Abstract
Cyclophosphamide (CP) could cause severe gonadotoxicity via imbalanced activation of primordial follicles through PI3K/AKT/mTOR activation. Whether metformin, a widely prescribed anti-diabetes agent with mTOR inhibitory effect, could preserve ovarian function against CP toxicity is unknown. Female C57BL/6 mice were randomized into seven groups (n = 11), including control, CP-alone, CP + metformin, CP + sirolimus or everolimus, metformin-alone and sirolimus-alone groups. The duration of pharmaceutical treatment was 4 weeks. CP treatment significantly impaired ovarian function and fertility in mice. CP + metformin treatment significantly attenuated the gonadotoxicity comparing to CP-alone treatment (primordial follicle count: 17.6 ± 4.2 versus 10.3 ± 2.7 follicles/high-power field; P = 0.027). CP + metformin treatment also tended to increase antral follicular count (5.4 ± 1.1 versus 2.5 ± 1.6 follicles/section), serum AMH levels (4.6 ± 1.2 versus 2.0 ± 0.8 ng/ml) and the litter size (4.2 ± 1.3 versus 1.5 ± 1.0 mice per pregnancy), compared with CP-alone group. Expression of phospho-mTOR and the number of TUNEL-positive granulosa cells increased after CP treatment and decreased in the CP + metformin groups, suggesting the mTOR inhibitory and anti-apoptotic effects of metformin. In in-vitro granulosa cell experiments, the anti-apoptotic effect of metformin was blocked after inhibiting p53 or p21 function, and the expression of p53 mRNA was blocked with AMPK inhibitor, suggesting that the anti-apoptotic effect was AMPK/p53/p21-mediated. In conclusion, concurrent metformin treatment during CP therapy could significantly preserve ovarian function and fertility and could be a promising novel fertility preserving agent during chemotherapy. The relatively acceptable cost and well-established long-term safety profiles of this old drug might prompt its further clinical application at a faster pace.
Keywords: cyclophosphamide, metformin, mTOR protein, AMP-activated protein kinases, fertility preservation, apoptosis, anti-Mullerian hormone, p53, p21
Introduction
During recent decades, advances in adjuvant chemotherapy and endocrine therapies have significantly improved the survival of young cancer patients, specifically by >80% in those suffering from breast cancer (Janssen-Heijnen et al., 2014). This provides the young survivors better life expectancy; however, quality of life is then important and fertility concerns are undoubtedly prominent in patients who desire to have children (Howard-Anderson et al., 2012). Even for patients with no fertility needs, early menopause-related physical deterioration and psychological stress still have significant impact on their quality of life (Anderson et al., 2011).
Furthermore, chemoprotection toward ovarian damage has not improved at the same pace as the widespread application of chemotherapy. Depending on the different regimens applied, chemotherapeutic agents were well-known to cause premature gonadal failure via various possible mechanisms, including direct damage to oocytes, the surrounding follicular somatic cells and the vascular system, and fibrosis of ovarian stroma (Morgan et al., 2012). Not all kinds of chemotherapeutic agents carry the same risk of gonadotoxicity. There is strong experimental and clinical evidence showing that alkylating agents or DNA intercalating agents induced more severe gonadal damage (Spears et al., 2019, Szymanska et al., 2020). Among them, cyclophosphamide (CP) is the first chemotherapeutic drug reported to impair ovarian function both in human studies (Miller et al., 1971) and animal models (Miller and Cole, 1970). It is an alkylating agent which is frequently used in many childhood cancers such as leukemia and lymphoma, breast cancer and nonmalignant autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus (Peng et al., 2016). It causes intrastrand and interstrand cross-link of DNA, and further inhibits DNA replication and cell division (Morgan et al., 2012). It has been demonstrated to damage both primordial follicles (Meng et al., 2014) and growing follicles (Yuksel et al., 2015). The reported underlying pathologies included granulosa cell apoptosis, stroma atresia (Yuksel et al., 2015), inflammation, vascular damage (Pascuali et al., 2018) and accelerated activation of primordial follicles (Goldman et al., 2017).
One of the most popular chemoprotective agents both in basic research and clinical practice is the administration of GnRH agonist (GnRHa) during chemotherapy, especially to breast cancer patients. Researchers believed that this treatment might suppress the hypothalamus–pituitary–ovary (HPO) axis and keep the ovaries in a more quiescent status, which might be less vulnerable to external damage (Morgan et al., 2012). Horicks et al. (2018) applied a unique FSHb-deficient (−/−) mouse model to mimic the profound pituitary suppression during GnRHa treatment and the ovarian damage by CP treatment remained tremendous, suggesting that inhibition of the pituitary–gonadal axis was not involved in ovarian protection during GnRHa treatment. Although the underlying mechanisms remained unclear, GnRHa administration was still shown to preserve ovarian function and possibly fertility in some previous human studies (Moore et al., 2015; Munhoz et al., 2016). However, the number of subjects was small. Besides, a randomized control trial reported similarly low serum anti-Mullerian hormone (AMH) levels, a serum marker of ovarian reserve, 2 years after chemotherapy in GnRHa-treated and control groups (Leonard et al., 2017), suggesting that the degree of truly preserved ovarian function might have been limited.
One of the proposed mechanisms of chemotherapy-mediated ovarian damage is the premature primordial follicle activation and follicular ‘burnout’ caused by alkylating agents like CP (Kalich-Philosoph et al., 2013). A persistent imbalanced activation of the phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway was indicated as the underlying mechanism for chemotherapy-mediated ovarian damage (Chang et al., 2015). Recently, interesting research conducted by Goldman et al. (2017) revealed that blocking the mTOR pathway with the selective mTOR complex 1 (mTORC1) inhibitor everolimus and another experimental mTORC1/2 inhibitor INK128 could preserve the ovarian function and fertility in a murine model. However, due to the high cost of everolimus therapy, (∼$342 USD per day) (Perrin et al., 2015) and the high frequency of adverse effects (Paplomata et al., 2013), such as stomatitis (50%) and grade 3/4 neutropenia (52%), its clinical application on fertility preservation might be limited.
Metformin, originally used as an anti-diabetic medication, has rapidly become a promising treatment for a wide spectrum of human diseases, such as polycystic ovarian syndrome (Ou et al., 2017), preeclampsia (Romero et al., 2017), chronic liver and kidney diseases (Crowley et al., 2017), autoimmune disorders (Nath et al., 2009) and even various types of cancer (Bost et al., 2012). Many of its anti-tumor effects were related to its involvement in adenosine monophosphate-activated protein kinase (AMPK) activation, phosphorylation and activation of tuberous sclerosis complex 2 (TSC2) and the downstream inhibition of mTORC1/2 (Wang et al., 2018). Although metformin might influence the activation of ovarian follicles through mTOR inhibition, no research has shown its effects on gonadal protection. Therefore, we conducted a murine study to test whether metformin could be a promising chemoprotective agent when coadministrated with CP. The relatively low cost and the well-established safety profile of metformin, combined with its role in preserving ovarian reserve and fertility during CP treatment, as shown in this manuscript, could constitute a novel promising option for fertility preservation with clinical application in the near future.
Materials and methods
Animal study
Female C57BL/6 mice at 6–8 weeks of age (from National Laboratory Animal Center, Taiwan) were housed in controlled conditions of humidity and temperature (25 ± 1°C) with a 12-/12-h light-dark cycle (lights on at 7:00 am). All protocols were approved by the Institutional Animal Care and Use Committee, the National Taiwan University College of Medicine and the College of Public Health. The female C57BL/6 mice were randomized into seven groups (n = 11 per group). The first group was the control group without any medical treatment. The second group received only CP (Endoxan®, 200 mg/vial, Baxter Oncology Gmbh, Germany) 75 mg/kg, proved in previous murine studies to cause subfertility (Goldman et al., 2017; Zhou et al., 2017). The third group received concomitant CP and metformin (Glucophage®, 850 mg/tab, MERCK SANTE S.A.S, France) 50 mg/kg/day, which was equivalent to the maximal daily dose of 3000 mg for a 60 kg adult. The fourth group received concomitant CP and sirolimus (a specific mTORC inhibitor, Rapamune®, 0.5 mg/tab, Pfizer Ireland Pharmaceuticals, Ireland) 0.67 mg/day/kg equivalent to the maximal adult dose of 40 mg/day. The fifth group was treated with concomitant CP and everolimus (a specific mTORC inhibitor, Afinitor®, 5 mg/tab, Novartis Pharma Stein AG, Switzerland) 0.167 mg/day/kg equivalent to the maximal adult dose of 10 mg/day. Metformin-only and sirolimus-only as control groups were run in separate experiments that included no treatment and CP-alone groups.
Metformin, sirolimus and everolimus were administered by gavage on Days 1 to 5 for 4 weeks. Metformin, sirolimus and everolimus were finely ground and suspended in 100 µl double-distilled water prior to gavage. CP 75 mg/kg was provided weekly by intraperitoneal injection for 3 weeks. The selective mTORC inhibitors everolimus and sirolimus were used as positive controls.
Tissue processing of mouse ovaries and blood sampling
Five mice in each group were sacrificed 1 week after the 4-week treatment course and their ovaries were harvested and preserved in 4% (w/v) paraformaldehyde and embedded in paraffin. Serial sections of 5-μm were obtained for following experiments. All mice were anesthetized with 5% (v/v) isoflurane and blood samples for hormonal profile analyses were collected by cardiac puncture.
Ovarian histology and follicle classification
The ovaries were longitudinally and serially sectioned at 5 μm and mounted on a glass slide and stained with hematoxylin and eosin (H&E; Sigma, St. Louis, MO, USA). The number of the follicular counts per sections was determined from observation of images with a conventional birefringence microscope by two observers who were blinded to the sample origin. The number of primordial, primary, secondary and tertiary follicles (antral follicles) as well as of corpus lutea was calculated. The classification of follicular stages was performed as previously established (Myers et al., 2004). Briefly, the primordial follicles were identified when an oocyte was clearly seen with a surrounding layer of flattened granulosa cells. The primary follicles were identified by a single layer of cuboid granulosa cells encircling the oocyte. The secondary follicles possessed more than one layer of granulosa cells without visible antrum. The tertiary follicles (antral follicles) were defined when an antral space could be identified. The counts of primordial and primary follicles were determined under ×400 magnification. The results were expressed as the average count of five selected high-power fields (HPF) per section, which was then averaged again for a total of five sections per ovary. Meanwhile, the counts of secondary and tertiary follicles and corpus luteum were determined under ×40 magnification. The results were expressed as the total follicular counts per section, which as then averaged again for a total of five sections per ovary.
Immunohistochemistry
The slides were rehydrated in PBS for 15 min and the endogenous peroxidase activity was inhibited by incubating the slides with 3% H2O2/methanol for 10 min at room temperature, followed by microwave antigen retrieval in 0.01 M citrate buffer (pH 6.0). For blocking unspecific binding of antibodies, the samples were incubated with 5% nonfat milk/PBS for 30 min at room temperature. Next, slides were incubated with specific antibodies against p-mTOR (sc-293133), p53 (sc-393031), p21 (sc-817) and Ki67(sc-7846) from Santa Cruz Biotechnology (Santa Cruz, CA, USA) or p-AMPK (Abcam, #ab194920) (1:100), or for the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay (R&D Systems, Minneapolis, MN, USA) for 16 h at 4°C, at the concentration recommended by the manufacturer’s protocol. After the removal of nonbound antibodies, the samples were incubated with the peroxidase-conjugated secondary antibody for 1 h at room temperature, followed by immersing the slides in 0.06% 3,3ʹ-diaminobenzidine tetrahydrochloride (DAKO) and counterstaining with Gill's Hematoxylin V solutions. The percentage of granulosa cells with positive staining was calculated by counting the number of positive staining/total number of granulosa cells in ovarian follicles under a microscopy at ×400 magnification. Five selected fields were counted per ovary section for each staining antibody, and the average percentage of positive staining was calculated. One ovary per mouse was collected for immunohistochemistry (IHC) analysis and in total five ovaries per group (n = 5 mice) were counted. The specificity of P-mTOR or p-AMPK staining was verified by phosphatase treatment on the Formalin-fixed paraffin-embedded tissue (FFPE) serial sections of ovaries from CP-treated mice. Mock phosphatase (reaction buffer only)-treated section and calf intestinal phosphatase (CIPP, Lambda Phosphatase: sc-200312 from Santa Cruz Biotechnology; working with 100 U according to the datasheet)-treated section was added for 1 h at 37 °C prior to anti-p-mTOR or p-AMPK staining.
Reproductive hormone determination
The serum levels of estradiol, progesterone and AMH were detected with Estradiol Parameter Assay Kit (R&D Systems, Minneapolis, MN, USA), Mouse Progesterone ELISA Kit (Cusabio Biotech Co., Houston, TX, USA) and Mouse AMH ELISA kit (Cusabio Biotech Co., Houston, TX, USA), respectively, and according to the manufacturer’s instructions.
Breeding test
A breeding test was conducted 1 week after the 4-week treatment of metformin of other mTOR inhibitors (Fig. 1A). All the oral medications were stopped 1 week before breeding test. Six female mice in each group were housed together with one healthy male mouse in the same cage for 24 h for mating. Before housing together, Whitten effect (Whitten, 1956) was achieved with male-urine-soaked bedding for estrus cycle synchronization. Only one round of timed mating was conducted per female mouse and the outcome of the first pregnancy was evaluated. The number of live-born litters in each female mouse was counted 20 days after mating.
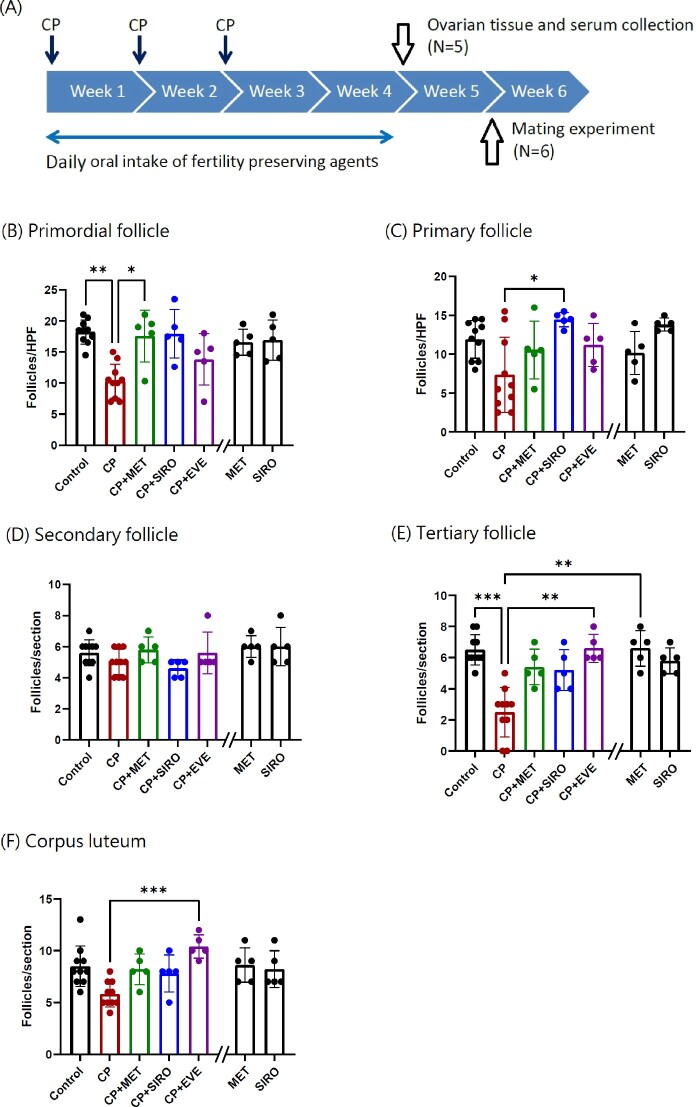
Figure 1.
The timeline of experiments and the number of ovarian follicles in different stages in each group. (A) The timeline of experiments was shown. Metformin (MET), sirolimus (SIRO) and everolimus (EVE) were administered by gavage on Days 1 to 5 for 4 weeks. Cyclophosphamide (CP) was provided after gavage on Day 1 by intraperitoneal injection weekly for 3 weeks. Other experimental details were described in Materials and methods section. (B–F) The numbers of ovarian follicles in different stages during folliculogenesis in C57BL/6 mice are shown. The mice were treated with CP-alone, MET-alone, SIRO-alone, or CP in combination with MET, SIRO or EVE. After 4 weeks of treatment, the ovaries were processed into paraffin blocks, sectioned, mounted and hematoxylin and eosin (H&E) stained for follicular counting. The number of primordial and tertiary follicles, and corpus luteum decreased in the CP-alone group compared with the control group (P = 0.0014, 0.00003, 0.073, respectively). The deleterious effects of CP on follicular counts were diminished when oral MET was given to mice (Primordial follicle: P = 0.0274). The other two specific mTOR inhibitors, SIRO and EVE, also exhibited significant protective effects against CP damage (primary follicles: CP-alone versus CP + SIRO: P = 0.024; tertiary follicles: CP-alone versus CP + EVE: P = 0.0046). (B) and (C) The Y axis represented the average follicular counts per high-power field (HPF). N = 10 mice in the control and CP-alone group, while n = 5 mice in the other groups. Each value represents the average of 2–3 HPF per animal. (D)–(F) The Y axis represented the total follicular counts per ovarian section. N = 10 mice in the control and CP-alone group, while n = 5 mice in the other groups. Data are expressed as the mean ± standard deviation. Statistical analyses were performed by nonparametric Kruskal–Wallis test with Dunn's post-hoc for multiple comparisons. *P < 0.05, **P < 0.01, ***P < 0.001. Note: The double slash mark on the X axis separated the MET-alone and SIRO-alone group from other groups because these two control groups were run in a separate experiment.
Primary culture of mouse ovarian granulosa cells
Mouse granulosa cells were isolated from freshly removed ovaries of 6-week-old C57BL/6 mice without prior treatment. Briefly, ovaries were dissected and incubated in cold serum-free Dulbecco modified Eagle's medium (DMEM)/F-12 medium with addition of 15 mM hydroxyethyl piperazineethanesulfonic acid (HEPES) (pH 7.4), human insulin (2 μg/ml), hydrocortisone (40 ng/ml) and 1× antibiotic antimycotic solution (Sigma-Aldrich) for 20 min. Then the ovaries were incubated in DMEM/F-12 medium with addition of 0.5 M sucrose and 10 mM ethylene glyco tetraacetic acid (EGTA) at 37°C for 30 min. After then, the ovaries were washed with fresh DMEM/F-12, and granulosa cells were removed from the ovaries via follicular puncture with a 25-gauge hypodermic needle. The collected cells were incubated in DMEM/F12 medium supplemented with 10% FBS (GIBCO BRL, Grand Island, NY, USA) under a humidified atmosphere of 5% CO2 at 37°C.
Western blot analyses
Mouse granulosa cells (1 × 106 cells/well) were cultured in 6-well plates. Cells were lysed using a lysis buffer (1% Triton X-100, 150 mM, NaCl, 1 mM EGTA, 1% NP-40, 1 mM NaF, 1 mM Na3VO4, 2 mM phenylmethylsulfonyl fluoride (PMSF) and 1 mg/ml aprotinin and leupeptin in PBS) and centrifuged at 13 200g (12 000rpm; Kubota Model 3500) for 25 min at 4°C. The cell lysates were quantified using the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA, USA). A total of 50-μg protein per sample was separated using SDS-PAGE, transferred onto a polyvinylidene difluoride (PVDF) membrane and immunoblotted with anti-p53 antibody (sc-393031, 1/1000), anti-p-p53 (S392) (sc-7997, 1/1000) or anti-p21 antibody (sc-817, 1/1000). Bound antibodies were detected using appropriate peroxidase-coupled secondary antibodies, an enhanced chemiluminescence detection system (ECL, Boehringer Mannheim, Indianapolis, IN, USA) and a Digital imaging system (Bio Pioneer Tech Co., New Taipei City, Taiwan). Furthermore, the membranes incubated with anti-p53 antibody were stripped in the following buffer (37.5 mM Tris, pH 6.8, 2% SDS and 1% β-mercaptoethanol) at 56°C for 20 min. Stripped membranes were washed three times using PBS with Tween (10 mM Tris, pH 7.5,150 mM NaCl, 0.05% Tween 20), followed by immunoblotting with anti-alpha-tubulin (α-t) antibody (sc-32233, Santa Cruz Biotechnology, Dallas, TX, USA).
Chemical reagents
For in-vitro studies, metformin hydrochloride (dissolved in PBS) and CP (dissolved in DMSO) was purchased from Sigma (St. Louis, MO, USA) and Tocris Bioscience (Bristol, UK), respectively. AMPK inhibitor BML-275 was purchased from Santa Cruz Biotechnology (Dallas, TX, USA) and was solubilized in DMSO. Sirolimus was purchased from Pfizer (New York, NY, USA) and dissolved in DMSO. Everolimus was purchased from Novartis Pharma Stein AG (Stein, Switzerland) and dissolved in DMSO. In all in-vivo mice experiments, the medications were finely grinded and suspended in deionization and distilled water to avoid toxicity of solution.
In IHC staining, antibodies against p-mTOR (sc-293133), p53 (sc-393031), p21 (sc-817) and Ki67(sc-7846) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The antibody against p-AMPK (#ab194920) was purchased from Abcam (Cambridge, UK). The antibody for the TUNEL assay was purchased from R&D systems (Minneapolis, MN, USA). In the primary culture of mouse granulosa cells, Dulbecco’s modified Eagle’s medium (DMEM)/F-12 and antibiotics were purchased from Life Technologies (Carlsbad, CA, USA). HEPES, human insulin, hydrocortisone, sucrose, EGTA were purchased from Sigma. In the western blot analyses, lysis buffer (1% Triton X-100, NaCl, EGTA, NP-40, NaF, Na3VO4, phenylmethylsulfonyl fluoride (PMSF), aprotinin, leupeptin and Tween were purchased from Sigma. anti-p53 antibody (sc-393031, 1/1000 in western blot), anti-p21 antibody (sc-817, 1/1000 in western blot), anti-GAPDH antibody (sc-365062, 1/5000 in western blot), were purchased from Santa Cruz Biotechnology. In the MTT assay, the MTT solution was purchased from Sigma.
Cell viability determination by MTT assay
Mouse granulosa cells were plated onto 96-well microplates at a density of 5 × 103 cells/well. After allowing adhesion for 6 h, cells were treated with different concentrations of metformin or CP. After 72 h, the cells were treated with 30 µl of MTT solution (5 mg/ml) and cultured at 37°C for 4 h in darkness. Formazan grains were dissolved in DMSO, and the absorbance at 570 nm was measured using an ELISA plate reader. The mean optical density (OD, absorbance) value was used to calculate the relative percentage of cell viability in each group by comparison to that of the control group.
Cell cycle analysis of apoptosis with flow cytometry
The cell cycles including subG1 (apoptotic phase), G1, S and G2/M of the assayed cells were determined by staining their DNA contents with propidium iodide (Sigma-Aldrich, St. Louis, MN, USA) and quantifying the degree of staining with a FACS scanner and Cell Quest software (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA). At least 10 000 cells were used for each analysis, and results were displayed as histograms.
Real‐time quantitative RT‐PCR
The p53 mRNA expression was quantified under various conditions using a fluorescein quantitative real‐time PCR detection system (Light Cycler DNA master SYBR Green I; Roche Molecular Biochemicals, Indianapolis, IN, USA). For p53: 5ʹ-TGCTCACCCTGGCTAAAGTT‐3ʹ and 5ʹ‐AATGTCTCCTGGCTCAGAGG‐3ʹ, (product of 208 bp); and for glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH): 5ʹ-CAAGGTCATCCATGACAACTTTG‐3ʹ and 5ʹ-GTCCACCACCCTGTTGCTGTAG‐3ʹ (product of 496 bp). Amplification was followed by melting curve analysis to verify the specificity of the amplicon. The levels of p53 mRNA were quantified by using GAPDH mRNA as an internal control. The changes in p53 transcript levels due to treatment were determined by comparing with those of the vehicle control.
RNA interference
Mouse granulosa cells were plated onto 6-well microplates at a density of 1 × 106 cells/well, and transfected with p53 or control siRNA at a concentration of 25 nM in serum‐free Opti‐MEM using the Oligofectamine method (Invitrogen, Carlsbad, CA, USA) for 6 h. Then the cells were cultured in DMEM/F12 medium supplemented with 10% FBS for 24 h and subjected to other experiments. The targeted siRNAs for p21, p53 and control were from Santa Cruz Biotechnology; sc-29428, sc-29436 and sc-36869, respectively. The request for siRNA sequences was declined by Santa Cruz.
Statistical analysis
Results from continuous variables were expressed as the mean ± standard deviation and were analyzed by a nonparametric Kruskal–Wallis test with Dunn's post hoc for multiple comparisons. All tests were two-tailed with a confidence level of 95% (P < 0.05). The statistical analysis was performed using the Statistical Program for Social Sciences (SPSS version 17; SPSS, Inc., Chicago, IL, USA) and Graphpad Prism (V9.0).
Results
Administrating metformin during CP treatment improved ovarian reserve and fertility function
The counts of different ovarian follicular stages in C57BL/6 female mice are shown in Fig. 1B–F. The numbers of primordial and tertiary follicles significantly decreased in the CP-alone group compared with those in the control group. The deleterious effects of CP were diminished when oral metformin was given to mice. The primordial follicle count was significantly higher in the CP + metformin group than in the CP-alone group (17.6 ± 4.2 versus 10.3 ± 2.7 follicles/high-power field; P = 0.027). The number of tertiary follicles and corpus luteum was also higher in the CP + metformin group than the CP-alone group; however, statistical significance was not reached using nonparametric Kruskal–Wallis analysis due to limited case number. Administration of the two specific mTOR inhibitors, sirolimus and everolimus, also exhibited protective effects on the ovarian follicular counts against CP damage. There were no significant differences in the number of ovarian follicles at different stages between the metformin-alone group, the sirolimus-alone group and control group.
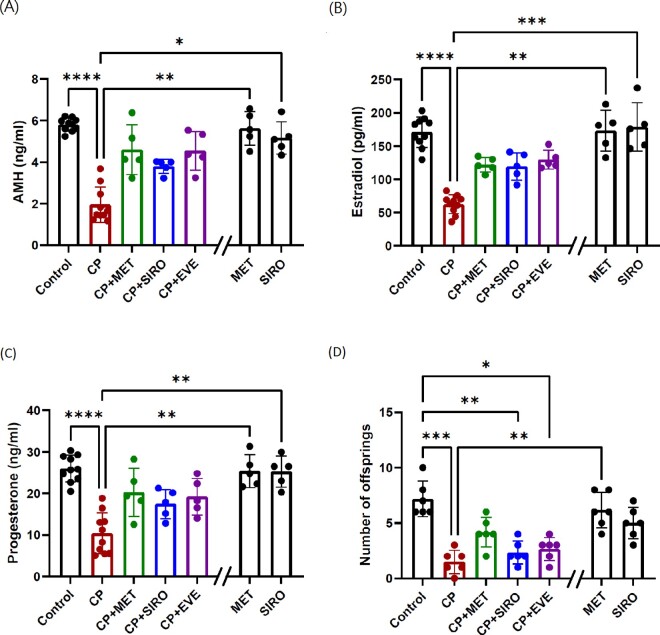
The function of ovarian granulosa cells, for instance the hormonal secretion, was also successfully preserved by metformin therapy. The serum levels of AMH (Fig. 2A), secreted by the preantral and antral follicles and was a reliable objective marker of ovarian reserve, were significantly decreased in the CP-alone group (2.0 ± 0.8 ng/ml) compared to the control group (5.8 ± 0.3 ng/ml), and reincreased in the CP + metformin group (4.6 ± 1.2 ng/ml). The serum levels of estradiol (Fig. 2B) and progesterone (Fig. 2C) were also significantly decreased by CP administration and were improved when coadministrating metformin (estradiol: control versus CP versus CP + metformin: 170.8 ± 22.8 versus 60.2 ± 14.3 versus 121.8 ± 11.0 pg/ml; progesterone: 26.0 ± 3.2 versus 10.3 ± 4.9 versus 20.2 ± 5.8 ng/ml). As for the fecundity (Fig. 2D), the number of offspring was significantly decreased in the CP-alone group but increased in the CP + metformin group, when compared to the control group (control versus CP versus CP + metformin: 7.2 ± 1.6 versus 1.5 ± 1.0 versus 4.2 ± 1.3 mice per pregnancy). Serum AMH, estradiol and progesterone levels were also increased when providing the other two specific mTOR inhibitors to mice. There were no differences in the serum concentration of AMH, estradiol and progesterone between the metformin-alone group, the sirolimus-alone group and control group.
Figure 2.
The serum hormone concentration and the number of offspring in each group. C57BL/6 mice were treated with CP-alone, metformin (MET)-alone, sirolimus (SIRO)-alone, or CP in combination with MET, SIRO or everolimus (EVE). After 4 weeks of treatment, the mice were sacrificed and serum was collected for hormonal determination. (A) The serum levels of anti-Müllerian hormone (AMH) were significantly decreased in the CP-alone group (compared with control group: P < 0.0001). Although the AMH levels increased in CP + MET, CP + SIRO and CP + EVE group, the data did not reach statistical significance probably due to limited case number. (B) The serum levels of estradiol were significantly decreased in the CP-alone group (P < 0.0001), and tended to increase in CP + MET group, CP + SIRO group and CP + EVE group. (C) The serum levels of progesterone were significantly decreased in the CP-alone group (P < 0.0001) and tended to increase in CP + MET group and CP + EVE group. (D) A breeding test was conducted 1 week after the 4-week treatment. Only one round of timed mating was conducted per female mouse and the outcome of the first pregnancy in each mouse was evaluated. The number of the offspring was significantly decreased in the CP-alone group (P < 0.0001) and tended to increase in the CP + MET group. In (A)–(C), n = 10 mice in the control and CP-alone group, while n = 5 mice in the other groups. In (D), n = 6 mice per group. Data are expressed as the mean ± standard deviation. Statistical analyses were performed by nonparametric Kruskal–Wallis test with Dunn's post-hoc for multiple comparisons. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Note: The double slash mark on the X axis separated the MET-alone and SIRO-alone group from other groups because these two control groups were run in a separate experiment.
There was a dose–response effect of metformin against the gonadotoxicity of CP (Supplementary Fig. S1). Comparing with the dose we used in initial experiments (50 mg/kg), the protective effects on the number of ovarian follicles could be achieved using a lower dose of 12.5 mg/kg, but there was no protective effect when using the dose of 2.5 mg/kg.
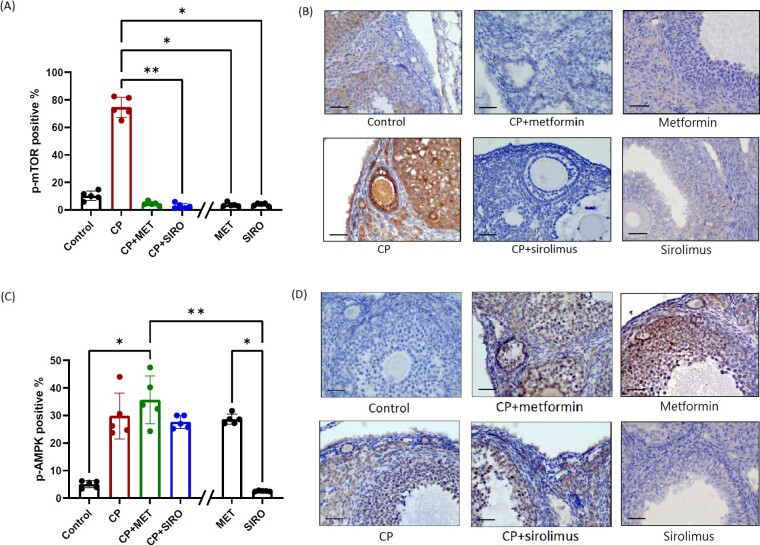
Metformin exerted mTOR inhibition and AMPK activation in ovarian tissue
To elucidate the underlying mechanisms of the chemoprotective effects of metformin, IHC staining of the molecules involved in the mTOR pathway was performed. The expression of phospho-mTOR protein in ovarian follicles was elevated after CP treatment, compared to that of the control group (Fig. 3A and B). Coadministration of metformin and any specific mTOR inhibitor exerted significant mTOR inhibitory effects on ovarian tissue thus significantly counteracting the increase of phospho-mTOR expression. The expression of upstream phospho-AMPK was increased in CP-alone, CP + metformin and CP + sirolimus groups compared with the control group; however, no difference was observed between these three treatment groups (Fig. 3C and D). There was also significantly increased phospho-AMPK expression in metformin-alone group, but not in sirolimus-alone group. The staining of phospho-mTOR and phospho-AMPK remained positive after mock phosphatase treatment, and there was no staining after calf intestinal phosphatase treatment (Supplementary Fig. S2), showing the specificity of anti-phospho-mTOR and anti-phospho-AMPK antibodies.
Figure 3.
Immunohistochemical (IHC) staining of the proteins involved in mTOR signaling pathway. C57BL/6 mice were treated with CP-alone, metformin (MET)-alone, sirolimus (SIRO)-alone or CP in combination with MET or SIRO. After 4 weeks of treatment, the ovaries were processed into paraffin sections for the IHC detection of p-mTOR (A, B) and p-AMPK (C, D) proteins. The percentage of granulosa cells with positive staining was calculated by dividing the number of positive stained cells with the total number of granulosa cells in ovarian follicles under a microscopy at ×400 magnification. One largest tertiary follicle in each section was selected to calculate the number of positively stained cells. Five sections per mice were counted from a total of 5 mice in each group (n = 5 mice). Data are expressed as the mean (%) ± standard deviation. Statistical analyses were performed by nonparametric Kruskal–Wallis test with Dunn's post-hoc for multiple comparisons. *P < 0.05, **P < 0.01. The scale bar is 50 μm. Note: The double slash mark on the X axis separated the MET-alone and SIRO-alone group from other groups because these two control groups were run in a separate experiment.
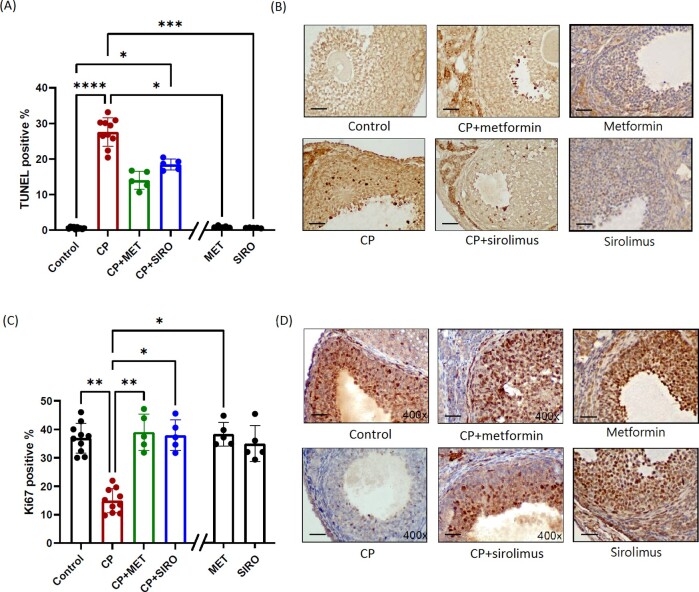
Metformin exerted an anti-apoptotic effect on ovarian granulosa cells via p53-mediated pathway
The number of TUNEL-positive cells within granulosa cells was significantly increased after CP treatment, especially in the secondary and tertiary follicles, indicating a direct cellular apoptotic effect of CP (Fig. 4A). Both metformin and sirolimus coadministration alleviated the CP-induced cellular apoptosis. Significantly lower expression of ki67 was also noted among the ovarian granulosa cells in these CP-treated mice, indicating their suppressed cellular proliferative activity (Fig. 4B). Cotreating mice with CP and either metformin or sirolimus successfully enhanced granulosa cell proliferation. The metformin-alone group and sirolimus-alone group did not have significant effects on cellular apoptosis and proliferation, comparing with the control group.
Figure 4.
IHC staining of the cellular apoptotic and proliferative markers. C57BL/6 mice were treated with CP-alone, metformin (MET)-alone, sirolimus (SIRO)-alone or CP in combination with MET or SIRO. After 4 weeks of treatment, the ovaries were processed into paraffin sections for the TUNEL assay (A, B) and Ki67 staining (C, D) to separately evaluate the degree of cellular apoptosis and proliferation. The percentage of granulosa cells with positive staining was calculated by dividing the number of positive stained cells with the total number of granulosa cells in ovarian follicles under a microscopy at ×400 magnification. One largest tertiary follicle in each section was selected to calculate the number of positively stained cells. Five sections per mice were counted. N = 10 mice in the control and CP-alone group, while n = 5 mice in the other groups. Data are expressed as the mean (%) ± standard deviation. Statistical analyses were performed by nonparametric Kruskal–Wallis test with Dunn's post-hoc for multiple comparisons. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. The scale bar is 50 μm. Note: The double slash mark on the X axis separated the MET-alone and SIRO-alone group from other groups because these two control groups were run in a separate experiment.
Cell cycle analysis by quantitation of DNA content with flow cytometry was further performed to elucidate the underlying molecular mechanism of granulosa cell apoptosis in vitro. A primary culture of mouse granulosa cells was used in these experiments. Cell viability assay was conducted to determine which concentration of CP was appropriate for these in-vitro experiments. The cellular viability slightly decreased when the CP concentration reached 1 μg/ml; therefore, this concentration was chosen (Supplementary Fig. S3A). As for the concentration of metformin, the cellular viability remained 100% up to 10 mM but started to decrease when using 20 mM (Supplementary Fig. S3B). Besides, 10 mM metformin has also been used in previous in-vitro experiments proving its mTOR1 inhibitory effect (Kalender et al., 2010). Therefore, 10 mM metformin was used in the in-vitro experiments. The CP treatment induced significant cellular apoptotic effect in the in-vitro analysis that the number of cells in the subG1 phase significantly increased in the CP-alone group (Fig. 5A and B). The coadministration of metformin with CP reversed the prolongation of subG1 phase, indicating the existence of anti-apoptotic effect among the cell line experiments. There was no significant difference in the cell cycle analysis between metformin-alone and control group.
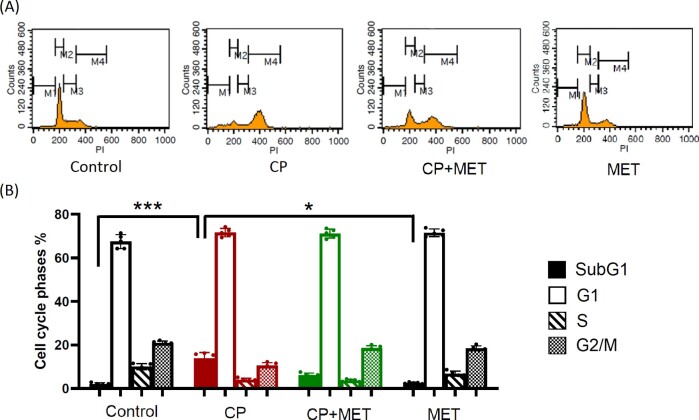
Figure 5.
Cell cycle phase analysis by flow cytometry using cultured mouse granulosa cells. Mouse granulosa cells were treated with CP-alone (1ug/ml) or with CP + metformin (MET) (10 mM) or with MET-alone for 72 h. Fresh granulosa cells were collected from five mice, and then mixed and cultured together. (A) The cell cycles, including subG1 (apoptotic phase), G1, S and G2/M of the assayed cells were determined by quantifying their DNA contents with propidium iodide (PI) staining and flow cytometry. The M1, M2, M3 and M4 bars represent the subG1, G1, S and G2/M phases, respectively. (B) The quantitative results of the cell cycle phases in each group were shown. The percentage of cells in the subG1 phase was significantly increased in the CP group (P = 0.0009) and tended to decrease when cells were cotreated with MET. There was no significant difference between control group and MET-alone group. All the experiments were repeated five times and therefore N = 5 per group. Statistical analyses were performed by nonparametric Kruskal–Wallis test with Dunn's post-hoc for multiple comparisons. *P < 0.05, ***P < 0.001.
The role of the tumor suppressor protein p53 activation was critical for cell cycle arrest and apoptosis in previous studies of tumor progression (Chen, 2016). In addition, AMPK activation and mTOR inhibition were involved in the p53-mediated apoptosis of cancer cells (Zhang et al., 2010).Therefore, whether the p53-mediated regulation was also involved in the anti-apoptotic effect of metformin, a well-known AMPK activator, was further evaluated. The expression of the cyclin-dependent kinase inhibitor p21 was also tested since it is a critical downstream effector protein of p53-induced anti-apoptosis pathway (Georgakilas et al., 2017). The apoptotic rate of cultured mouse granulosa cells significantly increased in presence of CP and decreased when coadministrating metformin (Fig. 6A). The cellular apoptosis rate reincreased after blocking p53 (Fig. 6A) or p21 (Fig. 6B) function with siRNA. Therefore, the anti-apoptotic effect of metformin on granulosa cells was p53/p21-dependent. The apoptotic toxicity of CP was not affected when blocking the p53 or p21 function. There was no difference in the apoptotic rate between the control, metformin-alone, p53 siRNA-alone and p21 siRNA-alone group (Fig. 6A and B). Sequential measurements of p53 mRNA expression in cultured granulosa cells significantly increased after 8 h of adding metformin, suggesting that metformin induced p53 mRNA transcription in a time-dependent manner (Fig. 6C). The induction of p53 by metformin was AMPK-dependent as the expression of p53 mRNA was blocked with the addition of cell-permeable AMPK inhibitor BML (Fig. 6C). The expression of p21 mRNA in cultured granulosa cells was not evident after 8 h of adding metformin but was significantly increased after 24 h, slower than the metformin-induced p53 expression (Fig. 6D). The metformin-induced p21 mRNA expression could be blocked by adding p53 siRNA, proving that p21 was the downstream effector of metformin-induced p53 expression. The western blot analysis of cultured mice granulosa cells revealed the same results. The expression of p21 protein induced by metformin treatment could be blocked when adding either p53 siRNA (Supplementary Fig. S4C) or p21 siRNA (Supplementary Fig. S4D). The metformin-induced p53 protein expression was phosphorylated on S392 by western blot analysis (Supplementary Fig. S4B). The p53/p21 activity was generally low in normal untreated ovarian tissue but was significantly elevated after CP treatment according to IHC staining (Fig. 6E and F). The coadministration of metformin with CP showed even higher expression of p53/p21 protein in the ovarian tissue, suggesting a p53/p21-dependent anti-apoptotic effect induced by metformin administration.
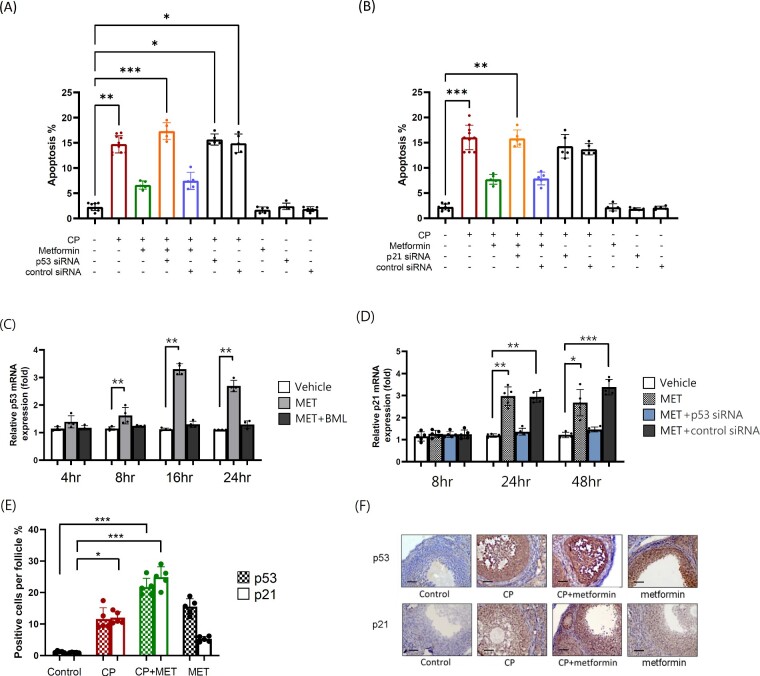
Figure 6.
Metformin exerted AMPK/p53/p21-mediated anti-apoptotic effect on cultured mouse granulosa cells. (A) Mouse granulosa cells were treated with p53 siRNA (25 nM) or control siRNA for 24 h prior CP (1ug/ml) or metformin (MET) (10 mM) treatment, after 72 h. Apoptotic cells were determined by flow cytometry with propidium iodide staining and quantified by the subG1 ratio. The experiments were repeated five times and therefore N = 5 per group. The apoptosis rate significantly increased in the CP-alone group (P = 0.0029) and tended to decrease after cotreating the cells with MET. The anti-apoptosis effect of MET was diminished after blocking the p53 activity with siRNA. (B) The experimental conditions were the same as A, except that p21 siRNA (25 nM) was applied instead of p53 siRNA. (C) Mouse granulosa cells were treated with AMPK inhibitor BML275(10 μM) for 30 min prior MET (10 mM) treatment. At indicated time periods, the expression of p53 mRNA was determined by qRT-PCR. The experiments were repeated four times and therefore N = 4 per group. Sequential measurement of p53 mRNA expression in culture granulosa cells significantly increased 8 h after the addition of MET (P = 0.0098). The expression of p53 mRNA was blocked with the addition of cell-permeable AMPK inhibitor BML. (D) The expression of p21 mRNA was determined by qRT-PCR and sequential measurement of p21 mRNA expression in cultured granulosa cells significantly increased 24 h after the addition of MET (P = 0.0095). The experiments were repeated five times and therefore N = 5 per group. (E) C57BL/6 mice were treated with CP-alone or in combination with MET or MET-alone. After 4 weeks of treatment, the ovaries were processed into paraffin sections for the p53 and p21 staining. Both p53 and p21 activity were generally low in normal untreated ovarian tissue but was elevated after CP treatment (p53: P = 0.6529; p21: P = 0.0452) as determined with IHC staining under microscopic examination at ×400 magnification. The coadministration of MET with CP increased p53 and p21 protein expression in the ovarian tissue even higher (P < 0.0004 in both). Statistical analyses were performed by nonparametric Kruskal–Wallis test with Dunn's post-hoc for multiple comparisons. *P < 0.05, **P < 0.01, ***P < 0.001. (F) Representative p53 and p21 IHC images of each group were shown. The scale bar is 50 μm.
Discussion
Our study successfully demonstrated for the first time the chemoprotective effects of metformin, a widely prescribed anti-diabetic agent, against the highly gonadotoxic CP treatment. The protective effects were evident in many aspects of ovarian function and fertility, including increased ovarian follicular counts by histology, elevated serum hormone concentrations and increased offspring number in mating experiments. Furthermore, the use of metformin might not result in major safety issues as it is well-tolerated and induces low levels of toxicity according to results obtained from long-term diabetic treatment. Furthermore, accumulated studies have shown an anti-tumor effect of metformin treatment, thus no interference of metformin with the cancer treatment is expected (Bost et al., 2012). The financial cost of metformin therapy is also more acceptable comparing to that of the currently used GnRH agonist therapy and previously proposed specific mTOR inhibitor treatment. Further studies will be needed to confirm its efficacy in humans to warrant its clinical application.
Recently, several murine studies were carried out to investigate new strategies for preserving ovarian function and fertility during chemotherapy. AMH is produced by granulosa cells in preantral and antral follicles and has been shown to be involved in the regulation of primordial follicular recruitment in in-vitro experiments (Carlsson et al., 2006; Yang et al., 2017). In mice studies, intraperitoneal delivery of recombinant AMH (rAMH) during CP treatment was shown to preserve primordial follicular count and to increase litter size (Kano et al., 2017; Roness et al., 2019; Sonigo et al., 2019), without diminishing the anti-tumor effect of CP. Direct DNA damage and subsequent cellular apoptosis of both oocytes and somatic cells are another important underlying mechanisms of chemotherapy-induced ovarian damage (Morgan et al., 2012; Luan et al., 2019; Nguyen et al., 2019), which therefore became therapeutic targets in several murine models. Several pro-apoptotic proteins, PUMA and NOXA, have been shown to be activated within mice primordial follicle oocytes after radiation exposure (Kerr et al., 2012) and the primordial follicle reserve could be effectively preserved during chemotherapy in the Puma-deficient−/− mouse model (Nguyen et al., 2018). In a prepubertal mice model, both p-AKT/p-FOXO3a and p-ATM/γH2AX signaling axes were activated in the nucleus of oocytes under CP exposure, and concomitant administration of an allosteric ABL inhibitor and CP could modify these signaling pathways and protect ovarian reserve (Bellusci et al., 2019). Finally, intraperitoneally delivered sphingolipids like sphingosine-1-phosphate (S1P) and ceramide-1-phosphate (C1P) also increased ovarian follicular counts, restored hormone levels and improved fertility during chemotherapy via inhibition of apoptosis and improvement of angiogenesis (Li et al., 2014; Pascuali et al., 2018). Although several strategies appeared effective and promising in murine models, all of the aforementioned agents were merely applied in animals and could not be considered to be used clinically in human soon.
Inspired by Goldman’s study (2017), we chose metformin based on its mTOR inhibitory effect to test if it alleviates ovarian damages against CP treatment. In our murine model, expression of phosphorylated mTOR protein and ovarian reserve were significantly increased and decreased, respectively, after CP treatment, as Goldman’s study. Furthermore, the elevation of mTOR activity in ovarian granulosa cells by CP was reversed when cotreating them with metformin, similar with the cotreatment with other two specific mTOR inhibitors. The mTOR signaling pathway can modulate multiple cellular processes, such as protein synthesis, ribosome biogenesis and autophagy (Laplante and Sabatini, 2012) and is crucially involved in the regulation of cell growth, energy and metabolism in response to nutrients, stress and growth factors (Zoncu et al., 2011). Accumulated evidence also revealed a significant involvement of mTOR signaling pathway in female reproduction, such as in ovarian folliculogenesis, steroidogenesis and ovarian aging (Guo and Yu, 2019). In the mutant mice lacking tuberous sclerosis complex 1 (TSC1) gene, a negative regulator of mTOR complex, the mTOR activity was elevated and the activation of primordial follicles was accelerated, leading to premature ovarian failure (Adhikari et al., 2010). The alkylating agents like CP have been shown to cause premature follicular depletion through similar molecular mechanisms in mouse studies (Kalich-Philosoph et al., 2013).
After >60 years of its clinical use as the first-line treatment for diabetes, metformin is currently well-known for its multiple complex molecular mechanisms with numerous sites of action (Romero et al., 2017), encouraging extensive research of its therapeutic applications on many other human diseases. Although not fully understood, its major molecular mechanisms have been suggested to mainly involve AMPK activation and AMPK-independent mitochondria complex inhibition (Rena et al., 2017). In our study, the expression of phosphorylated AMPK was generally low in the control group and significantly elevated in the CP group cotreated with metformin, as expected. However, the AMPK activity was also elevated in the CP-alone group, and no significant difference in the CP-alone and CP + metformin groups were observed. Apoptotic cells were reported to activate AMPK and inhibit cell growth (Patel et al., 2015), which might explain the increased AMPK expression by CP treatment.
Although we initially selected to use of metformin in our research due to its mTOR inhibitory effect, the protective effects of metformin against CP toxicity could exert through multiple regulatory mechanisms. AMPK activation and mTOR inhibition are only parts of the complex molecular mechanisms of metformin (Rena et al., 2017), and whether these pathways are the most critical mediators of ovarian protection is still unknown. This might be attributed to the broad pleiotropic effects and promiscuous interactions induced by metformin. More experiments will be needed to clarify its underlying molecular pathways for preserving ovarian function. In addition, CP induced ovarian damage not only through the overactivation of the dormant oocyte, but also through direct DNA damage and further cell cycle arrest or apoptosis in both oocytes and granulosa cells (Petrillo et al., 2011). Also, the use of other anti-apoptotic agents has been proposed to be effective in ovarian protection (Tan et al., 2014). With the coadministration of metformin, the increased apoptosis and anti-cellular proliferative effects of CP were reversed in our study. Therefore, the modulating effects of metformin on cell proliferation and apoptosis seemed to be diverse among distinct cellular conditions, thus it might depend on the cell type, stress (Safe et al., 2018), and different doses and treatment duration (Rena et al., 2017). Accumulated evidence reported that metformin could inhibit tumorigenesis and cancer progression by affecting cancer cell metabolism, promoting apoptosis and decreasing cell proliferation in various cancer cell types, such as in colon (Sena et al., 2018), ovarian (Zou et al., 2019), breast (Malki and Youssef, 2011) and prostate cancer (Demir et al., 2014). However, in our study conflicting results were noted when studying the cell proliferative and anti-apoptotic effects of metformin in the ovarian granulosa cells under the stress of chemotherapeutic agents, which agreed with another murine study, which reported a cardioprotective effect against apoptosis and inflammation during arsenic poisoning (Wang et al., 2020). It is possible that metformin is more likely to exert an anti-apoptotic effect under a preexisted stressful cellular condition. Whether the anti-apoptotic and cell proliferative effects of metformin against chemotoxicity vary with different doses and treatment duration, or different chemotherapeutic agents is unknown and thus requires further research.
The anti-apoptotic activity of metformin against chemotoxicity of ovarian granulosa cells was mediated via AMPK-dependent p53 signaling pathway according to our in-vitro experiments. The tumor suppressor p53 induced cell cycle arrest and apoptosis in numerous cell lines and animal studies and has been critically involved in anti-cancer mechanisms (Chen, 2016). It can regulate >500 target genes and affect a broad spectrum of cellular responses, and either a loss or gain of p53 function could impair genomic integrity and unbalance cell growth (Pietsch et al., 2008). The p53 activity was generally low in normal mouse granulosa cells (Fig. 6C) before any treatment and significantly activated after CP administration. Although p53-mediated apoptosis in normal cells/tissues was related the chemotherapy toxicity (Chen, 2016), the apoptotic effects of CP were still evident after blocking p53 function with siRNA, suggesting a p53-independent apoptotic activity, at least in part. Meanwhile, collective evidence supported p53-mediated anti-apoptosis effect through the regulation of a bunch of anti-apoptotic proteins involved in the DNA repair, cell cycle control, oxidative stress, response and DNA transcription (Jänicke et al., 2008), which was concordant with the p53-dependent anti-apoptotic activity of metformin found in our study.
Conclusions
In conclusion, our study demonstrated effective ovarian protection mediated by metformin during CP treatment. Metformin increased ovarian reserve and fertility through multiple regulatory mechanisms, including mTOR inhibitory pathway, enhanced cell proliferation and AMPK/p53/p21-mediated anti-apoptotic effect. Although the long-term chemoprotective efficacy and safety of the adjuvant metformin treatment during chemotherapy has not been studied, the relatively acceptable cost and previously established safety profiles of this widely used old drug still prompts its further clinical application at a faster pace. More studies will be needed to clarify the optimal treatment dose, to generalize the effects of other chemotherapeutic agents than CP, and to determine the long-term health impact for both the patients and the offspring when using this novel fertility preserving method during chemotherapy.
Supplementary data
Supplementary data are available at Molecular Human Reproduction online.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
Acknowledgements
English editorial assistance in the preparation of this manuscript was provided by Elsevier Language Editing Services, with financial support from the National Taiwan University Hospital.
Authors’ roles
C.-C.H. designed the study, analyzed and interpreted the data and wrote the article. C.-H.C. conducted the experiments and revised the article. H.-N.H., Y.-S.Y., S.-U.C. and M.-J.C. contributed to the study conception and design, acquisition of data, data analysis and article revision. C.-T. S. and W.-F. W. processed the section slides of the mouse ovaries and hematoxylin and eosin staining. All of the authors approved the submitted version of the article.
Funding
This study was supported by grants MOST 108-2314-B-002-142 (H.N. Ho), MOST105-2628-B-002-043-MY4 (M.J. Chen), MOST 109-2628-B-002-030- (C.C. Huang) and MOST 108-2314-B-002-146-MY3 (S.U. Chen) from the Ministry of Science and Technology of Taiwan and the National Taiwan University Hospital (108-004336, 109-004795).
Conflict of interest
None.
References
- Adhikari D, Zheng W, Shen Y, Gorre N, Hamalainen T, Cooney AJ, Huhtaniemi I, Lan ZJ, Liu K.. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum Mol Genet 2010;19:397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DJ, Yates P, McCarthy A, Lang CP, Hargraves M, McCarthy N, Porter-Steele J.. Younger and older women's concerns about menopause after breast cancer. Eur J Cancer Care 2011;20:785–794. [DOI] [PubMed] [Google Scholar]
- Bellusci G, Mattiello L, Iannizzotto V, Ciccone S, Maiani E, Villani V, Diederich M, Gonfloni S.. Kinase-independent inhibition of cyclophosphamide-induced pathways protects the ovarian reserve and prolongs fertility. Cell Death Dis 2019;10:726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bost F, Sahra IB, Le Marchand-Brustel Y, Tanti JF.. Metformin and cancer therapy. Curr Opin Oncol 2012;24:103–108. [DOI] [PubMed] [Google Scholar]
- Carlsson IB, Scott JE, Visser JA, Ritvos O, Themmen APN, Hovatta O.. Anti-Mullerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum Reprod 2006;21:2223–2227. [DOI] [PubMed] [Google Scholar]
- Chang EM, Lim E, Yoon S, Jeong K, Bae S, Lee DR, Yoon TK, Choi Y, Lee WS.. Cisplatin induces overactivation of the dormant primordial follicle through PTEN/AKT/FOXO3a pathway which leads to loss of ovarian reserve in mice. PLoS One 2015;10:e0144245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. The cell-cycle arrest and apoptotic functions of p53 in tumor initiation and progression. Cold Spring Harb Perspect Med 2016;6:a026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley MJ, Diamantidis CJ, McDuffie JR, Cameron CB, Stanifer JW, Mock CK, Wang X, Tang S, Nagi A, Kosinski AS. et al. Clinical outcomes of metformin use in populations with chronic kidney disease, congestive heart failure, or chronic liver disease: a systematic review. Ann Intern Med 2017;166:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir U, Koehler A, Schneider R, Schweiger S, Klocker H.. Metformin anti-tumor effect via disruption of the MID1 translational regulator complex and AR downregulation in prostate cancer cells. BMC Cancer 2014;14:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgakilas AG, Martin OA, Bonner WM.. p21: a two-faced genome guardian. Trends Mol Med 2017;23:310–319. [DOI] [PubMed] [Google Scholar]
- Goldman KN, Chenette D, Arju R, Duncan FE, Keefe DL, Grifo JA, Schneider RJ.. mTORC1/2 inhibition preserves ovarian function and fertility during genotoxic chemotherapy. Proc Natl Acad Sci USA 2017;114:3186–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Yu Q.. Role of mTOR signaling in female reproduction. Front Endocrinol 2019;10:692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horicks F, , Van Den Steen G, , Gervy C, , Clarke HJ, , Demeestere I. Both in vivo FSH depletion and follicular exposure to Gonadotrophin-releasing hormone analogues in vitro are not effective to prevent follicular depletion during chemotherapy in mice. Mol Hum Reprod 2018;24:221–232. [DOI] [PubMed] [Google Scholar]
- Howard-Anderson J, Ganz PA, Bower JE, Stanton AL.. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst 2012;104:386–405. [DOI] [PubMed] [Google Scholar]
- Jänicke RU, Sohn D, Schulze-Osthoff K.. The dark side of a tumor suppressor: anti-apoptotic p53. Cell Death Differ 2008;15:959–976. [DOI] [PubMed] [Google Scholar]
- Janssen-Heijnen ML, van Steenbergen LN, Voogd AC, Tjan-Heijnen VC, Nijhuis PH, Poortmans PM, Coebergh JW, van Spronsen DJ.. Small but significant excess mortality compared with the general population for long-term survivors of breast cancer in the Netherlands. Ann Oncol 2014;25:64–68. [DOI] [PubMed] [Google Scholar]
- Kalender A, Selvaraj A, Kim SY, Gulati P, Brûlé S, Viollet B, Kemp BE, Bardeesy N, Dennis P, Schlager JJ. et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab 2010;11:390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalich-Philosoph L, Roness H, Carmely A, Fishel-Bartal M, Ligumsky H, Paglin S, Wolf I, Kanety H, Sredni B, Meirow D.. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci Transl Med 2013;5:185–162. [DOI] [PubMed] [Google Scholar]
- Kano M, Sosulski AE, Zhang LH, Saatcioglu HD, Wang D, Nagykery N, Sabatini ME, Gao GP, Donahoe PK, Pépin D.. AMH/MIS as a contraceptive that protects the ovarian reserve during chemotherapy. Proc Natl Acad Sci USA 2017;114:E1688–E1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JB, Hutt KJ, Michalak EM, Cook M, Vandenberg CJ, Liew SH, Bouillet P, Mills A, Scott CL, Findlay JK. et al. Loss of PUMA protects the ovarian reserve during DNA-damaging chemotherapy and preserves fertility. Mol Cell 2012;48:343–352.23000175 [Google Scholar]
- Laplante M, Sabatini DM.. mTOR signaling in growth control and disease. Cell 2012;149:274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard RCF, Adamson DJA, Bertelli G, Mansi J, Yellowlees A, Dunlop J, Thomas GA, Coleman RE, Anderson RA.. Anglo Celtic Collaborative Oncology Group and National Cancer Research Institute Trialists. GnRH agonist for protection against ovarian toxicity during chemotherapy for early breast cancer: the Anglo Celtic Group OPTION trial. Ann Oncol 2017;28:1811–1816. [DOI] [PubMed] [Google Scholar]
- Li F, Turan V, Lierman S, Cuvelier C, De Sutter P, Oktay K.. Sphingosine-1-phosphate prevents chemotherapy-induced human primordial follicle death. Hum Reprod 2014;29:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan Y, Edmonds ME, Woodruff TK, Kim SY.. Inhibitors of apoptosis protect the ovarian reserve from cyclophosphamide. J Endocrinol 2019;240:243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malki A, Youssef A.. Antidiabetic drug metformin induces apoptosis in human MCF breast cancer via targeting ERK signaling. Oncol Res 2011;19:275–285. [DOI] [PubMed] [Google Scholar]
- Meng Y, Xu Z, Wu F, Chen W, Xie S, Liu J, Huang X, Zhou Y.. Sphingosine-1-phosphate suppresses cyclophosphamide induced follicle apoptosis in human fetal ovarian xenografts in nude mice. Fertil Steril 2014;102:871–877. [DOI] [PubMed] [Google Scholar]
- Miller JJ 3rd, Cole LJ.. Changes in mouse ovaries after prolonged treatment with cyclophosphamide. Proc Soc Exp Biol Med 1970;133:190–193. [DOI] [PubMed] [Google Scholar]
- Miller JJ, Williams GF, Leissring JC.. Multiple late complications of therapy with cyclophosphamide, including ovarian destruction. Am J Med 1971;50:530–535. [DOI] [PubMed] [Google Scholar]
- Moore HCF, Unger JM, Phillips K-A, Boyle F, Hitre E, Porter D, Francis PA, Goldstein LJ, Gomez HL, Vallejos CS, et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med 2015;372:923–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan S, Anderson RA, Gourley C, Wallace WH, Spears N.. How do chemotherapeutic agents damage the ovary? Hum Reprod Update 2012;18:525–535. [DOI] [PubMed] [Google Scholar]
- Munhoz RR, Pereira AA, Sasse AD, Hoff PM, Traina TA, Hudis CA, Marques RJ.. Gonadotropin-releasing hormone agonists for ovarian function preservation in premenopausal women undergoing chemotherapy for early-stage breast cancer: a systematic review and meta-analysis. JAMA Oncol 2016;2:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M, Britt KL, Wreford NG, Ebling FJ, Kerr JB.. Methods for quantifying follicular numbers within the mouse ovary. Reproduction 2004;127:569–580. [DOI] [PubMed] [Google Scholar]
- Nath N, Khan M, Paintlia MK, Singh I, Hoda MN, Giri S.. Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis. J Immunol 2009;182:8005–8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen QN, Zerafa N, Liew SH, Findlay JK, Hickey M, Hutt KJ.. Cisplatin- and cyclophosphamide-induced primordial follicle depletion is caused by direct damage to oocytes. Mol Hum Reprod 2019;25:433–444. [DOI] [PubMed] [Google Scholar]
- Nguyen QN, Zerafa N, Liew SH, Morgan FH, Strasser A, Scott CL, Findlay JK, Hickey M, Hutt KJ.. Loss of PUMA protects the ovarian reserve during DNA-damaging chemotherapy and preserves fertility. Cell Death Dis 2018;9:618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou HT, Chen PC, Wu MH.. Effect of metformin by employing 2-hour postload insulin for measuring insulin resistance in Taiwanese women with polycystic ovary syndrome. J Formos Med Assoc 2017;116:80–89. [DOI] [PubMed] [Google Scholar]
- Paplomata E, Zelnak A, O’Regan R.. Everolimus: side effect profile and management of toxicities in breast cancer. Breast Cancer Res Treat 2013;140:453–462. [DOI] [PubMed] [Google Scholar]
- Pascuali N, Scotti L, Di Pietro M, Oubiña G, Bas D, May M, Gómez Muñoz A, Cuasnicú PS, Cohen DJ, Tesone M. et al. Ceramide-1-phosphate has protective properties against cyclophosphamide-induced ovarian damage in a mice model of premature ovarian failure. Hum Reprod 2018;33:844–859. [DOI] [PubMed] [Google Scholar]
- Patel VA, Massenburg D, Vujicic S, Feng L, Tang M, Litbarg N, Antoni A, Rauch J, Lieberthal W, Levine JS.. Apoptotic cells activate AMP-activated protein kinase (AMPK) and inhibit epithelial cell growth without change in intracellular energy stores. J Biol Chem 2015;290:22352–22369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Wei SY, Li LT, He YX, Li B.. Comparison of different therapies in high-risk patients with idiopathic membranous nephropathy. J Formos Med Assoc 2016;115:11–18. [DOI] [PubMed] [Google Scholar]
- Perrin A, Sherman S, Pal S, Chua A, Gorritz M, Liu Z, Wang X, Culver K, Casciano R, Garrison LP.. Lifetime cost of everolimus vs axitinib in patients with advanced renal cell carcinoma who failed prior sunitinib therapy in the US. J Med Econ 2015;18:200–209. [DOI] [PubMed] [Google Scholar]
- Petrillo SK, Desmeules P, Truong TQ, Devine PJ.. Detection of DNA damage in oocytes of small ovarian follicles following phosphoramide mustard exposures of cultured rodent ovaries in vitro. Toxicol Appl Pharmacol 2011;253:94–102. [DOI] [PubMed] [Google Scholar]
- Pietsch EC, Sykes SM, McMahon SB, Murphy ME.. The p53 family and programmed cell death. Oncogene 2008;27:6507–6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rena G, Hardie DG, Pearson ER.. The mechanisms of action of metformin. Diabetologia 2017;60:1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Erez O, Hüttemann M, Maymon E, Panaitescu B, Conde-Agudelo A, Pacora P, Yoon BH, Grossman LI.. Metformin, the aspirin of the 21st century: its role in gestational diabetes mellitus, prevention of preeclampsia and cancer, and the promotion of longevity. Am J Obstet Gynecol 2017;217:282–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roness H, Spector I, Leichtmann-Bardoogo Y, Savino AM, Dereh-Haim S, Meirow D.. Pharmacological administration of recombinant human AMH rescues ovarian reserve and preserves fertility in a mouse model of chemotherapy, without interfering with anti-tumoural effects. J Assist Reprod Genet 2019;36:1793–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe S, Nair V, Karki K.. Metformin-induced anticancer activities: recent insights. Biol Chem 2018;399:321–335. [DOI] [PubMed] [Google Scholar]
- Sena P, Mancini S, Benincasa M, Mariani F, Palumbo C, Roncucci L.. Metformin induces apoptosis and alters cellular responses to oxidative stress in Ht29 colon cancer cells: preliminary findings. Int J Mol Sci 2018;19:1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonigo C, Beau I, Grynberg M, Binart N.. AMH prevents primordial ovarian follicle loss and fertility alteration in cyclophosphamide-treated mice. FASEB J 2019;33:1278–1287. [DOI] [PubMed] [Google Scholar]
- Spears N, Lopes F, Stefansdottir A, Rossi V, De Felici M, Anderson RA, Klinger FG.. Ovarian damage from chemotherapy and current approaches to its protection. Hum Reprod Update 2019;25:673–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanska KJ, Tan X, Oktay K.. Unraveling the mechanisms of chemotherapy-induced damage to human primordial follicle reserve: road to developing therapeutics for fertility preservation and reversing ovarian aging. Mol Hum Reprod 2020;26:553–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SJ, Lee LJ, Tzeng CR, Wang CW, Hsu MI, Chen CH.. Targeted anti-apoptosis activity for ovarian protection against chemotherapy-induced ovarian gonadotoxicity. Reprod Biomed Online 2014;29:612–620. [DOI] [PubMed] [Google Scholar]
- Wang L, Shi W, Gao X, SreeHarsha N, Zhang D.. Cardioprotective role of metformin against sodium arsenite-induced oxidative stress, inflammation, and apoptosis. IUBMB Life 2020;72:749–757. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xu W, Yan Z, Zhao W, Mi J, Li J, Yan H.. Metformin induces autophagy and G0/G1 phase cell cycle arrest in myeloma by targeting the AMPK/mTORC1 and mTORC2 pathways. J Exp Clin Cancer Res 2018;37:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitten WK. Modification of the oestrous cycle of the mouse by external stimuli associated with the male. J Endocrinol 1956;13:399–404. [DOI] [PubMed] [Google Scholar]
- Yang MY, Cushman RA, Fortune JE.. Anti-Mullerian hormone inhibits activation and growth of bovine ovarian follicles in vitro and is localized to growing follicles. Mol Hum Reprod 2017;23:282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuksel A, Bildik G, Senbabaoglu F, Akin N, Arvas M, Unal F, Kilic Y, Karanfil I, Eryılmaz B, Yilmaz P. et al. The magnitude of gonadotoxicity of chemotherapy drugs on ovarian follicles and granulosa cells varies depending upon the category of the drugs and the type of granulosa cells. Hum Reprod 2015;30:2926–2935. [DOI] [PubMed] [Google Scholar]
- Zhang WB, Wang Z, Shu F, Jin YH, Liu HY, Wang QJ, Yang Y.. Activation of AMP-activated protein kinase by temozolomide contributes to apoptosis in glioblastoma cells via p53 activation and mTORC1 inhibition. J Biol Chem 2010;285:40461–40471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Xie Y, Li S, Liang Y, Qiu Q, Lin H, Zhang Q.. Rapamycin prevents cyclophosphamide-induced over-activation of primordial follicle pool through PI3K/Akt/mTOR Signaling Pathway in vivo. J Ovarian Res 2017;10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM.. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 2011;12:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou G, Bai J, Li D, Chen Y.. Effect of metformin on the proliferation, apoptosis, invasion and autophagy of ovarian cancer cells. Exp Ther Med 2019;18:2086–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.