Abstract
Contrast-enhanced mammography (CEM) combines conventional mammography with iodinated contrast material to improve cancer detection. CEM has comparable performance to breast MRI without the added cost or time of conventional MRI protocols. Thus, this technique may be useful for indications previously reserved for MRI, such as problem-solving, determining disease extent in patients with newly diagnosed cancer, monitoring response to neoadjuvant therapy, evaluating the posttreatment breast for residual or recurrent disease, and potentially screening in women at intermediate- or high-risk for breast cancer. This article will provide a comprehensive overview on the past, present, and future of CEM, including its evolving role in the diagnostic and screening settings.
Keywords: contrast-enhanced mammography, contrast-enhanced digital mammography, contrast-enhanced spectral mammography, breast imaging
1. Introduction
Contrast-enhanced digital mammography (CEM) is a promising technique that uses iodinated contrast to detect tumor vascularity. The United States Food and Drug Administration (FDA) approved CEM for clinical use as an adjunct to mammography in 2011. The success of mammography, which is the most frequently used breast imaging modality for breast cancer detection, arises from its ability to detect morphologic abnormalities. However, in women with dense breasts who comprise nearly half of the screening population [1], mammography has decreased sensitivity of 62.9–69.4% compared to 81.5–87.0% in women with nondense breasts [2]. Breast cancers can be hidden by opaque dense tissue due to the lack of difference in x-ray attenuation between lesions and background tissue. CEM is similar to conventional mammography by being low cost and a relatively quick modality.
CEM provides morphologic and physiologic information similar to contrast-enhanced breast MRI, which has been demonstrated to be the most sensitive imaging technique in cancer detection with sensitivity of 96–99% [3–5]. Enhancement on MRI is related to tumor neovascularity, which may be apparent prior to morphologic changes on mammography or MRI. However, the widespread use of MRI has been limited due to its high cost, long scan times, and lack of availability [6].
This article will review the essential concepts of CEM, including technique, interpretation and reporting, clinical indications, advantages, disadvantages, and future directions.
2. CEM Technique
2.1. Historical Techniques
CEM technique has evolved over time. It was first introduced in 1985 with digital subtraction angiography of the breast [7,8]. The goal was to differentiate benign from malignant breast lesions to avoid surgical biopsy. This technique was performed by placing a catheter through an antecubital vein into the superior vena cava and obtaining multiple image exposures as contrast entered the vessels in the breast. However, given the invasive nature of this procedure and initial suboptimal results, its continued use was not pursued.
Subsequently, CEM was performed using temporal technique. In this technique, the breast is placed in compression and a pre-contrast image is acquired. Intravenous contrast material is then administered after which multiple postcontrast images are obtained over 5–7 minutes. The precontrast image is subtracted from postcontrast images, highlighting areas of contrast uptake. While the temporal technique is successful in detecting cancers [9,10], there are several disadvantages. It is subject to patient motion due to the long acquisition time. In addition, only one breast can be examined in one view limiting the ability to localize lesions that are detected. Any additional views of the ipsilateral or contralateral breast requires another dose of contrast. Finally, the breast is in compression during contrast administration, which may restrict blood flow and result in suboptimal tissue enhancement.
2.2. Current Technique
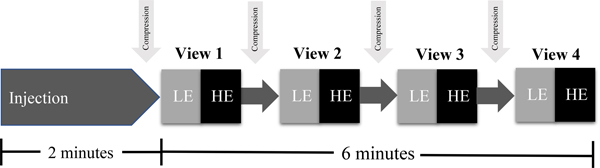
CEM using dual-energy energy technique, first described in 2003 by Lewin et. al. [11], was developed as an alternative to the temporal technique. Dual-energy imaging capitalizes on the different x-ray attenuation of breast tissue and iodine. It can be performed using standard mammography equipment with the addition of copper filtration and software upgrades to make the unit capable of dual-energy imaging. Prior to image acquisition, a non-ionic low-osmolar iodinated contrast material is administered intravenously via power injector at a dose of 1.5 ml/kg (maximum of 150 ml) at a rate of 3 ml/sec. Two minutes after completion of injection, the breast is placed into compression and paired low-energy and high-energy images are obtained in the standard craniocaudal and mediolateral oblique views (Fig. 1).
Fig. 1.
Steps of a contrast-enhanced mammography examination. Approximately two minutes after completion of contrast injection, the breast is placed in compression. Low-energy (LE) and high-energy (HE) images are sequentially acquired in the four standard mammographic views within the next six minutes.
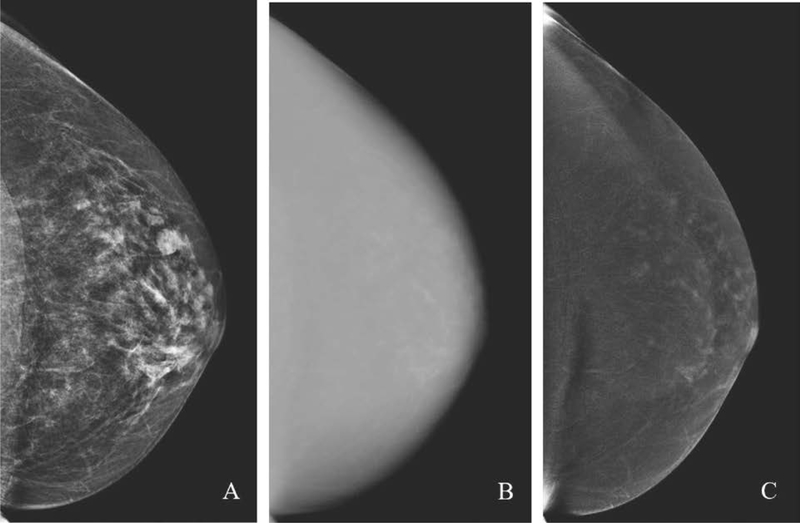
Low-energy images are acquired below the K-edge of iodine at a kVp of 28–33 and demonstrate only breast tissue, similar to full field digital mammography (FFDM). Studies have shown low-energy images are diagnostically equivalent to FFDM despite presence of intravenous contrast [12]. High-energy images are acquired above the K-edge of iodine at kVp of 45–49 and demonstrate iodine uptake and breast tissue but are noninterpretable. Post-processing generates recombined images which highlight areas of iodine uptake, i.e. enhancement, while suppressing signal from background tissue, similar to subtracted maximum intensity projection (MIP) images on MRI (Fig. 2). Iodine uptake in the breast is a depiction of tumor vascularity on CEM.
Fig. 2.
Paired low-energy (A) and high-energy (B) craniocaudal images are acquired after intravenous contrast agent administration. Post-processing generates a recombined (C) image, which highlights areas of iodine uptake. No suspicious findings are present on the recombined image shown here; scattered areas of enhancement are consistent with mild background parenchymal enhancement.
The optimal imaging window is 2–8 minutes after injection [13]. The images do not need to be obtained in a specific order with institutions varying in their practice [3]. Contrast remains present for up to 10 minutes, which allows for additional contrast enhanced views to be obtained. Diagnostic views, such as spot compression or magnification, can subsequently be performed if needed, typically with conventional mammographic technique.
3. CEM Interpretation and Reporting
A CEM-specific Breast Imaging Reporting and Data System (BI-RADS) lexicon does not currently exist for image interpretation but is under development. In the meantime, the BI-RADS mammography lexicon can be used to describe morphologic findings on low-energy images and the BI-RADS MRI lexicon sans kinetics to describe enhancing findings on recombined images [14]. Reporting should also include an assessment of breast density based on low-energy images and background parenchymal enhancement (BPE) using recombined images. A “malignancy potential score” (MPS) has been proposed by Tsigginou et al. [15] for final assessment reporting. This aggregate score combines the standard mammography BI-RADS score and a CEM score based on a 4-level scale of lesion enhancement ranging from negative to intense enhancement. They found the MPS score had improved diagnostic accuracy in breast cancer detection compared to FFDM or CEM scores alone.
When interpreting CEM, it is crucial to interpret low-energy and recombined images together with subsequent management based on both sets of images. Findings identified on low-energy images should be correlated with the recombined images and vice versa. When enhancement is present, the degree of enhancement may be an indicator of lesion invasiveness. Strong or medium enhancement is more frequently associated with invasive carcinomas [11,16]. However, lack of enhancement does not always exclude malignancy. It has been shown that enhancement may be decreased in invasive lobular carcinoma [17] and mucinous carcinoma [18]. Calcifications may be a manifestation of DCIS without associated enhancement, particularly if low-grade or small. CEM has the advantage of calcification detection on low-energy images, which is not possible with MRI. While the NPV of CEM has been reported to be as high as 93% in the evaluation of suspicious calcifications [19], calcifications with suspicious features on low-energy images should always undergo biopsy. When no enhancement is visualized at a site of low suspicion mammographic concern, such as a questioned asymmetry, CEM may be beneficial in excluding cancer.
If suspicious enhancement is identified on recombined images without a low-energy correlate, further workup is warranted with ultrasound (US) and/or MRI. These modalities can then be used to guide further management and image-guided biopsy if warranted as there is no CEM-guided biopsy system commercially available in the United States. CEM reports should include a management plan in case a correlate is not found on supplemental imaging. Six-month follow-up CEM can be considered for a low suspicion CEM finding without US or MRI correlate. Alternatively, to guide biopsy, a clip or seed is placed at the site of suspicious enhancement under CEM guidance followed by stereotactic needle biopsy targeting the clip or surgical excisional biopsy targeting the seed [20]. After biopsy using these multi-step methods, radiology-pathology correlation is crucial to determine if the area of concern was adequately sampled. Occasionally repeating CEM after clip placement may be of value.
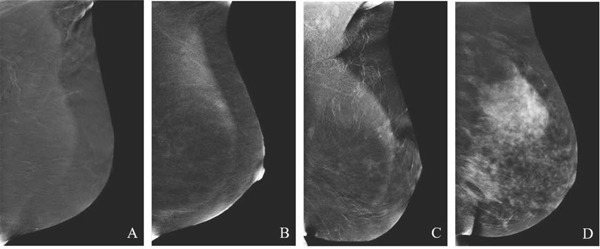
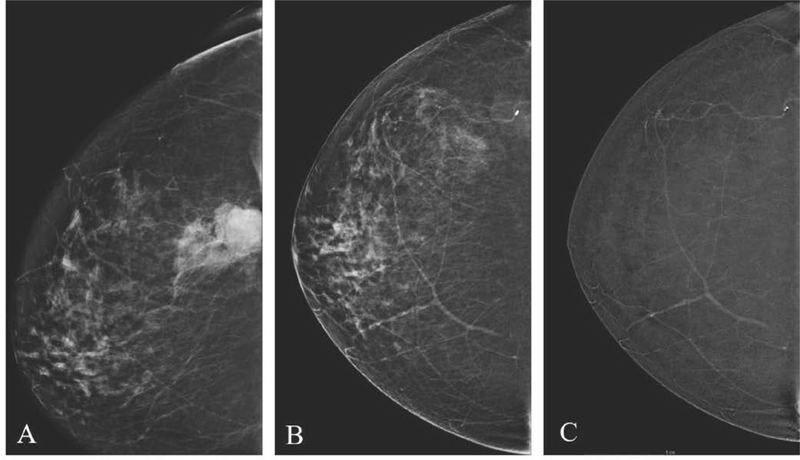
To identify pathologic enhancement on CEM, familiarity with the appearance of normal BPE is essential (Fig. 3). BPE on CEM is similar to MRI, and may lower sensitivity due to obscuration of enhancing lesions. Scheduling of CEM need not be based on timing of the menstrual cycle which appears to have minimal effect on BPE [21,22]. Similar to MRI, increased BPE levels on CEM may be an indication of breast cancer risk [23] and thus is important to report.
Fig. 3.
Background parenchymal enhancement (BPE) on CEM. Categories include minimal (A), mild (B), moderate (C), and marked (D).
Not all enhancing lesions on CEM are malignant, resulting in false-positive findings. Benign enhancing findings may include fibroadenoma, atypical ductal hyperplasia, papilloma, pseudoangiomatous stromal hyperplasia, abscess, and radial scar or complex sclerosing lesion [15,24].
Like other imaging modalities, CEM imaging artifacts can occur (Fig. 4), challenging interpretation [25–27]. Rim and skin line artifacts, due to scatter radiation from non-uniform thickness of breast tissue and skin, can mimic enhancement. Ripple artifact results from patient motion between acquisition of low-energy and high-energy images. Skin contamination from contrast material may occur, which presents as avid enhancement greater than seen with suspicious findings. Skin contamination may be visible even on low-energy images unlike contrast injected intravenously, which may be due to a higher concentration of contrast on skin leading to greater photon attenuation [28]. Additional artifacts include axillary line artifact due to use of small compression paddles and air artifact at sites of skin folds or scarring. Recognition of common imaging artifacts is essential for the interpreting radiologist to accurately identify suspicious enhancement.
Fig. 4.
Common artifacts seen on CEM. Rim artifact (A) and skin line artifact (B) from non-uniform scatter radiation in the breast and skin. Ripple artifact (C) from motion. Skin contamination artifact (D) from contrast spillage.
4. Clinical Performance of CEM
CEM has better sensitivity and specificity for breast cancer detection compared to FFDM and US and demonstrates a similar performance to MRI. In one of the earliest trials in 2011, Dromain et al. [24] performed unilateral CEM in 120 women who had suspected abnormalities on FFDM or US or had symptoms. They reported presence of enhancement in 75/80 (82%) of cancers. They concluded CEM had better diagnostic accuracy than FFDM alone or FFDM and US. In 2014, Jochelson et al. [3] evaluated bilateral CEM in 52 women with known cancer. They found that CEM detected known primary tumors at a rate comparable to MRI, each detecting 50/52 (96%) of the index lesions, which was significantly better than mammography, which detected 42/52 (81%). Comparing CEM and US ßfor breast cancer detection, Klang et al.[29] showed that CEM has significantly higher accuracy (64% versus 45%) and specificity (40% versus 8%) and greater sensitivity (97% versus 92%).
Since these initial studies, additional studies have demonstrated cancer detection sensitivities ranging from 92.7–100% and specificities ranging from 41–94% [18,30–35]. A recent meta-analysis by Zhu et al. [36] reported a pooled sensitivity and specificity of 89% (95% CI, 88–91) and 84% (95% CI, 82–85), respectively. The negative predictive value (NPV) of CEM is also high, ranging from 92–100% [18,19,32,37,38] with a positive predictive value (PPV) greater than MRI from 93–97% [3,5]. The available data support CEM as a high-quality alternative examination for patients unable to undergo MRI or for whom MRI is not recommended.
With the widespread adoption of digital breast tomosynthesis (DBT) in clinical practice, the combination of CEM and DBT has also been examined. In the largest study to date of 185 women, Chou et al. [39] showed that contrast-enhanced tomosynthesis (CET), CEM, and MRI had similar diagnostic accuracy in breast cancer detection and greater than FFDM and unenhanced DBT. However, the addition of CET to CEM did not result in a significant improvement in performance. Further studies will be necessary to evaluate the utility of CET.
5. Clinical Applications of CEM
Clinical applications of CEM include those that are currently accepted for MRI as both techniques are based on tumor enhancement (Table 1). A summary of studies evaluating the performance of CEM in the diagnostic and screening settings are provided in Tables 2 and 3.
Table 1:
Potential Clinical Indications for CEM
| Problem solving for inconclusive findings on screening mammography |
| Evaluation of the symptomatic patient |
| Preoperative assessment of disease extent |
| Neoadjuvant therapy response monitoring |
| Evaluation of the posttreatment breast |
| Screening in intermediate- and high-risk patients |
Table 2:
Evaluation of CEM in the Diagnostic Setting
| Clinical Indication | Study and Year | No. of Women | Modalities Compared | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Problem Solving | Lobbes et al, 2014 (33) | 113 | CEM vs FFDM | 100 | 88 | 76 | 100 |
| 97 | 42 | 40 | 97 | ||||
| Lalji et al, 2016 (18) | 199 | CEM vs LE | 97 | 70 | 58 | 98 | |
| 93 | 36 | 39 | 93 | ||||
|
| |||||||
| Symptomatic patient | Tennant et al, 2016 (34) | 100 | CEM vs LE | 95 | 81 | - | - |
| 84 | 63 | ||||||
| Luczynska et al, 2016 (32) | 116 | CEM vs FFDM vs US | 100 | 27 | 76 | 100 | |
| 90 | 22 | 73 | 47 | ||||
| 92 | 20 | 73 | 50 | ||||
| Sorin et al, 2020 (38) | 138 | CEM vs LE vs US | 100 | 73 | 57 | 100 | |
| 95 | 84 | 67 | 98 | ||||
| 100 | 68 | 52 | 100 | ||||
|
| |||||||
| Disease extent | Dromain et al, 2011 (24) | 120 | CEM vs FFDM vs FFDM + US | 93 | 56 | 73 | 85 |
| 80 | 50 | 67 | 66 | ||||
| 94 | 39 | 67 | 82 | ||||
| Jochelson et al, 2013 (3) | 52 | CEM vs FFDM vs MRI | 96 | 97 | |||
| 81 | - | - | - | ||||
| 96 | 85 | ||||||
| Fallenberg et al, 2014 (4) | 80 | CEM vs FFDM vs MRI | 100 | ||||
| 83 | - | - | - | ||||
| 97 | |||||||
| Lee-Felker et al, 2017 (5) | 52 | CEM vs MRI | 94 | 17 | 93 | 20 | |
| 99 | 4 | 60 | 67 | ||||
|
| |||||||
| Neoadjuvant therapy response | ElSaid et al, 2017 (50) | 21 | CEM | 100 | 83 | - | - |
| Iotti et al, 2017 (47) | 46 | CEM vs MRI | 100 | 84 | 57 | 100 | |
| 87 | 60 | 32 | 96 | ||||
| Barra et al, 2018 (48) | 33 | CEM vs FFDM vs MRI | 76 | 88 | 95 | 54 | |
| 76 | 63 | 86 | 45 | ||||
| 92 | 75 | 92 | 75 | ||||
| Patel et al, 2018 (49) | 65 | CEM vs MRI | 95 | 67 | 56 | 97 | |
| 95 | 69 | 58 | 97 | ||||
|
| |||||||
| Posttreatment breast | Helal et al, 2019 (51) | 76 | CEM vs FFDM | 91 | 75 | 78 | 90 |
| 50 | 22 | 38 | 32 | ||||
Note.–CEM = contrast-enhanced mammography, FFDM = full-field digital mammography, LE = low-energy imaging, MRI = magnetic resonance imaging, NPV = negative predictive value, PPV = positive predictive value.
Table 3:
Evaluation of CEM in the Screening Setting
| Study and Year | No. of Women | Modalities Compared | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
|
| ||||||
| Jochelson et al, 2017 (52) | 307 | CEM vs MRI | - | 95 | 15 (of biopsy) | - |
| 94 | 14 (of biopsy) | |||||
| Sorin et al, 2018 (53) | 611 | CEM vs CEM + US vs LE | 91 | 76 | 12 | 100 |
| 91 | 61 | 8 | 99 | |||
| 52 | 91 | 16 | 98 | |||
| Sung et al, 2019 (54) | 904 | CEM vs LE | 88 | 94 | 21 | 100 |
| 50 | 97 | 25 | 99 | |||
Note.–CEM = contrast-enhanced mammography, LE = low-energy imaging, MRI = magnetic resonance imaging, NPV = negative predictive value, PPV = positive predictive value. US = ultrasound.
5.1. Problem Solving for Inconclusive Findings
CEM can be a useful problem-solving tool in the diagnostic setting for patients with inconclusive findings on screening mammography. Lobbes et al. [33] performed a study in 113 women recalled from screening who underwent subsequent CEM. CEM improved diagnostic performance over FFDM with the most significant improvements in specificity and PPV, suggesting that CEM is particularly helpful in downgrading false-positive recalls. In a larger study with 199 women, Lalji et al. [18] demonstrated similar improved diagnostic performance of CEM. These results were observed for ten readers in the study regardless of their level of CEM experience, suggesting that CEM interpretation does not have a steep learning curve.
Zuley et al. [40] showed that CEM may also be valuable by reducing the number of biopsies of low suspicion lesions that do not enhance without compromising cancer detection. They found that the addition of CEM improved PPV of biopsy for low-intermediate suspicion lesions to 20.5% compared to 13.2% on FFDM [41]. Therefore, CEM may be beneficial in the diagnostic setting to not only detect more cancers but also to help eliminate unnecessary benign biopsies.
5.2. Evaluation of Symptomatic Patients
CEM may be beneficial for symptomatic patients. Tennant et al. [34] performed a retrospective analysis of 100 symptomatic women who underwent CEM on initial imaging. They found a statistically significant improvement in performance of CEM compared to low-energy images, with area under curve (AUC), 0.93 versus 0.83, respectively. CEM was helpful for diagnosis in 75% of the symptomatic cases. Luczynska et al. [32] compared performance of CEM, FFDM, and US in 116 symptomatic patients. CEM sensitivity was 100%, significantly higher than FFDM and US. CEM accuracy was also higher at 78% compared to FFDM (69%) and US (70%). While there was no significant difference between the AUC for CEM and US (both 0.83), they were significantly greater than that of FFDM at 0.69. More recently, Sorin et al. [38] demonstrated similar sensitivity and NPV for CEM with detection of fewer false-positive findings than US. CEM may potentially serve as a first-line imaging test in the symptomatic patient population (Fig. 5).
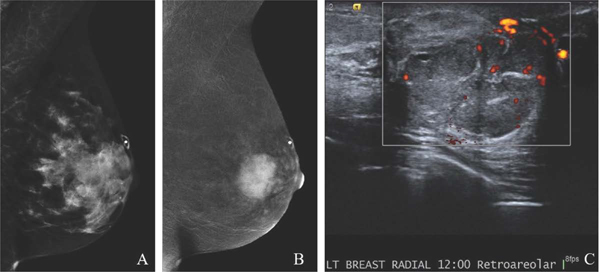
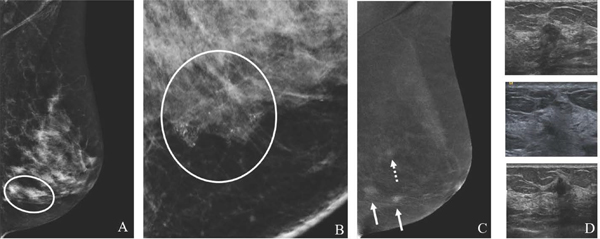
Fig. 5.
49-year-old with remote history of phyllodes tumor with new palpable left breast mass. MLO low-energy image (A) demonstrates an obscured mass underlying a triangular palpable skin marker. The full extent of the mass is not well delineated due to overlapping dense tissue. MLO recombined image (B) clearly shows a large avidly enhancing retroareolar mass. Targeted US image (C) demonstrates a solid vascular mass, which correlates to the CEM enhancement and palpable finding. US-guided biopsy yielded recurrent phyllodes tumor. CEM is beneficial in delineating the extent of suspicious abnormalities in areas of dense parenchymal breast tissue.
5.3. Preoperative assessment of disease extent
In patients with newly diagnosed breast cancer, additional imaging may be performed to determine disease extent or to identify disease in the contralateral breast (Fig. 6). While the value of supplemental imaging for staging, particularly MRI, remains controversial, CEM has proven to be superior to FFDM with or without US and comparable to or even better than MRI [3–5,24,42]. Jochelson et al. [3] found CEM was less sensitive than MRI for detection of additional ipsilateral disease, detecting 14 of 25 (56%) ipsilateral tumor foci compared to 22 (88%) foci with MRI. A subsequent study by Lee-Felker et al. [5] showed CEM to be as sensitive as MRI in detecting additional sites of disease with CEM identifying 11 of 11 (100%) of additional foci compared to 10 (91%) with MRI. Both studies found CEM had significantly fewer false-positives and higher PPV compared to MRI.
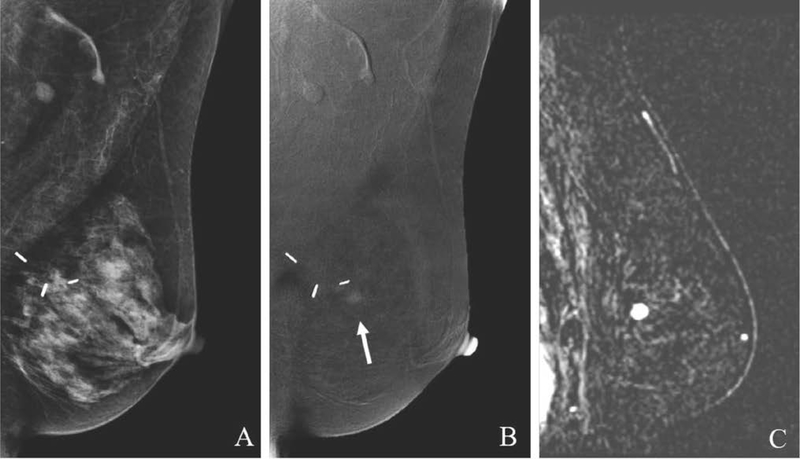
Fig. 6.
59-year-old women with history of LCIS with suspicious left breast calcifications on screening CEM. MLO low-energy image (A) and 2D ML magnification view (B) shows segmental fine pleomorphic calcifications in the lower breast (circle). MLO recombined image (C) demonstrates two enhancing masses (arrows) in the region of the calcifications and a superiorly located third mass (dashed arrow). Targeted US images (D) confirm three suspicious masses, two of which underwent US-guided biopsy yielding multicentric invasive ductal carcinoma. CEM may show greater extent of disease than conventional mammography, rendering more accurate staging.
Breast cancer staging not only consists of identifying multifocal, multicentric, or contralateral disease but also accurate tumor sizing for preoperative planning. Multiple studies have demonstrated CEM provides comparable or better tumor size estimation than MRI [4,24,43]. More recently, Patel et al. [44] compared tumor size correlation for CEM and FFDM in women with dense and nondense breasts. They found that CEM improved size estimation for both dense and non-dense breasts but the benefit was greater for dense breasts. The ability to have accurate staging information available immediately at the time of CEM may allow for a more expedited preoperative workup.
5.4. Monitoring Neoadjuvant Therapy Response
Neoadjuvant therapy is increasingly being used as initial treatment for locally advanced breast cancer and women with axillary adenopathy. Tumor size reduction to enable breast conservation is often an indication of neoadjuvant therapy. Thus, an accurate means to detect residual cancer is essential for surgical planning. While MRI is currently the most accurate imaging modality to evaluate response to neoadjuvant therapy in breast cancer patients [45,46], initial small studies show CEM may be comparable to MRI for this purpose [47–49].
ElSaid et al. [50] studied 21 women undergoing neoadjuvant chemotherapy and found CEM had 100% sensitivity and 83% specificity for detecting complete pathologic response. In a larger cohort of 46 women, Iotti et al. [47] prospectively compared CEM and MRI, which showed comparable performance metrics for complete response assessment and high correlation for tumor size measurement. Patel et al. [49] found similar performance values in the largest study to date in 65 women. These positive initial studies suggest a potential role for CEM in monitoring neoadjuvant therapy response (Fig. 7).
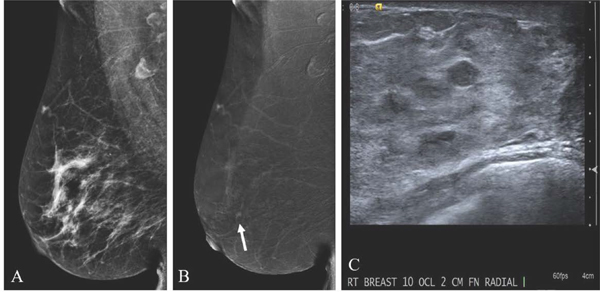
Fig. 7.
54-year-old with palpable right breast mass status post ultrasound-guided biopsy yielding invasive ductal carcinoma and subsequently treated with neoadjuvant chemotherapy. Pre-treatment digital mammogram (A) demonstrates a high density irregular mass in the posterior breast corresponding to the palpable finding and known cancer. Post-treatment low-energy image (B) performed six months later shows decreased mass surrounding the biopsy marker suggestive of residual disease. Post-treatment recombined image (C) shows no abnormal enhancement. Surgical pathology specimen from lumpectomy confirmed no residual carcinoma concordant with the lack of enhancement on CEM.
5.5. Evaluation of the Posttreatment Breast
Evaluation of patients after breast cancer treatment can be challenging due to overlap in appearances of benign post-treatment changes and cancer. Posttreatment changes, including parenchymal distortion and asymmetry, calcification, and seroma, may not be easily differentiated from cancer on FFDM, leading to missed cancers, additional imaging, and biopsies. CEM may confirm benign findings with the absence of enhancement in the postoperative bed. While enhancement in the postoperative bed should raise suspicion for residual or recurrent disease, it can also be the result of posttreatment inflammation, such as fat necrosis. Thus, assessment of the morphologic features of enhancement is crucial to determine need for further workup (Fig. 8).
Fig. 8.
70-year-old with remote history of left breast conservation therapy for invasive lobular carcinoma presenting for screening. Postsurgical changes are present in the upper posterior breast. MLO low-energy image (A) demonstrates no suspicious finding. MLO recombined image (B) shows an enhancing mass anterior to the lumpectomy site (arrow). Targeted ultrasound was negative (not shown). Sagittal MRI postcontrast subtraction image (C) shows an enhancing mass, which correlates to the CEM finding. MRI-guided biopsy yielded invasive lobular carcinoma recurrence.
There is little data assessing the role of CEM in cancer detection in the posttreatment breast. Helal et al. [51] found CEM improved the performance of FFDM with higher sensitivity, specificity, PPV, and NPV. False-positive findings on CEM were mostly due to enhancing fat necrosis. False-negative cases on CEM depicted minimal enhancement possibly due to extensive postoperative fibrosis limiting vascular perfusion and visualization of cancers.
5.6. Screening
CEM has primarily been used in the diagnostic setting and its role in screening remains to be determined [52–54]. The use of contrast material and its potential side effects deter the widespread acceptance of CEM in the general population. Patient populations at high risk of developing breast cancer, such as mutation carriers, benefit from annual breast MRI screening [55–57]. CEM may be an alternative for patients who are unable to undergo MRI. Additionally, there are intermediate-risk patient populations who do not qualify for MRI screening including women with personal history of breast cancer or high-risk lesion, family history of breast cancer, and dense breasts. Recently there has been concern about gadolinium contrast deposition in neuronal tissues [58]. While the clinical significance is unknown, it raises questions about using contrast-enhanced MRI annually for high-risk screening. Like any screening test, the potential risks of CEM will need to be weighed in relation to the benefits.
Several studies have evaluated the use of CEM in screening. A pilot study by Jochelson et al. [52] compared MRI to CEM in women at increased risk for breast cancer, including intermediate- and high-risk patients. Three cancers were detected in 307 patients with MRI identifying all three and CEM identifying two, none of which were visible on FFDM or low-energy images. The PPV for biopsy and specificity were similar for CEM and MRI. A larger follow-up study by Sung et al. [54] in 904 women with a similar risk profile found a cancer detection rate (CDR) of 15.5 of 1000 women screened, comparable to MRI at 14.9 of 1000 [57]. The incremental CDR was 6.7 of 1000. Sorin et al. [53] performed a study in 611 intermediate-risk women and observed a CDR of 31 of 1000 women screened with an incremental CDR of 13.1 of 1000.
As breast density notification laws continue to be adopted in more states, there is growing interest in supplemental screening for women with dense breasts. CEM has the advantage of not being limited by density given the ability of the recombination algorithm to suppress background tissue. Several studies have shown a greater benefit of CEM in women with dense breasts [30,31,42,53]. These initial studies suggest that CEM may have value as a supplemental screening tool in women at intermediate- and high-risk for breast cancer, leading to a more personalized approach to screening.
6. Advantages of CEM
Studies have consistently shown CEM to be superior to FFDM and comparable to MRI in terms of diagnostic accuracy. Thus, CEM may serve as an alternate modality for patients who are unable to undergo MRI due to contraindication or inaccessibility. Common contraindications to MRI, include metallic implants, claustrophobia, and weight limitations.
Other reasons to consider adopting CEM into clinical practice include patient comfort, cost, and accessibility. Several studies have shown that patients have a higher overall preference for CEM over MRI in both the diagnostic and screening settings [59,60]. Common reasons included shorter procedure time, greater comfort, and lower noise level [59]. Image acquisition time is much shorter for CEM at 7–10 minutes versus 30–60 minutes for MRI as fewer images are acquired for CEM. As a result, not only do patients better tolerate CEM but it also leads to increased throughput and less radiologist interpretation time. Even as abbreviated MRI protocols are becoming common, CEM can provide similar information more efficiently when performed at the time of the patient’s initial mammogram. CEM is less costly than a full or abbreviated MRI protocol with the total cost approximately four times less than a full screening MRI [6]. The costs and space and safety requirements of an MRI magnet limit its widespread availability. On the other hand, CEM can be implemented at pre-existing mammography sites with relative ease, resulting in increased patient access.
From an imaging standpoint, CEM may better depict cancers that tend to be missed on MRI due to its later acquisition. While most invasive cancers are thought to enhance due to the development of neoangiogenesis, DCIS may lack neoangiogenesis, especially at an early stage. Enhancement of DCIS on contrast-enhanced imaging has been proposed to be the result of contrast collecting inside ducts by diffusion [61]. The rate of diffusion is time-dependent, so the longer time delay between contrast injection and CEM exposure compared to MRI may result in stronger enhancement and better visualization of DCIS on CEM [4]. The differences in structure and mechanism of gadolinium and iodinated contrast agents may also contribute to variability in visibility of lesions on MRI and CEM [4].
7. Disadvantages of CEM
The greatest disadvantage of CEM limiting its widespread use is due to intravenous contrast material risks, including allergic reactions, extravasation events, and more rarely contrast-induced nephropathy. Adverse reactions have been reported in 0.6–0.8% of women undergoing CEM [62,63]; the majority of the cases were mild and self-limiting, suggesting that contrast agents are relatively safe. Nonetheless, staff members must have adequate training on appropriate contrast administration and management of reactions.
The radiation dose from CEM is higher than FFDM and DBT. Two separate exposures are performed per imaging position. The reported dose estimates for CEM vary from 20–80% higher than that of FFDM [24,64] and 20–42% higher than that of DBT [65,66]. Nonetheless, the radiation dose from CEM remains within the regulatory range for mammography.
A concern about implementation of CEM into a busy breast imaging practice is disruption of clinical workflow. Setting up the contrast injector, talking to the patient to assess for contraindications to contrast administration, creatinine testing, and placement and removal of an intravenous line are additional steps required to perform CEM, contributing to longer examination times [6,67]. Delegating these patient and equipment setup tasks between a trained nurse and mammography technologist has improved the efficiency of performing CEM in our practice.
A CEM-guided biopsy device is not yet commercially available in the United States, limiting efficiency of biopsy of CEM-only findings. As discussed previously, biopsy must currently be performed using an alternative imaging modality or two-procedure method with CEM-guided clip or seed placement followed by stereotactic-guided or surgical biopsy, respectively.
False-positive and false-negative findings may occur on CEM similar to other imaging modalities. Benign entities can demonstrate enhancement, which may lead to further workup with imaging and biopsy (Fig. 9). Cancers may be missed due to lack of enhancement, obscuration by background parenchymal enhancement, or exclusion from the field of view if located close to the chest wall or axilla (Fig. 10).
Fig. 9.
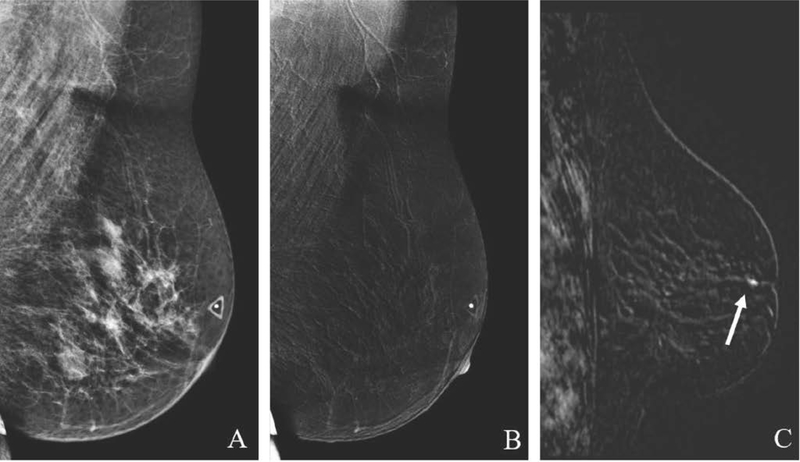
47-year-old woman with family history of breast cancer presents for screening with incidentally found false-positive finding in the right breast on CEM. MLO low-energy image (A) demonstrates no suspicious finding. MLO recombined image (B) reveals a subtle enhancing mass in the upper breast (arrow). Targeted US image (C) shows an oval mass with slightly indistinct margins. US-guided biopsy yielded benign fibroadenoma
Fig. 10.
47-year-old woman with left breast palpable mass with false-negative finding on CEM. MLO low-energy (A) and recombined (B) images demonstrate no suspicious finding. Ultrasound targeted to site of palpable concern was also negative (not shown). Given persistent palpable concern, MRI was performed. Sagittal MRI postcontrast subtraction image (C) shows an enhancing focus at the site of palpable concern in the retroareolar region. MRI-guided biopsy yielded ductal carcinoma in situ. CEM may not detect all cancers, particularly if they are small or low-grade.
8. Future Directions
Larger studies are necessary to evaluate the role of CEM in screening and are imminent. In 2019, the American College of Radiology (ACR), Breast Cancer Research Foundation, and GE Healthcare announced their plan for the Contrast Enhanced Mammography Imaging Screening Trial (CMIST). This large multicenter trial will compare the performance of CEM to a combination of DBT and whole breast US in screening women with dense breasts.
As the use of CEM is increasing in clinical practice, the need for a CEM-guided biopsy device has become apparent. In 2019, GE Healthcare revealed its CEM-guided biopsy system called Serena Bright. While FDA clearance in the United States is pending, the system has begun to be used in clinical practice in Spain [68]. GE Healthcare and the ACR are planning a clinical trial to compare CEM biopsy to other biopsy methods [69].
The rise of personalized medicine and advances in medical imaging have heralded the field of radiomics. Radiomics allows high-throughput extraction of quantitative features from medical images with the premise that they can be used to characterize tumors and predict outcomes in a non-invasive manner. While MRI has been the primary modality studied in radiomics research in breast imaging, CEM may also have a role. An initial study in 2019 by Marino et al. [70] demonstrated radiomics analysis using CEM has the potential for differentiation of cancers based on level of invasiveness, hormone receptor status, and tumor grade. They found accuracies as high as 79.6–87.4% using combinations of radiomic features on CEM to differentiate invasive and noninvasive breast cancers. Larger validation studies will be needed to identify significant imaging biomarkers on CEM that will allow accurate noninvasive characterization of breast cancers.
9. Conclusion
CEM is emerging as a multifaceted imaging modality for breast cancer detection. Diagnostic performance of CEM is superior to conventional mammography and similar to MRI with evidence of fewer false-positive findings. Compared to MRI, CEM has advantages of low cost, speed, and accessibility and is better tolerated by patients. As the popularity of CEM continues to grow, additional larger studies will be needed to validate its role as an acceptable contrast-enhanced alternative to MRI in the diagnostic and screening settings.
Highlights:
CEM is an emerging imaging technique for breast cancer detection.
CEM has primarily been used in the diagnostic setting.
CEM may have a role in screening intermediate- or high-risk women for breast cancer.
With similar diagnostic performance to MRI, CEM may be a comparable alternative.
References
- [1].Sprague BL, Gangnon RE, Burt V, Trentham-Dietz A, Hampton JM, Wellman RD, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst 2014;106. 10.1093/jnci/dju255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Carney PA, Miglioretti DL, Yankaskas BC, Kerlikowske K, Rosenberg R, Rutter CM, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med 2003;138:168–75. 10.7326/0003-4819-138-9-200305060-00025. [DOI] [PubMed] [Google Scholar]
- [3].Jochelson MS, Dershaw DD, Sung JS, Heerdt AS, Thornton C, Moskowitz CS, et al. Bilateral contrast-enhanced dual-energy digital mammography: feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology 2013;266:743–51. 10.1148/radiol.12121084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fallenberg EM, Dromain C, Diekmann F, Engelken F, Krohn M, Singh JM, et al. Contrast-enhanced spectral mammography versus MRI: Initial results in the detection of breast cancer and assessment of tumour size. Eur Radiol 2014;24:256–64. 10.1007/s00330-013-3007-7. [DOI] [PubMed] [Google Scholar]
- [5].Lee-Felker SA, Tekchandani L, Thomas M, Gupta E, Andrews-Tang D, Roth A, et al. Newly diagnosed breast cancer: comparison of contrast-enhanced spectral mammography and breast MR imaging in the evaluation of extent of disease. Radiology 2017;285:389–400. 10.1148/radiol.2017161592. [DOI] [PubMed] [Google Scholar]
- [6].Patel BK, Gray RJ, Pockaj BA. Potential cost savings of contrast-enhanced digital mammography. Am J Roentgenol 2017;208:W231–7. 10.2214/AJR.16.17239. [DOI] [PubMed] [Google Scholar]
- [7].Ackerman LV, Watt AC, Shetty P, Flynn MJ, Burke M, Kambouris A, Fine GWS. Breast lesions examined by digital angiography. Work in progress. Radiology 1985;155:65–68. 10.1097/00004424-198708000-00017. [DOI] [PubMed] [Google Scholar]
- [8].Watt AC, Ackerman LV, Shetty PC, Burke M, Flynn M, Grodsinsky C, Fine G, Wilderman S. Differentiation between benign and malignant disease of the breast using digital subtraction angiography of the breast. Cancer 1985;56:1287–1292. [DOI] [PubMed] [Google Scholar]
- [9].Jong RA, Yaffe MJ, Skarpathiotakis M, Shumak RS, Danjoux NM, Gunesekara A, et al. Contrast-enhanced digital mammography: initial clinical experience. Radiology 2003;228:842–50. 10.1148/radiol.2283020961. [DOI] [PubMed] [Google Scholar]
- [10].Diekmann F, Freyer M, Diekmann S, Fallenberg EM, Fischer T, Bick U, et al. Evaluation of contrast-enhanced digital mammography. Eur J Radiol 2011;78:112–21. 10.1016/j.ejrad.2009.10.002. [DOI] [PubMed] [Google Scholar]
- [11].Lewin JM, Isaacs PK, Vance V, Larke FJ. Dual-energy contrast-enhanced digital subtraction mammography: feasibility. Radiology 2003;229:261–8. 10.1148/radiol.2291021276. [DOI] [PubMed] [Google Scholar]
- [12].Francescone MA, Jochelson MS, Dershaw DD, Sung JS, Hughes MC, Zheng J, et al. Low energy mammogram obtained in contrast-enhanced digital mammography (CEDM) is comparable to routine full-field digital mammography (FFDM). Eur J Radiol 2014;83:1350–5. 10.1016/j.ejrad.2014.05.015. [DOI] [PubMed] [Google Scholar]
- [13].Smith A, Ph D. The Principles of Contrast Mammography 2014:8. [Google Scholar]
- [14].Kamal RM, Helal MH, Mansour SM, Haggag MA, Nada OM, Farahat IG, et al. Can we apply the MRI BI-RADS lexicon morphology descriptors on contrast-enhanced spectral mammography? Br J Radiol 2016;89. 10.1259/bjr.20160157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tsigginou A, Gkali C, Chalazonitis A, Feida E, Vlachos DE, Zagouri F, et al. Adding the power of iodinated contrast media to the credibility of mammography in breast cancer diagnosis. Br J Radiol 2016;89. 10.1259/bjr.20160397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Łuczyńska E, Niemiec J, Hendrick E, Heinze S, Jaszczyński J, Jakubowicz J, et al. Degree of enhancement on contrast enhanced spectral mammography (CESM) and lesion type on mammography (MG): comparison based on histological results. Med Sci Monit 2016;22:3886–93. 10.12659/MSM.900371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].van Nijnatten TJ, Jochelson MS, Pinker K, Keating DM, Sung JS, Morrow M, et al. Differences in degree of lesion enhancement on CEM between ILC and IDC. BJR|Open 2019;1:20180046. 10.1259/bjro.20180046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lalji UC, Houben IPL, Prevos R, Gommers S, van Goethem M, Vanwetswinkel S, et al. Contrast-enhanced spectral mammography in recalls from the Dutch breast cancer screening program: validation of results in a large multireader, multicase study. Eur Radiol 2016;26:4371–9. 10.1007/s00330-016-4336-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cheung YC, Tsai HP, Lo YF, Ueng SH, Huang PC, Chen SC. Clinical utility of dual-energy contrast-enhanced spectral mammography for breast microcalcifications without associated mass: a preliminary analysis. Eur Radiol 2016;26:1082–9. 10.1007/s00330-015-3904-z. [DOI] [PubMed] [Google Scholar]
- [20].Covington MF, Pizzitola VJ, Lorans R, Pockaj BA, Patel BK. The future of contrast-enhanced mammography. AJR Am J Roentgenol 2018;210:292–300. [DOI] [PubMed] [Google Scholar]
- [21].Sogani J, Morris EA, Kaplan JB, D’Alessio D, Goldman D, Moskowitz CS, et al. Comparison of background parenchymal enhancement at contrast-enhanced spectral mammography and breast MR imaging. Radiology 2017;282:63–73. 10.1148/radiol.2016160284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Savaridas SL, Taylor DB, Gunawardana D, Phillips M. Could parenchymal enhancement on contrast-enhanced spectral mammography (CESM) represent a new breast cancer risk factor? Correlation with known radiology risk factors. Clin Radiol 2017;72:1085.e1–1085.e9. 10.1016/j.crad.2017.07.017. [DOI] [PubMed] [Google Scholar]
- [23].Sorin V, Yagil Y, Shalmon A, Gotlieb M, Faermann R, Halshtok-Neiman O, et al. Background parenchymal enhancement at contrast-enhanced spectral mammography (CESM) as a breast cancer risk factor. Acad Radiol 2019:1–7. 10.1016/j.acra.2019.10.034. [DOI] [PubMed] [Google Scholar]
- [24].Dromain C, Thibault F, Muller S, Rimareix F, Delaloge S, Tardivon A, et al. Dual-energy contrast-enhanced digital mammography: Initial clinical results. Eur Radiol 2011;21:565–74. 10.1007/s00330-010-1944-y. [DOI] [PubMed] [Google Scholar]
- [25].Yagil Y, Shalmon A, Rundstein A, Servadio Y, Halshtok O, Gotlieb M, et al. Challenges in contrast-enhanced spectral mammography interpretation: artefacts lexicon. Clin Radiol 2016;71:450–7. 10.1016/j.crad.2016.01.012. [DOI] [PubMed] [Google Scholar]
- [26].Bhimani C, Li L, Liao L, Roth RG, Tinney E, Germaine P. Contrast-enhanced spectral mammography: modality-specific artifacts and other factors which may interfere with image quality. Acad Radiol 2017;24:89–94. 10.1016/j.acra.2016.08.024. [DOI] [PubMed] [Google Scholar]
- [27].Nori J, Gill MK, Vignoli C, Bicchierai G, De Benedetto D, Di Naro F, et al. Artefacts in contrast enhanced digital mammography: how can they affect diagnostic image quality and confuse clinical diagnosis? Insights Imaging 2020;11. 10.1186/s13244-019-0811-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gluskin J, Click M, Fleischman R, Dromain C, Morris EA, Jochelson MS. Contamination artifact that mimics in-situ carcinoma on contrast-enhanced digital mammography. Eur J Radiol 2017;95:147–54. 10.1016/j.ejrad.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Klang E, Krosser A, Amitai MM, Sorin V, Halshtok Neiman O, Shalmon A, et al. Utility of routine use of breast ultrasound following contrast-enhanced spectral mammography. Clin Radiol 2018;73:908.e11–908.e16. 10.1016/j.crad.2018.05.031. [DOI] [PubMed] [Google Scholar]
- [30].Mori M, Akashi-Tanaka S, Suzuki S, Daniels MI, Watanabe C, Hirose M, et al. Diagnostic accuracy of contrast-enhanced spectral mammography in comparison to conventional full-field digital mammography in a population of women with dense breasts. Breast Cancer 2017;24:104–10. 10.1007/s12282-016-0681-8. [DOI] [PubMed] [Google Scholar]
- [31].Cheung YC, Lin YC, Wan YL, Yeow KM, Huang PC, Lo YF, et al. Diagnostic performance of dual-energy contrast-enhanced subtracted mammography in dense breasts compared to mammography alone: interobserver blind-reading analysis. Eur Radiol 2014;24:2394–403. 10.1007/s00330-014-3271-1. [DOI] [PubMed] [Google Scholar]
- [32].Luczynska E, Heinze S, Adamczyk A, Janusz R, Mitus JW, Hendrick E. Comparison of the mammography, contrast-enhanced spectral mammography and ultrasonography in a group of 116 patients. Anticancer Res 2016;4366:4359–66. [PubMed] [Google Scholar]
- [33].Lobbes MBI, Lalji U, Houwers J, Nijssen EC, Nelemans PJ, Van Roozendaal L, et al. Contrast-enhanced spectral mammography in patients referred from the breast cancer screening programme. Eur Radiol 2014;24:1668–76. 10.1007/s00330-014-3154-5. [DOI] [PubMed] [Google Scholar]
- [34].Tennant SL, James JJ, Cornford EJ, Chen Y, Burrell HC, Hamilton LJ, et al. Contrast-enhanced spectral mammography improves diagnostic accuracy in the symptomatic setting. Clin Radiol 2016;71:1148–55. 10.1016/j.crad.2016.05.009. [DOI] [PubMed] [Google Scholar]
- [35].Luczyńska E, Heinze-Paluchowska S, Dyczek S, Blecharz P, Rys J, Reinfuss M. Contrast-enhanced spectral mammography: comparison with conventional mammography and histopathology in 152 women. Korean J Radiol 2014;15:689–96. 10.3348/kjr.2014.15.6.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhu X, Huang JM, Zhang K, Xia LJ, Feng L, Yang P, et al. Diagnostic value of contrast-enhanced spectral mammography for screening breast cancer: systematic review and meta-analysis. Clin Breast Cancer 2018;18:e985–95. 10.1016/j.clbc.2018.06.003. [DOI] [PubMed] [Google Scholar]
- [37].Patel BK, Naylor ME, Kosiorek HE, Lopez-Alvarez YM, Miller AM, Pizzitola VJ, et al. Clinical utility of contrast-enhanced spectral mammography as an adjunct for tomosynthesis-detected architectural distortion. Clin Imaging 2017;46:44–52. 10.1016/j.clinimag.2017.07.003. [DOI] [PubMed] [Google Scholar]
- [38].Sorin V, Faermann R, Yagil Y, Shalmon A, Gotlieb M, Halshtok-Neiman O, et al. Contrast-enhanced spectral mammography (CESM) in women presenting with palpable breast findings. Clin Imaging 2020;61:99–105. 10.1016/j.clinimag.2020.01.019. [DOI] [PubMed] [Google Scholar]
- [39].Chou CP, Lewin JM, Chiang CL, Hung BH, Yang TL, Huang JS, et al. Clinical evaluation of contrast-enhanced digital mammography and contrast enhanced tomosynthesis - comparison to contrast-enhanced breast MRI. Eur J Radiol 2015;84:2501–8. 10.1016/j.ejrad.2015.09.019. [DOI] [PubMed] [Google Scholar]
- [40].Zuley ML, Bandos AI, Abrams GS, Ganott MA, Gizienski TA, Hakim CM, et al. Contrast enhanced digital mammography (CEDM) helps to safely reduce benign breast biopsies for low to moderately suspicious soft tissue lesions. Acad Radiol 2019:1–8. 10.1016/j.acra.2019.07.020. [DOI] [PubMed] [Google Scholar]
- [41].Elezaby M, Li G, Bhargavan-Chatfield M, Burnside ES, Demartini WB. ACR BI-RADS assessment category 4 subdivisions in diagnostic mammography: utilization and outcomes in the National Mammography Database. Radiology 2018;287:416–22. 10.1148/radiol.2017170770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Fallenberg EM, Dromain C, Diekmann F, Renz DM, Amer H, Ingold-Heppner B, et al. Contrast-enhanced spectral mammography: does mammography provide additional clinical benefits or can some radiation exposure be avoided? Breast Cancer Res Treat 2014;146:371–81. 10.1007/s10549-014-3023-6. [DOI] [PubMed] [Google Scholar]
- [43].Lobbes MBI, Lalji UC, Nelemans PJ, Houben I, Smidt ML, Heuts E, et al. The quality of tumor size assessment by contrast-enhanced spectral mammography and the benefit of additional breast MRI. J Cancer 2015;6:144–50. 10.7150/jca.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Patel BK, Garza SA, Eversman S, Lopez-Alvarez Y, Kosiorek H, Pockaj BA. Assessing tumor extent on contrast-enhanced spectral mammography versus full-field digital mammography and ultrasound. Clin Imaging 2017;46:78–84. 10.1016/j.clinimag.2017.07.001. [DOI] [PubMed] [Google Scholar]
- [45].Rosen EL, Blackwell KL, Baker JA, Soo MS, Bentley RC, Yu D, et al. Accuracy of MRI in the detection of residual breast cancer after neoadjuvant chemotherapy. Am J Roentgenol 2003;181:1275–82. 10.2214/ajr.181.5.1811275. [DOI] [PubMed] [Google Scholar]
- [46].Marinovich ML, Macaskill P, Irwig L, Sardanelli F, Mamounas E, von Minckwitz G, et al. Agreement between MRI and pathologic breast tumor size after neoadjuvant chemotherapy, and comparison with alternative tests: Individual patient data meta-analysis. BMC Cancer 2015;15:1–12. 10.1186/s12885-015-1664-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Iotti V, Ravaioli S, Vacondio R, Coriani C, Caffarri S, Sghedoni R, et al. Contrast-enhanced spectral mammography in neoadjuvant chemotherapy monitoring: A comparison with breast magnetic resonance imaging. Breast Cancer Res 2017;19:1–13. 10.1186/s13058-017-0899-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Barra FR, Sobrinho AB, Barra RR, Magalhães MT, Aguiar LR, Albuquerque GFL, et al. Contrast-enhanced mammography (CEM) for detecting residual disease after neoadjuvant chemotherapy: A comparison with breast magnetic resonance imaging (MRI). Biomed Res Int 2018;2018. 10.1155/2018/8531916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Patel BK, Hilal T, Covington M, Zhang N, Kosiorek HE, Lobbes M, et al. Contrast-enhanced spectral mammography is comparable to MRI in the assessment of residual breast cancer following neoadjuvant systemic therapy. Ann Surg Oncol 2018;25:1350–6. 10.1245/s10434-018-6413-x. [DOI] [PubMed] [Google Scholar]
- [50].ElSaid NAE, Mahmoud HGM, Salama A, Nabil M, ElDesouky ED. Role of contrast enhanced spectral mammography in predicting pathological response of locally advanced breast cancer post neo-adjuvant chemotherapy. Egypt J Radiol Nucl Med 2017;48:519–27. 10.1016/j.ejrnm.2017.03.022. [DOI] [Google Scholar]
- [51].Helal MH, Mansour SM, Ahmed HA, Abdel Ghany AF, Kamel OF, Elkholy NG. The role of contrast-enhanced spectral mammography in the evaluation of the postoperative breast cancer. Clin Radiol 2019;74:771–81. 10.1016/j.crad.2019.06.002. [DOI] [PubMed] [Google Scholar]
- [52].Jochelson MS, Pinker K, Dershaw DD, Hughes M, Gibbons GF, Rahbar K, et al. Comparison of screening CEDM and MRI for women at increased risk for breast cancer: a pilot study. Eur J Radiol 2017;97:37–43. 10.1016/j.ejrad.2017.10.001. [DOI] [PubMed] [Google Scholar]
- [53].Sorin V, Yagil Y, Yosepovich A, Shalmon A, Gotlieb M, Neiman OH, et al. Contrast-enhanced spectral mammography in women with intermediate breast cancer risk and dense breasts 2018:1–8. 10.2214/AJR.17.19355. [DOI] [PubMed] [Google Scholar]
- [54].Sung JS, Lebron L, Keating D, D’Alessio D, Comstock CE, Lee CH, et al. Performance of dual-energy contrast-enhanced digital mammography for screening women at increased risk of breast cancer. Radiology 2019;293:81–8. 10.1148/radiol.2019182660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kuhl CK, Schmutzler RK, Leutner CC, Kempe A, Wardelmann E, Hocke A, et al. Breast MR imaging screening in 192 women proved or suspected to be carrier of a breast cancer susceptibility gene: preliminary results. Radiology 2000;215:267–79. 10.1148/radiology.215.1.r00ap01267. [DOI] [PubMed] [Google Scholar]
- [56].Leach MO, Boggis C, Dixon A, Easton D, Eeles R, Evans DG, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet 2005;365:1769–78. 10.1016/S0140-6736(05)66481-1. [DOI] [PubMed] [Google Scholar]
- [57].Kuhl C, Weigel S, Schrading S, Arand B, Bieling H, König R, et al. Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: the EVA trial. J Clin Oncol 2010;28:1450–7. 10.1200/JCO.2009.23.0839. [DOI] [PubMed] [Google Scholar]
- [58].McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, et al. Gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015;277:924–5. 10.1148/radiol.2015150697. [DOI] [PubMed] [Google Scholar]
- [59].Hobbs MM, Taylor DB, Buzynski S, Peake RE. Contrast-enhanced spectral mammography (CESM) and contrast enhanced MRI (CEMRI): Patient preferences and tolerance. J Med Imaging Radiat Oncol 2015;59:300–5. 10.1111/1754-9485.12296. [DOI] [PubMed] [Google Scholar]
- [60].Phillips J, Miller MM, Mehta TS, Fein-Zachary V, Nathanson A, Hori W, et al. Contrast-enhanced spectral mammography (CESM) versus MRI in the high-risk screening setting: patient preferences and attitudes. Clin Imaging 2017;42:193–7. 10.1016/j.clinimag.2016.12.011. [DOI] [PubMed] [Google Scholar]
- [61].Fan X, Vogt S, Krausz T, Newstead GM, Karczmar GS. Ductal carcinoma in situ: x-ray fluorescence microscopy and dynamic contrast-enhanced MR imaging reveal gadolinium uptake within neoplastic mammary ducts in a murine model 2009;253:399–406. 10.1148/radiol.2533082026/-/DC1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Houben IPL, Van de Voorde P, Jeukens CRLPN, Wildberger JE, Kooreman LF, Smidt ML, et al. Contrast-enhanced spectral mammography as work-up tool in patients recalled from breast cancer screening has low risks and might hold clinical benefits. Eur J Radiol 2017;94:31–7. 10.1016/j.ejrad.2017.07.004. [DOI] [PubMed] [Google Scholar]
- [63].Zanardo M, Cozzi A, Trimboli RM, Labaj O, Monti CB, Schiaffino S, et al. Technique, protocols and adverse reactions for contrast-enhanced spectral mammography (CESM): a systematic review. Insights Imaging 2019;10. 10.1186/s13244-019-0756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Jeukens CRLPN, Lalji UC, Meijer E, Bakija B, Theunissen R, Wildberger JE, et al. Radiation exposure of contrast-enhanced spectral mammography compared with full-field digital mammography. Invest Radiol 2014;49:659–65. 10.1097/RLI.0000000000000068. [DOI] [PubMed] [Google Scholar]
- [65].James JR, Pavlicek W, Hanson JA, Boltz TF, Patel BK. Breast radiation dose with CESM compared with 2D FFDM and 3D tomosynthesis mammography. Am J Roentgenol 2017;208:362–72. 10.2214/AJR.16.16743. [DOI] [PubMed] [Google Scholar]
- [66].Phillips J, Mihai G, Hassonjee SE, Raj SD, Palmer MR, Brook A, et al. Comparative dose of contrast-enhanced spectral mammography (CESM), digital mammography, and digital breast tomosynthesis. Am J Roentgenol 2018;211:839–46. 10.2214/AJR.17.19036. [DOI] [PubMed] [Google Scholar]
- [67].Phillips J, Steinkeler J, Talati K, Brook A, Dialani V, Fishman M, et al. Workflow considerations for incorporation of contrast-enhanced spectral mammography into a breast imaging practice. J Am Coll Radiol 2018;15:881–5. 10.1016/j.jacr.2018.02.012. [DOI] [PubMed] [Google Scholar]
- [68].Alcantara R Guided breast biopsies in contrast-enhanced spectral mammography. Diagnostic Imaging Eur 2020:56–9. [Google Scholar]
- [69].Palmer WJ. GE Healthcare introduces first contrast-enhanced mammography biopsy. Diagn Imaging, last modified December 3, 2019. https://www.diagnosticimaging.com/rsna-conference/ge-healthcare-introduces-first-contrast-enhanced-mammography-biopsy (accessed April 8, 2020).
- [70].Marino MA, Pinker K, Leithner D, Sung J, Avendano D, Morris EA, et al. Contrast-enhanced mammography and radiomics analysis for noninvasive breast cancer characterization: initial results. Mol Imaging Biol 2019. 10.1007/s11307-019-01423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]