] [Google Scholar]
- 25. Van de Velde S, Van Bergen T, Sijnave D, et al. AMA0076, a novel, locally acting Rho kinase inhibitor, potently lowers intraocular pressure in New Zealand white rabbits with minimal hyperemia. Invest Ophthalmol Vis Sci. 2014;55:1006‐1016. [DOI] [PubMed] [Google Scholar]
- 26. Inoue T, Tanihara H. Rho‐associated kinase inhibitors: a novel glaucoma therapy. Prog Retin Eye Res. 2013;37:1‐12. [DOI] [PubMed] [Google Scholar]
- 27. Tanihara H, Inoue T, Yamamoto T, et al. Intra‐ocular pressure‐lowering effects of a Rho kinase inhibitor, ripasudil (K‐115), over 24 hours in primary open‐angle glaucoma and ocular hypertension: a randomized, open‐label, crossover study. Acta Ophthalmol. 2015;93:e254‐e260. [DOI] [PubMed] [Google Scholar]
- 28. Ida Y, Hikage F, Ohguro H. ROCK inhibitors enhance the production of large lipid‐enriched 3D organoids of 3T3‐L1 cells. Sci Rep. 2021;11:5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Itoh K, Hikage F, Ida Y, Ohguro H. Prostaglandin F2α agonists negatively modulate the size of 3D organoids from primary human orbital fibroblasts. Invest Ophthalmol Vis Sci. 2020;61:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hikage F, Atkins S, Kahana A, Smith TJ, Chun TH. HIF2A‐LOX pathway promotes fibrotic tissue remodeling in thyroid‐associated orbitopathy. Endocrinology. 2019;160:20‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ida Y, Hikage F, Itoh K, Ida H, Ohguro H. Prostaglandin F2α agonist‐induced suppression of 3T3‐L1 cell adipogenesis affects spatial formation of extra‐cellular matrix. Sci Rep. 2020;10:7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ida Y, Hikage F, Umetsu A, Ida H, Ohguro H. Omidenepag, a non‐prostanoid EP2 receptor agonist, induces enlargement of the 3D organoid of 3T3‐L1 cells. Sci Rep. 2020;10:16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang J, Ruan F, Zheng Z. Ripasudil attenuates lipopolysaccharide (LPS)‐mediated apoptosis and inflammation in pulmonary microvascular endothelial cells via ROCK2/eNOS signaling. Med Sci Monit. 2018;24:3212‐3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liang H, Liao M, Zhao W, et al. CXCL16/ROCK1 signaling pathway exacerbates acute kidney injury induced by ischemia‐reperfusion. Biomed Pharmacother. 2018;98:347‐356. [DOI] [PubMed] [Google Scholar]
- 35. Diep DTV, Hong K, Khun T, et al. Anti‐adipogenic effects of KD025 (SLx‐2119), a ROCK2‐specific inhibitor, in 3T3‐L1 cells. Sci Rep. 2018;8:2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Noguchi M, Hosoda K, Fujikura J, et al. Genetic and pharmacological inhibition of Rho‐associated kinase II enhances adipogenesis. J Biol Chem. 2007;282:29574‐29583. [DOI] [PubMed] [Google Scholar]
- 37. Smith SM, Birk DE. Focus on molecules: collagens V and XI. Exp Eye Res. 2012;98:105‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc Res Tech. 2008;71:357‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yurchenco PD. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol. 2011;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lamande SR, Bateman JF. Collagen VI disorders: insights on form and function in the extracellular matrix and beyond. Matrix Biol. 2018;71–72:348‐367. [DOI] [PubMed] [Google Scholar]
- 41. Gregorio I, Braghetta P, Bonaldo P, Cescon M. Collagen VI in healthy and diseased nervous system. Dis Model Mech. 2018;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zollinger AJ, Smith ML. Fibronectin, the extracellular glue. Matrix Biol. 2017;60–61:27‐37. [DOI] [PubMed] [Google Scholar]
- 43. Pattabiraman PP, Maddala R, Rao PV. Regulation of plasticity and fibrogenic activity of trabecular meshwork cells by Rho GTPase signaling. J Cell Physiol. 2014;229:927‐942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang K, Sawaya MR, Eisenberg DS, Liao JC. Expanding metabolism for biosynthesis of nonnatural alcohols. Proc Natl Acad Sci USA. 2008;105:20653‐20658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ichioka H, Ida Y, Watanabe M, Ohguro H, Hikage F. Prostaglandin F2α and EP2 agonists, and a ROCK inhibitor modulate the formation of 3D organoids of Grave's orbitopathy related human orbital fibroblasts. Exp Eye Res. 2021;205:108489. [DOI] [PubMed] [Google Scholar]
- 46. Itoh K, Ida Y, Ohguro H, Hikage F. Prostaglandin F2α agonists induced enhancement in collagen1 expression is involved in the pathogenesis of the deepening of upper eyelid sulcus. Sci Rep. 2021;11:9002. [DOI] [PMC free article] [PubMed] [Google Scholar]


