Abstract
Introduction
The clinical significance of Blastocystis sp. and Dientamoeba fragilis in patients with gastrointestinal symptoms is a controversial issue. Since the pathogenicity of these protists has not been fully elucidated, testing for these organisms is not routinely pursued by most laboratories and clinicians. Thus, the prevalence of these organisms and the subtypes of Blastocystis sp. in human patients in Turkey are not well characterized. This study aimed to determine the prevalence of Blastocystis sp. and D. fragilis in the diarrheic stool samples of immunodeficient and immunocompetent patients using conventional and molecular methods and to identify Blastocystis sp. subtypes using next generation sequencing.
Material and methods
Individual stool specimens were collected from 245 immunodeficient and 193 immunocompetent diarrheic patients between March 2017 and December 2019 at the Gazi University Training and Research Hospital in Ankara, Turkey. Samples were screened for Blastocystis sp. and D. fragilis by conventional and molecular methods. Molecular detection of both protists was achieved by separate qPCRs targeting a partial fragment of the SSU rRNA gene. Next generation sequencing was used to identify Blastocystis sp. subtypes.
Results
The prevalence of Blastocystis sp. and D. fragilis was 16.7% and 11.9%, respectively as measured by qPCR. The prevalence of Blastocystis sp. and D. fragilis was lower in immunodeficient patients (12.7% and 10.6%, respectively) compared to immunocompetent patients (21.8% and 13.5%, respectively). Five Blastocystis sp. subtypes were identified and the following subtype distribution was observed: ST3 54.4% (n = 37), ST2 16.2% (n = 11), ST1 4.4% (n = 3), ST6 2.9% (n = 2), ST4 1.5% (n = 1), ST2/ST3 11.8% (n = 8) and ST1/ST3 8.8% (n = 6). There was no statistically significant difference in the distribution of Blastocystis sp. subtypes between immunocompetent and immunodeficient patients.
Conclusion and recommendation
Our findings demonstrated that Blastocystis sp. and D. fragilis are commonly present in immunocompetent and immunodeficient patients with diarrhea. This study is the first to use next generation sequencing to address the presence of Blastocystis sp. mixed subtypes and intra-subtype variability in clinical samples in Turkey.
Author summary
Blastocystis sp. and Dientamoeba fragilis are single-cell parasites of the human intestine which are common worldwide and reported in cases with gastrointestinal symptoms. However, the role of Blastocystis sp. and D. fragilis in patients with gastrointestinal symptoms is still controversial because their presence is not always associated with symptoms. As some intestinal parasitic infections can cause severe illness in immunocompromised individuals careful consideration of intestinal protist infection is warranted. However, testing for Blastocystis sp. and D. fragilis is not routinely carried out by most laboratories and clinicians. Therefore, this study aimed to determine the prevalence of Blastocystis sp. and D. fragilis in immunocompetent and immunosuppressed patients with diarrhea by conventional and molecular methods. Both Blastocystis sp. and D. fragilis were detected more frequently by quantitative polymerase chain reaction than by conventional methods. Next generation sequencing was used to characterize the diversity and frequency of Blastocystis sp. subtypes and mixed subtypes in patients in Turkey. Five Blastocystis sp. subtypes (ST1, ST2, ST3, ST4 and ST6) were detected. ST3 was the most frequent subtype in both immunocompetent and immunosuppressed patients. Mono-infections were more common than mixed subtype infections. Our findings showed that Blastocystis sp. and D. fragilis are commonly present in immunocompetent and immunodeficient patients with diarrhea.
Introduction
Diarrhea is one of the most widespread gastrointestinal symptoms and is a common problem in immunosuppressed patients. The spectrum of pathogens that cause diarrhea in immunosuppressed patients is significantly different from those in patients with a normal immune system. In fact, in immunocompromised individuals, there is a higher risk for opportunistic pathogen infections. Such pathogens include Cryptosporidium spp. and Cystoisospora belli, which are classical opportunistic parasites commonly found in immunosuppressed diarrheal patients [1–5]. Blastocystis sp. and Dientamoeba fragilis are cosmopolitan intestinal protists commonly reported in people with and without symptoms [6–9]. Gastrointestinal symptoms, such as diarrhea, abdominal pain, and irritable bowel syndrome, have been associated with their infections/colonizations [10–12]. However, because both protists are also commonly observed in asymptomatic people, their clinical significance is still controversial [8, 12, 13]. The pathogenic potential of Blastocystis sp. and D. fragilis is not clear, but there are reports of their presence in immunocompromised individuals (cancer or HIV-infected patients) associated with gastrointestinal symptoms suggesting that they could be a relevant threat to immunocompromised populations [14, 15].
Blastocystis sp. is estimated to colonize more than one billion people worldwide [16]. Prevalence ranges of 0.5% to 100% from developing countries [17–23] and 1.2% to 35.2% from developed countries [24–28] have been reported. Currently, based on analysis of the small subunit (SSU) of the ribosomal RNA (rRNA) gene, 28 subtypes (STs) have been proposed in birds and mammals [7, 29–31]. Of those, 24 subtypes (ST1-ST17, ST21, ST23-ST28) are currently acknowledged as valid subtypes [30, 32]. Twelve subtypes (ST1-ST10, ST12, and ST14) have been found in humans with different levels of prevalence [33–38]. It has generally been reported that ST1-ST4 are more commonly seen in humans, whereas ST5-ST10, ST12, and ST14 in humans likely represent the consequence of a zoonotic transmission event. [33, 35–38].
Dientamoeba fragilis has been reported in humans with a worldwide distribution [12, 39]. Most studies have been conducted in industrialized countries where prevalence ranged from 0.3% to 82.9% [40–44]. Less is known from the developing world, but prevalence is reported to range from 0% to 60.6% [45–49]. There are two described genotypes of D. fragilis, named 1 and 2, which were defined using molecular analysis of restriction fragment length polymorphisms in the SSU rRNA gene [50]. Potential for zoonotic transmission has been suggested based on the few reports of D. fragilis in non-human hosts that include non-human primates (gorilla), pigs, and companion animals (dogs and cats). [51–55].
The most common parasitological examination methods used in clinical laboratories to detect Blastocystis sp. and D. fragilis are based on microscopy: direct smear (Native-Lugol examination), formalin-ethyl acetate concentration technique (FECT), and permanent staining. However, these methods are known to be insufficient for the definitive diagnosis of these two protists [56–59]. For Blastocystis sp., culture from stool samples are significantly more sensitive than direct microscopic examination for the detection, but stool cultures can be time consuming making them not practical for diagnosis when a quick turnaround is needed [56, 60]. Molecular techniques are progressively replacing microscopy for diagnosis of intestinal parasites, and they are the first-line diagnostic method in laboratories particularly in industrialized countries [61]. Molecular epidemiology studies of Blastocystis sp. and D. fragilis have clearly demonstrated that molecular screening methods are needed in accurately detecting the presence of these protists in stool samples [12, 56, 57, 62, 63]. The use of molecular methods to improve detection of Blastocystis sp. and D. fragilis in stool samples is crucial as it is challenging to identify these parasites by microscopy. By improving detection, we also improve our understanding of their epidemiology.
For Blastocystis sp., it is also key to identify subtypes and intra-subtype variability to understand its public health significance and pathogenicity. Several methods have been used to describe Blastocystis sp. mixed subtype infections. Next generation sequencing (NGS) provides a powerful tool for Blastocystis sp. detection that allows: subtype identification, detection of mixed subtypes within a sample, detection of low-abundance subtypes, and intra-subtype variations [8, 30, 64]. The aim of this study was to detect the prevalence of Blastocystis sp. and D. fragilis in immunocompetent and immunosuppressed patients with diarrhea by conventional and molecular methods. We also use NGS to characterize the prevalence of Blastocystis sp. subtypes and mixed subtypes in these patients. This is the first study to use next-generation sequencing technology to investigate Blastocystis sp. subtypes in Turkey.
Material and methods
Ethics statement
All study procedures, informed consent forms, and epidemiological questionnaires involved in the study were approved by the Ethics Commission of Gazi University (09.05.2017/05). Written informed consent was obtained from the participants.
Study population and collection of samples
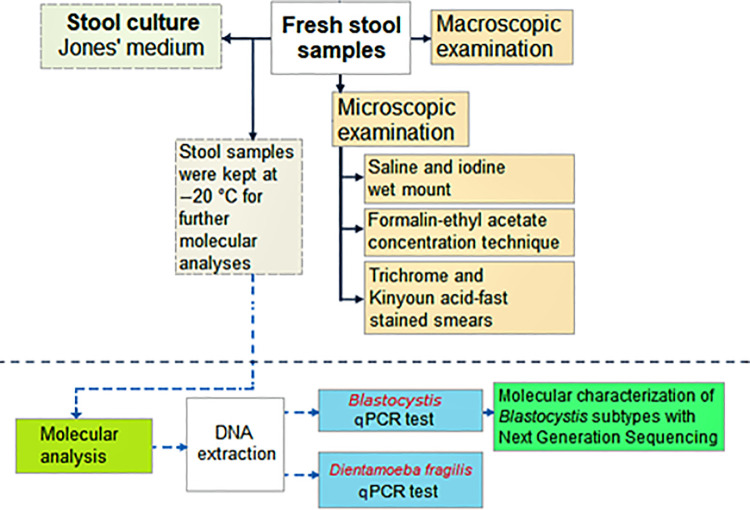
Individual stool samples from 438 outpatients were collected between March 2017 and December 2019 at the Gazi University Training and Research Hospital in Ankara, Turkey. The inclusion criteria for the study was to be adult patients (18 years and older) with diarrhea. Diarrhea was defined according to the Bristol Stool Form Scale. Stool samples were examined macroscopically and compared to the Bristol stool chart, and stools consistent with type 6 (fluffy pieces with ragged edges, a mushy stool) and type 7 (watery, no solid pieces, entirely liquid) were considered diarrhea [65]. Patients included in the immunodeficiency patient group were those patients treated at hematology, oncology, rheumatology, nephrology, and bone marrow transplantation units. Patients included in the immunocompetent patient group where those treated at gastroenterology and other clinics with the complaint of diarrhea and with no known immunodeficiency. Exclusions to participate in the study included patients who have inflammatory bowel disease, irritable bowel syndrome, urticaria, under antibiotic treatment, or those that had a colonoscopy in the last three months. Additionally, any patients found positive to Adenovirus and Rotavirus (RIDA QUICK Rotavirus/Adenovirus Combi test, R-biopharm, Germany) or Salmonella/Shigella (culture) were also excluded from the study. Of samples which met the criteria for inclusion in this study, 245 (56.3%) were immunodeficient diarrheal patients and 193 (43.7%) were immunocompetent diarrheal patients. Among immunodeficient patients, four were from rheumatology (immunosupresive treatment recipients), nine were from nephrology, 56 received bone marrow transplant, 70 were from medical oncology, and 106 were from hematology clinics (S1 Table). One hundred thirty-six (55.52%) patients with immunodeficiency were female and 109 (44.48%) were male. The age of the immunodeficient patients ranged from 18 to 85 years with a median age of 55 years. Immunocompetent patients consisted of 99 (51.30%) females and 94 (48.70%) males. The ages of the immunocompetent patients ranged from 1 to 84 years, and the median age was 41 years (p<0,001). All collected stool samples were independently screened for the presence of enteric parasites by conventional (Native-Lugol examination, FECT, trichrome, and acid-fast staining) and molecular methods for the detection of Blastocystis sp. and D. fragilis. A flow chart of parasite detection methods used in this study is depicted in Fig 1. Blastocystis sp. and D. fragilis positive stool samples were screened by ELISA test for E. histolytica, G. duodenalis and Cryptosporidium spp. according to manufacturer’s recommendations (E. HISTOLYTICA II, TECHLAB, USA; GIARDIA II, TECHLAB, USA; CRYPTOSPORIDIUM II, TECHLAB, USA, respectively).
Fig 1. Flow chart used to process stool specimens.
Diagram showing the flow of clinical samples, diagnostic, and molecular identification procedures followed in the present study.
Microscopy
Fresh stool samples were immediately examined microscopically by preparing saline and iodine wet mounts to test for the presence of enteric parasites. Saline and iodine wet mounts were prepared by mixing a small volume of stool with a drop of physiological saline or Lugol’s iodine (diluted 1:5 with distilled water) on a glass microscope slide and placing a coverslip over the mixture [66]. Entire coverslips were examined systematically at 10X and 40X magnification under a light microscope (CX31, Olympus, Japan).
The formalin-ethyl acetate concentration technique was conducted for all stool samples as previously described [67]. Briefly, for each sample, 3 ml of ethyl-acetate solution were added to 10 ml of filtered stool suspension and the tubes were vigorously shaken and centrifuged at 500 × g for 10 minutes. After centrifugation, the supernatant was discarded and the pellet was placed on a microscope slide, covered with a coverslip, and examined microscopically as described above. Additionally, trichrome and Kinyoun’s acid-fast stained smears were prepared from all stool samples, after parasite concentration, and examined using a 100X immersion objective by screening a minimum of 200–300 fields [66].
Blastocystis sp. culture
Fresh stool specimens were cultured in 2 ml Eppendorf tubes containing Jones’ medium with 10% horse serum and incubated at 37°C for 48–72 hours then examined microscopically to detect Blastocystis sp. [60].
DNA extraction
Genomic DNA was isolated from all stool samples. First, approximately 200 mg of each fecal sample was lysed using a tissue homogenizer (Qiagen TissueLyser LT, Hilden, Germany) for 15 minutes by adding 200 mg of acid-washed glass beads prior to DNA extraction then processed according to the manufacturer’s recommendation using a QiaAmp DNA Stool mini-kit (Qiagen, Hilden, Germany). DNA was eluted in 100 μl elution buffer provided with the kit. DNA extraction was performed weekly. DNA was stored at -20°C until molecular analyses were performed.
Molecular detection of Blastocystis sp. and D. fragilis
All stool samples were tested by quantitative polymerase chain reaction (qPCR) for both Blastocystis sp. and D. fragilis every two weeks. Molecular detection of Blastocystis sp. was achieved by a qPCR method to specifically amplify a 118-bp fragment of the SSU rRNA gene of the parasite [68]. Amplification reactions (25 μl) contained 12.5 μl of PCR Master Mix (Thermo Fisher Scientific, Waltham, MA, USA), 0.5 μM of the primer pair Blasto_FWD_F5/Blasto_R_F2, 0.3 μM of probe (S2 Table), and 2 μl of template DNA. Cycling parameters were 95°C for 3 min, and 40 cycles of denaturation at 95°C for 15 s followed by annealing and extension at 57°C for 1 min.
Detection of D. fragilis was achieved by a qPCR protocol amplifying a 78-bp fragment of the SSU rRNA gene of the parasite [69]. Reaction mixes (25 μl) consisted of 12.5 μl PCR of Master Mix (Thermo Fisher Scientific), 0.5 μM of the primer pair DF3/DF4, 0.3 μM of probe (S2 Table), and 2 μl of template DNA. Cycling parameters were the same as for Blastocystis sp.: 95°C for 3 min, and 40 cycles of denaturation at 95°C for 15 s followed by annealing and extension at 57°C for 1 min. For all Blastocystis sp. and D. fragilis negative qPCR results, these DNAs were diluted tenfold with 1xTE buffer and then qPCR was repeated.
Positive (target DNA previously identified as Blastocystis sp. or D. fragilis by DNA sequencing) and negative (sterile water) controls were included in each run. Amplification reaction was carried out in a Rotor-Gene 6000 real-time cycler (Rotor-Gene Q, Germantown, MD, USA).
Molecular characterization of Blastocystis sp. subtypes
To detect Blastocystis sp., a ca. 500 base pair fragment of the Blastocystis sp. SSU rRNA gene, which contains a variable region suitable for subtyping, was amplified by PCR [70]. PCR products were analyzed using a QIAxcel (Qiagen, Valencia, CA, USA). All positive samples were used to conduct next generation amplicon sequencing and libraries were prepared as previously described [64]. Briefly, all positive samples were amplified by PCR using primers ILMN_Blast505_532F and ILMN_Blast998_1017R. These primers amplify a region of the SSU rRNA gene and are identical to Blast505_532F/Blast998_1017R [70], except for containing the Illumina overhang adapter sequences on the 5′ end. PCR conditions were as follows: 95°C for 4 min, 35 cycles of 95°C for 30 s, 54°C for 30s, and 72°C for 30 s, and a final elongation step at 72°C for 5 min. Each 25 μl PCR reaction contained 2.5 μl template DNA, 12.5 μl 2X KAPA HiFi HotStart ReadyMix (KAPABioSystems, Cape Town, South Africa), 2.5 μl BSA (0.1 g/10 ml),1 μM of each primer, 7.75 μl H2O, and 1.25 ul of BSA (0.1 g/10 ml). Final libraries were quantified using the Quant-iT dsDNA Broad-Range Assay Kit (ThermoFisher, Waltham, MA, USA) on a SpectraMax iD5 (Molecular Devices, San Jose, CA, USA) prior to normalization. A final pooled library concentration of 8 pM with 20% PhiX control was sequenced using Illumina MiSeq 600 cycle v3 chemistry (Illumina, San Diego, CA, USA). Paired-end reads were processed and analyzed with an in-house pipeline that uses the BBTools package v38.22 [71], VSEARCH v2.8.0 [72], and BLAST+ 2.7.1. After removing singletons, clustering, and the assignment of centroid sequences to operational taxonomic units (OTU) was performed within each sample at a 98% identity threshold. Only those OTUs with a minimum of 100 sequences were retained. All OTUs were assigned a Blastocystis sp. subtype based on the best match by BLAST search in the GenBank database. The nucleotide sequences for unique sequences obtained in this study have been deposited in GenBank under the accession numbers MW728054- MW728093.
Data analysis
Between-group differences in baseline characteristics and parasite prevalence were calculated using Pearson chi-square test, Fisher’s exact test, and Kruskal-Wallis rank test in STATISTICA 12.0 (StatSoft, Tulsa, OK, USA). Proportion confidence limits were carried out using software available at http://openepi.com/Proportion/Proportion.htm. Cohen’s kappa index for intertest agreement was calculated using software available at (http://openepi.com/DiagnosticTest/DiagnosticTest.htm). Kappa considered values < 0 as indicative of no agreement,0–0.20 as slight agreement, 0.21–0.40 as fair agreement, 0.41–0.60 as moderate agreement, 0.61–0.80 as substantial agreement, and 0.81–1 as almost perfect agreement [73]. Medians and means of cycle threshold (Ct) values were calculated and a two-tailed Student t test for comparison of medians was carried out using STATISTICA version 12.0. Odds ratios (OR) and their 95% confidence intervals (CI) were calculated by univariate and multivariable analyses using logistic regression models to assess the association between potential risk factors. Exact logistic regression was used to calculate univariate odds ratios to avoid division by zero (https://stats.blue/Stats_Suite/logistic_regression_calculator.html). The qPCR was considered the reference test to compare results obtained by conventional methods for detection of Blastocystis sp. and D. fragilis. The statistical significance level was considered p <0.05 for all analyses.
Results
A total of 438 diarrheal patients, including immunocompromised (n = 245) and immunocompetent (n = 193) were recruited to participate in this study. There was no statistically significance between the two groups according to sex. However, there was a difference between two groups according to age. The median age was higher in patients with immunodeficiency than in immunocompetent patients. This observation could be due to the emergence of immunodeficiency as age progresses. Microscopic examination of the samples allowed for identification of other parasitic or commensal protozoans present in the samples in addition to Blastocystis sp. and D. fragilis. Using conventional and/or molecular methods it was determined that 26.7% (n = 117) of 438 patients were infected with one or more intestinal parasites in this study (Table 1). Protists identified were: Blastocystis sp. (16.7%, n = 73), D. fragilis (11.9%, n = 52), Giardia duodenalis (0.7%, n = 3), Cryptosporidium spp. (0.7%, n = 3), and Chilomaxtix mesnili (0.2%, n = 1). The prevalence of patients positive for these parasites was 37.8% (n = 73) and (24.1%) (n = 59) in immunocompetent and immunocompromised diarrheal patients, respectively(p = 0.001). Information for each protist detected in immunocompetent and immunocompromised diarrheal patients is shown in Table 1.
Table 1. Distribution of Blastocystis sp., D. fragilis, and other parasites in immunocompetent and immunodeficient patients with diarrhea.
| Protists | Immunodeficient (n = 245) | Immunocompetent (n = 193) | Total (n = 438) | |||
|---|---|---|---|---|---|---|
| N | % (95% CI) | N | % (95% CI) | N | % (95% CI) | |
| Blastocystis sp.a | 31 | 12.7 (8.9–17.3) | 42 | 21.8 (16.4–28) | 73 | 16.7 (13.4–20.4) |
| Dientamoeba fragilis a | 26 | 10.6 (7.2–14.9) | 26 | 13.5 (9.2–18.9) | 52 | 11.9 (9.1–15.2) |
| Giardia duodenalis b,c | 1 | 0.4 (0.02–2.0) | 2 | 1.03 (0.2–3.4) | 3 | 0.7 (0.2–1.9) |
| Cryprosporidium spp.d | 1 | 0.4 (0.02–2.0) | 2 | 1.03 (0.2–3.4) | 3 | 0.7 (0.2–1.9) |
| Chilomastix mesnili b,c | - | 1 | 0.5 (0.03–2.5) | 1 | 0.2 (0.01–1.1) | |
| Total | 59 | 24.1 (19–29.7) | 73 | 37.8 (31.2–44.8) | 132 | 30.1 (25.9–34.6) |
CI: Confidence Interval
a Data obtained by qPCR.
b Data obtained by direct microscopy.
c Data obtained by trichrome stain.
d Data obtained by modified Kinyoun’s acid-fast stain.
Infection with two or more parasites was reported in 15 (3.4%) patients. The most frequent combination found was Blastocystis sp.+D. fragilis (80.2%, 12/15), followed by Blastocystis sp.+G. duodenalis (6.6%, 1/15), Blastocystis sp.+Cryptosporidium spp. (6.6%, 1/15) and Blastocystis sp.+C. mesnili (6.6%, 1/15) (S3 Table). The rate of co-infection with Blastocystis sp.+D. fragilis was 4.15% (8/193) in immunocompetent and 1.63% (4/245) in immunodeficient study participants (χ2 = 2.55, p = 0.11).
Comparison of methods used in the diagnosis of Blastocystis sp. and D. fragilis
The diagnostic performance of saline, Lugol’s iodine, FECT, trichrome stain, and culture were compared with qPCR (Table 2). Both Blastocystis sp. and D. fragilis were detected more frequently by qPCR than by any other detection method.
Table 2. Comparison of saline, Lugol’s iodine, formol ethyl acetate concentration technique (FECT), trichrome staining (TS) and culture methods for detecting Blastocystis sp. and D. fragilis. qPCR was used as the reference method for comparing methods by statistical analyses.
| Saline | Lugol’s iodine | FECT | TS | Culture | |
|---|---|---|---|---|---|
| Blastocystis sp. (n/N) | 48/438 | 50/438 | 46/438 | 47/438 | 55/438 |
| Sensitivity | 57.3% | 61.8% | 58.8% | 64.7% | 77.9% |
| Specificity | 97.6% | 97.8% | 98.4% | 99.2% | 99.5% |
| Positive Predictive Value | 81.2% | 84.0% | 87.0% | 93.6% | 96.4% |
| Negative Predictive Value | 92.6% | 93.3% | 92.9% | 93.9% | 96.1% |
| Cohen’s kappa (Unweighted) | 0.62 | 0.66 | 0.66 | 0.73 | 0.84 |
| Dientamoeba fragilis (n/N)a | 10/438 | ||||
| Sensitivity | - | - | - | 19.2% | - |
| Specificity | - | - | - | 99% | - |
| Positive Predictive Value | - | - | - | 71.4% | - |
| Negative Predictive Value | - | - | - | 90.1% | - |
| Cohen’s kappa (Unweighted) | 0.27 |
a Only TS and qPCR methods were used for detection of D. fragilis.
When comparing conventional methods with Blastocystis sp. qPCR, kappa value was detected as the highest only in the culture method (k = 0.84, perfect agreement). Dientamoeba fragilis apart from qPCR could only be detected in trichrome staining and the kappa value was found very low (k = 0.27, fair) (Table 2).
Occurrence of Blastocystis sp. and D. fragilis determined by qPCR
Blastocystis sp. was detected using qPCR in 73 patients, including 31 (12.7%) patients with immunodeficiency and 42 (21.8%) immunocompetent patients. The frequency of Blastocystis sp. was significantly higher in immunocompetent patients than in immunodeficient patients (χ2 = 6.40, p = 0.01) (Table 3). Multiple logistic regression analysis revealed that male gender category [χ2 = 4.78, odds ratio (OR) = 2.33, 95% confidential interval (CI): (1.08,5.03)] and 64–74 age category [χ2 = 14.43, OR = 8.80, 95% CI: (2.68,28.91)] were risk factors for Blastocystis sp. infection among immunocompetent patients (Table 3). With respect to age groups, Blastocystis sp. positivity was significantly higher only in the 64–74 age group in immunocompetent patients (χ2 = 14.43, p<0.001) (Table 3). There were no statistically significant differences in the distribution of total Blastocystis sp.-positive patients by season (χ2 = 1.04, SD = 3, p = 0.79) (Table 3).
Table 3. Distribution of the prevalence of Blastocystis sp. and Dientamoeba fragilis in immunodeficient (n = 245) and immunocompetent patients (n = 193) by gender, age and season (Statistically significant values have been highlighted in bold).
| Immunodeficient patients n/N (%) | Immunocompetent patients n/N (%) | χ 2 | OR (95% CI) | p valuea | |
|---|---|---|---|---|---|
| Blastocystis sp. | 31/245 (12.7) | 42/193 (21.8) | 6.40 | 1.92 (1.15–3.19) | 0.01 |
| Stratified by gender | |||||
| Male | 12/109 (11.0) | 21/94 (22.3) | 4.78 | 2.33 (1.08–5.03) | 0.03 |
| Female | 19/136 (14.0) | 21/99 (21.2) | 2.10 | 1.66 (0.84–3.28) | 0.15 |
| χ2 = 1.87, SD = 1, p = 0.17 | |||||
| Stratified by age category (years) | |||||
| 18–29 | 5/31 (16.1) | 8/57 (14.0) | 0.07 | 0.85 (0.25–2.86) | 0.79 |
| 30–40 | 4/25 (16.0) | 5/36 (11.1) | 0.05 | 0.85 (0.20–3.23) | 0.82 |
| 41–52 | 6/49 (12.2) | 7/31 (22.6) | 1.45 | 2.09 (0.63–6.94) | 0.23 |
| 53–63 | 8/68 (11.8) | 9/33 (24.2) | 3.61 | 0.36 (0.12–1.03) | 0.06 |
| 64–74 | 5/60 (8.33) | 12/27 (44.4) | 14.43 | 8.80 (2.68–28.91) | <0.001 |
| 75–85 | 3/12 (25.0) | 1/9 (11.1) | 0.68 | 0.38 (0.03–4,37) | 0.41 |
| χ2 = 2.14, SD = 5, p = 0.83 | |||||
| Stratified by seasons category | |||||
| Spring | 16/97 (16.5) | 17/84 (20.2) | 0.42 | 0.78 (0.37–1.66) | 0.52 |
| Summer | 2/47 (4.2) | 5/30 (16.7) | 3.34 | 4.50 (0.81–24.91) | 0.06 |
| Autumn | 4/52 (7.7) | 7/36 (19.4) | 2,64 | 0,35 (0.09–1,28) | 0.10 |
| Winter | 9/49 (18.3) | 13/43 (30.2) | 1.77 | 1.93 (0.73–5.09) | 0.19 |
| χ2 = 1.04, SD = 3, p = 0.79 | |||||
| Dientamoeba fragilis | 26/245 (10.6) | 26/193 (13.5) | 0.84 | 1.31 (0.73–2.34) | 0.36 |
| Stratified by gender | |||||
| Male | 11/109 (10.1) | 11/94 (11.7) | 0.14 | 1.18 (0.49–2.86) | 0.71 |
| Female | 15/136 (11.0) | 15/99 (15.2) | 0.86 | 1.44 (0.67–3.10) | 0.35 |
| χ2 = 0, SD = 1, p = 0.99 | |||||
| Stratified by age category (years) | |||||
| 18–29 | 5/31 (16.1) | 6/57 (10.5) | 0.56 | 0.61 (0.17–2.19) | 0.45 |
| 30–40 | 3/25 (12.0) | 7/36 (19.4) | 0.62 | 1.77 (0.41–7.63) | 0.44 |
| 41–52 | 7/49 (14.3) | 4/31 (12.9) | 0.03 | 0.89 (0.24–3.33) | 0.86 |
| 53–63 | 4/68 (5.9) | 3/33 (9.1) | 0.34 | 1.60 (0.34–7.60) | 0.55 |
| 64–74 | 7/60 (11.7) | 4/27 (14.8) | 0.16 | 1.32 (0.35–4.94) | 0.68 |
| 75–85 | -/12 (-) | 2/9 (22.2) | 3.67 | ||
| χ2 = 5.47, SD = 5, p = 0.36 | |||||
| Stratified by seasons category | |||||
| Spring | 17/97 (17.5) | 10/84 (11.9) | 1.14 | 0.64 (0.27–1.48) | 0.29 |
| Summer | 2/47 (4.3) | 2/30 (6.7) | 0.21 | 1.61 (0.21–12.06) | 0.64 |
| Autumn | 3/52 (5.8) | 6/36 (16.7) | 2.70 | 3.27 (0.76–14.04) | 0.11 |
| Winter | 4/49 (8.2) | 8/43 (18.6) | 2.22 | 2.57 (0.72–9.24) | 0.14 |
| χ2 = 4.15, SD = 3, p = 0.25 | |||||
a Multiple logistic regression analysis
Cycle threshold (Ct) values of Blastocystis sp.-positive patients with immunodeficiency ranged from 16.7 to 34.6 (median: 21.6), and immunocompetent patients ranged from 17 to 34.8 (median: 24.9). The median of Blastocystis sp. Ct values was found to be lower in women with immunodeficiency compared to immunocompetent women, indicating a higher parasite load in immunodeficient women (p = 0.04). However, differences of the median Blastocystis sp. Ct values were not found statistically significant between immunodeficient (median: 21.6) and immunocompetent (median:24.9) males (p = 0.40) (Table 4).
Table 4. Median of qPCR cycle threshold (Ct) values of Blastocystis sp. and D. fragilis (Statistically significant values have been highlighted in bold).
| Blastocystis sp. | Dientamoeba fragilis | |||||
|---|---|---|---|---|---|---|
| Immuno deficient | Immuno competent | pa | Immuno deficient | Immuno competent | pa | |
| Gender | ||||||
| Male | 26.7 | 23.5 | 0.26 | 32.0 | 32.2 | 0.69 |
| Female | 21.4 | 26.5 | 0.04 | 31.4 | 32.1 | 0.83 |
| Age | ||||||
| 18–40 | 20.2 | 25.2 | 0.42 | 30.8 | 32.2 | 0.55 |
| 41–63 | 21.5 | 26.5 | 0.16 | 31.6 | 33.1 | 0.96 |
| >64 | 25.2 | 23.5 | 0.35 | 31.6 | 25.1 | 0.07 |
| Total | 21.6 | 24.9 | 0.40 | 31.6 | 32.2 | 0.53 |
aT-test for Independent Samples
Dientamoeba fragilis was detected using qPCR in 52 patients, including 26 (11.4%) patients with immunodeficiency and 26 (20.7%) immunocompetent patients. The frequency of D. fragilis between the two groups was not significantly different (χ2 = 0,84, p = 0.36) (Table 3). Similar Ct values were found in D. fragilis-positive patients with immunodeficiency (16.5 to 34.4; median: 31.6) and in immunocompetent patients (15.1 to 34.7; median: 32.2) (p = 0.53) (Table 4).
Blastocystis sp. subtypes identified using NGS
Out of the 73 positive qPCR samples, only 68 isolates were positive when PCR was done to prepare NGS library. The remaining five samples were not sequenced using the MiSeq platform. Clustering yielded 40 unique Blastocystis sp. OTUs across the 68 Blastocystis sp.-positive samples (Table 5). Five Blastocystis sp. subtypes (ST1, ST2, ST3, ST4, and ST6) were found. ST4 was detected only in an immunocompetent patient.
Table 5. Blastocystis sp. subtypes identified by next generation sequencing including information about number of variants per subtype and patients ID in which they were found. Bold denotes intra-subtype variability.
| Subtype | No. of unique subtype variants | GenBank Accession number | No. of samples containing variant | Patients ID1 |
|---|---|---|---|---|
| ST1 | 8 | MW728059 | 3 | ID/F42, IY/F82, IY/F114 |
| MW728064 | 3 | ID/F18, ID/F91, ID/F99 | ||
| MW728065 | 1 | ID/F184 | ||
| MW728079 | 1 | IY/F78 | ||
| MW728086 | 1 | ID/F165 | ||
| MW728088 | 1 | ID/F165 | ||
| MW728091 | 1 | ID/F42 | ||
| MW728092 | 1 | ID/F184 | ||
| ST2 | 20 | MW728061 | 6 | ID/F68, ID/F104, ID/F157, ID/F170, IY/F124, IY/F180, |
| MW728078 | 4 | ID/F16, ID/F100, IY/F34, IY/F109 | ||
| MW728066 | 2 | ID/F87, ID/F150 | ||
| MW728070 | 2 | ID/F68, ID/F170 | ||
| MW728072 | 2 | ID/F16, IY/F5 | ||
| MW728076 | 2 | ID/F125, IY/F108 | ||
| MW728083 | 2 | ID/F125, IY/F5 | ||
| MW728067 | 1 | ID/F180 | ||
| MW728068 | 1 | ID/F173 | ||
| MW728071 | 1 | ID/F104 | ||
| MW728075 | 1 | IY/F185 | ||
| MW728077 | 1 | IY/F34 | ||
| MW728080 | 1 | IY/F109 | ||
| MW728082 | 1 | ID/F157 | ||
| MW728084 | 1 | IY/F108 | ||
| MW728084 | 1 | IY/F180 | ||
| MW728087 | 1 | ID/F100 | ||
| MW728089 | 1 | IY/F109 | ||
| MW728090 | 1 | ID/F173 | ||
| MW728093 | 1 | ID/F43 | ||
| ST3 | 9 | MW728054 | 27 | ID/F18, ID/F33, ID/F42, ID/F43, ID/F47, ID/F71, ID/F85, ID/F89, ID/F99, ID/F100, ID/F158, ID/F164, ID/F169, IY/F1, IY/F6, IY/F34, IY/F59, IY/F82, IY/F85, IY/F100, IY/F115, IY/F121, IY/F133, IY/F170, IY/F206, ID/F165, ID/F170 |
| MW728055 | 6 | ID/F115, ID/F133, ID/F188, ID/F204, IY/F78, IY/F156 | ||
| MW728056 | 6 | ID/F37, ID/F38, ID/F72, ID/F87, IY/F136, IY/F171 | ||
| MW728057 | 4 | ID/F16, ID/F45, IY/F26, IY/F74 | ||
| MW728058 | 3 | ID/F67, ID/F68, ID/F129 | ||
| MW728060 | 2 | ID/F159, ID/F185 | ||
| MW728062 | 1 | ID/F210 | ||
| MW728063 | 1 | ID/F63 | ||
| MW728081 | 1 | IY/F180 | ||
| ST4 | 1 | MW728074 | 1 | ID/F151 |
| ST6 | 2 | MW728069 | 1 | ID/F109 |
| MW728073 | 1 | IY/F174 |
ID/F: Immunocompetent patient group; IY/F: Immunodeficient patient group.
Mono-subtype infections were more common than mixed infections representing 79.4% (n = 54) and 20.6% (n = 14) of the positive samples, respectively (Tables 6 and S4). ST3 was observed in 54.4% (n = 37) of Blastocystis sp. positive patients and was the most common subtype observed. The prevalence of other subtypes was as follows: ST2: 16.2% (n = 11), ST1: 4.4% (n = 3), ST6: 2.9% (n = 2), and ST4: 1.5% (n = 1) (Table 6). While mixed subtypes were higher in immunocompetent patients (25.0%) than in immunodeficient patients (14.2%) the difference was not statistically significant. Mixed ST2/ST3 and ST1/ST3 were observed with ST2/ST3 (11.8%) being the most common subtype combination (Table 6).
Table 6. Distribution of Blastocystis sp. subtypes in the immunodeficient (n = 28) and immunocompetent (n = 40) patient groups.
| Subtype | Number immunodeficient patients (%) | Number of immunocompetent patients (%) | Total number of patients (%) | χ 2 | pa |
|---|---|---|---|---|---|
| ST1 | 1 (3.6) | 2 (5) | 3 (4.4) | 0.07 | 0.78 |
| ST2 | 6 (21.4) | 5 (12.5) | 11 (16.2) | 0.97 | 0.33 |
| ST3 | 16 (57.2) | 21 (52.5) | 37 (54.4) | 0.14 | 0.71 |
| ST4 | - | 1 (2.5) | 1 (1.5) | 0.71 | 0.39 |
| ST6 | 1 (3.6) | 1 (2.5) | 2 (2.9) | 0.07 | 0.80 |
| Total mixed subtypes | 4 (14.2) | 10 (25.0) | 14 (20.6) | 1.16 | 0.28 |
| ST1/ST3 | 2 (7.1) | 4 (10.0) | 6 (8.8) | 0.17 | 0.68 |
| ST2/ST3 | 2 (7.1) | 6 (15.0) | 8 (11.8) | 0.98 | 0.32 |
a Statistical analysis using Chi-Square Test for two-way tables.
Blastocystis sp. intra-subtype variability
Forty unique OTUs were detected among the five Blastocystis sp. subtypes identified in this study. A high degree of intra-subtype diversity was observed for ST1 and ST2 with eight unique OTUs among the nine ST1-positive samples and 20 unique OTUs among the 19 ST2-positive samples (Table 5). ST1 and ST2 Blastocystis sp. positive samples frequently contained multiple unique OTUs. In fact, up to three unique OTUs were detected in a single ST2 sample (Table 5). Interestingly, ST3 displayed low intra-subtype diversity relative to the number of positive isolates, with only nine unique OTUs among 51 ST3-positive samples. ST4 had one unique OTU in only one ST4-positive sample. ST6 had two unique OTUs among two ST6-positive samples.
Discussion
In the present study, Blastocystis sp. and D. fragilis were investigated in immunodeficient and immunocompetent diarrheal patients using conventional and molecular methods. This study demonstrates that the successful diagnosis of Blastocystis sp. and D. fragilis infections depends on the detection method. Using conventional methods such as direct smear, it is possible to overlook protists, especially when few organisms are present. In fact, D. fragilis was not detected in any samples by direct smear. In the diagnosis of Blastocystis sp., the sensitivity of direct smear, FECT, and trichrome smear was significantly lower than qPCR, while culture had a similar diagnostic accuracy when compared to qPCR. Our findings are in agreement with other studies that have also shown low sensitivity of microscopic methods to detect Blastocystis sp. and D. fragilis [56, 74]. Studies that compared direct microscopy, culture and qPCR for Blastocystis sp. detection have reported that qPCR was the most sensitive method [75, 76]. Similarly, a study comparing just culture and qPCR to detect Blastocystis sp. reported a higher sensitivity of qPCR [67]. However, the effectiveness of the culture method for Blastocystis sp. diagnosis has also been demonstrated by other researchers [77, 78] and the mini-culture method is a practical method especially for diagnostic laboratories with a limited budget [79].
In this study, the overall prevalence of Blastocystis sp. was 16.7% (n = 73/438) by qPCR. The prevalence of Blastocystis sp. has been reported to vary widely among studies ranging from 0.54% to 88.8% [20, 26, 47, 80–82]. Prevalence variations could be related to many factors such as studies conducted in different geographical regions, different populations (socio-economic level, immune status, age…), or use of different diagnostic methods for detection [34]. In Turkey, Blastocystis sp. is the most common gastrointestinal parasite reported and prevalence ranges between 0.5% to 37.9% (S5 Table) [18, 21, 83–90]. Blastocystis sp. carriage was detected in limited studies from the same province in Turkey as 14.2–14.6% in school children [91, 92] and 15.5% in adults [75]. Both the present and the other studies from Turkey indicate no apparent differences between symptomatic and asymptomatic cases, supporting the commensal nature of Blastocystis sp. [75, 91, 92].
Blastocystis sp. was observed in 20.7% and 11.4% of immunocompetent and immunodeficient diarrheal patients examined in this study, respectively. One limitation of this study is a lack of a healthy comparison group (no diarrhea) as all samples were collected from hospital patients presenting with diarrhea. Thus, no comparison can be made of Blastocysits prevalence between symptomatic and asymptomatic individuals. The prevalence of Blastocystis sp. observed in immunodeficient patients in this study is similar to prevalence reported in cancer patients in Turkey (6.5–10.8%) (S5 Table) [84, 93]. However, studies in some neighbour countries have reported higher Blastocystis sp. prevalence in patients with cancer ranging from 22.3 to 27.5% [14, 94]. In a prospective study in France using qPCR, similar prevalence of Blastocystis sp. was found in immunodeficient patients (16%;15/94) and immunocompetent patients (13%;12/92) [76]. In our study, immunocompetent group had a significantly higher Blastocystis sp. prevalence than immunocompromised group. It is possible that immunocompromised patients that are under constant medical monitoring tend also to avoid contact with the external environment and behave carefully making them less likely to contract parasitic infections.
We did not observe significant differences in Blastocystis sp. prevalence between males and females. However, a previous study detected Blastocystis sp. more frequently in males than in females in immunocompromised patients in Iran [95, 96]. We found significant differences in Blastocystis sp. prevalence between immunocompetent males (22.3%) and immunodeficient males (11.0%). Additionally, a significantly higher Blastocystis sp. infection rate was observed in the age range of 64–74 years in immunocompetent patients (44.4%) than in immunocompromised patients (8.3%). This differs from results of a study conducted in Turkey in patients with gastrointestinal symptoms that found highest prevalence of Blastocystis sp. in the 20–29 age group (28.9%) [75]. Unfortunately, there was no information regarding to the immune statuses of those patients. An age-related epidemiological pattern was also reported in a study in France in which higher prevalence was observed in patients between 15 and 49 years of age (22.2%) than the patients over 50 years of age (16.6%) [26]. In the same study, the prevalence of Blastocystis sp. in immunocompromised subjects (12.4%) was significantly lower than in immunocompetent patients (24.2%). The patients were further divided into immunocompromised subgroups which were HIV, solid organ transplants, immunosuppressive therapy, solid cancer, and bone marrow transplants. The results of these subgroup analyses validated that the prevalence of Blastocystis sp. was considerably lower in subjects getting immunosuppressive treatment (8.4%) and bone marrow transplant (7.7%) but not significantly lower in the other subgroups [26]. The patients recieving bone marrow transplant had lower prevalence (7.1%) than the other immunodeficient patient subgroups (S1 Table).
Because qPCR allows quantification, it has been reported that the use of qPCR in large-scale surveys could assist in identifying whether the development of symptoms is related to infection intensity by simple analysis of Ct values [34]. We found a significantly lower median Ct value in immunocompromised females (Ct = 21.4) than in immunocompetent females (Ct = 26.5) (Table 4) that appears to indicate a higher burden of Blastocystis sp. in females with immunodeficiency. A multicenter case-control study in The Netherlands designed to clarify the clinical importance of qPCR in patients with gastroenteritis found a higher mean (SD) Ct value of Blastocystis sp. and D. fragilis in cases as compared to controls [6]. Interestingly, the hypothesis of this study was that during an episode of diarrhea, “a flush out effect” could decrease the load of Blastocystis sp.. However, no information was given about the immune status of the cases.
Dientamoeba fragilis prevalence has been reported to range from 0% to 82.9% in studies using conventional or molecular methods [12, 43, 97]. Differences have been attributed to variations associated to geographical region, socioeconomic status, and diagnostic methods [12, 51]. In Turkey, the prevalence of D. fragilis ranges from 0% to 18.3% [48, 49, 97–109] (S6 Table). The overall prevalence of D. fragilis found in this study (11.9%) falls within the previously reported range for Turkey. A limited number of studies exist on D. fragilis carriage in Turkey. However, D. fragilis was found in 3.4% of school children by trichrome staining in one study [91]. In another study, D. fragilis was detected in 12.04% of outpatients with gastrointestinal symptoms such as diarrhea, abdominal pain and nausea [48]. In the second study, real-time PCR was used and diarrhea was statistically more significant in patients with the presence of D. fragilis. In this study, although D. fragilis was detected more frequently (13.5%) in the immunocompetent patients with diarrhea than immunodeficient patients with diarrhea (10.6%), these differences were not statistically significant. In Turkey, using qPCR, two studies reported a prevalence for D. fragilis in patients with gastrointestinal symptoms, with unknown immune status, of 10.7% and 12.4% [48, 109]. There are only a few articles about the prevalence of D. fragilis in immunodeficient patients. In a study from Iran, D. fragilis was found 1.2% of 190 patients including primary immunodeficiency patients, cancer patients and organ transplants recipients [14]. A study in HIV positive men with diarrhea in Australia reported a prevalence of 0.3% for D. fragilis [44]. Dientamoeba fragilis was detected only by microscopy in both studies. In the Netherlands, using the multiplex real-time PCR method D. fragilis was detected in 25.8% and 37.6% of patients with gastrointestinal complaints and without complaints, respectively [6]. In our study, no statistically significant differences were found between the two groups in terms of prevalence, gender, age, and seasonal variables for D. fragilis (Table 3). Case-control studies with a large number of samples are needed to determine the relationship between D. fragilis and symptomatology.
In this study, co-infection with D. fragilis was detected in 12 (17.6%) of the of 73 Blastocystis sp. positives. This coinfection rate was lower than previously reported in other studies [48, 95, 110]. To investigate the relationship between D. fragilis colonization and specific gastrointestinal symptoms, and sociodemographic characteristic, a cross-sectional study that included 490 fecal specimens were collected from outpatient with gastrointestinal symptoms and tested with qPCR in Turkey [48]. Their results suggested that D. fragilis is a pathogenic parasite and that the most common clinical symptom found in infected patients is diarrhea [48]. They found that 23.7% were co-infected with Blastocystis sp. [48]. A study in Italy that included 756 patients suspected of harboring intestinal parasites were subjected to multiplex RT-PCRs to detect parasites [95]. The prevalance of Blastocytis was 34% and co-infection with D. fragilis was detected in 24% of patients [95]. Our study showed lower co-infection than both of those studies. The common occurrence of co-infection of these two parasites may indicate that there are shared sources of transmission.
Low infection rates were found for G. duodenalis and Cryptosporidium spp. in this study (<1%). This situation may be explained in part by the study location which is located in the center of Ankara, the second biggest city and the capital city of Turkey. Ankara is known also as a civil servant city, far from the places where agriculture and animal husbandry are made. The majority of the population lives in apartments and uses carboys or purified water. Healthcare workers like nurses and doctors also inform immunocompromised patients about protection from infections. Similar results were obtained in a study conducted in another university hospital in Ankara. The period of the study was 2003–2012 and G. duodenalis and Crptosporidium spp. prevalences were 1.3% and 0.003% respectively. Out of the 85,707 fecal samples examined, 3,681 (4.2%) were positive to parasites [108]. Another study also reported low prevalence of G. duodenalis (0.61%) and Cryptosporidium spp. (0%) from one of university hospitals in Izmir city (the third biggest city of Turkey) in the western region of Turkey [48]. To date, several methods based on specific primers for determining subtypes of Blastocystis sp. have been developed [60, 70, 111–115]. However, only a few of these methods can be used to determine mixed subtypes [111, 113, 115, 116]. The disadvantages of STS-PCR [113, 116] and ST-specific nested PCR assay [115] are being time-consuming, having a high risk of contamination, and being able to identify only certain STs. Recently, NGS was used for identification of Blastocystis sp. subtypes with the advantage of allowing identification of all currently known subtypes, the ability to evaluate mixed infections, and the ability to detect intra-subtype diversity within a single sample quickly and with only a small amount of input material conserving both time and valuable sample material. [8, 30]. Furthermore, the NGS method provides improved sensitivity for identifying subtypes with zoonotic potential that could be in low proportions within a specimen. This is the first study adopting an NGS method to investigate the genetic diversity of Blastocystis sp. in Turkey, as previous studies conducted in the country were based on STS-PCR and Sanger sequencing [21, 83–87]. NGS was used to study Blastocystis sp. genetic diversity in a rural human population from Mexico where 3 subtypes were reported, ST1-ST3, with the following frequencies: ST3 (67.7%), (ST2 11.3%), ST1 (7.3%), ST1/ST3 (7.3%), ST2/ST3 (4.0%), and ST1/ST2/ST3 (2.4%) [8]. Both the present study and the study in Mexico report ST1, ST2, and ST3, with ST3 being the most prevalent subtype. However, we have a higher proportion of mixed infections than the study in Mexico. Another difference between the two studies is that we identified two additional subtypes in patients in Turkey, ST4 and ST6. Previous molecular studies in Turkey have shown that ST1-ST4 represent 87.5% of Blastocystis sp. positive human samples, ST5-ST7 represent 3.5%, and mixed subtype infections represent 9% (S5 and S7 Tables).
Globally, ST1-ST4 have been identified as the most common subtypes in humans. Thus, the findings of our study are consistent with other studies carried out in Turkey and worldwide [10, 75, 83, 87, 117, 118]. ST2 was the second most common ST in our study. This is in agreement with other studies in humans that also report ST2 as the second most common ST after ST3 [8, 81, 119–121]. In Turkey, Dogruman-Al, et al. (2008) reported ST2 as the second most common subtype after ST3 with a statistically significant association to asymptomatic patients, suggesting that ST2 may be a non-pathogenic subtype of Blastocystis sp. in this population [117]. Another study in Turkey reported a relationship between ST1 and abdominal pain [122]. In our study, ST4, which is generally found across Europe, was detected in only one immunocompetent patient. In Turkey, reports in humans of ST4 are sporadic (S4 Table) [86, 118, 123]. In our study, ST6 was detected in one immunocompetent and one immunodeficient patient. There are other reports of ST6 in humans from Turkey, Poland and South America [30, 31, 33, 75, 87, 123, 124]. Because ST6 is mostly identified in birds, its presence in humans may indicate the potential for zoonotic transmission [31]. In the present study, mixed subtype infections of Blastocystis sp. represented 20.6% of the total subtype identified. The incidence of mixed subtypes in Turkey has been reported to range from 3.2% to 30.5% with the STS-PCR method (S7 Table). We found no statistically significant differences between immunocompetent and immunodeficient patients for both Blastocystis sp. positivity and mixed subtype distribution (Table 6). The importance of the mixed subtype infection of Blastocystis sp. is not clear yet for symptomatology or pathogenicity, and additional studies that include methodology to detect mixed subtype infections are needed.
Some researchers have suggested that intra-subtype variability could have a role in the transmission and pathogenicity of Blastocystis sp. [8, 29, 30, 64, 125]. This study showed intra-subtype variations only for ST2 and ST1. Other subtypes (ST3, ST4, and ST6) did not have within sample intra-subtype variations. However, result for ST4 and ST6 should be taken with caution because ST4 and ST6 were only identified in 1, and 2 patients, respectively. Intra-subtype variability observed for ST1-ST3 is consistent with intra-subtype variations reported in previous studies in Iran and Mexico [8, 126]. In the study in Mexico, intra-subtype variability was also more common in ST2 and ST1 than in ST3 [8]. However, there is still limited data and more studies reporting intra-subtype variability are needed to understand the potential role of this variability in pathogenicity, zoonotic potential, and transmission.
This study provides valuable information about Blastocystis sp. and D. fragilis in humans. However, because no healthy controls were available for comparison, conclusions about the role of these parasites in health and disease could not be drawn. In the future, further case-control studies implementing high-resolution molecular tools or functional genomic analysis are necessary to understand the role of intra-subtype variation of Blastocystis sp. in pathogenicity or symptomatology and the role of neglected protists in health and disease.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank Aleksey Molokin for assisting with bioinformatic analysis.
Data Availability
All data generated during this study are included in the article and its supplemental material. Sequencing data from this study are available in the GenBank database, under the accession numbers MW728054- MW728093.
Funding Statement
This research was partially funded by the Scientific Research Unit of Gazi University (Ankara, Turkey) under project number 01/2017-15 (FDA), the Scientific Research Unit of Hitit University (Corum, Turkey) under project number TIP19001.16.005 (ATO), and USDA-ARS Project No: 8042-32000-100-00-D (MS). The publication fee was provided by Akhmed Yassawi University (FS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Krones E, Högenauer C. Diarrhea in the immunocompromised patient. Gastroenterol Clin North Am. 2012;41(3):677–701. doi: 10.1016/j.gtc.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 2.Törnblom H, Holmvall P, Svenungsson B, Lindberg G. Gastrointestinal symptoms after infectious diarrhea: a five-year follow-up in a Swedish cohort of adults. Clin Gastroenterol Hepatol. 2007;5(4):461–464. doi: 10.1016/j.cgh.2007.01.007 [DOI] [PubMed] [Google Scholar]
- 3.Svenungsson B, Lagergren A, Ekwall E, Evengård B, Hedlund KO, Kärnell A, et al. Enteropathogens in adult patients with diarrhea and healthy control subjects: a 1-year prospective study in a Swedish clinic for infectious diseases. Clin Infect Dis. 2000;30(5):770–778. doi: 10.1086/313770 [DOI] [PubMed] [Google Scholar]
- 4.Dubey JP, Almeria S. Cystoisospora belli infections in humans: the past 100 years. Parasitology. 2019;146(12):1490–1527. doi: 10.1017/S0031182019000957 [DOI] [PubMed] [Google Scholar]
- 5.Laksemi DA, Suwanti LT, Mufasirin M, Suastika K, Sudarmaja M. Opportunistic parasitic infections in patients with human immunodeficiency virus/acquired immunodeficiency syndrome: A review. Vet World. 2019;13(4):716–725. doi: 10.14202/vetworld.2020.716-725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Boer MD, Schuurs TA, Vermeer M, Ruijs GJHM, van der Zanden AGM, Weel JF, et al. Distribution and relevance of Dientamoeba fragilis and Blastocystis species in gastroenteritis: results from a case-control study. Eur J Clin Microbiol Infect Dis. 2020;39(1):197–203. doi: 10.1007/s10096-019-03710-z [DOI] [PubMed] [Google Scholar]
- 7.Stensvold CR, Clark CG. Pre-empting Pandora’s Box: Blastocystis subtypes revisited. Trends Parasitol. 2020;36(3):229–232. doi: 10.1016/j.pt.2019.12.009 [DOI] [PubMed] [Google Scholar]
- 8.Rojas-Velázquez L, Maloney JG, Molokin A, Morán P, Serrano-Vázquez A, González E, et al. Use of next-generation amplicon sequencing to study Blastocystis genetic diversity in a rural human population from Mexico. Parasit Vectors. 2019;12(1):566. doi: 10.1186/s13071-019-3814-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barratt JL, Harkness J, Marriott D, Ellis JT, Stark D. A review of Dientamoeba fragilis carriage in humans: several reasons why this organism should be considered in the diagnosis of gastrointestinal illness. Gut Microbes. 2011;2(1):3–12. doi: 10.4161/gmic.2.1.14755 [DOI] [PubMed] [Google Scholar]
- 10.Dogruman-Al F, Kustimur S, Yoshikawa H, Tuncer C, Simsek Z, Tanyuksel M, et al. Blastocystis subtypes in irritable bowel syndrome and inflammatory bowel disease in Ankara, Turkey. Mem Inst Oswaldo Cruz. 2009;104(5):724–727. doi: 10.1590/s0074-02762009000500011 [DOI] [PubMed] [Google Scholar]
- 11.Ramírez JD, Sánchez LV, Bautista DC, Corredor AF, Flórez AC, Stensvold CR. Blastocystis subtypes detected in humans and animals from Colombia. Infect Genet Evol. 2014;22:223–228. doi: 10.1016/j.meegid.2013.07.020 [DOI] [PubMed] [Google Scholar]
- 12.Stark D, Barratt J, Chan D, Ellis JT. Dientamoeba fragilis, the neglected trichomonad of the human bowel. Clin Microbiol Rev. 2016;29(3):553–580. doi: 10.1128/CMR.00076-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stenzel DJ, Boreham PF. Blastocystis hominis revisited. Clin Microbiol Rev. 1996;9(4):563–584. doi: 10.1128/CMR.9.4.563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esteghamati A, Khanaliha K, Bokharaei-Salim F, Sayyahfar S, Ghaderipour M. Prevalence of intestinal parasitic infection in cancer, organ transplant and primary immunodeficiency patients in Tehran, Iran. Asian Pac J Cancer Prev. 2019;20(2):495–501. doi: 10.31557/APJCP.2019.20.2.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontanelli Sulekova L, Gabrielli S, Furzi F, Milardi GL, Biliotti E, De Angelis M, et al. Molecular characterization of Blastocystis subtypes in HIV-positive patients and evaluation of risk factors for colonization. BMC Infect Dis. 2019;19(1):876. doi: 10.1186/s12879-019-4537-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersen LO, Stensvold CR. Blastocystis in health and disease: Are we moving from a clinical to a public health perspective?. J Clin Microbiol. 2016;54(3):524–528. doi: 10.1128/JCM.02520-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belleza ML, Reyes JC, Tongol-Rivera PN, Rivera WL. Subtype analysis of Blastocystis sp. isolates from human and canine hosts in an urban community in the Philippines. Parasitol Int. 2016;65(3):291–294. doi: 10.1016/j.parint.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 18.Beyhan YE, Yilmaz H, Cengiz ZT, Ekici A. Clinical significance and prevalence of Blastocystis hominis in Van, Turkey. Saudi Med J. 2015;36(9):1118–1121. doi: 10.15537/smj.2015.9.12444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Safadi D, Gaayeb L, Meloni D, Cian A, Poirier P, Wawrzyniak I, et al. Children of Senegal River Basin show the highest prevalence of Blastocystis sp. ever observed worldwide. BMC Infect Dis. 2014;14:164. doi: 10.1186/1471-2334-14-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sánchez A, Munoz M, Gómez N, Tabares J, Segura L, Salazar Á, et al. Molecular Epidemiology of Giardia, Blastocystis and Cryptosporidium among Indigenous Children from the Colombian Amazon Basin. Front Microbiol. 2017;8:248. doi: 10.3389/fmicb.2017.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sankur F, Ayturan S, Malatyali E, Ertabaklar H, Ertug S. The distribution of Blastocystis subtypes among school-aged children in Mugla, Turkey. Iran J Parasitol. 2017;12(4):580–586. [PMC free article] [PubMed] [Google Scholar]
- 22.Seguí R, Muñoz-Antoli C, Klisiowicz DR, Oishi CY, Köster PC, De Lucio A, et al. Prevalence of intestinal parasites, with emphasis on the molecular epidemiology of Giardia duodenalis and Blastocystis sp., in the Paranaguá Bay, Brazil: a community survey. Parasit Vectors. 2018;11(1):490. doi: 10.1186/s13071-018-3054-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang W, Ren G, Zhao W, Yang Z, Shen Y, Sun Y, et al. Genotyping of Enterocytozoon bieneusi and subtyping of Blastocystis in cancer patients: Relationship to diarrhea and assessment of zoonotic transmission. Front Microbiol. 2017;8:1835. doi: 10.3389/fmicb.2017.01835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartolini A, Zorzi G, Besutti V. Prevalence of intestinal parasitoses detected in Padua teaching hospital, Italy, March 2011—February 2013. Infez Med. 2017;25(2):133–141. [PubMed] [Google Scholar]
- 25.Bednarska M, Jankowska I, Pawelas A, Piwczyńska K, Bajer A, Wolska-Kuśnierz B, et al. Prevalence of Cryptosporidium, Blastocystis, and other opportunistic infections in patients with primary and acquired immunodeficiency. Parasitol Res. 2018;117(9):2869–2879. doi: 10.1007/s00436-018-5976-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El Safadi D, Cian A, Nourrisson C, Pereira B, Morelle C, Bastien P, et al. Prevalence, risk factors for infection and subtype distribution of the intestinal parasite Blastocystis sp. from a large-scale multi-center study in France. BMC Infect Dis. 2016;16(1):451. doi: 10.1186/s12879-016-1776-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paulos S, Köster PC, De Lucio A, Hernández-De-Mingo M, Cardona GA, Fernández-Crespo JC, et al. Occurrence and subtype distribution of Blastocystis sp. in humans, dogs and cats sharing household in northern Spain and assessment of zoonotic transmission risk. Zoonoses Public Health. 2018;65(8):993–1002. doi: 10.1111/zph.12522 [DOI] [PubMed] [Google Scholar]
- 28.Scanlan PD, Knight R, Song SJ, Ackermann G, Cotter PD. Prevalence and genetic diversity of Blastocystis in family units living in the United States. Infect Genet Evol. 2016;45:95–97. doi: 10.1016/j.meegid.2016.08.018 [DOI] [PubMed] [Google Scholar]
- 29.Maloney JG, Lombard JE, Urie NJ, Shivley CB, Santin M. Zoonotic and genetically diverse subtypes of Blastocystis in US pre-weaned dairy heifer calves. Parasitol Res. 2019;118(2):575–582. doi: 10.1007/s00436-018-6149-3 [DOI] [PubMed] [Google Scholar]
- 30.Maloney JG, Molokin A, da Cunha MJR, Cury MC, Santin M. Blastocystis subtype distribution in domestic and captive wild bird species from Brazil using next generation amplicon sequencing. Parasite Epidemiol Control. 2020;9:e00138. doi: 10.1016/j.parepi.2020.e00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hublin JSY, Maloney JG, Santin M. Blastocystis in domesticated and wild mammals and birds. Res Vet Sci. 2020;S0034-5288(20)31032-8. doi: 10.1016/j.rvsc.2020.09.031 [DOI] [PubMed] [Google Scholar]
- 32.Stensvold CR, Tan KSW, Clark CG. Blastocystis. Trends Parasitol. 2020;36(3):315–316. doi: 10.1016/j.pt.2019.12.008 [DOI] [PubMed] [Google Scholar]
- 33.Ramírez JD, Sánchez A, Hernández C, Flórez C, Bernal MC, Giraldo JC, et al. Geographic distribution of human Blastocystis subtypes in South America. Infect Genet Evol. 2016;41:32–35. doi: 10.1016/j.meegid.2016.03.017 [DOI] [PubMed] [Google Scholar]
- 34.Stensvold CR, Clark CG. Current status of Blastocystis: A personal view. Parasitol Int. 2016;65(6 Pt B):763–771. doi: 10.1016/j.parint.2016.05.015 [DOI] [PubMed] [Google Scholar]
- 35.Mattiucci S, Crisafi B, Gabrielli S, Paoletti M, Cancrini G. Molecular epidemiology and genetic diversity of Blastocystis infection in humans in Italy. Epidemiol Infect. 2016;144(3):635–646. doi: 10.1017/S0950268815001697 [DOI] [PubMed] [Google Scholar]
- 36.Khaled S, Gantois N, Ly AT, Senghor S, Even G, Dautel E, et al. Prevalence and Subtype Distribution of Blastocystis sp. in Senegalese School Children. Microorganisms. 2020;8(9):1408. doi: 10.3390/microorganisms8091408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aynur ZE, Güçlü Ö, Yıldız İ, Aynur H, Ertabaklar H, Bozdoğan B, et al. Molecular characterization of Blastocystis in cattle in Turkey. Parasitol Res. 2019;118(3):1055–1059. doi: 10.1007/s00436-019-06243-8 [DOI] [PubMed] [Google Scholar]
- 38.Greige S, El Safadi D, Khaled S, Gantois N, Baydoun M, Chemaly M, et al. First report on the prevalence and subtype distribution of Blastocystis sp. in dairy cattle in Lebanon and assessment of zoonotic transmission. Acta Trop. 2019;194:23–29. doi: 10.1016/j.actatropica.2019.02.013 [DOI] [PubMed] [Google Scholar]
- 39.Stensvold CR. Blastocystis: Genetic diversity and molecular methods for diagnosis and epidemiology. Trop Parasitol. 2013;3(1):26–34. doi: 10.4103/2229-5070.113896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brands MR, Van de Vijver E, Haisma SM, Heida A, van Rheenen PF. No association between abdominal pain and Dientamoeba in Dutch and Belgian children. Arch Dis Child. 2019;104(7):686–689. doi: 10.1136/archdischild-2018-316383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crotti D, D’Annibale ML. [Role of Dientamoeba fragilis in human bowel infections]. Infez Med. 2007;15(1):30–39. Italian. [PubMed] [Google Scholar]
- 42.Pietilä J-P, Meri T, Siikamäki H, Tyyni E, Kerttula A-M, Pakarinen L, et al. Dientamoeba fragilis—the most common intestinal protozoan in the Helsinki Metropolitan Area, Finland, 2007 to 2017. Euro Surveill. 2019;24(29):1800546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Preiss U, Ockert G, Brömme S, Otto A. Dientamoeba fragilis infection, a cause of gastrointestinal symptoms in childhood. Klin Padiatr. 1990;202(2):120–123. doi: 10.1055/s-2007-1025503 [DOI] [PubMed] [Google Scholar]
- 44.Stark D, Fotedar R, van Hal S, Beebe N, Marriott D, Ellis JT, et al. Prevalence of enteric protozoa in human immunodeficiency virus (HIV)-positive and HIV-negative men who have sex with men from Sydney, Australia. Am J Trop Med Hyg. 2007;76(3):549–552. [PubMed] [Google Scholar]
- 45.Greigert V, Abou-Bacar A, Brunet J, Nourrisson C, Pfaff AW, Benarbia L, et al. Human intestinal parasites in Mahajanga, Madagascar: The kingdom of the protozoa. PLoS One. 2018;13(10):e0204576. doi: 10.1371/journal.pone.0204576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jimenez-Gonzalez DE, Martinez-Flores WA, Reyes-Gordillo J, Ramirez-Miranda ME, Arroyo-Escalante S, Romero-Valdovinos M, et al. Blastocystis infection is associated with irritable bowel syndrome in a Mexican patient population. Parasitol Res. 2012;110(3):1269–1275. doi: 10.1007/s00436-011-2626-7 [DOI] [PubMed] [Google Scholar]
- 47.Osman M, El Safadi D, Cian A, Benamrouz S, Nourrisson C, Poirier P, et al. Prevalence and risk factors for intestinal protozoan infections with Cryptosporidium, Giardia, Blastocystis and Dientamoeba among schoolchildren in Tripoli, Lebanon. PLoS Negl Trop Dis. 2016;10(3):e0004496. doi: 10.1371/journal.pntd.0004496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aykur M, Calıskan Kurt C, Dirim Erdogan D, Biray Avcı C, Vardar R, Aydemir S, et al. Investigation of Dientamoeba fragilis prevalence and evaluation of sociodemographic and clinical features in patients with gastrointestinal symptoms. Acta Parasitol. 2019;64(1):162–170. doi: 10.2478/s11686-018-00017-5 [DOI] [PubMed] [Google Scholar]
- 49.Karasartova D, Gureser AS, Zorlu M, Turegun-Atasoy B, Taylan-Ozkan A, Dolapci M. et al. Blastocystosis in post-traumatic splenectomized patients. Parasitol Int. 2016;65(6 Pt B):802–805. doi: 10.1016/j.parint.2015.12.004 [DOI] [PubMed] [Google Scholar]
- 50.Johnson JA, Clark CG. Cryptic genetic diversity in Dientamoeba fragilis. J Clin Microbiol. 2000;38(12):4653–4654. doi: 10.1128/JCM.38.12.4653-4654.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cacciò SM. Molecular epidemiology of Dientamoeba fragilis. Acta Trop. 2018;184:73–77. doi: 10.1016/j.actatropica.2017.06.029 [DOI] [PubMed] [Google Scholar]
- 52.Chan D, Barratt J, Roberts T, Phillips O, Šlapeta J, Ryan U, et al. Detection of Dientamoeba fragilis in animal faeces using species specific real time PCR assay. Vet Parasitol. 2016;227:42–47. doi: 10.1016/j.vetpar.2016.07.025 [DOI] [PubMed] [Google Scholar]
- 53.Cacciò SM, Sannella AR, Manuali E, Tosini F, Sensi M, Crotti D, et al. Pigs as natural hosts of Dientamoeba fragilis genotypes found in humans. Emerg Infect Dis. 2012;18(5):838–841. doi: 10.3201/eid1805.111093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stark D, Phillips O, Peckett D, Munro U, Marriott D, Harkness J, et al. Gorillas are a host for Dientamoeba fragilis: an update on the life cycle and host distribution. Vet Parasitol. 2008;151(1):21–26. doi: 10.1016/j.vetpar.2007.10.002 [DOI] [PubMed] [Google Scholar]
- 55.Lankester F, Kiyang JA, Bailey W, Unwin S. Dientamoeba fragilis: initial evidence of pathogenicity in the western lowland gorilla (Gorilla gorilla gorilla). J Zoo Wildl Med. 2010;41(2):350–352. doi: 10.1638/2009-0190.1 [DOI] [PubMed] [Google Scholar]
- 56.Dogruman-Al F, Simsek Z, Boorom K, Ekici E, Sahin M, Tuncer C, et al. Comparison of methods for detection of Blastocystis infection in routinely submitted stool samples, and also in IBS/IBD Patients in Ankara, Turkey. PLoS One. 2010;5(11):e15484. doi: 10.1371/journal.pone.0015484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gough R, Ellis J, Stark D. Comparison and recommendations for use of Dientamoeba fragilis real-time PCR assays. J Clin Microbiol. 2019. Apr 26;57(5):e01466–18. doi: 10.1128/JCM.01466-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roberts T, Barratt J, Harkness J, Ellis J, Stark D. Comparison of microscopy, culture, and conventional polymerase chain reaction for detection of Blastocystis sp. in clinical stool samples. Am J Trop Med Hyg. 2011;84(2):308–312. doi: 10.4269/ajtmh.2011.10-0447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stark D, Barratt J, Roberts T, Marriott D, Harkness J, Ellis J. et al. Comparison of microscopy, two xenic culture techniques, conventional and real-time PCR for the detection of Dientamoeba fragilis in clinical stool samples. Eur J Clin Microbiol Infect Dis. 2010;29(4):411–416. doi: 10.1007/s10096-010-0876-4 [DOI] [PubMed] [Google Scholar]
- 60.Stensvold CR, Arendrup MC, Jespersgaard C, Mølbak K, Nielsen HV. Detecting Blastocystis using parasitologic and DNA-based methods: a comparative study. Diagn Microbiol Infect Dis. 2007;59(3):303–307. doi: 10.1016/j.diagmicrobio.2007.06.003 [DOI] [PubMed] [Google Scholar]
- 61.van Lieshout L, Roestenberg M. Clinical consequences of new diagnostic tools for intestinal parasites. Clin Microbiol Infect. 2015;21(6):520–528. doi: 10.1016/j.cmi.2015.03.015 [DOI] [PubMed] [Google Scholar]
- 62.Stensvold CR, Clark CG. Molecular identification and subtype analysis of Blastocystis. Curr Protoc Microbiol. 2016;43:20A.2.1–20A.2.10. doi: 10.1002/cpmc.17 [DOI] [PubMed] [Google Scholar]
- 63.Jerez Puebla LE, Núñez-Fernández FA, Fraga Nodarse J, Atencio Millán I, Cruz Rodríguez I, Martínez Silva I, et al. Diagnosis of intestinal protozoan infections in patients in Cuba by microscopy and molecular methods: advantages and disadvantages. J Microbiol Methods. 2020;179:106102. doi: 10.1016/j.mimet.2020.106102 [DOI] [PubMed] [Google Scholar]
- 64.Maloney JG, Molokin A, Santin M. Next generation amplicon sequencing improves detection of Blastocystis mixed subtype infections. Infect Genet Evol. 2019;73:119–125. doi: 10.1016/j.meegid.2019.04.013 [DOI] [PubMed] [Google Scholar]
- 65.Chumpitazi BP, Self MM, Czyzewski DI, Cejka S, Swank PR, Shulman RJ. Bristol Stool Form Scale reliability and agreement decreases when determining Rome III stool form designations. Neurogastroenterol Motil. 2016;28(3):443–448. doi: 10.1111/nmo.12738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garcia LS. Diagnostic medical parasitology. 6th ed. Washington: ASM Press; 2017. [Google Scholar]
- 67.World Health Organization. Basic laboratory methods in medical parasitology: World Health Organization; 1991. [Google Scholar]
- 68.Stensvold CR, Ahmed UN, Andersen LO, Nielsen HV. Development and evaluation of a genus-specific, probe-based, internal-process-controlled real-time PCR assay for sensitive and specific detection of Blastocystis spp. J Clin Microbiol. 2012;50(6):1847–1851. doi: 10.1128/JCM.00007-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stark D, Beebe N, Marriott D, Ellis J, Harkness J. Evaluation of three diagnostic methods, including real-time PCR, for detection of Dientamoeba fragilis in stool specimens. J Clin Microbiol. 2006;44(1):232–235. doi: 10.1128/JCM.44.1.232-235.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Santín M, Gómez-Muñoz MT, Solano-Aguilar G, Fayer R. Development of a new PCR protocol to detect and subtype Blastocystis spp. from humans and animals. Parasitol Res. 2011;109(1):205–212. doi: 10.1007/s00436-010-2244-9 [DOI] [PubMed] [Google Scholar]
- 71.Bushnell B. BBMap: A fast, accurate, splice-aware aligner. Lawrence Berkeley National Lab.(LBNL), Berkeley, CA (United States), 2014. [Google Scholar]
- 72.Rognes T, Flouri T, Nichols B, Quince C, Mahé F. VSEARCH: A versatile open source tool for metagenomics. PeerJ. 2016;4:e2584. doi: 10.7717/peerj.2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 74.Stensvold CR, Arendrup MC, Mølbak K, Nielsen HV. The prevalence of Dientamoeba fragilis in patients with suspected enteroparasitic disease in a metropolitan area in Denmark. Clin Microbiol Infect. 2007;13(8):839–842. doi: 10.1111/j.1469-0691.2007.01760.x [DOI] [PubMed] [Google Scholar]
- 75.Dagci H, Kurt Ö, Demirel M, Mandiracioglu A, Aydemir S, Saz U, Epidemiological and diagnostic features of Blastocystis infection in symptomatic patients in Izmir province, Turkey. Iran J Parasitol. 2014;9(4):519–529. [PMC free article] [PubMed] [Google Scholar]
- 76.Poirier P, Wawrzyniak I, Albert A, El Alaoui H, Delbac F, Livrelli V. et al. Development and evaluation of a real-time PCR assay for detection and quantification of Blastocystis parasites in human stool samples: prospective study of patients with hematological malignancies. J Clin Microbiol. 2011;49(3):975–983. doi: 10.1128/JCM.01392-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moosavi A, Haghighi A, Mojarad EN, Zayeri F, Alebouyeh M, Khazan H, et al. Genetic variability of Blastocystis sp. isolated from symptomatic and asymptomatic individuals in Iran. Parasitol Res. 2012;111(6):2311–2315. doi: 10.1007/s00436-012-3085-5 [DOI] [PubMed] [Google Scholar]
- 78.Tungtrongchitr A, Manatsathit S, Kositchaiwat C, Ongrotchanakun J, Munkong N, Chinabutr P, et al. Blastocystis hominis infection in irritable bowel syndrome patients. Southeast Asian J Trop Med Public Health. 2004;35(3):705–710. [PubMed] [Google Scholar]
- 79.Yoshikawa H, Dogruman-Al F, Turk S, Kustimur S, Balaban N, Sultan N. et al. Evaluation of DNA extraction kits for molecular diagnosis of human Blastocystis subtypes from fecal samples. Parasitol Res. 2011;109(4):1045–1050. doi: 10.1007/s00436-011-2342-3 [DOI] [PubMed] [Google Scholar]
- 80.Nithyamathi K, Chandramathi S, Kumar S. Predominance of Blastocystis sp. Infection among School Children in Peninsular Malaysia. PLoS One. 2016;11(2):e0136709. doi: 10.1371/journal.pone.0136709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abdulsalam AM, Ithoi I, Al-Mekhlafi HM, Al-Mekhlafi AM, Ahmed A, Surin J. et al. Subtype distribution of Blastocystis isolates in Sebha, Libya. PLoS One. 2013;8(12):e84372. doi: 10.1371/journal.pone.0084372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stensvold CR, Christiansen DB, Olsen KE, Nielsen HV. Blastocystis sp. subtype 4 is common in Danish Blastocystis-positive patients presenting with acute diarrhea. Am J Trop Med Hyg. 2011;84(6):883–885. doi: 10.4269/ajtmh.2011.11-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dogruman-Al F, Yoshikawa H, Kustimur S, Balaban N. PCR-based subtyping of Blastocystis isolates from symptomatic and asymptomatic individuals in a major hospital in Ankara, Turkey. Parasitol Res. 2009;106(1):263–268. doi: 10.1007/s00436-009-1658-8 [DOI] [PubMed] [Google Scholar]
- 84.Yersal O, Malatyali E, Ertabaklar H, Oktay E, Barutca S, Ertug S. et al. Blastocystis subtypes in cancer patients: Analysis of possible risk factors and clinical characteristics. Parasitol Int. 2016;65(6 Pt B):792–796. doi: 10.1016/j.parint.2016.02.010 [DOI] [PubMed] [Google Scholar]
- 85.Coskun A, Malatyali E, Ertabaklar H, Yasar MB, Karaoglu AO, Ertug S. et al. Blastocystis in ulcerative colitis patients: Genetic diversity and analysis of laboratory findings. Asian Pac J Trop Med. 2016;9(9):916–919. doi: 10.1016/j.apjtm.2016.07.018 [DOI] [PubMed] [Google Scholar]
- 86.Dogan N, Aydin M, Tuzemen NU, Dinleyici EC, Oguz I, Dogruman-Al F. et al. Subtype distribution of Blastocystis spp. isolated from children in Eskisehir, Turkey. Parasitol Int. 2017;66(1):948–951. doi: 10.1016/j.parint.2016.10.008 [DOI] [PubMed] [Google Scholar]
- 87.Cakir F, Cicek M, Yildirim IH. Determination the subtypes of Blastocystis sp. and evaluate the effect of these subtypes on pathogenicity. Acta Parasitol. 2019;64(1):7–12. doi: 10.2478/s11686-018-00002-y [DOI] [PubMed] [Google Scholar]
- 88.Koltas IS, Eroglu F. Subtype analysis of Blastocystis isolates using SSU rRNA-DNA sequencing in rural and urban population in southern Turkey. Exp Parasitol. 2016;170:247–251. doi: 10.1016/j.exppara.2016.10.006 [DOI] [PubMed] [Google Scholar]
- 89.Seyer A, Karasartova D, Ruh E, Güreser AS, Turgal E, Imir T, et al. Epidemiology and prevalence of Blastocystis spp. in North Cyprus. Am J Trop Med Hyg. 2017;96(5):1164–1170. doi: 10.4269/ajtmh.16-0706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Karaman U, Koloren Z, Ayaz E, Gur U. Epidemiology of Blastocystis spp. in primary school students at a central village of Ordu province. Med Sci. 2019;8(1):77–80. [Google Scholar]
- 91.Ostan I, Kilimcioğlu AA, Girginkardeşler N, Ozyurt BC, Limoncu ME, Ok UZ. et al. Health inequities: lower socio-economic conditions and higher incidences of intestinal parasites. BMC Public Health. 2007;7:342. doi: 10.1186/1471-2458-7-342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aksoy U, Akisü C, Bayram-Delibaş S, Ozkoç S, Sahin S, Usluca S. et al. Demographic status and prevalence of intestinal parasitic infections in schoolchildren in Izmir, Turkey. Turk J Pediatr. 2007;49(3):278–282. [PubMed] [Google Scholar]
- 93.Tan TC, Ong SC, Suresh KG. Genetic variability of Blastocystis sp. isolates obtained from cancer and HIV/AIDS patients. Parasitol Res. 2009;105(5):1283–1286. doi: 10.1007/s00436-009-1551-5 [DOI] [PubMed] [Google Scholar]
- 94.Mohamed AM, Ahmed MA, Ahmed SA, Al-Semany SA, Alghamdi SS, Zaglool DA. et al. Predominance and association risk of Blastocystis hominis subtype I in colorectal cancer: a case control study. Infect Agent Cancer. 2017;12:21. doi: 10.1186/s13027-017-0131-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Piubelli C, Soleymanpoor H, Giorli G, Formenti F, Buonfrate D, Bisoffi Z, et al. Blastocystis prevalence and subtypes in autochthonous and immigrant patients in a referral centre for parasitic infections in Italy. PLoS One. 2019;14(1):e0210171. doi: 10.1371/journal.pone.0210171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rasti S, Hassanzadeh M, Hooshyar H, Momen-Heravi M, Mousavi SGA, Abdoli A. et al. Intestinal parasitic infections in different groups of immunocompromised patients in Kashan and Qom cities, central Iran. Scand J Gastroenterol. 2017;52(6–7):738–741. doi: 10.1080/00365521.2017.1308547 [DOI] [PubMed] [Google Scholar]
- 97.Mumcuoğlu I, Coşkun FA, Aksu N, Pürnak T, Güngör C. [Role of Dientamoeba fragilis and Blastocystis spp. in Irritable Bowel Syndrome]. Turkiye Parazitol Derg. 2013;37(2):73–77 (in Turkish). doi: 10.5152/tpd.2013.19 [DOI] [PubMed] [Google Scholar]
- 98.Taş Cengiz Z, Akbayram S, Ciçek M, Yilmaz H. [Intestinal parasitoses detected in primary schoolchildren in the Van province]. Turkiye Parazitol Derg. 2009;33(4):289–293 (in Turkish). [PubMed] [Google Scholar]
- 99.Calik S, Karaman U, Colak C. Prevalence of microsporidium and other intestinal parasites in children from Malatya, Turkey. Indian J Microbiol. 2011;51(3):345–349. doi: 10.1007/s12088-011-0107-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Karaman U, Atambay M, Aycan O, Yologlu S, Daldal N. [Incidence of intestinal parasites in municipal sanitary workers in Malatya]. Turkiye Parazitol Derg. 2006;30(3):181–183 (in Turkish). [PubMed] [Google Scholar]
- 101.Karaman U, Turan A, Depecik F, Gecit I, Ozer A, Karci E, et al. [Frequency of intestinal parasites among administrators and workers in sanitary and non-sanitary institutions]. Turkiye Parazitol Derg. 2011;35(1):30–33 (in Turkish). doi: 10.5152/tpd.2011.08 [DOI] [PubMed] [Google Scholar]
- 102.Doğan N, Oz Y, Kocman N, Nursal A. [Comparison of individual differences in the direct microscopic examination in the diagnosis of intestinal parasites]. Turkiye Parazitol Derg. 2012;36(4):211–214 (in Turkish). doi: 10.5152/tpd.2012.51 [DOI] [PubMed] [Google Scholar]
- 103.Ozçakir O, Güreser S, Ergüven S, Yilmaz YA, Topaloğlu R, Hasçelik G. et al. Characteristics of Blastocystis hominis infection in a Turkish university hospital. Turkiye Parazitol Derg. 2007;31(4):277–282. [PubMed] [Google Scholar]
- 104.Arserim SK, Limoncu ME, Gündüz T, Balcıoğlu IC. Investigation of intestinal parasites in living nursing home. Turkiye Parazitol Derg. 2019;43(2):74–77. doi: 10.4274/tpd.galenos.2019.6321 [DOI] [PubMed] [Google Scholar]
- 105.Tanyuksel M, Yilmaz H, Ulukanligil M, Araz E, Cicek M, Koru O, et al. Comparison of two methods (microscopy and enzyme-linked immunosorbent assay) for the diagnosis of amebiasis. Exp Parasitol. 2005;110(3):322–326. doi: 10.1016/j.exppara.2005.02.012 [DOI] [PubMed] [Google Scholar]
- 106.Maçin S, Kaya F, Çağdaş D, Hizarcioglu-Gulsen H, Saltik-Temizel IN, Tezcan I, et al. Detection of parasites in children with chronic diarrhea. Pediatr Int. 2016;58(6):531–533. doi: 10.1111/ped.12959 [DOI] [PubMed] [Google Scholar]
- 107.Girginkardeşler N, Coşkun S, Cüneyt Balcioğlu I, Ertan P, Ok UZ. Dientamoeba fragilis, a neglected cause of diarrhea, successfully treated with secnidazole. Clin Microbiol Infect. 2003;9(2):110–113. doi: 10.1046/j.1469-0691.2003.00504.x [DOI] [PubMed] [Google Scholar]
- 108.Gülmez D, Sarıbaş Z, Akyön Y, Ergüven S. [The results of Hacettepe University Faculty of Medicine Parasitology Laboratory in 2003–2012: evaluation of 10 years]. Turkiye Parazitol Derg. 2013;37(2):97–101 (in Turkish). doi: 10.5152/tpd.2013.23 [DOI] [PubMed] [Google Scholar]
- 109.Sivcan E, Charyyeva A, Ceylan Ş, Yürük M, Erdoğan E, Şahin İ. et al. [Dientamoeba fragilis infection in patients with gastrointestinal system complaints]. Mikrobiyol Bul. 2018;52(2):166–179 (in Turkish). doi: 10.5578/mb.66468 [DOI] [PubMed] [Google Scholar]
- 110.Burgaña A, Abellana R, Yordanov SZ, Kazan R, Pérez Ortiz AM, Ramos CC, et al. Paromomycin is superior to metronidazole in Dientamoeba fragilis treatment. Int J Parasitol Drugs Drug Resist. 2019;11:95–100. doi: 10.1016/j.ijpddr.2019.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Srichaipon N, Nuchprayoon S, Charuchaibovorn S, Sukkapan P, Sanprasert V. A Simple genotyping method for rapid differentiation of Blastocystis subtypes and subtype distribution of Blastocystis spp. in Thailand. Pathogens. 2019;8(1):38. doi: 10.3390/pathogens8010038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stensvold CR, Traub RJ, von Samson-Himmelstjerna G, Jespersgaard C, Nielsen HV, Thompson RC. et al. Blastocystis: subtyping isolates using pyrosequencing technology. Exp Parasitol. 2007;116(2):111–119. doi: 10.1016/j.exppara.2006.12.002 [DOI] [PubMed] [Google Scholar]
- 113.Yoshikawa H, Wu Z, Kimata I, Iseki M, Ali IKMD, Hossain MB, et al. Polymerase chain reaction-based genotype classification among human Blastocystis hominis populations isolated from different countries. Parasitol Res. 2004;92(1):22–29. doi: 10.1007/s00436-003-0995-2 [DOI] [PubMed] [Google Scholar]
- 114.Parkar U, Traub RJ, Kumar S, Mungthin M, Vitali S, Leelayoova S, et al. Direct characterization of Blastocystis from faeces by PCR and evidence of zoonotic potential. Parasitology. 2007;134(Pt 3):359–367. doi: 10.1017/S0031182006001582 [DOI] [PubMed] [Google Scholar]
- 115.Scanlan PD, Stensvold CR, Cotter PD. Development and application of a Blastocystis subtype-specific PCR assay reveals that mixed-subtype infections are common in a healthy human population. Appl Environ Microbiol. 2015;81(12):4071–4076. doi: 10.1128/AEM.00520-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yoshikawa H, Iwamasa A. Human Blastocystis subtyping with subtype-specific primers developed from unique sequences of the SSU rRNA gene. Parasitol Int. 2016;65(6 Pt B):785–791. doi: 10.1016/j.parint.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 117.Dogruman-Al F, Dagci H, Yoshikawa H, Kurt O, Demirel M. A possible link between subtype 2 and asymptomatic infections of Blastocystis hominis. Parasitol Res. 2008;103(3):685–689. doi: 10.1007/s00436-008-1031-3 [DOI] [PubMed] [Google Scholar]
- 118.Ozyurt M, Kurt O, Mølbak K, Nielsen HV, Haznedaroglu T, Stensvold CR. et al. Molecular epidemiology of Blastocystis infections in Turkey. Parasitol Int. 2008;57(3):300–306. doi: 10.1016/j.parint.2008.01.004 [DOI] [PubMed] [Google Scholar]
- 119.Forsell J, Granlund M, Samuelsson L, Koskiniemi S, Edebro H, Evengård B. et al. High occurrence of Blastocystis sp. subtypes 1–3 and Giardia intestinalis assemblage B among patients in Zanzibar, Tanzania. Parasit Vectors. 2016;9(1):370. doi: 10.1186/s13071-016-1637-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mohamed RT, El-Bali MA, Mohamed AA, Abdel-Fatah MA, El-Malky MA, Mowafy NM, et al. Subtyping of Blastocystis sp. isolated from symptomatic and asymptomatic individuals in Makkah, Saudi Arabia. Parasit Vectors. 2017;10(1):174. doi: 10.1186/s13071-017-2114-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sanpool O, Laymanivong S, Thanchomnang T, Rodpai R, Sadaow L, Phosuk I, et al. Subtype identification of human Blastocystis spp. isolated from Lao People’s Democratic Republic. Acta Trop. 2017;168:37–40. doi: 10.1016/j.actatropica.2017.01.006 [DOI] [PubMed] [Google Scholar]
- 122.Eroglu F, Genc A, Elgun G, Koltas IS. Identification of Blastocystis hominis isolates from asymptomatic and symptomatic patients by PCR. Parasitol Res. 2009;105(6):1589–1592. doi: 10.1007/s00436-009-1595-6 [DOI] [PubMed] [Google Scholar]
- 123.Adiyaman Korkmaz G, Dogruman Al F, Mumcuoglu I. [Investigation of the presence of Blastocystis spp. in stool samples with microscopic, culture and molecular methods]. Mikrobiyol Bul. 2015;49(1):85–97 (in Turkish). doi: 10.5578/mb.8439 [DOI] [PubMed] [Google Scholar]
- 124.Rudzińska M, Kowalewska B, Wąż P, Sikorska K, Szostakowska B. Blastocystis subtypes isolated from travelers and non-travelers from the north of Poland—A single center study. Infect Genet Evol. 2019;75:103926. doi: 10.1016/j.meegid.2019.103926 [DOI] [PubMed] [Google Scholar]
- 125.Clark CG. Extensive genetic diversity in Blastocystis hominis. Mol Biochem Parasitol. 1997;87(1):79–83. doi: 10.1016/s0166-6851(97)00046-7 [DOI] [PubMed] [Google Scholar]
- 126.Rezaei Riabi T, Mirjalali H, Haghighi A, Rostami Nejad M, Pourhoseingholi MA, Poirier P, et al. Genetic diversity analysis of Blastocystis subtypes from both symptomatic and asymptomatic subjects using a barcoding region from the 18S rRNA gene. Infect Genet Evol. 2018;61:119–126. doi: 10.1016/j.meegid.2018.03.026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All data generated during this study are included in the article and its supplemental material. Sequencing data from this study are available in the GenBank database, under the accession numbers MW728054- MW728093.