ZBP1-MLKL necroptotic signaling bridges tumor cell damage to antitumor immune responses after radiation.
Abstract
Necroptosis, a form of regulated necrosis, participates in tumor development and dying cell immunogenicity. However, it remains unclear how tumor cell–intrinsic necroptotic signaling contributes to radiation-induced antitumor immunity. Here, we found that the ZBP1-MLKL necroptotic cascade in irradiated tumor cells was essential for antitumor immunity. ZBP1-dependent activation of MLKL potentiated type I interferon responses following tumor cell irradiation. Mechanistically, the ZBP1-MLKL necroptotic cascade induced cytoplasmic DNA accumulation in irradiated tumor cells and, in turn, autonomously activated cGAS-STING signaling, thus creating a positive feedback loop between those two pathways to drive persistent inflammation. Accordingly, ablation of caspase-8 enhanced STING pathway activation and the antitumor effects of radiation by activating MLKL. These findings reveal that ZBP1-MLKL necroptosis signaling maximized radiation-induced antitumor immunity through mutual interaction with the tumor cell–intrinsic STING pathway. This study provides insight into how radiotherapy bridges tumor cell damage to antitumor immune responses and an alternative strategy to improve radiotherapy.
INTRODUCTION
Radiotherapy is widely used to treat various types of human cancers (1, 2). The antitumor effects of radiotherapy rely on the engagement of innate and adaptive immune components (1, 3). Moreover, the cross-priming capacity of dendritic cells (DCs), induced by radiation treatment, requires cytosolic DNA sensing and subsequent type I interferon (IFN) signaling (4). Cyclic guanosine monophosphate (GMP)–adenosine monophosphate (AMP) synthase (cGAS) is an essential cytosolic DNA sensor that catalyzes the formation of cyclic GMP-AMP (cGAMP) in response to DNA accumulation in the cytosol (5). After detection of DNA in the cytosol, cGAS triggers cGAMP binding to stimulator of IFN genes (STING). STING then interacts with TANK-binding kinase 1 (TBK1) and IFN regulator factor 3 (IRF3), leading to the induction of type I IFNs (5). cGAS is activated by the abundance of cytosolic DNA originating from micronuclei, mitochondria, or viruses in a sequence-independent manner (5). Regardless of the ability of mitochondrial DNA (mtDNA) in the cytosol to engage cGAS, the prevailing perspective is that chromatin fragments inside micronuclei are essential for cGAS-STING–mediated type I IFN responses and antitumor host immunity after radiation treatment (6–11). Moreover, the molecular events that trigger cytosolic DNA sensing by the cGAS-STING pathway in radiation-stressed tumor cells remain unclear.
Necroptosis, which requires receptor-interacting protein kinase 3 (RIPK3) activity and is executed by mixed-lineage kinase domain-like pseudokinase (MLKL) protein, has been considered to be a major modality for immunogenic cell death (12). However, the role of necroptosis in tumor immune response is controversial. In oncogene-induced spontaneous tumor models, necroptosis was found to contribute to lineage-commitment liver tumorigenesis and pancreatic oncogenesis by accelerating inflammation (13, 14). By contrast, in transplant tumor models, transduction of active RIPK3 or MLKL constructs into tumor cells was shown to potentiate antitumor adaptive immunity (15). Among the multiple signal transduction cascades that precipitate necroptosis in response to extracellular signals (e.g., ligand binding to death receptors) or intracellular stimuli (such as nucleic acids), the ZBP1-RIPK3-MLKL axis has been recently documented to aggravate chronic inflammation and influenza pathogenesis (16–19). In the tumor microenvironment, there are multiple spatiotemporal barriers preventing the extracellular dissemination of damage-associated molecular patterns (DAMPs) for activation of antitumor immune cell components (20). It is necessary to avoid potential immune escape while generating robust cross-priming of CD8+ T cells in cancer therapy (20). However, it remains unknown how the tumor cell–intrinsic necroptotic pathway is elicited and, in turn, activates an antitumor immune response to radiation.
Tumor relapses often occur after radiotherapy in multiple types of human cancers due to immune privilege induced by tumor-intrinsic and tumor-extrinsic factors (21). Recently, the escape of innate immune sensing was described as a key step for limiting the immunological “cold” to “hot” tumor switch after radiation. For instance, noncanonical nuclear factor κB (NF-κB) activation in tumor-infiltrating DCs was found to counteract STING-mediated type I IFN production following radiation treatment (22). In addition, monocytic myeloid-derived suppressor cells (MDSCs) can function as feedback circuits to sequester the STING-induced antitumor immune response to radiation therapy (23). Moreover, tumor cell–intrinsic autophagy and caspase-9 signaling give rise to radiation resistance by inhibiting STING-mediated cytosolic DNA sensing after irradiation (24, 25). This evidence strongly suggests that prosurvival and prodeath pathways in tumor cells both participate in the reconstitution of the tumor immune environment following radiotherapy (21). As a master regulator of cell death programs, caspase-8 promotes extrinsic apoptosis by inducing activation of the effector caspases 3, 6, and 7 while preventing ZBP1-dependent necroptosis via cleavage of ZBP1-RIPK3 complex components (26). In contrast to apoptosis, necroptosis is substantially more potent in triggering inflammation and adaptive immune responses (27). Thus, tumor cell death reprogramming from apoptosis to necroptosis may be a potential strategy to improve radiotherapy.
In this study, we found that the ZBP1-MLKL necroptotic signaling cascade governs antitumor immune responses after radiation by mutually interacting with STING-mediated cytosolic DNA sensing. Correspondingly, ablation of caspase-8 promoted cytosolic DNA sensing and radiotherapeutic effects by enhancing MLKL activity. Our findings provide new insight to understand the early events that alert the immune system upon radiation and therefore indicate an alternative strategy to improve radiotherapy via cell death reprogramming.
RESULTS
The tumor cell–intrinsic ZBP1-MLKL necroptotic cascade is required for antitumor effects of radiation treatment
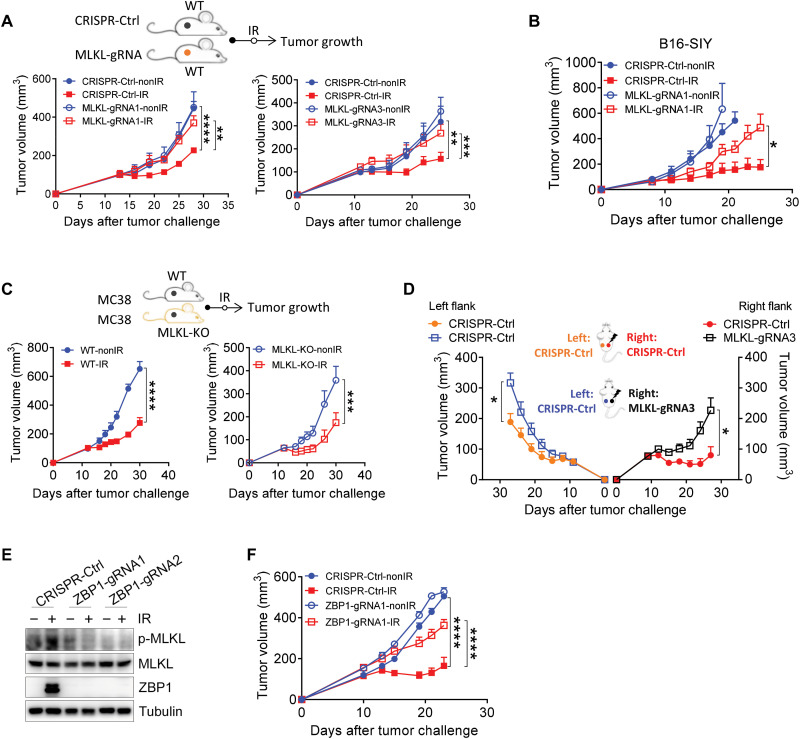
To define the role of the tumor-intrinsic necroptosis pathway in radiation, we generated an MC38 murine colon adenocarcinoma cell line deleted for MLKL using CRISPR-Cas9 technology with three different guide RNAs. MLKL protein expression in the MLKL-deficient (MLKL−/−) stable cell lines was remarkably decreased compared to that in the control cell line (CRISPR-Ctrl) (fig. S1, A and B). We next sought to confirm whether MLKL regulates necroptosis based on the uptake of annexin V staining and SytoxOrange dye by MLKL−/− cells following radiation. The results showed that loss of MLKL resulted in decreased cell death after radiation (fig. S1C). We then implanted MLKL-deficient or MLKL-sufficient MC38 (control) tumor cells on the flanks of wild-type (WT) mice and then monitored tumor growth after local radiation treatment. Tumors derived from two of the three MLKL−/− cell lines displayed notably greater resistance to the antitumor effects of radiation, exhibiting rapid relapse, whereas MLKL-sufficient tumors exhibited obvious growth retardation following radiation (Fig. 1A).
Fig. 1. The tumor cell–intrinsic ZBP1-MLKL necroptotic cascade is required for antitumor effects of radiation treatment.
Mice were subcutaneously inoculated with MC38 or B16-SIY tumor cells, and then established tumors were treated locally with one fraction of 15-Gy ionizing radiation (IR). (A) Tumor growth of Ctrl-MC38 and MLKL−/− MC38 tumors with distinct guide RNAs in WT mice after IR treatment. (B) Tumor growth of Ctrl-B16-SIY and MLKL−/− B16-SIY tumors in WT mice after IR treatment. (C) Tumor growth of MC38 tumors in WT and MLKL-KO mice after IR treatment. (D) Tumor growth of unirradiated secondary tumors (Ctrl-MC38, left flank) and irradiated primary tumors (Ctrl-MC38 or MLKL−/− MC38, right flank) in WT mice. (E) Phosphorylated MLKL (p-MLKL) expression in Ctrl-MC38 and ZBP1−/− MC38 tumor cells after IR treatment. (F) Tumor growth of Ctrl-MC38 and ZBP1−/− MC38 tumors in WT mice after IR treatment. Data are represented as means ± SEM. Representative data are shown from two or three independent experiments conducted with five to seven mice per group. Statistical analysis was performed by two-way ANOVA with Bonferroni’s multiple comparisons test (A, C, D, and F) or by two-tailed unpaired t test (B). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
We then used another tumor model, B16-SIY, a murine melanoma cell line, to determine whether MLKL functioned in post-radiation tumor regression in a generalized manner. Similarly, knockout (KO) of tumor cell–intrinsic MLKL in B16-SIY tumors also resulted in negation of the effects of radiation, similar to our observations in MC38 tumors after radiation (Fig. 1B), thus confirming that tumor cell–intrinsic MLKL is required for the maximal antitumor effects of radiation. To further assess whether tumor cell–extrinsic MLKL is also necessary for the antitumor efficacy of radiation, we injected MC38 primary tumor cells into the flanks of MLKL-KO and WT mice. We found that tumor growth was comparably restricted following radiation in both MLKL-KO and WT mice, indicating that MLKL in the host was redundant for the antitumor effects of radiation (Fig. 1C).
The abscopal effect of radiation has been observed in several types of human cancers and has been linked to host immune responses (1). We next examined whether tumor cell–intrinsic MLKL in primary tumors after radiation regulates the abscopal effects on secondary tumors that did not receive radiation. A delay in secondary tumor growth was observed in mice bearing MLKL-sufficient tumors compared with mice bearing MLKL-deficient tumors after radiation treatment (Fig. 1D). These results implied that, in primary tumors receiving radiation, tumor cell–intrinsic MLKL contributes to the restriction of unirradiated secondary tumors via induction of antitumor adaptive immune responses. Collectively, these findings suggest that the antitumor effects of radiation rely on tumor cell–intrinsic MLKL rather than host MLKL.
ZBP1 triggers RIPK3-MLKL–dependent necroptosis to promote the pathogenesis of chronic inflammation and influenza (17, 18). We noticed that ZBP1 expression was remarkably elevated in tumor cells after radiation (Fig. 1E). To determine whether ZBP1 is the upstream activator of MLKL-dependent necroptosis after radiation, we knocked out ZBP1 in MC38 tumor cells (Fig. 1E). We found that radiation increased the expression of phosphorylated MLKL, while ZBP1 deficiency led to impairment of MLKL activity (Fig. 1E). To further confirm that ZBP1 is essential for necroptosis after radiation, we measured cell death by SytoxOrange and annexin V staining and found that loss of ZBP1 inhibited radiation-induced tumor cell death (fig. S1D). Considering that ZBP1 was required for MLKL-dependent necroptosis after radiation, we next sought to assess whether tumor cell–intrinsic ZBP1 is indispensable for the antitumor effects of radiation. The results showed that the antitumor effects of radiation were compromised in ZBP1-deficient tumors compared to that of control primary tumors (Fig. 1F). Together, these results suggest that ZBP1-mediated necroptotic signaling is required for the antitumor effects of radiation.
The ZBP1-MLKL necroptotic cascade promotes radiation-induced antitumor immunity
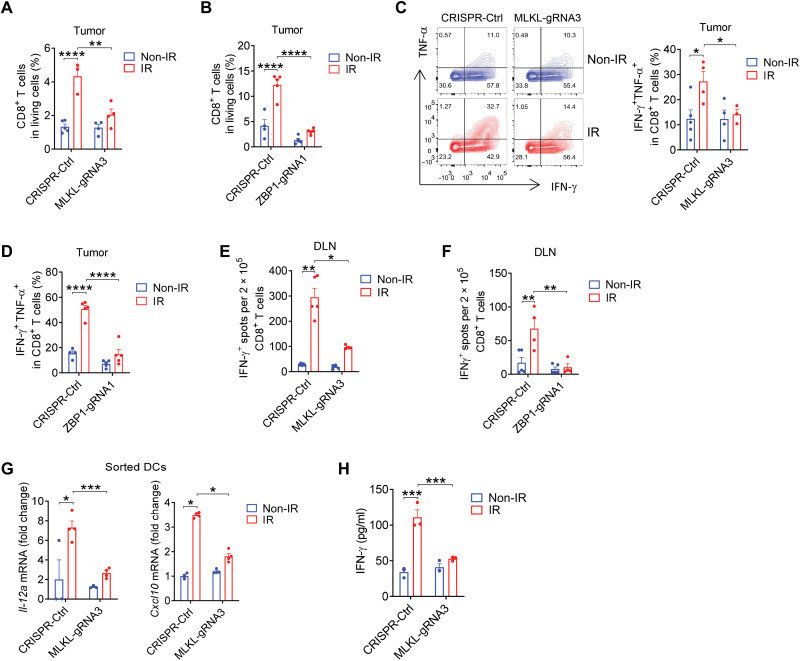
Our previous studies have demonstrated that CD8+ T cells play an essential role in tumor destruction after radiation because the depletion of CD8+ T cells reverses the antitumor effects of radiation (4). To assess whether the tumor cell–intrinsic ZBP1-MLKL cascade is required for the reconstitution of immune cell populations in tumors after radiation, we analyzed the proportion of CD8+ T cells in the tumor immune microenvironment using a flow cytometry gating strategy (fig. S2A). Compared to control counterpart tumors, both MLKL−/− tumors and ZBP1−/− tumors failed to increase the percentage of CD8+ T cells after radiation (Fig. 2, A and B). To further investigate whether the tumor cell–intrinsic ZBP1-MLKL cascade exerts any effects on tumor-infiltrating CD8+ T cell effector functions, we performed intracellular cytokine staining in CD8+ T cells with the stimulation by phorbol 12-myristate 13-acetate (PMA) and ionomycin. The loss of MLKL or ZBP1 inhibited the enrichment of the IFN-γ+TNF-α+CD8+ T cells in tumors after radiation (Fig. 2, C and D), thereby revealing that the ZBP1-MLKL cascade in tumor cells is required for CD8+ T cell effector functions after radiation.
Fig. 2. The ZBP1-MLKL necroptotic cascade promotes radiation-induced antitumor immune responses.
(A and B) Quantification analysis of the percentage of CD8+ T cells in living cells from Ctrl and MLKL−/− MC38 (A) or ZBP1−/− MC38 (B) tumors on day 7 after IR treatment. (C and D) Representative data and quantification analysis of the percentage of IFN-γ+TNF-α+CD8+ T cells from Ctrl and MLKL−/− MC38 (C) or ZBP1−/− MC38 (D) tumors. (E and F) Function of tumor antigen–specific CD8+ T cells from the DLNs of Ctrl tumor–bearing mice compared to MLKL−/− MC38 (E) or ZBP1−/− MC38 (F). (G) Il-12a and Cxcl10 mRNA expression in DCs from tumors on day 3 after IR treatment. (H) DCs from Ctrl or MLKL−/− B16-SIY tumors 3 days after IR were cocultured with 2C-TCR CD8+ T cells for 72 hours. The supernatant IFN-γ was evaluated. Data are represented as means ± SEM. Representative data are shown from two or three independent experiments. Statistical analysis was performed by one-way ANOVA test (A, B, D, and H), by Brown-Forsythe and Welch ANOVA tests (E), by two-tailed unpaired t test (C, F, and G, left panel), or by Mann-Whitney test (G, right panel). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
We next investigated whether impaired function of antigen-specific CD8+ T cells was responsible for the failure of MLKL−/− and ZBP1−/− tumors to respond to radiation. To this end, we used an enzyme-linked immunospot (ELISPOT) assay with purified CD8+ T cells from tumor inguinal draining lymph nodes (DLNs) and spleens and found that radiation resulted in a robust tumor antigen–specific CD8+ T cell response in control counterpart tumors, whereas the response was substantially attenuated among antigen-specific CD8+ T cells in MLKL- or ZBP1-deficient tumors (Fig. 2, E and F, and fig. S2, B and C). To investigate the translational value of our observations in human samples, we systematically examined the relationship between infiltration of CD8+ T cells and ZBP1 or MLKL expression levels through Spearman correlation analysis in publicly available data from a cohort in The Cancer Genome Atlas (TCGA). We found that the infiltration of CD8+ T cells was notably positively correlated with the expression of MLKL in 25 cancer types (median Rs = 0.44) and with the expression of ZBP1 in 31 cancer types (median Rs = 0.61) (fig. S2D). Collectively, these results suggest that the tumor cell–intrinsic ZBP1-MLKL necroptotic cascade is essential for tumor-specific CD8+ T cell responses after radiation.
DCs are highly potent in the cross-priming of CD8+ T cells in response to radiotherapy, chemotherapy, and immune checkpoint blockade (28). We hypothesized that the tumor cell–intrinsic ZBP1-MLKL cascade promoted CD8+ T cell responses through enhanced cross-priming by DCs after radiation. It has been demonstrated that interleukin-12 (IL-12) contributes central functions to DC cross-priming of CD8+ T cells, whereas CXCL10 serves as a key mediator for the migration of antigen-specific CD8+ T cells (28). Next, to examine whether tumor cell–intrinsic MLKL regulates the functions of DCs after radiation, using a gating strategy, we sorted out CD11c+ DCs for real-time polymerase chain reaction (PCR) quantification of IL-12 and CXCL10 expression (fig. S3A). The loss of MLKL in tumor cells decreased IL-12 transcriptional expression in tumor-infiltrating DCs after radiation compared to that in DCs of primary tumors (Fig. 2G). Similarly, the absence of MLKL in tumor cells also impaired CXCL10 transcriptional expression in sorted DCs from tumors after radiation (Fig. 2G).
Cross-priming is a robust process involved in CD8+ T cell response to tumor cells. This critical function is mediated by DCs when they acquire antigens from dying tumor cells and subsequently present the associated antigenic peptides on self–major histocompatibility complex (MHC) class I molecules (28). To determine whether MLKL in irradiated tumor cells is essential for DC-mediated cross-priming of CD8+ T cells, we performed a cross-priming assay in which we measured IFN-γ production in cocultures of sorted tumor-infiltrating DCs after radiation and CD8+ T cells isolated from naïve 2C-TCR transgenic mice. We found that functional capacity of DCs for CD8+ T cells cross-priming was augmented by the stimulation with irradiated tumors compared to DCs exposed to nonirradiated tumors. In contrast, tumor-infiltrating DCs isolated from MLKL-deficient tumors after radiation failed to cross-prime CD8+ T cells (Fig. 2H). To further substantiate this observation, a coculture system of bone marrow–derived DCs (BMDCs) and irradiated tumor cells were performed in vitro. Similarly, IL-12 mRNA and CXCL10 mRNA expression in BMDCs, as well as the cross-priming activity by BMDCs, were both impaired in the absence of MLKL in tumor cells (fig. S3, B and C). Thus, these results suggest that tumor cell–intrinsic MLKL is required to maintain the cross-priming ability of DCs in tumors after radiation.
The ZBP1-MLKL necroptotic cascade triggers type I IFN responses in irradiated tumor cells
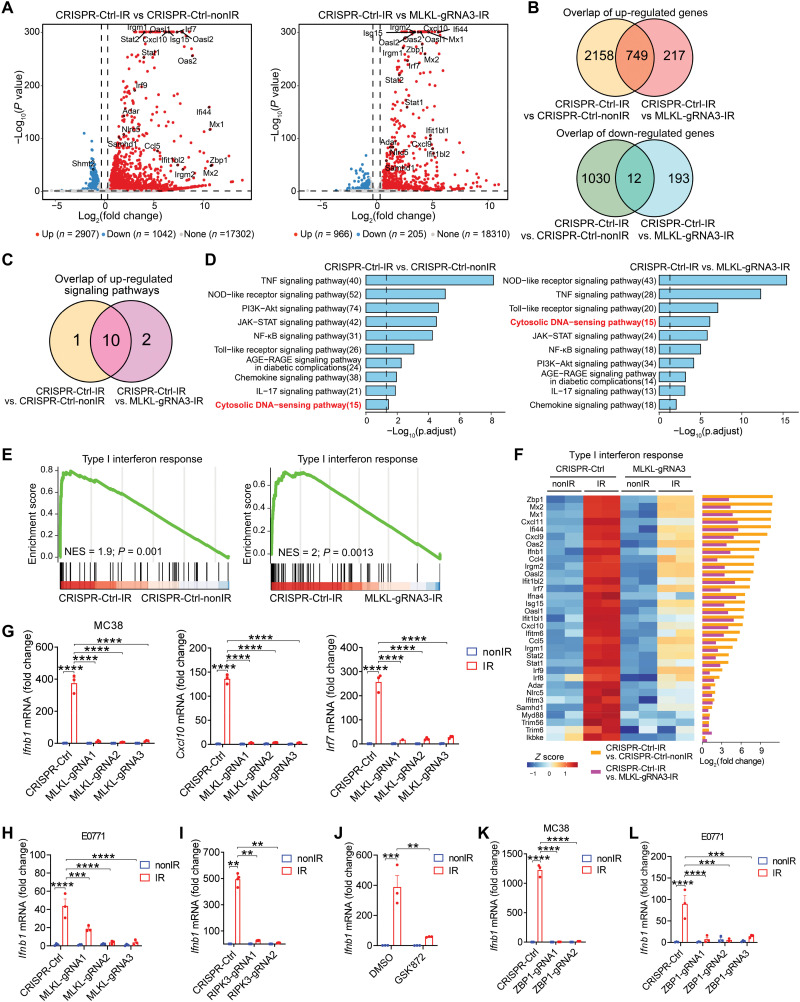
To gain insight into the immunological aspects of tumor cell–intrinsic necroptosis in response to radiation, we performed RNA sequencing (RNA-seq) using tumor cells with or without MLKL. RNA-seq analysis showed that 2907 genes were differentially up-regulated, and 1042 genes were differentially down-regulated in irradiated MC38 tumor cells compared to unirradiated tumor cells, while 966 genes were up-regulated and 205 genes were down-regulated in comparison between Ctrl-MC38 and MLKL−/− MC38 after radiation (Fig. 3A). Among the up- and down-regulated genes, 749 genes and 12 genes overlapped, respectively, between those two sets of comparisons, likely attributable to the presence of MLKL in MC38 with radiation (Fig. 3B). Similarly, among the top-ranking up-regulated signaling pathways (hypergeometric tests, P < 0.05), 10 overlapped signaling pathways were revealed in the comparison of MC38 tumor cells after radiation in the presence or absence of functional MLKL (Fig. 3C).
Fig. 3. The ZBP1-MLKL necroptotic cascade triggers type I IFN responses in irradiated tumor cells.
(A) Volcano plot exhibiting the differentially expressed genes from RNA-seq data. (B) Venn diagram showing notably up-regulated (top) or downregulated (bottom) genes. (C) Venn diagram displaying the notably up-regulated signaling pathways. (D) KEGG pathways enriched of the up-regulated genes. (E) GSEA of type I IFN response gene. (F) Heat map and bar plots from RNA-seq revealing the normalized expression (left) and log2-transformed fold changes (right) of type I IFN response genes. (G) Ifnb1, Cxcl10, and Irf7 mRNA expression in Ctrl-MC38 and MLKL−/− MC38 cells with or without IR. (H to L) Ifnb1 mRNA expression in MLKL−/− E0771 (H), RIPK3−/− MC38 (I), MC38 with GSK′872 (J), ZBP1−/− MC38 (K), and ZBP1−/− E0771 (L) cells with or without IR. Data are represented as means ± SEM. Representative data are shown from three independent experiments. Statistical analysis was performed by one-way ANOVA with Bonferroni’s multiple comparisons test [left and right panels of (G), (H), and (J) to (L)], by Brown-Forsythe and Welch ANOVA tests with Tamhane’s T2 multiple comparisons test (I), or by two-tailed unpaired t test [middle panel of (G)]. **P < 0.01; ***P < 0.001; ****P < 0.0001.
Functional pathway enrichment analysis showed that alteration of cytosolic DNA–sensing pathway ranked among the top four enriched pathways in the comparison of Ctrl-MC38 and MLKL−/− MC38 after radiation (Fig. 3D). Moreover, gene set enrichment analysis (GSEA) revealed that the up-regulated genes involved in type I IFN response were substantially enriched in MLKL-sufficient tumor cells upon radiation compared to the counterpart groups (Fig. 3E). As a further analysis, the normalized heatmap of gene expression showed that the gene signature of type I IFN response was predominantly increased in tumor cells after radiation, whereas MLKL absence limited the induction of type I IFN response–relevant genes in irradiated tumor cells (Fig. 3F). Consistent with these findings, the abovementioned volcano plot showed that the type I IFN response–relevant genes were also substantially up-regulated in MLKL-expressing tumor cells after radiation (Fig. 3A).
Type I IFNs have been demonstrated to play a critical role in bridging DNA damage in tumor cells and antitumor adaptive immunity following radiation (6, 7). To further validate whether MLKL is required for induction of type I IFN in irradiated tumor cells, we conducted real-time PCR assay with MC38 cells. In agreement with our observations from RNA-seq, we found a marked induction of IFN-β and IFN-stimulated genes (ISGs), including Cxcl10 and Irf7, after radiation, whereas the lack of MLKL abolished the induction of those genes in irradiated tumor cells (Fig. 3G). To confirm that this observation was generalized, we analyzed IFN-β production in an MLKL−/− E0771 murine breast cancer cell line (fig. S4A). Again, consistent with our previous results, the lack of MLKL resulted in impaired induction of IFN-β in E0771 tumor cells after radiation (Fig. 3H). In addition, MLKL-associated IFN-β production after radiation occurred in the longer time points as well, indicating that the pattern is not temporally restricted (fig. S4B). Together, these results suggest that tumor cell–intrinsic MLKL may engage in the delayed but sustained type I IFN production after radiation through a reciprocal cross-talk.
It has been well documented that MLKL-mediated necroptosis requires phosphorylation of the protein kinase RIPK3 (19). MLKL also contributes to endosomal trafficking and generation of extracellular vesicles in a RIPK3-independent manner (29). Although many tumor cell lines are refractory to necroptosis induced by common stimuli, we observed that stimulation with radiation typically activates MLKL-dependent necroptosis in tumor cells. To determine whether tumor cells expressed RIPK3, we first measured its expression and that of relevant genes in multiple tumor cell lines, including MC38, B16-SIY, and E0771. We determined that RIPK3 mRNA and protein expression were detectable in all three of those tumor cell lines (fig. S4, C and D), raising the possibility that RIPK3 may participate in MLKL-mediated type I IFN responses induced by radiation. We then found that loss of RIPK3 substantially decreased the induction of IFN-β in tumor cells after radiation (Fig. 3I). To confirm this observation, we treated tumor cells with a selective RIPK3 inhibitor (GSK′872) starting before radiation. Consistent with previous observations, treatment with RIPK3 inhibitor resulted in defective IFN-β induction by tumor cells after radiation (Fig. 3J). These results thus indicated that RIPK3, as a mediator of necroptotic signaling, facilitates IFN-β production in tumor cells after radiation.
Next, to assess whether ZBP1 regulates MLKL-mediated type I IFN induction after radiation, we used quantitative PCR (qPCR) to quantify Ifnb1 expression in MC38 tumor cells. The loss of ZBP1 abolished transcriptional induction of IFN-β by radiation treatment in tumor cells (Fig. 3K), showing that ZBP1 is a key mediator for MLKL-dependent necroptosis in terms of type I IFN response to radiation. We then tested the role of ZBP1 in another tumor cell line, E0771, also deficient for ZBP1 (fig. S4E). Similarly, loss of ZBP1 predominantly compromised IFN-β induction by radiation in E0771 tumor cells (Fig. 3L). These results thus indicate that ZBP1 acts as the upstream regulator of RIPK3-MLKL necroptotic signaling to control radiation-induced type I IFN expression.
ZBP1-dependent necroptotic cascade enhances the activation of STING pathway in tumor cells after radiation treatment
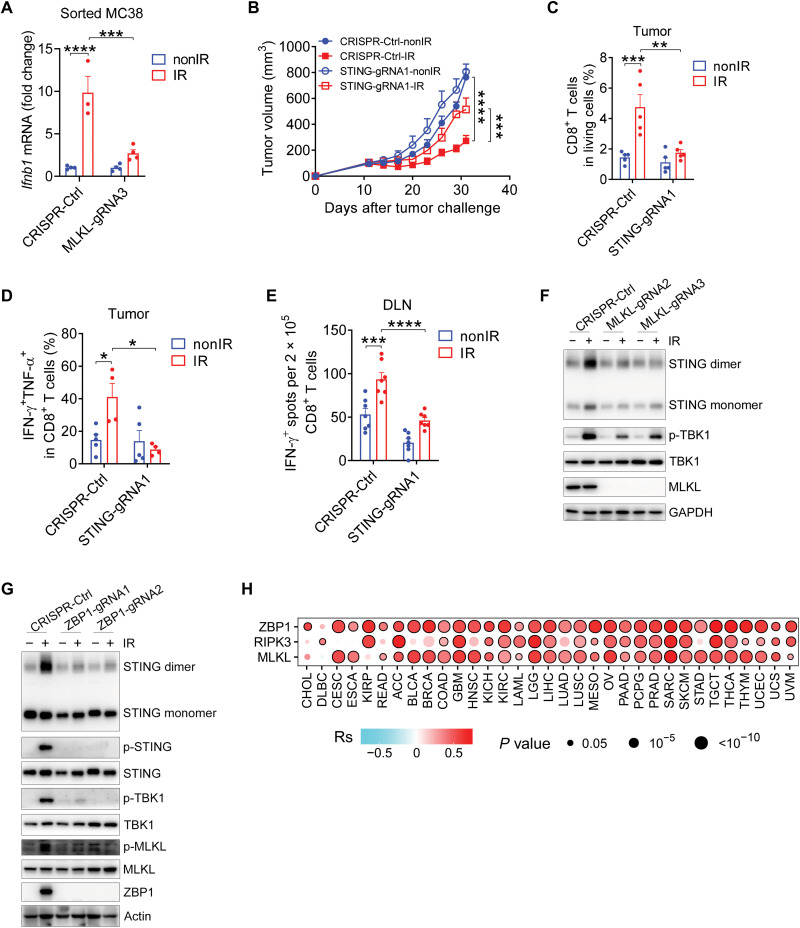
Given that tumor cells cultured alone in vitro cannot recapitulate the tumor microenvironment, we therefore validated whether tumor cell–intrinsic MLKL is responsible for type I IFN production in tumors upon radiation in vivo. Tumor cell lines expressing tdTomato with or without MLKL were inoculated into WT mice. tdTomato-positive cells from tumors receiving radiation were then sorted out by the gating strategy shown in fig. S5A to determine the levels of Ifnb1 mRNA expression. As expected, we found that IFN-β transcription in tumor cells was decreased in MLKL-deficient tumors compared with that in MLKL-sufficient tumors after radiation (Fig. 4A). This result indicates that tumor cell–intrinsic MLKL is a prerequisite for IFN-β production in vivo after radiation, which is consistent with our observations in vitro.
Fig. 4. The ZBP1-MLKL necroptotic cascade promotes the activation of STING pathway in tumors after radiation treatment.
(A) Ifnb1 mRNA expression in CD45−tdTomato+ tumor cells from Ctrl and MLKL−/− MC38 tumors after IR treatment. (B) Tumor growth of Ctrl and STING−/− MC38 tumors in WT mice after IR. (C) Percentage of CD8+ T cells from Ctrl or STING−/− MC38 tumors after IR. (D) Percentage of IFN-γ+TNF-α+CD8+ T cells from tumors after IR. (E) Function of tumor antigen–specific CD8+ T cells from the DLNs of tumor-bearing mice after IR. (F) STING dimer and p-TBK1 expression in tumor cells with or without IR. (G) STING dimer, p-STING, p-TBK1, and p-MLKL expression in tumor cells with or without IR. (H) Spearman correlation of the enrichment score of cytosolic DNA–sensing pathway and the ZBP1, RIPK3, or MLKL mRNA expression across 33 human cancer types. Data are represented as means ± SEM. Representative data are shown from two or three independent experiments conducted with four to seven mice per group. Statistical analysis was performed by one-way ANOVA (A and C to E) or by two-way ANOVA (B) with Bonferroni’s multiple comparisons test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Nucleic acid recognition by cGAS has been demonstrated to dictate type I IFN response in cells exposed to radiation (7, 30). To further examine whether the cGAS-STING pathway is responsible for induction of type I IFN in tumor cells upon radiation, we individually knocked out cGAS and STING in tumor cells. The protein expression of cGAS and STING were both significantly decreased in different clones with distinct guide RNAs (fig. S5, B and C). As anticipated, the loss of cGAS notably decreased both TBK1 phosphorylation and Ifnb1 mRNA expression in irradiated tumor cells (fig. S5, B and D). Likewise, the deficiency of STING also abolished the increase in TBK1 phosphorylation and IFN-β mRNA expression in tumor cells after radiation (fig. S5, C and E). These results indicated that both cGAS and STING are essential for the radiation-mediated induction of type I IFN, implying that MLKL may potentially trigger the activation of cGAS-STING cascade in tumor cells after radiation.
We previously demonstrated that local blockade of type I IFN signaling using neutralizing antibodies reversed antitumor effects of radiation, implying an essential role for type I IFN signaling in the tumor microenvironment after radiation (4). Thus, we presumed that in tumor cell, the STING–IFN-β cascade remodels the immune contexture of the tumor microenvironment to achieve antitumor effects of radiation. To investigate whether tumor cell–intrinsic STING is indispensable for the efficacy of radiation, we implanted STING-deficient tumor cells and control tumor cells into the flanks of WT mice, respectively. Tumor cells lacking STING exhibited remarkably impaired tumor control after radiation, confirming that tumor cell–intrinsic STING is essential for the antitumor effects of radiation (Fig. 4B). This finding is consistent with a previous study that showed that tumor cell–intrinsic STING determines the synergy between radiation and immune checkpoint blockade in the control of the secondary tumors. Moreover, we observed a reduced percentage of CD8+ T cells in living cells of STING−/− tumors after radiation, compared with that in parental tumors (Fig. 4C). Correspondingly, following radiation treatment, the lack of STING also decreased the proportion of IFN-γ+TNF-α+CD8+ T cells in tumors (Fig. 4D). In addition, STING deficiency in tumor cells resulted in compromised responses of tumor-specific CD8+ T cells after radiation (Fig. 4E). These results suggest that tumor cell–intrinsic STING signaling plays a vital role in regulating antitumor adaptive immune responses after exposure to radiation.
Considering that the cGAS-STING cascade is required for type I IFN induction and antitumor adaptive immunity after radiation, we then asked whether MLKL is required for tumor cell maintenance of STING activity upon radiation. Therefore, we analyzed the dimerization of STING in tumor cells with or without MLKL after radiation and found that the amount of STING dimers was elevated in tumor cells after radiation, while the loss of MLKL led to substantially impaired formation of STING dimers in irradiated tumor cells (Fig. 4F). Likewise, loss of MLKL in tumor cells prevented TBK1 phosphorylation after radiation (Fig. 4F). Given that ZBP1 is an inducer of MLKL-mediated necroptosis after radiation, we next sought to assess whether ZBP1 regulates STING activity. ZBP1 deficiency resulted in decreased STING dimerization and TBK1 phosphorylation after radiation (Fig. 4G), indicating that ZBP1 mediates type I IFN responses through regulation of MLKL and STING activation.
Given emerging evidence that STING could also be activated by cGAS-independent factors, we next test whether cGAS is catalytically active under our conditions. We measured cGAMP production in irradiated tumor cells with or without MLKL and found that MLKL was necessary for cGAMP production in irradiated tumor cells (fig. S5F). To test whether alternative methods of activating the ZBP1-MLKL pathway also activate the cGAS-STING pathway, we used 5-aza-2′-deoxycytidine (5-AZA), a DNA methylation inhibitor, to treat tumor cells. Consistently, we found that 5-AZA was able to activate the STING pathway in a ZBP1-dependent manner (fig. S5G), suggesting that the mechanism that the ZBP1-MLKL pathway initiates the activation of the STING pathway is not restricted in radiation treatment. In addition, cGAS loss resulted in decreased STING phosphorylation after radiation (fig. S5H). To further explore whether there is a cross-talk between the cGAS-STING pathway and the ZBP1-MLKL pathway, we measured the expression of phosphorylated MLKL and cell death by SytoxOrange and annexin V staining. Loss of the cGAS-STING pathway resulted in decreased MLKL activity and less cell death after radiation (fig. S5, H to K), indicating a role of the cGAS-STING pathway in expanding ZBP1-MLKL signaling activation. Together, these results suggest that there is a reciprocal and positive cross-talk between the ZBP1-MLKL pathway and the cGAS-STING pathway after radiation, which contributes to prolonged innate immune responses for maximal antitumor immunity.
Taking into account that the ZBP1-RIPK3-MLKL cascade was observed to regulate STING-mediated cytosolic DNA sensing in murine tumor cells after radiation, we next investigated whether these two factors were correlated in human cancers from TCGA database with treatment naïve. Our analysis revealed a strong correlation of ZBP1, RIPK3, or MLKL with cytosolic DNA–sensing pathway across most types of cancers from TCGA datasets (fig. S6). Overall, we observed a positive association between the expression of ZBP1, RIPK3, or MLKL and the enrichment score of cytosolic DNA–sensing pathway in most of those 33 types of cancer from TCGA cohort (Fig. 4H). This finding indicates that the ZBP1-MLKL necroptotic cascade may exert effects on cytosolic DNA sensing in human tumors with treatment naïve probably through a similar reciprocal cross-talk, supporting our findings in preclinical models with radiation treatment.
The ZBP1-MLKL necroptotic cascade induces cytoplasmic accumulation of mtDNA in irradiated tumor cells
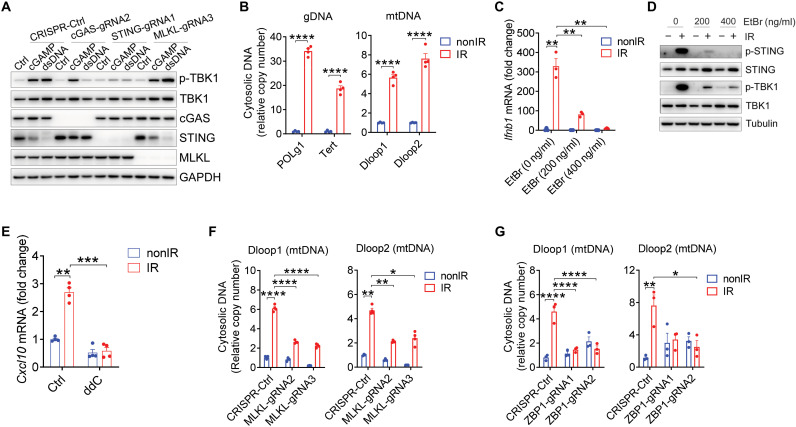
To further explore whether MLKL directly interacts with STING in tumor cells, we stimulated tumor cells with double-stranded DNA (dsDNA) and 2′3′-cGAMP, which can activate cGAS and STING, respectively. In agreement with previous studies (31), the absence of cGAS resulted in failed TBK1 phosphorylation after stimulation with dsDNA instead of cGAMP, while the deficiency of STING led to decreased TBK1 phosphorylation after either dsDNA or cGAMP stimulation (Fig. 5A). In contrast, the loss of MLKL did not affect TBK1 phosphorylation following exposure to either stimulator (Fig. 5A), which indicated that MLKL is unable to directly interact with cGAS and STING regarding radiation-induced type I IFN response. Likewise, the absence of MLKL could not prevent an increase of TBK1 phosphorylation with stimulation of STING agonists (i.e., DMXAA and ABZI-C3) in tumor cells (fig. S7A). To examine whether STING can function as an adaptor protein in the absence of MLKL after radiation, we set up a bypassing experiment in which MLKL−/− MC38 tumor cells were restimulated with STING agonist ABZI-C3 following radiation. The results showed that treatment with ABZI-C3 after radiation rescued IFN-β production in the absence of MLKL (fig. S7B), indicating that STING signaling remains intact in irradiated tumor cells regardless of the absence of MLKL. Together, these results suggest that STING activation mediated by ZBP1-MLKL cascade relies on a particular radiation-related setting, whereas MLKL is unable to directly participate in the signaling events elicited by agonists of cGAS or STING without radiation treatment.
Fig. 5. The ZBP1-MLKL necroptotic cascade induces cytoplasmic accumulation of mtDNA in irradiated tumor cells.
(A) p-TBK1 expression in Ctrl-MC38, cGAS−/− MC38, STING−/− MC38, and MLKL−/− MC38 4 hours after transfection with either cGAMP or dsDNA. (B) Cytosolic amount of gDNA and mtDNA in MC38 tumor cells after IR treatment. (C) Ifnb1 mRNA expression in EtBr-pretreated MC38 tumor cells after IR treatment. (D) p-STING and p-TBK1 expression in EtBr-pretreated MC38 tumor cells after IR treatment. (E) CXCL10 expression in DCs with the stimulation of ddC-pretreated tumor cells upon IR treatment. (F and G) Cytosolic amount of mtDNA in MC38 tumor cells in the absence or presence of MLKL (F) or ZBP1 (G) after IR treatment. Data are represented as means ± SEM. Representative data are shown from two or three independent experiments with at least three culture replicates. Statistical analysis was performed by one-way ANOVA with Bonferroni’s multiple comparisons test [B; left panel of (F) and (G)], by unpaired t test with Welch’s correction (C), or by Brown-Forsythe and Welch ANOVA tests with Tamhane’s T2 multiple comparisons test [right panel of (F) and (E)]. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
We next sought to examine how ZBP1-MLKL necroptotic cascade promotes STING activation in tumor cells following radiation. Abnormal cytosolic DNA abundance can act as a DAMP to trigger cGAS-STING pathway activation and antitumor adaptive immunity in response to various tumor therapies. To determine whether cytosolic DNA accumulates in tumor cells upon radiation, we purified cytosolic extracts and then conducted qPCR quantification of cytosolic DNA content in tumor cells after radiation treatment. We observed a high abundance of cytosolic genomic DNA (gDNA) and mtDNA within the cytosol of irradiated tumor cells (Fig. 5B). Micronuclei display compromised membrane integrity, thereby allowing cGAS access to the chromatin fragments. To assess whether micronuclei or cytoplasmic DNA from nuclear-origin contributes to the activation of cGAS-STING pathway initiated by the ZBP1-MLKL pathway after radiation, we first established the stable cell lines with flag-cGAS overexpression. The exogenous cGAS expression was comparable between Ctrl-MC38 and MLKL−/− MC38 (fig. S7C). We next measured localization of cGAS to micronuclei and found that cGAS-positive micronuclei were not affected by MLKL deficiency after radiation (fig. S7D). To further explore whether MLKL promotes the appearance of micronuclei after radiation, we visualized nuclei and micronuclei with the staining of DAPI (4′,6-diamidino-2-phenylindole) and γH2AX, a marker of DNA double-strand breaks. The appearance of micronuclei was increased in tumor cells after radiation over time, and the expression of γH2AX was up-regulated in nuclei and micronuclei after radiation as well (fig. S7E). However, MLKL loss could not affect the appearance of micronuclei and the expression of γH2AX after radiation (fig. S7E). To validate this observation, we measured the expression of γH2AX with flow cytometry. Consistently, lack of MLKL was unable to alter the expression of γH2AX in irradiated tumor cells (fig. S7, F and G). These results indicate that the ZBP1-MLKL pathway is redundant for the micronucleation after radiation.
We thus focused on determining whether cytosolic enrichment with mtDNA is a critical event in determining the activation of cytosolic DNA sensing after radiation. To this end, we generated mtDNA-depleted tumor cells by culturing MC38 in the presence of ethidium bromide (EtBr) according to a well-established protocol. The addition of low concentrations of EtBr (200 to 400 ng/ml) selectively inhibited mtDNA replication without affecting gDNA replication (fig. S8A). At the same time, we examined whether EtBr treatment had other effects on tumor cells. The expression of all relevant components (MLKL, RIPK3, cGAS, STING, TBK1, and ZBP1) was not compromised after EtBr treatment (fig. S8, B and C). The levels of cell death were not altered by EtBr treatment (fig. S8D). Technically, the cell cycle was slightly affected by EtBr treatment (fig. S8E). The percentage of cells in G0-G1 phase was increased after EtBr treatment, while the percentage of cells in S phase was decreased. In addition, there was no significant difference in the percentage of cells in G2-M phases between the three groups (fig. S8E). The shape of nuclei was not aberrant after EtBr exposure (fig. S8F). When we treated mtDNA-depleted tumor cells with radiation, the production of IFN-β was strongly inhibited compared with that in control cells (Fig. 5C). Moreover, STING and TBK1 activity were both abolished in mtDNA-depleted tumor cells versus control tumor cells after radiation (Fig. 5D), strongly suggesting that mtDNA is a major inducer for cGAS-STING–mediated type I IFN production following radiation treatment. Likewise, we observed decreased CXCL10 production in DCs when DCs were cocultured with mtDNA-depleted tumor cells pretreated with dideoxycytidine (ddC) versus control tumor cells after radiation (Fig. 5E).
We next sought to determine whether MLKL is necessary for tumor cell cytosolic accumulation of mtDNA in response to radiation. In MLKL−/− tumor cells, cytosolic mtDNA was substantially reduced compared to its accumulation in control tumor cells after radiation (Fig. 5F). Consistent with this result, mtDNA enrichment inside the cytosol of tumor cells after radiation was dependent on the presence of ZBP1 (Fig. 5G). Unexpectedly, loss of MLKL did not adversely affect mitochondrial reactive oxygen species (ROS) production or mitochondrial membrane potentials (fig. S8, G and H), indicating that MLKL does not contribute to mitochondrial damage leading to mtDNA leakage. To rule out the possibility that any paracrine factors secreted into the medium promote MLKL-mediated type I IFN production after radiation, we performed medium transfer experiment and transwell coculturing experiments. The supernatants from irradiated tumor cells could not induce type I IFN production in nonirradiated tumor cells in both settings (fig. S8, I and J), indicating that paracrine factors are not involved in the activation of MLKL and STING pathways in tumor cells after radiation. Together, these results suggest that the ZBP1-MLKL necroptotic cascade in irradiated tumor cells leads to the accumulation of cytosolic mtDNA, which is required for activation of the cGAS-STING pathway.
Ablation of caspase-8 promotes STING pathway activation and therapeutic effects of radiation
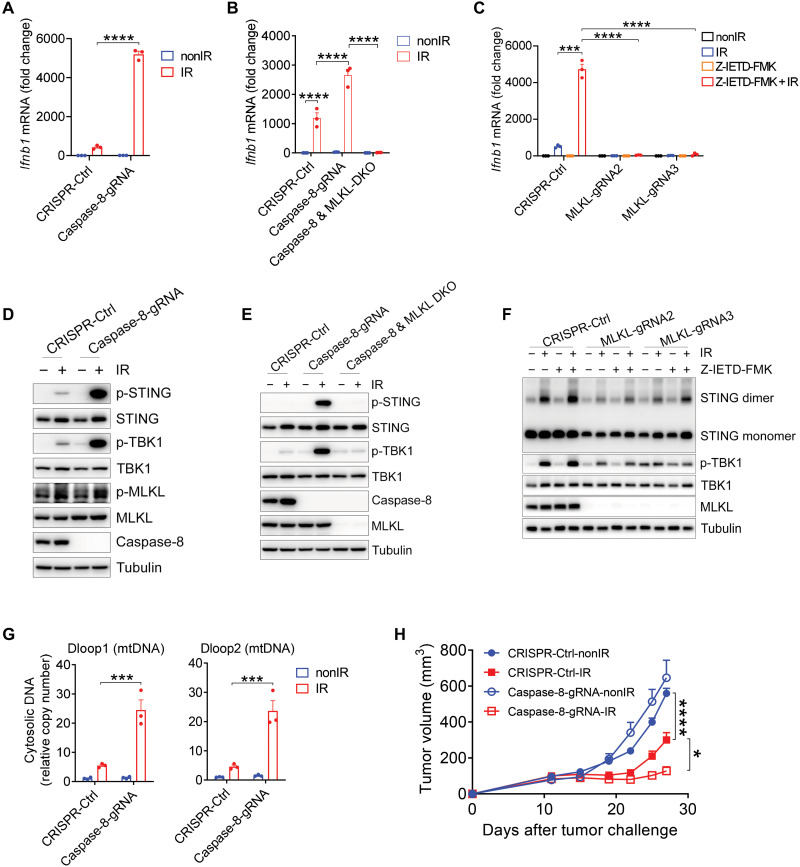
Caspase-8 protease negatively regulates ZBP1-dependent activation of RIPK3-MLKL–mediated necroptosis by cleaving components of the ZBP1-RIPK3 complex (17). To test whether caspase-8 deletion would augment type I IFN induction in tumor cells upon irradiation, we deleted caspase-8 from MC38 tumor cells (fig. S9A). Notably, the ablation of caspase-8 prominently enhanced type I IFN production after radiation treatment (Fig. 6A). To validate whether caspase-8 deficiency amplifies IFN-β production after irradiation through MLKL-dependent necroptosis, we deleted both MLKL and caspase-8 from tumor cells or treated MLKL−/− MC38 tumor cells with caspase-8 pharmacological inhibitor (Z-IETD-FMK). Genetic ablation of MLKL completely abolished the induction of IFN-β in caspase-8–deficient tumor cells after radiation treatment (Fig. 6B). Consistent with this result, caspase-8 inhibitor with radiation treatment synergistically increased IFN-β production in tumor cells, whereas MLKL deletion completely abolished IFN-β production in tumor cells after combination treatment of caspase-8 inhibitor and radiation (Fig. 6C). Thus, our findings indicate that caspase-8 is a regulator for IFN-β production in irradiated tumor cells through disruption of ZBP1-RIPK3-MLKL necroptosis signaling.
Fig. 6. Ablation of caspase-8 enhances STING pathway activation and therapeutic effects of radiation.
(A and B) Ifnb1 mRNA expression in Ctrl, caspase-8−/−, and caspase-8−/-MLKL−/− MC38 tumor cells after IR treatment. (C) Ifnb1 mRNA expression in Ctrl or MLKL−/− MC38 tumor cells after IR treatment with or without caspase-8 inhibitor (10 μM Z-IETD-FMK). (D) p-STING, p-TBK1, and p-MLKL expression in Ctrl and caspase-8−/− MC38 tumor cells after IR treatment. (E) p-STING and p-TBK1 expression in Ctrl, caspase-8−/−, and caspase-8−/−MLKL−/− MC38 tumor cells after IR treatment. (F) STING dimer and p-TBK1 expression in Ctrl and MLKL−/− MC38 tumor cells after IR treatment with or without caspase-8 inhibitor. (G) Cytosolic amount of mtDNA in Ctrl and caspase-8−/− MC38 cells after IR treatment. (H) Tumor growth of Ctrl and caspase-8−/− MC38 tumors in WT mice after IR treatment. Data are represented as means ± SEM. Representative data are shown from two or three independent experiments with at least three culture replicates. Statistical analysis was performed by one-way ANOVA with Bonferroni’s multiple comparisons test (A, B, and G), by two-tailed unpaired t test (C), or by two-way ANOVA with Bonferroni’s multiple comparisons test (H). *P < 0.05; ***P < 0.001; ****P < 0.0001.
To assess whether caspase-8 deficiency also enhances activation of the STING pathway for increased IFN-β production in irradiated tumor cells, we again measured STING dimerization and TBK1 phosphorylation. This assay confirmed that ablation of caspase-8 markedly increased the expression of STING dimerization and TBK1 phosphorylation after irradiation (Fig. 6D). In addition, MLKL phosphorylation was elevated in caspase-8–deficient tumor cells compared to control tumor cells after irradiation (Fig. 6D), revealing that caspase-8 loss amplifies STING-mediated type I IFN production largely by increasing MLKL-dependent necroptosis. Genetic ablation of MLKL limited the activity of STING and TBK1 that were induced by loss of caspase-8 in tumor cells after radiation treatment (Fig. 6E).
To further verify the effect of caspase-8 on cytosolic DNA sensing, we treated tumor cells with the combination of radiation and caspase-8 inhibitor. As we observed in caspase-8–deficient tumor cells, caspase-8 inhibitor synergized with radiation treatment to up-regulate STING and TBK1 activation (Fig. 6F). In contrast, the lack of MLKL greatly attenuated STING dimerization and TBK1 phosphorylation after the combination treatment (Fig. 6F). These results suggested that MLKL is essential for type I IFN production following treatment with radiation and caspase-8 inhibitor, probably due to the plasticity between apoptosis and necroptosis in tumor cells. Furthermore, we observed that caspase-8 deletion increased the cytosolic enrichment of mtDNA in irradiated tumor cells (Fig. 6G). In addition, type I IFN induction was completely diminished in cGAS- or STING-deficient tumor cells following combination treatment with radiation and caspase-8 inhibitor Z-IETD-FMK (fig. S9, B and C), thus demonstrating that cGAS-STING signaling is indispensable for type I IFN production after combined treatment with radiation and caspase-8 inhibitor in vitro. Together, these results suggest that tumor endogenous caspase-8 is a barrier for the ZBP1-RIPK3-MLKL necroptotic pathway in the context of radiation-induced cytosolic DNA sensing.
Although radiotherapy is widely used to treat various types of human cancers, tumor relapses commonly occur following radiotherapy (2). Our findings indicate that the accurate ablation of caspase-8 in tumor cells may be a strong potential therapeutic strategy to improve the efficacy of radiotherapy. To this end, we injected caspase-8−/− MC38 and Ctrl-MC38 cells into mice, and the established tumors were treated with local radiation. Tumors lacking caspase-8 were notably restricted in their growth compared to the control group after irradiation (Fig. 6H). This finding thus demonstrates that tumor cell–intrinsic caspase-8 ablation may be a viable avenue for improvement of the effectiveness of radiotherapy.
DISCUSSION
Understanding the immunological aspects of radiotherapy aids the design of new therapeutic combinations. In this study, we found that the ZBP1-MLKL necroptotic cascade facilitated antitumor immunity of radiation through increasing cytosolic DNA sensing. In addition, caspase-8 ablation and radiation treatment synergistically increased mtDNA release and antitumor effects. Our findings highlight the importance of the ZBP1-MLKL necroptotic cascade in radiotherapy and the regulatory module to bridge tumor cell damage and antitumor immunity.
Necroptosis has been considered to be one modality for immunological cell death to cross-prime CD8+ T cells (12). RIPK3-containing necrosome promotes the release of DAMPs and induces the maturation of DCs (15, 32). Breaking from the above model, our findings provide a new perspective that the tumor cell–intrinsic STING-mediated DNA sensing relays the action of ZBP1-MLKL necroptotic signaling for the cross-priming of CD8+ T cells after radiation treatment. By preserving the duration of plasma membrane integrity, ESCRT-III–mediated prolonged necroptotic cell death has been shown to promote cross-priming of CD8+ T cells (33). It is possible that the ESCRT-III machinery regulates cytosolic DNA sensing after radiation by preventing the efflux of mtDNA from the dying cells. A recent study has demonstrated that the accumulation of MLKL at intercellular junctions potentiates necroptosis between neighboring cells (34). It is therefore possible that necroptotic tumor cells may recruit their neighboring tumor cells to expand the necroptotic signaling and subsequent cytosolic DNA sensing by tight junction proteins after radiation treatment.
ZBP1-MLKL necroptotic signaling plays a critical role in driving chronic inflammation and influenza virus pathogenesis (17, 18). Besides exerting a necroptotic function, MLKL partly participates in endosomal trafficking mediated by ESCRT machinery in a cell death–independent pattern (29). We observed the engagement of RIPK3 and MLKL was comparable in IFN-β induction after radiation, suggesting that MLKL mainly functions as the molecular machinery for necroptosis in the context of IFN-β induction after radiation treatment. Although Toll-like receptor 3 (TLR3) ligation is one of regulators involved in initiating the activation of molecular machinery for necroptosis, TLR3 mediates type I IFN production of tumor cells upon chemotherapy in an MLKL-irrelevant pattern (35). ZBP1 is able to trigger both apoptosis and necroptosis by sensing influenza A virus genomic RNA (36). We cannot rule out the possibility that ZBP1 simultaneously interacts with molecular machineries of apoptosis and necroptosis after radiation. At least, ZBP1 is sufficient for MLKL-dependent necroptosis regarding type I IFN production in tumor cells after radiation treatment. Of note, it has been demonstrated that MLKL can localize to both nucleus and mitochondria, most likely to induce nuclear envelope rupture and mitochondrial pore formation after radiation (18, 37). In addition, it will be interesting to determine how ZBP1 is up-regulated and activated by radiation.
gDNA aggregated in micronuclei is essential for cGAS-STING–mediated antitumor immunity following genotoxicity therapy and poly(adenosine 5′-diphosphate–ribose) polymerase (PARP) inhibitor treatment (9–11, 38). In this study, we found that both cGAS-positive micronuclei and DNA damage were not affected by MLKL loss after radiation. However, we observed that the removal of mtDNA abrogated type I IFN induction in irradiated tumor cells, indicating that mtDNA may play a paralleled role with gDNA in triggering the cGAS-STING pathway in response to radiation. It has been demonstrated that HMGB/TFAM-bound U-turn DNA is efficient to bind to cGAS by forming protein-DNA ladders (39). Therefore, TFAM may provide assistance to maintain the stability of mtDNA binding to cGAS. The release of mtDNA is the result of the inner mitochondrial membrane herniation during caspase-inhibited apoptosis (24). Part of the RIPK3-MLKL–containing necrosome has been observed to translocate to the mitochondria in a dynamic and transient manner (37). So one possible interpretation is that MLKL is required for the release of mtDNA instead of mitochondrial collapse after radiation; however, the relevant molecular mechanism of how MLKL regulates the release of mtDNA needs to be further determined.
Tumor radiation resistance often occurs in multiple types of human cancers, due to tumor cell–intrinsic or tumor cell–extrinsic factors (21). Previously, we identified that PD-L1 and MDSCs act as two major barriers of antitumor immunity after radiation (23, 40). Given the findings in this study, the attenuation of ZBP1-RIPK3-MLKL necroptotic signaling and cGAS-STING signaling may be cell-intrinsic factors for radiation resistance. It is noticeable that caspase-8 is also involved in the regulation of cytosolic mtDNA sensing by inhibiting MLKL activation. According to the reciprocal interaction between MLKL-dependent necroptosis and caspase-8–dependent apoptosis, it would be feasible to interrupt caspase-8 to improve radiotherapy. Despite the additional role of catalytically inactive caspase-8 in modulating pyroptosis (41), caspase-8 is evidently a major barrier for the ZBP1-RIPK3-MLKL cascade in tumor cells after radiation treatment in terms of STING-mediated type I IFN production. Because MLKL in tumor cells rather than in host cells is required for antitumor effects of radiation treatment, the fine-tuning of MLKL-dependent necroptosis by caspase-8 intervention is appreciated in tumor cells to improve radiotherapy. In addition, ISGs in tumor cells contribute to radiation resistance through limiting both adaptive and innate immune killing (42). Genetic abolition of type I IFN signaling in tumor cells improves radiation or treatment naïve (42–44). These evidences raise a possibility that the ISG signature affects antitumor immune responses of radiation in a cell type–dependent pattern.
Our study does have certain limitations, such as the reason of ZBP1 up-regulation and the localization of MLKL in tumor cells after radiation treatment. Whether MLKL localizes to the mitochondrial membrane to trigger mtDNA release into the cytoplasm after radiation treatment needs to be further determined. Whether ZBP1-RIPK3-MLKL axis and caspase-8 can be an indicator of tumor prognosis in patients with radiotherapy also needs to be investigated. Although the positive correlation between the DNA-sensing signature and ZBP1/MLKL expression is observed in mentioned TCGA-Pan Cancer with treatment naïve, whether the correlation implies that all those tumors should have a better response to irradiation or respond to irradiation through the same pathway needs to be further investigated.
In summary, our results define a previously unrecognized cross-talk between ZBP1-MLKL necroptotic cascade and STING-mediated cytosolic DNA sensing, which governs cross-priming of CD8+ T cells after radiation treatment. Increasing MLKL activity by caspase-8 inhibition greatly augments STING activation and antitumor effects of radiation. Moreover, these data specify a new modality of danger signal recognition that alters immunological aspects of radiotherapy, and indicate an alternative strategy to improve radiotherapy.
MATERIALS AND METHODS
Experimental design
The objective of this study was to identify the potential mechanism of how necroptotic signaling cascade initiates antitumor immune responses to radiation. We generated MLKL-, ZBP1-, cGAS-, or STING-deficient tumor cell lines by CRISPR-Cas9 technology with distinct guide RNAs and implanted these tumor cell lines on the flanks of WT mice. Then, we monitored tumor growth and analyzed the percentage and function of CD8+ T cells in the tumors, spleens, and DLNs after receiving local radiation. To investigate how ZBP1-MLKL axis induces antitumor host immunity after radiation, we performed RNA-seq using tumor cells with or without MLKL. In addition, the association between the expression of ZBP1 or MLKL and the enrichment score of cytosolic DNA–sensing pathway was analyzed in human cancers from TCGA. To investigate whether ZBP1-MLKL cascade is essential for type I IFN responses in tumor cells after radiation, we detected phosphorylation of STING and TBK1 by Western blotting and Ifnb1 mRNA expression by real-time PCR. To determine whether MLKL regulates cytosolic DNA releasing in irradiated tumor cells, cytosolic DNA was extracted and measured by real-time PCR. To further explore whether mtDNA is responsible for radiation-induced type I signaling in tumor cells, we generated mtDNA-depleted tumor cells by culturing MC38 in the presence of EtBr. Last, to assess whether overactivation of MLKL improves therapeutic effects of radiation, we generated caspase-8–deficient tumor cells to enhance the activity of MLKL and STING and antitumor effects after radiation.
In our study, for cell-based experiments, at least biological triplicates were performed in each single experiment, unless otherwise stated. Animals were randomized into different groups after tumor cell inoculation and at least four to six mice were used for each group; then, one fraction of local radiation was administrated for tumor treatment. Three to five mice were used for tumor microenvironment analysis. For cell-based experiments, the number of biological replicates was illustrated in each figure. Whenever possible, the investigators were blinded to group allocation during the experiments and when assessing outcomes. In some cases, selected samples were excluded from specific analyses because of technical flaws during sample processing or data acquisition. Analytical studies were typically performed according to two or three independent experiments.
Mice
Six- to 8-week-old female C57BL/6J (WT) mice were purchased from Shanghai Slac Laboratory Animal Co. Ltd., China. MLKL-KO mice were purchased from the National Resource Center of Model Mice (NRCMM), Nanjing, China. All the mice were maintained under specific pathogen–free conditions at the animal facility of Shanghai Jiao Tong University School of Medicine. All the animal studies were conducted in compliance with the protocol approved by the Institutional Animal Care and Use Committee of Shanghai Jiao Tong University School of Medicine.
Cell lines and culture conditions
MC38, B16-SIY, and A549 were contributed by the laboratory of Y.-X. Fu; 293T was purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) (catalog no. SCSP-502); and E0771 was from the laboratory of B. Zhou. These cell lines were maintained at 37°C with 5% CO2 in Dulbecco’s modified Eagle’s medium–high glucose medium (HyClone, catalog no. SH30243.01) containing 10% fetal bovine serum (FBS) (Gemini, catalog no. 900-108), penicillin (100 U/ml), streptomycin (100 μg/ml), and 10 mM Hepes (Thermo Fisher Scientific, catalog nos. 15140122 and 15630080). All cell lines were tested to be mycoplasma free. MLKL−/− MC38, ZBP1−/− MC38, RIPK3−/− MC38, cGAS−/− MC38, STING−/− MC38, caspase-8−/− MC38, MLKL−/− E0771, ZBP1−/− E0771, and MLKL−/− B16-SIY cell lines were generated using CRISPR-Cas9 plasmid lenti-crispr-V2 (from the laboratory of X.-Y. Liu). The annealed single-guide RNA (sgRNA) oligos (see table S1) were cloned into pLenti-CRISPR-V2 and packaged in 293T cells (Cell Bank of the Chinese Academy of Sciences, catalog no. SCSP-502). Supernatants containing virus particles were collected 24 and 48 hours after transfection and then added to preplated cells with polybrene (2 μg/ml; Genomeditech, catalog no. GM-040901B). The transduced cells were selected by puromycin (Beyotime Biotechnology, catalog no. ST551) at 2 μg/ml to acquire the gene-deleted stable cell lines. The efficiency of gene knockdown was determined via Western blotting. In addition, tdTomato-expressing Ctrl-MC38 cells and tdTomato-expressing MLKL−/-MC38 cells were generated using pLenti-CMV-tdTomato (from the laboratory of J. Wang). Briefly, Ctrl-MC38 or MLKL−/− MC38 cells were infected by lentivirus supernatants of tdTomato with polybrene (2 μg/ml). tdTomato+ cells were sorted out with a cell sorter (BD Biosciences) and cultured for further experiments.
In vivo tumor models
To explore the effects of radiation on tumor growth, WT mice were subcutaneously inoculated with 2 × 106 Ctrl-MC38, MLKL−/− MC38, ZBP1−/− MC38, STING−/− MC38, or caspase-8−/− MC38, which were resuspended in the mixture of phosphate-buffered saline (PBS) and Matrigel matrix (Corning BioCoat, catalog no. 356234) (100 μl, v/v = 1:1). In B16-SIY model, WT mice were subcutaneously inoculated with 1 × 106 Ctrl-B16-SIY or MLKL−/− B16-SIY tumor cells. To investigate the role of host MLKL in tumor growth, WT and MLKL-KO mice were subcutaneously inoculated with 1 × 106 MC38 cells resuspended in 100 μl of PBS (HyClone, catalog no. SH30256.01). Tumors were measured, randomly grouped, and locally irradiated at a single fraction of 15 Gy on the indicated day, and then tumors were monitored twice a week afterward. For the detection of abscopal effects of radiation, WT mice were subcutaneously inoculated with 1.5 × 106 Ctrl-MC38 or MLKL−/− MC38 cells resuspended in the mixture of PBS and Matrigel matrix on the right flank and with 1 × 106 Ctrl-MC38 cells resuspended in 100 μl of mixture of PBS and Matrigel matrix on the corresponding opposite flank of the same mice. Tumors on the right flank were subjected to local radiation at a single fraction of 15 Gy, while tumors on the left flank were shielded from radiation. Tumors on both flanks were monitored twice a week subsequently.
Quantitative reverse transcription PCR
Total RNA for real-time PCR assay was extracted and purified using the TRIzol Reagent (Thermo Fisher Scientific, catalog no. 15596018). Reverse transcription reactions were performed with ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo, catalog no. FSQ-301) following the standard protocol. Quantitative reverse transcription PCR was performed with SYBR Green Realtime PCR Master Mix (Toyobo, catalog no. QPK-201) in the ViiA 7 Real-Time PCR System with 384-well block (Applied Biosystems, Life Technologies). The expression of mRNA was normalized against glyceraldehyde-3-phosphate dehydrogenase (Gapdh) by the change in cycling threshold (ΔCt) method. Primers used in this study are included in table S1.
Western blotting
For whole-cell protein extraction, pretreated cells were washed with cold PBS and lysed in cell lysis buffer for Western blotting and immunoprecipitation (Beyotime Biotechnology, catalog no. P0013). The protein samples were quantified using a BCA protein assay kit (Beyotime Biotechnology, catalog no. P0012), loaded on SDS–polyacrylamide gel electrophoresis (SDS-PAGE) gels, electrophoresed, transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, catalog no. IPVH00005), and further incubated with the primary and secondary antibodies. For STING dimer detection, the pretreated cells were washed with cold PBS and lysed in cell lysis buffer for 2 hours at 4°C with gentle rocking. Then, the protein samples were diluted with nondenatured gel sample loading buffer [4×; formulation: 40 ml of glycerol, 20 ml of 1 M tris (pH 6.8), 1 ml of 2% bromophenol blue, and 40 ml of 20% SDS] without heating and loaded on SDS-PAGE gels. Last, the blots were developed following protocols as above. The following primary antibodies were obtained from Cell Signaling Technology: anti–phospho-MLKL (Ser345) (D6E3G) (catalog no. 37333), anti-STING (D2P2F) (catalog no. 13647), anti–phospho-STING (Ser365) (D8F4W) (catalog no. 72971), anti-cGAS (D3O8O) (catalog no. 31659), rabbit anti-TBK1 (catalog no. 3013), anti–phospho-TBK1(S172) (D52C2) (catalog no. 5483), and anti–caspase-8 (catalog no. 4927); from Millipore: anti-MLKL (3H1) (catalog no. MABC604); from AdipoGen: anti-ZBP1 (Zippy-1) (catalog no. AG-20B-0010); from Prosci: anti-RIPK3 (catalog no. 2283); and from Proteintech: anti-tubulin (catalog no. 10094-1-AP) and anti-GAPDH (catalog no. 10494-1-AP).
Flow cytometry
Subcutaneous tumors with or without radiation were removed on indicated days, cut into small pieces with ophthalmic forceps, digested with collagenase I (1 mg/ml; Worthington Biochemical, catalog no. LS004196) and deoxyribonuclease (DNase) I (0.2 mg/ml; Sigma-Aldrich, catalog no. DN25) for 30 min, and filtered with 70-μm cell strainers to obtain the single-cell suspensions followed by cell staining and flow cytometry analysis. Briefly, for surface marker staining, single cells prepared as above were incubated with anti-mouse CD16/32 (2.4G2) (Bio X Cell, catalog no. CUS-HB-197) at 4°C for 15 min, washed with fluorescence-activated cell sorting (FACS) buffer (2% FBS in PBS), and then incubated at 4°C for 30 min with antibody cocktail against anti-CD45–BV785 (104) (BioLegend, catalog no. 109839), anti-CD8–AF700 (53-6.7) (BioLegend, catalog no. 100730), and live/dead (Fixable Viability Stain 780, BD Biosciences, catalog no. 565388). When intracellular staining was needed, single-cell suspensions were fixed with fixation/permeabilization buffer (A:B = 1:3) (Invitrogen, catalog nos. 00-5223-56 and 00-5123-43) at 4°C for 1 hour, washed twice with permeabilization buffer (Invitrogen, catalog no. 00-8333-56), and incubated in corresponding antibody cocktails (in permeabilization buffer) in the dark at 4°C for 40 min to 1 hour. To evaluate the function of T cells in tumor microenvironments, single-cell suspensions were treated with Cell Stimulation Cocktail (plus protein transport inhibitors) (eBioscience, catalog no. 00-4975-03) for 3 hours before cell staining, and the percentages of IFN-γ+TNF-α+CD8+ T cells in tumor microenvironments were determined with flow cytometry. Last, multicolor flow cytometry was performed with either LSR Fortessa or LSR X20, while cell sorting was performed with FACSAria III (BD Biosciences). The data acquired would be further analyzed with FlowJo software (Tree Star).
Cell sorting
Single-cell suspensions from tumor tissues were obtained as mentioned above. For the isolation of tdTomato-labeled MC38 tumor cells, single-cell suspensions were blocked with anti-mouse CD16/32 and then stained with anti-CD45 and live/dead. For CD11c+ DC isolation, single-cell suspensions were blocked with anti-mouse CD16/32 and then stained with antibodies anti-CD11c–allophycocyanin (N418) (BioLegend, catalog no. 117310), anti-mouse I-A/I-E-PerCP/Cy5.5 (M5/114.15.2) (BioLegend, catalog no. 107626), anti-CD45.2–AF700 (104) (eBioscience, catalog no. 56045481), and live/dead. The cells were performed with FACSAria III Cell Sorter (BD Biosciences).
ELISPOT assay
For tumor-specific CD8+ T cell functional assay, DLNs or spleens from tumor-bearing mice were removed 7 days after radiation, cut into small pieces, grinded, and filtered through 70-μm cell strainers to obtain the single-cell suspensions. Subsequently, CD8+ T cells were selected by EasySep Mouse CD8a Positive Selection Kit II (STEMCELL, catalog no. 18953). MC38 tumor cells were preexposed to murine IFN-γ (20 ng/ml) for 24 hours before plating with purified CD8+ T cells. CD8+ T cells (2 × 105) were incubated with MC38 at a ratio of 10:1 for 48 hours, and ELISPOT assays were performed to detect IFN-γ spots according to product protocol (BD Biosciences, catalog no. 551083).
Cross-priming activity by DCs
MC38-SIY cells (3 × 105) were plated into a six-well plate overnight, then treated with radiation at 15 Gy, and incubated for 48 hours. For the control group, 1.5 × 105 MC38-SIY cells were plated into a six-well plate. BMDCs were harvested on day 6, and then 1.2 × 106 BMDCs were added and cocultured with MC38-SIY cells in the presence of fresh granulocyte-macrophage colony-stimulating factor (GM-CSF) at 5 ng/ml for additional 12 hours. Subsequently, 2 × 104 CD11c+ cells purified with EasySep Mouse CD11c Positive Selection Kit II (STEMCELL, catalog no. 18780) were incubated with isolated CD8+ T cells from naïve 2C-TCR transgenic mice using EasySep Mouse CD8a Positive Selection Kit II (STEMCELL, catalog no. 18953) for 3 days at a ratio of 1:10. For tumor-infiltrating DCs, CD11c+ DCs were sorted with a BD FACSAria III cell sorter from tumors 3 days after radiation. The sorted CD11c+ cells were cocultured with isolated CD8+ T cells from naïve 2C-TCR transgenic mice for 3 days at a ratio of 1:10. The supernatants were collected for IFN-γ detection using Cytometric Bead Array (CBA) Mouse IFN-γ Flex Set (RUO) (BD Biosciences, catalog no. 558296) according to standard protocols.
RNA sequencing
Briefly, Ctrl-MC38 (CRISPR-Ctrl) or MLKL−/-MC38 (MLKL-gRNA3) tumor cells were subjected to a single fraction of 15 Gy, and total RNA from each sample was extracted using TRIzol Reagent (Thermo Fisher Scientific) following standard protocols 60 hours later. The concentration and quality of RNA samples were determined with a NanoDrop 2000 spectrophotometer (NanoDrop Technologies) and the Agilent 2100 Bioanalyzer (Agilent Technologies). Qualified RNA samples were used for RNA-seq and data analysis (Novogene, Shanghai, China). Data analysis was performed using R software (R Foundation for Statistical Computing). RNA-seq data generated in this paper were deposited at the Gene Expression Omnibus (accession number GSE168016).
Detection of cytosolic DNA
MC38 cells were cultured in a 10-cm dish and subjected to radiation at 15 Gy. Fifty-four hours later, total DNA and cytosolic DNA were extracted and detected as described previously (45). Briefly, MC38 cells (2 × 106 to 1 × 107) were divided into two equal aliquots. One aliquot was resuspended in 300 μl of 50 mM NaOH and boiled for 30 min to solubilize DNA. Thirty microliters of 1 M tris-HCl (pH 8.0) was added to neutralize the pH and then centrifuged at 12,000 rpm for 10 min to pellet intact cells. In addition, these extracts served as normalization controls for total gDNA and mtDNA. The second equal aliquots were resuspended in about 300 μl of buffer containing 150 mM NaCl, 50 mM Hepes (pH 7.4), and digitonin (15 to 25 mg/ml; Sigma-Aldrich, catalog no. 300410). The homogenates were incubated end over end for 10 min on ice to allow selective plasma membrane permeabilization and then centrifuged at 980g for 3 min three times to pellet intact cells. Last, the cytosolic supernatants were transferred to fresh tubes and spun at 17,000g for 10 min to pellet any remaining cellular debris, yielding cytosolic preparations free of nuclear, mitochondrial, and endoplasmic reticulum contamination. The cytosolic DNA and whole-cell DNA were purified using DNA Clean & Concentrator-5 (ZYMO RESEARCH, catalog no. D4013). Quantitative real-time PCR was performed on both whole-cell extracts and cytosolic fractions using gDNA primers (Tert and Plog1) and mtDNA primers (Dloop1 and Dloop2), and the Ct values for whole-cell extracts served as normalization controls for the values obtained from the cytosolic fractions.
mtDNA depletion
MC38 cells were developed in the presence or absence of EtBr (200 to 400 ng/ml) (Sigma-Aldrich, catalog no. E7637) or 50 μM ddC (Sigma-Aldrich) for 6 days. The content of mitochondrial and nuclear DNA in EtBr- or ddC-treated tumor cells was confirmed by real-time PCR. Afterward, tumor cells were collected and cultured without EtBr or ddC followed by radiation treatment.
Analysis of RNA-seq and TCGA datasets
RNA-seq was performed by Illumina NovaSeq 6000 with 150–base paired-end reads. All reads were aligned to the mouse reference genome (mm10) using hisat2 v2.0.5 with the default setting. HTSeq was used to count the read numbers mapped to each gene. DESeq2 was used to normalize the raw counts and identify differentially expressed genes [|fold change| ≥ 1.5; false discovery rate (FDR) < 0.05]. Gene ontology enrichment analysis was performed by R package clusterProfiler, where the differentially expressed genes identified as described above were supplied as the input for genes by function enrichKEGG. Hypergeometric tests were used to calculate P values, which were then subjected to multiple testing adjustments by Benjamini-Hochberg correction. GSEA was performed to test whether interested gene sets are substantially enriched in corresponding conditions.
Gene-level RNA-seq expression data [normalized RSEM (RNA-seq by expectation-maximization) value] of 33 cancer types were downloaded from TCGA data portal (https://portal.gdc.cancer.gov/). Normalized gene expression values were then log2-transformed. To generate gene expression signature, we took the mean of log2-transformed expression values of signature genes. The signature genes of cytosolic DNA–sensing pathway signature (Zbp1, Ifnb1, Ccl4, Irf7, Ifna4, Cxcl10, Ccl5, Il6, Ddx58, Casp1, Il18, Adar, Nfkbia, Polr1c, Polr3e, Polr3h, Polr2h, and Polr3g) and type I IFN response (Zbp1, Mx2, Mx1, Cxcl11, Ifi44, Cxcl9, Oas2, Ifnb1, Ccl4, Irgm2, Oasl2, Ifit1bl2, Irf7, Ifna4, Isg15, Oasl1, Ifit1bl1, Cxcl10, Ifitm6, Ccl5, Irgm1, Stat2, Stat1, Irf9, Irf8, Adar, Nlrc5, Ifitm3, Samhd1, Myd88, Trim56, Trim6, and Shmt2) are obtained from The Molecular Signatures Database (MSigDB; http://software.broadinstitute.org/gsea/msigdb/). Gene set variation analysis (GSVA) was performed to calculate the score of these two pathways. The infiltration of CD8+ T cells was determined by CIBERSORT (http://cibersort.stanford.edu/). We calculated the Spearman correlation between pathway GSVA score or the infiltration of CD8+ T cells and the RNA expression candidate genes, considering |Rs| > 0.2 and FDR < 0.05 for statistical significance.
Statistical analysis
No statistical method was used to predetermine sample size. Mice were assigned at random to treatment groups for all mouse studies. Experiments were repeated two to three times. Group sizes and number of replications are provided in the figure legends. Statistical analysis was performed using Prism software (GraphPad Prism8 software). Tumor growth monitor, flow cytometry, and quantitative real-time PCR were analyzed using one-way analysis of variance (ANOVA) with Bonferroni’s multiple comparisons test, Brown-Forsythe and Welch ANOVA tests with Tamhane’s T2 multiple comparisons test, two-tailed unpaired t test, unpaired t test with Welch’s correction, or two-way ANOVA with Bonferroni’s multiple comparisons test as indicated in the figure legends. Data were presented as mean values ± SEM. We indicated significance corresponding to the following: *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Acknowledgments
We thank B. Su, Y.-X. Fu, and S. Wang for helpful scientific discussion. Funding: This work was supported by the National Natural Science Foundation of China (81771682 and 82071741 to L.D., 81702804 to W.L., 31900649 to M.W., and 82030082 to J.Y.), National Thousand Youth Talents Program (to L.D.), Science and Technology Commission of Shanghai Municipality (16JC1406000 to L.D.), Innovative research team of high-level local universities in Shanghai (to L.D. and W.L.), Shanghai Municipal Commission of Health and Family Planning (20194Y0625 to W.L.), and the Interdisciplinary Program of Shanghai Jiao Tong University (YG2021ZD03 to L.D.). Author contributions: Y.Ya. and M.W. performed the experiments and wrote the paper. D.C. and C.Y. performed part of experiments. J.J. and C.Y. aided in the experiments of flow cytometry assay. L.W., X.H., W.L., and J.L. aided in some experiments of real-time PCR and Western blot. Y.Ya., M.W., L.L., X.W., and Y.Ye. analyzed the data. X.M., Z.Z., Y.Ye., H.X., and J.Y. helped in editing the manuscript. L.D. conceived and supervised all experiments and the writing of the manuscript. All authors approved the paper. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Supplementary Materials and Methods
Table S1
Figs. S1 to S9
REFERENCES AND NOTES
- 1.Herrera F. G., Bourhis J., Coukos G., Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J. Clin. 67, 65–85 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Citrin D. E., Recent developments in radiotherapy. N. Engl. J. Med. 377, 1065–1075 (2017). [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin M., Patin E. C., Pedersen M., Wilkins A., Dillon M. T., Melcher A. A., Harrington K. J., Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat. Rev. Cancer 20, 203–217 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Deng L., Liang H., Xu M., Yang X., Burnette B., Arina A., Li X. D., Mauceri H., Beckett M., Darga T., Huang X., Gajewski T. F., Chen Z. J., Fu Y. X., Weichselbaum R. R., STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 41, 843–852 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ablasser A., Chen Z. J., cGAS in action: Expanding roles in immunity and inflammation. Science 363, eaat8657 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Demaria O., Cornen S., Daeron M., Morel Y., Medzhitov R., Vivier E., Harnessing innate immunity in cancer therapy. Nature 574, 45–56 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Kwon J., Bakhoum S. F., The cytosolic DNA-sensing cGAS–STING pathway in cancer. Cancer Discov. 10, 26–39 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.West A. P., Khoury-Hanold W., Staron M., Tal M. C., Pineda C. M., Lang S. M., Bestwick M., Duguay B. A., Raimundo N., MacDuff D. A., Kaech S. M., Smiley J. R., Means R. E., Iwasaki A., Shadel G. S., Mitochondrial DNA stress primes the antiviral innate immune response. Nature 520, 553–557 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mackenzie K. J., Carroll P., Martin C. A., Murina O., Fluteau A., Simpson D. J., Olova N., Sutcliffe H., Rainger J. K., Leitch A., Osborn R. T., Wheeler A. P., Nowotny M., Gilbert N., Chandra T., Reijns M. A. M., Jackson A. P., cGAS surveillance of micronuclei links genome instability to innate immunity. Nature 548, 461–465 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harding S. M., Benci J. L., Irianto J., Discher D. E., Minn A. J., Greenberg R. A., Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 548, 466–470 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dou Z., Ghosh K., Vizioli M. G., Zhu J., Sen P., Wangensteen K. J., Simithy J., Lan Y., Lin Y., Zhou Z., Capell B. C., Xu C., Xu M., Kieckhaefer J. E., Jiang T., Shoshkes-Carmel M., Tanim K., Barber G. N., Seykora J. T., Millar S. E., Kaestner K. H., Garcia B. A., Adams P. D., Berger S. L., Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature 550, 402–406 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galluzzi L., Buque A., Kepp O., Zitvogel L., Kroemer G., Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 17, 97–111 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Seifert L., Werba G., Tiwari S., Giao Ly N. N., Alothman S., Alqunaibit D., Avanzi A., Barilla R., Daley D., Greco S. H., Torres-Hernandez A., Pergamo M., Ochi A., Zambirinis C. P., Pansari M., Rendon M., Tippens D., Hundeyin M., Mani V. R., Hajdu C., Engle D., Miller G., The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression. Nature 532, 245–249 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seehawer M., Heinzmann F., D’Artista L., Harbig J., Roux P.-F., Hoenicke L., Dang H., Klotz S., Robinson L., Dore G., Rozenblum N., Kang T.-W., Chawla R., Buch T., Vucur M., Roth M., Zuber J., Luedde T., Sipos B., Longerich T., Heikenwalder M., Wang X. W., Bischof O., Zender L., Necroptosis microenvironment directs lineage commitment in liver cancer. Nature 562, 69–75 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snyder A. G., Hubbard N. W., Messmer M. N., Kofman S. B., Hagan C. E., Orozco S. L., Chiang K., Daniels B. P., Baker D., Oberst A., Intratumoral activation of the necroptotic pathway components RIPK1 and RIPK3 potentiates antitumor immunity. Sci. Immunol. 4, eaaw2004 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang R., Li H., Wu J., Cai Z. Y., Li B., Ni H., Qiu X., Chen H., Liu W., Yang Z. H., Liu M., Hu J., Liang Y., Lan P., Han J., Mo W., Gut stem cell necroptosis by genome instability triggers bowel inflammation. Nature 580, 386–390 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Jiao H., Wachsmuth L., Kumari S., Schwarzer R., Lin J., Eren R. O., Fisher A., Lane R., Young G. R., Kassiotis G., Kaiser W. J., Pasparakis M., Z-nucleic-acid sensing triggers ZBP1-dependent necroptosis and inflammation. Nature 580, 391–395 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang T., Yin C., Boyd D. F., Quarato G., Ingram J. P., Shubina M., Ragan K. B., Ishizuka T., Crawford J. C., Tummers B., Rodriguez D. A., Xue J., Peri S., Kaiser W. J., Lopez C. B., Xu Y., Upton J. W., Thomas P. G., Green D. R., Balachandran S., Influenza virus Z-RNAs induce ZBP1-mediated necroptosis. Cell 180, 1115–1129.e13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galluzzi L., Kepp O., Chan F. K.-M., Kroemer G., Necroptosis: Mechanisms and relevance to disease. Annu. Rev. Pathol. 12, 103–130 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruni D., Angell H. K., Galon J., The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat. Rev. Cancer 20, 662–680 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Ruiz M. E., Vitale I., Harrington K. J., Melero I., Galluzzi L., Immunological impact of cell death signaling driven by radiation on the tumor microenvironment. Nat. Immunol. 21, 120–134 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Hou Y., Liang H., Rao E., Zheng W., Huang X., Deng L., Zhang Y., Yu X., Xu M., Mauceri H., Arina A., Weichselbaum R. R., Fu Y. X., Non-canonical NF-κB antagonizes STING sensor-mediated DNA sensing in radiotherapy. Immunity 49, 490–503.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang H., Deng L., Hou Y., Meng X., Huang X., Rao E., Zheng W., Mauceri H., Mack M., Xu M., Fu Y. X., Weichselbaum R. R., Host STING-dependent MDSC mobilization drives extrinsic radiation resistance. Nat. Commun. 8, 1736 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han C., Liu Z., Zhang Y., Shen A., Dong C., Zhang A., Moore C., Ren Z., Lu C., Cao X., Zhang C. L., Qiao J., Fu Y. X., Tumor cells suppress radiation-induced immunity by hijacking caspase 9 signaling. Nat. Immunol. 21, 546–554 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Yamazaki T., Kirchmair A., Sato A., Buque A., Rybstein M., Petroni G., Bloy N., Finotello F., Stafford L., Navarro Manzano E., Ayala de la Pena F., Garcia-Martinez E., Formenti S. C., Trajanoski Z., Galluzzi L., Mitochondrial DNA drives abscopal responses to radiation that are inhibited by autophagy. Nat. Immunol. 21, 1160–1171 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Crawford E. D., Wells J. A., Caspase substrates and cellular remodeling. Annu. Rev. Biochem. 80, 1055–1087 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Pasparakis M., Vandenabeele P., Necroptosis and its role in inflammation. Nature 517, 311–320 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Wculek S. K., Cueto F. J., Mujal A. M., Melero I., Krummel M. F., Sancho D., Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 20, 7–24 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Yoon S., Kovalenko A., Bogdanov K., Wallach D., MLKL, the protein that mediates necroptosis, also regulates endosomal trafficking and extracellular vesicle generation. Immunity 47, 51–65.e7 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Li T., Chen Z. J., The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J. Exp. Med. 215, 1287–1299 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun L., Wu J., Du F., Chen X., Chen Z. J., Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yatim N., Jusforgues-Saklani H., Orozco S., Schulz O., Barreira da Silva R., Reis e Sousa C., Green D. R., Oberst A., Albert M. L., RIPK1 and NF-κB signaling in dying cells determines cross-priming of CD8+ T cells. Science 350, 328–334 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gong Y. N., Guy C., Olauson H., Becker J. U., Yang M., Fitzgerald P., Linkermann A., Green D. R., ESCRT-III acts downstream of MLKL to regulate necroptotic cell death and its consequences. Cell 169, 286–300.e16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samson A. L., Zhang Y., Geoghegan N. D., Gavin X. J., Davies K. A., Mlodzianoski M. J., Whitehead L. W., Frank D., Garnish S. E., Fitzgibbon C., Hempel A., Young S. N., Jacobsen A. V., Cawthorne W., Petrie E. J., Faux M. C., Shield-Artin K., Lalaoui N., Hildebrand J. M., Silke J., Rogers K. L., Lessene G., Hawkins E. D., Murphy J. M., MLKL trafficking and accumulation at the plasma membrane control the kinetics and threshold for necroptosis. Nat. Commun. 11, 3151 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sistigu A., Yamazaki T., Vacchelli E., Chaba K., Enot D. P., Adam J., Vitale I., Goubar A., Baracco E. E., Remédios C., Fend L., Hannani D., Aymeric L., Ma Y., Niso-Santano M., Kepp O., Schultze J. L., Tuting T., Belardelli F., Bracci L., La Sorsa V., Ziccheddu G., Sestili P., Urbani F., Delorenzi M., Lacroix-Triki M., Quidville V., Conforti R., Spano J.-P., Pusztai L., Poirier-Colame V., Delaloge S., Penault-Llorca F., Ladoire S., Arnould L., Cyrta J., Dessoliers M.-C., Eggermont A., Bianchi M. E., Pittet M., Engblom C., Pfirschke C., Préville X., Uzè G., Schreiber R. D., Chow M. T., Smyth M. J., Proietti E., André F., Kroemer G., Zitvogel L., Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat. Med. 20, 1301–1309 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Thapa R. J., Ingram J. P., Ragan K. B., Nogusa S., Boyd D. F., Benitez A. A., Sridharan H., Kosoff R., Shubina M., Landsteiner V. J., Andrake M., Vogel P., Sigal L. J., tenOever B. R., Thomas P. G., Upton J. W., Balachandran S., DAI senses influenza A virus genomic RNA and activates RIPK3-dependent cell death. Cell Host Microbe 20, 674–681 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Z., Wang Y., Zhang Y., He X., Zhong C. Q., Ni H., Chen X., Liang Y., Wu J., Zhao S., Zhou D., Han J., RIP3 targets pyruvate dehydrogenase complex to increase aerobic respiration in TNF-induced necroptosis. Nat. Cell Biol. 20, 186–197 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Pantelidou C., Sonzogni O., De Oliveria Taveira M., Mehta A. K., Kothari A., Wang D., Visal T., Li M. K., Pinto J., Castrillon J. A., Cheney E. M., Bouwman P., Jonkers J., Rottenberg S., Guerriero J. L., Wulf G. M., Shapiro G. I., PARP inhibitor efficacy depends on CD8+ T-cell recruitment via intratumoral STING pathway activation in BRCA-deficient models of triple-negative breast cancer. Cancer Discov. 9, 722–737 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andreeva L., Hiller B., Kostrewa D., Lassig C., de Oliveira Mann C. C., Jan Drexler D., Maiser A., Gaidt M., Leonhardt H., Hornung V., Hopfner K. P., cGAS senses long and HMGB/TFAM-bound U-turn DNA by forming protein-DNA ladders. Nature 549, 394–398 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Deng L., Liang H., Burnette B., Beckett M., Darga T., Weichselbaum R. R., Fu Y. X., Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Invest. 124, 687–695 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Newton K., Wickliffe K. E., Maltzman A., Dugger D. L., Reja R., Zhang Y., Roose-Girma M., Modrusan Z., Sagolla M. S., Webster J. D., Dixit V. M., Activity of caspase-8 determines plasticity between cell death pathways. Nature 575, 679–682 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Benci J. L., Johnson L. R., Choa R., Xu Y., Qiu J., Zhou Z., Xu B., Ye D., Nathanson K. L., June C. H., Wherry E. J., Zhang N. R., Ishwaran H., Hellmann M. D., Wolchok J. D., Kambayashi T., Minn A. J., Opposing functions of interferon coordinate adaptive and innate immune responses to cancer immune checkpoint blockade. Cell 178, 933–948.e14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu H., Golji J., Brodeur L. K., Chung F. S., Chen J. T., deBeaumont R. S., Bullock C. P., Jones M. D., Kerr G., Li L., Rakiec D. P., Schlabach M. R., Sovath S., Growney J. D., Pagliarini R. A., Ruddy D. A., MacIsaac K. D., Korn J. M., McDonald E. R. III, Tumor-derived IFN triggers chronic pathway agonism and sensitivity to ADAR loss. Nat. Med. 25, 95–102 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Chen J., Cao Y., Markelc B., Kaeppler J., Vermeer J. A., Muschel R. J., Type I IFN protects cancer cells from CD8+ T cell-mediated cytotoxicity after radiation. J. Clin. Invest. 129, 4224–4238 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li W., Lu L., Lu J., Wang X., Yang C., Jin J., Wu L., Hong X., Li F., Cao D., Yang Y., Wu M., Su B., Cheng J., Yang X., Di W., Deng L., cGAS-STING-mediated DNA sensing maintains CD8+ T cell stemness and promotes antitumor T cell therapy. Sci. Transl. Med. 12, eaay9013 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials and Methods
Table S1
Figs. S1 to S9