Abstract
Multisystem inflammatory syndrome in children (MIS-C) can cause a myriad of cardiac manifestations, including coronary dilation and aneurysms; giant aneurysms are infrequent. We describe 3patients with giant coronary aneurysms associated with MIS-C, including the youngest case reported to date, treated with intravenous immunoglobulin, corticosteroids, and biologic agents. (Level of Difficulty: Intermediate.)
Key Words: COVID-19, giant coronary aneurysms, multisystem inflammatory syndrome in children, SARS-CoV-2
Abbreviations and Acronyms: IgG, immunoglobulin G; IVIG, intravenous immunoglobulin; IVMP, intravenous methylprednisolone; KD, Kawasaki disease; LAD, left anterior descending; MIS-C, multisystem inflammatory syndrome in children; PCR, polymerase chain reaction; RCA, right coronary artery; SARS-CoV-2, severe acute respiratory distress syndrome-coronavirus-2
Central Illustration
Introduction
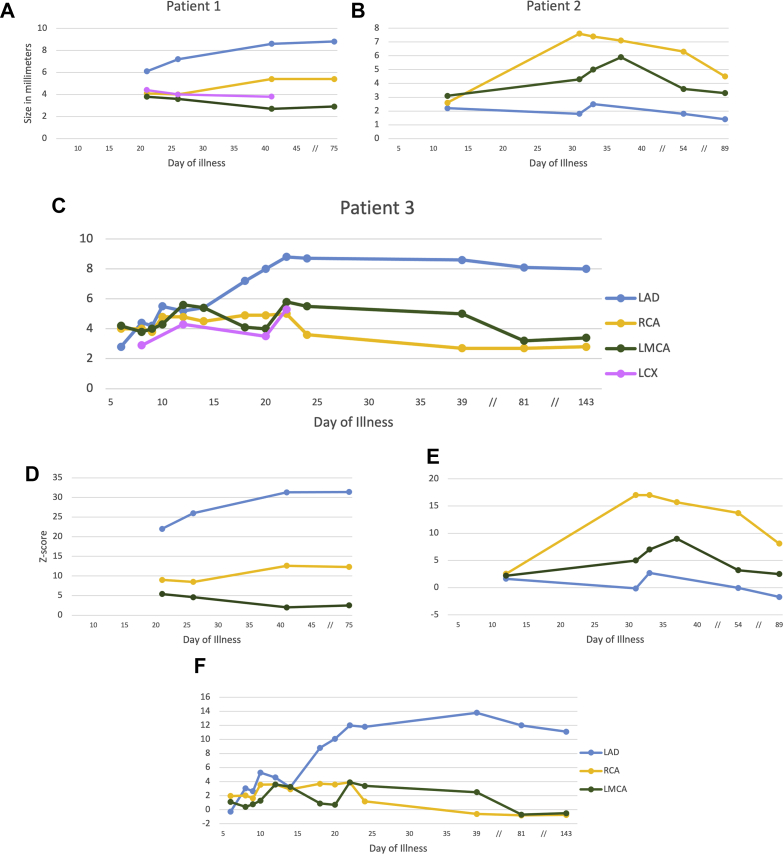
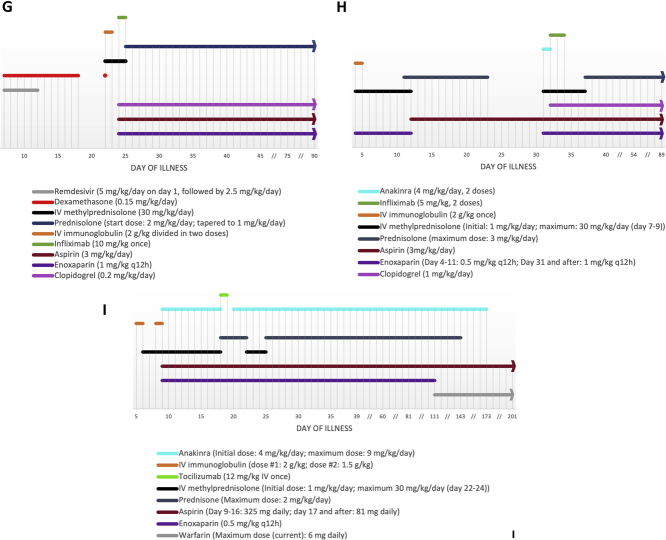
Multisystem inflammatory syndrome in children (MIS-C) following severe acute respiratory distress syndrome-coronavirus-2 (SARS-CoV-2) infection has been linked to various cardiac manifestations (1,2). We report 3 cases of MIS-C complicated by giant coronary aneurysms. Table 1 summarizes patients’ characteristics. Figures 1A to 1I detail coronary size evolution and medications throughout the disease course (tracked in days following symptom onset). Relevant laboratory values are shown in Table 2.
Learning Objectives
-
•
To identify the cardiac complications of MIS-C.
-
•
To highlight the importance of cardiac imaging in patients with MIS-C.
Table 1.
Patient Demographics, Clinical Features, and Management Before and After Diagnosis of Giant Coronary Aneurysms
| Categories | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Age | 4 mo | 2 y | 12 y |
| Sex | Male | Male | Female |
| Race or ethnicity | African American | African American | Hispanic or Latino |
| Medical history | 33 wk premature, twin | Negative | Obesity |
| Met CDC case definition of MIS-C(9) | Yes | Yes | Yes |
| Age <21 y | Yes | Yes | Yes |
| Fever | Yes | Yes | Yes |
| Inflammation (laboratory) | Yes | Yes | Yes |
| Severe illness (hospitalized) | Yes | Yes | Yes |
| Multiorgan involvement (>2) | Dermatologic, gastrointestinal, respiratory, cardiac, hematologic | Dermatologic, gastrointestinal, respiratory, cardiac, renal, hematologic | Dermatologic, neurologic, respiratory, cardiac, hematologic |
| Positive SARS-CoV-2 test | PCR and serology | Serology | Serology |
| Met criteria for KD | Incomplete | Complete | Incomplete |
| Fever >5 d | Yes | Yes | Yes |
| Conjunctivitis | Yes | Yes | Yes |
| Extremity changes | Yes | Yes | Yes |
| Rash | Yes | Yes | Yes |
| Oral mucosal changes | No | Yes | No |
| Cervical lymphadenopathy | No | No | No |
| Laboratory criteriaa | 5/6 | N/A | 5/6 |
| CRP >3mg/dLa | Yes | N/A | Yes |
| ESR >40 mm/ha | No | N/A | Yes |
| Giant CAA diagnosis | Day of illness 21 | Day of illness 31 | Day of illness 20 |
| Maximum z-score | +31.4 (LAD) | +17 (RCA) | +13.8 (LAD) |
| Therapy pre–giant CAA diagnosis | Dexamethasone, remdesivir | IVIG, IVMP/prednisolone Aspirin, enoxaparin |
IVIG, IVMP/prednisone, anakinra Aspirin, enoxaparin |
| Therapy post–giant CAA diagnosis | IVIG, IVMP/prednisolone, infliximab Aspirin, enoxaparin, clopidogrel |
IVMP/prednisolone, infliximab Aspirin, enoxaparin, clopidogrel |
Tocilizumab, anakinra, IVMP/prednisone Aspirin, enoxaparin, warfarin |
| Coronary thrombosis | Suspected | No | No |
| Follow-up | |||
| Time to last encounter | 3 mo | 3 mo | 6 mo |
| Coronary aneurysm size | Decreased, except for mid-RCA (increased) | Decreased | Decreased |
| Ongoing therapy | Prednisolone taper, aspirin, enoxaparin, clopidogrel | Prednisolone taper, aspirin, enoxaparin, clopidogrel | Aspirin, warfarin |
CAA = coronary artery aneurysms; CDC = Centers for Disease Control and Prevention; CRP = C-reactive protein; ESR = sedimentation rate; IVIG = intravenous immunoglobulin, IVMP = intravenous methylprednisolone; KD = Kawasaki disease; LAD = left anterior descending coronary artery; MIS-C = multisystem inflammatory syndrome in children; N/A = not applicable: PCR = polymerase chain reaction; RCA = right coronary artery; SARS-CoV-2 = severe acute respiratory syndrome-coronavirus-2.
Supportive of Kawasaki disease diagnosis if criteria for incomplete Kawasaki disease were met.
Figure 1.
Coronary Size and Medications
Evolution of echocardiographic coronary diameter in (A to C) millimeters and (D to F)z-score vs time for each patient. (G to I) The medication timeline for each patient. IV = intravenous; LAD = left anterior descending coronary artery; LCX = left circumflex coronary artery; LMCA = left main coronary artery; RCA = right coronary artery; // = nonlinear scale.
Table 2.
Most Representative Laboratory Values Reflective of Inflammation and Cardiac Injury Throughout the Disease Course for All Patients
| Test | Patient 1 |
Patient 2 |
Patient 3 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 7 | Day 21 | Day 23 | Day 4 | Day 9 | Day 31 | Day 37 | Day 6 | Day 17 | Day 22 | Day 25 | |
| WBC (103/μL) | 6.55 | 17.52 | 9.06 | 9.09 | 18.61 (↑) | 13.9 | 24 (↑) | 8.3 | 13.5 | 14.8 (↑) | 14.1 (↑) |
| Platelets (103/μL) | 327 | 585 (↑) | 714 (↑) | 126 (↓) | 101 (↓) | 855 (↑) | 1140 (↑) | 111 (↓) | 302 | 594 (↑) | 404 |
| CRP (mg/dL) | 3.1 (↑) | 5.6 (↑) | 0.9 | 15.7 (↑) | 3.4 (↑) | 10.3 (↑) | <0.3 | >20 (↑) | 1.2 (↑) | <0.3 | <0.3 |
| Ferritin (ng/mL) | 75.74 | — | 9.45 | 513.8 (↑) | 357.6 (↑) | 196.7 (↑) | 19.2 | 516 (↑) | 567.2 (↑) | 411.4 (↑) | 270.7 (↑) |
| D-dimer (ng/mL) | 971 (↑) | 1,359 (↑) | — | 4,211 (↑) | — | 2,474 (↑) | — | 1,749 (↑) | — | 271 (↑) | 186 |
| BNP (pg/mL) | 80.6 | 101.4 (↑) | 35.2 | 556.2 (↑) | 1,422.8 (↑) | 10.6 | 11.1 | <10 | 1,422.8 (↑) | — | 18.8 |
| Troponin (ng/mL) | <0.015 | <0.015 | <0.015 | <0.015 | 0.039 | <0.015 | <0.015 | <0.015 | 0.039 | 0.456 (↑) | 0.072 (↑) |
Abnormal values are indicated by arrows: (↑) = above the upper limit of normal for age; (↓) = below the lower limit of normal for age.
BNP = B-type natriuretic peptide; CRP = C-reactive protein; WBC = white blood cell count.
Patient 1
A 4-month-old male infant presented with acute respiratory distress, preceded by 5 days of fever, 7 days of nasal congestion, 1 day of rash, hand and foot swelling, conjunctivitis, and diarrhea. Respiratory failure prompted hospitalization for noninvasive positive-pressure ventilation. The SARS-CoV-2 polymerase chain reaction (PCR) result was positive; the immunoglobulin G (IgG) antibody test result was negative. Remdesivir and dexamethasone resulted in partial improvement. Fever persisted until day 13. The patient was transitioned to room air on day 18.
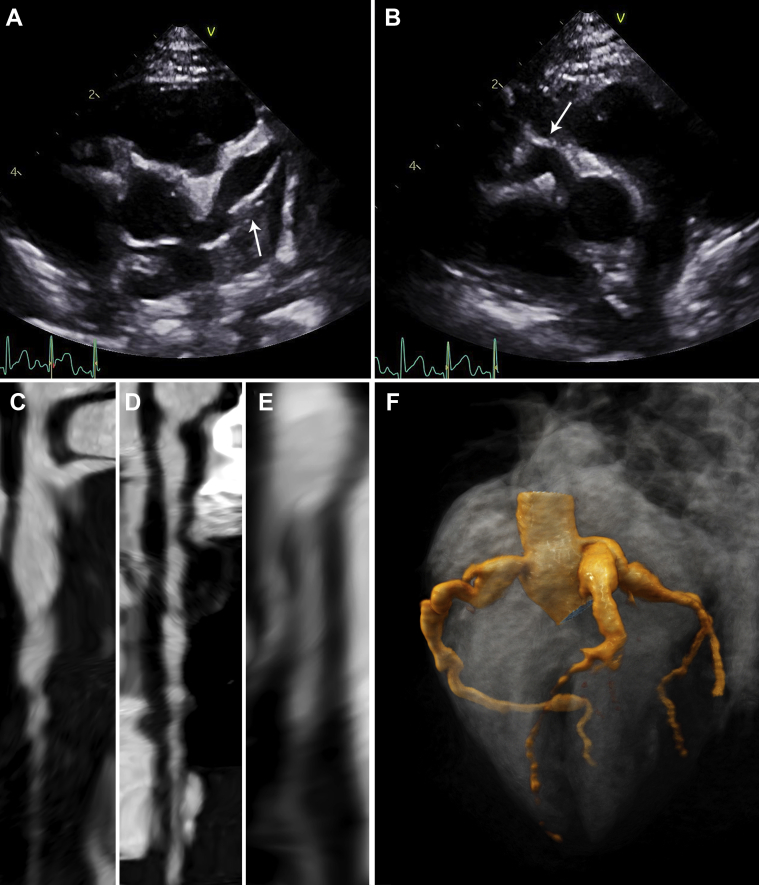
Fever and intermittent dyspnea recurred on day 19. The result of repeat SARS-CoV-2 PCR testing was negative; the IgG antibody test result became positive. On day 22, the infant’s distal extremities demonstrated peeling. An echocardiogram revealed giant left anterior descending (LAD) coronary artery (6.1 mm; z-score +22) and large right coronary artery (RCA) aneurysms; the left main and circumflex coronary arteries were dilated (Figures 2A to 2F).
Figure 2.
Early Cardiac Imaging (Patient 1)
Echocardiography (day 21) showing (A) a giant (z-score +26) left anterior descending artery aneurysm and (B) a large (z-score +8.5) right coronary artery aneurysm (arrow). Computed tomography angiography (day 24), showing straightened multiplanar views of (C) the left main and left anterior descending coronary arteries, (D) the right coronary artery, and (E) the left circumflex artery with aforementioned dilations. (F) A 3-dimensional reconstruction of coronary architecture.
MIS-C was diagnosed. Intravenous immunoglobulin (IVIG), pulse intravenous methyl-prednisolone (IVMP) (days 22-24), enoxaparin, and aspirin were started. By day 23, he was afebrile.
Cardiac computed tomography (day 24) also demonstrated coronary involvement, with possible LAD artery mural thrombus (Figures 2A to 2F, Video 1). He received infliximab 10 mg/kg. Clopidogrel was added. An echocardiogram (day 26) showed coronary aneurysm enlargement. On the basis of improved clinical features and laboratory values, he was discharged.
Cardiac magnetic resonance imaging (day 90) showed LAD, circumflex, and distal RCA size improvement, whereas the mid-RCA aneurysm diameter was 1 mm larger (Figures 3A to 3C). He remains asymptomatic on prednisolone and triple anticoagulation.
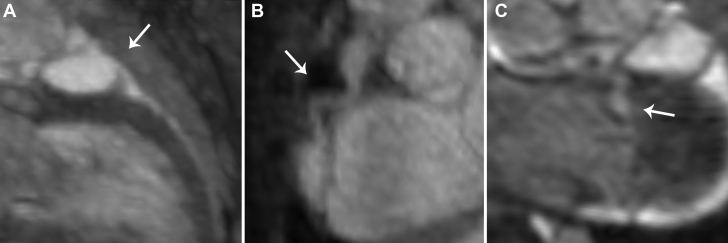
Figure 3.
Follow-Up Cardiac Imaging (Patient 1)
Magnetic resonance angiography images showing (A) a giant proximal left anterior descending artery aneurysm (arrow), (B) a proximal and distal right coronary artery aneurysm(arrow), and (C) a proximal left circumflex artery aneurysm (arrow).
Patient 2
A 2-year-old male child presented with dehydration following 2 days of fever, vomiting, diarrhea, rhinorrhea, rash, and palmoplantar erythema and edema. On day 4, the SARS-CoV2 PCR result was negative, and the IgG antibody test result was positive. An echocardiogram (day 5) showed normal findings. MIS-C was diagnosed. Treatment with IVMP, IVIG, and enoxaparin was instituted.
Hypotension and desaturations during IVIG infusion required transfer to intensive care and a low-flow nasal cannula. Pulse IVMP was initiated (days 7-9) for persistent fever. By day 9, he was on room air, hemodynamically stable, and afebrile. An echocardiogram (day 12) showed mild RCA and left main coronary artery dilation; therapy was not escalated given his improved clinical manifestations and laboratory values. He was discharged on a prednisolone taper and aspirin.
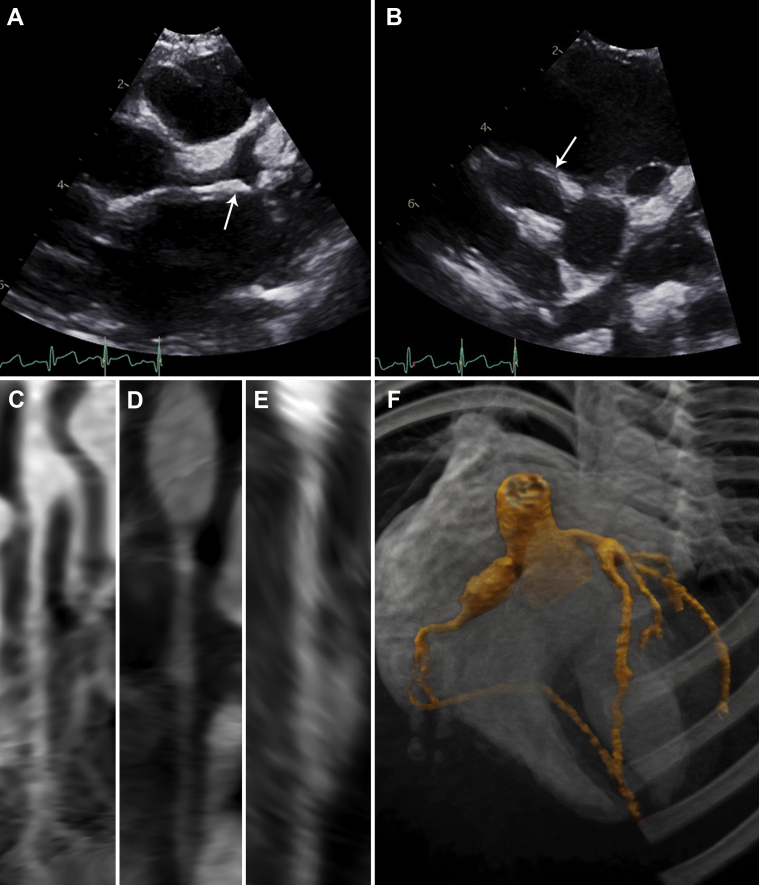
On day 31 he was readmitted with fever and bilateral hip pain and effusions. An echocardiogram revealed giant RCA (7.6 mm; z-score +17) and moderate left main coronary aneurysms (Figures 4A to 4F). He received IVMP pulses (days 31-33), anakinra (2 doses), infliximab 5 mg/kg, and enoxaparin. By day 32 fever resolved. An echocardiogram on day 33 showed worsening coronary changes prompting redosing with infliximab 5 mg/kg. Clopidogrel was started.
Figure 4.
Early Cardiac Imaging (Patient 2)
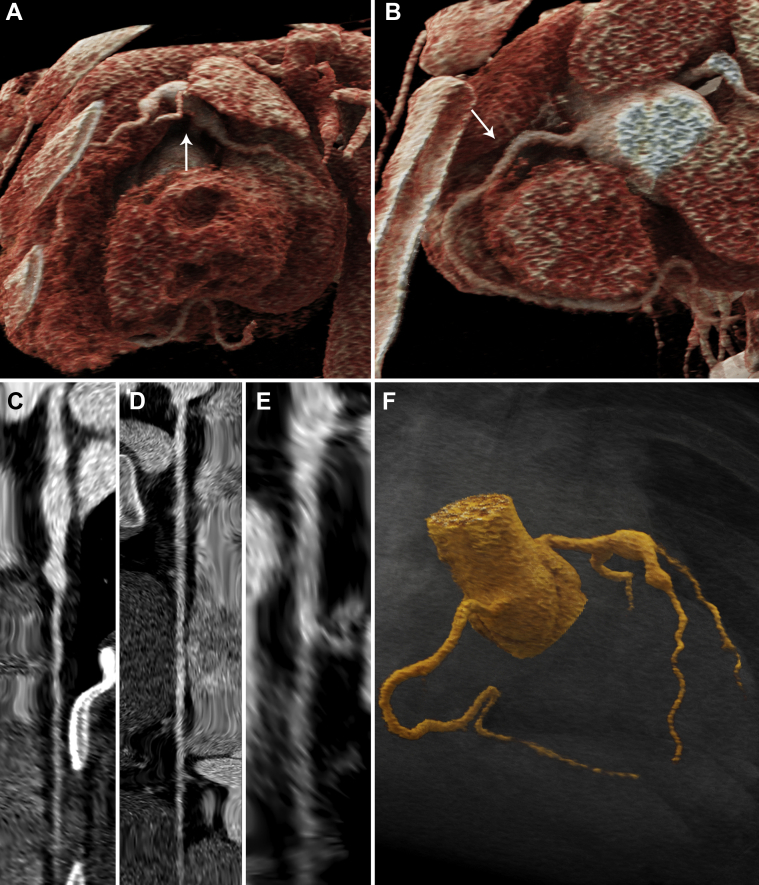
Echocardiography (day 31) showing (A) a large (z-score +5) left main coronary artery aneurysm (arrow) at the bifurcation and (B) a giant (z-score +17) proximal right coronary artery aneurysm (arrow). Computed tomography angiography (day 34) showing straightened multiplanar views of (C) the left main and left anterior descending arteries, (D) the right coronary artery, and (E) the left circumflex artery with aforementioned dilations. (F) A 3-dimensional reconstruction of coronary architecture.
Whereas cardiac computed tomography (day 34) (Figures 4A to 4F, Video 2) showed stable coronary size, a follow-up echocardiogram (day 89) revealed improvement. He remains on prednisolone and triple anticoagulation.
Patient 3
A 12-year-old female child presented with 5 days of fever and headache and 1 day of hand swelling and erythema, conjunctivitis, rash, dyspnea, and chest pain. The SARS-CoV-2 PCR result was negative; the IgG antibody test result was positive. MIS-C was diagnosed. IVIG and enoxaparin were started. An echocardiogram (day 6) showed mild RCA dilation prompting IVMP initiation.
On days 7 to 8, hypotension, altered mental status, and respiratory failure developed, requiring noninvasive positive-pressure ventilation and transfer to intensive care. An echocardiogram (day 9) revealed severely depressed left ventricular function (ejection fraction 35%) that required epinephrine and milrinone administration. IVIG was redosed.
On day 9, extreme agitation led to intubation for optimized sedation. Out of concern for ongoing inflammation, anakinra was started. Aspirin was initiated. Fever subsided by day 13. She was extubated by day 14; vasoactive drugs were discontinued by day 15. Anakinra and corticosteroids were gradually decreased until day 17.
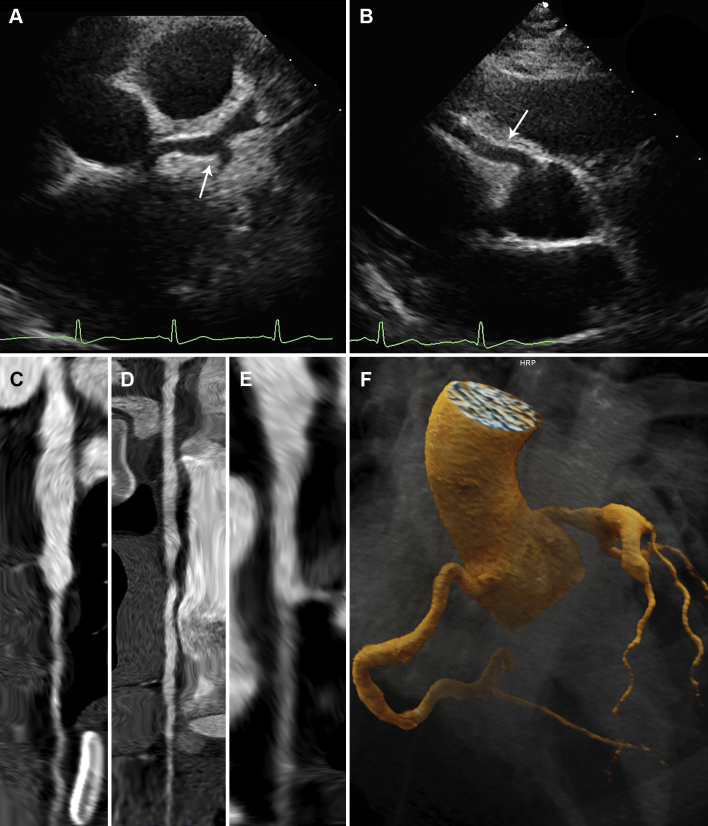
On day 18, an echocardiogram demonstrated large LAD artery and moderate RCA aneurysms. She received tocilizumab; anakinra was discontinued. On day 20, an echocardiogram and cardiac computed tomography (Figures 5A to 5F, Video 3) showed further LAD artery enlargement with giant aneurysm formation (8 mm; z-score +10.1); anakinra was restarted.
Figure 5.
Early Cardiac Imaging (Patient 3)
Echocardiography (day 22) showing (A) moderate (z-score +4) left main coronary artery dilation (arrow), a proximal aneurysm (z-score +12) of the left anterior descending artery, and (B) mild (z-score +3.9) right coronary artery dilation (arrow). Computed tomography angiography (day 20) showing straightened multiplanar views of the (C) the left main and left anterior descending arteries, (D) the right coronary artery, and (E) the left circumflex artery with aforementioned dilations. (F) A 3-dimensional reconstruction of coronary architecture.
An echocardiogram (day 22) showed worsening coronary enlargement (Figures 5A to 5F). Pulse IVMP was added (days 22-24), with coronary size stabilization. She remained afebrile, and her laboratory values improved. Prednisone and anakinra were gradually tapered on an outpatient basis.
Cardiac computed tomography (day 173) showed improving coronary aneurysms (Figures 6A to 6F, Video 3). She remains well on aspirin and warfarin.
Figure 6.
Follow-Up Cardiac Imaging (Patient 3)
Follow-up computed tomography angiography (day 173) showing 3-dimensional reconstruction of (A) the left main and left anterior descending arteries (arrow) and (B) the right coronary artery (arrow), with mild interval improvement. Straightened multiplanar views of (C) the left main and left anterior descending arteries, (D) the right coronary artery, and (E) the left circumflex artery, with mild interval improvement. (F) A 3-dimensional reconstruction of coronary architecture.
Discussion
We report 3 cases of giant coronary aneurysms secondary to MIS-C. MIS-C has been associated with cardiac complications in up to 87% of cases, manifesting as various degrees of myocardial dysfunction, pericardial effusion, arrhythmias, valvulopathies, and/or coronary artery involvement. Coronary artery aneurysms and dilation, usually mild or moderately sized, occur in 6% to 24% of cases (1, 2, 3). The z-score, a normalized measurement of coronary artery diameter on the basis of body surface area, and diameter in millimeters are used to classify coronary dilation and aneurysms by size. Reports of giant coronary aneurysms (z-score ≥10 or diameter ≥8 mm) in MIS-C are scarce (4, 5, 6, 7, 8). Patient 1 is the youngest case of MIS-C–related giant coronary aneurysms described to date.
Given the broad definition of MIS-C (Table 1), distinguishing between MIS-C and alternative diagnoses can be difficult, as exemplified by patient 1, whose therapy initially targeted acute coronavirus disease-19, and the determination of MIS-C depended on coronary abnormalities and seroconversion. Differentiating between Kawasaki disease (KD) and MIS-C is particularly challenging, considering that up to 64% of patients with MIS-C meet criteria for complete or atypical KD, as did our cases (2). KD was a consideration for our patients, more so for Patient 1 given his age. However, African and Hispanic heritage and gastrointestinal and neurologic symptoms are features more frequent in MIS-C than in KD, and their presence favored MIS-C in our cases (9). Our patients’ respiratory compromise and need for intensive care are also uncommon in KD. Laboratory parameters, including higher C-reactive protein levels, lymphopenia, and thrombocytopenia, may help differentiate MIS-C from KD (3,9). Interestingly, all our patients exhibited thrombocytosis at the time of giant coronary aneurysm diagnosis or evolution, despite initial thrombocytopenia (Patients 2 and 3). Laboratory value measurements in relation to the time of development of coronary changes have not been analyzed and warrant further exploration. Similarly, predictors of complications in MIS-C remain under investigation. Mucocutaneous manifestations and severe inflammation, as observed in our patients, have been proposed as risk factors for coronary abnormalities in MIS-C, along with conjunctival injection and higher peak interleukin-6 and pro–B-type natriuretic peptide levels (3,5,7,10).
Data from randomized trials addressing therapy-based outcomes for MIS-C are lacking. Given the similarities between MIS-C and KD, their management also overlaps. Corticosteroids, IVIG, and biologic agents have been used in MIS-C management (2,3,7,9). IVIG and/or corticosteroids are favored as first-line therapy, especially in patients with KD-like features, although a protective effect of IVIG against adverse coronary outcomes has not been established in MIS-C (9). Importantly, coronary aneurysms developed in Patients 2 and 3 after IVIG administration. Furthermore, clinical status was not predictive of echocardiographic aneurysm progression in our patients; thus, serial echocardiography proved essential in their management.
As evidenced by our case series, the use of biologic agents for MIS-C has not been standardized. Infliximab has been used for MIS-C–related aneurysms, with partial or complete reversibility of the coronary changes (4,5,7). Interleukin-1 (anakinra) and interleukin-6 (tocilizumab) blockade has been used in refractory MIS-C; these agents were used successfully in Patient 3 (2). Multicenter studies are needed to determine the most effective therapies for MIS-C. Long-term outcomes are still to be determined, and multispecialty follow-up is essential.
Funding Support and Author Disclosures
Dr. Prahalad has received partial support from the Marcus Foundation Inc.; and has served on a Macrophage Activation Syndrome Adjudication Committee for Novartis Pharmaceuticals. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Dynamic Early Cardiac Imaging (Patient #1). Cine 3-dimensional reconstruction of prospectively-gated computed tomography angiography images (day 24), showing (A) left anterior descending artery and (B) Right coronary artery aneurysms. (C) A 3-dimensional reconstruction of coronary architecture. (D) Proximal left anterior descending artery mural thrombus manifesting as a filling defect on 3-dimensional reconstruction (black arrow).
Dynamic Early Cardiac Imaging (Patient #2). Cine 3-dimensional reconstruction of prospectively-gated computed tomography angiography images (day 34) showing (A) a left main coronary artery aneurysm at the bifurcation, and (B) a giant proximal right coronary artery aneurysm. (C) A 3-dimensional reconstruction of coronary architecture.
Dynamic Computed Tomography Images (Patient #3). Computed tomography angiography showing 3-dimensional reconstruction of coronary architecture during (A) early presentation (day 20) and (B) at follow-up (day 173) with mild interval improvement.
References
- 1.Sperotto F., Friedman K.G., Son M.B.F., VanderPluym C.J., Newburger J.W., Dionne A. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur J Pediatr. 2021;180(2):307–322. doi: 10.1007/s00431-020-03766-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alsaied T., Tremoulet A.H., Burns J.C. Review of cardiac involvement in multisystem inflammatory syndrome in children. Circulation. 2021;143:78–88. doi: 10.1161/CIRCULATIONAHA.120.049836. [DOI] [PubMed] [Google Scholar]
- 3.Godfred-Cato S., Bryant B., Leung J. COVID-19–associated multisystem inflammatory syndrome in children—United States, March–July 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shahbaznejad L., Navaeifar M.R., Abbaskhanian A., Hosseinzadeh F., Rahimzadeh G., Rezai M.S. Clinical characteristics of 10 children with a pediatric inflammatory multisystem syndrome associated with COVID-19 in Iran. BMC Pediatr. 2020;20:513. doi: 10.1186/s12887-020-02415-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whittaker E., Bamford A., Kenny J. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bautista-Rodriguez C., Sanchez-de-Toledo J., Clark B.C. Multisystem inflammatory syndrome in children: an international survey. Pediatrics. 2021;147(2) doi: 10.1542/peds.2020-024554. [DOI] [PubMed] [Google Scholar]
- 8.Valverde I., Singh Y., Sanchez-de-Toledo J. Acute cardiovascular manifestations in 286 children with multisystem inflammatory syndrome associated with COVID-19 infection in Europe. Circulation. 2021;143:21–32. doi: 10.1161/CIRCULATIONAHA.120.050065. [DOI] [PubMed] [Google Scholar]
- 9.Henderson L.A., Canna S.W., Friedman K.G. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 2. Arthritis Rheumatol. 2021;73(4):e13–e29. doi: 10.1002/art.41616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abrams J.Y., Oster M.E., Godfred-Cato S.E. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: a retrospective surveillance study. Lancet Child Adolesc Health. 2021;5(5):323–331. doi: 10.1016/S2352-4642(21)00050-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dynamic Early Cardiac Imaging (Patient #1). Cine 3-dimensional reconstruction of prospectively-gated computed tomography angiography images (day 24), showing (A) left anterior descending artery and (B) Right coronary artery aneurysms. (C) A 3-dimensional reconstruction of coronary architecture. (D) Proximal left anterior descending artery mural thrombus manifesting as a filling defect on 3-dimensional reconstruction (black arrow).
Dynamic Early Cardiac Imaging (Patient #2). Cine 3-dimensional reconstruction of prospectively-gated computed tomography angiography images (day 34) showing (A) a left main coronary artery aneurysm at the bifurcation, and (B) a giant proximal right coronary artery aneurysm. (C) A 3-dimensional reconstruction of coronary architecture.
Dynamic Computed Tomography Images (Patient #3). Computed tomography angiography showing 3-dimensional reconstruction of coronary architecture during (A) early presentation (day 20) and (B) at follow-up (day 173) with mild interval improvement.