Abstract
Extracellular vesicles (EVs) are released by many different cell types throughout the body and play a role in a diverse range of biological processes. EVs circulating in blood as well as in other body fluids undergo dramatic alterations over an organism's lifespan that are only beginning to be elucidated. The exact nature of these changes is an area of active and intense investigation, but lacks clear consensus due to the substantial heterogeneity in EV subpopulations and insufficiencies in current technologies. Nonetheless, emerging evidence suggests that EVs regulate systemic aging as well as the pathophysiology of age‐related diseases. Here, we review the current literature investigating EVs and aging with an emphasis on consequences for the maintenance of human healthspan. Intriguingly, the biological utility of EVs both in vitro and in vivo and across contexts depends on the states of the source cells or tissues. As such, EVs secreted by cells in an aged or pathological state may impose detrimental consequences on recipient cells, while EVs secreted by youthful or healthy cells may promote functional improvement. Thus, it is critical to understand both functions of EVs and tip the balance toward their beneficial effects as an antiaging intervention.
Keywords: ageing, aging, exosome, extracellular vesicle, longevity, NAD+ metabolism
1. INTRODUCTION
The once mythological idea that young blood may confer antiaging benefits has had legitimate scientific support for more than 60 years. Despite the intrigue of these early parabiosis experiments, potential mechanisms for how young blood may be exerting rejuvenating effects remained elusive until much more recently. 1 Historically characterized as “platelet dust” with a role in thrombin formation, 2 or simply as a cellular waste disposal system, 3 , 4 the involvement of extracellular vesicles (EVs) in a wide array of biological processes, including aging, is becoming increasingly appreciated. 5 , 6 , 7 There is an already substantial and quickly growing body of evidence attesting to the importance of EVs in the regulation of systemic aging as well as in a number of age‐related detriments including inflammaging (the pathophysiologies caused by the manifestation of chronic inflammation with increasing age), cellular senescence, metabolic dysfunction, cardiovascular disease, cancer, and neurodegeneration. 5 , 7 , 8 , 9 Additionally, the utilization of age‐related changes in EVs as easily accessible aging biomarkers is an attractive strategy. 10 However, the small size of EVs, the insufficiency of current methodologies, and the heterogeneity of EV producers, recipients, and affected biological processes have created extraordinary complexity in the EV field. 11 , 12 Significant efforts have been put forth to standardize EV collection methods, characterization, and use to increase reproducibility. 12 , 13 While these standardized recommendations offer a reasonable foundation, much work is needed to unravel the physiological significance of EVs in different biological paradigms. Aging is characterized by a number of complex pathophysiological alterations, 14 some of which are also intensive research topics in the field of EV biology. The intersection of these two fields offers considerable promise, constituting a new and exciting research arena. Recent work in this arena suggests that characterization and manipulation of age‐related changes in EVs has the potential to provide a unique window through which to view, and perhaps one‐day treat, the systemic deterioration that accompanies aging.
“Extracellular vesicles” is the general term for particles of approximately 40–1000 nm in diameter bound by a lipid bilayer membrane, but without a nucleus that are released by virtually every cell in the body. 5 , 13 As strict methods for the characterization of EV subpopulations such as “exosomes” or “microvesicles” are the matter of some debate in the field, the term “EVs” will be used throughout this text with the terms “small EVs” referring to the colloquial “exosomes” (<~150 nm diameter) and “large EVs” referring to “ectosomes/microparticles/microvesicles” (>~150 nm in diameter) unless otherwise noted. 13 Biogenesis of small versus large EVs occurs via divergent pathways that nonetheless involve some of the same regulatory molecules. Small EVs are generated by a secondary invagination of the luminal membrane of endosomes following endocytosis, forming multivesicular bodies (MVBs). These MVBs then fuse with the cellular outer plasma membrane, emptying their contents including the nascent small EVs into the extracellular space. In contrast, large EVs form by directly budding from the external cellular plasma membrane. 15 Description of current knowledge regarding the biogenesis, uptake, and basic biological functions of EVs are beyond the scope of this review, but are reviewed in detail elsewhere. 5 , 15 Importantly, EVs can serve as both local and long‐range transport and intertissue signaling mechanisms for mRNA, miRNA, protein, and lipids and are a major source for cell‐free DNA and RNA in humans. 15 , 16 , 17 , 18 , 19 , 20 In this review article, we will focus on the biological significance of EVs in the systemic regulation of mammalian aging and longevity and also in the pathogenesis of age‐associated dysfunctions.
2. CHANGES TO EVs WITH AGE
2.1. EV quantification
Recent evidence suggests that there are substantial changes to EVs with age. Nevertheless, the extent of these changes and whether they represent a cause or effect of aging remain largely unanswered questions in the field. Such changes could be viewed as potential boons to our understanding of the aging process as well as in our search for viable aging biomarkers in humans. Analysis of plasma from 74 humans including young (30–35 years old), middle‐aged (40–55 years old), and old (55–64 years old) revealed gradually decreasing quantities of circulating EVs over age as measured cross sectionally as well as longitudinally (average of 4.6 year follow‐up). 21 No sex differences were detected between male and female participants. However, there was a positive correlation between both smoking and BMI with EV concentration, although only during one of the timepoints examined. Interestingly, EVs from older participants were more readily taken up by and induced activation in B cells and monocytes, but not other immune cells, irrespective of the age of the cell donor. 21 Another group reported a similar decrease in total circulating EVs between young 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 and old 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 subjects. 22 Taken in context of the increased production of EVs by senescent cells 8 and data that the number of senescent cells increases substantially with age 14 (see below section), this decrease in concentration and concomitant increase in EV uptake with age may be even more dramatic. On the other hand, other reports have found no change in the concentration of serum EVs (enriched for EVs ~50–300 nm in diameter) in humans ranging from 21 to 92 years of age. 23 While these studies focused on small EVs, another report demonstrated an increase in the quantities of large EVs (diameter of approximately 1 um) over age between individuals 20–28 years old compared with those 75–83 years old. 24 All of these studies used nanoparticle tracking analysis (NTA) to measure EV concentration and examined both sexes, although one study had substantially more women in the elderly group than in the young group. 22 Data from mice suggest that plasma particle number decreases, but EV marker (CD63, CD81, and TSG101) protein levels measured by western blot increase with age. 25 The authors of this study attributed their conflicting results to the concomitant age‐related decrease in plasma lipoproteins (APOA1, APOB‐100, and APOB‐48) that may be falsely counted as EVs by NTA analysis. Another explanation could be that the concentration of EV‐associated proteins in each EV increases with age, leading to an overestimate in the marker analysis. Another group found a dramatic accumulation of EV marker CD63, which also colocalized with complement protein C3, in the retinal pigment epithelium (RPE) in old mice and in age‐related macular degeneration patients. 26 These data suggest a possible increase in exocytosis of damaged intracellular proteins with age and may facilitate immune cell recruitment to the aged RPE. It remains unclear whether these conflicting data represent biological challenges such as differences in the EV populations studied or a considerable variability in aging rates or comorbid pathologies between individuals. Given the inconsistent EV isolations, the inadequacy of quantification technologies and the incompletely categorized EV heterogeneity, it is also possible that the disagreement between studies represents a technical shortcoming. In any case, further investigation of age‐related changes to EV production, uptake, and degradation, including the development of more precise technology for measuring EV concentration, are needed to resolve the discrepancies in current studies.
2.2. EV composition
Substantial changes in EV composition and accompanying functional consequences are also characteristic of aging. Preliminary proteomic analyses in humans 21 and mice 27 reveal that a subset of EV proteins changes with age. For instance, Galectin‐3, which normally promotes osteogenic differentiation, is decreased in EVs from elderly people. 28 Relatedly, increased levels of miR‐31 found in circulating large EVs of elderly humans and secreted by senescent fibroblasts can suppress osteogenic differentiation. 29 Changes in the miRNA content of bone marrow‐derived EVs from old mice, especially increased miR‐183‐5p, also suppress the osteogenic differentiation and increase senescence of bone marrow stromal cells in vitro, potentially through increased reactive oxygen species (ROS) production. 30 These findings suggest that altered EV content may play a role in age‐related bone frailty. 8 One group found decreases in a panel of EV marker proteins between nonsmokers and smokers as well as with age, although several of these changes were sex‐dependent. 31 Platelet‐released EVs may also decrease with age. However, this decrease is accompanied by enrichment of the chemokines CXCL4 and CXCL7 as well as HMGB1. 32 Large EVs circulating in the plasma of elderly individuals or isolated from senescent endothelial cells can promote the calcification of smooth muscle cells in vitro, potentially through correlated increases in Annexin A2, Annexin A6, or BMP2. 24 Interestingly, small EVs derived from young primary human fibroblasts possess intrinsic glutathione‐S‐transferase activity, a property that declines with age and is mediated at least partially through GSTM2 within EVs. 33 Together, these studies suggest that age‐related alterations in circulating EV protein content, and perhaps number, represent an amalgamation of heterogeneous cell‐type‐specific changes to secreted EVs. Further investigation is needed to characterize the weighted contributions of age‐related changes in various cell types as drivers of systemic changes in EV content.
There are marked age‐related changes documented in the miRNA content of small EVs derived from cerebral spinal fluid (CSF), plasma, and serum of rodents and saliva of humans. 25 , 34 , 35 , 36 For example, EVs derived from the sera of aged rats contain decreased levels of miR‐219. In contrast, supplementation of serum EVs derived from either young rats or aged rats housed in an enriched environment can promote oligodendrocyte precursor differentiation and myelination in a miR‐219‐dependent manner. 37 In humans, the expression of circulating serum miRNAs change with age 38 and certain miRNA signatures may be associated with particularly long‐lived individuals, suggesting that these miRNAs may play a role in regulating the aging process. 39 Although these studies did not specifically isolate EVs, it is likely that the majority of circulating miRNAs in plasma are encapsulated within EVs. 19 miRNA changes in human plasma EVs were also negatively correlated with cognitive function in older individuals (age 6–89), 40 implying a potential role for EVs in regulating cognitive aging. Many of the changes in CSF miRNAs in mice can be recapitulated by blocking small EV (exosome) release through Rab27 knockdown in hypothalamic neural stem cells, suggesting that EVs secreted by these cells may be responsible for the age‐related deficits observed in CSF EVs. 34 However, substantial alterations in cellular miRNA content of senescent cells, 41 dramatic increases in the number of senescent cells, 14 and the depletion of certain cell populations as with stem cells in aging 42 , 43 makes it difficult to ascertain the source of these changes in EV miRNA content. Altogether, these data suggest that marked changes to the overall EV composition in a variety of body fluids accompanies aging. Possibilities that these changes represent increased secretion from deteriorating or pathological tissues, decreased secretion from and/or depletion of healthy tissue, or differential uptake by, for example, activated immune cells remain unclear. A more comprehensive characterization of EV contents, including proteomics, transcriptomics, and lipidomics, especially in humans in aging is needed. Moreover, the tracing and identification of the tissue and/or cellular sources and sinks for EVs and how these roles may be altered in aging will be critical to the development of interventions to stem possible negative consequences of EV‐related changes in aging. Relatedly, changes to EV content in the context of aging, but in the absence of overt pathology are still being revealed. Thus, more work is needed to clarify inconsistencies between previous studies and to further examine whether these changes in EV composition, and potentially EV number, could drive age‐associated pathophysiologies or simply be the result of aging (Figure 1).
FIGURE 1.
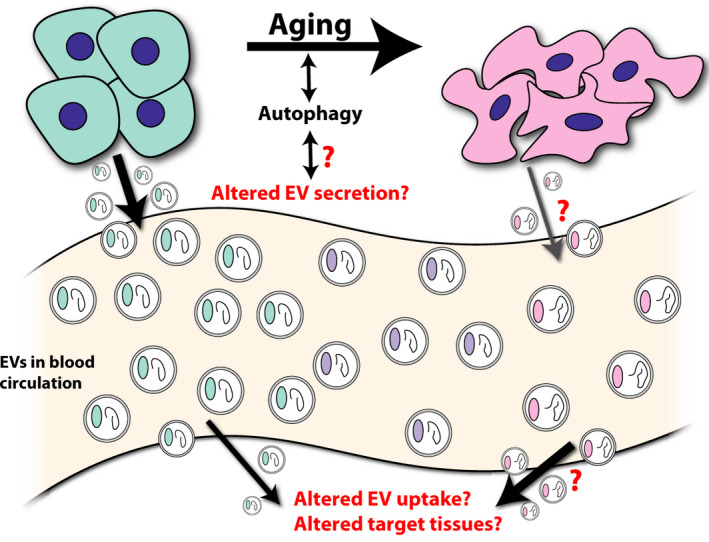
Changes to circulating extracellular vesicles (EVs) with age. Together, studies suggest that there may be an age‐dependent decrease in the concentration of circulating EVs and concomitant changes to EV protein (colored oval within EVs) and EV nucleic acid (curled line within EVs) with age. It remains unknown whether these changes reflect altered secretion, uptake, or rerouting of EVs with age. Autophagy is an important regulator of aging and shares substantial regulatory overlap with EV biogenesis and degradation, but potential interactions between these relationships have not been thoroughly investigated
2.3. EVs and autophagy
The biogenesis of small EVs and subsequent secretion from MVBs is counterbalanced by autophagy and lysosomal degradation of intraluminal vesicles. 15 , 44 For example, conditions such as starvation that stimulate autophagy inhibit the release of EVs in favor of autophagic degradation of MVBs 45 while inhibition of autophagy with bafilomycin promotes EV secretion. 46 , 47 Furthermore, the suppression of phosphoinositide kinase PIKfyve, which may regulate vesicular transport, reduces autophagy, but increases secretion of small EVs enriched with autophagy components LC3 and p62 as well as overall EV secretion. 48 These data suggest overlapping, but opposing regulation of autophagy and EV secretion. However, in non‐starvation conditions, the autophagy proteins ATG12 and ATG3 interact with EV biogenesis protein ALIX such that the absence of ATG12‐ATG3 impairs EV generation while the absence of ALIX impairs autophagy. 49 Moreover, DNA damage or bacterial infection that increases EV secretion can also lead to increased autophagy, 47 , 50 demonstrating that these processes are not always antagonistic. EVs have also been known to transfer mRNA encoding autophagy‐related proteins, thereby upregulating autophagy in recipient cells. 51 For a more detailed overview on the relationship between autophagy and EVs, please see the accompanying article in this special collection. 52 This relationship, the importance of autophagic pathways in the regulation of stem cell and systemic aging, 53 , 54 , 55 and the regulation of autophagy and EV release by longevity factor SIRT1, 56 , 57 suggest that the maintenance of this autophagy–EV balance may be crucial in the context of aging (Figure 1). The interconnectedness of these processes constitutes an important and largely unexplored area for future investigation. In total, the substantial alterations to circulating EV number and content as well as in overlapping regulatory changes in many cell populations that secrete EVs with age suggest EVs as potential mediators of systemic aging.
3. EVs AS A CRITICAL REGULATORY COMPONENT FOR MAMMALIAN AGING AND LONGEVITY
3.1. Blood‐based antiaging therapies
A considerable number of studies suggest that blood‐based therapies may benefit aging‐related deficits. For instance, heterochronic parabiosis, or joining the circulatory systems of old and young mouse pairs, has revealed a series of factors in old blood that promote aging and in young blood that mitigate aging phenotypes. Exposure to a young circulatory system rejuvenates muscle regenerative capacity by activating satellite cells and restores the proliferative capacity of hepatic progenitors. 42 Heterochronic parabiosis also affects neurogenesis and associated learning and memory, improving these capacities in old mice, 43 , 58 , 59 while impairing them in young mice. 60 , 61 , 62 CyPA 61 and chemokines including CCL11 62 have been identified as negative regulators, while TIMP2 58 and GPLD1 59 were identified as positive regulators of these functions. Outside of parabiosis, studies have demonstrated that plasma extracted from exercised animals confers antiaging cognitive benefits 59 and that there are substantial changes to the plasma EV pool, including increased numbers of EVs during exercise in humans. 63 In combination, these findings implicate the potential involvement of EVs in the benefits of blood‐based therapies to maintain normal physiological functions with increasing age. In a promising development, the safety and tolerability of young human plasma in the treatment of Alzheimer disease (AD) patients was recently demonstrated in an exploratory clinical trial (ClinicalTrials.gov Identifier: NCT02256306). 64 However, the extent to which EVs may be mediating both the positive and negative consequences of heterochronic parabiosis or other blood‐based therapies needs more direct investigation.
3.2. EVs ameliorate age‐related pathology
It has been reported that EVs may play a complex regulatory role in age‐associated pathophysiologies. It has also been reported that EVs are capable of ameliorating deterioration associated with healthy aging. Indeed, treatment of primary fibroblasts from old or Hutchison–Gilford progeria syndrome patients with EVs derived from young, but not old, primary fibroblasts was able to reduce senescence as measured by SA‐β‐galactosidase staining, increase proliferation, and reduce oxidative stress by replenishing reduced glutathione in culture. Injection of young fibroblast‐derived EVs reduced senescence markers, oxidative stress including lipid peroxidation, and inflammatory factors in multiple tissues of old mice. 33 Another recent study demonstrated that EVs derived from neonatal umbilical cord mesenchymal stem cells (MSCs), but not those in adult bone MSCs, rescued senescence in culture and mitigated multiple aging‐associated deficits, including in wound healing, kidney structure integrity and trabecular bone architecture at least partially through delivery of PCNA. 65 Treatment with MSC‐derived EVs can also mitigate damage in a mouse liver injury model by improving hepatocyte viability and proliferative capacity. 66 Small EVs derived from human mesenchymal stromal cells or large EVs derived from endothelial progenitor cells were able to reduce tubular necrosis and improve renal function in a mouse model of acute kidney injury, potentially due to their unique delivery of miRNA and growth factor contents. 67 , 68 , 69 The overexpression of Nrf2 in EV source cells may also improve the proliferative and wound healing benefits of EVs through the reduction of inflammation and oxidative stress in recipient cells. 70 Treatment of aged hematopoietic stem cells with large EVs derived from young MSCs was able to improve successful engraftment following bone marrow transplantation in mice. This benefit may be conferred by the transfer of mRNAs that bolster autophagy as well as expression of pro‐longevity factors such as sirtuins and FOXO3. 51
3.3. Effects of EVs on healthspan and lifespan
Extracellular vesicles may have a profound function to directly regulate healthspan and lifespan in mammals. It has been demonstrated that the hypothalamus functions as the control center of aging. 34 , 71 , 72 Supplementation with hypothalamic neural stem cell‐derived EVs can mitigate aging‐related deficiencies in locomotion, muscle coordination, sociality, and memory as measured by novel object recognition and Morris Water Maze. 34 These benefits are likely conferred by the miRNA content within stem cell‐derived EVs, suggesting that depletion of these miRNAs may drive some aging phenotypes. Aspects of this study have been corroborated by the attenuation of aging‐related phenotypes including muscle and bone deterioration through alternate means of suppressing hypothalamic stem cell senescence. 73 These investigations underscore the importance of hypothalamic homeostasis in the regulation of systemic aging.
Another recent study demonstrated that extracellular nicotinamide phosphoribosyltransferase (eNAMPT), a key NAD+ biosynthetic enzyme in mammals, is packaged in small EVs and secreted into blood circulation by adipose tissue. 27 The quantity of EV‐contained eNAMPT in plasma decreases with age in mice and humans and, interestingly, is predictive of remaining lifespan in mice. Adipose tissue‐specific overexpression of NAMPT rescued the age‐related decline in eNAMPT levels, improved median lifespan in females by 13.4%, and ameliorated a series of aging‐related deficits including activity levels and sleep fragmentation. Improvements in glucose tolerance, insulin secretion, memory performance, and eye function were also observed in 20‐month‐old animals. 27 Remarkably, weekly intraperitoneal injections with eNAMPT‐containing EVs purified from young mouse plasma starting at 26 months of age extended median and maximal lifespan by 10.5% and 16.3%, respectively, in female mice at the completion of the study. Furthermore, EV injection restored youthful appearance and activity levels. Given that eNAMPT enhances NAD+ biosynthesis, SIRT1 activity, and neuronal activity in the hypothalamus, 74 it has been speculated that the hypothalamus is one of the major targets for eNAMPT‐containing EVs secreted from adipose tissue. In this regard, it is likely that the inter‐tissue communication between the hypothalamus and adipose tissue plays a critical role in aging/longevity control in mammals. 75 It is now accepted that a systemic decline in NAD+ availability is a critical driver of systemic aging. 75 Thus, these recent findings suggest that the depletion of circulating eNAMPT during aging bears partial responsibility for the shortage in NAD+ availability. Together, these studies strongly support a role for EVs in the regulation of systemic aging and suggest exploration of EV treatment as a potential antiaging intervention.
4. EVs AND PATHOPHYSIOLOGICAL ASPECTS OF AGING
4.1. EVs and cellular senescence
Cellular senescence is the essentially irreversible exit from the cell cycle intended to curtail tumor growth in response to potentially oncogenic damage, especially to DNA. 14 While senescence provides an important benefit in acute tumor suppression, the steady accumulation of senescent cells characteristic of aging can drive chronic inflammation as well as cause damage to and, counterintuitively, promote tumor growth in neighboring cells. 14 , 76 These detriments are primarily driven by the senescence‐associated secretory phenotype (SASP). Despite a large range of individual SASP components, the secretion of pro‐inflammatory cytokines and chemokines as well as growth factors, survival factors, ROS, and matrix metalloproteinases occurs across cell types. 77 , 78 The accumulation of senescent cells and by extension, SASP, may drive age‐associated pathophysiologies. 14 , 78 Strong support for this hypothesis can be found in a fair number of recent studies in which elimination of senescent cells by genetic ablation or senolytic treatment led to abatement of aging symptoms including muscle weakness, bone frailty, activity levels, kidney dysfunction, cardiac stress, and inflammation, and the extension of healthspan. 79 , 80 , 81 , 82 It remains unclear what proportion of EVs in aging are actually derived from senescent cells and the physiological relevance of such EVs in vivo. However, recent studies demonstrating that treatment with senolytics can restore aging‐related changes in EV miRNA content suggest that senescent cell‐derived EVs may make up a considerable portion of the aged EV pool. 25 , 83
It has recently been suggested that EVs constitute a component of the SASP. 8 However, proteomic analysis reveals that the protein changes observed in senescence‐associated EVs target substantially distinct pathways from those affected by non‐EV soluble factors. 84 Nevertheless, senescent cells secrete more EVs and EVs secreted from senescent cells have a substantially altered composition. 8 For instance, Radiation‐induced or proliferative senescence can increase the number of EVs secreted by fibroblasts and prostate cancer cell lines in a p53‐dependent manner. 85 The miRNA content of senescent platelet EVs may also be substantially altered with potential changes affecting target genes involved in metabolic, antiapoptotic, inflammatory, and vascular disease pathways among others. 86 , 87
Rab27b, 88 as well as p53 targets Tsap6, 89 , 90 Caveolin‐1 (Cav1), and Chmp4c 91 all of which may enable or enhance small EV secretion, are upregulated in senescence, suggesting a possible mechanism for the increase of EVs from senescent cells. Alternatively, the accumulation of damaged DNA in cytoplasmic chromatin fragments can drive inflammation, senescence, and SASP through the cGAS‐STING DNA sensing pathway. 92 , 93 This DNA damage may activate ceramide synthesis, triggering the release of additional EVs in order to dispose of the damaged DNA as a protective mechanism. 47 However, in an Erff/− mouse model with constitutively deficient DNA repair machinery, increased EV secretion from macrophages eventually leads to chronic systemic inflammation. 50 On the other hand, preventing excess EV secretion during senescence may block the secretion of damaged DNA fragments in these EVs. Thus, the accumulation of damaged DNA induces the DNA damage response and eventually cell death. 94 Together, these data suggest that the secretion of EVs containing damaged DNA fragments in aging may serve to circumvent such a stress response within the cell, only to inadvertently trigger inflammation and/or senescence by activating the cGAS‐STING pathway in neighboring cells. However, a more recent report contests the hypothesis that DNA is secreted within small EVs as the presence of cell‐free DNA was DNase digestible and was not found to colocalize with EV markers CD81 and CD63. Instead, it is proposed that cell‐free DNA is secreted in association with non‐membranous particles. 20 Clearly, further investigation is needed to resolve these inconsistencies.
Like other components of the SASP, EVs may play a role in paracrine senescence 8 (Figure 2). EVs secreted from senescent cells or derived from the plasma of acute coronary syndrome patients were sufficient to induce senescence, including oxidative stress and upregulation of p21 and p16, in naïve endothelial cells. 95 This transference of senescence may involve the expression of the downstream interferon signaling molecule IFITM3, which is located on the EV membrane and enriched during senescence. 96 Induction of senescence by EVs may also depend on activation of nuclear factor‐kappa B (NF‐κB) in recipient cells. 97 Furthermore, a deficiency in the activity of SIRT1, which is decreased in multiple tissues during aging 98 and regulates longevity, 71 can induce secretion of EVs or endothelial microparticles. 56 , 99 , 100 This microparticle population, which is comprised to a significant degree by large EVs, is sufficient to induce paracrine senescence in cultured endothelial cells. 100 In light of systemic decreases in NAD+ with age, 75 these data suggest a potential pathway whereby loss of NAD+ positively reinforces the EV‐mediated propagation of senescence through declining SIRT1 activity.
FIGURE 2.
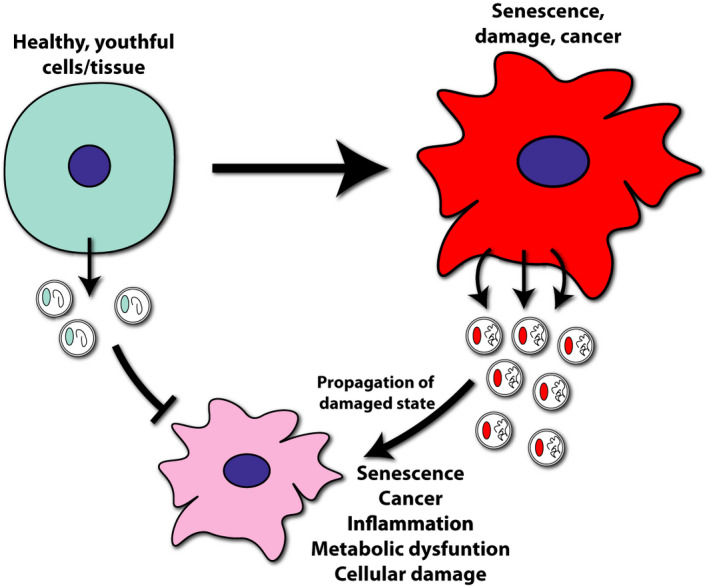
Extracellular vesicles (EVs) propagate the state of their source cell. As cells become senescent or enter a damaged state, EV secretion increases. EVs secreted by these unhealthy cells may induce inflammation or damage responses in the recipient cells, eventually inducing a similar unhealthy state in these cells. In contrast, EVs secreted by healthy tissue provide trophic support and promote the maintenance of homeostasis in recipient cells
However, EVs may also transfer protection to recipient cells in the context of stress. For instance, small EV mRNA changes due to oxidative stress can confer resistance against cell death in EV recipient mast cells under hydrogen peroxide exposure. 101 Treatment of senescent MSCs with EVs derived from either induced pluripotent stem cells (iPSCs) or MSCs partially rescued senescence phenotypes including proliferation and ROS production in culture. The authors of this study attributed a portion of this rescue to EV delivery of peroxiredoxins PRDX1 and PRDX2. 102 iPSC‐derived small EVs have also been shown to reduce senescence markers and modestly preserve proliferation of human dermal fibroblasts challenged by radiation and serial passage to induce senescence. 103
4.2. EVs and cancer
There have been a tremendous number of investigations reporting connections between EVs and cancer. This topic is beyond the main scope of this review, but has been reviewed elsewhere. 5 , 9 , 104 Nonetheless, it should be noted that in a setting of aging, senescence, cancer, or other pathology, EVs can provide factors that positively reinforce the applicable disease state, whereas EVs are also capable of providing profound rejuvenation effects on target tissues. This dichotomy is perhaps best epitomized in a study investigating the effects of bone marrow mesenchymal stromal cell (BMMSC)‐derived EVs on multiple myeloma (MM) progression. 105 It was found that EVs derived from MM BMMSCs promoted, but EVs derived from normal BMMSCs inhibited, tumor growth in vivo. Changes in EV content including enrichment of inflammatory factors such as IL6 and depletion of tumor suppressing miR‐15a in MM BMMSC EVs potentially mediated this effect. 105
4.3. EVs and inflammaging
Chronic inflammation is a critical component of age‐associated pathophysiologies and this important aspect of aging is embodied by the term “inflammaging”. EVs are well known to play prominent roles in both the activation and suppression of the immune system. 106 , 107 Indeed, EVs secreted from senescent or cancer cells induce inflammation and telomere damage. In breast cancer cells, cell‐free DNA secreted largely in EVs‐activated TLR9 and stabilized NF‐κB subunit p65 in EV recipient cells. 108 However, these pro‐inflammatory actions of EV‐contained DNA may also trigger antitumor immunity through STING‐dependent activation of dendritic cells. 109 EVs in media conditioned by irradiated cancer cells decreased both telomere length and telomerase activity in recipient breast cancer epithelial cells. 110 Telomere dysfunction may increase the enrichment of smaller form telomeric repeats‐containing RNA (TERRA) in small EVs, potentially upregulating inflammation, including Tnfa, Il6, and Cxcl10 in recipient cells. 111 , 112 While telomeric dysfunction is common in senescence, 14 the extent to which TERRA may be increased in EVs secreted from senescent cells in vivo remains unclear. 8 Pathway enrichment analysis of protein changes observed in senescence‐associated EVs reveals increases in proteins involved in inflammatory, cellular signaling transduction, including RAS and G‐protein signaling, and membrane organization pathways. 84
Interestingly, small EVs can also transport mRNA encoding a number of pro‐inflammatory cytokines. In one report, quantities of Il6, Tnfa, and Il12 mRNA in serum EVs enriched for macrophage‐secreted EVs were increased in aged (≥65 years of age) human subjects. A similar upregulation was also present in EVs derived from macrophages exposed to pro‐inflammatory stimulants amyloid beta (Aβ) or lipopolysaccharide (LPS). 113 Additionally, the immune signaling proteins ICAM‐1 and IL‐6R are enriched in large EVs secreted by senescent fibroblasts 114 or serum EVs, 115 respectively. EVs derived from the ex vivo adipose tissue of ob/ob obese model mice can promote inflammatory macrophage activation, including upregulation of cytokines IL6 and tumor necrosis factor alpha. 116 Together, these studies suggest that EVs secreted under pathological conditions may serve to positively reinforce systemic inflammation and disease pathogenesis (Figure 2). Direct evaluation of this hypothesis and the extent to which EVs may contribute to inflammaging represent promising areas for future research.
Crucially, EVs can also mitigate inflammatory pathologies. For instance, enrichment by source cell overexpression of miR‐26a‐5p or miR‐140‐5p in EVs derived from MSCs can mitigate osteoarthritic damage by targeting Ptgs2 (COX2) 117 or even induce cartilage regeneration, respectively, in rats. 118 Additionally, administration of EVs secreted by adipose‐derived MSCs can protect against senescence, including pro‐inflammatory cytokine production and DNA damage in osteoarthritis osteoblasts. 119
4.4. EVs and metabolic dysfunction
There is evidence that EVs have potentially important roles as biomarkers and as regulators of metabolic processes, including in age‐associated conditions such as obesity, insulin resistance, and diabetes. 5 , 120 Accordingly, in rats, there are considerable age‐related changes to the levels of EV‐contained miRNAs targeting multiple metabolic pathways including those targeting insulin and growth hormone receptor signaling. 35 Furthermore, miRNAs secreted from adipose tissue in EVs can regulate gene expression in distal tissues, such as hepatic FGF21 expression. 121 The treatment of WT mice with adipose‐derived EVs from obese model mice can drive insulin resistance, glucose intolerance, increased triglyceride levels, dyslipidemia, inflammatory macrophage activation, and hepatic steatosis, potentially through the transfer of EV‐contained miRNAs. 116 , 122 EVs derived from pancreatic cancer patients can induce adipocyte lipolysis through EV‐contained adrenomedullin activation of MAPK signaling, mediating phosphorylation of hormone sensitive lipase, 123 and suppressing insulin secretion from pancreatic β‐cells. 124 Interestingly, increased EV secretion from macrophages induced by the DNA damage response can cause glucose uptake in recipient cells, leading to metabolic dysfunction and systemic inflammation. 50 The increased delivery of miR‐29b‐3p by bone marrow MSC‐derived small EVs to various tissues, including adipose and hepatic tissues, may induce age‐related insulin resistance in a SIRT1‐dependent manner. 125 Depletion of EV‐contained miR‐26a in obesity may drive diabetes‐like symptoms including insulin resistance and glucose intolerance in mice and is associated with Type 2 diabetes in humans. 126 Conversely, pancreatic β‐cells secrete small EVs containing miR‐26a, which may promote insulin sensitivity both locally and distally. Adipose tissue‐specific Nampt‐knockout (ANKO) mice, which show a significant reduction in circulating eNAMPT levels, develop severe insulin resistance in multiple tissues, suggesting that eNAMPT containing EVs play an important role in maintaining insulin sensitivity at a systemic level. 127 Additionally, treatment of old mice with young plasma‐derived EVs can reduce mTOR and IGF1R in the lung and liver, potentially through delivery of miRNAs targeting these genes. 128 Together, these studies indicate that maintaining EV content, such as miRNAs and eNAMPT, associated with youth and metabolic fitness may be important in suppressing age‐related metabolic dysfunction.
4.5. EVs and neurological disease
There are emerging data that EVs target the brain and may mediate the pathogenesis of neurological disease. 5 , 9 Treatment with MSC‐derived EVs following induction of traumatic brain injury and hemorrhagic shock in a Yorkshire swine model reduced the severity of neurologic impairment and improved recovery time. 129 The local transfer of miR‐124‐3p in small EVs from microglia to hippocampal neurons was also found to serve a protective function in a setting of traumatic brain injury. 130 While the injuries in these models involved loss of blood‐brain barrier (BBB) integrity, a number of studies suggest that administered EV treatments can pass through the BBB. This ability is essential in the context of aging because certain brain regions, especially the hypothalamus, 34 , 71 , 75 , 131 may exert control of the aging process and also because the brain is particularly vulnerable to age‐related deterioration. For example, the delivery of BDNF to the brain via macrophage‐derived EVs may be mediated by surface expression of ICAM‐1 and LFA‐1 and is increased in the setting of brain inflammation induced by intracranial LPS injection. 132 However, uptake of EVs to the brain may be less pronounced than to other organs including the liver, spleen, lung, kidney, and heart at least at 10 min post‐EV injection. 132
Data describing the function of EVs in AD models is context‐dependent. A preliminary study found a benefit of stereotactic MSC‐derived EV injection directly to the dentate gyrus of Aβ aggregate‐injected mice on cognitive performance. This effect is correlated with stimulation of neurogenesis in the subventricular zone by EV treatment. 133 Another study found that intranasal injection of MSC‐derived EVs could attenuate microglial activation and modestly increase hippocampal dendritic spine density in the 3xTg AD mouse model. 134 Supplementation of EVs derived from human MSCs or hypoxic mouse mesenchymal stromal cells may rescue Aβ aggregate pathology as well as the associated inflammation and cognitive dysfunction in a beta‐amyloidosis model mouse. 135 , 136 However, the inhibition of EV secretion reduced Aβ plaque burden, while injecting EVs increased plaque burden in a different beta‐amyloidosis mouse model. The effects in this latter study may have been mediated through astrocytes as astrocyte‐derived EVs inhibited glial Aβ uptake in culture. 137 EVs may also contribute to spreading of tau pathology. 138 Again, differences in models as well as the heterogeneity of EV populations and phenotypes examined suggest that more work is needed to resolve these discrepancies. Moreover, the relative contribution of local in contrast to peripherally derived EVs in the regulation of AD pathogenesis remains relatively unexplored. While intriguing, future work should more comprehensively evaluate the robustness of these and other findings in a range of neurodegenerative settings. Additionally, the investigation of possible common regulatory roles undertaken by EVs in the pathogenesis and amelioration of disease, including EV trafficking and uptake mechanisms, between neurological conditions would be advantageous in the development of therapeutics.
4.6. EVs and cardiovascular disease
Extracellular vesicles may also be influential in regulating age‐related cardiovascular dysfunction. For example, in contrast to large EVs, platelet‐derived small EVs may mitigate atherothrombosis by directing both platelet and macrophage CD36 to proteasomal degradation, thereby limiting thrombosis. 139 On the other hand, EVs secreted by vascular smooth muscle cells may promote calcification and thrombosis in a setting of an atherosclerotic plaque. 140 Additionally, EVs secreted by platelets in septic, but not healthy patients, are sufficient to depress left ventricular pressure in ex vivo rabbit hearts, suggesting a role for EVs in promoting myocardial dysfunction. 141
However, treatment with EVs from healthy tissue or cell culture may also confer vascular benefits. For instance, large EVs derived from endothelial progenitor cells may induce pro‐angiogenic and antiapoptotic effects through delivery of mRNA that activates PI3K/AKT signaling. 142 The pro‐angiogenic properties of adipose stem cell‐derived EVs may be abolished during obesity and dependent on delivery of miR‐126 and, to a lesser extent, VEGF and MMP‐2 to endothelial cells. 143 Endothelial EV transfer of miR‐214 may also confer pro‐angiogenic properties while modestly reducing senescence in recipient cells. 144 Administration of MSC‐derived small EVs 5 min before reperfusion of myocardial ischemia in a mouse model markedly reduced infarct size. 145 Serum‐derived EVs can promote angiogenesis and limit muscle damage in a mouse model of acute hind limb ischemia. 146 As in other contexts, 101 oxidative stress can induce a beneficial priming effect by increasing miR‐21 content in EVs derived from cardiac progenitor cells. This transfer of miR‐21 may play a protective role in cardiomyocytes by targeting apoptosis promoting PDCD4. 147 Relatedly, ischemic preconditioning, which involves cycles of oxygen deprivation, in MSCs can alter EV content such that delivery of conditioned EVs can confer protection against apoptosis in vitro and cardiac fibrosis in vivo. Among the changes driving this benefit is an increase in miR‐22 in MSC‐derived EVs, which targets Mecp2 in recipient cardiomyocytes. 148 miR‐21‐5p in MSC‐derived EVs promotes calcium handling and procontractile force in cultured engineered cardiac tissue, at least partially through PI3K signaling. 149 Consistent with investigations in other disease states, these data suggest that the effect of EVs may be a direct consequence of the fitness of the source cells or tissue (Figure 2).
5. EVs IN CLINICS
5.1. EVs as biomarkers
The unique cargo including protein, mutated DNA, mRNA, and miRNA contained in circulating EVs during aging and disease pathogenesis affords an opportunity for the measurement of such EV content as a non‐invasive diagnostic tool. 5 , 7 For example, microRNAs such as miR‐24‐3p were found to be upregulated by more than fourfold in the salivary small EVs of older humans. 36 EVs may also serve as tools for evaluation of aging‐relevant interventions. For instance, changes in EV miRNA content observed in aged plasma as well as in synovial fluid extracted from mouse knee joints can be partially rescued with senolytic treatment. 25 , 83 One group found that in men with prostate cancer, 1 month of protein restricted diet increased leptin receptor levels in plasma EVs and LCAM1+ EVs (suggestive of a neuronal origin). An increase in the ratio of pan‐tyrosine to serine 312 phosphorylation on IRS, indicative of improved insulin sensitivity, was also observed in LCAM+ EVs. 150 These data indicate that dietary interventions relevant to age‐associated pathology such as obesity affect neuronally released EVs and suggest that such changes may serve as proxies for measuring neuronal homeostasis.
Tumor‐derived EVs may contain considerable amounts of double stranded DNA and, to a lesser extent, single stranded DNA fragments. 151 Thus, it may be possible to analyze these fragments for oncogenic mutations as a cancer diagnostic tool. For example, the presence of mutated p53 and KRAS DNA fragments 18 , 152 or the enrichment of membrane‐associated Glypican‐1 153 in serum EVs from pancreatic cancer patients and EVs secreted from pancreatic cancer cell lines could serve as cancer biomarkers. Biomarkers for glioblastoma can even be detected in large EVs isolated from serum. 154 Together, these data suggest that EV content could be measured as both an indicator of pathology and as a benchmark for treatment efficacy. Additionally, the relative protection afforded miRNA species within EVs against RNases would help ensure reliability and reduce variability in these measurements. 155
5.2. EVs as therapies in humans
While the molecules that target EVs to specific tissues are still being actively investigated, biologically produced or engineered EVs may already have built in protection against degradation. For instance, inclusion of CD47 on the EV surface may confer protection against phagocytosis and degradation of injected EVs by immune cells including macrophages and monocytes. 156 Importantly, injection of cell‐culture‐derived EVs also does not appear to induce overt toxicity or pro‐inflammatory effects. 157 Given these properties, the overt use of EVs as therapeutics has been explored in the clinic. For instance, treatment of a graft‐versus‐host disease patient with MSC‐derived EVs‐induced abatement of symptoms including diarrhea and skin pathology. Treatment of peripheral blood mononuclear cells derived from the same patient with MSC‐derived EVs also reduced inflammatory cytokine release in culture. 158 In a phase‐II clinical trial, treatment of patients with chemotherapy‐responsive inoperable non‐small cell lung carcinoma with dendritic cell‐derived EVs enriched for MHC‐I and MHC‐II was able to enhance natural killer (NK) cell function through NKp30. Although this study did not reach its primary endpoint (50% of patients with 4 months progression‐free survival), the treatment was well tolerated and showed biological efficacy in activating NK cells. 159 EVs derived from human foreskin fibroblasts and loaded with either siRNA or shRNA expression plasmid targeting oncogenic KRASG12D successfully mitigated pancreatic tumor growth in several mouse models. 156 , 160 These EVs, termed “iExosomes” are currently being tested in a clinical trial with an estimated completion date of 31 March 2022 (ClinicalTrials.gov Identifier: NCT03608631). These studies suggest that clinical treatment with EVs is feasible, but further optimization will be necessary to increase specificity and efficacy.
6. CONCLUSIONS AND PERSPECTIVES
It is now clear that EVs play active roles in the maintenance and eventual breakdown of physiological robustness in aging 75 and in a wide range of age‐related morbidities from cellular senescence, inflammaging, and cancer to metabolic, cardiovascular, and neurological dysfunction 5 , 8 , 9 (Figure 2). Moreover, EVs derived from plasma and a variety of culture sources have been utilized to successfully ameliorate a diverse array of pathological phenotypes in both preclinical and clinical settings. In combination, these studies suggest that in aging, EVs serve as a key instrument through which a series of interlocking regulatory feedback loops positively or negatively regulate the process of aging. Thus, we propose a model in which EVs play a central regulatory role in mammalian aging and longevity control (Figure 3). Gradually, chronic cellular stress may induce the accumulation of oxidative, inflammatory, and DNA damage. This damage may alter the nucleotide and protein content and increase the secretion of EVs, inducing inflammation and propagating damage to proximal and distal cell populations. These EVs then disrupt the homeostasis of recipient cells, initiating a damage response including mitigation measures such as autophagy. When these compensatory mechanisms eventually become overwhelmed and break down, EV contents and secretion again change, manifesting the pathological state from the secondary cell and continuing the cycle. Meanwhile, EVs secreted by healthy or “youthful” tissues carry growth factors, protective enzymes, antioxidants, metabolites, and beneficial miRNAs that mediate healthy intertissue communication pathways and bolster the resilience of recipient tissues. The resultant EV pool then reflects an amalgamation of cells at different points in this continuum from healthy to pathological. Over time, the decline in beneficial EVs and/or the accumulation of EVs secreted by senescent and otherwise damaged cells supplants homeostatic/rejuvenating EVs, impairing maintenance of tissue functions, and driving aging‐related physiological deterioration. In order to test this theory, a more thorough characterization of EV subpopulations including tracing of cellular origins, destinations, and alterations to these subpopulations with age is needed.
FIGURE 3.
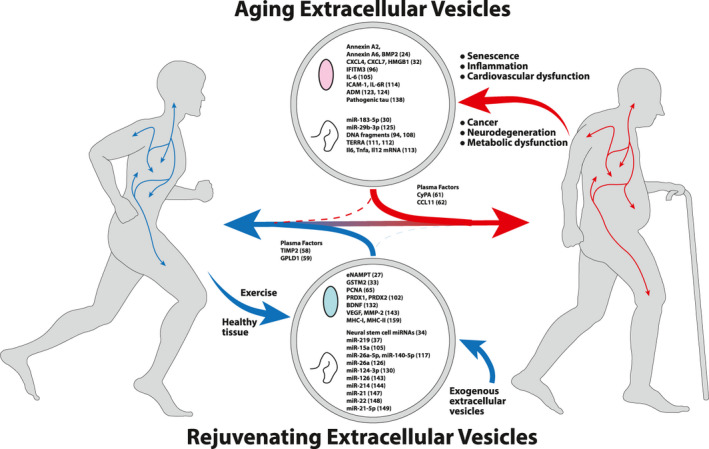
An extracellular vesicle theory of aging. Early in life, extracellular vesicles (EVs) serve as beneficial signaling molecules and promote tissue health. As senescent/damaged cells accumulate over the lifespan of an organism, these cells secrete EVs carrying detrimental cargo. These aging‐promoting EVs circulate throughout the body, positively reinforcing aging‐related tissue deterioration. At the same time, as an organism ages, the number of rejuvenating or supportive EVs decreases. Beneficial effects seen with EV treatments may then reflect a shift of this balance toward rejuvenating EVs and may be one strategy for circumventing age‐related deterioration. Proteins are denoted by colored ovals and nucleic acids are denoted by curled lines. Examples of potential effector molecules found to have detrimental effects (top, aging) or beneficial effects (bottom, rejuvenating) are depicted along with supporting citation
If support is uncovered for a consequential regulatory role for EVs in systemic aging, then strategies to intervene in this process would fall into two broad categories. First, systematic repair or removal of damaged cells from EV source tissues would tip the balance in favor of rejuvenating EVs. Senolytics would constitute such an intervention. This approach would have the advantage of more acutely clearing potentially deleterious EVs by removing their source. However, such an approach may lack pervasiveness or specificity, possibly leaving many sources of contaminated EVs to accumulate and propagate damage. Second, supplementing the EV pool with exogenous, supportive EVs would also skew the balance in favor of rejuvenation and promote homeostasis. This approach has the advantage of leveraging endemic targeting and delivery mechanisms to provide trophic support to a range of deteriorating tissues spread across the body. However, this approach may take time, requiring repeated treatments to placate sources of detrimental EVs. Importantly, the ability to enhance the efficacy and potentially the targeting precision of EVs by supplementing EV content prior to delivery presents a compelling justification for pursuing the second option. Of course, a multifaceted approach incorporating both strategies simultaneously may also prove most efficacious. In any case, substantial future work is required to elucidate the specific molecules that dictate EV targeting and uptake as well as how these molecules may be altered with age. Also critical is the identification of effector cell populations that may particularly benefit from EV‐mediated support in aging. Once we understand and harness the power of this endogenous system, including pivotal components involved in the antiaging effects of EVs, we have the exciting potential to undermine several facets of aging at once, improving resilience against age‐related dysfunctions and extending healthspan in humans.
CONFLICT OF INTEREST
S.I. receives a part of patent‐licensing fees from MetroBiotech (USA) and Teijin Limited (Japan) through Washington University. B.V.L. declares no competing interests.
AUTHOR CONTRIBUTIONS
Brian V. Lananna and Shin‐ichiro Imai researched the literature and wrote the paper.
ACKNOWLEDGMENTS
The authors apologize to those whose works were not cited due to space limitation. This work was mainly supported by grants to S.I. from the National Institute on Aging (AG037457, AG047902) and the Tanaka Fund. B.V.L. is supported as a W.M. Keck Fellow. S.I. is also supported by the Project for Elucidating and Controlling Mechanisms of Aging and Longevity (21gm5010002s0003), organized by the Japan Agency for Medical Research and Development (AMED), and by the Uehara Memorial Foundation at the Institute of Biomedical Research and Innovation (IBRI) in Kobe, Japan.
Lananna BV, Imai S‐I. Friends and foes: Extracellular vesicles in aging and rejuvenation. FASEB BioAdvances. 2021;3:787–801. 10.1096/fba.2021-00077
This article is part of the Extracellular Vesicles and Homeostasis Special Collection.
REFERENCES
- 1. Prattichizzo F, Giuliani A, Sabbatinelli J, et al. Extracellular vesicles circulating in young organisms promote healthy longevity. J Extracell Vesicles. 2019;8:1656044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13:269‐288. [DOI] [PubMed] [Google Scholar]
- 3. Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967‐978. [DOI] [PubMed] [Google Scholar]
- 4. Harding C, Heuser J, Stahl P. Receptor‐mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mahmoudi S, Xu L, Brunet A. Turning back time with emerging rejuvenation strategies. Nat Cell Biol. 2019;21:32‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prattichizzo F, Giuliani A, Sabbatinelli J, et al. Extracellular vesicles circulating in young organisms promote healthy longevity. J Extracell Vesicles. 2019;8:1656044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Takasugi M. Emerging roles of extracellular vesicles in cellular senescence and aging. Aging Cell. 2018;17:e12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shah R, Patel T, Freedman JE. Circulating extracellular vesicles in human disease. N Engl J Med. 2018;379:958‐966. [DOI] [PubMed] [Google Scholar]
- 10. Robbins PD. Extracellular vesicles and aging. Stem Cell Investig. 2017;4:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bobrie A, Colombo M, Krumeich S, Raposo G, Théry C. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles. 2012;1:18397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA. 2016;113:E968‐E977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. López‐Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The Hallmarks of Aging. Cell. 2013;153:1194‐1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213‐228. [DOI] [PubMed] [Google Scholar]
- 16. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome‐mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654‐659. [DOI] [PubMed] [Google Scholar]
- 17. Fernando MR, Jiang C, Krzyzanowski GD, Ryan WL. New evidence that a large proportion of human blood plasma cell‐free DNA is localized in exosomes. PLoS One. 2017;12:e0183915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kahlert C, Melo SA, Protopopov A, et al. Identification of double‐stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem. 2014;289:3869‐3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gallo A, Tandon M, Alevizos I, Illei GG. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One. 2012;7:e30679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jeppesen DK, Fenix AM, Franklin JL, et al. Reassessment of exosome composition. Cell. 2019;177:428‐445.e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eitan E, Green J, Bodogai M, et al. Age‐related changes in plasma extracellular vesicle characteristics and internalization by leukocytes. Sci Rep. 2017;7(1). 10.1038/s41598-017-01386-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Forest A, Pautas E, Ray P, et al. Circulating microparticles and procoagulant activity in elderly patients. J Gerontol A Biol Sci Med Sci. 2010;65A:414‐420. [DOI] [PubMed] [Google Scholar]
- 23. Alberro A, Sáenz‐Cuesta M, Muñoz‐Culla M, et al. Inflammaging and frailty status do not result in an increased extracellular vesicle concentration in circulation. Int J Mol Sci. 2016;17:1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alique M, Ruíz‐Torres MP, Bodega G, et al. Microvesicles from the plasma of elderly subjects and from senescent endothelial cells promote vascular calcification. Aging. 2017;9:778‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alibhai FJ, Lim F, Yeganeh A, et al. Cellular senescence contributes to age‐dependent changes in circulating extracellular vesicle cargo and function. Aging Cell. 2020;19(3):e13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang AL, Lukas TJ, Yuan M, Du N, Tso MO, Neufeld AH. Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to drusen formation and age‐related macular degeneration. PLoS One. 2009;4:e4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoshida M, Satoh A, Lin JB, et al. Extracellular vesicle‐contained eNAMPT delays aging and extends lifespan in mice. Cell Metab. 2019;30(2):329‐342.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weilner S, Keider V, Winter M, et al. Vesicular Galectin‐3 levels decrease with donor age and contribute to the reduced osteo‐inductive potential of human plasma derived extracellular vesicles. Aging. 2016;8:16‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weilner S, Schraml E, Wieser M, et al. Secreted microvesicular miR‐31 inhibits osteogenic differentiation of mesenchymal stem cells. Aging Cell. 2016;15:744‐754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Davis C, Dukes A, Drewry M, et al. MicroRNA‐183‐5p increases with age in bone‐derived extracellular vesicles, suppresses bone marrow stromal (stem) cell proliferation, and induces stem cell senescence. Tissue Eng Part A. 2017;23:1231‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bæk R, Varming K, Jørgensen MM. Does smoking, age or gender affect the protein phenotype of extracellular vesicles in plasma? Transfus Apheres Sci. 2016;55:44‐52. [DOI] [PubMed] [Google Scholar]
- 32. Goetzl EJ, Goetzl L, Karliner JS, Tang N, Pulliam L. Human plasma platelet‐derived exosomes: effects of aspirin. FASEB J. 2016;30:2058‐2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fafian‐Labora JA, Rodriguez‐Navarro JA, O'Loghlen A. Small extracellular vesicles have GST activity and ameliorate senescence‐related tissue damage. Cell Metab. 2020;32:71‐86.e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang Y, Kim MS, Jia B, et al. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature. 2017;548:52‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang H, Jin K. Peripheral circulating exosomal mirnas potentially contribute to the regulation of molecular signaling networks in aging. Int J Mol Sci. 2020;21:1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Machida T, Tomofuji T, Ekuni D, et al. MicroRNAs in salivary exosome as potential biomarkers of aging. Int J Mol Sci. 2015;16:21294‐21309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pusic AD, Kraig RP. Youth and environmental enrichment generate serum exosomes containing miR‐219 that promote CNS myelination. Glia. 2014;62:284‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dluzen DF, Noren Hooten N, De S, et al. Extracellular RNA profiles with human age. Aging Cell. 2018;17:e12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Elsharawy A, Keller A, Flachsbart F, et al. Genome‐wide miRNA signatures of human longevity. Aging Cell. 2012;11:607‐616. [DOI] [PubMed] [Google Scholar]
- 40. Rani A, O'Shea A, Ianov L, Cohen RA, Woods AJ, Foster TC. miRNA in circulating microvesicles as biomarkers for age‐related cognitive decline. Front Aging Neurosci. 2017;9. 10.3389/fnagi.2017.00323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li G, Luna C, Qiu J, Epstein DL, Gonzalez P. Alterations in microRNA expression in stress‐induced cellular senescence. Mech Ageing Dev. 2009;130:731‐741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760‐764. [DOI] [PubMed] [Google Scholar]
- 43. Villeda SA, Plambeck KE, Middeldorp J, et al. Young blood reverses age‐related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20:659‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Baixauli F, López‐Otín C, Mittelbrunn M. Exosomes and autophagy: coordinated mechanisms for the maintenance of cellular fitness. Front Immunol. 2014;5. 10.3389/fimmu.2014.00403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fader CM, Sánchez D, Furlán M, Colombo MI. Induction of autophagy promotes fusion of multivesicular bodies with autophagic vacuoles in K562 cells. Traffic. 2007;9:230‐250. [DOI] [PubMed] [Google Scholar]
- 46. Miao Y, Li G, Zhang X, Xu H, Abraham SN. A TRP channel senses lysosome neutralization by pathogens to trigger their expulsion. Cell. 2015;161:1306‐1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hitomi K, Okada R, Loo TM, Miyata K, Nakamura AJ, Takahashi A. DNA damage regulates senescence‐associated extracellular vesicle release via the ceramide pathway to prevent excessive inflammatory responses. Int J Mol Sci. 2020;21:3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hessvik NP, Øverbye A, Brech A, et al. PIKfyve inhibition increases exosome release and induces secretory autophagy. Cell Mol Life Sci. 2016;73:4717‐4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Murrow L, Malhotra R, Debnath J. ATG12–ATG3 interacts with Alix to promote basal autophagic flux and late endosome function. Nat Cell Biol. 2015;17:300‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Goulielmaki E, Ioannidou A, Tsekrekou M, et al. Tissue‐infiltrating macrophages mediate an exosome‐based metabolic reprogramming upon DNA damage. Nat Commun. 2020;11. 10.1038/s41467-019-13894-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kulkarni R, Bajaj M, Ghode S, Jalnapurkar S, Limaye L, Kale VP. Intercellular transfer of microvesicles from young mesenchymal stromal cells rejuvenates aged murine hematopoietic stem cells. Stem Cells. 2018;36:420‐433. [DOI] [PubMed] [Google Scholar]
- 52. Leidal AM, Debnath J. Emerging roles for the autophagy machinery in extracellular vesicle biogenesis and secretion. FASEB BioAdv. 2021;3(5):377‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ho TT, Warr MR, Adelman ER, et al. Autophagy maintains the metabolism and function of young and old stem cells. Nature. 2017;543:205‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. García‐Prat L, Martínez‐Vicente M, Perdiguero E, et al. Autophagy maintains stemness by preventing senescence. Nature. 2016;529:37‐42. [DOI] [PubMed] [Google Scholar]
- 55. Hansen M, Rubinsztein DC, Walker DW. Autophagy as a promoter of longevity: insights from model organisms. Nat Rev Mol Cell Biol. 2018;19:579‐593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Latifkar A, Ling L, Hingorani A, et al. Loss of sirtuin 1 alters the secretome of breast cancer cells by impairing lysosomal integrity. Dev Cell. 2019;49:393‐408.e397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee IH, Cao L, Mostoslavsky R, et al. A role for the NAD‐dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci USA. 2008;105:3374‐3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Castellano JM, Mosher KI, Abbey RJ, et al. Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature. 2017;544:488‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Horowitz AM, Fan X, Bieri G, et al. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science. 2020;369:167‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yousef H, Czupalla CJ, Lee D, et al. Aged blood impairs hippocampal neural precursor activity and activates microglia via brain endothelial cell VCAM1. Nat Med. 2019;25:988‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Smith LK, Verovskaya E, Bieri G, et al. The aged hematopoietic system promotes hippocampal‐dependent cognitive decline. Aging Cell. 2020;19(8). 10.1111/acel.13192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Villeda SA, Luo J, Mosher KI, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Brahmer A, Neuberger E, Esch‐Heisser L, et al. Platelets, endothelial cells and leukocytes contribute to the exercise‐triggered release of extracellular vesicles into the circulation. J Extracell Vesicles. 2019;8:1615820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sha SJ, Deutsch GK, Tian L, et al. Safety, tolerability, and feasibility of young plasma infusion in the plasma for Alzheimer symptom amelioration study. JAMA Neurol. 2019;76:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lei Q, Gao F, Liu T, et al. Extracellular vesicles deposit PCNA to rejuvenate aged bone marrow‐derived mesenchymal stem cells and slow age‐related degeneration. Sci Transl Med. 2021;13:eaaz8697. [DOI] [PubMed] [Google Scholar]
- 66. Tan C, Lai R, Wong W, Dan Y, Lim S‐K, Ho H. Mesenchymal stem cell‐derived exosomes promote hepatic regeneration in drug‐induced liver injury models. Stem Cell Res Ther. 2014;5:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bruno S, Tapparo M, Collino F, et al. Renal regenerative potential of different extracellular vesicle populations derived from bone marrow mesenchymal stromal cells. Tissue Eng Part A. 2017;23:1262‐1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Collino F, Bruno S, Incarnato D, et al. AKI recovery induced by mesenchymal stromal cell‐derived extracellular vesicles carrying microRNAs. J Am Soc Nephrol. 2015;26:2349‐2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cantaluppi V, Gatti S, Medica D, et al. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia–reperfusion injury by microRNA‐dependent reprogramming of resident renal cells. Kidney Int. 2012;82:412‐427. [DOI] [PubMed] [Google Scholar]
- 70. Li X, Xie X, Lian W, et al. Exosomes from adipose‐derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018;50:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Satoh A, Brace CS, Rensing N, et al. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013;18:416‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Xiao YZ, Yang M, Xiao Y, et al. Reducing hypothalamic stem cell senescence protects against aging‐associated physiological decline. Cell Metab. 2020;31(3):534–548.e5. [DOI] [PubMed] [Google Scholar]
- 73. Xiao Y‐Z, Yang M, Xiao Y, et al. Reducing hypothalamic stem cell senescence protects against aging‐associated physiological decline. Cell Metab. 2020;31:534‐548.e535. [DOI] [PubMed] [Google Scholar]
- 74. Yoon MJ, Yoshida M, Johnson S, et al. SIRT1‐mediated eNAMPT secretion from adipose tissue regulates hypothalamic NAD+ and function in mice. Cell Metab. 2015;21:706‐717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Imai S. The NAD World 2.0: the importance of the inter‐tissue communication mediated by NAMPT/NAD(+)/SIRT1 in mammalian aging and longevity control. NPJ Syst Biol Appl. 2016;2:16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tchkonia T, Zhu Y, Van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966‐972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Coppé J‐P, Patil CK, Rodier F, et al. Senescence‐associated secretory phenotypes reveal cell‐nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Coppé J‐P, Desprez P‐Y, Krtolica A, Campisi J. The senescence‐associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Baker DJ, Childs BG, Durik M, et al. Naturally occurring p16Ink4a‐positive cells shorten healthy lifespan. Nature. 2016;530:184‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a‐positive senescent cells delays ageing‐associated disorders. Nature. 2011;479:232‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Farr JN, Xu M, Weivoda MM, et al. Targeting cellular senescence prevents age‐related bone loss in mice. Nat Med. 2017;23:1072‐1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Xu M, Pirtskhalava T, Farr JN, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24:1246‐1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jeon OH, Wilson DR, Clement CC, et al. Senescence cell–associated extracellular vesicles serve as osteoarthritis disease and therapeutic markers. JCI Insight. 2019;4(7):e125019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Basisty N, Kale A, Jeon OH, et al. A proteomic atlas of senescence‐associated secretomes for aging biomarker development. PLoS Biol. 2020;18:e3000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lehmann BD, Paine MS, Brooks AM, et al. Senescence‐associated exosome release from human prostate cancer cells. Can Res. 2008;68:7864‐7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pienimaeki‐Roemer A, Konovalova T, Musri MM, et al. Transcriptomic profiling of platelet senescence and platelet extracellular vesicles. Transfusion. 2017;57:144‐156. [DOI] [PubMed] [Google Scholar]
- 87. Terlecki‐Zaniewicz L, Lammermann I, Latreille J, et al. Small extracellular vesicles and their miRNA cargo are anti‐apoptotic members of the senescence‐associated secretory phenotype. Aging (Albany NY). 2018;10:1103‐1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fujii M, Kawai Y, Endoh M, Hossain MN, Nakabayashi K, Ayusawa D. Expression of RAB27B is up‐regulated in senescent human cells. Mech Ageing Dev. 2006;127:639‐642. [DOI] [PubMed] [Google Scholar]
- 89. Lespagnol A, Duflaut D, Beekman C, et al. Exosome secretion, including the DNA damage‐induced p53‐dependent secretory pathway, is severely compromised in TSAP6/Steap3‐null mice. Cell Death Differ. 2008;15:1723‐1733. [DOI] [PubMed] [Google Scholar]
- 90. Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Can Res. 2006;66:4795‐4801. [DOI] [PubMed] [Google Scholar]
- 91. Yu X, Riley T, Levine AJ. The regulation of the endosomal compartment by p53 the tumor suppressor gene. FEBS J. 2009;276:2201‐2212. [DOI] [PubMed] [Google Scholar]
- 92. Dou Z, Ghosh K, Vizioli MG, et al. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature. 2017;550:402‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Glück S, Guey B, Gulen MF, et al. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat Cell Biol. 2017;19:1061‐1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Takahashi A, Okada R, Nagao K, et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun. 2017;8:15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Abbas M, Jesel L, Auger C, et al. Endothelial microparticles from acute coronary syndrome patients induce premature coronary artery endothelial cell aging and thrombogenicity. Circulation. 2017;135:280‐296. [DOI] [PubMed] [Google Scholar]
- 96. Borghesan M, Fafian‐Labora J, Eleftheriadou O, et al. Small extracellular vesicles are key regulators of non‐cell autonomous intercellular communication in senescence via the interferon protein IFITM3. Cell Rep. 2019;27(13):3956‐3971.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mato‐Basalo R, Morente‐López M, Arntz OJ, Van De Loo FAJ, Fafián‐Labora J, Arufe MC. Therapeutic potential for regulation of the nuclear factor kappa‐B transcription factor p65 to prevent cellular senescence and activation of pro‐inflammatory in mesenchymal stem cells. Int J Mol Sci. 2021;22:3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Xu C, Wang L, Fozouni P, et al. SIRT1 is downregulated by autophagy in senescence and ageing. Nat Cell Biol. 2020;22(10):1170–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Han L, Long Q, Li S, et al. Senescent stromal cells promote cancer resistance through SIRT1 loss‐potentiated overproduction of small extracellular vesicles. Can Res. 2020;80:3383‐3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Simoncini S, Chateau A‐L, Robert S, et al. Biogenesis of pro‐senescent microparticles by endothelial colony forming cells from premature neonates is driven by SIRT1‐dependent epigenetic regulation of MKK6. Sci Rep. 2017;7(1). 10.1038/s41598-017-08883-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Eldh M, Ekström K, Valadi H, et al. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS One. 2010;5:e15353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Liu S, Mahairaki V, Bai H, et al. Highly purified human extracellular vesicles produced by stem cells alleviate aging cellular phenotypes of senescent human cells. Stem Cells. 2019;37:779‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Oh M, Lee J, Kim YJ, Rhee WJ, Park JH. Exosomes derived from human induced pluripotent stem cells ameliorate the aging of skin fibroblasts. Int J Mol Sci. 2018;19:1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ. Extracellular vesicles in cancer—implications for future improvements in cancer care. Nat Rev Clin Oncol. 2018;15:617‐638. [DOI] [PubMed] [Google Scholar]
- 105. Roccaro AM, Sacco A, Maiso P, et al. BM mesenchymal stromal cell–derived exosomes facilitate multiple myeloma progression. J Clin Invest. 2013;123:1542‐1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Robbins PD, Dorronsoro A, Booker CN. Regulation of chronic inflammatory and immune processes by extracellular vesicles. J Clin Invest. 2016;126:1173‐1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581‐593. [DOI] [PubMed] [Google Scholar]
- 108. Wang W, Kong P, Ma G, et al. Characterization of the release and biological significance of cell‐free DNA from breast cancer cell lines. Oncotarget. 2017;8:43180‐43191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kitai Y, Kawasaki T, Sueyoshi T, et al. DNA‐containing exosomes derived from cancer cells treated with topotecan activate a STING‐dependent pathway and reinforce antitumor immunity. J Immunol. 2017;198:1649‐1659. [DOI] [PubMed] [Google Scholar]
- 110. Al‐Mayah AHJ, Bright SJ, Bowler DA, Slijepcevic P, Goodwin E, Kadhim MA. Exosome‐mediated telomere instability in human breast epithelial cancer cells after X irradiation. Radiat Res. 2017;187:98‐106. [DOI] [PubMed] [Google Scholar]
- 111. Wang Z, Lieberman PM. The crosstalk of telomere dysfunction and inflammation through cell‐free TERRA containing exosomes. RNA Biol. 2016;13:690‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wang Z, Deng Z, Dahmane N, et al. Telomeric repeat‐containing RNA (TERRA) constitutes a nucleoprotein component of extracellular inflammatory exosomes. Proc Natl Acad Sci USA. 2015;112:E6293‐E6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Mitsuhashi M, Taub DD, Kapogiannis D, et al. Aging enhances release of exosomal cytokine mRNAs by Aβ 1–42 ‐stimulated macrophages. FASEB J. 2013;27:5141‐5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Effenberger T, Heyde J, Bartsch K, et al. Senescence‐associated release of transmembrane proteins involves proteolytic processing by ADAM17 and microvesicle shedding. FASEB J. 2014;28:4847‐4856. [DOI] [PubMed] [Google Scholar]
- 115. Schumacher N, Meyer D, Mauermann A, et al. Shedding of Endogenous Interleukin‐6 Receptor (IL‐6R) is governed by A Disintegrin and Metalloproteinase (ADAM) proteases while a full‐length IL‐6R isoform localizes to circulating microvesicles. J Biol Chem. 2015;290:26059‐26071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Deng ZB, Poliakov A, Hardy RW, et al. Adipose tissue exosome‐like vesicles mediate activation of macrophage‐induced insulin resistance. Diabetes. 2009;58:2498‐2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Jin Z, Ren J, Qi S. Human bone mesenchymal stem cells‐derived exosomes overexpressing microRNA‐26a‐5p alleviate osteoarthritis via down‐regulation of PTGS2. Int Immunopharmacol. 2020;78:105946. [DOI] [PubMed] [Google Scholar]
- 118. Tao SC, Yuan T, Zhang YL, Yin WJ, Guo SC, Zhang CQ. Exosomes derived from miR‐140‐5p‐overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7:180‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Tofino‐Vian M, Guillen MI, Perez Del Caz MD, Castejon MA, Alcaraz MJ. Extracellular vesicles from adipose‐derived mesenchymal stem cells downregulate senescence features in osteoarthritic osteoblasts. Oxid Med Cell Longev. 2017;2017:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Pardo F, Villalobos‐Labra R, Sobrevia B, Toledo F, Sobrevia L. Extracellular vesicles in obesity and diabetes mellitus. Mol Aspects Med. 2018;60:81‐91. [DOI] [PubMed] [Google Scholar]
- 121. Thomou T, Mori MA, Dreyfuss JM, et al. Adipose‐derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542:450‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Castaño C, Kalko S, Novials A, Párrizas M. Obesity‐associated exosomal miRNAs modulate glucose and lipid metabolism in mice. Proc Natl Acad Sci USA. 2018;115:12158‐12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Sagar G, Sah RP, Javeed N, et al. Pathogenesis of pancreatic cancer exosome‐induced lipolysis in adipose tissue. Gut. 2016;65:1165‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Javeed N, Sagar G, Dutta SK, et al. Pancreatic cancer‐derived exosomes cause paraneoplastic β‐cell dysfunction. Clin Cancer Res. 2015;21:1722‐1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Su T, Xiao Y, Xiao Y, et al. Bone marrow mesenchymal stem cells‐derived exosomal MiR‐29b‐3p regulates aging‐associated insulin resistance. ACS Nano. 2019;13:2450–2462. [DOI] [PubMed] [Google Scholar]
- 126. Xu H, Du X, Xu J, et al. Pancreatic β cell microRNA‐26a alleviates type 2 diabetes by improving peripheral insulin sensitivity and preserving β cell function. PLoS Biol. 2020;18:e3000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Stromsdorfer KL, Yamaguchi S, Yoon MJ, et al. NAMPT‐mediated NAD(+) biosynthesis in adipocytes regulates adipose tissue function and multi‐organ insulin sensitivity in mice. Cell Rep. 2016;16:1851‐1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Lee B‐R, Kim J‐H, Choi E‐S, Cho JH, Kim E. Effect of young exosomes injected in aged mice. Int J Nanomed. 2018;13:5335‐5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Williams AM, Dennahy IS, Bhatti UF, et al. Mesenchymal stem cell‐derived exosomes provide neuroprotection and improve long‐term neurologic outcomes in a swine model of traumatic brain injury and hemorrhagic shock. J Neurotrauma. 2019;36:54‐60. [DOI] [PubMed] [Google Scholar]
- 130. Ge X, Guo M, Hu T, et al. Increased microglial exosomal miR‐124‐3p alleviates neurodegeneration and improves cognitive outcome after rmTBI. Mol Ther. 2020;28:503‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zhang G, Li J, Purkayastha S, et al. Hypothalamic programming of systemic ageing involving IKK‐β, NF‐κB and GnRH. Nature. 2013;497:211‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Yuan D, Zhao Y, Banks WA, et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials. 2017;142:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Canales‐Aguirre A, Reza‐Zaldivar E, Hernández‐Sapiéns M, et al. Mesenchymal stem cell‐derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer's disease. Neural Regen Res. 2019;14:1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Losurdo M, Pedrazzoli M, D'Agostino C, et al. Intranasal delivery of mesenchymal stem cell‐derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer's disease. Stem Cells Transl Med. 2020;9:1068‐1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Cui GH, Wu J, Mou FF, et al. Exosomes derived from hypoxia‐preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J. 2018;32:654‐668. [DOI] [PubMed] [Google Scholar]
- 136. Ding M, Shen Y, Wang P, et al. Exosomes isolated from human umbilical cord mesenchymal stem cells alleviate neuroinflammation and reduce amyloid‐beta deposition by modulating microglial activation in Alzheimer's disease. Neurochem Res. 2018;43:2165‐2177. [DOI] [PubMed] [Google Scholar]
- 137. Dinkins MB, Dasgupta S, Wang G, Zhu G, Bieberich E. Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer's disease. Neurobiol Aging. 2014;35:1792‐1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Winston CN, Aulston B, Rockenstein EM, et al. Neuronal exosome‐derived human tau is toxic to recipient mouse neurons in vivo. J Alzheimers Dis. 2019;67:541‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Srikanthan S, Li W, Silverstein RL, McIntyre TM. Exosome poly‐ubiquitin inhibits platelet activation, downregulates CD36 and inhibits pro‐atherothombotic cellular functions. J Thromb Haemost. 2014;12:1906‐1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kapustin AN, Schoppet M, Schurgers LJ, et al. Prothrombin loading of vascular smooth muscle cell‐derived exosomes regulates coagulation and calcification. Arterioscler Thromb Vasc Biol. 2017;37:e22‐e32. [DOI] [PubMed] [Google Scholar]
- 141. Azevedo L, Janiszewski M, Pontieri V, et al. Platelet‐derived exosomes from septic shock patients induce myocardial dysfunction. Crit Care. 2007;11:R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Deregibus MC, Cantaluppi V, Calogero R, et al. Endothelial progenitor cell–derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110:2440‐2448. [DOI] [PubMed] [Google Scholar]
- 143. Togliatto G, Dentelli P, Gili M, et al. Obesity reduces the pro‐angiogenic potential of adipose tissue stem cell‐derived extracellular vesicles (EVs) by impairing miR‐126 content: impact on clinical applications. Int J Obes. 2016;40:102‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. van Balkom BW, de Jong OG, Smits M, et al. Endothelial cells require miR‐214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood. 2013;121(19):3997–4006. [DOI] [PubMed] [Google Scholar]
- 145. Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214‐222. [DOI] [PubMed] [Google Scholar]
- 146. Cavallari C, Ranghino A, Tapparo M, et al. Serum‐derived extracellular vesicles (EVs) impact on vascular remodeling and prevent muscle damage in acute hind limb ischemia. Sci Rep. 2017;7:8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Xiao J, Pan Y, Li XH, et al. Cardiac progenitor cell‐derived exosomes prevent cardiomyocytes apoptosis through exosomal miR‐21 by targeting PDCD4. Cell Death Dis. 2016;7:e2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Feng Y, Huang W, Wani M, Yu X, Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR‐22. PLoS One. 2014;9:e88685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Mayourian J, Ceholski DK, Gorski PA, et al. Exosomal microRNA‐21‐5p mediates mesenchymal stem cell paracrine effects on human cardiac tissue contractility. Circ Res. 2018;122:933‐944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Eitan E, Tosti V, Suire CN, et al. In a randomized trial in prostate cancer patients, dietary protein restriction modifies markers of leptin and insulin signaling in plasma extracellular vesicles. Aging Cell. 2017;16:1430‐1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Thakur BK, Zhang H, Becker A, et al. Double‐stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24:766‐769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Allenson K, Castillo J, San Lucas FA, et al. High prevalence of mutantKRAS in circulating exosome‐derived DNA from early‐stage pancreatic cancer patients. Ann Oncol. 2017;28:741‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Melo SA, Luecke LB, Kahlert C, et al. Glypican‐1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Skog J, Würdinger T, Van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470‐1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Cheng L, Sharples RA, Scicluna BJ, Hill AF. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell‐free blood. J Extracell Vesicles. 2014;3:23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Kamerkar S, Lebleu VS, Sugimoto H, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Zhu X, Badawi M, Pomeroy S, et al. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J Extracell Vesicles. 2017;6:1324730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Kordelas L, Rebmann V, Ludwig AK, et al. MSC‐derived exosomes: a novel tool to treat therapy‐refractory graft‐versus‐host disease. Leukemia. 2014;28:970‐973. [DOI] [PubMed] [Google Scholar]
- 159. Besse B, Charrier M, Lapierre V, et al. Dendritic cell‐derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. OncoImmunology. 2016;5:e1071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Mendt M, Kamerkar S, Sugimoto H, et al. Generation and testing of clinical‐grade exosomes for pancreatic cancer. JCI Insight. 2018;3(8):e99263. [DOI] [PMC free article] [PubMed] [Google Scholar]


