Abstract
Two hundred ninety isolates of Escherichia coli were investigated for the production of extended-spectrum β-lactamases (ESBLs). Fourteen (4.8%) of the 290 strains were found to produce ESBLs. Each of the 14 strains produced one or two ESBLs, as follows: 10 strains produced TEM-52, 1 strain produced SHV-2a, 1 strain produced SHV-12, 1 strain produced a CMY-1-like enzyme, and 1 strain expressed SHV-2a and a CMY-1-like enzyme. Another two strains for which the MICs of ceftazidime and cefoxitin were high, were probable AmpC enzyme hyperproducers. Because of the high prevalence of TEM-52 in E. coli isolates, we further investigated the TEM-type ESBLs produced by Klebsiella pneumoniae in order to observe the distribution of TEM-52 enzymes among Enterobacteriaceae in Korea. All TEM enzymes produced by 12 strains of K. pneumoniae were identified as TEM-52. To evaluate the genetic relatedness among the organisms, ribotyping of TEM-52-producing E. coli and K. pneumoniae was performed. The ribotyping profiles of the organisms showed similar but clearly different patterns. In conclusion, TEM-52 is the most prevalent TEM-type ESBL in Korea.
Extended-spectrum β-lactamases (ESBLs) in gram-negative organisms have been implicated as the enzymes responsible for resistance to β-lactam antibiotics such as cefotaxime, ceftazidime, and aztreonam (4, 10). Since these enzymes were first identified in western Europe, more than 70 ESBLs have been found worldwide (5, 10). ESBLs such as plasmid-encoded class A TEM- and SHV-type enzymes have been developed from successive mutations in their structural genes, resulting in either single or multiple amino acid changes in the encoded enzymes (4, 5, 10, 15).
The incidence of resistance to extended-spectrum β-lactam antibiotics is increasing in Korea. We have previously reported that SHV-12 and SHV-2a have been the most commonly identified ESBLs, and CMY-1, a plasmid-encoded AmpC enzyme, is found frequently in Klebsiella pneumoniae isolates (14). However, Escherichia coli isolates usually show different resistance patterns for oxyimino-cephalosporins and monobactam, yet no data have been published about the prevalence and characteristics of ESBLs among isolates of E. coli in Korea. Thus, we carried out a survey of ESBL-producing clinical isolates of E. coli from two major Korean hospitals to assess the prevalence of ESBLs and to investigate the ESBL-producing isolates for phenotypical and genotypical characteristics. To further study the TEM-type ESBLs in Enterobacteriaceae, we also characterized the TEM-type ESBLs produced by K. pneumoniae clinical isolates.
(This report was presented at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, 24 to 27 September 1998 [21a].)
MATERIALS AND METHODS
Bacterial strains.
Two hundred ninety strains of E. coli were collected in Ulsan University Hospital and Seoul National University Hospital during July and August 1997. The strains were initially isolated and identified in the microbiology laboratories, and E. coli isolates collected from different patients during the period were included in this study. For K. pneumoniae, we selected 13 isolates which were TEM enzyme producers, screened by isoelectric focusing (IEF) and TEM-specific PCR (see below), from among 53 ESBL-producing isolates which we have investigated before (14). Those strains of K. pneumoniae were collected from clinical laboratories at Dankook University Hospital and Seoul National University Hospital during March and April 1996 and at Yonsei University Hospital in 1994 and 1996.
E. coli J53 Azir was used as a recipient for mating experiments (11). Strains carrying plasmids encoding β-lactamases TEM-1 (R1), TEM-3 (pCFF04), TEM-4 (pUD16), SHV-2 (pMG229), SHV-5 (pAFF2), and CMY-1 (pMVP-1) served as the IEF standards (3, 11), and strains carrying plasmid RP4 or R6K were used as controls for plasmid-sizing studies (9, 22).
Antibiotics.
Antimicrobials tested were ampicillin and cephalothin (Young Jin Pharmaceutical Co., Seoul, Korea), cefoxitin and imipenem (Choongwae Pharma Co., Seoul, Korea), cefotaxime (Handok Pharmaceuticals Co., Seoul, Korea), ceftazidime (Glaxo Korea Co., Seoul, Korea), aztreonam (Dong-A Biotech Co., Seoul, Korea), clavulanic acid (Il-Sung Pharmaceuticals, Seoul, Korea), cloxacillin (Yuhan Co., Seoul, Korea), and moxalactam (Il Dong Pharma Co., Seoul, Korea).
Susceptibility tests and double-disk synergy test.
The MICs of the antibiotics were determined by agar dilution on Mueller-Hinton agar (Difco, Detroit, Mich.) with a Steers multiple inoculator according to the approved method of the National Committee for Clinical Laboratory Standards (19). E. coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as quality reference strains.
Double-disk synergy tests were performed with 30-μg cefotaxime, 30-μg ceftazidime, 30-μg aztreonam, and amoxicillin-clavulanic acid (amoxicillin at 20 μg and clavulanic acid at 10 μg) disks on unsupplemented Mueller-Hinton agar plates (Difco), and the results were interpreted as described previously (12). Aztreonam disks and amoxicillin-clavulanic acid disks (30 μg each) were purchased from Oxoid, Basingstoke, Hampshire, England, and 30-μg disks containing cefotaxime and ceftazidime were purchased from Becton Dickinson Microbiology Systems, Cockeysville, Md. Moxalactam disks were made by adding moxalactam (40 μg/20 μl/disk) to blank disks (Becton Dickinson).
Analytical IEF and enzyme inhibition assay.
Crude preparations of β-lactamases from clinical isolates or their transconjugants were obtained by two sonications for 30 s each time at an amplitude of 12 μm, with intermittent cooling on ice, in 0.1 M phosphate buffer (pH 7.0) (6). IEF was performed by the method of Matthew et al. (18) with an LKB Multiphor apparatus on prepared PAG plates (pH 3.5 to 9.5; Pharmacia Biotech Asia Pacific, Hong Kong) or with a Mini IEF cell system (Bio-Rad, Hercules, Calif.). Enzyme activities were detected by overlaying the gel with 0.5 mM nitrocefin in 0.1 M phosphate buffer, pH 7.0. β-Lactamases were identified by comparison to reference enzymes run in tracks adjacent to the test samples.
Inhibition assay was performed by overlaying the gels with 0.5 mM nitrocefin with and without 0.3 mM cloxacillin or 0.3 mM clavulanic acid in 0.1 M phosphate buffer, pH 7.0 (8).
Transfer of resistance and plasmid analysis.
Logarithmic-phase cells of each isolate were mated with similar cultures of E. coli J53 Azir on Trypticase soy agar plates (Becton Dickinson). After overnight incubation, transconjugants were selected on Trypticase soy agar containing 100 μg of sodium azide (Sigma, St. Louis, Mo.) per ml and 10 μg of ceftazidime or 64 μg of cefoxitin per ml (11). To confirm the presence of plasmids and to estimate their sizes, plasmids from clinical isolates and transconjugants were extracted by the method of Kado and Liu (13) and electrophoresed at 100 V for 4 h in a 0.7% agarose gel (Sigma).
TEM- or SHV-specific PCR and DNA sequencing.
To amplify TEM-related genes from clinical isolates or their transconjugants, the following oligonucleotide primers were synthesized and used for PCR: T1 (5′-ATA AAA TTC TTG AAG ACG AAA-3′) and T2 (5′-GAC AGT TAC CAA TGC TTA ATC-3′). The PCR amplification was carried out as described elsewhere (16). For SHV-specific PCR, primers S1 (5′-TGG TTA TGC GTT ATA TTC GCC-3′) and S2 (5′-GGT TAG CGT TGC CAG TGC T-3′), corresponding to nucleotides 120 to 140 and 990 to 972, respectively, of the SHV gene, were synthesized, and the PCR amplification was carried out as described elsewhere (14). The amplified PCR products were purified with a QIAEX gel extraction kit (Qiagen, Chatsworth, Calif.) and were used as templates for sequencing with a dideoxy termination cycle sequencing kit (Perkin-Elmer Cetus, Norwalk, Conn.).
CMY-1-specific PCR and Southern hybridization.
To amplify CMY-1-related genes from clinical isolates or their transconjugants, the primers C1 (5′-ATG CAA CAA CGA CAA TCC ATC-3′) and C2 (5′-GTT GGG GTA GTT GCG ATT GG-3′), corresponding to nucleotides 1 to 21 and 1098 to 1078, respectively, of the CMY-1 gene, were used (14). After agarose gel electrophoresis, PCR products were transferred to a nylon membrane by the vacuum transfer method as described previously (17) and hybridized with a digoxigenin (DIG DNA labelling and detection kit; Boehringer Mannheim, Mannheim, Germany)-labelled probe [the probe was a PCR product of the blaCMY-1 gene of E. coli C600 R+(pMVP-1) (3)].
Ribotyping.
The chromosomal DNA of the organisms was extracted and purified as described previously (17). The DNA of E. coli was digested by EcoRI or HindIII restriction enzyme (Promega, Madison, Wis.) (25). EcoRI or BstEII restriction enzyme was used for restriction of K. pneumoniae DNA (2, 20). The digests of chromosomal DNA were electrophoresed at 35 V for 14 h in a 0.8% agarose gel, transferred to a nylon membrane by the vacuum transfer method, and then hybridized with a digoxigenin-labelled cDNA copy of E. coli rRNA (Boehringer Mannheim) manufactured by reverse transcription with avian myeloblastosis virus reverse transcriptase (Promega).
RESULTS
Isolate selection.
The 290 strains of E. coli were screened for susceptibility to ceftazidime. We selected the isolates for which the MIC of ceftazidime was ≥2 μg/ml for further study. Among the 290 strains, there were 19 isolates for which the MIC of ceftazidime was ≥2 μg/ml, and we tested those isolates by a double-disk synergy test. Clavulanic acid enhanced the oxyimino-β-lactam susceptibilities of 13 of the 19 isolates. Three out of the six strains which showed no clavulanic acid synergy were included in this study because the MICs of ceftazidime and cefoxitin for them were high. For K. pneumoniae, we studied 13 isolates which were TEM-type enzyme producers, screened by IEF and TEM-specific PCR, from among 53 ESBL-producing isolates which we have studied before (14). Thus, a total of 16 isolates of E. coli and 13 strains of K. pneumoniae were further investigated.
IEF and inhibition test.
In an attempt to classify the β-lactamases produced by each isolate, the isoelectric points of the β-lactamases were determined by IEF. Among the 13 strains of E. coli whose susceptibilities to oxyimino-β-lactams were enhanced by clavulanic acid, 10 produced β-lactamases with a pI of 5.9 and 3 isolates expressed other enzymes with pIs of 5.4 and 8.2 (Ec133); 7.6 (Ec145); and 5.4, 7.6, and 8.0 (Ec132). Of the three strains for which the MICs of ceftazidime and cefoxitin were high but which gave a negative double-disk synergy test result, one strain (Ec108) produced β-lactamases with pIs of 5.4 and 8.0 and two strains (Ec12 and Ec102) produced β-lactamases with pIs of 8.5 and >8.5, respectively. To classify the β-lactamases with pIs of 8.0, 8.5, and >8.5, which were suspected to mediate cefoxitin and ceftazidime resistance in the isolates, we tested β-lactamase inhibition by overlaying the IEF gels with 0.5 mM nitrocefin with and without 0.3 mM cloxacillin or 0.3 mM clavulanic acid in 0.1 M phosphate buffer, pH 7.0. The β-lactamases with pIs of 8.0, 8.5, and >8.5 were all inhibited by 0.3 mM cloxacillin but not by 0.3 mM clavulanic acid; thus, those enzymes were considered AmpC enzymes belonging to Bush-Jacoby-Medeiros group 1 (4).
Among the 13 K. pneumoniae isolates, 12 strains produced β-lactamases with a pI of 5.9. Four of these strains expressed other kinds of β-lactamases in addition to pI 5.9 β-lactamases, as follows: two strains (KpS18 and KpY5) expressed the pI 8.0 AmpC enzymes and two strains (KpY25 and KpY26) expressed the pI 8.2 β-lactamases. Another strain (KpS36) expressed β-lactamases with pIs of 7.6 and 5.4.
On the basis of the pIs of the β-lactamases and their profiles of inhibition by cloxacillin or clavulanic acid, we tentatively classified the β-lactamases of the isolates into TEM-related, SHV-related, and AmpC enzymes as follows: pI of 5.4 or 5.9, TEM related; pI of 7.6 or 8.2, SHV related; and pI of 8.0, 8.5, or >8.5, AmpC enzymes.
Transfer of resistance and plasmid analysis.
To test the transmissibility of the ceftazidime or cefoxitin resistance of the isolates, a conjugation experiment was performed with E. coli J53 Azir as a recipient and with selective media containing 100 μg of sodium azide and either 10 μg of ceftazidime or 64 μg of cefoxitin per ml. For E. coli strains, ceftazidime resistance was transferred from 10 of 10 isolates which produced β-lactamases with a pI of 5.9 and from 2 of 2 isolates producing pI 7.6 β-lactamases. However, cefoxitin resistance was transferred in one strain producing a pI-8.0 AmpC enzyme (Ec108). Electrophoresis of the plasmid DNAs from each individual E. coli wild type and its transconjugant showed that transconjugants acquired plasmids with sizes ranging from 41 to 135 kb (Table 1). However, we did not further clarify the plasmids harboring β-lactamase genes in three strains (Ec111, Ec118, and Ec108) which transferred two different-sized plasmids on conjugation.
TABLE 1.
Antimicrobial susceptibilities, IEF of β-lactamases, and plasmid sizes of E. coli clinical isolates and their transconjugants
| Strain | MIC (μg/ml)a
|
Isoelectric point(s) | Plasmid size(s) (kb) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PIP | CEP | CFX | CTX | CAZ | ATM | IMP | |||
| Ec11 | >256 | 32 | 8 | 16 | 16 | 8 | <0.5 | 5.9 | 85, 63 |
| Transconjugant | 16 | 8 | 4 | 8 | 8 | 2 | <0.5 | 5.9 | 63 |
| Ec1 | 16 | 32 | 4 | 32 | 32 | 16 | <0.5 | 5.4, 5.9 | 115, 102 |
| Transconjugant | 16 | 16 | 4 | 16 | 16 | 4 | <0.5 | 5.9 | 102 |
| Ec15 | >256 | 32 | 4 | 32 | 32 | 8 | <0.5 | 5.9 | 103 |
| Transconjugant | >256 | 32 | 4 | 32 | 32 | 8 | <0.5 | 5.9 | 103 |
| Ec7 | >256 | 16 | 8 | 32 | 16 | 4 | <0.5 | 5.4, 5.9 | 130, 90 |
| Transconjugant | >256 | 8 | 4 | 8 | 8 | 4 | <0.5 | 5.4, 5.9 | 130 |
| Ec8 | >256 | 16 | 8 | 32 | 16 | 4 | <0.5 | 5.4, 5.9 | 130 |
| Transconjugant | >256 | 16 | 8 | 8 | 8 | 4 | <0.5 | 5.4, 5.9 | 130 |
| Ec101 | 128 | 16 | 16 | 8 | 8 | 2 | <0.5 | 5.4, 5.9 | 103 |
| Transconjugant | 128 | 16 | 32 | 8 | 8 | 2 | <0.5 | 5.4, 5.9 | 103 |
| Ec106 | >256 | 32 | 16 | 32 | 32 | 8 | 1 | 5.4, 5.9 | 91 |
| Transconjugant | >256 | 16 | 16 | 32 | 16 | 8 | <0.5 | 5.4, 5.9 | 91 |
| Ec111 | >256 | 64 | 16 | 32 | 64 | 16 | <0.5 | 5.4, 5.9 | 135, 97 |
| Transconjugant | >256 | 64 | 16 | 32 | 64 | 16 | <0.5 | 5.4, 5.9 | 135, 97 |
| Ec118 | 256 | 16 | 32 | 16 | 16 | 8 | <0.5 | 5.4, 5.9 | 103, 84 |
| Transconjugant | 128 | 8 | 4 | 32 | 16 | 4 | <0.5 | 5.4, 5.9 | 103, 84 |
| Ec147 | >256 | 64 | 32 | 128 | 128 | 64 | <0.5 | 5.4, 5.9 | 63, 47 |
| Transconjugant | 128 | 2 | 4 | 8 | 8 | 2 | <0.5 | 5.4, 5.9 | 47 |
| Ec133 | >256 | >256 | 32 | 128 | >256 | >256 | 4 | 5.4, 8.2 | 77, 44 |
| Transconjugant | 128 | 32 | 8 | 4 | 16 | 16 | <0.5 | 5.4 | 44 |
| Ec145 | 256 | 1 | 16 | 2 | 1 | 1 | <0.5 | 7.6 | 59, 41 |
| Transconjugant | 16 | 0.25 | 16 | 1 | 2 | 0.25 | <0.5 | 7.6 | 41 |
| Ec132 | >256 | 32 | >256 | 64 | 32 | 8 | 1 | 5.4, 7.6, 8.0 | 104, 56 |
| Transconjugant | >256 | 16 | 4 | 8 | 16 | 4 | <0.5 | 5.4, 7.6 | 104 |
| Ec108 | >256 | 32 | >256 | 256 | 32 | 4 | 0.5 | 5.4, 8.0 | 77, 52 |
| Transconjugant | >256 | 8 | 256 | 128 | 8 | 2 | <0.5 | 5.4, 8.0 | 77, 52 |
| Ec12 | >256 | 64 | 256 | 32 | 64 | 8 | 2 | 5.4, 8.5 | 71, 50 |
| Ec102 | >256 | 128 | 64 | 256 | 128 | 64 | 2 | 5.4, >8.5 | 64 |
Abbreviations: PIP, piperacillin; CEP, cephalothin; CFX, cefoxitin; CTX, cefotaxime; CAZ, ceftazidime; ATM, aztreonam; IMP, imipenem.
For the strains of K. pneumoniae, the transfer of ceftazidime resistance was achieved for 11 of 12 isolates which produced pI-5.9 enzymes (transfer was not achieved for KpY26). On electrophoresis of plasmid DNAs, we found that the transconjugants acquired ceftazidime resistance via a plasmid with a size of 71 to 100 kb (Table 2).
TABLE 2.
Antimicrobial susceptibilities, IEF of β-lactamases, and plasmid sizes of K. pneumoniae clinical isolates and their transconjugants
| Strain | MIC (μg/ml)a
|
Isoelectric point(s) | Plasmid size(s) (kb) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AMP | PIP | CEP | CFX | CTX | CAZ | ATM | IMP | |||
| KpY25 | >512 | >512 | 512 | 16 | 64 | 256 | 512 | <1 | 7.6, 5.9, 8.2 | |
| Transconjugant | >512 | 128 | 8 | 4 | 2 | 2 | <1 | 5.9 | 77 | |
| KpS15 | >512 | >512 | 512 | 8 | 64 | 32 | 32 | <1 | 7.6, 5.4, 5.9 | |
| Transconjugant | >512 | 64 | 16 | 4 | 4 | 4 | 2 | 5.9 | 77 | |
| KpB35 | >512 | 512 | 256 | 4 | 32 | 16 | 4 | <1 | 7.6, 5.4, 5.9 | |
| Transconjugant | >512 | >512 | 32 | 4 | 32 | 8 | 8 | 5.4, 5.9 | 81 | |
| KpY36 | >512 | >512 | 64 | 4 | 16 | 16 | 16 | <1 | 7.6, 5.4, 5.9 | |
| Transconjugant | >512 | 512 | 32 | 4 | 8 | 4 | 2 | 5.4, 5.9 | 100 | |
| KpY9 | >512 | 512 | 128 | 4 | 64 | 8 | 4 | <1 | 7.6, 5.4, 5.9 | |
| Transconjugant | >512 | 512 | 32 | 4 | 16 | 4 | 2 | 5.4, 5.9 | 81 | |
| KpY10 | >512 | 512 | 128 | 4 | 32 | 8 | 8 | <1 | 7.6, 5.4, 5.9 | |
| Transconjugant | >512 | 128 | 4 | 4 | 4 | 4 | 2 | 5.4 5.9 | 81 | |
| KpY40 | >512 | >512 | 256 | 16 | 32 | 16 | 32 | <1 | 7.6, 5.4, 5.9 | |
| Transconjugant | >512 | 256 | 64 | 4 | 32 | 16 | 2 | 5.4, 5.9 | 100 | |
| KpS37 | >512 | >512 | 512 | 4 | <1 | 8 | <1 | <1 | 7.6, 5.4, 5.9 | |
| Transconjugant | >512 | >512 | 512 | 32 | <1 | 8 | 2 | 5.4, 5.9 | 71 | |
| KpY27 | >512 | 512 | 64 | 16 | 64 | 16 | 32 | <1 | 7.6, 5.4, 5.9 | |
| Transconjugant | >512 | 128 | 16 | 4 | 4 | 8 | 1 | 5.4, 5.9 | 100 | |
| KpS36 | >512 | 512 | 512 | 4 | 32 | 16 | 8 | <1 | 7.6, 5.4 | |
| Transconjugant | >512 | >512 | 128 | 4 | 16 | 8 | 2 | 7.6, 5.4 | 71 | |
| KpS18 | >512 | 512 | >512 | >512 | 128 | 128 | 512 | <1 | 7.6, 5.4, 5.9, 8.0 | |
| Transconjugant | >512 | 128 | 32 | 4 | 4 | 8 | 2 | 5.4, 5.9 | 100 | |
| KpY5 | >512 | 512 | >512 | >512 | >512 | 512 | 512 | 32 | 7.6, 5.4, 5.9, 8.0 | |
| Transconjugant | >512 | 512 | 32 | 4 | 16 | 8 | 2 | 5.4, 5.9 | 100 | |
| KpY26 | >512 | 512 | 128 | 8 | 64 | 256 | 512 | <1 | 5.9, 7.6, 8.2 | |
Abbreviations: PIP, piperacillin; CEP, cephalothin; CFX, cefoxitin; CTX, cefotaxime; CAZ, ceftazidime; ATM, aztreonam; IMP, imipenem.
MICs of β-lactam antibiotics.
In order to find an antibiotic resistance mediated by ESBLs, we determined and compared the MICs of β-lactams for each individual E. coli wild type and its transconjugant (Table 1). For eight strains of E. coli which produced β-lactamases with pIs of 5.4 and 5.9, the MICs at which 50% of the isolates were inhibited for cefoxitin, cefotaxime, ceftazidime, and aztreonam were 16, 32, 32, and 8 μg/ml, respectively. The MICs of the β-lactams for the transconjugants were similar to those for each wild-type isolate except Ec147. We suspected that other mechanisms of resistance besides ESBL production, such as a change of permeability, might play a role in the antimicrobial resistance of Ec147. The isolate producing a β-lactamase with a pI of 8.2 (Ec133) expressed a high level of resistance to ceftazidime and aztreonam, but a strain producing a pI 7.6 ESBL (Ec145) had a relatively low level of resistance. For strains producing pI 8.0 enzymes (Ec132 and Ec108), the MIC of cefotaxime as well as cefoxitin was high. For another two strains which produced β-lactamases with pIs of 8.5 and >8.5, the MICs of cefoxitin and oxyimino-cephalosporins were relatively high, and the MICs of imipenem for these strains were also elevated.
The MICs for 13 strains of K. pneumoniae and their transconjugants were determined (Table 2). For eight strains producing β-lactamases with pIs of 5.4 and 5.9, the MICs of cefotaxime, ceftazidime, and aztreonam at which 50% of the isolates were inhibited were similar to those for eight E. coli strains which expressed the same enzymes: 32, 16, and 8 μg/ml. Two strains expressing β-lactamases with a pI of 8.2 showed a high level of resistance, especially to ceftazidime and aztreonam, and the presence of the pI 8.0 β-lactamases raised the MICs of cefoxitin up to >512 μg/ml for two strains.
PCR and sequencing of the blaTEM and blaSHV genes.
In order to identify the TEM-related enzymes, TEM-specific PCR and the nucleotide sequencing of blaTEM genes were performed for the strains or their transconjugants which produced pI 5.4 or 5.9 β-lactamases. blaTEM genes were amplified from 15 clinical isolates of E. coli and their transconjugants (all except Ec145). Using amplified PCR products, we sequenced the blaTEM genes of the transconjugants of Ec1, Ec11, and Ec15, which produced pI 5.9 β-lactamases, and two strains expressing pI 5.4 β-lactamases (Ec108 and Ec133). DNA sequencing and deduced amino acid sequence analysis revealed TEM-52-specific mutations in all three strains expressing pI 5.9 β-lactamases: lysine 104, threonine 182, serine 238 (numbering according to the scheme of Ambler et al. [1]) (Table 3) (23). The sequenced TEM-52 genes contained three additional silent point mutations within the open reading frames, compared to known sequences of TEM-1 genes (Table 3). The pI 5.4 β-lactamases in two strains (Ec108 and Ec133) were confirmed as TEM-1 by nucleotide sequencing. The genes for these TEM-1 enzymes harbored the same silent point mutations, and the TEM-1 gene of strain Ec133 showed an additional point mutation at position 211 (GCG → GCA; alanine). For K. pneumoniae, blaTEM genes were amplified from all 13 clinical isolates and their transconjugants by TEM-specific PCR. DNA sequencing of blaTEM genes was performed as described above for 11 of the 13 strains (i.e., all except KpY26 and KpS37). The sequences revealed that the β-lactamases with a pI of 5.9 were TEM-52, and the pI 5.4 enzyme produced by KpS36 was TEM-1. The blaTEM genes of K. pneumoniae strains had the same nucleotide changes as those of E. coli isolates.
TABLE 3.
Amino acid changes and silent point mutations of β-lactamase TEM-52 compared to TEM-1
| β-Lactamase | Amino acid at position:
|
Codon for residue:
|
||||
|---|---|---|---|---|---|---|
| 104 | 182 | 238 | Phe-8 | Gly-78 | Ala-134 | |
| TEM-1a | Glu (GAG) | Met (ATG) | Gly (GGT) | TTC | GGC | GCG |
| TEM-52 | Lys (AAG) | Thr (ACG) | Ser (AGT) | TTT | GGT | GCT |
Sequences are from reference 24.
To identify SHV-related genes, we performed SHV-specific PCR and nucleotide sequencing with three strains of E. coli or their transconjugants which produced pI 7.6 or 8.2 β-lactamases. The nucleotide sequencing revealed that the pI 7.6 β-lactamases from Ec145 and Ec132 were SHV-2a (glutamine 35 and serine 238; numbering according to the scheme of Ambler et al. [1]). In Ec133, expressing the pI 8.2 β-lactamase, SHV-12-specific mutations in the bla gene were detected (glutamine 35, serine 238, and lysine 240) (20).
Test of clavulanic acid enhancement of moxalactam activity in TEM-52-producing strains.
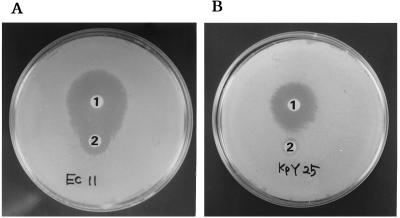
The TEM-52 enzyme had been reported to hydrolyze moxalactam (23). To find out whether our TEM-52-producing isolates had that characteristic feature, we performed a double-disk synergy test for TEM-52-producing isolates, using amoxicillin-clavulanic acid (amoxicillin at 20 μg and clavulanic acid at 10 μg) disks and 40-μg moxalactam disks. Synergies were observed between amoxicillin-clavulanic acid and moxalactam in TEM-52-producing strains (Fig. 1A). However, the strains which produced other enzymes as well as TEM-52 revealed a reduced clavulanic acid effect with the double-disk synergy test (Fig. 1B).
FIG. 1.
Detection of the synergy between moxalactam and amoxicillin-clavulanic acid. The bacterial strains were wild-type E. coli Ec11, producing TEM-52, and K. pneumoniae KpY25, producing TEM-52 and SHV-12. Disks: 1, 40 μg of moxalactam; 2, amoxicillin-clavulanic acid (amoxicillin at 20 μg and clavulanic acid at 10 μg).
Characterization of the AmpC β-lactamases.
Four strains which were resistant to cefoxitin and ceftazidime produced AmpC enzymes (Ec132, Ec108, Ec12, and Ec102). On conjugation to E. coli J53 Azir, Ec108 transferred cefoxitin resistance as described above. Because the AmpC enzymes produced by Ec108 and Ec132 showed a pI of 8.0, and one of the two strains transferred cefoxitin resistance, we suspected that the enzymes were plasmid-encoded CMY-1 enzymes. Thus, CMY-1-specific PCR was performed for Ec108, its transconjugant, and Ec132, and the blot of amplified PCR products was hybridized with a CMY-1 probe. This experiment showed that the pI 8.0 β-lactamase was CMY-1-like.
For the Ec12 and Ec102 strains, which produced the AmpC enzyme, with pIs of 8.5 and >8.5, respectively, cefoxitin resistance could not be transferred conjugatively. Attempt to transfer cefoxitin resistance after insertion of R641 transfer factor into the isolates was also unsuccessful. Therefore, these two isolates were presumptively considered AmpC hyperproducers.
Ribotyping pattern.
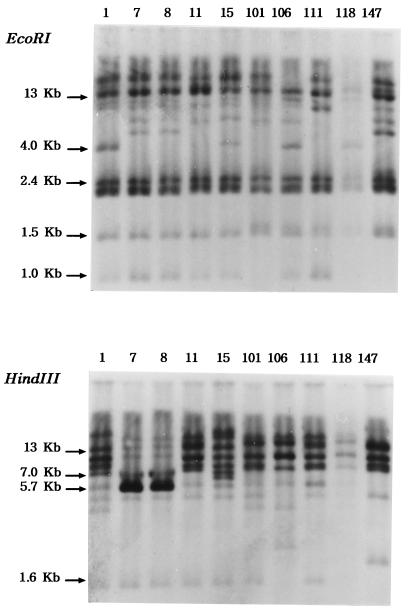
Clinical isolates of E. coli and K. pneumoniae in this study expressed only a single kind of TEM-type ESBL, TEM-52. Thus, the clonal spread of the organisms producing TEM-52 in or between the hospitals was suspected. In order to exclude the possibility of clonal spread, we performed ribotyping of E. coli and K. pneumoniae strains which produced TEM-52 ESBLs (Fig. 2). The ribotyping with chromosomal DNA digested with EcoRI and HindIII led to nine different patterns from 10 isolates of E. coli. Ec7 and Ec8, sharing the same ribotype, were isolated in the same hospital and harbored similar plasmids. The ribotyping of K. pneumoniae isolates which expressed TEM-52 revealed relationships between KpY9 and KpY10 and between KpY25 and KpY26. K. pneumoniae KpY9 and KpY10 were collected from Yonsei University Hospital in 1994, and KpY25 and KpY26 were collected from the same hospital in 1996. These data clearly showed that these organisms were not from single clone.
FIG. 2.
Ribotyping profiles of E. coli clinical strains which produced TEM-52 β-lactamases. Clinical isolates Ec7 and Ec8 revealed the same ribotyping pattern. The number above each lane is the isolate number (Table 1).
In conclusion, of the 290 E. coli isolates, 14 produced ESBLs and 2 were presumably AmpC enzyme hyperproducers. Thus, the prevalence of ESBL producers was 4.8% in E. coli isolates. Of the 14 ESBL-producing E. coli isolates, 10 produced TEM-52 β-lactamases (71%). All 12 isolates of K. pneumoniae producing TEM-type ESBLs produced TEM-52. With these data, we conclude that TEM-52 is the most prevalent ESBL in E. coli and the most prevalent TEM-type ESBL in K. pneumoniae in Korea.
DISCUSSION
The prevalence of ESBL producers among E. coli strains in this study was 4.8%. On the other hand, the prevalence of ESBL producers among K. pneumoniae strains in our previous study was 22% (21). The prevalence of β-lactamase producers among and the types of extended-spectrum β-lactamases produced by gram-negative bacilli vary from country to country and between institutions within a country. However, our data clearly showed that, in Korea, most TEM-type ESBLs from E. coli and K. pneumoniae were TEM-52. In addition, TEM-52 genes from different isolates had the same silent mutations. Another report also showed that most TEM enzymes had a pI of 5.9 in K. pneumoniae strains in Korea (7). However, blaTEM genes were not sequenced in that study. We have previously shown that most SHV-type ESBLs in K. pneumoniae isolated in Korea were SHV-2a or SHV-12 (14). In this study, three E. coli strains had the same SHV-type ESBLs, SHV-12 or SHV-2a. Another important plasmid-encoded enzyme, CMY-1, was also found in E. coli. Therefore, we now speculate that TEM-52, SHV-12, SHV-2a, and CMY-1 are the most common ESBLs in Korean isolates of Enterobacteriaceae.
SHV-12 enzymes have rarely been found in other countries. Two of 34 SHV-type ESBL-producing strains in Switzerland have been reported to produce SHV-12 (20). TEM-52 is rarely found in other countries and was only recently discovered. One strain producing this enzyme was found in France (23). It is interesting that the enzymes rarely found in other countries are the most prevalent in Korea. Moxalactam and cefotaxime were both available in the early 1980s in Korea, but ceftazidime began to be used in the early 1990s. The early exposure of the microorganisms to moxalactam and cefotaxime could partially explain the prevalence of TEM-52 in Korea.
TEM-52 conferred a relatively low level of resistance to cefotaxime and lower levels of resistance to ceftazidime and aztreonam. However, in a previous study this enzyme showed a very characteristic feature, moxalactam hydrolysis (23). The strains which produced TEM-52 enzymes clearly showed the synergy between clavulanic acid and moxalactam. Thus, with a positive double-disk synergy test with moxalactam and clavulanic acid, we could presumably predict the production of TEM-52 enzymes in the strains which produced pI 5.9 enzymes in Korea. However, because with the isolates which produce several kinds of enzymes the enhancement of moxalactam activity by clavulanic acid was reduced, the interpretation of double-disk synergy test results for those strains should be done carefully.
Another noteworthy finding was that the isolates collected from different hospitals during different periods had the same ESBL types. The reason for the common ESBL types in Enterobacteriaceae isolated in different hospitals during different periods remains to be determined. The ribotyping profiles of clinical isolates which produced TEM-52 ESBLs showed similar but clearly different patterns. Thus, clonal spread between hospitals was not probable. Plasmids or other movable elements might have spread among the clinical isolates through the patients, and the selective pressure by similar antimicrobial use in each hospital may have helped the organisms which contained these resistant plasmids to survive.
Although we studied a limited number of clinical strains, we think that TEM-52 is the most common ESBL in E. coli strains and the most common TEM-type ESBL in K. pneumoniae. These findings suggest that plasmids or other movable elements have spread among the organisms isolated in Korea. Therefore, more prudent use of antibiotics and control of the spread of these resistant organisms are necessary.
ACKNOWLEDGMENTS
We are very grateful to G. A. Jacoby, who provided the five strains carrying plasmids encoding TEM-1, TEM-3, TEM-4, SHV-2, and SHV-5 β-lactamases. We thank Y. Chong and K. Lee for giving us the strain producing CMY-1 β-lactamase and clinical isolates of K. pneumoniae. And we thank E. C. Kim for providing the clinical isolates of E. coli.
REFERENCES
- 1.Ambler R P, Coulson A F W, Frere J M, Ghuysen J M, Joris B, Forsman M, Levesque R C, Tiraby G, Waley S G. A standard numbering scheme for the class A β-lactamases. Biochem J. 1991;276:269–270. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arlet G, Rouveau M, Casin I, Bouvet P J M, Lagrange P H, Philippon A. Molecular epidemiology of Klebsiella pneumoniae strains that produce SHV-4 β-lactamase and which were isolated in 14 French hospitals. J Clin Microbiol. 1994;32:2553–2558. doi: 10.1128/jcm.32.10.2553-2558.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauernfeind A, Chong Y, Schweighart S. Extended broad spectrum β-lactamase in Klebsiella pneumoniae including resistance to cephamycins. Infection. 1989;17:316–321. doi: 10.1007/BF01650718. [DOI] [PubMed] [Google Scholar]
- 4.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bush K, Jacoby G. Nomenclature of TEM β-lactamases. J Antimicrob Chemother. 1997;39:1–3. doi: 10.1093/jac/39.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Chen H Y, Yuan M, Livermore D M. Mechanisms of resistance to β-lactam antibiotics amongst Pseudomonas aeruginosa isolates collected in the UK in 1993. J Med Microbiol. 1995;43:300–309. doi: 10.1099/00222615-43-4-300. [DOI] [PubMed] [Google Scholar]
- 7.Chong Y, Lee K, Ryoichi O, Inoue M. Characteristics of extended-spectrum β-lactam hydrolyzing activity of Klebsiella pneumoniae and Escherichia coli strains isolated from clinical specimens. Korean J Infect Dis. 1997;29:477–485. [Google Scholar]
- 8.Danel F, Hall L M C, Gur D, Livermore D M. OXA-15, an extended-spectrum variant of OXA-2 β-lactamase, isolated from a Pseudomonas aeruginosa strain. Antimicrob Agents Chemother. 1997;41:785–790. doi: 10.1128/aac.41.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Datta N, Kontomichalou P. Penicillinase synthesis controlled by infectious R factors in Enterobacteriaceae. Nature. 1965;208:239–244. doi: 10.1038/208239a0. [DOI] [PubMed] [Google Scholar]
- 10.Jacoby G A, Medeiros A A. More extended-spectrum β-lactamases. Antimicrob Agents Chemother. 1991;35:1697–1704. doi: 10.1128/aac.35.9.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacoby G A, Han P. Detection of extended-spectrum β-lactamases in clinical isolates of Klebsiella pneumoniae and Escherichia coli. J Clin Microbiol. 1996;34:908–911. doi: 10.1128/jcm.34.4.908-911.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarlier V, Nicolas M H, Fournier G, Philippon A. Extended broad-spectrum β-lactamase conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev Infect Dis. 1988;10:867–878. doi: 10.1093/clinids/10.4.867. [DOI] [PubMed] [Google Scholar]
- 13.Kado C I, Liu S-T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145:1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J, Kwon Y, Pai H, Kim J-W, Cho D-T. Survey of Klebsiella pneumoniae strains producing extended-spectrum β-lactamases: prevalence of SHV-12 and SHV-2a in Korea. J Clin Microbiol. 1998;36:1446–1449. doi: 10.1128/jcm.36.5.1446-1449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kliebe C, Nies B A, Meyer J F, Tolxdorff-Neutzling R M, Wiedemann B. Evolution of plasmid-coded resistance to broad-spectrum cephalosporins. Antimicrob Agents Chemother. 1985;28:302–307. doi: 10.1128/aac.28.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mabilat C, Goussard S. PCR detection and identification of genes for extended-spectrum β-lactamases. In: Persing D H, editor. Diagnostic molecular biology principles and applications. Rochester, Minn: Mayo Foundation; 1993. pp. 553–559. [Google Scholar]
- 17.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 18.Matthew M, Harris M, Marshall M J, Rose G W. The use of analytical isoelectric focusing for detection and identification of β-lactamases. J Gen Microbiol. 1975;88:169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 3rd ed. Approved standard M7-A3. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 20.Nüesch-Inderbinen M T, Kayser F H, Hächler H. Survey and molecular genetics of SHV β-lactamases in Enterobacteriaceae in Switzerland: two novel enzymes, SHV-11 and SHV-12. Antimicrob Agents Chemother. 1997;41:943–949. doi: 10.1128/aac.41.5.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pai H, Kim J, Kwon Y, et al. Characterization of extended-spectrum β-lactamases in Klebsiella pneumoniae isolated in Korea. Korean J Infect Dis. 1997;29:93–103. [Google Scholar]
- 21a.Pai H J, Yu S, Lee J H, Kim J M. Abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. Survey of extended-spectrum β-lactamases produced by Escherichia coli and Klebsiella pneumoniae: prevalence of TEM-52 in Korea, abstr. E-110; p. 202. [Google Scholar]
- 22.Paul G, Barthélémy M, Philippon A, Peduzzi J, Gilly L, Labia R, Névot P. Immunological comparison of constitutive β-lactamases of gram-negative bacteria by neutralization in zymogram gels: properties of anti-TEM-1 and anti-TEM-2 sera. Ann Inst Pasteur/Microbiol (Paris) 1988;139:435–451. [PubMed] [Google Scholar]
- 23.Poyart C, Mugnier P, Quesne G, Berche P, Trieu-Cuot P. A novel extended-spectrum TEM-type β-lactamase (TEM-52) associated with decreased susceptibility to moxalactam in Klebsiella pneumoniae. Antimicrob Agents Chemother. 1998;42:108–113. doi: 10.1128/aac.42.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutcliffe J G. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci USA. 1978;75:3732–3736. doi: 10.1073/pnas.75.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tarkka E, Ahman H, Siitonen A. Ribotyping as an epidemiologic tool for Escherichia coli. Epidemiol Infect. 1994;112:263–274. doi: 10.1017/s0950268800057678. [DOI] [PMC free article] [PubMed] [Google Scholar]