Abstract
Electrocardiogram (ECG) monitoring of the fetus during pregnancy, before and during labor, can provide crucial information for the assessment of fetal well-being and development, as well as labor progress. An out-of-clinics fetal ECG monitoring system may pave the way for instant diagnosis, suggesting immediate intervention, which could help reduce the fetal mortality rate. In this paper, we present an unobtrusive fetal maternal ECG monitoring system which can operate in the home setting. The acquisition of the mother’s abdominal ECG is done using the non-contact electrode approach. The extraction of the fetal ECG from the combined fetal/maternal ECG signal is investigated using both Fast Independent Component Analysis (FastICA) and RobustICA algorithms. An accelerometer is integrated for motion artifact detection which would help reduce interferences due to movement. The device also is connected to a cloud server, allowing doctors to access the data in real time.
Keywords: Fetal/maternal Electrocardiogram, Fetal Mortality, Blind Source Separation, Independent Component Analysis
I. Introduction
Despite high healthcare expenditures, prenatal care for women is often inadequate, with the maternal-fetal care system being overburdened. A recent report by the CDC showed that the U.S. fetal mortality rate remained unchanged from 2006 through 2012 at 6.05 per 1,000 births [1]. In the 1970s, continuous monitoring of fetal heartrate (fHR) using Doppler ultrasound (known as cardiotocograph – CTG) was introduced into clinical practice, holding promise that the technology would help reduce incidences of intra-partum fetal injury and death. Unfortunately, a study has revealed that the technique increased the use of cesarean surgery, forceps, and vacuum delivery, but actually did not reduce perinatal morbidity or mortality [2]. Nevertheless, CTG is still a common practice and utilized in over 85% of labor episodes in the U.S [3]. Home-based fHR monitoring systems were introduced lately but it is extremely difficult to operate by non-professional personnel. Further, the side effects of prolonged ultrasound exposure to fetus is a subject of controversy, as warned by the FDA especially with home devices (FDA 2014) [4].
There are existing ECG-based fHR monitoring systems, such as MERIDIAN M110 by MindChild Medical and the GE Monica Novii wireless patch system. However, M110 is wireconnected, bulky, non-mobile and costly whilst the Monica Novii is limited to the labor and delivery areas in the hospital. The Pregsense belt for mobile fHR monitoring is bulky, and it is sold at $250/unit. Further, it would be a break-through if fullfeature fetal ECG (fECG) can be achieved. It has been reported that the changes in morphological parameters of fECG are associated with the level of fetal oxygenation, which greatly affects the development of the fetus [5]. Thus, numerous researchers have aimed to develop optimal signal processing algorithms to extract fECG for the high-noise fetal/maternal ECG (f/mECG). Adaptive filter and deep learning are among promising methods [6].
Our team has been developing patch-based f/mECG monitoring systems [7, 8]. We extensively investigated the use of the non-contact electrode (NCE) approach for signal acquisition [9]. We have demonstrated that the NCE patch can record abdominal ECG signal through the shirt, yielding favorable signal to noise ratio (SNR). In this paper, we present the use of the blind source separation (BSS) method under independent analysis component (ICA) framework to extract fECG from the combined f/mECG signal. Both FastICA and RobustICA were implemented and the extraction of fECG using simulated data and online data was achieved and compared.
II. Design and Implementation
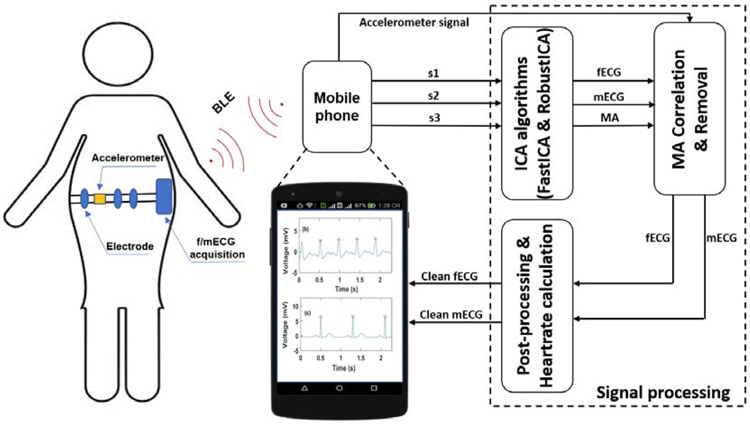
Fig. 1 describes the conceptual view of the f/mECG monitoring system. It comprises of f/mECG acquisition with three ECG channels and an integrated MPU 6050 accelerometer sensor (InvenSense, CA). A system-on-chip Bluetooth Low Energy (BLE) is used to digitize the data and transmit them to a smartphone where an application (App) was developed for further display, logging and analysis. The f/mECG system was designed for expectant mothers to wear as an elastic band to minimize the effects of motion artifacts (MA), thus improving the signal quality. The accelerometer is used to measure MA. We speculate that the signal collected by each ECG channel contains a mixture of independent signals including MA, fetal ECG and maternal ECG. Under this assumption, we leveraged independent component analysis algorithms (FastICA and RobustICA) to extract the fetal ECG.
Fig. 1.
The conceptual overview of the fetal/maternal ECG monitoring system. The s1, s2 and s3 are mixed abdominal ECG signals (aECG) including fetal ECG (fECG), maternal ECG (mECG) and motion artifacts (MA).
A). Circuit Design and Mobile Application
As described in our previous work [7], the circuit was upgraded to have three ECG channels and a motion sensor, in order to facilitate signal processing and extraction. It includes three pairs of non-contact electrodes (NCEs), analog signal processing circuitry and the system-on-chip BLE nRF52832 (Nordic Semiconductor) which is in charge of digitalizing the data and transmitting them to the Android App. Compared with that of the previous work [7], the new BLE module is more capable owing to the direct memory access (DMA) module which independently works with the central processing unit.
In the mobile App, six values chronically-transmitted via BLE are s1, s2, s3, acc_x, acc_y, and acc_z. Where s1, s2, s3 are three mixed ECG signals and acc_x, acc_y, acc_z are the values recorded by the accelerometer corresponding to x axis, y axis and z axis, respectively. Before reaching the App, each value is stored in a number of buffers with 8-bit length each. As the value is 12-bit (by the ADC), it is partitioned and stored in 2 buffers with 8-bit and 4-bit segments, respectively. In the App, the data are received by merging the two buffers. To do so, the first buffer with the 8-bit segment is multiplied by 256, then the value of second buffer with the 4-bit segment is added. Thus, the 12-bit data would be fully reconstructed. The data are collected in real time and preprocessed with a bandpass filter (0.5 Hz to 40 Hz) which is a Chebyshev IIR filter with 4th order.
B). Fetal ECG Extraction with Independent Component Analysis
Blind source separation (BSS) approaches have been applied for fetal/maternal ECG analysis by effectively segregating the unobservable set of signals [10]. Here, we define S = [s1, s2, s3] representing three ECG channels (three vectors) among which each channel is a combination of fetal ECG, maternal ECG and MA. We aim to find a matrix M = [m1, m2, m3] which contains three separated components (fECG, mECG and MA). This matrix could be found via some unknown mixing matrix A with size of 3 × 3 as following
| (1) |
Under the assumption that each component is independent in the mixed signal, the elements of matrix A were found through the process of maximizing the statistical independence of sources. This could be done by maximizing the non-Gaussianity or minimizing the mutual information between among sources [11]. To demonstrate the efficacy of the independent component analysis (ICA) framework, FastICA and RobustICA algorithms have been implemented [11, 12]. The FastICA algorithm simplifies the kurtosis function which aims to measure the non-Gaussianity. However, there are some tradeoffs: 1) Pre-processing is needed (i.e. centering and whitening); 2) If there are sudden changes in the signals, the simplified equations are subject to inaccuracies; and 3) the step size in the kurtosis equations for updating weights is fixed, leading to the potential of being trapped in saddle areas and local extrema [12]. Compared with FastICA, the RobustICA algorithm is an alternative method to overcome such issues. Specifically, the original kurtosis equation is used thus preprocessing is not needed and RobustICA can deal with all signal types. Additionally, an adaptive step size is used with RobustICA, ensuring the weights converge to the actual convergence point, thus avoiding getting trapped as the former algorithm does [12].
C). Cloud System
We have been developing a cloud system to work with all human wearable devices as well as data collection systems with animal models. The system utilized an IoT architecture, opting for direct connection to the cloud via a Wi-Fi module. This provides each peripheral device with enough throughput to easily stream recorded signals in real time. Each peripheral device (smartphone in this case) could be connected to the cloud via a standard home or enterprise internet access point (router). Utilizing a direct connection to the systems servers by each device sidesteps possible data bottlenecking issues present with a hub-and-spoke approach. Most commercial and residential wireless access points (routers) have a theoretical limit of approximately 250 devices which may be connected at any one time. This architecture allows for scaling of the device pool up to the limit of the router and the bandwidth of the internet connection. Each streaming connection utilized a relatively small amount of data, so even with a large number of connections, the system consumes a relatively small amount of bandwidth. Each peripheral monitoring device utilizes a 32-bit microcontroller (MCU) running FreeRTOS. These peripherals can be coupled directly to additional monitoring equipment or sensors via cable or BLE which allows for flexible deployment in a laboratory setting. Users can utilize cloud-based multichannel signal recording and processing.
III. Experiment and Results
The efficacy of ICA methods was examined using both simulated data and the online database from PhysioNet [13]. Fetal ECG and maternal ECG with a sampling rate of 1000 Hz were generated by using a subVI from Lab View software (National Instruments, Austin, TX) within one minute and MAs were collected by the MPU sensor (MPU6050). A matrix A with the size of 3×3 for mixing all signals was also created by randn() function (Matlab 2018, Matlab Works). The simulated data, then were formed by multiplying matrix A and a matrix of fECG, fECG and MAs. For the online database from PhysioNet, Abdominal and Direct Fetal Electrocardiogram Database (ADFECGDB) was utilized [13]. It has data packets with 5 min of recordings (4 abdominal channels and the scalp ECG) from 5 women in labor (38 to 41 weeks of gestation) and with reference FQRS annotation derived from the scalp electrocardiogram (sECG).
For the simulated data, spectrogram was used to assess the power per frequency of fECG after extraction. Several statistical values were used to evaluate the two ICA algorithms. A set of binary classification, including the sensitivity (Se), positive predictive value (PPV) and the accuracy measure (F1), was used to evaluate fECG peak detection in ADFECGDB database as the following:
| (2) |
| (3) |
| (4) |
where TP, FP and FN are true positive, false positive and false negative in fECG peak detection, respectively.
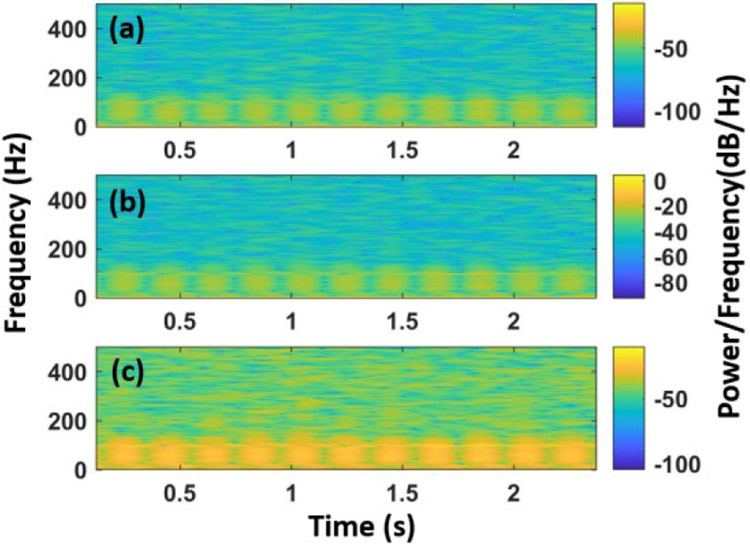
Fig. 2 illustrates the spectrum of frequencies of the original fECG signal, followed by the extracted one by FastICA and RobustICA, respectively. Compared with the original fECG, the one extracted by FastICA algorithm (Fig. 2b) has the most identical spectrum with the major frequencies within 0 to 100 Hz. In contrast, the spectrum of the signal acquired by RobustICA (Fig. 2c) shows additional components. It may stem from other signals as RobustICA did not completely suppressed them.
Fig. 2.
The spectrogram of original fECG (a), the extracted fECG by FastICA (b), and RobustICA (c), respectively.
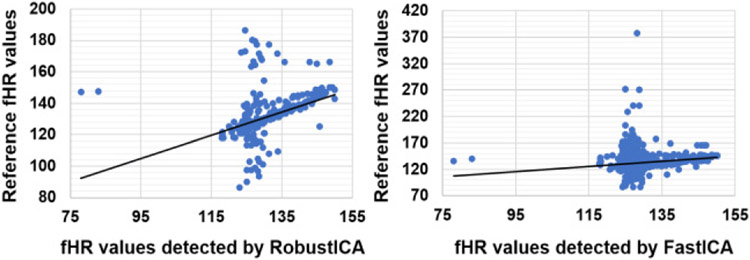
Signals from the ADFECGDB database first underwent preprocessing process by applying a bandpass filter of 10 Hz - 150 Hz, and then fHR values were extracted by both algorithms. Table I summarizes the performance of each algorithm. FastICA showed the best results on r01 and r08 with the F1 score of ~0.92 and ~0.9, respectively. Compared with this algorithm on the same sets, the RobustICA outperformed with the FI score of ~0.96 for rOl and ~0.99 for r08. However, both algorithms got low F1 scores (<0.51) on r01 and r04. This may be explained by the inconsistency of the signal in the dataset. Fig. 3 depicts the validation of the fetal heartrate (fHR) values of the database record r01 detected by RobustICA (Fig. 3a) and FastICA (Fig. 3b) algorithms with the reference fHR collected by using scalp electrodes. Two algorithms yielded fHR with great correlation compared with the reference fHR. Regarding the algorithm run time (with 1-minute-long data), it took 0.087 second for FastICA and 0.1 second for RobustICA. This did not show a significant difference, though RobustICA slightly outperformed FastICA.
TABLE I.
The Performance of Fastica and Robustica on the ADFECGDB Database
| Record | FastICA | RobustICA | ||||
|---|---|---|---|---|---|---|
| Se | PVV | F1 | Se | PVV | F1 | |
| r01 | 0.923 | 0.9115 | 0.9172 | 0.9654 | 0.9624 | 0.9639 |
| r04 | 0.1458 | 0.2121 | 0.1728 | 0.1442 | 0.2098 | 0.1709 |
| r07 | 0.664 | 0.6246 | 0.6437 | 0.2036 | 0.2258 | 0.2141 |
| r08 | 0.9033 | 0.8853 | 0.8942 | 0.9922 | 0.9876 | 0.9899 |
| r10 | 0.5717 | 0.4705 | 0.5162 | 0.5621 | 0.4777 | 0.5165 |
Fig. 3.
The correlation of reference fHR and the fHR detected by RobustICA (a) and FastICA (b) algorithm (Unit: BPM).
IV. Conclusion
A home-based and cloud-enabled fetal/matcrnal ECG monitoring system has been developed. The extraction algorithms of FastICA and RobustICA were validated and compared with both simulated data and online data. We are applying for Institutional Review Board (IRB) protocols to conduct validations with pregnant women in our participating clinics in both UC Irvine, CA and the Institute of Population, Health and Development, Hanoi, Vietnam. This will further help us characterize and improve our next-generation systems.
Acknowledgment
This work is supported by the National Science Foundation CAREER Award under #1652818 (H. Cao) and the National Institute of Health Award #OD 024874 – 01A1 (H. Cao and M.P.H. Lau).
References
- [1].Gregory E, MacDonnan MF, and Martin JA, "Trends in fetal and perinatal mortality in the United States, 2006-2012," NCHS data brief, pp. 1–8, 2014. [PubMed] [Google Scholar]
- [2].Vintzileos AM, Nochimson DJ, Guzman ER, Knuppel RA, Lake M, and Schifrin BS, "Intrapartum electronic fetal heart rate monitoring versus intermittent auscultation: a meta-analysis," Obstetrics & Gynecology, vol. 85, pp. 149–155, 1995. [DOI] [PubMed] [Google Scholar]
- [3].Macones G, "Intrapartum fetal heart rate monitoring: nomenclature, interpretation, and general management principles," Obstetrics and Gynecology, vol. 114, pp. 192–202, 2009. [DOI] [PubMed] [Google Scholar]
- [4].FDA. (2014). Avoid Fetal "Keepsake" Images, Heartbeat Monitors. Available: https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm095508.htm
- [5].Martens SM, Rabotti C, Misclii M, and Sluijter RJ, "A robust fetal ECG detection method for abdominal recordings," Physiological measurement, vol. 28, p. 373, 2007. [DOI] [PubMed] [Google Scholar]
- [6].Wei Z, Lijuan L, Xuemei G, and Guoli W, "A deep learning approach for fetal QRS complex detection," Physiological Measurement, vol. 39, p. 045004, 2018. [DOI] [PubMed] [Google Scholar]
- [7].Le T, Moravec A, Huerta M, Lau MPH, and Cao H, "Unobtrusive Continuous Monitoring of Fetal Cardiac Electrophysiology in the Home Setting," presented at the IEEE; Sensors, New Delhi, India, 2018. [Google Scholar]
- [8].Sharma M, Ritchie P, Ghirmai T, Cao H, and Lau MP, "Unobtrusive acquisition and extraction of fetal and maternal ECG in the home setting," in SENSORS, 2017. IEEE, 2017, pp. 1–3. [Google Scholar]
- [9].Griggs D, Sharma M, Naghibi A, Wallin C, Ho V, Barbosa K et al. , "Design and development of continuous cuff-less blood pressure monitoring devices," in SENSORS, 2016 IEEE, 2016, pp. 1–3. [Google Scholar]
- [10].Behar J, "Extraction of Clinical Information From the Non-Invasive Fetal Electrocardiogram," PhD, University of Oxford, Oxford, UK, 2014. [Google Scholar]
- [11].Hyvärinen A and Oja E, "Independent component analysis: algorithms and applications," Neural Networks, vol. 13, pp. 411–430, 2000/06/01/2000. [DOI] [PubMed] [Google Scholar]
- [12].Zarzoso V and Comon P, "Robust Independent Component Analysis by Iterative Maximization of the Kurtosis Contrast With Algebraic Optimal Step Size," IEEE Transactions on Neural Networks, vol. 21, pp. 248–261, 2010. [DOI] [PubMed] [Google Scholar]
- [13].Goldberger A, Amaral L, Glass L, Hausdorff J, Ivanov P, Mark RG, et al. , PhysioBank, PhysioToolkit, and PhysioNet : Components of a New Research Resource for Complex Physiologic Signals vol. 101, 2000 [DOI] [PubMed] [Google Scholar]