Abstract
The density of nerves in cancer is emerging as a relevant clinical parameter for patient survival. Nerves in the tumor microenvironment have been associated with poor survival and recurrence, particularly if involved in perineural invasion. However, usually only a few nerves inside a tumor are affected by perineural invasion, while most nerves are not. Mechanistic studies have shown nerve‐secreted factors promote tumor growth and invasion thereby making tumors more aggressive. Therefore, the overall number of nerves in the tumor microenvironment should be more representative of the nerve‐tumor biological interaction than perineural invasion. This review summarizes the available clinical information about nerve density as a measure of clinical outcome in cancer and explores the mechanisms underlying nerve density in cancer, specifically, neurogenesis, axonogenesis, and neurotropism.
Keywords: neoplasms, nerve tissue/pathology, review, tumor microenvironment
Abbreviations
- AJCC
American Joint Committee on Cancer
- BDNF
brain‐derived neurotrophic factor
- CAM
chorioallantoic membrane
- CGRP
calcitonin GENE‐related peptide
- DCX
doublecortin
- DRG
dorsal root ganglia
- GALR2
galanin receptor 2
- GAP43
growth‐associated protein
- H&E
hematoxylin and eosin
- HNSCC
head and neck squamous cell carcinoma
- miRNAs
microRNAs
- NF‐H
heavy neurofilament
- NF‐L
light neurofilament
- NGF
nerve growth factor
- PET/CT
positron emission tomography–computed tomography
- PGP9.5
protein gene product 9.5
- PNI
perineural invasion
- PNS
peripheral nervous system
- S4F
semaphorin 4F
- TCGA
The Cancer Genome Atlas
- TH
tyrosine hydroxylase
- TMA
tissue microarrays
- TUJ‐1
beta‐III tubulin
- VAChT
vesicular acetylcholine transporter
1. INTRODUCTION
From a clinical viewpoint, perineural invasion (PNI) is a well‐recognized phenotype involving nerves in cancer, 1 and is associated with poor prognosis in multiple cancers. Consequently, cancers with PNI are treated more aggressively than tumors without PNI. 1 , 2 This role in treatment selection emphasizes the importance of recognizing PNI in cancer. PNI is currently defined as nerves invaded by tumor cells or in close proximity to tumor, provided that at least 33% of the nerve circumference is surrounded by tumor. 2 However, the definition of PNI has been debated for many decades with continued challenges, and there is variability between pathologists in interpretation of PNI. 3 , 4 , 5 Many factors contribute to the variability in opinions among pathologists when diagnosing PNI, including variation in histologic appearance of nerves in tissue sections and variable distance between tumor and nerve. 5 The underlying confounding issue is that none of the proposed definitions of PNI has incorporated the rapidly evolving knowledge of the mechanism involved. In part, this may be due to the challenge of translating complex biologic investigations into clinical practice. Our recent study emphasized the importance of bridging this gap between biology and clinical practice. 6 Our findings showed that proximity between cancer and nerves, even when nerves are “PNI‐negative” according to current criteria, is associated with higher death rates of patients with head and neck cancer. 6
Nerves are an important and previously understudied component of the tumor microenvironment. Despite the fact that PNI is not a perfect prognostic feature, the attention given to the biology of PNI over the last two decades has enhanced our understanding of neural regulation in the tumor microenvironment. 7 This has led to exploration of other neural phenotypes that impact the clinical course of a tumor. Of importance, nerve density or neural infiltration in the tumor stroma has been associated with poor clinical outcomes. In this review, we will discuss and summarize the findings on nerve density as an assessment of patient outcomes in cancer, and the current understanding of the biologic basis for this link.
2. NEURAL INFILTRATION IN TUMORS
Nerve density is a measurement of nerve infiltration in a given tissue; the possible causes for neural infiltration are discussed in this section.
A nerve is a cord‐like bundle of axons that function as a conduit for electrochemical impulses. 8 Axons or nerve fibers are neuronal projections that are enveloped by a myelin sheath or unmyelinated. Regardless of myelination, axons are wrapped in a connective tissue layer known as endoneurium. This layer contains nerve‐supporting cells such as Schwann cells and their subtypes that are associated with unmyelinated and myelinated axons. 9 Groups of axons, each surrounded by endoneurium, are encircled by another membrane, the perineurium, to constitute a fascicle. 10 Finally, several fascicles, wrapped by a protective layer called epineurium, form a typical peripheral nerve trunk (Figure 1). Histologically, nerves are observed as individual segments depending on plane of section and spatial orientation. These nerve segments contain one or a few nerve fibers, or one or many fascicles, depending on the size and branching pattern of the nerve.
FIGURE 1.
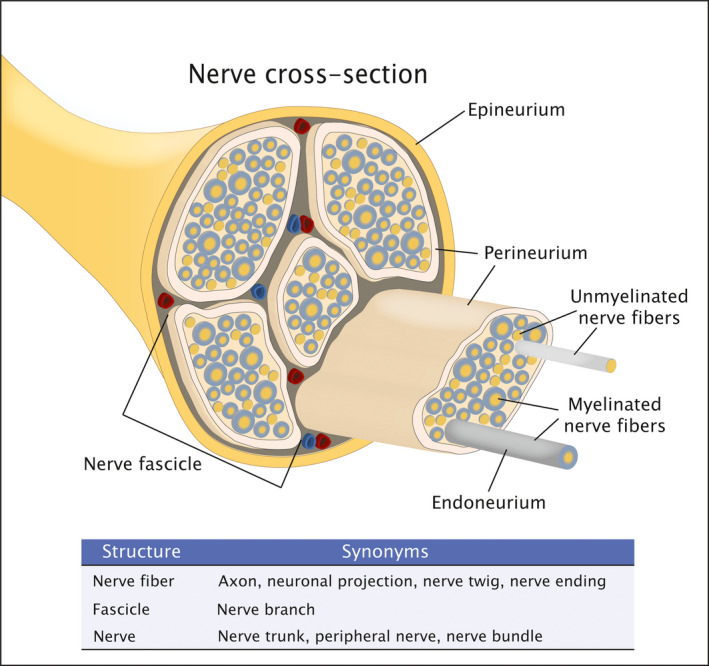
Schematic of the histologic structure of a peripheral nerve
The peripheral nervous system (PNS) consists of nerves, such as cranial and spinal nerves, outside the central nervous system. The PNS connects the central nervous system, comprised of the brain and spinal cord, to the rest of the body. 8 The PNS is divided into somatic and autonomic nervous system. The somatic nervous system controls voluntary skeletal muscle to perform daily functions. It consists of afferent (sensory) nerves which carry signals from the periphery toward the central nervous system, while efferent (motor) nerves transport signals from the central nervous system to the periphery. 11 In contrast, the autonomic nervous system controls involuntary physiological functions of innervated organs and tissues except skeletal muscle. The autonomic nervous system is comprised of the sympathetic, parasympathetic, and enteric nervous system. 12 Sympathetic nerves originate from the lateral horn of the spinal cord while parasympathetic nerves arise from the brainstem (cranial nerves III, VII, IX, and X) and sacral spinal cord.
There are different explanations for the presence of nerves in tumor stroma: nerves are formed de novo or actively grow inside tumors, or tumors grow in proximity to existing nerves, incorporating them into stroma. Axonogenesis or axon morphogenesis is the growth of axonal projections from a neuronal cell. 13 Also known as neurite outgrowth or neurite sprouting, axonogenesis in cancer is described both in vitro and in vivo. 14 , 15 , 16 , 17 , 18 , 19
Neurogenesis, the formation of new neurons 20 was demonstrated in prostate cancer. 16 , 21 In contrast, neurotropism is the ability to invade neural tissue. 22 Neurotropic tumors grow toward and ultimately invade pre‐existing nerves, a phenotype known as perineural invasion. In this situation, tumor growth pattern, rather than axonogenesis or neurogenesis would cause an accumulation of nerves in the tumor stroma. It is likely that all three phenotypes, axonogenesis, neurogenesis, and neurotropism, contribute to the high nerve density in tumors. The extent of involvement of each phenotype is influenced by differences in tumor biology across distinct tumors. A summary of the mechanisms implicated in nerve density is presented in Table 1.
TABLE 1.
Mechanisms involved in intratumoral nerve density
| Mechanism | Findings | References |
|---|---|---|
| Neurogenesis |
Migration of neurons from the subventricular zone of the brain to the tumor that can differentiate in adrenergic neo‐neurons. Higher density of neuronal progenitors (DCX‐positive) cells in prostate tumors in human. |
Mauffrey et al., 2019 21 |
| Prostate cancer patients presented an increased number of neurons in all prostatic ganglia compared with control patients. | Ayala et al., 2008 16 | |
| Axonogenesis | ||
| Cancer‐derived exosomes | Exosomal microRNAs (miR‐21 and miR‐324) enhanced axonogenesis in trigeminal ganglia neurons. | Amit et al., 2020 15 |
| Exosomes from plasma and tumor of HNSCC patients enhanced neurite outgrowth from PC‐12 cells. | ||
| EphrinB1 increased exosome‐induced axonogenesis. | Madeo et al., 2018 14 | |
| Cancer‐derived molecules | Adrenergic signaling (β2‐adrenergic receptors) increased neurotrophins which incremented sympathetic neurite outgrowth in pancreatic cancer. | Renz et al., 2018 18 |
| NGF promoted cholinergic nerve growth in gastric tumor. | Hayakawa et al., 2017 17 | |
| proNGF produced by prostate cancer cells (PC‐3) induced PC12 and 50B11 neuronal differentiation and axonogenesis. | Pundavela et al., 2014 19 | |
| Semaphorin 4F (S4F) contained in the supernatant of prostate cancer cells (DU145/S4F) induced more axonogenesis in N1E‐115 cells. | Ayala et al., 2008 16 |
2.1. Axonogenesis
Early reports of tumor axonogenesis were inconclusive. While electron microscopy supports active nerve growth inside ocular adenomas, 23 hepatocellular carcinomas have greater innervation in the capsule compared to tumor stroma; only preexisting portal tract innervation was observed in the latter, suggesting these tumors do not stimulate axonogenesis. 24 The discussion about whether tumor innervation is pre‐existing or from newly formed terminals was fueled by the identification of nerves in exophytic urinary bladder tumors 25 ; this suggests a remarkable capacity of the PNS to remodel and populate tumor stroma.
One of the mechanisms by which tumor cells regulate axonogenesis is through extracellular vesicles (exosomes). Most cells release vesicles that transport proteins and genetic information to facilitate intercellular communication. Exosomes play critical roles in several early and late events associated with tumor development and metastasis. Exosomes from plasma and tumor of patients with head and neck squamous cell carcinoma (HNSCC) enhanced neurite outgrowth from PC‐12 cells. 14 This phenotype was also observed with exosomes in a murine model of HNSCC, and was attenuated with exosome inhibitors. 14 EphrinB1, an axonal guidance molecule, potentiated exosome‐induced axonogenesis. 14 Additionally, MicroRNAs (miRNAs), contained within exosomes, may modulate neurite outgrowth. miRNAs are a class of non‐coding RNAs that regulate gene expression, usually to prevent protein production. miRNA expression profiles are altered in specific tumors and may be involved in cancer development. 26 Inhibition of miR‐21 and miR‐324 reduced axonogenesis in neurons of the trigeminal ganglia that were co‐cultured with exosomes derived from miR34a‐deficient/p53‐null oral tumor cells. These findings show that miRNAs modulate the neural element within the tumor microenvironment. 15
Cancer cells can release neurotrophins and axonal guidance molecules that increase neurite outgrowth. Neurotrophins are growth factors that stimulate neuron survival and differentiation and control proliferation and migration in non‐neuronal cells, including cancer cells. 27 Prostate cancer cells produce and release a nerve growth factor precursor (proNGF) which can promote tumor innervation. 19 Likewise, brain‐derived neurotrophic factor (BDNF) and nerve growth factor (NGF), that are upregulated in cancer cells, increase nerve density through axonogenesis in pancreatic ductal adenocarcinoma 18 and in gastric cancer. 17 Esophageal tumors with higher NGF expression were infiltrated by nerves more often than low NGF‐expressing counterparts. 28 These results suggest that nerve infiltration could be triggered by tumor‐derived NGF. In contrast, semaphorins, a large family of extracellular signaling proteins, modulate axonal guidance. Furthermore, they regulate angiogenesis, tumor growth, and metastatic dissemination. 29 Supernatant from co‐cultures of DU145 cells and DRGs (dorsal root ganglia) (cancer cells and neurons), significantly increased neurite sprouting of PC‐12 (rat pheochromocytoma cell line) and N1E‐115 (mouse neuroblastoma cell line), suggesting a tumor‐related axonogenesis. 16 Interestingly, Semaphorin 4F (S4F) was overexpressed in DU145/ DRG co‐culture samples. In addition, supernatant of DU145 cells infected with S4F retrovirus (DU145/S4F) increased neurite outgrowth in N1E‐115 cells. In DU145/S4F cells, siRNA‐mediated inhibition of S4F abrogated this effect. 16
2.2. Neurogenesis
Formation of new neurons from neural precursor cells was described in prostate cancer. 21 Specimens from human prostate cancer were stained with doublecortin (DCX), a marker of neuronal progenitors. High intratumoral density of DCX‐positive cells was significantly associated with tumor aggressiveness. Furthermore, using a murine model, this study demonstrated that these cells migrate from the subventricular zone of the brain to the tumor, showing distant communication to recruit neurons necessary for tumor growth. 21 Another study reported that total neurons in all prostatic ganglia and total area of those ganglia were increased in patients with prostate cancer compared to control patients, suggesting cancer‐related neurogenesis. 16
2.3. Neurotropism
Neurotropism may be another explanation for intratumoral nerve density. In vitro and in vivo studies simulating PNI interactions have shown cancer cell migration toward nerves. 30 , 31 , 32 In co‐cultures of mouse DRGs with prostate cancer cells, neurites from DRGs migrated toward cancer cells, and after physical contact, cancer cells migrated in the opposite direction toward DRGs, suggesting that reciprocal attraction, and nerves guide prostate cancer cells after physical interaction. 32 Similarly, co‐culture of human pancreatic cancer cells with DRG showed that Schwann cells guided cancer cells to migrate through nerve fibers to the DRG body/center in a contact‐dependent manner. 31 An in vivo model of nerve‐tumor interaction in which rat DRG and human oral cancer cells were grafted onto the chick embryo chorioallantoic membrane (CAM), 33 revealed that cancer cells overexpressing GALR2 (galanin receptor 2) were more invasive toward DRG than control cells; this effect was blocked by anti‐galanin antibody. 30
Although we discuss these mechanisms independently, there is crosstalk between nerves and tumor cells, and many processes occur simultaneously building cancer‐related nerve networks that nurture the tumor microenvironment thereby regulating tumorigenesis and cancer dissemination. Since nerves and tumors have reciprocal attraction, and “nerve fibers without a function do not exist”, 23 it is likely that nerves within a tumor are important for tumor biology. To illustrate the significance of nerves in innervated tissues, nerve‐derived molecules such as NGF promote proliferation to increase limb length and regeneration speed in severed salamander limbs. 34 In this regard, the impact of innervation on cancer growth was assessed with respect to tumor and nerve fiber type. Co‐culture of enteric primary neurons with a gastric organoid model enhanced neuronal outgrowth (axonogenesis), and promoted tumor growth. A muscarinic receptor agonist stimulated upregulation of gastric stem cell markers and Wnt genes; however, this effect was not detected with gastric organoids from type 3 muscarinic receptor (M3) knockout mice, suggesting that parasympathetic cholinergic nerves contribute to gastric tumorigenesis by activating M3‐induced Wnt signaling. 35 Similarly, parasympathetic cholinergic fibers promote tumor cell invasion, migration, and distant metastases via type 1 muscarinic receptors in an animal model of prostate cancer. 36 In contrast, parasympathetic nerves decelerate tumor progression in a murine breast cancer model. 37 Sympathetic fibers acting via β2‐ and β3‐adrenergic receptors promote prostate cancer cell survival 36 ; increased nerve density promotes tumor growth via β2‐adrenergic receptors in pancreatic ductal adenocarcinoma. 18 Both parasympathetic and sympathetic signals may play a role in tumor development or progression; however, they may have contrasting effects in different tumors.
All the aforementioned findings indicate participation of nerves in tumor progression via diverse mechanisms, suggesting that quantifying nerves within tumors could be of greater importance than evaluating PNI for treatment selection. Consequently, nerve density has been explored as a measure of nerve infiltration in cancer and as a predictor of poor prognosis.
3. NERVE DENSITY
There are multiple definitions for nerve density with no apparent consensus. Nerve density is often defined as the number of nerve segments (nerve trunks, nerve fascicles and/or individual nerve fibers) divided by the area analyzed, using tissue sections stained with immunohistochemistry 16 , 24 , 38 , 39 , 40 , 41 or hematoxylin and eosin (H&E). 15 Nerve density has also been defined as the area occupied by nerves divided by the total area analyzed. 36 , 42 , 43 , 44 If nerve density is used to assess axonogenesis or neurogenesis, it should evaluate delicate nerve fibers instead of large nerve trunks or nerve fascicles. Conversely, if nerve trunks or large fascicles are assessed, nerve density likely reflects neurotropism. Therefore, the size of nerves evaluated will highlight a different aspect of nerve‐tumor biology. However, all nerve‐related phenotypes, including axonogenesis, neurogenesis, and neurotropism, are likely important for tumor biology, considering the various nerve‐secreted factors that reach tumor cells.
There is considerable variation in methods to assess nerve density; these methods are summarized in Table 2 and Figure 2. For example, nerves are counted in a varying number of observation fields in tissue microarrays 45 or in resection specimens. 16 , 39 , 40 More subjectively, some studies evaluated nerve density by observation of the amount of immunohistochemical staining, dividing it into low, intermediate, or high density. 46 , 47 Regarding the size of nerve segments included in the analysis of nerve density, some studies limited the analysis to nerve trunks <100 µm in diameter 48 or to nerves presenting 3 or more axons, 49 with no clear rationale for the parameters used.
TABLE 2.
Nerve density definitions and methods of assessment in cancer, organized by the type of nerve structure analyzed
| Nerve density definition | Disease | Nerve IHC marker | Exclusions | Reference |
|---|---|---|---|---|
| 1. Nerve fibers | ||||
| Number of nerve fibers per 1 mm2. | Liver cancer | S100 | None | Terada & Matsunaga, 2011 24 |
| Area of nerves per 1 cm2. | Pancreatic cancer | GAP43 | None | Gao et al., 2015 41 |
| Area of nerve fibers per observation field. | Breast cancer | TH, VAChT and NF‐L | None | Kamiya et at., 2019 37 |
| 2. Nerve trunks/fascicles | ||||
| Number of nerves/high power field | Pancreatic cancer | S100 | None | Renz et al., 2018 18 |
| Number of nerves per observation field. | Prostate cancer | S100, TH and VAChT | None | Reeves et al., 2019 57 |
| Number of nerves divided by the total area of tissue present on the slide. | Thyroid cancer | PGP9.5 | Nerves with less than 3 axon fibers | Rowe et al., 2020 49 |
| 3. Nerve fibers + nerve trunks/fascicles | ||||
| Number of nerves per 20 high power fields, grouped as 1) negative: no nerve fascicles or nerve fibers; 2) weak: 1 to 10 nerve fascicles; and 3) moderate/strong: >10 nerves fascicles. | Breast cancer | S100 and PGP9.5 | Nerve trunks >100 µm in diameter | Zhao et al., 2014 48 |
| 4. Not specified if nerve fibers or nerve trunks/fascicles | ||||
| Number of nerves per 10 mm2 or average area of nerves per 10 mm2. | Pancreatic cancer/Diabetes Mellitus | PGP9.5 | None | Li et al., 2011 61 |
| Area of stained nerves in nerve hotspot areas. | Prostate cancer | PGP9.5 | None | Ayala et al., 2008 16 |
| Nerve density: Number of nerve fibers per 1 mm2. | Pancreatic cancer | PGP9.5 | None | Ceyhan et al., 2010 40 |
| Undefined. | Pancreatic cancer | TH and VAChT | None | Ceyhan et al., 2009 53 |
| Number of nerves per high power field as 0, 1–20 or >20 nerves. | Colorectal cancer | PGP9.5 | None | Albo et al., 2011 64 |
| Area of nerves per observation field. | Prostate cancer | TH, VAChT, NF‐L, NF‐H | None | Magnon et al., 2013 36 |
| Expression of immunohistochemistry stain (automated measurement). | Prostate cancer | PGP9.5 | None | Olar et al., 2014 45 |
| “Low and high expression of PGP9.5 were defined with respect to the median of the volume density of PGP9.5.” | Gastric cancer | PGP9.5 | None | Zhao et al., 2014 35 |
| Area of nerves per total tissue area. | Liver cancer | PGP9.5 | None | Wang et al., 2015 42 |
| Pixel area of nerves | Pancreatic cancer | PGP9.5 | None | He at al., 2016 43 |
| Cases were classified as low expressing or high expressing TH or VAChT based on observation. | Liver cancer | TH and VAChT | None | Zhang et al., 2017 46 |
| Neural density: sum of the nerve area per unit area; nerve number: the total number of nerves per unit area. | Pancreatic cancer | GAP43 | None | Iwasaki et al., 2019 44 |
| Number of nerves per observation field (H&E); area of TH or VAChT stain per observation field. | Head and neck cancer | H&E, TH and VAChT | None | Amit et al., 2020 15 |
| Nerve density scored as low, intermediate or high based on observation. | Prostate cancer | PGP9.5, panNeurofilament | None | Hänze et al., 2020 47 |
FIGURE 2.
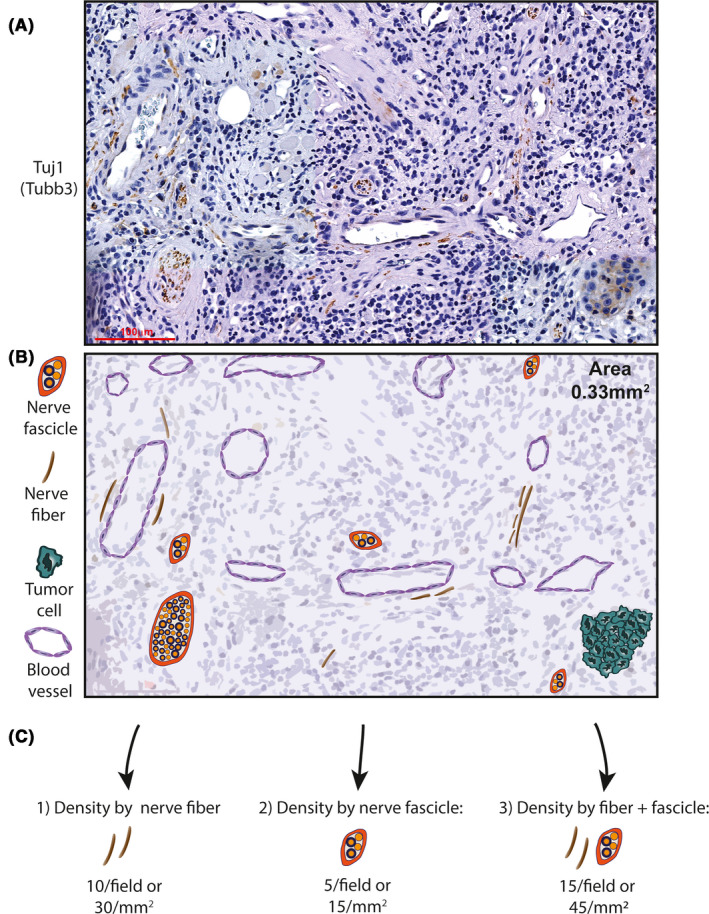
Methods of quantification of nerve density. (A) Tuj1 immunohistochemistry stain in human oral cavity squamous cell carcinoma. (B) Schematic representation of A highlighting the different nerve structures present in the tissue (0.33 mm2 area of tissue, scale bar = 100 µm). (C) Different methods of nerve density assessment based on histologic observation; refer to Table 2 for references
Exploratory studies that first showed axonogenesis inside tumor stroma used electron microscopy. 23 , 25 Not surprisingly, many delicate nerve fibers visualized with electron microscopy are not seen with regular histologic preparations and immunohistochemistry for protein gene product 9.5 (PGP9.5), a universal nerve marker, 25 imposing a problem for using immunohistochemistry to evaluate true axonogenesis. However, precise, electron microscopy is not cost‐ or time‐effective for routine use in evaluation of clinical specimens. All subsequent clinical studies evaluating nerve density in tumors used light microscopy combined with various immunohistochemical markers for nerves.
S100 protein is used as a nerve marker, 16 , 24 , 48 alone or in combination with PGP9.5. 16 , 48 Both proteins are generic nerve markers; while S100 stains for Schwann cells in the nerve sheath, PGP9.5 is supposedly a neuronal/neuroendocrine‐specific marker. 50 PGP9.5 is the preferred nerve marker in studies evaluating nerve density, despite the fact that it stains tumors parenchyma in multiple cancers. 47 , 51 Heavy (NF‐H) and light (NF‐L) neurofilaments, which are structural proteins in neuronal cells, are popular in mouse studies with immunofluorescence. 36 , 38 , 52 Pan‐neurofilament is more specific than PGP9.5 in immunohistochemistry for nerve density in prostate cancer. 47 Density of specific types of nerves is evaluated by markers such as tyrosine hydroxylase (TH) for sympathetic nerves, 15 , 36 , 53 vesicular acetylcholine transporter (VAChT) for cholinergic/parasympathetic nerves, 15 , 36 , 53 and calcitonin gene‐related peptide (CGRP) 38 or substance P 49 for sensory nerves.
Due to lack of specificity of nerve markers such as PGP9.5, careful morphologic examination of the tissue is necessary to separate nerves from non‐specific signals, hindering the use of automated systems to quantify nerve area and number. The same is true for H&E stain. He et al. 43 addressed this issue by using a combination of image deconvolution and segmentation to separate tumor cells from stroma in order to single out nerve‐derived PGP9.5 stain in the stroma from tumor‐associated PGP9.5 when calculating nerve density.
Another factor to consider when evaluating nerves in a tumor is the variability in morphological appearance of the nerve segments. Some studies showed that nerves within tumors are larger than nerves in normal tissues. 40 , 53 , 54 Another study showed that nerve size is comparable between chronic pancreatitis and pancreatic cancer, and that nerves are reduced in size when tumor stroma is desmoplastic. 44 These size differences in nerves are taken into account when evaluating nerve density as a measurement of nerve area, but not when the number of nerves is evaluated independent of size.
Importantly, despite the large variation in assessment methods, nerve density has been mostly associated with poor patient outcomes, possibly reflecting the biology behind nerve‐tumor interactions, that is, more nerves provide greater potential for interactions with cancer. In a similar way, tissue regeneration is dependent on nerve‐secreted factors such as neuropeptides, neurotransmitters, growth factors, and morphogens. 55 Experimental denervation impairs tissue regeneration in various different organisms and tissues. 55
4. CLINICAL RELEVANCE OF NERVE DENSITY IN CANCER
Nerve density was evaluated in many different cancers, especially those with a high incidence of PNI, such as prostate, pancreatic, and head and neck cancers. A genetic study of pancreatic cancer in mice assessed nerve density during tumor development 38 and showed that nerve infiltration in tumors is associated with tumor growth. The density of sensory CGRP‐positive fibers and sympathetic TH‐positive fibers is increased with tumor progression and tumor‐associated pain. 38 Prostate cancer mouse xenografts show axonogenesis measured by the larger ratio of NF‐L‐positive fibers compared to NF‐H‐positive ones; tumors showed reduced progression when autonomic nerves were blocked pharmacologically or genetically. 36 While mouse studies are advantageous for experimental manipulation in mechanistic studies, they are inadequate for clinical outcomes. Clinical studies have assessed the relevance of nerve density in cancers at different sites.
4.1. Prostate cancer
Ayala et al. 16 conducted a detailed assessment of nerves in prostate using radical prostatectomy specimens. The peripheral zone of non‐cancer prostate tissue has high nerve density compared to the intermediate zone, where most tumors arise. In prostate cancer specimens, nerve density within tumor areas was higher than normal peripheral zones. Pre‐neoplastic lesions also display increased nerve density compared to histologically normal prostate. High nerve density measured as area of PGP9.5‐positive nerves was significantly associated with extracapsular extension, a pathological indicator of aggressive behavior in prostate cancer. Likewise, high nerve density was associated with recurrence.
When evaluating nerve density in 434 patients as area of PGP 9.5‐positivity in prostate cancer stroma, a nerve density of ≥0.09 was associated with poor recurrence‐free survival and increased proliferative potential, measured by Ki‐67 expression. 45 However, multivariate analysis failed to find significant associations between nerve density and survival. In a companion study published by the same group, 56 both nerve density and diameter of PNI‐affected nerves had similar effects on tumor biology. For example, both phenotypes were associated with high Ki‐67 score and expression of other molecules related to proliferation and survival.
Patients with high‐risk prostate cancer have increased nerve density measured by the area of NF‐H and NF‐L immunofluorescence staining, both in tumor areas and normal adjacent tissues. 36 In the same cohort, nerve density of sympathetic and parasympathetic fibers was significantly associated with high tumor recurrence. The cutoffs used for sympathetic and parasympathetic fibers was 2000 µm2 and 300 µm2/field, respectively; it is unclear why these cut‐offs were selected. Another study characterized nerve types in prostate cancer and showed that nerve density, measured as number of S100‐positive nerve bundles per observation field, is not associated with biochemical recurrence. 57 In contrast, higher density of exclusively TH‐positive nerves was an independent predictor of biochemical recurrence (high PSA after treatment), 57 emphasizing the importance of the autonomic nervous system in prostate cancer progression.
Due to the clinical importance of identifying nerves in prostate cancer, two recent studies focused on developing techniques for nerve identification that do not rely on histology. 52 , 58 Using a previously validated imaging method to detect acetylcholinesterase (11C‐donepezil PET/CT scan), 58 , 59 parasympathetic innervation was detected in 26 cancer patients. Interestingly, high PET signals, representing high innervation, were associated with high grade tumors and 11C‐donepezil accumulated within tumors and metastatic lesions compared to normal tissues. 58 Magnetic resonance coupled with nanoparticles to enhance image contrast was also used for nerve density imaging. 52 By coupling nanoparticles with NP41, a nerve‐binding peptide, nerves could be detected and imaged because of the contrast characteristics of the nanoparticles. This approach allowed highly specific and sensitive detection of nerve density in mice, and has potential as an alternative to histological evaluation of nerve density in cancer patients. 52
4.2. Head and neck cancer
To investigate the role of p53 mutations in nerve infiltration and tumor progression in head and neck cancer, nerve density was evaluated in H&E stained tissue sections available in the TCGA database. 15 Nerve segments were counted; higher nerve density was associated with p53 mutations. Using a different cohort of patients, the same group evaluated density of TH‐ and VAChT‐positive nerves as the area of positive immunofluorescence per field of observation. Higher density of TH‐positive nerves was independently associated with poor overall and recurrence‐free survival. 15 Another study in head and neck cancer evaluated density of PNI‐positive nerves only, by counting the number of PNI foci per slide 60 ; a density >1 was associated with poor disease‐specific survival. However, since the majority of nerves in any sample are PNI‐negative, the value of this assessment is unclear.
Rowe et al. 49 investigated the clinical relevance of nerves in thyroid cancer. A meticulous assessment of nerve density was performed in papillary and follicular thyroid cancers, and in normal thyroid tissue from unrelated patients. Nerve density was calculated as the number of nerve segments per cm2 of tissue for the entirety of the specimen. No nerve fibers were counted since all nerves with less than three axons were excluded. The nerve density of papillary tumors (12.4 nerves/cm2) was almost double that of normal thyroid tissues (6.6 nerves/cm2). No increase in nerve density was observed in follicular tumors. In papillary tumors, nerve density was higher in the tumor than adjacent histologically normal tissue. Interestingly, adjacent normal areas had an increased density compared to unrelated normal samples. Higher nerve density in papillary tumors is independently associated with extra‐thyroidal invasion and inversely correlated with tumor size, while no correlation with lymph node metastasis was found. Since head and neck cancers are highly heterogeneous in biology and site, it is hard to draw conclusions about the clinical impact of nerve density from the limited available evidence.
4.3. Pancreatic cancer
Nerve density, measured by PGP9.5, is increased in pancreatic cancer compared to normal pancreas. 40 , 43 Histologically normal pancreatic tissue adjacent to pancreatic ductal carcinoma shows increased nerve density when compared to normal tissue from unrelated patients; nerve density was assessed by both nerve area and number of nerves per tissue area. 40 Interestingly, nerve density is also increased in pancreatitis, an inflammatory condition, suggesting that the increase is not cancer‐specific. 39 Pancreatic cancers in hyperglycemic patients have increased nerve density, measured by area and number, 61 presumably due to overlap between cancer and chronic inflammation associated with hyperglycemia.
Contradicting these findings, Iwasaki et al. 44 showed that normal pancreas has increased nerve density compared to pancreatic ductal adenocarcinoma. When subdividing tumor samples in concentric areas, central areas of the tumor contained fewer nerves than the periphery. Interestingly, when nerve density (nerve area per tissue area) and nerve number (per tissue area) were used to evaluate patient outcomes, lower nerve density was independently associated with worse survival. PNI was significantly associated with lower nerve density within tumors, presumably due to physical damage in invaded nerves. A density of 7 nerves was suggested as a cut‐off for predicting patient prognosis, since patients with ≤7 intra‐pancreatic nerves showed significantly worse overall survival and disease‐free survival. One particular difference between this study and others is the use of growth‐associated protein (GAP43) to visualize nerves. GAP43 is a marker of axonal growth that is expressed only in outgrowing nerves. Our experience with GAP‐43 in head and neck cancer is that it does not highlight all nerves in contrast to a universal marker; in fact, GAP‐43 expression is higher in nerves closer to tumor. 6 Therefore, it is unclear why the authors saw less nerves in the inner tumor area. It remains to be seen if this parameter will be replicated in similar studies with different cohorts.
Using a TMA with 99 pancreatic cancer and 71 normal pancreas patient samples, investigators assessed the presence or absence of nerves in the samples considering all three markers, S100, PGP9.5, and GAP43, in consecutive sections. 54 Only 10% of pancreatic cancers and 13% of normal pancreas samples had nerves in the TMA sections. From these nerve‐positive samples, pancreatic cancers had significantly larger nerves. Furthermore, cancer patients with mean nerve area >3200 µm2 survived significantly less than patients with smaller nerves. The cut‐off was calculated based on the median nerve area across all samples. Although this study did not directly address nerve density, it showed that larger nerves associate with poor survival, which can be interpreted as equivalent to a nerve density measurement. It also showed a significant association between the presence of nerves and poor survival for patients ≤50 years of age. However, the small sample size of 11 patients did not allow for definitive conclusions. 54
Analysis of autonomic nerve density in cancer and pancreatitis showed a reduction in sympathetic and no change in parasympathetic nerves compared to normal tissue, 53 contradicting previous pre‐clinical mouse data. 38 Renz et al. 18 investigated the role of stress and β‐adrenergic receptors in the progression of pancreatic ductal adenocarcinoma. These receptors are involved in response to sympathetic nerve‐derived norepinephrine during stress. Patients taking nonselective β‐blockers had decreased nerve density in tumor samples and improved survival, compared to patients who did not take β‐blockers. Nerve density was evaluated as number of S100‐stained nerves in 10 histologic fields, using three tumor sections per patient in a limited sample of 13 patients.
Although pancreatic cancer patients often experience abdominal pain, 40 , 53 only one study verified the association between nerve density and pain, 53 reporting that pancreatic cancer patients with less sympathetic and cholinergic nerve fiber infiltration have increased pain. This association was not statistically significant. It is possible that sensory nerves were increased in these tumors, but this hypothesis was not tested. Due to limited and contradictory findings among studies in pancreatic cancer, the contribution of nerve density to clinical outcome requires more investigation.
4.4. Breast cancer
To investigate neural infiltration in breast cancer, tissues from normal mammary glands, fibroadenomas, ductal carcinomas‐in‐situ, and invasive ductal carcinomas, were stained for PGP9.5. 48 While all normal tissues presented nerve fibers and/or nerve bundles, only 61.8% of the carcinomas and none of the fibroadenomas or carcinoma‐in‐situ lesions had nerves in the stroma. Nerve diameter was similar between groups (average ~20 µm); nerves >100 µm in diameter (6% of all nerves) were excluded from the analysis. Nerve density was measured by quantifying nerve fibers or trunks per field and categorized as negative (no nerves), weak (1–10 nerves), or moderate/strong (>10 nerves). Higher nerve density was significantly associated with higher microvessel density, assessed by CD‐34 stain, possibly due to molecular interactions between the nervous system and vasculature. PGP9.5 positivity was associated with significantly lower 3‐year disease‐specific survival in a bivariate analysis.
In a limited cohort of 29 patients, using a combination of NF‐L and TH or VAChT immunofluorescence, Kamyia et al. 37 showed that a density of TH+NF‐L+ fibers >12,000 µm2/field was associated with poor recurrence‐free survival, while a density of VAChT+NF‐L+ fibers >2,000 µm2/field was associated with better recurrence‐free survival; it is unclear why these cut‐offs were selected. This evidence suggests that higher density of sympathetic nerves portend worse outcomes for breast cancer while parasympathetic nerves have the opposite effect.
Another study in breast cancer evaluated nerve fibers in a larger cohort of ductal carcinomas‐in‐situ, invasive ductal carcinomas, and invasive lobular carcinomas. 62 Using TMAs and IHC for PGP9.5, only nerve fibers were assessed as opposed to larger nerve trunks. Because TMAs provide limited information about the overall histologic characteristics, nerve density was not measured and nerve fibers were scored only as present/absent. Interestingly, only 8% of invasive lobular carcinomas and 12% of ductal carcinomas‐in‐situ were positive for nerve fibers, while 28% of invasive ductal carcinomas had nerve fibers. The presence of nerve fibers was significantly associated with lymph node metastasis when both carcinomas were grouped, but no survival analysis was performed. Because of the limited scope of the available studies, it is still unclear if the presence of nerves has any prognostic value in breast cancer.
4.5. Gastric, esophageal, and colorectal cancers
Nerve density was evaluated in gastric cancer using PGP9.5 immunohistochemistry. Density was measured as low and high based on the “median of the volume density of PGP9.5”. High nerve density was associated with more advanced tumors, invading the subserosal connective tissue, deeper in the gastric wall. 35 Furthermore, TH‐positive nerve fibers around arterioles were investigated in gastric cancer. 63 Interestingly, there is a marked loss of peri‐arteriolar nerve fibers in cancer compared to normal tissues. This reduction was also associated with depth of invasion, lymph node metastasis, and poorer disease‐specific survival. The underlying hypothesis is that reduced sympathetic innervation would increase blood supply to the tumor due to the role of sympathetic fibers in vasoconstriction. It would be interesting to know how overall nerve density compares with this reduction in peri‐arteriolar sympathetic nerves.
Esophageal cancers and normal esophageal tissues have nerve infiltration by both nerve fibers and nerve trunks, detected by PGP9.5 immunostaining. 28 Taking the presence or absence of nerves in TMAs as a dichotomous variable, 96% of normal samples had nerves, 40% of squamous type cancers, and 18% of adenocarcinomas were infiltrated by nerves. While there was no association between the presence of nerves and clinical outcome variables, the presence of PNI was significantly associated with poor survival (n = 9 PNI‐positive patients). However, no measurement of nerve density was performed, likely due to the limitations of using TMA tissue. 28
Colorectal cancers were evaluated for nerve density and the presence of nerve fibers. 64 For nerve density, whole mount tissues were stained with PGP9.5, evaluated for number of nerve segments per high‐power field, and scored as no nerves, low (1–20), or high (>20) nerve density. The presence of nerve fibers was evaluated using a TMA and an automated image analysis tool to measure PGP9.5‐positive pixels. Sixty‐three percent of colorectal cancer specimens had nerves fibers in TMA cores. From these, only 11% had high nerve density, which was significantly correlated with poorer disease‐specific survival. Rectal tumors presented more neurogenesis than colon tumors. When analysis was controlled for age, number of involved lymph nodes, tumor grade, AJCC (American Joint Committee on Cancer) stage, and adjuvant therapy utilized, the presence of nerve fibers was an independent predictor of poor survival, suggesting that nerve presence is a strong predictor of poor outcomes for colorectal cancer.
5. CONCLUDING REMARKS
Nerve density in cancer is emerging as an important parameter with potential to predict patient outcomes. The available clinical and mechanistic evidence indicates an association between high nerve density and poor survival, recurrence, and worsening of other outcome parameters, such as extra‐prostatic extension (Table 3). In contrast, only a few clinical studies showed correlation between lower nerve density and poor outcome. Specifically, pancreatic tumor patients had better survival when presenting higher nerve density assessed by GAP43 immunohistochemistry, 44 and higher density of parasympathetic nerves in breast cancer was correlated with better recurrence‐free survival. 37 In contrast to nerve density, a large body of literature is available for PNI, which is also associated with poor patient survival. However, even after many decades of clinical studies, the definition of PNI is still under debate. 1 , 4
TABLE 3.
Clinical significance of nerve density in cancer
| Disease | Clinical findings | Patient n | Reference |
|---|---|---|---|
| Pancreatic cancer | Low nerve density associated with the use of non‐selective β‐adrenergic receptor inhibitors and better survival | 13 | Renz et al., 2018 18 |
| High nerve density associated with better survival while PNI associated with worse survival. | 256 | Iwasaki et al., 2019 44 | |
| Prostate cancer | High nerve density associated with recurrences and extraprostatic extension. | 27 | Ayala et al., 2008 16 |
| High nerve density associated with extraprostatic extension; highsympathetic and parasympathetic nerve densities associated with poor recurrence‐free survival. | 43 | Magnon et al., 2013 36 | |
| High nerve density associated with worse recurrence‐free survival. | 435 (TMA) | Olar et al., 2014 45 | |
| High nerve density of pure sympathetic nerves associated with biochemical recurrence. | 98 | Reeves et al., 2019 57 | |
| Breast cancer | High nerve density associated with poor 3‐year disease‐free survival and higher tumor grade. | 162 | Zhao et al., 2014 48 |
| High density of sympathetic fibers and low density of parasympathetic fibers associated with poor recurrence‐free survival. | 29 | Kamiya et al., 2019 37 | |
| Liver cancer | High expression of TH and VAChT associated with lymph node metastasis, vascular invasion, higher clinical stages, and worse survival. High TH expression associated with recurrences. | 30 | Zhang et al., 2017 46 |
| Colorectal cancer | High nerve density associated with lymph node metastasis, with decreased disease specific survival and increased recurrence. Nerve density was a more powerful predictor of poor prognosis than lymph node status in adjusted analyses. | 236 | Albo et al., 2011 64 |
| Gastric cancer | Reduced TH‐positive nerve density around arterioles associated with poor survival, increased lymph node metastasis and higher depth of invasion. | 82 | Myiato et al., 2011 63 |
| High nerve density associated with lymph node metastasis and advanced tumor stage. | 120 | Zhao et al., 2014 35 | |
| Head and neck cancer | High nerve density associated with worse overall survival (H&E). High TH‐positive nerve density independently associated with worse overall survival and recurrence‐free survival. | 70 (TH) & 231 (H&E) | Amit et al., 2020 15 |
| Thyroid cancer | High nerve density and PNI were both independently associated with extra‐thyroidal invasion. | 75 | Rowe et al., 2020 49 |
From a biological standpoint, there is rationale for using nerve density and/ or PNI as predictors of prognosis. Molecular mechanisms of PNI are related to tumor invasiveness and the ability of nerve‐secreted factors to attract tumor cells (neurotropism). 30 , 31 , 32 Similarly, nerve density mechanisms, described in this review, involve axonogenesis, neurogenesis, and neurotropism. The likelihood of finding PNI increases as nerves grow via axonogenesis and neurogenesis. Consequently, nerve density and PNI are mechanistically associated; an increase in nerve density could lead to an increase in PNI.
Clinically, a patient is defined as PNI‐positive if one or more nerves has PNI, evaluated by histology. However, from the entire population of nerves in a given tumor, typically only a few have PNI. As an example, only 3.8% of all nerves in a head and neck cancer cohort of 71 patients with 2879 nerves were PNI‐positive. 6 Efforts have been made to increase the clinical significance of PNI by separating patients into unifocal (one PNI‐positive nerve) versus multifocal PNI (more than one PNI‐positive nerve) 65 ; however, validation studies are needed. Since nerves have the ability to secrete factors to promote a cancer growth‐friendly environment, 55 why would only PNI‐positive nerves be important? Nerve density evaluation takes into consideration PNI‐positive and ‐negative nerves in the tumor, better representing the biological crosstalk between nerves and tumor.
There are many challenges to incorporating nerve density in the histopathologic examination of tumors. As highlighted in this review, there are many definitions and methods of assessment of nerve density (Table 2). Starting with histologic assessment, using a nerve marker is crucial. A H&E stain should not be used exclusively for histopathologic evaluation, as immunohistochemistry for nerves increase nerve detection. 6 However, the choice of immunohistochemistry marker can dictate which type of nerve segments are being observed (nerve fibers versus nerve bundles or fascicles). For example, S100 immunohistochemistry is suitable for detection of nerve fascicles, but does not highlight all small fibers. Also, some nerve markers stain tumor parenchyma, hindering the ability to discern small nerve fibers. We have successfully used Tuj1 (β‐tubulin III) to specifically detect nerve tissue, and have observed very similar detection of larger nerves compared to S100, 6 and more reliable detection of delicate fibers, as illustrated in Figure 2. Regarding the use of specific nerve type markers such as TH and VAChT, further investigation is required to understand if density of different types of nerves is relevant for clinical outcome.
Another important challenge to the use of nerve density as a clinical parameter is to standardize how much of the tissue should be analyzed for each sample. While some studies used large tissue specimens 16 , 40 and looked for nerve hotspots throughout the tumor, 16 , 64 others used TMAs 45 or analyzed a specific number of representative fields. 15 , 36 Importantly, analysis of adjacent normal tissue relative to cancer tissue could be of importance in understanding tumoral nerve density relative to normal anatomical nerve distribution. 49 While evaluating nerves in the entire tumor and surrounding tissue is preferred, it is time consuming and not feasible for routine histopathologic examination. Digital pathology is rapidly evolving to incorporate artificial intelligence tools that detect tumor areas and stromal areas with minimal human interference. 66 This method could be used to detect and count nerves in an automated manner, facilitating the use of nerve density in patient outcome in the future. However, current lack of specificity of automated models in discerning true nerves from non‐specific immunostaining is an obstacle that must be overcome. 67
In conclusion, nerve density is a biologically relevant clinical parameter to assess nerves in tumor relative to patient outcomes. However, there are many challenges to routine application as a treatment selection tool. To adjust for variations in nerve density by anatomic location, we believe that tumoral nerve density should be evaluated in comparison to normal surrounding tissues. Although this comprehensive pathological examination is time consuming, it could be performed and used as a gold standard for comparison to less inclusive methods of analysis, such as TMAs or selected fields of examination. Expanded, rigorous, and well‐defined studies will help determine the true value of nerve density for each specific type of tumor, to produce a unified method of assessment. Additionally, to understand which types of nerve segments are more relevant to tumor biology (larger nerve fascicles or delicate nerve fibers), a better understanding about the molecular mechanisms of nerve participation in the tumor microenvironment is required.
CONFLICT OF INTEREST
The authors have declared that no conflict of interest exists.
AUTHOR CONTRIBUTIONS
LB Schmitd, C Perez‐Pacheco, and NJ D`Silva designed and wrote the manuscript. LB Schmitd prepared the figures.
ACKNOWLEDGMENTS
This work was funded by NIH/NIDCR DE027551 and DE022567 (NJD).
Schmitd LB, Perez‐Pacheco C, D'Silva NJ. Nerve density in cancer: Less is better. FASEB BioAdvances. 2021;3:773–786. 10.1096/fba.2021-00046
This article is part of the Neuroscience of Cancer, Regeneration, and Immunity special collection.
REFERENCES
- 1. Schmitd LB, Scanlon CS, D'Silva NJ. Perineural invasion in head and neck cancer. J Dent Res. 2018;97(7):742‐750. 10.1177/0022034518756297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115(15):3379‐3391. 10.1002/cncr.24396 [DOI] [PubMed] [Google Scholar]
- 3. Chi AC, Katabi N, Chen HS, Cheng YL. Interobserver variation among pathologists in evaluating perineural invasion for oral squamous cell carcinoma. Head Neck Pathol. 2016;10(4):451‐464. 10.1007/s12105-016-0722-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yan F, Cheng YL, Katabi N, et al. Interobserver variation in evaluating perineural invasion for oral squamous cell carcinoma: phase 2 survey study. Head Neck Pathol. 2021; 10.1007/s12105-021-01321-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Egevad L, Delahunt B, Samaratunga H, et al. Interobserver reproducibility of perineural invasion of prostatic adenocarcinoma in needle biopsies. Virchows Arch. 2021;478(6):1109‐1116. 10.1007/s00428-021-03039-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmitd LB, Beesley LJ, Russo N, et al. Redefining perineural invasion: integration of biology with clinical outcome. Neoplasia. 2018;20(7):657‐667. 10.1016/j.neo.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Monje M, Borniger JC, D'Silva NJ, et al. Roadmap for the emerging field of cancer neuroscience. Cell. 2020;181(2):219‐222. 10.1016/j.cell.2020.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang RB. Optogenetic control of the peripheral nervous system. Cold Spring Harb Perspect Med. 2019;9(12):a034397. 10.1101/cshperspect.a034397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deborde S, Wong RJ. How Schwann cells facilitate cancer progression in nerves. Cell Mol Life Sci. 2017;74(24):4405‐4420. 10.1007/s00018-017-2578-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Möller I, Miguel M, Bong DA, Zaottini F, Martinoli C. The peripheral nerves: update on ultrasound and magnetic resonance imaging. Clin Exp Rheumatol. 2018;36(Suppl 114(5)):145‐158. [PubMed] [Google Scholar]
- 11. Akinrodoye MA, Lui F. Neuroanatomy, somatic nervous system. StatPearls. StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021. [PubMed] [Google Scholar]
- 12. Wehrwein EA, Orer HS, Barman SM. Overview of the anatomy, physiology, and pharmacology of the autonomic nervous system. Compr Physiol. 2016;6(3):1239‐1278. [DOI] [PubMed] [Google Scholar]
- 13. The Zebrafish Information Network. University of Oregon. Accessed March, 2021, 2021. https://zfin.org/GO:0007409
- 14. Madeo M, Colbert PL, Vermeer DW, et al. Cancer exosomes induce tumor innervation. Nat Commun. 2018;9(1):4284. 10.1038/s41467-018-06640-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Amit M, Takahashi H, Dragomir MP, et al. Loss of p53 drives neuron reprogramming in head and neck cancer. Nature. 2020;578(7795):449‐454. 10.1038/s41586-020-1996-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ayala GE, Dai H, Powell M, et al. Cancer‐related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res. 2008;14(23):7593‐7603. 10.1158/1078-0432.Ccr-08-1164 [DOI] [PubMed] [Google Scholar]
- 17. Hayakawa Y, Sakitani K, Konishi M, et al. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell. 2017;31(1):21‐34. 10.1016/j.ccell.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Renz BW, Takahashi R, Tanaka T, et al. β2 adrenergic‐neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell. 2018;33(1):75‐90.e7. 10.1016/j.ccell.2017.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pundavela J, Demont Y, Jobling P, et al. ProNGF correlates with gleason score and is a potential driver of nerve infiltration in prostate cancer. Am J Pathol. 2014;184(12):3156‐3162. 10.1016/j.ajpath.2014.08.009 [DOI] [PubMed] [Google Scholar]
- 20. Owji S, Shoja MM. The history of discovery of adult neurogenesis. Clin Anat. 2020;33(1):41‐55. 10.1002/ca.23447 [DOI] [PubMed] [Google Scholar]
- 21. Mauffrey P, Tchitchek N, Barroca V, et al. Progenitors from the central nervous system drive neurogenesis in cancer. Nature. 2019;569(7758):672‐678. 10.1038/s41586-019-1219-y [DOI] [PubMed] [Google Scholar]
- 22. National Cancer Institute . NCI Dictionaries. Accessed March 2021, 2021. https://www.cancer.gov/publications/dictionaries/cancer‐terms/def/neurotropism
- 23. Seifert P, Spitznas M. Tumours may be innervated. Virchows Arch. 2001;438(3):228‐231. 10.1007/s004280000306 [DOI] [PubMed] [Google Scholar]
- 24. Terada T, Matsunaga Y. S‐100‐positive nerve fibers in hepatocellular carcinoma and intrahepatic cholangiocarcinoma: an immunohistochemical study. Pathol Int. 2001;51(2):89‐93. 10.1046/j.1440-1827.2001.01172.x [DOI] [PubMed] [Google Scholar]
- 25. Seifert P, Benedic M, Effert P. Nerve fibers in tumors of the human urinary bladder. Virchows Arch. 2002;440(3):291‐297. 10.1007/s004280100496 [DOI] [PubMed] [Google Scholar]
- 26. Macfarlane L‐A, Murphy PR. MicroRNA: biogenesis, function and role in cancer. Curr Genomics. 2010;11(7):537‐561. 10.2174/138920210793175895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Griffin N, Faulkner S, Jobling P, Hondermarck H. Targeting neurotrophin signaling in cancer: the renaissance. Pharmacol Res. 2018;135:12‐17. 10.1016/j.phrs.2018.07.019 [DOI] [PubMed] [Google Scholar]
- 28. Griffin N, Rowe CW, Gao F, et al. Clinicopathological significance of nerves in esophageal cancer. Am J Pathol. 2020;190(9):1921‐1930. 10.1016/j.ajpath.2020.05.012 [DOI] [PubMed] [Google Scholar]
- 29. Gurrapu S, Tamagnone L. Semaphorins as regulators of phenotypic plasticity and functional reprogramming of cancer cells. Trends Mol Med. 2019;25(4):303‐314. 10.1016/j.molmed.2019.01.010 [DOI] [PubMed] [Google Scholar]
- 30. Scanlon CS, Banerjee R, Inglehart RC, et al. Galanin modulates the neural niche to favour perineural invasion in head and neck cancer. Nat Commun. 2015;6:6885. 10.1038/ncomms7885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Deborde S, Omelchenko T, Lyubchik A, et al. Schwann cells induce cancer cell dispersion and invasion. J Clin Invest. 2016;126(4):1538‐1554. 10.1172/jci82658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ayala GE, Wheeler TM, Shine HD, et al. In vitro dorsal root ganglia and human prostate cell line interaction: redefining perineural invasion in prostate cancer. Prostate. 2001;49(3):213‐223. 10.1002/pros.1137 [DOI] [PubMed] [Google Scholar]
- 33. Schmitd LB, Liu M, Scanlon CS, Banerjee R, D'Silva NJ. The chick chorioallantoic membrane in vivo model to assess perineural invasion in head and neck cancer. J Vis Exp. 2019;148. 10.3791/59296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Farkas JE, Monaghan JR. A brief history of the study of nerve dependent regeneration. Neurogenesis (Austin). 2017;4(1):e1302216. 10.1080/23262133.2017.1302216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao C‐M, Hayakawa Y, Kodama Y, et al. Denervation suppresses gastric tumorigenesis. Sci Transl Med. 2014;6(250):250ra115. 10.1126/scitranslmed.3009569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Magnon C, Hall SJ, Lin J, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341(6142):1236361. 10.1126/science.1236361 [DOI] [PubMed] [Google Scholar]
- 37. Kamiya A, Hayama Y, Kato S, et al. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat Neurosci. 2019;22(8):1289‐1305. 10.1038/s41593-019-0430-3 [DOI] [PubMed] [Google Scholar]
- 38. Lindsay TH, Jonas BM, Sevcik MA, et al. Pancreatic cancer pain and its correlation with changes in tumor vasculature, macrophage infiltration, neuronal innervation, body weight and disease progression. Pain. 2005;119(1–3):233‐246. 10.1016/j.pain.2005.10.019 [DOI] [PubMed] [Google Scholar]
- 39. Ceyhan GO, Bergmann F, Kadihasanoglu M, et al. The neurotrophic factor artemin influences the extent of neural damage and growth in chronic pancreatitis. Gut. 2007;56(4):534‐544. 10.1136/gut.2006.105528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ceyhan GO, Schäfer KH, Kerscher AG, et al. Nerve growth factor and artemin are paracrine mediators of pancreatic neuropathy in pancreatic adenocarcinoma. Ann Surg. 2010;251(5):923‐931. 10.1097/SLA.0b013e3181d974d4 [DOI] [PubMed] [Google Scholar]
- 41. Gao L, Bo H, Wang Y, Zhang J, Zhu M. Neurotrophic factor artemin promotes invasiveness and neurotrophic function of pancreatic adenocarcinoma in vivo and in vitro. Pancreas. 2015;44(1):134‐143. 10.1097/mpa.0000000000000223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang X, Lan H, Shen T, et al. Perineural invasion: a potential reason of hepatocellular carcinoma bone metastasis. Int J Clin Exp Med. 2015;8(4):5839‐5846. [PMC free article] [PubMed] [Google Scholar]
- 43. He D, Manzoni A, Florentin D, et al. Biologic effect of neurogenesis in pancreatic cancer. Hum Pathol. 2016;52:182‐189. 10.1016/j.humpath.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 44. Iwasaki T, Hiraoka N, Ino Y, et al. Reduction of intrapancreatic neural density in cancer tissue predicts poorer outcome in pancreatic ductal carcinoma. Cancer Sci. 2019;110(4):1491‐1502. 10.1111/cas.13975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Olar A, He D, Florentin D, Ding Y, Ayala G. Biologic correlates and significance of axonogenesis in prostate cancer. Hum Pathol. 2014;45(7):1358‐1364. 10.1016/j.humpath.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang L, Wu LL, Huan HB, et al. Sympathetic and parasympathetic innervation in hepatocellular carcinoma. Neoplasma. 2017;64(6):840‐846. 10.4149/neo_2017_605 [DOI] [PubMed] [Google Scholar]
- 47. Hänze J, Rexin P, Jakubowski P, et al. Prostate cancer tissues with positive TMPRSS2‐ERG‐gene‐fusion status may display enhanced nerve density. Urol Oncol. 2020;38(1):3.e7‐3.e15. 10.1016/j.urolonc.2018.07.019 [DOI] [PubMed] [Google Scholar]
- 48. Zhao Q, Yang Y, Liang X, et al. The clinicopathological significance of neurogenesis in breast cancer. BMC Cancer. 2014;14(1):484. 10.1186/1471-2407-14-484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rowe CW, Dill T, Griffin N, et al. Innervation of papillary thyroid cancer and its association with extra‐thyroidal invasion. Sci Rep. 2020;10(1):1539. 10.1038/s41598-020-58425-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thompson RJ, Doran JF, Jackson P, Dhillon AP, Rode J. PGP 9.5–a new marker for vertebrate neurons and neuroendocrine cells. Brain Res. 1983;278(1–2):224‐228. 10.1016/0006-8993(83)90241-x [DOI] [PubMed] [Google Scholar]
- 51. Campbell LK, Thomas JR, Lamps LW, Smoller BR, Folpe AL. Protein gene product 9.5 (PGP 9.5) is not a specific marker of neural and nerve sheath tumors: an immunohistochemical study of 95 mesenchymal neoplasms. Mod Pathol. 2003;16(10):963‐969. 10.1097/01.MP.0000087088.88280.B0 [DOI] [PubMed] [Google Scholar]
- 52. You H, Shang W, Min X, et al. Sight and switch off: Nerve density visualization for interventions targeting nerves in prostate cancer. Science Advances. 2020;6(6):eaax6040. 10.1126/sciadv.aax6040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ceyhan GO, Demir IE, Rauch U, et al. Pancreatic neuropathy results in "neural remodeling" and altered pancreatic innervation in chronic pancreatitis and pancreatic cancer. Am J Gastroenterol. 2009;104(10):2555‐2565. 10.1038/ajg.2009.380 [DOI] [PubMed] [Google Scholar]
- 54. Ferdoushi A, Griffin N, Marsland M, et al. Tumor innervation and clinical outcome in pancreatic cancer. Sci Rep. 2021;11(1):7390. 10.1038/s41598-021-86831-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Boilly B, Faulkner S, Jobling P, Hondermarck H. nerve dependence: from regeneration to cancer. Cancer Cell. 2017;31(3):342‐354. 10.1016/j.ccell.2017.02.005 [DOI] [PubMed] [Google Scholar]
- 56. Olar A, He D, Florentin D, Ding Y, Wheeler T, Ayala G. Biological correlates of prostate cancer perineural invasion diameter. Hum Pathol. 2014;45(7):1365‐1369. 10.1016/j.humpath.2014.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Reeves FA, Battye S, Roth H, et al. Prostatic nerve subtypes independently predict biochemical recurrence in prostate cancer. J Clin Neurosci. 2019;63:213‐219. 10.1016/j.jocn.2019.01.052 [DOI] [PubMed] [Google Scholar]
- 58. Nielsen MM, Tolbod LP, Borre M, et al. The relationship between tumor aggressiveness and cholinergic PET imaging in prostate cancer tissue. A proof‐of‐concept study. Am J Nucl Med Mol Imaging. 2019;9(3):185‐192. [PMC free article] [PubMed] [Google Scholar]
- 59. Gjerløff T, Jakobsen S, Nahimi A, et al. In vivo imaging of human acetylcholinesterase density in peripheral organs using 11C‐donepezil: dosimetry, biodistribution, and kinetic analyses. J Nucl Med. 2014;55(11):1818‐1824. 10.2967/jnumed.114.143859 [DOI] [PubMed] [Google Scholar]
- 60. Cracchiolo JR, Xu B, Migliacci JC, et al. Patterns of recurrence in oral tongue cancer with perineural invasion. Head Neck. 2018;40(6):1287‐1295. 10.1002/hed.25110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li J, Ma Q, Liu H, et al. Relationship between neural alteration and perineural invasion in pancreatic cancer patients with hyperglycemia. PLoS One. 2011;6(2):e17385. 10.1371/journal.pone.0017385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pundavela J, Roselli S, Faulkner S, et al. Nerve fibers infiltrate the tumor microenvironment and are associated with nerve growth factor production and lymph node invasion in breast cancer. Mol Oncol. 2015;9(8):1626‐1635. 10.1016/j.molonc.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Miyato H, Kitayama J, Ishigami H, Kaisaki S, Nagawa H. Loss of sympathetic nerve fibers around intratumoral arterioles reflects malignant potential of gastric cancer. Ann Surg Oncol. 2011;18(8):2281‐2288. 10.1245/s10434-011-1562-1 [DOI] [PubMed] [Google Scholar]
- 64. Albo D, Akay CL, Marshall CL, et al. Neurogenesis in colorectal cancer is a marker of aggressive tumor behavior and poor outcomes. Cancer. 2011;117(21):4834‐4845. 10.1002/cncr.26117 [DOI] [PubMed] [Google Scholar]
- 65. Aivazian K, Ebrahimi A, Low TH, et al. Perineural invasion in oral squamous cell carcinoma: quantitative subcategorisation of perineural invasion and prognostication. J Surg Oncol. 2015;111(3):352‐358. 10.1002/jso.23821 [DOI] [PubMed] [Google Scholar]
- 66. Classe M, Lerousseau M, Scoazec JY, Deutsch E. Perspectives in pathomics in head and neck cancer. Curr Opin Oncol. 2021;33(3):175‐183. 10.1097/cco.0000000000000731 [DOI] [PubMed] [Google Scholar]
- 67. Astono I, Rowe CW, Welsh J, Jobling P. MON‐535 deep‐machine learning for objective quantification of nerves in immunohistochemistry specimens of thyroid cancer. J Endocrine Soc. 2020;4(Supplement_1): 10.1210/jendso/bvaa046.1335 [DOI] [Google Scholar]


