Assessment of response to COVID-19 vaccines in patients with inflammatory and autoimmune diseases showed that impaired response is more associated with the type of disease modifying anti-rheumatic drugs (DMARDs) the patient is using than the underlying disease.1, 2, 3 As found by Laura Boekel and collaegues4 and other study groups,1 rituximab is associated with deeply impaired immune responses after COVID-19 vaccination. Data remain sparse for more modern targeted DMARDs, such as JAK inhibitors. Here, we report immune responses to COVID-19 vaccination in patients treated with JAK inhibitors.
The MAJIK-SFR Registry is a nationwide, multicentre, prospective study (NCT04602091) including adult patients initiating JAK inhibitors for rheumatoid arthritis or psoriatic arthritis at 59 rheumatology centres in France that has been ongoing since October, 2019. Treatment was chosen by the recruiting physicians and patients are being followed-up for 5 years, even if they change treatment during this time. Here, we report on patients in this cohort who were being treated with JAK inhibitors at the time of COVID-19 vaccination and who had a serological assessment at least 2 weeks after completion of their full vaccination scheme. A full vaccination scheme was defined as two dose of BNT162b2 (tozinameran; Pfizer–BioNTech), CX-024414 (elasomeran; Moderna) or ChAdOx1 nCoV-19 (AstraZeneca); one dose of Ad.26.COV2.S (Janssen); or previous SARS-CoV-2 infection followed by one dose of any of those vaccines. Serological assessment for concentrations of IgG (or total) anti-spike antibodies was done at each centre using commercially available assays (appendix p 2). We used the cutoff value indicated in the manufacturers' instructions to define response. To identify factors associated with non-response, we compared characteristics of responders and non-responders using Fisher's exact test for categorical variables and the Mann–Whitney U test and the Kruskall-Wallis test for variance on ranks for continuous variables. A two-sided p value of 0·05 or less was considered to be significant. Ethical approval for this study was granted by the local ethics committee (CPP Sud Méditerranée II, ID-RCB-2018-A02671-54). Patients gave written informed consent.
We included 113 patients from 13 centres in this analysis, for whom COVID-19 serology was done between March 16 and July 22, 2021. Of 113 patients, 98 (87%) had rheumatoid arthritis and 15 (13%) had psoriatic arthritis. The mean age was 61·8 years (SD 12·5) and 81 (72%) patients were female and 32 (28%) were male. 56 (50%) were taking baricitinib, 30 (27%) were taking tofacitinib, and 27 (24%) were taking upadacitinib (appendix pp 3–4). Except for two (2%) patients, JAK inhibitor treatment was not stopped before or after vaccination. Nine (8%) patients previously had a PCR-confirmed SARS-CoV-2 infection: four (44%) of nine received one dose of vaccine, as recommended in France, and five (56%) received two doses. In the 104 patients without previous SARS-CoV-2 infection, five (5%) received a third dose of vaccine. Mean interval between the two doses (or the first two doses in those who received three) was 4·5 weeks (SD 0·96) for BNT162b2 and CX-024414 and 11·3 weeks (2·0) for ChAdOx1 nCoV-19. Serological assessment was done after a mean of 8·7 weeks (SD 5·2) after the last dose of vaccine.
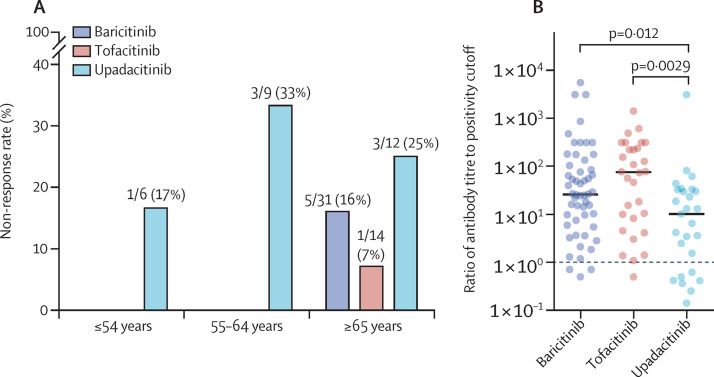
The overall response rate (ie, the proportion of patients with detectable anti-spike antibodies per manufacturer's cutoff values) was 88% (100 of 113). Non-responders were older than responders (p=0·020). The rate of non-response was higher with upadacitinib (seven [26%] of 27 patients) than with baricitinib (five [9%] of 56) or tofacitinib (one [3%] of 30), but mean age at the time of vaccination did not differ between upadacitinib and other JAK inhibitors (61·4 years [SD 11·5] vs 61·9 years [12·8]; p=0·51). All non-responders were aged 65 years or older, except for four of the seven non-responders receiving upadacitinib (figure ). Antibody titres (measured by the ratio of anti-spike titres to the threshold of positivity) were higher in patients treated with tofacitinib and baricitinib than in those treated with upadacitinib (figure). The interval between last vaccine dose and serological assessment was slightly longer in non-responders than in responders (11·3 weeks [SD 5·9] vs 8·3 [5·0]; p=0·099). No other parameters, including concomitant use of methotrexate, corticosteroids, dose of JAK inhibitor, disease activity, or type of vaccine were associated with non-response. Previous use of rituximab (18 [16%]) was not associated with non-response, although last rituximab injections occurred more than 6 months before vaccination (appendix p 3).
Figure.
Immune response to COVID-19 vaccination for patient treated with JAK inhibitor
(A) Proportion of non-responders in each age group (≤54, 55–64, and ≥65 years) according to their JAK inhibitor. (B) Antibody titres (measured as ratio of antibody titre to assay's positivity cutoff) for each patient according to JAK inhibitor. Solid horizontal bars show the median for each inhibitor group and the horizontal dashed line shows the threshold for positive response to the vaccines. Comparisons are based on Kruskall-Wallis analysis of variance on ranks (n=111, the antibody titres were not available for two patients, the result of the serology was only indicated as being positive or negative).
Methotrexate negatively affects response to influenza and pneumococcal vaccines,2, 3 and so probably also negatively affects response to COVID-19 vaccines, but to a lesser extent than rituximab.1, 5 Here, in combination with a JAK inhibitor, methotrexate did not affect serological responses. As in Boekel and colleagues' study, we observed that older age was associated with an impaired response to COVID-19 vaccination. Interestingly, apart from age, the only factor associated with non-response was the use of upadacitinib. Vaccine responses in patients treated with JAK inhibitors have been little investigated. Data from a placebo-controlled trial showed diminished responsiveness to pneumococcal vaccine but not influenza vaccine in patients treated with tofacitinib.6 An uncontrolled study of patients treated with baricitinib showed satisfactory responses to pneumococcal vaccine, while tetanus vaccine responses were less robust.7 No data exist on vaccine responses in patients treated with upadacitinib.
Our study is the first to assess response to COVID-19 vaccines in a comparatively large number of patients on JAK inhibitors. However, our study has some limitations, such as the use of several different serological assays, and different timepoints for assessment. Nevertheless, all assays were approved by the US Food and Drug Administration.8 Also, most of our patients received mRNA vaccines, thus we cannot draw conclusions on the immunogenicity of viral vector vaccines. However, in Boekel and colleagues' study, vaccine type did not seem to affect the rate of seroconversion.4
Our data indicate that the overall response rate to COVID-19 vaccine in patients treated with JAK inhibitors remained high, in line with rates reported with other immunosuppressants.1, 4 However, non-response might occur principally in older patients. Additionally, upadacitinib was the JAK inhibitor associated with the highest rate of non-response. These results need to be confirmed in a prospective trial but suggest that in patients aged 65 years and older or treated with upadacitinib, or both, serological assessment might be recommended to guide clinical decision in non-responders (eg, whether a third dose or vaccination of family members might be needed, or both). Assessment of cellular immune response in the non-responders is also warranted to determine if cellular immunity might have been acquired.9
RS has received consulting fees from GSK, BMS, Fresenius Kabi, Boerhinger, Jansen, Amgen, Pfizer, and Roche. AB has received honoraria from AbbVie, BMS, Celgene, Janssen, Eli Lilly, MSD, Novartis, Biogen, and UCB. M-ET has received honoraria or participated on advisory boards for Galapagos, Eli Lilly, SOBI, Boehringer Ingelheim, Fresenius Kabi, MSD, and AbbVie and had received research support from Gilead. AR has received honoraria from AbbVie and Pfizer. J-EG has received honoraria or participated on advisory boards for AbbVie, BMS, Eli Lilly, Galapagos, Gilead, Pfizer, Roche, Sanofi, Novartis, MSD, CSL-Behrin,g and Genzyme and received research support from BMS. RF has received honoraria or participated on advisory boards for AbbVie, Eli Lilly, and Pfizer. AC has received honoraria from Chugai and Pfizer. JA has received consultancy fees from AbbVie, Sanofi, and Nordic Pharma; honoraria from Galapagos, AbbVie, BMS, Sanofi, Roche-Chugai, Nordic Pharma, Biogen, Fresenius Kabi, and MSD; and research support from Pfizer, BMS, and Novartis. XM has received consulting fees from BMS, Galapagos, Gilead, Janssen, Eli Lilly, Novartis, Pfizer, Sanofi, and UCB and research support from Ose Pharmaceutical and Pfizer. CP has received honoraria from UCB, Eli Lilly, AbbVie, Sandoz, Pfizer, and Novartis; support for attending meetings from Chugai, AbbVie, Sandoz, and Amgen; and a research grant from Pfizer. J-HS has received consulting fees or honoraria from Eli Lilly, Pfizer, AbbVie, and Galapagos. ED has received honoraria from AbbVie, Amgen, Celgene, BMS, UCB, Eli Lilly, Janssen, MSD, and Novartis. All other authors declare no competing interests. We thank the French Society of Rheumatology, the sponsor of this study. We also thank the MAJIK scientific committee and the group of MAJIK Registry investigators and Pascale Thevenot for her great help. Funding of the MAJIK Registry is supported by the French Rheumatology Society. The French Rheumatology Society received unrestricted institutional grants for conducting the MAJIK Registry from AbbVie, Galapagos, Eli Lilly, and Pfizer. The French Rheumatology Society, AbbVie, Galapagos, Eli Lilly, and Pfizer were not involved in the study design, data collection, data analysis, data interpretation, or writing of the manuscript. RS and MC contributed to study conception, study design, data collection, statistical analysis, interpretation of data and writing - original draft. RS and MC had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. J-HS, CR, ED, AB, VG, CL, SB, M-ET, AR, J-EG, RF, FC, AC, CP, XM, and JA contributed to interpretation of data and writing - critical review for intellectual concept and editing.
Supplementary Material
References
- 1.Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 2.Hua C, Barnetche T, Combe B, Morel J. Effect of methotrexate, anti-tumor necrosis factor α, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2014;66:1016–1026. doi: 10.1002/acr.22246. [DOI] [PubMed] [Google Scholar]
- 3.Subesinghe S, Bechman K, Rutherford AI, Goldblatt D, Galloway JB. A systematic review and metaanalysis of antirheumatic drugs and vaccine immunogenicity in rheumatoid arthritis. J Rheumatol. 2018;45:733–744. doi: 10.3899/jrheum.170710. [DOI] [PubMed] [Google Scholar]
- 4.Boekel L, Steenhuis M, Hooijberg F, et al. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in the Netherlands: a substudy of data from two prospective cohort studies. Lancet Rheumatol. 2021 doi: 10.1016/S2665-9913(21)00222-8. published online Aug 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bugatti S, De Stefano L, Balduzzi S, et al. Methotrexate and glucocorticoids, but not anticytokine therapy, impair the immunogenicity of a single dose of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic inflammatory arthritis. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220862. published online June 25. [DOI] [PubMed] [Google Scholar]
- 6.Winthrop KL, Silverfield J, Racewicz A, et al. The effect of tofacitinib on pneumococcal and influenza vaccine responses in rheumatoid arthritis. Ann Rheum Dis. 2016;75:687–695. doi: 10.1136/annrheumdis-2014-207191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winthrop KL, Bingham CO, 3rd, Komocsar WJ, et al. Evaluation of pneumococcal and tetanus vaccine responses in patients with rheumatoid arthritis receiving baricitinib: results from a long-term extension trial substudy. Arthritis Res Ther. 2019;21:102. doi: 10.1186/s13075-019-1883-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.US Food and Drug Administration EUA authorized serology test performance. Aug 18, 2021. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance
- 9.Bonelli MM, Mrak D, Perkmann T, Haslacher H, Aletaha D. SARS-CoV-2 vaccination in rituximab-treated patients: evidence for impaired humoral but inducible cellular immune response. Ann Rheum Dis. 2021;80:1355–1356. doi: 10.1136/annrheumdis-2021-220408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.