Patients with immunosuppression are at risk for prolonged infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In several case reports, investigators have indicated that multimutational SARS-CoV-2 variants can arise during the course of such persistent cases of coronavirus disease 2019 (Covid-19).1-4 These highly mutated variants are indicative of a form of rapid, multistage evolutionary jumps (saltational evolution; see Glossary), which could preferentially occur in the milieu of partial immune control.2,3 The presence of a large number of mutations is also a hallmark of the variants of concern — including B.1.1.7 (alpha), B.1.351 (beta), P.1 (gamma), and B.1.617.2 (delta)5 — which suggests that viral evolution in immunocompromised patients may be an important factor in the emergence of such variants. Since a large number of persons globally are living with innate or acquired immunosuppression, the association between immunosuppression and the generation of highly transmissible or more pathogenic SARS-CoV-2 variants requires further delineation and mitigation strategies.
Rapid viral evolution has been described in immunosuppressed patients with persistent SARS- CoV-2 infection. Choi et al. described an immunosuppressed patient with antiphospholipid syn- drome who was hospitalized in August 2020 and treated with anticoagulants, glucocorticoids, cyclophosphamide, intermittent rituximab, and eculizumab.2 During 152 days of persistent SARS-CoV-2 infection in this patient, the investigators identified 31 substitutions and three deletions in genome sequences. Twelve spike mutations were found, including seven in a segment of the receptor-binding domain consisting of 24 amino acids, some at sites linked to immune evasion (478, 484, and 493).6,7 The patient eventually died of severe Covid-19–related pneumonia. In another immunocompromised patient, Kemp and colleagues analyzed SARS-CoV-2 sequences at 23 time points over 101 days.4 Viral diversification was limited during the first 2 months but subsequently increased after the patient received an infusion of convalescent plasma on days 63 and 65, leading to rapid shifts in the frequency of the different variants. As such, sequences that were sampled on days 89, 93, and 102 showed distinct combinations of spike mutations. Truong et al. recently described the cases of three patients with B-cell acute lymphoblastic leukemia (including one with B-cell aplasia) after the receipt of chimeric antigen receptor (CAR) T cells. These patients were found to have multiple escape variants over the course of persistent Covid-19 infection.3 Some case reports have not identified highly divergent variants but have documented variants with mutations (e.g., V483A1-4 and E484K8) that alter antibody recognition.
Features Shared by Sequences from Case Patients and Emerging Variants
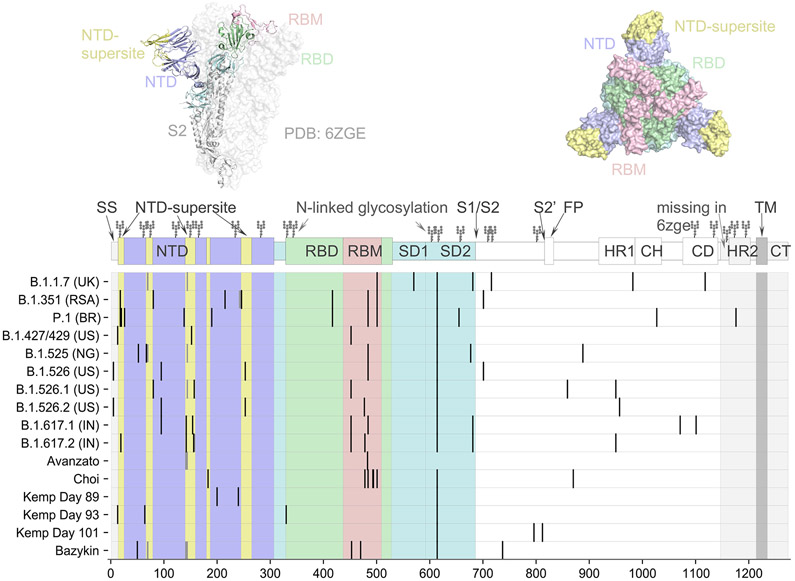
The mutational patterns that are described in these case reports have several key commonalities with the variants of concern and variants of interest that have become widespread. The variants B.1.1.7, B.1.351, and P.1 have at least three times as many mutations as the viruses that were circulating when these variants emerged. Likewise, investigators have detected 45 substitutions and deletions (including 17 in the spike protein) in B.1.617.2 as compared with B.1.1.7, the variant that was prevalent when B.1.617.2 emerged. A high percentage of these polymorphisms (>40%) are in the spike protein, which constitutes only 13% of the proteome. Because the spike protein is the prime target of the protective antibody response and mediates viral entry, a preponderance of mutations in this protein is consistent with adaptive evolution.
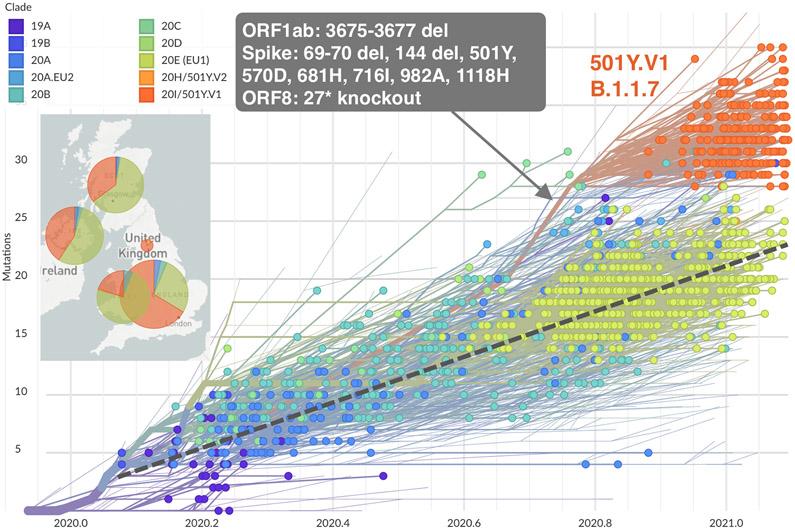
In addition, selection pressure is also shown by evidence of convergent evolution. Convergent mutations are seen in variants of concern and interest and in sequences obtained from immunocompromised patients, in particular deletions in the N terminal domain (NTD) (69-70del, Y144del, and 157-158del), the NTD supersite, and the receptor-binding domain (RBD) (K417N and E484K). These domains were associated with antibody escape or mutations that were probably associated with increased transmissibility (N501Y9 and P681H/R) (Fig. 1A).10 Such convergent mutations have been reported in both immunocompetent and immunosuppressed populations.11 The selective advantage of these convergent mutational alterations is supported by the pace at which these variants, particularly B.1.1.7, displaced previously circulating viruses. The evolutionary pattern of the B.1.1.7 variant in the United Kingdom illustrates the rapid evolution of multiple mutations in the spike protein and its subsequent rapid spread throughout the populace (Fig. 1B). The succession of new variants of concern or interest and the emergence of sublineages in these variants (i.e., variants of variants) illustrate a shifting evolutionary landscape in which new variants can rapidly become dominant, as suggested by the recent swift dissemination of B.1.617.2 that was originally identified in India.12 This variant shows a constellation of mutations that were identified in previous variants of concern or interest.
Figure 1. Spike Protein Structure and Phylogenetic Tree of SARS-CoV-2 Viruses.
Panel A shows the spike protein structure of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (entry 6ZGE in the Worldwide Protein Data Bank [PDB]). Domains are colored according to their role in viral entry and antibody recognition; the terminal domain that is missing in 6ZGE is shaded in gray. As shown in the graphic above the chart, the spike protein consists of two subunits, with subunit 1 (S1) (the portions in yellow, purple, blue, green, and pink) containing the receptor-binding domain (RBD), the N-terminal domain (NTD), and several other subdomains and subunit 2 (S2) (the portion in gray) mediating fusion of the virus-cell membrane. Also shown are the spike mutations that are representative of circulating variants of concern (B.1.1.7, B.1.351, P.1, and B.1.617.2) or variants of interest (B.1.427/429, B.1.525, and B.1.526), as well as patterns of mutations identified over the course of persistent infections in immunocompromised patients, as described in case reports. Representative sequences from the case reports are listed according to the first author’s name (1,2,4,8,10). Mutations in the NTD super- site are located nearby on the protein structure. Mutations are also recurring in the receptor-binding motif (RBM) and around the furin cleavage site. The symbols at the top of the columns indicate the N-linked glycosylation sites. CD denotes connector domain, CH center helix, CT cytoplasmic tail, HR1 heptad repeat 1, HR2 heptad repeat 2, SD1 subdomain 1, SD2 subdomain 2, S2′FP S2 fusion peptide, and TM transmembrane.
Panel B shows the phylogenetic tree of 1510 SARS-CoV-2 viruses detected in patients in the United Kingdom between January 2020 and February 2021. The phylogeny is embedded as a root-to-tip plot, in which the x axis represents the date of sample collection and the y axis represents the number of genomewide mutations that have occurred since the phylogeny root. The phylogeny is colored according to the clade, as listed in the Nextstrain database (an online site for the sharing of sequencing and genomic data about SARS-CoV-2). Clade 20I/501Y.V1 in this database corresponds to lineage B.1.1.7 in the Phylogenetic Assignment of Named Global Outbreak Lineages (PANGO) and is colored in orange and annotated in the figure. The branch leading to lineage B.1.1.7 is annotated with selected mutations that are present along this single branch. Lineage B.1.1.7 has a higher number of mutations than most other circulating viruses and has rapidly displaced existing genetic diversity in the United Kingdom. The phylogeny is constructed from sequence data shared to GISAID (a database of genomic information regarding influenza viruses and coronaviruses) by means of methods implemented by Nextstrain.
These compilations of case reports indicate the need to identify whether certain forms of immunosuppression are associated with an in- creased risk of such multimutational escape patterns — for instance, specific cancers or specific therapies, such as the development of B-cell aplasia related to CAR T-cell or anti-CD20 therapy, prolonged use of glucocorticoids, long-term chemotherapy, or radiotherapy. Similarly, organ transplant recipients and those with untreated or poorly controlled human immunodeficiency virus infection may also have prolonged SARS- CoV-2 infection and could constitute a reservoir of divergent escape variants that can spread in the general community. Prolonged viral replication in the context of an inadequate immune response facilitates the emergence of immune- pressure escape mutations.
Public Health Implications of Variant Evolution
The findings that immunocompromised patients with persistent SARS-CoV-2 infection may generate more transmissible or more pathogenic SARS-CoV-2 variants have a number of medical and public health implications. Heightened precautions should be taken to avert nosocomial transmission of Covid-19 among immunocompromised patients.8 Such patients should be prioritized for anti-Covid-19 immunization not only to protect them from SARS-CoV-2 but also to mitigate persistent SARS-CoV-2 infections. Immunologic studies to evaluate antibody and T-cell immune responses to currently approved vaccines should be undertaken in a variety of populations of immunocompromised patients to evaluate the efficacy of these approaches to mitigate disease and cur- tail prolonged viral replication. Early data suggest that a substantial number of solid-organ trans- plant recipients may have a poor antibody response to the spike protein.13 Whether this reduction in response alters the efficacy of vaccination in preventing severe Covid-19 or prolonged SARS- CoV-2 infection is currently unknown. Developing next-generation vaccines that are effective against SARS-CoV-2 variants and that can block escape pathways is needed.
Immunocompromised patients with Covid-19 should be informed about the importance of self-isolation until viral shedding is documented to be negative. Reactivation of symptomatic infection in compromised hosts has been reported, and reemergence of high-titered shedding can occur and should be a signal to look for potential escape mutants. Household and close contacts of such persons should be vaccinated regardless of age, since asymptomatic infection with these escape variants among healthy contacts may facilitate community spread of new transmissible variants. Until data regarding vaccine dose, safety, and immunogenicity are available for children under 12 years of age, these recommendations may lead to incomplete coverage of close contacts by contact vaccination.
Evolving Therapies for Immunocompromised Patients
The recent demonstration of the use of combination monoclonal antibodies for preventing SARS- CoV-2 acquisition in nursing homes and home settings suggests that passive antibody prophylaxis may be another approach for immunosuppressed patients who do not have an adequate immune response to vaccination or for patients and their family members with high-risk exposure.14 Monoclonal antibodies with mutations in the Fc portion of the formulation that extend the half-life and the likely effective concentration for several months or that enhance the effective immune clearance of SARS-CoV-2 may be an alternative form of Covid-19 prevention for immunocompromised patients who do not have a response to a vaccine.15 Monoclonal antibodies have already been approved for emergency use as treatment for high-risk persons and can reduce disease progression, viral load, and the duration of viral shedding. However, variants of concern have shown in vitro escape from some monoclonal antibodies, including bamlanivimab and casirivimab. (The latter along with imdevimab constitutes the REGN- COV2 antibody cocktail.)16 The effects of these mutations on clinical outcomes among immunocompromised patients are not known. New mono- clonal antibodies that are resistant to the current recognized mutations are in development.17
Antiviral agents that inhibit SARS-CoV-2 replication offer an alternative mechanism of protection that should be unaffected by mutations that compromise monoclonal antibodies. Molnupiravir (EIDD-2801) was reported to suppress replication of SARS-CoV-2 in 203 patients with early infection, and two larger clinical trials are in progress18,19 (ClinicalTrials.gov numbers, NCT04575584 and NCT04575597); whether antiviral escape variants emerge remains to be defined. Stopping the replication of SARS-CoV-2 in a compromised host by means of an effective monoclonal antibody or small molecule offers an opportunity to halt the development of mutations and the spread of SARS-CoV-2 to close contacts. Strategies to ensure that appropriate selection and monitoring for rapid detection of resistance to these therapies will become a key part of medical and public health management.
Viral adaptation to the human host and viral escape variants are a threat to patient health and Covid-19 pandemic control. Scientific attention to the intersection of SARS-CoV-2 biologic features, host immunologic characteristics, and the unique concerns of immunocompromised hosts is warranted. Additional considerations for immunization and other mitigation strategy guidelines for immunocompromised patients and their close contacts are also needed.
Acknowledgments
The views expressed are those of the authors and do not necessarily represent the views of the U.S. Army or the Department of Defense. We thank Dr. Mindy Miner for technical editing and support with an earlier version of the manuscript and Dr. Hongjun Bai for assistance in the preparation of an earlier version of Figure 1A; the authors of the originating and submitting laboratories of the sequences from the GISAID (Global Initiative on Sharing All Influenza Data) EpiCoV database on which this research is based; and the Covid-19 Genomics UK Consortium for open sequence sharing.
GLOSSARY
- Variants of interest and variants of concern
are variants with properties that warrant further evaluation. Distinct SARS- CoV-2 variants are circulating across the world. Certain variants are specific to a geographic area, and new variants are emerging. The Centers for Disease Control and Prevention (CDC) has classified some variants as variants of interest and those with specific attributes (e.g., evidence of increased transmissibility or disease severity or a potential effect on diagnostics, therapeutics, or vaccines) as variants of concern. Current variants of interest include B.1.526 (which was originally detected in New York), B.1.525 (detected in Indiana and New York), and P.2 (detected in Brazil). Current variants of concern include B.1.1.7 (or alpha, originally detected in the United Kingdom), B.1.351 (or beta, detected in South Africa), P.1 (or gamma, detected in Brazil), and B.1.617.2 (or delta, detected in India).
- Adaptive evolution
is the process in which advantageous mutations become dominant in circulating viruses through positive selection. Nucleotide substitutions occur when the virus replicates, and some of these nonsynonymous substitutions result in amino acid changes. If the viruses with these mutations have a selective advantage, the mutations will become more prevalent over time. The viruses carrying these mutations are better adapted to their environment. For example, at the beginning of the pandemic, when most populations had not been exposed to SARS-CoV-2, adaptive evolution potentially favored viruses with increased transmissibility. As the pandemic progresses, the virus is diversifying so that many slightly different variants are cocirculating and competing against one another. In parallel, a larger number of persons are immune to the virus from either previous infection or vaccination. This environment with a partially immune population can favor the selection of viruses that escape immune recognition by the host.
- Convergent evolution
is the process in which the same advantageous mutations are selected through positive selection in independent (not monophyletic) viral lineages. Similar mutations are identified in independent viral lineages. For example, the spike mutation E484K is found in the B.1.351 and P.1 variants, and it also appeared in other viral backgrounds, including in the B.1.1.7 lineage. This mutation was also selected during persistent infection in immunocompromised patients. E484K is known to provide a selective advantage since it impairs antibody recognition.
- Saltation or saltational evolution
is the process in which evolution proceeds with multimutational jumps. This activity contrasts with the key tenet of the gradualness of evolution, in which evolution proceeds with incremental rounds — that is, mutations accrue in a stepwise fashion. Saltation is thought to be a rare event. The sudden appearance of distant SARS- CoV-2 variants is evocative of saltation. The variants of concern showed multimutational leaps that are larger than those associated with the variants that were circulating when the variants of concern emerged. Likewise, sequences that were isolated during persistent infection in some immunocompromised patients showed a sudden burst of mutations.
REFERENCES
- 1.Avanzato VA, Matson MJ, Seifert SN, et al. Prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell 2020;183(7):1901.e9–1912.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi B, Choudhary MC, Regan J, et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med 2020;383:2291–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Truong TT, Ryutov A, Pandey U, et al. Persistent SARS-CoV-2 infection and increasing viral variants in children and young adults with impaired humoral immunity. March 2, 2021. (https://www.medrxiv.org/content/10.1101/2021.02.27.21252099v1). preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kemp SA, Collier DA, Datir RP, et al. SARS-CoV-2 evolution during treatment of chronic infection. Nature 2021;592:277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdool Karim SS, de Oliveira T. New SARS-CoV-2 variants — clinical, public health, and vaccine implications. N Engl J Med 2021; 384:1866–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Addetia A, Crawford KHD, Dingens A, et al. Neutralizing anti- bodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J Clin Micro- biol 2020;58(11):e02107–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lumley SF, O’Donnell D, Stoesser NE, et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med 2021;384:533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khatamzas E, Rehn A, Muenchhoff M, et al. Emergence of multiple SARS-CoV-2 mutations in an immunocompromised host. January 15, 2021. (https://www.medrxiv.org/content/10.1101/2021.01.10.20248871v1). preprint. [Google Scholar]
- 9.Wang P, Nair MS, Liu L, et al. Antibody resistance of SARS- CoV-2 variants B.1.351 and B.1.1.7. Nature 2021;593:130–5. [DOI] [PubMed] [Google Scholar]
- 10.Bazykin GA, Stanevich O, Danilenko D, et al. Emergence of Y453F and Δ69-70HV mutations in a lymphoma patient with long- term COVID-19. 2021. (https://virological.org/t/emergence-of-y453f-and-69-70hv-mutations-in-a-lymphoma-patient-with-long-term-covid-19/580). [Google Scholar]
- 11.Tegally H, Wilkinson E, Giovanetti M, et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature 2021;592: 438–43. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira I, Datir R, Kemp S, et al. SARS-CoV-2 B.1.617 emergence and sensitivity to vaccine-elicited antibodies. 2021. (https://www.biorxiv.org/content/10.1101/2021.05.08.443253v2.full). preprint. [Google Scholar]
- 13.Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA 2021;325:1784–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen MS, Nirula A, Mulligan M, et al. Bamlanivimab prevents Covid-19 morbidity and mortality in nursing-home setting. Presented at the virtual Conference on Retroviruses and Opportunistic Infections, March 9, 2021:121. abstract. (https://www.croiconference.org/abstract/bamlanivimab-prevents-covid-19-morbidity-and-mortality-in-nursing-home-setting/). [Google Scholar]
- 15.Ko S-Y, Pegu A, Rudicell RS, et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature 2014;514:642–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Starr TN, Greaney AJ, Addetia A, et al. Prospective mapping of viral mutations that escape antibodies used to treat COVID-19. Science 2021;371:850–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dejnirattisai W, Zhou D, Supasa P, et al. Antibody evasion by the P.1 strain of SARS-CoV-2. Cell 2021;184(11):2939.e9–2954.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Service RF. Researchers race to develop antiviral weapons to fight the pandemic coronavirus. Science. March 11, 2021. (https://www.sciencemag.org/news/2021/03/researchers-race-develop-antiviral-weapons-fight-pandemic-coronavirus). [Google Scholar]
- 19.Painter WP, Sheahan T, Baric R, et al. Reduction in infectious SARS-CoV-2 in a treatment study of COVID-19 with molnupiravir. Presented at the virtual Conference on Retroviruses and Opportunistic Infections, March 6, 2021:777. abstract. (https://www.croiconference.org/abstract/reduction-in-infectious-sars-cov-2-in-treatment-study-of-covid-19-with-molnupiravir/). [Google Scholar]