Executive summary
At the end of 2019, the first reports of a new respiratory virus appeared in China. The subsequent COVID-19 pandemic has affected every person, in every country, in the world. One early lesson was the crucial importance of timely accurate diagnosis. A second lesson was the widespread scarcity of such diagnostic capacity and capability.
The second lesson supported the findings of the 2018 Lancet Series on Pathology and Laboratory Medicine in Low-Income and Middle-Income Countries, namely that despite diagnostics being central to health care, access to diagnostic testing in pathology and laboratory medicine (PALM) is poor and inequitable in many parts of the world. In diagnostic imaging (DI), the other major diagnostic discipline, data are scarce, but what data are available suggest the situation is similar or even worse.
Poor accessibility of diagnostics is not a new issue. In 2008, the Maputo Declaration on Strengthening of Laboratory Systems identified the need to address the problems of poor accessibility to diagnostic testing. Although progress has been slow, there is now a conjunction of factors that has the potential to accelerate change. First, three major global health priorities—universal health coverage, antimicrobial resistance, and global health security—all require better access to diagnostics. Second, the publication of an essential diagnostics list (EDL) for priority settings by WHO in 2018 has been a key step in recognising the importance of diagnostics. Third, the COVID-19 pandemic has greatly raised awareness of the crucial importance of diagnostics. Lastly, within the past 15 years, extraordinary innovations in technology and informatics promise transformation across all aspects of diagnostics. The combination of all these factors can fuel political will to accelerate change.
This Lancet Commission on Diagnostics was set up with the remit of analysing the issues and identifying solutions for both PALM and DI, in part because these are the two major diagnostic disciplines and in part because, increasingly, optimum patient care (eg, in cancer) depends on the integration and synthesis of the results of both disciplines. Also, both disciplines share many of the same issues; for example, insufficient financial support, staff shortages, infrastructure problems, and low visibility and, hence, low priority.
In this Commission, we analyse the current status of diagnostics with the use of the six WHO building blocks of health systems, namely health service delivery, health workforce, health information systems, access to diagnostics (analogous to essential medicines), financing, and leadership and governance, as the basis. Given the dearth of reliable and comprehensive data, the Commission's first step was to quantify, where possible, the current state of diagnostics globally. We use six tracer conditions (diabetes, hypertension, HIV, and tuberculosis in the overall population, plus hepatitis B virus infection and syphilis for pregnant women) and show that the diagnostic gap (ie, the proportion of the population with the condition who remain undiagnosed) is, at 35–62%, the single largest gap in the care pathway (the cascade of care comprising screening, diagnosis, treatment, and cure or successful management). We also examine the current availability of diagnostics by level of health care facility, geography, and socioeconomic group. The diagnostic gap is most severe at the level of primary health care, in which only about 19% of populations in low-income and lower-middle-income countries have access to the simplest of diagnostic tests (other than those for HIV and malaria). Even in hospitals, this figure only rises to 60–70%. DI is essentially absent outside of hospitals. People who are poor, marginalised, young, or less educated have the least access to diagnostics.
Key messages.
-
1
47% of the global population has little to no access to diagnostics.
-
2
Diagnostics are central and fundamental to quality health care. This notion is under-recognised, leading to underfunding and inadequate resources at all levels.
-
3
The level of primary health care is the diagnostic so-called last mile and particularly affects poor, rural, and marginalised communities globally; appropriate access is essential for equity and social justice.
-
4
The COVID-19 pandemic has emphasised the crucial role of diagnostics in health care and that without access to diagnostics, delivery of universal health coverage, antimicrobial resistance mitigation, and pandemic preparedness cannot be achieved.
-
5
Innovations within the past 15 years in many areas (eg, in financing, technology, and workforce) can reduce the diagnostic gap, improve access, and democratise diagnostics to empower patients.
-
6
As an example of the potential impact, 1·1 million premature deaths in low-income and middle-income countries could be avoided annually by reducing the diagnostic gap for six priority conditions: diabetes, hypertension, HIV, and tuberculosis in the overall population, and hepatitis B virus infection and syphilis for pregnant women.
-
7
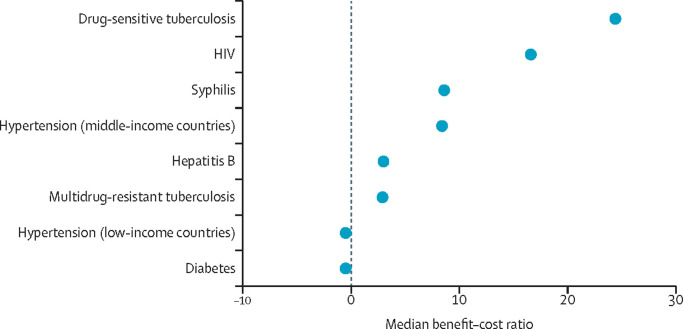
The economic case for such investment is strong. The median benefit–cost exceeds one for five of the six priority conditions in middle-income countries, and exceeds one for four of the six priority conditions in low-income countries, with a range of 1·4:1 to 24:1.
Given the depth and breadth of the problems, sustained access to quality, affordable diagnostics will require multi-decade prioritisation, commitment, and investment. Incorporating diagnostics into universal health coverage packages will begin this process.
Our conclusion is that just under half (47%) of the world's population have little to no access to diagnostics. We estimate that reducing the diagnostic gap for the six tracer conditions from 35–62% to 10% would reduce the annual number of premature deaths in low-income and middle-income countries (LMICs) by 1·1 million (2·5% of total annual deaths in LMICs), and annual disability-adjusted life-year (DALY) losses by 38·5 million (1·8% of losses from all conditions).
In this Commission, we examine the policy environment and conclude that the fundamental cause for the current situation is the low visibility and prioritisation of diagnostics. Diagnostics are not explicitly mentioned in proposals for universal health coverage and are largely missing from national strategic plans for health, and the focus on diagnostics in the National Action Plans for Health Security is limited primarily to epidemic infectious diseases. Although corruption is a problem across any health system, diagnostics are particularly susceptible because they require acquisition of expensive equipment and supplies.
Although data are particularly scarce at the operational level, the necessary physical infrastructure is clearly deficient in many facilities, resulting in weak services of inadequate quality. Similarly, support capabilities, such as management and procurement systems, technical support, information technology, and supply chains, are widely insufficient. Regarding workforce, we estimate there is a global shortfall of around 840 000 diagnostics staff (using the UK as the benchmark), noting that current education and training is not even enough to maintain current levels. Quality and safety mechanisms for standards are scarce, particularly for LMICs. For example, a 2019 study suggested that India has only 1151 accredited medical laboratories, whereas the USA, with a quarter of India's population, has 260 000 accredited medical laboratories.
Because low political prioritisation is the key cause of poor access to diagnostics, we explore how we can use the framework of Shiffman and Smith to achieve political change. With the importance of diagnostics fresh in people's minds from the COVID-19 pandemic, and with the 2018 EDL (a useful tool for prioritisation and a way forward), there might now be an opportunity for progress.
This Commission offers potential solutions to the problems associated with the poor access to diagnostics. We have developed an evidence-based template for a national EDL as the basic core of all integrated tiered networks, designed to meet the diagnostic needs of the predicted top 20 conditions in the global burden of disease for 2030 and 2040 (the GBD-20 EDL).
Because technology is an enabler of many of the putative solutions in this Commission, we discuss the crucial role of technological innovation and also propose solutions via changes in policy, governance, and finance, and in infrastructure, workforce, and quality. The key aspects of the solutions proposed are summarised in following paragraphs under the relevant recommendation.
This Commission also outlines the economic case for investing in diagnostics. We provide a benefit–cost analysis for the same aforementioned six tracer diagnostic tests. Although costs are relatively simple to calculate, measuring the benefits is difficult and the benefit–cost is context-specific, varying with several factors, such as country income, disease prevalence, and availability of more effective treatment. Although little work has been done in this area, by making several assumptions, we show that the median benefit–cost in LMICs for all but one of the six tracer tests exceeds one, with a range of 1·4:1 to 24:1. Our conclusion is that there is a strong case for investment to improve access to diagnostics.
There is no single effective means (eg, technology) to address the multiplicity of challenges in improving the access to diagnostics. As solutions, we propose 10 recommendations. Although each recommendation is important in its own right, they are also highly interdependent. If implemented as a group, these recommendations will make a substantial difference.
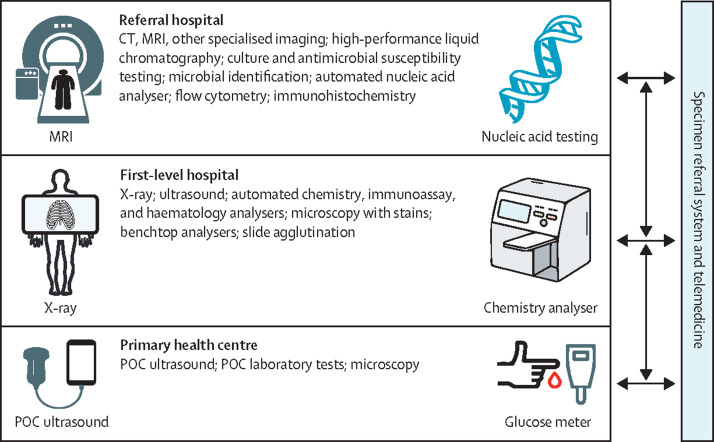
In the relative absence of national strategic plans for diagnostics, it is unsurprising that access is poor for many countries. Therefore, we recommend that countries develop a national diagnostics strategy and do so with an evidenced-based integrated and tiered network and a national EDL (this EDL can be based on our template) as the model (recommendation 1). Diagnostics would be allocated across the different health system tiers: point-of-care investigations to primary health care, basic analysers and x-ray to first-level hospitals, and more sophisticated diagnostics (eg, MRI, CT, flow cytometers, nucleic acid analysers, and microbial identification) to higher level facilities. Implementation of this model would serve to drive investment in all of the resources (eg, staff, equipment, and finance) of an effective diagnostics system. Because each country will have different existing facilities and varying disease prevalence, countries can adapt this template to their own context. However, it is key that whatever model is adopted is evidence-based.
Given that the biggest gap is in provision of diagnostics at the level of primary health care, which is also the entry point to the care cascade, we also recommend that, as a priority, a set of key point-of-care diagnostics (point-of-care tests and point-of-care ultrasound) be made available at all primary health-care centres (recommendation 2).
Health workforce expansion is key to improving access to diagnostics and diagnostic services. Expansion of the health workforce with current approaches alone will be insufficient. New approaches are needed to ensure expansion of workforce capacity and acquisition of contemporary skills, including more competency-based education, greatly expanded access to continuing professional development, telehealth for remote services, and greater use of task shifting and sharing. We recommend that each country use these approaches to expand the size and effective capacity of its health workforce (recommendation 3).
Without systems to ensure diagnostic safety and quality, expanded access is of questionable value, potentially causing harm and wasting resources. A national regulatory framework that addresses safety and quality is essential. Device regulation could be simplified by regional harmonisation or by expansion of programmes such as WHO prequalification. The implementation of quality services will need regulation for both laboratory accreditation and for professional standards and competencies. We recommend each country develops an appropriate governance and regulation framework (recommendation 4).
Without adequate infrastructure, the provision of diagnostic services will always be insufficient. A number of approaches supporting improvement are outlined in this Commission. These approaches include more efficient use of current resources through better management, regional pooled procurement and equipment standardisation, fostering of regional and national manufacturing capacity, and development of public–private partnerships with manufacturers. However, additional financing for diagnostics more generally is essential, for which the majority will need to be domestic and primarily public. Higher taxes on tobacco (so-called sin taxes) are one possibility. Other potential sources include financing instruments, such as Social Impact Bonds or Development Impact Bonds, which have rarely been used for diagnostics, and borrowing from multilateral banks. We recommend that each country develops mechanisms to finance sustainable diagnostics (recommendation 5).
Complementing improved financing, there also needs to be national and international action to increase the affordability of diagnostics generally. Supporting more production in LMICs and pooled procurement (market shaping) can increase affordability. Therefore, we recommend global action to improve the affordability of diagnostics (recommendation 6).
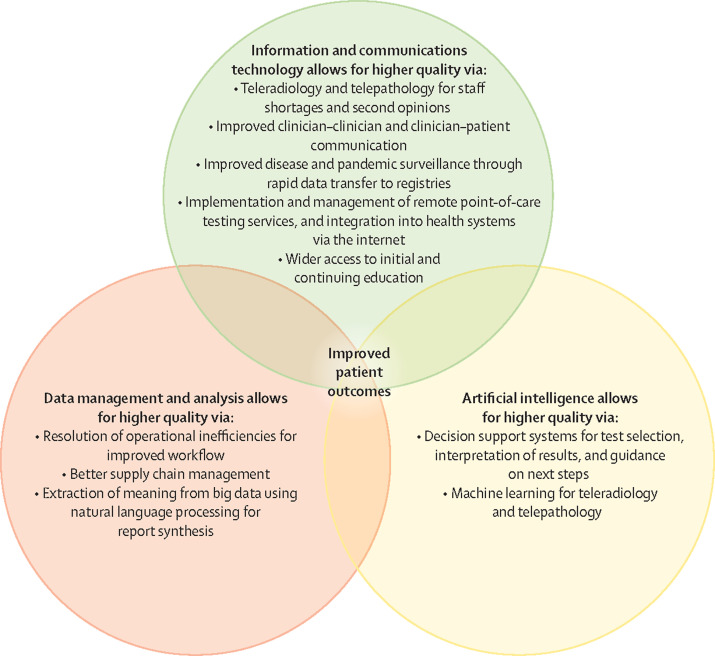
A key reason why now is an apposite time to address the issues with the accessibility of diagnostics is the transformative potential of innovation in many areas of diagnostics. In this Commission, we identify three broad approaches relating to technology that offer the greatest potential—namely, digitalisation, point-of-care diagnostics, and democratisation of diagnostics. By enabling diagnostic testing outside of the hospital (eg, self-testing or self-sampling), the first two approaches democratise diagnostics and empower the patient, particularly those patients who are marginalised. To ensure equity, privacy, and alignment with other social and political factors, we briefly review the general principles of implementation. These principles include designing technologies with, and for, the end user, generating data that can be integrated into the patient record and into national monitoring indicators, and a standards-based approach to increase system interoperability and reduce potential for conflict and confusion. As many of the Commission's recommendations depend on innovation in education, management, communications, and financing, as well as technology, to achieve their transformative effect, one of our main recommendations is the continued fostering of innovation, especially in LMICs (recommendation 7).
A particular challenge is the provision of diagnostics for that third of the world's population living in fragile and conflict situations. These are complex, challenging settings and have very different health actors involved. Within the past 15 years, innovations in areas such as information technology and point-of-care testing can address some of the challenges, but more coordination of the civilian and security sector is needed, and humanitarian staff and affected populations need to be involved to define needs (recommendation 8).
Considering that low visibility is probably the single most important global barrier to the adequate resourcing of diagnostics, there will need to be a major advocacy drive, combining efforts at both national and international levels and alignment of the activities of diverse stakeholders. Therefore, we recommend a coordinated advocacy programme for diagnostics at national and international levels, including adopting a World Health Assembly resolution on diagnostics (recommendation 9).
Finally, as the effort in transforming diagnostics will need to be focused, persistent, multi-year, and sustainable, we recommend the creation of an international Diagnostics Alliance to work with relevant national and international agencies to promote and support this effort (recommendation 10).
To build on the findings of this Commission, key next steps should be the initiation of national and international advocacy programmes, the creation of an international Diagnostics Alliance as an advocate, and the adoption of a World Health Assembly resolution on the need for diagnostics to be an integral part of any universal health coverage programme. Continued research is also needed to fill key data gaps; for example, research on the health workforce and the benefit–cost of diagnostics.
The COVID-19 pandemic must be a turning point. Implementation of our recommendations over the next 20 years would transform the world from one where close to half of the population has little to no access to diagnostics, to one where the great majority does.
Introduction
Diagnostics are central to effective health care. The importance of diagnostics has been brought into focus by the COVID-19 pandemic (panel 1 ), which has shown not only the crucial need of timely and accurate diagnostics, but also that there are problems in virtually every aspect of the provision of diagnostics, including in the health workforce, the equipment, the supply chain, the quality, the communications, and the regulation. The pandemic has also shown that deficiencies and inequities apply in virtually every country and that the current methods for measuring preparedness for a pandemic are flawed.
Panel 1. COVID-19.
The COVID-19 pandemic has highlighted the centrality of access to diagnostics as a key component of detecting and controlling emerging infectious diseases. Early issues in developing accurate tests hampered the understanding of, and response to, the initial stages of the COVID-19 outbreak, resulting in the rapid emergence of unreliable tests that led to confusion in clinical care and epidemiological reporting about rates of infection, erroneous messages to the public, and widespread deployment of inaccurate (or even fake) tests that quickly had to be replaced with accurate tests.1, 2, 3 At the same time, widespread media reports noted that lack of SARS-CoV-2 testing capacity resulted in prolonged delays in obtaining test results, delays that severely affected the clinical and epidemiological usefulness of the tests.
Pressures to develop high-quality testing at scale did drive innovation in test development and deployment. First was leveraging of extant public health laboratory systems before commercial tests became available, such as the Centers for Disease Control and Prevention RT-PCR assay that was made available to public health laboratories early in the COVID-19 pandemic. Next, commercial companies quickly developed SARS-CoV-2 PCR assays for use on existing test platforms that, when combined with existing manufacturing and distribution systems, allowed for relatively rapid deployment of assays and eliminated dependence on a single assay or manufacturer. When supply chain issues did develop during the COVID-19 pandemic, access to multiple assays allowed laboratories to partially mitigate shortages by shifting between alternative assays.
Development of rapid PCR assays allowed health systems to decentralise some testing through the use of smaller instruments suitable for near-patient settings, such as outpatient clinics. The ability to use existing laboratory infrastructure and systems enabled testing to be tailored to specific needs, such as the use of rapid assays (turnaround times of less than 2 h) for symptomatic patients being considered for admission to hospital or for quarantine, or the use of high-throughput batch assays (slower turnaround times of up to 8 h per batch) for testing asymptomatic patients with a history of exposure to infected people, pre-procedure screening, or health-care employee screening.
However, these successes occurred in high-income countries (HICs) where public health, hospital, clinic, and commercial laboratories with necessary resources already existed and operate within well established public health and health-care systems. Of equal importance is that these laboratory and health-care systems had ready access to the commercial manufacturers of assays and other testing supplies (and the associated technical support); in too many parts of the world, these resources do not exist, creating substantial barriers to dealing with the COVID-19 pandemic.
Additionally, although in HICs pre-existing mechanisms for financing were in place, global investment in SARS-CoV-2 testing has lagged. Despite development of the Access to COVID-19 Tools Accelerator partnership,4tests, medical oxygen, and protective equipment for health workers are badly underfunded”5 and “[c]ountries with no resources to buy tests are left without. A lack of laboratory facilities for RT-PCR tests and the paucity of trained laboratory specialists leave many low-income and middle-income countries (LMICs) disadvantaged in reaching full testing capacity”.6 Prioritisation of diagnostics and appropriate allocation of resources remains a substantial barrier to global access to diagnostics—even in the setting of a global COVID-19 pandemic—and highlights the disparities in access to diagnostics between HICs and LMICs.
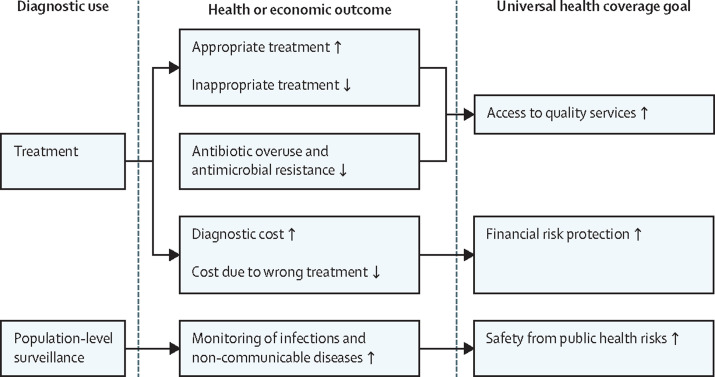
However, the centrality of diagnostics extends far beyond testing for a pandemic virus. The reach of diagnostics is broad and deep. For the patient, diagnostics are used not just to establish diagnoses in communicable and non-communicable diseases, they are also used to guide therapy, prognosticate, monitor progress, and measure response to therapy. More broadly, diagnostics are crucial to universal health coverage, public health, epidemiology, and global health security, being vital for disease detection and surveillance (figure 1 ). For example, addressing the challenges of antimicrobial resistance and the maintenance of disease registries, such as cancer registries, depends on good diagnostics.
Figure 1.
Diagnostics are essential for universal health coverage
Diagnostics are used to guide treatment of patients as well as population-level surveillance, both of which affect health and economic outcomes. These outcomes, in turn, have a multifaceted and substantial impact on achieving specific goals of universal health coverage.
Global access to high-quality diagnostics is poor, and even when diagnostics are available they are often of variable quality.7, 8 Access is also inequitable, with diagnostics often being more readily available in larger urban areas, and for people of higher socioeconomic status. Because of this circumstance, low-income and middle-income countries (LMICs), with their larger proportion of poor and rural populations compared with high-income countries (HICs), are particularly affected. However, even in HICs, access to diagnostic services is often physically and economically difficult for rural, poor, and marginalised communities.
Diagnostic systems are urgently needed, with the preparedness and resilience to deal with future epidemics and with the capacity and capability to provide equitable and effective services under normal conditions. The scarcity of high-quality diagnostics has serious and even deadly outcomes for patients (panel 2 ), and applies equally to all diseases and conditions, not just COVID-19. For instance, currently, about 30% of patients with tuberculosis are not diagnosed or reported, contributing to the 1·5 million annual deaths globally.14 In pregnancy, clinical misdiagnosis can be fatal. A 2020 study from Mozambique showed that, in 35 (38%) of 91 maternal deaths, the mother's life would probably have been saved if a clinical misdiagnosis had not occurred.15 Furthermore, malaria is commonly over-diagnosed clinically. For example, in Angola in 2017, only 90 (15·7%) of 573 patients clinically diagnosed with malaria actually had malaria—an error rate of 84·3%.16 This over-diagnosis and consequent neglect of alternative diagnoses leads to avoidable morbidity and mortality. Treatment without a confirmed diagnosis also results in high rates of empirical and unnecessary use of antimicrobials, because without diagnostics health-care providers rely on clinical judgement (syndromic diagnosis), with great uncertainty as to the diagnosis.17 This approach is especially true at the level of primary care.18
Panel 2. Impact of diagnostic error on patients in low-income and middle-income countries—a case study in Latin America of unnecessary hardship due to delayed treatment.
Rarely are the voices of patients, especially those of rural and lower socioeconomic status, amplified and brought into medical journals. However, we know from articles in the media that patients across the world suffer a variety of negative impacts due to diagnostic error. Many patients lose trust in their health-care providers following a misdiagnosis or other diagnostic error, as well as experiencing anguish and misery.9, 10, 11, 12, 13 The voices of patients who have experienced these negative outcomes need to be heard. When patients come together to advocate for better care and the right diagnosis, they can have a massive positive impact on health care as a whole.
Bertha Aguilar, Lancet Commissioner, describes her journey to becoming a patient advocate:
“Many patient groups start as a result of a personal experience, when patients feel the system has failed them. In my case, a very well known gynaecologist never asked for my family history and never performed a breast examination until after I had found a lump that turned out to be a 5 cm tumour. I had a radical mastectomy. Not one more woman will die in my family due to negligence.
I co-founded an organisation [Fundación Cimab, now called Fundación Cima] to create awareness and raise funds to help patients. The response was immediate due to the teamwork of many people. Then, we realised that being Pink [raising awareness and supporting patients] was not enough, we needed access to the right treatment. Through the help of an American Cancer Society grant to participate in American Cancer University [a programme launched in 2008 by the American Cancer Society for select countries from Latin America to receive training] that is when everything started to change. The networking was amazing. We modelled our efforts after a programme from Brazil called Femama, which coordinates and empowers local non-governmental organisations from all over the country to promote, formulate, and monitor public policies. This approach gave the movement a whole new perspective: first, it ensured that all non-governmental organisations sent the same and correct messages to the public, and second, it ensured that we could focus on what was vital.
It is very frustrating when you act responsibly regarding health issues but your country does not even have a National Health Plan. Patients can start by giving support to each other. Then, through advocacy, they reach a point where they have raised their voice sufficiently to be heard and to begin seeing changes. Then again, although agreements on paper can look very nice, it is important to create Citizen Observatories to monitor such agreements.
The economic burden for patients having to navigate the health system to get a right diagnostic is unacceptable. They lose work so they do not get a salary, they pay out of their own resources, and yet the emotional impact when you feel sick, when you know something is wrong but cannot have access to appropriate diagnostic testing, is devastating. Health should not be a matter of postal code, it is a right we have, and we will keep fighting to make a difference.”
Delayed diagnosis is also an important factor that leads to avoidable morbidity and mortality. In England, for only four major cancer types, it has been estimated that the delay in diagnosis due to the COVID-19 pandemic will be associated with over 3000 additional deaths in the next 5 years and with around 60 000 additional years of life lost.19
Given the scale of the challenges, incremental increases in the current approaches to providing access to diagnostics are unlikely to work. To take one example, staff are the most important component of any diagnostics system. In sub-Saharan Africa, just to address the shortfall in pathologists at the present rate of training, it will take more than 400 years to reach around the same ratio of pathologists per 100 000 population that currently exists in the USA and in the UK.8 Similar shortfalls exist for radiologists and for all other staff in both was pathology and laboratory medicine (PALM) and diagnostic imaging (DI) disciplines. Although accepting that the level of staffing in the USA and in the UK is not necessarily the appropriate target, particularly with more widespread use of telehealth, redistribution of health-care tasks, and changes in clinical practice and service delivery, these are the best documented data currently available to provide some quantified measure of the scale of the shortfall.
Fortunately, the extraordinary innovations in diagnostics of the past decade or so provide opportunities to address the challenges in improving the accessibility of diagnostics. Technological developments in, for example, digitalisation, artificial intelligence (AI), electronic data transfer, and mobile technologies alone, and in combination with developments in education and training, workflow organisation, and data and supply chain management, are facilitating the transformation of diagnostics. Many of these developments, particularly point-of-care testing and examinations, patient self-testing, and patient self-collection of specimens, are starting to address what has been, and remains, one of the key barriers—namely, access sufficiently close to the patient to be convenient, which is often described as the so-called last mile problem.
Although there have been several initiatives to improve diagnostics over the past decade or so—from the Maputo Declaration on Strengthening of Laboratory Systems in 2008,20 to the creation of the first WHO essential diagnostic list (EDL) in 2018—progress has been slow.21 However, the commitment by all UN member countries to provide universal health coverage by 2030 provides an ideal opportunity to accelerate the pace of change. However, none of the available plans for implementation of universal health coverage explicitly show provision and financing of diagnostics.22 Unless the gap in diagnostic provision is addressed and investment is expanded, the opportunities and promise of universal health coverage will be undermined. Similarly, substantial and sustainable improvement in antimicrobial resistance and global health security will be much constrained.
The contrast of investment in diagnostics with the enormous investment devoted to developing pharmaceuticals could not be more striking. However, if the patient's diagnosis is wrong, then even the most effective drug will be useless, and the resources invested to produce it will have been wasted. The inappropriate treatment might also be harmful to the patient. The fundamental nature of this linkage is shown by the drive to identify actionable mutations in cancer, for which companion diagnostics are crucial for the identification of mutations that will or will not be affected by a particular drug.23 Wider recognition of this linkage will result in a more appropriate ratio of investment, less waste, and better outcomes for the patient.
In response to these urgent issues, the Lancet Commission on Diagnostics was initiated in 2018 to document the scale of the challenge and to propose solutions for the attention of, and adoption by, policy makers, funders, clinicians, and patients. As the issues are global, affecting HICs as well as LMICs (albeit with important differences), the Commission has had a global perspective and has not been confined to LMICs.
Setting the stage
During and following the publication of the Lancet Series on Pathology and Laboratory Medicine in Low-Income and Middle-Income Countries in April, 2018, it became evident that not only was PALM poorly accessible globally and under-represented in the discourse on universal health coverage, it also became clear that DI, the other major diagnostic discipline, was in a similar situation. For this and other reasons, it was decided that, to assess and address the issues effectively, the Lancet Commission on Diagnostics should consider both disciplines together (panel 3 ).
Panel 3. The Lancet Commission on Diagnostics.
The Lancet Commission on Diagnostics was initiated in 2018. It brought together 25 Commissioners from 16 countries, from a range of disciplines, including pathology and laboratory medicine, radiology and diagnostic imaging, surgery, internal medicine, health policy, health systems, and health economics research, as well as former ministers of health, a patient advocate, and participants from key international diagnostic networks. Following an initial planning meeting at Harvard University (Cambridge, MA, USA) in November, 2018, the first full meeting was held at Oxford University (Oxford, UK) in April, 2019, and the second at the Brocher Villa (Geneva, Switzerland) in January, 2020. The third planned meeting in India was replaced by online meetings due to the COVID-19 pandemic, a global health crisis which dramatically underscored the key importance of diagnostics.
We created working groups on patient focus, workforce, health system (at a macro level), economics, policy and governance, advocacy, and innovation. The chairs of the working groups formed a Steering Committee of seven Commissioners that met regularly by email or teleconference. The material from the working groups was synthesised by the Steering Committee supplemented with a number of Commissioners, and all Commissioners participated in teleconferences and email discussions of the Commission.
A roundtable consultation with 14 representatives from the private sector was held in January, 2020, in Geneva, Switzerland, and online consultations took place between February and May, 2020, with 11 individuals from eight different international and non-governmental organisations, when in-person consultations became no longer possible. A small group of more junior colleagues undertook various new modelling exercises for the Commission.
At the first meeting, Commissioners discussed and agreed on the scope of the Commission. Additionally, the Commissioners adopted specific definitions for specific key words and phrases that crucially have considerable variation in the understanding between different disciplinary groups (eg, radiologists and pathologists) and countries. The Commissioners also agreed that moving towards coordinated or integrated reporting by both PALM and DI disciplines, centred on maximising the outcome for the patient, would be a guiding principle for the Commission.
Commission scope and definitions
Diagnostics can be used for numerous purposes, ranging from obtaining a diagnosis, to monitoring the efficacy of therapeutic interventions, to population-based disease surveillance. The Commission focused on the steps involved in diagnosis and patient management, and the term diagnostic investigations is used throughout (appendix p 2) for the diagnostic tests and examinations involved.
The diagnostic process is a complex, iterative, collaborative activity with the goal of narrowing down the diagnostic possibilities and developing a more precise and complete understanding of a patient's health problem. The process consists of performing a clinical history and interview, doing a physical examination and diagnostic testing, and referring or consulting with other clinicians.24
The two major diagnostic disciplines are PALM and DI. PALM includes anatomical pathology and the disciplines within clinical pathology, such as biochemistry, haematology, microbiology, and immunology. By use of microscopy or a variety of other instruments, these disciplines involve the analysis of samples of tissue (sometimes acquired under imaging guidance) or fluids (eg, blood or urine) outside of the body (ie, in-vitro diagnostics). Although anatomical pathology includes autopsy and forensic investigation, these approaches were not within the Commission's scope. Medical imaging involves the use of several technologies, (eg, x-rays, ultrasound, MRI, CT, and nuclear medicine) to obtain visual images of the structure and function of the body and its organs. The field of medical imaging is further divided into DI and interventional imaging. Although there is an overlap, interventional imaging primarily involves the use of image-guided interventions to provide treatment or to obtain tissue sampling. The Commission's focus was solely on DI.
Both for laboratory medicine and for DI, technology now permits some diagnostics to be used at the bedside, in the doctor's office and, for laboratory medicine, even by the patient themselves. The role of such point-of-care diagnostics and self-testing is discussed in this Commission.
A number of medical professionals specialise in diagnostics. Pathologist is the term used for physicians who specialise in PALM. Although other specialists can use imaging in their practice, radiologist is the term used for physicians who specialise in DI. There are also highly trained non-physician scientists involved (eg, laboratory scientists and medical physicists) as well as specialised technical staff. Although PALM uses the term diagnostic tests, DI uses the term diagnostic examinations; diagnostic investigations encompasses both disciplines.
Although in several countries the majority of diagnostics provision is by the private sector, in most countries there is a mix of public and private provision. In the era of universal health coverage, antimicrobial resistance, and health security, the Commission's focus was primarily on the public sector provision. Uptake of diagnostics involves both the supply side (including aspects such as geographical availability, cost, quality, and accessibility; appendix p 3) and the demand side. The Commission's focus was primarily on the supply side of access to diagnostics. The drivers of the demand side include a number of factors: clinician ordering; patient behaviour, needs, and perceptions; household income; education in general and education about specific health issues; cultural beliefs; individual characteristics, such as age and sex; initiatives intended to improve access to health care, such as universal health coverage; and rapidly changing demand driven by issues such as the COVID-19 pandemic (panel 1). We touch on a few of these aspects while discussing democratisation of diagnostics, but addressing all of these was considered beyond the scope of the Commission.
There are several important aspects of diagnosis and diagnostics that this Commission specifically excluded. The issues around how to improve clinical diagnosis (ie, history and examination during the encounter between clinician and patient) are important,25, 26 but the Commission's focus was on the diagnostic investigations that follow clinical examination. Accordingly, we did not consider devices used in physical examinations, such as thermometers and ophthalmoscopes. Specialised examinations, such as endoscopy and electrocardiograms, although fundamental for patient care, involve disciplines other than DI and PALM and, therefore, we did not address these examinations. However, we did consider blood pressure monitors, whose use is closely linked to laboratory testing for cardiovascular disease. Additionally, although the availability of and access to diagnostics generally have important implications at the level of public health, given the scale of the issues, diagnostics for surveillance, for organised population screening for prevention, and for the new area of precision public health were beyond this Commission's scope.27
Patient-centred diagnostics
Although synthesis of the results of the two major diagnostic disciplines by the patient's clinician has long been standard practice, modern medicine has markedly increased this requirement. Deeper understanding of the pathobiological complexities of many diseases is revealing unsuspected variants with therapeutic and prognostic importance. Cancer is the prototypical example. Increasingly, the results of PALM and DI investigations need to be combined and even integrated to solve the complexities of diagnosis and management.28 Advanced imaging technology, including CT and MRI, combined with PET, match functional imaging to anatomy to stage a patient's tumour. Integrating this imaging information with data from PALM on cancer type, grade, and molecular analysis allows for optimal prediction of disease progression, selection of targeted therapy, and prediction of the patient's response to therapy—ie, predictive medicine. This integration also reduces the likelihood of over-diagnosis and over-treatment. Given that predictive medicine extends beyond cancer, it seems probable that this model of having integration or even co-reporting of the results of diagnostic tests, centring on the patient's needs, will apply in other complex diseases. Because this approach clearly increases both efficiency and efficacy, such close correlation and integration should become the standard of care throughout health-care systems.
However, notwithstanding the benefits of integration, the reality is that, in many places, both disciplines function in separate silos. One of the reasons for defining specific key words and phrases at the outset of the Commission was that it was clear that the pathologists and radiologists were either not aware of, or did not understand, key terminology from the other discipline, largely arising from the little experience of working together. Aside from the resultant clinical disadvantages, there are also operational disadvantages. The infrastructure of both DI and PALM is under-resourced in many countries. Due to the considerable overlap in some infrastructure requirements (eg, power, supply chain, information technology, communications, telereporting, and use of AI to interpret images), cooperation on, or even joint management of, such infrastructure could be beneficial to both disciplines.29 More importantly, the central role of DI and PALM in high-quality health care is under-recognised and under-resourced by decision makers of funding and policy. Jointly making the case that diagnostics will help patients and improve efficiency is more likely to be successful in garnering support for investment (especially in LMIC settings), than if done separately and, to some extent, in competition.
For all the aforementioned reasons, the driving concept of this Commission was that overcoming the global challenges to providing good access to accurate timely diagnostics would be best served by addressing the issues for DI and PALM jointly, centring on the optimum outcome for the patient.
Structure of the Commission
To provide a conceptual structure for the Commission, we adapted the WHO building blocks as the areas on which to focus, namely health service delivery, health workforce, health information systems, access to essential diagnostics (analogous to essential medicines), financing, and leadership and governance. Availability of data was also a determining factor.
In this Commission, we describe the current status and the underlying causal issues in terms of access, policy environment (ie, visibility, planning, and leadership and governance), and operational factors (ie, infrastructure including information, workforce, quality, and safety). These areas cover the six WHO building blocks of health systems. We also discuss how the current situation of poor access to diagnostics might be changed by use of the political prioritisation framework of Shiffman and Smith30 and why now is a propitious moment for change. We propose the implementation of an EDL within integrated and tiered networks as a powerful mechanism to drive provision of equitable access to high-quality diagnostics. Additionally, we discuss how to develop an evidence-based template for an EDL, allocating diagnostics to three levels of the health-care system (ie, point-of-care tests to primary health centres; basic analysers and x-ray to first-level hospitals; and more sophisticated diagnostics, such as MRI, CT, flow cytometers, nucleic acid analysers, and microbial identification, to facilities of a higher level). Furthermore, we identify three main areas of innovation in technology (ie, digitalisation, point-of-care testing, and democratisation of diagnostics), with particular promise in addressing the challenges of providing access to high-quality diagnostics, and outline specific solutions to operational issues as well as finance and governance. This Commission also refers to the economic case for investments. We conclude on how COVID-19 has highlighted the current deficiencies in the accessibility of diagnostics globally and that the raised awareness resulting from the COVID-19 pandemic provides an opportunity for change that should not be wasted. We propose key next steps, particularly the creation of a Diagnostics Alliance, to provide long-term and continuing focus to the effort of improving the accessibility of diagnostics globally.
Current status of diagnostic availability, accessibility, and impacts on health
In order for people who need diagnostics to be able to use them, diagnostics first need to be available (ie, present and working at a location). Furthermore, to minimise the delay in getting the right treatment to the patient, diagnostic investigations must be accessible by being reasonably close to the patient's home. Importantly, the diagnostics must be affordable. We assess these criteria using six tracer conditions, which as a group account for an important share of the global burden of disease, and all but one of which are included in WHO's primary care priority testing recommendations for antenatal care.31 The six tracer conditions are diabetes, hypertension, HIV, tuberculosis, syphilis, and hepatitis B virus infection. We explore how missing diagnoses compare with the gaps in each of the three other stages in the cascade of care, to see where the largest gap lies, and then model the impact on health if the diagnostic gap were to be substantially reduced.
Diagnosis is the biggest gap in the cascade of care
The cascade of care model describes the sequential steps a patient should navigate to receive appropriate care for any health condition. Typically, the four major steps in any care cascade are screening, diagnosis, treatment, and treatment completion or control of condition. In some situations, there is no screening of asymptomatic individuals, and patients who are symptomatic proceed immediately to diagnosis. Ideally, any patient who enters the care cascade should follow through the entire cascade. However, that is frequently not the case and most patients drop out of these steps because of various factors, such as complex patient pathways, poor access to diagnostics and treatments, affordability, and poor adherence to therapy. Our analysis quantifies where the greatest gaps exist worldwide in the care cascades for the six tracer conditions.
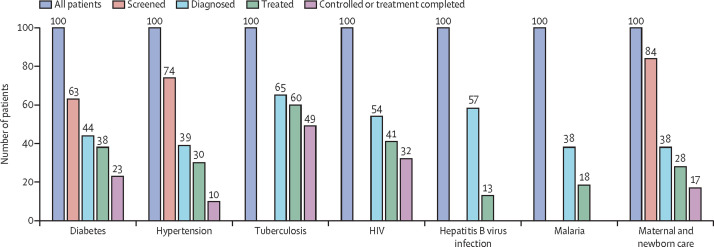
Figure 2 summarises the results of a scoping review done on MEDLINE for tracer conditions, including communicable and non-communicable diseases. One study of maternal and child health care across multiple countries was also included, since this is an important opportunity for diagnosing and treating undetected conditions. The data, with details of all included studies, as well as definitions, are included in the appendix (pp 4–5). All studies with global or multi-country data or data for LMICs were included; a total of 16 studies for the six conditions, plus maternal and newborn care. For maternal and newborn care, diagnosis was defined as having four antenatal care visits, at which WHO recommends eight key diagnostic tests,31 and treatment was defined as at least having a skilled attendant at the birth. No global or LMIC data regarding the cascade-of-care studies were available for hepatitis B virus infection, other than one study for Australia (figure 2 includes data for hepatitis B for Australia only).
Figure 2.
Cascade of care for different health conditions
Figure shows that the diagnostic gap is the largest gap for five of the six conditions. For hepatitis B virus infection, the only study is of Australia. Further details regarding the data shown are included in the appendix (pp 4–5).
The diagnostic gap (ie, the proportion of the population with the condition who are undiagnosed) is the biggest gap in the cascade of care across all conditions, across all settings. The only exception is hepatitis B virus infection, for which the diagnostic gap and the treatment gap (ie, the proportion of the population who have been diagnosed but not treated) are virtually the same. Across all conditions, the diagnostic gap ranges from 35% to 62%, indicating that, on average, about half of all people with these conditions are undiagnosed.
Modelling the health impact of reducing diagnostic and treatment gaps in LMICs
Having shown that diagnostics are the greatest gap in the cascade of care, we did a modelling exercise to estimate the health impact in LMICs of reducing this gap. Similar global modelling exercises often use 90% coverage as a goal, given that achieving 100% coverage is recognised as difficult. We examined the impact of increasing the proportion of people with the condition who are diagnosed to 90% (ie, the diagnostic gap decreasing to 10%). Subsequently, we examined the impact of reducing both the diagnostic and the treatment gaps to 10%. The health measures were mortality and disability-adjusted life-years (DALYs) averted. We investigated diabetes, hypertension, HIV, and tuberculosis in the population overall, plus hepatitis B virus infection and syphilis for pregnant women. For these six conditions, the main diagnostics involved were PALM, with the exception that chest x-ray was included for screening for tuberculosis. Cascade-of-care studies did not exist for all conditions for all LMICs; most sources used in this Commission cover at least 40 LMICs. The exception was hepatitis B virus infection, for which the only data found relating to the cascade of care were from Australia.32 The median value of risk reduction from available studies was used and applied to all LMICs.
For each condition, we used published studies that provided the relative reduction of DALY loss or of premature death for people who were having treatment (after diagnosis), compared with those with the diagnosis who were not receiving treatment (references are shown in table 1 ). The same value of risk reduction was used for all LMICs.
Table 1.
Estimated reduction in burden of disease in low-income and middle-income countries by reducing the diagnostic gap
| Current diagnostic gap | Current treatment gap | Current number of annual deaths | Current burden in DALYs | Relative risk for those treated vs untreated |
Diagnostics at 90% coverage |
Diagnostics and treatment at 90% coverage |
|||
|---|---|---|---|---|---|---|---|---|---|
| Number of annual deaths averted | Number of DALYs averted | Number of annual deaths averted | Number of DALYs averted | ||||||
| Diabetes | 56% | 13% | 839 682 | 46 747 576 | 0·78 | 82 073 | 4 569 268 | 88 835 | 4 945 684 |
| Hepatitis B (congenital) | 62% | 26% | 6225 | 548 019 | 0·29 | 1993 | 175 443 | 2838 | 249 834 |
| HIV | 46% | 24% | 938 891 | 53 567 471 | 0·37 | 253 351 | 14 454 678 | 349 411 | 19 935 277 |
| Hypertension | 61% | 23% | 6 605 400 | 127 662 800 | 0·58 | 526 164 | 10 169 187 | 949 864 | 18 358 058 |
| Syphilis (congenital) | 62% | 26% | 53 245 | 4 679 046 | 0 | 20 225 | 1 777 312 | 34 620 | 3 042 286 |
| Tuberculosis | 35% | 26% | 1 167 623 | 44 666 899 | 0·50 | 192 465 | 7 362 676 | 192 465 | 7 362 676 |
| Total, six conditions | .. | .. | 9 611,066 | 277 871 811 | .. | 1 076 271 | 38 508 564 | 1 618 033 | 53 893 815 |
| Total, all conditions | .. | .. | 43 236 034 | 2 158 810 815 | .. | .. | .. | .. | .. |
We made several simplifying assumptions. We assumed that the proportion of people diagnosed who received treatment did not change as the numbers of those who were diagnosed increased (ie, the treatment gap was unchanged). Due to insufficient data, we made the assumption that people who were undiagnosed had the same severity of the condition as those who were diagnosed. However, those people who were undiagnosed might have had the condition for a shorter period of time than those people who were diagnosed. Additionally, people who were poor, less educated, or living in remote areas were less likely to be diagnosed. We did not take account of the effect on reducing transmission of the disease for HIV and tuberculosis, and for hepatitis B virus and syphilis we included only the reduction in transmission from mothers to newborn babies. We used the 2017 data for DALY losses and mortality. For hypertension, we included the burden associated with essential hypertension and all the other components of cardiovascular disease (primarily ischaemic heart disease and stroke), for which hypertension is a risk factor. The equations that were used for the calculations are given in the appendix (p 6).
The results suggest that reducing the diagnostic gap has an important effect on reducing morbidity and mortality for people with these six conditions (table 1). The largest absolute effect was for hypertension, followed by HIV and tuberculosis. Overall, narrowing the diagnostic gap for these six conditions would reduce the annual number of premature deaths in LMICs by 1·1 million (2·5% of total annual deaths from all conditions in LMICs), and the annual DALY losses by 38·5 million (1·8% of losses from all conditions). The lower percentage reduction in DALYs than in deaths is largely due to those deaths averted primarily being those of adults (particularly older adults for the chronic conditions), rather than children. In the second model, in which, additionally, 90% of diagnosed patients received treatment, the number of deaths averted increased to 1·6 million (3·7% of total annual deaths in LMICs) and DALYs averted increased to 53·9 million (2·5% of the current disease burden in LMICs).
The results of the modelling exercise help underscore that, in any care cascade, reducing the diagnostic gap and providing appropriate and timely diagnosis will help in averting deaths and DALYs. Additionally, if the treatment gap can also be substantially reduced, even more deaths and DALYs can be averted. Although diagnosis alone cannot decrease the disease burden, it is the most crucial first step.
Limitations of this model include that it relies on simplistic assumptions and focuses only on six conditions, using only PALM and the only use of DI being x-ray for tuberculosis. Furthermore, this model does not take account of additional reductions in infection associated with lower transmission, unlike more sophisticated models (eg, for tuberculosis).39 Furthermore, the model used is a comparative statics model—ie, we did not model how changes in DALY losses and deaths occur in real time; instead, we compared the current population to a hypothetical alternative, in which 90% of the population had been diagnosed throughout their lives. Thus, the decrease in DALY losses and deaths is different from the annual reductions estimated in real-time dynamic models, such as those using the target ratio of 90 to 90 to 90 for HIV.40 Furthermore, the model does not incorporate comorbidities (eg, HIV in combination with tuberculosis, and diabetes in combination with hypertension are relatively common), which might overstate the benefits. The model assumes that disease severity does not differ between individuals who have been diagnosed and undiagnosed. The model provides point estimates only, and does not apply sensitivity analysis.
Current availability of diagnostics
A large proportion of the world's population has little to no access to diagnostics. A frequently cited statistic originating from WHO that dates back to the 1980s is that two-thirds of the world's population does not have access to DI,41 although this figure seems to depend on expert opinion rather than robust data. One study identified that only 2% of health centres in ten countries (nine in low-income and lower-middle-income countries, and one upper-middle-income country) had the resources to do all of eight basic laboratory tests at the time of the survey.42 Surveys for India, Peru, and Ethiopia from 2017–19 support that there is poor availability of tests listed on the WHO EDL at the level of primary care.43, 44, 45
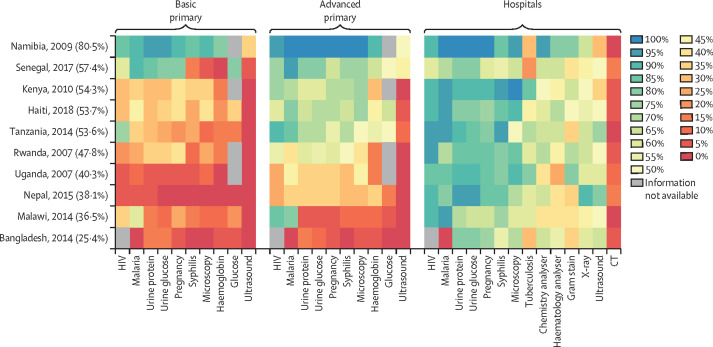
To estimate the general availability of diagnostics, we analysed the availability of a set of basic diagnostics at different levels in the health system in ten countries, as documented in available Service Provision Assessments of the Demographic and Health Surveys (figure 3 ). At the time of data extraction, the Service Provision Assessments surveys were only available in a recoded format for a subset of countries, in some cases only for 1 year, in other cases for 2 years or more. For the present analysis, survey data were used from ten countries between 2004 and 2018.
Figure 3.
Availability of basic diagnostics by tier in ten low-income and middle-income countries in various years, 2007–18
The heat map provides information on the proportion of facilities at each of the two levels that had specific diagnostic investigations available. Countries were ranked in descending order of average availability of all investigations, taking the average across both tiers. Average availability was calculated first by weighting facilities to be representative of their numbers nationally, and then simple averages of availability were taken, omitting those investigations for which no information was available. Availability was also sorted left to right in decreasing order of availability across countries (ie, the most readily available diagnostics were at the left of each of the two panels, and the least available at the right). At both levels of primary care, ten basic tests and examinations were included, while four more advanced investigations were added at hospital level, which require laboratories or more advanced imaging.
Data are from public and private facilities, from three different levels in the health system, namely basic primary care (ie, pharmacies and health posts or similar, where no qualified doctor or nurse is available), advanced primary care (ie, health centres usually staffed by a doctor or a nurse), and secondary and tertiary care in hospitals (all hospital service levels combined). To be considered available, the facility had to have the equipment for the particular investigation, the person doing the survey had to see the equipment, and the equipment had to be in working order. Further details of methodology are provided elsewhere.46
Availabilities of diagnostics were lowest in basic primary health-care facilities, with the greatest availability being for malaria at 40%, but only around 15% for urine glucose and urine protein, and 5% for ultrasound. At the advanced level of primary health care, HIV and malaria were the most readily available tests (65% and 62% availability, respectively) and investigations included as part of WHO recommendations for antenatal care had variable, but low, availability—namely, syphilis testing (49%), urine dipsticks (52%), haemoglobin testing (37%), blood glucose testing (32%), and ultrasound (12%). Even in hospitals, the availability ranged from 54% to 86% for these most basic of investigations. Generally, patients must travel to hospitals for investigations, such as a complete blood count, blood chemistry, basic bacteriology, and any form of imaging. Although 36–87% of hospitals had a working x-ray, only 2–29% had a CT scanner, although another study documents that access to CT across a broad range of LMICs is slowly increasing.47
The limitations on diagnostic availability at health centres, particularly in LMICs, make it harder to fulfil WHO's (2016) recommendations for antenatal care, namely that all pregnant women should receive six PALM tests (ie, testing for urine protein, haemoglobin, HIV, glucose, syphilis and, where prevalence warrants, tuberculosis), plus a blood pressure measurement and one ultrasound. Data showed that women can obtain the PALM tests about half of the time and have to go to a hospital for the ultrasound.
The availability of diagnostics is somewhat correlated with country income, particularly at the level of primary care. Both Namibia and Senegal, the two countries with the highest per capita income, had the greatest availability. The countries with lowest availability were also those with lowest income: Malawi, Uganda, Rwanda, and Nepal. Bangladesh is an exception, with the lowest availability in primary care despite having a relatively high income.
Trends over time for Haiti (between 2013 and 2018), Kenya (between 2004 and 2010), Senegal (between 2012 and 2017), and Tanzania (between 2006 and 2014), the four countries with at least two surveys (Demographic and Health Surveys and Service Provision Assessments) each, showed that there has been a modest improvement over time in each country. Availability of tests increased at both basic and advanced levels of primary care for at least 80% of tests studied at each level in all four countries; however, the actual increase in mean availability over a period of 5 years or 6 years, averaged over all diagnostics in primary care, was modest. The mean availability increased by 17·6 percentage points in basic primary care and 7·6 percentage points in advanced primary care for Senegal, with the corresponding increases being 6·1 percentage points and 9·4 percentage points for Haiti, 12·2 percentage points and 15·0 percentage points for Tanzania, and 6·3 percentage points and 25·0 percentage points for Kenya.46
Geographical access to diagnostics
The analysis of test availability by facility does not indicate how the population is distributed relative to facilities with available diagnostics. One scenario might be that diagnostics are more readily available in remote rural areas (eg, if stocks have run out in more densely populated areas). This scenario has very different implications than the scenario in which diagnostics are readily available in densely populated urban areas, but remote and rural areas are poorly served.
Analysis was done for this Commission by examining access of the population to a primary health facility within a 2 h walking distance of their home, for nine LMICs and two states in the USA. For Malawi and Senegal, the population data were then linked to the availability of specific diagnostics at these health facilities. Distance was defined as a travel time of 2 h on foot (8 km in rural areas, 10 km in urban areas) using WHO-CHOICE's health economics GeoAccess work.48 Numerous studies document that, in general, greater distance is associated with less uptake of health services,49 less uptake of diagnostics in particular (Hoxha K, University of Waterloo Waterloo, ON, Canada, personal communication), and worse health outcomes50, 51 in a range of countries and for a range of health conditions. The rare exceptions are usually cases where people are willing to travel further for specialist or high-quality services, or both.
Table 2 shows the proportion of the population living within 2 h of a primary health centre in nine LMICs and two US states. Our analysis showed that more than 70% of the population in each country or state (with the sole exception of Namibia) had geographical accessibility to a primary health centre or hospital. Wealthier countries and states generally reported higher coverage, although Malawi (the lowest-income country) had surprisingly high coverage, and Namibia, a sparsely populated, upper-middle-income country, reported low coverage, presumably due to its vast sprawl and concentration of health-care services in the northern part of the country, which are isolated geographically from much of the population. These findings show that, despite differences in resources and population density, most countries have managed to make their primary health services geographically accessible to their populations.
Table 2.
Proportion of the population in countries living within 2 h of a primary health facility
| Gross domestic product per capita, US$*† | Total population, thousands† | Population density, people per km2 | Median district-level travel time (min) to nearest health centre, median (IQR)‡ | Median district-level travel time (min) to nearest district hospital, median (IQR)‡ | Coverage§ | ||
|---|---|---|---|---|---|---|---|
| USA | |||||||
| Texas | 53 795§ | 28 996 | 40·0 | 19·4 (14·5–26·4) | NA | 98·6% | |
| Colorado | 52 795§ | 5759 | 20·0 | 33·5 (26·8–45·4) | NA | 99·2% | |
| South Africa | 13 687 | 57 780 | 47·6 | 42·1 (32·9–52·6) | 79·8 (50·3–112·2) | 97·0% | |
| Mexico | 9763 | 126 191 | 64·9 | NA | 96·7 (56·3–153·6) | 70·8% | |
| Bangladesh | 4372 | 161 356 | 1239·6 | 34·3 (29·2–41·3) | 41·1 (35·9–48·1) | 94·4% | |
| Kenya | 3468 | 51 393 | 90·3 | 44·8 (20·0–74·5) | 37·0 (17·9–102·2) | 84·2% | |
| Tanzania | 3240 | 56 318 | 63·6 | 84·9 (53·4–132·7) | 97·6 (64·4–160·5) | 67·9% | |
| Senegal | 3783 | 15 854 | 82·3 | 93·0 (45·9–126·4) | 121·6 (77·8–196·5) | 81·0% | |
| Namibia | 11 102 | 2448 | 3·0 | 194·3 (94·8–275·87) | 182·9 (98·3–261·3) | 23·8% | |
| Rwanda | 2252 | 12 302 | 498·7 | 19·5 (14·4–26·9) | 35·8 (27·8–58·9) | 76·1% | |
| Malawi | 1311 | 18 143 | 192·4 | 55·7 (49·2–67·2) | 134·5 (110·6–169·0) | 93·5% | |
NA is shown for US states because the closest facility is a hospital and for Mexico because the data are not available. Sources of facility data: Bangladesh;52 Mexico;53 USA, using North American Industry Classification System codes to identify hospitals;54 and all other countries.55 NA=not applicable.
2018, US Bureau of Economic Analysis.
2018, World Bank Development Indicators.
Estimated using least cost–distance based on AccessMod 5 geographical information systems.
Percent of the total population within 2 h of a primary hospital (Texas or Colorado, USA), district hospital (Mexico), or health centre (primary care facility in other countries).
Finally, information on actual test availability at facilities was combined with the population and distance analyses. The specific diagnostic investigations examined are the same basic diagnostics covered in the Demographic and Health Surveys and Service Provision Assessments, analysed for availability. These tests include the minimum set of eight point-of-care PALM tests plus a single ultrasound included in the WHO recommendations on antenatal care for a positive pregnancy experience,31 which would ideally be available at a primary health centre, plus some basic diagnostics that would be desirable to have at a first-level hospital. Further details of the methodology used are in the appendix (pp 7–9).
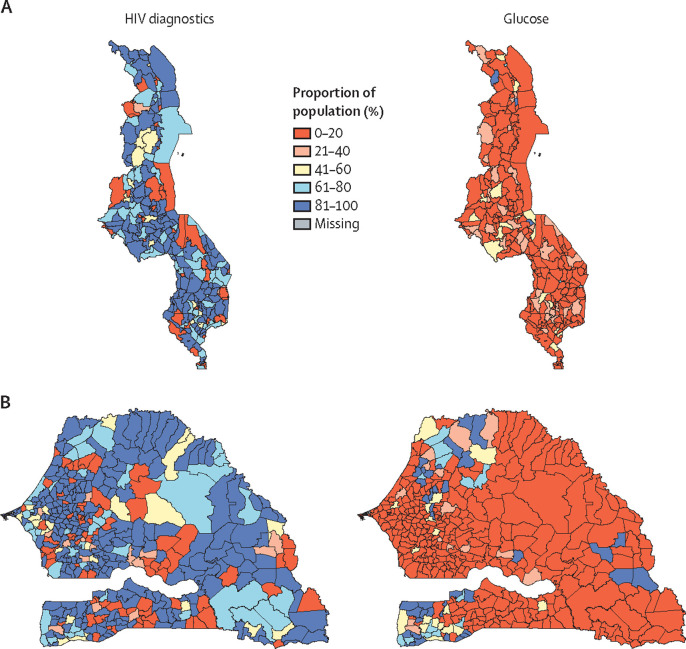
Table 3 shows that, while 74–80% of the population have reasonable geographical access to tests for HIV and malaria (diseases covered by vertical programmes, often with external funding), only 10–20% have similar access to almost all of the other laboratory tests, and less than 10% have access to imaging of any kind. Moreover, there is very little difference between low-income Malawi and lower-middle-income Senegal (figure 4 ), which presents a much bleaker picture than table 2. Some geographical variability exists: counties or communes on land borders with other countries in some cases have worse access than other areas, and this finding is also true for the eastern (more sparsely populated) part of Senegal, compared with at least two of the regions in the western half of the country with access to ocean ports.
Table 3.
Estimated population within 2 h of essential diagnostics in Malawi and Senegal
|
Malawi (Service Provision Assessments 2013–14) |
Senegal (Service Provision Assessments 2012–13) |
|||
|---|---|---|---|---|
| Facilities | Population within 2 h | Facilities | Population within 2 h | |
| HIV dry blood spot | 44% | 51% | 6% | 5% |
| HIV diagnostics* | 78% | 80% | 82% | 74% |
| Malaria | 85% | 79% | 83% | 75% |
| Tuberculosis rapid diagnostic test | 7% | 2% | NA | NA |
| Pregnancy | 22% | 19% | 24% | 17% |
| Syphilis | 23% | 18% | 1% | 1% |
| Glucose | 20% | 16% | 23% | 11% |
| Urine protein | 15% | 14% | 23% | 64% |
| Haemoglobin | 21% | 19% | 11% | 10% |
| Blood pressure | 78% | 63% | NA | NA |
| X-ray | 5% | 0·4% | 3% | 8% |
| Ultrasound | 7% | 6% | 6% | 5% |
| CT scan | 1% | 0·6% | 3% | 3% |
Travel times were estimated using the WHO AccessMod 5 algorithm.48 In Malawi, the 2013–14 Service Provision Assessments database provided a comprehensive list of facilities, their locations, and testing availability.56 In Senegal, we linked data from the 2012–13 Service Provision Assessments database,57 with a published available database of facility types.53 We assumed that facilities at the same level of the health system in the same geographical regions would have the same testing availability.
HIV diagnostics include nucleic acid tests, antigen tests, and antibody tests. NA=not applicable.
Figure 4.
Maps of Malawi and Senegal population access to HIV and glucose tests
Proportion of the population in Malawi (county level; A) and Senegal (commune level; B) that have access to HIV and glucose tests. Access is defined as being within 2 h travel of a facility offering a test: data for Malawi are for 2013–14, grouped into 256 counties; data for Senegal are for 2012–13, grouped into 433 communes.
To estimate the overall global access, we used the median estimate that only 19% of the population in low-income and lower-middle-income countries have access to the key diagnostics needed for a healthy pregnancy and for non-communicable diseases. Although comprehensive data could not be identified for upper-middle-income countries, data were available on the proportion of pregnant women receiving an antenatal syphilis test (mandated at the first antenatal visit in the large majority of countries).31 The median coverage was 75% for 2010 or 2011 across 11 South American countries, China, Malaysia, and Iraq (data from small island nations were not included). Even if 100% of the population in HICs could have had access (a generous estimate, since marginalised communities are often not covered), 47% (almost half) of the world's population had little to no access to basic diagnostics at the baseline year for the Sustainable Development Goals in 2015.58
Inequalities in diagnosis by socioeconomic status and other factors
Although there is a clear diagnostic gap worldwide, the extent of unmet need for diagnostics varies by socioeconomic and other factors, including age, gender, and race or ethnicity. Understanding this variation is important for developing targeted policies to meet the needs of different groups effectively. We focused primarily on socioeconomic differentials in use of diagnostics because these can indicate barriers due to cost or lack of insurance coverage, or both.
Systematic reviews across a variety of countries have documented associations between lower socioeconomic status and lower participation rates in cancer screening programmes. This finding was true for screening for cervical cancer in a global survey,59 and for a survey for breast cancer across Latin American countries:60 both reviews used individual income or education, or both, as indicators of socioeconomic status. Similarly, systematic reviews across HICs that used area-level deprivation rather than individual socioeconomic status reported a similar association for breast cancer screening across seven European countries,61 and prostate cancer screening across four HICs.62 A similar association was also reported in a study of colorectal cancer screening in three HICs that used individual socioeconomic status.63
A series of cascade-of-care studies of diabetes and hypertension similarly documented an association between lower socioeconomic status and a lower probability of being tested. Nationally representative studies were done in a number of LMICs. As part of these studies, in addition to being asked questions about sociodemographic status and whether they had been tested, diagnosed, and treated for the condition, participants also provided a blood sample to be tested (for the diabetes study) or their blood pressure was measured (for the hypertension study).
One such multi-country study of 38 311 adults in 12 countries reported a clear education gradient with being tested for diabetes,64 and an updated, even larger study of 847 413 adults in 28 countries reported that both more education and household wealth were associated with being tested.65 Another study for India, again, reported that being more educated and coming from a higher wealth quintile was associated with having been tested.66
For hypertension, analysis of nationally representative pooled data for 1·1 million adults in 44 LMICs for hypertension diagnosis yielded similar findings.67 An association of education and of household wealth with the odds of being tested for hypertension was reported. Similar results were obtained from the analysis of national-level and state-level representative survey data of 731 864 individuals in India. States with higher gross domestic product per capita tended to do better on testing and diagnosing hypertension and, although no education gradient was observed, there was an observed wealth gradient in blood pressure having been measured.68
For cervical cancer screening, an analysis of nationally representative surveys done between 2005 and 2018 in 55 LMICs representing 1 129 404 women for lifetime access to cervical cancer screening showed that women who lived in rural areas, had low educational attainment, or had low household wealth were generally least likely to self-report to have ever been screened.
In addition to disparities in diagnosis by socioeconomic status, there are disparities by gender and race. Data on numbers of COVID-19 cases reported in June, 2020, showed that, while the numbers were roughly similar for men and women in most countries, in 13 of 130 countries, more than 70% of diagnosed infections were in men, and in the two countries with the most extreme differences, 88% of diagnosed infections were in men. Although it is possible that this finding is due to unusual patterns of infection, unequal access to testing is another possible explanation.69
In the USA, there are multiple studies of access to health care by race, including access to diagnostics. Time delays from symptom recognition to diagnosis for breast cancer were reported to be substantially longer for African American women than for White women.70 Black and Hispanic women were more likely to be diagnosed with locally advanced breast cancer than White women, even when controlling for disadvantages due to less coverage by health insurance, and other socioeconomic factors.71 There are possible biological differences in the progression of cancers by race which complicate interpretation. The COVID-19 pandemic has laid bare racial inequalities in testing as well as health outcomes,72 while underlying inequalities in exposure, again, complicate interpretation.
The disparities presented suggest that affordability and socioeconomic characteristics of individuals are issues in diagnosis. Some of these disparities might result from differences in health-seeking behaviour, but affordability could also deter more marginalised groups, including racialised and immigrant groups. Although there is a substantial literature on affordability of medicines, much less empirical work has been done on affordability of diagnostics. For example, a systematic review of availability and affordability of diagnostics and medicine for chronic obstructive pulmonary disease in sub-Saharan Africa reported nine eligible studies, but only a single measurement of affordability of a diagnostic test in all these studies.73 Affordability of diagnostics is an area in which future work would be beneficial.
The global burden of disease and future diagnostic needs
A forecasting platform developed in 2018 provides estimates of the future global burden of disease,74 including rankings of years of life lost and mortality from 2017 to 2040. Table 4 presents the data for years of life lost for 2030 and 2040 globally and for LMICs. The analysis shows that, unlike the coverage in the current WHO EDL, it will be important for the list to substantially expand its diagnostics for non-communicable diseases, such as neurodegenerative disorders and management of mental health conditions (although these conditions might primarily require questionnaire screening tools rather than laboratory or imaging diagnostics). It will also be vital to include DI. We used the global burden of disease rankings to develop a diagnostics template and to identify how diagnostics should be made available by tier within the health system.
Table 4.
Top 20 conditions responsible for YLL in 2030 and 2040 by rank
|
Global data (by YLLs) |
Data from low-income and middle-income countries (by YLLs) |
|||
|---|---|---|---|---|
| 2030 | 2040 | 2030 | 2040 | |
| 1 | Ischaemic heart disease | Ischaemic heart disease | Ischaemic heart disease | Ischaemic heart disease |
| 2 | Stroke | Stroke | Cerebrovascular disease | Cerebrovascular disease |
| 3 | Lower respiratory infections | Lower respiratory infections | Lower respiratory infections | Lower respiratory infections |
| 4 | Road injuries | COPD | Road injuries | COPD |
| 5 | COPD | Chronic kidney disease | COPD | Road injuries |
| 6 | Diarrhoeal diseases | Alzheimer's disease | Diarrhoeal diseases | Chronic kidney disease |
| 7 | Lung cancer | Diabetes | HIV/AIDS | Diabetes mellitus |
| 8 | Diabetes | Road injuries | Diabetes mellitus | Diarrhoeal diseases |
| 9 | Chronic kidney disease | Lung cancer | Chronic kidney disease | Alzheimer's disease and other dementias |
| 10 | HIV/AIDS | Diarrhoeal diseases | Self-harm | HIV/AIDS |
| 11 | Self-harm | Self-harm | Neonatal preterm birth complications | Tracheal, bronchus, and lung cancer |
| 12 | Alzheimer's disease | HIV/AIDS | Tracheal, bronchus, and lung cancer | Self-harm |
| 13 | Neonatal preterm birth | Liver cancer | Malaria | Liver cancer |
| 14 | Malaria | Hypertensive heart disease | Tuberculosis | Hypertensive heart disease |
| 15 | Congenital defects | Colorectal cancer | Congenital birth defects | Tuberculosis |
| 16 | Liver cancer | Tuberculosis | Neonatal encephalopathy due to birth asphyxia and trauma | Congenital birth defects |
| 17 | Tuberculosis | Congenital defects | Liver cancer | Neonatal preterm birth complications |
| 18 | Neonatal encephalopathy | Neonatal preterm birth | Alzheimer's disease and other dementias | Neonatal encephalopathy due to birth asphyxia and trauma |
| 19 | Colorectal cancer | Breast cancer | Hypertensive heart disease | Colon and rectum cancer |
| 20 | Hypertensive heart disease | Falls | Interpersonal violence | Malaria |
Further details of methodology are provided in the appendix (p 10). In some cases, the database uses slightly different terms for essentially similar conditions. For this analysis, we retained the terms used in the database but treated these as the same condition. COPD=chronic obstructive pulmonary disease. YLL=years of life lost.
To summarise the inequalities in diagnosis, we highlight three key messages. First, just under half the world's population has little to no access even to the most key basic diagnostics. Although governments have invested to provide primary health centres within reasonable reach of most of their population, people might have to walk for up to 2 h to reach a facility, only to find that the tests they need are not available, and for pregnant women needing an ultrasound, this procedure is only attainable at a hospital. Second, this paucity of access particularly affects people who are poorer and more marginalised. Lastly, the diagnostic gap has adverse consequences for the burden of disease.
Current status of the policy environment
There is a low availability of, and poor access to, diagnostics globally. Notably, it is important to consider factors in the policy environment that contribute to this situation, beginning with the power of the actors involved,30 and subsequently other aspects, namely the absence of diagnostics from national health strategy plans, the detrimental effect of under-regulation and over-regulation, the disadvantageous structure of the global diagnostics market (especially for LMICs) and the effect of corruption.
Low visibility
COVID-19 has brought a great deal more saliency to diagnostics. Historically, however, diagnostics have been under-appreciated, and their importance in universal health coverage and antimicrobial resistance has received insufficient attention.75 Although countries and donors have traditionally emphasised access to vaccines and essential medicines (which are mentioned explicitly three and four times, respectively, in the targets for Sustainable Development Goal 3 on health), there has never been a push for access to diagnostics (beyond those diagnostics in the key vertical programmes, such as HIV, tuberculosis, and malaria). This factor, in part, explains why it took years for WHO to develop an EDL (first edition in 2018), whereas the first essential medicines list was released in 1977. WHO's measurement framework for universal health coverage includes 16 essential services; however, although diagnostics are implicitly included (in treatment of three key infectious diseases, in prevention and treatment of two non-communicable diseases, and in screening for cervical cancer), diagnostics are not mentioned explicitly.76 Content analysis in the Lancet Series on Pathology and Laboratory Medicine in Low-Income and Middle-Income Countries showed that professionals in PALM have had little or no visibility in a number of advisory bodies, such as WHO, in research programmes, or in health systems overall.77 A bibliometric analysis undertaken for this Commission showed that there was virtually no representation of radiologists on international committees and policy making panels. The databases Web of Science, PubMed, Airiti, BASE, and Scopus were searched and open-source search engines (Google and Bing) were searched for documents relevant to the political science of radiology or imaging, or both, using a number of search terms with wildcard root [RADIOL*; IMAG*]. No relevant studies were identified, although there were a number of national and international strategic reviews concerned with the science of radiology or imaging, or both. This low visibility regarding diagnostics in turn results in an inability to develop effective advocacy for national strategic plans, sustainable funding, development of regulatory frameworks, and prioritisation of diagnostics within health systems.
To our knowledge, the reasons for this low visibility and ineffective advocacy have not been studied systematically, but possible causes include the small proportion of health-care workers whose primary role is in diagnostics (less than 4% for each of PALM and DI in the USA and in the UK) and the relatively small proportion of health-care expenditures in PALM and DI (3–6% of health-care expenditures in a number of LMICs and HICs for PALM; comparable estimates could not be found for DI; limited OECD data are shown in the appendix (pp 11–13).77 Little or no experience or training is also an important factor with regard to PALM and DI among patients and policy makers. Although patients interact with technicians involved in DI, they might not meet with the individual who interprets the image, and they do not meet with the laboratory professionals. In LMICs, there is the additional barrier that physicians in primary care have had either insufficient access to diagnostics or, where diagnostic tests have been available, negative experiences that make them question whether the tests are accurate or available in a reliable manner.17, 78, 79, 80 The net result has been low use of laboratory tests in those settings. Although we could not identify any studies that showed that low use of laboratory tests also resulted in a decrease or absence of advocacy for better tests, it would not be expected that these practitioners would advocate for something for which they have little experience or confidence in using.
National strategic plans for diagnostics
Developing national laboratory strategic plans to improve laboratory services was part of the 2008 Maputo Declaration.20 These plans are necessary for countries to successfully develop integrated and tiered laboratory systems that can support national health systems over time. Important components of such plans include identifying a network structure, developing a health workforce that can work effectively within that structure, creating a regulatory framework (including strategies for accreditation), defining sustainable systems of finance, and establishing practical metrics to monitor and evaluate performance. In one analysis of national strategic laboratory plans in sub-Saharan Africa collected in 2012, a number of countries had developed national strategic laboratory plans, although these plans differed substantially in the issues that were addressed, and only a small minority (five of 39 countries) addressed financing.81 In particular, “roadmaps explaining how countries intend to move toward accreditation across diseases, at different tiers of the laboratory system and given the resources available[,] were rarely provided or referred to” and “[c]ertification of laboratories based on compliance to national standards was never mentioned as a strategy to ensure the quality of laboratory services at lower tiers”.81 WHO does provide some guidance for such plans for PALM, but similar guidance for DI is scarce.82 A recent analysis of national Health Strategy Plans for 79 low-income and lower-middle-income countries reported that 36 of these countries had current Plans (of which all but two could be accessed).83 Of these 36, 30 mentioned laboratories but only eight mentioned imaging, and only one specifically referenced a national strategic laboratory plan. Although WHO's advice on national plans for health security does include considering laboratories but not DI, the focus is restricted to infectious disease and environment hazards.84 Furthermore, having a plan is not enough. Plans need to be appropriately funded and implemented to achieve their aims.
National regulatory authorities and frameworks for diagnostic devices
While most countries have national regulatory authorities for medicines, low-income countries, in particular, are less likely to have national regulatory authorities for medical devices (the category under which PALM diagnostics and DI devices fall). Where the authority exists, its enforcement abilities are often limited. This deficiency can have a detrimental effect in three broad areas: pre-market evaluation, to ensure that diagnostics are safe and of good quality;85 marketing controls, to ensure that diagnostics are used appropriately and that advertising is truthful and accurate; and post-marketing controls, to monitor any issues that arise in use.86
Although regulations are needed, it is important not to put unnecessary barriers in place. If each country requires different submission dossiers to obtain approval, its own in-country testing before authorisation, or its own certification of manufacturing quality, this requirement makes entry into the country more complex and costly,86 with the end result that people within the country either do not get access or face delays in access to diagnostics, and have to pay more for them.
Regulatory issues are not only of concern for diagnostics entering the country, but also for how diagnostics are handled domestically. It is not appropriate to apply the same regulations to diagnostics as to medicines, considering that diagnostics have different associated risks, different speed of change in technology, and the sheer numbers of different diagnostics for the same condition. In certain cases, regulations that are well intentioned have unanticipated outcomes. For example, some countries, such as Peru, require a local distributor for diagnostics,87 which can ensure accountability for maintenance and training. However, in small markets with little to no competition, a local distributor can unnecessarily inflate diagnostic cost.
Size of the global market for diagnostics
In 2019, the global market size for in-vitro diagnostics (including both laboratory and point-of-care PALM diagnostics) was US$60·8 billion, of which 31% was for point-of-care tests, whereas for DI this value was $34·7 billion.88, 89, 90 In-vitro diagnostics plus DI combined are just over 10% of the size of the global pharmaceutical market, which was $843 billion.91 The markets for in-vitro diagnostics, medical imaging, and pharmaceuticals are expected to grow strongly at cumulative annual nominal growth rates between 4·4% and 6·9% over 5 year projections to 7 year projections.88, 90 Markets for new technologies are expected to grow even faster, namely 10·4% for point-of-care tests, and 8·4% for molecular tests (appendix p 14).92, 93
HICs dominate the global purchases of diagnostics, medical technologies, and pharmaceuticals, with North America and Europe accounting for 75% of global purchases of in-vitro diagnostics, 64% of medical imaging equipment, and 58% of pharmaceuticals, while HICs in the Asia-Pacific account for 13% of global purchases of pharmaceuticals (no data available for global purchases of diagnostics by HICs in the Asia-Pacific; appendix p 14; figure 5 ). HICs also dominate the global supply of diagnostics: around half of the market for in-vitro diagnostics is accounted for by four companies from the USA and Europe, and three-quarters of the market for medical imaging is accounted for by four companies from the USA, Europe, and Japan (appendix p 14; figure 6 ).
Figure 5.
Global market shares of diagnostics and pharmaceutical purchases in 2015–19 by world region
The majority of global purchases of diagnostics and pharmaceuticals are from high-income countries in North America and Europe. *Germany, the Netherlands, Italy, Hungary, and Denmark. †China, Japan, South Korea, Australia, and Saudi Arabia. ‡Latin America, Russia, India, Indonesia, and rest of the world. Sources for this figure are included in the appendix (p 14).
Figure 6.
Global market share for four top suppliers of diagnostics and pharmaceuticals, by region of headquarters in 2015–19
Manufacturers with headquarters in high-income countries dominate global supply of diagnostics, but provide a much smaller proportion globally of pharmaceuticals.
By contrast, the much larger market for pharmaceuticals is less concentrated: the top four suppliers (all from the USA and Europe) control just less than a quarter of the market (appendix p14; figure 6). India, in particular, has a major role in supplying pharmaceuticals to other LMICs, being the third largest producer globally by volume, and the 14th by value.94 Market concentration can lead to higher prices and to a low number of products oriented towards smaller niche markets (eg, tropical and neglected diseases, and options which are more appropriate for resource-constrained environments).
Suppliers from LMICs are, however, gaining a foothold in fast-growing sectors. China accounts for around 14% of in-vitro diagnostics and 20% of DI of the global total, with only a small share accounted for by all the other LMICs.95, 96, 97 One of the top ten suppliers in each of point-of-care tests, ultrasound devices, MRI scanners, and nuclear medicine comes from China.98, 99 This factor might help to improve the affordability of diagnostics in LMICs.
Corruption and self-interest
Corruption is a problem for health systems generally, but some aspects of diagnostics (eg, acquisition of expensive equipment and supplies) increase the risk. Globally, corruption in health care causes an estimated yearly loss of $500 billion, the death of 140 000 children, and extensive morbidity and mortality by thwarting programmes to prevent and contain disease, leading patients all over the world to bear the physical, mental, and financial brunt.100, 101 Transparency International, a leading international organisation on anti-corruption efforts, has termed corruption in health care “the Ignored Pandemic”.101
Corruption has been lurking in health systems for a long time. However, in recent years, corruption has been identified as a major impediment in achieving Sustainable Development Goals that relate to health.102 In 2003, resolution 58/4 of the United Nations Convention against Corruption was adopted by the UN General Assembly and has been ratified by all but a very few countries.103 Non-signatories include Eritrea, North Korea, Syria, and Somalia and some small, largely island, countries. However, there has been only limited success in reducing and preventing corruption.
In LMICs, no reliable data are available, but reports have described the prevailing culture of corruption at all levels of the health system. International agencies are not immune from this blight: the International Federation of Red Cross and Red Crescent Societies launched investigations in 2017 into fraud schemes in three countries during its Ebola operations, totalling over 6 million Swiss francs.104
Within health systems, diagnostics is an area open to corruption, characterised by the importance of human resources and attractive technological inputs whose uses are difficult to monitor. In PALM, there are recurring expenditures and, therefore, an ongoing source of illicit revenue. However, in DI, there are large equipment purchases, which might make incentive and side payments attractive to those involved. Anecdotal examples abound, such as so-called sink tests where unscrupulous laboratories take samples which they pour down the sink and then generate completely unfounded reports.105 Diagnostic investigations are often not available at public facilities (and sometimes public diagnostic equipment is even deliberately damaged),106 and doctors and laboratory staff instead refer patients to their own private laboratories and DI centres where the same test is available at a higher price. Some private laboratories use reagents pilfered from the public laboratories, compounding the problem.106 Patients frequently rely on their doctor or laboratory personnel to suggest a good quality source for their test, which can lead to issues of conflict of interest. Although settlements in lawsuits against multinational suppliers of pharmaceuticals tend to be larger and better publicised than cases involving diagnostics, Siemens, for example, paid a $1·6 billion settlement in 2007 over a worldwide bribery scheme, which included $14·4 million in bribes involved in sales of medical equipment to five Chinese state-run hospitals.107
Although not as extreme as corruption, overuse of diagnostic tests (which fee-for-service payments might encourage) is a serious issue in many countries when patients have generous private health insurance, or when (as in the USA) malpractice liability leads to defensive medicine. Rapid growth in the use of expensive DI examinations was a particular issue in the USA in the early 2000s,108 with potential harms to patients including excessive exposure to radiation.
Entrenched professional interests might prevent shifting to newer, better, or less expensive means of offering diagnostic investigations. Obstetrician and gynaecologist professionals in some Latin American countries have vested interests in revenue from cytology (eg, Pap smears) and have resisted the shift to self-sampling for human papillomavirus molecular testing, which could be done instead by nurses and midwives.109
The key message with regard to policy is the limited recognition of the central importance of diagnostics, which results in major underfunding and insufficient resources at all levels. There is a need for greatly increased, strong, continuing advocacy for diagnostics. Highlighting the key contribution of diagnostics to universal health coverage, and enhancing its visibility in the universal health coverage agenda, is very important.
Current status of operational barriers to access
Notably, there are several factors at the operational level that hinder access to timely, accurate diagnostics globally. There are three main barriers: widespread deficiencies in infrastructure; marked shortfalls in global health workforce capacity, including insufficient and inadequate systems for education and training; and insufficient systems to ensure diagnostic quality and safety. In this Commission, we use the term infrastructure in two ways: first, the physical infrastructure needed to support diagnostic investigations, including space, power and water supplies, and equipment; and second, the operational infrastructure, including management systems, supply chain, and information technology.
Physical infrastructure
Globally, many health-care facilities, especially in LMICs, do not have an adequate physical infrastructure that is needed to support diagnostic services for both DI and PALM.8, 110, 111 Although the infrastructure challenges to providing diagnostic services might differ in type and degree between rural and urban areas, there are common infrastructure barriers in both settings: access to stable electrical supplies, clean water, reliable internet connections, and systems for transporting specimens. The poor access to systems for transporting specimens is more of a barrier in rural settings due to the distances between facilities, but this issue can also be a barrier in congested urban areas. Control of ambient climate, particularly to mitigate the effects of high temperatures and humidity, is necessary for optimal performance of laboratory and DI equipment, and for storage of reagents and supplies, and therefore can be an important barrier to providing diagnostic services in tropical or semi-tropical climates. Moreover, clinics and small hospitals in rural areas typically do not have the space to support most PALM and DI services.
In the absence of adequate physical infrastructure to support diagnostics, health-care facilities are left with a few options: providing no services; outsourcing to external vendors, which delays results, is typically more expensive, and creates further barriers to access because patients need to travel to these external facilities; collecting laboratory specimens locally and then forwarding them to other facilities for laboratory testing, or using telepathology or teleradiology; requiring patients to travel to facilities where laboratory testing or DI is available, which for many patients is a substantial barrier to care; or relying entirely on point-of-care testing, which cannot fully substitute for centralised laboratory facilities. For PALM, most point-of-care testing is designed to support primary care through a narrower scope of tests and, therefore, is not sufficient for hospital care that requires access to a broader scope of testing. When an imaging facility is not on site, point-of-care ultrasound is the only imaging modality that lends itself to true point-of-care service provision.
Operational infrastructure
Most health-care facilities in LMICs, and even many facilities in rural areas of HICs, also do not have the operational infrastructure that is needed to support diagnostic services.112, 113 This includes adequate management systems for workflow, procurement, and supply chain (particularly a cold supply chain). Similarly, key technologies are not available, including information technology for laboratory information systems, laboratory management information systems, radiology information systems, digital image management systems (eg, picture archiving and communication systems), technology necessary to support radiation safety, and electronic health records to support diagnostic services from order to report. Adequate and sufficient information systems are becoming of increasing importance due to several reasons: the need for data sharing between the tiers of an integrated and tiered diagnostic network; increasing use of digital technology in DI and PALM; a need to move from paper-based systems; and increasing use of mobile technologies in health care. In addition to their benefits for improving patient care and provider experience, robust information technology systems enable data collection that facilitates better management systems, disease tracking and reporting, and development of quality metrics used to assess the performance of health systems. Facilities need reliable access to high-speed internet connectivity to make full use of information systems, allow for integration between facilities, and make use of teleradiology or telepathology.
For all equipment, but especially imaging, appropriate maintenance and updating of both hardware and software are major issues.47 WHO and others have identified that insufficient access to technology support, such as biomedical engineers, is a crucial gap in health-care capacity.114 Even where diagnostic equipment might be available for purchase, or is donated (panel 4 ), technical support from manufacturers might not be available at all or might not be available in a timely or affordable manner. Depending on the specific equipment, regional, national, or local technical support is not available in much of the world.117 In LMICs, because there frequently is no technical support available regionally or nationally, it is costly and time-consuming to obtain this service from abroad. Often, tenders for equipment do not include costs for training and software upgrades, which holds down costs but at the expense of quality over the long term. Even when local technology support is available, local contractors do not have the expertise required to service a wide variety of instruments, nor do they have access to replacement parts locally. For complex equipment, buying parts from a third party is common, to reduce costs and also because parts might be more readily available. The result is use of replacement parts that might not fully meet the manufacturers' specifications, creation of multiple supply chains, and adverse effects on warranties.
Panel 4. Donated diagnostic imaging equipment—a case study of Africa.
Donations of diagnostic equipment have been a mainstay of global efforts at increasing access to diagnostics; however, such donations can actually make the situation worse. By some estimates, 40–96% of donated medical equipment is at any point in time out of service, although most analyses of this issue were not focused on diagnostic equipment and are not recent.115 As recently reviewed for donated diagnostic imaging equipment (the principles apply equally to diagnostic equipment used for pathology and laboratory medicine), equipment donations create a number of problems at the facility level:47
-
•
Donated equipment is often incomplete (ie, missing parts), with limited availability to acquire missing parts or replace parts as needed for maintenance
-
•
Longer lifecycles of equipment used in high-income countries can result in donation of equipment that is well beyond the normal end of service
-
•
For larger pieces of equipment, site preparation might be inadequate
-
•
Installation by qualified technical staff might not be available or might be done poorly
-
•
Inconsistent power supply often leads to frequent breakdowns of sensitive equipment; a systematic review116 reported that, in ten countries in sub-Saharan Africa, only 39·1% of hospitals surveyed had access to reliable electricity, compared with 58·1% overall in the 21 countries with available data
-
•
User manuals or training materials might not be available (or printed in languages inappropriate for the setting), resulting in little or no applications training
-
•
Software updates that are necessary for the operation of instruments, as well as for interfaces with other information systems, are often not available due to cost or because equipment is beyond the point at which software updates are compatible
-
•
Donated equipment often cannot be supported due to the scarcity of service contracts or access to qualified service engineers and physicists
Unless donors, recipients, and governments work together to follow best practices, donated diagnostic equipment can also create systemic problems at the health system level:117
-
•
Many donations are made without previous assessment or appreciation of the immediate or long-term needs of recipients
-
•
Typically, donations are not coordinated, resulting in the donation of equipment from different vendors, often from different countries, creating problems for procurement programmes due to the need to support multiple supply chains
-
•
User-driven donations can run counter to country-specific procurement programmes, contracts, and regulations
-
•
Donations from different vendors can create additional needs for qualified technical staff due to the multiplicity of platforms
-
•
User manuals and training materials from different countries create language barriers
-
•
User training and competency programmes require familiarity with multiple systems
-
•
Management and quality systems become more complex (eg, there is a need for different standard operating procedures) or unaffordable
-
•
Most countries lack adequate mechanisms to track and monitor donations, conduct inventories of donated equipment, and assess their effects, resulting in poor transparency and accountability
In summary, although donations might still be useful for some supplies and furniture, there are severe disadvantages for complex diagnostic equipment.
Inadequate infrastructure presents a challenge for basic diagnostic testing; it is even more of a barrier for complex testing. Equipment for imaging modalities, such as CT, MRI, or PET, cannot be installed or maintained in low-resource settings where physical infrastructure is insufficient. In the same way, complex diagnostic testing, such as that done in microbiology laboratories, requires adequate infrastructure, such as biological safety cabinets for testing and to protect laboratory staff from laboratory-acquired infections.118 Without the necessary infrastructure, testing, such as the detection and monitoring of antimicrobial resistance, becomes impossible (panel 5 ).
Panel 5. Antimicrobial resistance and ineffective antimicrobial therapy—consequences of insufficient or inadequate diagnostic information.
Antimicrobial resistance occurs naturally at low rates in the pathogens that cause human infections. Over time, pathogens can develop increasing rates of antimicrobial resistance, the most important cause being exposure to antimicrobial agents (known as selection pressure, which occurs because pathogens without resistance to an antimicrobial agent are eliminated, whereas those with resistance rapidly increase in number). A number of factors drive selection pressure in clinical settings: (1) treating infections with antimicrobial agents to which causative pathogens are resistant, or for which use of antimicrobial agents is not appropriate (eg, viral upper respiratory tract infections); (2) treating infections with wrong antimicrobial agents due to not knowing the cause of infections, use of presumptive diagnosis and treatment, or no antibiograms to guide empirical use of antimicrobial agents; and (3) inadequate treatment (eg, wrong dose, wrong route of administration, or lack of treatment compliance). As antimicrobial resistance develops for one class of antimicrobial agent, providers switch to another class to treat infections, which increases selection pressure for the new class and, eventually, emergence of pathogens that are resistant to more than one antimicrobial agent (multidrug resistance). Perhaps the best example of the development of a drug-resistant pathogen is the emergence of extensively drug-resistant tuberculosis, which has now been reported from 131 countries.13
Presumptive or syndromic diagnosis occurs when clinicians do not have access to, or do not use, diagnostics. One consequence of presumptive diagnosis is overuse of medicines for common conditions, including inappropriate use of antibiotics. The inappropriate use of antibiotics is particularly severe at the level of primary health care in many countries, especially in low-income and middle-income countries.118, 119 Studies have shown that the availability of rapid diagnostic tests for malaria reduced over-prescription of antimalarials, but increased presumptive prescription of antibiotics;120 that increased availability of tests for malaria and helminths decreased prescription of antimalarials and anthelmintics;121 and that (in a clinical scenario rather than a study of actual behaviour) the availability of laboratory test results reduced presumptive treatment with antibiotics across six different conditions by more than 90% (Horton S, unpublished).
The results of antimicrobial resistance are devastating. At the patient level, antimicrobial resistance results in increased morbidity and mortality, prolonged treatment in hospital, the need to use more expensive (and often more toxic) antimicrobial agents, and, for some multidrug-resistant infections, an inability to treat with antimicrobial agents. At the global level, WHO estimates that antimicrobial resistance results in at least 700 000 deaths per year due to the inability to successfully treat bacterial infections, a figure that could increase to as many as 10 million deaths per year by 2050.122 By 2050, the cumulative global cost due to decreased productivity caused by these infections is estimated to be as high as US$100 trillion.122
An underlying theme for the factors driving the development of antimicrobial resistance is insufficient or inadequate information on which to base treatment decisions, particularly the inability to access quality diagnostics. Preventing antimicrobial resistance requires a correct diagnosis, accurate identification of causative pathogens to inform treatment options, and use of antimicrobial susceptibility testing to identify antimicrobial susceptibility or resistance. Because of the importance of antimicrobial resistance to health-care systems globally, providing access to the necessary diagnostic tests must be a component of any national essential diagnostics list.
Health workforce capacity
Providing effective health-care services to attain the goal of universal health coverage will require the right numbers and types of health-care workers, including those providing diagnostic services.123, 124, 125 Some estimate of the shortfall in diagnostic workforce capacity can be calculated on the basis of the projected shortfall in global health-care workforce capacity, which has been estimated to range from 15 million to 18 million by 2030 (appendix pp 15–18).122, 124 Although the proportion of that workforce involved in diagnostics (eg, medical, non-medical scientist, radiographer, physicist, and technician) varies from country to country, based on estimates from select HICs there is probably a need for an additional 480 000–576 000 staff to support diagnostic testing in PALM and another 360 000–432 000 to support medical imaging, for a total increase of 840 000–1 008 000 workers.126
Because even HICs struggle to maintain their health workforce capacity at current levels, both overall,127 and in diagnostics,106, 128 attaining the necessary workforce capacity globally to meet future diagnostic needs will require substantial, long-term political commitment and financial investment. To guide such efforts, estimates of projected workforce capacity should be guided by country-specific diagnostic needs, better national workforce capacity data, and projections on how the diagnostic workforce will function. For example, the ratios of pathologists to population in most sub-Saharan Africa countries are less than one per million population,8, 129, 130 and for radiologists less than five per million population.130 Although there are no comparable data for many LMICs, and it can be difficult to obtain these data even for many HICs, comparing the number of pathologists and radiologists from a small set of countries for which data are available is striking. In the USA, the UK, and Canada, the number of pathologists per 1 million population is 39, 46, and 48, and the number of radiologists is 85, 46, and 37, respectively. By contrast, in Nigeria and Brazil, the number of pathologists per 1 million population is 0·9 and 7, and the number of radiologists is 2·3 and 5·8, respectively. 131, 132, 133, 134
A major cause of the diagnostic workforce capacity gap is that existing global capacity to educate and train a diagnostic workforce is inadequate.135 In some countries, the number of people taking part in training in the diagnostic disciplines, specifically PALM and DI, is insufficient even to maintain current workforce capacity levels, let alone allow for future growth.107, 136, 137, 138 For example, in sub-Saharan Africa, the number of medical schools is insufficient to support the growing needs for workforce capacity such that the numbers of radiologists and pathologists cannot increase.139, 140 The same is true for non-physician doctoral scientists, for whom the number of postgraduate training programmes in diagnostic specialties is small (or even non-existent) in many countries.129, 141
The lengthy time frame associated with building professional workforce capacity also creates challenges for obtaining sustainable funding.142 Furthermore, without appropriate circumstances and incentives, trainees do not have reason to stay in their country of training, or to stay in the public sector, both of which adversely affect efforts to build health workforce capacity in the public sector within countries.8 Although countries need to maintain and expand their workforce capacity in all health-care sectors, it is unrealistic to suggest that projected workforce capacity needs will be met solely by expanding existing education and training programmes. Not only are these programmes highly variable from country to country, both in terms of content and duration due to an absence of standardised or harmonised curricula, this situation will probably worsen with the introduction of new diagnostic technology, particularly digitisation, as it increasingly will be difficult for health-care workers in many (especially resource-limited) settings to keep up with changes in technology. In order to make the current and future health workforce more efficient and effective, education and training programmes need to be updated and aligned with fundamental changes in the roles of available health-care workers.
The shortfall in global health workforce capacity is also due to various other causes: work environments that do not allow for professional growth or job satisfaction, partly because of a scarcity of attractive career pathways in many health-care systems; inability of workers to use their full scope of education, training, and skills; insufficient infrastructure and supplies that are needed to provide services on a regular basis, leading to gaps in service provision; an absence of regulatory systems to ensure high-quality service provision; poor administrative systems (including human resources management); inadequate financial incentive; and inability to access Continuing Professional Development programmes.123, 143, 144, 145, 146
Quality and safety
Diagnostics testing in DI and PALM involves technical and interpretive components. In many instances, the two disciplines are inextricably linked, such as the need for high-quality images to enable radiologists and histopathologists to optimally interpret data. However, for some laboratory medicine tests, there is no need for professional interpretation per se, but rather it is the technical aspects of the test that determine if accurate test results are generated on a consistent basis.
To ensure quality, PALM and DI require robust oversight and training of staff and both rely extensively on continuing maintenance and calibration of complex technical equipment. Oversight of staff is usually through professional certification, whereas oversight of technical equipment and procedures is through accreditation. Professional certification is a standardised process by which individuals demonstrate that they have the necessary education, training, skills, and experience to qualify them to do specific roles. Professional certification is typically granted by professional societies or organisations and not by regulatory bodies. By contrast, accreditation is granted to organisations, not individuals, and in many countries is granted by regulatory bodies.
Maintaining professional certification typically includes requirements for Continuing Professional Development as well as demonstrating ongoing competency in the specific roles for which professionals are certified. Access to Continuing Professional Development programmes is often severely limited in many countries, particularly LMICs. Maintaining standards and competency in PALM and DI in much of the world is made challenging by issues such as inadequate access to Continuing Professional Development programmes, affordability, and insufficient specialty and subspecialty workforce precluding professional consultation and collaboration. Although many parts of the world have national or regional professional societies, membership in these societies, and travel to and participation in meetings, often is unaffordable. Attendance at regional or international meetings often requires use of bursaries or other forms of financial support that are insufficient for more than a limited number of participants. More importantly, with minimal staffing in many health-care facilities, providers often cannot take the time to attend professional meetings or programmes without causing gaps in service provision.
Quality systems in PALM
In addition to professional certification, PALM relies on accreditation and External Quality Assurance programmes as the basis of quality systems. Not surprisingly, in most of the world, these types of quality systems are either not available or, where they are available, not affordable. For example, a 2011 survey of 954 laboratories in Kampala, Uganda, noted that only 45 (5%) of identified laboratories met the lowest standards of the laboratory quality checklist developed by the WHO Regional Office for Africa.147 A subsequent 2018 analysis showed no improvement, with only 4 (5%) of the 78 laboratories originally surveyed (40 laboratories with moderate-complexity to high-complexity testing, and 38 laboratories with low-complexity testing) now being accredited.148 The survey results also showed that 23% of basic test results were inaccurate; only 42% and 38% of serum glucose and urea nitrogen test results were accurate.148
Achieving accreditation in laboratories in many countries has been limited by inadequate access to accreditation programmes or cost. In India, for example, only 1151 medical laboratories are accredited for a population of 1·3 billion; by contrast, the USA has 260 000 accredited laboratory entities for a population of 328 million.149, 150 Only a few international accreditation organisations exist, such as Joint Commission International, but these organisations do not extend their services to every country. Examples of regional accreditation services include Jamaica National Agency for Accreditation in the Caribbean, whereas the South African National Accreditation System operates in south, east, and west Africa. Many parts of the world do not have regional accreditation services. A few accreditation services operate only in a specific country: China, Kenya, Malaysia, Pakistan, and Taiwan have national or local systems.
Even where available, accreditation programmes are often not affordable for many facilities. In addition to the time and effort required to achieve accreditation, which often takes several years, there are the direct costs of subscribing to accreditation programmes and, for PALM, participating in External Quality Assurance programmes that are a requirement for accreditation. There can be other costs to achieve accreditation: for example, a study from Mozambique showed that the biggest cost for the National Tuberculosis Reference Laboratory to become accredited was associated with laboratory renovation, followed by technical assistance and mentorship, and equipment maintenance.151 The laboratory spent around US$1·5 million in renovations and equipment costs to achieve accreditation first to an African standard (ie, Strengthening Laboratory Management Toward Accreditation) and then to an international standard, such as those provided by the International Standards Organization. The estimated cost for the same laboratory to maintain equipment and laboratory renovations is just over US$200 000 annually.151 In Rwanda, limited access to resources such as water and waste disposal combined with infrastructure constraints has made it difficult for older hospitals to attain the standards needed for accreditation.152 In Bangladesh, local governments are responsible for overseeing these infrastructure issues within laboratories. The local governments are dependent on the central government for funding. These funds, unfortunately, are strictly given in accordance to rules and regulations instead of demand and severity of the situation,153 which results in many laboratories not getting the financial aid they desperately need.
A key component of PALM accreditation is participation in External Quality Assurance programmes. As defined by WHO, “external quality assessment (EQA) is used to describe a method that allows for comparison of a laboratory's testing to a source outside the laboratory. This comparison can be made to the performance of a peer group of laboratories or to the performance of a reference laboratory”.154 These programmes typically consist of a vendor providing specimens to laboratories to be tested and interpreted using that laboratory's standard methods, with the results reported to the vendor, who then provides a report comparing the laboratory's results against those of the peer group or reference laboratory.154 For many laboratories, participation in these programmes is hampered by lack of accessibility and affordability: there are no universally accessible External Quality Assurance programmes because even the most widely available ones do not extend their services to all countries. Very few regional or national External Quality Assurance programmes exist outside of HICs, and many are for a single disease or condition only.155 These programmes are expensive and even if they were available in LMICs they would be unaffordable in most resource-limited settings. Other barriers to widespread use of these programmes in LMICs include that they often require a cold supply chain for shipping the test reagents, many are not available in local languages, and many facilities do not have the necessary administrative systems to support their ongoing use.
Quality systems in DI
Imaging service delivery requires a systemic approach to quality management. Complex technology, processes, and infrastructure demand a highly coordinated team to produce intended outcomes in imaging diagnosis, treatment, follow-up, and meaningful communication. To provide DI services, the required professional personnel include radiologists, technologists, radiographers, sonographers, medical physicists, biomedical engineers, and information technology specialists. Because of the need for a highly specialised workforce, the workforce capacity gaps described previously have a particularly marked effect on the quality of DI available in many parts of the world. Maintaining professional standards and competency is challenging in many locations for the same reasons as described for PALM. Additionally, because of the partially subjective nature of medical image interpretation, it is essential for radiologists to have access to systems of audit by other radiologists. In many countries, the absence of digital transmission systems, as well as the dearth of radiologists, makes this access difficult or impossible. Approaches to quality management in DI have been published but are voluntary in many countries, and it is not clear how widely these approaches are used, particularly in LMICs.156, 157 In the same way, systems of accreditation for DI exist in many HICs but are less common in LMICs.
Radiation safety is of paramount concern in DI, to protect patients as well as health-care workers. In HICs, national governments provide regulations and guidance for radiation exposure.158, 159, 160, 161 Additionally, the International Atomic Energy Agency (IAEA) provides guidance for radiation safety,162 particularly through adoption of the International Basic Safety Standards,163 although country compliance with this guidance is voluntary.162 The IAEA also provides technical support for national and regional efforts to improve medical radiation safety, such as the Ibero-American Forum of Radiological and Nuclear Regulatory Agencies, and also supports individual member states.164 In some HICs, there are guidelines for limiting unnecessary radiation exposure from DI procedures;165 the Pan African Congress of Radiology and Imaging has promoted a similar initiative in Africa called AFROSAFE,166 although it is not clear how widely these standards and guidelines are followed due to the small number of trained professionals to implement and follow them, or the few available administrative mechanisms to track compliance.
Programmes for the safe use of MRI and ultrasound are described in HICs.167, 168, 169 As with quality programmes for PALM, a major barrier to the use of these programmes results from weak or even non-existent regulatory frameworks for these medical devices, and enforcing safety guidelines is particularly difficult because there are no standards to which people are held.
We have outlined the widespread, key deficiencies in the provision of adequate infrastructure, workforce, and quality assurance. The challenges are deep and wide-ranging. Overcoming these challenges will require a coherent multi-year approach, but the scale of the issues is such that incremental solutions alone will be inadequate. Radical and transformative approaches will be needed.
Achieving change
There is no single effective means to improving the access to diagnostics. Addressing the complexities of the disparate but interactive problems we have highlighted in this Commission will require a multifaceted approach, ranging across policy, regulation, financing, workforce, and infrastructure. Political scientists have analysed how change can occur, and one such study by Shiffman and Smith,30 which applied to global health, pointed to four key groups of factors: the characteristics of the issue; the power of actors involved; the degree of coalescence around ideas about the issue; and the political context. This framework allows us to analyse why, despite the serious shortcomings, change in the case of diagnostics has not yet occurred, and how current circumstances now provide an opening for change. We summarise how the diagnostics agenda ranks on the 11 component factors that Shiffman and Smith identify as affecting political priority.30
Three factors define the characteristics of the issue. First, a credible indicator of the severity of the problem is required, which can also be used to measure progress: a key message of our Commission is that 47% of the world's population does not have adequate access even to the most basic diagnostics. Second, severity of the problem is important: we have identified that as many as 1·1 million deaths annually could potentially be averted by better access to basic diagnostics leading to treatment. Lastly, a cost-effective, evidence-based intervention needs to be available, which we discuss in following sections on the EDL, technology, and on the benefit–cost of diagnostics. To our knowledge, none of these data have been available before.
Four factors underpin the power of actors involved, namely policy community cohesion (ie, coalescence among the stakeholders at a global level), leadership (ie, strong champions), guiding institutions, and civil society mobilisation. We have analysed the weaknesses of the diagnostics agenda in all these areas and believe that there is a need for a Global Alliance to address them.
The degree of coalescence around ideas involveddepends on how the ideas are shared by the policy community, and how these ideas resonate with external audiences. Although the diagnostics community was already well aware of the issues involved, the COVID-19 pandemic has moved the importance of diagnosing diseases and conditions markedly up in priority, both to the global health community and to the general public (who in turn influence politicians). Inability to test adequately for a new, deadly infectious disease has meant that inadequate diagnostics has become a threat not only to survival but also to global prosperity.
Lastly, the political context matters, and the opening for change currently exists. First, the pandemic has shown why diagnostics are essential in avoiding deadly diseases globally (not just in poor countries). Second, the effective implementation of universal health coverage and the avoidance of antimicrobial resistance both require good access to quality diagnostics. Both issues can provide the impetus for embedding diagnostics in global governance; for example, explicitly recognising diagnostics along with vaccines and medicines in policy statements, such as the Sustainable Development Goals.
Having used the aforementioned framework of Shiffman and Smith to analyse the issues, we propose change through innovation in policy, governance, and finance, and through removal of barriers in infrastructure, workforce, and quality. Considering that technology is an enabler of many of these changes, we have a previous discussion on the explosion in innovation in technology. In combination, we believe these changes have the potential to transform access to accurate, affordable, and timely diagnostics globally.
Finding a mechanism, or mechanisms, to drive these changes successfully across such a diverse set of fields is a formidable challenge. However, the Commission believes that if every country successfully implemented an evidence-based national EDL and worked towards including this list in the universal health coverage benefits package, this would be a key mechanism of achieving the necessary transformation. This notion is based on the recognition that successful implementation and maintenance of an EDL requires much more than just a list of diagnostics; trained staff, equipment, supply chain, regulation, finance, quality control, and engagement of relevant stakeholders (especially local and regional) are also examples of key requirements. In other words, an effective diagnostics system is needed.
Therefore, we developed a template for a national EDL, which any country could adapt to their own needs and situation. Such an EDL is based on having an integrated and tiered network of diagnostics, with a minimum set of point-of-care diagnostics at a primary health-care level and a community level, linked with a broader set of diagnostics at first-level hospitals, and the broadest set at referral hospitals. Having such an integrated system could allow for many advantages, including in terms of scale for workforce, training, purchase, and maintenance of equipment. This system could also ensure that relevant patient information is available across all levels of the health-care system.
Solutions based on the future top 20 global burden of disease conditions (GBD-20 EDL)—a template for a tiered national EDL
National governments regulate the importation and marketing of all diagnostics used in both public and private sectors. The national EDL refers to a narrower list of diagnostics approved for use in public institutions in the health system. Ideally, all diagnostics on the national EDL would be publicly funded and either provided at no cost to patients, or patients would be entitled to reimbursement from national health insurance. However, in countries with lower income, they might start with a subset of the national EDL being publicly funded, and gradually expand as national income increases.
The national EDL includes diagnostics used for a range of purposes. A large proportion are associated with the diagnosis of conditions, but there are many other indications for diagnostics; for example, monitoring progress of treatment, dosing medications, or identifying medication toxicity. Another set of indications are for functions of public health, such as disease surveillance and ensuring safety of the blood supply. India, for example, includes tests for water supply monitoring in its national EDL.
We modelled an evidence-based list of diagnostics using the top 20 global burden of disease conditions globally for 2030 and 2040, using mortality and years of life lost as the metric (23 conditions in total). This list, termed GBD-20 EDL, could be used as a template for the construction of any national EDL, to which additional diagnostics could be added that are important for local epidemiology and national priorities.
We also modelled an allocation of those diagnostics by tier. At primary health-care facilities, we assumed a minimal infrastructure, such that only a few diagnostic investigations could potentially be done, primarily using point-of-care tests and possibly point-of-care ultrasound as the only imaging capability. At first-level hospitals, we assumed the potential for using basic automated analysers, as well as ultrasound and x-ray. More advanced laboratory and imaging capabilities were allowed at higher level hospitals. It is assumed that higher level facilities can undertake all tests available at lower levels.
The methodology for constructing our GBD-20 EDL relies on several sets of assumptions and evidence. First, we categorised the management of conditions into two levels, namely management of the uncomplicated condition and management of the condition with complications. Additionally, we added a level in the health system at which the condition cannot be treated, but where it is important to identify those individuals who potentially have the condition, to refer them to a higher level facility for diagnosis and management. We called this triage, a term that is sometimes used for treatment. We did not use the term screening because organised screening has very specific connotations.
Second, we made a key assumption that conditions should generally be diagnosed and monitored at the health system tier at which they can be managed, given that this probably permits having a crucial mass of medical expertise, experience, and resources available. However, occasionally, a patient with a condition might be ideally managed at a particular tier according to guidelines and expert opinion (figure 7 ), but the diagnostic procedure required is sufficiently specialised that it was judged not feasible to do at that tier due to the complexity of resources required. There are at least four alternatives that are possible. One is to use a send-out test, if there is sufficient capability to send a specimen and have results returned in a timely way (this option is incorporated into the Indian national EDL and is a reasonable solution for non-urgent use cases). A second alternative is for the patient to go to a higher level hospital for diagnosis as well as developing a management plan, which can then be implemented at a more local hospital; this approach is used in some circumstances for cancer treatment. A third alternative is to move condition management up to the next tier level. The final option is to investigate the possibility of developing another version of the diagnostic investigation that can be done at the lower tier, for example, the development of a rapid diagnostic test, benchtop analyser, or point-of-care imaging device.
Figure 7.
Allocation of management of conditions, by tier and level of acuity
These 19 conditions are derived from predicted top 20 conditions in the global burden of disease for 2030 and 2040 (a total of 23 distinct conditions) in table 4. Trauma (here) combines four global burden of disease conditions, namely falls, interpersonal violence, road traffic injuries, and self-harm (table 4). Antenatal care refers to neonatal preterm birth (table 4). Neonatal encephalopathy (table 4) is not included here (the level of care required is only at referral hospitals in most LMICs, and not widely available). Congenital defects (table 4) is also not included here, given that more guidance is required for LMICs. Hence, 19 distinct conditions are shown. LMIC=low-income and middle-income country.
Third, we made assumptions to model which condition is managed at which health system tier (figure 7). This model is based on our understanding of current practice in an archetype low-income or lower-middle-income country, which could be in sub-Saharan Africa or south Asia. The model is also based on our understanding of the type of treatment resources that might be available in a resource-limited environment where infectious diseases have been prevalent in the past three decades or more but non-communicable diseases are now beginning to increase. Availability of diagnostic and treatment resources is generally related to local epidemiology, such that resources for locally prevalent infectious diseases (eg, malaria) are often more readily available at a more basic tier in the health systems than would be the case in an HIC. Conversely, resources for complicated versions of non-communicable diseases are often only available at higher tier facilities than would be the case in an HIC.
Last, we used the link between conditions and tiers to identify the diagnostics required at each level. For PALM diagnostics, this link was based on a model which links the WHO essential medicines list to conditions and, subsequently, to PALM diagnostic tests (using evidence-based expert databases).170 Published guidelines were then used to help allocate the management of conditions to tier levels (Schroeder L, unpublished). This model was expanded to include radiology, using published guidelines.171 Other conditions from the top 20 GBD diseases were added to the model that require management and care but not necessarily medicines, including antenatal care to address healthy pregnancy outcomes, and trauma care.
The basic capabilities that are required at each tier are given in figure 8 , including primary health centres, first-level hospitals, and higher level hospitals (appendix pp 19–20). Notably, the diagnostic procedure lists are cumulative: higher level facilities need to be able to do all procedures from lower level facilities (but not necessarily using the same methods and equipment). Detailed tables of which conditions require which procedures at which levels are given in a separate study (Schroeder L, unpublished).
Figure 8.
Diagnostics capabilities required at each tier, to address top 20 future global burden of disease conditions in national EDL (ie, GBD-20 EDL)
More complex equipment needs (and associated workforce skills) are required at higher tier facilities. EDL=essential diagnostics list. POC=point-of-care.
Primary health centres are assumed to have the ability to run rapid diagnostic tests, dipstick tests, tests requiring basic equipment (eg, haemoglobinometer or glucometer), and (preferably) ultrasound, which is part of WHO's recommendations on antenatal care for a positive pregnancy experience.31 First-level hospitals in our model need automated haematology, chemistry, and immunoassay analysers and x-ray, as well as basic microbiology and histopathology capability and capability to support blood supply and banking. A variety of other more specialised capabilities are desirable. Upper-level hospitals need all the capabilities to support advanced treatment, including advanced imaging capabilities (eg, CT, PET, and MRI), as well as the more specialised imaging capabilities required for ischaemic heart disease and stroke. For cancer, referral hospitals need immunohistopathology and molecular diagnostics, as well as specialised imaging for breast cancer and bone scans for metastatic cancer. Due to the expensive equipment required, some countries have grouped equipment at hospitals with specialised units (eg, cancer centres and cardiac centres), while first-level hospitals in some countries use a hub-and-spoke model for some of the more specialised equipment and procedures.
If the GBD-20 EDL is compared with the WHO EDL and the Indian national EDL, we observe many similarities as well as some differences (Schroeder L, unpublished). There are 97 PALM tests included in at least one of the three lists. The WHO EDL includes 82 PALM tests; the Indian national EDL includes 67 PALM tests, whereas our GBD-20 list includes 58 PALM tests. Combined, these three lists include 97 unique PALM tests. Because diagnostic tests are not well standardised internationally by name or format, some simplifying assumptions were required: for example, the Indian national EDL includes the test for thyroid stimulating hormone, but also individual tests for components T3 and T4, and we treated these three as one test. The WHO list does not include any imaging tests, whereas the GBD-20 and the Indian national EDL include essentially the same imaging tests. The Indian national EDL includes other diagnostic tests not appearing on the WHO or GBD-20 lists (eg, endoscopy) from disciplines other than PALM and DI. The Indian list also includes other public health tests (eg, water supply tests).
The three EDLs have somewhat different purposes. The Indian national EDL aims to meet national needs and is tailored to Indian epidemiology, including a number of tests for infectious conditions that are not as common in other world regions. The WHO EDL evolved initially from WHO guidelines for priority infectious diseases, to which various expert groups, including WHO working groups, have proposed the addition of additional diagnostics. The GBD-20 EDL has been developed from a narrower list of conditions as an illustration of a template as to how to use evidence to construct a list. Hence, although there are strong commonalities (37 of the 97 PALM tests appear on all three lists), there are also differences (31 of the tests appear only on two lists, and 29 on only one list). The 37 PALM tests common to all lists are also very widely used tests: almost all appear on the top 25 tests by volume in a study of six referral hospitals in five countries.172
Limitations of the model for constructing the GBD-20 EDL include that it only focuses on the top 20 conditions globally by burden of disease for two different future years (23 conditions in total), and does not include many locally important conditions. Importantly, the GBD-20 EDL does not include the expanding range of investigations done outside of primary health care under the democratisation agenda, but such investigations should be linked into the system in the future. Childhood conditions resulting in mortality are weighted heavily in the years of life lost analysis. However, conditions causing disability are underweighted because the global burden of disease dates of 2030 and 2040 are only available to date by years of life lost rather than by DALYs averted. Equipment needs are estimated according to technologies that are currently available: it is possible that, by 2030 and 2040, there will be new types of equipment permitting diagnosis and diagnostic procedures to occur at different tiers in the health system than is currently possible. Moreover, the model does not include functions other than the management of conditions; for example, surveillance for infectious diseases or testing for safety of the blood system are not included, which might or might not occur in hospital laboratories. The model also does not incorporate population density. In densely populated regions, larger volumes of required tests or imaging examinations might make it feasible to purchase larger analysers or more expensive imaging equipment at facilities lower in the health system.
The model is presently built using an archetype low-income or lower-middle-income country. A future enhancement could be to develop a fully computerised model allowing the user to change the assignment of condition management to tier level, and hence see how diagnostic requirements change.
The key point of our GBD-20 EDL is that expanded public provision (and financial coverage) of diagnostics needs to be prioritised and evidence-based. No single model is appropriate for all countries, but our GBD-20 EDL provides a framework that can be used as guidance to use evidence to make decisions for individual country situations.
Solutions: innovation in technology
In the past decade or so, there has been an explosive expansion in diagnostic innovation involving almost every aspect—technology, education and training, workflow organisation, data analysis and management, supply chain management, and even governance and finance. Innovation in all these areas is necessary to address the challenges, but innovation that is based primarily on technology, which is the area in which expansion has been most dramatic, is considered in this Commission. We are not going to discuss the advantages of any particular test or technology because this will vary by local needs, staff availability, and cost, but the approaches that such technologies allow. There are other groups and commercial entities that do periodic evaluations of the state of diagnostic technologies (eg, the Broadband Commission for Sustainable Development).173
Three technology-based approaches have the greatest potential for diagnostic transformation. The first two, digitalisation and point-of-care diagnostics, bring diagnostics closer to the patient, enabling the third, democratisation, which puts diagnostic testing in the control of the patient.
Digitalisation
Arguably, digitalisation has been the fundamental driver and facilitator of the majority of the most important, recent, innovations in diagnostics (figure 9 ). In 2018, a World Health Assembly resolution recognised the enormous potential of digital health to contribute to advancing the objectives of the Sustainable Development Goals and universal health coverage.174 Subsequently, in 2019, WHO released the first formal guidelines recommending the use of digital health for health system strengthening.175 Several of the recommended applications were relevant to diagnostics: targeted client communication via mobile phone; client-to-provider and provider-to-provider telemedicine; stock notification and commodity management; training and education; decision support tools; and tracking for treatment initiation and monitoring.176
Figure 9.
Digitalisation facilitates innovative tools to support diagnostic testing
Digitalisation and its potential benefits rely on the integration of two particular technologies, namely information and communications technology and artificial intelligence. This figure was produced by Catherine Hidalgo for this Commission.
Information and communications technology is fundamental to digitalisation, so all aspects rely on effective information and communications technology systems being in place. Within DI and PALM departments, information and communications technology-based management and reporting systems, especially when combined with digitised images, can improve workflow.177, 178 These systems can also improve quality and safety through increased speed of reporting, reduced errors, such as those arising via data entry, and increased completeness of reports through use of synoptic reporting.179, 180 The data produced by these systems can also be used to manage the supply chain and can be linked to outside organisations for public health epidemiological purposes, such as disease surveillance and registries.
The use of digital images in DI and PALM is changing education, quality assurance, and clinical practice, but both disciplines are dependent on the availability of information and communications technology. Digital teleradiology is widely used and has become standard globally. Notably, digital teleradiology is used to address shortages of radiologists, especially in remote locations. Although digital pathology has been less widely used globally, it is now becoming more common,181 and is being increasingly used for primary diagnosis (ie, routine clinical reporting without referral elsewhere). For example, in 2018, in the UK, 31% of pathologists reported having used digital pathology for primary diagnostics.182 In Switzerland, this number was 20%.183 Interestingly, this trend is being markedly accelerated by the use of telehealth to deal with the off-site requirements of COVID-19 lockdown. The breakthrough has been the development of digital slide scanners, which provide images of sufficient quality to allow primary reporting. This development has allowed for the transmission and interpretation of digitised slide images in a process similar to that used by digital imaging. Furthermore, digital slide scanners have similar potential to that of digital imaging for provision of services, including sharing cases for multidisciplinary meetings, obtaining a second opinion, archiving cases, and servicing remote locations.184
Outside of the hospital setting, information and communications technology is essential for the implementation and management of point-of-care diagnostics at remote sites and their integration into the health system via mobile connectivity. Furthermore, one of the great benefits of digitalisation and information and communications technology is the ability to provide much more rapid and secure communications between clinicians, and between patients and clinicians, than with analogue systems. Although this contribution of digital health towards equitable delivery of health services is still impeded by insufficient mobile network coverage in rural and remote locations globally, advances in mobile networking technology, such as the introduction of 5G wireless systems, will support the implementation of digital health in settings that have previously had inadequate access to mobile network services.
Progress in technology, AI, and available data are creating unprecedented opportunities for prediction models to inform, personalise, and improve care.185, 186 This progress is already affecting practice in DI.187, 188 For example, AI can provide workflow enhancements that integrate with an electronic medical record to identify patients who are at high risk or critically ill and prioritise them for care.178 An area of great interest is use of computer-aided detection algorithms to help radiologists identify easy-to-miss abnormalities. There are numerous publications showing at least equivalent outcomes to radiologists in the identification of such abnormalities.189 However, at this early stage, most people see AI as having more of a triage role, filtering out obvious normal and abnormal images, allowing trained staff to focus on the difficult cases. In PALM, AI is much less established. AI has been used in cytopathology for some time,190 and more recently, in clinical microbiology.191 Notably, AI is increasingly being assimilated into histopathology.192, 193 In histopathology, examples of areas of interest include biomarker detection and scoring (eg, via immunohistochemistry) and tumour detection and grading of cancers.194 A particularly valuable use is for exclusion of negative cases.
In both disciplines, a further use is in enhancing the diagnostic report. A final report is not a simple record of the result—the pathologist or radiologist incorporates information from several other sources, such as clinical and demographic data and data from other diagnostics, to rule out certain conditions, provide a definitive or differential diagnosis, and give advice on possible therapy or next steps. Most of these data are currently in free text format. Although natural language processing can extract relevant information from these other sources and tie them to the case in point, AI can help to filter and resolve these other data more accurately to provide a more comprehensive and useful report.195
The uses of AI might differ slightly between HICs and LMICs in a variety of ways; for example, prioritising between AI applications to extract actionable data and intelligence on health system performance, or automating burdensome processes that require specialists who are in short supply (eg, radiologists). In LMICs, data quality might be an important issue that is preventing the widespread and rapid adoption of AI. The use and outputs of AI systems will be limited by the quality and accuracy of the data being fed into the system. In HICs, AI use will be driven by commercially licensed systems. LMICs might need to rely on subsidised global goods made available without the support of large medical technology corporations to support the systems. LMICs might have a lower bar for acceptance of AI as a primary diagnostic system simply because the need is more urgent. Each country will, however, make its own decision. UN-led groups like I-DAIR are helping to set milestones and lower the bar for high-quality AI systems to be introduced in LMIC health systems.
Decision support systems are increasingly using AI. These systems help clinicians on clinical decision making and provide guidance for best practice and evidence-based management, based on a patient's clinical presentation. The functions of such systems range over initial diagnosis, management of subsequent disease, treatment, and prevention. These systems are already in use in DI with benefits; for example, in quality of care and patient safety and increased cost-effectiveness.196, 197 To date, in PALM, these systems are largely used in the non-anatomic pathology disciplines.198
Given the transformative potential of digitalisation, the Global Digital Health Index is an interactive digital resource that has been created in an effort to benchmark country-level capacity for information and communications technology, to inform and facilitate further implementation.199 This Global Digital Health Index evaluates the use of digital technology for health within and across countries, allowing national decision makers to make better decisions about resource allocation. The Global Digital Health Index also evaluates infrastructure readiness for mobile-enabled technologies. For example, as new technologies are introduced, their capacity to scale and remain sustainable depends heavily on the extrinsic factors, such as human capital, national standards, and interoperability frameworks, as well as the policy environment and regulatory environments.200 Without adequate investment in these essential cognate systems, on which end user and facility-based systems rely, innovations will struggle to stay active beyond the pilot phase. Government incentives to digitalise systems, as shown in the USA with the Affordable Care Act and Meaningful Use legislation, might be important to drive ecosystem maturity.201
Point-of-care diagnostics
One of the key barriers to diagnostic provision has been access at the so-called last mile; that is, in the community, away from the hospitals and clinics where imaging and laboratory services are located. This barrier particularly affects poor people and people in rural locations and illustrates why point-of-care diagnostics are important.
Although simple point-of-care diagnostics have been around for many years (eg, a dipstick urine test for glucose in 1956), addressing this need has driven much recent innovation and expansion.202 The essential advantage of point-of-care diagnostics is the provision of diagnostics beside or close to the patient, to allow treatment and next-step decisions to be made rapidly and as part of the same clinical encounter.203 Although the primary use is outside of hospitals, point-of-care tests can, of course, be used at any level in the health system (eg, in the home, community, and outpatient setting but also in the emergency room, intensive care unit, or operating room of a hospital). A common situation is to deliver a result (often within 15 min) to guide acute emergency treatment. For example, handheld and mobile ultrasound as well as x-ray can be used in emergency rooms and in intensive care units, in which patients cannot readily be moved. In less acute situations, point-of-care diagnostics can allow a so-called one-stop shop approach, in which the patient is seen, tested, diagnosed, and treated during one health centre or outpatient visit. Point-of-care diagnostics can also be used to screen for a disease and to triage those people who need further testing. Lastly, through self-testing, self-sampling, and community use, point-of-care diagnostics are key enablers of democratisation of diagnostics and patient-centred care by putting diagnostics more in the control of the patient.
In 2004, WHO defined the desired characteristics of any point-of-care diagnostic with the ASSURED criteria (ie, affordable, sensitive, specific, user-friendly, rapid and robust, equipment-free or simple, deliverable to end users). In 2019, Land and colleagues204 proposed updating these to include real-time connectivity and ease of specimen collection—REASSURED criteria. These authors also expanded the equipment-free criterion to include environmentally friendly.
Point-of-care PALM technologies can be split into two categories. The first category is small handheld devices, such as dipsticks, lateral flow strips, and microfluidic devices, providing a limited (but increasing) range of tests. Since around 2007, there has been much interest in moving to paper-based devices for cost, stability, and environmental reasons. The second category is larger, often bench-top devices, which are essentially laboratory instruments that have been reduced in size and complexity. For example, the move over the past 10 years to nucleic acid detection for infectious diseases and for some cancer genomics has been accelerated by the use of PCR and loop-mediated isothermal amplification technology in small desktop instruments (eg, GeneXpert). Another focus has been the development of multiplex devices (ie, devices that can analyse, detect, and measure a number of different analytes from the one sample). The range of PALM point-of-care tests is large and increasing, with use in both hospital care and primary and community care.
Point-of-care DI is becoming more prevalent in the marketplace, with even more advanced technology coming down the pipeline. The technology most widely available is point-of-care ultrasound. This technology is now used in a multitude of settings for many clinical indications, from maternal and fetal health, to trauma, and to communicable and non-communicable diseases.205 Although not represented in formal guidelines, because handheld ultrasound devices can be powered by batteries for use in situations where time is of the essence (emergency room, intensive care), or the location favours portable devices, authors have described effective use of ultrasound in primary clinic facilities. Many experts agree that ultrasound is a necessary baseline diagnostic imaging intervention for all tiers of health delivery in LMICs. When country rules and regulations allow, task shifting for ultrasound might be considered with appropriate supervision, attention to quality, and continued education. Modern handheld ultrasound devices are cheap, portable, user-friendly, and do not use radiation. Some devices have the ability to diagnose specific cancers, even small breast and liver cancers; others are less sensitive and are used for disease processes that are easier to detect, such as pleural effusion or pericardial effusion, or for procedural guidance.
Both PALM and DI point-of-care handheld and benchtop devices are increasingly electronically linked (eg, via mobile phones) for quality control and for workforce and supply chain management. Electronic linking also permits linking to the patient's health record for disease management, and to public health surveillance registries for epidemiology and research. The devices can also be linked to the Cloud to allow the user in a remote location to stream a real-time image for expert advice. Increasingly, AI and decision support systems are being incorporated into point-of-care procedures to allow better result interpretation and treatment recommendations (eg, AI algorithms to position the ultrasound probe correctly by correcting angles and settings automatically).206
In addition to ultrasound, x-ray units are still an important diagnostic tool in many health-care settings. Due to infrastructure limitations, many clinics have turned to mobile x-ray units and even mobile stroke units fitted with CT scanners that can be used to diagnose strokes and allow for more timely clinical intervention. In future, mobile CT and MRI technology will probably become available.
The advantages of point-of-care diagnostics include patient-centredness, rapidity, lower staff requirements, reduced hospital attendance and, in the case of PALM, avoidance of transport issues. The most important disadvantage of point-of-care diagnostics is limited and poor reliability.207 Reliability depends not just on the performance characteristics of the actual test, but on all elements involved in the testing (ie, sample collection and preparation [PALM], result interpretation, and result communication). All these elements rely on users being adequately trained and having ongoing access to quality control materials and technical support and maintenance for instruments. Therefore, it is vital to incorporate adequate quality assurance and quality control into the point-of-care testing protocols. Additionally, these point-of-care diagnostics should only be used in situations in which there are referral pathways and there is health-care provider buy-in and patient trust. The other major disadvantage is cost. Generally, the cost per point-of-care laboratory test is considerably higher than tests done in a central location, although the clinical advantages might well outweigh the increased cost. However, in the case of imaging, ultrasound is both highly portable and less expensive than other forms of imaging.
Point-of-care procedures are best used in situations in which speed is required or where access to a laboratory or radiology department is not available, or both. Point-of-care diagnostics have enabled enormous expansion in access to diagnostics, especially for the populations with least access to conventional diagnostics. This expansion in access to diagnostics will only continue to grow in the future, reinforcing their major role in solving the challenges of delivering timely accurate diagnostics.
Democratisation of diagnostics
A major benefit of moving diagnostics into the community is that of democratisation—the enabling and empowering of patients to be more in control of the diagnostic process.208 For PALM, this approach can involve self-testing (when an individual collects their own specimen, performs the test, and interprets the result—eg, home diabetes care and COVID-19 testing) and self-sampling (when an individual collects their own specimen and sends it away for testing). For both PALM and DI, institutional testing and examinations (in which testing or examinations take place in a pharmacy, school, correctional facility, or other institution) is another means of putting the diagnostic process more in the control of the patient. As mobile health technologies mature and become more widely used, patients and providers will have better access to information regarding the availability, cost, and quality of tests. All possibilities improve uptake by giving people more choice around when, where, and how to access diagnostic services.209, 210, 211 These methods are already beginning to transform diagnosis in LMICs for diseases, such as HIV and human papillomavirus.209, 210, 211
The process is being driven by several factors. For example, for the top 20 diseases that are expected to contribute to years of life lost in LMICs in 2020, a diagnostic test that can be done at the level of primary care exists for less than 50% of these diseases, and 90% of existing tests require laboratories or hospital imaging facilities (Kohli M, Adam P, unpublished). As a result, many people delay or avoid seeking diagnosis due to concerns over transportation, time taken out of work or daily duties, and costs.212, 213 Similarly, stigmatisation linked with particular diseases and marginalisation, including sexual minorities and those in remote areas, can also prevent people from seeking care.212, 214
Democratisation clearly has the potential to transform access to diagnostic tests in populations with the least access at present. This approach can encourage early diagnosis by expanding diagnostic procedures into communities and homes. Furthermore, democratisation can also relieve the health-care burden through triaging of individual care and allowing appointments with primary care physicians to be reserved for patients who, for instance, have tested positive.208, 214 Ultimately, this approach can lead to reduced health-care costs and improved patient outcomes. Another benefit of this process is that it facilitates involvement of local communities in developing innovative solutions for their own problems relating to health-care delivery. Innovation incorporating such community involvement provides a means to address the broad and complex range of factors affecting health and, thus, successful delivery of universal health coverage.215
The concerns around reliability and quality control,216, 217 affordability, linkage to the care system,218, 219, 220 and trust, including confidentiality and privacy, which apply to point-of-care procedures, equally apply to the democratisation process. However, given the strength of demand and supporting scientific evidence for democratisation of diagnostics, efforts are warranted to implement and scale up existing procedures and programmes, and to develop out-of-clinic options for other disease areas outside of HIV and sexually transmitted diseases.
General principles for implementation of innovation
The above innovations will only lead to transformative diagnostic solutions if implemented in accordance with the local social, epidemiological, health system, and political contexts.221 Addressing these constraints is urgent and crucial. The WHO's formal guidelines for digital health and the Principles for Digital Development outline key practices which provide a roadmap and advice on how to do so.175 There are several practices included: designing technologies with and for the end user; understanding the ecosystem into which the technology is being introduced; designing for scale and building for sustainability; emphasising the meaningful use of data, especially by ensuring that data generated can be integrated into the patient record and into national monitoring indicators; and addressing privacy, confidentiality, and security.
Furthermore, a standards-based approach is vital to increase system interoperability at all levels and to reduce potential for conflict and confusion. International standards, such as Digital Imaging and Communications in Medicine have achieved near-universal adoption among medical imaging equipment and software manufacturers since the late 1980s. Breakthroughs in digital health record standards, such as Fast Healthcare Interoperability Resources developed by Health Level 7 International in 2011, have further expanded interoperability and standardisation across systems, reducing barriers in sharing and exchanging information. A particularly important requirement is the leveraging of open technologies (eg, Open Source or Open Standards approaches), thereby increasing the ability of low-resource environments to absorb and adapt technologies. In 2020, WHO has issued further guidance on addressing the many issues around implementation of digital interventions in health care.222
Diagnostics innovation in LMICs
One problem in the field of global health is an excessive reliance on the so-called trickle-down model, in which products and innovations are developed in HICs and after a decade or two, they slowly trickle down to the LMICs where the biggest needs are, and where technologies often have the greatest impact.223 As a result, today, almost all LMICs are still highly dependent on laboratory reagents, diagnostic kits, and devices (both PALM and DI) imported from HICs. This reliance drives up the cost of diagnostics, made worse by fluctuating disadvantageous foreign currency exchange rates, which also pose budgetary, supply chain, and sustainability challenges. This dependence also has far reaching implications for preparedness and resilience to disasters and epidemics. Although implementation has been another matter, the current COVID-19 pandemic has exposed very clearly how only countries with strong research and innovation capacity (mainly HICs) were able to rapidly develop viral diagnostic kits.224, 225 Those countries with no or limited capability or capacity had to depend on sending samples away, purchase services, or depend on aid from the international community, all at high costs and delay.
Additionally, diagnostics developed without cognisance of geographical differences in disease biology and health-care logistics might not be the best fit for LMICs. Emerging research has shown genomic differences in breast cancer between Asian and White women, underscoring the need to validate test profiles and predictive rules developed in White women before implementing them in Asia.226 Design of laboratory information systems that are unnecessarily complex for LMIC needs, or of DI equipment vulnerable to unstable electricity supply, are examples of prohibitive logistical challenges.
Building capacity in research and innovation in diagnostics in LMICs will help to address some of these issues. There are emerging examples (panel 6 ); however, the challenges are large. In 2020, data from WHO Global Observatory on Health R&D showed that HICs have 73 times more health researchers per million inhabitants than the low-income countries and 7 times more than lower-middle-income countries.234 World Bank Data (2017–18) shows that the HIC spend, on average, is 2·56% of gross domestic product on research and development compared with 1·51% LMIC spend, 0·60% by south Asia and 0·47% by sub-Saharan Africa.235 Despite this finding, success is possible.
Panel 6. Country success stories in developing capacity in research and innovation.
Several countries, such as India, China, South Korea, and Singapore, have shown a substantial increase in technological advances. Strengthening the research and innovation enterprise through international collaborations (be it North–South or South–South), increasing the number of PhD students, creating biobanks, and forming partnerships with industry are some well used approaches in capacity building. Another strategic move is to proceed with new technologies of promise, such as next-generation sequencing, artificial intelligence, and machine learning. A 2018 workshop convened in the Philippines on improving the development and deployment of diagnostics in southeast Asia described the bottlenecks and barriers faced by researchers.227 Some encouraging successes were shared, arising from the development of national laboratories and the Association of Southeast Asian Nations Network for Drugs, Diagnostics and Vaccines Innovation, cross-disciplinary collaborations, local manufacturers, and harmonisation of regulations and shared diagnostic development by disease. Moves by international research funders to ringfence grants for capacity building (eg, the newly minted Medical Research Council UK Applied Global Health Research Board 2020) continue to address the so-called 10/90 gap, in which only 10% of global health research resources are applied to the conditions that form 90% of global disease burden.228
A major outcome of developing capacity in research and innovation is the creation of a pool of competent researchers and laboratories on which the country can rely for solutions and crucial advice. For example, in 2020, an Indian molecular test for tuberculosis was endorsed by WHO,229 while China has several indigenous tuberculosis molecular diagnostics.230 These products are already being scaled up in India and China. In diagnostic imaging, an Indian artificial intelligence tool for CT scans of the head was approved by the US Food and Drug Administration.231
Such research capability is crucial during epidemic and pandemic crises. For example, the containment of the Malaysian Nipah virus outbreak in 1998–99 depended on the discovery by a researcher at the University of Malaya (Kuala Lumpur, Malaysia) of a novel Nipah virus as the agent, in what had been deemed a Japanese B encephalitis outbreak.232 In the current COVID-19 pandemic, the capability of China to identify the infective agent rapidly and share its genomic sequences was invaluable in the rapid design and implementation of needed PCR-based diagnostic tests. The technology on which the molecular diagnostics tests for tuberculosis are based has been repurposed to also test for COVID-19. Similarly, the rapid re-organisation and planning of the diagnostic imaging services to adapt to local conditions during COVID-19 in Singapore shows the value of such local expertise.233
Mostly, building national research and innovation capacity in all countries depends heavily on the political will of governments in creating and sustaining a research and entrepreneurship culture within their nations. The importance of advocacy for the development, manufacture, and distribution of local diagnostics cannot be understated. When governments are key stakeholders, geopolitical collaborations, harmonisation of regulations, funder confidence, community acceptance, and scaling up are more likely to be achieved. Without government involvement, it is probable that little or none of this change will happen.
We have outlined three of the most powerful and promising areas of technology innovation for diagnostics. One particular advantage of all three is that, as cross-cutting technologies, they can address the situation across many problem areas. Additionally, and crucially, these areas address the greatest need, namely provision of diagnostics at the last mile, for which diagnostics are largely absent. These innovative solutions might be capable of providing the transforming power needed for the delivery of universal health coverage and attaining the Sustainable Development Goals. Therefore, we recommend that countries should, as part of any national strategic plan for diagnostics, particularly focus on innovations in digitalisation, democratisation, and point-of-care diagnostics. These innovations provide excellent opportunities for those countries with less well-developed diagnostics systems. However, technology innovations on their own cannot, and will not, be sufficient to address the many challenges to providing good access to diagnostics globally.236 This provision also requires changes in policy, governance, finance, and in removing operational barriers.
Solutions: removing operational barriers
We described the various barriers at the operation level that result in little to no access to diagnostics for at least 47% of the global population. We propose approaches to each of the main components—infrastructure, workforce, and quality and safety— which will address the issues and close this inequitable gap.
Infrastructure
The gaps in infrastructure needed to support diagnostics cannot be solved by piecemeal approaches. Countries will need systemic solutions. Not only will this approach be more efficient, it will also be more affordable because solutions can be standardised across many sites and lead, through volume purchasing, to better pricing. Standardised approaches would also permit tiered and integrated diagnostic networks to function more smoothly and efficiently with the use of standardised equipment and technical support, improved supply chains and operating procedures, and digitalisation. As part of developing these standardised approaches, countries and health systems need to develop comprehensive, long-term systems of finance to plan, build, and sustain the infrastructure needed to provide diagnostics. Smarter purchasing methods could help to make infrastructure more affordable.
More favourable infrastructure prices could result from reducing the domination of the diagnostics and imaging markets by a few firms based in HICs (Figure 5, Figure 6). Incentivising new entry into this sector by firms based in LMICs is a possibility, and international agencies, such as the Global Fund, and non-profit organisations, such as the Foundation for Innovative New Diagnostics (FIND), are playing a role initiating this change. Countries can also borrow techniques from the private sector, such as online procurement and tendering, to improve their supply chain (eg, implementing technology and reviewing procedures regularly).
Managed Equipment Services partnerships are being used in some countries. Under Managed Equipment Services, firms enter into agreements with health-care organisations to cover their needs over a multi-year period via equipment leasing and provision of training. Whether applied to imaging, laboratory, or intensive care equipment, the general framework is the same, in that these are performance-based contracts, which demand that the instruments are functioning 98% of the time. This mechanism has been used in several HICs and was used in Zambia to supply equipment to 71 hospitals over 12 years,237 and in Kenya more recently since 2014 to supply equipment to county hospitals.238 Although the management of hospitals has been decentralised to the counties, for the assurance of quality service, the Kenyan Government adheres to a capitated fee arrangement (ie, the Ministry of Health pays X amount per patient per quarter), which improves the facilities' ability to forecast costs. The result is that individual facilities now have access to advanced digital technologies, and the capacity to transmit images for external review. The Ministry of Health is kept aware of the functionality, rate of use, and application of all machines in all facilities. The Managed Equipment Services model ensures that governments are finally dealing directly with vendors as opposed to aid agencies or third-party re-sellers motivated solely by increasing their sales volume. Vendors are motivated to excel in their service provision, because they correctly see LMICs as emerging markets, and because not doing so would adversely affect their reputations.
The Managed Equipment Services model also has downsides. Although the government gets a better price by negotiating with a single supplier, there are risks of over-reliance on this supplier over the long-term and awarding big tenders can be associated with corruption because of the high stakes involved. This model tends to entrench the hold of existing major equipment suppliers in the market while new entrants might find it hard to compete in large-scale tenders, making it harder for new suppliers from LMICs to establish themselves. Writing the contracts for large tenders requires considerable expertise; in Kenya, this process required an external legal advisory firm and an external accounting and consulting firm to help draft the contracts.236 Even if Managed Equipment Services contracts include training of those operating the equipment, there remains a need for training of clinicians as to how to interpret images and incorporate them into clinical practice, which is a national responsibility. Lack of training has meant that equipment is underused in some counties in Kenya.
Public–private partnerships have also been used, whereby the private sector incurs the capital costs and then operates medical facilities and, depending on the local circumstances, receives payment from private individuals, private insurers, or from the national health insurance; for example, in Ghana, this type of partnership has been used.239 Brazil is another example where public–private partnerships in health care have been widely used.240 In Zimbabwe and Zambia, public laboratory facilities are underfinanced. A substantial amount of medical laboratory testing goes through the private laboratories and there are a couple of well established private laboratory chains operating internationally accredited laboratories in southern and eastern regions of Africa.241 A successful public–private partnership in Moldova brought access to DI services to 100 000 people annually.242 Another public–private partnerships model (ie, Initiative for Promoting Affordable and Quality Tuberculosis Tests) in India reduced the price of WHO-endorsed tuberculosis tests by nearly 50%,243 and the model is now being replicated in Pakistan and the Philippines.
Although public–private partnerships might have benefits for access and quality, there are equity issues. If, as often happens, the reimbursement from the national health insurance is delayed or the reimbursement rate for tests is too low to cover the full cost of tests, the tests will not be accessible, and only wealthier households will be able to pay out of pocket for diagnostic investigations. With public–private partnerships, the key issue is to design the incentives very carefully, such that there are no unexpected adverse outcomes.
Irrespective of the investment mechanisms used to address gaps in diagnostic infrastructure, solving infrastructure problems in different health-care settings should be a component of national strategic diagnostic plans and should be tailored to local contexts.244 Assessments of the current states of different infrastructure components, combined with clearly defined target states, would allow for realistic planning, budgeting, and implementation. When aligned with other parts of a national diagnostic plan, this planning would allow for acquisition and deployment of affordable, clinically appropriate and resource-appropriate technology.
Expansion of the global diagnostics workforce capacity
Given the size of the shortfall, and the cost, required infrastructure, and length of time needed to educate and train a global workforce, using existing models of education and training is unlikely to succeed in expanding the global diagnostics workforce in any reasonable time frame. To do so, novel approaches are needed in education and training: use of telehealth and AI; redistribution of health-care tasks (ie, task shifting); and changes in clinical practice and service delivery. In combination, these approaches will increase both the size as well as the efficiency of the existing and future diagnostics workforce.
In order to expand the global diagnostic workforce in a timely manner, fundamental shifts in traditional approaches to education and training will be necessary:245 increased use of remote learning opportunities, which have the potential to provide access to education and training in areas where traditional learning is unavailable; modification of curricula so they are driven by development of competency-based skills reflecting the local health and health-care context, built on a set of core competencies that are applicable in all settings;245 emphasis on education and training programmes that prepare individuals for lifelong learning and, in particular, provide the ability to quickly adapt to new technologies as they emerge; establishment of robust and adaptable Continuing Professional Development programmes developed to help individuals continue and build on their education, training, and experience and to provide opportunities for skill enhancement; use of group learning as an effective way of educating and training professionals and promoting teamwork;245 and implementation of regulatory systems to oversee and monitor the effectiveness of these new models of education and training, to ensure quality care.
Although the scope and scale of new education and training systems intended to meet the needs for future diagnostic capacity would vary from country to country, there is precedent for the concept of progressive scaling up of DI training in Myanmar (panel 7 ).
Panel 7. The story of radiology training in Myanmar—progressive scaling up of training that is fit for purpose.
Yangon General Hospital (then known as Rangoon General Hospital) in Yangon, Myanmar, was opened in 1911,246 equipped with the latest modern medical facilities of its time, including operating theatres. It was not until after World War 2 that a Radiology Department headed by Dr Tha Din was recorded. Dr Kyaw Aung, a young medical graduate, was sent to the UK to train, and when he obtained the Diploma in Medical Radio Diagnosis, he returned to Yangon General Hospital in the mid-1950s and assumed headship of the department.247 The few radiologists who followed continued to be trained in the UK or Canada.247
In the late 1960s, a School of Radiology was set up in Yangon and, from 1972, a summative examination called the Diploma in Medical Radio Diagnosis was introduced. Training for a radiologist took 2 years, with the first 6 months spent on radiation physics and radiographic anatomy. The remaining 18 months were spent on training in general radiology and radiography, fluoroscopy, and conventional angiography. The training later incorporated conventional CT and MRI as they became available. The intake for trainees was once every 2 years (Lin TT, Past Head, Department of Radiology, Yangon General Hospital, and Past President, Myanmar Radiological Society, Yangon, Myanmar, personal communication).
In 1992, the Master of Medical Sciences degree in Radiology was established but the training continued to be for 2 years, although the intake increased to annually (Lin TT, Past Head, Department of Radiology, Yangon General Hospital, and Past President, Myanmar Radiological Society, Yangon, Myanmar, personal communication). The curriculum was progressively expanded with the introduction of spiral CT and digital angiography. As a result, in 2014, the Master of Medical Sciences degree in Radiology course became 3 years long to fit in this expanded curriculum. With the advancement of imaging technology and the increased access to imaging equipment in Myanmar, this course had increased to become a 4 year course in 2018. Currently, with this programme, approximately 90 radiologists are trained every year. There are presently more than 650 active radiologists, serving a population of 54 million (Lin TT, Past Head, Department of Radiology, Yangon General Hospital and Past President, Myanmar Radiological Society, Yangon, Myanmar, personal communication).
The story of how the training of radiologists evolved in Myanmar over the past 50 years can be a template for other low-income and middle-income countries. At every stage, the training was designed to train the radiologist to be fit for purpose, based on the available imaging modalities within the health system. As more advanced imaging technology became accessible, training was then ramped up progressively, both in duration and in the number of radiology trainees.
Telehealth can mitigate workforce deficiencies in a variety of ways. This service allows for rapid professional consultation and better patient care, and technology can be incorporated into educational systems with the goal of providing Continuing Professional Development programmes to areas where they are otherwise unavailable. Furthermore, access to integrated and tiered diagnostic networks through telehealth leverages existing workforce capacity for more timely referrals, and access to such networks could also improve recruitment and retention in rural areas, resulting in an upward spiral of care for the community. Lastly, telehealth allows for optimal use of resources that otherwise might be used at less than full capacity.
Although some critics argue that centralised diagnostic services will lead to brain drain and lack of capacity building in remote areas, the potential benefits outweigh these points.
In DI, advances in equipment and information technology, especially the picture archiving and communication system, have propelled teleradiology as the foundation for remote imaging support all around the world. Teleradiology services have been used for years to support after-hours imaging for sites that contract with radiologists who live in a different, suitable time zone to interpret studies with rapid turnaround times. In recent years, after-hours teleradiology services have become a standard of practice for many DI groups, both private and academic. Teleradiology services are now both ubiquitous and expected.248, 249
Teleradiology has proven to be a valuable tool to mitigate workforce shortages in LMICs. The World Federation of Paediatric Imaging has supported remote expert interpretation of paediatric x-rays from underserved locations for almost 10 years. Médecins Sans Frontières started a telemedicine platform in 2010, with the first DI case sent in June, 2011. Teleradiology programmes have shown important intermediate patient outcomes.250
In contrast with teleradiology, which has had widespread use of digital-based interpretation for more than a decade, telepathology is only now emerging as a common tool for routine clinical practice due to the more recent emergence of commercial digital systems.183, 251 Telepathology has the potential to expand access to laboratory diagnostics much in the same way as teleradiology already has done.
More widespread use of AI technologies, particularly clinical decision support, will cause shifts in the roles of diagnostic workers in both PALM and DI and has the potential to expand the effective workforce capacity, helping mitigate projected shortages in the absolute number of workers in global diagnostics.252, 253
Task shifting has been defined as “a process whereby specific tasks are moved, where appropriate, to health workers with shorter training and fewer qualifications. By reorganising the workforce in this way, task shifting can make more efficient use of existing human resources and ease bottlenecks in service delivery. Where further additional human resources are needed, task shifting may also involve the delegation of some clearly delineated tasks to newly created cadres of health workers who receive specific, competency-based training”.254
Task shifting of diagnostic image interpretation and reporting from radiologists to radiographers has been used since the 1990s, primarily in the form of annotating or reporting appendicular skeletal x-rays from emergency departments in countries such as the UK and Australia, with acceptable accuracy and fast turnaround times.255 Role extension of radiographers also is being tested and is taking new directions in LMICs. In Ghana, radiographer-led reporting is thought to be the professional practice most likely to deliver local patient benefit, and there is evidence that radiographers can report select radiographs as accurately as radiologists.256 Senior radiographers reporting appendicular skeletal trauma radiographs in a South African hospital achieved higher reporting accuracy and sensitivity than medical officers.255 Task shifting for breast ultrasound has been initiated as an alternative to mammography in low-resource areas and is considered an appropriate option considering the absence of infrastructure for mammography programmes.257, 258 WHO recommends at least one ultrasound during pregnancy and notes that the intervention can be task shifted from sonographers or physicians to trained nurses, midwives, and clinical officers, provided that on-going training, staff retention, quality improvement, and supervision are ensured.31, 254
Task shifting also could have a greater role in PALM as countries move towards tiered and integrated diagnostic networks. The base tier, in which patient care and diagnostic investigations occur in the community or outpatient clinic setting, will require a limited set of diagnostic investigations with rapid turnaround time so that test results can be used to guide treatment during that visit. This point-of-care testing often uses technologically straightforward tests, such as urinalysis, that do not require the expertise of trained laboratory personnel. Robust systems for quality oversight are needed, considering that most people doing the tests are not fully trained laboratory personnel and most point-of-care tests are less accurate compared with tests done by large analysers in clinical laboratories. Pre-analytic variables, such as self-collection or sample size, can cause pre-analytic errors. Despite those challenges, shifting the task of basic laboratory testing to the clinic level has been done successfully for several tests and can result in amplification of the diagnostic workforce without the need for additional personnel in many settings.259
However, there is not full support for task shifting in every setting. In China, for example, despite the majority of health workers having positive attitudes towards task shifting, numerous barriers to implementation included financial support, perceived heavy workloads, low wages, and high turnover of providers.260 In the Philippines, a survey showed that delegating HIV counselling and testing to lower level health workers was feasible but not acceptable due to inadequate skills and patient perceptions.261 Moreover, in many countries, it is not clear that there are sufficient numbers of other people to whom tasks could be shifted or shared. Without excess capacity among those people, task shifting or sharing could have the unintended consequence of reducing the time available for other equally important activities and, by adding job complexity and increasing workload, decrease work quality.261, 262 Furthermore, merely shifting emphasis away from one health-care activity to another will probably have adverse effects on other parts of health systems that are already strained to meet existing demands.263 However, when implemented along with other changes in health systems, including proper training and supervision (and certification where appropriate), financial remuneration, access to the diagnostic equipment and supplies necessary to do jobs adequately, physician oversight and support, and effective management, task shifting has the potential to reduce costs and improve the efficiency of health systems.262, 264, 265, 266, 267
To maximise professional satisfaction, provide optimal patient care, and make health systems efficient, individuals need to work at the top of their licence—make full use of their educational background, skill set, and experience.266, 268 However, there is wide recognition that there is a mismatch between the skills of many health-care workers and what they do in their roles in health systems.269 This mismatch includes both over-skilled workers (which wastes their education, training, and experience) and under-skilled workers (which creates risks to quality of care and patient safety).269 In one analysis of OECD countries, “around 70% of doctors and 80% of nurses reported being overskilled for some aspects of their work, while about 50% of doctors and 40% of nurses reported being underskilled for other tasks”.269 In reality, many practitioners in LMICs actually practice at the bottom of their licence and in very limited scopes of practice: specialists end up reading plain films instead of MRI scans, pathologists have access only to basic histology, and laboratorians provide only rudimentary laboratory tests. Solving this problem will require the development of fully functional health systems, including integrated diagnostic systems that are well resourced and align diagnostic roles with local health-care needs so that individuals can make full use of their skills. Minimal improvements in service delivery or health outcomes will be achieved by expanding global diagnostic workforce capacity if the workforce cannot work to its fullest ability. Allowing the workforce to work to its fullest ability also is important for “promoting greater return on the substantial investment of time and money in educating and training health professionals”.269 This change is also likely to improve staff retention by increasing job satisfaction.
Because of the lengthy time horizon and large investments required to develop and scale solutions to increase global diagnostic workforce capacity, sustained commitment will be crucial. Country involvement will be needed to align local needs with potential solutions and educational curricula. In many countries, ministries of health and education have overlapping jurisdictions over educational and training systems for health care, and ministries of finance allocate funding. Long-term agreement and coordination among these bodies will be essential for strategic alignment and to develop sustainable funding for education and training systems. Furthermore, multinational involvement will be vital, considering that such organisations could endorse actions such as development and reporting of an updated WHO framework for scaling workforce capacity. Additionally, the 2006 WHO document remains useful but does not include a description for adapting to rapid changes in technology.270 In addition to the points made in the 2006 WHO document, WHO could: require member states to report country-specific workforce data on a periodic basis; create a forum for public and private partners to develop recommendations for staffing models and to help guide the development of education and training systems; and encourage development of common education and training curricula and standards to facilitate rapid deployment to countries as is appropriate.
Quality and safety
It is counterproductive to expand access to low-quality diagnostics. Not only does this have the potential of harming patients, practitioners can lose confidence and discontinue use of what they perceive to be of low value in clinical care.13, 17, 271, 272 The use of low-quality diagnostics also results in waste of resources, which is a concern particularly in low-resource settings.
For PALM, exporting systems for standards and accreditation systems from HICs to LMICs is impractical due to their complexity and cost. For LMICs to have access to these systems, they need national strategic plans for diagnostics that include systems for standards and accreditation as part of their regulatory frameworks. These frameworks need to be adaptable to local needs, especially to reflect the different circumstances between urban and rural health systems. Development of country-specific and resource-specific External Quality Assurance programmes that can be implemented and maintained in low-resource settings should be a priority. An example of how these programmes can be developed is that of China's system of medical laboratory accreditation (panel 8 ).
Panel 8. Evolution of External Quality Assurance programmes and accreditation for medical laboratories in mainland China—a focus on clinical pathology.
China's system of medical laboratory accreditation has evolved considerably over the past 40 years. In 1981, when the National Centre for Clinical Laboratories was founded, medical laboratories had few tests, reagents, and professional staff, as well as suboptimal management methods.273 The National Centre for Clinical Laboratories mandate includes organising External Quality Assurance, developing reference methodology for tests (mainly related to ISO 15195), and External Quality Assurance programmes provided by the National Centre for Clinical Laboratories, in which more than 6000 medical laboratories participate;274 many provincial-level clinical laboratory centres also develop External Quality Assurance programmes with an even larger number of participants. Stringent laboratory assessment, including satisfactory performance in national External Quality Assurance programmes, is an integral requirement for accreditation of public grade 3 (ie, the higher level) hospitals, and grade 2 hospitals must similarly participate in the provincial programmes. 275, 276 Participation of private laboratories in External Quality Assurance programmes is encouraged everywhere and is compulsory in some provinces.277, 278, 279, 280
What is now called the China National Accreditation Service for Conformity Assessment was established in 1994 and joined the International Laboratory Accreditation Cooperation in 1996. The first international accreditation of a medical laboratory in China was received in 2003 under ISO/IEC 17025, which covers industrial laboratories,281 and the first international accreditation of a hospital laboratory was received in 2005 under ISO 15189, which applies to medical laboratories.282 In 2006, CNAS-CL02 was issued as the official document for medical laboratory accreditation, which is updated continually according to the latest update of ISO 15189.283 That same year, the National Centre for Clinical Laboratories Regulation for Management of Medical Laboratories was approved. This regulation is compulsory for all medical laboratories; however, the requirements are more basic than those of laboratory accreditation.284 In 2015, the China National Health Commission issued 15 quality indicators for clinical laboratory medicine that cover the pre-analytical, analytical, and post-analytical phases of testing.285
As of April, 2020, 431 medical laboratories had received ISO 15189 accreditation, primarily clinical pathology laboratories in tertiary-level hospitals located in cities.286 An impressive rising trend is observed, yet there are major regional differences: a substantial proportion of accredited laboratories are located in the relatively more affluent and populated major cities and provinces in eastern China. A similar pattern is also observed among the 71 laboratories in China that are accredited by the College of American Pathologists.
China's example shows that it is possible to make substantial progress on quality assurance and accreditation but also that it takes time to extend this effort across a large country.
For DI, programmes need to be developed that ensure regular and systematic review of all diagnostic processes, procedures, and safety standards necessary to provide quality services. For example, quality control testing on all equipment should be regularly done and radiation, MRI, and ultrasound safety programmes should be guided by national and international regulatory standards. Interpreting providers should undergo a regular comprehensive clinical audit, or peer review, to check performance against standards. Executing this highly matrixed systemic approach to quality is costly in terms of financial and human resources.287
Systems to maintain quality in DI and PALM are most similar at the stage of professional interpretation by radiologists and pathologists (ie, professional competency). In order to assure quality at this stage, regulatory frameworks will need to mandate how health-care organisations and facilities verify the education, training, and certification of professionals, as well as how professionals are assessed for competence on an ongoing basis through participation in Continuing Professional Development programmes, through their professional organisations, or other mechanisms. As the use of digital diagnostic systems becomes more widespread and teleradiology or telepathology are used for diagnosis, the quality of images, adequate transmission of images, turnaround time, patient confidentiality, and availability of relevant previous images are paramount. As digital diagnostic systems become more integrated with AI systems, particularly for providing primary diagnosis, it will be imperative that this integration be done in conjunction with quality systems designed to ensure accurate interpretation.
The novel approaches outlined address some of the operational barriers to expanding access to diagnostics. For infrastructure, there are some possible options for innovations in purchasing. For workforce, we recommend major changes in content and delivery of curricula to provide staff who are more flexible and digitally literate. This recommended change, along with telepathology, teleradiology, AI, and task shifting, could mitigate workforce shortages. For quality and safety, we recommend that countries should develop appropriate regulatory frameworks to ensure both professional competence and quality assurance and control of equipment, particularly as use of digital diagnostic systems grows and becomes more closely linked to AI systems for interpretation.
Solutions: improving policy, governance, and financing
Governance and financing are the two key underpinning factors in the WHO building blocks for health systems. We take up solutions to some of the key impediments relating to the current status of governance, and then examine ways to increase financing for diagnostics. However, to address the low visibility as the key cause of diagnostics under-resourcing, we first address the need for a powerful advocacy programme.
Advocacy
The crucial role of diagnostics in good health care is under-recognised and under-resourced. The inclusion of universal health coverage in the Sustainable Development Goals provides an opportunity to highlight the importance of diagnostics, and the COVID-19 pandemic has made the topic top of mind. An advocacy programme directed at national governments to develop, fund, and implement national strategic diagnostic plans is, therefore, vital. All levels need to be involved: national governments, diagnostics professionals, clinicians (especially those who currently use presumptive treatment, due to limited access to quality diagnostics), patients, and the public.
Marshalling this effort globally over a long period of time would be best served by the creation of an international partnership model involving the national advocacy groups, other interested parties, and a global coordinating body. There are many examples of such an international partnership model, including Scaling Up Nutrition, Nursing Now, the Global Fund, and Gavi, the Vaccine Alliance. The coordinating body can help to provide leadership and continuity, advocate both by itself, but also facilitate advocacy by others, foster transformative change, provide advice to governments and other relevant bodies, build partnerships for market shaping, collect accurate data to underpin the case, monitor progress, raise necessary funding, and more.
One such multi-stakeholder partnership with diagnostics for COVID-19 as one its four pillars, was launched in April, 2020, the Access to COVID-19 Tools.288 However, what is needed is a broad Diagnostics Alliance, covering both infectious and non-infectious diseases and ensuring that diagnostics are appropriately incorporated into, for example, universal health coverage programmes. Such an alliance would not conflict with organisations such as WHO, which have a responsibility for the totality of health, but rather, by focusing on the issues relevant to diagnostics, can inform their decisions. Several calls for such a broad Diagnostics Alliance have been made in the past few years by academics,289 and in response a new partnership between FIND and WHO was created in 2020.290 An early objective should be the adoption by the World Health Assembly of a resolution on the need for countries to develop a funded strategic plan for diagnostics.
Using governance to make diagnostics more accessible and available
There is a scarcity of national strategic plans for diagnostics. Clearly in the absence of such a planning framework, it is difficult to ensure comprehensive national diagnostics provision. Such a strategy is essential and should cover all the required elements, including workforce, education, infrastructure, supply chain, and regulation. The strategy should also incorporate an EDL. We envisage that an evidence-based, integrated, and tiered network for diagnostics would form the fundamental basis of such a plan, consisting of a network of laboratories or radiology departments, distributed across various tiers of hospitals and primary care, and running services as an integrated whole. The network would share patients, staff, equipment, supply chain, purchasing, communications, education, quality control, and specimen transport, and could be at national or regional level.
Such a network has been widely recognised as an efficient and effective model for the delivery of care in several specialties, including PALM (Horton S, unpublished), surgery,291 cancer,292 and primary care.293 Among the advantages are continuity of care and improved patient experience, interoperable systems, reduced variance, and sharing of resources, knowledge, capacity building, and advocacy. A key element of the network should be the adoption of innovations, such as digitalisation, point-of-care diagnostics, and democratisation, to address the crucial last-mile gap. However, having a plan is not enough. The national strategic plans need to be appropriately funded to allow for sustained and effective implementation.
Using governance to make diagnostics safer
Having a national regulatory framework is an essential precondition for making diagnostic devices safer. However, if national frameworks are too differentiated, then it makes it harder for manufacturers to conform to multiple formulations and requirements. Two main solutions are to rely on international recognised authorities to inform national decisions or to use regional harmonisation among countries with broadly similar needs. Among the HICs, mutual recognition agreements have led to the US Food and Drug Administration and the European Medicines Agency Conformité Européenne mark as indicators of quality and safety of medical devices.294 For LMICs, WHO prequalification has often been used.
Prequalification by WHO is important because LMICs have different diagnostic and medicine needs from the USA and European countries, due both to major differences in epidemiology and resource availability.295 Increasing funding for this WHO function would permit faster processing and expanding of the focus of prequalification beyond infectious diseases and medicines that are essential for reproductive health. Prequalification could also be expanded to cover diagnostics for bacterial and fungal culture, which would be helpful for antimicrobial susceptibility testing. In areas for which there are no prequalified diagnostics, LMICs have sometimes relied on purchasing decisions by other international agencies, such as UNICEF or the Global Fund, to advise national purchasing selection decisions.
The second main regulatory option has been to encourage regional harmonisation of regulations to simplify the process of supplying diagnostics, and to use pooled expertise to improve quality and safety. Similar to the efforts for medicines, there are various regional organisations for harmonisation in diagnostics, including the International Medical Device Regulators Forum and the Asian Harmonisation Working Party. The International Medical Device Regulators Forum has ten members, mainly HICs with the addition of Brazil and China, whereas the Asian Harmonisation Working Party has 22 members. Although Latin America and Africa have harmonisation initiatives for medicines, they are not yet well established for medical devices. In Africa, the harmonisation effort even for medicines has been somewhat slow to produce successful results.296 Latin America has longer experience in this area and has been able via the Pan American Health Organization to negotiate group prices for some vaccines.
There are four key areas in which harmonisation for medical devices would be valuable, including a common submission dossier for new products (the EU being the only successful example of this so far), convergence in the auditing of quality systems, reducing duplication in studies of clinical effectiveness (ie, being prepared to recognise trials in another country with similar demographic and epidemiological characteristics), and participating in networks of information on post-market surveillance; for example, using regional networks of accredited laboratories and imaging departments.297 It is probable that some combination of better funding for the WHO prequalification process, combined with steady efforts at regional harmonisation, could be beneficial. Progress on this agenda could be facilitated by a Diagnostics Alliance.
Technical assistance in designing appropriate regulations is needed, from technical agencies such as WHO. An example of a country that has experience in strengthening the governance framework for diagnostics is India (panel 9 ).
Panel 9. Strengthening governance around diagnostics—India's experience.
Over the past decade, the Government of India has taken steps to give diagnostics a suitable place in the health system. Under the National Health Mission, India launched the Free Diagnostic Services Initiative programme in 2015 to improve the overall quality of health care and patient experience by ensuring availability of a minimum set of diagnostics.298 One immediate aim of this programme is to screen patients for non-communicable diseases and to enable secondary prevention measures. A longer term aim is to reduce out-of-pocket expenditure. The Free Diagnostic Initiative has been taken up by almost all states in India and, using the national essential diagnostics list, will help standardise implementation of the ongoing state-level initiatives, with corresponding operational advantages.
Capacity to undertake diagnostic testing at different levels of health care requires resources, trained staff, and investment to strengthen the laboratories. The Indian Public Health Standards provide the benchmarks for public health care infrastructure planning and upgrading.299 The Indian Public Health Standards are revised every few years to incorporate the latest needs and requirements and are currently being revised to accommodate the requirements of the Free Diagnostic Initiative.
India also has a large number of private diagnostic laboratories (offering pathology and laboratory tests or diagnostic imaging services, or both) that cater to a sizable proportion of the Indian population. The scarcity of regulation in the private sector resulted in deficiencies in service quality and a proliferation of laboratories not following standard protocols, leading not only to compromised patient safety but also problems of accountability in health-care costs. To standardise the health-care services, the Government of India enacted the Clinical Establishments (Registration and Regulation) Act, 2010, which makes registration compulsory for running a clinical establishment, including a diagnostic laboratory. The minimum standards for medical diagnostics laboratories have been revised further in the Clinical Establishments (Central Government) Amendment Rules, 2018.300 The latest amendment specifies the minimum standards of facilities and services for all clinical establishments relating to diagnosis or treatment of diseases, dealing with pathological, bacteriological, genetic, radiological, chemical, biological, or other diagnostic or investigative services.
In the absence of a stringent regulatory process for diagnostics in the past, poor-quality diagnostic tests made their way into the Indian market. To circumvent the issue of poor-quality tests, the Government of India introduced the Medical Devices Rules in 2017,301 and laboratory tests and diagnostic imaging devices are now regulated under this new rule, including risk classification for laboratory tests. Most laboratory tests now require approval for use in India, as well as import licences and performance evaluations, to receive approval for marketing. The new rules are well defined and are intended to boost manufacturing capabilities, reduce import product dependence, and increase market size.
Distinct from devices, practice recommendations and practice guidelines for appropriate use of diagnostics are another aspect of governance involving health professionals and are important to guard against both under-testing and over-testing. Recommendations and guidelines might be voluntary (eg, established by the medical profession to improve practice) or mandatory (eg, to achieve accreditation, or to avoid litigation, or to receive reimbursement from insurance). Many hospitals worldwide have stewardship committees to monitor the use of laboratories and the more expensive imaging devices. The Choosing Wisely campaign aims to promote discussion between patients and health professionals to avoid unnecessary tests and treatments, but so far is active primarily in HICs.
Using governance to make diagnostics more affordable
National governments might be able to make diagnostics more accessible by focusing on a national EDL that helps to prioritise resources. India has developed the first such national list,302 building on the WHO's list that was issued first in 2018 (panel 10 ). National governments also increase affordability of diagnostics by borrowing aspects of the organisational mindset from the private sector. South Africa's National Health Laboratory Service has a very large capacity for HIV viral load testing (10 million assays per year)303 and, due to efficiency and volume, achieves one of the lowest prices in the world for purchasing these tests. One technique the National Health Laboratory Service uses for efficiency is to require supplier registration and to offer framework agreements (tenders) for periods of up to 5 years. Kenya used a similar tendering system to upgrade imaging systems across 47 counties,304 which can help to get better maintenance and training than if counties used equipment from different suppliers. However, with long-term tenders it is important to rotate preferred suppliers periodically to retain some competition among suppliers. This is similar to Managed Equipment Supply.
Panel 10. The making of India's national essential diagnostics list (EDL).
The Indian Council of Medical Research, the nodal body for medical research in India, steered and coordinated the process of building a national consensus on a national EDL. The national burden of disease and endemicity of diseases like dengue, cholera, and Japanese encephalitis virus infection were taken into consideration while drafting the list. Considering that the feasibility of conducting tests at every level depends on adequate logistic support, the equipment and human resources which were being provided at different levels of health care by the Indian Public Health Standards were referred to before recommending the diagnostic tests for each level of health care in the public sector. Stakeholders (including all the programme managers for HIV, tuberculosis, malaria, and dengue programmes, to name a few, experts from the Free Diagnostic Initiative, National Health Systems Resource Centre, New Delhi, India) and independent experts (including clinicians, microbiologists, pathologists, and radiologists) were part of the consultative decision making process. A key innovation was the inclusion of diagnostic imaging in India's national EDL, which differs from the WHO EDL.
The first draft of the national EDL was posted on the Indian Council of Medical Research website for public comments in December, 2019. The responses received were examined by an expert group and all suggestions were discussed for their merit. The suggestions that qualified the requirements of the EDL were included in the final EDL list, which was released in August, 2020. The implementation of the EDL list is the responsibility of the Ministry of Health. The Free Diagnostic Initiative is the programme under the National Health Mission to roll out the diagnostic programme and was part of all the discussions, and subsequently adopted all the tests proposed in the national EDL.
Recommendations for countries wanting to have their own national EDL:
-
•
Understand the burden of disease in the country and local needs like endemicity of any region-specific diseases (eg, malaria, dengue, and chikungunya).
-
•
Assess the current availability and existing programmes to deliver diagnostics, and willingness by the government to support diagnostics.
-
•
Initiate discussions at different fora around the importance of making diagnostics an integral part of health care.
-
•
Identify champions for diagnostics within the country and seek their help in initiating discussion and mobilising the process.
-
•
Finalisation of the national EDL list should be a participatory process, in which concerns of most stakeholders should be taken into consideration. It is not possible to please everyone.
-
•
Build a case for having a comprehensive system for delivering diagnostics rather than one with silos and present it to the Ministry of Health. Suggest how having single laboratories for all programmes will lead to shared equipment and human resources, thereby saving resources.
-
•
Push for a regulatory framework to ensure that only quality diagnostics are registered for sale in the country.
-
•
For sustainability, have government funding committed to this effort and do not initiate this with donor funding alone.
This same emphasis on strategic use of procurement is used by international organisations such as the Pan American Health Organization, UNICEF, and the United States Agency for International Development, and many of the organisations established in 2000 onwards (eg, Clinton Health Access Initiative, the Global Fund, Unitaid, and Gavi, the Vaccine Alliance, all of which purchase diagnostics). These organisations use pooled procurement (one component of a so-called market-shaping strategy), combining purchasing power across multiple countries and multiple years to leverage better prices. The Global Fund, for example, also uses other mechanisms, such as long-term framework agreements with suppliers and an online procurement tool, Wambo.305 Of the $821·6 million in products procured through the Global Fund's pooled procurement mechanism in 2018, 13% was for diagnostics. Empirical studies suggest that pooled procurement has indeed been associated with declines in price and improvements in quality of malaria rapid diagnostic tests, as well as reductions in the price of malaria treatments and insecticide-treated nets.306 One issue with such mechanisms is that when countries graduate from eligibility to accessing the pooled procurement, they face price increases. Although graduation is typically associated with increased income, there can remain pockets of vulnerable groups that lose access when prices increase. Individual countries have also used such market-shaping policies to make diagnostics more affordable, such as the Indian Initiative for Promoting Affordable and Quality Tuberculosis Tests involving tuberculosis diagnostics.307
Other international organisations have focused on the innovation space to increase affordability. International organisations, such as FIND and the Coalition for Epidemic Preparedness Innovations, can help smaller manufacturers, often in LMICs, to bring products to market. A recent example of local production is the partnership between the Pasteur Institute of Dakar and a British biotechnology company with support from the UK and the Bill & Melinda Gates Foundation, which aims to produce inexpensive COVID-19 point-of-care tests in Africa.308
Although market-based solutions are a viable option, some other organisations eschew the focus on market mechanisms, in favour of a more equity-focused approach. Existing efforts have been primarily directed at medicines; however, these efforts can equally apply to diagnostics. Several organisations use a rights-based approach rather than incremental change. Médecins San Frontières' Access Campaign website states “[w]hat we as a civil society movement demand is change, not charity”.309 The Ecumenical Pharmaceutical Network's vision is “[a] valued global partner for just compassionate quality pharmaceutical services for all”.310 The Treatment Action Group was key in the movement to getting equitable pricing for antiretrovirals for HIV/AIDS and, more recently, has applied similar efforts to diagnostics, such as the Time for $5 campaign for GeneXpert cartridges.311
Experience with medicines suggests that some combination of all the tools we have addressed is needed to make diagnostics more affordable—ie, technical efforts at improving the supply chain, market shaping involving international organisations, and grassroots, rights-based advocacy.
When governance breaks down: diagnostics in fragile and conflict states and global health security
Diagnostics is a key part of global health security. Nearly a third of the world's population now live in fragile and conflict settings. There are now over 52 active conflicts in 36 different countries, which has led to over 70 million forcibly displaced people (refugees and internally displaced persons); the vast majority of these people have been displaced as a result of armed conflicts, political violence, and human rights violations.312, 313 This finding includes around 25·9 million refugees and stateless people who are living in fragile contexts in other countries. These dynamic and complex therapeutic geographies mean that diagnostic innovation needs to reflect more complex, challenging settings (eg, humanitarian camps, formal refugees, settled poor rural-based, urban-based, and internally displaced people) with very different health actors (such as military, private, public, community and hospital actors).314
Diagnostics are also crucial for pandemic preparedness and response. The COVID-19 pandemic has exposed fundamental deficits.315 In parallel, several infectious disease outbreaks are ongoing in the poorest parts of the world, with, at the time of writing, Ebola and monkeypox in the Republic of the Congo, cholera across eastern Africa, and Lassa fever in Nigeria.316 New and emerging threats of infectious diseases have moved to the centre of national security responses with diagnostics fast becoming a central part of defence and security doctrines, in which the security sector is having a growing role in biosecurity preparedness and response; for example, the US Department of Defense (Uniformed Services University and Veterans Affairs), the UK Ministry of Defence (Defence Medical Services and Defence Science and Technology Laboratory) and Totalförsvarets forskningsinstitut (Swedish Defence Agency—FOI). Learnings from security sector UN deployments (eg, South Sudan and Afghanistan) span not just rapid needs-driven technical developments (eg, the portable genome sequencer MinION for fever of unknown origin) but wider issues of procedures, processes, and scale-up.317 Beyond infectious diseases, point-of-care tests are increasingly required for rapid front-line point-of-injury stabilisation or treatment (eg, handheld focused assessment with sonography for trauma, also known as FAST).318
Global health security also encompasses a wider humanitarian–security sector interface. Diagnostics is a major part of humanitarian medicine, but it is fragmented and under-researched.319 From a range of interviews done with key opinion leaders, the humanitarian sector's focus on diagnostics in global health security broadly falls into two technical platforms: mini laboratories, or mini-labs, which are small-scale, transportable, affordable, autonomous laboratories), and multiplexing, in which a single specimen can be simultaneously tested for the presence of more than one pathogen. There is also considerable interest in the use of AI and mini-labs for electrocardiogram interpretation (to enable point-of-care heart failure diagnosis), and mobile technology device AI for ophthalmic differential diagnosis (eg, distinguishing cerebral malaria from diabetic retinopathy). Considerable work and insight has been put into task shifting, decentralised care, and certification (eg, compliance with International Organisation for Standardization standards) as a driver for diagnostic innovation.320
The COVID-19 pandemic, wider focus on global health security, and integration of humanitarian with security sector research agendas321 will drive new innovation and pathways to market. However, beyond urgent and complex demand-driven aspects of the ecosystems that global health security encompasses, there are currently few incentives to bring focused development and scale-up together. Furthermore, the COVID-19 pandemic has also exposed that diagnostic innovation alone will be insufficient to deliver impact. Equally crucial will be the command-led intelligence and operational systems in which they are embedded.322
Solutions will require additional resources. Initiating efforts now is of great importance, to increase funding for applied research and to develop diagnostics that can be rolled out in conflict and displacement situations. Innovative technologies exist that can be incorporated. Co-development with humanitarian staff and affected populations will ensure that what is developed is relevant and useful. Better coordination within the humanitarian sector is vital, involving international agencies and non-governmental organisations, and drawing on and adapting solutions developed from research by the security sector.
Financing
New sources of financing are needed to expand access to effective and affordable diagnostics in LMICs. In most countries, the largest proportion of funding for scaling up diagnostic capability will come from domestic sources, as part of country investments in meeting the Sustainable Development Goals' aim regarding universal health coverage. The sources are likely to be from public financing (government budget allocated to health) with complementary financing from the private sector, including private investments in new diagnostic capacity and private health insurance. Additionally, there will be external investments or, in the case of low-income countries, Official Development Assistance.
The level of public financing for any sector is determined by the so-called fiscal space available to the government, defined as “the availability of budgetary room that allows a government to provide resources for a desired purpose without any prejudice to the sustainability of a government's financial position”.323
Fiscal space depends on various sources of finances: economic growth that creates favourable macroeconomic conditions for increased government revenues and budget; strengthening tax administration; reprioritisation of health within the government budget; borrowing from domestic and international sources or development assistance to invest in health; more effective and efficient allocation of available health resources; and innovative domestic and international financing.324, 325
Economic growth is generally seen as an important source for finance for health, particularly as the share of health spending in national income tends to increase with income. However, given the pandemic and the negative effects on growth and poverty, this is unlikely to be a major source of finance for diagnostics in the very near future.
Strengthening tax collection and judicious increase in taxes can provide a potentially substantial source of government revenues to expand the fiscal space. In LMICs, government revenues from tax are low: on average, 15% of gross domestic product in low-income countries, 25% of gross domestic product in lower-middle-income countries, and 30% of gross domestic product in upper-middle-income countries, compared with 40% of gross domestic product in HICs.326 Modelling studies have estimated that an increase in tax revenue, in which at least one-third of newly raised revenues is allocated to health, could, on average, increase public expenditure on health in LMICs by 78%.327 Increased taxes on tobacco and alcohol, which are highly cost-effective public policies, also offer potentially substantial additional revenues for investing in the health system. Egypt, the Philippines, and Thailand have successfully used tobacco taxes to generate earmarked funding for the health sector.328 Modelling studies have estimated that, beyond health benefits, a 20% and 50% price increase in tobacco prices could generate over 50 years additional tax revenues of US$1987 billion (uncertainty interval: $1613 to $2297 billion) and $3625 billion (uncertainty interval: $2534 billion to $4599 billion), respectively, and in low-income countries an average of 0·17% additional revenue of gross domestic product each year in the 50% scenario.329
Demonstration of the health and economic benefits for health investments could provide the necessary evidence to persuade governments to shift budget priorities in favour of health. Modelling estimates that budget reprioritisation could potentially increase funds allocated to health by 72%.327
Concessional financing with low interest rates and generous grace periods for repayments could be mobilised from international development banks, to invest in health and expansion of diagnostics capacity. In 2017, the World Bank had 45 active projects for a total sum of $470 million for medical equipment procurement.330 In April, 2020, the World Bank announced a dedicated COVID-19 Fast-Track Facility, amounting to $1·9 billion to assist 25 LMICs, with a further $1·6 billion made available by June, 2020, for a further 75 LMICs. These projects aimed at strengthening preparedness and response to COVID-19 also include development of diagnostic and testing capacity,331 as does the African Health Diagnostic Platform launched in October, 2019.332 The European Investment Bank launched in May, 2020, €6 billion for investments in health systems for COVID-19, including €1·5 billion for companies that include diagnostics. A further €5 billion is for countries outside of the EU for medical infrastructure and research, or other vaccine-related and treatment-related financing to support health system infrastructure and to maintain access to finance for affected firms.333 Other regional development banks might be a possible financing source.
More effective and efficient allocation of available health resources in health systems could increase funding for diagnostics. WHO has estimated that, worldwide, around 20–40% of all health spending in health systems is not used efficiently or effectively.334 With priority setting, and more efficient allocation and use of resources, governments could generate a 26% increase in public expenditure on health.327
Innovative financing (ie, funding mobilised from non-traditional sources) is another potential source of funding for diagnostics. Innovative financing mechanisms, such as the Global Fund, Unitaid, and Gavi, the Vaccine Alliance (which link different elements of the financing value chain—namely, resource mobilisation, pooling, channelling, resource allocation, and implementation), have channelled more than $55 billion to LMICs for health.335 These mechanisms have used new innovative financing instruments, such as Airline Solidarity Levy, Advance Market Commitments, sovereign debt conversion, and targeted bonds to mobilise more than $12 billion in funding.336
Social Impact Bonds or Development Impact Bonds are other promising innovative financing instruments that could be used to finance expansion of diagnostics capability. A Social or Development Impact Bond is created by a government agency (or external funder, such as a development agency or a charitable foundation) that wishes to achieve a desired social or health outcome. The initiating agency engages an external organisation to achieve the outcome.337, 338 A third-party investor provides upfront working capital to the external organisation as an at-risk investment. If the desired social outcome is achieved, the initiating agency releases payment to the external organisation, based on terms specified in an upfront contract, which repays its investors their principal, plus a return on the investment. If the outcome is not met, the initiating agency disburses no payment. Development Impact Bonds are still relatively new and time-consuming to develop; however, one such Bond has helped to incentivise accreditation of small private health-care facilities providing antenatal and delivery care, and could work similarly for laboratory accreditation.339
There are scarce data on what countries need to spend on diagnostics. The Lancet Series on Pathology and Laboratory Medicine in Low-Income and Middle-Income Countries found data on spending on PALM in a range of countries (mainly HICs but including a couple of LMICs) was around 4% of total health expenditures (the range was 3–6%).77 Expenditures on imaging are harder to obtain and tend to vary more over time as diffusion of new technologies require large investments. Examining Medicare expenditures on physician-provided services to outpatients (Medicare is the largest single provider of care in the USA), the share of imaging was 21% in 2008 and declined gradually to 12% in 2017 due to legislation designed to curb these expenditures.340 The USA is, however, probably an outlier in the share of health spending on imaging. Data from the OECD for 2018 show that the USA provides substantially more CT examinations per 100 000 population than other OECD countries, often by a significant margin, and more MRI examinations (using the same metric) than all but two other OECD countries (appendix pp 11–13).341 It was not possible to find data on the share of imaging in total health expenditures in LMICs.
The economic case for investment
Although data are not easy to come by, diagnostic investigations provide excellent value for money. The COVID-19 pandemic provides an important illustration of this: a comparison of South Korea's early and rapid efforts in testing, combined with intensive contact tracing, enabled the country to be able to resume economic activity more quickly and suffer smaller economic losses than Italy, the UK, and the USA.342 In imaging, a recent microsimulation model of the 11 most common cancers showed that the scale-up of imaging diagnostics alone would avert 3·2% (2·46 million) of the 76·0 million projected cancer deaths worldwide between 2020 and 2030, saving 54·92 million life-years and yielding a net return of $179·19 per $1 invested.343
To show the benefit–cost ratio of diagnostics, we have analysed the benefit–cost ratios for basic tests for the six key tracer conditions (ie, for hypertension, type 2 diabetes, HIV, and tuberculosis in the overall population, and syphilis and hepatitis B virus infection in pregnant women), which were examined in the cascade-of-care analysis and the associated modelling of death and DALY losses previously. The benefit–cost estimates for the six tracer conditions complement 2020–21 work on imaging diagnostics for cancer, which shows the benefits of DI for improved overall survival as well as substantial return on investment.344, 345, 346
Calculating the benefit–cost ratio of diagnostics is not an easy task. A diagnostic test or examination per se does not usually directly affect the health outcome without some form of treatment. Additionally, much medical care involves the use of multiple tests repeated over a period of time, and the pre-test probability of testing heavily influences the interpretation of results. Thus, some tests confirm a particular diagnosis and indicate the appropriate treatment. Other tests rule out alternative diagnoses and rule out inappropriate treatment. Some tests monitor toxicity of the treatment itself. Moreover, some diagnostic tests are used for screening purposes in asymptomatic patients and only a fraction of these tests lead to treatment.
We make a few assumptions about the benefit–cost analysis. Costs include both the cost of the diagnostic test and the accompanying treatment. Estimated benefits include avoided direct medical costs as well as a monetary value assigned to the morbidity and mortality averted. Future costs and benefits are discounted at 3% and a DALY averted is evaluated at one time per capita gross domestic product. Existing disease models are used as far as possible. These models are more complex for infectious diseases because preventing transmission to others is an important benefit, and transmission depends on behaviour. Cascade data are used to make estimates of how many diagnostic tests are required to encounter one positive case, as well as the probabilities that a positive diagnosis leads to treatment, treatment is complied with, and the treatment is successful (where appropriate). Benefit–cost ratios for the same condition vary according to context, given that treatment costs, per capita income, and prevalence all vary. Hence, estimates are provided for countries at different income levels where possible, and important context information is provided to assist in interpreting these findings. Further details are shown in the appendix (pp 21–23).
The results of our benefit–cost analysis (table 5 , figure 10 ) show that benefit–cost ratios vary from less than one (ie, the benefits are worth less than the costs) to 24·4:1. Context is important in this case. Benefit–cost ratios tend to be higher if country income is higher (since morbidity and mortality averted are evaluated using per capita income), if disease prevalence is higher (a higher proportion of those tested need treatment), if treatment has a higher probability of success, if loss to follow-up is lower, and if diagnostic costs and preventive treatment costs are lower. In general, benefit–cost ratios for infectious disease are higher in low-income and lower-middle-income countries where infectious conditions are more prevalent, whereas those ratios for non-communicable disease increase as country income rises.
Table 5.
Benefit–cost ratios for selected key diagnostic tests and associated treatment
| Benefit–cost ratio | Context and sources used | |
|---|---|---|
| Hypertension* | 4·1:1 (the Middle East and north Africa regions), 12·7:1 (Latin America and Caribbean region), <1:1 (South Asia), <1:1 (sub-Saharan Africa) | Calculations from Gaziano and colleagues;36 a blood pressure test was used (in conjunction with tests of lipids and blood glucose) to calculate absolute risk and prescribe a preventive four-drug regimen to adults with no previous cardiac event if appropriate, for four regions (using selected countries to represent each region) |
| Diabetes† | <1:1 | Calculation from a cost-of-illness study for South Africa347 and estimates of long-term effects of metformin or intensive lifestyle interventions for adults33 |
| HIV‡ | 16·6:1 | Estimates for diagnosing HIV and treating those patients with a CD4 count of less than 350 cells per μL with antiretroviral therapy,348 in sub-Saharan Africa, assuming that regional per capita income averages US$5000 when weighted by population prevalence of HIV |
| Syphilis | 2·1:1 to 62:1 (median 8·6:1) | Estimates of cost-effectiveness for eight archetype regions of low-income and middle-income countries with calculations from Kahn and colleagues37 to attach a monetary value to disability-adjusted life-years averted; intervention is to screen pregnant women and treat those diagnosed with syphilis, hence preventing mother-to-child transmission |
| Tuberculosis | 24·4:1 (drug-susceptible), 2·9:1 (multidrug-resistant) | Estimates for drug-susceptible and multidrug-resistant tuberculosis in Bangladesh from Vassall349 (the case-finding method was used for drug-susceptible tuberculosis); for multidrug-resistant tuberculosis, a shorter drug course (which WHO permits under specific conditions) or treatment at a community health centre increased the benefit–cost ratio to 3·8 and 5·3, respectively; Vassall has also suggested that the range in benefit–cost ratio for drug-susceptible tuberculosis and for multidrug-resistant tuberculosis is 11:1 to 192:1 and 0·5:1 to 5:1, respectively, across different countries350 |
| Hepatitis B | 1·4:1 (The Gambia), 4:1 to 5:1 (Ghana and Nigeria) | Calculations from Nayagam and colleagues;351 a strategy of screen and treat in pregnant women for 4 months after week 28, to prevent mother-to-child transmission, as outlined in a study of South Africa, but applied to The Gambia, and extrapolated to Ghana and Nigeria assuming similar costs, higher benefits (higher per capita income), and higher prevalence of hepatitis B virus |
Based on a model using data for 2001.33 Prevalence has risen since then, particularly in South Asia and sub-Saharan Africa, and income has grown substantially in South Asia; hence, the benefit–cost ratio probably increased particularly in South Asia. In sub-Saharan Africa, a key constraint is that only a small proportion of people who are hypertensive are able to control their blood pressure.
The main issue is that annual treatment is relatively expensive and has beneficial effects in the short term, but these effects generally do not persist.
Since the study used here was published,348 WHO guidance has changed such that all people living with HIV are now eligible for treatment irrespective of CD4 cell count.352 This change lowers the benefit–cost ratio (since morbidity and mortality averted are lower at higher CD4 cell counts); however, it is not possible to calculate by how much, since cost depends on take-up of therapy by CD4 cell count.
Figure 10.
Median benefit–cost ratios to diagnose and treat six conditions in low-income and middle-income countries
Data are from table 5. Middle-income regions include Latin America, and the Middle East and north Africa. Low-income regions include South Asia and sub-Saharan Africa.
Although there is much variation in the median benefit–cost ratios ranging from less than one to 24, the ratios provide a strong case for investment in diagnostics and associated treatment. At one extreme, diagnostic tests accompanied by treatment actually save the health system money for one of the six conditions (syphilis). A review for the USA came to a similar finding, in that only one in three screen-and-treat interventions examined actually saved the health system money.353 One would expect more interventions in the USA than in LMICs to be cost-saving for the health system, given the proportionately higher spending on curative care. At the other extreme, it costs more to diagnose and treat diabetes than the societal benefits, largely due to the fact that current interventions using lifestyle modification or metformin, or both, are only modestly effective for type 2 diabetes.
Limitations of the methods used include that we used existing disease models, and cost and cascade data for varying countries and dates, depending on what was available, and we did not undertake sensitivity analysis. Future work on the benefit–cost ratio of using DI, particularly in the emergency room, would be useful.
Transforming access to diagnostics —recommendations
Based on the suggested solutions in improving policy, governance, and finance, we have 10 recommendations (panel 11 ); recommendations 1–5 are aimed primarily at the national level, whereas recommendations 6–10 are international in focus. Advocacy at all levels is key. A main focus should be making the role of diagnostics in universal health coverage explicit. A key national-level action is developing a strategic plan for diagnostics, an essential component of which should be an integrated and tiered network for diagnostics that addresses the last-mile gap. An international partnership in terms of a Diagnostics Alliance can help to coordinate the national programmes, raise international awareness, and help shape markets and innovations in ways that are inclusive for all.
Panel 11. Recommendations from this Commission.
1 National diagnostics strategy, based on an integrated and tiered network, including an evidence-based essential diagnostics list (EDL), with a prioritised subset for universal health coverage
-
•
Actions: develop a costed national diagnostics strategy that is based on an integrated and tiered network. As part of this strategy, with the use of evidence-based disease guidelines, countries should develop and periodically review a national EDL, linked to their national essential medicines list. A prioritised subset of the diagnostics on this list should be provided as part of the universal health coverage benefits package, and this subset should be expanded over time as resources permit.
-
•
Who: national governments, with technical support from international organisations.
-
•
Measure: existence of a diagnostic strategy, tiered and integrated network, and EDL; diagnostics are integrated into universal health coverage benefits packages.
2 Primary health centre diagnostic availability and accessibility
-
•
Actions: make point-of-care tests for key conditions available at all primary health centres.
-
•
Who: ministries of health, with support from other relevant ministries (eg, finance and industry).
-
•
Measure: proportion of the population with access to an appropriate set of basic diagnostics at all primary health centres, including rapid tests for about 10 key conditions and ultrasound.
3 Health workforce expansion and upskilling for contemporary diagnostic skills
-
•
Actions: expand workforce capacity as appropriate for the needs of the health-care system, change the content and delivery of curricula to ensure staff are digitally literate, increase the use of digital diagnostics and artificial intelligence to support the workforce in a better manner, develop high-quality task-shifting programmes, and exploit the full capability and skills of all staff.
-
•
Who: ministries of health and education.
-
•
Measure: expanded workforce capacity, existence of contemporary curricula for education and training, increased use of digital diagnostics and artificial intelligence, particularly in low-income and middle-income countries, and development of effective task-shifting programmes.
4 Governance and regulatory frameworks to support and oversee diagnostic quality and safety
-
•
Actions: build and enforce a national regulatory framework covering the market for diagnostics (eg, as part of the regulation of medical devices) and the registration, accreditation, and oversight of laboratories; national professional bodies concerned with diagnostics should have a framework of standards for their members. Additionally, coordinate national regulations concerning medical devices, via regional harmonisation or expansion of the WHO prequalification process.
-
•
Who: national governments, national associations of diagnostics professionals, and WHO, which needs to be funded to broaden the prequalification process.
-
•
Measure: monitor regulations nationally and internationally using selected key tests.
5 National financing strategy to provide sufficient, long-term financing to plan and implement diagnostics, including infrastructure
-
•
Actions: create comprehensive, sustainable mechanisms of finance to design, implement, and sustain diagnostics, including infrastructure, and explore new sources of global and domestic financing, including innovative instruments (eg, Social or Development Impact Bonds).
-
•
Who: ministries of health and finance.
-
•
Measure: use of new financing sources; specific line item for diagnostics within the budgets for universal health coverage.
6 Improve the affordability of diagnostics
-
•
Actions: promote global action to encourage production of good-quality diagnostics in low-income and middle-income countries, innovate to produce diagnostics suited to low-resource settings, create an international forum to share best practices on diagnostics, use market-shaping mechanisms internationally, and collect ongoing information on the affordability of diagnostics similar to the WHO–Health Action International initiative on affordability of medicines, for selected key diagnostics.
-
•
Who: national governments; international organisations (WHO) for collecting information; multiple international organisations (eg, Global Fund, UNICEF, Foundation for Innovative New Diagnostics, Coalition for Epidemic Preparedness Innovations, Clinton Health Access Initiative, and Médecins Sans Frontières) for innovations; a convenor (eg, the Global Alliance for Diagnostics) for an international forum to share best practices; and a forum that includes national actors, non-governmental organisations, and industry.
-
•
Measure: creation of a forum; compare domestic prices of selected key diagnostics internationally.
7 Foster development and appropriate use of technology to benefit everyone
-
•
Actions: promote local research and development, manufacturing, and distribution capacity; harness the opportunities to use digitalisation, point-of-care diagnostics, and democratisation in national diagnostics strategies to benefit patients; engage local communities in this process; and instigate an international forum for joint meetings of clinicians and technology innovators.
-
•
Who: international organisations, national governments, non-governmental organisations, researchers, private sector, and donors.
-
•
Measure: the share of the global market for diagnostics produced by low-income and middle-income countries; monitored in the Global Digital Health Index.
8 Address the diagnostics needs of populations living in fragile and conflict situations
-
•
Actions: use innovative technologies to provide suitable solutions (better coordination and funding of research and implementation science are needed), ensure that populations living in the fragile and conflict situations and humanitarian staff are part of the co-development process, and fund research that bridges civilian and security sector research.
-
•
Who: Specialised civilian and security international agencies working with these populations.
-
•
Measure: increase the portfolio of clinical trials, studies, projects, and programmes dedicated to fragile and conflict populations by 10% annually for the next decade. Work needs to start now given the urgency of the need.
9 Advocate at all levels to ensure that diagnostics receive appropriate recognition and funding
-
•
Actions: develop and implement an advocacy programme for each country and instigate adoption of a World Health Assembly resolution on the importance of diagnostics.
-
•
Who: diagnostics professionals need to engage in health policy discussions and need to be included in ministry-level discussion; reach needs to extend to patients, the public, policy makers, and other clinicians; professional societies of diagnostics professionals need to inform and engage members with regard to the role of diagnostics in global health.
-
•
Measure: the number of countries with a national integrated, tiered network and EDL; the degree of integration with use of the Human Service Integration Scale; the number of countries where diagnostics are integrated into universal health coverage benefits packages; bibliometric measures (mentions of diagnostics in the media); and numbers of pathology and laboratory medicine and radiology professionals in the international policy arena.
10 International Diagnostics Alliance to support and monitor the effort in transforming diagnostics
-
•
Actions: establish an international Diagnostics Alliance to have a key role in advocacy, setting specific targets, and monitoring progress, convening an international forum for stakeholders to share best practices.
-
•
Who: a broad coalition of national policy makers, representatives from international and non-government organisations, representatives from professional societies (including those representing workers in the sector), and patient representatives.
-
•
Measure: creation of the Alliance
-
•
Timing: urgent, by the end of 2022.
From late March, 2020, it has been impossible to look at media reports anywhere in the world without being overwhelmed by the need to have faster, more accurate diagnostics for COVID-19, the rationale being that by diagnosing accurately and as early as possible, the appropriate isolation and intervention can be initiated, and the pandemic controlled. This rationale applies equally to all other diseases. Yet, as shown in this Commission, almost half the world's population does not have access to accurate and timely diagnosis of any sort (other than for HIV and malaria). This circumstance is because diagnostics have been under-resourced in most countries and at all levels, but particularly at the levels of primary and community care. This under-resourcing especially affects the disadvantaged. The over-riding reason for the poor global access to diagnostics has been the relatively low attention and priority given to diagnostics by policy makers and funders, especially in comparison with the prominence given to pharmaceuticals or vaccines. It appears that the centrality of diagnostics, which has been recognised when dealing with COVID-19, is not recognised for other conditions.
It is often said that a crisis should never be wasted. In the past decade or so, there have been several reports and papers, which like this Commission, have highlighted the importance of diagnostics, described the serious deficiencies, and proposed solutions. However, progress has been modest. The COVID-19 pandemic can, and must, be a turning point. When COVID-19 has fallen from the headlines, there should not be a return to business as usual. In the future, it must be unacceptable to build health-care programmes and systems, particularly universal health coverage programmes, without integrating the necessary diagnostic resources. To ensure implementation of our recommendations, there needs to be a series of next steps following our Commission.
Given that the key barrier to improving the global access to diagnostics is the inadequate resourcing of diagnostics due to low prioritisation, the over-riding aim must be to reverse this. In the case of diagnostics, the message of COVID-19 should be noted, namely that when the case is strong, resources are allocated. There now needs to be a long-term major advocacy programme, backed by evidence and data (especially economic data) on the benefits of good diagnostics. This Commission has provided some key data on access, on the impact of this poor access on mortality, and on how developing a prioritised EDL can drive change. Internationally, a champion for diagnostics (the Diagnostics Alliance) can mobilise and coordinate support for this effort, and complement the key work of national governments.
A second and related crucial action is the adoption of a World Health Assembly resolution on the need for diagnostics to be an integral part of any universal health coverage programme. This approach would be both a powerful statement and a driver of progress. It should be a key priority of the Diagnostics Alliance.
In parallel, there are a number of issues that this Commission does not address, either because data are not available or from constraints of time (eg, demand-side issues), but to make the case for diagnostics, these will need addressing in the near future. Arguably, one of the most important areas is data collection. One of the many downsides of the low priority for diagnostics is that essential data are scarce, especially compared with medicines. For example, we found it difficult to obtain accurate (or even any) data on fundamental issues, such as workforce numbers or national estimates of spending on DI and PALM. There are very few data on the benefit–cost ratios of PALM and particularly of DI, yet these data are important for making the case for diagnostics. Similarly, there are few data on affordability of diagnostics and its effect on patient care. More generally, more research is needed, including not only on new technology or equipment, but also on more mundane but crucial aspects like supply chain, developing an effective and affordable regulatory framework, market shaping, procurement, and key performance indicators to monitor and evaluate.
We recognise that diagnostics are part of a system of health care and that the provision of appropriate diagnostics should not be at the expense of, for instance, treatment. However, without accurate diagnosis, treatment might very well be wasted by treating the wrong disease or condition, or by delaying or not giving appropriate treatment. Diagnosis and treatment are interdependent and equally necessary. Given the potential health, social, and economic benefits of improving diagnostics, it is time for change. Implementing actions and recommendations described in this Commission will transform access to diagnostics.
This online publication has been corrected. The corrected version first appeared at thelancet.com on November 8, 2021
Declaration of interests
TD declares minor shareholdings and an unpaid directorship at CapeRay Medical, which was an institution-based activity. WC declares he is the chief executive officer of a telemedicine company providing general medical consultation. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
Funding for the first meeting of this Commission was provided by the Bill & Melinda Gates Foundation. Funding for a meeting of the Commission Steering Committee was provided by the Wellcome Trust. Funding and in-kind support for the second meeting of this Commission was provided by the Brocher Foundation, the Canadian Institutes for Health Research, and King's College London. Other funding for the Commission has been received from the British Division of the International Academy of Pathology, the World Pathology Foundation, African Strategies for Advancing Pathology, Oxford University, University of Vermont, University of Waterloo, and Harvard University. The funders did not have a role in the research methods, decision to publish findings, or the content of the Commission. All authors had full access to the full data in the study and accept responsibility to submit for publication. We would like to thank the following individuals for their contributions to the Commission. However, they did not have a role in the research methods, decision to publish findings, and are not responsible for the content or any statements made in this Commission. Participants in the Industry Roundtable included the following: Duncan Blair, Paul Baum, Mark Davis, Eugen Emmentraut, Mark Holmshaw, Philippe Jacon, Ammar Jagirdar, Jan Kimpen, Ricardo Lampariello, Ian McLane, Mark Miller, Bernd Ohnesorge, and Davide Pedrazzini. Participants in the virtual non-governmental organisation and international organisation consultations included the following: David Branigan, Arlene Chua, Yogesh Jain, Shalini Jayasekar-Zurn, Sonali Johnson, Lindsay McKenna, Sushil Patil, Pablo Perel, Trevor Peter, Rangarajan Sampath, and Mohammed Yassin. Thanks to the following professional societies or members of such societies for helpful comments on the essential diagnostics list: Renato Mendonca, President, on behalf of the officers of The International Society of Radiology; Dianabasi Eduwmen, President, on behalf of the Executive and members of the Association of Radiologists of West Africa; Michael H M Chan, Chemical Pathologist, Sammy P L Chen, Chemical Pathologist, and Christopher KC Lai, Clinical Microbiologist, all from Hong Kong; and Phaik Leng Cheah, Anatomical Pathologist, Malaysia. The following individuals provided helpful input on diagnostic innovations: Michael Feldman, Fergus Gleeson, Kiran Jobanputra, Ramanan Laxminarayan, Bernd Ohnesorge, Yuri Quintana, Arunan Skandarajah, and David Walt. Thanks to the following for helpful comments on earlier drafts of the Commission: Barnabas Alayande, Adam Ammar, Alexandra Buda, Gabrielle Cahill, Kashmira Chawla, Scott Corlew, Zachary Fowler, Deen Garba, Anusha Jayaram, Tarinee Kucchal, Ian Maclane, Craig McLain, Elizabeth Miranda, Isioma Okolo, Manon Pigeolet, Rennie Qin, Makela Stankey, and Paul Truche. Individuals who provided research support to the Commission included the following: Greg Flanigan, Angela Mutuku, and Jiajun Su. Catherine Hidalgo, Klesta Hoxha, and Laura Jimenez provided helpful assistance in the process of producing some sections of the Commission.
Editorial note: the Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Contributors
All authors contributed to the key messages, conclusions, recommendations, writing, and editing of the Commission. Substantial contributions to the research, data gathering, and assembly of the final Commission material were supported by a research team listed in the Acknowledgments section. Additional helpful counsel and advice were supplied by several key individuals and organisations, who are also listed in the Acknowledgments. All research was done under the leadership of the Commission Chair and Deputy Chairs (KAF, SH, and MLW) and the other members of the Steering Committee, (KD, SS, RA, and JF). All members of the Steering Committee also chaired or co-chaired working groups, as did two other members of the Commission (JGM and MP). All members of the Steering Committee were also members of the Writing Group, as were four other members of the Commission (ABL, CB, MP, and RS). The following authors verified data: PA and MK (cascades of care); LS, DS, and SH (availability of diagnostics by facility); HSI and JF (geospatial data); PA, MK, and MP (future global burden of disease); MK and SH (projected health benefits of reducing the diagnostic gap); SH and JGM (benefit–cost of diagnostics); and SH and DS (global market for diagnostics).
Supplementary Material
References
- 1.US Food and Drug Administration Coronavirus (COVID-19) update: FDA alerts consumers about unauthorized fraudulent COVID-19 test kits. 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-alerts-consumers-about-unauthorized-fraudulent-covid-19-testkits
- 2.Shuren J, Stenzel T. The FDA's experience with Covid-19 antibody tests. N Engl J Med. 2021;384:592–594. doi: 10.1056/NEJMp2033687. [DOI] [PubMed] [Google Scholar]
- 3.Chmura C, Jackson J. Feds work to snag fake COVID-19 test kits, bogus virus products. 2020. https://www.nbcbayarea.com/investigations/consumer/feds-work-to-snag-fake-covid-19-test-kits-bogus-virusproducts/2282195/
- 4.The Global Fund Access to COVID-19 Tools Accelerator. 2021. https://www.theglobalfund.org/en/covid-19/act-a/
- 5.Usher AD. ACT Accelerator strains donors' aid budgets. Lancet. 2021;397 doi: 10.1016/S0140-6736(21)01284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Lancet The ACT Accelerator: heading in the right direction? Lancet. 2021;397 doi: 10.1016/S0140-6736(21)00835-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kosack CS, Page AL, Klatser PR. A guide to aid the selection of diagnostic tests. Bull World Health Organ. 2017;95:639–645. doi: 10.2471/BLT.16.187468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson ML, Fleming KA, Kuti MA, Looi LM, Lago N, Ru K. Access to pathology and laboratory medicine services: a crucial gap. Lancet. 2018;391:1927–1938. doi: 10.1016/S0140-6736(18)30458-6. [DOI] [PubMed] [Google Scholar]
- 9.Anne C, Rajeh F. Miscarriages and malnourishment: the perils of pregnancy in Yemen. 2018. https://www.aljazeera.com/indepth/features/miscarriages-malnourishment-perils-pregnancy-yemen-181103003049587.html
- 10.Qidwai W, Ali SS, Ayub S, Ayub S. Healing during physician-patient consultation. J Coll Physicians Surg Pak. 2005;15:689–692. [PubMed] [Google Scholar]
- 11.Meyers J. Distrusting China's medical system, patients turn to U.S. doctors online. 2018. https://www.latimes.com/world/asia/la-fg-china-cancer-us-20180111-story.html
- 12.Amexo M, Tolhurst R, Barnish G, Bates I. Malaria misdiagnosis: effects on the poor and vulnerable. Lancet. 2004;364:1896–1898. doi: 10.1016/S0140-6736(04)17446-1. [DOI] [PubMed] [Google Scholar]
- 13.Babalola L. Doctors of death: Nigeria's medical misdiagnosis crisis. 2019. https://www.pmnewsnigeria.com/2019/10/26/doctors-of-death-nigerias-medical-misdiagnosis-crisis/
- 14.WHO Global tuberculosis report 2019. 2019. https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1
- 15.Menéndez C, Quintó L, Castillo P, et al. Quality of care and maternal mortality in a tertiary-level hospital in Mozambique: a retrospective study of clinicopathological discrepancies. Lancet Glob Health. 2020;8:e965–e972. doi: 10.1016/S2214-109X(20)30236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manguin S, Foumane V, Besnard P, Fortes F, Carnevale P. Malaria overdiagnosis and subsequent overconsumption of antimalarial drugs in Angola: consequences and effects on human health. Acta Trop. 2017;171:58–63. doi: 10.1016/j.actatropica.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Polage CR, Bedu-Addo G, Owusu-Ofori A, et al. Laboratory use in Ghana: physician perception and practice. Am J Trop Med Hyg. 2006;75:526–531. [PubMed] [Google Scholar]
- 18.Sulis G, Daniels B, Kwan A, et al. Antibiotic overuse in the primary health care setting: a secondary data analysis of standardised patient studies from India, China and Kenya. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2020-003393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21:1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization Regional Office for Africa The Maputo Declaration on Strengthening of Laboratory Systems. 2008. http://www.who.int/diagnostics_laboratory/Maputo-Declaration_2008.pdf
- 21.WHO First WHO model list of essential in-vitro diagnostics. 2018. https://apps.who.int/iris/bitstream/handle/10665/311567/9789241210263-eng.pdf?ua=1
- 22.Nicholson D, Yates R, Warburton W, Fontana G. Delivering universal health coverage: a guide for policymakers. 2015. https://www.imperial.ac.uk/media/imperial-college/institute-of-global-health-innovation/public/Universal-health-coverage.pdf
- 23.Carr TH, McEwen R, Dougherty B, et al. Defining actionable mutations for oncology therapeutic development. Nat Rev Cancer. 2016;16:319–329. doi: 10.1038/nrc.2016.35. [DOI] [PubMed] [Google Scholar]
- 24.National Academies of Sciences, Engineering, and Medicine . National Academies Press; Washington, DC: 2015. Improving diagnosis in health care. [Google Scholar]
- 25.Verghese A, Brady E, Kapur CC, Horwitz RI. The bedside evaluation: ritual and reason. Ann Intern Med. 2011;155:550–553. doi: 10.7326/0003-4819-155-8-201110180-00013. [DOI] [PubMed] [Google Scholar]
- 26.Kugler J, Verghese A. The physical exam and other forms of fiction. J Gen Intern Med. 2010;25:756–757. doi: 10.1007/s11606-010-1400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chowkwanyun M, Bayer R, Galea S. “Precision” public health—between novelty and hype. N Engl J Med. 2018;379:1398–1400. doi: 10.1056/NEJMp1806634. [DOI] [PubMed] [Google Scholar]
- 28.Sorace J, Aberle DR, Elimam D, Lawvere S, Tawfik O, Wallace WD. Integrating pathology and radiology disciplines: an emerging opportunity? BMC Med. 2012;10:100. doi: 10.1186/1741-7015-10-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lippi G, Plebani M. Integrated diagnostics: the future of laboratory medicine? Biochem Med (Zagreb) 2020;30 doi: 10.11613/BM.2020.010501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiffman J, Smith S. Generation of political priority for global health initiatives: a framework and case study of maternal mortality. Lancet. 2007;370:1370–1379. doi: 10.1016/S0140-6736(07)61579-7. [DOI] [PubMed] [Google Scholar]
- 31.WHO WHO recommendations on antenatal care for a positive pregnancy experience. 2016. https://www.who.int/reproductivehealth/publications/maternal_perinatal_health/anc-positive-pregnancy-experience/en/ [PubMed]
- 32.Allard NL, MacLachlan JH, Cowie BC. The cascade of care for Australians living with chronic hepatitis B: measuring access to diagnosis, management and treatment. Aust N Z J Public Health. 2015;39:255–259. doi: 10.1111/1753-6405.12345. [DOI] [PubMed] [Google Scholar]
- 33.Diabetes Prevention Program Research Group Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3:866–875. doi: 10.1016/S2213-8587(15)00291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mokaya J, Burn EAO, Tamandjou CR, et al. Modelling cost-effectiveness of tenofovir for prevention of mother to child transmission of hepatitis B virus (HBV) infection in South Africa. BMC Public Health. 2019;19:829. doi: 10.1186/s12889-019-7095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanser F, Bärnighausen T, Grapsa E, Zaidi J, Newell M-L. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339:966–971. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaziano TA, Opie LH, Weinstein MC. Cardiovascular disease prevention with a multidrug regimen in the developing world: a cost-effectiveness analysis. Lancet. 2006;368:679–686. doi: 10.1016/S0140-6736(06)69252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kahn JG, Jiwani A, Gomez GB, et al. The cost and cost-effectiveness of scaling up screening and treatment of syphilis in pregnancy: a model. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oxlade O, Piatek A, Vincent C, Menzies D. Modeling the impact of tuberculosis interventions on epidemiologic outcomes and health system costs. BMC Public Health. 2015;15:141. doi: 10.1186/s12889-015-1480-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dowdy DW, Chaisson RE, Maartens G, Corbett EL, Dorman SE. Impact of enhanced tuberculosis diagnosis in South Africa: a mathematical model of expanded culture and drug susceptibility testing. Proc Natl Acad Sci USA. 2008;105:11293–11298. doi: 10.1073/pnas.0800965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maddali MV, Gupta A, Shah M. Epidemiological impact of achieving UNAIDS 90-90-90 targets for HIV care in India: a modelling study. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-011914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan American Health Organization. WHO World Radiography Day: two-thirds of the world's population has no access to diagnostic imaging. 2012. https://www.paho.org/hq/index.php?option=com_content&view=article&id=7410:dia-de-la-radiografia-dos-tercios-de-la-poblacion-mundial-no-tiene-acceso-al-diagnostico-por-imagen&Itemid=0&lang=en
- 42.Leslie HH, Spiegelman D, Zhou X, Kruk ME. Service readiness of health facilities in Bangladesh, Haiti, Kenya, Malawi, Namibia, Nepal, Rwanda, Senegal, Uganda and the United Republic of Tanzania. Bull World Health Organ. 2017;95:738–748. doi: 10.2471/BLT.17.191916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kohli M, Walia K, Mazumdar S, Boehme CC, Katz Z, Pai M. Availability of essential diagnostics in primary care in India. Lancet Infect Dis. 2018;18:1064–1065. doi: 10.1016/S1473-3099(18)30539-5. [DOI] [PubMed] [Google Scholar]
- 44.Sánchez-Sánchez J, Alarcón-Loayza J, Villa-Castillo L, et al. Availability of essential diagnostics at primary care public clinics in Peru. Microbes Infect. 2021;23 doi: 10.1016/j.micinf.2020.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Shumbej T, Menu S, Gebru T, et al. Essential in-vitro laboratory diagnostic services provision in accordance with the WHO standards in Guragae zone primary health care unit level, South Ethiopia. Trop Dis Travel Med Vaccines. 2020;6:4. doi: 10.1186/s40794-020-0104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yadav H, Shah D, Sayed S, Horton S, Schroeder LF. Availability of essential diagnostics in ten low-income and middle-income countries: results from national health facility surveys. Lancet Glob Health (in press). [DOI] [PMC free article] [PubMed]
- 47.DeStigter K, Horton S, Atalabi OM, et al. Equipment in the global radiology environment: why we fail, how we could succeed. J Glob Radiol. 2019;5:3. [Google Scholar]
- 48.WHO AccessMod: geographic access to health care. 2017. https://www.who.int/tools/accessmod-geographic-access-to-health-care
- 49.Wong KLM, Benova L, Campbell OMR. A look back on how far to walk: systematic review and meta-analysis of physical access to skilled care for childbirth in sub-Saharan Africa. PLoS One. 2017;12 doi: 10.1371/journal.pone.0184432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelly C, Hulme C, Farragher T, Clarke G. Are differences in travel time or distance to healthcare for adults in global north countries associated with an impact on health outcomes? A systematic review. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-013059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lankowski AJ, Siedner MJ, Bangsberg DR, Tsai AC. Impact of geographic and transportation-related barriers on HIV outcomes in sub-Saharan Africa: a systematic review. AIDS Behav. 2014;18:1199–1223. doi: 10.1007/s10461-014-0729-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.USAID The Demographic and Health Surveys. 2020. https://dhsprogram.com/pubs/pdf/FR344/FR344.pdf
- 53.INEGI Directorio Estadístico Nacionale de Unidades Económicas. 2020. www.inegi.org.mx/app/mapa/denue/default.aspx
- 54.ESRI ArcGIS business analyst: data and infographics. 2020. https://www.esri.com/en-us/arcgis/products/arcgis-business-analyst/data-reports
- 55.Maina J, Ouma PO, Macharia PM, et al. A spatial database of health facilities managed by the public health sector in sub Saharan Africa. Sci Data. 2019;6:134. doi: 10.1038/s41597-019-0142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malawi Ministry of Health and ICF International Malawi: Service Provision Assessment (MSPA) 2013–14 key findings. 2014. https://dhsprogram.com/pubs/pdf/SR217/SR217.pdf
- 57.Agence Nationale de la Statistique et de la Demographie. ICF International Continuous Service Provision Assessment survey (SCSPA) in Senegal 2012–2013. 2014. https://dhsprogram.com/pubs/pdf/SPA18/SPA18.eng.pdf
- 58.The World Bank Population, total. 2020. https://data.worldbank.org/indicator/sp.pop.totl
- 59.Chidyaonga-Maseko F, Chirwa ML, Muula AS. Underutilization of cervical cancer prevention services in low and middle income countries: a review of contributing factors. Pan Afr Med J. 2015;21:231. doi: 10.11604/pamj.2015.21.231.6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nuche-Berenguer B, Sakellariou D. Socioeconomic determinants of cancer screening utilisation in Latin America: a systematic review. PLoS One. 2019;14 doi: 10.1371/journal.pone.0225667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith D, Thomson K, Bambra C, Todd A. The breast cancer paradox: a systematic review of the association between area-level deprivation and breast cancer screening uptake in Europe. Cancer Epidemiol. 2019;60:77–85. doi: 10.1016/j.canep.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dasgupta P, Baade PD, Aitken JF, Ralph N, Chambers SK, Dunn J. Geographical variations in prostate cancer outcomes: a systematic review of international evidence. Front Oncol. 2019;9:238. doi: 10.3389/fonc.2019.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Essink-Bot M-L, Dekker E. Equal access to colorectal cancer screening. Lancet. 2016;387:724–726. doi: 10.1016/S0140-6736(15)01221-0. [DOI] [PubMed] [Google Scholar]
- 64.Manne-Goehler J, Atun R, Stokes A, et al. Diabetes diagnosis and care in sub-Saharan Africa: pooled analysis of individual data from 12 countries. Lancet Diabetes Endocrinol. 2016;4:903–912. doi: 10.1016/S2213-8587(16)30181-4. [DOI] [PubMed] [Google Scholar]
- 65.Manne-Goehler J, Geldsetzer P, Agoudavi K, et al. Health system performance for people with diabetes in 28 low- and middle-income countries: a cross-sectional study of nationally representative surveys. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prenissl J, Jaacks LM, Mohan V, et al. Variation in health system performance for managing diabetes among states in India: a cross-sectional study of individuals aged 15 to 49 years. BMC Med. 2019;17:92. doi: 10.1186/s12916-019-1325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Geldsetzer P, Manne-Goehler J, Marcus ME, et al. The state of hypertension care in 44 low-income and middle-income countries: a cross-sectional study of nationally representative individual-level data from 1·1 million adults. Lancet. 2019;394:652–662. doi: 10.1016/S0140-6736(19)30955-9. [DOI] [PubMed] [Google Scholar]
- 68.Prenissl J, Manne-Goehler J, Jaacks LM, et al. Hypertension screening, awareness, treatment, and control in India: a nationally representative cross-sectional study among individuals aged 15 to 49 years. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Global Health 5050. International Center for Research on Women. African Population and Research Center The COVID-19 sex-disaggregated data tracker. 2020. https://globalhealth5050.org/the-sex-gender-and-covid-19-project/the-data-tracker/?explore=country
- 70.George P, Chandwani S, Gabel M, et al. Diagnosis and surgical delays in African American and white women with early-stage breast cancer. J Womens Health (Larchmt) 2015;24:209–217. doi: 10.1089/jwh.2014.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ko NY, Hong S, Winn RA, Calip GS. Association of insurance status and racial disparities with the detection of early-stage breast cancer. JAMA Oncol. 2020;6:385–392. doi: 10.1001/jamaoncol.2019.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Whittle RS, Diaz-Artiles A. An ecological study of socioeconomic predictors in detection of COVID-19 cases across neighborhoods in New York City. BMC Med. 2020;18:271. doi: 10.1186/s12916-020-01731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kibirige D, Sanya RE, Nantanda R, Worodria W, Kirenga B. Availability and affordability of medicines and diagnostic tests recommended for management of asthma and chronic obstructive pulmonary disease in sub-Saharan Africa: a systematic review. Allergy Asthma Clin Immunol. 2019;15:14. doi: 10.1186/s13223-019-0329-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Institute for Health Metrics and Evaluation GBD foresight visualization. 2018. http://www.healthdata.org/data-visualization/gbd-foresight-visualization
- 75.Pai M, Walia K, Boehme CC. Essential medicines and essential diagnostics: a package deal. Lancet Public Health. 2019;4:e492. doi: 10.1016/S2468-2667(19)30165-3. [DOI] [PubMed] [Google Scholar]
- 76.WHO Universal health coverage. 2019. https://www.who.int/news-room/fact-sheets/detail/universal-health-coverage-(uhc)#:~:text=UHC%20means%20that%20all%20individuals,%2C%20rehabilitation%2C%20and%20palliative%20care
- 77.Horton S, Sullivan R, Flanigan J, et al. Delivering modern, high-quality, affordable pathology and laboratory medicine to low-income and middle-income countries: a call to action. Lancet. 2018;391:1953–1964. doi: 10.1016/S0140-6736(18)30460-4. [DOI] [PubMed] [Google Scholar]
- 78.Van't Hoog AH, Sarr A, Koster W, et al. A study to better understand under-utilization of laboratory tests for antenatal care in Senegal. PLoS One. 2020;15 doi: 10.1371/journal.pone.0225710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pai NP, Wilkinson S, Deli-Houssein R, et al. Barriers to implementation of rapid and point-of-care tests for human immunodeficiency virus infection. Findings from a systematic review (1996–2014) Point Care. 2015;14:81–87. doi: 10.1097/POC.0000000000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moyo K, Porter C, Chilima B, et al. Use of laboratory test results in patient management by clinicians in Malawi. Afr J Lab Med. 2015;4:277. doi: 10.4102/ajlm.v4i1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ondoa P, van der Broek A, Jansen C, de Bruijn H, Schultsz C. National laboratory policies and plans in sub-Saharan African countries: gaps and opportunities. Afr J Lab Med. 2017;6:578. doi: 10.4102/ajlm.v6i1.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.World Health Organization Regional Office for Africa. Centers for Disease Control and Prevention Guidance for Development of National Laboratory Strategic Plans. 2008. https://www.who.int/hiv/amds/amds_guide_dev_nat_lab_strat.pdf
- 83.Shah DR, Abimbola L, DeStigter K, van de Broek-Altenburg E, Horton S, Dahn B. Medical imaging: the missing element of National Health Plans. J Glob Radiol (in press).
- 84.WHO . World Health Organization; Geneva: 2018. NAPHS for all: a 3 step strategic framework for national action plan for health security. [Google Scholar]
- 85.Newton PN, Bond KC, Newton P, et al. Global access to quality-assured medical products: the Oxford Statement and call to action. Lancet Glob Health. 2019;7:e1609–e1611. doi: 10.1016/S2214-109X(19)30426-7. [DOI] [PubMed] [Google Scholar]
- 86.McNerney R, Sollis K, Peeling RW. Improving access to new diagnostics through harmonised regulation: priorities for action. Afr J Lab Med. 2014;3:123. doi: 10.4102/ajlm.v3i1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.US Department of Commerce Medical devices. 2020. https://www.trade.gov/knowledge-product/peru-medical-equipment
- 88.Grand View Research In vitro diagnostics market size, share & trends analysis report by product, by application, by technology (immunochemistry, molecular diagnostics), by end-use, by region, and segment forecasts, 2021–2027. 2020. https://www.grandviewresearch.com/industry-analysis/in-vitro-diagnostics-ivd-market
- 89.Grand View Research Point of care diagnostics market size, share & trends analysis report by product (infectious diseases, cancer marker), by end-use (home, clinics), by region, and segment forecasts, 2021–2028. 2020. https://www.grandviewresearch.com/industry-analysis/point-of-care-poc-diagnostics-industry
- 90.Industry Stats Report Medical imaging equipment market 2019 by product type (magnetic resonance imaging equipment, compound tomography equipment, x-ray equipment, ultrasound equipment, molecular equipment), by application (cardiology, neurology, orthopedics, gynecology, oncology, others), by end user (hospitals, specialty clinics, diagnostic imaging centers, others) forecast to 2025. 2020. https://industrystatsreport.com/Medical-Devices-and-Consumables/Medical-Imaging-Equipment-Market-Size-and-Growth/Summary
- 91.EvaluatePharma World preview 2019, outlook to 2024. 2019. https://info.evaluate.com/rs/607-YGS-364/images/EvaluatePharma_World_Preview_2019.pdf
- 92.Markets and Markets Point of care diagnostics market by product (glucose, infectious disease (hepatitis c, influenza, respiratory), coagulation), platform (microfluidics, immunoassays), mode (prescription & OTC), end-user (hospitals, home care)—global forecast to 2024. 2019. https://www.marketsandmarkets.com/Market-Reports/point-of-care-diagnostic-market-106829185.html
- 93.Markets and Markets Molecular diagnostics market worth $15·94 billion by 2027, at a CAGR of 8·4% 2018. https://www.marketsandmarkets.com/Market-Reports/molecular-diagnosticmarket-833.html
- 94.India Brand Equity Foundation Pharmaceuticals. https://www.ibef.org/industry/pharmaceutical-india.aspx
- 95.ReportLinker. China In Vitro Diagnostic (IVD) Industry Report, 2019–2025. 2019. https://www.reportlinker.com/p05916125/China-In-Vitro-Diagnostics-Market-Outlookto-Cardiac-Disease-Clinical-Chemistry-Hematological-Disorders-and-Others-.html
- 96.Technology OMDIA. Global medical imaging market hit $25 billion in 2017. 2019. https://technology.informa.com/610963/global-medical-imaging-market-hit-25-billion-in-2017
- 97.Statista Share of pharmaceutical revenue worldwide in 2017, by country. 2020. https://www.statista.com/statistics/784420/share-of-worldwide-pharma-revenue-by-country/
- 98.Grand View Research Asia Pacific PoC diagnostics market analysis and segment forecasts to 2020. 2014. https://www.grandviewresearch.com/industry-analysis/asia-pacific-poc-diagnostics-market
- 99.The World Bank Medical Diagnostic Imaging (MDI) equipment: understanding how to procure Medical Diagnostic Imaging equipment. 2019. http://pubdocs.worldbank.org/en/258511553620191211/Procurement-Guidance-MDI-Equipment-Buyers.pdf
- 100.Hanf M, Van-Melle A, Fraisse F, Roger A, Carme B, Nacher M. Corruption kills: estimating the global impact of corruption on children deaths. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bruckner T. The ignored pandemic. 2019. http://ti-health.org/wp-content/uploads/2019/03/IgnoredPandemic-WEB-v3.pdf
- 102.UN Sustainable Development Goals. Goal 3: ensure healthy lives and promote well-being for all at all ages. 2020. https://www.un.org/sustainabledevelopment/health/
- 103.United Nations Office of Drugs and Crime United Nations Convention against Corruption. 2017. https://www.unodc.org/unodc/en/corruption/uncac.html
- 104.International Federation of Red Cross and Red Crescent Societies IFRC statement on fraud in Ebola operations. 2017. https://media.ifrc.org/ifrc/ifrc-statement-fraud-ebola-operations/
- 105.Gadre A, Shukla A. Penguin Random House India; Gurgaon: 2016. Dissenting diagnosis: voices of conscience from the medical profession. [Google Scholar]
- 106.Garcia E, Kundu I, Kelly M, Soles R. The American Society for Clinical Pathology's 2018 vacancy survey of medical laboratories in the United States. Am J Clin Pathol. 2019;152:155–168. doi: 10.1093/ajcp/aqz046. [DOI] [PubMed] [Google Scholar]
- 107.Rose-Ackerman S, Tan Y. Corruption in the procurement of pharmaceuticals and medical equipment in China: the incentives facing multinationals, domestic firms and hospital officials. Pacific Basin Law Journal. 2014;32:1–53. [Google Scholar]
- 108.Rao VM, Levin DC. The overuse of diagnostic imaging and the Choosing Wisely initiative. Ann Intern Med. 2012;157:574–576. doi: 10.7326/0003-4819-157-8-201210160-00535. [DOI] [PubMed] [Google Scholar]
- 109.Binagwaho A, Garcia PJ, Gueye B, et al. Eliminating deaths from cervical cancer—report of a panel at the 7th Annual Symposium on Global Cancer Research, a Satellite Meeting at the Consortium of Universities for Global Health 10th Annual Meeting. J Glob Oncol. 2019;5:1–7. doi: 10.1200/JGO.19.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.African Strategies for Advancing Pathology Group Members Quality pathology and laboratory diagnostic services are key to improving global health outcomes: improving global health outcomes is not possible without accurate disease diagnosis. Am J Clin Pathol. 2015;143:325–328. doi: 10.1309/AJCP6K0DZCNVCSCI. [DOI] [PubMed] [Google Scholar]
- 111.Denkinger CM, Nicolau I, Ramsay A, Chedore P, Pai M. Are peripheral microscopy centres ready for next generation molecular tuberculosis diagnostics? Eur Respir J. 2013;42:544–547. doi: 10.1183/09031936.00081113. [DOI] [PubMed] [Google Scholar]
- 112.Ndihokubwayo JB, Maruta T, Ndlovu N, et al. Implementation of the World Health Organization Regional Office for Africa Stepwise Laboratory Quality Improvement Process Towards Accreditation. Afr J Lab Med. 2016;5:280. doi: 10.4102/ajlm.v5i1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fonjungo PN, Kebede Y, Messele T, et al. Laboratory equipment maintenance: a critical bottleneck for strengthening health systems in sub-Saharan Africa? J Public Health Policy. 2012;33:34–45. doi: 10.1057/jphp.2011.57. [DOI] [PubMed] [Google Scholar]
- 114.WHO . World Health Organization; Geneva: 2017. Human resources for medical devices, the role of biomedical engineers. [Google Scholar]
- 115.Perry L, Malkin R. Effectiveness of medical equipment donations to improve health systems: how much medical equipment is broken in the developing world? Med Biol Eng Comput. 2011;49:719–722. doi: 10.1007/s11517-011-0786-3. [DOI] [PubMed] [Google Scholar]
- 116.Chawla S, Kurani S, Wren SM, et al. Electricity and generator availability in LMIC hospitals: improving access to safe surgery. J Surg Res. 2018;223:136–141. doi: 10.1016/j.jss.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 117.WHO Medical devices: donation of medical equipment. 2020. https://www.who.int/medical_devices/management_use/manage_donations/en/
- 118.Centers for Disease Control and Prevention. National Institute of Health Biosafety in microbiological and biomedical laboratories, 5th edn. 2009. https://www.cdc.gov/labs/pdf/CDC-BiosafetymicrobiologicalBiomedicalLaboratories-2009-P.pdf
- 119.Sulis G, Adam P, Nafade V, et al. Antibiotic prescription practices in primary care in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bruxvoort KJ, Leurent B, Chandler CIR, et al. The impact of introducing malaria rapid diagnostic tests on fever case management: a synthesis of ten studies from the ACT Consortium. Am J Trop Med Hyg. 2017;97:1170–1179. doi: 10.4269/ajtmh.16-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Carter JY, Lema OE, Wangai MW, Munafu CG, Rees PH, Nyamongo JA. Laboratory testing improves diagnosis and treatment outcomes in primary health care facilities. Afr J Lab Med. 2012;1:8. doi: 10.4102/ajlm.v1i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.O'Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. 2014. https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf
- 123.Liu JX, Goryakin Y, Maeda A, Bruckner T, Scheffler R. Erratum to: Global health workforce labor market projections for 2030. Hum Resour Health. 2017;15:18. doi: 10.1186/s12960-017-0193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Scheil-Adlung X. Health workforce benchmarks for universal health coverage and sustainable development. Bull World Health Organ. 2013;91:888–889. doi: 10.2471/BLT.13.126953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Campbell J, Dussault G, Buchan J, et al. A universal truth: no health without a workforce. 2013. http://www.who.int/workforcealliance/knowledge/resources/hrhreport2013/
- 126.US Bureau of Labor Statistics Occupational Employment Statistics. 2020. https://www.bls.gov/oes/current/oes_nat.htm#29-0000/
- 127.The Health Foundation. The King's Fund. Nuffield Trust . The Health Foundation, The King's Fund, Nuffield Trust; London: 2018. The health care workforce in England. Make or break? [Google Scholar]
- 128.Silvestrin C. Europe's looming radiology capacity challenge. A comparative study. 2016. https://www.telemedicineclinic.com/wp-content/uploads/2016/11/Europes_looming_radiology_capacity_challenge-A_comparitive_study.pdf
- 129.Nelson AM, Milner DA, Rebbeck TR, Iliyasu Y. Oncologic care and pathology resources in Africa: survey and recommendations. J Clin Oncol. 2016;34:20–26. doi: 10.1200/JCO.2015.61.9767. [DOI] [PubMed] [Google Scholar]
- 130.Rosman DA, Bamporiki J, Stein-Wexler R, Harris RD. Developing diagnostic radiology training in low resource countries. Curr Radiol Rep. 2019;7:27. [Google Scholar]
- 131.Association of American Medical Colleges 2019 State Physician Workforce Data Report. 2019. https://www.aamc.org/data-reports/workforce/report/state-physician-workforce-data-report/
- 132.Metter DM, Colgan TJ, Leung ST, Timmons CF, Park JY. Trends in the US and Canadian pathologist workforces from 2007 to 2017. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Statista Number of licensed clinical pathology specialists in Brazil between 2013 and 2018. 2020. https://www.statista.com/statistics/962717/number-licensed-clinical-pathology-specialists-brazil/
- 134.Statista Number of licensed radiologists and diagnostic imaging professionals in Brazil between 2013 and 2018. 2020. https://www.statista.com/statistics/962206/number-licensed-radiologists-brazil/#:~:text=Brazil%3A%20number%20of%20radiologists%202013%2D2018&text=This%20statistic%20shows%20the%20number,from%209%2C670%20specialists%20in%202015/
- 135.WHO Regional Office for Africa WHO Guide for the Stepwise Laboratory Improvement Process Towards Accreditation in the African Region (with checklist) 2013. http://www.who.int/tb/laboratory/afro-slipta-checklist-guidance.pdf
- 136.Kinfu Y, Dal Poz MR, Mercer H, Evans DB. The health worker shortage in Africa: are enough physicians and nurses being trained? Bull World Health Organ. 2009;87:225–230. doi: 10.2471/BLT.08.051599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bennett A, Garcia E, Schulze M, et al. Building a laboratory workforce to meet the future: ASCP Task Force on the Laboratory Professionals Workforce. Am J Clin Pathol. 2014;141:154–167. doi: 10.1309/AJCPIV2OG8TEGHHZ. [DOI] [PubMed] [Google Scholar]
- 138.The King's Fund The health care workforce in England: make or break? 2018. https://www.kingsfund.org.uk/sites/default/files/2018-11/The%20health%20care%20workforce%20in%20England.pdf
- 139.Mullan F, Frehywot S, Omaswa F, et al. Medical schools in sub-Saharan Africa. Lancet. 2011;377:1113–1121. doi: 10.1016/S0140-6736(10)61961-7. [DOI] [PubMed] [Google Scholar]
- 140.Chen C, Buch E, Wassermann T, et al. A survey of sub-Saharan African medical schools. Hum Resour Health. 2012;10:4. doi: 10.1186/1478-4491-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rehani MM. Patient radiation exposure and dose tracking: a perspective. J Med Imaging (Bellingham) 2017;4 doi: 10.1117/1.JMI.4.3.031206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cancedda C, Cotton P, Shema J, et al. Health professional training and capacity strengthening through international academic partnerships: the first five years of the Human Resources for Health Program in Rwanda. Int J Health Policy Manag. 2018;7:1024–1039. doi: 10.15171/ijhpm.2018.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen L, Evans T, Anand S, et al. Human resources for health: overcoming the crisis. Lancet. 2004;364:1984–1990. doi: 10.1016/S0140-6736(04)17482-5. [DOI] [PubMed] [Google Scholar]
- 144.Jimba M, Cometto G, Yamamoto T, Shiao L, Huicho L, Sheikh M. Health workforce: the critical pathway to universal health coverage. 2010. http://healthsystemsresearch.org/hsr2010/images/stories/10health_workforce.pdf
- 145.Mwaikambo L, Ohkubo S, Cassaniti J. Collaborative learning and stakeholder engagement: lessons and implications of the revitalization of the Continuing Professional Development policy for health workers in Nigeria. J Knowl. 2013;9:63–78. [Google Scholar]
- 146.Kasvosve I, Ledikwe JH, Phumaphi O, et al. Continuing professional development training needs of medical laboratory personnel in Botswana. Hum Resour Health. 2014;12:46. doi: 10.1186/1478-4491-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Elbireer AM, Jackson JB, Sendagire H, Opio A, Bagenda D, Amukele TK. The good, the bad, and the unknown: quality of clinical laboratories in Kampala, Uganda. PLoS One. 2013;8 doi: 10.1371/journal.pone.0064661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Amukele TK, Jones R, Elbireer A. Test cost and test accuracy in clinical laboratories in Kampala, Uganda. Am J Clin Pathol. 2018;149:522–529. doi: 10.1093/ajcp/aqy017. [DOI] [PubMed] [Google Scholar]
- 149.National Accreditation Board for Testing & Calibration Laboratories NABL Newsletter. 2019. https://nabl-india.org/wp-content/uploads/2020/03/12-December-2019.pdf
- 150.Centers for Medicare and Medicaid Services Clinical Laboratory Improvement Amendments (CLIA) 2020. https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA#:~:text=The%20Centers%20for%20Medicare%20%26%20Medicaid,covers%20approximately%20260%2C000%20laboratory%20entities/
- 151.Viegas SO, Azam K, Madeira C, et al. Mozambique's journey toward accreditation of the National Tuberculosis Reference Laboratory. Afr J Lab Med. 2017;6:491. doi: 10.4102/ajlm.v6i2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Binagwaho A. Changes in global health governance are needed for sustainable health development in Africa. Afr Pol J. 2019;14:3–8. [Google Scholar]
- 153.Ahmed S, Begum T, Smith O. Diagnostic study of public financial management: to strengthen health financing and service delivery in Bangladesh. 2019. http://documents.worldbank.org/curated/en/856271558671301506/pdf/Diagnostic-Study-of-Public-Financial-Management-To-Strengthen-Health-Financing-and-Service-Delivery-in-Bangladesh.pdf
- 154.WHO Content Sheet 10–1: Overview of External Quality Assessment (EQA) https://www.who.int/ihr/training/laboratory_quality/10_b_eqa_contents.pdf
- 155.Carter JY. External quality assessment in resource-limited countries. Biochem Med (Zagreb) 2017;27:97–109. doi: 10.11613/BM.2017.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.International Atomic Energy Agency Comprehensive clinical audits of diagnostic radiology practices: a tool for quality improvement: Quality Assurance Audit for Diagnostic Radiology Improvement and Learning (QUAADRIL) 2010. https://www-pub.iaea.org/MTCD/Publications/PDF/Pub1425_web.pdf
- 157.Kruskal JB, Eisenberg R, Sosna J, Yam CS, Kruskal JD, Boiselle PM. Quality initiatives: quality improvement in radiology: basic principles and tools required to achieve success. Radiographics. 2011;31:1499–1509. doi: 10.1148/rg.316115501. [DOI] [PubMed] [Google Scholar]
- 158.United States Department of Labor Occupational Health and Safety Administration: ionizing radiation. 2019. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1096
- 159.United States Environmental Protection Agency. Interagency Working Group on Medical Radiation Radiation protection guidance for diagnostic and interventional X-ray procedures: Federal Guidance Report No. 14. 2014. https://www.epa.gov/sites/production/files/2015-05/documents/fgr14-2014.pdf
- 160.New Zealand Legislation Radiation Safety Act 2016. 2020. http://www.legislation.govt.nz/act/public/2016/0006/latest/DLM6339517.html
- 161.Singapore Statutes Online Radiation Protection Act (chapter 262) 2008. https://sso.agc.gov.sg/Act/RPA2007/
- 162.International Atomic Energy Agency Radiation protection and safety in medical uses of ionizing radiation. 2018. https://www-pub.iaea.org/MTCD/Publications/PDF/PUB1775_web.pdf
- 163.International Atomic Energy Agency Radiation protection and safety of radiation sources: International Basic Safety Standards. 2014. https://www-pub.iaea.org/MTCD/Publications/PDF/Pub1578_web-57265295.pdf
- 164.International Atomic Energy Agency Strengthening radiation safety infrastructure in Latin America and the Caribbean: concrete project activities to begin in 2020. 2020. https://www.iaea.org/newscenter/news/strengthening-radiation-safety-infrastructure-in-latin-america-and-the-caribbean-concrete-project-activities-to-begin-in-2020#_ftnref1
- 165.US Food and Drug Administration Initiative to reduce unnecessary radiation exposure from medical imaging. 2019. https://www.fda.gov/radiation-emitting-products/radiation-safety/initiative-reduce-unnecessary-radiation-exposure-medical-imaging/
- 166.WHO To x-ray or not to x-ray? 2016. https://www.who.int/news-room/feature-stories/detail/to-x-ray-or-not-to-x-ray-
- 167.Medicines and Healthcare Products Regulatory Agency London. Safety guidelines for magnetic resonance imaging equipment in clinical use. 2014. http://www.ismrm.org/smrt/files/con2033065.pdf
- 168.American College of Radiology Committee on MR Safety ACR manual on MR safety. 2020. https://www.acr.org/-/media/ACR/Files/Radiology-Safety/MR-Safety/Manual-on-MR-Safety.pdf
- 169.Ter Haar G. Ultrasonic imaging: safety considerations. Interface Focus. 2011;1:686–697. doi: 10.1098/rsfs.2011.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schroeder LF, Guarner J, Amukele TK. Essential diagnostics for the use of World Health Organization essential medicines. Clin Chem. 2018;64:1148–1157. doi: 10.1373/clinchem.2017.275339. [DOI] [PubMed] [Google Scholar]
- 171.DeStigter K, Pool K-L, Leslie A, Hussain S, Tan BS. Donoso Bach L, Andronikou S. Optimizing integrated imaging service delivery by tier in low-resource health systems. Insights Imaging (in press). [DOI] [PMC free article] [PubMed]
- 172.Horton S, Fleming KA, Kuti M, et al. The top 25 laboratory tests by volume and revenue in five different countries. Am J Clin Pathol. 2019;151:446–451. doi: 10.1093/ajcp/aqy165. [DOI] [PubMed] [Google Scholar]
- 173.Broadband Commission for Sustainable Development Working group on digital and AI in health reimagining global health through artificial intelligence: the roadmap to AI maturity. 2020. https://www.broadbandcommission.org/Documents/working-groups/AIinHealth_Report.pdf
- 174.WHO Seventy-first World Health Assembly: digital health. 2018. https://apps.who.int/gb/ebwha/pdf_files/WHA71/A71_R7-en.pdf?ua=1
- 175.WHO WHO guideline: recommendations on digital interventions for health system strengthening. 2019. https://www.who.int/reproductivehealth/publications/digital-interventions-health-system-strengthening/en/ [PubMed]
- 176.Labrique A, Agarwal S, Tamrat T, Mehl G. WHO Digital Health Guidelines: a milestone for global health. NPJ Digit Med. 2020;3:1–3. doi: 10.1038/s41746-020-00330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Siegel EL, Reiner B. Work flow redesign: the key to success when using PACS. 2002. J Digit Imaging. 2003;16:164–168. doi: 10.1007/s10278-002-6006-9. discussion 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ho J, Ahlers SM, Stratman C, et al. Can digital pathology result in cost savings? A financial projection for digital pathology implementation at a large integrated health care organization. J Pathol Inform. 2014;5:33. doi: 10.4103/2153-3539.139714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Schwartz LH, Panicek DM, Berk AR, Li Y, Hricak H. Improving communication of diagnostic radiology findings through structured reporting. Radiology. 2011;260:174–181. doi: 10.1148/radiol.11101913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Baidoshvili A, Bucur A, van Leeuwen J, van der Laak J, Kluin P, van Diest PJ. Evaluating the benefits of digital pathology implementation: time savings in laboratory logistics. Histopathology. 2018;73:784–794. doi: 10.1111/his.13691. [DOI] [PubMed] [Google Scholar]
- 181.Stathonikos N, Nguyen TQ, Spoto CP, Verdaasdonk MAM, van Diest PJ. Being fully digital: perspective of a Dutch academic pathology laboratory. Histopathology. 2019;75:621–635. doi: 10.1111/his.13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Williams BJ, Lee J, Oien KA, Treanor D. Digital pathology access and usage in the UK: results from a national survey on behalf of the National Cancer Research Institute's CM-Path initiative. J Clin Pathol. 2018;71:463–466. doi: 10.1136/jclinpath-2017-204808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Unternaehrer J, Grobholz R, Janowczyk A, Zlobec I. Current opinion, status and future development of digital pathology in Switzerland. J Clin Pathol. 2020;73:341–346. doi: 10.1136/jclinpath-2019-206155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pantanowitz L, Sinard JH, Henricks WH, et al. Validating whole slide imaging for diagnostic purposes in pathology: guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2013;137:1710–1722. doi: 10.5858/arpa.2013-0093-CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pencina MJ, Goldstein BA, D'Agostino RB. Prediction models—development, evaluation, and clinical application. N Engl J Med. 2020;382:1583–1586. doi: 10.1056/NEJMp2000589. [DOI] [PubMed] [Google Scholar]
- 186.Schwalbe N, Wahl B. Artificial intelligence and the future of global health. Lancet. 2020;395:1579–1586. doi: 10.1016/S0140-6736(20)30226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ahuja AS. The impact of artificial intelligence in medicine on the future role of the physician. PeerJ. 2019;7 doi: 10.7717/peerj.7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yasaka K, Abe O. Deep learning and artificial intelligence in radiology: current applications and future directions. PLoS Med. 2018;15 doi: 10.1371/journal.pmed.1002707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Liu X, Faes L, Kale AU, et al. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: a systematic review and meta-analysis. Lancet Digit Health. 2019;1:e271–e297. doi: 10.1016/S2589-7500(19)30123-2. [DOI] [PubMed] [Google Scholar]
- 190.Wilbur DC. Digital cytology: current state of the art and prospects for the future. Acta Cytol. 2011;55:227–238. doi: 10.1159/000324734. [DOI] [PubMed] [Google Scholar]
- 191.Smith KP, Wang H, Durant TJ, et al. Applications of artificial intelligence in clinical microbiology diagnostic testing. Clin Microbiol Newsl. 2020;42:61–70. [Google Scholar]
- 192.Pell R, Oien K, Robinson M, et al. The use of digital pathology and image analysis in clinical trials. J Pathol Clin Res. 2019;5:81–90. doi: 10.1002/cjp2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Niazi MKK, Parwani AV, Gurcan MN. Digital pathology and artificial intelligence. Lancet Oncol. 2019;20:e253–e261. doi: 10.1016/S1470-2045(19)30154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Colling R, Pitman H, Oien K, et al. Artificial intelligence in digital pathology: a roadmap to routine use in clinical practice. J Pathol. 2019;249:143–150. doi: 10.1002/path.5310. [DOI] [PubMed] [Google Scholar]
- 195.Pons E, Braun LM, Hunink MG, Kors JA. Natural language processing in radiology: a systematic review. Radiology. 2016;279:329–343. doi: 10.1148/radiol.16142770. [DOI] [PubMed] [Google Scholar]
- 196.Stivaros SM, Gledson A, Nenadic G, Zeng XJ, Keane J, Jackson A. Decision support systems for clinical radiological practice—towards the next generation. Br J Radiol. 2010;83:904–914. doi: 10.1259/bjr/33620087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Karami M, Safdari R. From information management to information visualization: development of radiology dashboards. Appl Clin Inform. 2016;7:308–329. doi: 10.4338/ACI-2015-08-RA-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jackups R, Jr, Szymanski JJ, Persaud SP. Clinical decision support for hematology laboratory test utilization. Int J Lab Hematol. 2017;39(suppl 1):128–135. doi: 10.1111/ijlh.12679. [DOI] [PubMed] [Google Scholar]
- 199.Global Digital Health Index The state of digital health. 2019. https://static1.squarespace.com/static/5ace2d0c5cfd792078a05e5f/t/5d4dcb80a9b3640001183a34/1565379490219/State+of+Digital+Health+2019.pdf
- 200.Labrique A, Vasudevan L, Mehl G, Rosskam E, Hyder AA. Digital health and health systems of the future. Glob Health Sci Pract. 2018;6(suppl 1):S1–S4. doi: 10.9745/GHSP-D-18-00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hinrichs SH, Zarcone P. The Affordable Care Act, meaningful use, and their impact on public health laboratories. Public Health Rep. 2013;128(suppl 2):7–9. doi: 10.1177/00333549131280S202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Abel G. Current status and future prospects of point-of-care testing around the globe. Expert Rev Mol Diagn. 2015;15:853–855. doi: 10.1586/14737159.2015.1060126. [DOI] [PubMed] [Google Scholar]
- 203.Pai NP, Vadnais C, Denkinger C, Engel N, Pai M. Point-of-care testing for infectious diseases: diversity, complexity, and barriers in low- and middle-income countries. PLoS Med. 2012;9 doi: 10.1371/journal.pmed.1001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Land KJ, Boeras DI, Chen XS, Ramsay AR, Peeling RW. REASSURED diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nat Microbiol. 2019;4:46–54. doi: 10.1038/s41564-018-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Melgarejo S, Schaub A, Nobel VE. Point of care ultrasound: an overview. 2017. https://www.acc.org/latest-in-cardiology/articles/2017/10/31/09/57/point-of-care-ultrasound
- 206.Akkus Z, Cai J, Boonrod A, et al. A survey of deep-learning applications in ultrasound: artificial intelligence-powered ultrasound for improving clinical workflow. J Am Coll Radiol. 2019;16:1318–1328. doi: 10.1016/j.jacr.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 207.Shaw JLV. Practical challenges related to point of care testing. Pract Lab Med. 2015;4:22–29. doi: 10.1016/j.plabm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bose R, Saxon LA. The democratization of diagnosis: bringing the power of medical diagnosis to the masses. EClinicalMedicine. 2019;8:6–7. doi: 10.1016/j.eclinm.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ingold H, Mwerinde O, Ross AL, et al. The Self-Testing AfRica (STAR) Initiative: accelerating global access and scale-up of HIV self-testing. J Int AIDS Soc. 2019;22(suppl 1) doi: 10.1002/jia2.25249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Oketch SY, Kwena Z, Choi Y, et al. Perspectives of women participating in a cervical cancer screening campaign with community-based HPV self-sampling in rural western Kenya: a qualitative study. BMC Womens Health. 2019;19:75. doi: 10.1186/s12905-019-0778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Asiimwe S, Ross JM, Arinaitwe A, et al. Expanding HIV testing and linkage to care in western Uganda with community health extension workers. J Int AIDS Soc. 2017;20(suppl 4) doi: 10.7448/IAS.20.5.21633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Samal J. Health seeking behaviour among tuberculosis patients in India: a systematic review. J Clin Diagn Res. 2016;10:LE01–LE06. doi: 10.7860/JCDR/2016/19678.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lama TP, Khatry SK, Katz J, LeClerq SC, Mullany LC. Illness recognition, decision-making, and care-seeking for maternal and newborn complications: a qualitative study in Sarlahi District, Nepal. J Health Popul Nutr. 2017;36(suppl 1):45. doi: 10.1186/s41043-017-0123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Nymark C, Mattiasson AC, Henriksson P, Kiessling A. Emotions delay care-seeking in patients with an acute myocardial infarction. Eur J Cardiovasc Nurs. 2014;13:41–47. doi: 10.1177/1474515113475953. [DOI] [PubMed] [Google Scholar]
- 215.Dako-Gyeke P, Amazigo UV, Halpaap B, Manderson L. Social innovation for health: engaging communities to address infectious diseases. Infect Dis Poverty. 2020;9:98. doi: 10.1186/s40249-020-00721-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ortblad KF, Kibuuka Musoke D, Ngabirano T, et al. HIV self-test performance among female sex workers in Kampala, Uganda: a cross-sectional study. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2018-022652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Abou Tayoun AN, Burchard PR, Malik I, Scherer A, Tsongalis GJ. Democratizing molecular diagnostics for the developing world. Am J Clin Pathol. 2014;141:17–24. doi: 10.1309/AJCPA1L4KPXBJNPG. [DOI] [PubMed] [Google Scholar]
- 218.Johnson CC, Kennedy C, Fonner V, et al. Examining the effects of HIV self-testing compared to standard HIV testing services: a systematic review and meta-analysis. J Int AIDS Soc. 2017;20 doi: 10.7448/IAS.20.1.21594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tucker JD, Bien CH, Peeling RW. Point-of-care testing for sexually transmitted infections: recent advances and implications for disease control. Curr Opin Infect Dis. 2013;26:73–79. doi: 10.1097/QCO.0b013e32835c21b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Stevens DR, Vrana CJ, Dlin RE, Korte JE. A global review of HIV self-testing: themes and implications. AIDS Behav. 2018;22:497–512. doi: 10.1007/s10461-017-1707-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Howitt P, Darzi A, Yang GZ, et al. Technologies for global health. Lancet. 2012;380:507–535. doi: 10.1016/S0140-6736(12)61127-1. [DOI] [PubMed] [Google Scholar]
- 222.WHO Digital implementation investment guide (DIIG): integrating digital interventions into health programmes. 2020. https://apps.who.int/iris/handle/10665/334306/
- 223.Pai M. Global Health Technologies: time to re-think the ‘trickle down’ model. 2020. https://www.forbes.com/sites/madhukarpai/2020/02/17/global-health-technologies-time-to-re-think-the-trickle-down-model/#2104824e44d9/
- 224.The Royal College of Pathologists of Australasia Pathology update 2020: cracking the code. 2020. https://www.rcpa.edu.au/getattachment/09f70fb7-44b7-49ac-8ede-7377fc2fa1e2/Pathology-Update-2020-Preliminary-Scientific-Progr.aspx
- 225.Pang J, Wang MX, Ang IYH, et al. Potential rapid diagnostics, vaccine and therapeutics for 2019 Novel Coronavirus (2019-nCoV): a systematic review. J Clin Med. 2020;9:9. doi: 10.3390/jcm9030623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bhoo-Pathy N, Yip CH, Hartman M, et al. Adjuvant! Online is overoptimistic in predicting survival of Asian breast cancer patients. Eur J Cancer. 2012;48:982–989. doi: 10.1016/j.ejca.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 227.The Academy of Medical Sciences Improving the development and deployment of diagnostics in southeast Asia: workshop report. 2018. https://acmedsci.ac.uk/file-download/94242103
- 228.Medical Research Council MRC Applied Global Health Research Board. 2020. https://mrc.ukri.org/funding/browse/mrc-applied-global-health-research/applied-global-health-research-board/
- 229.FIND World Health Organization endorses Truenat Tests for initial diagnosis of tuberculosis and detection of rifampicin resistance. 2020. https://www.finddx.org/newsroom/pr-02jul20/
- 230.Deng S, Sun Y, Xia H, et al. Accuracy of commercial molecular diagnostics for the detection of pulmonary tuberculosis in China: a systematic review. Sci Rep. 2019;9 doi: 10.1038/s41598-019-41074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Cision PR Newswire Qure.ai bags industry's first 4-in-1 FDA clearance for medical imaging AI. 2020. https://www.prnewswire.com/news-releases/qureai-bags-industrys-first-4-in-1-fda-clearance-for-medical-imaging-ai-301085918.html
- 232.Looi LM, Chua KB. Lessons from the Nipah virus outbreak in Malaysia. Malays J Pathol. 2007;29:63–67. [PubMed] [Google Scholar]
- 233.Tsou IYY, Liew CJY, Tan BP, et al. Planning and coordination of the radiological response to the coronavirus disease 2019 (COVID-19) pandemic: the Singapore experience. Clin Radiol. 2020;75:415–422. doi: 10.1016/j.crad.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.WHO Global Observatory on Health R&D: health researchers (in full-time equivalent) per million inhabitants, by income group (second set of charts) 2020. https://www.who.int/observatories/globalobservatory-on-health-research-and-development/indicators/health-researchers-in-full-time-equivalent-per-millioninhabitants-by-income-group-second-set-of-charts
- 235.The World Bank Research and development expenditure (% of GDP) 2020. https://data.worldbank.org/indicator/GB.XPD.RSDV.GD.ZS?view=map
- 236.Ondoa P, Ndlovu N, Keita MS, et al. Preparing national tiered laboratory systems and networks to advance diagnostics in Africa and meet the continent's health agenda: insights into priority areas for improvement. Afr J Lab Med. 2020;9 doi: 10.4102/ajlm.v9i2.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lusaka Times Ministry of Health signs a five-year “multi-million euro” contract with Philips Electronics (detailed) 2010. https://www.lusakatimes.com/2010/06/16/ministry-health-signs-fiveyear-multimillion-euro-contract-philips-electronics-detailed/
- 238.Olotch C. Managed equipment services (MES)—healthcare for sustainable development: the Kenya MES experience. https://docplayer.net/57372467-Managed-equipment-services-meshealthcare-for-sustainable-development-the-kenya-mesexperience-cynthia-olotch.html
- 239.Suchman L, Hart E, Montagu D. Public–private partnerships in practice: collaborating to improve health finance policy in Ghana and Kenya. Health Policy Plan. 2018;33:777–785. doi: 10.1093/heapol/czy053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Barral W, Haas A. Public–private partnership in Brazil. Int Lawyer. 2007;3:957–973. [Google Scholar]
- 241.Schroeder LF, Amukele T. Medical laboratories in sub-Saharan Africa that meet international quality standards. Am J Clin Pathol. 2014;141:791–795. doi: 10.1309/AJCPQ5KTKAGSSCFN. [DOI] [PubMed] [Google Scholar]
- 242.International Finance Corporation Public–private partnership stories—Moldova: Radiology and Diagnostic Imaging Center. 2012. https://www.ifc.org/wps/wcm/connect/fb59d94b-03de-4e1e-bb39-2b932527e545/PPPStories_Moldova_RadiologyDiagnosticsImagingCenter.pdf?MOD=AJPERES&CVID=lHoReFL/
- 243.Dabas H, Deo S, Sabharwal M, et al. Initiative for Promoting Affordable and Quality Tuberculosis Tests (IPAQT): a market-shaping intervention in India. BMJ Glob Health. 2019;4 doi: 10.1136/bmjgh-2019-001539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Sayed S, Cherniak W, Lawler M, et al. Improving pathology and laboratory medicine in low-income and middle-income countries: roadmap to solutions. Lancet. 2018;391:1939–1952. doi: 10.1016/S0140-6736(18)30459-8. [DOI] [PubMed] [Google Scholar]
- 245.Crisp N, Chen L. Global supply of health professionals. N Engl J Med. 2014;370:950–957. doi: 10.1056/NEJMra1111610. [DOI] [PubMed] [Google Scholar]
- 246.Architectural Guide Yangon Yangon General Hospital. https://www.yangongui.de/yangon-general-hospital/
- 247.Kyaw A. self-published; Yangon: 2013. A turbulent life: Personal memoirs of Dr Kyaw Aung. [Google Scholar]
- 248.Otero HJ, Andronikou S, Grassi DC, Silva CT. Providing expert pediatric teleradiology services around the globe: the World Federation of Pediatric Imaging experience. J Am Coll Radiol. 2020;17:53–55. doi: 10.1016/j.jacr.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 249.Rosenkrantz AB, Hanna TN, Steenburg SD, Tarrant MJ, Pyatt RS, Friedberg EB. The current state of teleradiology across the United States: a national survey of radiologists' habits, attitudes, and perceptions on teleradiology practice. J Am Coll Radiol. 2019;16:1677–1687. doi: 10.1016/j.jacr.2019.05.053. [DOI] [PubMed] [Google Scholar]
- 250.Bashshur RL, Krupinski EA, Thrall JH, Bashshur N. The empirical foundations of teleradiology and related applications: a review of the evidence. Telemed J E Health. 2016;22:868–898. doi: 10.1089/tmj.2016.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.US Food and Drug Administration FDA allows marketing of first whole slide imaging system for digital pathology. 2017. https://www.fda.gov/news-events/press-announcements/fda-allows-marketing-first-whole-slide-imaging-system-digital-pathology
- 252.Britnell M. Oxford University Press; Oxford: 2019. Human: solving the global workforce crisis in healthcare. [Google Scholar]
- 253.Salto-Tellez M, Maxwell P, Hamilton P. Artificial intelligence—the third revolution in pathology. Histopathology. 2019;74:372–376. doi: 10.1111/his.13760. [DOI] [PubMed] [Google Scholar]
- 254.WHO Task shifting: global recommendations and guidelines. 2008. https://www.who.int/workforcealliance/knowledge/resources/taskshifting_guidelines/en/
- 255.du Plessis J, Pitcher R. Towards task shifting? A comparison of the accuracy of acute trauma-radiograph reporting by medical officers and senior radiographers in an African hospital. Pan Afr Med J. 2015;21:308. doi: 10.11604/pamj.2015.21.308.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Wuni AR, Courtier N, Kelly D. Opportunities for radiographer reporting in Ghana and the potential for improved patient care. Radiography (Lond) 2020;26:e120–e125. doi: 10.1016/j.radi.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 257.Galukande M, Kiguli-Malwadde E. Rethinking breast cancer screening strategies in resource-limited settings. Afr Health Sci. 2010;10:89–92. [PMC free article] [PubMed] [Google Scholar]
- 258.Torres-Mejía G, Smith RA, Carranza-Flores ML, et al. Radiographers supporting radiologists in the interpretation of screening mammography: a viable strategy to meet the shortage in the number of radiologists. BMC Cancer. 2015;15:410. doi: 10.1186/s12885-015-1399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Simmonds FM, Cohn JE, Mafaune HW, Nyamundaya TH, Mahomva A, Chadambuka A. Task shifting for point-of-care early infant diagnosis: a comparison of the quality of testing between nurses and laboratory personnel in Zimbabwe. Hum Resour Health. 2020;18:4. doi: 10.1186/s12960-020-0449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Liu R, Huang L, Yang Q, et al. Investigation on task shifting of HIV/AIDS follow-up management workers in new launched areas, China. Int J Environ Res Public Health. 2018;15 doi: 10.3390/ijerph15102132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Sy TRL, Padmawati RS, Baja ES, Ahmad RA. Acceptability and feasibility of delegating HIV counseling and testing for TB patients to community health workers in the Philippines: a mixed methods study. BMC Public Health. 2019;19:185. doi: 10.1186/s12889-019-6497-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Philips M, Zachariah R, Venis S. Task shifting for antiretroviral treatment delivery in sub-Saharan Africa: not a panacea. Lancet. 2008;371:682–684. doi: 10.1016/S0140-6736(08)60307-4. [DOI] [PubMed] [Google Scholar]
- 263.Jaskiewicz W, Tulenko K. Increasing community health worker productivity and effectiveness: a review of the influence of the work environment. Hum Resour Health. 2012;10:38. doi: 10.1186/1478-4491-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Berer M. Task-shifting: exposing the cracks in public health systems. Reprod Health Matters. 2009;17:4–8. doi: 10.1016/S0968-8080(09)33458-8. [DOI] [PubMed] [Google Scholar]
- 265.Mumtaz Z, Patterson P. Does task shifting among parts of a weak health system help? Lancet Glob Health. 2017;5:e734–e735. doi: 10.1016/S2214-109X(17)30256-5. [DOI] [PubMed] [Google Scholar]
- 266.Seidman G, Atun R. Does task shifting yield cost savings and improve efficiency for health systems? A systematic review of evidence from low-income and middle-income countries. Hum Resour Health. 2017;15:29. doi: 10.1186/s12960-017-0200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Fitzgerald L, Gathara D, McKnight J, Nzinga J, English M. Are health care assistants part of the long-term solution to the nursing workforce deficit in Kenya? Hum Resour Health. 2020;18:79. doi: 10.1186/s12960-020-00523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Smith S, Deveridge A, Berman J, et al. Task-shifting and prioritization: a situational analysis examining the role and experiences of community health workers in Malawi. Hum Resour Health. 2014;12:24. doi: 10.1186/1478-4491-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.WHO Health employment and economic growth: an evidence base. 2017. https://www.who.int/hrh/resources/WHO-HLC-Report_web.pdf?ua=1
- 270.WHO . World Health Organization; Geneva: 2006. Scaling up health workforce production: a concept paper towards the implementation of World Health Assembly resolution WHA 59.23. [Google Scholar]
- 271.Petti CA, Polage CR, Quinn TC, Ronald AR, Sande MA. Laboratory medicine in Africa: a barrier to effective health care. Clin Infect Dis. 2006;42:377–382. doi: 10.1086/499363. [DOI] [PubMed] [Google Scholar]
- 272.Bates I, Bekoe V, Asamoa-Adu A. Improving the accuracy of malaria-related laboratory tests in Ghana. Malar J. 2004;3:38. doi: 10.1186/1475-2875-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Shen Z, Li S, Wang Z. The government regulation of clinical laboratory quality in China [J] Chin J Lab Med. 2006;29:6–8. (in Chinese). [Google Scholar]
- 274.National Center for Clinical Laboratories Introduction of NCCL [EB/OL] 2020. https://www.nccl.org.cn/jggkCn in Chinese.
- 275.China National Health Commission Implementation Checklist for Grade 3 Hospital Accreditation (2011 edition) [EB/OL] 2011. http://www.nhc.gov.cn/wjw/gfxwj/201304/0404f9cd71764ab29b2365e069cfbf2d/files/02b5187a195c42e0855e4e27445fc2f0.pdf in Chinese.
- 276.China National Health Commission Implementation Checklist for Grade 2 Hospital Accreditation (2012 edition) [EB/OL] 2012. http://www.nhc.gov.cn/cmsresources/mohylfwjgs/cmsrsdocument/doc14985.pdf in Chinese.
- 277.China National Center for Clinical Laboratories Arrangement and precautions for 2020 national EQA of quality indicators for clinical laboratory medicine [EB/OL] 2020. http://www.nccl.org.cn/showMeetDetail?code=09&id=139 in Chinese.
- 278.China National Health Commission Response to No. 3795 advice in the fifth session of the 12th National People's Congress [EB/OL] 2017. http://www.nhc.gov.cn/wjw/jiany/201712/aa7cb4c327bc4ac38ce6375ab41debba.shtml in Chinese.
- 279.Henan Health Commission Clinical laboratories must participate in EQA[EB/OL] 2007. http://www.hnwsjsw.gov.cn/contents/111/7775.shtml in Chinese.
- 280.Jiangxi Center for Clinical Laboratories Announcement on applying for 2018 clinical laboratory medicine EQA in Jiangxi Province [EB/OL] 2017. http://www.jxccl.com/webnotice/?type=detail&id=221 in Chinese.
- 281.Zhang S, Chu Y. Problems and solutions during implementation of ISO 17025 in medical laboratory [J] Shanghai Quality. 2004;2:60–63. (in Chinese). [Google Scholar]
- 282.Zhai P. A decade of devotion along with joys and hardships: memories of the past ten years on China accreditation of medical laboratory. Chin J Clin Lab Mgt. 2014;2:193–197. [Google Scholar]
- 283.Lyu J. What is China National Accreditation Service for Conformity Assessment and the application criteria for accreditation? Chin J Lab Med. 2007;30:275. (in Chinese). [Google Scholar]
- 284.Yang Z. The laboratory accreditation and regulations of clinical laboratory in China. Clin Biochem. 2009;42:310. doi: 10.1016/j.clinbiochem.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 285.Duan M, Kang F, Zhao H, et al. Analysis and evaluation of the external quality assessment results of quality indicators in laboratory medicine all over China from 2015 to 2018. Clin Chem Lab Med. 2019;57:812–821. doi: 10.1515/cclm-2018-0983. [DOI] [PubMed] [Google Scholar]
- 286.China National Accreditation Service for Conformity Assessment Statistics of accredited laboratories by 2020–4-30 [EB/OL] 2020. https://www.cnas.org.cn/rkxx/systjxx/images/2020/05/12/1589263721593077691.doc in Chinese.
- 287.Patel V, Sindhwani G, Gupta M, et al. A comprehensive approach towards quality and safety in diagnostic imaging services: our experience at a rural tertiary health care center. J Clin Diagn Res. 2017;11:TC10–TC16. doi: 10.7860/JCDR/2017/29545.10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.WHO The Access to COVID-19 Tools (ACT) Accelerator. 2020. https://www.who.int/initiatives/act-accelerator#:~:text=The%20ACT%2DAccelerator%20brings%20together,united%20response%20against%20COVID%2D19.&text=The%20ACT%2DAccelerator%20is%20organized,vaccines%20and%20health%20system%20strengthening
- 289.Mugambi ML, Palamountain KM, Gallarda J, Drain PK. Exploring the case for a Global Alliance for Medical Diagnostics Initiative. Diagnostics (Basel) 2017;7:8. doi: 10.3390/diagnostics7010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.WHO WHO and FIND formalize strategic collaboration to drive universal access to essential diagnostics. 2020. https://www.who.int/news-room/detail/10-02-2020-who-and-find-formalize-strategic-collaboration-to-drive-universal-access-to-essential-diagnostics
- 291.Meara JG, Leather AJ, Hagander L, et al. Global Surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Lancet. 2015;386:569–624. doi: 10.1016/S0140-6736(15)60160-X. [DOI] [PubMed] [Google Scholar]
- 292.Atun R, Bhakta N, Denburg A, et al. Sustainable care for children with cancer: a Lancet Oncology Commission. Lancet Oncol. 2020;21:e185–e224. doi: 10.1016/S1470-2045(20)30022-X. [DOI] [PubMed] [Google Scholar]
- 293.WHO Continuity and coordination of care: a practice brief to support implementation of the WHO Framework on integrated people-centred health services. 2018. https://apps.who.int/iris/handle/10665/274628/
- 294.European Medicines Agency Human regulatory: medical devices. 2019. https://www.ema.europa.eu/en/human-regulatory/overview/medical-devices/
- 295.WHO Prequalification. 2020. https://www.who.int/rhem/prequalification/en/
- 296.Dansie LS, Odoch WD, Årdal C. Industrial perceptions of medicines regulatory harmonization in the East African Community. PLoS One. 2019;14 doi: 10.1371/journal.pone.0218617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Rugera SP, McNerney R, Poon AK, et al. Regulation of medical diagnostics and medical devices in the East African community partner states. BMC Health Serv Res. 2014;14:524. doi: 10.1186/s12913-014-0524-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Ministry of Health & Family Welfare Government of India National Health Mission: NHM Free Drugs Service Initiative. 2020. https://nhm.gov.in/index1.php?lang=1&level=2&sublinkid=1218&lid=192
- 299.Ministry of Health & Family Welfare Government of India National Health Mission: Indian Public Health Standards. 2020. https://nhm.gov.in/index1.php?lang=1&level=2&sublinkid=971&lid=154
- 300.The Gazette of India Clinical Establishment Rule. 2018. http://clinicalestablishments.gov.in/WriteReadData/4161.pdf in Hindi and English.
- 301.The Gazette of India Health Ministry notifies medical devices rules. 2017. https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/download_file_division.jsp?num_id=MTAwOQ== in Hindi and English.
- 302.Indian Council of Medical Research National Essential Diagnostics List. 2019. main.icmr.nic.in/sites/default/files/guidelines/NEDL_2019.pdf
- 303.National Health Laboratory Service Annual performance plan—fiscal year: 2019/20. 2019. https://www.nhls.ac.za/wp-content/uploads/2019/11/NHLS_APP_2019.pdf
- 304.BusinessWire Kenyan Ministry of Health Selects GE Healthcare as a strategic partner to support healthcare modernization program. 2015. https://www.businesswire.com/news/home/20150224006553/en/Kenyan-Ministry-Health-Selects-GE-Healthcare-Strategic
- 305.The Global Fund Sourcing and management of health products: health product procurement. 2020. https://www.theglobalfund.org/en/sourcing-management/health-products/
- 306.Wafula F, Agweyu A, Macintyre K. Regional and temporal trends in malaria commodity costs: an analysis of Global Fund data for 79 countries. Malar J. 2013;12:466. doi: 10.1186/1475-2875-12-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Affordable and Quality Tuberculosis Tests (IPAQT): a market-shaping intervention in India. BMJ Glob Health. 2019;4 doi: 10.1136/bmjgh-2019-001539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Rosman R. Senegal: 10-minute coronavirus test may be on its way—for $1. 2020. https://www.aljazeera.com/news/2020/03/senegal-10-minute-coronavirus-test-1-200327053901231.html
- 309.Médecins sans Frontières Access campaign. 2020. https://msfaccess.org/about-us
- 310.Devex Ecumenical Pharmaceutical Network. 2020. https://www.devex.com/organizations/ecumenical-pharmaceutical-network-114465
- 311.Treatment Action Group Treatment Action Group statement on the high price of Cepheid's Xpert test for COVID-19. 2020. https://www.treatmentactiongroup.org/statement/treatment-action-group-statement-on-the-high-price-of-cepheids-xpert-test-for-covid-19/
- 312.Strand H, Rustad SA, Urdal H, Nygård HM. Trends in armed conflict. 1946–2018. https://reliefweb.int/sites/reliefweb.int/files/resources/Strand%2C%20Rustad%2C%20Urdal%2C%20Nyg%C3%A5rd%20-%20Trends%20in%20Armed%20Conflict%2C%201946%E2%80%932018%2C%20Conflict%20Trends%203-2019.pdf
- 313.UNHCR Global trends: forced displacement in 2018. 2018. https://www.unhcr.org/globaltrends2018/
- 314.Dewachi O, Skelton M, Nguyen VK, et al. Changing therapeutic geographies of the Iraqi and Syrian wars. Lancet. 2014;383:449–457. doi: 10.1016/S0140-6736(13)62299-0. [DOI] [PubMed] [Google Scholar]
- 315.Kandel N, Chungong S, Omaar A, Xing J. Health security capacities in the context of COVID-19 outbreak: an analysis of International Health Regulations annual report data from 182 countries. Lancet. 2020;395:1047–1053. doi: 10.1016/S0140-6736(20)30553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Ilunga Kalenga O, Moeti M, Sparrow A, Nguyen V-K, Lucey D, Ghebreyesus TA. The ongoing Ebola epidemic in the Democratic Republic of Congo, 2018–2019. N Engl J Med. 2019;381:373–383. doi: 10.1056/NEJMsr1904253. [DOI] [PubMed] [Google Scholar]
- 317.Bricknell MCM, Nadin M. Lessons from the organisation of the UK medical services deployed in support of Operation TELIC (Iraq) and Operation HERRICK (Afghanistan) J R Army Med Corps. 2017;163:273–279. doi: 10.1136/jramc-2016-000720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Brooks AJ, Price V, Simms M. FAST on operational military deployment. Emerg Med J. 2005;22:263–265. doi: 10.1136/emj.2004.014308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Bowsher G, Papamichail A, El Achi N, et al. A narrative review of health research capacity strengthening in low and middle-income countries: lessons for conflict-affected areas. Global Health. 2019;15:23. doi: 10.1186/s12992-019-0465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Brown V, Guerin PJ, Legros D, Paquet C, Pécoul B, Moren A. Research in complex humanitarian emergencies: the Médecins Sans Frontières/Epicentre experience. PLoS Med. 2008;5:e89. doi: 10.1371/journal.pmed.0050089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.HM Government National Security Capability Review. 2018. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/705347/6.4391_CO_National-Security-Review_web.pdf
- 322.Bernard R, Sullivan R. The use of HUMINT in epidemics: a practical assessment. Intell Natl Secur. 2020;35:493–501. [Google Scholar]
- 323.Heller PS. Understanding fiscal space. 2005. https://www.imf.org/external/pubs/ft/pdp/2005/pdp04.pdf
- 324.Tandon A, Cashin C. Assessing public expenditure on health from a fiscal space perspective. 2010. https://openknowledge.worldbank.org/bitstream/handle/10986/13613/560530WP0Box341penditureFiscalSpace.pdf?sequence=1&isAllowed=y
- 325.Reddy CL, Peters AW, Jumbam DT, et al. Innovative financing to fund surgical systems and expand surgical care in low-income and middle-income countries. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2020-002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Glenday G, Bharali I, Wang Z. Enhancing domestic revenues: constraints and opportunities. A cross-country comparative study of tax capacity, effort and gaps. 2019. http://centerforpolicyimpact.org/wp-content/uploads/sites/18/2019/04/CPIGH-Report_Tax-report_Enhancing-Domestic-Revenues__April-2019_FINAL.pdf
- 327.Barroy H, Sparkes S, Dale E, Mathonnat J. Can low- and middle-income countries increase domestic fiscal space for health: a mixed-methods approach to assess possible sources of expansion. Health Syst Reform. 2018;4:214–226. doi: 10.1080/23288604.2018.1441620. [DOI] [PubMed] [Google Scholar]
- 328.WHO Earmarked tobacco taxes: lessons learnt from nine countries. 2015. http://apps.who.int/iris/bitstream/10665/206007/1/9789241510424_eng.pdf?ua=1
- 329.Summan A, Stacey N, Birckmayer J, Blecher E, Chaloupka FJ, Laxminarayan R. The potential global gains in health and revenue from increased taxation of tobacco, alcohol and sugar-sweetened beverages: a modelling analysis. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2019-002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.The World Bank Industry engagement program. Large medical diagnostic imaging equipment market review and action plan. 2017. http://pubdocs.worldbank.org/en/452061518466768717/Procurement-Market-Review-and-Action-Plan.pdf
- 331.The World Bank World Bank Group's operational response to COVID-19. 2020. https://www.worldbank.org/en/about/what-we-do/brief/world-bank-group-operational-response-covid-19-coronavirus-projects-list
- 332.European Commission EU and Bill & Melinda Gates Foundation join forces to support health services in Africa. 2018. https://ec.europa.eu/commission/presscorner/detail/en/IP_18_6135
- 333.The European Investment Bank Coronavirus outbreak: EIB Group's response. 2020. https://www.eib.org/en/about/initiatives/covid-19-response/index.htm
- 334.WHO Health systems financing. The path to universal health coverage. World Health Report 2010. 2010. https://www.who.int/whr/2010/10_summary_en.pdf?ua=1 [DOI] [PMC free article] [PubMed]
- 335.Atun R, Knaul FM, Akachi Y, Frenk J. Innovative financing for health: what is truly innovative? Lancet. 2012;380:2044–2049. doi: 10.1016/S0140-6736(12)61460-3. [DOI] [PubMed] [Google Scholar]
- 336.Atun R, Silva S, Knaul FM. Innovative financing instruments for global health 2002–15: a systematic analysis. Lancet Glob Health. 2017;5:e720–e726. doi: 10.1016/S2214-109X(17)30198-5. [DOI] [PubMed] [Google Scholar]
- 337.Social Finance Social Impact Bonds. 2020. http://www.socialfinance.org.uk/wp-content/uploads/2016/07/SIBs-Early-Years_Social-Finance_2016_Final3.pdf
- 338.Social Finance Investing in social outcomes: Development Impact Bonds. 2020. http://www.socialfinance.org.uk/investing-in-social-outcomes-development-impact-bonds/
- 339.United States Agency for International Development The Utkrisht Development Bond. 2017. https://www.usaid.gov/sites/default/files/documents/1864/Utkrish-Impact-Bond-Brochure-November-2017.pdf
- 340.Medpac June 2018 data book: healthcare spending and the Medicare program. 2020. http://www.medpac.gov/docs/default-source/data-book/jun18_databookentirereport_sec.pdf
- 341.Organisation for Economic Co-operation and Development Health care utilization: diagnostic exams. 2020. https://stats.oecd.org/index.aspx?queryid=30160#Date
- 342.Hasel J. Testing early, testing late: four countries' approaches to COVID-19 testing compared. 2020. https://ourworldindata.org/covid-testing-us-uk-korea-italy
- 343.Hricak H, Abdel-Wahab M, Atun R, et al. Medical imaging and nuclear medicine: a Lancet Oncology Commission. Lancet Oncol. 2021;22:e136–e172. doi: 10.1016/S1470-2045(20)30751-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Ward ZJ, Grover S, Scott AM, et al. The role and contribution of treatment and imaging modalities in global cervical cancer management: survival estimates from a simulation-based analysis. Lancet Oncol. 2020;21:1089–1098. doi: 10.1016/S1470-2045(20)30316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Ward ZJ, Scott AM, Hricak H, et al. Estimating the impact of treatment and imaging modalities on 5-year net survival of 11 cancers in 200 countries: a simulation-based analysis. Lancet Oncol. 2020;21:1077–1088. doi: 10.1016/S1470-2045(20)30317-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Ward ZJ, Scott AM, Hricak H, Atun R. Global costs, health benefits, and economic benefits of scaling up treatment and imaging modalities for survival of 11 cancers: a simulation-based analysis. Lancet Oncol. 2021;22:341–350. doi: 10.1016/S1470-2045(20)30750-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Erzse A, Stacey N, Chola L, Tugendhaft A, Freeman M, Hofman K. The direct medical cost of type 2 diabetes mellitus in South Africa: a cost of illness study. Glob Health Action. 2019;12 doi: 10.1080/16549716.2019.1636611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Geldsetzer P, Bloom DE, Humair S, Bärnighausen T. In: Prioritizing development: a cost–benefit analysis of the UN's Sustainable Development Goals. Lomborg B, editor. Cambridge University Press; Cambridge: 2018. Targets for the Post-2015 Development Agenda Post-2015 Consensus. [Google Scholar]
- 349.Vassall A. Analyzing the benefit-cost ratio of interventions to improve the outcomes for those with drug-susceptible and multi-drug resistant tuberculosis. 2016. https://www.copenhagenconsensus.com/sites/default/files/vassall_tb.pdf
- 350.Vassall A. Benefits and costs of the tuberculosis targets for the post 2015 development agenda. 2014. https://www.copenhagenconsensus.com/sites/default/files/health_perspective_tb_-_vassall.pdf
- 351.Nayagam S, Conteh L, Sicuri E, et al. Cost-effectiveness of community-based screening and treatment for chronic hepatitis B in The Gambia: an economic modelling analysis. Lancet Glob Health. 2016;4:e568–e578. doi: 10.1016/S2214-109X(16)30101-2. [DOI] [PubMed] [Google Scholar]
- 352.WHO Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach—second edition. 2016. https://www.paho.org/en/documents/consolidated-guidelines-use-antiretroviral-drugs-treating-and-preventing-hivinfection [PubMed]
- 353.Messonier ML, Corso PS, Teutsch SM, Haddix AC, Harris JR. An ounce of prevention...what are the returns? Second edition, 1999. Am J Prev Med. 1999;16:248–263. doi: 10.1016/s0749-3797(99)00002-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.