Abstract
The protein kinase Gcn2 stimulates translation of the yeast transcription factor Gcn4 upon amino acid starvation. Using genetic and biochemical approaches, we show that Gcn2 is regulated by the molecular chaperone Hsp90 in budding yeast Saccharomyces cerevisiae. Specifically, we found that (i) several Hsp90 mutant strains exhibit constitutive expression of a GCN4-lacZ reporter plasmid; (ii) Gcn2 and Hsp90 form a complex in vitro as well as in vivo; (iii) the specific inhibitors of Hsp90, geldanamycin and macbecin I, enhance the association of Gcn2 with Hsp90 and inhibit its kinase activity in vitro; (iv) in vivo, macbecin I strongly reduces the levels of Gcn2; (v) in a strain expressing the temperature-sensitive Hsp90 mutant G170D, both the accumulation and activity of Gcn2 are abolished at the restrictive temperature; and (vi) the Hsp90 cochaperones Cdc37, Sti1, and Sba1 are required for the response to amino acid starvation. Taken together, these data identify Gcn2 as a novel target for Hsp90, which plays a crucial role for the maturation and regulation of Gcn2.
In the budding yeast Saccharomyces cerevisiae, starvation for an amino acid triggers the transcription of more than 40 genes involved in amino acid biosynthesis. This “general amino acid control” requires the expression of the transcriptional activator Gcn4. Gcn4 expression is increased at the translational level by a regulatory mechanism involving phosphorylation of the α subunit of translation initiation factor eIF-2 (eIF-2α) by the protein kinase Gcn2 (18, 30, 31). When amino acids are abundant, four short open reading frames (uORFs) in the GCN4 mRNA leader sequence act in cis to repress translation of the GCN4 ORF. According to a model proposed by Abastado et al. (1), ribosomes translate the first encountered uORF (uORF1) and then resume scanning. Under normal conditions, essentially all ribosomes reinitiate translation at one of the remaining uORFs (uORF2 to uORF4) and fail to reinitiate translation at the GCN4 start codon. In amino acid-starved cells, ribosomes translate the first uORF and reinitiate at the GCN4 start codon instead of translating uORF2 to -4 because reinitiation is less efficient due to the reduction of functional eIF-2 levels.
It has been proposed (31) that Gcn2 is activated in amino acid-starved cells by direct binding of uncharged tRNA to a regulatory region located C-terminal to the kinase domain. This region has homology to the entire sequence of histidyl-tRNA synthetase (HisRS) (68, 69, 77). Consistent with this model, the HisRS-related domain in Gcn2 binds tRNAs in vitro and mutations in motifs characteristic of class II aminoacyl-tRNA synthetases abolish the phosphorylation of eIF-2α upon amino acid starvation (70).
Gcn2 is a serine/threonine protein kinase which belongs to the family of eIF-2α kinases, together with the heme-regulated inhibitor (HRI) (11), double-stranded RNA-activated protein kinase (PKR) (52, 67), Drosophila Gcn2 (44, 53), and cpc-3 from Neurospora crassa (54). The eIF-2α kinases are activated by various specific stress conditions: HRI is activated by heme deficiency, heat-shock, or heavy metal; PKR is activated by viral infection; and Gcn2 is activated by amino acid or purine starvation (for a review, see reference 16). The vertebrate eIF-2α kinase HRI has been shown to interact with the heat shock protein 90 (Hsp90) in rabbit reticulocyte lysates, and the activity of this molecular chaperone is required for full kinase activity (65).
Hsp90, a protein of the heat shock protein family, is expressed at high levels even under nonstress conditions and is required for viability in eukaryotes (for reviews see references 12, 33, and 49). Two genes encode closely related isoforms in mammals as well as in budding yeast. Deletion experiments with yeast have shown that the expression of at least one of the two Hsp90 isoforms, either Hsp82 or Hsc82, is essential for viability (7). Hsp90 can act as a molecular chaperone in vitro to promote refolding of denatured proteins, to hold denatured proteins in a folding-competent state for other chaperones, and to prevent protein unfolding and aggregation (see, for example, references 27, 34, and 75). A remarkably large subset of known Hsp90 substrates are signaling molecules, notably kinases and ligand-regulated transcription factors (see, for example, references 2, 48, 57, 63, and 76).
In this study, we have investigated the potential role of Hsp90 in yeast with respect to the eIF-2α kinase Gcn2. We have taken advantage of yeast genetics by using different strains containing mutations in HSP90. We present here genetic and biochemical evidence that Gcn2 requires Hsp90 for proper regulation. Moreover, Hsp90 is the first characterized protein interacting with Gcn2.
MATERIALS AND METHODS
Plasmids. (i) Reporter plasmids.
The GCN4-lacZ plasmids p180 and p227 have been described previously (29). In plasmid pLG/LUC, firefly luciferase coding sequences are under the control of a galactose-inducible promoter; the parent vector is plasmid pLGSD5-ATG (56) with the 2μ replicon and the URA3 marker.
(ii) Hsp90 plasmids.
Wild-type (WT) Hsp82, Hsp82 with a G313N mutation (Hsp82 G313N) and Hsp82 T525I (6), and Hsp82 G170D were expressed from plasmids pTCA/Hsp82 (6) and pHCA/Hsp82, pHCA/Hsp82 G313N and pHCA/Hsp82 T525I, and pTGPD/G170D (41), respectively, or various derivatives thereof with other auxotrophic markers. Human Hsp90β was expressed from plasmid p2HG/hHsp90β (36) or from plasmid p2U/hHsp90β, which was constructed as follows: the coding sequence of human Hsp90β was excised as a BamHI-SacI fragment from plasmid p2TG/hHsp90β (36) and cloned into the BamHI-SacI sites of p2U (45). Plasmids pHCA/Hsp82 G313N and pHCA/Hsp82 T525I are identical to plasmid pHCA/Hsp82 (36) except for the point mutations; they are the HIS3 versions of plasmids pTCA/Hsp82 G313N and pTCA/Hsp82 T525I (4) and were obtained by substituting the backbone of shuttle vector pRS313 for that of pRS314 (59). Plasmid p2TG/flag.Hsp82wt or G313N served to express WT Hsp82 or Hsp82 G313N with a FLAG epitope at the N terminus (36). Unless indicated, the strong constitutive promoter from the glyceraldehyde-3-phosphate dehydrogenase (GPD) gene TDH3 was used to drive expression. To obtain reduced levels of Hsp82 (about 10% of the level of Hsp82 plus Hsc82 in a wild-type strain), Hsp82 was expressed from a construct containing the leaky GAL1 promoter from strain GRS4 (47) fused to HSP82 coding sequences in plasmid pRS304 (59). On medium with 2% glucose, repression of this mutant GAL1 promoter construct is only partial and low levels of Hsp82 accumulate.
(iii) Gcn2 plasmids.
For galactose-inducible expression of Gcn2, plasmid pYES/Gcn2 was constructed by inserting the Gcn2 coding sequence into the BamHI-SphI sites of pYES2 (Invitrogen) as two fragments: a PCR-generated BamHI-XhoI fragment (with the sequence GGATCCCCGGGGCG preceding the AUG of GCN2) and an XhoI-SphI fragment from plasmid c-102-2 coding for the remainder of Gcn2 (68). To construct plasmid pYes/Gcn2K559V, the PCR fragment was generated with plasmid p530 (69) as the template. Plasmid pYES/Gcn2ΔN was constructed like pYES/Gcn2 except that the BamHI-XhoI PCR fragment started at codon 438 (beginning of the kinase domain). Plasmid p2U/GSTGcn2 was constructed by inserting the sequences encoding glutathione S-transferase (GST) and Gcn2 between the SacI and NaeI sites of the yeast expression vector p2U (45). It contains the GST coding sequence as a SacI-BglII fragment (with the sequence GAGCTCAAAGC preceding the AUG of GST) fused to the same GCN2 sequences present in plasmid pYES/Gcn2. Note that this results in an in-frame fusion of the BglII and BamHI sites of the GST and GCN2 moieties, respectively. Plasmid YcpGCN2:TRP1 was constructed from plasmid c-102-2. A SnaBI-PvuII insert was removed from plasmid c-102-2 and exchanged with the SnaBI-NaeI fragment containing the TRP1 gene from plasmid pRS314 (59). Note that the clones used in this study encode the 1,590-amino-acid version of Gcn2, which can complement a gcn2 deletion strain as an overexpressed GST fusion protein (see below and Table 2); a potential N-terminal extension of 69 amino acids has recently been revealed by the yeast genome project (yeast ORF: YDR283C).
TABLE 2.
Growth tests on 3-ATa
| Strainb | Growth on 3-AT |
|---|---|
| Hsp90 | |
| Hsp82wt | ++ |
| hHsp90β | ++ |
| 10% Hsp82wt | ++ |
| Hsp82 G313N | ++ |
| Hsp82 T525I | ++ |
| Cochaperones | |
| cdc37-1 strain | −/+ |
| cdc37-34 strain | −/+ |
| Δsba1 strain | −/+ |
| Δsti1 strain | −/+ |
| Gcn2 | |
| gcn2-1 strain | − |
| gcn2-1 strain with GSTGcn2 | ++ |
Strains were plated on medium with 30 mM 3-AT and scored for growth after 2 to 3 days. Note that growth is similar for all strains in the absence of 3-AT. ++, confluent growth; −/+, very weak growth; −, no visible growth.
The prefix HH1a-pHCA/ is omitted throughout.
Strains.
The parent strains and some of the derivatives are listed in Table 1. The yeast strain background HH1a (36) was used to replace the endogenous Hsp82/Hsc82 with Hsp90 mutants by plasmid shuffling. Plasmids were introduced into yeast by the LiAc/polyethylene glycol method and selected for on appropriate minimal media. The GCN2 deletion was introduced into strains HH1a-pHCA/Hsp82wt (36) and HH1a-pHCA/Hsp82 G313N with the ClaI-SphI insert of the plasmid YcpGCN2:TRP1 to yield strains OD1 and OD2, respectively. Following transformation, strains auxotrophic for tryptophan were selected and checked for GCN2 deletion by PCR and immunoblot analysis.
TABLE 1.
Yeast strains
| Strain | Genotypeb | Source or reference |
|---|---|---|
| HH1aa | MATa ade2-1 can1-100 his3-12, 16 leu2-3, 112 trp1-1 ura3-1 Δhsc82::LEU2 Δhsp82::LEU2 | 36 |
| HH1a-pHCA/Hsp82wt | HH1a/HSP82-CEN/ARS-HIS3 [pHCA/Hsp82] | 36 |
| HH1a-pHCA/Hsp82 G313N | HH1a/HSP82 G313N-CEN/ARS-HIS3 [pHCA/Hsp82 G313N] | This paper |
| HH1a-pHCA/Hsp82 T525I | HH1a/HSP82 T525I-CEN/ARS-HIS3 [pHCA/Hsp82 T525I] | This paper |
| HH1a-pHCA/10% Hsp82wt | HH1a/GAL*HSP82-CEN/ARS-His3 [pHCA/gal* Hsp82] | This paper |
| HH1a-p2HG/hHsp90β | HH1a/hHsp90β-2μ-HIS3 [p2HG/hHsp90β] | This paper |
| HH1a-G170D | HH1a/HSP82 G170D-CEN/ARS-TRP1 [pTGPD/G170D] | This paper |
| OD1 | HH1a/Δgcn2::TRP1 / HSP82-CEN/ARS-HIS3 [pHCA/Hsp82] | This paper |
| OD2 | HH1a/Δgcn2::TRP1 / HSP82 G313N-CEN/ARS-HIS3 [pHCA/Hsp82 G313N] | This paper |
| H748 | MATa leu2-3, 112 ura3-52 gcn2-1 | 26 |
| CN11 | MATa his3-11, 15 leu2-3, 112 lys1 lys2 Δtrp1 ura3-52 sti1-1::HIS3 | 43 |
| RD249-2D | MATa ura3 leu2 trp1 cdc37-1 bar1::LEU2 Δpep4::TRP1 | 28 |
| 8A7 | MATα lys2 leu2 ura3 trp1 cdc37-34 | 25 |
| YNK234 | MATα ura3-Δ152 lys2-801 amber ade2-101 ochre trp1-Δ1 his3-Δ200 leu2-Δ1 pdr5-101 Δsba1::HIS3 | 5 |
HH1a represents the genotypic background of other strains rather than a real strain (strains are not viable without the Hsp90 function being provided, for example, by a plasmid).
Episomes are indicated after a slash, with the name of the plasmid in brackets.
Immunoprecipitation experiments. (i) Yeast extracts.
Coimmunoprecipitation experiments using the FLAG tag were done as follows: extracts from strain HH1a-pHCA/Hsp82wt transformed with plasmid p2TG/flag.Hsp82wt or p2TG/flag.HSP82 G313N and with plasmid p2U/GSTGcn2 were lysed in buffer A (10 mM Tris-HCl [pH 7.5], 50 mM NaCl, 1 mM dithiothreitol, 10 mM sodium molybdate, 1 mM EDTA, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 3 μg of chymostatin/ml, 1.5 μg of pepstatin A/ml, 0.75 μg of leupeptin/ml, 3.8 μg of antipain/ml). After the extracts were adjusted to 0.1% Triton X-100, they were incubated at 4°C with the anti-FLAG monoclonal antibody M2 (Kodak) for 2 h, followed by 1 h with protein G-Sepharose (Pharmacia). Immunoprecipitates were washed four times for 10 min at 4°C with buffer A containing 0.1% Triton X-100, solubilized in sodium dodecyl sulfate (SDS) sample buffer, and loaded onto SDS–8% polyacrylamide gels. The same protocol was used for immunoprecipitation by a Gcn2-specific rabbit polyclonal antiserum (77) (a kind gift from Ronald Wek, Indiana University) of overexpressed Gcn2 from 0.5 mg of extracts from strain HH1a-p2HG/hHsp90β (36). Immunoprecipitation of human Hsp90 from strain HH1a-p2HG/hHsp90β was done with the monoclonal antibody H90-10 (a kind gift from David O. Toft, Mayo Clinic). For the coimmunoprecipitation of endogenous Gcn2, strains HH1a-pHCA/Hsp82wt and OD1 transformed with plasmid p2TG/flag.Hsp82 or strains HH1a-p2HG/hHsp90β and OD1 transformed with plasmid p2U/hHsp90β were grown in the presence of 30 mM 3-aminotriazole (3-AT) and lysed in buffer A with 100 mM NaCl. 3-AT increases the levels of Flag.Hsp82 and human Hsp90 in the extract thereby facilitating the analysis; this unexplained effect is unrelated to Gcn2 and does not affect the coprecipitation of Gcn2 with Hsp90 (data not shown). Immunoprecipitations were done as described above.
(ii) Reticulocyte lysate.
Coimmunoprecipitation of rabbit Hsp90 and Gcn2 from rabbit reticulocyte lysate was performed with the monoclonal antibody H90-10. After synthesis of Gcn2 from plasmid pYES/Gcn2 with the TNT RRL kit (Promega), Hsp90 was immunoprecipitated in buffer A with Triton X-100. After addition of protein G-Sepharose, the beads containing the immune complex were washed three times with buffer A with Triton X-100, followed by a wash with buffer A without Triton X-100. The proteins were analyzed by gel electrophoresis on an SDS–8% polyacrylamide gel.
In vitro kinase assay.
Gcn2 protein was synthesized from plasmid pYES/Gcn2 in rabbit reticulocyte lysate (TNT RRL kit; Promega) containing [35S]methionine according to the manufacturer’s recommendations with or without geldanamycin (GA) or macbecin I (provided by J. Johnson, Drug Synthesis and Chemistry Branch, National Cancer Institute). Gcn2 was immunoprecipitated by adding a rabbit polyclonal anti-Gcn2 serum to the reticulocyte lysate plus buffer A and 0.5% Triton X-100. After addition of protein A-Sepharose to harvest the antibody complex, immune pellets were washed with buffer A with Triton X-100 three times, followed by two washes with kinase buffer (20 mM Tris-HCl [pH 7.9], 50 mM NaCl, 10 mM MgCl2, 1 mM dithiothreitol). A 30-μl slurry of Sepharose-bound Gcn2 was incubated with 10 μl of [γ-32P]ATP (10 mCi/mmol) at a final concentration of 10 μM ATP for 20 min at 30°C. Beads were washed twice with kinase buffer, and SDS sample buffer was added to each sample to terminate the reaction. Samples were boiled for 2 min and analyzed on an SDS–8% polyacrylamide gel.
Western blot experiments.
After transfer of proteins from SDS-polyacrylamide gels to nitrocellulose membranes, the membranes were blocked with Tris-buffered saline–0.2% Tween-20 (TBST) containing 5% (wt/vol) milk powder and probed with appropriate antibodies (HS90-10, rabbit antisera against Gcn2, and firefly luciferase) in TBST-milk powder at room temperature for 1 h. Membranes were washed three times for 10 min with TBST. The secondary antibodies were alkaline phosphatase-conjugated goat anti-rabbit (Bio-Rad) or anti-chicken (Promega) antibodies and horseradish peroxidase-conjugated anti-mouse antibodies (Cappel). They were used in TBST-milk powder at room temperature for 1 h. After three washes with TBST, the blots were developed either with the nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate (NBT/BCIP) reagent for alkaline phosphatase or with the enhanced chemiluminescence reagent (Amersham) for horseradish peroxidase.
Assay of GCN4-lacZ reporter plasmid.
β-Galactosidase assays were conducted with cultures grown in minimal SD medium containing only the required supplements (66). For repressing conditions, saturated cultures were diluted 1:50 and harvested in mid-logarithmic phase after 6 h of growth at 30°C. For derepressing conditions, cultures were grown for 2 h under repressing conditions and then for 6 h after the addition of 3-AT to 40 mM. β-Galactosidase assays were corrected for cell density.
Luciferase assay.
Strain HH1a-pHCA/Hsp82wt was transformed with the plasmid pLG/LUC. Cells were grown with 2% glucose or galactose in the presence or absence of macbecin I (50 μM) for 24 h. Luciferase activity was measured according to the manufacturer’s recommendations (Promega).
Growth tests.
Strains containing mutations in HSP90 or deletions in different cochaperone genes were tested for their ability to grow on 3% agar plates containing histidine dropout medium supplemented with 30 mM 3-AT and excess (40 mM) leucine at 30°C (69). Note that all strains have the HIS3 marker.
RESULTS
HSP82 mutations affect translation regulated by the 5′-untranslated region of the GCN4 mRNA.
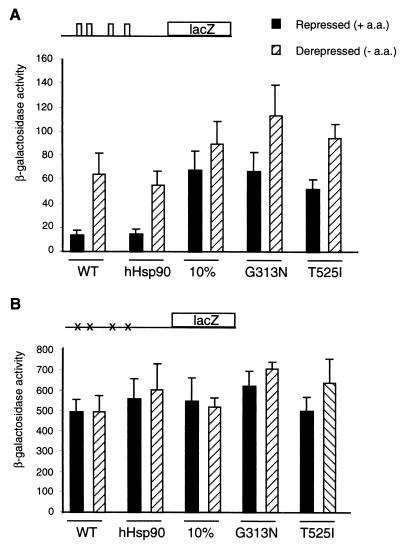
To determine whether yeast Hsp90 is involved in Gcn2 regulation, a GCN4-lacZ reporter plasmid containing the GCN4 translational control elements (uORFs) in the mRNA leader region (plasmid p180) (39) was introduced into yeast strains carrying different Hsp90 mutants. Some of the HSP90 mutations used here are known to produce defects in steroid receptor regulation (6, 47). Reporter gene activity was measured in the presence of amino acids (repressed conditions) or upon histidine starvation (derepressed), which was induced by 3-AT, a chemical inhibitor of the histidine biosynthetic enzyme His3 (70). As shown in Fig. 1A, GCN4-lacZ activity in the parent WT strain was stimulated fivefold by addition of 3-AT (derepressed). In cells expressing human Hsp90β instead of the yeast complement, regulation was similar to that for the parental strain, indicating that human Hsp90β can substitute for the yeast homologs in this assay. Surprisingly, compared with the activity in isogenic WT cells, β-galactosidase activity was higher in the Hsp90 mutant strains under repressed conditions (Fig. 1A). In a strain with Hsp82 levels reduced to 10% those of the WT (strain 10% Hsp82wt) (47), the activity observed in the repressed state was fivefold higher. In both strains with Hsp82 point mutations (Gly-313 to Asn [G313N] and Thr-525 to Ile [T525I]) (6), the activity of the GCN4-lacZ reporter was upregulated in the repressed state as observed for strain 10% Hsp82wt. In all mutant strains, the expression of β-galactosidase was further enhanced under conditions of amino acid limitations (Fig. 1A), reaching a level of activity slightly above that for the WT.
FIG. 1.
Hsp90 mutants stimulate GCN4-lacZ expression under repressed conditions. (A) β-Galactosidase activity assayed in extracts prepared from the different yeast strains expressing Hsp90 mutants transformed with the GCN4-lacZ reporter plasmid p180 (schematically represented). Enzyme activities are given in units. (B) β-Galactosidase activity assayed for the panel A strains transformed with the control reporter plasmid p227 containing point mutations (×) in the ATGs of the four uORFs in the 5′-untranslated region of the GCN4 mRNA. The values are the means of three to four independent experiments. + a.a., with amino acids; − a.a., without amino acids; hHsp90, human Hsp90β; 10%, strain 10% Hsp82wt; G313N, HH1a-pHCA/Hsp82 G313N; T525I, HH1a-pHCA/Hsp82 T525I.
In principle, the increase in GCN4-lacZ expression seen with the different HSP82 mutations could occur at the transcriptional or at the translational levels. To distinguish between these two possibilities, the different strains were transformed with plasmid p227 containing a GCN4-lacZ reporter in which all four uORFs have been inactivated by point mutations (39). Removal of the uORFs eliminates translational regulation of GCN4; thus, any increase in expression from p227 is due to transcriptional induction. Figure 1B shows that β-galactosidase expression levels from p227 are similar in all strains. These data indicate that stimulation of GCN4 expression in the three different Hsp82 mutant strains occurs at the translational level. The fact that different independent Hsp82 mutants show the same phenotype for GCN4 expression suggests that Hsp90 plays a role directly or indirectly in the translational regulation of GCN4.
The increased expression of GCN4-lacZ in Hsp82 mutant strains requires the kinase Gcn2.
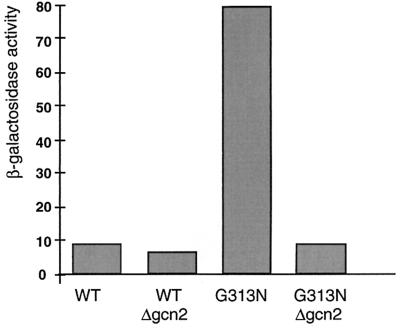
It has been reported that GCN4 translational regulation requires the protein kinase Gcn2 (30). To determine whether the derepression of β-galactosidase expression observed in the different Hsp82 mutant strains requires Gcn2, the GCN2 gene was deleted from the parental strain and the strain with the G313N mutation (WTΔgcn2 and G313NΔgcn2). Expression from the reporter plasmid p180 was checked under repressed conditions in both strains. As shown in Fig. 2, strain G313NΔgcn2 fails to display increased GCN4-lacZ expression under repressed conditions, indicating that Gcn2 is involved in this process. Conversely, the increased basal activity in the different Hsp90 mutants is not due to higher levels of Gcn2. Levels of endogenous Gcn2 in the mutant strains were observed to be similar to the levels in the WT strain (data not shown).
FIG. 2.
Gcn2 is required for the increased expression of the GCN4-lacZ reporter. β-Galactosidase activities of WT and G313N strains with a deletion of the gcn2 gene (strains OD1 and OD2) transformed with plasmid p180 are shown. Cells were grown only under repressed conditions (with amino acids). The experiments were carried out twice with less than 20% difference.
HSP90 mutations are directly responsible for the increased expression of GCN4-lacZ.
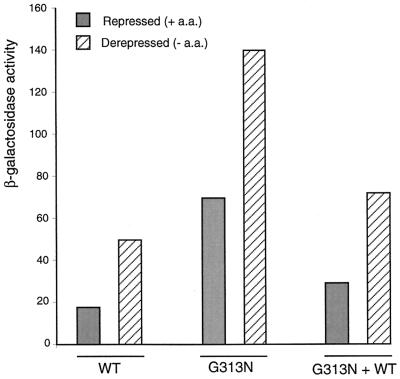
The increased expression of GCN4-lacZ was observed in different independent mutants of Hsp82, which led us to conclude that the observed phenotype was indeed due to an alteration of Hsp90. To exclude the formal possibility that the altered phenotype could be due to another genomic mutation, we reintroduced the WT allele of HSP82 into the cells carrying the G313N mutation (Fig. 3). This restores a WT pattern, indicating that hsp82G313N is responsible for the enhancement of translation through the GCN4 leader and that the G313N mutation is recessive relative to the WT HSP82.
FIG. 3.
The G313N mutation is responsible for increased GCN4-lacZ expression under repressed conditions. β-Galactosidase activity from reporter plasmid p180 was assayed in extracts prepared from the different yeast strains (WT or Hsp82 G313N or Hsp82 G313N transformed with the plasmid pTCA/Hsp82 which codes for the WT allele of Hsp82). The experiments were carried out twice. + a.a., with amino acids; − a.a., without amino acids.
Gcn2 binds to Hsp90 in vivo.
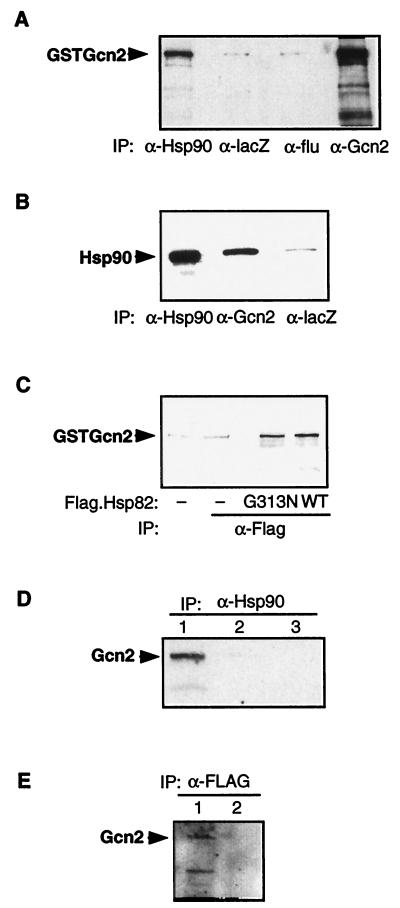
The genetic data suggested that Gcn2 is regulated by Hsp90. This could occur by the formation of a complex between Hsp90 and the kinase. We therefore examined whether overexpressed Gcn2 could be coimmunoprecipitated from yeast whole-cell extracts with an anti-Hsp90 antibody. As reported above, Gcn2 regulation is normal in a strain with human Hsp90 antibody. As reported above, Gcn2 regulation is normal in a strain with human Hsp90. Since a good monoclonal antibody to immunoprecipitate the human Hsp90 (monoclonal antibody H90-10) is available, we examined this interaction first. We overexpressed it as a GST fusion protein (GSTGcn2). We ascertained that GSTGcn2 is functional by showing that it can complement a gcn2 deletion strain (see Table 2). Extracts expressing GSTGcn2 were immunoprecipitated with the anti-Hsp90 antibody, and the immune complexes were probed by immunoblot analysis with an antiserum against Gcn2. Figure 4A shows that GSTGcn2 was coimmunoprecipitated with Hsp90 and not by control antibodies. GST alone does not coprecipitate with Hsp90 (data not shown), indicating that the Gcn2 moiety is responsible for this interaction. As an additional negative control, the immunoprecipitation was performed with the anti-human Hsp90 antibody with extracts from the parental strain (containing yeast and not human Hsp90); no GSTGcn2 could be coimmunoprecipitated in this experiment (data not shown). The inverse experiment was also performed: GSTGcn2 was immunoprecipitated with a rabbit polyclonal antibody directed against Gcn2, and human Hsp90 was revealed by immunoblot analysis. As shown in Fig. 4B, Hsp90 is coimmunoprecipitated with GSTGcn2. The experiments were done with extracts from cells grown under repressed or derepressed conditions with identical results (data not shown; see also Discussion).
FIG. 4.
Gcn2 binds Hsp90 in vivo. (A) Antibodies to human Hsp90β coprecipitate Gcn2. Equal amounts of yeast extracts (0.5 mg) from cells expressing human Hsp90β transformed with the plasmid p2U/GSTGcn2 were used with different antibodies as indicated below the panel. GSTGcn2 was revealed by immunoblotting with an anti-Gcn2 antiserum. α-Hsp90, antibody against human Hsp90; α-lacZ, antibody against β-galactosidase (Promega); α-flu, antibody against flu-tag (Aves Laboratory); α-Gcn2, polyclonal antibody against Gcn2 (77). IP, immunoprecipitation. (B) Antibodies to Gcn2 coprecipitate human Hsp90β. Equal amounts of yeast extracts (0.5 mg) from cells expressing the human Hsp90β transformed with the plasmid p2U/GSTGcn2 were used with different antibodies as indicated below the panel. Hsp90 was revealed by immunoblotting with the monoclonal antibody against Hsp90 (H90-10). (C) FLAG-tagged Hsp82 associates with GSTGcn2. Equal amounts of yeast extracts (0.5 mg) from isogenic strains expressing Hsp82wt or G313N with or without the FLAG tag were used with the FLAG antibody. All strains also expressed GSTGcn2 from plasmid p2U/GSTGcn2. Gcn2 was revealed by immunoblotting with an anti-Gcn2 antiserum. (D) Endogenous Gcn2 interacts with human Hsp90. Equal amounts of yeast extracts from cells expressing the human Hsp90β were used with the monoclonal anti-human Hsp90 antibody. Gcn2 was revealed as described for panel A. Lane 1, strain expressing human Hsp90; lane 2, strain expressing human Hsp90 with a gcn2 deletion; lane 3, strain expressing the yeast Hsp82. (E) Endogenous Gcn2 interacts with FLAG-tagged Hsp82. Equal amounts of yeast extracts from cells expressing the FLAG-tagged yeast Hsp82 were used with the FLAG antibody. Lane 1, strain with GCN2; lane 2, strain carrying a gcn2 deletion.
To show that overexpressed Gcn2 also interacts with yeast Hsp90, we performed coimmunoprecipitation experiments using Hsp82 tagged with a FLAG epitope at the N terminus. Figure 4C shows that GSTGcn2 is coprecipitated with FLAG-tagged Hsp82 when an anti-FLAG antibody is used. The GST moiety does not coprecipitate with FLAG-tagged Hsp82 (36). The results were similar with the G313N mutant version of FLAG-tagged Hsp82.
To ascertain that the Hsp90–Gcn2 interaction was not due to an artifact of Gcn2 overexpression, coimmunoprecipitation of endogenous Gcn2 with either human Hsp90 or yeast Hsp82, expressed at physiological levels, was assessed. Figure 4D and E show that endogenous Gcn2 is coimmunoprecipitated with both human Hsp90 and FLAG-tagged yeast Hsp82. Note that the anti-FLAG antibody alone does not nonspecifically precipitate overexpressed Gcn2 as a GST fusion (Fig. 4C) and that endogenous Gcn2 does not precipitate with an unrelated monoclonal antibody (Fig. 4D). Taken together, these experiments indicate that the eIF-2α kinase Gcn2 forms a complex with Hsp90. The steady-state proportion of Gcn2 molecules that are in complexes with the molecular chaperone Hsp90 remains to be determined. However, such complexes are likely to be highly dynamic and inherently unstable (see also Discussion).
Hsp90 inhibitors prevent the maturation of the kinase Gcn2 in vitro.
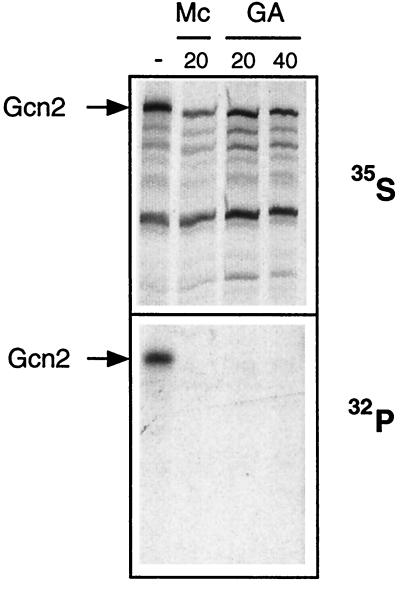
Hsp90 can be specifically inhibited by the benzoquinone ansamycins geldanamycin (GA) (64, 72) and macbecin I (3, 24), the latter being more effective in yeast. We postulated that if Gcn2 activity depends on Hsp90, then addition of these compounds might block its kinase activity. To test this hypothesis, we synthesized Gcn2 de novo in rabbit reticulocyte lysate and performed immunoprecipitation experiments with an antiserum against Gcn2. The immunoprecipitates were subsequently subjected to an in vitro kinase assay (77), which is based on the ability of Gcn2 to autophosphorylate (69). As presented in Fig. 5 (top), Gcn2 is translated to a similar extent in the presence or absence of GA, macbecin I, or vehicle (dimethyl sulfoxide). Increasing the concentration of GA further to 50 μM decreased Gcn2 levels but not those of an unrelated protein (data not shown). Following immunoprecipitation, [γ-32P]ATP was added for an in vitro kinase reaction and the products were separated on an SDS–8% polyacrylamide gel (Fig. 5, bottom). Incorporation of phosphates into Gcn2 was seen only in the absence of drug (Fig. 5, bottom, left lane). Addition of 20 μM GA or macbecin I completely inhibited the kinase reaction. The same results were observed in the presence of 10 μM GA (data not shown). Addition of GA after completion of Gcn2 translation (during the immunoprecipitation reaction or kinase assay) did not inhibit the kinase reaction, indicating that Hsp90 function is only required during synthesis of Gcn2. GA does not act unspecifically in rabbit reticulocyte lysate, since luciferase synthesized de novo in the presence or absence of 20 μM GA showed the same activity (data not shown) (55). These results demonstrate that Hsp90 activity is required during synthesis of Gcn2 and for it to become an active kinase.
FIG. 5.
Ansamycin benzoquinones block kinase activity of Gcn2 in vitro. Gcn2 was translated at 30°C in rabbit reticulocyte lysate. Macbecin I (Mc) and GA were added during synthesis, where indicated. After immunoprecipitation with an anti-Gcn2 antiserum, the samples were used for in vitro kinase assays and electrophoresed on an SDS-polyacrylamide gel electrophoresis gel. The two panels represent the same gel with differential detection of incorporated 35S (upper panel) and 32P (lower panel) by using two sheets of X-ray film. The numbers above the panels represent the concentrations of compounds used (in micromolar). Note that the slightly reduced levels of immunoprecipitated Gcn2 in the presence of Mc were specific to this particular experiment and are not generally observed. −, no drug added.
The association of Hsp90 with Gcn2 is stabilized in vitro in the presence of GA or macbecin I.
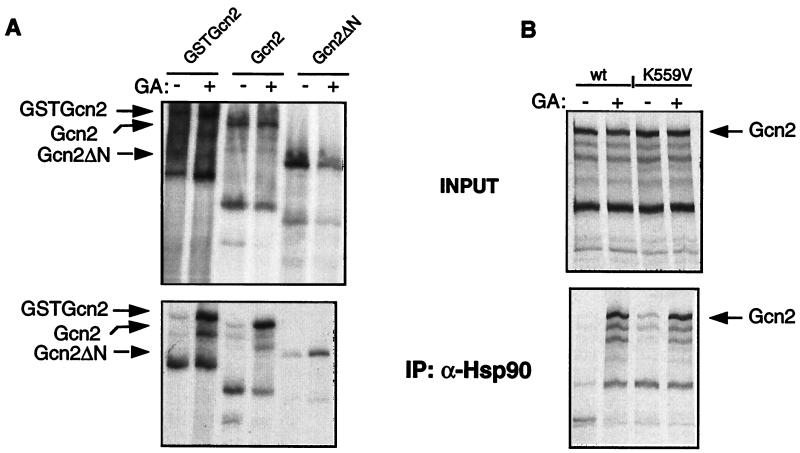
We next investigated the in vitro association of Gcn2 with Hsp90. Since Hsp90 binds to GSTGcn2 in vivo and since benzoquinone ansamycins have an effect on Gcn2 activity (see above), we assessed the coimmunoprecipitation of rabbit reticulocyte Hsp90 with in vitro-synthesized Gcn2 by using the anti-Hsp90 monoclonal antibody in the presence or absence of the Hsp90-specific inhibitors, GA and macbecin I. As shown in Fig. 6A, the amount of Gcn2 that is coimmunoprecipitated is much larger in the presence of GA than with the vehicle dimethyl sulfoxide alone. The amount of immunoprecipitated Hsp90 was the same in the presence or absence of Hsp90 inhibitors (data not shown). The same results were also observed with macbecin I. The interaction was specific for Gcn2 since unrelated control proteins did not interact with Hsp90 in the presence or absence of GA (data not shown). The effect was similar with three different constructs coding for Gcn2, GSTGcn2, Gcn2, and Gcn2 ΔN. The result with the last construct indicates that the N-terminal domain (amino acids [aa] 1 to 437) of Gcn2, which is dispensable for in vitro autophosphorylation (69), is not required for the association with Hsp90. A deletion of the C-terminal domain (aa 1024 to 1590) also did not affect Hsp90 binding (data not shown), suggesting that the kinase domain alone is sufficient to bind Hsp90.
FIG. 6.
GA increases the association of Hsp90 with Gcn2 in vitro. Gcn2 was translated at 30°C in rabbit reticulocyte lysate. GA was added during synthesis at a concentration of 20 μM. The top sections represent the input, and the bottom sections represent the immune pellets after coimmunoprecipitation (IP) with the anti-Hsp90 monoclonal antibody. (A) Plasmids pYes/GSTGcn2, pYes/Gcn2, and pYes/Gcn2ΔN were used for in vitro translation; (B) plasmids pYes/Gcn2 and pYes/Gcn2K559V were used. The migration positions of the different Gcn2 variants are indicated with arrows.
The data presented in Fig. 6A suggest that the release of Hsp90 is blocked by GA. As an inhibitor of Hsp90, GA could conceivably block this release at two steps during Gcn2 activation: (i) the binding of uncharged tRNAs to Gcn2 and (ii) the autophosphorylation of Gcn2. To distinguish between these two possibilities, we performed a series of in vitro translation and immunoprecipitation experiments using an inactive kinase mutant (lysine 559 mutated to valine) (71). The fact that the mutant does not incorporate 32P upon immunoprecipitation shows that phosphorylation of the WT is due to autophosphorylation and not to a copurifying kinase (77) (data not shown). The Gcn2 mutant was translated as well as WT Gcn2 (Fig. 6B). Gcn2K559V is bound more tightly to Hsp90 in the presence of GA, as observed for the WT Gcn2 (Fig. 6B). This result indicates that autophosphorylation is not the triggering signal for the release of Hsp90. Instead, in the absence of GA, binding of uncharged tRNAs is probably sufficient for this release. This is reminiscent of the hormone-induced dissociation of Hsp90 from a steroid receptor (49).
Inhibition of Hsp90 activity reduces Gcn2 levels in vivo.
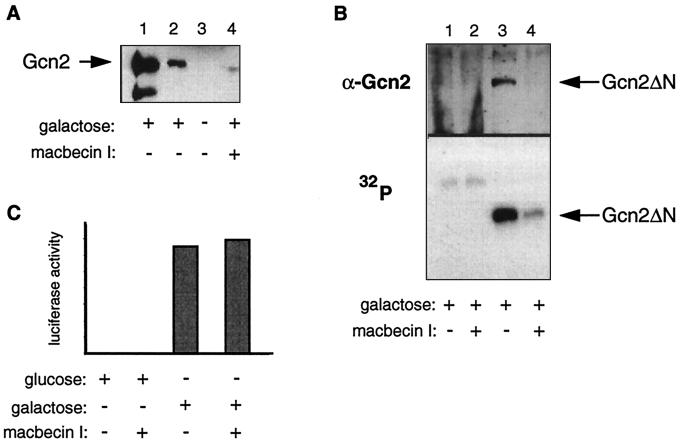
To investigate the effect of macbecin I in vivo, we transformed a WT strain with plasmid pYES/Gcn2, which allows the expression of Gcn2 to be induced by addition of galactose in the presence or absence of 50 μM macbecin I. After a 24-h induction, Gcn2 levels were monitored by Western blot analysis using an antiserum against Gcn2. As shown in Fig. 7A (lanes 1 and 2), Gcn2 is only expressed upon galactose induction; no protein is seen when cells are grown in glucose (lane 3). Addition of an inhibitor of Hsp90 macbecin I reduced the levels of Gcn2. As presented in Fig. 7B, in the presence of macbecin I, levels of Gcn2ΔN (lacking the N-terminal region flanking the kinase domain) were also strongly reduced. An in vitro kinase assay was performed with Gcn2ΔN after immunoprecipitation with an anti-Gcn2 antiserum. In Fig. 7B (bottom), we show that autophosphorylation is reduced in the presence of macbecin I to the same extent as are the levels of Gcn2 (Fig. 7B, top). As a control to confirm that macbecin I does not affect the galactose induction per se, we tested the induction and the activity of firefly luciferase induced by galactose in the presence or absence of macbecin I (Fig. 7C). No effect of macbecin I on luciferase activity was observed, indicating that the ansamycin does not affect galactose induction and that the effect is specific for Gcn2. Thus, in yeast, macbecin I at our experimental concentrations leads to a drop of Gcn2 levels, but the remaining Gcn2 molecules are still functional (see below). Therefore we conclude that Hsp90 is required for Gcn2 accumulation in vivo, confirming our in vitro results that Gcn2 maturation depends on Hsp90. Moreover the N-terminal region of Gcn2 is not necessary for regulation by Hsp90 both in vitro and in vivo.
FIG. 7.
Macbecin I reduces Gcn2 levels in vivo. Strain HH1a-pHCA/Hsp82 was transformed with plasmids pYes/Gcn2 (A) and pYes/Gcn2ΔN (B). Gcn2 variants were induced for 24 h by 2% galactose in the presence or absence of macbecin I (50 μM) and immunoprecipitated with an anti-Gcn2 antiserum. (A) Gcn2 was revealed by immunoblotting with an antiserum against Gcn2. Lanes 1 and 2, induction in the presence of galactose (10-fold less material was loaded in lane 2 than in lane 1; lane 3, cells grown with 2% glucose; lane 4, induction with galactose in the presence of 50 μM macbecin I. (B) Gcn2ΔN was either visualized by immunoblot analysis with an antibody against Gcn2 or used for an in vitro kinase assay. Lanes 1 and 2, parent strain; lanes 3 and 4, strain transformed with the plasmid pYes/Gcn2ΔN. (C) The WT strain was transformed with the plasmid pLG/LUC and grown with glucose or galactose in the presence or absence of macbecin I for 24 h. The plotted luciferase activity represents the mean values from two independent experiments.
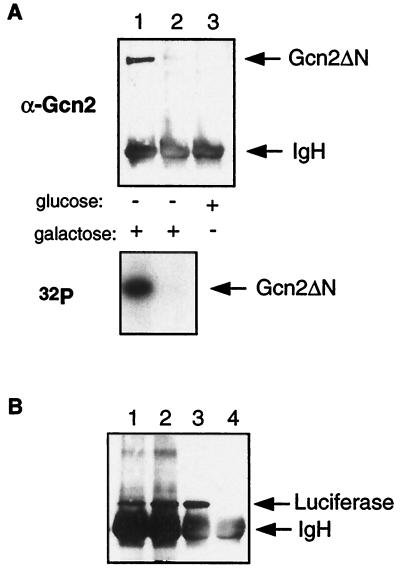
As an alternative to the pharmacological inhibition, we inactivated Hsp90 function in a yeast strain expressing the temperature-sensitive Hsp90 G170D mutant by a temperature shift. This mutant, in contrast to the mutants used above, is WT at 25 to 30°C but rapidly loses function when cells are shifted to 34°C; Hsp90 function is greatly reduced at 34°C and completely absent at 37°C (41). The G170D mutant cells were transformed with the plasmid expressing Gcn2ΔN under a galactose-inducible promoter. At the permissive temperature (30°C), Gcn2ΔN is expressed only upon galactose induction as determined by immunoblotting with an antiserum against Gcn2 (Fig. 8A; compare lanes 1 and 3). When cells are shifted simultaneously to galactose medium and 37°C for 6 h, the accumulation of Gcn2ΔN is severely decreased (Fig. 8A, lane 2). An in vitro kinase assay was performed with Gcn2ΔN after immunoprecipitation with an anti-Gcn2 antiserum. In the lower panel of Fig. 8A, autophosphorylation is observed only at the permissive temperature of 30°C. This indicates that at the restrictive temperature, in the temperature-sensitive G170D mutant, no functional Gcn2 molecules accumulate. To establish the specificity of the effect of inactivating Hsp90, we performed the same experiment with luciferase under the control of a galactose-inducible promoter (Fig. 8B). Following immunoprecipitation with an antiserum against luciferase, the protein levels were monitored by immunoblotting with the same antibody. The luciferase protein accumulated normally under all conditions. Together with the data obtained with macbecin I in vivo, these results indicate that Hsp90 function is required for full activity and accumulation of Gcn2 activity.
FIG. 8.
Inactivation of Hsp90 abolishes Gcn2 accumulation and activity in vivo. Strain HH1a-G170D was transformed with plasmids pYes/Gcn2ΔN (A) and pLG/LUC (B). Cells grown to mid-logarithmic phase at 30°C in glucose were shifted to galactose and 37°C simultaneously for 6 h to induce the expression of Gcn2ΔN (A) or luciferase (B). (A) Cell extracts from the different treatments were immunoprecipitated with an anti-Gcn2 antiserum, and an immunoblotting experiment with the same antibody (top) and a kinase assay (bottom) were performed. Lanes 1 and 2, shift to galactose. Cells were grown at 30°C (lanes 1 and 3) or at 37°C (lane 2). (B) Cells were grown with galactose (lanes 1, 2, and 3) or glucose (lane 4) at 30°C (lanes 1, 3, and 4) or at 37°C (lane 2) for 6 h. Lane 3, cells treated also with macbecin I (50 μM) for 24 h. Following immunoprecipitation with an anti-luciferase antiserum, luciferase was revealed with the same antibody. IgH, immunoglobulin heavy chain.
The cochaperones Cdc37, Sba1, and Sti1 are required for an intact general amino acid control.
Hsp90 acts in collaboration with a series of other proteins, some of which have intrinsic chaperone activity themselves (49). The function of these factors, notably p50 (Cdc37 in yeast), p23 (Sba1), and p60/Hop (Sti1) remains unclear (48). It has been proposed that p50/Cdc37 and Hsp90 could act as a specific chaperone complex for protein kinases (13, 15, 19, 28, 35, 46). Sba1 has been shown to be associated with several proteins, including transcription factors, protein kinases, and a viral reverse transcriptase (32, 40, 74). Deletion of the SBA1 gene in yeast results in no striking phenotype (5, 24). Sti1 (the S. cerevisiae homolog of p60) is required for the assembly of the Hsp90–glucocorticoid receptor (GR) complex in vitro and contributes to the maturation of both GR and the tyrosine kinase pp60v-src in budding yeast (8, 61). To investigate whether Cdc37, Sba1, and Sti1 are involved in regulating Gcn2 activity, we tested the general amino acid control in different Cdc37 mutant strains, in a strain lacking the SBA1 gene, and in cells lacking the STI1 gene (Table 2). We used a simple growth assay to monitor general control of amino acid biosynthesis, which involves growing the different strains on plates containing 3-AT, the inhibitor of histidine biosynthesis (17). Cells with an intact general control response will grow on medium containing the drug; however, the growth of yeast strains lacking any one of the factors required to mediate the induction of GCN4 expression will be defective under these starvation conditions. The different strains harboring Hsp90 mutants grow as well as the WT (Table 2), confirming that the Gcn2/Gcn4 pathway is not impaired under derepressed conditions (see above). Unlike the Hsp90 mutant cells, the two strains harboring cdc37 mutations, the Δsba1 cells, and the Δsti1 cells, have a slow-growth phenotype on plates containing 3-AT. These results show that three proteins known to interact with Hsp90, Cdc37, Sba1, and Sti1, are involved in the regulation of the general amino acid control, possibly by affecting the regulation of Gcn2.
DISCUSSION
We report that in the yeast S. cerevisiae the eIF-2α kinase Gcn2 requires the molecular chaperone Hsp90 for proper regulation. Hsp90 forms a complex with Gcn2 in vitro as well as in vivo. Using different Hsp90 mutants, we observed an enhancement of the translation of the Gcn2 target GCN4-lacZ under repressing conditions for GCN4 expression, suggesting that Hsp90 inhibits Gcn2 activity. The specific inhibitors of Hsp90, the benzoquinone ansamycins GA and macbecin I, block Gcn2 activity in vitro and lower Gcn2 levels in vivo. Gcn2 is thus one of the few known targets of Hsp90 in budding yeast, along with Hap1 (76) and Ste11 (36), and Hsp90 is the first characterized protein interacting with Gcn2.
Gcn2 activity and levels are dependent on Hsp90.
In vitro, Gcn2 synthesized in the presence of the inhibitors of Hsp90 (GA or macbecin I), or at the restrictive temperature in a strain expressing a temperature-sensitive Hsp90 mutant (G170D mutant), is nonfunctional, showing that the chaperone activity of Hsp90 is required for Gcn2 maturation and/or activation. The binding of Gcn2 to Hsp90 is increased when Gcn2 is synthesized in the presence of the Hsp90 inhibitors, indicating that immature Gcn2 binds more tightly to Hsp90 and that Gcn2 forms a complex with Hsp90 during Gcn2 synthesis. Similarly, one of the vertebrate members of the Gcn2 family, HRI, also binds to Hsp90 in vitro in its inactive form (in the presence of hemin) (37, 65, 74). However, although both kinases belong to the same family (eIF-2α kinases) and both bind to Hsp90, the effects of Hsp90 inhibitors are different (65): (i) GA disrupts the interaction of Hsp90 with HRI, while it enhances the interaction with Gcn2 and (ii) release of Hsp90 from HRI depends on autophosphorylation (an inactive mutant of HRI binds Hsp90 in the presence or absence of the activating molecule hemin), while Gcn2 does not need autophosphorylation to dissociate from Hsp90 (an inactive kinase mutant of Gcn2 can still release Hsp90). The effects of GA are specific for Gcn2 since GA does not influence folding and the activity of unrelated proteins such as luciferase and does not force the binding of Hsp90 to these proteins. Thus, the stronger binding of Hsp90 to Gcn2 in the presence of the Hsp90 inhibitors suggests that the release of Hsp90 is blocked.
While autophosphorylation of the kinase does not seem to be the triggering signal for the release of the chaperone from Gcn2, the binding of uncharged tRNAs might be. Several studies of budding yeast have clearly established that Gcn2, through its tRNA synthetase-related domain (HisRS region), binds to and is activated by uncharged tRNAs (51, 66, 68, 70, 77, 78). In vitro, Gcn2 is constitutively activated while being synthesized. The signal that activates Gcn2 in vitro has not been characterized, but uncharged tRNAs may be present in sufficient amounts (77). Similarly, we and others could not see any difference between the activity of Gcn2 in extracts from cells grown under repressed or derepressed conditions by using an in vitro kinase assay. tRNA charging may be substantially reduced in a cell lysate. Moreover, breakage of cells with glass beads may release activating RNA, such as immature tRNA, from cellular compartments not accessible to Gcn2 in vivo. The constitutive presence of an “activating signal” in cell-free systems precluded experiments in which Gcn2 is synthesized in the inactive form.
In vivo, in a strain with the temperature-sensitive Hsp90 mutant (HH1a-G170D) at restrictive temperature or in a WT strain in the presence of macbecin I, the levels of newly synthesized Gcn2 are strongly decreased. In the presence of macbecin I, the few remaining molecules are still functional, perhaps because it is difficult to block all Hsp90 molecules pharmacologically. In contrast, in the HH1a-G170D strain in which Hsp90 activity is efficiently destroyed at 37°C (42), no Gcn2 activity is detected. These in vivo data might reflect a higher sensitivity of cells in sensing and degrading misfolded proteins than that of the in vitro system where Gcn2, although not functional in the presence of macbecin I, is more stable. Moreover, this mirrors the effect of GA treatment on Hsp90 client proteins in vertebrate cells, such as Raf-1 (57), pp60v-src (72), and GR (14, 58, 71).
Certain mutations in HSP90 can derepress Gcn2.
Gcn4 regulation is influenced by mutations in HSP90. In several Hsp90 mutant strains (10% Hsp82wt, Hsp82 G313N, Hsp82 T525I) that, unlike the temperature-sensitive mutant strain HH1a-G170D, have Hsp90 defects even at low temperature, GCN4-lacZ is constitutively expressed even under repressed conditions. This regulation requires the kinase Gcn2. The phenotypes of the derepressed HSP90 mutations might appear contradictory to the biochemical results obtained with the Hsp90 mutant HH1a-G170D and with macbecin I, and to the growth phenotypes of the cochaperone mutant strains. However, these differences are probably due to the nature of the mutations themselves. The activities of the three viable Hsp90 mutants (as listed above) are only partially defective and still support proper folding, but not repression, of the kinase domain of Gcn2. In contrast, a more severe block of Hsp90 functions as with HH1a-G170D and macbecin I or with defective cochaperones might even affect the maturation of the kinase activity.
Recently, it has been reported that lowering the levels of Hsp90 in yeast induces a heat shock response (23). Moreover, one kinase in the Gcn2 family, HRI, is known to be activated by heat shock (10). Thus, it was conceivable that the increased GCN4-lacZ expression in the Hsp90 mutant strains was due to an indirect effect of the heat shock response and not directly to the alteration of Hsp90. To rule out this possibility, we stressed a WT strain by incubation at 42°C in the presence or absence of 3-AT. The regulation of the GCN4-lacZ reporter plasmid was not affected by heat shock treatment (data not shown), indicating that the phenotype of Hsp90 mutant strains is not due to a heat shock response.
The same set of Hsp90 mutant strains (10% Hsp82wt, G313N, T525I) has been used previously to study the functions of Hsp90 for other client proteins. Low levels of Hsp90 such as that in strain 10% Hsp82wt impair the functions of steroid receptors (47), the vertebrate tyrosine kinase pp60v-src (73), the yeast mitogen-activated protein kinase kinase kinase Ste11 (36), and the yeast transcription factor Hap1 (76). Mutations at codon 313 of Hsp82 (either G313N or G313S) have been shown to affect steroid receptors (6), pp60v-src (41), and Ste11 (36). The Hsp82 point mutation T525I results in a mutant that is defective for steroid receptor function (6) and normal for Ste11 (36) signaling. Strains that live on human Hsp90β support normal steroid receptor function, whereas pheromone signaling, which depends on Ste11, is defective (36). Interestingly, for the two kinases pp60v-src and Ste11, defective function seems to correlate with reduced accumulation, which is not the case for the steroid receptors, Hap1, and, as shown here, Gcn2. It is remarkable that the effects on steroid receptors and Gcn2 correlate for all of the above-mentioned HSP90 mutations, suggesting that their requirements for Hsp90 are very similar, if not identical, and different from those of the other client proteins.
Model for the regulation of Gcn2 by Hsp90.
The mechanism of inhibition of Gcn2 during repressed conditions (in the presence of amino acids) is still unclear. Qiu and collaborators have proposed a model (50) which postulates that Gcn2 is kept in an inactive conformation by intramolecular interactions between the kinase domain and its flanking regions. Binding of uncharged tRNA to the HisRS region would trigger a conformational change that relieves these inhibitory interdomain interactions. Following this step, autophosphorylation, binding, and phosphorylation of the substrate eIF-2α is thought to occur.
The data presented here together with earlier reports on Gcn2 can be integrated into a model for Gcn2 regulation in which Hsp90 plays a crucial role for the folding and the regulation of the kinase (Fig. 9). This model is reminiscent of the regulation of steroid receptors by Hsp90 (49, 60) in several respects. (i) While Gcn2 is being synthesized or shortly thereafter, it is bound by the Hsp90 chaperone complex. (ii) At first, folding of Gcn2 is incomplete and the complex is an unstable intermediate; as for steroid receptors, it may contain Hsp90 cochaperones such as Sti1 that are only transiently associated and required during assembly of the Hsp90 complex. (iii) In the mature Hsp90-Gcn2 complex, the Hsp90 complex maintains Gcn2 inactive but ready to respond to the activating signal (uncharged tRNAs). Inhibition may also involve intramolecular interactions within Gcn2 as mentioned above. (iv) Upon amino acid starvation, uncharged tRNA binds to the HisRS domain of Gcn2 and induces a conformational change that results in the release of Hsp90. (v) Following the release from Hsp90, Gcn2 can be autophosphorylated and can phosphorylate its endogenous substrate eIF-2α.
FIG. 9.
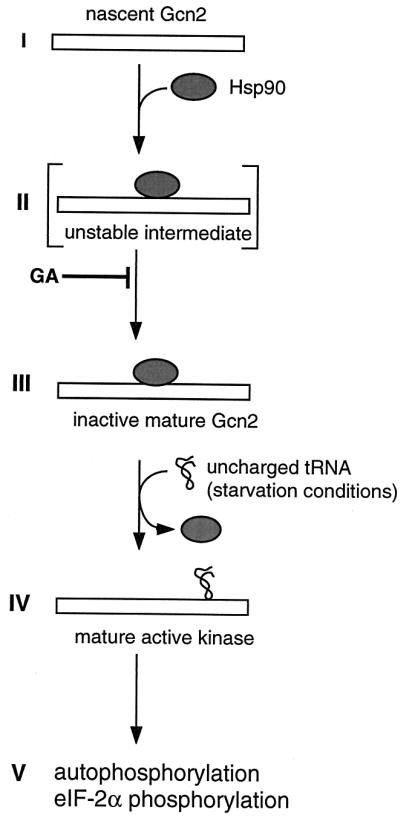
Model for the role of Hsp90 in the maturation and inhibition of Gcn2. GA may block the transition from step II to III.
The assembly of Hsp90-steroid receptor complexes has been extensively analyzed and even reconstituted in vitro with purified components. It is a unidirectional multistep process that involves at least eight proteins in addition to Hsp90 (49, 60, 62). While we have shown that several Hsp90 cochaperones are required for general amino acid control, the precise role of these factors remains to be established. By analogy to steroid receptors (9, 21), it can be speculated that Sti1, the yeast homolog of Hop, is an essential component of the unstable intermediate (step II in Fig. 9; Table 2). Indeed, GA has been shown to stabilize the equivalent of this complex in the steroid receptor assembly pathway and as a result to block the maturation to the hormone-binding-competent form (20, 22, 62). Similarly, we found that GA stabilizes Gcn2–Hsp90 complexes and blocks the maturation of Gcn2 to an active kinase in vitro, perhaps by preventing Gcn2 from adopting a conformation that is capable of binding uncharged tRNA. The fact that Gcn2 autophosphorylation, unlike that of HRI (65), is not required to release Hsp90 is consistent with our hypothesis that this is triggered by a ligand-induced conformational change of the protein kinase. For a steroid receptor, its cognate ligand, the steroid hormone, induces this release.
Despite all the remarkable similarities between Gcn2 and steroid receptors, one should not overlook potentially important, albeit not fundamental, differences. Kinases and steroid receptors may not use exactly the same set of Hsp90 cochaperones. As indicated above, it has been suggested that Cdc37 may be a kinase-specific Hsp90 cochaperone. Consistent with a role for Cdc37 in regulating Gcn2, general amino acid control is defective in strains with cdc37 mutations (Table 2). Although Cdc37 may be preferentially dedicated to kinases, it may also play an accessory role for steroid receptors since their responses are at least partially defective in a Cdc37 mutant strain (25). The most obvious difference between Gcn2 and steroid receptors lies in the effects that HSP90 mutations have on their activities. Gcn2 is constitutively activated even in the absence of the inducing stimulus (see discussion above), whereas steroid receptors still require the addition of ligand but respond to ligand less efficiently. There is evidence that the Hsp82 mutant G313N may be less tightly bound to the GR (4). As in the strain that expresses limiting levels of Hsp82 (47), a larger fraction of steroid receptor molecules might therefore not be complexed with Hsp90 at any given time. It is likely that constitutive steroid receptor activity is not observed because induction of Hsp90 release is not the sole role of ligand binding (38). In contrast, for Gcn2, weakened binding or an altered nature of the complex with Hsp90 mutants may be sufficient to allow constitutive activity or low levels of uncharged tRNAs present even under repressed conditions to activate the kinase.
As the first yeast kinase that is both ligand regulated and handled by the Hsp90 complex, Gcn2 will be a particularly interesting model to study how protein folding and assembly overlap with signaling.
ACKNOWLEDGMENTS
We thank A. Caplan, S. P. Bohen, K. R. Yamamoto, E. A. Craig, M. Foiani, J. Johnson, S. Lindquist, S. Mader, P. Mueller, David O. Toft, and R. C. Wek for plasmids, strains, chemicals, and antibodies. We are indebted to J.-F. Louvion for establishing an impressive collection of Hsp90 plasmids. We are grateful to B. Cenni and T. Abbas-Terki for critical comments on the manuscript. We acknowledge the sequencing service of the Department of Molecular Biology.
This work was supported by the Swiss National Science Foundation and the Canton de Genève.
REFERENCES
- 1.Abastado J P, Miller P F, Jackson B M, Hinnebusch A G. Suppression of ribosomal reinitiation at upstream open reading frames in amino acid-starved cells forms the basis for GCN4 translational control. Mol Cell Biol. 1991;11:486–496. doi: 10.1128/mcb.11.1.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aligue R, Akhavan-Niak H, Russell P. A role for Hsp90 in cell cycle control: Wee1 tyrosine kinase activity requires interaction with Hsp90. EMBO J. 1994;13:6099–6106. doi: 10.1002/j.1460-2075.1994.tb06956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blagosklonny M V, Toretsky J, Bohen S, Neckers L. Mutant conformation of p53 translated in vitro or in vivo requires functional HSP90. Proc Natl Acad Sci USA. 1996;93:8379–8383. doi: 10.1073/pnas.93.16.8379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohen S P. Hsp90 mutants disrupt glucocorticoid receptor ligand binding and destabilize aporeceptor complexes. J Biol Chem. 1995;270:29433–29438. doi: 10.1074/jbc.270.49.29433. [DOI] [PubMed] [Google Scholar]
- 5.Bohen S P. Genetic and biochemical analysis of p23 and ansamycin antibiotics in the function of Hsp90-dependent signaling proteins. Mol Cell Biol. 1998;18:3330–3339. doi: 10.1128/mcb.18.6.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohen S P, Yamamoto K R. Isolation of Hsp90 mutants by screening for decreased steroid receptor function. Proc Natl Acad Sci USA. 1993;90:11424–11428. doi: 10.1073/pnas.90.23.11424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borkovich K A, Farrelly F W, Finkelstein D B, Taulien J, Lindquist S. hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol Cell Biol. 1989;9:3919–3930. doi: 10.1128/mcb.9.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang H C, Nathan D F, Lindquist S. In vivo analysis of the Hsp90 cochaperone St1 (p60) Mol Cell Biol. 1997;17:318–325. doi: 10.1128/mcb.17.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C F, Chen Y, Dai K, Chen P L, Riley D J, Lee W H. A new member of the hsp90 family of molecular chaperones interacts with the retinoblastoma protein during mitosis and after heat shock. Mol Cell Biol. 1996;16:4691–4699. doi: 10.1128/mcb.16.9.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J J, London I M. Regulation of protein synthesis by heme-regulated eIF-2 alpha kinase. Trends Biochem Sci. 1995;20:105–108. doi: 10.1016/s0968-0004(00)88975-6. [DOI] [PubMed] [Google Scholar]
- 11.Chen J J, Throop M S, Gehrke L, Kuo I, Pal J K, Brodsky M, London I M. Cloning of the cDNA of the heme-regulated eukaryotic initiation factor 2 alpha (eIF-2 alpha) kinase of rabbit reticulocytes: homology to yeast GCN2 protein kinase and human double-stranded-RNA-dependent eIF-2 alpha kinase. Proc Natl Acad Sci USA. 1991;88:7729–7733. doi: 10.1073/pnas.88.17.7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Csermely P, Schnaider T, Soti C, Prohaszka Z, Nardai G. The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol Ther. 1998;79:129–168. doi: 10.1016/s0163-7258(98)00013-8. [DOI] [PubMed] [Google Scholar]
- 13.Cutforth T, Rubin G M. Mutations in Hsp83 and cdc37 impair signaling by the sevenless receptor tyrosine kinase in Drosophila. Cell. 1994;77:1027–1036. doi: 10.1016/0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]
- 14.Czar M J, Galigniana M D, Silverstein A M, Pratt W B. Geldanamycin, a heat shock protein 90-binding benzoquinone ansamycin, inhibits steroid-dependent translocation of the glucocorticoid receptor from the cytoplasm to the nucleus. Biochemistry. 1997;36:7776–7785. doi: 10.1021/bi970648x. [DOI] [PubMed] [Google Scholar]
- 15.Dai K, Kobayashi R, Beach D. Physical interaction of mammalian CDC37 with CDK4. J Biol Chem. 1996;271:22030–22034. doi: 10.1074/jbc.271.36.22030. [DOI] [PubMed] [Google Scholar]
- 16.de Haro C, Mendez R, Santoyo J. The eIF-2alpha kinases and the control of protein synthesis. FASEB J. 1996;10:1378–1387. doi: 10.1096/fasebj.10.12.8903508. [DOI] [PubMed] [Google Scholar]
- 17.Dever T E. Using GCN4 as a reporter of eIF2 alpha phosphorylation and translational regulation in yeast. Methods. 1997;11:403–417. doi: 10.1006/meth.1996.0437. [DOI] [PubMed] [Google Scholar]
- 18.Dever T E, Feng L, Wek R C, Cigan A M, Donahue T F, Hinnebusch A G. Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell. 1992;68:585–596. doi: 10.1016/0092-8674(92)90193-g. [DOI] [PubMed] [Google Scholar]
- 19.Dey B, Lightbody J J, Boschelli F. CDC37 is required for p60v-src activity in yeast. Mol Biol Cell. 1996;7:1405–1417. doi: 10.1091/mbc.7.9.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dittmar K D, Demady D R, Stancato L F, Krishna P, Pratt W B. Folding of the glucocorticoid receptor by the heat shock protein (hsp) 90-based chaperone machinery. The role of p23 is to stabilize receptor.hsp90 heterocomplexes formed by hsp90.p60.hsp70. J Biol Chem. 1997;272:21213–21220. doi: 10.1074/jbc.272.34.21213. [DOI] [PubMed] [Google Scholar]
- 21.Dittmar K D, Hutchison K A, Owens-Grillo J K, Pratt W B. Reconstitution of the steroid receptor.hsp90 heterocomplex assembly system of rabbit reticulocyte lysate. J Biol Chem. 1996;271:12833–12839. doi: 10.1074/jbc.271.22.12833. [DOI] [PubMed] [Google Scholar]
- 22.Dittmar K D, Pratt W B. Folding of the glucocorticoid receptor by the reconstituted Hsp90-based chaperone machinery. The initial hsp90.p60.hsp70-dependent step is sufficient for creating the steroid binding conformation. J Biol Chem. 1997;272:13047–13054. doi: 10.1074/jbc.272.20.13047. [DOI] [PubMed] [Google Scholar]
- 23.Duina A A, Kalton H M, Gaber R F. Requirement for Hsp90 and a CyP-40-type cyclophilin in negative regulation of the heat shock response. J Biol Chem. 1998;273:18974–18978. doi: 10.1074/jbc.273.30.18974. [DOI] [PubMed] [Google Scholar]
- 24.Fang Y, Fliss A E, Rao J, Caplan A J. SBA1 encodes a yeast hsp90 cochaperone that is homologous to vertebrate p23 proteins. Mol Cell Biol. 1998;18:3727–3734. doi: 10.1128/mcb.18.7.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fliss A E, Fang Y, Boschelli F, Caplan A J. Differential in vivo regulation of steroid hormone receptor activation by Cdc37p. Mol Cell Biol. 1997;8:2501–2509. doi: 10.1091/mbc.8.12.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foiani M, Cigan A M, Paddon C J, Harashima S, Hinnebusch A G. GCD2, a translational repressor of the GCN4 gene, has a general function in the initiation of protein synthesis in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:3203–3216. doi: 10.1128/mcb.11.6.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freeman B C, Toft D O, Morimoto R I. Molecular chaperone machines: chaperone activities of the cyclophilin Cyp-40 and the steroid aporeceptor-associated protein p23. Science. 1996;274:1718–1720. doi: 10.1126/science.274.5293.1718. [DOI] [PubMed] [Google Scholar]
- 28.Gerber M R, Farrell A, Deshaies R J, Herskowitz I, Morgan D O. Cdc37 is required for association of the protein kinase Cdc28 with G1 and mitotic cyclins. Proc Natl Acad Sci USA. 1995;92:4651–4655. doi: 10.1073/pnas.92.10.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hinnebusch A G. A hierarchy of trans-acting factors modulates translation of an activator of amino acid biosynthetic genes in Saccharomyces cerevisiae. Mol Cell Biol. 1985;5:2349–2360. doi: 10.1128/mcb.5.9.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hinnebusch A G. The eIF-2 alpha kinases: regulators of protein synthesis in starvation and stress. Semin Cell Biol. 1994;5:417–426. doi: 10.1006/scel.1994.1049. [DOI] [PubMed] [Google Scholar]
- 31.Hinnebusch A G. Translational regulation of yeast GCN4. A window on factors that control initiator-tRNA binding to the ribosome. J Biol Chem. 1997;272:21661–21664. doi: 10.1074/jbc.272.35.21661. [DOI] [PubMed] [Google Scholar]
- 32.Hu J, Toft D O, Seeger C. Hepadnavirus assembly and reverse transcription require a multi-component chaperone complex which is incorporated into nucleocapsids. EMBO J. 1997;16:59–68. doi: 10.1093/emboj/16.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jakob U, Buchner J. Assisting spontaneity: the role of Hsp90 and small Hsps as molecular chaperones. Trends Biochem Sci. 1994;19:205–211. doi: 10.1016/0968-0004(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 34.Jakob U, Lilie H, Meyer I, Buchner J. Transient interaction of Hsp90 with early unfolding intermediates of citrate synthase. Implications for heat shock in vivo. J Biol Chem. 1995;270:7288–7294. doi: 10.1074/jbc.270.13.7288. [DOI] [PubMed] [Google Scholar]
- 35.Kimura Y, Rutherford S L, Miyata Y, Yahara I, Freeman B C, Yue L, Morimoto R I, Lindquist S. Cdc37 is a molecular chaperone with specific functions in signal transduction. Genes Dev. 1997;11:1775–1785. doi: 10.1101/gad.11.14.1775. [DOI] [PubMed] [Google Scholar]
- 36.Louvion J F, Abbas-Terki T, Picard D. Hsp90 is required for pheromone signaling in yeast. Mol Cell Biol. 1998;9:3071–3083. doi: 10.1091/mbc.9.11.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mendez R, Moreno A, de Haro C. Regulation of heme-controlled eukaryotic polypeptide chain initiation factor 2 alpha-subunit kinase of reticulocyte lysates. J Biol Chem. 1992;267:11500–11507. [PubMed] [Google Scholar]
- 38.Moras D, Gronemeyer H. The nuclear receptor ligand-binding domain: structure and function. Curr Opin Cell Biol. 1998;10:384–391. doi: 10.1016/s0955-0674(98)80015-x. [DOI] [PubMed] [Google Scholar]
- 39.Mueller P P, Hinnebusch A G. Multiple upstream AUG codons mediate translational control of GCN4. Cell. 1986;45:201–207. doi: 10.1016/0092-8674(86)90384-3. [DOI] [PubMed] [Google Scholar]
- 40.Nair S C, Toran E J, Rimerman R A, Hjermstad S, Smithgall T E, Smith D F. A pathway of multi-chaperone interactions common to diverse regulatory proteins: estrogen receptor, Fes tyrosine kinase, heat shock transcription factor Hsf1, and the aryl hydrocarbon receptor. Cell Stress Chaperones. 1996;1:237–250. doi: 10.1379/1466-1268(1996)001<0237:apomci>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nathan D F, Lindquist S. Mutational analysis of Hsp90 function: interactions with a steroid receptor and a protein kinase. Mol Cell Biol. 1995;15:3917–3925. doi: 10.1128/mcb.15.7.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nathan D F, Vos M H, Lindquist S. In vivo functions of the Saccharomyces cerevisiae Hsp90 chaperone. Proc Natl Acad Sci USA. 1997;94:12949–12956. doi: 10.1073/pnas.94.24.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicolet C M, Craig E A. Isolation and characterization of STI1, a stress-inducible gene from Saccharomyces cerevisiae. Mol Cell Biol. 1989;9:3638–3646. doi: 10.1128/mcb.9.9.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olsen D S, Jordan B, Chen D, Wek R C, Cavener D R. Isolation of the gene encoding the drosophila melanogaster homolog of the saccharomyces cerevisiae GCN2 eIF-2alpha kinase. Genetics. 1998;149:1495–1509. doi: 10.1093/genetics/149.3.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palmer G, Louvion J F, Tibbetts R S, Engman D M, Picard D. Trypanosoma cruzi heat-shock protein 90 can functionally complement yeast. Mol Biochem Parasitol. 1995;70:199–202. doi: 10.1016/0166-6851(95)00007-n. [DOI] [PubMed] [Google Scholar]
- 46.Perdew G H, Wiegand H, Vanden Heuvel J P, Mitchell C, Singh S S. A 50 kilodalton protein associated with raf and pp60(v-src) protein kinases is a mammalian homolog of the cell cycle control protein cdc37. Biochemistry. 1997;36:3600–3607. doi: 10.1021/bi9612529. [DOI] [PubMed] [Google Scholar]
- 47.Picard D, Khursheed B, Garabedian M J, Fortin M G, Lindquist S, Yamamoto K R. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990;348:166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- 48.Pratt W B. The hsp90-based chaperone system: involvement in signal transduction from a variety of hormone and growth factor receptors. Proc Soc Exp Biol Med. 1998;217:420–434. doi: 10.3181/00379727-217-44252. [DOI] [PubMed] [Google Scholar]
- 49.Pratt W B, Toft D O. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocrinol Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- 50.Qiu H, Garcia-Barrio M T, Hinnebusch A G. Dimerization by translation initiation factor 2 kinase GCN2 is mediated by interactions in the C-terminal ribosome-binding region and the protein kinase domain. Mol Cell Biol. 1998;18:2697–2711. doi: 10.1128/mcb.18.5.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramirez M, Wek R C, Vazquez de Aldana C R, Jackson B M, Freeman B, Hinnebusch A G. Mutations activating the yeast eIF-2α kinase GCN2: isolation of alleles altering the domain related to histidyl-tRNA synthetases. Mol Cell Biol. 1992;12:5801–5815. doi: 10.1128/mcb.12.12.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samuel C E, Kuhen K L, George C X, Ortega L G, Rende-Fournier R, Tanaka H. The PKR protein kinase—an interferon-inducible regulator of cell growth and differentiation. Int J Hematol. 1997;65:227–237. doi: 10.1016/s0925-5710(96)00544-0. [DOI] [PubMed] [Google Scholar]
- 53.Santoyo J, Alcalde J, Mendez R, Pulido D, de Haro C. Cloning and characterization of a cDNA encoding a protein synthesis initiation factor-2alpha (eIF-2alpha) kinase from Drosophila melanogaster. Homology to yeast GCN2 protein kinase. J Biol Chem. 1997;272:12544–12550. doi: 10.1074/jbc.272.19.12544. [DOI] [PubMed] [Google Scholar]
- 54.Sattlegger E, Hinnebusch A G, Barthelmess I B. cpc-3, the Neurospora crassa homologue of yeast GCN2, encodes a polypeptide with juxtaposed eIF2alpha kinase and histidyl-tRNA synthetase-related domains required for general amino acid control. J Biol Chem. 1998;273:20404–20416. doi: 10.1074/jbc.273.32.20404. [DOI] [PubMed] [Google Scholar]
- 55.Schneider C, Sepp-Lorenzino L, Nimmesgern E, Ouerfelli O, Danishefsky S, Rosen N, Hartl F U. Pharmacologic shifting of a balance between protein refolding and degradation mediated by Hsp90. Proc Natl Acad Sci USA. 1996;93:14536–14541. doi: 10.1073/pnas.93.25.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schneider J C, Guarente L. Vectors for expression of cloned genes in yeast: regulation, overproduction, and underproduction. Methods Enzymol. 1991;194:373–388. doi: 10.1016/0076-6879(91)94028-b. [DOI] [PubMed] [Google Scholar]
- 57.Schulte T W, Blagosklonny M V, Ingui C, Neckers L. Disruption of the Raf-1-Hsp90 molecular complex results in destabilization of Raf-1 and loss of Raf-1-Ras association. J Biol Chem. 1995;270:24585–24588. doi: 10.1074/jbc.270.41.24585. [DOI] [PubMed] [Google Scholar]
- 58.Segnitz B, Gehring U. The function of steroid hormone receptors is inhibited by the hsp90-specific compound geldanamycin. J Biol Chem. 1997;272:18694–18701. doi: 10.1074/jbc.272.30.18694. [DOI] [PubMed] [Google Scholar]
- 59.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith D F. Dynamics of heat shock protein 90-progesterone receptor binding and the disactivation loop model for steroid receptor complexes. Mol Endocrinol. 1993;7:1418–1429. doi: 10.1210/mend.7.11.7906860. [DOI] [PubMed] [Google Scholar]
- 61.Smith D F, Sullivan W P, Marion T N, Zaitsu K, Madden B, McCormick D J, Toft D O. Identification of a 60-kilodalton stress-related protein, p60, which interacts with hsp90 and hsp70. Mol Cell Biol. 1993;13:869–876. doi: 10.1128/mcb.13.2.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith D F, Whitesell L, Nair S C, Chen S, Prapapanich V, Rimerman R A. Progesterone receptor structure and function altered by geldanamycin, an hsp90-binding agent. Mol Cell Biol. 1995;15:6804–6812. doi: 10.1128/mcb.15.12.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stancato L F, Silverstein A M, Owens-Grillo J K, Chow Y H, Jove R, Pratt W B. The hsp90-binding antibiotic geldanamycin decreases Raf levels and epidermal growth factor signaling without disrupting formation of signaling complexes or reducing the specific enzymatic activity of Raf kinase. J Biol Chem. 1997;272:4013–4020. doi: 10.1074/jbc.272.7.4013. [DOI] [PubMed] [Google Scholar]
- 64.Uehara Y, Hori M, Takeuchi T, Umezawa H. Phenotypic change from transformed to normal induced by benzoquinonoid ansamycins accompanies inactivation of p60src in rat kidney cells infected with Rous sarcoma virus. Mol Cell Biol. 1986;6:2198–2206. doi: 10.1128/mcb.6.6.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uma S, Hartson S D, Chen J J, Matts R L. Hsp90 is obligatory for the heme-regulated eIF-2alpha kinase to acquire and maintain an activatable conformation. J Biol Chem. 1997;272:11648–11656. doi: 10.1074/jbc.272.17.11648. . (Erratum, 272:16068.) [DOI] [PubMed] [Google Scholar]
- 66.Vazquez de Aldana C R, Wek R C, Segundo P S, Truesdell A G, Hinnebusch A G. Multicopy tRNA genes functionally suppress mutations in yeast eIF-2α kinase GCN2: evidence for separate pathways coupling GCN4 expression to unchanged tRNA. Mol Cell Biol. 1994;14:7920–7932. doi: 10.1128/mcb.14.12.7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wek R C. eIF-2 kinases: regulators of general and gene-specific translation initiation. Trends Biochem Sci. 1994;19:491–496. doi: 10.1016/0968-0004(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 68.Wek R C, Jackson B M, Hinnebusch A G. Juxtaposition of domains homologous to protein kinases and histidyl-tRNA synthetases in GCN2 protein suggests a mechanism for coupling GCN4 expression to amino acid availability. Proc Natl Acad Sci USA. 1989;86:4579–4583. doi: 10.1073/pnas.86.12.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wek R C, Ramirez M, Jackson B M, Hinnebusch A G. Identification of positive-acting domains in GCN2 protein kinase required for translational activation of GCN4 expression. Mol Cell Biol. 1990;10:2820–2831. doi: 10.1128/mcb.10.6.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wek S A, Zhu S, Wek R C. The histidyl-tRNA synthetase-related sequence in the eIF-2α protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids. Mol Cell Biol. 1995;15:4497–4506. doi: 10.1128/mcb.15.8.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Whitesell L, Cook P. Stable and specific binding of heat shock protein 90 by geldanamycin disrupts glucocorticoid receptor function in intact cells. Mol Endocrinol. 1996;10:705–712. doi: 10.1210/mend.10.6.8776730. [DOI] [PubMed] [Google Scholar]
- 72.Whitesell L, Mimnaugh E G, De Costa B, Myers C E, Neckers L M. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci USA. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu Y, Lindquist S. Heat-shock protein hsp90 governs the activity of pp60v-src kinase. Proc Natl Acad Sci USA. 1993;90:7074–7078. doi: 10.1073/pnas.90.15.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu Z, Pal J K, Thulasiraman V, Hahn H P, Chen J J, Matts R L. The role of the 90-kDa heat-shock protein and its associated cohorts in stabilizing the heme-regulated eIF-2alpha kinase in reticulocyte lysates during heat stress. Eur J Biochem. 1997;246:461–470. doi: 10.1111/j.1432-1033.1997.t01-1-00461.x. [DOI] [PubMed] [Google Scholar]
- 75.Yonehara M, Minami Y, Kawata Y, Nagai J, Yahara I. Heat-induced chaperone activity of HSP90. J Biol Chem. 1996;271:2641–2645. doi: 10.1074/jbc.271.5.2641. [DOI] [PubMed] [Google Scholar]
- 76.Zhang L, Hach A, Wang C. Molecular mechanism governing heme signaling in yeast: a higher-order complex mediates heme regulation of the transcriptional activator HAP1. Mol Cell Biol. 1998;18:3819–3828. doi: 10.1128/mcb.18.7.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu S, Sobolev A Y, Wek R C. Histidyl-tRNA synthetase-related sequences in GCN2 protein kinase regulate in vitro phosphorylation of eIF-2. J Biol Chem. 1996;271:24989–24994. doi: 10.1074/jbc.271.40.24989. [DOI] [PubMed] [Google Scholar]
- 78.Zhu S, Wek R C. Ribosome-binding domain of eukaryotic initiation factor-2 kinase GCN2 facilitates translation control. J Biol Chem. 1998;273:1808–1814. doi: 10.1074/jbc.273.3.1808. [DOI] [PubMed] [Google Scholar]