Abstract
Health-related quality of life (HRQoL) is a multidimensional concept including physical, emotional, social, and cognitive functions, disease symptoms, and side effects of treatment. Differences in HRQoL due to gender, existence of comorbidities, and number of chemotherapy cycles are little explored in diffuse large B-cell lymphoma (DLBCL) survivors. Our objective was to investigate whether differences in HRQoL in function of these factors exist 1 year after the diagnosis of DLBCL. One hundred and one patients, enrolled in the RT3 (Real-Time Tailored Therapy) Study, answered self-administrated European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30), EORTC High-Grade Non-Hodgkin Lymphoma (NHL-HG29), Hospital Anxiety and Depression Scale (HADS), Post Traumatic Growth Inventory (PTGI), and Multidimensional Fatigue Inventory (MFI) questionnaires. Adjusted means of scores were calculated in multivariate linear regression models. Fifty-seven survivors (mean age of 58.5 years) answered all questionnaires. Women have significantly higher scores of posttraumatic growth and lower physical functioning than men (P < 0.04). Survivors with comorbidities have increased physical fatigue and symptom burden, increased emotional impact, mental fatigue and depression, and reduced physical functioning and global health status (all P < 0.05). A greater number of cycles of chemotherapy increase the level of symptoms (pain, neuropathy, and dyspnoea; P < 0.05). The various aspects related to HRQoL should be discussed with DLBCL patients and investigated, with the aim of developing strategies to ensure appropriate psychosocial and supportive care and to improve the HRQoL in these patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00277-021-04689-4.
Keywords: Lymphoma, Survivors, Comorbidities, Chemotherapy, Quality of life
Introduction
Non-Hodgkin lymphoma (NHL) includes various subtypes, with distinct epidemiological, immune-phenotypic, genetic, clinical features, and response to therapy [1]. Among those, diffuse large B-cell lymphoma (DLBCL) constitutes a heterogeneous clinicopathologic entity accounting for 30% of NHL [2]. In metropolitan France, DLBCL is the most common histological subtype of NHL and the second most common haematological malignancy after multiple myeloma/plasmacytoma, with a number of incident cases estimated at 5,071 in 2018 (2,778 in male and 2,293 in female), at a median age at diagnosis of 69 and 71 years in men and women, respectively [3]. The treatment of DLBCL varies, depending on tumour stage and patient factors (e.g. age and performance status) [1]. DLBCL is classically treated with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) and rituximab (R-CHOP) [4–6] that can have long-term consequences on the quality of life of patients.
Health-related quality of life (HRQoL) is a multidimensional concept that covers the subjective perceptions of the positive and negative aspects of cancer patients’ symptoms, including physical, emotional, social, and cognitive functions, disease symptoms, and side effects of treatment [7]. With chemotherapy treatments, patients may report more anxiety and depression compared to a normative population [8, 9], high levels of psychological distress [10], decreased physical and emotional well-being [11], more fatigue [9, 10, 12, 13], sleep problems [10], and lower HRQoL [9]. NHL chemotherapy-specific toxicities include neuropathy [14], cardiotoxicity, and secondary cancers [6].
Some studies have investigated HRQoL in patients with DLBCL after completion of chemotherapy and used one or two psychometric scales to measure some components of quality of life [8, 9, 12, 15–18]. In addition, differences in certain areas of quality of life due to gender, to presence of comorbidities, or linked to treatments are still little explored. In this context, we proposed a cross-sectional study to evaluate several facets of the quality of life, by administering various psychometric scales in survivors, a year after the diagnosis of their DLBCL. Our main objective was to investigate whether differences in the HRQoL between women and men exist, in particular in terms of symptoms, fatigue, mood disorder, and overall health status. The second objective was to investigate if other factors such as the presence of comorbidities affect the DLBCL survivors’ quality of life scores and if the number of chemotherapy cycles affects particularly the survivors’ symptoms scores.
Material and methods
Study population
This study is part of the RT3 (Real-Time Tailored Therapy) project conducted by the Lymphoma Academic Research Organisation (LYSARC) (Lyon South Hospital Center, Pierre-Bénite, France) [clinicaltrial.gov NCT03104478]. The RT3 is a multicentre longitudinal Study on the “Real-time molecular characterization of Diffuse Large B-Cell Lymphoma (DLBCL)” in patients and covers the follow-up of these patients over 24 months from the time of diagnosis.
Among 201 patients enrolled in the RT3 Study [19], 138 patients had a detailed histological characterization. The DLBCL patients included in the present project were enrolled in the RT3 Study between November 2018 and September 2019, were distributed all over France, and were followed in 15 hospitals specializing in the treatment of haematological cancers (Supplementary Information 1). Twelve months after diagnosis, the 101 eligible DLBCL patients participated in a routine medical examination and were invited to answer a self-administrated “Lymphoma Quality of Life” (LQL) questionnaire.
The planned medical examination occurred in the majority of cases in 2020, during the COVID-19 pandemic. In France, this is the period between March 15 and December 31, 2020, and included two confinements of populations (between March 17 and May 11, 2020, and between October 30 and November 28, 2020). The medical team has proposed to each patient to answer the LQL questionnaire, either online on the study website (https://calym-main.ches.pro) or in print, according to their choice. All the patients who gave their consent to participate received an explanatory note and those who opted for the online version received personal, confidential codes for connection to the study platform.
Also, to be eligible to answer the LQL questionnaire, volunteers had to live in metropolitan France, be 18 years old or above, and not to have experienced a relapse/progression or their lymphoma. Data on sex, age of the patients at the time of inclusion in the RT3 Study, reported comorbidities (type 2 diabetes, arterial hypertension, cardiovascular illnesses, other previous cancer), the Ann Arbor staging system at diagnosis (I, II, III, IV) [20], the Hans classification [21], the ICD-O Lymph Node Site Codes [22], the followed therapies, and the number of treatment cycles were prospectively collected and included from the RT3 study database. The main therapies used were monoclonal antibodies (Rituximab, R) associated with chemotherapy: R-CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) cycles over 14 days (R-CHOP-14) or 21 days (R-CHOP-21), R-mini-CHOP (reduced CHOP dose in elderly patients), R-ACVBP (doxorubicin: Adriamycin, cyclophosphamide, vindesine, bleomycin, and prednisone), or associated with experimental therapies. The mini-CHOP and CHOP-21 correspond to a moderate conventional chemotherapy, and CHOP-14 and ACVBP to a more intensive chemotherapy [23].
Questionnaire and study measures
The LQL questionnaire was structured in two parts which collected information on (1) the socio-economic and familial characteristics of the participants, such as marital status, financial level, and to be professionally active; and (2) the answers to 5 psychometric scales (French validated versions) concerning the HRQoL after lymphoma.
The five psychometric scales proposed (more details are given in Supplementary Information 2) were:
The French-validated version of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) version 3 was used to assess HRQoL of patients with lymphoma. The questionnaire includes the global health status, five functional scales, and nine symptom scales or items [24, 25].
-
2.
The French version of the High-Grade Non-Hodgkin Lymphoma (NHL-HG29) is a module with 29 items that completes the EORTC QLQ-C30. It incorporates five multi-item scales to assess symptom burden due to disease and/or treatment, neuropathy, physical condition/fatigue, emotional impact, and worries/fears about health and functioning [25, 26].
-
3.
The Hospital Anxiety and Depression Scale (HADS) is an instrument for detecting states of anxiety and depression that would facilitate the large task of detection and management of emotional disorder in patients under investigation and treatment in medical and surgical departments [27, 28]. The French version was validated in a population of hospitalized cancer patients [29].
-
4.
The Post Traumatic Growth Inventory (PTGI) is an instrument for assessing positive outcomes reported by persons who have experienced traumatic events [30], which has been validated in French [31]. The PTGI is composed of 5 subscales, which include factors of new possibilities, relating to others, personal strength, spiritual change, and appreciation of life.
Statistical analyses
Comparisons were made between the demographic and health-related characteristics of people who agreed to participate in the study and those who refused to participate, and between female and male participants (main objective), using the Chi2 test, Fisher’s exact test, and Student’s t test, depending on the variables. The median was used to dichotomize the variables: age at entry into the study, number of months between entry into the study and the response to the LQL questionnaire, and number of treatment cycles followed, because the linearity of the distribution of these variables was not assumed.
On the other hand, the median age of 60 also corresponds to the International Prognostic Index (IPI) which predicts the prognosis of patients with aggressive non-Hodgkin lymphoma [34], and the median of 6 chemotherapy cycles corresponds to the usual number of treatment cycles that DLBCL stage I–II patients receive.
The scores of the different psychometric scales were calculated according to the algorithms published by their authors. Pearson correlation coefficients were calculated between the subscales of the five psychometric scales. Spearman’s correlation coefficients were calculated between the different QoL subscales and the other categorical variables recorded in the study, in order to identify strongly correlated factors.
Adjusted means of scores and their 95% confidence intervals (95% CI) by gender (female/male), presence of comorbidities (at least one, yes/no), and number of treatment cycles (> 6 cycles vs. ≤ 6 cycles) were calculated in multivariate linear regression models. Analyses concerning the QoL according to gender and presence of comorbidities were performed for all the psychometric scales/subscales, while analyses according to the number of treatment cycles were performed only for psychometric subscales that include symptoms. Adjustments were made for several variables (forced in the model): age at inclusion in the study (≥ 60 years vs. < 60 years), number of months between inclusion and response to the LQL questionnaire (> 13 months vs. ≤ 13 months), Ann Arbor stage (III–IV vs. I–II), type of therapy followed (R-CHOP-14, R-ACVBP, and R-Experiment therapy vs. mini-R-CHOP and R-CHOP-21), and response obtained during the COVID-19 pandemic (yes/no).
Reference values in the general population were used for comparison purposes. Age- and sex-matched French normative population [35] was used to compare and interpret EORTC QLQ-C30 scores. Scores of DLBCL survivors were considered ‘clinically relevant’, if the difference from the scores recorded in the reference population was greater than 10 points [36, 37].
Statistical tests were 2-sided, and statistical significance was defined as a P < 0.05. All analyses were performed using the SAS statistical software package (version 9.4; SAS Institute, Cary, NC).
Ethical approvals
All participants received a written briefing note and gave their consent to participate, first in the RT3 study and then in the Lymphoma Quality of Life study. The project has been approved by the Person Protection Committee 8 Ile-de-France, on July 9, 2019.
Results
Characteristics of participants and nonparticipants
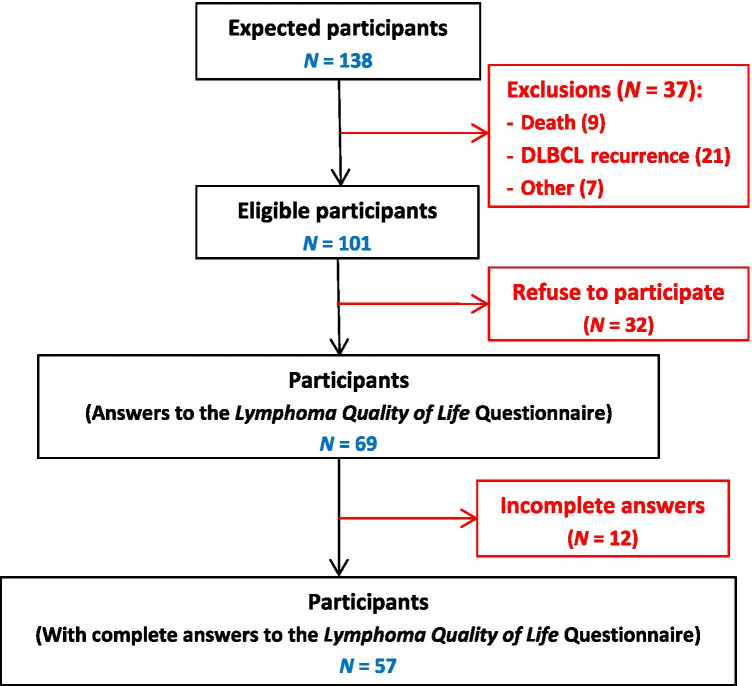
Sixty-eight percent (69/101) of potentially eligible patients answered the LQL questionnaire, and 82% of them have responded to all the proposed psychometric scales (Fig. 1). Participants and nonparticipants did not differ on all the characteristics taken into consideration (Table 1). However, the nonparticipants were slightly older, reported slightly more comorbidities, had more high (III–IV) Ann Arbor Stage (72% vs. 62%), and were slightly more likely to have received more than 6 cycles of treatment than the participants (41.9% vs. 33.3%).
Fig. 1.
Study diagram
Table 1.
Comparison of characteristics between participants (N = 69) and nonparticipants (N = 32) in the Lymphoma Quality of Life Study
| Characteristics | Participants (N = 69) |
Nonparticipants (N = 32) |
Comparison (p-Value) |
|
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| Range | Range | |||
| Age at inclusion in the ‘RT3’ study (years) | 60.3 (14.8) | 64.3 (14.9) | 0.211a | |
| 20–84 | 29–92 | |||
| N (%) | N (%) | |||
| Sex | Male | 38 (55.07) | 21 (65.63) | 0.317b |
| Female | 31 (44.93) | 11 (34.38) | ||
| Health-related characteristics | ||||
| Ann Arbor Stage | I–II | 26 (37.68) | 9 (28.12) | 0.348b |
| III–IV | 43 (62.32) | 23 (71.88) | ||
| (N = 62) | (N = 31) | |||
| HANS results | Germinal centre B-cell–like (GCB) | 33 (53.23) | 17 (54.84) | 0.883b |
| Non-GCB | 29 (46.77) | 14 (45.16) | ||
| ICD-Oc | (N = 63) | (N = 30) | ||
| DLBCL | 51 (80.95) | 24 (80.00) | 0.841d | |
| Primary mediastinal large B-cell lymphoma (PMBL) | 6 (9.52) | 2 (6.67) | ||
| High grade B-cell lymphoma with double/triple-hit or NOS (HGBL)/DLBCL not otherwise specified (DLBCL-NOS) | 6 (9.52) | 4 (13.33) | ||
| Comorbidities | ||||
| Type 2 diabetes | Yes | 6 (8.70) | 7 (21.88) | 0.107d |
| No | 63 (91.30) | 25 (78.13) | ||
| Arterial hypertension | Yes | 21 (30.43) | 10 (31.25) | 0.934b |
| No | 48 (69.57) | 22 (68.75) | ||
| Cardiovascular illnesses | Yes | 12 (17.39) | 4 (12.50) | 0.770d |
| No | 57 (82.61) | 28 (87.50) | ||
| Cancer (other) | Yes | 4 (5.80) | 5 (15.73) | 0.137d |
| No | 65 (94.20) | 27 (84.38) | ||
| Comorbidities (at least one) | Yes | 31 (44.93) | 16 (50.00) | 0.673d |
| No | 38 (55.07) | 16 (50.00) | ||
| Treatments | ||||
| Chemotherapy | Moderate (conventional) e | 43 (62.32) | 18 (58.06) | 0.687b |
| Intensivef | 26 (37.68) | 13 (41.94) | ||
| Number of treatment cycles | ≤ 6 | 46 (66.67) | 18 (58.06) | 0.500d |
| > 6 | 23 (33.33) | 13 (41.94) | ||
aStudent’s t-test
bChi2 test
cICD-O: Diffuse large B-cell lymphoma (DLBCL), B27; Diffuse large B-cell lymphoma EBV +, B28; Follicular lymphoma grade 3B, B41; Plasmablatic lymphoma, B45; Association of diffuse large cell and follicular B cell NHL, C05; Aggressive B-cell lymphoma unclassifiable, C14; Large B-cell lymphoma with IRF4 rearrangement, C18
dFisher’s exact test
eModerate chemotherapy: R-mini-CHOP, R-CHOP-21
fIntensive chemotherapy: R-CHOP-14, R-ACVBP, and R-Experimental therapy
Characteristics of female and male participants
The majority of participants (73.7%) responded in the midst of the COVID-19 pandemic. Female and male participants did not differ from each other for all the characteristics considered (Table 2). However, more men had received moderate conventional chemotherapy compared to women (64.5% vs. 53.9%). Although men reported more comorbidities than women (54.8% vs. 30.8%), frequencies were statistically not significant (Table 2). The frequency of mood disorders like anxiety and depression was also no different in women and men. However, compared to men, women tended to have more doubtful symptomatology, with scores between 8 and 10 [28] (anxiety: 15.4% vs. 9.7% and depression: 26.9 vs. 12.9%) (Table 2).
Table 2.
Characteristics of female (N = 26) and male (N = 31) participants who answered the Lymphoma Quality of Life questionnaire
| Characteristics | Female (N = 26) |
Male (N = 31) |
p-Value | |
|---|---|---|---|---|
| Socio-demographic characteristics | ||||
| Mean (SD) | Mean (SD) | |||
| Range | Range | |||
| Age at entry into the RT3 Study (years) | 58.2 (15.8) | 58.7 (15) | 0.886a | |
| 26–84 | 20–79 | |||
| Duration between inclusion in the RT3 Study and the answer to the LQL questionnaire (months) | 12.9 (2.2) | 13.4 (2.7) | 0.410a | |
| 7–19 | 7–21 | |||
| N (%) | N (%) | |||
| Age | N = 26 | N = 31 | ||
| < 60 | 12 (46.15) | 14 (45.16) | 0.940c | |
| ≥ 60b | 14 (53.85) | 17 (54.84) | ||
| Living | N = 26 | N = 30 | ||
| Alone | 6 (23.08) | 6 (20) | 0.780c | |
| As a couple | 20 (76.92) | 24 (80) | ||
| Professionally active | N = 26 | N = 30 | ||
| Yes | 10 (38.46) | 13 (43.33) | 0.712c | |
| No | 16 (61.54) | 17 (56.67) | ||
| Actual financial level | N = 26 | N = 30 | ||
| Comfortable | 11 (42.31) | 9 (30) | 0.644d | |
| Neither at ease nor in difficulty | 13 (50) | 19 (63.33) | ||
| In difficulty or in great difficulty | 2 (7.69) | 2 (6.67) | ||
| Questionnaire | N = 26 | N = 31 | ||
| Version | Online | 7 (26.92) | 6 (19.35) | 0.498c |
| Paper | 19 (73.08) | 25 (80.65) | ||
| Response time after enrolment in the study (month) | ≤ 13 | 19 (73.08) | 18 (58.06) | 0.237c |
| > 13 | 7 (26.92) | 13 (41.94) | ||
| Responses given during the COVID-19 period | Yes | 19 (73.08) | 23 (74.19) | 0.924c |
| No | 7 (26.92) | 8 (25.81) | ||
| Health-related characteristics | ||||
| Ann Arbor Stage | N = 26 | N = 31 | ||
| I–II | 9 (34.62) | 14 (45.16) | 0.419c | |
| III–IV | 17 (65.38) | 17 (54.84) | ||
| HANS results | N = 23 | N = 28 | ||
| Germinal centre B-cell–like (GCB) | 11 (47.83) | 12 (42.86) | 0.723c | |
| Non-GCB | 12 (52.17) | 16 (57.14) | ||
| ICD-Oe | N = 23 | N = 29 | ||
| DLBCL | 15 (65.22) | 26 (89.66) | 0.110d | |
| Primary mediastinal large B-cell lymphoma (PMBL) | 4 (17.39) | 2 (6.90) | ||
| High grade B-cell lymphoma with double/triple-hit or NOS (HGBL) / DLBCL not otherwise specified (DLBCL-NOS) | 4 (17.39) | 1 (3.45) | ||
| Comorbidities | N = 26 | N = 31 | ||
| Type 2 diabetes | Yes | 0 (0) | 4 (12.90) | 0.118d |
| No | 26 (100) | 27 (87.10) | ||
| Arterial hypertension | Yes | 5 (19.23) | 11 (35.48) | 0.174c |
| No | 21 (80.77) | 20 (64.52) | ||
| Cardiovascular illnesses | Yes | 3 (11.54) | 6 (19.35) | 0.488d |
| No | 23 (88.46) | 25 (80.65) | ||
| Cancer (other) | Yes | 1 (3.85) | 2 (6.45) | 0.999d |
| No | 25 (96.15) | 29 (93.55) | ||
| Comorbidities (at least one) | Yes | 8 (30.77) | 17 (54.84) | 0.068c |
| No | 18 (69.23) | 14 (45.16) | ||
| Mood disorders | N = 26 | N = 31 | ||
| HADS Anxiety | ≤ 7 (no cases) | 17 (65.38) | 22 (70.97) | 0.918d |
| 8 to 10 (doubtful cases) | 4 (15.38) | 3 (9.68) | ||
| ≥ 11 (severe cases) | 5 (19.23) | 6 (19.35) | ||
| HADS Depression | ≤ 7 (no cases) | 17 (65.38) | 24 (77.42) | 0.462d |
| 8 to 10 (doubtful cases) | 7 (26.92) | 4 (12.9) | ||
| ≥ 11 (severe cases) | 2 (7.69) | 3 (9.68) | ||
| Treatments | N = 26 | N = 31 | ||
| Chemotherapy | Moderate (conventional)f | 14 (53.85) | 20 (64.52) | 0.414c |
| Intensiveg | 12 (46.15) | 11 (35.48) | ||
| Number of treatment cycles | ≤ 6 | 17 (65.38) | 20 (64.52) | 0.945c |
| > 6 | 9 (34.62) | 11 (35.48) |
aStudent’s t-test
bWomen: N = 3 ≥ 80 years
cChi2 test
dFisher’s exact test
eICD-O: Diffuse large B-cell lymphoma (DLBCL), B27; Diffuse large B-cell lymphoma EBV +, B28; Follicular lymphoma grade 3B, B41; Plasmablatic lymphoma, B45; Association of diffuse large cell and follicular B-cell NHL, C05; Aggressive B-cell lymphoma unclassifiable, C14; Large B-cell lymphoma with IRF4 rearrangement, C18
fModerate (conventional) chemotherapy: R-mini-CHOP, R-CHOP-21
gIntensive chemotherapy: R-CHOP-14, R-ACVBP, and Rituximab + Experimental therapy
HRQoL scores
HRQoL according to gender
EORTC QLQ-C30
The adjusted means of scores of global health status and functional scales were slightly higher (better) in men than in women and significantly different only for the physical functioning (82.1 in men vs. 70.9 in women, P = 0.031). The adjusted means of symptom scores were slightly higher (poor) in women compared to men and statistically significant only for constipation (adjusted scores of 27.9 in women vs. 11.7 in men, P = 0.025) (Table 3).
Table 3.
Comparisons of the different psychometric scales between female (N = 26) and male (N = 31) participants in the Lymphoma Quality of Life Study
| Scales/subscales | Sex | Comparison | |
|---|---|---|---|
| Female (N = 26) | Male (N = 31) | p-Value | |
| Adjusted mean [95% CI]a | Adjusted mean [95% CI]a | ||
| EORTC QLQ-C30 (v3) | |||
| Global health statusb | 64.37 [55.04–73.70] | 67.95 [60.13–75.78] | 0.504 |
| Functional scalesc | |||
| Physical functioning | 70.86 [61.99–79.73] | 82.10 [74.66–89.53] | 0.031 |
| Role functioning | 74.76 [63.57–85.95] | 84.85 [75.47–94.23] | 0.120 |
| Emotional functioning | 70.08 [56.98–83.18] | 78.52 [67.54–89.50] | 0.263 |
| Cognitive functioning | 76.45 [65.05–87.85] | 79.24 [69.68–88.80] | 0.670 |
| Social functioning | 76.29 [64.93–87.64] | 80.42 [70.91–89.94] | 0.525 |
| Symptom scales/itemsd | |||
| Fatigue | 48.46 [35.32–61.60] | 37.25 [26.23–48.26] | 0.141 |
| Nausea and vomiting | 4.39 [(−2.35)–11.11] | 2.20 [(−3.44)–7.84] | 0.572 |
| Pain | 32.12 [18.04–46.19] | 20.00 [8.20–31.79] | 0.137 |
| Dyspnoea | 29.08 [15.58–42.57] | 24.83 [13.52–36.14] | 0.584 |
| Insomnia | 32.81 [16.82–48.80] | 19.37 [5.96–32.77] | 0.147 |
| Appetite loss | 14.26 [0.53–27.99] | 5.12 [(−6.39)–16.63] | 0.248 |
| Constipation | 27.94 [15.66–40.23] | 11.69 [1.39–21.98] | 0.025 |
| Diarrhoea | 13.92 [5.36–22.48] | 7.55 [0.37–14.72] | 0.198 |
| Financial difficulties | 12.74 [2.75–22.73] | 17.37 [9.00–25.74] | 0.420 |
| EORTC QLQ-NHL-HG29e | |||
| Symptom burden | 31.82 [21.52–42.11] | 21.22 [12.59–29.85] | 0.077 |
| Neuropathy | 27.67 [13.59–41.76] | 36.91 [25.10–48.71] | 0.256 |
| Physical condition / fatigue | 37.43 [23.71–51.15] | 28.90 [17.40–40.41] | 0.281 |
| Emotional impact | 30.50 [17.91–43.09] | 23.40 [12.84–33.95] | 0.327 |
| Worries / fears about health and functioning | 37.00 [25.23–48.78] | 36.00 [26.13–45.87] | 0.882 |
| HADSf | |||
| Anxiety | 7.62 [5.45–9.80] | 5.76 [3.94–7.59] | 0.141 |
| Depression | 6.67 [4.76–8.58] | 5.89 [4.29–7.49] | 0.473 |
| PTGIg | |||
| Relating to others | 21.63 [18.36–24.90] | 17.22 [14.48–19.96] | 0.022 |
| New possibilities | 11.25 [8.45–14.05] | 9.08 [6.74–11.43] | 0.181 |
| Personal strength | 10.47 [8.03–12.90] | 9.34 [7.30–11.38] | 0.421 |
| Spiritual changes | 4.12 [2.64–5.60] | 3.37 [2.13–4.61] | 0.379 |
| Appreciation of life | 9.60 [7.98–11.23] | 7.78 [6.42–9.15] | 0.056 |
| PTGI total score | 57.06 [47.63–66.50] | 46.79 [38.88–54.70] | 0.062 |
| MFI-20h | |||
| General fatigue | 12.14 [9.96–14.32] | 11.28 [9.45–13.11] | 0.494 |
| Physical fatigue | 11.76 [9.46–14.05] | 12.11 [10.19–14.03] | 0.788 |
| Mental fatigue | 9.38 [7.33–11.42] | 9.70 [7.98–11.41] | 0.783 |
| Reduction of activities | 10.15 [7.99–12.31] | 9.27 [7.46–11.08] | 0.478 |
| Reduction of motivation | 11.66 [9.60–13.72] | 11.34 [9.62–13.07] | 0.787 |
| MFI-20 total score | 55.09 [45.78–64.40] | 53.71 [45.90–61.51] | 0.796 |
aModel adjusted on age at entry into the RT3 study (1: ≥ 60, 0: < 60 years), duration between inclusion in the RT3 study and the answer to the questionnaire (1: > 13, 0: ≤ 13 months), number of treatment cycles (1: > 6, 0: ≤ 6 cycles), Ann Arbor stage (1: III–IV, 0: I–II), comorbidities (type 2 diabetes/arterial hypertension/cardiovascular diseases/other cancer: 1: yes / 0: no), therapy followed (1: R-CHOP-14, R-ACVBP, R-Experimental therapy; 0: mini-R-CHOP and R-CHOP-21), and response during COVID-19 period (1: yes, 0: no)
bA high score for the Global health status represents a high QoL [Fayers et al., 2001]
cA high score for a functional scale represents a high/healthy level of functioning [Fayers et al., 2001]
dA high score for a symptom scale or item represents a high level of symptomatology or problems [Fayers et al., 2001]
eA high score for all of the multi-item scales represents a high level of symptomatology or problems [van de Poll-Franse et al., 2018; Fayers et al., 2001]
fA score of 0 to 7 for either subscale could be regarded as being in the normal range, a score of 8 to 10 being just suggestive of a doubtful symptomatology, and a score of 11 or higher indicating presence of the mood disorder [Snaith, 2003]
gPost-traumatic development increases with the calculated score [Tedeschi et al., 1996]
hHigh scores represent high levels of fatigue. The total score is calculated by adding the scores of 5 subscales
EORTC QLQ-NHL-HG29
The adjusted means scores were not significantly different between women and men; however, men tended to have higher neuropathy scores than women (36.9 vs. 27.7), and women to have higher scores for symptom burden than men (31.8 vs. 21.2) (Table 3).
HADS
The adjusted means of anxiety and depression scores were not different between women and men. However, women tended to have a higher average anxiety score than men (7.6 vs. 5.8) (Table 3).
PTGI
For all 5 subscales, as well as for the total PTGI score, the adjusted means obtained were higher in women (more severely affected) than in men. The adjusted means obtained for the relating to others subscale were significantly higher in women than in men (21.6 vs. 17.2, P = 0.022) (Table 3).
MFI-20
The adjusted means of the scores of 5 subscales were not significantly different between women and men (Table 3).
HRQoL according to comorbidities
EORTC QLQ-C30
For the global health status and functional scales, the presence of comorbidities ensured lower adjusted scores than those recorded in patients without comorbidities. The global health status and the physical functioning were significantly lower, also deteriorated in patients who reported at least one comorbidity (58.9 vs. 73.5, P = 0.017 and 70.4 vs. 82.5, P = 0.035, respectively) (Table 4).
Table 4.
Comparison of adjusted HRQoL scores from different psychometric scales according to comorbidities (N = 57)
| Comorbiditiesa | |||
|---|---|---|---|
| Scales/subscales | Without comorbidities (N = 32) | With comorbidities (N = 25) | |
|
Adjusted mean [95% CI of adjusted mean] |
Adjusted mean [95% CI of adjusted mean] |
p-Value | |
| EORTC QLQ-C30 (v3)b | |||
| Global health status | 73.45 [65.11–81.78] | 58.88 [49.33–68.42] | 0.017 |
| Functional scalesc | |||
| Physical functioning | 82.54 [74.61–90.47] | 70.42 [61.35–79.49] | 0.035 |
| Role functioning | 81.29 [71.29–91.29] | 78.32 [66.87–89.77] | 0.675 |
| Emotional functioning | 76.05 [64.34–87.75] | 72.56 [59.16–85.95] | 0.674 |
| Cognitive functioning | 79.58 [69.40–89.77] | 76.11 [64.45–87.77] | 0.630 |
| Social functioning | 80.40 [70.25–90.55] | 76.31 [64.70–87.92] | 0.569 |
| Items/scalesd | |||
| Fatigue | 38.56 [26.82–50.30] | 47.15 [33.71–60.59] | 0.303 |
| Nausea and vomiting | 1.62 [(−4.39)–7.63] | 4.96 [(−1.92)–11.84] | 0.434 |
| Pain | 24.80 [12.23–37.37] | 27.31 [12.92–41.70] | 0.778 |
| Dyspnoea | 21.70 [9.64–33.75] | 32.21 [18.41–46.01] | 0.221 |
| Insomnia | 22.96 [8.67–37.25] | 29.22 [12.87–45.57] | 0.536 |
| Appetite loss | 5.79 [(−6.48)–18.06] | 13.59 [(−0.45)–27.63] | 0.370 |
| Constipation | 15.80 [4.83–26.78] | 23.83 [11.27–36.39] | 0.303 |
| Diarrhoea | 9.36 [1.71–17.01] | 12.10 [3.35–20.86] | 0.612 |
| Financial difficulties | 10.32 [1.39–19.24] | 19.79 [9.58–30.01] | 0.138 |
| EORTC QLQ-NHL-HG29e | |||
| Symptom burden | 18.79 [9.59–27.99] | 34.24 [23.71–44.77] | 0.021 |
| Neuropathy | 27.04 [14.46–39.63] | 37.54 [23.13–51.94] | 0.242 |
| Physical condition / fatigue | 23.47 [11.21–35.73] | 42.87 [28.83–56.90] | 0.029 |
| Emotional impact | 20.04 [8.79–31.29] | 33.86 [20.98–46.73] | 0.087 |
| Worries/fears about health and functioning | 30.72 [20.20–41.24] | 42.28 [30.24–54.33] | 0.125 |
| HADSf | |||
| Depression | 4.83 [3.12–6.53] | 7.73 [5.78–9.68] | 0.019 |
| Anxiety | 6.74 [4.79–8.69] | 6.65 [4.42–8.87] | 0.946 |
| PTGIg | |||
| Relating to others | 19.23 [16.31–22.15] | 19.62 [16.28–22.97] | 0.848 |
| New possibilities | 10.58 [8.08–13.08] | 9.75 [6.89–12.62] | 0.641 |
| Personal strength | 10.06 [7.88–12.24] | 9.74 [7.25–12.24] | 0.837 |
| Spiritual changes | 3.71 [2.38–5.03] | 3.78 [2.26–5.29] | 0.941 |
| Appreciation of life | 9.17 [7.72–10.63] | 8.22 [6.55–9.88] | 0.354 |
| PTGI total score | 52.75 [44.32–61.18] | 51.11 [41.46–60.76] | 0.784 |
| MFI-20h | |||
| General fatigue | 10.65 [8.70–12.60] | 12.77 [10.54–15.01] | 0.128 |
| Physical fatigue | 10.59 [8.54–12.63] | 13.28 [10.94–15.62] | 0.068 |
| Mental fatigue | 8.24 [6.42–10.07] | 10.83 [8.74–12.92] | 0.049 |
| Reduction of activities | 9.39 [7.46–11.32] | 10.04 [7.83–12.25] | 0.637 |
| Reduction of motivation | 11.02 [9.18–12.86] | 11.99 [9.88–14.09] | 0.460 |
| MFI-20 total score | 49.89 [41.57–58.21] | 58.90 [49.38–68.43] | 0.130 |
aModel adjusted on age at entry into the RT3 study (1: ≥ 60, 0: < 60 years), sex (1: male, 0: female), duration between inclusion in the RT3 study and the answer to the questionnaire (1: > 13, 0: ≤ 13 months), number of treatment cycles (1: > 6, 0: ≤ 6 cycles), Ann Arbor stage (1: III–IV, 0: I–II), therapy followed (1: R-CHOP-21, R-ACVBP, and R-Experimental therapy; 0: R-mini-CHOP and R-CHOP14), and response during COVID-19 period (1: yes, 0: no)
bA high score for the Global health status represents a high QoL [Fayers et al., 2001]
cA high score for a functional scale represents a high/healthy level of functioning [Fayers et al., 2001]
dA high score for a symptom scale or item represents a high level of symptomatology or problems [Fayers et al., 2001]
eA high score for all of the Multi-item scales represents a high level of symptomatology or problems [van de Poll-Franse et al., 2018; Fayers et al., 2001]
fA score of 0 to 7 for either subscale could be regarded as being in the normal range, a score of 8 to 10 being just suggestive of a doubtful symptomatology, and a score of 11 or higher indicating presence of the mood disorder [Snaith, 2003]
gPost-traumatic development increases with the calculated score [Tedeschi et al., 1996]
hHigh scores represent high levels of fatigue. The total score is calculated by adding the scores of 5 subscales
EORTC QLQ-NHL-HG29
Presence of comorbidities ensured significant higher scores of symptom burden, and a more deteriorated physical condition or fatigue compared to absence of comorbidities (34.2 vs. 18.8, P = 0.021; and 42.9 vs. 23.5, P = 0.029, respectively) (Table 4).
HADS
Patients with comorbidities had significantly higher scores of depression compared to those without comorbidities (7.7 vs. 4.8, P = 0.019) (Table 4).
PTGI
The adjusted means of the scores of 5 subscales were not significantly different according to comorbidities (Table 4).
MFI-20
Patients with comorbidities had significantly higher mental fatigue score compared to those without comorbidities (10.8 vs. 8.2, P = 0.049) (Table 4).
Symptoms scores according to number of treatment cycles
Patients with higher number of treatment cycles (> 6) had slightly higher EORTC QLQ-C30 symptoms scores such as fatigue, appetite loss, and constipation, and significantly higher pain and dyspnoea scores compared to those who had ≤ 6 cycles of treatment (35.5 vs. 16.6, P = 0.046 and 36.7 vs. 17.3, P = 0.033, respectively). Also, patients with more than 6 treatment cycles had higher EORTC QLQ-NHL-HG29 symptom burden and significantly higher neuropathy scores compared to those who had ≤ 6 cycles of treatment (44.7 vs.19.9, P = 0.010) (Table 5).
Table 5.
Comparison of adjusted symptom scores measured with EORTC QLQ-C30 and QLQ-NHL-HG29 scales according to number of chemotherapy cycles (N = 57)
| Number of treatment cyclesa | |||
|---|---|---|---|
| ≤ 6 cycles (N = 37) | > 6 cycles (N = 20) | ||
| Symptom scales/itemsb | Adjusted mean [95% CI] | Adjusted mean [95% CI] | p-Value |
| Fatiguec | 37.10 [24.86–49.34] | 48.60 [35.16–62.05] | 0.188 |
| Painc | 16.62 [3.51–29.73] | 35.49 [21.09–49.88] | 0.046 |
| Dyspnoeac | 17.26 [4.69–29.83] | 36.65 [22.85–50.46] | 0.033 |
| Insomniac | 31.02 [16.12–45.91] | 21.16 [4.80–37.51] | 0.351 |
| Nausea and vomitingc | 3.60 [(−2.67)–9.87] | 2.98 [(−3.90)–9.86] | 0.889 |
| Appetite lossc | 8.32 [(−4.47)–21.11] | 11.06 [(−2.98)–25.11] | 0.762 |
| Constipationc | 17.43 [5.99–28.88] | 22.20 [9.63–34.76] | 0.557 |
| Diarrhoeac | 10.96 [2.98–18.93] | 10.51 [1.75–19.26] | 0.936 |
| Neuropathyd | 19.93 [6.81–33.05] | 44.65 [30.24–59.06] | 0.010 |
| Symptom burdend | 21.99 [12.40–31.58] | 31.05 [20.51–41.58] | 0.186 |
aModel adjusted on age at entry into the RT3 study (1: ≥ 60, 0: < 60 years), sex (1: male, 0: female), duration between inclusion in the RT3 study and the answer to the questionnaire (1: > 13, 0: ≤ 13 months), comorbidities (1: yes, 0: no), Ann Arbor stage (1: III–IV, 0: I–II), therapy followed (1: R-CHOP-21, R-ACVBP, and R-Experimental therapy; 0: R-mini-CHOP and R-CHOP14), and response during COVID-19 period (1: yes, 0: no)
bA high score for a symptom scale or item represents a high level of symptomatology or problems [Fayers et al., 2001; van de Poll-Franse et al., 2018]
cMeasured with EORTC QLQ-C30 (v3)
dMeasured with EORTC QLQ-NHL-HG29
HRQoL of DLBCL patients compared to reference values in general population
EORTC QLQ-C30
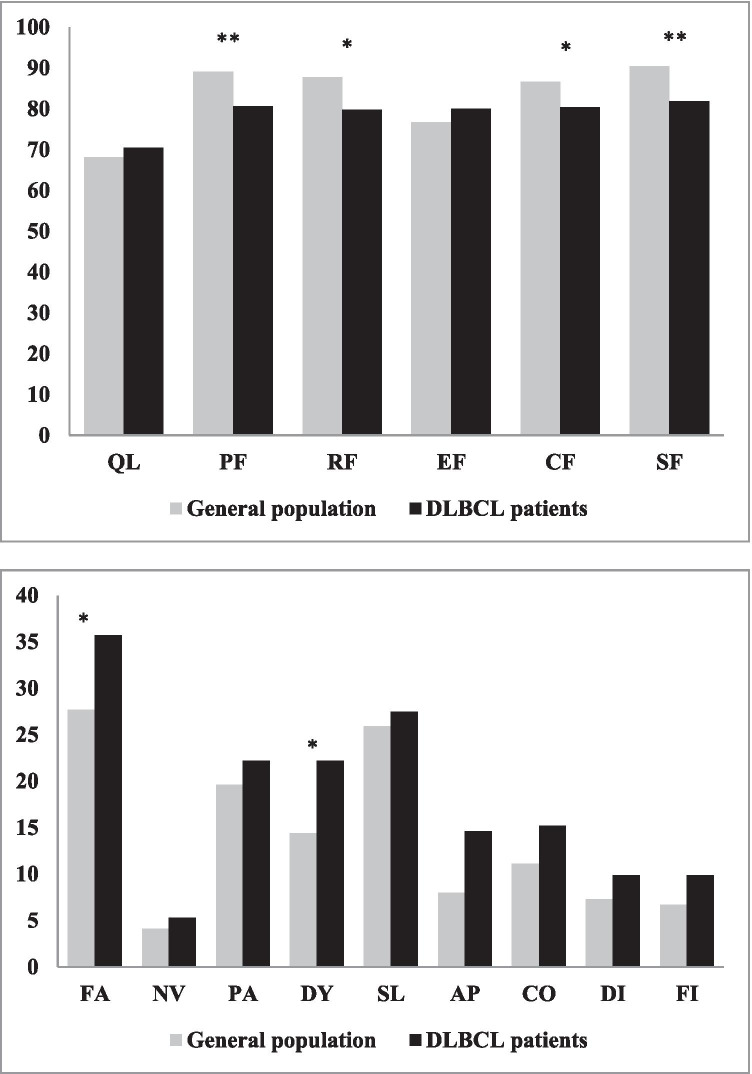
Compared to a French normative population [35], DLBCL patients exhibited on average similar global quality of life score. However, DLBCL patients have statistically significant but clinically marginally relevant (< 10 points) [36, 37] worse scores on physical functioning, role functioning, cognitive, and social functioning. They also reported more (P < 0.05) but clinically marginally relevant fatigue and dyspnoea compared to the general French population (Fig. 2).
Fig. 2.
Comparisons of EORTC QLQ-C30 scores of DLBCL patients participating in the Lymphoma Quality of Life Study and general French population reference values. EORTC QLQ-C30 mean scores in DLBCL patients and reference values in France [Nolte et al., 2019]. Abbreviations: QL, global health status; PF, physical functioning; RF, role functioning; EF, emotional functioning; CF, cognitive function; SF, social functioning; FA, fatigue; NV, nausea and vomiting; PA, pain; DY, dyspnoea; SL, insomnia; AP, appetite loss; CO, constipation; DI, diarrhoea; FI, financial difficulties. ** P < 0.01; * P < 0.05
Discussion
Our study shows that in women, PTGI (relating to others) and some EORTC QLQ-C30 subscales (physical functioning and constipation) following lymphoma were more affected than in men. The existence of chronic comorbidities increases physical fatigue, symptom burden, and impacts on physical functioning and global health status. Comorbidities also play a role on the psychological level: increase the emotional impact, the mental fatigue, and the level of depression of patients, regardless of sex, age, and other factors related to lymphoma and these treatments. A greater number of treatment cycles affect certain symptoms, leading to an increase in the level of pain, neuropathy, and dyspnoea.
It is known that cancer survivors are at risk of experiencing adverse physical and psychosocial effects of their cancer and its treatment that can affect their HRQoL [38]. It was reported that NHL survivors showed the most defects in EORTC QLQ-C30 physical functioning, appetite loss, and financial problems [38], and to our knowledge, a relatively small number of studies investigated the HRQoL specifically in DLBCL patients. The results of population-based PHAROS registry in the Netherlands showed that patients with DLBCL exhibited on average statistically significant and clinically relevant worse scores on EORTC QLQ-C30 physical, role, cognitive, and social functioning compared to a normative population [9]. DLBCL patients reported more fatigue, dyspnoea, sleeping problems, appetite loss, and financial problems compared to the general population [9]. These results are similar with those obtained in our study, except for the last three elements, where the differences compared to the general French population were not significant. In addition, in our study, the differences between the means of the EORTC QLQ-C30 scores in DLBCL patients and those in the general French population (physical, cognitive, social, and role functioning; fatigue and dyspnoea), which were statistically significant, correspond only to small relevant effects.
Having had chemotherapy was negatively associated with HRQoL in NHL survivors [38]. The type of therapy followed by patients with DLBCL can have a major role in the HRQoL of patients some time afterwards. The prospective study of Tholstrup et al. (2001) showed that the QoL of DLBCL Danish patients was substantially affected during treatment with CHOP-14 (physical, role, and emotional functioning were significantly decreased and patients reported fatigue and diarrhoea), but only for a short time period of 3 months. Afterwards, patients had generally recovered fully, and scores were found to be equal to those of the reference population or better [15]. However, in another study, even 2 years later, the DLBCL patients treated with R-CHOP-14 reported more neuropathic symptoms, more fatigue, and a lower HRQoL than patients treated with R-CHOP-21 [9]. It is possible that the lack of significant results in our study was due to the small number of subjects per treatment group. To our knowledge, the number of treatment cycles has not been studied in relation to any symptoms in DBLCL survivors. In our study, we showed that in patients who received more than 6 treatment cycles (patients with ACVBP chemotherapy received the greatest number of cycles, 9 to 12 cycles; data not shown), the adjusted means for symptoms such as pain, neuropathy, and dyspnoea were statistically significant and the difference between the categories is wide. The greatest difference was recorded for neuropathy. Moreover, it is known that neuropathy is a common squeal of chemotherapy protocols that contain vincristine [14] that our patients have received.
Fatigue is one of the most common side effects in patients with cancer [39, 40]. Fatigue can be a consequence of active treatment, but it may also persist into posttreatment periods and may be responsible for a reduced QoL. In the study of Hinz et al. (2020), all the dimensions of fatigue measured with MFI-20 were higher (more affected) in German patients with cancer (several cancer sites including haematological malignancies) than in the general population [41]. In this study, female patients reported significantly higher levels of fatigue (general and mental fatigue and MFI total score) than men [41]. Similar results were obtained by Mounier et al. (2019), who reported in his cross-sectional study on NHL survivors after 11 years of follow-up after initial treatment of their lymphoma that women reported significantly higher fatigue than men, including general and physical fatigue and reduced motivation [12]. In this study, it was also mentioned that long-term fatigue in NHL survivors was independent of disease characteristics and treatment [12]. Unlike these studies, the adjusted means of the MFI-20 scores that we presented by sex showed no difference. However, in agreement with these studies, we did not find any differences in scores related to the Ann Arbor stage of the lymphoma, or the therapy followed (data not shown).
Mounier et al. (2019) specified that obesity and comorbidities play a role in the development of fatigue (MFI-20) that persists or develops early after treatment completion [12]. A similar conclusion was drawn by Busson et al. (2019) in a cross-sectional study, who showed that after a follow-up of 5 years or more, the NHL survivors who reported health disorders displayed higher levels of fatigue (all 5 dimensions) than those who did not (P < 0.001) [42]. Our results point in the same direction. DLBCL survivors with comorbidities had higher mental and physical fatigue scores compared to those without comorbidities. However, we did not collect any data related to body weight or BMI. Our study also showed that DLBCL survivors with comorbidities had a significantly higher adjusted mean fatigue score measured with EORTC QLQ-NHL-HG29 and lower physical functioning (EORTC QLQ-C30) than those without comorbidities (P < 0.04).
The diagnosis and treatment of cancer can be extremely stressful. So cancer survivors often report posttraumatic growth (PTG) [43, 44], and it seems that compared to males, female survivors are more prone to PTG [45]. To our knowledge, PTGI has not yet been administered to DLBCL patients. In our study, gender disparity on PTG was also found. So significantly higher (more affected) adjusted scores (relating to others) or close to the significance level (appreciation of life and PTGI total, P < 0.07) were obtained in female survivors compared to men.
Cancer patients may experience anxiety and depression. Hinz et al. (2019) reported that patients with cancer are also more anxious but slightly less depressed than age- and gender-matched individuals of the general population [46]. The authors found gender difference in anxiety for all tumour types analysed [46]. Likewise, in the longitudinal study of the PROFILES registry, Oerlemans et al. (2014) reported that both anxiety and depression were more often declared by lymphoma (NHL and DLBCL) patients compared to the age- and sex-matched normative Dutch population (P < 0.05) [8]. Unfortunately, in France, we do not have normative data in the general population to make similar comparisons.
Because women experience more anxiety, posttraumatic growth, or posttraumatic stress symptoms than men, it seems important that these issues be more approached with female DLBCL patients, and to plan strategies that would reduce their levels and thus improve their QoL. In addition, in our cross-sectional study, 13 months after diagnosis, depression scores were significantly higher in survivors with comorbidities compared to those without (P = 0.02). As reported by Oerlemans et al. (2014), DLBCL patients with comorbidity reported particularly higher depression scores (P < 0.01) and also anxiety over time, between the first and the last assessment (first year and the 4th year) [8].
In parallel, the SARS-CoV-2 pandemic and the lockdown phases during the year 2020, also led to an increase in the levels of posttraumatic stress disorder, depression, anxiety, insomnia, and perceived stress in the general population [47, 48], and particularly in the NHL patients under treatment [49]. It is therefore very likely that the findings of our study were influenced to a certain extent by the experience of participants related to the SARS-CoV-2 pandemic.
To our knowledge, few studies on the HRQoL of NHL survivors have been carried out, and our study is one of the few studies performed in DLBCL patients. Another strong point of our study is that we looked at several aspects related to the QoL of DLBCL survivors, by administering five psychometric scales. Also, our study is one of the few studies that investigated the QoL of DLBCL patients in a gender-differentiated manner, depending on the presence of comorbidities and the number of treatment cycles received, and presenting mean scores adjusted for several factors (demographic, health-, and treatment-related).
However, our study has some limitations. First, this study has a cross-sectional design which does not allow a causal link to be established. We measured the QoL at a single point in time, on average 13 months after diagnosis of lymphoma, and we do not have the data, neither at the time of diagnosis (before the treatments) nor afterwards, at several points in time. Second, we have a relatively small number of patients who responded to all of the proposed psychometric scales, a fact that did not allow us to perform stratified analyses according to the Ann Arbor stages and the categories of treatments received. Third, it is possible that there was a certain selection bias. Patients suffering from severe anxiety, depression, fatigue, and who felt much diminished may not have answered the questionnaires. Fourth, we do not have normative data on the quality of life in the general French population (COVID-19 period and not) in order to make comparisons, excepting for EORTC QLQ-C30. In addition, the QoL, especially at the emotional level can be influenced by the difficult situation to live for some people who responded to the QL questionnaire during the pandemic COVID-19 and lockdown. During our study which spanned over a year, the very rapid evolution of the health situation did not allow the creation and administration of an additional focused COVID-19 questionnaire that could have clarified some of these aspects. Given the very low number of participants especially in the ‘non-COVID period’ category, stratified multivariate analyses could not be performed. However, to overcome this drawback, all multivariate models have also been adjusted for the COVID period (or not).
In conclusion, our study shows that differences in the HRQoL between female and male survivors exist 1 year after diagnosis of their lymphoma and that women are more prone to posttraumatic growth and lower physical functioning than men. In both sexes, other factors independent of lymphoma, such as the presence of comorbidities, or related to the disease, such as the higher number of treatment cycles followed, can affect quality of life physically, mentally, or emotionally. At the mental and emotional level, patterns of change in depression, anxiety, and posttraumatic growth over time after diagnosis and treatment in DLBCL survivors need to be further investigated, preferably in longitudinal studies, with repeated measurements over time, to track HRQoL developments. A reduction in the number of chemotherapy cycles (i.e. < 7) could lead to an improvement in HRQoL scores. Studies are also needed to ensure appropriate psychosocial and supportive care interventions in individuals with different haematological cancers and particularly in DLBCL patients where studies are still few.
Supplementary Information
(PDF 520 kb)
Acknowledgments
Availability of data and material
The dataset generated during the current study is available from the corresponding author on reasonable request and after permission of LYSARC and INSERM.
Code availability
Not applicable
Author contribution
ACP and CB designed the Lymphoma Quality of Life Study. CCB, CH, and FJ designed the RT3 study. SM, SLG, VR, KB, and DS collected the data. ACP and CB analysed and interpreted the data. ACP and CB wrote the paper. MP and NM contributed to the design and the interpretation of the quality of life study. GR designed the software used in the quality of life data collection. All authors read and approved the manuscript.
Funding
This work was supported by the CALYM Carnot Institute funded by the French National Research Council (ANR).
Declarations
Ethics approval
The project has been approved by the Person Protection Committee 8 Ile-de-France, on July 9, 2019.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
All patients consented to the research and its publication.
Competing interests
The authors declare no competing interests.
Footnotes
The original version of this article was revised: This article was originally published with inverted last names and first names of all the authors.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
10/25/2021
A Correction to this paper has been published: 10.1007/s00277-021-04705-7
Contributor Information
Alexandra-Cristina Paunescu, Email: alexandra-cristina.paunescu@gustaveroussy.fr.
Caroline Besson, Email: cbesson@ch-versailles.fr.
References
- 1.Sapkota S, Shaikh H (2021) Non-Hodgkin lymphoma. In: StatPearls [Internet] Treasure Island (FL): StatPearls Publishing [PubMed]
- 2.Lossos IS. Molecular pathogenesis of diffuse large B-cell lymphoma. J Clin Oncol. 2005;23(26):6351–6357. doi: 10.1200/JCO.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Defossez G, Le Guyader-Peyrou S, Uhry Z, Grosclaude P, Colonna M, Dantony E, et al. Overview. Saint-Maurice: Santé publique France; 2019. National estimates of cancer incidence and mortality in metropolitan France between 1990 and 2018. [Google Scholar]
- 4.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 5.Chaganti S, Illidge T, Barrington S, Mckay P, Linton K, Cwynarski K, et al. Guidelines for the management of diffuse large B-cell lymphoma. Br J Haematol. 2016;174(1):43–56. doi: 10.1111/bjh.14136. [DOI] [PubMed] [Google Scholar]
- 6.Lewis WD, Lilly S, Jones KL. Lymphoma: diagnosis and treatment. Am Fam Physician. 2020;101(1):34–41. [PubMed] [Google Scholar]
- 7.Bottomley A. The cancer patient and quality of life. The Oncologist. 2002;7:120–125. doi: 10.1634/theoncologist.7-2-120. [DOI] [PubMed] [Google Scholar]
- 8.Oerlemans S, Mols F, Nijziel MR, Zijlstra WP, Coebergh JW, van de Poll-Franse LV. The course of anxiety and depression for patients with Hodgkin’s lymphoma or diffuse large B cell lymphoma: a longitudinal study of the PROFILES registry. J Cancer Surviv. 2014;8(4):555–564. doi: 10.1007/s11764-014-0367-1. [DOI] [PubMed] [Google Scholar]
- 9.Oerlemans S, Issa DE, van den Broek EC, Nijziel MR, Coebergh JW, Huijgens PC, et al. Health-related quality of life and persistent symptoms in relation to (R-)CHOP14, (R-)CHOP21, and other therapies among patients with diffuse large B-cell lymphoma: results of the population-based PHAROS-registry. Ann Hematol. 2014;93(10):1705–1715. doi: 10.1007/s00277-014-2099-8. [DOI] [PubMed] [Google Scholar]
- 10.Mehnert A, Hartung TJ, Friedrich M. One in two cancer patients is significantly distressed: prevalence and indicators of distress. Psychooncology. 2018;27:75–82. doi: 10.1002/pon.4464. [DOI] [PubMed] [Google Scholar]
- 11.Oberoi D, White V, Seymour J, Prince HM, Harrison S, Jefford M, et al. The course of anxiety, depression and unmet needs in survivors of diffuse large B cell lymphoma and multiple myeloma in the early survivorship period. J Cancer Surviv. 2017;11(3):329–338. doi: 10.1007/s11764-016-0591-y. [DOI] [PubMed] [Google Scholar]
- 12.Mounier N, Anthony S, Busson R, Thieblemont C, Ribrag V, Tilly H, et al. Long-term fatigue in survivors of non-Hodgkin lymphoma: The Lymphoma Study Association SIMONAL cross-sectional study. Cancer. 2019;125(13):2291–2299. doi: 10.1002/cncr.32040. [DOI] [PubMed] [Google Scholar]
- 13.Schoormans D, Jansen M, Mols F, Oerlemans S. Negative illness perceptions are related to more fatigue among haematological cancer survivors: a PROFILES study. Acta Oncol. 2020;59(8):959–966. doi: 10.1080/0284186X.2020.1759823. [DOI] [PubMed] [Google Scholar]
- 14.Dorchin M, Masoumi Dehshiri R, Soleiman S, Manashi M. Evaluation of neuropathy during intensive vincristine chemotherapy for non-Hodgkin's lymphoma and Acute Lymphoblastic Leukemia. Iran. J Ped Hematol Oncol. 2013;3(4):138–142. [PMC free article] [PubMed] [Google Scholar]
- 15.Tholstrup D, Brown Pde N, Jurlander J, Bekker Jeppesen P, Groenvold M. Quality of life in patients with diffuse large B-cell lymphoma treated with dose-dense chemotherapy is only affected temporarily. Leuk Lymphoma. 2011;52(3):400–408. doi: 10.3109/10428194.2010.541310. [DOI] [PubMed] [Google Scholar]
- 16.Heutte N, Haioun C, Feugier P, Coiffier B, Tilly H, Ferme C, et al. Quality of life in 269 patients with poor-risk diffuse large B-cell lymphoma treated with rituximab versus observation after autologous stem cell transplant. Leuk Lymphoma. 2011;52(7):1239–1248. doi: 10.3109/10428194.2011.566951. [DOI] [PubMed] [Google Scholar]
- 17.Jung HA, Park S, Cho JH, Kim S, Ko YH, Kim SJ, et al. Prognostic relevance of pretreatment quality of life in diffuse large B-cell lymphoma patients treated with rituximab-CHOP: results from a prospective cohort study. Ann Hematol. 2012;91(11):1747–1756. doi: 10.1007/s00277-012-1516-0. [DOI] [PubMed] [Google Scholar]
- 18.van der Poel MW, Oerlemans S, Schouten HC, Mols F, Pruijt JF, Maas H, et al. Quality of life more impaired in younger than in older diffuse large B cell lymphoma survivors compared to a normative population: a study from the population-based PROFILES registry. Ann Hematol. 2014;93(5):811–819. doi: 10.1007/s00277-013-1980-1. [DOI] [PubMed] [Google Scholar]
- 19.Copie Bergman C, Bohers E, Dartigues-Cuillères P, Viailly P-J, Ruminy P, Marchand V, et al. (2020) 2118 real time pathological and molecular characterization of aggressive B-cell lymphomas based on a national network. A Lysa Project. American Society of Hematology December 6, Session: 627. Aggressive Lymphoma (Diffuse Large B-Cell and Other Aggressive B-Cell Non-Hodgkin Lymphomas) Poster II Hematology Disease Topics & Pathways
- 20.Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. AJCC Cancer Staging Manual (8th edition) New York: Springer; 2017. [Google Scholar]
- 21.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 22.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, Whelan S (2013) editor. International classification of diseases for oncology (ICD-O) - 3rd edition, 1st revision: WHO
- 23.Zelenetz AD, Gordon LI, Wierda WG, Abramson JS, Advani RH, Andreadis CB, et al. Non-Hodgkin’s lymphomas, Version 4.2014: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2014;12(9):1282–1303. doi: 10.6004/jnccn.2014.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quality of Life Unit, EORTC Data Center (2001) EORTC QLQ-C30. Brussels, Belgium ; Third: [Available from: https://qol.eortc.org/questionnaire/eortc-qlq-c30/
- 25.Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A, et al. EORTC QLQ-C30 Scoring Manual (3rd edition) Brussels: EORTC; 2001. [Google Scholar]
- 26.van de Poll-Franse L, Oerlemans S, Bredart A, Kyriakou C, Sztankay M, Pallua S, et al. International development of four EORTC disease-specific quality of life questionnaires for patients with Hodgkin lymphoma, high- and low-grade non-Hodgkin lymphoma and chronic lymphocytic leukaemia. Qual Life Res. 2018;27(2):333–345. doi: 10.1007/s11136-017-1718-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 28.Snaith RP (2003) The Hospital Anxiety And Depression Scale. Health Qual Life Outcomes 1(29). 10.1186/1477-7525-1-29 [DOI] [PMC free article] [PubMed]
- 29.Razavi D, Delvaux N, Farvacques C, Robaye E. Validation de la version française du HADS dans une population de patients cancéreux hospitalisés [Validation of the French version of the Hospital Anxiety and Depression Scale (HADS) in a population of hospitalized cancer patients] Revue de Psychologie Appliquée. 1989;39(4):295–307. [Google Scholar]
- 30.Tedeschi RG, Calhoun LG. Posttraumatic Growth Inventory: measuring the positive legacy of trauma. J Traumatic Stress. 1996;9:455–471. doi: 10.1002/jts.2490090305. [DOI] [PubMed] [Google Scholar]
- 31.Cadell S, Suarez E, Hemsworth D. Reliability and validity of a French version of the Posttraumatic Growth Inventory. Open Journal of Medical Psychology. 2015;4:53–65. doi: 10.4236/ojmp.2015.42006. [DOI] [Google Scholar]
- 32.Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) Psychometric properties of an instrument to asses fatigue. Journal of Psychosomatic Research. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 33.Gentile S, Delarozière JC, Favre F, Sambuc R, San Marco JL. Validation of the French ‘multidimensional fatigue inventory’ (MFI 20) Eur J Cancer Care. 2003;12:58–64. doi: 10.1046/j.1365-2354.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- 34.International Non-Hodgkin’s Lymphoma Prognostic Factors Project A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329(14):987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 35.Nolte S, Liegl G, Petersen MA, Aaronson NK, Costantini A, Fayers PM, et al. General population normative data for the EORTC QLQ-C30 health-related quality of life questionnaire based on 15,386 persons across 13 European countries, Canada and the Unites States. Eur J Cancer Care (Engl) 2019;107:153–163. doi: 10.1016/j.ejca.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 36.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 37.Cocks K, King MT, Velikova G, Martyn St-James M, Fayers PM, Brown JM. Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J Clin Oncol. 2010;29(1):89–96. doi: 10.1200/JCO.2010.28.0107. [DOI] [PubMed] [Google Scholar]
- 38.Oerlemans S, Mols F, Nijziel MR, Lybeert M, van de Poll-Franse LV. The impact of treatment, socio-demographic and clinical characteristics on health-related quality of life among Hodgkin’s and non-Hodgkin’s lymphoma survivors: a systematic review. Ann Hematol. 2011;90(9):993–1004. doi: 10.1007/s00277-011-1274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berger AM, Mooney K, Alvarez-Perez A, Breitbart WS, Carpenter KM, Cella DCC, et al. Cancer-related fatigue, version 2.2015. J Natl Compr Canc Netw. 2015;13(8):1012–1039. doi: 10.6004/jnccn.2015.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebede CC, Jang Y, Escalante CP. Cancer-related fatigue in cancer survivorship. Med Clin North Am. 2017;101(6):1085–1097. doi: 10.1016/j.mcna.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 41.Hinz A, Weis J, Brähler E, Härter M, Geue K, Ernst J. Fatigue in cancer patients: comparison with the general population and prognostic factors. Support Care Cancer. 2020;28(9):4517–4526. doi: 10.1007/s00520-019-05260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Busson R, van der Kaaij M, Mounier N, Aleman BMP, Thiéblemont C, Stamatoullas A, et al. Fatigue level changes with time in long-term Hodgkin and non-Hodgkin lymphoma survivors: a joint EORTC-LYSA cross-sectional study. Health Qual Life Outcomes. 2019;17(1):115. doi: 10.1186/s12955-019-1186-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cormio C, Muzzatti B, Romito F, Mattioli V, Annunziata MA. Posttraumatic growth and cancer: a study 5 years after treatment end. Support Care Cancer. 2017;25(4):1087–1096. doi: 10.1007/s00520-016-3496-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jim HS, Jacobsen PB. Posttraumatic stress and posttraumatic growth in cancer survivorship: a review. Cancer J. 2008;14(6):414–419. doi: 10.1097/PPO.0b013e31818d8963. [DOI] [PubMed] [Google Scholar]
- 45.Zwahlen D, Hagenbuch N, Carley MI, Jenewein J, Buchi S. Posttraumatic growth in cancer patients and partners--effects of role, gender and the dyad on couples’ posttraumatic growth experience. Psychooncology. 2010;19(1):12–20. doi: 10.1002/pon.1486. [DOI] [PubMed] [Google Scholar]
- 46.Hinz A, Herzberg PY, Lordick F, Weis J, Faller H, Brähler E, et al. Age and gender differences in anxiety and depression in cancer patients compared with the general population. Eur J Cancer Care (Engl) 2019;28(5):e13129. doi: 10.1111/ecc.13129. [DOI] [PubMed] [Google Scholar]
- 47.Rossi R, Socci V, Talevi D, Mensi S, Niolu C, Pacitti F, et al. COVID19 pandemic and lockdown measures impact on mental health among the general population in Italy An N = 18147 web-based survey. JAMA Netw Open. 2020;3:e2010185. doi: 10.1101/2020.04.09.20057802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang C, Pan R, Wan X, Tan Y, Xu L, Ho CS, et al. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. Int J Environ Res Public Health. 2020;17:E1729. doi: 10.3390/ijerph17051729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romito F, Dellino M, Loseto G, Opinto G, Silvestris E, Cormio C, et al. Psychological distress in outpatients with lymphoma during the COVID-19 pandemic. Front Oncol. 2020;10:1270. doi: 10.3389/fonc.2020.01270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 520 kb)
Data Availability Statement
The dataset generated during the current study is available from the corresponding author on reasonable request and after permission of LYSARC and INSERM.