Abstract
Ferroptosis is an iron-dependent regulated necrosis characterized by the peroxidation damage of lipid molecular containing unsaturated fatty acid long chain on the cell membrane or organelle membrane after cellular deactivation restitution system, resulting in the cell membrane rupture. Ferroptosis is biochemically and morphologically distinct and disparate from other forms of regulated cell death. Recently, mounting studies have investigated the mechanism of ferroptosis, and numerous proteins play vital roles in regulating ferroptosis. With detailed studies, emerging evidence indicates that ferroptosis is found in multiple lung diseases, demonstrating that ferroptosis appears to be particularly important for lung diseases. The mounting interest in ferroptosis drugs specifically targeting the ferroptosis mechanism holds substantial therapeutic promise in lung diseases. The present review emphatically summarizes the functions and integrated molecular mechanisms of ferroptosis in various lung diseases, proposing that multiangle regulation of ferroptosis might be a promising strategy for the clinical treatment of lung diseases.
1. Background
The systematic analysis for the Global Burden of Disease Study 2017 indicates that lung cancer and chronic obstructive pulmonary disease (COPD) are the leading causes of death worldwide [1]. With the outbreak of the 2019 coronavirus disease (COVID-19) in 2020, the surge of confirmed cases poses a severe threat to public health globally. The pathogenesis and epidemiological characteristics of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) have recently attracted considerable attention [2]. Ferroptosis is a new form of iron-dependent regulated cell death (RCD), different from necrosis, apoptosis, autophagy, necroptosis, and pyroptosis [3]. As a stress response, ferroptosis mediates the occurrence and development of neurological diseases, tumors, renal injury, ischemia-reperfusion (I/R) injury, and lung diseases [4–6]. Recent studies have suggested that various lung diseases display ferroptosis of lung cells and progressive damage of lung function in the process of chronic development, which is life-threatening ultimately. The role and mechanism of ferroptosis in lung diseases have attracted much attention recently [7]. In the studies of COVID-19, acute lung injury (ALI), pulmonary fibrosis (PF), COPD, lung cancer, and other lung diseases; unstable iron accumulation; and enhanced lipid peroxidation (LPO) are observed in lung cells, accompanied by the downregulation of glutathione peroxidase 4 (GPX4), suggesting that ferroptosis in lung tissue plays a vital role in various lung diseases [8–11]. Thus, it is imperative to investigate further the regulatory mechanism of ferroptosis and its role in lung diseases. Targeting ferroptosis in lung cells has broad prospects for lung diseases therapy.
2. Introduction
Ferroptosis is iron-catalyzed regulated necrosis, and it occurs when the intracellular antioxidant systems are inhibited by pharmacological inhibition or genetic knockdown, such as GPX4, ferroptosis suppressor protein 1 (FSP1), cystine/glutamate antiporter SLC7A11 (also commonly known as xCT), and guanosine triphosphate cyclohydrolase 1 (GCH1). The nature of ferroptosis is the peroxidation damage of the cell membrane or organelle membrane caused by the inactivation of the intracellular reduction system, resulting in cell membrane rupture [12, 13]. Ferroptosis requires regulation of other intracellular signaling molecules, as exemplified by protein 53 (p53), nuclear factor erythroid-2-related factor 2 (Nrf2), and mitogen-activated protein kinase (MAPK) [4, 14, 15]. But the specific mechanism is unclear. Mounting evidence indicates multiple oxidation and antioxidant systems can be activated simultaneously and run in parallel to adjust the ferroptosis threshold. The role of ferroptosis in growth and development has remained largely uncharacterized. Still, the disorder of ferroptosis regulation triggers diseases as stress response and participates in various lung diseases [8–11].
3. Mechanism and Indicators of Ferroptosis
3.1. Quantitative Variation-Accumulation Period of Iron-Dependent Lipid Peroxides
3.1.1. Abnormal Iron Metabolism
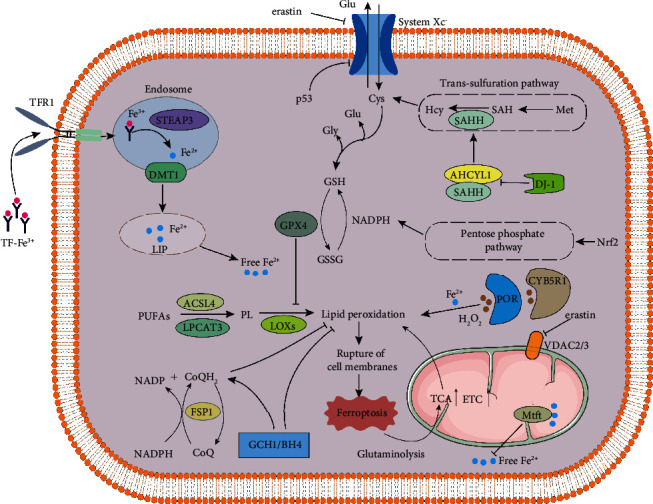
In physiological situations, duodenal cytochrome B (DCYTB), a small intestinal cell membrane reductase, reduces Fe3+ to Fe2+ and transports Fe2+ into intestinal epithelial cells through the divalent metal transporter (DMT1) [16]. Fe2+ in intestinal epithelial cells is transported to the blood through membrane iron transporters and is oxidized into Fe3+ by ferrous oxidase. In the blood circulation, Fe3+ binds to transferrin (TF) and undergoes various tissues and organs [16]. The iron reductase six-transmembrane epithelial antigen of prostate 3 (STEAP3) reduces Fe3+ to Fe2+ in the endosome. Fe2+ is transferred to the labile iron pool through DMT1, and excessive iron is transported to the bloodstream or stored in ferritin [11]. Under pathological conditions, excessive free Fe2+ will gather in the cytoplasm (iron overload), and Fe2+ produces vast amounts of hydroxyl radicals and reactive oxygen species (ROS) through the Fenton reaction, which destroys the cell membrane, DNA, and proteins in the cells and triggers ferroptosis [17]. The labile iron pool is strictly controlled at multiple levels by the p62/keap1/Nrf2 axis. The upregulation of Nrf2 expression promotes the expression of antioxidant-related genes NAD(P)H:quinone oxidoreductase 1 (NQO1), heme oxygenase 1 (HO-1), and ferritin heavy chain 1 (FTH1), which diminishes the production of free Fe2+ and LPO and inhibits the occurrence of ferroptosis [15].
3.1.2. Abnormal Lipid Metabolism
Polyunsaturated fatty acids (PUFAs) are one kind of linear fatty acids with two or more double bonds and 18~22 carbon atoms in length [18], which participate in synthesizing lipid signaling molecules. Moreover, PUFAs are esterified into membrane phospholipids and integrated into the cell membrane to regulate cell membrane fluidity. Most investigators concur that PUFAs are easily oxidized to lipid peroxides to induce ferroptosis in cells because of their unstable double bonds [19]. Acyl-CoA synthetase long-chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3) related to lipid remodeling play a central role in the synthesis of PUFAs. With the knockdown of ACSL4 or LPCAT3, the synthesis of PUFAs decreases, and ferroptosis is significantly inhibited [20]. In addition, lipoxygenases (LOXs) catalyze PUFAs to form lipid hydroperoxides, aggravate toxic LPO, and induce ferroptosis [18].
3.1.3. Mitochondrial Dysfunction
Mitochondria are the prominent organelle of intracellular ROS generation and contribute to the regulation of ferroptosis [21]. Inhibiting the activity of GPX4 by RAS selective lethal 3 (RSL3) results in decreased mitochondrial volume, diminished cristae number, and disruption of the outer membrane [22]. The ferroptosis inducer erastin binds to the voltage-dependent anion channel (VDAC2/3) of the mitochondrial outer membrane, downregulates the mitochondrial outer membrane permeability, reduces the NADH oxidation rate, and promotes ferroptosis [23]. In addition, the inhibition of the mitochondrial tricarboxylic acid (TCA) cycle or electron transport chain (ETC) attenuates mitochondrial membrane potential hyperpolarization and lipid peroxide accumulation [21]. Lowering cysteine (Cys) levels promotes intracellular glutamine catabolism, leading to the expansion of lipid peroxides and ferroptosis [21]. Mitochondrial ferritin (MtFt) stores intracellular free Fe2+ and inhibits intracellular iron overload, thereby diminishing LPO [24].
3.1.4. GPX4 and System Xc−
With the reductant glutathione (GSH), GPX4 catalyzes lipid hydroperoxide and free hydrogen peroxide to produce lipid alcohols (L-OH) and water, inhibits the accumulation of lipid peroxide, and negatively regulates ferroptosis [25]. GSH engages in the precise regulation of GPX4 activity, which functions as a positive regulator in maintaining intracellular redox balance [26]. Cys is essential to the synthesis of intracellular GSH, and its intracellular content depends on the functional activity of the cystine/glutamate antiporter (system Xc−) [27, 28]. When the intracellular Cys content is insufficient, GSH synthesis encounters a barrier and GPX4 activity decreases, resulting in an accumulation of intracellular lipid peroxide [27]. Moreover, the transsulfuration pathway is another important source of intracellular GSH synthesis. Inhibition of transsulfuration is a leading cause of the imbalance of the intracellular antioxidant system [29]. When system Xc− is inhibited by erastin, DJ-1 (also known as Parkinson disease type 7, PARK7) inhibits the interaction between adenosylhomocysteinase like 1 (AHCYL1) and S-adenosyl-L-homocysteine hydrolase (SAHH), maintains the activity of SAHH, and sustains the essential synthesis of Cys [30]. Thus, inhibition of the system Xc− appears to be particularly important for reducing GSH, precisely regulating cells' antioxidant function.
3.1.5. FSP1
FSP1 is a novel coenzyme Q10 (CoQ10) plasma membrane oxidoreductase, protecting cells from glutathione-dependent ferroptosis [31, 32]. FSP1 is localized to the cytoplasmic membrane after acylation modification. It promotes the transformation of CoQ10 to Ubiquinol-10 (CoQH2) with the assistance of NAD(P)H to generate radical-trapping antioxidants (RTA), which prevented the proliferation of LPO [33, 34]. In some cases, FSP1 directly activates the endosomal sorting complex required for transport-III (ESCRT-III) to promote cell membrane repair and inhibit ferroptosis [35]. With detailed studies, increased FSP1 expression reduces LPO and inhibits ferroptosis when GPX4 is inactivated [36]. Consequently, the FSP1/CoQ10/NAD(P)H pathway is an antioxidant system parallel to GPX4, which cooperates with GPX4 and glutathione to upregulate antiferroptotic defense.
3.1.6. GCH1
GCH1 is a rate-limiting enzyme in the synthesis of tetrahydrobiopterin (BH4), which maintains vital antioxidant activity [37, 38]. GCH1 enhances the synthesis of BH4/BH2 to induce lipid remodeling and inhibits phospholipid consumption containing two polyunsaturated fatty acyl groups to diminish ferroptosis [39]. In addition, the overexpression of GCH1 favors the formation of CoQ10 and synergistically exerts the inhibitory effect of ferroptosis. BH4 is a cofactor for dopamine key enzymes that attenuates erastin-induced ferroptosis [40]. The GCH1-BH4-phospholipid axis functions as a negative regulator of ferroptosis by promoting the synthesis of endogenous BH4 and CoQ10 and reducing the formation of lipid peroxides [39].
3.1.7. NADPH
NADPH is a ferroptosis inhibitor produced by the pentose phosphate pathway, which suppresses ferroptosis induced by erastin. The increase of the NADP/NADPH ratio promotes ferroptosis sensitivity, ferroptosis-resistant cell lines are associated with the high expression of NADPH, and NADPH oxidase- (NOX-) mediated NADPH oxidation triggers ferroptosis [41]. Nrf2 is an essential nuclear transcription factor of intracellular antioxidants, enhancing NADPH synthesis by regulating the pentose phosphate pathway [42]. NADPH functions as a positive regulator of the antioxidant activity of GPX4 and FSP1 by regulating electron transport and plays a vital role in attenuating ferroptosis.
3.1.8. Others
Multiple intracellular signaling molecules regulate ferroptosis. For example, p53 is the negative regulator of the system Xc− activity and intracellular antioxidant GSH production. In addition, p53 promotes the expression of spermidine/spermine N1-acetyltransferase (SAT1), increases the expression of arachidonic acid 15-LOX, and accelerates intracellular lipid peroxide accumulation [43]. Heat shock protein beta-1 (HSPB1) is phosphorylated by protein kinase C in Hela cells, blocking cytoskeleton-mediated iron uptake and subsequent ROS production and inhibiting ferroptosis [44]. Nuclear receptor coactivator 4 (NCOA4) is a selective freighter receptor that promotes selective autophagy of ferritin. Knockdown of NCOA4 inhibits ferritin degradation and prevents ferroptosis [45]. High mobility group protein B1 (HMGB1) is another crucial ferroptosis regulator, promoting erastin-induced ROS production through an iron-mediated lysosome pathway. Moreover, HMGB1 increases the level of TFR through the RAS-JNK/p38 pathway and exacerbates intracellular iron overload and lethal accumulation of lipid peroxides [46, 47]. Thus, multiple intracellular signaling molecules participate in ferroptosis, and the specific role and mechanism need to be further elucidated [47].
3.2. Quality Change-Oxidative Damage of Liposome Membrane
NADPH-cytochrome P450 reductase (POR) and cytochrome b5 reductase (CYB5R1) transmit electrons from NADPH/NADH to oxygen; the generated H2O2 undergoes Fenton reaction with iron ions to produce hydroxyl radicals, which deprives the hydrogen atoms between the double bonds in the unsaturated fatty acid chain, triggers LPO, and causes membrane damage [48, 49]. Oxidative stress in the process of ferroptosis comes from the “accidental” electron leakage of intracellular oxidoreductase during electron transfer. The intracellular antioxidant systems alleviate oxidative damage under physiological conditions. The cell membrane damage during ferroptosis is a nondependent lipid peroxidation process of membrane perforation protein, which is different from necroptosis and pyroptosis [50, 51] (Figure 1).
Figure 1.
3.3. Indicators of Ferroptosis
Ferroptosis is accompanied by a series of molecular biological changes. Morphologically, transmission electron microscopy (TEM) observes that cells undergoing ferroptosis mainly manifest as volume reduction, mitochondrial density increase, ridge reduction or disappearance, and mitochondrial outer membrane disruption [52]. More recent evidence indicates ferroptosis is accompanied by decreased cell viability with cholecystokinin octapeptide (CCK-8). In terms of iron metabolism, the PGSK probe is considered to display the increase of cell iron concentration. Quantitative PCR (qPCR), Western blot, and immunoblotting are used to detect the decrease of ferritin expression and the increase of TFR1 and hepcidin expression. In terms of lipid metabolism, GPX4, SLC7A11, COX2, and ACSL4 are considered as specific biomarkers of ferroptosis. qPCR or Western blot show that GPX4 and SLC7A11 are significantly downregulated, while the expression of COX2 and ACSL4 increased. Mitochondrial function has been proposed as another ferroptosis biomarker. qPCR and Western blot suggest that DHODH is significantly upregulated in ferroptosis [53]. VDAC2 and VDAC3 are necessary and insufficient conditions for erastin-induced ferroptosis. Infection with shRNA targeting VDAC2 has been used as a positive control for ferroptosis research [3].
4. Differences and Connections between Ferroptosis and Other RCDs
4.1. Ferroptosis and Necrosis
Necrosis is a passive cell death under substantial physical and chemical or biological factors. It is morphologically characterized by increased cell swelling, organelle deformation or swelling, and eventually cell rupture. Necrotic cell division explains the release of inclusions and causes an inflammatory response, often accompanied by fibrosis of tissues and organs during healing. With detailed studies, multiple proteins participate in the signal regulation of necrosis. Ferroptosis is a newly described caspase-independent regulatory necrosis, featuring iron-dependent necrosis which resulted in lethal accumulation of lipid peroxides. In the glioblastoma (GBM) model, neutrophils transfers myeloperoxidase particles into tumor cells, inducing iron-dependent accumulation of lipid peroxides in tumor cells and promoting iron-dependent necrosis of glioblastoma [54]. Since ferroptosis is crucial regulatory necrosis, triggering ferroptosis to induce cancer cell necrosis plays an increasingly important role in the clinical treatment of malignant tumors.
4.2. Ferroptosis and Apoptosis
Apoptosis is a programmed cell death in that cells “end” their lives automatically under certain physiological or pathological conditions, controlled by an internal genetic mechanism, morphologically manifested as cell shrinkage and the formation of an apoptosome [55]. Under certain conditions, apoptosis and ferroptosis promote each other in positive feedback. p53, an apoptosis-related tumor suppressor gene, upregulates ferroptosis [56]. Ferroptosis further advances cells' sensitivity to apoptosis, while the ferroptosis inducer promotes the expression of the p53 upregulated modulator of apoptosis (PUMA) by protein-folding reaction [57, 58]. The above-mentioned results indicate the mutual promotion between ferroptosis and apoptosis, and it is of great significance in modulating ferroptosis by adjusting apoptosis in clinical antitumor therapy.
4.3. Ferroptosis and Autophagy
Autophagy is a process in which cytoplasmic proteins or organelles are phagocytized and coated into vesicles, forming autolysosomes and degrading contents, morphologically manifested as cytoplasmic vacuolization and autophagosome formation [59]. Most investigators concur that autophagy is a necessary step of ferroptosis and promotes the occurrence of ferroptosis. On the one hand, autophagy degrades ferritin, and ferritinophagy is a critical factor in ferroptosis [60]. Autophagy-dependent lysosome ROS formation stimulates ferroptosis and provides unstable iron through NCOA4-mediated iron autophagy [61]. On the other hand, intracellular Fe2+ and LPO reduction happened after targeted knockdown autophagy-related gene 5 (Atg5) and Atg7 genes [62, 63]. Thus, the induction of autophagy is necessary for ferroptosis, and ferroptosis is essentially a process of autophagy cell death [45, 64, 65]. Regulating ferroptosis by targeting autophagy-related pathways is potentially an essential method for clinically treating ferroptosis-related diseases.
4.4. Ferroptosis and Necroptosis
Necroptosis is a kind of cell death with similar morphological necrosis and signaling pathways of apoptosis [66], characterized by necrosis-like cell death [67]. Ferroptosis and necroptosis coincide under ferroptosis inducer erastin in human promyelocytic leukemia HL-60 cells [4]. Ferroptosis and necroptosis are mutually substituted and reciprocal. Ferroptosis resistance induces necroptosis, while necroptosis resistance promotes ferroptosis [68]. Inhibition of ferroptosis marker ACSL4 promotes mixed lineage kinase domain-like (MLKL) expression and downregulates MLKL-induced ferroptosis [68]. The murine embryonic fibroblast cell line (NIH3T3) with targeted MLKL gene knockdown shows specific resistance to necroptosis, and ferroptosis occurs immediately after treatment with erastin [69]. In addition, the change of intracellular NADPH content is correlated with the substitution between ferroptosis and necroptosis [70]. Therefore, targeted inhibition of MLKL to obtain necroptosis resistance enhances the clinical antitumor effect of the ferroptosis inducer to some extent.
4.5. Ferroptosis and Pyroptosis
Pyroptosis is a distinct RCD [71]. Pyroptosis is accompanied by the forming of caspase-1-dependent plasma membrane pores, leading to the release of proinflammatory cytokines and cell fragmentation, morphologically characterized by pyroptosis formation bodies [72]. The early ferroptosis stage shows a similar pattern to pyroptosis, which releases inflammatory injury-related factors to control the inflammatory response and coordinate antibacterial defense [73, 74]. Ferroptosis is a caspase-independent regulatory death. The mitochondrial permeability increased by Bcl-2-associated X protein/Bcl-2-associated K protein (Bax/Bak) is unnecessary [75]. Synergistic enhancement of antitumor activity and sensitivity to drug-resistant tumors happened when inducing pyroptosis and ferroptosis simultaneously [76]. Therefore, the synergistic antitumor effect of inducing pyroptosis and ferroptosis offers current knowledge to cancer treatment (Table 1).
Table 1.
Comparison of characteristics of ferroptosis and necrosis, apoptosis, autophagy, necroptosis, and pyroptosis.
| Cell death manner | Morphologic features | Biochemical characteristics | Key controlling genes | References |
|---|---|---|---|---|
| Ferroptosis | Membrane rupture or blistering, mitochondrial atrophy, crista reduction, lack of chromatin condensation | Lipid peroxidation in cells induced by ferrous or esterase | GPX4, p53, Ras, Nox, SLC7A11, TFR1 | [3, 4, 52] |
| Necrosis | Lipid peroxidation in cells induced by ferrous or esterase | Inflammatory response | — | |
| Apoptosis | Cell membrane foaming, cell contraction, the formation of apoptotic bodies | DNA degradation | Bcl-2 family, caspase family, C-myc, p53 | [55, 56] |
| Autophagy | Cytoplasmic vacuolization to form autophagosomes | Increase activity of lysosomes | ATG5, ATG7, Beclin1 | [59, 199] |
| Necroptosis | Cell volume enlargement, organelle swelling, cell membrane perforation, with necrotic cell characteristics | Cell collapse, release contents, triggering an immune response, and clearing the dead cells through macropinocytosis bodies | RIPK1, RIPK3, TNFR1, caspase-8 | [66, 67] |
| Pyroptosis | Cell swelling, with a large number of bubble-like protrusions; a large number of vesicles, pyroptosis bodies, formed before the rupture of the plasma membrane | Formation of inflammatory bodies, activation of caspase and gasdermin, the release of a large number of proinflammatory factors | Caspase family, NLRP3, ASC | [71–73] |
5. Pulmonary Manifestations of Ferroptosis
5.1. Ferroptosis-Induced Inflammatory Response
The changes in the extracellular immune microenvironment play vital roles in the pathophysiology of lung diseases. COVID-19 is characterized by an invasive inflammatory response, which strongly leads to airway damage [77]. ALI undergoes alveolar epithelial dysfunction caused by acute inflammation and tissue injury [78]. COPD is associated with an abnormal inflammatory response induced by harmful particle deposition [79]. The research on the pathogenesis of idiopathic pulmonary fibrosis (IPF) mainly focuses on chronic inflammation [80]. The above provides evidence that lung inflammation participates in the development of lung diseases.
Lung inflammation is accompanied by lung cells' ferroptosis, which further promotes the inflammatory response. In the early stage of ferroptosis, cells release inflammatory injury-related factors to control the inflammatory response and coordinate host defense [73, 74]. Ferroptotic cells release damage-associated molecular patterns (DAMPs) and activate the advanced glycation end product specific receptor (AGER) to trigger the inflammatory response of peripheral macrophages and activate NF-κB in innate immunity [46, 81]. Hemin, which is chemically similar to heme, causes ROS accumulation and lipid peroxidation in platelets through proteasome activity and inflammasome activation and induces cell ferroptosis [82]. Patients with COVID-19 showed high concentrations of proinflammatory CD4+ T cells and cytotoxic particle CD8+ T cells and dysregulation of iron homeostasis [83, 84]. The iron chelator lactoferrin (LF) inhibits iron overload to reduce inflammation and prevent SARS-CoV-2 from entering host cells [85]. The release of inflammatory factors in ALI enhances the accumulation of ROS and induces ferroptosis. The accumulated ROS functions as a positive regulator of inflammatory response and promotes the progression of ALI [86]. Early release of DAMPs from ferroptotic cells in COPD triggers an inflammatory response, and GPX4 knockdown favors the development of chronic inflammation induced by cigarette smoke [8]. Ferroptosis inhibitor ferrstatin-1 (Fer-1) inhibits the fibrogenic factor TGF-β1 during the progression of IPF to play the protective role against IPF [87]. Collectively, ferroptosis promotes lung inflammation through DAMPs and ROS, and targeted inhibition of inflammatory response mediated by lung cell ferroptosis is a potential strategy for lung disease therapy.
5.2. Ferroptosis in Pulmonary I/R Injury
Lung I/R injury (LIRI) is a common clinical-pathological phenomenon with high mortality, which can be caused by lung transplantation, atherosclerosis, and trauma [88–90]. Ischemia leads to an insufficient energy supply, intracellular calcium, and lactic acid accumulation and eventually cell death [91]. ROS and proinflammatory neutrophils after reperfusion increase lung tissue damage [91, 92]. Oxidative stress, inflammatory response, and toxic substances play crucial roles in the progression of lung disease during LIRI, in which NOX and labile iron are involved [93–95].
The LIRI process is accompanied by obvious ferroptosis of lung cells. Inefficient oxidative phosphorylation, ROS production in hypoxanthine metabolism, increase of labile iron caused by ischemia, and NOX expression can all trigger ferroptosis [89, 96]. In addition, LIRI contributes to the decrease of GSH and GPX4, the increase of ASCL4 expression, and the change of mitochondrial morphology, which favors the accumulation of lipid peroxidation [88]. Ferroptosis participates in the injury of the lung and other tissues and organs, which is related to the promotion of ferroptosis by I/R [97]. Ferroptosis is the primary form of cell death during IR [98], and ferroptosis of lung tissue cells aggravates LIRI. With detailed studies, ferroptosis exacerbates hepatic I/R injury, intestinal I/R injury, and ER stress-related myocardial I/R injury [6, 99, 100], and the induction of ferroptosis deteriorates I/R-related lung injury and pulmonary edema [97]. Most investigators concur that inhibiting ferroptosis can alleviate LIRI. Given the vital role of ferroptosis in I/R, diminishing ferroptosis and reversing LIRI are beneficial to COVID-19 treatment [101]. The inhibitor of the apoptosis-stimulating protein of p53 can inhibit ferroptosis and alleviate IR-induced ALI [102]. Upregulating Nrf2 attenuates ferroptosis and plays a protective role against intestinal IR-induced ALI [97]. In addition, pirfenidone ameliorates LIRI, ALI, and PF by modulation of inflammation and oxidative stress [103], indicating that the inhibition of ferroptosis may also be involved. In summary, LIRI is the pathological process of various lung diseases, and inhibiting ferroptosis alleviates LIRI-mediated lung diseases effectively.
5.3. Ferroptosis Triggers Immunological Changes
Normal lung function is closely related to immune homeostasis, and the congenital immune response is the first line of defense for early pulmonary infection [104]. Patients with COVID-19 have decreased lymphocytes, increased neutrophils [105, 106], and necrosis of the spleen, lymph nodes, and other lymphoid tissues, as well as lymphocyte infiltration in the alveolar septa [83, 106]. In ALI and PF, alveolar macrophages are activated [107], and neutrophils trigger the cascade of inflammation and fibrosis in the lungs [108]. A significant feature of COPD is inflammation, with increased macrophages, neutrophils, T and B lymphocytes, and dendritic cells (DC) [109]. Lymphocyte infiltration is associated with lung cancer [110].
Ferroptosis holds great therapeutic potential in many lung diseases related to ferroptosis-mediated immune changes in the lung and whole body. There are many immune cells, such as macrophages and DC in the lungs, which play a crucial role in maintaining the immune homeostasis of the lung [107]. The signals released by ferroptotic cells can recruit antigen-presenting cells (APCs), such as macrophages, DC, and neutrophils, to the site of ferroptosis and promote antigen presentation to initiate and regulate immune response [111, 112]. When ferroptosis occurs in lung tissue, the APCs mentioned above migrate to the damaged area [113] and start specific immunity by recognizing, phagocytizing, and directly killing the pathogen or promoting antigen presentation. At present, lung cancer has been mainly studied for ferroptosis-mediated immune changes. Ferroptosis favors the activation of macrophages [74], which is beneficial for pulmonary immune monitoring [114]. Nonetheless, GPX4 diminishes lipid peroxidation to maintain activation of T regulatory cells and attenuate antitumor immunity [115]. Current knowledge argues that interferon-γ (IFNγ) produced by immune cells downregulates SLC3A2 and SLC7A11 to trigger ferroptosis [116], and the induction of ferroptosis contributes to the antitumor efficacy of immunotherapy [117]. With detailed studies, an increase in tumor-associated macrophages (TAM) in the tumor microenvironment is associated with poor prognosis of patients with lung adenocarcinoma [117], and ferroptosis attenuates metabolic and inflammatory regulation of TAM to elicit antitumor activity [118]. Ferroptotic cancer cells release immunomodulatory molecules to induce systemic immune change, including DAMP, oxidized lipid mediators, and prostaglandin E2 (PGE2). DAMP triggers the toll-like receptor 4 (TLR4) signals of neutrophils and DC, activating the innate immune system [111]. Oxidized lipid mediators induce GPX4 depletion to promote T cell ferroptosis [119]; oxidized phosphatidylcholine inhibits DC maturation through Nrf2 activation and inhibits the differentiation of T helper cell 17 (Th17) [120]. PGE2 plays an essential role in both innate and regulatory immunity. PGE2 directly attenuates the function of cytotoxic T cells and conventional type 1 dendritic cells (cDC1) or diminishes the accumulation of cDC1 in tumor sites by inhibiting the natural killer (NK) cell-chemokine CC chemokine ligand 5 (CCL5)/chemokine lymphotactin (XCL1) pathway [25, 121].
Therefore, the immune response is crucial in the lungs and the whole body, and the pathophysiological process of lung diseases is accompanied by the ferroptosis of lung cells. Ferroptosis-mediated immune changes precisely regulate the progression of lung diseases. The regulation of ferroptosis in lung cells and ferroptosis-mediated immune changes holds broad prospects in treating lung diseases.
6. Ferroptosis and Lung Disease
6.1. Ferroptosis and COVID-19
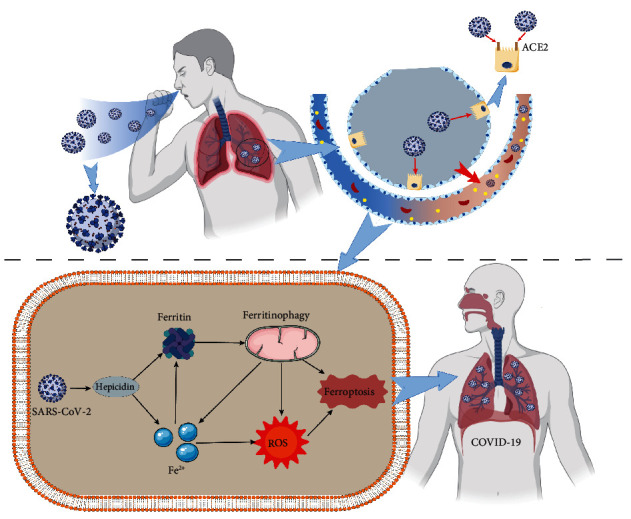
COVID-19 is a new type of fulminant epidemic disease caused by SARS-CoV-2 infection, clinically manifested with pneumonia, acute respiratory distress syndrome (ARDS), and sepsis, leading to multiple organ failure [122, 123]. More recent evidence indicates that SARS-CoV-2 induces iron overload by promoting the overexpression of hepcidin [124, 125]. COVID-19 infection leads to an inflammatory state, including cytokine storm, in which IL-6 stimulates the synthesis of ferritin and hepcidin. Both upregulation of hepcidin and downregulation of ferritin significantly impede iron output, leading to iron overload and ferroptosis in cells [126]. With detailed studies, SARS-CoV-2 simulates hepcidin to increase serum ferritin content significantly. Hyperferritinemia enriches high oxidative stress and LPO and ultimately increases mitochondrial autophagy leading to ferroptosis [127, 128]. The overexpression of hepcidin and iron overload play critical roles in COVID-19, and hepcidin has been proposed as a specific biomarker to measure the effectiveness of COVID-19 therapy. Studies have indicated that a large amount of phospholipid oxide accumulates in myocardial cells of COVID-19 patients. Ferroptosis aggravates myocardial I/R injury, which contributes to myocardial injury and multiple organ failure of COVID-19 [129–131]. Overall, ferroptosis may take a vital part in promoting COVID-19, and inhibition of ferroptosis sheds new light on reliable treatments of COVID-19 (Figure 2).
Figure 2.
6.2. Ferroptosis and ALI
ALI is an acute and fatal illness after severe infection, trauma, and harmful gas inhalation, associated with pathological features, namely, pulmonary edema and atelectasis caused by diffuse alveolar-capillary membrane injury. The clinical manifestations are respiratory distress and refractory hypoxemia [132]. Of note, uncontrolled lung inflammation and oxidative/antioxidant imbalance are the two main mechanisms in the complex pathogenesis of ALI [133]. Ferroptosis is reportedly crucial in various ALI models, including lipopolysaccharide- (LPS-) induced ALI [134], intestinal I/R injury-induced ALI [102], seawater drowning-induced ALI [135], and oleic acid-induced ALI [136], as well as pseudomonas aeruginosa- (PA-) induced ALI [137]. The development of ALI stimulates ferroptosis, which further aggravates ALI [9].
In multiple ALI models, the release of inflammatory cytokines contributes to oxidative damage and uncontrolled inflammation and increases intracellular lipid ROS, myeloperoxidase (MPO), and malondialdehyde (MDA) levels which confer resistance to GSH content GPX4 activity, eventually leading to severe mitochondrial damage, presented as obvious ferroptosis [138, 139]. Inflammatory out-of-control and cytokine storm in ALI facilitated ROS accumulation in lung tissue, which further boosted ferroptosis [134, 140]. ROS-induced oxidative damage and inflammatory activity in ferroptosis further promote the release of inflammatory factors and aggravate LPS-induced ALI [141]. Ferroptosis and LPS-induced ALI show positive feedback to each other. Thus, inhibition of ferroptosis is expected to be useful in ALI therapy.
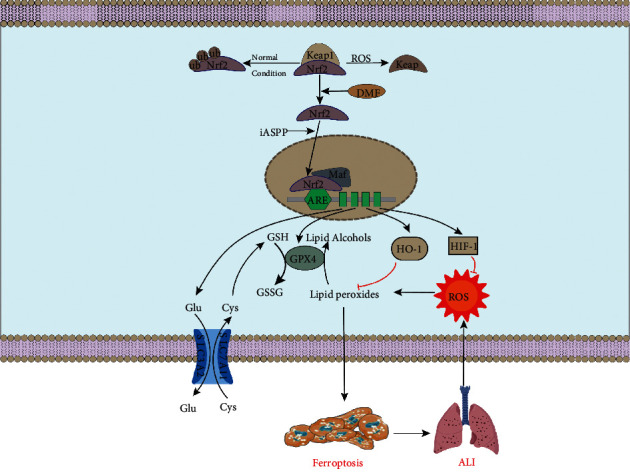
Nrf2 functions as a positive regulator of ALI by inhibiting ferroptosis. With detailed studies, exogenous Nrf2 agonist dimethyl fumarate (DMF) promotes nuclear translocation of Nrf2 through the p62-Keap1-Nrf2 axis [135]. Nrf2 enhances the inhibition of ferroptosis-related genes HIF-1α, HO-1, and SLC7A11 by reducing GSH loss; inhibiting MDA, ROS, and lipid ROS accumulation; and enhancing mitochondrial function to diminish ferroptosis and alleviate ALI [86, 142]. HIF-α plays a vital role in upregulating antiferroptotic defense, which resists iron overload and increases GPX4 expression [143]. HO-1, a ROS detoxifying enzyme, has a protective effect against oxidative stress by upregulating the Nrf2/ARE pathway [144]. The SLC7A11 gene encodes cystine/glutamate xCT transporter, which reduces oxidative stress in epithelial cells by increasing the intracellular cystine level and inhibits the Nrf2/HO-1 signaling pathway by negative feedback to maintain cellular antioxidant balance [142]. Nrf2/HIF-1α and Nrf2/HO-1/SLC7A11 signaling pathways have recently attracted widespread attention in inhibiting ferroptosis and alleviating ALI [102, 145].
The inhibitor of apoptosis stimulating p53 protein (iASPP) is a p53 inhibitor that inhibits ferroptosis by promoting Nrf2 aggregation and nuclear translocation and further alleviates ALI [146, 147]. Fer-1 attenuates ALI by inhibiting LPO and reducing total iron levels in cells [148]. Thus, inhibition of ferroptosis in lung tissue is a promising therapy of ALI (Figure 3).
Figure 3.
6.3. Ferroptosis and PF
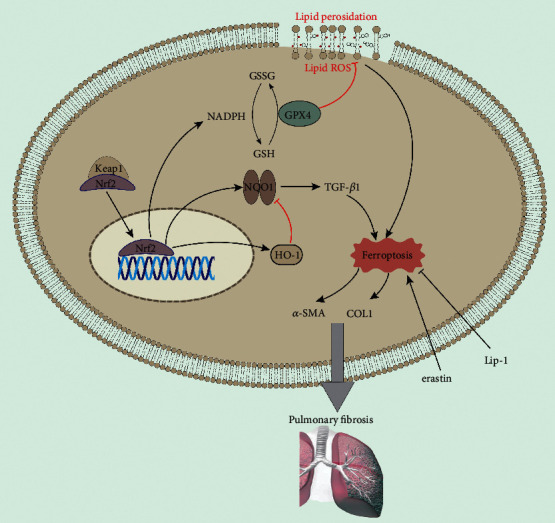
PF is a chronic progressive interstitial lung disease accompanied by the proliferation of myofibroblasts [149]. Recent studies indicate that ferroptosis in lung tissue promotes the development of PF. In the IPF model, erastin decreases the activity of GPX4 in human fetal lung fibroblasts and contributes to the accumulation of lipid peroxides, α-SMA, COL1, and fibroblast differentiation [150]. Ferroptosis inhibitor Fer-1 enhances GPX4 expression and reduces LPO. Moreover, Fer-1 eliminates the effect of TGF-β1 and erastin on cell ferroptosis [87], inhibits the differentiation of fibroblasts into myofibroblasts, and delays the process of PF.
Ferroptosis promotes the occurrence and development of radiation-induced lung fibrosis (RILF) [151]. In the irradiation group in the RILF mouse model, the volume of lung tissue decreases, the mitochondrial density increases, the crista reduces, and the outer membrane is interrupted, showing typical ferroptosis characteristics. Ferroptosis inhibitor liproxstatin-1 (Lip-1) has a significant therapy effect on RILF mice. Lip-1 can increase GPX4 level, inhibit collagen deposition, and reduce inflammatory cytokines and ROS in RILF mice [10]. In addition, Lip-1 attenuates RILF by activating Nrf2, promoting HO-1 and NQO1 expression, downregulating TGF-β1, and inhibiting ferroptosis in lung tissue [10]. Consequently, these findings provide evidence for the clinical treatment of PF by inhibiting ferroptosis (Figure 4).
Figure 4.
6.4. Ferroptosis and COPD
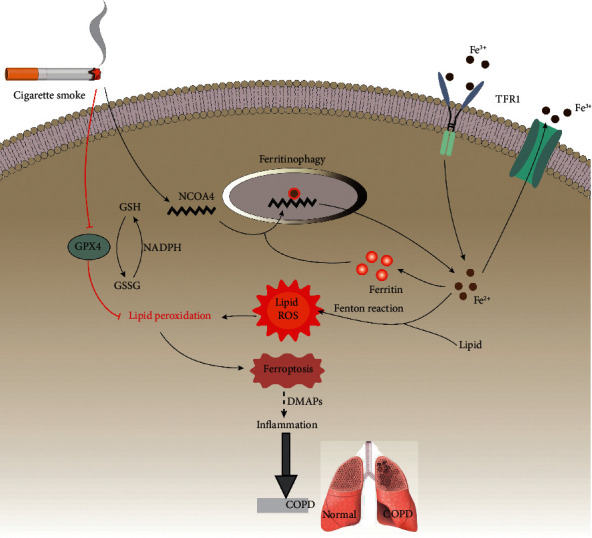
COPD is chronic bronchitis or emphysema, characterized by airflow obstruction, further developing into a common chronic disease of pulmonary heart disease and respiratory failure. COPD is associated with abnormal inflammation caused by harmful gases and particle deposition, with high morbidity and mortality [79]. Although an initial study indicates that ferroptosis occurs in the lung tissue of COPD mice, the current knowledge on the integrated molecular mechanism underlying ferroptosis-involved COPD is controversial.
Some investigators concur that chronic inflammation caused by long-term smoking is one of the pathogenesis of COPD [152]. At the core of the process, free radicals in cigarette smoke induce cell iron overload, promote ferroptosis, and accelerate the development of COPD [153]. Studies have shown that cigarette smoke induces ferroptosis in bronchial epithelial cells by promoting endoplasmic reticulum stress and mitochondrial homeostasis disorder [154]. NCOA4 is a selective receptor for autophagy recognition of ferritin. Cigarette smoke increases the expression of NCOA4, thereby increasing the selective autophagy of ferritin to trigger iron overload and inducing ferroptosis [61]. Moreover, cigarette smoke increases 4-hydroxy-2-nominal (4-HNE) production and downregulates GPX4 expression to accelerate intracellular LPO [8]. DAMPs are released early in ferroptosis to promote chronic inflammation during COPD progression [155].
Inhibition of ferroptosis has a good prospect in the clinical treatment for COPD. Ferroptosis inhibitors, deferoxamine and Fer-1, alleviate cigarette smoke-induced ferroptosis in bronchial epithelial cells [153]. Targeting lipid metabolism-related DAMP signaling might be a promising strategy for treating COPD related to ferroptotic damage (Figure 5).
Figure 5.
6.5. Ferroptosis and Lung Cancer
Lung cancer is the leading cause of death worldwide in males and females [156], usually divided into small cell lung cancer (SCLC) and non-small cell lung cancer cells (NSCLC). Currently, the clinical treatments of lung cancer are mainly chemotherapy drugs and targeted drugs, which achieve the therapeutic purpose by inducing apoptosis [157], but limitations in application still exist. Cancer cells require higher iron metabolism levels and are more susceptible to ferroptosis [158]. In general, ferroptosis resistance is widespread in cancer cells, and inducing ferroptosis in lung cells is expected to become a new target for clinical treatment of lung cancer [159, 160].
6.5.1. The Antiferroptotic Defense of Lung Cancer Cells
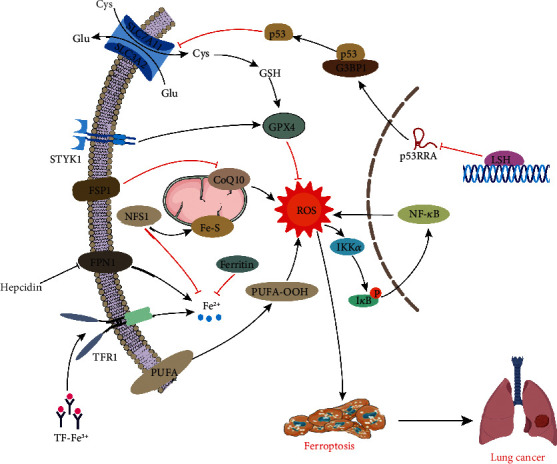
Inhibition of p53 activity is prevalent in tumor cells [161], and acetylation is crucial to p53-mediated ferroptosis and tumor inhibition [43]. SLC7A11 and SLC3A2 are highly expressed in tumor cell lines and increase cellular antioxidant capacity. High expression of serine-threonine tyrosine kinase 1 (STYK1) in NSCLC cell line SW900 promotes the expression of GPX4 and the proliferation of lung cancer cells [162]. In addition, high expression of FSP1 in lung cancer cells promotes lipophilic antioxidant production and inhibits ferroptosis [33, 34].
Iron metabolism is closely related to the tumor microenvironment (TME), which accelerates tumor progression by promoting DNA replication [163, 164]. More recent evidence indicates that high ferritin expression in lung cancer tissues of NSCLC patients attenuates the Fenton reaction to reduce ferroptosis. Iron-sulfur cluster synthase 1 (NFS-1) is generally highly expressed in lung cancer tissue and cell lines. Moreover, NFS-1 collects more sulfur elements from Cys to produce iron-sulfur clusters, which diminishes the release of iron from cells and significantly alleviates the high oxygen-induced cell ferroptosis [165]. Hepcidin and ferroportin-1 (FPN1) constitute important regulators of iron homeostasis in vivo. Indeed, increased plasma hepcidin promotes tumor progression by inhibiting FPN1-induced NF-κB and Wnt signaling pathways and iron-dependent ROS production.
Lung cancer cells fight against ferroptosis by regulating lipid metabolism through lymphoid-specific helicase (LSH). As a DNA methylation modifier, LSH inhibits ferroptosis by activating lipid metabolism-related genes, including glucose transporter 1 (GLUT1), ferroptosis-related genes sterol-CoA desaturase 1 (SCD1), and fatty acid desaturase 2 (FADS2). Thus, LSH is a novel antiferroptotic molecule, which inhibits ferroptosis by reducing intracellular iron and ROS levels [166, 167] (Figure 6).
Figure 6.
6.5.2. Ferroptosis and Lung Cancer Treatment
There is a broad prospect of treating lung cancer by using a ferroptosis inducer [160]. Cisplatin is an inducer of ferroptosis and apoptosis in NSCLC A549 cells, promoting the depletion of reduced GSH and inactivation of GPX4. Ginkgo flavonoids enhance cisplatin's efficacy in treating NSCLC by downregulating the Nrf2/HO-1 pathway [142]. GPX4 inhibitor enhances cisplatin's antitumor activity, and cisplatin combined with erastin has a significant synergistic effect on antitumor activity [168, 169]. Erastin inhibits cell proliferation, promotes G2/M phase arrest, triggers iron transport, and inhibits cell migration in lung cancer cells. Natural product erastin has the potential of treating lung cancer by upregulating p53 expression and promoting the Ca2+/CaM pathway to induce ferroptosis [169–174]. In addition, erastin combined with acetaminophen (APAP) inhibits NSCLC cell viability and promotes ferroptosis and apoptosis by suppressing Nrf2 nuclear translocation [175]. The lysosomal instability drug siramesine and dual tyrosine kinase inhibitor lapatinib induce ferroptosis in A549 cells by decreasing the HO-1 level [176]. Dihydroartemisinin (DHA) inhibits lung cancer cell proliferation and colony formation, enhances cell death, and induces ferroptosis by inactivating the polypeptide 2 (PRIM2)/SLC7A11 axis, indicating that the inhibitory expression of PRIM2 may be a potential therapeutic target for lung cancer [177]. Ferroptosis induced by STYK1 inhibits the development of cancer cells. Zinc poisoning promotes ferroptosis in cancer cells and upregulates antiferroptotic defense [178], which indicates that targeting ferroptosis has been mounting interest in the treatment for NSCLC [162].
Long noncoding RNA (lncRNA) LINC00336 reduces iron concentration, lipid ROS, and mitochondrial superoxide and increases mitochondrial membrane potential, consistent with its role in ferroptosis [179]. Metallothionein 1D pseudogene (MT1DP) is a lncRNA that intensifies oxidative stress by inhibiting the antioxidant effect of Nrf2 [180]. P53RRA is a standard tumor suppressor that inhibits lung cancer by activating the p53 pathway and promoting ferroptosis [181]. LSH is directly recruited into the promoter region of p53RRA to silence p53RRA, and DNA methylation is involved in the inhibition of p53RRA. Ferroptosis is epigenetically regulated by LSH and promotes lipid metabolism genes, including SCD1 and FADS2 expression [167]. The prospect of ferroptosis induction in cancer treatment is growing, but its therapeutic effect is low due to the reduction of Fenton reaction in the TME. Mixed semiconductor nanozymes (HSN) produce heat under light to enhance cytotoxicity and Fenton reaction and increase hydroxyl radical production to promote ferroptosis in tumor cells [182].
After tumor immunotherapy, IFNγ released by activated CD8 + T cells downregulates the expression of SLC7A11 and SLC3A2, thereby inhibiting the uptake of cystine in tumor cells and enhancing LPO and ferroptosis. Inhibition of synergistic effect of system Xc− and GPX4 by ferroptosis inducers and radiation promotes ferroptosis by enhancing LPO in the cytoplasm. Ferroptosis inducer may be an effective radiosensitizer to expand the efficacy and indications of radiotherapy [183]. Drug resistance has become a significant problem in the chemotherapy treatment of lung cancer. The combined use of ferroptosis inducers and chemotherapy drugs may become a new strategy for lung cancer treatment. After combined treatment, tumor cells showed typical morphological changes of ferroptosis [168]. The magnetic field (MF) induces oxidative stress and activates the DNA damage repair pathway through ROS-induced DNA damage, eventually leading to apoptosis and ferroptosis and inhibiting tumor growth [184]. MF is being used for antitumor treatment, but its potential biological mechanism is still unclear.
Clinical treatment of patients with tumor metastasis is a global problem. Targeted ferroptosis may be a promising method to prevent lung cancer metastasis [185]. Compared with local injections, nanoparticles have more tremendous advantages in treating metastasis-associated cancer with relatively low risk [186]. Based on the coordination between ferric ion (Fe3+) and tannic acid (TA), a p53 plasmid encapsulated metal-organic network (MON-p53) is constructed. MON-p53 plasmid inhibits the growth of cancer cells by promoting ferroptosis. The MON-p53 complex has broad prospects in targeting ferroptosis of tumor cells [56]. Lung cancer cells are prone to metastasis and drug resistance. The antagonistic Merlin-yes-associated protein (YAP) pathway promotes ferroptosis by upregulating ferroptosis regulators such as ACSL4 and transferrin receptor (TFRC) [187]. Together, these findings provide evidence that metastatic cancer cells are susceptible to ferroptosis, which indicates excellent treatment prospects in lung cancer by upregulating ferroptosis (Table 2).
Table 2.
The role and mechanism of ferroptosis in pulmonary diseases.
| Disease | Research object | Biochemical features | Regulation mechanism | Inhibitors/inducers | References |
|---|---|---|---|---|---|
| COVID-19 | Serum | Thrombosis, accumulation of oxidized phospholipids | Hepcidin | — | [124, 125, 130] |
| ALI | Human bronchial epithelial cells BEAS-2B and mouse lung cells | Lung inflammation out of control, oxidation/antioxidant imbalance | Nrf2/HIF-1, Nrf2/HO-1/SLC7A11, p62/Keap1/Nrf2 axis, PTGS2 | Inhibitor Fer-1 | [102, 133, 135, 137, 145] |
| PF | Human fetal lung fibroblasts HFL1, RILF mouse lung tissue cells | Increased ROS, lipid peroxidation, and fibroblast differentiation | α-SMA, COLI, Nrf2/HO-1/NQO1 | Inhibitor Fer-1, Lip-1 | [87, 151] |
| COPD | Human bronchial epithelial cells and mouse mode | Lipid peroxidation increased production of 4-HNE and DAMPs | NCOA4, GPX4 | Inhibitor, deferoxamine, and Fer-1 | [154, 155] |
| Lung cancer | NSCLC cell SW900, human plasma | Upregulation of GPX4/GSH pathway, reduction of iron, inhibition of lipid synthesis, and upregulation of FSP1 | NF2-YAP, ACSL4, PRIM2/SLC7A11 axis, Nrf2/HO-1 axis, Ca2+/CaM, P53RRA | Inducer cisplatin, erastin, DHA, STYK1 | [142, 169–174, 177, 178, 187] |
6.6. Ferroptosis and Other Lung Diseases
6.6.1. Ferroptosis and Asthma
Ferroptosis has been implicated in the pathogenesis of asthma recently. The phosphatidylethanolamine-binding protein 1/15 lipoxygenase (PEBP1/15-LO) complex plays a vital role in regulating ferroptosis. Compared with regular patients, there were increased colocalization levels of PEPBP1 and 15-LO in human airway epithelial cells (HAECs) of asthmatic patients, which suggests that ferroptosis may occur in asthma. Blocking PEBP1 in HAECs decreases the sensitivity to ferroptosis [188]. Moreover, the 15LO1-PEBP1-generated ferroptotic phospholipid, 15-hydroperoxy-arachidonoyl-phosphatidylethanolamine (15-HpETE-PE), promoted light chain3-I (LC3-1) to stimulate autophagy. In type 2 Hi asthma, there were high levels of both 15LO1-PEBP1 and LC3-II in HAECs, which are associated with low bronchoalveolar lavage fluid mitochondrial DNA and more severe disease [189]. Therefore, inhibiting ferroptosis in HAECs may be an effective asthma treatment.
6.6.2. Ferroptosis and Allergic Airway Inflammation
Allergic airway inflammation (AAI) is a chronic inflammation characterized by increased airway responsiveness to various stimuli, which causes recurrent asthma, chest tightness, and cough [190]. Eosinophils play a vital role in the development of AAI. After ferroptosis-inducing agent (FIN) treatment, eosinophils shrink and mitochondria are damaged, showing typical ferroptosis morphology [3, 191]. Further study suggests that erastin and RSL3 inhibit GSH production in eosinophils and decrease GPX4 activity, while artesunate (ART) induces ferroptosis in eosinophils by promoting Fenton reaction [25, 192]. In addition, FINs combined with dexamethasone increase ROS production in the cytoplasm, induce eosinophilic ferroptosis, and alleviate AAI [193]. Thus, it is feasible to treat AAI by inducing eosinophilic ferroptosis.
6.6.3. Ferroptosis and Infectious Lung Diseases
Ferroptosis plays an adverse role in infectious lung diseases. Pseudomonas aeruginosa (PA), a prokaryotic bacterium, can express lipoxygenase (pLoxA) to oxidize host acid-phosphatidylethanolamines (AA-PE) to 15-hydroperoxy-AA-PE (15-HOO-AA-PE), triggering ferroptosis in human bronchial epithelial cells [194]. Further study suggested that PA can activate the lysosomal chaperone-mediated autophagy (CMA) to decrease the host GPX4 defense. Meanwhile, the host can stymie lipid peroxidation and protect GPX4/GSH-deficient cells by stimulating the inducible nitric oxide synthase (iNOS)/NO-driven antiferroptotic mechanism [195]. Therefore, promoting the iNOS/NO-driven antiferroptotic mechanism may provide a new strategy for the host against PA-induced ferroptosis. In addition, mycobacterium tuberculosis (Mtb) can cause macrophage necrosis, which is associated with reduced GSH and GPX4, with increased lipid peroxidation, mitochondrial superoxide, and free ion. Moreover, Fer-1 treatment can inhibit necrotic cell death in Mtb-infected macrophage and pulmonary necrosis in acutely infected mice and reduce bacterial load in infected animals [196]. These findings suggest that ferroptosis plays an essential role in Mtb infection and can be a novel target for the treatment of tuberculosis.
6.6.4. Ferroptosis and Radiation-Induced Lung Injury
Radiation-induced lung injury (RILI) is a severe and life-threatening complication of thoracic radiotherapy, including acute radiation pneumonitis and RILF [197]. ROS accumulation induced by irradiation in lung tissue is a critical factor in the pathogenesis of RILI, which is also the leading cause of ferroptosis. In acute RILI mice, the level of ROS is upregulated and the level of GPX4 is downregulated in the lungs, which is reversed by treatment with Lip-1. Moreover, treated with Lip-1, RILI mice exhibit lower serum levels of inflammatory cytokines, mitigatory structure damage, and hemorrhage and declined mitochondria shrinkage [198]. Together, ferroptosis plays an essential role in acute RILI, and inhibition of ferroptosis may be an effective therapy for acute RILI.
7. Conclusion and Foresight
The exploration on the fine mechanism of regulating ferroptosis is developing rapidly, and the research on ferroptosis is being ushered in an outbreak. LPO accumulation and iron dependency contribute to ferroptosis, which is distinct from other types of RCDs. Recent studies indicate that cell ferroptosis sensitivity is regulated by the extrinsic or transporter-dependent pathway and the intrinsic or enzyme-regulated pathway (SLC7A11/GPX4, NADPH/FSP1/CoQ10, and GCH1/BH4), and the sensitivity of lung cells to ferroptosis is closely related to the progression of lung diseases. With detailed studies, the pathological process of lung diseases is accompanied by ferroptosis in lung cells, which promotes lung inflammation and lung I/R injury, and mediates lung and systemic immune changes. The targeted inhibition of ferroptosis alleviates lung inflammation and lung I/R injury, which is beneficial to clinical treatment of lung diseases such as COVID-19, ALI, PF, and COPD. In addition, inducing ferroptosis in lung cancer cells and enhancing antitumor immunity have broad prospects in the diagnosis and treatment of lung cancer. Many precise targets for inducing and inhibiting ferroptosis have been identified, but the targeted regulation of ferroptosis for lung diseases still needs to be further explored.
The research progress on the ferroptosis mechanism is rapid, and opportunities and challenges coexist. Ferroptosis is one of the crucial RCDs that regulate the body's environment's stability and plays a vital role in human health. In the early stage, combined and regular application of ferroptosis inducers and control of appropriate doses are conducive to improving the specificity of ferroptosis inducers and enhancing the effect of regulating ferroptosis in treating pulmonary diseases. The heterogeneity and plasticity of cancer cells may lead to the variation of ferroptosis sensitivity. It is necessary to hold the sensitivity of cancer cells to ferroptosis to avoid tumor escape. The regulation of endogenous Fe2+, GSH, GPX4, NADPH, PUFAs, and lipid antioxidants has been proposed as the key of ferroptosis, further clarifying that the molecular mechanism of the different molecular regulations of ferroptosis remains a challenge.
With further understanding of the mechanism of ferroptosis, it is possible to improve lung diseases' therapeutic effect by simultaneously regulating multiple cell death pathways. The occurrence of ferroptosis may be influenced by some immune processes, whose emerging regulatory network may be crucial in lung disease therapy. It is still in the infancy of the research on targeted therapy and drug design of lung diseases based on ferroptosis regulation. We may focus on targeting drugs and biological carriers' development in future work to make it a historic breakthrough in the clinical treatment of lung diseases through interdisciplinary cooperation.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (91949110, 82170096), the Open Project of the State Key Laboratory of Trauma, Burns and Combined Injury, Army Medical University (SKLKF201702), the Major Research Plan in the Field of Social Development of the Hunan Province (2020SK3024), the National University Student Innovation Program (2020105330257), and the High School Innovation Fund of Hunan Province (18K009, 19K103).
Abbreviations
- 15-HOO-AA-PE:
15-Hydroperoxy-AA-PE
- 15-HpETE-PE:
15-Hydroperoxy-arachidonoyl-phosphatidylethanolamine
- AAI:
Allergic airway inflammation
- AA-PE:
Acid-phosphatidylethanolamines
- ACSL4:
Acyl-CoA synthetase long-chain family member 4
- AGER:
Advanced glycation end product specific receptor
- AHCYL1:
Adenosylhomocysteinase like 1
- ALI:
Acute lung injury
- APAP:
Acetaminophen
- APCs:
Antigen-presenting cells
- ARDS:
Acute respiratory distress syndrome
- ART:
Artesunate
- Atg5:
Autophagy-related gene 5
- Bax/Bak:
Bcl-2-associated X protein/Bcl-2-associated K protein
- BH4:
Tetrahydrobiopterin
- CCK-8:
Cholecystokinin octapeptide
- CCL5:
Chemokine CC chemokine ligand 5
- cDC1:
Conventional type 1 dendritic cells
- CMA:
Chaperone-mediated autophagy
- COPD:
Chronic obstructive pulmonary disease
- CoQ10:
Coenzyme Q10
- COVID-19:
2019 coronavirus disease
- CYB5R1:
Cytochrome b5 reductase
- Cys:
Cysteine
- DAMPs:
Damage-associated molecular patterns
- DC:
Dendritic cells
- DCYTB:
Duodenal cytochrome B
- DHA:
Dihydroartemisinin
- DMF:
Dimethyl fumarate
- DMT1:
Divalent metal transporter
- ESCRT-III:
Endosomal sorting complex required for transport-III
- ETC:
Electron transport chain
- FADS2:
Fatty acid desaturase 2
- Fer-1:
Ferrstatin-1
- FINs:
Ferroptosis-inducing agents
- FPN1:
Ferroportin-1
- FSP1:
Ferroptosis suppressor protein 1
- FTH1:
Ferritin heavy chain 1
- GBM:
Glioblastoma
- GCH1:
Guanosine triphosphate cyclohydrolase 1
- GLUT1:
Glucose transporter 1
- GPX4:
Glutathione peroxidase 4
- GSH:
Glutathione
- HAECs:
Human airway epithelial cells
- HMGB1:
High mobility group protein B1
- HO-1:
Heme oxygenase 1
- HSPB1:
Heat shock protein beta-1
- I/R:
Ischemia-reperfusion
- iASPP:
Inhibitor of apoptosis stimulating p53 protein
- IFNγ:
Interferon-γ
- iNOS:
Inducible nitric oxide synthase
- IPF:
Idiopathic pulmonary fibrosis
- LC3-1:
Promoted light chain3-I
- LF:
Chelator lactoferrin
- Lip-1:
Liproxstatin-1
- LIRI:
Lung I/R injury
- lncRNA:
Long noncoding RNA
- L-OH:
Lipid alcohols
- LOXs:
Lipoxygenases
- LPCAT3:
Lysophosphatidylcholine acyltransferase 3
- LPO:
Lipid peroxidation
- LPS:
Lipopolysaccharide
- LSH:
Lymphoid-specific helicase
- MAPK:
Mitogen-activated protein kinase
- MDA:
Malondialdehyde
- MF:
Magnetic field
- MLKL:
Mixed lineage kinase domain-like
- MON:
Metal-organic network
- MPO:
Myeloperoxidase
- MT1DP:
Metallothionein 1D pseudogene
- Mtb:
Mycobacterium tuberculosis
- MtFt:
Mitochondrial ferritin
- NCOA4:
Nuclear receptor coactivator 4
- NFS-1:
Iron-sulfur cluster synthase 1
- NK:
Natural killer
- NOX:
NADPH oxidase
- NQO1:
NAD(P)H:quinone oxidoreductase 1
- Nrf2:
Nuclear factor erythroid-2-related factor
- NSCLC:
Non-small cell lung cancer cells
- p53:
Protein 53
- PA:
Pseudomonas aeruginosa
- PEBP1/15-LO:
Phosphatidylethanolamine-binding protein 1/15 lipoxygenase
- PF:
Pulmonary fibrosis
- PGE2:
Prostaglandin E2
- pLoxA:
Lipoxygenase
- POR:
P450 reductase
- PUFAs:
Polyunsaturated fatty acids
- PUMA:
p53 upregulated modulator of apoptosis
- qPCR:
Quantitative PCR
- RCDs:
Regulated cell deaths
- RILF:
Radiation-induced lung fibrosis
- RILI:
Radiation-induced lung injury
- ROS:
Reactive oxygen species
- RSL3:
RAS selective lethal 3
- RTA:
Radical-trapping antioxidant
- SAHH:
S-Adenosyl-L-homocysteine hydrolase
- SARS-CoV-2:
Severe acute respiratory syndrome coronavirus-2
- SAT1:
Spermidine/spermine N1-acetyltransferase
- SCD1:
Sterol-CoA desaturase 1
- SCLC:
Small cell lung cancer
- STEAP3:
Six-transmembrane epithelial antigen of prostate 3
- STYK1:
Serine-threonine tyrosine kinase 1
- System Xc−:
Cystine/glutamate antiporter
- T:
Helper cell 17
- TA:
Tannic acid
- TAM:
Tumor-associated macrophages
- TCA:
Tricarboxylic acid
- TEM:
Transmission electron microscopy
- TF:
Transferrin
- TFR1:
Transferrin receptor protein 1
- TFRC:
Transferrin receptor
- TGF-β1:
Transforming growth factor beta 1
- TLR4:
Toll-like receptor 4
- Th17:
T helper cell 17
- TME:
Tumor microenvironment
- VDAC2/3:
Voltage-dependent anion channel
- XCL1:
Chemokine lymphotactin
- YAP:
Yes-associated protein.
Contributor Information
Yong Zhou, Email: zhouyong421@csu.edu.cn.
Cha-Xiang Guan, Email: gcxdzd91@163.com.
Conflicts of Interest
The authors declared no conflict of interest.
Authors' Contributions
Tian-Liang Ma wrote the original draft. Tian-Liang Ma, Yong Zhou, Ci Wang, Lu Wang, Jing-Xian Chen, Hui-Hui Yang, and Chen-Yu Zhang participated in writing and editing the review. Tian-Liang Ma and Yong Zhou prepared the figures. Yong Zhou and Cha-Xiang Guan edited the manuscript. All authors read and approved the final manuscript.
References
- 1.Zhou M., Wang H., Zeng X., et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet . 2019;394(10204):1145–1158. doi: 10.1016/S0140-6736(19)30427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li H., Liu S. M., Yu X. H., Tang S. L., Tang C. K. Coronavirus disease 2019 (COVID-19): current status and future perspectives. International Journal of Antimicrobial Agents . 2020;55(5):p. 105951. doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon S. J., Lemberg K. M., Lamprecht M. R., et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell . 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie Y., Hou W., Song X., et al. Ferroptosis: process and function. Cell Death and Differentiation . 2016;23(3):369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magtanong L., Dixon S. J. Ferroptosis and Brain Injury. Developmental Neuroscience . 2018;40(5-6):382–395. doi: 10.1159/000496922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W., Li W., Leng Y., Xiong Y., Xia Z. Ferroptosis is involved in diabetes myocardial ischemia/reperfusion injury through endoplasmic reticulum stress. DNA and Cell Biology . 2020;39(2):210–225. doi: 10.1089/dna.2019.5097. [DOI] [PubMed] [Google Scholar]
- 7.Musani A. I. Pulmonary disease. The Medical Clinics of North America . 2019;103(3):xix–xx. doi: 10.1016/j.mcna.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida M., Minagawa S., Araya J., et al. Involvement of cigarette smoke-induced epithelial cell ferroptosis in COPD pathogenesis. Nature Communications . 2019;10(1):p. 3145. doi: 10.1038/s41467-019-10991-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou H., Li F., Niu J. Y., et al. Ferroptosis was involved in the oleic acid-induced acute lung injury in mice. Sheng Li Xue Bao . 2019;71(5):689–697. [PubMed] [Google Scholar]
- 10.Li X., Duan L., Yuan S., Zhuang X., Qiao T., He J. Ferroptosis inhibitor alleviates radiation-induced lung fibrosis (RILF) via down-regulation of TGF-β1. Journal of Inflammation . 2019;16(1):p. 11. doi: 10.1186/s12950-019-0216-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuang Y., Wang Q. Iron and lung cancer. Cancer Letters . 2019;464:56–61. doi: 10.1016/j.canlet.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Cao J. Y., Dixon S. J. Mechanisms of ferroptosis. Cellular and Molecular Life Sciences . 2016;73(11-12):2195–2209. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venkatesh D., O'Brien N. A., Zandkarimi F., et al. MDM2 and MDMX promote ferroptosis by PPARα-mediated lipid remodeling. Genes & Development . 2020;34(7-8):526–543. doi: 10.1101/gad.334219.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu Y., Xie Y., Cao L., et al. The ferroptosis inducer erastin enhances sensitivity of acute myeloid leukemia cells to chemotherapeutic agents. Molecular & Cellular Oncology . 2015;2(4, article e1054549) doi: 10.1080/23723556.2015.1054549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdalkader M., Lampinen R., Kanninen K. M., Malm T. M., Liddell J. R. Targeting Nrf2 to suppress ferroptosis and mitochondrial dysfunction in neurodegeneration. Frontiers in Neuroscience . 2018;12:p. 466. doi: 10.3389/fnins.2018.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torti S. V., Torti F. M. Iron and cancer: more ore to be mined. Nature Reviews. Cancer . 2013;13(5):342–355. doi: 10.1038/nrc3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conrad M., Kagan V. E., Bayir H., et al. Regulation of lipid peroxidation and ferroptosis in diverse species. Genes & Development . 2018;32(9-10):602–619. doi: 10.1101/gad.314674.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang W. S., Kim K. J., Gaschler M. M., Patel M., Shchepinov M. S., Stockwell B. R. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proceedings of the National Academy of Sciences of the United States of America . 2016;113(34):E4966–E4975. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kagan V. E., Mao G., Qu F., et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nature Chemical Biology . 2017;13(1):81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doll S., Proneth B., Tyurina Y. Y., et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nature Chemical Biology . 2017;13(1):91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao M., Yi J., Zhu J., et al. Role of mitochondria in ferroptosis. Molecular Cell . 2019;73(2):354–363.e3. doi: 10.1016/j.molcel.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ingold I., Berndt C., Schmitt S., et al. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell . 2018;172(3):409–422.e21. doi: 10.1016/j.cell.2017.11.048. [DOI] [PubMed] [Google Scholar]
- 23.Yagoda N., von Rechenberg M., Zaganjor E., et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature . 2007;447(7146):865–869. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y. Q., Chang S. Y., Wu Q., et al. The protective role of mitochondrial ferritin on erastin-induced ferroptosis. Frontiers in Aging Neuroscience . 2016;8:p. 308. doi: 10.3389/fnagi.2016.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang W. S., SriRamaratnam R., Welsch M. E., et al. Regulation of ferroptotic cancer cell death by GPX4. Cell . 2014;156(1-2):317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maiorino M., Conrad M., Ursini F. GPx4, lipid peroxidation, and cell death: discoveries, rediscoveries, and open issues. Antioxidants & Redox Signaling . 2018;29(1):61–74. doi: 10.1089/ars.2017.7115. [DOI] [PubMed] [Google Scholar]
- 27.Ursini F., Maiorino M. Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radical Biology & Medicine . 2020;152:175–185. doi: 10.1016/j.freeradbiomed.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 28.Lewerenz J., Hewett S. J., Huang Y., et al. The cystine/glutamate antiporter system x (c)(-) in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxidants & Redox Signaling . 2013;18(5):522–555. doi: 10.1089/ars.2011.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayano M., Yang W. S., Corn C. K., Pagano N. C., Stockwell B. R. Loss of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death and Differentiation . 2016;23(2):270–278. doi: 10.1038/cdd.2015.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao J., Chen X., Jiang L., et al. DJ-1 suppresses ferroptosis through preserving the activity of S-adenosyl homocysteine hydrolase. Nature Communications . 2020;11(1):p. 1251. doi: 10.1038/s41467-020-15109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hadian K. Ferroptosis suppressor protein 1 (FSP1) and coenzyme Q10 cooperatively suppress ferroptosis. Biochemistry . 2020;59(5):637–638. doi: 10.1021/acs.biochem.0c00030. [DOI] [PubMed] [Google Scholar]
- 32.Du M. Y., Duan J. X., Zhang C. Y., et al. Psoralen attenuates bleomycin-induced pulmonary fibrosis in mice through inhibiting myofibroblast activation and collagen deposition. Cell Biology International . 2020;44(1):98–107. doi: 10.1002/cbin.11205. [DOI] [PubMed] [Google Scholar]
- 33.Bersuker K., Hendricks J. M., Li Z., et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature . 2019;575(7784):688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doll S., Freitas F. P., Shah R., et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature . 2019;575(7784):693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 35.Dai E., Zhang W., Cong D., Kang R., Wang J., Tang D. AIFM2 blocks ferroptosis independent of ubiquinol metabolism. Biochemical and Biophysical Research Communications . 2020;523(4):966–971. doi: 10.1016/j.bbrc.2020.01.066. [DOI] [PubMed] [Google Scholar]
- 36.Santoro M. M. The Antioxidant Role of Non-mitochondrial CoQ10: Mystery Solved! Cell Metabolism . 2020;31(1):13–15. doi: 10.1016/j.cmet.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Cronin S. J. F., Seehus C., Weidinger A., et al. The metabolite BH4 controls T cell proliferation in autoimmunity and cancer. Nature . 2018;563(7732):564–568. doi: 10.1038/s41586-018-0701-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soula M., Weber R. A., Zilka O., et al. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nature Chemical Biology . 2020;16(12):1351–1360. doi: 10.1038/s41589-020-0613-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kraft V. A. N., Bezjian C. T., Pfeiffer S., et al. GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Central Science . 2020;6(1):41–53. doi: 10.1021/acscentsci.9b01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang D., Peng Y., Xie Y., et al. Antiferroptotic activity of non-oxidative dopamine. Biochemical and Biophysical Research Communications . 2016;480(4):602–607. doi: 10.1016/j.bbrc.2016.10.099. [DOI] [PubMed] [Google Scholar]
- 41.Shimada K., Hayano M., Pagano N. C., Stockwell B. R. Cell-line selectivity improves the predictive power of pharmacogenomic analyses and helps identify NADPH as biomarker for ferroptosis sensitivity. Cell Chemical Biology . 2016;23(2):225–235. doi: 10.1016/j.chembiol.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun X., Ou Z., Chen R., et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology . 2016;63(1):173–184. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang S. J., Li D., Ou Y., et al. Acetylation is crucial for p53-mediated ferroptosis and tumor suppression. Cell Reports . 2016;17(2):366–373. doi: 10.1016/j.celrep.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun X., Ou Z., Xie M., et al. HSPB1 as a novel regulator of ferroptotic cancer cell death. Oncogene . 2015;34(45):5617–5625. doi: 10.1038/onc.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hou W., Xie Y., Song X., et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy . 2016;12(8):1425–1428. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wen Q., Liu J., Kang R., Zhou B., Tang D. The release and activity of HMGB1 in ferroptosis. Biochemical and Biophysical Research Communications . 2019;510(2):278–283. doi: 10.1016/j.bbrc.2019.01.090. [DOI] [PubMed] [Google Scholar]
- 47.Ye F., Chai W., Xie M., et al. HMGB1 regulates erastin-induced ferroptosis via RAS-JNK/p38 signaling in HL-60/NRASQ61L cells. American Journal of Cancer Research . 2019;9(4):730–739. [PMC free article] [PubMed] [Google Scholar]
- 48.Zou Y., Li H., Graham E. T., et al. Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nature Chemical Biology . 2020;16(3):302–309. doi: 10.1038/s41589-020-0472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan B., Ai Y., Sun Q., et al. Membrane damage during ferroptosis is caused by oxidation of phospholipids catalyzed by the oxidoreductases POR and CYB5R1. Molecular Cell . 2020;81(2):355–369.e10. doi: 10.1016/j.molcel.2020.11.024. [DOI] [PubMed] [Google Scholar]
- 50.Ding J., Wang K., Liu W., et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature . 2016;535(7610):111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 51.Wang H., Sun L., Su L., et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Molecular Cell . 2014;54(1):133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 52.Yu H., Guo P., Xie X., Wang Y., Chen G. Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. Journal of Cellular and Molecular Medicine . 2017;21(4):648–657. doi: 10.1111/jcmm.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mao C., Liu X., Zhang Y., et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature . 2021;593(7860):586–590. doi: 10.1038/s41586-021-03539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yee P. P., Wei Y., Kim S. Y., et al. Neutrophil-induced ferroptosis promotes tumor necrosis in glioblastoma progression. Nature Communications . 2020;11(1):p. 5424. doi: 10.1038/s41467-020-19193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cotter T. G. Apoptosis and cancer: the genesis of a research field. Nature Reviews. Cancer . 2009;9(7):501–507. doi: 10.1038/nrc2663. [DOI] [PubMed] [Google Scholar]
- 56.Zheng D. W., Lei Q., Zhu J. Y., et al. Switching apoptosis to ferroptosis: metal-organic network for high-efficiency anticancer therapy. Nano Letters . 2017;17(1):284–291. doi: 10.1021/acs.nanolett.6b04060. [DOI] [PubMed] [Google Scholar]
- 57.Mou Y., Wang J., Wu J., et al. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. Journal of Hematology & Oncology . 2019;12(1):p. 34. doi: 10.1186/s13045-019-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee Y. S., Lee D. H., Choudry H. A., Bartlett D. L., Lee Y. J. Ferroptosis-induced endoplasmic reticulum stress: cross-talk between ferroptosis and apoptosis. Molecular Cancer Research . 2018;16(7):1073–1076. doi: 10.1158/1541-7786.Mcr-18-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.di Fazio P., Waldegger P., Jabari S., et al. Autophagy-related cell death by pan-histone deacetylase inhibition in liver cancer. Oncotarget . 2016;7(20):28998–29010. doi: 10.18632/oncotarget.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang M., Chen Z., Wu D., Chen L. Ferritinophagy/ferroptosis: iron-related newcomers in human diseases. Journal of Cellular Physiology . 2018;233(12):9179–9190. doi: 10.1002/jcp.26954. [DOI] [PubMed] [Google Scholar]
- 61.Dowdle W. E., Nyfeler B., Nagel J., et al. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nature Cell Biology . 2014;16(11):1069–1079. doi: 10.1038/ncb3053. [DOI] [PubMed] [Google Scholar]
- 62.Lu B., Chen X. B., Ying M. D., He Q. J., Cao J., Yang B. The role of ferroptosis in cancer development and treatment response. Frontiers in Pharmacology . 2018;8:p. 992. doi: 10.3389/fphar.2017.00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshida G. J. Therapeutic strategies of drug repositioning targeting autophagy to induce cancer cell death: from pathophysiology to treatment. Journal of Hematology & Oncology . 2017;10(1):p. 67. doi: 10.1186/s13045-017-0436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao M., Monian P., Pan Q., Zhang W., Xiang J., Jiang X. Ferroptosis is an autophagic cell death process. Cell Research . 2016;26(9):1021–1032. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bauckman K. A., Mysorekar I. U. Ferritinophagy drives uropathogenic Escherichia coli persistence in bladder epithelial cells. Autophagy . 2016;12(5):850–863. doi: 10.1080/15548627.2016.1160176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bai Y., Lam H. C., Lei X. Dissecting programmed cell death with small molecules. Accounts of Chemical Research . 2020;53(5):1034–1045. doi: 10.1021/acs.accounts.9b00600. [DOI] [PubMed] [Google Scholar]
- 67.Degterev A., Huang Z., Boyce M., et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nature Chemical Biology . 2005;1(2):112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 68.Müller T., Dewitz C., Schmitz J., et al. Necroptosis and ferroptosis are alternative cell death pathways that operate in acute kidney failure. Cellular and Molecular Life Sciences . 2017;74(19):3631–3645. doi: 10.1007/s00018-017-2547-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wenz C., Faust D., Linz B., et al. t-BuOOH induces ferroptosis in human and murine cell lines. Archives of Toxicology . 2018;92(2):759–775. doi: 10.1007/s00204-017-2066-y. [DOI] [PubMed] [Google Scholar]
- 70.Tonnus W., Linkermann A. "Death is my heir"--ferroptosis connects cancer pharmacogenomics and ischemia-reperfusion injury. Cell Chemical Biology . 2016;23(2):202–203. doi: 10.1016/j.chembiol.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 71.Case C. L., Kohler L. J., Lima J. B., et al. Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila. Proceedings of the National Academy of Sciences of the United States of America . 2013;110(5):1851–1856. doi: 10.1073/pnas.1211521110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Danthi P. Viruses and the diversity of cell death. Annual Review of Virology . 2016;3(1):533–553. doi: 10.1146/annurev-virology-110615-042435. [DOI] [PubMed] [Google Scholar]
- 73.Xiao Q., Yu J. S., Wang Y., Ma D., Zhou J., Lou X. 3-Difluoroalkyl quaternary oxindoles inhibit macrophage pyroptosis by blocking inflammasome recruitment of caspase-1. ACS Medicinal Chemistry Letters . 2020;11(7):1392–1401. doi: 10.1021/acsmedchemlett.0c00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Proneth B., Conrad M. Ferroptosis and necroinflammation, a yet poorly explored link. Cell Death and Differentiation . 2019;26(1):14–24. doi: 10.1038/s41418-018-0173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reed J. C., Pellecchia M. Ironing out cell death mechanisms. Cell . 2012;149(5):963–965. doi: 10.1016/j.cell.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 76.Tang R., Xu J., Zhang B., et al. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. Journal of Hematology & Oncology . 2020;13(1):p. 110. doi: 10.1186/s13045-020-00946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tay M. Z., Poh C. M., Rénia L., MacAry P. A., Ng L. F. P. The trinity of COVID-19: immunity, inflammation and intervention. Nature Reviews. Immunology . 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gouda M. M., Bhandary Y. P. Acute lung injury: IL-17A-mediated inflammatory pathway and its regulation by curcumin. Inflammation . 2019;42(4):1160–1169. doi: 10.1007/s10753-019-01010-4. [DOI] [PubMed] [Google Scholar]
- 79.Aldonyte R., Bagdonas E., Raudoniute J., Bruzauskaite I. Novel aspects of pathogenesis and regeneration mechanisms in COPD. International Journal of Chronic Obstructive Pulmonary Disease . 2015;10:995–1013. doi: 10.2147/COPD.S82518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Phan T. H. G., Paliogiannis P., Nasrallah G. K., et al. Emerging cellular and molecular determinants of idiopathic pulmonary fibrosis. Cellular and Molecular Life Sciences . 2021;78(5):2031–2057. doi: 10.1007/s00018-020-03693-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kang R., Chen R., Zhang Q., et al. HMGB1 in health and disease. Molecular Aspects of Medicine . 2014;40:1–116. doi: 10.1016/j.mam.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.NaveenKumar S. K., Hemshekhar M., Kemparaju K., Girish K. S. Hemin-induced platelet activation and ferroptosis is mediated through ROS-driven proteasomal activity and inflammasome activation: protection by melatonin. Biochimica et Biophysica Acta - Molecular Basis of Disease . 2019;1865(9):2303–2316. doi: 10.1016/j.bbadis.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 83.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respiratory Medicine . 2020;8(4):420–422. doi: 10.1016/s2213-2600(20)30076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen C., Zhang X. R., Ju Z. Y., He W. F. Advances in the research of mechanism and related immunotherapy on the cytokine storm induced by coronavirus disease 2019. Zhonghua Shao Shang Za Zhi . 2020;36:471–475. doi: 10.3760/cma.j.cn501120-20200224-00088. [DOI] [PubMed] [Google Scholar]
- 85.Habib H. M., Ibrahim S., Zaim A., Ibrahim W. H. The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomedicine & Pharmacotherapy . 2021;136:p. 111228. doi: 10.1016/j.biopha.2021.111228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shin D., Kim E. H., Lee J., Roh J. L. Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radical Biology & Medicine . 2018;129:454–462. doi: 10.1016/j.freeradbiomed.2018.10.426. [DOI] [PubMed] [Google Scholar]
- 87.Zilka O., Shah R., Li B., et al. On the mechanism of cytoprotection by ferrostatin-1 and liproxstatin-1 and the role of lipid peroxidation in ferroptotic cell death. ACS Central Science . 2017;3(3):232–243. doi: 10.1021/acscentsci.7b00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu Y., Li X., Cheng Y., Yang M., Wang R. Inhibition of ACSL4 attenuates ferroptotic damage after pulmonary ischemia-reperfusion. The FASEB Journal . 2020;34(12):16262–16275. doi: 10.1096/fj.202001758R. [DOI] [PubMed] [Google Scholar]
- 89.Oyaizu T., Fung S. Y., Shiozaki A., et al. Src tyrosine kinase inhibition prevents pulmonary ischemia-reperfusion-induced acute lung injury. Intensive Care Medicine . 2012;38(5):894–905. doi: 10.1007/s00134-012-2498-z. [DOI] [PubMed] [Google Scholar]
- 90.Qu L., Yan J., Jiang Z., Song Z., Luo F., Peng Q. Low-intensity pulsed ultrasound pretreatment inhibits HMGB1 expression and attenuates lung ischemia-reperfusion injury in rats via the cholinergic anti-inflammatory pathway. Nan Fang Yi Ke Da Xue Xue Bao . 2018;38(9):1061–1065. doi: 10.12122/j.issn.1673-4254.2018.09.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kalogeris T., Baines C. P., Krenz M., Korthuis R. J. Cell biology of ischemia/reperfusion injury. International Review of Cell and Molecular Biology . 2012;298:229–317. doi: 10.1016/b978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zimmerman B. J., Granger D. N. Mechanisms of reperfusion injury. The American Journal of the Medical Sciences . 1994;307(4):284–292. doi: 10.1097/00000441-199404000-00009. [DOI] [PubMed] [Google Scholar]
- 93.Ovechkin A. V., Lominadze D., Sedoris K. C., Robinson T. W., Tyagi S. C., Roberts A. M. Lung ischemia-reperfusion injury: implications of oxidative stress and platelet-arteriolar wall interactions. Archives of Physiology and Biochemistry . 2007;113(1):1–12. doi: 10.1080/13813450601118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fei L., Jingyuan X., Fangte L., et al. Preconditioning with rHMGB1 ameliorates lung ischemia-reperfusion injury by inhibiting alveolar macrophage pyroptosis via the Keap1/Nrf2/HO-1 signaling pathway. Journal of Translational Medicine . 2020;18(1):p. 301. doi: 10.1186/s12967-020-02467-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iadecola C., Anrather J. The immunology of stroke: from mechanisms to translation. Nature Medicine . 2011;17(7):796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Singh A., Kukreti R., Saso L., Kukreti S. Oxidative stress: a key modulator in neurodegenerative diseases. Molecules . 2019;24(8):p. 1583. doi: 10.3390/molecules24081583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qiang Z., Dong H., Xia Y., Chai D., Hu R., Jiang H. Nrf2 and STAT3 alleviates ferroptosis-mediated IIR-ALI by regulating SLC7A11. Oxidative Medicine and Cellular Longevity . 2020;2020:16. doi: 10.1155/2020/5146982.5146982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yan H.-F., Tuo Q. Z., Yin Q. Z., Lei P. The pathological role of ferroptosis in ischemia/reperfusion-related injury. Zoological Research . 2020;41(3):220–230. doi: 10.24272/j.issn.2095-8137.2020.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yamada N., Karasawa T., Wakiya T., et al. Iron overload as a risk factor for hepatic ischemia-reperfusion injury in liver transplantation: potential role of ferroptosis. American Journal of Transplantation . 2020;20(6):1606–1618. doi: 10.1111/ajt.15773. [DOI] [PubMed] [Google Scholar]
- 100.Li Y., Feng D., Wang Z., et al. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death and Differentiation . 2019;26(11):2284–2299. doi: 10.1038/s41418-019-0299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wichniak A., Kania A., Siemiński M., Cubała W. J. Melatonin as a potential adjuvant treatment for COVID-19 beyond sleep disorders. International Journal of Molecular Sciences . 2021;22(16):p. 8623. doi: 10.3390/ijms22168623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Y., Cao Y., Xiao J., et al. Inhibitor of apoptosis-stimulating protein of p53 inhibits ferroptosis and alleviates intestinal ischemia/reperfusion-induced acute lung injury. Cell Death and Differentiation . 2020;27(9):2635–2650. doi: 10.1038/s41418-020-0528-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pourgholamhossein F., Rasooli R., Pournamdari M., et al. Pirfenidone protects against paraquat-induced lung injury and fibrosis in mice by modulation of inflammation, oxidative stress, and gene expression. Food and Chemical Toxicology . 2018;112:39–46. doi: 10.1016/j.fct.2017.12.034. [DOI] [PubMed] [Google Scholar]
- 104.Zhang H., He F., Li P., Hardwidge P. R., Li N., Peng Y. The role of innate immunity in pulmonary infections. BioMed Research International . 2021;2021:14. doi: 10.1155/2021/6646071.6646071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mathew D., Giles J. R., Baxter A. E., et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science . 2020;369(6508):p. eabc8511. doi: 10.1126/science.abc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yuan X., Huang W., Ye B., et al. Changes of hematological and immunological parameters in COVID-19 patients. International Journal of Hematology . 2020;112(4):553–559. doi: 10.1007/s12185-020-02930-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Misharin A. V., Morales-Nebreda L., Mutlu G. M., Budinger G. R. S., Perlman H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. American Journal of Respiratory Cell and Molecular Biology . 2013;49(4):503–510. doi: 10.1165/rcmb.2013-0086MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ding L., Yang J., Zhang C., Zhang X., Gao P. Neutrophils modulate fibrogenesis in chronic pulmonary diseases. Frontiers in Medicine . 2021;8:p. 616200. doi: 10.3389/fmed.2021.616200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Caramori G., Casolari P., Barczyk A., Durham A. L., di Stefano A., Adcock I. COPD immunopathology. Seminars in Immunopathology . 2016;38(4):497–515. doi: 10.1007/s00281-016-0561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Luo C., Lei M., Zhang Y., et al. Systematic construction and validation of an immune prognostic model for lung adenocarcinoma. Journal of Cellular and Molecular Medicine . 2020;24(2):1233–1244. doi: 10.1111/jcmm.14719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Friedmann Angeli J. P., Krysko D. V., Conrad M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nature Reviews. Cancer . 2019;19(7):405–414. doi: 10.1038/s41568-019-0149-1. [DOI] [PubMed] [Google Scholar]
- 112.Tu G., Peng W., Cai Q., et al. Construction and validation of a 15-gene ferroptosis signature in lung adenocarcinoma. PeerJ . 2021;9, article e11687 doi: 10.7717/peerj.11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Futosi K., Fodor S., Mócsai A. Neutrophil cell surface receptors and their intracellular signal transduction pathways. International Immunopharmacology . 2013;17(3):638–650. doi: 10.1016/j.intimp.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xu W., Deng H., Hu S., et al. Role of ferroptosis in lung diseases. Journal of Inflammation Research . 2021;14:2079–2090. doi: 10.2147/jir.S307081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xu C., Sun S., Johnson T., et al. The glutathione peroxidase Gpx4 prevents lipid peroxidation and ferroptosis to sustain Treg cell activation and suppression of antitumor immunity. Cell Reports . 2021;35(11):p. 109235. doi: 10.1016/j.celrep.2021.109235. [DOI] [PubMed] [Google Scholar]
- 116.Wang W., Green M., Choi J. E., et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature . 2019;569(7755):270–274. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhu G., Huang H., Xu S., et al. Prognostic value of ferroptosis-related genes in patients with lung adenocarcinoma. Thoracic Cancer . 2021;12(12):1890–1899. doi: 10.1111/1759-7714.13998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gu Z., Liu T., Liu C., et al. Ferroptosis-strengthened metabolic and inflammatory regulation of tumor-associated macrophages provokes potent tumoricidal activities. Nano Letters . 2021;21(15):6471–6479. doi: 10.1021/acs.nanolett.1c01401. [DOI] [PubMed] [Google Scholar]
- 119.Friedmann Angeli J. P., Schneider M., Proneth B., et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nature Cell Biology . 2014;16(12):1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rothe T., Gruber F., Uderhardt S., et al. 12/15-Lipoxygenase-mediated enzymatic lipid oxidation regulates DC maturation and function. The Journal of Clinical Investigation . 2015;125(5):1944–1954. doi: 10.1172/JCI78490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang D., DuBois R. N. Immunosuppression associated with chronic inflammation in the tumor microenvironment. Carcinogenesis . 2015;36(10):1085–1093. doi: 10.1093/carcin/bgv123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Galanopoulos M., Gkeros F., Doukatas A., et al. COVID-19 pandemic: pathophysiology and manifestations from the gastrointestinal tract. World Journal of Gastroenterology . 2020;26(31):4579–4588. doi: 10.3748/wjg.v26.i31.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cavezzi A., Troiani E., Corrao S. COVID-19: hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clinics and Practice . 2020;10(2):24–30. doi: 10.4081/cp.2020.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang H., Li H., Jiang X., Shi W., Shen Z., Li M. Hepcidin is directly regulated by insulin and plays an important role in iron overload in streptozotocin-induced diabetic rats. Diabetes . 2014;63(5):1506–1518. doi: 10.2337/db13-1195. [DOI] [PubMed] [Google Scholar]
- 125.Banchini F., Vallisa D., Maniscalco P., Capelli P. Iron overload and hepcidin overexpression could play a key role in COVID infection, and may explain vulnerability in elderly, diabetics, and obese patients. Acta Biomed . 2020;91(3, article e2020013) doi: 10.23750/abm.v91i3.9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Edeas M., Saleh J., Peyssonnaux C. Iron: innocent bystander or vicious culprit in COVID-19 pathogenesis? International Journal of Infectious Diseases . 2020;97:303–305. doi: 10.1016/j.ijid.2020.05.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hirschhorn T., Stockwell B. R. The development of the concept of ferroptosis. Free Radical Biology & Medicine . 2019;133:130–143. doi: 10.1016/j.freeradbiomed.2018.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ganz T., Nemeth E. Hepcidin and iron homeostasis. Biochimica et Biophysica Acta . 2012;1823(9):1434–1443. doi: 10.1016/j.bbamcr.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jacobs W., Lammens M., Kerckhofs A., et al. Fatal lymphocytic cardiac damage in coronavirus disease 2019 (COVID-19): autopsy reveals a ferroptosis signature. ESC Heart Failure . 2020;7(6):3772–3781. doi: 10.1002/ehf2.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bayır H., Anthonymuthu T. S., Tyurina Y. Y., et al. Achieving life through death: redox biology of lipid peroxidation in ferroptosis. Cell Chemical Biology . 2020;27(4):387–408. doi: 10.1016/j.chembiol.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Linkermann A., Skouta R., Himmerkus N., et al. Synchronized renal tubular cell death involves ferroptosis. Proceedings of the National Academy of Sciences of the United States of America . 2014;111(47):16836–16841. doi: 10.1073/pnas.1415518111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Feng Y., Wang L., Ma X., et al. Effect of hCMSCs and liraglutide combination in ALI through cAMP/PKAc/β-catenin signaling pathway. Stem Cell Research & Therapy . 2020;11(1):p. 2. doi: 10.1186/s13287-019-1492-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 133.Mokra D., Mikolka P., Kosutova P., Mokry J. Corticosteroids in acute lung injury: the dilemma continues. International Journal of Molecular Sciences . 2019;20(19):p. 4765. doi: 10.3390/ijms20194765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu P., Feng Y., Li H., et al. Ferrostatin-1 alleviates lipopolysaccharide-induced acute lung injury via inhibiting ferroptosis. Cellular & Molecular Biology Letters . 2020;25(1):p. 10. doi: 10.1186/s11658-020-00205-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Qiu Y. B., Wan B. B., Liu G., et al. Nrf2 protects against seawater drowning-induced acute lung injury via inhibiting ferroptosis. Respiratory Research . 2020;21(1):p. 232. doi: 10.1186/s12931-020-01500-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nishi K. ABO blood group typing in forensic autopsies. Nihon Hōigaku Zasshi . 2005;59(2):111–117. [PubMed] [Google Scholar]
- 137.Deshpande R., Zou C. Pseudomonas aeruginosa induced cell death in acute lung injury and acute respiratory distress syndrome. International Journal of Molecular Sciences . 2020;21(15):p. 5356. doi: 10.3390/ijms21155356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Guzmán E. A. Regulated cell death signaling pathways and marine natural products that target them. Marine Drugs . 2019;17(2):p. 76. doi: 10.3390/md17020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Muthumalage T., Rahman I. Cannabidiol differentially regulates basal and LPS-induced inflammatory responses in macrophages, lung epithelial cells, and fibroblasts. Toxicology and Applied Pharmacology . 2019;382:p. 114713. doi: 10.1016/j.taap.2019.114713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yang H., Lv H., Li H., Ci X., Peng L. Oridonin protects LPS-induced acute lung injury by modulating Nrf2-mediated oxidative stress and Nrf2-independent NLRP3 and NF-κB pathways. Cell Communication and Signaling: CCS . 2019;17(1):p. 62. doi: 10.1186/s12964-019-0366-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wei S., Bi J., Yang L., et al. Serum irisin levels are decreased in patients with sepsis, and exogenous irisin suppresses ferroptosis in the liver of septic mice. Clinical and Translational Medicine . 2020;10(5, article e173) doi: 10.1002/ctm2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lou J. S., Zhao L. P., Huang Z. H., et al. Ginkgetin derived from Ginkgo biloba leaves enhances the therapeutic effect of cisplatin via ferroptosis-mediated disruption of the Nrf2/HO-1 axis in EGFR wild-type non-small-cell lung cancer. Phytomedicine . 2021;80:p. 153370. doi: 10.1016/j.phymed.2020.153370. [DOI] [PubMed] [Google Scholar]
- 143.Xiao X., Lu Z., Lin V., et al. MicroRNA miR-24-3p reduces apoptosis and regulates Keap1-Nrf2 pathway in mouse cardiomyocytes responding to ischemia/reperfusion injury. Oxidative Medicine and Cellular Longevity . 2018;2018:9. doi: 10.1155/2018/7042105.7042105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mahmoud A. M., Hussein O. E., Abd el-Twab S. M., Hozayen W. G. Ferulic acid protects against methotrexate nephrotoxicityviaactivation of Nrf2/ARE/HO-1 signaling and PPARγ, and suppression of NF-κB/NLRP3 inflammasome axis. Food & Function . 2019;10(8):4593–4607. doi: 10.1039/c9fo00114j. [DOI] [PubMed] [Google Scholar]
- 145.Dong H., Qiang Z., Chai D., et al. Nrf2 inhibits ferroptosis and protects against acute lung injury due to intestinal ischemia reperfusion via regulating SLC7A11 and HO-1. Aging (Albany NY) . 2020;12(13):12943–12959. doi: 10.18632/aging.103378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ge W., Zhao K., Wang X., et al. iASPP is an antioxidative factor and drives cancer growth and drug resistance by competing with Nrf2 for Keap1 binding. Cancer Cell . 2017;32(5):561–573.e6. doi: 10.1016/j.ccell.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 147.Wang C. Y., Zhang Q., Xun Z., et al. Increases of iASPP-Keap1 interaction mediated by syringin enhance synaptic plasticity and rescue cognitive impairments via stabilizing Nrf2 in Alzheimer's models. Redox Biology . 2020;36:p. 101672. doi: 10.1016/j.redox.2020.101672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li N., Wang W., Zhou H., et al. Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radical Biology & Medicine . 2020;160:303–318. doi: 10.1016/j.freeradbiomed.2020.08.009. [DOI] [PubMed] [Google Scholar]
- 149.Huang Z., Li H., Zhang Q., et al. Discovery of indolinone-based multikinase inhibitors as potential therapeutics for idiopathic pulmonary fibrosis. ACS Medicinal Chemistry Letters . 2017;8(11):1142–1147. doi: 10.1021/acsmedchemlett.7b00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gong Y., Wang N., Liu N., Dong H. Lipid peroxidation and GPX4 inhibition are common causes for myofibroblast differentiation and ferroptosis. DNA and Cell Biology . 2019;38(7):725–733. doi: 10.1089/dna.2018.4541. [DOI] [PubMed] [Google Scholar]
- 151.Zanoni M., Cortesi M., Zamagni A., Tesei A. The role of mesenchymal stem cells in radiation-induced lung fibrosis. International Journal of Molecular Sciences . 2019;20(16):p. 3876. doi: 10.3390/ijms20163876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chiappori A., Folli C., Balbi F., et al. CD4+CD25highCD127− regulatory T-cells in COPD: smoke and drugs effect. World Allergy Organization Journal . 2016;9:p. 5. doi: 10.1186/s40413-016-0095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Park E. J., Park Y. J., Lee S. J., Lee K., Yoon C. Whole cigarette smoke condensates induce ferroptosis in human bronchial epithelial cells. Toxicology Letters . 2019;303:55–66. doi: 10.1016/j.toxlet.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 154.Kang M. J., Lee C. G., Lee J. Y., et al. Cigarette smoke selectively enhances viral PAMP- and virus-induced pulmonary innate immune and remodeling responses in mice. The Journal of Clinical Investigation . 2008;118:2771–2784. doi: 10.1172/jci32709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Linkermann A., Stockwell B. R., Krautwald S., Anders H. J. Regulated cell death and inflammation: an auto-amplification loop causes organ failure. Nature Reviews. Immunology . 2014;14(11):759–767. doi: 10.1038/nri3743. [DOI] [PubMed] [Google Scholar]
- 156.Torre L. A., Siegel R. L., Jemal A. Lung cancer statistics. Advances in Experimental Medicine and Biology . 2016;893:1–19. doi: 10.1007/978-3-319-24223-1_1. [DOI] [PubMed] [Google Scholar]
- 157.Mohammad R. M., Muqbil I., Lowe L., et al. Broad targeting of resistance to apoptosis in cancer. Seminars in Cancer Biology . 2015;35(Suppl):S78–S103. doi: 10.1016/j.semcancer.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li D., Li Y. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduction and Targeted Therapy . 2020;5(1):p. 108. doi: 10.1038/s41392-020-00216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xia X., Fan X., Zhao M., Zhu P. The relationship between ferroptosis and tumors: a novel landscape for therapeutic approach. Current Gene Therapy . 2019;19(2):117–124. doi: 10.2174/1566523219666190628152137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wu Y., Zhang S., Gong X., et al. The epigenetic regulators and metabolic changes in ferroptosis-associated cancer progression. Molecular Cancer . 2020;19(1):p. 39. doi: 10.1186/s12943-020-01157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Berkers C. R., Maddocks O. D. K., Cheung E. C., Mor I., Vousden K. H. Metabolic regulation by p53 family members. Cell Metabolism . 2013;18(5):617–633. doi: 10.1016/j.cmet.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lai Y., Zhang Z., Li J., et al. STYK1/NOK correlates with ferroptosis in non-small cell lung carcinoma. Biochemical and Biophysical Research Communications . 2019;519(4):659–666. doi: 10.1016/j.bbrc.2019.09.032. [DOI] [PubMed] [Google Scholar]
- 163.Pfeifhofer-Obermair C., Tymoszuk P., Petzer V., Weiss G., Nairz M. Iron in the tumor microenvironment-connecting the dots. Frontiers in Oncology . 2018;8:p. 549. doi: 10.3389/fonc.2018.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Thanan R., Oikawa S., Yongvanit P., et al. Inflammation-induced protein carbonylation contributes to poor prognosis for cholangiocarcinoma. Free Radical Biology & Medicine . 2012;52(8):1465–1472. doi: 10.1016/j.freeradbiomed.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 165.Alvarez S. W., Sviderskiy V. O., Terzi E. M., et al. NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature . 2017;551(7682):639–643. doi: 10.1038/nature24637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jiang Y., Mao C., Yang R., et al. EGLN1/c-Myc induced lymphoid-specific helicase inhibits ferroptosis through lipid metabolic gene expression changes. Theranostics . 2017;7(13):3293–3305. doi: 10.7150/thno.19988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jiang Y., He Y., Liu S., Tao Y. Chromatin remodeling factor lymphoid-specific helicase inhibits ferroptosis through lipid metabolic genes in lung cancer progression. Chinese Journal of Cancer . 2017;36(1):p. 82. doi: 10.1186/s40880-017-0248-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Guo J., Xu B., Han Q., et al. Ferroptosis: a novel anti-tumor action for cisplatin. Cancer Research and Treatment . 2018;50(2):445–460. doi: 10.4143/crt.2016.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang X., Sui S., Wang L., et al. Inhibition of tumor propellant glutathione peroxidase 4 induces ferroptosis in cancer cells and enhances anticancer effect of cisplatin. Journal of Cellular Physiology . 2020;235(4):3425–3437. doi: 10.1002/jcp.29232. [DOI] [PubMed] [Google Scholar]
- 170.Chen P., Wu Q., Feng J., et al. Erianin, a novel dibenzyl compound in dendrobium extract, inhibits lung cancer cell growth and migration via calcium/calmodulin-dependent ferroptosis. Signal Transduction and Targeted Therapy . 2020;5(1):p. 51. doi: 10.1038/s41392-020-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Li Y., Yan H., Xu X., Liu H., Wu C., Zhao L. Erastin/sorafenib induces cisplatin-resistant non-small cell lung cancer cell ferroptosis through inhibition of the Nrf2/xCT pathway. Oncology Letters . 2020;19:323–333. doi: 10.3892/ol.2019.11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Huang C., Yang M., Deng J., Li P., Su W., Jiang R. Upregulation and activation of p53 by erastin-induced reactive oxygen species contribute to cytotoxic and cytostatic effects in A549 lung cancer cells. Oncology Reports . 2018;40:2363–2370. doi: 10.3892/or.2018.6585. [DOI] [PubMed] [Google Scholar]
- 173.Pan X., Lin Z., Jiang D., et al. Erastin decreases radioresistance of NSCLC cells partially by inducing GPX4-mediated ferroptosis. Oncology Letters . 2019;17:3001–3008. doi: 10.3892/ol.2019.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kwon O. S., Kwon E. J., Kong H. J., et al. Systematic identification of a nuclear receptor-enriched predictive signature for erastin-induced ferroptosis. Redox Biology . 2020;37:p. 101719. doi: 10.1016/j.redox.2020.101719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gai C., Yu M., Li Z., et al. Acetaminophen sensitizing erastin-induced ferroptosis via modulation of Nrf2/heme oxygenase-1 signaling pathway in non-small-cell lung cancer. Journal of Cellular Physiology . 2020;235(4):3329–3339. doi: 10.1002/jcp.29221. [DOI] [PubMed] [Google Scholar]
- 176.Villalpando-Rodriguez G. E., Blankstein A. R., Konzelman C., Gibson S. B. Lysosomal destabilizing drug siramesine and the dual tyrosine kinase inhibitor lapatinib induce a synergistic ferroptosis through reduced heme oxygenase-1 (HO-1) levels. Oxidative Medicine and Cellular Longevity . 2019;2019:14. doi: 10.1155/2019/9561281.9561281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yuan B., Liao F., Shi Z. Z., et al. Dihydroartemisinin inhibits the proliferation, colony formation and induces ferroptosis of lung cancer cells by inhibiting PRIM2/SLC7A11 axis. Oncotargets and Therapy . 2020;13:10829–10840. doi: 10.2147/OTT.S248492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Palmer L. D., Jordan A. T., Maloney K. N., et al. Zinc intoxication induces ferroptosis in A549 human lung cells. Metallomics . 2019;11(5):982–993. doi: 10.1039/c8mt00360b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang M., Mao C., Ouyang L., et al. Long noncoding RNA LINC00336 inhibits ferroptosis in lung cancer by functioning as a competing endogenous RNA. Cell Death and Differentiation . 2019;26(11):2329–2343. doi: 10.1038/s41418-019-0304-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gai C., Liu C., Wu X., et al. MT1DP loaded by folate-modified liposomes sensitizes erastin-induced ferroptosis via regulating miR-365a-3p/NRF2 axis in non-small cell lung cancer cells. Cell Death & Disease . 2020;11(9):p. 751. doi: 10.1038/s41419-020-02939-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mao C., Wang X., Liu Y., et al. A G3BP1-interacting lncRNA promotes ferroptosis and apoptosis in cancer via nuclear sequestration of p53. Cancer Research . 2018;78:canres.3454.2017–canres.3454.3496. doi: 10.1158/0008-5472.CAN-17-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jiang Y., Zhao X., Huang J., et al. Transformable hybrid semiconducting polymer nanozyme for second near- infrared photothermal ferrotherapy. Nature Communications . 2020;11(1):p. 1857. doi: 10.1038/s41467-020-15730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ye L. F., Chaudhary K. R., Zandkarimi F., et al. Radiation-induced lipid peroxidation triggers ferroptosis and synergizes with ferroptosis inducers. ACS Chemical Biology . 2020;15(2):469–484. doi: 10.1021/acschembio.9b00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yuan L. Q., Wang C., Lu D. F., Zhao X. D., Tan L. H., Chen X. Induction of apoptosis and ferroptosis by a tumor suppressing magnetic field through ROS-mediated DNA damage. Aging (Albany NY) . 2020;12(4):3662–3681. doi: 10.18632/aging.102836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jiang M., Qiao M., Zhao C., Deng J., Li X., Zhou C. Targeting ferroptosis for cancer therapy: exploring novel strategies from its mechanisms and role in cancers. Translational Lung Cancer Research . 2020;9(4):1569–1584. doi: 10.21037/tlcr-20-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sun W., Du Y., Liang X., et al. Synergistic triple-combination therapy with hyaluronic acid-shelled PPy/CPT nanoparticles results in tumor regression and prevents tumor recurrence and metastasis in 4T1 breast cancer. Biomaterials . 2019;217:p. 119264. doi: 10.1016/j.biomaterials.2019.119264. [DOI] [PubMed] [Google Scholar]
- 187.Wu J., Minikes A. M., Gao M., et al. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature . 2019;572(7769):402–406. doi: 10.1038/s41586-019-1426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wenzel S. E., Tyurina Y. Y., Zhao J., et al. PEBP1 wardens ferroptosis by enabling lipoxygenase generation of lipid death signals. Cell . 2017;171(3):628–641.e26. doi: 10.1016/j.cell.2017.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhao J., Dar H. H., Deng Y., et al. PEBP1 acts as a rheostat between prosurvival autophagy and ferroptotic death in asthmatic epithelial cells. Proceedings of the National Academy of Sciences of the United States of America . 2020;117(25):14376–14385. doi: 10.1073/pnas.1921618117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ha E. H., Choi J. P., Kwon H. S., et al. Endothelial Sox17 promotes allergic airway inflammation. The Journal of Allergy and Clinical Immunology . 2019;144(2):561–573.e6. doi: 10.1016/j.jaci.2019.02.034. [DOI] [PubMed] [Google Scholar]
- 191.Geering B., Stoeckle C., Conus S., Simon H. U. Living and dying for inflammation: neutrophils, eosinophils, basophils. Trends in Immunology . 2013;34(8):398–409. doi: 10.1016/j.it.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 192.Gaschler M. M., Andia A. A., Liu H., et al. FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation. Nature Chemical Biology . 2018;14(5):507–515. doi: 10.1038/s41589-018-0031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wu Y., Chen H., Xuan N., et al. Induction of ferroptosis-like cell death of eosinophils exerts synergistic effects with glucocorticoids in allergic airway inflammation. Thorax . 2020;75(11):918–927. doi: 10.1136/thoraxjnl-2020-214764. [DOI] [PubMed] [Google Scholar]
- 194.Dar H. H., Tyurina Y. Y., Mikulska-Ruminska K., et al. Pseudomonas aeruginosa utilizes host polyunsaturated phosphatidylethanolamines to trigger theft-ferroptosis in bronchial epithelium. The Journal of Clinical Investigation . 2018;128(10):4639–4653. doi: 10.1172/JCI99490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Dar H. H., Anthonymuthu T. S., Ponomareva L. A., et al. A new thiol-independent mechanism of epithelial host defense against Pseudomonas aeruginosa: iNOS/NO• sabotage of theft-ferroptosis. Redox Biology . 2021;45:p. 102045. doi: 10.1016/j.redox.2021.102045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Amaral E. P., Costa D. L., Namasivayam S., et al. A major role for ferroptosis in Mycobacterium tuberculosis-induced cell death and tissue necrosis. The Journal of Experimental Medicine . 2019;216(3):556–570. doi: 10.1084/jem.20181776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ghafoori P., Marks L. B., Vujaskovic Z., Kelsey C. R. Radiation-induced lung injury. Assessment, management, and prevention. Oncology (Williston Park) . 2008;22:37–47. [PubMed] [Google Scholar]
- 198.Li X., Zhuang X., Qiao T. Role of ferroptosis in the process of acute radiation-induced lung injury in mice. Biochemical and Biophysical Research Communications . 2019;519(2):240–245. doi: 10.1016/j.bbrc.2019.08.165. [DOI] [PubMed] [Google Scholar]
- 199.Mortezavi A., Salemi S., Kranzbühler B., et al. Inhibition of autophagy significantly increases the antitumor effect of abiraterone in prostate cancer. World Journal of Urology . 2019;37(2):351–358. doi: 10.1007/s00345-018-2385-5. [DOI] [PubMed] [Google Scholar]


