Abstract
Background & Aims:
Recent literature has implicated a key role for mast cells in murine models of colonic inflammation, but their role in human ulcerative colitis (UC) is not well-established. A major advance has been the identification of mrgprb2 (human orthologue, MRGPX2) as mediating IgE-independent mast cell activation. We sought to define mechanisms of mast cell activation and MRGPRX2 in human UC.
Methods:
Colon tissues were collected from UC patients for bulk RNAseq and lamina propria cells were isolated for MRGPRX2 activation studies and single-cell RNA sequencing (scRNAseq). Genetic association of all protein altering GPCR SNPs was performed in an Ashkenazi Jewish UC case-control cohort. Variants of MRGPRX2 were transfected into CHO and HMC-1.1 cells to detect genotype-dependent effects on β-arrestin recruitment, IP-1 accumulation, and phosphoERK.
Results:
Mast cell-specific mediators and ADM (adrenomedullin, proteolytic precursor of PAMP-12, an MRGPRX2 agonist) are upregulated in inflamed compared to uninflamed UC. MRGPRX2 stimulation induces carboxypeptidase secretion from inflamed UC. Of all protein-altering GPCR alleles, a unique variant of MRGPRX2, Asn62Ser, was most associated, bioinformatically predicted to alter arrestin recruitment. We validated that the UC protective serine allele enhances beta-arrestin recruitment, decreases IP-1, and increases phosphoERK with MRGPRX2 agonists. scRNASeq defines that ADM is expressed by activated fibroblasts and epithelial cells, and that IFNG is a key, upstream regulator of mast cell gene expression.
Conclusion:
Inflamed UC regions are distinguished by MRGPRX2-mediated activation of mast cells, with decreased activation observed with a UC-protective genetic variant. These results define cell modules of UC activation and a new therapeutic target.
Keywords: ulcerative colitis, mast cells, G-protein coupled receptors, Single Cell Sequencing
Short summary:
Inflamed ulcerative colitis regions demonstrate MRGPRX2-mediated degranulation of activated mast cells, implicating a positive feedback inflammatory loop compared to uninflamed colon. The identification and functional validation of a loss-of-function, protective serine allele in MRGPRX2 defines a new therapeutic target for ulcerative colitis.
Lay Summary:
Inflamed ulcerative colitis regions demonstrate G-protein coupled receptor (GPCR)-mediated degranulation of mast cells. The identification of a loss-of-function, protective GPCR allele defines a new therapeutic target for ulcerative colitis.
Introduction
Upon activation, mast cells can release a variety of proinflammatory mediators of both acute and chronic effects. Such mediators include histamine, tryptase, and carboxypeptidase, which induce acute allergic responses and chronic inflammation through autocrine and paracrine pathways1. Targeting mast cell mediators has been highly effective in allergic and IgE-mediated diseases, including asthma. Beyond anti-histamines, approaches to block tryptase activity have been recently proposed to target selected asthma patients2.
While a growing literature is implicating mast cells in murine models of colitis3,4, its role in human ulcerative colitis (UC) is not well established5. Conflicting data exist as to whether mast cell numbers are increased6 or decreased7 in inflamed UC. The under-studied status of mast cells in IBD may result in part from the relative difficutly of isolating mast cells, especially activated ones, which are more prone to rapid cell death resulting from release of proteolytic enzymes. A major advance in the biology of mast cells was the identification of an essential role for mrgprb2 in murine models of injection site, pseudo-allergic drug reactions8. IgE-independent activation of mast cells via mrgprb2 (or its human ortholog, MRGPRX2) also mediates bacterial quorum-sensing, via activation by CSP-1 (colony-stimulating peptide)9. A variety of both host and microbial peptide triggers have been reported to activate MRGPRX2 and other G-protein coupled receptors (GPCRs), with the bioactive agent often resulting from proteolytic cleavage of inactive precursors.
GPCRs disproportionately contribute as targets for present medical therapies10. This reflects the broad range of biologic processes for which GPCRs play an essential role, combined with a well-studied biology, including ligand-receptor mappings and receptor desensitization via arrestin recruitment. Cell-cell communications mediated via GPCR ligand-receptor pairings are finely-tuned through ligand concentration gradients, and by highly variable activities to endogenous ligands11. Accelerated development of specific and high affinity synthetic ligands has recently been achieved by leveraging sophisticated and comprehensive 3D structure information12. These high affinity ligands provide powerful probes to more precisely delineate GPCR functions.
In this study, we explored the role of mast cells and MRGPRX2 in human UC through direct ex-vivo, bulk RNASeq expression, genetic and single-cell RNASeq (scRNASeq) studies. The superficial, continuous and often sharply demarcated distribution of injection site drug reactions8 is reminiscent of the frequent demarcation of inflammation observed in UC; we therefore compared acutely inflamed and uninflamed UC tissue to establish MRGPRX2 specific activation solely in inflamed tissues. Among protein-altering alleles in GPCRs, we observe the most significant association to an Asn62Ser allele in MRGPRX2 in an Ashkenazi Jewish UC exome chip case-control cohort, with the minor serine allele predicted to protect from UC. Prior studies have reported that MRGPRX2 stimulation by LL-37 does not result in internalization of MRGPRX213. By applying sequence motif patterns predicting arrestin recruitment gleaned from crystallography studies of high affinity GPCR-ligand binding14, we predicted that the minor, serine allele in MRGPRX2 would result in a gain of arrestin binding relative to the asparagine allele. We validated this prediction across a variety of MRGPRX2 agonists using arrestin recruitment and IP-1 (inositol monophosphate) accumulation assays. Both bulk RNASeq and scRNASeq implicate a key role for ADM (adrenomedullin) and its proteolytic product, PAMP-12, in perpetuating UC inflammation. UC is characterized by a failure to downregulate inflammation in response to incompletely defined triggers; the present study defines a module of different cells types that together may converge on the perpetuation of mast cell activation, defining a novel therapeutic candidate for the treatment of UC.
Materials and Methods
Bulk RNAseq analysis
The raw counts from 401 UC bulk mRNASeq samples in the Mount Sinai Crohn’s and Colitis Registry were obtained after mapping15. Removal of low expression transcripts, normalization and differential expression was performed using DESeq2 with criteria of adjusted P < 0.05 and |Fold change|> 1.41. For mast cell-specific tryptases, histamine, and carboxypeptidase (Figure 1A), the data consisted of 312 (144 inflamed and 168 uninflamed) rectums and 89 (48 inflamed and 41 uninflamed) sigmoids. For ADM expression (Figure 1B), the data consisted of paired inflamed and non-inflamed tissues of 163 rectum (75 inflamed 88 uninflamed) and 89 (48 inflamed and 41 uninflamed) sigmoids.
Figure 1. Mast cell activation in inflamed and uninflamed UC tissues and genetic associations implicate MRGPRX2 and altered arrestin recruitment.
(A-B) Analysis of 401 UC bulk mRNASeq samples from the Mount Sinai Crohn’s and Colitis Registry15 (A) Bulk mRNA analysis shows upregulation of mast cell-specific tryptases, histamine, and carboxypeptidase in non-inflamed and inflamed sigmoid and rectum, respectively. (B) The carboxypeptidase enzyme-substrate, ADM, is more highly expressed in inflamed compared to uninflamed UC. The UC non-inflamed sigmoid and inflamed rectum comparisons reflect paired samples from the same patients. (C) Carboxypeptidase secretion from immune cells isolated from three, paired inflamed and uninflamed UC tissues. MRGPRX2 specific ligand (R)-ZINC3573 stimulation was performed for 90 minutes, and supernatants were analyzed with CPA3 ELISA. One, two, and three asterisks correspond to p-values less than 0.05, 0.01, and 0.001, respectively. (D) Quantile-quantile association plot of protein-altering GPCR alleles from Ashkenazi Jewish UC patients from an exome chip case-control cohort16,17. (E) Model for ADM-CPA3-PAMP12-MRGPRX2 positive feedback inflammatory loop in inflamed colon. (F) Sequence motifs predicted to alter arrestin recruitment, with the 62Ser allele predicted to result in a gain of arrestin recruitment.
Human specimens for scRNASeq and ELISA studies
Patients were identified by screening surgical programs at Mount Sinai Hospital. Protocols were reviewed and approved by the Institutional Review Board (IRB) at the Icahn School of Medicine at Mount Sinai (HSM#14–00210). The demographic and clinical information of patients is shown in Table S1. All tissues included in the study were confirmed by pathological examination as active colitis.
GPCR UC association
Association testing was conducted in AJ ancestry samples for 559 UC cases, and 2614 controls genotyped using the exome chip as in Chuang16 and Hui17. The association results were filtered for human GPCR genes, and the p-values were plotted. The replication cohort was comprised of 685 independent AJ UC cases and 4,746 AJ controls.
Lamina propria single-cell suspensions
Biopsies were obtained from both the inflamed and non-inflamed regions and stored in RPMI-1640 (Corning), followed by incubation in 1mL dissociation medium (HBSS w/o Ca2+ Mg2+ with HEPES 10mM and EDTA 5mM (Life Technologies)) at room temperature for 30 minutes. Following by two washes in RPMI, biopsies were digested in digestion medium (HBSS with Ca2+ Mg2+, FBS 2%, DNase I 0.5mg/mL (Sigma-Aldrich) and Collagenase IV 0.5mg/mL (Sigma-Aldrich)) for 40 min at 37°C under 100 rpm agitation.
CPA3 ELISA
The lamina propria cells were seeded in 5000 cells per 20ul for each treatment. Cells were incubated with (R)-Zinc3573 at a final concentration of 120uM for 90 minutes at 37C. ELISAs were performed with LifeSpan BioSciences Human-CPA3 Elisa Kit with optical density readout at 450nm.
Flow cytometry and fluorescent activated cell sorting (FACS)
Cells were pelleted and washed in FACS buffer with 2% FBS in DPBS (Gibco). Cells were stained with PE anti-human MRGX2 Antibody (359003; BioLegend) or PE Mouse IgG2b k Isotype Control (400314; BioLegend) at a concentration of 1.25 ul of antibody per 1 million cells in a final volume of 100 ul for 20 minutes on ice and wash twice. Cells were resuspended in 0.1% FBS in DPBS with a 1:1000 dilution of Zombie Aqua Fixable Viability stain (423101; BioLegend) and sorted by BD FACSAria III (BD Biosciences).
β-Arrestin recruitment assay
Both Asn62 and 62Ser variants were synthesized and subcloned into pCMV-ProLink1 by Invitrogen GeneArt Gene Synthesis. PathHunter CHO-K1 β-Arrestin2-EA cells (DiscoverX) were electroporated with Amaxa_SF_Cell_Line 4D-Nuclefector Kit (Lonza) to introduce Asn62, 62Ser, and GFP positive transfection control. The media was supplemented with 800μg/mL G418 and renewed every 2 days for 14 days. Transfected cells were sorted by flow cytometry for high expressing MRGPRX2 cells using PE anti-human MRGX2 Antibody. 5,000 cells per 20μL of CHO-Asn62 and CHO-62Ser were seeded in quadruplicates into each experimental well of a 384-well opaque bottom, white plate and incubated overnight. Cells were treated with ligands and vehicle, and incubated for 90 minutes. 12.5μL of detection reagent (DiscoverX) was added and incubated at room temperature for 1 hour in the dark. Luminescence was read using EnVision Multimode Plate Reader (PerkinElmer). Each condition was repeated in triplicate.
IP-One Gq bioassay
We subcloned Asn62 and 62Ser plasmids from Gateway pDONR vectors into pEZYmyc-His. pEZYmyc-His was a gift from Yu-Zhu Zhang (Addgene plasmid #18701). CHO cells were cultured, expanded, and electroporated with Amaxa Kit. The transfectants were maintained in Ham’s F12-K Medium (Gibco) and 10% FBS for 48 hours. Growth media was supplemented with 900μg/mL G418 for selection and renewed every 2 days for 10 days. Transfected cells were sorted by flow cytometry. Experiments were conducted using IP-One_Gq Kit (Cisbio). After optimization, 40,000 cells per 20μL of CHO cells were seeded and treated as described in β-Arrestin assay. After 16 hours, 20μL media was removed and replaced with 7μL of fresh growth media. Each experimental well was treated with 7μL of 2X agonist for 2 hours. Fluorescence was read using EnVision Multimode Plate Reader (PerkinElmer).
HMC cell culture, transfection, selection and stimulation
HMC-1.1 (Sigma-Aldrich) were grown in Iscove’s Dulbecco’s Modified Medium (Gibco) supplemented with 10% FBS (Sigma), 1.2mM α-thioglycerol (Sigma), and either 1x penicillin/streptomycin for untransfected HMCs or 900 ug/ml Geneticin (Gibco) for MRGPRX2 transfected HMCs. Cells were transfected with the Amaxa_SE_Cell_Line 4D-Nucleofector X Kit L (Lonza) and grew at 900 ug/ml G418 for 14 days. Cells were sorted by flow cytometry. After expansion, 200,000 cells per well were stimulated at 37°C with PAMP-12, (R)-Zinc3573, and their respective vehicles. Cells were stimulated for 0.5 minutes, 1 minute, or 5 minutes and immediately pelleted and lysed using RIPA Lysis Buffer (Millipore) with added Halt Protease and Phosphatase Inhibitor Cocktail (Life Technologies), and placed on 4°C rocker for 30 minutes.
Immunoblotting
2–5 ug of protein samples obtained from HMC-1.1 stimulation were mixed with 4X sample buffer (Bio-Rad) containing 10% beta-mercaptoethanol (American Bioanylitical). The proteins were separated on a 4–15% Mini-PROTEAN TGX (BioRad) and transferred to a polyvinylidene difluoride membrane using Trans-Blot Turbo (Bio-Rad), and blocked in 5% bovine serum albumin in 1x tris buffered saline with 0.1% Tween20 (BioRad & Sigma-Aldrich). The blot was then probed with the following primary antibodies: 1:1000 p44/42 MAPK (ERK1/2) (4695S; Cell Signaling) or 1:1500 Phoshpo-p44/42 MAPK (pERK1/2) (9101; Cell Signaling) and rocked overnight at 4°C. A secondary incubation of a 1:5000 and 1:20,000 dilution of horseradish-peroxidase conjugated Goat anti-Rabbit IgG (31462; Pierce). Bound antibody was detected using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific). Blots were stripped using Restore PLUS Western Blot Stripping Buffer (Thermo Scientific) and re-probed with 1:1000 dilution of α/β - Tubulin (2148S; Cell Signaling). Following overnight rocking at 4°C, we performed a secondary incubation of a 1:5000 diluted antibody. Band intensity was quantified using ImageJ and mathematically normalized to α/β tubulin. The normalized values were divided by pERK/ERK and converted relative to the highest raw data value.
Taqman genotyping
The total DNA from cells was extracted using QIAamp DNA Mini Kit (Qiagen). Taqman_Custom SNP-Genotyping Assay with Taqman Genotyping Master Mix (Life Technologies) was used to confirm genotypes. Plasmids were sequence confirmed via Sanger sequencing (GeneWiz). Primer sequences are listed in Table S2
Immunohistochemistry (IHC)
A Discovery ULTRA Staining Module (Roche Diagnostics) was used with RUO DISCOVERY Multimer_V2 following the manufacture’s protocol. In short, we performed antigen retrieval by steaming slides in 0.01 M sodium citrate buffer at 100°C for 20 minutes. Incubating slides for 20 minutes and rinsed in 1x TBST for 1 minute at room temperature. Anti-MRGPRX2 or anti-CPA3 primary antibody (Sigma) was applied at 1:350 or 1:225 dilution, respectively, and anti-Mast Cell Tryptase (Fisher Scientific) at 1:2000 dilution for 60 minutes. We blocked with 2% BSA in PBS for 32 minutes. For anti-MRGPRX2 and CPA3, we applied secondary antibody anti-Rabbit HQ, amplified with anti-HQ-HRP and detected using Discovery Purple Kit (RUO). For anti-mast cell tryptase, we applied secondary antibody OmniMap-DAB anti-Mouse HRP and detected using Discovery ChromoMap DAB Kit.
The scRNAseq and analysis
Single cells were processed through the 10X Chromium Single-Cell Platform using the Chromium Single-Cell 3′ Library, Gel_Bead_Kit_v2 and the Single-Cell A Chip Kit (10X Genomics). Libraries were sequenced on Illumina NextSeq 500. We analyzed paired inflamed rectum (n = 4) and uninflamed sigmoid (n = 5) from 4 UC patients. Sequences were aligned to the GRCh38 using the Cell Ranger v.2.1.0 (10X). We excluded genes expressed in fewer than three cells, cells that expressed fewer than 500 genes, and unique molecular identifier (UMI) count less than 500 or greater than 60k. Per cell, we normalized by dividing UMI per gene by total UMI and log-transforming. We used Seurat (Seurat_R_package_v.3.0.1) integrated model to generate a combined UC model with cells from both inflamed and uninflamed samples retaining their group identity. We used shared nearest neighbor graph-based clustering with 15 principal components. UMAP visualizations, violin plots were produced using Seurat318 functions in conjunction with ggplot2. We conducted DEG in mast cell cluster (cluster 15) using FindMarkers function in Seurat, which is based on Wilcoxon test.
Upstream regulator analysis
The analysis was performed on the list of the significant differentially expressed gene between inflamed and uninflamed cells within the Mast cell cluster with p-value <0.005 using Ingenuity Pathway Analysis software (Ingenuity Systems)19. The results were visualized using gplots package in R with the average expression across each of the clusters in the combined UC integrated dataset.
LAD2 cell culture and stimulation
LAD2 cells were maintained in StemPro-34 serum-free media (Invitrogen) supplemented with penicillin (100 IU/ml), streptomycin (100 μg/ml), L-glutamine (2 mM), and recombinant human stem cell factor (100 ng/ml, Sigma). 3,000,000 cells per well with 3 replications were treated with 20 ng/mL TNFα, IFNγ, or IL-13 (all from R&D systems). After 4 hours of cytokine incubation, cells were pelleted and lysed for qPCR.
Real-time quantitative PCR
RNA was isolated using Trizol-LS (Invitrogen) and cDNA was synthesized with SuperScript III First-Strand Synthesis SuperMix (Invitrogen). qPCRs were prepared with SYBR Green PCR MasterMix (Applied Biosystems). All reactions were run for 40 cycles in duplicates and analyzed using the ViiA 7 (Applied Biosystems). Gene expression was normalized to 18S and fold changes were calculated relative to untreated controls. Primer sequences for IRF1 (interferon regulatory factor 1), PIM1 (proto-oncogene, serine/threonine kinase) and PSMB8 (proteasome 20S subunit beta 8) are listed in Table S2.
Results
Mast cell mediators and activators are transcriptionally induced in inflamed UC compared to uninflamed regions.
During inflammation, mast cells degranulate and release mediators such as histamine, tryptase, and carboxypeptidase in response to activating signals. We first sought to determine whether transcriptional induction of these mast cell predominant, abundant mediators was also present. We re-analyzed 401 UC bulk mRNASeq samples from the Mount Sinai Crohn’s and Colitis Registry (MSCCR)15. For tryptase alpha/beta 1 (TPSAB1), tryptase beta 2 (TPSB2), histidine decarboxylase (HDC), and carboxypeptidase 3 (CPA3) highly significant induction of mRNA levels (P-values < 0.001) were present in inflamed tissues comparing to uninflamed colon (Figure 1A). We next analyzed whether upstream, mast cell activators are also transcriptionally induced in inflamed colon. We tested for induction of IGHE (immunoglobulin heavy constant epsilon) or proteolytic precursors to MRGPRX2 activation were induced. Specifically, ADM (adrenomedullin, cleaved to PAMP1220), CAMP (cathelicidin antimicrobial peptide, hydrolyzed to produce LL-3721), and TAC1 (tachykinin precursor 1, proteolytic precursor to substance P22) were compared. Of these upstream activators of mast cells, no significant transcriptional induction was present in inflamed compared to uninflamed tissues, except for with ADM.
Comparing across patients sampled at the same location, we observed substantially higher ADM expression in inflamed compared to uninflamed colonic regions (adjusted p-values of 1.0 × 10−3 and 2.92 × 10−3, for the rectum and sigmoid, respectively) (Figure 1B). Within the same patients, paired analyses comparing inflamed rectum and uninflamed sigmoid, we observed significantly higher ADM expression in the inflamed rectum (adjusted p-value = 2.07 × 10−4). Finally, in healthy controls, we observed no significant difference between rectal and sigmoid expression of ADM, even though rectal expression trended higher. ADM is an enzyme-substrate of CPA320, so we next sought to test whether MRGPRX2 agnonism might be different in inflamed compared to uninflamed UC.
MRGPRX2-mediated induction of CPA3 observed in inflamed with UC regions, with nominal genetic association observed at a unique, protein-altering allele.
MRGPRX2 is a member of the Mas-related GPR gene family. A synthetic ligand, (R)-ZINC3573, was found to be specific to MRGPRX2 across a number of GPCRs by PRESTO-Tango and by testing the parent scaffold using a kinome screen23. We sampled lamina propria mononuclear cells attained from inflamed compared to uninflamed UC biopsies (Table S1; ID:1–3). In all patients analyzed, we observed substantially greater CPA3 secretion with (R)-ZINC3573 stimulation (Figure 1C), indicating that differential mast cell activation is one feature that distinguishes inflamed from uninflamed UC.
Analyses of all human GPCR protein-altering SNPs from our exome chip data of Ashkenazi Jewish IBD16,17 demonstrated that the Asn62Ser (rs10833049) allele of MRGPRX2 was the most associated protein-altering allele in UC (Figure 1D, P-value = 3.71× 10−5), with increased association evidence observed compared to the expected distribution by quantile-quantile plot. We saw similar trends (P-value = 0.019) in an independent, Jewish UC case-control cohort. Carboxypeptidase has been reported to proteolyze ADM to various endogenous peptide products, including PAMP-1220; a positive feedback loop between a) activated mast cells and b) increased production of ADM and PAMP-12, can be postulated (Figure 1E). Phosphorylation of serine or threonine residues of intracellular residues of GPCRs can alter arrestin recruitment and the precise amino acid and spacing features that modulate arrestin recruitment have been recently defined through crystallography14. In previous MRGPRX2 stimulation studies with LL-37, an antimicrobial peptide, had not demonstrated any evidence for receptor desensitization13.
The more common and ancestral asparagine allele at codon 62 would not be predicted to recruit beta-arrestin; however, the serine allele is predicted to result in a gain of a phosphorylation site, thereby enhancing beta-arrestin recruitment (Figure 1F). The presence of both highly potent and specific synthetic agonists, as well as a range of previously reported natural MRGPRX2 agonists13,23,24 provides a foundation upon which genotype-dependent differences in GPCR signaling can be tested.
The serine allele at Asn62Ser in MRGPRX2 increases arrestin recruitment compared to the ancestral asparagine allele.
Two main signaling pathways of MRGPRX2 are calcium release/IP1 for activation, and β-Arrestin recruitment for desensitization (Figure 2A). Besides down-regulating phospholipase C signaling, beta-arrestin recruitment to GPCRs activates MAP kinase pathways25. To test the desensitization ability of serine allele on MRGPRX2, we synthesized expression vectors of the two alleles tagged with a β-Gal ProLink Peptide Tag. Electroporation of Asn62 and 62Ser MRGPRX2 expression vectors was performed into CHO cells containing PathHunter β-Arrestin tagged to inactive beta-galactosidase. Following G418 selection for 14 days, FACS of the Asn62 and 62Ser MRGPRX2 stably expressing cells was performed to ensure comparable expression (Figure S1) of the receptor prior to their use in the β-Arrestin recruitment assay.
Figure 2. Dose-response stimulation with synthetic and natural MRGPRX2 ligands shows that the serine allele enhances beta-arrestin recruitment compared to the asparagine allele.
(A) Ligands and downstream signaling pathways of MRGPRX2. (B-C) pEC50 (B) and Emax (C) of MRGPRX2 ligands between Asn62 (red) and 62Ser (green) (D) Electroporation of Asn62 (red), 62Ser (green) MRGPRX2 or empty (blue) expression vectors with a β-Gal ProLink Peptide Tag was performed into CHO cells containing PathHunter β-Arrestin containing inactive beta-galactosidase. Beta-galactosidase luminescence reflects the recruitment of β-arrestin to MRGPRX2. (E) Difference in luminescence between serine and asparagine alleles. One, two, three and four asterisks correspond to p-values less than 0.05, 0.01, 0.001, and 0.0001.
Following time-course optimization studies (Figure S2), we performed dose-response assays using both the high affinity (R)-ZINC3573 synthetic agonist, its S-enantiomer 23, and previously reported natural ligands, including LL-3713, PAMP-12 24, and CSP-1 (competence stimulating peptide 1) 9. For all selected ligands, except LL-37, we observed significantly greater pEC50 for the serine allele, indicative of the greater capacity for beta-arrestin recruitment to MRGPRX2 (Figure 2B). There was no significant differences of Emax between alleles (Figure 2C). We tested all ligands throughout a broad range of doses, and consistently observed greater luminensence for the serine allele (except LL-37), indicative of higher levels of β-arrestin recruitment to MRGPRX2. LL-37 showed a modestly higher recruitment observed for the serine allele only at the highest dose tested. (Figure 2D-E). CSP-1 is a recently reported quorum-sensing molecule found in gram-positive bacteria including Enterococcus fecalis, which activates MRGPRX29.
Activation of MRGPRX2 as measured by IP1 accumulation is decreased with 62Ser compared to Asn62.
As a Gq-coupled receptor which activates phospholipase C, we next sought to test for genotype-dependent differences in activation as measured by inositol monophosphate (IP1), a downstream metabolite of inositol trisphosphate (IP3). Electroporation of Asn62 and 62Ser into CHO cells was performed, followed by G418 selection and flow cytometry. Addition of LiCl blocks breakdown of IP1, thereby providing sensitive and reproducible measurements of cumulative receptor signaling through phospholipase C. Stimulation with (R)-ZINC3573, PAMP-12, CSP-1, (S)-ZINC3573, and LL-37 was performed. With (R)-ZINC3573, PAMP-12 and CSP-1 stimulation, we observed substantially higher accumulation of IP1 with the asparagine allele with multiple activating doses, indicative of greater cumulative activation of the phospholipase C pathway compared with the serine allele at Asn62Ser in MRGPRX2 (Figure 3A-B). With (S)-ZINC3573, higher IP1 levels were observed at the highest activating dose with the asparagine allele, whereas with LL-37, only modest IP1 levels were observed, with no genotype dependent differences noted (Figure 3: (S)-ZINC3573 & LL-37). These results are consistent with the finding of greater β-arrestin recruitment with the serine allele.
Figure 3. Dose-response stimulation shows enhancement of MRGPRX2 activation as measured by IP1 accumulation for the asparagine allele.
(A) Stable transfectants of CHO cells were sorted and normalized for equivalent expression vector expression for Asn62 (red), 62Ser (green) or empty vector (blue). Stimulation of (R)-ZINC3573, PAMP-12, CSP-1, S-Zinc3573, and LL-37 was performed for 120 minutes and IP1 concentration was measured through a FRET based assay read on an HTRF plate reader. (B) Net change in IP1 between alleles. One, two, three and four asterisks correspond to p-values less than 0.05, 0.01, 0.001 and 0.0001.
Increased pERK activation with 62Ser compared to Asn62 allele in mast cells.
Because MRGPRX2 is most highly expressed in mast cells26, we performed stable transfections of HMC-1.1 (human mast cells), which do not express endogenous MRGPRX213. Stable transfections of Asn62 and 62Ser MRGPRX2 expression were created. MRGPRX2 expression was validated by flow cytometry. Western blotting of pERK was performed after 10 and 20 uM of PAMP-12 stimulation, and time-course analysis (0, 0.5, 1 and 5 minutes) was conducted (Figure 4A-B). Maximal pERK (normalized to total ERK and α/β tubulin) was observed at the 0.5 and 1 minute time points (Figure 4C, Figure S3). A significant genotype-dependent difference in pERK expression were observed at 1 minute, with the serine demonstrating higher pERK compared to the asparagine allele (Figure 4C, Figure S3). Taken together, the serine allele is associated with increased β-arrestin recruitment (Figure 2), decreased IP1 accumulation (Figure 3) and increased pERK expression (Figure 4) with agonist stimulation, all indicative of substantial genotype-dependent differences in receptor function.
Figure 4. Genotype-differences in pERK expression in mast cells.
(A-B) Representative blot of stable transfectants of human mast cells (HMCs) was performed for Asn62 and 62Ser. Stimulation of PAMP-12 was performed at 10 μM and 20 μM. Cell lysates were collected and pERK expression was quantified through western blot analysis. Immunoblotting of pERK (A), ERK (B) and loading control α/β tubulin (Figure S3 B-C). (C) Quantification of pERK of Asn62 (red) and 62Ser (green). All pERK expression levels were normalized to total ERK and α/β Tubulin. n = 3 independent replicates. *, p < 0.05 (Figure S3 D).
scRNASeq shows increased expression of the MRGPRX2 activating ligand precursor, ADM, in active UC in epithelial and activated fibroblasts.
Having established that PAMP-12 (proteolytic cleavage product of ADM) is a more potent agonist for MRGPRX2 than LL-37, we next sought to confirm expression of MRGPRX2 in inflamed and uninflamed UC tissue by immunohistochemistry (IHC) (Figure 5A). We observed MRGPRX2 (purple) and tryptase (brown) were expressed in the same cells, indicating expression of MRGPRX2 in colonic mast cells, many in close apposition to colonic epithelial crypts.
Figure 5. Comparative gene expression in uninflamed and inflamed UC by immunohistochemistry and scRNAseq.
(A) Co-localization of MRGPRX2 (purple) with tryptase (brown) in inflamed and uninflamed UC colon. Bar = 100 and 20 microns. (B) scRNASeq TSNE plot from four UC patients having active disease and a clear demarcation separating inflamed and uninflamed tissues. (C) Violin plots of selected transcripts, including mast cell proteases (CPA3, TPSAB1) and MRGPRX2 mRNA precursors (ADM, CAMP) of reported endogenous ligands (PAMP-12, LL-37, respectively).
We next sought to define the cellular sources of ADM that could activate mast cells. We performed scRNASeq of inflamed and uninflamed UC using 10X Genomics Chromium platform in four UC patients with active, ongoing inflammation, and evidence for a clear demarcation between inflamed and uninflamed colon (Table S1: ID:4–7). Altogether, we analyzed 34,157 high quality transcriptomes. Clustering of cells was performed jointly using Seurat318 (Figure 5B). Using 10X Genomics and Seurat318 QC metrics and combining inflamed and uninflamed colon samples, we identified 26 clusters, including a mast cell cluster (Figure 5B), characterized by high expression of TPSAB1 (tryptase alpha/beta1), FCER1A (Fc Fragment Of IgE Receptor Ia), and KIT (Kit proto-oncogene, receptor tyrosine kinase). Despite localization of MRGPRX2 in mast cells by protein expression (Figure 5A), we did not observe its expression by scRNASeq, reflecting the sparse transcriptome analyzed. We did observe substantial induction of CPA3 (carboxypeptidase 3) and TPSAB1 in inflamed compared to uninflamed colon (Figure 5C). We confirmed the presence of CPA3 positive cells with IHC of healthy and UC (inflamed and uninflamed) colon (Figure S4).
Activated and unactivated fibroblasts were distinguished using markers as we have reported previously27, and epithelial cells showing high EPCAM (epithelial cell adhesion molecule) were divided between secretory (showing high AGR228(p2), anterior gradient homolog 2 expression in uninflamed tissues) and primarily non-secretory cells (Figure 5C). Among fibroblasts, ADM was uniquely expressed by activated cells, and consistent with the bulk RNASeq data (Figure 1B), was substantially higher in cells taken from inflamed compared to uninflamed colon. In epithelial cells, ADM expression was restricted to secretory epithelial clusters. By marked contrast, we did not observe expression of CAMP (cathelicidin antimicrobial peptide), the gene precursor to LL-37.
Upstream regulator analysis of differentially expressed mast cell genes implicates IFNG-mediated transcriptional regulation with inflammation.
Comparing inflamed and uninflamed UC tissues, we did not observe an induction of mast cell numbers, but did observe a substantial number of differentially induced mast cell transcripts within the inflamed compared to the uninflamed tissues. Genes differentially expressed by scRNASeq from mast cells taken from inflamed vs. uninflamed tissues are shown by heat map (Figure 6A, Table S3). Upregulated genes (Figure 6B, red genes) far out-numbered down-regulated genes (Figure 6B, green genes). Using the Ingenuity Pathway Analysis software testing for upstream regulators of differential mast cell gene expression19 we observed two highly significant upstream pathways, namely TNF and IFNG, with gene regulation by these mediators substantially overlapping (Figure 6B; Table S4). By scRNASeq, these upstream regulators were primarily expressed by T cell subsets (Figure 6C). Violin plots of IRF1 (a transcription factor), PIM1 (a serine-threonine kinase) and PSMB8 (proteosome subunit) gene expression in inflamed vs. uninflamed mast cells from UC colon are summarized in Figure 6D. We next sought to validate gene regulation of these mast cell activation markers by TNF, IFNG (Fig 6B) and IL-13 (Th2 cytokine secreted by activated mast cells) by RT-PCR of LAD2 human mast cells. In LAD2 cells, only IFNG induced IRF1, PIM1 and PSMB8 gene expression by RT-PCR (Figure 6E). Despite the classic association of mast cells with Th2 disease, our present findings of IFNG-mediated regulation of mast cell gene expression are consistent with IFNG regulation of mast cell function in murine models of chronic ashma29. Taken together, our findings define a module of activated mast cells, activated fibroblasts and epithelial cells and upstream T cell activators that together contribute to ongoing inflammation in UC (Figure 7).
Figure 6. Mast cell genes demonstrate primarily induction of gene expression in inflamed UC tissues, with upstream regulators expressed primarily by T cell clusters.

(A) Heatmap of differentially expressed genes in the mast cell cluster of scRNAseq. (B) Circos plot of genes differentially expressed by mast cells between inflamed and uninflamed UC tissues. Shown are the mast cell genes differentially expressed by scRNASeq and regulated by the most significant predicted upstream regulators using Ingenuity Pathway Analysis package, namely TNF and IFNG. Red and green transcripts designate up- and down-regulated transcripts in inflamed and uninflamed tissues, respectively. (C) Heat-map of TNF and IFNG ligands in the composite UC scRNASeq dataset. (D) Violin plots of IRF1, PIM1, and PSMB8 from scRNASeq mast cell cluster of scRNAseq comparing inflamed and uninflamed UC. The expression level is shown as log2 normailized expression. (E) RT-PCR of IRF1, PIM1 and PSMB8 from LAD2 mast cells treated with TNF, IFNG or IL-13 treatment for 4 hours. One, two, three and four asterisks correspond to p-values less than 0.05, 0.01, 0.001 and 0.0001.
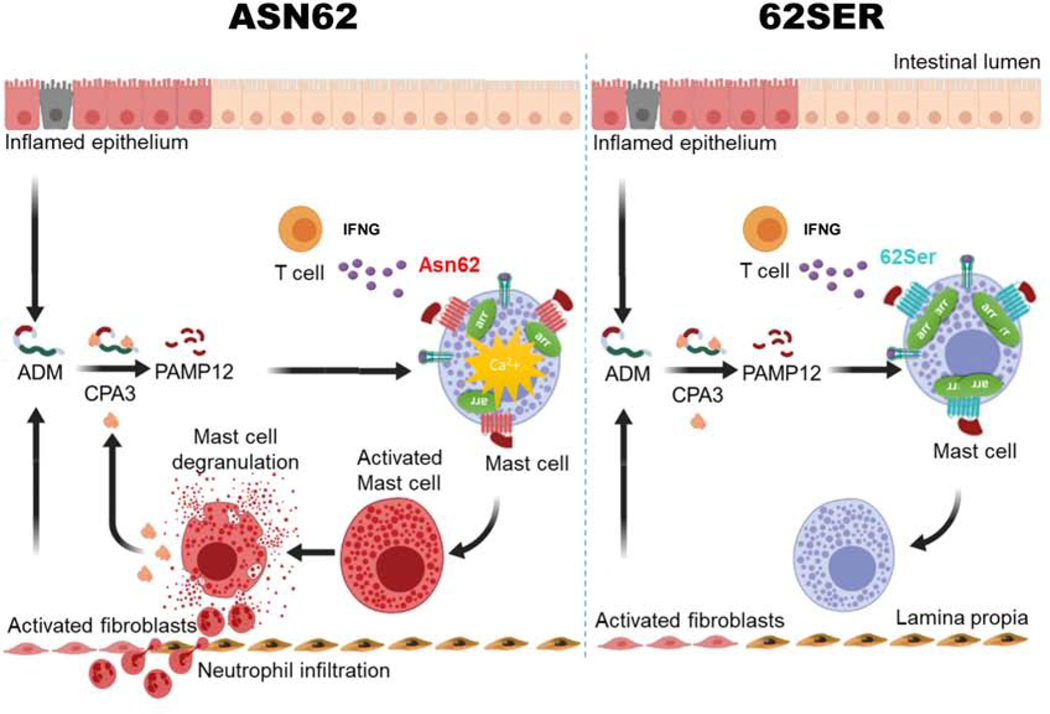
Figure 7. Model for MRGPRX2 mediated, genotype-dependent differences in mast cell activation, contributing to UC inflammation.
T cell derived IFNG modulates mast cell gene expression. Model for ADM production from inflamed epithelial cells and activated fibroblasts from inflamed regions being proteolyzed by CPA3, resulting in a feedback loop of continued mast cell activation.
Discussion
Differences in regulation of immune and stromal cell activation states between inflamed and uninflamed regions in UC represents one of its major unsolved mysteries. Patients with proctitis, left-sided and pancolitis29 have significant differences in clinical outcomes, with more extensive disease associated with more frequent need for colectomy30. Genetic factors15 and age31 have been reported to contribute to variable disease extent in UC. Our prior scRNASeq studies in Crohn’s disease implicated modules of different cell types, together, as contributing to anti-TNF treatment non-response (GIMATS high: IgG producing plasmablasts, inflammatory mononuclear phagocytes, activated T and stromal cells)27. In this study, we hypothesized that mast cell activation might play a particularly important role in defining new mechanisms for UC inflammation, given the recent understanding of the key role that the MRGPRX2 pathway plays in mast cell activation. UC’s continuous distribution, starting with the rectum, with variable proximal extension, is mirrored by ADM expression (Figure 1B), as well as the pseudo-allergic drug reactions that mrgprb2−/− mice are protected from8. Our results suggest a novel therapeutic approach of mimicking the protective serine allele by blocking mast cell degranulation, thereby blocking the positive feedback inflammatory loop comprised of activated mast cells, fibroblasts, epithelial cells and upstream T cell activators (Figure 7).
Mast cells promote a wide range of immediate and chronic inflammatory responses. In the colon, mast cells are found principally in mucosae and connective tissue, generally clustered at epithelial surfaces (Figure 5A). With specific MRGPRX2 ligand stimulation, there is a marked induction of carboxypeptidase secretion in the inflamed tissue (Figure 1C). Among all GPCR protein-altering alleles, we observe the highest association of Asn62Ser (P value = 9.8 × 10−4) in MRGPRX2 in Ashkenazi Jewish, but not non-Jewish, European ancestry UC. As the population with the highest IBD prevalence, Ashkenazi Jews are characterized by having the highest polygenic risk32. This observation of a higher effect size for a given allele in Jews33, has also been observed for the functionally decreased Asn360Asp association within NOX1 (NADPH oxidase 1)34. In the present study, we have shown that the serine allele at Asn62Ser in MRGPRX2 protects against UC in Jewish populations and reduces Gαs signaling. We did not observe MRGPRX2 in our scRNASeq data from UC samples, but we used the 10X Genomics Chromium panel, which captures only modest fraction of the transcriptome. By immunohistochemistry, we observe MRGPRX2 and tryptase were expressed in the same cells, confirming its presence within UC colon, including cells closely apposed to epithelial crypts (Figure 5A).
We demonstrate using three complementary functional assays (Figures 2–4), the expected genotype differences between the asparagine and serine alleles at Asn62Ser in MRGPRX2. While numerous natural ligands have been reported for MRGPRX2, we were struck by the greater functional effects, including genotype-dependent differences, observed with PAMP-12 compared to LL-37. The highly significant induction of ADM (Figure 1B) and CPA3 secretion (Figure 1C) in inflamed compared to uninflamed colonic tissues indicates that ADM induction and CPA3 secretion plays a key role in perpetuation of the chronic inflammatory process in UC. We observed consistent, genotype dependent differences for both the high affinity, synthetic ligand, (R)-ZINC3573, and a relevant natural ligand, PAMP-12, for beta-arrestin recruitment and IP1 accumulation in CHO cells. Specifically, the minor, serine allele enhances beta-arrestin recruitment and decreases IP1 accumulation, and can therefore be considered as a loss of function variant with respect to heterotrimeric Gq signaling. Enhanced beta-arrestin recruitment might also result in enhanced pERK upon stimulation. We tested this in the HMC-1.1 mast cell line using PAMP-12, and observed significantly increased phosphorylation with the serine allele after 1 minute of PAMP-12 stimulation.
The role of mast cells has been relatively under-studied in murine models of intestinal colitis. One recent study implicated an essential role for mast cells in TNBS (2,4,6-trinitrobenzene sulphonic acid) colitis via the ATP-dependent purinoreceptor, P2X735. We did not observe a difference in mast cell numbers between inflamed and uninflamed tissues by scRNASeq; however, this observation must be tempered by the understanding that granulocytic cells, by virtue of their release of proteolytic enzymes, may be destroyed in the cell isolation and processing procedures. Neutrophils are not well-captured by most extant scRNASeq datasets; importantly activated mast cells release histamine that drives neutrophil recruitment in DSS and oxazolone-induced colitis3. TNF and IFNG have long been implicated in colitis, T cells represent a major source of their expression (Figure 6C). Our study advances a model of differential genotype effects within MRGPRX2 and immune and stromal cell modules of activation in human ulcerative colitis (Figure 7).
The identification of loss-of-function, protective alleles is particularly promising for therapeutic targeting36, given the generally greater feasibility of blocking, as opposed to activating, therapeutic target pathways. In this regard, loss-of-function alleles in PCSK937 and IL23R38 protect against high LDL cholesterol levels and inflammatory bowel disease, respectively; antibodies blocking PCSK932 and the IL-2339 pathways are now in use for the treatment of these two respective traits. Here, we report that Ser62 allele in MRGPRX2 protects against UC and is a relative loss-of-function allele with respect to mast cell activation. Therefore, blocking MRGPRX2 activation and protease release represents a promising therapeutic targeting approach. The complex activation and de-activation states of GPCRs12 indicates that testing specific therapeutic hypotheses for lead compounds or antibodies developed for MRGPRX2 and UC will require in vivo studies utilizing the mrgprb2 knockout, with humanized MRGPRX2 knock-in studies.
Supplementary Material
BACKGROUND AND CONTEXT
The role of mast cells in human ulcerative colitis is not well established. G-protein coupled receptors represent attractive candidate for therapeutic targeting and recent literature has highlighted a mast-cell predominant GPCR (MRGPRX2) activated independently of immunoglobulin E.
NEW FINDINGS
Proteolytic precursors of MRGPRX2 activators demonstrate increased expression in inflamed compared to uninflamed ulcerative colitis. Furthermore, secretion of mast cell tryptases (e.g. carboxypeptidase) is increased from cells isolated from inflamed human colitis upon MRGPRX2 activation. Association to a genetic variant that alters MRGPRX2 functional activity is shown. Specifically, a variant that demonstrates decreased mast cell activity protects against developing ulcerative colitis.
LIMITATIONS
Additional support for blocking the MRGPRX2 pathway to treat ulcerative colitis will require murine studies before studies in humans can be considered.
IMPACT
These studies define a very new approach for treating ulcerative colitis that does not involve decreasing pro-inflammatory cytokine blockade (e.g. anti-TNF agents).
Acknowledgements:
We gratefully acknowledge the patients who participated in these studies. LAD2 cells were a generous gift from NIAID40.
Funding: Funding was provided by U01 DK062422, U24 DK052429, R01 DK123758, R01 DK106593 (JHC), U01 DK062431 (SRB), U01 DK062420 (RHD), U01 DK 062413 (DPB), U01 DK062432 (JDR), U01 DK062423 (MSS)
Funding: U01 DK062422, U24 DK052429, R01 DK123758 (JHC), R01 DK106593 (JHC), U01 DK062431 (SRB), U01 DK062420 (RHD), U01 DK062413 (DPB), U01 DK062432 (JDR), U01 DK062423 (MSS) and Regeneron Pharmaceuticals (JHC. No funding was provided for this study, but JHC is collaborating on antibody development).
EC, LC, MG, NV, JC. Experimental design and implementation. Data analyses and interpretation, and writing of manuscript NH, KS, SJ, ZC, IA. Experimental studies and quality control KG, SN, ZC, AJ, AM, GB. Data analyses and interpretation CC, UK. Sample quality and phenotypic interpretation SD, TT. Pathology interpretation RD, MS, SB, JR, IP, LPS, TH, DPM, YI. Study design, interpretation
Footnotes
Conflict of interests: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Wernersson S, Pejler G. Mast cell secretory granules: armed for battle. Nat Rev Immunol 2014;14:478–494. [DOI] [PubMed] [Google Scholar]
- 2.Maun HR, Jackman JK, Choy DF, et al. An Allosteric Anti-tryptase Antibody for the Treatment of Mast Cell-Mediated Severe Asthma. Cell 2019;179:417–431.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wechsler JB, Szabo A, Hsu CL, et al. Histamine drives severity of innate inflammation via histamine 4 receptor in murine experimental colitis. Mucosal Immunol 2018;11:861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamilton MJ, Sinnamon MJ, Lyng GD, et al. Essential role for mast cell tryptase in acute experimental colitis. Proc Natl Acad Sci U S A 2011;108:290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boeckxstaens G. Mast cells and inflammatory bowel disease. Curr Opin Pharmacol 2015;25:45–49. [DOI] [PubMed] [Google Scholar]
- 6.Sasaki Y, Tanaka M, Kudo H. Differentiation between ulcerative colitis and Crohn’s disease by a quantitative immunohistochemical evaluation of T lymphocytes, neutrophils, histiocytes and mast cells. Pathol Int 2002;52:277–285. [DOI] [PubMed] [Google Scholar]
- 7.Andoh A, Deguchi Y, Inatomi O, et al. Immunohistochemical study of chymase-positive mast cells in inflammatory bowel disease. Oncol Rep 2006;16:103–107. [PubMed] [Google Scholar]
- 8.McNeil BD, Pundir P, Meeker S, et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2015;519:237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pundir P, Liu R, Vasavda C, et al. A Connective Tissue Mast-Cell-Specific Receptor Detects Bacterial Quorum-Sensing Molecules and Mediates Antibacterial Immunity. Cell Host Microbe 2019;26:114–122.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hauser AS, Chavali S, Masuho I, et al. Pharmacogenomics of GPCR Drug Targets. Cell 2018;172:41–54.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster SR, Hauser AS, Vedel L, et al. Discovery of Human Signaling Systems: Pairing Peptides to G Protein-Coupled Receptors. Cell 2019;179:895–908.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wacker D, Stevens RC, Roth BL. How Ligands Illuminate GPCR Molecular Pharmacology. Cell 2017;170:414–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subramanian H, Gupta K, Guo Q, et al. Mas-related gene X2 (MrgX2) is a novel G protein-coupled receptor for the antimicrobial peptide LL-37 in human mast cells: resistance to receptor phosphorylation, desensitization, and internalization. J Biol Chem 2011;286:44739–44749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou XE, He Y, de Waal PW, et al. Identification of Phosphorylation Codes for Arrestin Recruitment by G Protein-Coupled Receptors. Cell 2017;170:457–469.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cleynen I, Boucher G, Jostins L, et al. Inherited determinants of Crohn’s disease and ulcerative colitis phenotypes: a genetic association study. Lancet Lond Engl 2016;387:156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chuang L-S, Villaverde N, Hui KY, et al. A Frameshift in CSF2RB Predominant Among Ashkenazi Jews Increases Risk for Crohn’s Disease and Reduces Monocyte Signaling via GM-CSF. Gastroenterology 2016;151:710–723.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hui KY, Fernandez-Hernandez H, Hu J, et al. Functional variants in the LRRK2 gene confer shared effects on risk for Crohn’s disease and Parkinson’s disease. Sci Transl Med 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stuart T, Butler A, Hoffman P, et al. Comprehensive Integration of Single-Cell Data. Cell 2019;177:1888–1902.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krämer A, Green J, Pollard J, et al. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014;30:523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zudaire E, Portal-Núñez S, Cuttitta F. The central role of adrenomedullin in host defense. J Leukoc Biol 2006;80:237–244. [DOI] [PubMed] [Google Scholar]
- 21.Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med 2007;13:975–980. [DOI] [PubMed] [Google Scholar]
- 22.Harmar AJ, Armstrong A, Pascall JC, et al. cDNA sequence of human beta-preprotachykinin, the common precursor to substance P and neurokinin A. FEBS Lett 1986;208:67–72. [DOI] [PubMed] [Google Scholar]
- 23.Lansu K, Karpiak J, Liu J, et al. In silico design of novel probes for the atypical opioid receptor MRGPRX2. Nat Chem Biol 2017;13:529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamohara M, Matsuo A, Takasaki J, et al. Identification of MrgX2 as a human G-protein-coupled receptor for proadrenomedullin N-terminal peptides. Biochem Biophys Res Commun 2005;330:1146–1152. [DOI] [PubMed] [Google Scholar]
- 25.Srivastava A, Gupta B, Gupta C, et al. Emerging Functional Divergence of β-Arrestin Isoforms in GPCR Function. Trends Endocrinol Metab TEM 2015;26:628–642. [DOI] [PubMed] [Google Scholar]
- 26.Tatemoto K, Nozaki Y, Tsuda R, et al. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem Biophys Res Commun 2006;349:1322–1328. [DOI] [PubMed] [Google Scholar]
- 27.Martin JC, Chang C, Boschetti G, et al. Single-Cell Analysis of Crohn’s Disease Lesions Identifies a Pathogenic Cellular Module Associated with Resistance to Anti-TNF Therapy. Cell 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park S-W, Zhen G, Verhaeghe C, et al. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc Natl Acad Sci U S A 2009;106:6950–6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu M, Eckart MR, Morgan AA, et al. Identification of an IFN-γ/mast cell axis in a mouse model of chronic asthma. J Clin Invest 2011;121:3133–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol J Can Gastroenterol 2005;19 Suppl A:5A–36A. [DOI] [PubMed] [Google Scholar]
- 31.Hoie O, Wolters FL, Riis L, et al. Low colectomy rates in ulcerative colitis in an unselected European cohort followed for 10 years. Gastroenterology 2007;132:507–515. [DOI] [PubMed] [Google Scholar]
- 32.Quezada SM, Cross RK. Association of age at diagnosis and ulcerative colitis phenotype. Dig Dis Sci 2012;57:2402–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ridker PM, Tardif J-C, Amarenco P, et al. Lipid-Reduction Variability and Antidrug-Antibody Formation with Bococizumab. N Engl J Med 2017;376:1517–1526. [DOI] [PubMed] [Google Scholar]
- 34.Hayes P, Dhillon S, O’Neill K, et al. Defects in NADPH Oxidase Genes NOX1 and DUOX2 in Very Early Onset Inflammatory Bowel Disease. Cell Mol Gastroenterol Hepatol 2015;1:489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwerd T, Bryant RV, Pandey S, et al. NOX1 loss-of-function genetic variants in patients with inflammatory bowel disease. Mucosal Immunol 2018;11:562–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurashima Y, Amiya T, Nochi T, et al. Extracellular ATP mediates mast cell-dependent intestinal inflammation through P2X7 purinoceptors. Nat Commun 2012;3:1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson MR, Tipney H, Painter JL, et al. The support of human genetic evidence for approved drug indications. Nat Genet 2015;47:856–860. [DOI] [PubMed] [Google Scholar]
- 38.Cohen J, Pertsemlidis A, Kotowski IK, et al. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet 2005;37:161–165. [DOI] [PubMed] [Google Scholar]
- 39.Duerr RH, Taylor KD, Brant SR, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 2006;314:1461–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med 2019;381:1201–1214. [DOI] [PubMed] [Google Scholar]
- 41.Kirshenbaum AS, Akin C, Wu Y, et al. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcepsilonRI or FcgammaRI. Leuk Res 2003;27:677–682. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.