Abstract
Introduction:
The optimum physical activity dose to achieve during young adulthood to prevent hypertension using the 2017 American College of Cardiology/American Heart Association guidelines remains undefined. This study aims to determine the association between level and change in physical activity through the adult life course and the onset of hypertension using these 2017 definitions.
Methods:
In 2020, prospective community-based cohort data of 5,115 Coronary Artery Risk Development in Young Adults study participants were analyzed. The cohort included Black and White men and women aged 18–30 years at baseline (1985–1986) at 4 urban sites, collected through 30 years of follow-up (2015–2016). Individualized physical activity trajectories were developed for each participant using linear mixed models.
Results:
Black women reported the lowest physical activity levels from young adulthood through middle age. Lower physical activity score (per 100 units) at age 18 years was associated with 4% (95% CI=1, 7, p=0.002) higher odds of hypertension incidence. Each additional 1-unit reduction per year in physical activity score was associated with 2% (95% CI=1, 3, p=0.001) higher annual odds of hypertension incidence. Meeting approximately the current minimum physical activity guideline levels at age 18 years and through follow-up was not protective of hypertension onset; however, meeting approximately twice the current minimum physical activity guideline level at age 18 years and through follow-up was protective of hypertension onset.
Conclusions:
Moderate physical activity levels may need to exceed current minimum guidelines to prevent hypertension onset using 2017 American College of Cardiology/American Heart Association definitions.
INTRODUCTION
Hypertension is a major modifiable risk factor for cardiovascular disease, the leading cause of death in the U.S.1 Physical activity (PA) is an important nonpharmacological intervention recommended for the prevention of hypertension, as reinforced by the 2017 American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for high blood pressure (BP) in adults. These guideline lowered the threshold for systolic and diastolic BP to define hypertension (130/80 mmHg from 140/90 mmHg), which expanded the number of adults with hypertension.2 Adequate PA is associated with lower odds of having hypertension,3–5 but there remains a need to better understand how PA affects hypertension through the entire life course, starting in young adulthood. Young adulthood may set PA trajectories (i.e., levels and slope over age and time) for the rest of adulthood and may, therefore, represent an important window for early intervention and primordial prevention.6,7
Data from national surveys estimate that two-thirds of adults aged 55–64 years in the U.S. have hypertension according to 2017 ACC/AHA definitions.2 The 2018 HHS Physical Activity Guidelines Scientific Report identified observational research examining the relationship between PA and BP using the 2017 ACC/AHA hypertension definitions as an important need for future research.8 Furthermore, few studies have examined the relationship between PA and hypertension starting in young adulthood through the life course given limited data on the young adult to older adult time periods. The Coronary Artery Risk Development in Young Adults (CARDIA) study is a cohort with data spanning the adult life course.9 A previous CARDIA study found an association between PA and hypertension in young adulthood;3 however, this study used older definitions of hypertension, was limited to 15 years of follow-up, and used the average of multiple PA measures rather than calculating slopes or trajectories. Previous studies in CARDIA have shown associations between PA and incident hypertension in young adulthood3 and middle age4 but have not conducted analyses spanning young adulthood through middle age. One study demonstrated the feasibility of PA trajectory analyses but used group-based rather than individualized trajectories and examined coronary artery calcium rather than hypertension as an outcome.10 Thus, the relationship between individual PA trajectories through the adult life course and hypertension using 2017 ACC/AHA definitions warrants examination.2
The objective of this study is to determine the associations between PA trajectories (level in young adulthood and slope through the adult lifespan) and the onset of hypertension defined by ACC/AHA through the adult life course. This study builds on previous literature by defining individualized longitudinal PA assessments for each participant, starting in young adulthood with a 30-year follow-up, and applying 2017 ACC/AHA hypertension definitions toward identifying incident cases. A secondary objective of the study is to determine whether meeting the adult PA guidelines are differentially associated with hypertension onset. The hypothesis is that both PA level in young adulthood and slope through the adult life course are associated with hypertension onset and that meeting the PA guidelines is protective of hypertension onset.
METHODS
Study Population
The CARDIA study is a prospective community-based cohort study that included 5,115 Black and White adults at the baseline examination (1985–1986). Participants were recruited from 4 urban sites (Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA) and have been followed for >30 years with high retention (90% in Year 2, 86% in Year 5, 81% in Year 7, 77% in Year 10, 74% in Year 15, 72% in Year 20, 72% in Year 25, and 71% in Year 30). The cohort was designed to be approximately balanced within center by sex, race (Black and White), age (18–24 years and 25–30 years at baseline), and educational level (high school or less and higher than high school). After the baseline examination, 1 participant requested to be excluded from all further analyses. Further details about the study design have been previously published.9 The study was approved by the institutional review board at each site, and written informed consent was obtained from all participants.
Measures
Self-reported leisure-time PA was ascertained by the interviewer-administered CARDIA Physical Activity History Questionnaire at each of the 9 examinations.10,11 Participants were asked about the frequency of participation in 13 different activity categories (8 vigorous and 5 moderate intensity) of recreational sports, exercise, leisure, and occupational activities over the previous 12 months. Each activity’s intensity was expressed as METs, where 1 MET is defined as the energy expended at rest (approximately an oxygen consumption of 3.5 mL/1 kg of body weight/minute). Vigorous-intensity activities (≥6 METs) included running, racquet sports, bicycling faster than 10 miles per hour, swimming, vigorous exercise classes, sports (e.g., basketball, football), heavy lifting, carrying or digging on the job, and home activities such as snow shoveling and lifting heavy objects. Moderate-intensity activities (3–5 METs) included nonstrenuous sports (e.g., softball), walking, bowling/golf, home maintenance (e.g., gardening, raking), and calisthenics.12 Each activity was assigned a frequency on the basis of whether it was performed for ≥1 hour or during any 1 month in the past year, the number of months it was performed at that level, and the number of months the activity was performed frequently. Intensity scores (3–8 METs) and duration thresholds (2–5 hours per week) were assigned to each activity; anything above these levels of participation was considered frequent.10 A moderate to vigorous–intensity PA score (referred to as PA score in the remaining part of this paper) was computed by multiplying the frequency (number of months) of participation by the intensity (METs) of the activity with a weighting factor for the months of more frequent participation.13 The total activity score was the sum of all activities expressed in exercise units (EUs). For reference, a PA score of 300 EUs approximates the HHS recommendations of approximately 150 minutes of moderate-intensity activity per week.14,15 The CARDIA PA questionnaire demonstrated a test–retest reliability of 0.77–0.84,13 similar to those of other activity questionnaires.11
BP was measured at each of the 9 CARDIA examinations. Resting systolic and diastolic BPs were measured 3 times at 1-minute intervals. Calibrated BP values were used to ensure the comparability of the measurements for Years 0–15 (random 0) and Years 20–30 (Omron) (Appendix Methods, available online).16,17 The average of the second and third BP measurements was used. Hypertension was defined per 2017 ACC/AHA guidelines as systolic BP >130 mmHg, diastolic BP >80 mmHg,2 or participants’ report of taking antihypertensive medications.
Age (in years), race (Black or White), sex (male or female), smoking status (never, former, or current smoker), alcohol use (mL of alcohol consumed per day), educational attainment (the highest grade of school completed), family history of hypertension or cardiovascular disease (yes or no), medical history, and medications were reported through the questionnaire. The use of antihypertensive medications was assessed by self-report at each examination. BMI was calculated on the basis of measured height and weight at each examination. Diabetes mellitus (yes/no) was defined as a self-reported physician diagnosis of diabetes, fasting glucose ≥126 mg/dL, or the use of hypoglycemic agents.9,10 Plasma concentrations of total cholesterol and triglycerides were measured using enzymatic methods.9 High-density lipoprotein cholesterol levels were measured after dextran-magnesium precipitation,18 and serum low-density lipoprotein cholesterol levels were calculated using the Friedewald equation.19
Statistical Analysis
PA trajectories were modeled among all CARDIA participants. A linear mixed model for repeated measures of PA to generate succinct summaries of exercise patterns was developed. The linear mixed model included fixed effects for a 4-level categorization of sex and race, age as continuous, and their interactions as well as random effects for participant and age, with unstructured covariance (age was included as both a fixed and random effect). From the fixed and random effects estimates provided by this model, the expected PA level at age 18 years and annual change for each participant was calculated. For ease of interpretation, the sign of both summaries was changed to capture the associations of a lower-level and faster decline in PA with increased hypertension risk.
Unadjusted cumulative incidence of hypertension by sex and race/ethnicity was estimated using Kaplan–Meier methods. The data for each participant were then expanded to include a record for each age between study entry and either at hypertension onset, assumed to occur at the first visit at which it was detected, or at censoring by the end of the study of loss to follow-up. Pooled logistic models were used to estimate the independent associations of the expected PA at age 18 years and subsequent annual change with onset of hypertension, adjusting for potential confounders, including sex, race, family history of hypertension or cardiovascular disease, years of education, smoking status, alcohol use, and BMI (smoking status, alcohol use, and BMI were time varying, with the last observation carried forward).10 A further model additionally adjusted for diabetes, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglycerides (all were time varying, with the last observation carried forward).3,10 Pooled logistic models estimated the associations of meeting approximately the minimum PA guideline (>300 EUs) or twice the minimum PA guideline (>600 EUs) at age 18 years and through follow-up with onset of hypertension, adjusting for potential confounders. Analyses used Stata, version 16.0, and were conducted in 2020. Additional sensitivity analyses are listed in the Appendix (Appendix Tables 1‒3, available online, show the retention and loss to follow-up; Appendix Tables 4 and 5, available online, show the interactions of level and slope of PA with hypertension onset).
RESULTS
Of the 5,114 participants included in the sample, 51.6% were Black, and 45.5% were men. Demographic and health characteristics of the sample at baseline are shown in Table 1. Figure 1 shows the average of race- and sex-specific population trajectories using locally weighted scatterplot smoothing. From age 18 to 60 years, PA, on average, was lowest among Black women than among other groups. Black men, on average, reported high levels of PA at age 18 years; however, the levels declined considerably from young adulthood to the end of middle age. Figure 2 shows the cumulative incidence of hypertension by race and sex. By age 60 years, 73.1% of participants had developed hypertension. Black men had the highest incidence of hypertension from young adulthood through middle age.
Table 1.
Baseline Demographic and Health Characteristics of Participants in the CARDIA Study
| Variables | Total N = 5,114 |
White women n = 1,307 |
Black women n = 1,480 |
White men n = 1,170 |
Black men n = 1,157 |
p-value |
|---|---|---|---|---|---|---|
| Baseline demographic characteristics | ||||||
| Age, years, median (IQR) | 25.0 (22.0‒28.0) | 26.0 (23.0‒28.0) | 24.0 (21.0‒28.0) | 26.0 (23.0‒28.0) | 24.0 (21.0‒28.0) | <0.001 |
| Highest grade of school completed, median (IQR) | 13.0 (12.0‒16.0) | 15.0 (12.0‒16.0) | 13.0 (12.0‒14.0) | 15.0 (12.0‒16.0) | 12.0 (12.0‒14.0) | <0.001 |
| Family history of hypertension, n (%) | 2,670 (52.2) | 619 (47.4) | 889 (60.1) | 522 (44.6) | 640 (55.3) | <0.001 |
| Family history of cardiovascular disease, n (%) | 1,022 (20.0) | 250 (19.1) | 310 (20.9) | 227 (19.4) | 235 (20.3) | 0.62 |
| BMI, median (IQR) | 23.4 (21.2‒26.4) | 22.0 (20.3‒24.6) | 24.2 (21.2‒28.9) | 23.7 (21.9‒26.0) | 23.7 (21.7‒26.4) | <0.001 |
| <25 kg/m2, n (%) | 3,328 (65.3) | 1,012 (77.7) | 823 (55.8) | 761 (65.2) | 732 (63.5) | |
| 25‒30 kg/m2, n (%) | 1,170 (23.0) | 195 (15.0) | 337 (22.8) | 334 (28.6) | 304 (26.4) | |
| >30 kg/m2, n (%) | 599 (11.8) | 95 (7.3) | 315 (21.4) | 72 (6.2) | 117 (10.1) | |
| Smoking status, n (%) | <0.001 | |||||
| Never | 2,856 (56.2) | 685 (52.7) | 885 (60.1) | 670 (57.8) | 616 (53.8) | |
| Former | 676 (13.3) | 261 (20.1) | 127 (8.6) | 182 (15.7) | 106 (9.3) | |
| Current | 1,546 (30.4) | 355 (27.3) | 461 (31.3) | 307 (26.5) | 423 (36.9) | |
| Alcohol (mL of alcohol consumed per day), median (IQR) | 5.4 (0.9‒15.5) | 4.8 (0.9‒12.1) | 1.8 (0.0‒6.9) | 11.1 (3.7‒23.2) | 10.2 (2.0‒25.2) | <0.001 |
| Total physical activity score at enrollment (EU), median (IQR) | 360.0 (197.0‒578.0) | 351.0 (207.0‒543.0) | 228.0 (103.0‒396.0) | 462.0 (288.0‒672.0) | 472.0 (271.0‒723.0) | <0.001 |
| Total physical activity score at age 18 (EU), median (IQR) | 373.7 (251.9‒536.0) | 340.0 (243.0‒472.2) | 238.5 (163.8‒337.4) | 480.7 (352.3‒613.9) | 516.0 (384.3‒673.9) | <0.001 |
| Annual reduction in total physical activity score (EU), median (IQR) | 2.3 (0.6‒4.5) | 1.3 (‒0.3‒3.1) | 1.3 (0.2‒2.8) | 2.5 (1.1‒4.2) | 5.1 (3.7‒7.3) | <0.001 |
| Expected total physical activity score always >300 EUs, n (%) | 1,658 (55.8) | 545 (53.9) | 230 (27.1) | 500 (77.5) | 383 (82.5) | <0.001 |
| Expected total physical activity score always >600 EUs, n (%) | 347 (11.7) | 63 (6.2) | 25 (3.0) | 131 (20.3) | 128 (27.6) | <0.001 |
| Hypertension, n (%) | 732 (14.3) | 86 (6.6) | 157 (10.6) | 240 (20.5) | 249 (21.5) | |
| Systolic blood pressure, median (IQR) | 110.0 (103.0‒118.0) | 104.0 (99.0‒110.0) | 107.0 (101.0‒114.0) | 114.0 (107.0‒121.0) | 115.0 (109.0‒122.0) | <0.001 |
| Diastolic blood pressure, median (IQR) | 68.0 (62.0‒75.0) | 66.0 (61.0‒71.0) | 67.0 (62.0‒73.0) | 71.0 (65.0‒77.0) | 71.0 (64.0‒77.0) | <0.001 |
| Diabetes, n (%) | 32 (0.6) | 7 (0.5) | 14 (1.0) | 6 (0.5) | 5 (0.4) | 0.29 |
| LDL cholesterol, median (IQR) | 106.0 (87.0‒127.0) | 102.0 (85.0‒123.0) | 107.0 (88.0‒129.0) | 109.0 (89.0‒129.0) | 106.0 (85.0‒127.0) | <0.001 |
| HDL cholesterol, median (IQR) | 52.0 (44.0‒61.0) | 55.0 (47.0‒64.0) | 54.0 (46.0‒64.0) | 45.0 (40.0‒53.0) | 52.0 (44.0‒61.0) | <0.001 |
| Triglycerides, median (IQR) | 62.0 (45.0‒84.0) | 62.0 (45.0‒81.5) | 56.0 (42.0‒75.0) | 72.0 (52.0‒103.0) | 60.0 (45.0‒82.0) | <0.001 |
Note: Boldface indicates statistical significance (p<0.05).
A total physical activity score of 300 EUs approximates the HHS recommendations of approximately 150 minutes of moderate-intensity activity per week.
CARDIA, Coronary Artery Risk Development in Young Adults Study; EU, exercise unit; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Figure 1. Average physical activity trajectories.
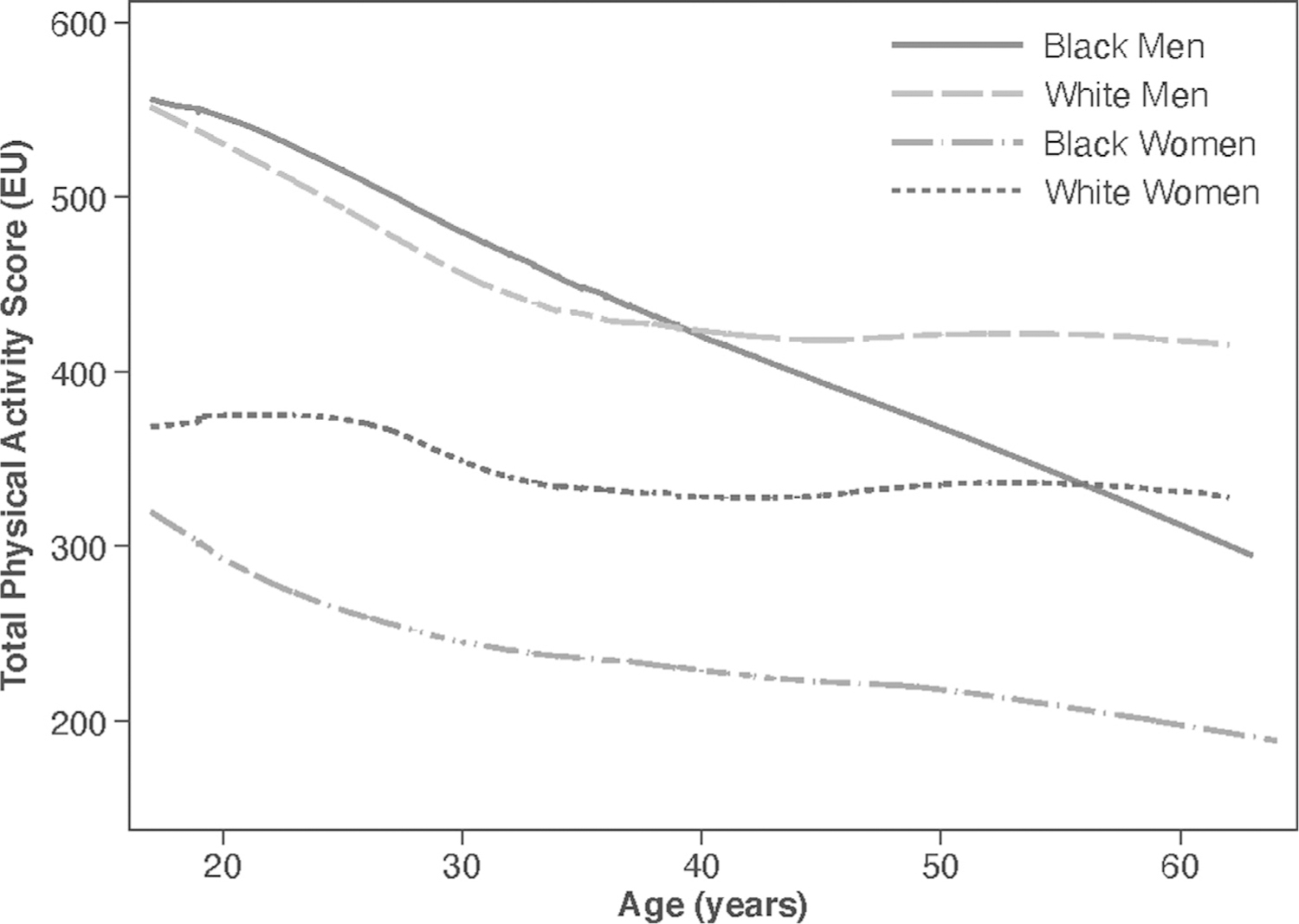
Note: A total physical activity score of 300 EUs approximates the HHS recommendations of approximately 150 minutes of moderate-intensity activity per week. The figure represents the group average of total physical activity score by race and sex groups using locally Weighted Scatterplot smoothing. Appendix Figure 1 (available online) shows the physical activity trajectories with 95% prediction intervals by race and sex. Appendix Figure 2 (available online) shows the average physical activity trajectories when physical activity observations after hypertension onset do not contribute. EU, exercise unit.
Figure 2.
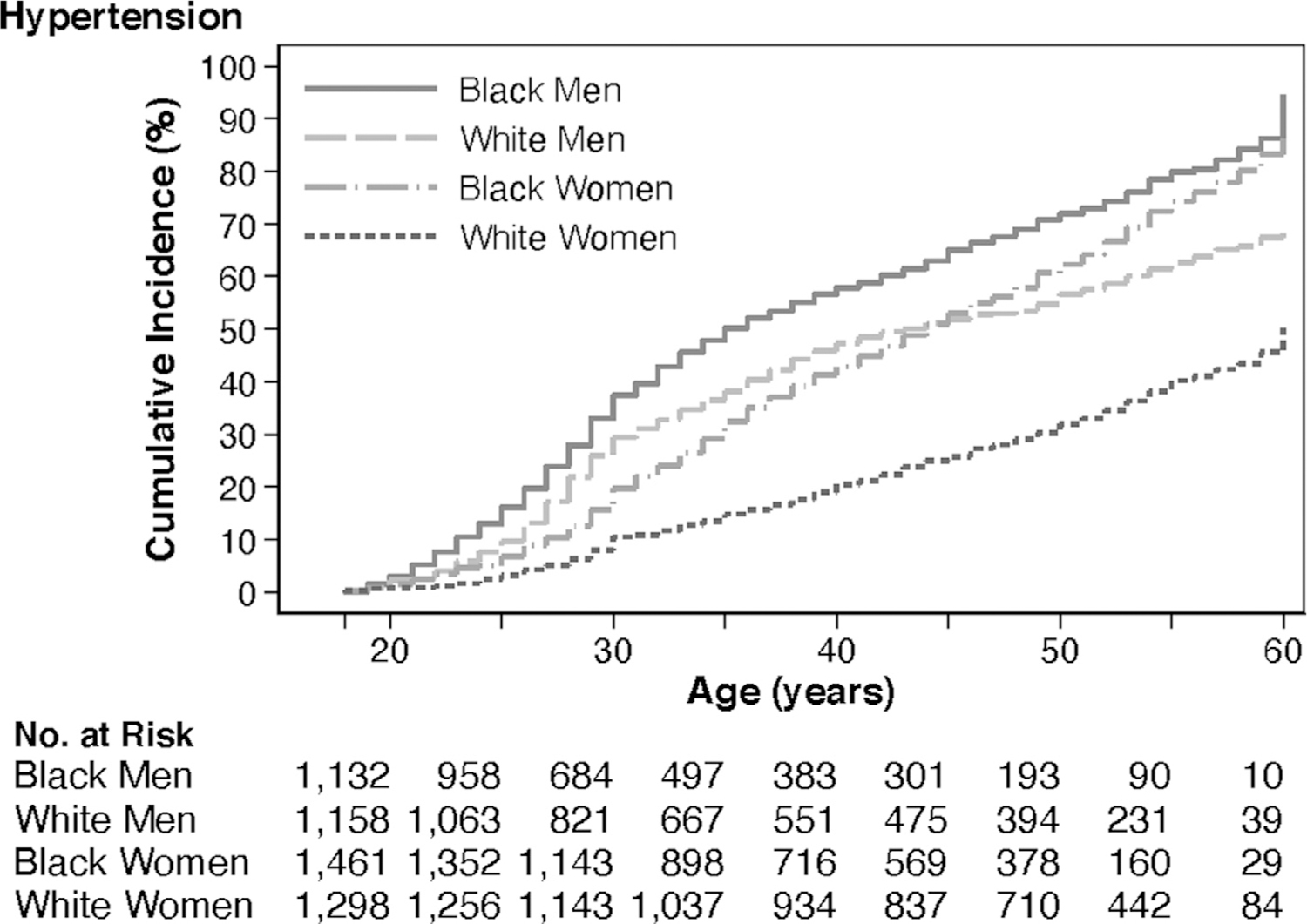
Hypertension incidence by race and sex.
No., number.
Table 2 shows pooled logistic regression model estimates for the associations of the 2 PA summaries (level at age 18 years and slope) with hypertension onset. Model 1 adjusted for age only, Model 2 adjusted for demographics and health behaviors, and Model 3 additionally adjusted for diabetes and cholesterol. In the fully adjusted model including diabetes and cholesterol (Model 3), a lower total PA score (per 100 units) at age 18 years was associated with 4% (95% CI=1%, 7%, p=0.002) higher odds of hypertension onset. In addition, each additional 1-unit reduction in the total PA score per year was associated with 2% (95% CI=1, 3, p=0.001) higher annual odds of hypertension onset. In a sensitivity analysis, interactions between PA and race/sex or BMI categories were not statistically significant (p>0.05).
Table 2.
Associations Between Physical Activity Through the Follow-Up Period and Onset of Hypertension in the CARDIA Study
| Variables | OR (95% CI) | p-value |
|---|---|---|
| Model 1 (adjusted for age) | ||
| Expected total physical activity score at age 18 yearsa | 1.03 (1.01, 1.05) | 0.007 |
| Expected annual reduction in total physical activity scoreb | 1.05 (1.04, 1.06) | <0.001 |
| Model 2 (fully adjusted)c | ||
| Expected total physical activity score at age 18 yearsa | 1.03 (1.01, 1.06) | 0.005 |
| Expected annual reduction in total physical activity scoreb | 1.01 (1.00, 1.02) | 0.017 |
| Model 3 (fully adjusted with diabetes and cholesterol)d | ||
| Expected total physical activity score at age 18 yearsa | 1.04 (1.01, 1.07) | 0.002 |
| Expected annual reduction in total physical activity scoreb | 1.02 (1.01, 1.03) | 0.001 |
| Physical activity guideline threshold models, fully adjusted with diabetes and cholesterol | ||
| Meeting physical activity guideline (>300 EUs) at age 18 yearsd | 1.08 (0.98, 1.19) | 0.14 |
| Meeting physical activity guideline (>300 EUs) through follow-upd | 1.02 (0.92, 1.12) | 0.66 |
| Meeting twice physical activity guideline (>600 EUs) at age 18 yearsd | 0.82 (0.71, 0.93) | 0.002 |
| Meeting twice physical activity guideline (>600 EUs) through follow-upd | 0.78 (0.66, 0.91) | 0.002 |
Note: Boldface indicates statistical significance (p<0.05).
A total physical activity score of 300 EUs approximates the HHS recommendations of approximately 150 minutes of moderate-intensity activity per week.
In 100s of EUs from high to low.
In EUs.
Covariates: age, race, sex, education, family history of hypertension, family history of cardiovascular disease, smoking status, alcohol use, and BMI.
Covariates: age, race, sex, education, family history of hypertension, family history of cardiovascular disease, smoking status, alcohol use, BMI, diabetes, LDL cholesterol, HDL cholesterol, and triglycerides.
CARDIA, Coronary Artery Risk Development in Young Adults Study; EU, exercise unit; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Meeting approximately twice the minimum HHS PA guidelines at age 18 years (OR=0.82, 95% CI=0.71, 0.93, p=0.002) and through the course of the follow-up (OR=0.78, 95% CI=0.66, 0.91, p=0.002) was associated with lower odds of hypertension onset (Table 2). Meeting approximately the minimum PA guidelines at age 18 years and through the course of the follow-up was not associated with lower odds of hypertension onset. Among participants with high levels of PA at age 18 years (>600 EUs), dramatic subsequent decreases (loss >2.5 EU/year) in PA compared with gains were associated with hypertension onset (AOR=4.11, 95% CI=2.58, 6.52) (Appendix Table 6, available online). Among participants with low levels of PA at age 18 years (<300 EU), subsequent increases in PA compared with rapid decreases (loss >2.5 EU/year) were not significantly protective of hypertension onset (AOR=1.08, 95% CI=0.89, 1.31).
DISCUSSION
In this prospective observational study with 30-year follow-up, total PA level at age 18 years and declines in PA over time were each independently associated with the onset of hypertension. On average, PA declined in all sex and race groups from age 18 to 40 years. However, after age 40 years, PA levels in Black participants continued to decline, whereas PA levels remained relatively constant in White participants. Recommended levels of PA may need to exceed current minimum guidelines to prevent hypertension onset using 2017 ACC/AHA definitions.
These findings align with those of previous studies that have shown an association between PA and hypertension.3–5,10 This study adds to the literature by incorporating 30 years of follow-up data in the CARDIA cohort and using ACC/AHA 2017 hypertension definitions (130/80 mmHg).2 The prevalence of hypertension in CARDIA using 2017 ACC/AHA definitions is similar to national data from the National Health and Nutrition Examination Surveys, which found that two-thirds of adults aged 55–64 years have hypertension according to 2017 ACC/AHA definitions.20 It is notable that both PA level and slope were independently associated with hypertension onset, even after adjusting for potential confounders.
Nearly half of the participants had suboptimal PA levels at baseline, and PA levels at age 18 years were significantly associated with hypertension onset. The annual reduction of PA is more strongly associated with hypertension onset than PA level at age 18 years. This suggests the need to emphasize, prioritize, and fund interventions to promote PA early in life to prevent the development of hypertension in later adulthood.
The findings also indicate that young adulthood is an important window when PA declines in all race and sex groups, and these declines are associated with hypertension onset. The HHS Physical Activity Guidelines Scientific Committee Report acknowledged that although young adults share growth and development features with adolescents, there was insufficient literature to confirm or support a change to the current approach for young adults.8 These findings indicate that a higher minimum threshold for PA than that of the current guidelines (150 minutes of moderate-intensity PA per week) may be beneficial for the prevention of hypertension onset and could inform future guidance. These findings may differ from previous literature owing to the use of the 2017 ACC/AHA hypertension definitions, which expanded the number of adults with hypertension.2 The HHS guidelines did not include studies using this newer hypertension definition and did not take into account additional health outcomes, including mortality and cardiovascular disease, to inform their recommendations.8 Young adulthood is associated with important economic, educational, and social transitions that may lead to less PA.21,22 For instance, after having a physical education or team sports requirements in high school, most young adults no longer have similar PA opportunities or requirements as they transition to college or the workforce.23 Furthermore, the transition to parenthood and additional social and economic responsibilities may displace leisure time for PA.24
Given the trends in declining PA and associated health consequences, young adulthood is also an important time period for interventions to prevent declines in PA. Clinicians and public health campaigns should focus messaging on maintaining adequate levels of PA. The AHA has developed Life’s Simple 7, consisting of 7 risk factors that people can improve through lifestyle changes to achieve ideal cardiovascular health, including BP and activity level.25 However, the AHA notes that although most of the Life’s Simple 7 are evaluated routinely in clinical practice (BP, glucose and lipid profiles, obesity, and smoking), PA is typically not assessed despite evidence on the feasibility, validity, and effectiveness of assessing and promoting PA in clinical settings.26 Messaging that promotes optimizing health beyond just the need to avoid risk may be particularly salient for young adults.27 Furthermore, PA intervention programs at schools,28 colleges,29 workplaces,30 churches,31 and community organizations32,33 may promote PA among young people and adults. Furthermore, digital interventions, including text messages, e-mails, social media, games, and multicomponent interventions, may promote PA in the short term among young people,34,35 but further development of digital interventions for PA promotion is needed.
This study identified notable PA patterns by race and sex through the life course. Black women have high rates of obesity and smoking and low levels of PA from age 18 to 60 years and thus may be an important group for early targeted interventions. Although Black men report the highest PA levels among all race/sex groups in young adulthood, they have the greatest declines through middle age, similar to accelerometer-based findings.36 Although 1 previous CARDIA study did not find an association between geocoded neighborhood PA resources and PA,37 another study found that self-reported neighborhood cohesion and resources are associated with more PA, particularly in Black men.38 Racial disparities in PA may be related to neighborhood environments, SES, family responsibilities, smoking status, access to healthcare professionals, and health status.39,40
Black men have a higher burden of incident hypertension from young adulthood through the life course, similar to findings from other population-based studies in the U.S.20 By age 45 years, Black women surpass White men in the incidence of hypertension, whereas White women have the lowest incidence of hypertension through the adult life course.
Limitations
Limitations of this study should be noted. The self-reported PA questionnaire may be subject to participant recall and social desirability and did not collect information regarding activity intensity. However, the strength is that the same measure was used across the 30-year follow-up period. The sampling design of CARDIA selected participants identifying as Black or White race9 but was not representative of all races or ethnicities in the U.S., which may limit generalizability. Although models adjusted for several potential confounders, there is the possibility of unmeasured confounders. Given the larger proportion of the sample with hypertension by age 60 years, the number of eligible participants in the analysis drops with age.
CONCLUSIONS
These findings have clinical and public health implications. First, this study supports the 2017 ACC/AHA guidelines, which recommend nonpharmacological interventions, such as increasing PA, for the prevention and management of high BP.2 This association is important to confirm using the new hypertension definition of 130/80 mmHg. Second, achieving at least twice the current minimum adult PA guidelines may be more beneficial for the prevention of hypertension than simply meeting the minimum guidelines. Public health interventions may emphasize longer durations of PA to prevent hypertension.
Supplementary Material
ACKNOWLEDGMENTS
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute, NIH, or HHS.
JMN is supported by the NIH (K08HL159350), was a participant in the Pediatric Scientist Development Program (K12HD00085033), funded by the American Academy of Pediatrics and the American Pediatric Society, and a recipient of the American Heart Association Career Development Award (CDA34760281). KBD is supported by the NIH (K24DK103992). The Coronary Artery Risk Development in Young Adults study is conducted and supported by the National Heart, Lung, and Blood Institute in collaboration with the University of Alabama at Birmingham (HHSN268201800005I and HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). This manuscript has been reviewed by Coronary Artery Risk Development in Young Adults for the scientific content.
Footnotes
No financial disclosures were reported by the authors of this paper.
SUPPLEMENTAL MATERIAL
Supplemental materials associated with this article can be found in the online version at https://doi.org/10.1016/j.amepre.2020.12.018.
REFERENCES
- 1.Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–e596. 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines [published correction appears in Hypertension. 2018;71(6):e140–e144]. Hypertension. 2018;71(6):e13–e115. 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 3.Parker ED, Schmitz KH, Jacobs DR Jr, Dengel DR, Schreiner PJ. Physical activity in young adults and incident hypertension over 15 years of follow-up: the CARDIA study. Am J Public Health. 2007;97(4):703–709. 10.2105/AJPH.2004.055889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sternfeld B, Gabriel KP, Jiang SF, et al. Risk estimates for diabetes and hypertension with different physical activity methods. Med Sci Sports Exerc. 2019;51(12):2498–2505. 10.1249/MSS.0000000000002083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diaz KM, Shimbo D. Physical activity and the prevention of hypertension. Curr Hypertens Rep. 2013;15(6):659–668. 10.1007/s11906-013-0386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon S, Janz KF, Letuchy EM, Burns TL, Levy SM. Developmental trajectories of physical activity, sports, and television viewing during childhood to young adulthood: Iowa bone development study. JAMA Pediatr. 2015;169(7):666–672. 10.1001/jamapediatrics.2015.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li K, Haynie D, Lipsky L, Iannotti RJ, Pratt C, Simons-Morton B. Changes in moderate-to-vigorous physical activity among older adolescents. Pediatrics. 2016;138(4):e20161372. 10.1542/peds.2016-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.HHS, Office of Disease Prevention and Health Promotion. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: HHS, Office of Disease Prevention and Health Promotion. XXX. Published XXX. Accessed XXX. [Google Scholar]
- 9.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 10.Laddu DR, Rana JS, Murillo R, et al. 25-year physical activity trajectories and development of subclinical coronary artery disease as measured by coronary artery calcium: the Coronary artery Risk Development in Young Adults (CARDIA) study. Mayo Clin Proc. 2017;92(11):1660–1670. 10.1016/j.mayocp.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pereira MA, FitzerGerald SJ, Gregg EW, et al. A collection of Physical Activity Questionnaires for health-related research. Med Sci Sports Exerc. 1997;29(6)(suppl):S1–S205. [PubMed] [Google Scholar]
- 12.Sidney S, Jacobs DR Jr, Haskell WL, et al. Comparison of two methods of assessing physical activity in the Coronary artery Risk Development in Young Adults (CARDIA) study. Am J Epidemiol. 1991;133 (12):1231–1245. 10.1093/oxfordjournals.aje.a115835. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs DR Jr, Hahn LP, Haskell WL, Pirie P, Sidney S. Validity and reliability of short physical activity history: CARDIA and the Minnesota Heart Health Program. J Cardiopulm Rehabil. 1989;9(11):448–459. 10.1097/00008483-198911000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.HHS. Physical activity guidelines for Americans. 2nd edition Washington, DC: HHS; 2018. https://health.gov/paguidelines/second-edi-tion/pdf/Physical_Activity_Guidelines_2nd_edition.pdf. Published 2018. Accessed January 15, 2021. [Google Scholar]
- 15.Gabriel KP, Sidney S, Jacobs DR, et al. Convergent validity of a brief self-reported physical activity questionnaire. Med Sci Sports Exerc. 2014;46 (8):1570–1577. 10.1249/MSS.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yano Y, Reis JP, Levine DA, et al. Visit-to-visit blood pressure variability in young adulthood and hippocampal volume and integrity at middle age: the CARDIA study (Coronary artery risk development in Young Adults). Hypertension. 2017;70(6):1091–1098. 10.1161/HYPERTENSIONAHA.117.10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.HHS NIH, The University of Alabama Birmingham. National Heart Lung and Blood Institute. CARDIA Y20 manual of operations ver. 1.2: blood pressure and pulse, section 3. Birmingham, AL: HHS, NIH, The University of Alabama Birmingham, National Heart Lung and Blood Institute; November 2, 2006. https://www.cardia.dopm.uab.edu/images/more/pdf/mooy20/Y20.pdf. Published November 2, 2006. Accessed October 19, 2020. [Google Scholar]
- 18.Warnick GR. High-density lipoproteins: the neglected stepchildren whose importance as a risk factor continues to be defined. Clin Chem. 2008;54(5):923–924. 10.1373/clinchem.2007.097758. [DOI] [PubMed] [Google Scholar]
- 19.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 20.Muntner P, Carey RM, Gidding S, et al. Potential U.S. population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation. 2018;137(2):109–118. 10.1161/CIRCULATIONAHA.117.032582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vespa J The changing economics and demographics of young adulthood: 1975–2016. Suitland-Silver Hill, MD: U.S. Census Bureau; April 2017. https://www.census.gov/library/publications/2017/demo/p20-579.html. Published April 2017. Accessed January 15, 2021. [Google Scholar]
- 22.Stroud C, Walker LR, Davis M, Irwin CE Jr.. Investing in the health and well-being of young adults. J Adolesc Health. 2015;56(2):127–129. 10.1016/j.jadohealth.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Kwan MY, Cairney J, Faulkner GE, Pullenayegum EE. Physical activity and other health-risk behaviors during the transition into early adulthood: a longitudinal cohort study. Am J Prev Med. 2012;42 (1):14–20. 10.1016/j.amepre.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 24.Bellows-Riecken KH, Rhodes RE. A birth of inactivity? A review of physical activity and parenthood. Prev Med. 2008;46(2):99–110. 10.1016/j.ypmed.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez E Life’s simple 7: vital but not easy. J Am Heart Assoc. 2018;7 (11):e009324. 10.1161/JAHA.118.009324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lobelo F, Rohm Young D, Sallis R, et al. Routine assessment and promotion of physical activity in healthcare settings: a scientific statement from the American Heart Association. Circulation. 2018;137(18): e495–e522. 10.1161/CIR.0000000000000559. [DOI] [PubMed] [Google Scholar]
- 27.Gooding HC, Milliren C, Shay CM, Richmond TK, Field AE, Gillman MW. Achieving cardiovascular health in young adulthood-which adolescent factors matter? J Adolesc Health. 2016;58(1):119–121. 10.1016/j.jadohealth.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Covelli MMC. Efficacy of a school-based cardiac health promotion intervention program for African-American adolescents. Appl Nurs Res. 2008;21(4):173–180. 10.1016/j.apnr.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Brown DM, Bray SR, Beatty KR, Kwan MY. Healthy Active Living: a residence community-based intervention to increase physical activity and healthy eating during the transition to first-year university. J Am Coll Health. 2014;62(4):234–242. 10.1080/07448481.2014.887572. [DOI] [PubMed] [Google Scholar]
- 30.Jirathananuwat A, Pongpirul K. Promoting physical activity in the workplace: a systematic meta-review. J Occup Health. 2017;59(5):385–393. 10.1539/joh.16-0245-RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lemacks JL, James RE, Abbott L, et al. The church bridge project: an academic–community perspective of a church-based weight management pilot intervention among young adult African Americans. Prog Community Health Partnersh. 2018;12(1S):23–34. 10.1353/cpr.2018.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garbers S, Hunersen K, Nechitilo M, et al. Healthy weight and cardiovascular health promotion interventions for adolescent and young adult males of color: a systematic review. Am J Mens Health. 2018;12 (5):1328–1351. 10.1177/1557988318777923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deatrick JA, Klusaritz H, Atkins R, et al. Engaging with the community to promote physical activity in urban neighborhoods. Am J Health Promot. 2019;33(5):718–726. 10.1177/0890117118807405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lau PW, Lau EY, Wong del P, Ransdell L. A systematic review of information and communication technology-based interventions for promoting physical activity behavior change in children and adolescents. J Med Internet Res. 2011;13(3):e48. 10.2196/jmir.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rose T, Barker M, Maria Jacob C, et al. A systematic review of digital interventions for improving the diet and physical activity behaviors of adolescents. J Adolesc Health. 2017;61(6):669–677. 10.1016/j.jadohealth.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pettee Gabriel K, Sidney S, Jacobs DR Jr, et al. Ten-year changes in accelerometer-based physical activity and sedentary time during mid-life: the CARDIA Study. Am J Epidemiol. 2018;187(10):2145–2150. 10.1093/aje/kwy117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer KA, Boone-Heinonen J, Duffey KJ, et al. Combined measure of neighborhood food and physical activity environments and weight-related outcomes: the CARDIA study. Health Place. 2015;33:9–18. 10.1016/j.healthplace.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitaker KM, Pettee Gabriel KP, Buman MP, et al. Associations of accelerometer-measured sedentary time and physical activity with prospectively assessed cardiometabolic risk factors: the CARDIA study. J Am Heart Assoc. 2019;8(1):e010212. 10.1161/JAHA.118.010212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmed NU, Smith GL, Flores AM, et al. Racial/ethnic disparity and predictors of leisure-time physical activity among U.S. men. Ethn Dis. 2005;15(1):40–52. XXX . [PubMed] [Google Scholar]
- 40.Orr MG, Kaplan GA, Galea S. Neighbourhood food, physical activity, and educational environments and black/white disparities in obesity: a complex systems simulation analysis. J Epidemiol Community Health. 2016;70(9):862–867. 10.1136/jech-2015-205621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


