Abstract
Study Objectives:
Solriamfetol, a dopamine/norepinephrine reuptake inhibitor, is approved in the United States and European Union for excessive daytime sleepiness in adults with narcolepsy (75–150 mg/day) or obstructive sleep apnea (OSA; 37.5–150 mg/day). In 12-week studies, solriamfetol was associated with improvements in quality of life in participants with narcolepsy or OSA. These analyses evaluated the long-term effects of solriamfetol on quality of life.
Methods:
Participants with narcolepsy or OSA who completed previous solriamfetol studies were eligible. A 2-week titration was followed by a maintenance phase ≤ 50 weeks (stable doses: 75, 150, or 300 mg/day). Quality of life assessments included Functional Outcomes of Sleep Questionnaire short version, Work Productivity and Activity Impairment Questionnaire: Specific Health Problem, and 36-Item Short Form Health Survey version 2. Mean (standard deviation) changes from baseline to end of study were evaluated. Data were summarized descriptively. Adverse events were assessed.
Results:
Safety population comprised 643 participants (417 OSA, 226 narcolepsy). Solriamfetol improved Functional Outcomes of Sleep Questionnaire short version Total scores (mean change [standard deviation], 3.7 [3.0]) and 36-Item Short Form Health Survey version 2 Physical and Mental Component Summary scores (3.1 [6.9] and 4.3 [8.4], respectively); improvements were sustained throughout treatment. On Work Productivity and Activity Impairment Questionnaire: Specific Health Problem, solriamfetol reduced (improved) % presenteeism, % overall work impairment, and % activity impairment by a minimum of 25%. Common adverse events (≥ 5%): headache, nausea, nasopharyngitis, insomnia, dry mouth, anxiety, decreased appetite, and upper respiratory tract infection.
Conclusions:
Long-term solriamfetol treatment was associated with clinically meaningful, sustained improvements in functional status, work productivity, and quality of life for up to 52 weeks. Adverse events were similar between narcolepsy and OSA.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Name: A Long-Term Safety Study of JZP-110 in the Treatment of Excessive Sleepiness in Subjects with Narcolepsy or OSA; Identifier: NCT02348632; URL: https://clinicaltrials.gov/ct2/show/NCT02348632
Citation:
Weaver TE, Pepin J-L, Schwab R, et al. Long-term effects of solriamfetol on quality of life and work productivity in participants with excessive daytime sleepiness associated with narcolepsy or obstructive sleep apnea. J Clin Sleep Med. 2021;17(10):1995–2007.
Keywords: JZP-110, Sunosi, HRQoL, sleep disorders, OSA, functional status, work productivity, narcolepsy, quality of life
BRIEF SUMMARY
Current Knowledge/Study Rationale: Short- and long-term treatment with solriamfetol have been shown to improve excessive daytime sleepiness in participants with narcolepsy or obstructive sleep apnea. In addition, data have demonstrated that short-term treatment has been associated with improvements in quality of life. The current study evaluated whether improvements in quality of life are sustained during long-term treatment.
Study Impact: Long-term solriamfetol treatment was associated with sustained improvements in functional status, work productivity, and quality of life for up to 52 weeks. These findings demonstrate the clinical relevance of the sustained efficacy findings observed during long-term solriamfetol treatment.
INTRODUCTION
Excessive daytime sleepiness (EDS) is a prominent symptom of narcolepsy and obstructive sleep apnea (OSA). EDS affects all patients with narcolepsy, and population-based studies estimate that 9–22% of patients with OSA continue to experience EDS despite treatment with continuous positive airway pressure (CPAP).1,2 EDS has been shown to have many adverse consequences in participants with narcolepsy and OSA, including impairments in daytime functioning, reduced quality of life (QoL), and increased risk of workplace and driving accidents.3–9 Furthermore, a recent study demonstrated a relationship between severity of EDS (as measured by the Epworth Sleepiness Scale) and degree of impairment in QoL and functioning such that increasing severity of EDS is associated with a greater degree of impairment.10
Solriamfetol (Sunosi; Jazz Pharmaceuticals, Palo Alto, CA) is a dopamine and norepinephrine reuptake inhibitor approved in the United States and the European Union to improve wakefulness in adults with EDS associated with narcolepsy (75 to 150 mg/day) or OSA (37.5 to 150 mg/day).11,12 Solriamfetol has been shown to have short-term and long-term efficacy in the treatment of EDS in patients with narcolepsy or OSA.13–16 In two 12-week, phase 3 studies, solriamfetol was also associated with improvements in functioning, work productivity, and health-related QoL (HRQoL) in participants with EDS associated with narcolepsy or OSA6,17; however, it is unknown whether these improvements are sustained with long-term treatment. The most common treatment-emergent adverse events (TEAEs) in solriamfetol-treated participants with narcolepsy or OSA in clinical trials were headache, nausea, decreased appetite, nasopharyngitis, dry mouth, insomnia, and anxiety.13,14,16
The current analyses examined whether long-term treatment with solriamfetol had sustained benefits with regard to functional status, work/activity impairment, and HRQoL. Adverse events (AEs) were also evaluated in this long-term study.
METHODS
Study design
The full methods of this study have been reported previously16 and are briefly summarized here. This was a long-term (up to 52 weeks), open-label extension phase 3 clinical trial that evaluated the efficacy and safety of solriamfetol for the treatment of EDS in adult participants with narcolepsy or OSA. The study was conducted at 79 clinical investigative sites in North America (63 sites) and Europe (16 sites) between May 26, 2015, and December 8, 2017. The study was approved by institutional review boards or ethics committees at each institution and was performed in accordance with the Declaration of Helsinki. All participants provided written informed consent. Results of the primary analyses of this study have been reported previously16; results of the secondary analyses are presented below.
Participants
Participants with narcolepsy or OSA who had previously completed a phase 2 or 3 clinical trial of solriamfetol (including NCT02806895/EudraCT 2015-003930-28 and NCT02806908/EudraCT 2015-003931-36 and several trials with published results13–15,18,19) were eligible for enrollment in the open-label extension study. Due to differences in time between prior study completion and enrollment in the current study, there were 2 groups. Group A was enrolled in the open-label extension study immediately after completion of 12-week phase 3 studies. Participants in Group B historically completed phase 2 studies or the 6-week phase 3 study and were subsequently enrolled in the open-label extension study (the time between studies could range from 0 to 48 months, depending on the timing of the completion of the parent study).
In addition to having met the inclusion criteria and completing a previous clinical trial of solriamfetol, participants also had a usual nightly total sleep time of at least 6 hours, body mass index between 18 and 45 kg/m2, and were, in the opinion of the investigator, able to take solriamfetol for 40 (Group A) or 52 weeks (Group B). Key exclusion criteria included usual bedtime later than 1am, nighttime or variable shiftwork, or having experienced any serious AE related to solriamfetol or any AE in a previous study that may prevent safe participation in the current study. Use of prescription or over-the-counter medications that could impact the evaluation of EDS was prohibited during the study.
Treatment
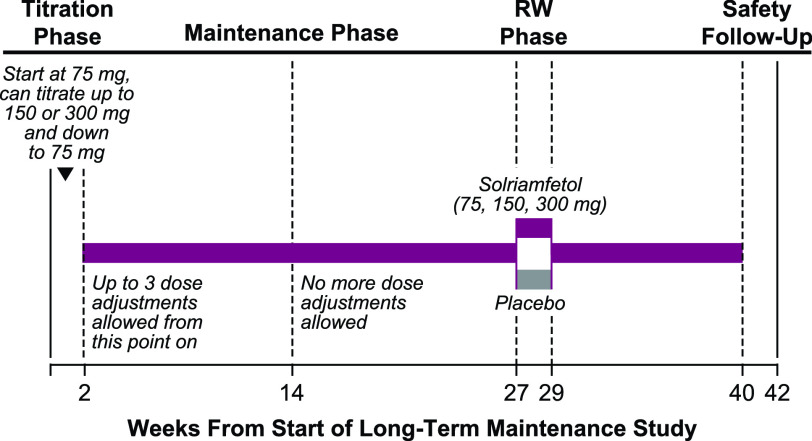
Open-label solriamfetol treatment for all participants in both Group A and Group B, regardless of treatment assignment in the parent study, was initiated at 75 mg during a 2-week titration phase, during which the dose could be titrated up 1 dose level every 3 days (to 150 mg and then a maximum dose of 300 mg) (Figure 1).16 Investigators were instructed to titrate solriamfetol to the maximum dose tolerated by each participant to maximize efficacy based on their clinical judgment of patients’ self-reported response. No objective measurements were used to determine dosage levels. During the titration phase, down-titration was permitted at any time for safety reasons. After the titration phase, up to 3 dose adjustments were allowed within the first 12 weeks of the maintenance phase. Participants whose doses could not be successfully adjusted were discontinued from the study. The titration phase was followed by an open-label maintenance phase (75, 150, or 300 mg), with a total study duration of 40 weeks for Group A and 52 weeks for Group B. After approximately 6 months of open-label treatment with solriamfetol, a subgroup (approximately 300 participants from Groups A and B were planned; there were no randomization criteria with respect to group) entered a 2-week placebo-controlled, randomized withdrawal phase (Figure 1), and the maintenance phase was resumed after the completion of the randomized withdrawal phase.
Figure 1. Study design.a.
aStudy design for Group A only; Group B was similar except total duration was 52 weeks. Adapted from Malhotra et al.16 RW = randomized withdrawal.
Functional outcomes, QoL, and work productivity measures
The impact of EDS on functional status, work/activity impairment related to narcolepsy or OSA, and general HRQoL was assessed with the Functional Outcomes of Sleep Questionnaire short version (FOSQ-10), Work Productivity and Activity Impairment Questionnaire: Specific Health Problem (WPAI: SHP; specified as narcolepsy or OSA), and 36-item Short Form Health Survey Version 2 (SF-36v2), respectively. These assessments were administered at weeks 14, 27, 29 (FOSQ-10 only), and 40 for participants in Group A and at weeks 14, 26, 28 (FOSQ-10 only), 39, and 52 for participants in Group B, and at any early termination visits that occurred after week 2. Changes in FOSQ-10, WPAI:SHP, and SF-36v2 endpoints were assessed during the open-label phase as changes from baseline of the parent study (Group A) or baseline of the current study (Group B) to the end of the open-label phase; baseline of the parent study was used for Group A, as this represents the true pretreatment baseline for these participants. Changes in FOSQ-10 from the beginning to the end of the randomized withdrawal phase (Groups A and B combined) were also assessed; other outcomes were not assessed for the randomized withdrawal phase.
The FOSQ-10 is a 10-item questionnaire that assesses how daytime sleepiness affects daily functioning,20 with higher scores representing better functioning, and a total score < 17.9 indicating abnormal functioning.20,21
The WPAI:SHP is a 6-item questionnaire that estimates how a specific health problem has impacted work productivity and activity impairment outside of work over the previous week.22 Specifically, percent work time missed (absenteeism), percent impairment while working (presenteeism), percent overall work impairment (absenteeism + presenteeism), and percent activity impairment (ability to do regular daily activities other than work at a job) due to a specific health problem (narcolepsy or OSA) were analyzed. Work impairment was evaluated in employed participants, whereas activity impairment was evaluated in all participants. Normative values have not been established for the WPAI:SHP in narcolepsy or OSA.
The SF-36v2 is a 36-question health survey that estimates a functional health status profile with Physical Component Summary and Mental Component Summary scores, as well as 8 subscales (Physical Functioning, Role Physical, Bodily Pain, General Health, Vitality, Social Functioning, Role Emotional, and Mental Health).23,24 Each subscale has a normative mean value of 50 with a standard deviation of 10 in the US population.25
Safety
Safety and tolerability of solriamfetol were evaluated across the entire study and based on TEAEs.
Statistical analysis
All data during the open-label phase were analyzed for the safety population, defined as participants who received at least 1 dose of solriamfetol. Changes in efficacy endpoints during the open-label phase were analyzed with descriptive statistics; no formal statistical testing, including missing data imputation, was performed. Summary statistics were reported for the overall study population and by indication (narcolepsy or OSA) for the combined solriamfetol dose group. For the purposes of this report, results reported for the assessments in the open-label period focus on Group A, which comprised the largest cohort of participants with long-term exposure to solriamfetol (data for Group B are provided in supplemental figures).
Changes in FOSQ-10 total score from beginning to end of the randomized withdrawal phase were analyzed for the modified intent-to-treat population (ie, all participants who were randomized in the randomized withdrawal phase, took at least 1 dose of study treatment in the randomized withdrawal phase, and had evaluable data at the end of the randomized withdrawal phase), overall and by indication, using analysis of covariance models; because no adjustments for multiplicity were used, P values are nominal. Results from the randomized withdrawal phase include data from participants in Groups A and B combined.
Baseline patient demographics, baseline clinical characteristics, and adverse events were summarized descriptively and are reported for the full safety population (Groups A and B combined).
RESULTS
Participant population
A total of 651 participants were screened for eligibility (Figure 2). Of these, 643 participants, 417 (64.9%) with OSA and 226 (35.1%) with narcolepsy (114/226 [50.4%] with cataplexy) were included in the safety population. Groups A and B consisted of 519 (80.7%) and 124 (19.3%) participants, respectively. A total of 458 participants (overall, 71.2%; OSA, 73.9%; narcolepsy, 66.4%) completed the study, with the most frequently reported reasons for discontinuation being AEs (narcolepsy, n = 23; OSA, n = 38) and lack of efficacy (narcolepsy, n = 39; OSA, n = 15) (Figure 2).16
Figure 2. Participant disposition.
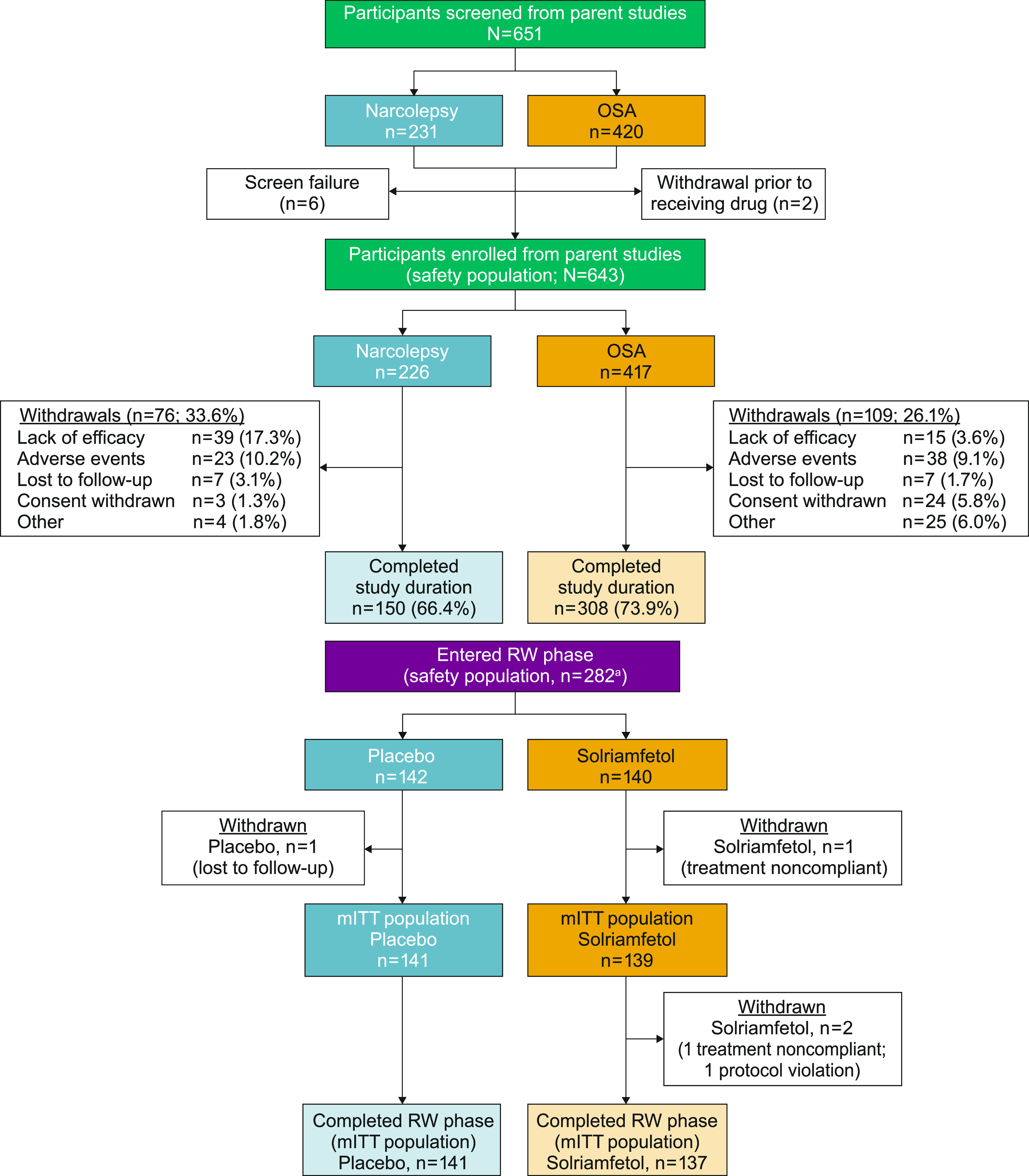
aA total of 226 from Group A, 56 from Group B. Adapted from Malhotra et al.16 mITT = modified intent-to-treat, OSA = obstructive sleep apnea, RW = randomized withdrawal.
Participants with OSA were, on average, older, predominately male, and had a higher body mass index compared with participants with narcolepsy (Table 1). In general, participants with narcolepsy had greater impairments in functional status and work productivity/activity impairment at baseline compared with participants with OSA (Table 2).
Table 1.
Baseline demographic characteristics of the safety population.a
| Variable | Overall (N = 643) | Narcolepsy (n = 226) | OSA (n = 417) |
|---|---|---|---|
| Age, years, mean (SD) | 49.3 (14.2) | 38.7 (13.5) | 55.1 (10.7) |
| Male, n (%) | 337 (52.4) | 80 (35.4) | 257 (61.6) |
| Race, n (%) | |||
| White | 506 (78.7) | 181 (80.1) | 325 (77.9) |
| Black | 109 (17.0) | 33 (14.6) | 76 (18.2) |
| Asian | 15 (2.3) | 4 (1.8) | 11 (2.6) |
| Native Hawaiian/Other Pacific Islander | 3 (0.5) | 1 (0.4) | 2 (0.5) |
| American Indian/Alaska Native | 2 (0.3) | 1 (0.4) | 1 (0.2) |
| BMI, kg/m2, mean (SD) | 31.7 (5.9) | 28.3 (5.8) | 33.5 (5.1) |
| Medical history, n (%) | |||
| Hypertension | 242 (37.6) | 39 (17.3) | 203 (48.7) |
| Hyperlipidemia | 98 (15.2) | 8 (3.5) | 90 (21.6) |
| Type 2 diabetes | 90 (14.0) | 7 (3.1) | 83 (19.9) |
aGroups A and B combined. BMI = body mass index, OSA = obstructive sleep apnea, SD = standard deviation.
Table 2.
Clinical characteristics of the safety population.
| Variable | Overall (N = 643) | Narcolepsy (n = 226) | OSA (n = 417) |
|---|---|---|---|
| Baseline FOSQ-10 total score,a mean (SD) | n = 518 | n = 185 | n = 333 |
| 13.1 (3.2) | 11.6 (3.0) | 13.9 (3.0) | |
| Baseline WPAI:SHP,a mean (SD) | |||
| % Work time missed (absenteeism) | n = 332 | n = 111 | n = 221 |
| 5.3 (11.8) | 9.8 (16.3) | 3.1 (7.9) | |
| % Impairment while working (presenteeism) | n = 326 | n = 108 | n = 218 |
| 43.5 (26.6) | 58.9 (21.1) | 35.9 (25.7) | |
| % Overall work impairment | n = 324 | n = 107 | n = 217 |
| 52.6 (26.5) | 67.5 (19.0) | 45.2 (26.5) | |
| % Activity impairment | n = 516 | n = 184 | n = 332 |
| 49.6 (26.8) | 64.1 (22.4) | 41.6 (25.7) | |
| Baseline SF-36v2,a mean (SD) | n = 519 | n = 186 | n = 333 |
| Physical component score | 46.2 (8.5) | 46.2 (8.7) | 46.1 (8.4) |
| Mental component score | 48.6 (9.2) | 45.4 (9.5) | 50.4 (8.5) |
| Role physical | 42.8 (10.6) | 39.5 (11.3) | 44.7 (9.6) |
| General health | 49.1 (9.3) | 48.7 (9.6) | 49.4 (9.2) |
| Vitality | 42.8 (9.5) | 39.7 (9.3) | 44.6 (9.2) |
| Physical functioning | 48.4 (8.1) | 49.4 (8.0) | 47.9 (8.1) |
| Bodily pain | 49.2 (9.7) | 50.3 (10.2) | 48.5 (9.3) |
| Role emotional | 48.8 (9.8) | 47.4 (10.4) | 49.5 (9.4) |
| Mental health | 51.2 (7.9) | 49.5 (8.7) | 52.1 (7.3) |
| Social functioning | 46.1 (10.4) | 41.6 (11.5) | 48.6 (8.9) |
aBaseline in the parent study. FOSQ-10 = Functional Outcomes of Sleep Questionnaire short version, OSA = obstructive sleep apnea, SD = standard deviation, SF-36v2 = Short Form Health Survey Version 2, WPAI:SHP = Work Productivity and Activity Impairment Questionnaire: Specific Health Problem.
Functional outcomes, QoL, and work productivity endpoints
For Group A, mean (standard deviation [SD]) FOSQ-10 total scores at parent study baseline were 13.1 (3.2), 11.6 (3.0), and 13.9 (3.0) in the overall, narcolepsy, and OSA populations, respectively. Mean (SD) changes from baseline of the parent study to week 40 in FOSQ-10 total scores were 3.7 (3.0), 3.7 (3.2), and 3.8 (2.9) for the overall, narcolepsy, and OSA populations, respectively (Figure 3). Improvements were sustained for the duration of solriamfetol treatment. Group B showed similar results (Figure S1A (266KB, pdf) in the supplemental material).
Figure 3. Change in FOSQ-10 Total score during the open-label phase.a.
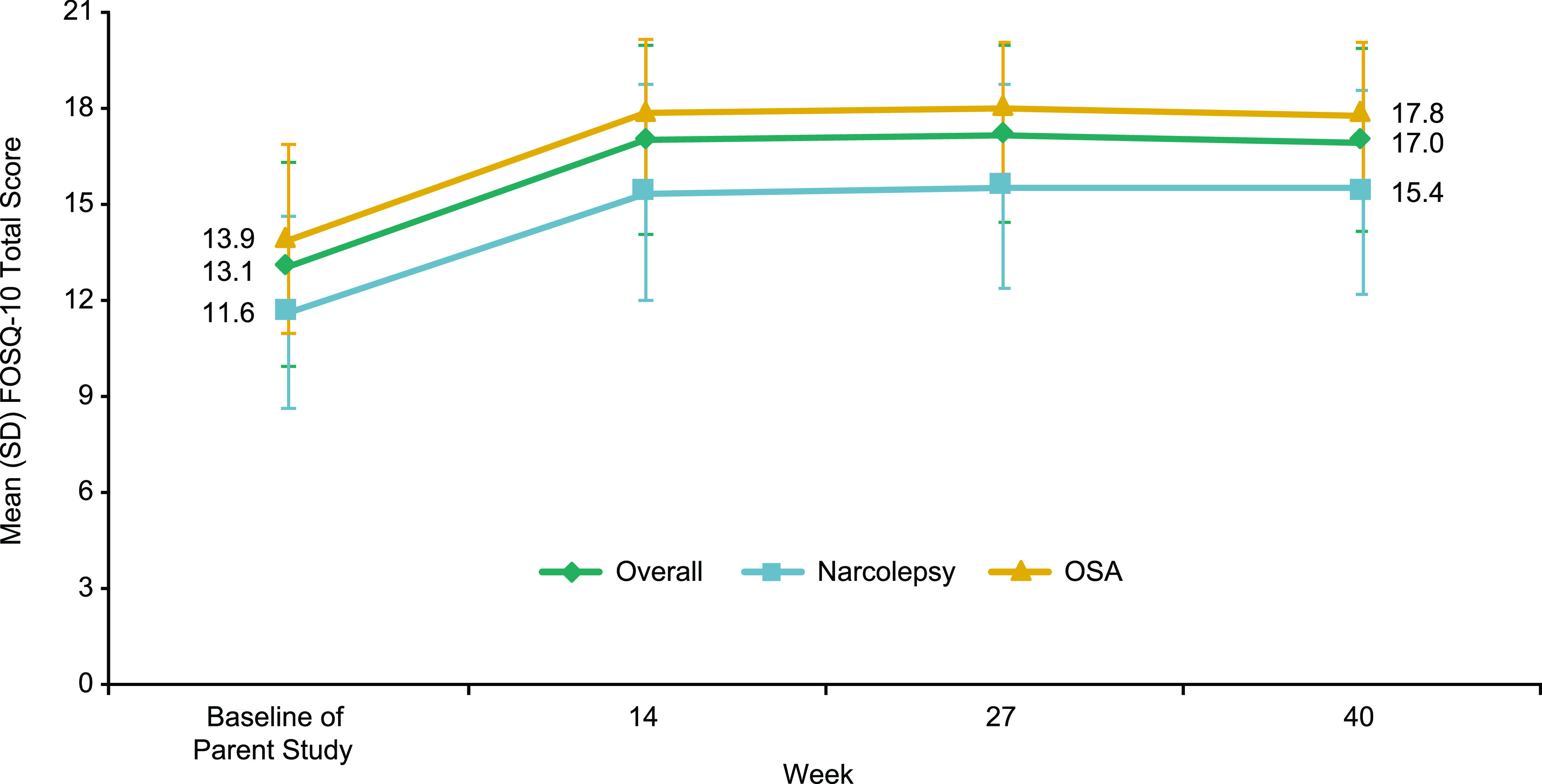
aGroup A only. A positive change from baseline indicates improvement. FOSQ-10 = Functional Outcomes of Sleep Questionnaire short version, OSA = obstructive sleep apnea, SD = standard deviation.
At the beginning of the randomized withdrawal phase (Figure 4), mean (SD) FOSQ-10 total scores were 17.1 (2.7) and 17.4 (2.8) for the placebo and solriamfetol groups, respectively. Participants randomized to placebo showed greater worsening on the FOSQ-10 (mean [SD] change: −2.5 [3.14]) compared with participants who remained on solriamfetol (mean [SD] change: −0.82 [2.09]; least-squares mean difference [95% confidence interval]: 1.7 [1.11, 2.37]; P < .0001). These effects were similar in the subgroups of participants with narcolepsy (mean [SD] changes: placebo, −2.92 [2.8]; solriamfetol, −0.78 [1.6] and OSA (mean [SD] changes: placebo, −2.4 [3.3]; solriamfetol, −0.83 [2.3]).
Figure 4. Change in FOSQ-10 total score during the randomized withdrawal phase.a.
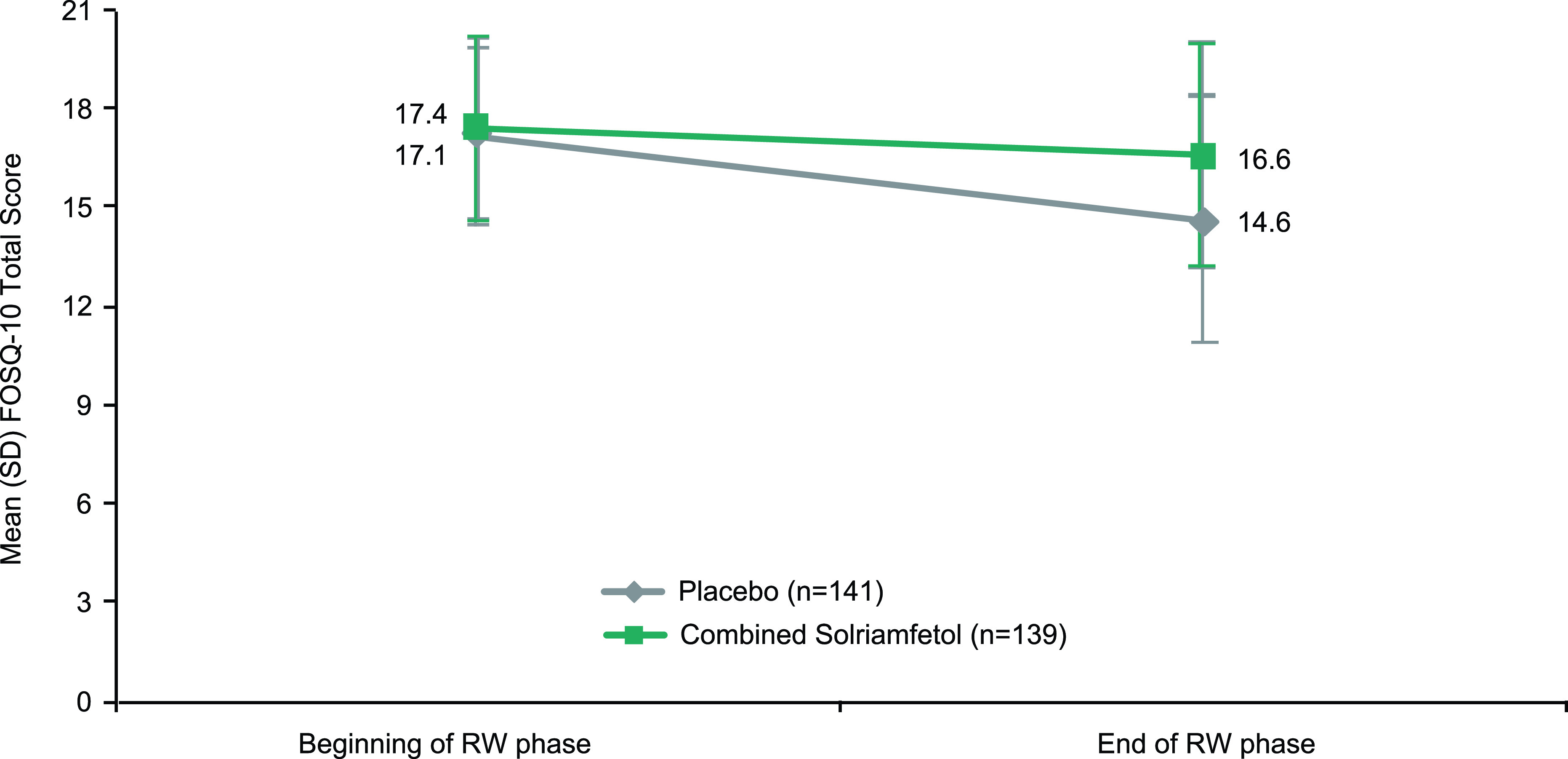
aGroups A and B combined. The end of the RW phase was scheduled at week 29 for Group A and week 28 for Group B. FOSQ-10 = Functional Outcomes of Sleep Questionnaire short version, OSA = obstructive sleep apnea, RW = randomized withdrawal, SD = standard deviation.
For Group A, at week 40, FOSQ-10 scores ≥ 17.9 (in the normal range) were reported for 47.1%, 21.6%, and 59.8% of participants in the overall, narcolepsy, and OSA populations, respectively (Figure 5). Data for Group B at week 52 are shown in Figure S1B (266KB, pdf) .
Figure 5. Percentage of participants with FOSQ-10 total scores in normal range (≥ 17.9).a.
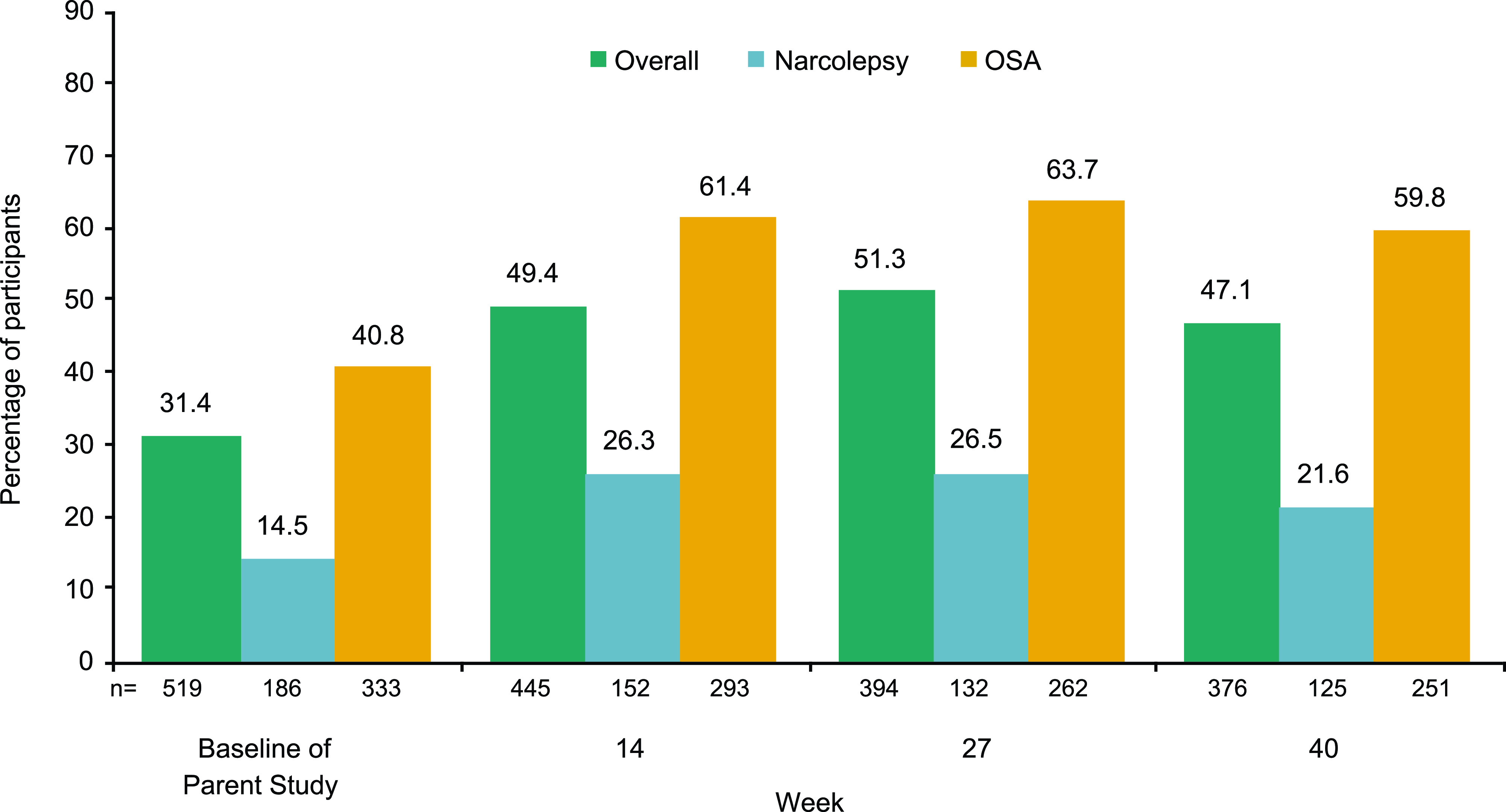
aGroup A only. Percentages are based on the number of participants with no missing data at a specific visit. FOSQ-10 = Functional Outcomes of Sleep Questionnaire short version, OSA = obstructive sleep apnea.
Approximately 63% of participants in Group A and 52% of participants in Group B were employed based on the number of participants who provided responses to the WPAI:SHP work-related items. At baseline, rates of absenteeism were low overall and higher among participants with narcolepsy compared with participants with OSA (Figure 6A). Specifically, at baseline, participants with narcolepsy reported missing 9.8% of work hours per week, whereas participants with OSA reported missing 3.1%. Likely due to the higher rate of absenteeism at baseline in participants with narcolepsy, a reduction (improvement) in the percentage of absenteeism during solriamfetol treatment was more apparent in the population with narcolepsy than those with OSA. Participants with narcolepsy and those with OSA reported high levels of impairment at baseline based on mean percent impairment while working (presenteeism), mean percent overall work impairment, and mean percent activity impairment (Figure 6B–D). In Group A (overall population), the percentage of presenteeism, overall work impairment, and activity impairment during solriamfetol treatment were reduced (improved) by ≥ 25% from baseline of the parent study; these improvements were maintained for the duration of treatment and generally greater in the narcolepsy population (Figure 6B–D). Specifically, from baseline of the parent study to week 40, mean percent presenteeism improved [mean change (SD)] by 25.2% (26.3), 29.5% (25.5), and 23.3% (26.6) for the overall, narcolepsy, and OSA populations, respectively. Mean percent overall work impairment improved by 26.1% (28.6), 29.5% (28.8), and 24.6% (28.4), respectively. Mean percent activity impairment improved by 26.7% (28.0), 26.7% (27.9), and 26.8% (28.0), respectively. Group B showed similar results (Figure S2A–D (266KB, pdf) in the supplemental material).
Figure 6. Change in EDS-related work/activity impairment on the WPAI:SHP.a.
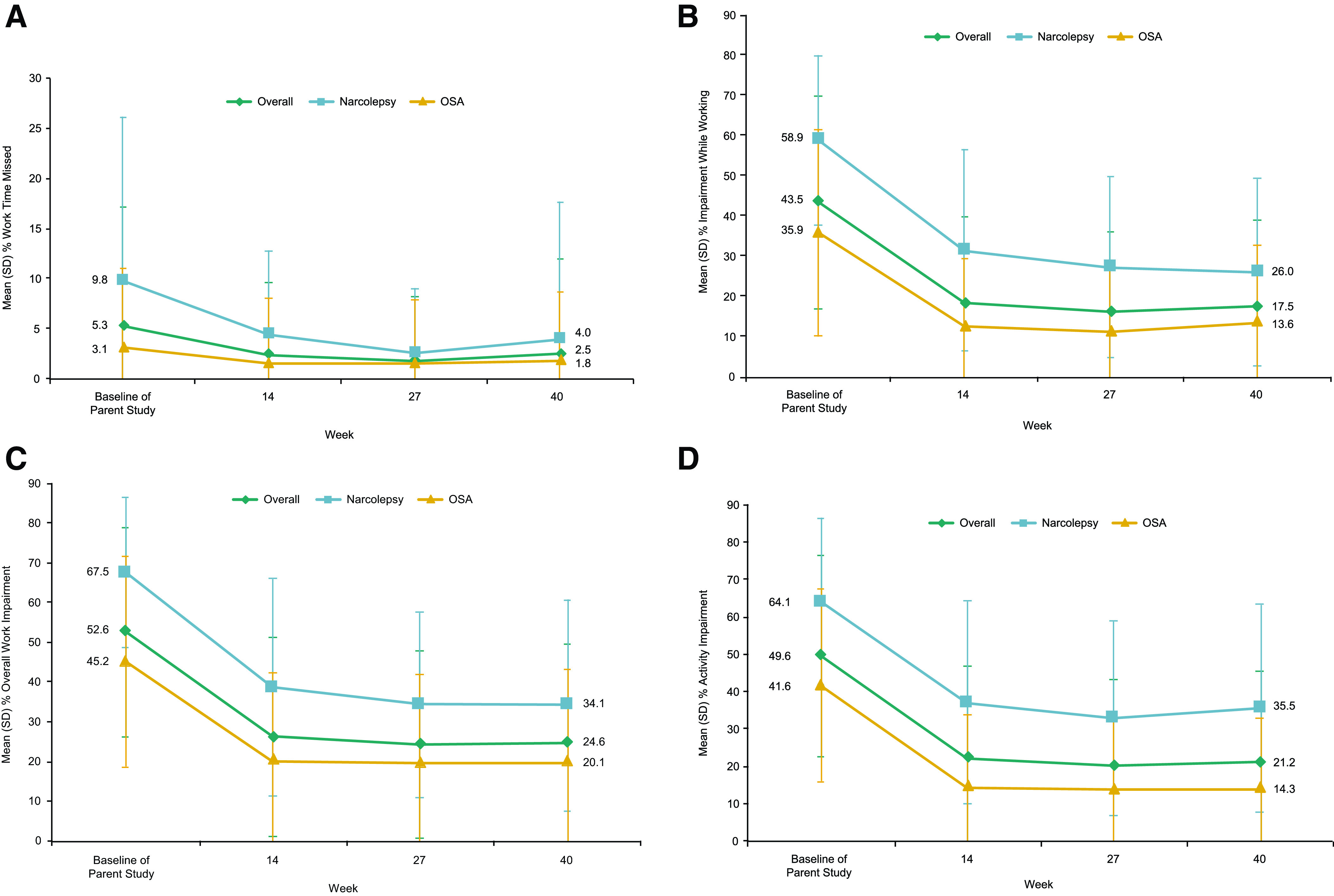
(A) Absenteeism (work time missed). (B) Presenteeism (impairment while working). (C) Overall work impairment. (D) Activity impairment. aGroup A only. bRegular daily activities other than work at a job. A negative change from baseline indicates improvement. EDS = excessive daytime sleepiness, OSA = obstructive sleep apnea, SD = standard deviation, WPAI:SHP = Work Productivity and Activity Impairment Questionnaire: Specific Health Problem.
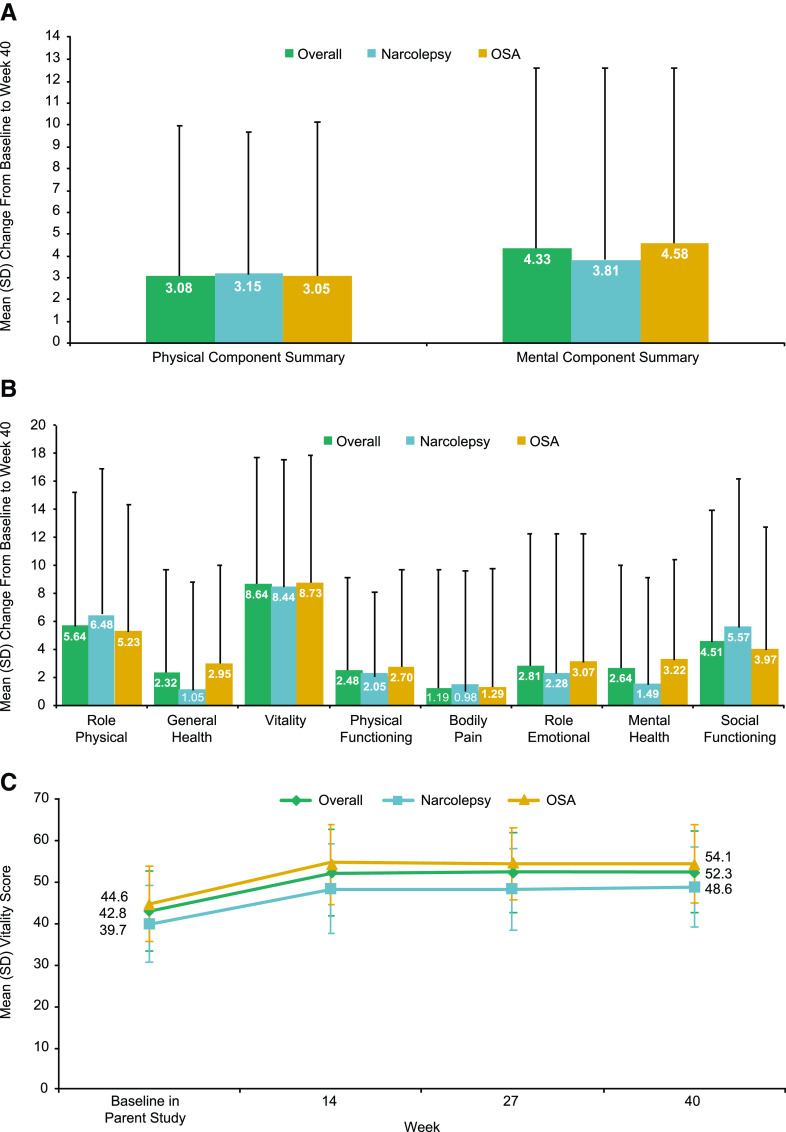
For the SF-36v2, baseline mean (SD) Physical Component Summary scores for Group A were 46.2 (8.5), 46.2 (8.7), and 46.2 (8.5) for the overall, narcolepsy, and OSA populations, respectively. Baseline mean (SD) Mental Component Summary scores were 48.6 (9.2), 45.4 (9.5), and 50.4 (8.5), respectively. Physical Component Summary and Mental Component Summary scores increased (improved) with solriamfetol treatment, and these increases were maintained for the duration of treatment (Figure 7A). There was high variability between participants on the individual subscale scores of the SF-36v2 (Figure 7B); the largest magnitude of change was observed in the vitality domain for both participants with narcolepsy and OSA (Figure 7C). Group B showed similar results on SF-36v2 measures (Figure S3A-C (266KB, pdf) in the supplemental material).
Figure 7. Change in general HRQoL scores on the SF-36v2.a.
(A) Physical and Mental Component Summary scores. (B) Subscale scores. (C) Vitality domain. aGroup A only. A positive change from baseline indicates improvement. HRQoL = health-related quality of life, OSA = obstructive sleep apnea, SD = standard deviation, SF-36v2 = Short Form Health Survey version 2.
Safety
TEAEs were experienced by 169/226 (74.8%) participants with narcolepsy and 313/417 (75.1%) participants with OSA. The most commonly reported TEAEs (≥ 5%) were headache, nausea, nasopharyngitis, insomnia, dry mouth, and anxiety in participants with narcolepsy and OSA and decreased appetite in participants with narcolepsy and upper respiratory tract infection in participants with OSA (Table 3). Serious TEAEs were reported in 27 (4.2%) participants, 6 with narcolepsy (2.7%) and 21 with OSA (5.0%). Five participants, 1 with narcolepsy and 4 with OSA, had a serious TEAE that was considered related to the study drug by the investigator. These events included cerebrovascular accident, stillbirth, atrial fibrillation, spontaneous abortion, and retinal vein occlusion. There was 1 death due to sepsis: a 70-year-old immunosuppressed male with OSA on solriamfetol 300 mg who had a history of diabetes mellitus, rheumatoid arthritis, pulmonary fibrosis, coronary artery disease, and bipolar disorder. The death was considered unrelated to the study drug by the investigator. A total of 59 (9.2%) participants, 23 (10.2%) with narcolepsy and 36 (8.6%) with OSA, had a TEAE leading to discontinuation from the study.
Table 3.
TEAEs across the entire study.16
| TEAEs | Number (%) of Participants in Combined Solriamfetol Groups | ||
|---|---|---|---|
| Overall (N = 643) | Narcolepsy (n = 226) | OSA (n = 417) | |
| At least 1 TEAE | 482 (75.0) | 169 (74.8) | 313 (75.1) |
| Serious TEAE | 27 (4.2) | 6 (2.7) | 21 (5.0) |
| TEAEs leading to discontinuation | 59 (9.2) | 23 (10.2) | 36 (8.6) |
| Death | 1 (0.2)a | 0 | 1 (0.2) |
| Most common TEAEsb | |||
| Headache | 71 (11.0) | 31 (13.7) | 40 (9.6) |
| Nausea | 57 (8.9) | 26 (11.5) | 31 (7.4) |
| Nasopharyngitis | 54 (8.4) | 19 (8.4) | 35 (8.4) |
| Insomnia | 51 (7.9) | 16 (7.1) | 35 (8.4) |
| Dry mouth | 47 (7.3) | 14 (6.2) | 33 (7.9) |
| Anxiety | 46 (7.2) | 21 (9.3) | 25 (6.0) |
| Decreased appetite | 32 (5.0) | 18 (8.0) | 14 (3.4) |
| Upper respiratory tract infection | 32 (5.0) | 10 (4.4) | 22 (5.3) |
aDue to sepsis. b≥5% in combined solriamfetol groups for any indication. OSA = obstructive sleep apnea, TEAE = treatment-emergent adverse event.
DISCUSSION
Baseline assessments from the current study demonstrated that narcolepsy and OSA were associated with impairments in daily functioning and work/activity in this study population. Specifically, baseline FOSQ-10 total scores were lower than normative values (normal, 17.9–20.0)20 for both participants with narcolepsy and OSA, suggesting difficulties with productivity, activity level, attention, social outcomes, and relationships due to EDS. Self-reported impairment in completing activities at work and outside of work may be considered in the context of data from matched controls for patients with narcolepsy or OSA reported in population-based studies. For instance, in matched controls without narcolepsy from a population-based study, rates of presenteeism, overall work impairment, and activity impairment were estimated to be 22%, 25%, and 34%, respectively.26 In the current study, participants with narcolepsy reported rates of presenteeism, overall work impairment, and activity impairment of 59%, 68%, and 64%, respectively, at baseline. In matched controls without OSA from a population-based study, rates of presenteeism, overall work impairment, and activity impairment were 15%, 17%, and 20%, respectively.27 In the current study, participants with OSA reported rates of presenteeism, overall work impairment, and activity impairment of 36%, 45%, and 42%, respectively, at baseline. These data support the high level of functional impairment associated with EDS in these 2 conditions.
The current analyses demonstrated sustained benefits in functional status, HRQoL, and work/activity impairment with long-term solriamfetol treatment for up to 52 weeks, as assessed by the FOSQ-10, SF-36v2, and WPAI:SHP. Notably, mean improvements in functional status on the FOSQ-10 exceeded the threshold for a minimally important difference (change of 1.7–2.028), suggesting clinically meaningful improvements, and were sustained for the duration of solriamfetol treatment for some participants. Results during the randomized withdrawal phase also demonstrated long-term maintenance of efficacy of solriamfetol on the FOSQ-10 under double-blind, placebo-controlled conditions. On the SF-36v2 scales for role physical, vitality, and social functioning, mean changes from baseline also exceeded the threshold for minimal clinically important differences (2 points for role physical and 3 points for vitality and social functioning29), reflecting clinically meaningful improvement in these domains. In addition, solriamfetol led to sustained improvements in work and activity impairment as assessed by the WPAI:SHP. In addition to the observation of sustained improvements during the study, it is important to note that mean FOSQ-10 total scores at the end of the long-term study for the total population and for the OSA subgroup approached normative values.20
The safety profile observed in the present study was similar in participants with narcolepsy and those with OSA, with roughly 9.2% of participants discontinuing the study due to AEs, a rate consistent with those seen in other long-term treatment studies in populations with EDS associated with narcolepsy, OSA, or shift work disorder (9–13%).30–32 Although no new common TEAEs were reported in either patient subset of this long-term study, overall incidence of any TEAE (74.8%) and any serious TEAE (2.7%) in the narcolepsy group was slightly higher than that reported in the 12-week phase 3 study of solriamfetol for patients with narcolepsy (68.4% and 0.6%, respectively).13 Similarly, the incidence of any TEAE (75.1%) and any serious TEAE (5.0%) in the present study was higher than that observed in the 12-week phase 3 study of solriamfetol for patients with OSA (67.9% and 0.8%, respectively).14 In addition to AEs, the other most common reason for discontinuation was lack of efficacy. A higher percentage of participants with narcolepsy discontinued due to lack of efficacy than participants with OSA (17.3% vs 3.6%),16 which may reflect the greater severity of disease and/or be due to the fact that other medications used to treat EDS were prohibited.
There is a paucity of available information regarding long-term risks and benefits of established therapies for EDS associated with narcolepsy and residual EDS in treated OSA. Although short-term studies are important, long-term data are essential to inform clinical decision making for long-term care of patients with chronic disease. Therefore, a strength of the current study is the 1-year duration. Furthermore, short-term studies with stimulants have shown improvements in EDS; however, it is not clear whether long-term reductions in sleepiness translate to other clinically meaningful improvements in functioning, HRQoL, or work productivity. For example, caffeine has been shown to improve alertness in the setting of sleep deprivation,33 but data are lacking to support the use of caffeine as a long-term strategy for improving QoL or work place performance. The current analyses demonstrate that solriamfetol improves daily functioning, HRQoL, and work productivity, in addition to EDS, with long-term treatment and in a similar manner as what has been previously reported with shorter-term treatment.6,16,17
Importantly, pharmacologic treatment for EDS should not replace primary treatment for the underlying cause of EDS. Solriamfetol is not indicated to treat the underlying airway obstruction in OSA; therefore, it is critical to encourage OSA patients to use a therapy that treats the underlying airway obstruction.11 CPAP is the gold standard treatment for OSA, but adherence to therapy is variable.34 Data support the optimization of CPAP adherence such that greater improvements in outcomes are observed with increased CPAP usage.35 Notably, a separate analysis of participants in the current study indicated that solriamfetol did not have a clinically meaningful effect on primary OSA therapy adherence.36 However, even with optimal adherence, some OSA patients will continue to experience residual EDS.1,2 The mechanism underlying this observation has been debated and may involve injury to the periaqueductal gray region.37 Human studies have also shown white matter compromise in OSA patients with residual EDS, including myelin diffusivity and axonal shrinkage.38,39 Regardless of the mechanism underlying persistent EDS in OSA, population estimates indicate that 9–22% of CPAP-treated patients may have residual EDS.1,2 In addition, many OSA patients have nonspecific complaints, including fatigue, nonrestorative sleep, and the need for caffeine, and many patients with narcolepsy have persistent symptoms that could benefit from the use of 1 or more medications.
Despite the strengths of the current study, there were a number of limitations. First, no direct comparisons with other stimulant or wake-promoting therapies were conducted; therefore, it is inconclusive whether one therapy is more effective and/or has a more tolerable side effect profile than another. A number of wake-promoting agents have been evaluated in open-label long-term studies and have demonstrated long-term efficacy under open-label conditions. For example, modafinil and armodafinil have been investigated in open-label, long-term extension studies in participants with narcolepsy or OSA, with data suggesting long-term efficacy in reducing EDS.30,40 Long-term studies of these agents in populations with narcolepsy or OSA have also demonstrated improvements in QoL measures, including the SF-36v2, FOSQ-10, and Brief Fatigue Inventory.30,41,42 Pitolisant has also demonstrated long-term efficacy in reducing EDS in participants with narcolepsy, as well as improvement in patient-reported health status as assessed by the European Quality of Life Questionnaire visual analog scale.43 Future research evaluating the comparative effectiveness of these various pharmacotherapies may be beneficial.
In addition, based on the long-term extension study design, participants were recruited from different studies and, as a result, participants who did not complete the parent studies were, by design, not eligible for long-term extension assessments. In theory, participants who discontinued the parent studies are likely to be less amenable to therapy, based on either lack of efficacy or occurrence of side effects. Although the design of the parent studies (ie, randomization to a specific treatment arm and inclusion of a placebo group) may affect the extent to which such an assumption holds in the current study, it should be noted that including only participants who completed a prior clinical trial may overestimate treatment effects and minimize intolerability and side effects in a long-term extension study. Despite this, the current study population is generalizable to the real-world patient population seeking treatment for EDS with a wake-promoting agent based on baseline demographics, disease characteristics, and underlying comorbidities. Future subgroup analyses to identify patients who are likely to be maximally responsive with minimal side effects would be informative. Despite these limitations, these findings provide important insights into the sustained treatment of patients with narcolepsy or OSA.
CONCLUSIONS
Long-term treatment with solriamfetol was associated with clinically meaningful, sustained improvements in functional status, work productivity, and HRQoL in participants with narcolepsy and OSA for up to 52 weeks. TEAEs associated with long-term solriamfetol treatment included headache, nausea, nasopharyngitis, insomnia, dry mouth, anxiety, decreased appetite, and upper respiratory tract infection and were similar between participants with narcolepsy and those with OSA.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. This study was funded by Jazz Pharmaceuticals. Jazz Pharmaceuticals has worldwide development, manufacturing, and commercialization rights to solriamfetol, excluding certain jurisdictions in Asia. SK Biopharmaceuticals, the discoverer of the compound (also known as SKL-N05), maintains rights in 12 Asian markets, including Korea, China, and Japan. T.E. Weaver has received royalties for use of the FOSQ from Philips Respironics, ResMed, ResMed Germany, Nyxoah, Jazz Pharmaceuticals, Cook Medical, RWS, Stratevi, WCG MedAvante Prophase, Bayer AG, LivaNova, and Evidation Health. J.-L. Pepin has received lecture fees or conference traveling grants from ResMed, Perimetre, Philips, Fisher and Paykel, AstraZeneca, Jazz Pharmaceuticals, Agiradom, and Teva and has received unrestricted research funding from ResMed, Philips, GlaxoSmithKline, Bioprojet, Fondation de la Recherche Medicale (Foundation for Medical Research), Direction de la Recherche Clinique du CHU de Grenoble (Research Branch Clinic CHU de Grenoble), and fond de dotation “Agir pour les Maladies Chroniques” (endowment fund “Acting for Chronic Diseases”). R. Schwab has received research funding from the National Institutes of Health and Inspire Medical Systems, ResMed, and CryOSA. He has received income from Merck for medical education and royalties from Up-To-Date. C. Shapiro has received research funding from the National Institutes of Health and the Canadian Institutes of Health Research and has served on the speakers’ bureau for Jazz Pharmaceuticals. J. Hedner has served on the speakers’ bureaus for AstraZeneca, Philips Respironics, Itamar Medical, and BresoTec and serves as a board member for Cereus Pharma. N. Foldvary-Schaefer served as a consultant for Jazz Pharmaceuticals and receives research support from Jazz Pharmaceuticals, Suven Life Sciences, and Takeda Pharmaceuticals. P.J. Strollo Jr has received consultancy fees and honoraria from Inspire Medical Systems, ResMed, Philips-Respironics, Emmi Solutions, Jazz Pharmaceuticals, and Itamar; has received research funding from the National Institutes of Health and Inspire Medical Systems; and has a provisional patent for positive airway pressure with integrated oxygen. G. Mayer has served on the speakers’ bureau for UCB Pharma, Jazz Pharmaceuticals, and Janssen Pharma and is a board member of the International REM Sleep Behavior Study Group. K. Sarmiento serves on advisory boards for Jazz Pharmaceuticals and has received research funding from ResMed. M. Baladi is an employee of Jazz Pharmaceuticals who, in the course of this employment, has received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc. M. Bron and L. Lee are former employees of Jazz Pharmaceuticals who, in the course of this employment, received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc. P. Chandler is an employee of Jazz Pharmaceuticals who, in the course of this employment, has received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc. A. Malhotra has served as a principal investigator for a Jazz Pharmaceuticals study but receives no personal income from Jazz Pharmaceuticals. He received income from Livanova, Equilium, and Corvus for medical education on topics unrelated to this article. ResMed gave a philanthropic donation to the University of California San Diego in support of a sleep center. M. Ahmed reports no conflicts of interest.
ACKNOWLEDGMENTS
T. Weaver and A. Malhotra wrote the first draft of the manuscript. Under the direction of the authors, Hannah Ritchie, PhD, and Jeannette Fee of Peloton Advantage, LLC, an OPEN Health company, provided medical writing and editorial support for this article, which was funded by Jazz Pharmaceuticals.
ABBREVIATIONS
- AE,
adverse event
- CPAP,
continuous positive airway pressure
- EDS,
excessive daytime sleepiness
- FOSQ-10,
Functional Outcomes of Sleep Questionnaire short version
- HRQoL,
health-related quality of life
- OSA,
obstructive sleep apnea
- QoL,
quality of life
- SD,
standard deviation
- SF-36v2,
Short Form Health Survey version 2
- TEAE,
treatment-emergent adverse event
- WPAI:SHP,
Work Productivity and Activity Impairment Questionnaire: Specific Health Problem
REFERENCES
- 1. Gasa M , Tamisier R , Launois SH , et al. ; Scientific Council of the Sleep Registry of the French Federation of Pneumology-FFP . Residual sleepiness in sleep apnea patients treated by continuous positive airway pressure . J Sleep Res . 2013. ; 22 ( 4 ): 389 – 397 . [DOI] [PubMed] [Google Scholar]
- 2. Pépin JL , Viot-Blanc V , Escourrou P , et al . Prevalence of residual excessive sleepiness in CPAP-treated sleep apnoea patients: the French multicentre study . Eur Respir J . 2009. ; 33 ( 5 ): 1062 – 1067 . [DOI] [PubMed] [Google Scholar]
- 3. Dodel R , Peter H , Spottke A , et al . Health-related quality of life in patients with narcolepsy . Sleep Med . 2007. ; 8 ( 7-8 ): 733 – 741 . [DOI] [PubMed] [Google Scholar]
- 4. Hirsch Allen AJM , Bansback N , Ayas NT . The effect of OSA on work disability and work-related injuries . Chest . 2015. ; 147 ( 5 ): 1422 – 1428 . [DOI] [PubMed] [Google Scholar]
- 5. Philip P , Sagaspe P , Lagarde E , et al . Sleep disorders and accidental risk in a large group of regular registered highway drivers . Sleep Med . 2010. ; 11 ( 10 ): 973 – 979 . [DOI] [PubMed] [Google Scholar]
- 6. Emsellem HA , Thorpy MJ , Lammers GJ , et al . Measures of functional outcomes, work productivity, and quality of life from a randomized, phase 3 study of solriamfetol in participants with narcolepsy . Sleep Med . 2020. ; 67 : 128 – 136 . [DOI] [PubMed] [Google Scholar]
- 7. Omachi TA , Claman DM , Blanc PD , Eisner MD . Obstructive sleep apnea: a risk factor for work disability . Sleep . 2009. ; 32 ( 6 ): 791 – 798 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nena E , Steiropoulos P , Constantinidis TC , Perantoni E , Tsara V . Work productivity in obstructive sleep apnea patients . J Occup Environ Med . 2010. ; 52 ( 6 ): 622 – 625 . [DOI] [PubMed] [Google Scholar]
- 9. Mulgrew AT , Ryan CF , Fleetham JA , et al . The impact of obstructive sleep apnea and daytime sleepiness on work limitation . Sleep Med . 2007. ; 9 ( 1 ): 42 – 53 . [DOI] [PubMed] [Google Scholar]
- 10. Jennum P , Coaquira JP , Mettam S , Kharkevitch T , Bron M , Cambron-Mellott J . Socioeconomic and humanistic burden of illness of EDS associated with OSA in the EU5: analysis of National Health and Wellness Survey Data [poster 096]. Presented at: Biennial World Sleep Congress; September 20-25, 2019; Vancouver, Canada.
- 11. Sunosi® (solriamfetol) tablets Prescribing Information. Palo Alto, CA: : Jazz Pharmaceuticals, Inc; ; 2019. [Google Scholar]
- 12. Sunosi® (solriamfetol) tablets Summary of Product Characteristics. Dublin, Ireland: : Jazz Pharmaceuticals Ireland Ltd; ; 2020. [Google Scholar]
- 13. Thorpy MJ , Shapiro C , Mayer G , et al . A randomized study of solriamfetol for excessive sleepiness in narcolepsy . Ann Neurol . 2019. ; 85 ( 3 ): 359 – 370 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schweitzer PK , Rosenberg R , Zammit GK , et al. ; TONES 3 Study Investigators . Solriamfetol for excessive sleepiness in obstructive sleep apnea (TONES 3): A randomized controlled trial . Am J Respir Crit Care Med . 2019. ; 199 ( 11 ): 1421 – 1431 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Strollo PJ Jr , Hedner J , Collop N , et al. ; Tones 4 Study Investigators . Solriamfetol for the treatment of excessive sleepiness in OSA: a placebo-controlled randomized withdrawal study . Chest . 2019. ; 155 ( 2 ): 364 – 374 . [DOI] [PubMed] [Google Scholar]
- 16. Malhotra A , Shapiro C , Pepin JL , et al . Long-term study of the safety and maintenance of efficacy of solriamfetol (JZP-110) in the treatment of excessive sleepiness in participants with narcolepsy or obstructive sleep apnea . Sleep . 2020. ; 43 ( 2 ): zsz220 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weaver TE , Drake CL , Benes H , et al . Effects of solriamfetol on quality of life measures from a 12-week phase 3 randomized trial . Ann Am Thorac Soc . 2020. ; 17 ( 8 ): 998 – 1007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bogan RK , Feldman N , Emsellem HA , et al . Effect of oral JZP-110 (ADX-N05) treatment on wakefulness and sleepiness in adults with narcolepsy . Sleep Med . 2015. ; 16 ( 9 ): 1102 – 1108 . [DOI] [PubMed] [Google Scholar]
- 19. Ruoff C , Swick TJ , Doekel R , et al . Effect of oral JZP-110 (ADX-N05) on wakefulness and sleepiness in adults with narcolepsy: a phase 2b study . Sleep . 2016. ; 39 ( 7 ): 1379 – 1387 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weaver TE , Maislin G , Dinges DF , et al . Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning . Sleep . 2007. ; 30 ( 6 ): 711 – 719 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chasens ER , Ratcliffe SJ , Weaver TE . Development of the FOSQ-10: a short version of the Functional Outcomes of Sleep Questionnaire . Sleep . 2009. ; 32 ( 7 ): 915 – 919 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reilly MC , Zbrozek AS , Dukes EM . The validity and reproducibility of a work productivity and activity impairment instrument . Pharmacoeconomics . 1993. ; 4 ( 5 ): 353 – 365 . [DOI] [PubMed] [Google Scholar]
- 23. Hays RD , Stewart AL . Construct validity of MOS health measures . In: Stewart AL , Ware JE , eds. Measuring Functioning and Well-Being: The Medical Outcomes Study Approach . Durham, NC: : Duke University Press; ; 1992. : 325 – 342 . [Google Scholar]
- 24. Ware JE Jr , Sherbourne CD . The MOS 36-Item Short-Form health survey (SF-36). I. Conceptual framework and item selection . Med Care . 1992. ; 30 ( 6 ): 473 – 483 . [PubMed] [Google Scholar]
- 25. Maglinte GA , Hays RD , Kaplan RM . US general population norms for telephone administration of the SF-36v2 . J Clin Epidemiol . 2012. ; 65 ( 5 ): 497 – 502 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Flores NM , Villa KF , Black J , Chervin RD , Witt EA . The humanistic and economic burden of narcolepsy . J Clin Sleep Med . 2016. ; 12 ( 3 ): 401 – 407 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stepnowsky C , Sarmiento KF , Bujanover S , Villa KF , Li VW , Flores NM . Comorbidities, health-related quality of life, and work productivity among people with obstructive sleep apnea with excessive sleepiness: findings from the 2016 US National Health and Wellness Survey . J Clin Sleep Med . 2019. ; 15 ( 2 ): 235 – 243 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weaver TE , Crosby RD , Bron M , Menno D , Mathias SD . Using multiple anchor-based and distribution-based estimates to determine the minimal important difference (MID) for the FOSQ-10 [abstract 0612]. Sleep . 2018. ; 41 ( Suppl 1 ): A227 . [Google Scholar]
- 29. Yarlas A , Miller K , Wen W , et al . A randomized, placebo-controlled study of the impact of the 7-day buprenorphine transdermal system on health-related quality of life in opioid-naïve patients with moderate-to-severe chronic low back pain . J Pain . 2013. ; 14 ( 1 ): 14 – 23 . [DOI] [PubMed] [Google Scholar]
- 30. Black JE , Hull SG , Tiller J , Yang R , Harsh JR . The long-term tolerability and efficacy of armodafinil in patients with excessive sleepiness associated with treated obstructive sleep apnea, shift work disorder, or narcolepsy: an open-label extension study . J Clin Sleep Med . 2010. ; 6 ( 5 ): 458 – 466 . [PMC free article] [PubMed] [Google Scholar]
- 31. Mitler MM , Harsh J , Hirshkowitz M , Guilleminault C . Long-term efficacy and safety of modafinil (PROVIGIL((R))) for the treatment of excessive daytime sleepiness associated with narcolepsy . Sleep Med . 2000. ; 1 ( 3 ): 231 – 243 . [DOI] [PubMed] [Google Scholar]
- 32. U.S. Xyrem Multicenter Study Group . A 12-month, open-label, multicenter extension trial of orally administered sodium oxybate for the treatment of narcolepsy . Sleep . 2003. ; 26 ( 1 ): 31 – 35 . [PubMed] [Google Scholar]
- 33. Wesensten NJ , Killgore WD , Balkin TJ . Performance and alertness effects of caffeine, dextroamphetamine, and modafinil during sleep deprivation . J Sleep Res . 2005. ; 14 ( 3 ): 255 – 266 . [DOI] [PubMed] [Google Scholar]
- 34. Rotenberg BW , Murariu D , Pang KP . Trends in CPAP adherence over twenty years of data collection: a flattened curve . J Otolaryngol Head Neck Surg . 2016. ; 45 ( 1 ): 43 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Antic NA , Catcheside P , Buchan C , et al . The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA . Sleep . 2011. ; 34 ( 1 ): 111 – 119 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schweitzer PK , Strohl KP , Mayer G , et al . Effects of solriamfetol in a long-term trial of participants with obstructive sleep apnea who are adherent or nonadherent to airway therapy . J Clin Sleep Med . 2021. ; 17 ( 4 ): 659 – 668 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhu Y , Fenik P , Zhan G , et al . Selective loss of catecholaminergic wake active neurons in a murine sleep apnea model . J Neurosci . 2007. ; 27 ( 37 ): 10060 – 10071 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xiong Y , Zhou XJ , Nisi RA , et al . Brain white matter changes in CPAP-treated obstructive sleep apnea patients with residual sleepiness . J Magn Reson Imaging . 2017. ; 45 ( 5 ): 1371 – 1378 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang J , Weaver TE , Zhong Z , et al . White matter structural differences in OSA patients experiencing residual daytime sleepiness with high CPAP use: a non-Gaussian diffusion MRI study . Sleep Med . 2019. ; 53 : 51 – 59 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moldofsky H , Broughton RJ , Hill JD . A randomized trial of the long-term, continued efficacy and safety of modafinil in narcolepsy . Sleep Med . 2000. ; 1 ( 2 ): 109 – 116 . [DOI] [PubMed] [Google Scholar]
- 41. Hirshkowitz M , Black J . Effect of adjunctive modafinil on wakefulness and quality of life in patients with excessive sleepiness-associated obstructive sleep apnoea/hypopnoea syndrome: a 12-month, open-label extension study . CNS Drugs . 2007. ; 21 ( 5 ): 407 – 416 . [DOI] [PubMed] [Google Scholar]
- 42. Beusterien KM , Rogers AE , Walsleben JA , et al . Health-related quality of life effects of modafinil for treatment of narcolepsy . Sleep . 1999. ; 22 ( 6 ): 757 – 765 . [DOI] [PubMed] [Google Scholar]
- 43. Dauvilliers Y , Arnulf I , Szakacs Z , et al. ; HARMONY III study group . Long-term use of pitolisant to treat patients with narcolepsy: Harmony III Study . Sleep . 2019. ; 42 ( 11 ): zsz174 . [DOI] [PMC free article] [PubMed] [Google Scholar]