Abstract
The purpose of this study is to describe a surgical technique and report the functional outcome of treating nonarteritic central retinal artery occlusion (CRAO) in 2 eyes of two consecutive male patients. Two males (A) and (B) presented 6 days and few hours, respectively, after symptom onset with the clinical features typical of CRAO, including sudden severe deterioration of vision to counting fingers and a cherry-red spot in the left and right fovea, respectively. While A had been on recent treatment for hypertension and hyperlipidemia, B had no significant medical history. Both patients underwent surgery, A, 9 days, and B, <15 h after symptom onset. Surgery involved a vitrectomy, lowering of intraocular pressure (<3 mmHg), and gradual increase of arterial pressure to 165/100 mmHg (through the slow injection of intravenous adrenaline). Intraoperatively, this resulted in immediate perfusion and visible dilation of the blood-filled central retinal artery (CRA) and retinal arteriolar network and dilatation of the central retinal vein. The final vision settled to 6/60 in A and 6/36+1 in B. Although preoperative macular infarction persisted in both eyes as demonstrated by optical coherence tomography angiography, both patients claim a convincing subjective visual benefit. The procedure appeared to have positively changed the natural history of the disease in both eyes. Vitrectomy with the manipulation of intraocular and arterial pressures significantly increases ocular perfusion along the entire CRA, which can dislodge CRA thrombo-emboli and has the potential to restore retinal perfusion and improve visual outcome if undertaken before irreversible retina damage. This is a relatively straightforward technique and should join the list of surgical techniques for treating CRAO.
Keywords: Arterial perfusion, central retinal artery, embolus, intraocular pressure, vaso-occlusion, vitrectomy
Introduction
The central retinal artery (CRA) is the primary source of blood supply to the retina and supplies the inner two-third of the retina. Obstruction of this artery often results in devastating consequences for the eye and vision and presents as a sudden loss of vision.[1] In their report on central retinal artery occlusion (CRAO), Hayreh and Zimmerman noted that only 22% of eyes with counting fingers or worse vision improve.[2] Initial nonsurgical efforts at the treatment of occlusion of the CRA using paracentesis and inhalation of carbogen with prospects of reversing occlusion and improving vision did not yield much progress as the majority of the eyes treated had similar visual outcome as nontreated eyes.[3] Therefore, there has been interest in developing surgical remedies. Treatment outcome is complicated by the fact that therapy has to be instituted before the onset of irreversible retinal ischemia which has been reported to be approximately 4 hours.[4]
Cho et al. reported on thrombi movement in 52 eyes that suffered retinal artery occlusion (RAO) and established that the mechanisms of vascular reperfusion in relation to the emboli include complete degradation, peripheral migration, partial dislodgment, angiophagy, and collateral circulation.[5] We report a surgical technique that facilitates dislodgment and peripheral migration of thromboemboli of the CRA.
Methodology
Surgical technique for the treatment of CRAO
A 25G three-port pars plana vitrectomy (Constellation vitrectomy; Alcon, Fort Worth, TX, USA) was performed utilizing a general anesthetic. A local anesthetic using a regional block could work just as well.
Intraocular pressure (IOP) compensated infusion system was used.
Core vitrectomy was performed at the onset, and triamcinolone assisted the highlighting of the cortical vitreous lamina. Induction of a posterior vitreous detachment and extension to the vitreous base was performed.
After this, infusion pressure was set at <3 mmHg for 3 min, and later infusion was completely stopped for another 6 min. During this time, the arterial blood pressure (BP) was gradually raised by the anesthetist who was on hand to monitor the patient's systemic intraoperative parameters.
Over a 30 seconds interval, intravenous adrenaline was administered at a dose of 0.1 mg to raise the arterial BP to a maximum level of 165/100 mmHg for about 6 min. The CRA reperfusion began at the point of reduction of the IOP to <3 mmHg. We noticed a perfusion of the CRA at this point. However, CRA perfusion greatly increased, with observed dilated CRA and central retinal vein (CRV) at the point of rise in intraarterial BP and cessation of intraocular infusion.
During this period, the CRA was perfused and engorged with blood. There was an obvious dilatation of the CRA and branches and the CRV also. The optic disc was massaged with a soft silicone tipped cannula in the form of gentle strokes applied at the bifurcation of the CRA and along the branches which at this time maintained significant blood flow.
After approximately 10 min of BP increase, the systemic BP was gradually lowered to previtrectomy levels. The three ports of the saline-filled eye were closed with size 8–0 vicryl sutures, and care was taken to keep the eye slightly hypotonic.
This surgical technique was performed in the two eyes of two consecutive males who presented with a complaint of sudden loss of vision involving one eye each and clinical diagnosis was CRAO in both cases. Both patients gave informed consent for their case management and outcome to be reported.
Approval was obtained from the Eye Foundation Institutional Review Board (IRB) for this surgical technique. Similarly, approval was obtained from the same IRB to report this work. Every effort has been made to anonymize the details of the patient.
Case Presentation
Case 1
A 40-year-old male presented with a 6-day history of sudden loss of vision in the right eye. He had been diagnosed to have a right eye artery occlusion and had practiced digital ocular massage and ingested blood thinners a day after this happened with no improvement in his vision. He had undergone extensive cardiology workup and was only recently commenced on oral hypotensive and oral statins. His ocular examination was significant for reduced vision in the right eye to counting fingers, a cherry-red spot in the central macula, diffuse macula edema, retinal arterioles were significantly narrowed, and there was optic disc swelling [Figure 1].
Figure 1.
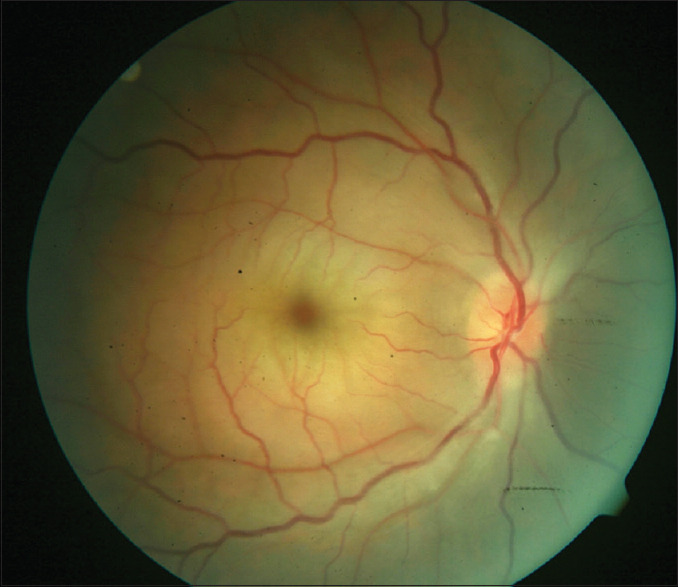
Fundus picture of the right eye showing the cherry red appearance of the fovea and opaque edematous macula. Arteries are significantly narrowed, and there is some disc swelling
IOP was 6 mmHg (he was on topical ocular hypotensive; G. Alphagan and G. Cosopt). He had macula optical coherence tomography (OCT), OCT angiography (OCTA), and fundus fluorescein angiography (FFA) which were all consistent with a diagnosis of right eye CRAO. Erythrocyte sedimentation rate (ESR) was normal, suggesting a nonarteritic form of CRAO. All blood works for thrombophilia were also normal.
Three days after this presentation (which was 9 days after he suffered the CRAO), he consented to and had right eye vitrectomy for CRAO. The surgical technique was as described earlier in this report. He was monitored postoperatively to determine the change in Snellen visual acuity, retinal microstructure using OCT, and retinal perfusion using the OCTA.
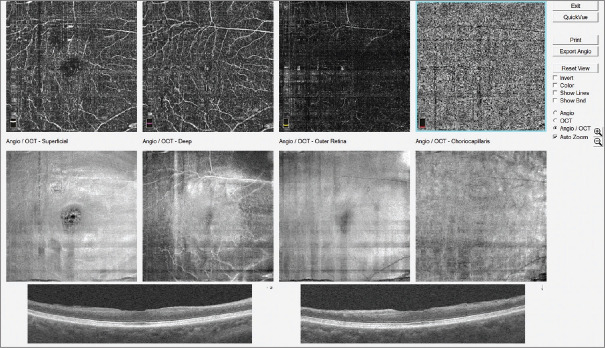
At 6 months postsurgery, his vision was stable at 6/60; OCT showed significant thinning of the inner retina, but relative preservation of the outer retina. There was postoperative superficial and inner retina vascular perfusion on OCTA [Figure 2]; however, the foveal avascular zone (FAZ) was significantly enlarged. Optic disc pallor was also present.
Figure 2.
Optical coherence tomography angiography showing significant blood flow in the deep capillary plexus, but an enlarged and irregular foveal avascular zone
Case 2
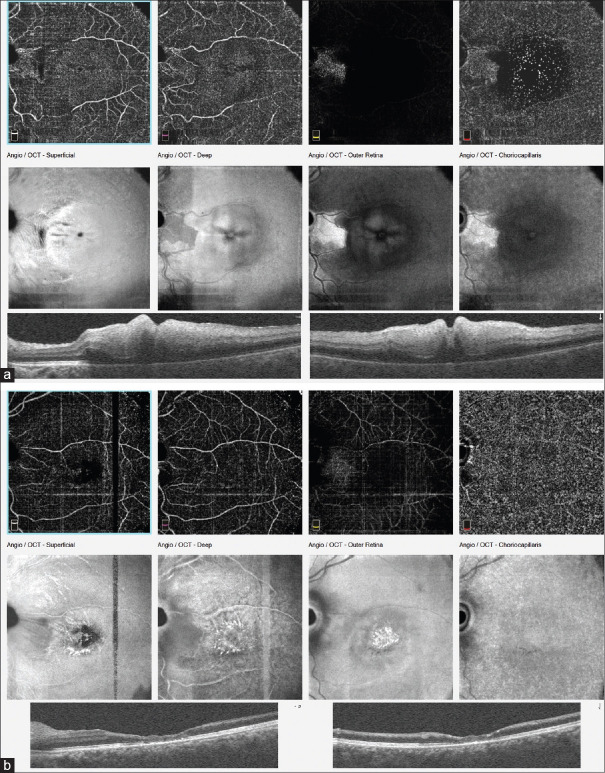
A 29-year-old male presented with sudden loss of vision in the left eye of some hours duration after engaging in vigorous physical activity (exercise) and profuse sweating. He had no contributory or relevant past medical or ocular history. His ocular examination was significant for decreased vision in the left eye to counting fingers and exaggerated pallor and severe edema of the macula, with a central cherry-red spot in the central fovea and narrowed retinal arterioles [Figure 3]. His IOP was 14 mmHg. FFA showed the absence of parafoveal vasculature and a very enlarged FAZ [Figure 4]. OCTA findings were similar to FFA. OCT revealed significant macular edema with disorganization of the entire macular microstructure, seen as hyperreflectivity without defined neurosensory layer or outer retinal layers [Figure 5a]. A diagnosis of left eye ischemic CRAO was made. ESR was normal, suggesting a nonarteritic form of CRAO. All blood works for thrombophilia was also normal.
Figure 3.
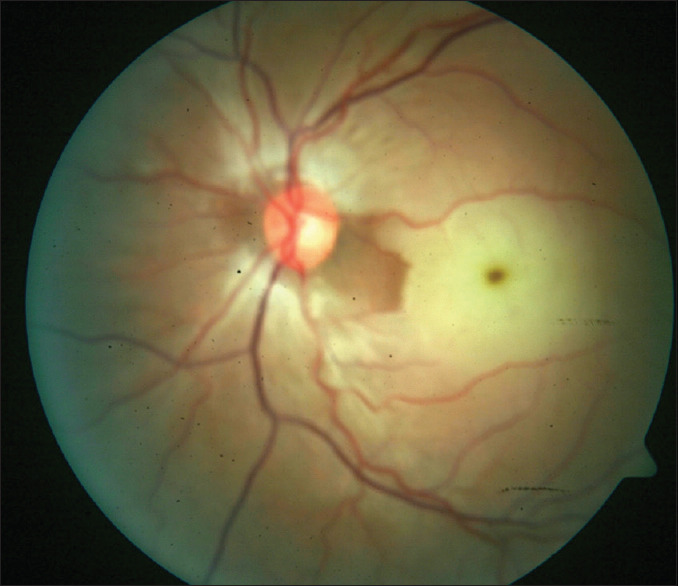
Fundus picture of the left eye with intensely pale and edematous macular and cherry red spot in the fovea. The nerve fiber layer surrounding the disc is also affected. Arteries are narrowed
Figure 4.
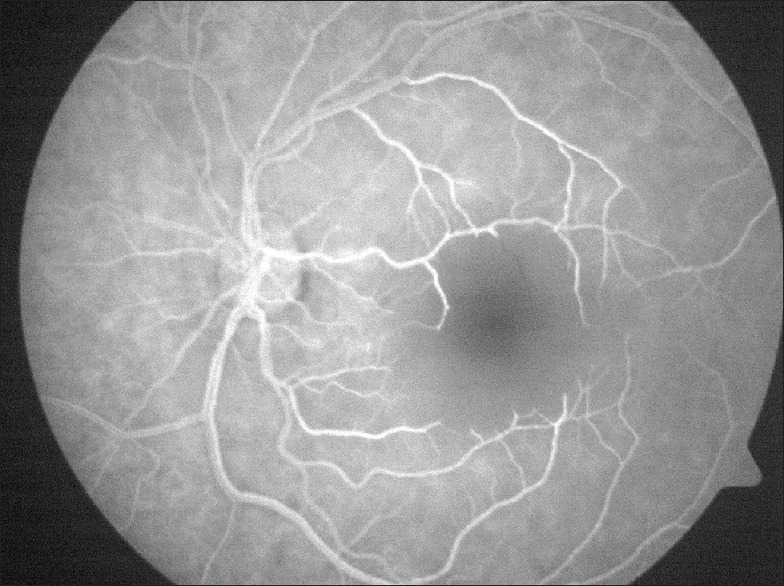
Fundus fluorescein angiography of patient B's left eye showing severe avascularity of the macular
Figure 5.
(a) Optical coherence tomography and optical coherence tomography angiography of the same patient, in the acute phase, with significant swelling of the macular and avascularity of the fovea. (b) Optical coherence tomography and optical coherence tomography angiography, 2 months after surgery with resolution of the macular edema and improved superficial and deep capillary vascular perfusion of the macular area. Foveal avascular zone remains abnormally enlarged and irregular
He gave an informed consent for the surgery. He had surgery as described above about 15 h after presentation. Postoperative monitoring was done to determine the change in Snellen visual acuity, retinal microstructure using OCT, and retinal perfusion using the OCTA.
At 2 months postsurgery, his vision was stable at 6/36+1, OCT showed severe thinning of the entire foveomacular area; there was an improved superficial and deep retina capillary vascular perfusion when compared to preoperatively on OCTA [Figure 5b]. FAZ was still abnormally enlarged.
Discussion
The young age of both patients in this report should be emphasized. Both patients were below the mean age for CRAO patients. Hayreh and Zimmerman reported age range in nonarteritic CRAO to be between 26.5 and 90.4 years. (Mean ± standard deviation [SD] = 67.7 years ± 12.3); 58% were males.[2] In our local experience, the mean age of RAO patients was 52.4 years (range; 31–60 years, SD = 9.0), 44.4% were males. Young age in CRAO has been well reported, some researchers reporting ages as low as 18 years while there are reports of CRAO even in infants.[6] The risk factors for nonarteritic CRAO in young patients below 40 years have been investigated by Greven et al. and found to be multifactorial.[7] They reported valvular defects as the most common finding. Our two patients underwent extensive cardiology workup including echocardiography and electrocardiography, and no valvular defects or heart defects were detected. Furthermore, screening for thrombophilia did not yield positive results. Greven demonstrated that various associated factors leading to a hypercoagulable state or embolic condition were identified in 91% of their cases. We hypothesize that in both patients an embolic phenomenon was the cause of acute obstruction to the CRA.
The site of embolic occlusion in CRAO has been thought to be at the narrowest lumen of the artery, which is where it pierces the dura mater of the optic nerve sheath before entering the optic nerve. Although embolic CRAO is likely most common at this point being the narrowest, however, there is evidence to support the notion that CRAO due to thrombus formation occurs at the level of the lamina cribrosa.[8] Dislodging and migration of emboli causing CRAO is well-recognized and has been demonstrated. Fibrinoplatelet emboli are known to be the most mobile, followed by the cholesterol (hollenhorst emboli), and the calcific emboli migrate least.[5] The migration of thrombus or embolus, to a more distal site, is the basis of the surgical technique we have described.
Other described vitrectomy techniques for treating RAO include retinal artery massage, CRA cannulation with microneedle, bloodletting, embolectomy, and regulation of intraocular hypotony.[9,10,11,12,13] Our surgical technique appears straightforward and can be undertaken by any vitreoretinal surgeon, requiring no additional surgical maneuvers or skill other than performing a standard removal of the vitreous. Although both patients opted for and had a general anesthesia, this procedure can be safely performed utilizing a local monitored anesthesia. There was a preoperative assessment and a medical clearance for general anesthesia by the medical team and anesthetist before the anesthesia was commenced as is the protocol of the hospital. It was determined that the anesthetic risk was no more than the usual for any other patient. The exaggerated anxiety state of the patients was another factor favoring a general anesthesia.
Nadal et al. reported a case of CRAO in a 65-year-old male and described a technique for the treatment of CRAO in which intraocular control of hypotony was used to induce the distal migration of thromboemboli with complete return of vision.[14] In their case, a more localized IOP -lowering approach was achieved after vitrectomy using a localized vacuum application. The team clearly demonstrated the visible migration of the emboli. In our 2 cases, emboli migration was not observed. The migration of nonvisible emboli has been well reported with the restoration of retinal perfusion.[14] The perfusion blood pressure, in the CRA is equal to the mean BP in the artery minus the IOP. Manipulation of these two pressures can result in a considerable increase in perfusion pressure within the CRA. Therefore, lowering the IOP would increase CRA perfusion and cause a dislodgment and migration of emboli. Nadal et al. lowered the IOP by the use of localized vacuum around the artery (vacuum was created using the cutter, which was placed closed to the artery and visible emboli). In our technique, the global ocular IOP was reduced first by reducing the vitrectomy machine compensated infusion to 3 mmHg, and then, eventually stopping the infusion entirely. The other difference in our technique is the further facilitation of retinal perfusion by momentary gradual elevation of the arterial BP to a level judged to be safe for the patient. This among other things would have the advantage of increased perfusion and blood flow at the more posterior narrowest point of the CRA (point of penetration through the optic nerve dura) and dislodge impacted emboli at this point. An increase in arterial BP was performed in our patients since they were relatively young and had no demonstrable, or known cardiac risks. Greater caution in systemic arterial BP elevation would be warranted in older patients. We advocate that a risk assessment be performed before arterial BP elevation.
CRAO is an ocular emergency with devastating consequence for vision. The hope of reversal of an occlusion and return of vision depends largely on the time between the onset of the CRAO and initiation of treatment. Experimental models have demonstrated that the retina can withstand 97 min of ischemia, but irreversible damage occurs after 4 h.[4] In our local experience, 86% of CRAO eyes were blind without treatment; vision ranged from hand motion to no perception of light. Therefore, our natural history experience of eyes from our previous local observation was the basis for surgical intervention in our 2 most recent CRAO patients. Both patients had a subjective feeling of visual benefit from the surgery, despite the occurrence of irreversible ischemia. Patient A recovered 6/60 after 1-month postoperative and patient B regained 6/36 after 2 months. This recovered vision was maintained. The very severe degree of macular edema and macular infarction seen in patient B [Figure 3] would have limited the outcome since interstitial tissue pressure has been shown to be a significant factor in foveal perfusion postocclusion. The mechanism of their visual gain is hypothesized to be the use of the perifoveal fixation since the protracted foveal ischemia would render the ultrastructure of the fovea incapable of normal function. However, we theorize that the dislodgment of the emboli and subsequent intraoperative perfusion of the retina could facilitate collateral retinal perfusion that improves perifoveal retina function.
Autoregulation is known to be active in the retina. This raises the following questions; can increasing BP cause further vasoconstriction of retinal vessels because of autoregulation and will intravenous adrenaline use for raising arterial pressure also cause vessel constriction; and could the vessel constriction further deteriorate the CRA obstruction? These were carefully considered. It was determined that the total period of intraarterial pressure rise by the use of intravenous adrenaline (which was approximately 15–20 min.) was too short to have a significant effect on the retinal arterial constriction. More so, the increased blood flow which occurred intraoperatively was observed to have a mechanical vasodilatory effect, the aim of which was to dislodge the impacted emboli. Afterward, the intra-arterial BP was reduced to the normal pre anesthesia levels, and so any triggered compensatory autoregulatory mechanisms would be abolished. Furthermore, it has been established that autoregulation works only within a critical range of perfusion pressure; therefore, autoregulation breaks down with any rise or fall of the perfusion pressure beyond the critical autoregulatory range. We believe the perfusion pressure we achieved likely exceeded the autoregulatory range.
The exact site of autoregulation is still being debated. Perhaps, this might be at the level of the terminal arterioles, and the pericytes of the capillaries may also play a role in retina autoregulation. These terminal arterioles would be affected by the ischemia arising from the CRAO and may not respond as expected. Consequently, in the setting of a CRAO, the retinal vascular response to autoregulation is abnormal because of acute ischemia and may not respond to the increased arterial pressure as predicted.
Finally, it is also known that the metabolic needs of the retina drive autoregulation and this may play an important role in the setting of post-CRAO ischemia; which may create a metabolic drive for increased blood flow to the retina.
Retinal ischemic reperfusion injury (IRI) after CRAO was first reported by Saxena et al. and is thought to be responsible for acute damage and cell death; occurring at about 7 days after the CRAO in their report.[15] This manifest as a decrease in retinal nerve fiber layer and macular thickness which was documented using OCT. Similar reperfusion injury has been reported following reperfusion in cases of myocardial infarction and acute cerebral strokes. It is likely that IRI will play an important role following the reperfusion of the retina in the technique we have described and may consequently limit the visual recovery. We, therefore, advice a more gradual reperfusion as has been advocated by Xu et al. to limit the occurrence of IRI.[16] They suggest that gradual flow reperfusion is superior to more rapid flow restoration in alleviating cerebral IRIs in the experimental rats. This is an important area of future research for this promising technique.
There are few reports on the visual improvement after CRAO. A previous study showed that CRAO patients presenting with initial poor vision had a tendency to have final poor vision despite treatment, after 3 months of follow-up.[17] On the other hand, Hayreh and Zimmerman reported that 22% of nonarteritic CRAO patients improved.[2] They also noted that visual acuity improvement essentially occurs during the first 7 days, with minimal chance of any appreciable improvement thereafter.
It is possible that the level of improvement after CRAO in younger patients (<40 years) could be different from the adult population considering that they may have more resilient cardiovascular system and retina vascular network. There is a scarcity of literature on the visual outcome of CRAO in patients <40 years. However, Greven et al. reported on RAO in patients <40 years.[7] In their report, four out of 5 CRAO eyes presented with a vision of no light perception, whereas one eye had a vision of counting fingers (CF). Of this number, only one eye (20%) had an improved vision. Greven's finding of 20% improvement in visual acuity is similar to Hayreh's 22%, inclusive of adult eyes. This suggests that the rates of improvement in vision after a CRAO are similar for both young and adult population and show no age discrimination. We report an improvement in the two consecutive eyes that had this surgical intervention.
In our opinion, whether the visual gain in these two consecutive patients is as a result of surgical intervention or natural history is open to debate. However, the procedure clearly greatly improves intraoperative retinal perfusion and increases the chances of thromboemboli dislodgment and has a potential for reperfusion and return of vision if performed before irreversible retina ischemia. It deserves listing among the surgical techniques for the treatment of acute nonarteritic CRAO.
Conclusion
Efforts to significantly improve the visual outcome after CRAO has occurred could yield results following the investigation of surgical treatments. The technique we describe has not been previously described; it utilizes a dual approach of creating global ocular hypotony and increasing CRA blood perfusion through the increase of systemic arterial BP. It therefore greatly facilitates perfusion along the entire length of the CRA and has the potential for dislodging the thromboemboli at any point along the CRA responsible for its occlusion. It is likely that this procedure will significantly reverse the loss in vision if performed before the onset of irreversible ischemia, in a number of eyes. The mechanism of the modest improvement in vision, even after irreversible retina damage has occurred, as seen in our two consecutive patients deserves further investigation. Discussion on IRI would greatly benefit this technique.
Declaration of patient consent
The authors certify that they have obtained all appropriate patients consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors declare that there are no conflicts of interests of this article.
References
- 1.Beatty S, Au Eong KG. Acute occlusion of the retinal arteries: Current concepts and recent advances in diagnosis and management. J Accid Emerg Med. 2000;17:324–9. doi: 10.1136/emj.17.5.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayreh SS, Zimmerman MB. Central retinal artery occlusion: Visual outcome. Am J Ophthalmol. 2005;140:376–91. doi: 10.1016/j.ajo.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 3.Atebara NH, Brown GC, Cater J. Efficacy of anterior chamber paracentesis and Carbogen in treating acute nonarteritic central retinal artery occlusion. Ophthalmology. 1995;102:2029–34. doi: 10.1016/s0161-6420(95)30758-0. [DOI] [PubMed] [Google Scholar]
- 4.Hayreh SS, Zimmerman MB, Kimura A, Sanon A. Central retinal artery occlusion.Retinal survival time. Exp Eye Res. 2004;78:723–36. doi: 10.1016/s0014-4835(03)00214-8. [DOI] [PubMed] [Google Scholar]
- 5.Cho KH, Ahn SJ, Cho JH, Jung C, Han MK, Park SJ, et al. The characteristics of retinal emboli and its association with vascular reperfusion in retinal artery occlusion. Invest Ophthalmol Vis Sci. 2016;57:4589–98. doi: 10.1167/iovs.16-19887. [DOI] [PubMed] [Google Scholar]
- 6.Hayreh SS. Acute retinal arterial occlusive disorders. Prog Retin Eye Res. 2011;30:359–94. doi: 10.1016/j.preteyeres.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greven CM, Slusher MM, Weaver RG. Retinal arterial occlusions in young adults. Am J Ophthalmol. 1995;120:776–83. doi: 10.1016/s0002-9394(14)72731-x. [DOI] [PubMed] [Google Scholar]
- 8.Satoh H, Maruyama-Inoue M, Sato S, Yamane S, Kadonosono K. Thrombus in lamina cribrosa seen using swept-source optical coherence tomography in eye with central retinal artery occlusion. Retina. 2018;38:e26–7. doi: 10.1097/IAE.0000000000002030. [DOI] [PubMed] [Google Scholar]
- 9.Lu N, Wang NL, Wang GL, Li XW, Wang Y. Vitreous surgery with direct central retinal artery massage for central retinal artery occlusion. Eye (Lond) 2009;23:867–72. doi: 10.1038/eye.2008.126. [DOI] [PubMed] [Google Scholar]
- 10.Kadonosono K, Yamane S, Inoue M, Yamakawa T, Uchio E. Intra-retinal arterial cannulation using a microneedle for central retinal artery occlusion. Sci Rep. 2018;8:1360. doi: 10.1038/s41598-018-19747-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin CJ, Su CW, Chen HS, Chen WL, Lin JM, Tsai YY. Rescue vitrectomy with blocked artery massage and bloodletting for branch retinal artery occlusion. Indian J Ophthalmol. 2017;65:323–5. doi: 10.4103/ijo.IJO_698_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almeida DR, Mammo Z, Chin EK, Mahajan VB. Surgical embolectomy for fovea-threatening acute retinal artery occlusion. Retin Cases Brief Rep. 2016;10:331–3. doi: 10.1097/ICB.0000000000000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen X, Yuan M, Li C, Zeng J, Duan F, Lou B, et al. Effect of vitrectomy with intrasurgical regulation of intraocular pressure in a rabbit model of central retinal artery occlusion. Exp Eye Res. 2019;189:107779. doi: 10.1016/j.exer.2019.107779. [DOI] [PubMed] [Google Scholar]
- 14.Nadal J, Ding Wu A, Canut M. Vitrectomy with intrasurgical control of ocular hypotony as a treatment for central retina artery occlusion. Retina. 2015;35:1704–5. doi: 10.1097/IAE.0000000000000702. [DOI] [PubMed] [Google Scholar]
- 15.Saxena S, Mishra N, Meyer CH, Akduman L. Ischaemia-reperfusion injury in central retinal artery occlusion. BMJ Case Rep 2013. 2013:201415. doi: 10.1136/bcr-2013-201415. doi: org/10.1136/bcr-2013-20141524145508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu WW, Zhang YY, Su J, Liu AF, Wang K, Li C, et al. Ischemia reperfusion injury after gradual versus rapid flow restoration for middle cerebral artery occlusion rats. Sci Rep. 2018;8:1638. doi: 10.1038/s41598-018-20095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuzurihara D, Iijima H. Visual outcome in central retinal and branch retinal artery occlusion. Jpn J Ophthalmol. 2004;48:490–2. doi: 10.1007/s10384-004-0102-y. [DOI] [PubMed] [Google Scholar]