Abstract
The Xenopus tadpole visual system exhibits an extraordinary extent of developmental and visual experience-dependent plasticity, establishing sophisticated neuronal response properties that guide essential survival behaviors. The external development and access to the developing visual circuit of Xenopus tadpoles make them an excellent experimental system in which to elucidate plastic changes in neuronal properties and their capacity to encode information about the visual scene. The temporal structure of neural activity encodes a significant amount of information, access to which requires recording methods with high temporal resolution. Conversely, elucidating changes in the temporal structure of neural activity requires recording over extended periods. It is challenging to maintain patch clamp recordings over extended periods and Ca++ imaging has limited temporal resolution. Extracellular recordings have been used in other systems for extended recording, however spike amplitudes in the developing Xenopus visual circuit are not large enough to be captured by distant electrodes. Here we describe a juxtacellular tetrode recording method for continuous long-term recordings from neurons in intact tadpoles, which can also be exposed to diverse visual stimulation protocols. Electrode position in the tectum is stabilized by the large contact area in the tissue. Contamination of the signal from neighboring neurons is minimized by the tight contact between the glass capillaries and the dense arrangement of neurons in the tectum. This recording method enables analysis of developmental and visual experience-dependent plastic changes in neuronal response properties at higher temporal resolution and over longer periods than current methods.
MATERIALS
It is essential that you consult the appropriate Material Safety Data Sheets and your institution’s Environmental Health and Safety Office for proper handling of equipment and hazardous materials used in this protocol.
Reagents
Albino or wild-type Xenopus laevis tadpoles (obtained by in-house fertilization or from XEN EXPRESS; stage 46–48 (Nieuwkoop and Faber, 1956))
3-Aminobenzoic acid ethyl ester (Tricane or MS222; Sigma; w/v = 0.01%, dissolved in Steinberg’s Solution. Adjust pH to pH7.4 after adding MS222
Bleach
External and internal solutions (115 mM NaCl, 4 mM KCl, 3 mM CaCl2, 3 mM MgCl2, 5 mM HEPES, 10 mM glucose, 10 μM glycine, pH 7.2 with NaOH, osmolality 255). The internal and external solutions are the same.
Pancuronium bromide (10μM)
Steinberg’s solution (100%) <R>
Equipment
Glass pipette (tip diameter~20–30μm)
- Materials for the glass tetrode electrode holder
- Acrylic blocks
- This is the material of the electrode holder.
- Ag-AgCl Electrode Pellet, 2 × 4 mm (Warner Instruments, E201, Cat#: 64-1305)
- Ceramic tile (SUTTER, #CTS)
- Co-axial cables
- Compression Cap Polycarb for 2mm glass holder (Warner Instruments, QC-20, Cat#: 64-1296)
- Cross theta capillaries made of borosilicate glass (outer diameter = 2 mm, inner diameter = 1.4 mm, septum = 0.0088 mm, length=100 mm; Hilgenberg GmbH Code# 1401731)
- Gold Plated 1 mm Pin (four; Warner Instruments, WC1-10, Cat#: 64-1325)
- Gold Plated 2 mm Pin (four; Warner Instruments, WC2-5, Cat#: 64-1326)
- Jack assembly, 2 mm (four; Warner Instruments, HC-21, Cat#: 64-1286)
- M3 long nut, 2cm
- This is part of the handle for the electrode holder.
- M3 threaded rod, 2cm (five)
- This is part of the handle for the electrode holder.
- Silver wire
- Materials for recording setup
- Back projecting screen
- Digitizer (Molecular Device, DIGIDATA 1440A)
- Faraday cage with front doors
- Headstage (four; Molecular Device, CV-7B)
- Patch Clamp Amplifier (two; Molecular Device, Multiclamp 700A/700B)
- Perfusion system
- Photodiode
- Rear projection screen (Alternative Screen Solutions, Sticky Screen, https://store.gooscreen.com/stickyscreen-rear-projection-screen-window-film)
- Visual stimulation equipment: miniprojector, etc.
- Peristaltic pump
- Silicon tube (ID 1mm, OD 3mm) × 3m
- 60ml syringe
- Air pump and air stone
- Petri dish
- Sutter P-97 pipette puller with a 3.0 mm × 3.0 mm square box filament (Sutter; FB-330B)
- Sylgard
- Vacuum grease
METHOD
This technique employs a pulled four-channel glass capillary pipette based on the electrode design used in industry and patch clamp recording instrumentation available in many laboratories. A flat blunt end on the glass capillary creates an electrically shielded space that can enclose from one to several neurons for recording. This excludes convergence of weak signals from distant cells that increase uncertainty in the spike-sorting process. Contact with the shaft of the glass electrode stabilizes the electrode in the tectum over extended recording sessions. Aeration and perfusion extend survival up to 1 or 2 days, which has not been achieved with previous recording methods. The four-channel electrodes collect signals from several cells, which enables the study of components in a local circuit.
Make a glass tetrode electrode holder
-
1
Prepare the holder for the four-channel glass electrode (Fig. 1A–C), which is a key component of this setup. This holds the cross-theta glass capillary (Hilgenberg-GmbH), originally designed for industrial use. The diameter of the glass electrode tip is ~40μm and covers ~3–5 neurons in the tectum (Fig1. D, E).
Our custom-made holder (Fig. 1A) is modified from the basic design of the theta-capillary holder, THS-M20PH,from Warner Instruments, which is commonly used for focal stimulation of neural tissues. We increased the number of channels from two to four and increased the length from the shank to the tip needs to be longer than 13mm. If the length of L3 is too short, the four Ag-AgCl wires are difficult to insert.
-
2
Insert a 2 mm pin into a 2 mm Jack Assembly (Fig. 1A left, four circles). A tube (internal diameter=1mm) is attached to the pipe protruding from the holder (Figure 1A, left). The other end of the tube is connected to a 1ml syringe. The pressure in the canal is controlled through the tube.
-
3
Set the silver wires in the holder. Cut the length of the four wires to different lengths corresponding to the different channels. This helps in inserting the wires into the four channels of the capillary. The longest wire should reach the shoulder of the pipette. Soak the silver wires in bleach for 5 min.
Figure 1.
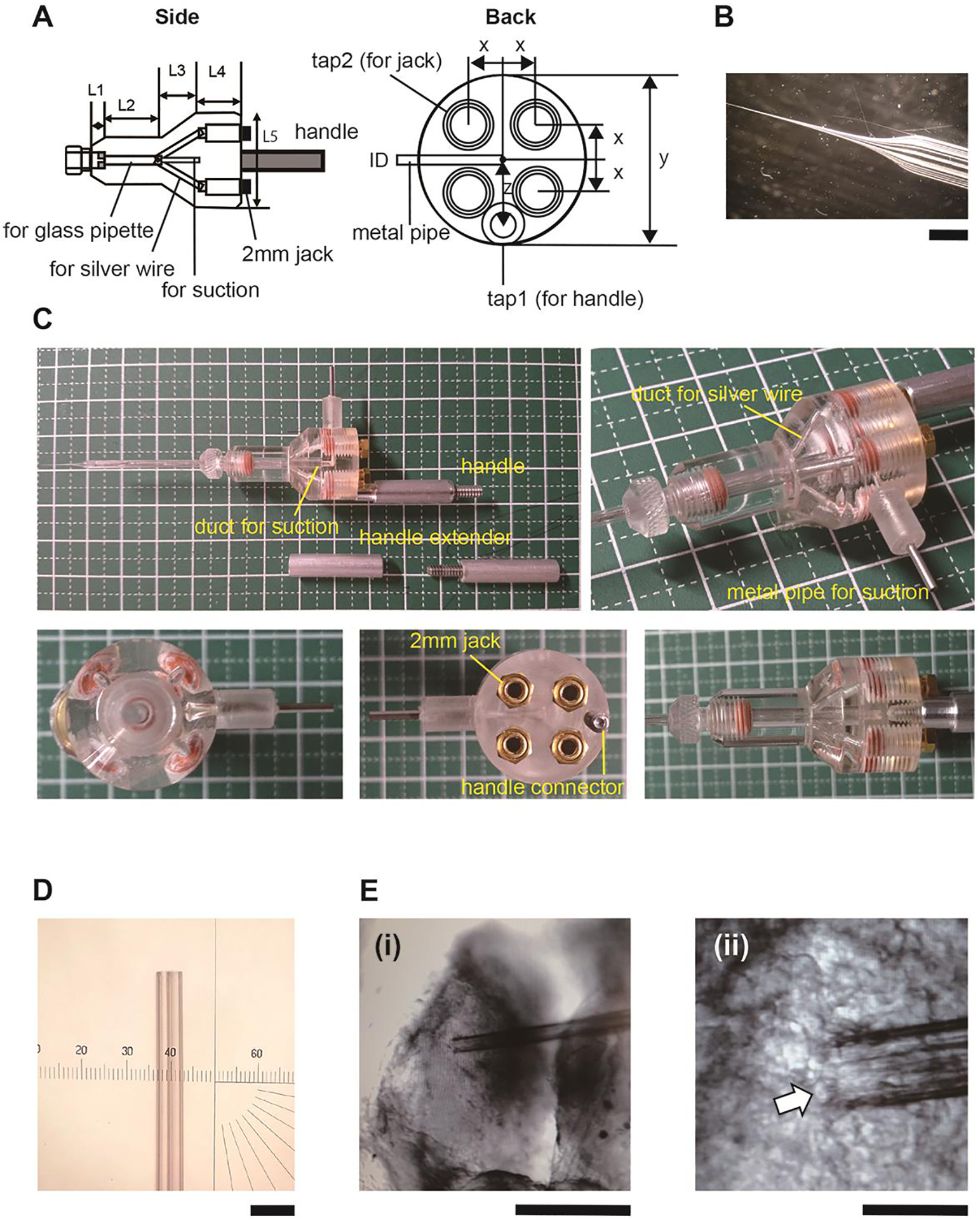
A. Diagram of the custom-designed four channel glass electrode holder. Left: projection from the side view, Right: projection from the connector side. The design is modified from a Warnnenr Instrumennts theta glass electrode holder (https://www.warneronline.com/theta-glass-holders). It has a duct for a glass pipette and five branches (left, three branches are visible). The four branches are for silver wires and the other duct at the center (left) is for a suction line, as labeled in the figure. Although the four branches for the wires merge, the wires do not touch each other unless they are twisted. The suction line is connected to a metal pipe (right). Each silver wire passes through a ϕ2mm compression cap (Warner, QC-20) and contacts a 2 mm jack (Warner, HC-21), which is screwed into the top-side of the capillary holder. A 2 mm pin connector (Warner, WC2–5) is connected to a 1 mm pin connector (Warner, 1–10) through a shielded cable. The 1mm pin connector is inserted into the 1mm jack on the patch clamp headstage (Molecular Device, CV-7B). The other end of the 2mm pin connector is inserted into the 2mm jack on the capillary holder. The ground terminals on the four headstages are connected to a single wire. The wire is soldered to a ground pellet (Warner, E201). Size (mm) x: 3.8, y: 20, z: 5.4, ID: 1, tap1: 4–40×4.7, tap2: 10–32×6, L1: 3, L2: 13, L3: 8, L4: 9 L5: 19 B. Photo of a four channel glass electrode pulled on a Sutter P-97 micropipette puller (shank=13mm, The setting on Sutter P-97: 3mm box heater, heat=RAMP, pull=60, velocity=83, delay=135, P=200) C. Photos of the electrode holder. D. Glass electrodes with a blunt end. E. Electrode positioned on the tectum. Arrow points to a tectal cell. Note diameter of the neuron compared with the pipette tip. The pipette tip has four openings corresponding to the 4 channels. Scale bars (i) 400 μm, (ii) 40 μm.
Making the recording setup
-
4
Make a recording chamber with clear acrylic plates (4mm thickness). This chamber has rear projection screens on both sides. The screen size is 60mm (width) × 55mm (height). Paste the rear projecting screens on both sides. The distance from the tadpole’s eye to the screen is ~20mm. These screen parts are glued to two trapezoid side panels (Figure 2A). The height of the trapezoid is 60mm. The sides of the trapezoid (Figure 2A) are 64mm and 35mm. The size of the bottom panel is 35mm × 60mm. To make a stage for tadpoles, penetrate an acrylic round rod (ϕ=7mm) through the two trapezoid plates at 18mm from the upper side. Flatten the upper side of the round rod. The holes are sealed with epoxy glue or O-rings. Make a 3mm hole on the bottom panel and glue a short pipe (OD 3mm × 5mm). Connect the recording chamber and 60ml syringe with a silicon tube (ID 2mm × 1m). Make a 2mm hole on the trapezoid panel and glue a short pipe (OD2mm × 5mm). Connect a silicon tube (ID 1mm) with an outlet from a peristatic pump. The inlet to the peristatic pump is connected to a silicon tube (ID 1–2mm × 20cm). Set the silicon tube inside of the 60ml syringe. Set an air stone connected to an air pump in the 60ml syringe.
-
5
For the tetrode recording system, use the same amplifiers and digitizer as used for patch clamp recording (Fig. 2 A, B). Use four channels for amplification (Fig. 2C). Connect the electrodes to the input terminals of the headstages using shielded wires, which should be as short as possible.
-
6
Connect the ground lines to a single Ag-AgCl pellet. To record visually evoked activity, place the animal on the stage in the recording chamber (Fig. 2C).Project images for visual stimulation from the miniprojector to the back projecting screen. Image details depend on the purpose of the experiment and images can be generated using a variety of software, including MATLab. To identify the time of stimulus presentation, a small dot is presented on a photodiode located outside of the back-projecting screen when the stimulus is presented.
Figure 2.
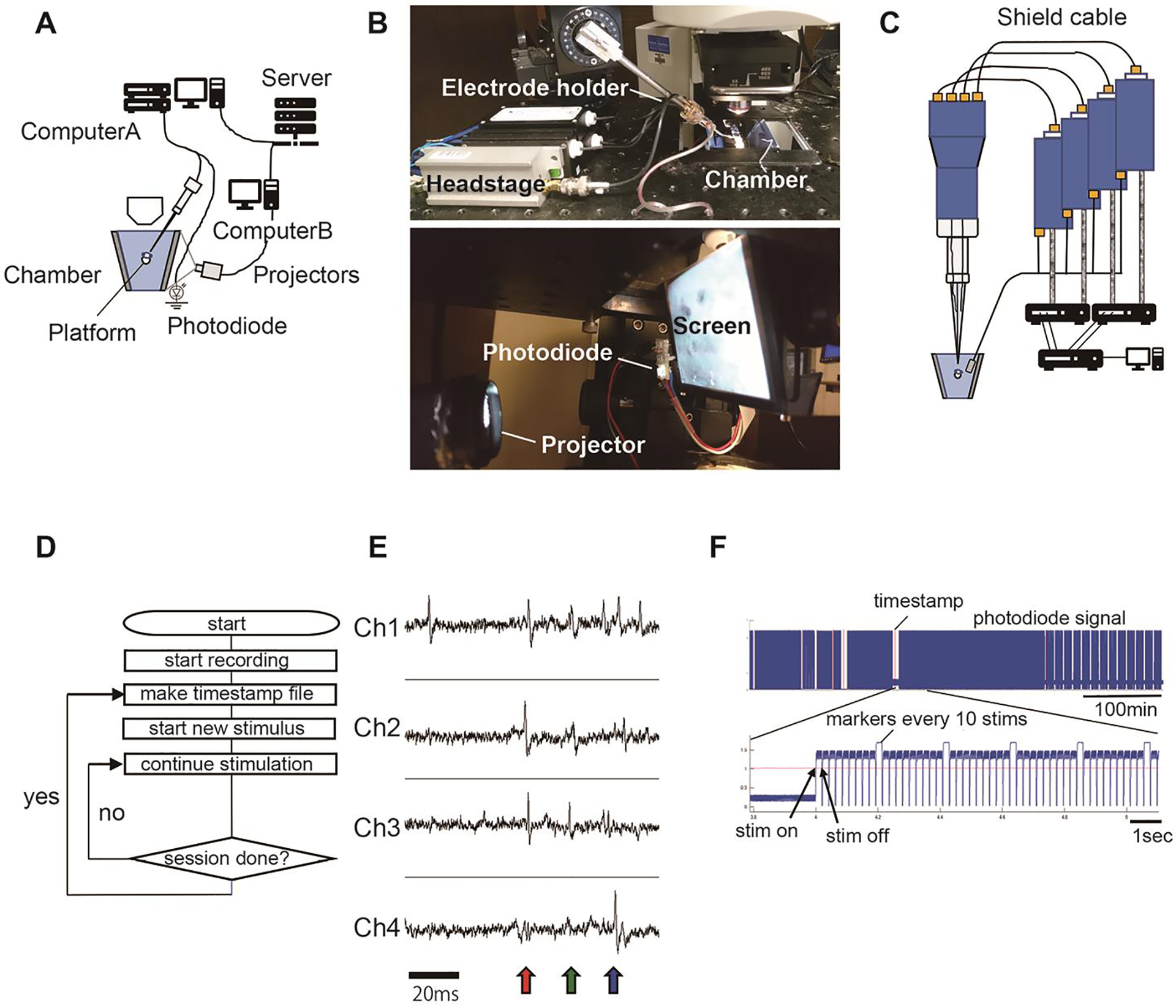
A. Diagram of setup. Tetrode data are recorded in computer A. A back-projection screen and a platform to hold the animal are attached to a recording chamber. Images are generated in computer B and projected onto the back-projection screen. Stimulus timing is monitored by a photodiode. B. Top: Picture of the mounted electrode and headstage on the manipulator. Bottom: Picture of the miniprojector, photodiode and screen on the side of the recording chamber. C. Wiring of the four-channel glass electrode with the headstage assembly and amplifier (MultiClamp 700A/B). Bottom: Picture of the recording chamber and the projector. The photodiode is placed to the left of the screen. D. A flow chart of continuous recording. Neural activity is recorded continuously. Temporal boundaries of recording sessions are marked with a timestamp file generated by the image presentation computer (computer B). E. Spike waveforms recorded by individual channels. Spike signals from the same cells are detected by multiple channels (colored arrows). F. An example of the photodiode signal. The red lines identify session start times marked by the timestamp file. Timing of each stimulus is identified by the rising edge of the photodiode signal.
Recording procedure
-
7
Pull the cross-theta glass electrodes with a Sutter P-97 puller with a 3.0 mm × 3.0 mm square box filament using the following parameters: Temp: RAMP+20, Pull=30, Vel=140, Time=20.
-
8
Insert the Ag-AgCl wires into the cross-theta capillary. Make sure the wires in the holder are dry. Insert the longest wire into the first canal, then set the second longest one into the neighboring canal. Insert the other wires this way. If the wires become bent many times during this process, replace the wires.
-
9
When the wires are set, check the isolation between electrodes by measuring electric resistance between the gold plated 2 mm jacks using an electrical tester. If the resistance is less than 100ohm, the wires contact each other in the holder. Remove the pipette and insert the wires again.
-
10Break the glass electrode. Follow these steps to generate a flat cutting plane (Fig. 1D) at the electrode tip for tight contact with the tissue:
- Break the very end of the tip with forceps.
- Dip the tip into the external solution to fill the end of the capillary to 5 mm.
- Make a scratch at the ϕ=40μm position on the capillary using a ceramic tile. The tip should break when the tile scratches the capillary. If the tip doesn’t break, the ceramic tile is not sharp enough. Ceramic tiles dull easily.
- Attach the electrode to the headstage assembly and dip the tip into the external solution applying negative pressure to fill the capillary. Monitor voltage in current clamp mode (I=0). The voltage changes when the external solution comes into the capillary. Wait until the baseline stabilizes.
-
11
Prepare the animal for recording. Anesthetize a tadpole in a Petri dish containing 0.01% MS222 for less than 1 min. Transfer the animal to a strip of Sylgard in a Petri dish filled with external solution containing 10μM pancuronium bromide. Hold the animal on the Sylgard using insect pins.
-
12
The tectal neurons are located on the ventral side of the tectal lobe. The neurons need to be exposed for recording. To do so, cut the dorsal midline of the tectum through the skin using a 30-gauge needle attached to a syringe. Then, flip one of the tectal lobes. Remove the membrane covering the ventricular surface of the tectum using suction through a glass pipette (tip diameter~20–30μm). This exposes the neurons. Recordings will be collected from the exposed neurons.
-
13
Transfer the tadpole on the Sylgard strip to the recording chamber made of clear acrylic plates as described in Figure 2A. Fill the chamber with external solution. Attach the Sylgard to the platform bar in the recording chamber using vacuum grease. Place the electrode tip on the exposed surface of the tectum.
-
14
Apply weak negative pressure (−0.73psi) through the syringe connected to the pipe on the electrode holder (Fig. 1A, C). Use the scale on the syringe to control the pressure. Close the front door of the Faraday cage. Wait until the baseline and the spiking are stabilized (~10 min). If clear spikes are not observed, start the process over with a new glass pipette. Contact between the glass electrodes and tissue will be stable for a long time, from 8 h to 1–2 d.
-
15
Turn on the peristatic pump and the air pump for aeration. The peristatic pump makes a spike-like periodic noise. Only turn on the pump only between recording sessions or use a gravity feed system. It is likely that tadpole tissues can tolerate less oxygen in the external solution compared to mammalian tissue because the animals live in poorly oxygenated water. We observed visual stimulation-evoked neuronal activity over 15–24 h without aeration when the chamber volume was 100cc. Nevertheless, aeration ensures a longer recording time.
-
16
Start recording. Perform recording in current clamp mode (I=0). Select the gap-free mode on the recording software. Spikes are usually detected in the first several trials. Failure to detect spikes may be due to poor positioning of the tetrode. If this occurs, reposition the tetrode. In successful trials, the amplitude of spikes becomes larger over the first ~10 min of recording because the contact between the pipette and neurons stabilizes and the electric shield between the pipette and tissue increases.
-
17Carry out a desired protocol. For instance, use the following protocol to test whether tectal cell visual response properties change in response to exposure to particular stimuli (Fig. 2D):
- Present image stimuli to characterize response properties of recorded neurons. Signals from the same cells are detected through the four channels (Fig. 2E).
- The computer for image presentation makes a setting data file in which stimulus information is described. The timestamp of the file also indicates the approximate start time of the stimulus.
- Identify the exact start time of the visual stimulus based on the photodiode signal and the timestamp of the setting file data (Fig. 2F).
-
18
Perform spike sorting with wave_clus (https://github.com/csn-le/wave_clus) (Fig. 3) (Chaure et al., 2018) (Quiroga, 2012). We modified the code to use the same waveform in sorting spikes in different sessions. In our experiment, the stringency (temperature) of the clustering condition was set so that sizes of major clusters were at the local maximum (Fig. 3). Change the stringency to match the purpose of the analysis.
Figure 3.
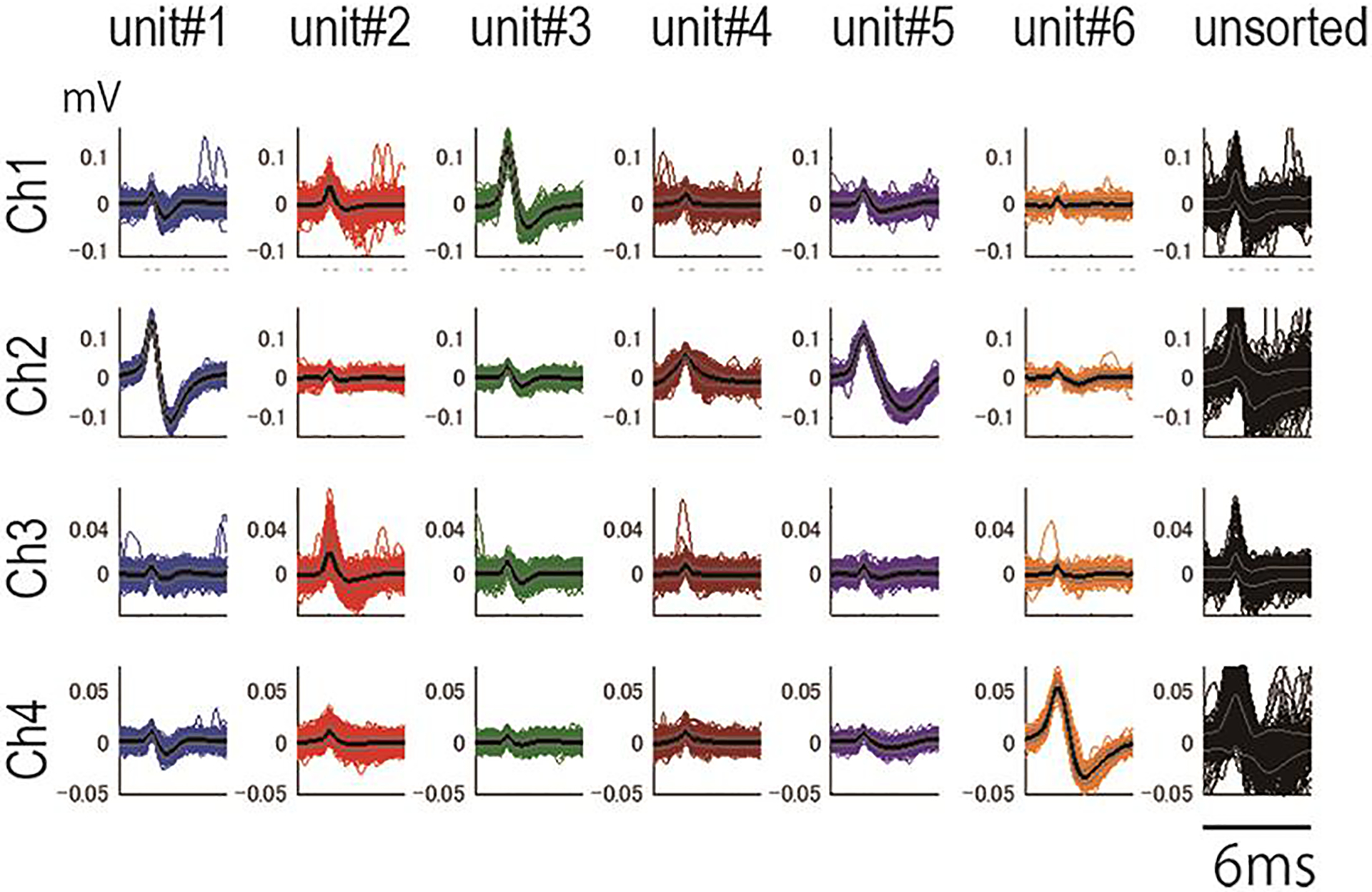
A. Waveforms of sorted spikes. Unit #5 and Unit #1 are likely the same cells. The rightmost column shows units that are not grouped into the clusters. The wave data are digitized at 10KHz.
TROUBLESHOOTING
-
Problem (Step 9): The Ag wires cannot be inserted during electrode preparation.
Solution: This is likely due to metal fatigue of wires. Use new wires.
-
Problem (Step 11): The electrode tip is not flat after breaking.
Solution: This can occur if the ceramic tile is old or dirty. Either use a new tile or clean the existing tile with 70% ethanol.
-
Problem (Step 11): The baseline voltage reading is unstable during electrode preparation.
Solution: An unstable baseline can occur due to incomplete soldering or lost Cl coating on the wire because of multiple attempts to insert Ag wires. Check the connection with a tester.
-
Problem (Step 17): No spikes were observed during recording.
Solution: This can occur because the electrode tip size is too small. Replace with an electrode with a larger tip. It can also occur due to poor recording position. Reposition the electrode in the tectum.
-
Problem (Step 17): Only small spikes were observed during recording.
Solution: This can occur because the electrode tip size is too large. Replace with an electrode with a smaller tip.
-
Problem (Step 17): A large local field potential is observed during recording.
Solution: This can occur when the pipette tip is not flat. Remake the electrode with a flat tip. To make the tip flat, make a deeper scratch, or use a new and clean ceramic tile.
-
Problem (Step 18): Spikes were only recorded for a short time.
Solution: This can occur due to unstable contact between neurons and the pipette. If this is the case, use a clean pipette. It can also occur due to animal movement. If the tadpole is young (<stage 44), use a higher concentration (20μM) of pancuronium dibromide or use other immobilization methods.
-
Problem (Step 18): Animals do not live long during recording.
Solution: Animals may die during recording sessions because they were damaged during preparation for recording. In future preparations, be more careful in animal preparation, taking care to not damage the tail. Another possible explanation for animals dying during the recording session is infection. Sterilize the chamber with 70% ethanol to prevent infection. A third explanation is that the animals experienced a lack of oxygen. For future experiments, increase intervals between recording sessions for longer aeration periods.
DISCUSSION
This protocol has been approved by The Scripps Research Institute IACUC (Protocol# 08-0083).
The Xenopus visual circuit is an excellent system in which to study fundamental principles of plasticity (Sin et al., 2002; Zhang et al., 1998) (Constantine-Paton and Law, 1978; Engert et al., 2002; Meyer, 1998). Currently, neural activity in the Xenopus tectum is mainly studied by patch clamp recording (Aizenman et al., 2003; Ciarleglio et al., 2015; Pratt et al., 2008; Wu et al., 1996) and Ca++ imaging (Dunfield and Haas, 2009; Hiramoto and Cline, 2009; Podgorski et al., 2012; Xu et al., 2011). These techniques have drawbacks: the duration of patch clamp recordings is limited and Ca++ imaging has poor temporal resolution, limiting the study of circuit organization and plasticity.. Methods that are amenable to longer recording duration would allow investigations of forms of plasticity that occur over longer time scales (Hiramoto and Cline, 2014; Lim et al., 2010; Ruthazer et al., 2003). Extracellular recording is such a method. Single unit extracellular recording with metal electrodes has been used in adult Xenopus retinotectal mapping studies (Gaze, 1958). Juxtacellular recordings with metal-filled glass with platinum-plated tips electrodes has also been used in Xenopus (Udin and Scherer, 1990), however these metal electrodes are less sensitive than glass electrodes. Multichannel glass electrode recording therefore offers multiple advantages over other recording methods. Because tetrodes are typically used in systems with relatively large amplitude spikes, we modified the tetrode design to detect the small amplitude spikes in developing Xenopus optic tectal neurons. This modification is particularly valuable for investigating development and plasticity of neuronal and circuit properties, because in many cases, neurons with rich plasticity are immature and do not generate large spikes. The large contact area between the shaft of the glass electrode and the tissue stabilizes the electrode within the tissue over extended periods of time. The tight seal between the neurons and the multiple openings of the pipette increases the sensitivity of the recording.
We wished to develop multi-unit recording capacity for longer time scales, with the following parameters in mind: the ability to collect stable recordings over time; the ability to perform reliable spike-sorting by limiting contaminating signals from distant cells; and the capacity to collect spikes from multiple neighboring cells. This protocol is optimized to collect long-term recordings to study changes in visual response properties of individual neighboring neurons.
The success of the glass tetrode recordings depends on high quality data, which is essential for accurate spike sorting and data analysis. While recordings with metal electrodes detect signals from many neurons, the glass pipette electrode records activity from relatively few neurons, based on several factors. First, the glass pipette records cells in a limited area in the tissue because the pipette and the tissue make an electric shield, excluding signals outside of the pipette. Excluding signals outside of the pipette contact area makes spike sorting easy. In addition, neurons within the cell-dense layers of the Xenopus tectum are closely packed. Usually, only 3–5 units are sorted from the tectal recording, roughly matching the expected number of the tectal cells in the shielded area (cell body=~10μm, each pipette tip =~15μm). Since only 3–5 cells are in the recording area, the wave forms across the channels are usually distinct between units. The spike waveform can change gradually over a long recording session. This is partly due to movement of the pipette location or changes in the property of the neurons. This recording method reduces the likelihood that changes in waveform over time are due to pipette movement because of the stable contact of the glass electrode shaft with the tissue. Sometimes changes in the waveform may be caused by changes in the biophysical properties of the neurons. To track these units over the recording sessions, methods by Dhawale et al, (Dhawale et al., 2017) in which they track the changes of waveforms and identify units that change waveforms over time, may be useful.
Since this electrode shields signals from other regions, local field potentials (LFP) are not typically detected. To record LFPs from larger groups of neurons, pipettes with larger diameters (>70μm) can be used. This will decrease signal amplitudes from single neurons.
In summary, we present a method for multichannel juxtacellular glass electrode recording from neurons in Xenopus optic tectum. The advantages of this method are its sensitivity to detect small spikes and the ability to maintain stable recordings in an intact animal over extended periods of time. Tetrode recording using the four-channel capillaries are useful to study developmental plasticity in the Xenopus visual system and may also be useful to study plasticity in other circuits.
REFERENCES
- Aizenman CD, Akerman CJ, Jensen KR, and Cline HT (2003). Visually driven regulation of intrinsic neuronal excitability improves stimulus detection in vivo. Neuron 39, 831–842. [DOI] [PubMed] [Google Scholar]
- Chaure FJ, Rey HG, and Quian Quiroga R (2018). A novel and fully automatic spike-sorting implementation with variable number of features. J Neurophysiol 120, 1859–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarleglio CM, Khakhalin AS, Wang AF, Constantino AC, Yip SP, and Aizenman CD (2015). Multivariate analysis of electrophysiological diversity of Xenopus visual neurons during development and plasticity. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantine-Paton M, and Law MI (1978). Eye-specific termination bands in tecta of three-eyed frogs. Science 202, 639–641. [DOI] [PubMed] [Google Scholar]
- Dhawale AK, Poddar R, Wolff SB, Normand VA, Kopelowitz E, and Olveczky BP (2017). Automated long-term recording and analysis of neural activity in behaving animals. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunfield D, and Haas K (2009). Metaplasticity governs natural experience-driven plasticity of nascent embryonic brain circuits. Neuron 64, 240–250. [DOI] [PubMed] [Google Scholar]
- Engert F, Tao HW, Zhang LI, and Poo MM (2002). Moving visual stimuli rapidly induce direction sensitivity of developing tectal neurons. Nature 419, 470–475. [DOI] [PubMed] [Google Scholar]
- Gaze RM (1958). The representation of the retina on the optic lobe of the frog. Q J Exp Physiol Cogn Med Sci 43, 209–214. [DOI] [PubMed] [Google Scholar]
- Hiramoto M, and Cline HT (2009). Convergence of multisensory inputs in Xenopus tadpole tectum. Dev Neurobiol 69, 959–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramoto M, and Cline HT (2014). Optic flow instructs retinotopic map formation through a spatial to temporal to spatial transformation of visual information. Proc Natl Acad Sci U S A 111, E5105–5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim BK, Cho SJ, Sumbre G, and Poo MM (2010). Region-specific contribution of ephrin-B and Wnt signaling to receptive field plasticity in developing optic tectum. Neuron 65, 899–911. [DOI] [PubMed] [Google Scholar]
- Meyer RL (1998). Roger Sperry and his chemoaffinity hypothesis. Neuropsychologia 36, 957–980. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, and Faber J (1956). Normal Table of Xenopus laevis (Daudin) (Amsterdam,: Elsevier-North Holland Publishing Company; ). [Google Scholar]
- Podgorski K, Dunfield D, and Haas K (2012). Functional clustering drives encoding improvement in a developing brain network during awake visual learning. PLoS Biol 10, e1001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt KG, Dong W, and Aizenman CD (2008). Development and spike timing-dependent plasticity of recurrent excitation in the Xenopus optic tectum. Nat Neurosci 11, 467–475. [DOI] [PubMed] [Google Scholar]
- Quiroga RQ (2012). Spike sorting. Curr Biol 22, R45–46. [DOI] [PubMed] [Google Scholar]
- Ruthazer ES, Akerman CJ, and Cline HT (2003). Control of axon branch dynamics by correlated activity in vivo. Science 301, 66–70. [DOI] [PubMed] [Google Scholar]
- Sin WC, Haas K, Ruthazer ES, and Cline HT (2002). Dendrite growth increased by visual activity requires NMDA receptor and Rho GTPases. Nature 419, 475–480. [DOI] [PubMed] [Google Scholar]
- Udin SB, and Scherer WJ (1990). Restoration of the plasticity of binocular maps by NMDA after the critical period in Xenopus. Science 249, 669–672. [DOI] [PubMed] [Google Scholar]
- Wu G, Malinow R, and Cline HT (1996). Maturation of a central glutamatergic synapse. Science 274, 972–976. [DOI] [PubMed] [Google Scholar]
- Xu H, Khakhalin AS, Nurmikko AV, and Aizenman CD (2011). Visual experience-dependent maturation of correlated neuronal activity patterns in a developing visual system. J Neurosci 31, 8025–8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LI, Tao HW, Holt CE, Harris WA, and Poo M (1998). A critical window for cooperation and competition among developing retinotectal synapses. Nature 395, 37–44. [DOI] [PubMed] [Google Scholar]


