Abstract
The gene encoding hepatocyte nuclear factor 1β (HNF1B), a transcription factor involved in the development of the kidney and other organs, is located on chromosome 17q12. Heterozygous deletions of chromosome 17q12, which involve 15 genes including HNF1B, are known as 17q12 deletion syndrome and are a common cause of congenital anomalies of the kidneys and urinary tract (CAKUT) and may also present as a multisystem disorder. Autosomal recessive polycystic kidney disease (ARPKD), on the other hand, is a severe form of polycystic kidney disease caused by mutations in PKHD1 (polycystic kidney and hepatic disease 1). It is important to differentiate between these two diseases because they differ significantly in inheritance patterns, renal prognosis, and extrarenal manifestations. Here we report a case of 17q12 deletion syndrome that clinically mimicked ARPKD in which genetic testing was essential for appropriate genetic counseling and monitoring of possible extrarenal manifestations. The patient presented antenatally with markedly enlarged kidneys and showed bilaterally hyperechoic kidneys with poor corticomedullary differentiation and multiple cysts on ultrasonography. There was no family history of renal disease. ARPKD was clinically suspected and genetic testing was performed to confirm diagnosis, resulting in an unexpected finding of 17q12 deletion including HNF1B. While some research has been done to identify patients that should be tested for HNF1B anomalies, this case illustrates the difficulty of recognizing HNF1B-related disease and the importance of genetic testing in appropriately managing CAKUT cases.
Keywords: Hepatocyte nuclear factor 1β (HNF1B), 17q12 deletion syndrome, HNF1B-associated disease, Autosomal recessive polycystic kidney disease (ARPKD), Congenital anomalies of the kidneys and urinary tract (CAKUT), Genetic testing
Introduction
Hepatocyte nuclear factor 1β (HNF1B) is a transcription factor involved in the development of the kidney, urogenital tract, pancreas, liver, and parathyroid gland [1]. Heterozygous mutations in HNF1B are the most common monogenic cause of developmental renal disease and may also present as a variable multisystem disorder. Heterozygous deletions of 17q12, which involve 15 genes including HNF1B, are known as 17q12 deletion syndrome and result in similar renal and extrarenal manifestations. Renal phenotypes of HNF1B-associated disease are highly heterogenic with cystic disease, including cystic dysplasia, being predominant. Other renal phenotypes include congenital anomalies of the kidneys and urinary tract (CAKUT) such as renal hypoplasia, unilateral renal agenesis, horseshoe kidney, and collecting system abnormalities. Renal function can range from normal to end-stage renal disease (ESRD). Extrarenal phenotypes of HNF1B-associated disease include early-onset diabetes mellitus (known as maturity-onset diabetes of the young type 5), pancreatic hypoplasia, elevated liver enzymes, genital tract malformations, and in the case of 17q12 deletions, autism spectrum disorders and cognitive impairment [2].
Autosomal recessive polycystic kidney disease (ARPKD), on the other hand, is a severe form of polycystic kidney disease caused by mutations in PKHD1 (polycystic kidney and hepatic disease 1). ARPKD usually presents antenatally or in early childhood with enlarged, hyperechoic kidneys and impaired renal function which in most cases progresses to ESRD. Developmental biliary defects are invariable features of ARPKD and can lead to portal hypertension with resulting esophageal varices, splenomegaly, and thrombocytopenia. Ascending cholangitis is also an important complication in ARPKD with high morbidity and mortality [3].
Here we report a case of 17q12 deletion syndrome that clinically mimicked ARPKD and presented antenatally with markedly enlarged kidneys. Genetic testing enabled to differentiate between the two diseases and was essential for accurate genetic counseling and appropriate monitoring for renal and extrarenal symptoms.
Case report
The mother, G3P2, was referred to our hospital at 26 weeks of gestation because of enlarged fetal kidneys. The parents were nonconsanguineous with no family history of renal disease. Fetal magnetic resonance imaging (MRI) at 30 weeks (Fig. 1a) showed bilaterally enlarged and polycystic kidneys. Amniotic fluid levels were normal throughout the pregnancy. Vaginal delivery occurred at 37 weeks 6 days. The male newborn weighed 3513 g and had Apgar scores of 8 and 8 at 1 and 5 min, respectively. He was admitted to the NICU because of respiratory distress and was diagnosed with transient tachypnea of the newborn.
Fig. 1.
Imaging findings of the patient's kidneys. a Fetal magnetic resonance imaging at 30 weeks gestation. The kidneys were bilaterally enlarged with lengths of 63 mm and 55 mm in the left and right kidney, respectively. Multiple small cysts were detected in both kidneys. No abnormalities were found in the lungs and the amniotic fluid level was normal. b Renal ultrasonography on day 16 of life. The kidneys were enlarged and hyperechoic with poor corticomedullary differentiation. The left and right renal lengths were 72 mm and 66 mm, respectively. Bilateral multiple cysts of maximum 10 mm in diameter were found
Clinical course in the NICU was unremarkable except for a mildly elevated urinary output. His blood pressure was normal and stable. He was discharged on day 17 of life with good feeding and weight gain.
Postnatal ultrasonography (Fig. 1b) revealed bilaterally hyperechoic and enlarged kidneys with poor corticomedullary differentiation and multiple cysts. No abnormal findings in the liver or biliary tract was found on ultrasonography. No abnormalities in brain structure were observed in cranial MRI. Serum creatinine levels were mildly elevated at 0.42 mg/dL at 1 month of age. No electrolyte imbalance, proteinuria or hematuria was observed.
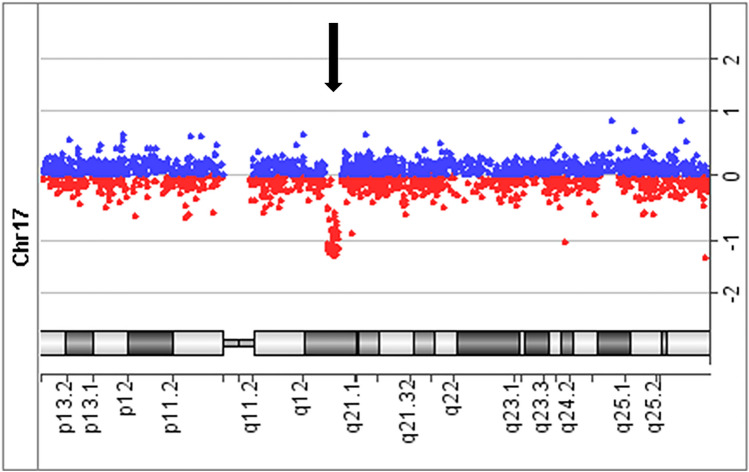
The ultrasonography findings and the clinical course suggested ARPKD and genetic testing was performed with informed consent of the parents. The CAKUT gene panel analysis of the patient detected no known pathogenic variants in PKHD1 or in any of the genes analyzed. Array comparative genomic hybridization identified a heterozygous 1.35-magabase(Mb) deletion in the patient at 17q12 including HNF1B (Fig. 2). Multiplex ligation-dependent probe amplification analysis of the patient and his parents revealed that the deletion was de novo. The genetic analyses were approved by the Central Ethics Board of the University of Tokyo and Kobe University.
Fig. 2.
Array comparative genomic hybridization (CGH) of the patient. Array CGH showed a heterozygous deletion in the patient at 17q12 including HNF1B
The patient has been followed up for 2 years and is showing normal growth and development. He has not developed diabetes or elevations in liver enzymes thus far. His serum creatinine levels remain at around 0.4 mg/dL and no further deterioration in kidney function has been observed.
Discussion
In our patient, hyperechoic and markedly enlarged kidneys presenting before birth clinically suggested ARPKD and genetic testing was performed to confirm diagnosis, resulting in an unexpected finding of 17q12 deletion including HNF1B. Studies that performed genetic testing on patients clinically diagnosed with ARPKD have also detected deletions or mutations in HNF1B. Szabó T et al. analyzed 36 unrelated patients with the clinical diagnosis of ARPKD and identified three patients with de novo heterozygous HNF1B deletion [4]. Similarly, Krall et al. analyzed 59 patients diagnosed or suspected with ARPKD and found 2 cases with HNF1B mutation [5]. These findings suggest that in some cases, HNF1B-associated disease may clinically mimic ARPKD and be misdiagnosed as such. It is important to differentiate between these two diseases, however, because they differ significantly in inheritance patterns, renal prognosis, and extrarenal manifestations. The absence of arterial hypertension and electrolyte imbalance in our patient was not typical of ARPKD and may serve as a differential point against ARPKD in future cases.
Clinically, there are other genes whose mutations may cause ARPKD-like kidneys. Mutations in DZIP1L, which encodes a ciliary protein that localizes to centrioles and the ciliary basal bodies, has recently been reported to demonstrate an ARPKD-like phenotype with a moderate course [6]. Either dominant or recessive mutations in PKD1 and PKD2, genes that generally cause autosomal dominant polycystic kidney disease, can mimic ARPKD in cases that manifest prenatally or during childhood. Nephronophthisis and other ciliopathies should also be considered as differential diagnoses for ARPKD [7]. Table 1 provides a summary of gene mutations that may clinically present as ARPKD-like kidneys.
Table 1.
Summary of gene mutations that may clinically present as ARPKD-like kidneys
| Gene(s) | Disease | Renal presentation | Extrarenal manifestations |
|---|---|---|---|
| HNF1B | HNF1B-associated disease | Highly variable, including cystic dysplasia, unilateral renal agenesis, renal hypoplasia, horseshoe kidney [2] | Early-onset diabetes mellitus, pancreatic hypoplasia, elevated liver enzymes, genital tract malformations |
| DZIP1L | ARPKD-like | Enlarged polycystic kidneys with poor cortico-medullary differentiation [6] | Hypertension from early childhood |
| PKD1, PKD2 | ADPKD | Cysts of variable sizes along all regions of the nephron | Polycystic liver disease, intracranial aneurysms, hypertension |
| NPHP1-NPHP20, NPHP1L, NPHP2L [8] | Nephronophthisis | Hyperechoic kidneys with poor corticomedullary differentiation; multiple cysts at the corticomedullary junction and the medulla | Retinitis pigmentosa, skeletal defects, hepatic fibrosis, situs inversus |
| INPP5E, TMEM216, AHI1, NPHP1, CEP290, TMEM67 and others [9] | Jubert Syndrome | Nephronophthisis or cystic dysplasia | Cerebellar vermis hypoplasia, hypotonia in infancy with later ataxia, intellectual disability, episodec tachypnea and/or apnea, abnormal eye movements |
| BBS1-BBS18 [9] | Bardet-Biedl Syndrome | Nephronophthisis or cystic dysplasia | Retinal dystrophy, obesity, postaxial polydactyly, intellectual disability, hypogonadism |
| MKS1, TMEM216, TMEM67, CEP290, RPGRIP1L, CC2D2A and others [9] | Meckel-Gruber Syndrome | Nephronophthisis or cystic dysplasia | Occipital encephalocele, hepatic ductal plate malformation, perinatal death |
ARPKD autosomal recessive polycystic kidney disease, ADPKD autosomal dominant polycystic kidney disease
Renal function in HNF1B-associated disease varies widely from normal renal function in adulthood to prenatal renal failure leading to oligohydraminos, pulmonary hypoplasia and prenatal death [10]. In our patient, his renal function was relatively preserved compared to those of early-onset ARPKD patients, but the histology behind his condition is unclear. Few renal biopsies have been performed in cases of HNF1B-associated disease. Clissold et al. reviewed histological data of 19 patients with HNF1B-associated renal disease and reported considerable variation in the histopathological findings, including hypoplastic glomerulocystic kidney disease in 6 patients, oligomeganephronia in 3 patients, and cystic renal dysplasia in 2 patients [2]. While these findings suggest abnormal nephron development in the patients, there is not sufficient evidence to rule out the involvement of ARPKD-like collecting duct dilatations in other cases of HNF1B-associated renal disease.
Research in mouse models indicates an association between Hnf1b and Pkhd1 in kidney development. Renal-specific inactivation of Hnf1b in mice results in polycystic kidneys. Pkhd1, along with cystic disease genes Umod and Pkd2, contains Hnf1b binding sites and is drastically downregulated in Hnf1b-inactivated cystic cells [11]. Hnf1b directly activates the Pkhd1 promoter in mice and renal-specific expression of a dominant-negative mutant Hnf1b develops renal cysts and reduces Pkhd1 mRNA in cystic collecting ducts but not in the surrounding noncystic tubules [12, 13].
Although these transcriptional networks may explain the mechanism of ARPKD-like cyst formation in some cases, little is known about the reasons for the high heterogeneity of phenotypes in HNF1B-associated disease. No clear genotype–phenotype correlation has been established and phenotypes may vary even within family members that have the same mutation [14]. The heterogeneity of phenotypes, incomplete penetrance, and the high frequency (50–60%) of de novo mutations make it difficult to clinically diagnose HNF1B-associated disease [15–17]. Faguer et al. described a scoring system as a screening tool to determine which patients should be genetically tested for HNF1B anomalies [18]. The HNF1B score was developed based on a weighted combination of the most typical findings according to the frequency or specificity reported in the literature. Characteristics that were considered most distinctive and thus attributed the highest score were hyperechoic kidneys, renal cysts, maturity-onset diabetes of the young, pancreatic hypoplasia or pancreatic exocrine insufficiency, and genital tract abnormalities. In the case of our patient, his HNF1B score adds up to 18 with bilateral hyperechoic kidneys, renal cysts and antenatal renal abnormalities, which is above the threshold of 8 for HNF1B gene testing proposed in the study (sensitivity 98.2%, specificity 41.1%, positive predictive value 19.6%, and negative predictive value > 99%). However, the authors also state that other recognized causes of renal cysts such as ARPKD should be excluded before evaluating the HNF1B score, and therefore cases mimicking other diseases may still be overlooked [18].
Virtually all patients with a whole-gene deletion of HNF1B is found to have a 1.4-Mb 17q12 deletion involving 15 genes [19]. This region of chromosome 17 is susceptible to genomic rearrangement mediated by non-allelic homologous recombination between segmental duplications [20]. Verbitsky et al. performed a genome-wide analysis of copy number variants in 2824 CAKUT cases and found that out of 112 patients carrying a known genomic disorder, 23 (20.5%) had a 17q12 deletion [21]. In addition to the various renal and extrarenal manifestations of HNF1B-associated disease, 17q12 deletion syndrome is associated with an increased risk of neurodevelopmental disorders such as autism spectrum disorders and cognitive impairment [2]. Although whether HNF1B is responsible for these neurological features remain unclear, patients with HNF1B deletions should be monitored for neurodevelopmental problems. These findings also highlight the need for both sequence and dosage analyses for genetic testing in CAKUT.
In summary, we have reported a case of 17q12 deletion including HNF1B that clinically mimicked ARPKD and illuminated the importance of genetic testing in appropriately managing CAKUT cases. Further research is required to improve the recognition of HNF1B-related disease and to refine the identification of patients that should be tested for HNF1B anomalies.
Acknowledgements
We gratefully acknowledge the hospital staff for their help and support.
Declarations
Conflict of interest
The authors declared that they have no conflict of interests.
Human and animal rights statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from the patient’s parents.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Verhave JC, Bech AP, Wetzels JF, Nijenhuis T. Hepatocyte nuclear factor 1β-associated kidney disease: more than renal cysts and diabetes. J Am Soc Nephrol. 2016;27:345–353. doi: 10.1681/ASN.2015050544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clissold RL, Hamilton AJ, Hattersley AT, Ellard S, Bingham C. HNF1B-associated renal and extra-renal disease-an expanding clinical spectrum. Nat Rev Nephrol. 2015;11:102–112. doi: 10.1038/nrneph.2014.232. [DOI] [PubMed] [Google Scholar]
- 3.Hartung EA, Guay-Woodford LM. Autosomal recessive polycystic kidney disease: a hepatorenal fibrocystic disorder with pleiotropic effects. Pediatrics. 2014;134:e833–e845. doi: 10.1542/peds.2013-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szabó T, Orosz P, Balogh E, Jávorszky E, Máttyus I, Bereczki C, Maróti Z, Kalmár T, Szabó AJ, Reusz G, Várkonyi I, Marián E, Gombos É, Orosz O, Madar L, Balla G, Kappelmayer J, Tory K, Balogh I. Comprehensive genetic testing in children with a clinical diagnosis of ARPKD identifies phenocopies. Pediatr Nephrol. 2018;33:1713–1721. doi: 10.1007/s00467-018-3992-5. [DOI] [PubMed] [Google Scholar]
- 5.Krall P, Pineda C, Ruiz P, Ejarque L, Vendrell T, Camacho JA, Mendizábal S, Oliver A, Ballarín J, Torra R, Ars E. Cost-effective PKHD1 genetic testing for autosomal recessive polycystic kidney disease. Pediatr Nephrol. 2014;29:223–234. doi: 10.1007/s00467-013-2657-7. [DOI] [PubMed] [Google Scholar]
- 6.Lu H, Galeano MCR, Ott E, Kaeslin G, Kausalya PJ, Kramer C, Ortiz-Brüchle N, Hilger N, Metzis V, Hiersche M, Tay SY, Tunningley R, Vij S, Courtney AD, Whittle B, Wühl E, Vester U, Hartleben B, Neuber S, Frank V, Little MH, Epting D, Papathanasiou P, Perkins AC, Wright GD, Hunziker W, Gee HY, Otto EA, Zerres K, Hildebrandt F, Roy S, Wicking C, Bergmann C. Mutations in DZIP1L, which encodes a ciliary-transition-zone protein, cause autosomal recessive polycystic kidney disease. Nat Genet. 2017;49:1025–1034. doi: 10.1038/ng.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Early BC, Disease SPK, Ciliopathies R. An emerging field of interest. Nephron. 2019;141:50–60. doi: 10.1159/000493532. [DOI] [PubMed] [Google Scholar]
- 8.Luo F, Tao YH. Nephronophthisis: a review of genotype-phenotype correlation. Nephrology (Carlton) 2018;23:904–911. doi: 10.1111/nep.13393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parisi MA. The molecular genetics of Joubert syndrome and related ciliopathies: the challenges of genetic and phenotypic heterogeneity. Transl Sci Rare Dis. 2019;4:25–49. doi: 10.3233/TRD-190041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heidet L, Decramer S, Pawtowski A, Morinière V, Bandin F, Knebelmann B, Lebre AS, Faguer S, Guigonis V, Antignac C, Salomon R. Spectrum of HNF1B mutations in a large cohort of patients who harbor renal diseases. Clin J Am Soc Nephrol. 2010;5:1079–1090. doi: 10.2215/CJN.06810909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gresh L, Fischer E, Reimann A, Tanguy M, Garbay S, Shao X, Hiesberger T, Fiette L, Igarashi P, Yaniv M, Pontoglio M. A transcriptional network in polycystic kidney disease. EMBO J. 2004;23:1657–1668. doi: 10.1038/sj.emboj.7600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiesberger T, Bai Y, Shao X, McNally BT, Sinclair AM, Tian X, Somlo S, Igarashi P. Mutation of hepatocyte nuclear factor-1beta inhibits Pkhd1 gene expression and produces renal cysts in mice. J Clin Invest. 2004;113:814–825. doi: 10.1172/JCI200420083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiesberger T, Shao X, Gourley E, Reimann A, Pontoglio M, Igarashi P. Role of the hepatocyte nuclear factor-1beta (HNF-1beta) C-terminal domain in Pkhd1 (ARPKD) gene transcription and renal cystogenesis. J Biol Chem. 2005;280:10578–10586. doi: 10.1074/jbc.M414121200. [DOI] [PubMed] [Google Scholar]
- 14.Edghill EL, Bingham C, Ellard S, Hattersley AT. Mutations in hepatocyte nuclear factor-1beta and their related phenotypes. J Med Genet. 2006;43:84–90. doi: 10.1136/jmg.2005.032854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Decramer S, Parant O, Beaufils S, Clauin S, Guillou C, Kessler S, Aziza J, Bandin F, Schanstra JP, Bellanné-Chantelot C. Anomalies of the TCF2 gene are the main cause of fetal bilateral hyperechogenic kidneys. J Am Soc Nephrol. 2007;18:923–933. doi: 10.1681/ASN.2006091057. [DOI] [PubMed] [Google Scholar]
- 16.Ulinski T, Lescure S, Beaufils S, Guigonis V, Decramer S, Morin D, Clauin S, Deschênes G, Bouissou F, Bensman A, Bellanné-Chantelot C. Renal phenotypes related to hepatocyte nuclear factor-1β (TCF2) mutations in a pediatric cohort. J Am Soc Nephrol. 2006;17:497–503. doi: 10.1681/ASN.2005101040. [DOI] [PubMed] [Google Scholar]
- 17.Edghill EL, Oram RA, Owens M, Stals KL, Harries LW, Hattersley AT, Ellard S, Bingham C. Hepatocyte nuclear factor-1beta gene deletions–a common cause of renal disease. Nephrol Dial Transplant. 2008;23:627–635. doi: 10.1093/ndt/gfm603. [DOI] [PubMed] [Google Scholar]
- 18.Faguer S, Chassaing N, Bandin F, Prouheze C, Garnier A, Casemayou A, Huart A, Schanstra JP, Calvas P, Decramer S, Chauveau D. The HNF1B score is a simple tool to select patients for HNF1B gene analysis. Kidney Int. 2014;86:1007–1015. doi: 10.1038/ki.2014.202. [DOI] [PubMed] [Google Scholar]
- 19.Laffargue F, Bourthoumieu S, Llanas B, Baudouin V, Lahoche A, Morin D, Bessenay L, De Parscau L, Cloarec S, Delrue MA, Taupiac E, Dizier E, Laroche C, Bahans C, Yardin C, Lacombe D, Guigonis V. Towards a new point of view on the phenotype of patients with a 17q12 microdeletion syndrome. Arch Dis Child. 2015;100:259–264. doi: 10.1136/archdischild-2014-306810. [DOI] [PubMed] [Google Scholar]
- 20.Mefford HC, Clauin S, Sharp AJ, Moller RS, Ullmann R, Kapur R, Pinkel D, Cooper GM, Ventura M, Ropers HH, Tommerup N, Eichler EE, Bellanne-Chantelot C. Recurrent reciprocal genomic rearrangements of 17q12 are associated with renal disease, diabetes, and epilepsy. Am J Hum Genet. 2007;81:1057–1069. doi: 10.1086/522591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verbitsky M, Westland R, Perez A, Kiryluk K, Liu Q, Krithivasan P, Mitrotti A, Fasel DA, Batourina E, Sampson MG, Bodria M, Werth M, Kao C, Martino J, Capone VP, Vivante A, Shril S, Kil BH, Marasà M, Zhang JY, Na YJ, Lim TY, Ahram D, Weng PL, Heinzen EL, Carrea A, Piaggio G, Gesualdo L, Manca V, Masnata G, Gigante M, Cusi D, Izzi C, Scolari F, van Wijk JAE, Saraga M, Santoro D, Conti G, Zamboli P, White H, Drozdz D, Zachwieja K, Miklaszewska M, Tkaczyk M, Tomczyk D, Krakowska A, Sikora P, Jarmoliński T, Borszewska-Kornacka MK, Pawluch R, Szczepanska M, Adamczyk P, Mizerska-Wasiak M, Krzemien G, Szmigielska A, Zaniew M, Dobson MG, Darlow JM, Puri P, Barton DE, Furth SL, Warady BA, Gucev Z, Lozanovski VJ, Tasic V, Pisani I, Allegri L, Rodas LM, Campistol JM, Jeanpierre C, Alam S, Casale P, Wong CS, Lin F, Miranda DM, Oliveira EA, Simões-E-Silva AC, Barasch JM, Levy B, Wu N, Hildebrandt F, Ghiggeri GM, Latos-Bielenska A, Materna-Kiryluk A, Zhang F, Hakonarson H, Papaioannou VE, Mendelsohn CL, Gharavi AG, Sanna-Cherchi S. The copy number variation landscape of congenital anomalies of the kidney and urinary tract. Nat Genet. 2019;51:117–127. doi: 10.1038/s41588-018-0281-y. [DOI] [PMC free article] [PubMed] [Google Scholar]