Abstract
Despite the benefits of yearly influenza vaccination, accumulating evidence suggests that diminished vaccine efficacy may be related to repeated vaccination. Although studied at the level of B-cell responses, CD4 T-cell responses have not yet been examined. In this study, we analyze CD4 T-cell responses to influenza vaccination in subjects who differ in their vaccine history. We find a striking disparity in their responses, with previously vaccinated subjects exhibiting significantly blunted CD4 T-cell responses and diminished antibody responses. These results suggest that limiting CD4 T-cell help mteaserrlie the diminished or altered antibody responses in repeatedly vaccinated subjects.
Keywords: CD4 T cells, cell-mediated immunity, influenza vaccine, influenza virus
Antibody responses to influenza vaccines are often diminished in individuals who are repeatedly vaccinated. Here, we discovered that requisite CD4 T-cell responses are similarly reduced, suggesting that early events in vaccine-induced immunity are blunted in repeatedly vaccinated subjects.
(See the Editorial Commentary by Nilsson and Chiodi, on pages 176–9.)
Sterilizing immunity to influenza virus relies on hemagglutinin (HA)-specific antibodies in the host that block infection by binding to sites on HA that control binding and internalization of virions by host cells. Variant influenza viruses can escape from this interference if they possess mutations in HA that diminish recognition by the antibodies generated by infection or vaccination. Mutation and selection of variant viruses, termed antigenic drift [1], necessitates regular vaccination with vaccines containing the variant HA proteins (reviewed in [2]). Because of drift and waning of serum antibodies over time, many countries recommend annual influenza vaccination. These vaccines provide protection that varies among individuals and depends in part on the match with the strains that ultimately emerge in the subsequent influenza season (reviewed in [3]).
Despite the benefits of yearly influenza vaccination, there has been increasing evidence that repeated vaccination can lead to diminished vaccine efficacy (reviewed in [4]). Recent publications have considered the mechanisms that underlie these deficiencies [5, 6]. These generally relate to either a viral antigen-clearance model, where pre-existing antibodies lead to rapid clearance of vaccine antigens, a FcR-mediated B-cell negative signaling event, where the circulating HA-specific antibodies bind to the vaccine components, inhibiting B-cell activation and, finally, an epitope masking model, where circulating antibodies bind to and mask specific epitopes on HA, blocking engagement of B cells with specificity for those or nearby HA epitopes, while allowing stimulation of B cells with specificity for more distal epitopes.
Although production of high-affinity, class-switched antibodies by B cells requires CD4 T cells, CD4 T-cell responses to influenza vaccination in subjects vaccinated in previous seasons have not yet been compared with those without this recent vaccination history. The role of CD4 T cells in facilitating the antibody responses to influenza vaccination have been well documented [7, 8]. Our previous studies involving human responses to monovalent H1N1 vaccines [9] and H5N1 vaccines [10] showed positive correlations between HA-specific CD4 T-cell responses and the magnitude of the antibody response, suggesting that deficient CD4 T-cell responses in repeatedly vaccinated individuals may underlie defects in the elicited antibody response. In this study, we recorded vaccine recipients’ self-reported history of influenza vaccination in the previous year and analyzed all vaccine recipients for their CD4 T-cell responses to vaccination and serum HA-specific antibodies. We found striking disparities in their response magnitude for both types of immunity, suggesting a global blunting of vaccine-induced immunity.
MATERIALS AND METHODS
Influenza Study Group and Sampling
After approval by Division of Microbiology and Infectious Diseases (DMID) and the University of Rochester Research Subjects Review Board (protocol l5-0055), blood was obtained from a group of 78 healthy adults, who provided informed consent, on day 0 and days 7, 14, and 28 after vaccination with licensed influenza vaccines including Fluzone, Flucelvax, and Flublok. In 2 successive seasons, subjects were enrolled in a study to evaluate both their CD4 T-cell and B-cell responses to influenza vaccination. The response patterns in the 2 successive years showed the same trends but gained more statistical power by combining the results from the 2 vaccine seasons. At the time of enrollment, subjects self-reported their previous year’s influenza vaccine history. The age range in these 2 groups was 18–48 and 18–47 years old, means 29 and 27 years old in the vaccinated and nonvaccinated group, respectively, with no statistical differences in the ages. For isolation of peripheral blood mononuclear cells (PBMCs), plasma was removed and frozen in aliquots. Peripheral blood mononuclear cells were isolated and frozen in fetal calf serum (Gibco) containing 10% dimethyl sulfoxide until use.
Synthetic Peptides
Peptide libraries encompassing the entire translated sequences of viral proteins were obtained from BEI Research Repository, National Institute of Allergy and Infectious Diseases, National Institutes of Health and included A/California/04/09(H1N1) HA (pH1), A/New York/384/2005(H3N2) HA (H3), and B/Florida/04/2006 HA (HA-B). A negative pool encompassing peptides from Sin Nombre virus glycoprotein precursor protein was used to subtract background. Peptide pools contained each peptide at a final concentration of 1 μM.
Enzyme-Linked Immunospot Assay
T-cell enzyme-linked immunospot (ELISPOT) assays were performed as previously described [11]. In brief, CD4-enriched PBMCs were cultured with peptides or control antigen (tetanus toxoid [EMD Millipore], 5 μg/mL and Rubeola [Meridian], 7 μg/mL) on plates coated with antihuman interferon (IFN)-γ, washed, and incubated with biotinylated antihuman IFN-γ, then developed for detection of cytokine-producing cells, using an Immunospot reader (series 5.2).
Detection of T Follicular Helper Cells
Peripheral blood mononuclear cells from day 7 postvaccination were thawed, rested at room temperature for 2 hours, stained for 30 minutes with Fixable Live/Dead Aqua (Life Technologies) for 30 minutes, then stained with a pool of antibodies including CD4 (RPA-T4), CD3 (SK7), CD45RA (HI100), CXCR5 (RF8B2), PD-1 (EH12), and ICOS (DX29). Flow cytometry data were acquired using a BD LSR-II instrument, configured with 488, 633, 407, and 532-nm lasers using FACS DIVA software (BD Bioscience).
Hemagglutination Inhibition Assay
Hemagglutination inhibition assay (HAI) testing was performed as previously described [9]. The virus strains used included H1N1 A/California/07/09, H3N2 A/Switzerland/9715293/13, and Influenza B strains B/Phuket/3073/13 and B/Brisbane/60/08, obtained from BEIR. H3N2 A/Hong Kong/4801/14 was also tested, with results equivalent to the A/Switzerland/9715293/13 results (data not shown).
Enzyme-Linked Immunosorbent Assay
Plates were coated with 200 ng/well purified HA proteins, prepared as 2 μg/mL, from A/California/04/09 (H1), A/Wisconsin/67/05 (H3), and B/Florida/04/06 (HA-B) (obtained from BEIR), rinsed, and blocked with 3% bovine serum albumin in phosphate-buffered saline. Diluted plasma samples were added to the plates, incubated for 2–3 hours at room temperature, then washed and incubated sequentially with 100 μL/well alkaline phosphatase-conjugated antihuman immunoglobulin G (MT78) (MabTech) and 1 p-nitrophenyl phosphate substrate, and scored at 405 nm.
Statistics
Data are presented as the frequency of cytokine-producing cells per million CD8- and CD56-depleted PBMCs or for sera, fold-change of relative titers. Statistical differences between cohorts were calculated as P values using the Mann-Whitney U test in Prism 8. The likelihood-ratio test [12] suggests that vaccine formulations did not affect vaccine response (P > .4 for all responses). This allows us to conclude that the same patterns of responses we observed in subjects who were vaccinated versus nonvaccinated were independent of the vaccine formulation administered. Thus, we analyzed the vaccine responses in aggregate, combining the results from the 3 different vaccine formulations and increasing the statistical power of the study.
RESULTS
The Role of Previous Vaccination in CD4 T-Cell Responses to Influenza Vaccination
Although there have been reports characterizing the B-cell and antibody response to influenza vaccination and the impact of repeated vaccination on protection from infection (reviewed in [4]), the characteristics of the CD4 T-cell response have not yet been evaluated. Therefore, in our studies of responses to licensed influenza vaccination, we recorded vaccine recipients’ self-reported history of influenza vaccination in the previous year. After completion of the experiments, we comparatively analyzed the vaccine recipients’ responses based on whether they had received an influenza vaccine in the previous season.
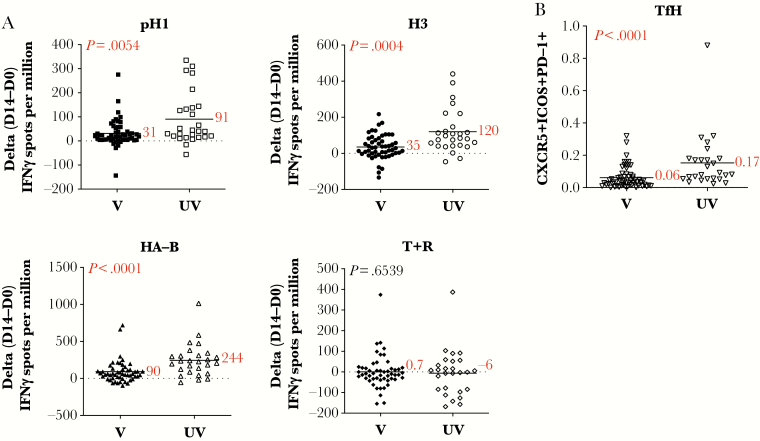
To quantify HA-specific CD4 T cells, we first used peptide-stimulated cytokine ELISPOTs, as we have previously described [11]. Figure 1A shows the results of these experiments, where the expansion of CD4 T cells specific for epitopes derived from H1, H3, and HA-B are displayed. These experiments showed that CD4 T-cells reactive with each of the HA-derived epitopes tested were significantly more amplified by day 14 postvaccination in subjects not previous vaccinated, by 2- to 3-fold (P = .0054, P = .004, P < .0001, respectively). Responses to control antigens Tetanus and Rubeola were equivalent (P = .6539). Supplemental Figure 1A shows the CD4 T-cell ELISPOT values for day 0 and day 14 in both the vaccinated and unvaccinated groups, whereas Supplemental Figure 1B shows the CD4 T-cell responses represented as “fold-change” from day 14 relative to day 0.
Figure 1.
CD4 T-cell expansion and T follicular helper cell (Tfh) are more robust in subjects not vaccinated in the prior season. Healthy subjects vaccinated with seasonal influenza vaccine were stratified into 2 groups based on self-reported vaccination status from the previous season. Those subjects that received influenza vaccine in the previous season are indicated here as “V” (closed symbols), and subjects that had not received influenza vaccine in the previous season are indicated here as “UV” (open symbols). In A, CD4-enriched populations were stimulated with pools of peptides from H1 (top left), H3 (top right), HA-B (bottom left), or tetanus and rubeola (T + R, bottom right) as a control antigen and evaluated for interferon (IFN)γ production using enzyme-linked immunospot analyses. Shown is the change in the response between day 0 and day 14 (D14-D0) with the average response indicated by a black line and the mean value reported in red. The P value calculated using the Mann-Whitney U test is indicated in the top left corner for each stimulation condition. In B, circulating Tfh cells, defined as CD4+CD45RA−CXCR5+ICOS+PD1+ cells, were quantified at day 7-postinfluenza vaccination by flow cytometry. Shown is the frequency of cells at day 7. The average, indicated by a black line and the mean value, reported in red text, are shown. The P value calculated using the Mann-Whitney U test is indicated in the top left corner.
Human CD4 T-cell responses to vaccination can also be assessed by the presence of circulating CD4 T cells that express markers associated with the T follicular helper cell (Tfh) response at day 7 postvaccination. These cells have been shown to be a correlate of the elicited B-cell response (reviewed in [7]) and likely reflect the emergence of cells that have engaged antigen-specific B cells in the vaccine-draining lymph node. Therefore, we quantified CD4 T cells at day 7 postvaccination expressing CXCR5, PD1, and ICOS, markers that are used to identify Tfh (Figure 1B). By this criterion as well, the unvaccinated subjects had a more robust CD4 T helper-cell response to vaccination, averaging approximately 3-fold greater Tfh cells in the blood than those who were previously vaccinated (P < .0001). These data, taken together, indicate that vaccine recipients vaccinated in the previous year mount a significantly less robust HA-specific CD4 T-cell response than those who were not.
Hemagglutinin-Specific Antibody Responses in Previously Vaccinated Verus Nonvaccinated Subjects
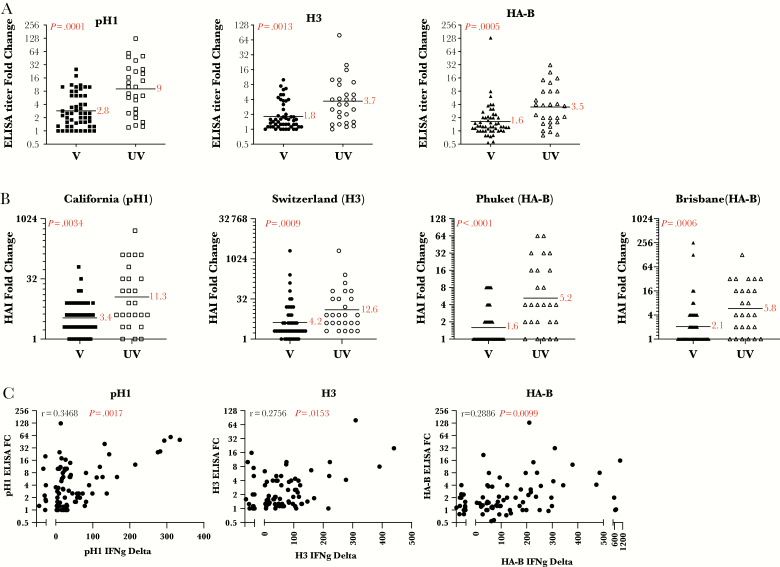
With the evidence for blunted CD4 T-cell responses to vaccination, we sought to quantify antibody responses. The full spectra of circulating HA-specific antibodies were measured before and after vaccination by enzyme-linked immunosorbent assays (ELISAs). It is becoming increasingly clear that many antibodies to HA contribute to protective immunity by a variety of mechanisms [13, 14]. Sera were tested in ELISAs using full-length HA proteins. Figure 2A shows the results of these assays, where the fold-change from day 0 to day 14 is presented. The results show a striking and significant disparity in the magnitude of the total antibody response to each of the HA proteins tested. Depending on the HA type, vaccinated subjects exhibited approximately a 2- to 4-fold gain in serum antibody to HA, whereas subjects who were not vaccinated in the previous year exhibited a 6- to 10-fold increase (H1, P < .0001; H3, P = .0013; HA-B, P = .005). A similar pattern of disparity in HA-specific responses was observed when HAI titers, reflecting antibodies that detect the specific regions on the head HA protein associated with binding to red blood cells, the most commonly used correlate of protection [15], were measured. As shown in Figure 2B, gains in HAI titers after vaccination were also significantly less robust in subjects vaccinated in the previous year (H1N1, P = .034; H3N2, P = .0009; B-Phuket, P < .0001; B-Brisbane, P = .0006). Supplemental Figure 2 shows the day-0 HAI values. These results, taken together, indicate that an influenza vaccination in the previous season diminishes the vaccine-induced antibody response in the following year. Data supporting the view that lower CD4 T-cell responses underlie the observed diminished antibody responses to influenza vaccination are the correlation relationship shown in Figure 2C. These data demonstrate that the elicited antibody responses are statistically and positively correlated with the elicited CD4 T-cell response to each of the HA proteins (pH1, H3, and HA-B, P = .0017, P = .0153, P = .0099, respectively).
Figure 2.
Subjects not vaccinated in the prior season respond with higher gains in hemagglutinin (HA)-specific antibody responses. Subjects that received influenza vaccine in the previous season are indicated here as “V” (closed symbols), and subjects who reported that they had not received influenza vaccine in the previous season are indicated here as “UV” (open symbols). In A, serum antibody from day 0, before vaccination, and day 14 postvaccination is measured by HA-specific immunoglobulin G enzyme-linked immunosorbent assay (ELISA). The relative titers were determined based on the serum dilution at a fixed OD405 signal on the linear portion of the curve. Shown is the fold change between day 0 and day 14 (D14/D0). A black line indicates the geometric mean and the value is reported in red next to it. The p value calculated using the Mann-Whitney U test is indicated in the top left corner for each HA protein. In panel B, the neutralizing antibody response measured at day 0, before vaccination, and day 28-post vaccination was measured by HAI assay. Shown is the fold change between day 0 and day 28 (D28/D0). The geometric mean, indicated by a black line and the value, reported in red text, are shown. The P value calculated using the Mann-Whitney U test is indicated in the top left corner for each HA protein. In C, the correlation between the CD4 T-cell response and the serum antibody response to H1 (left), H3 (middle), and HA-B (right), represented as the change in interferon (IFN)γ-secreting cells between D0 and D14, and the serum antibody response of the matched protein, represented as the fold change (D14/D0), are shown. The r and P values shown in the top left corner of each panel were calculated by the Spearman-rank correlation test.
DISCUSSION
The data here, taken together, show that both influenza-specific CD4 T-cell expansion and Tfh responses postvaccination are significantly diminished in vaccine recipients who were vaccinated in the previous year, relative to those who were not. This muting of the CD4 T-cell response was accompanied by weaker protective antibody responses in the previously vaccinated subjects. Because CD4 T-cell activation generally precedes B-cell responses, our results suggest that early events in vaccine-induced responses are blunted in repeatedly vaccinated subjects and that diminished recruitment of CD4 T-cell helper cells may underlie the attenuated or modified B-cell response to influenza vaccination.
CONCLUSIONS
The simplest mechanism to explain the effects on CD4 T-cell responses is consistent with a model that HA-specific antibodies that accumulate in repeatedly vaccinated individuals bind the vaccine and either target it for degradation or toward a dead-end path that is not immunogenic; for example, through uptake by a nonstimulatory antigen-presenting cell. This model is in agreement with the antigen clearance mechanism, although our results to do not address the role of epitope-blocking antibodies. Antibody-mediated clearance of the vaccine antigens would lower the effective dose of vaccine delivered, blunting elicitation of CD4 T cells, which, in turn, would negatively impact the elicited B-cell responses. Lower CD4 T-cell helper responses might also diminish elicitation of B cells specific for novel epitopes in the vaccine, thus providing less sterilizing immunity to novel emerging seasonal influenza vaccines. It is clear that more work is needed on this topic to dissect the complex events that may be involved in the diminished antibody responses to influenza vaccines exhibited by those subjects who were vaccinated in the previous season.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: Annual Meeting of the Centers of Excellence in Influenza, New York, NY, July 2018.
Acknowledgments. We acknowledge the contribution of the New York Influenza Center of Excellence clinical core in enrolling study subjects as well as obtaining and processing all clinical samples. We also thank Theresa Fitzgerald for technical expertise with the hemagglutination inhibition assays (HAI). Finally, we are also very grateful to our study participants, whose willingness to contribute to science made this research possible. The following reagents were obtained through BEIR Resources, National Institute of Allergy and Infectious Diseases, National Institutes of Health: Peptide arrays influenza virus A/California/04/09 Hemagglutinin, NR-15433, A/New York/384/2005 Hemagglutinin, NR-2603, B/Florida/04/06 Hemagglutinin, NR-18972, and Sin Nombre virus (NM H10) glycoprotein precursor protein, NR-4764; Hemaggluttinin Proteins from Influenza Virus A/California/04/09, Recombinant from Baculovirus, NR-44074, A/Wisconsin/67/05, Recombinant from Baculovirus, NR-15171, and B/Florida/04/06, Recombinant from Baculovirus, NR-15169.
Financial support. This work was supported with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under CEIRS contract (HHSN272201400005C; to A. J. S. and J.L.N.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Kim H, Webster RG, Webby RJ. Influenza virus: dealing with a drifting and shifting pathogen. Viral Immunol 2018; 31:174–83. [DOI] [PubMed] [Google Scholar]
- 2. Krammer F. The human antibody response to influenza A virus infection and vaccination. Nat Rev Immunol 2019; 19:383–97. [DOI] [PubMed] [Google Scholar]
- 3. Poland GA. Influenza vaccine failure: failure to protect or failure to understand? Expert Rev Vaccines 2018; 17:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines 2017; 16:1–14. [DOI] [PubMed] [Google Scholar]
- 5. Zarnitsyna VI, Lavine J, Ellebedy A, Ahmed R, Antia R. Multi-epitope models explain how pre-existing antibodies affect the generation of broadly protective responses to influenza. PLoS Pathog 2016; 12:e1005692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ellebedy AH. Immunizing the immune: can we overcome influenza’s most formidable challenge? Vaccines 2018; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ueno H. Tfh cell response in influenza vaccines in humans: what is visible and what is invisible. Curr Opin Immunol 2019; 59:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koutsakos M, Nguyen TH, Kedzierska K. With a little help from t follicular helper friends: humoral immunity to influenza vaccination. J Immunol 2019; 202:360–7. [DOI] [PubMed] [Google Scholar]
- 9. Nayak JL, Fitzgerald TF, Richards KA, Yang H, Treanor JJ, Sant AJ. CD4+ T-cell expansion predicts neutralizing antibody responses to monovalent, inactivated 2009 pandemic influenza A(H1N1) virus subtype H1N1 vaccine. J Infect Dis 2013; 207:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nayak JL, Richards KA, Yang H, Treanor JJ, Sant AJ. Effect of influenza A(H5N1) vaccine prepandemic priming on CD4+ T-cell responses. J Infect Dis 2015; 211:1408–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Richards KA, Treanor JJ, Nayak JL, Sant AJ. Overarching immunodominance patterns and substantial diversity in specificity and functionality in the circulating human influenza A and B virus-specific CD4 T-cell repertoire. J Infect Dis 2018; 218:1169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li X, Li H, Jin M, D Goldberg J. Likelihood ratio and score tests to test the non-inferiority (or equivalence) of the odds ratio in a crossover study with binary outcomes. Stat Med 2016; 35:3471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sicca F, Neppelenbroek S, Huckriede A. Effector mechanisms of influenza-specific antibodies: neutralization and beyond. Expert Rev Vaccines 2018; 17:785–95. [DOI] [PubMed] [Google Scholar]
- 14. Neu KE, Henry Dunand CJ, Wilson PC. Heads, stalks and everything else: how can antibodies eradicate influenza as a human disease? Curr Opin Immunol 2016; 42:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Katz JM, Hancock K, Xu X. Serologic assays for influenza surveillance, diagnosis and vaccine evaluation. Expert Rev Anti Infect Ther 2011; 9:669–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.