Abstract
Preeclampsia and superimposed preeclampsia usually develop after 20 weeks of gestation. We report a case of a 35-year-old Japanese woman who developed hypertensive disorders of pregnancy (HDP) before 20 weeks of gestation. She presented with hypertension and proteinuria at 9 and 11 weeks of gestation, respectively, and developed nephrotic syndrome at 17 weeks of gestation. She did not have definite hypertension or urinary abnormalities before pregnancy. The patient was serologically positive for the antinuclear antibody. However, the complement levels were normal and anti-phospholipid antibody was not detected. A renal biopsy performed at 18 weeks of gestation showed diffuse endotheliosis and tip lesions of secondary focal segmental glomerulosclerosis but no hypertensive changes of the arterioles. Although electron microscopic examination showed electron-dense deposits in the subendothelial lesions, they were considered nonspecific plasma exudation by mass spectrometry. An abortion was performed at 20 weeks gestation because the patient’s congestive symptoms due to nephrotic syndrome had worsened and marked fetal growth restriction was observed. After delivery, the patient’s symptoms resolved immediately without any additional treatment; however, continuous antihypertensive medication was required. Finally, the patient was diagnosed with HDP based on the renal biopsy findings and her clinical course after delivery. Compared to previous reports, this case describes the earliest onset of HDP. Thus, HDP should be considered as a differential diagnosis in pregnant women with hypertension or proteinuria presenting with symptoms before 20 weeks of gestation.
Keywords: Preeclampsia, Hypertensive disorders of pregnancy, 20 weeks of gestation, Early onset
Introduction
Hypertensive disorders of pregnancy (HDP) are defined as the development of hypertension during pregnancy and are classified into four types; namely, chronic hypertension (CH), preeclampsia (PE), gestational hypertension (GH), and superimposed preeclampsia (SPE) [1]. A diagnosis of PE is made when hypertension appears after 20 weeks of gestation with maternal organ damage such as kidney injury, liver involvement, stroke, neurological complications, hematological complications, or uteroplacental insufficiency. In contrast, SPE is diagnosed in women with hypertension and/or proteinuria before 20 weeks of gestation with worsening of those with new onset of maternal organ damage or uteroplacental insufficiency such as PE after 20 weeks of gestation. Therefore, hypertension and proteinuria newly observed and worsening before 20 weeks of gestation are usually considered to be caused by renal diseases rather than HDP. However, some cases of HDP have been diagnosed before 20 weeks of gestation without renal disease [2–6]. Here, we report a case of HDP that was confirmed by renal biopsy and describe the clinical course after elective abortion. Although her blood pressure (BP) was slightly elevated only once before pregnancy, her BP increased from 9 weeks of gestation. This report describes the earliest onset of HDP to date.
Case report
We report the case of a 35-year-old Japanese woman with subclinical hypothyroidism treated with levothyroxine. She had previously miscarried twice with the same partner at 6 and 8 weeks of gestation, respectively, and had presented to the obstetrics clinic for infertility treatment. However, the cause of infertility was not identified. Although she did not regularly measure her BP, no abnormalities were noted in her annual periodic examination. She became pregnant by artificial insemination and the transfer of cryopreserved-warmed embryos. She presented to the endocrinology clinic for the treatment of hypothyroidism 1 week before embryo transfer. Only once was her diastolic BP confirmed to be slightly high (136/98 mmHg), although she was negative for proteinuria and her serum creatinine (sCr) concentration was 0.57 mg/dL. Afterward, prominent hypertension (170/110 mmHg) and proteinuria (2 +) appeared at 9 and 11 weeks of gestation, respectively. She started to take amlodipine from 12 weeks of gestation. Her liver function was within normal units (aspartate aminotransferase [AST], 24 IU/L; alanine aminotransferase [ALT], 26 IU/L; lactate dehydrogenase [LDH], 208 IU/L; gamma-glutamyl transpeptidase, 14 IU/L; and alkaline phosphatase, 188 IU/L). In addition, her platelet counts were within normal levels (29.3 × 104 /μL) at 12 weeks of gestation. However, proteinuria increased and leg edema appeared. She was diagnosed with nephrotic syndrome at 17 weeks of gestation (serum albumin 2.5 g/dL and urinary protein 7.5 g/gCr) and was admitted to our nephrology department for renal biopsy at 18 weeks of gestation.
Her height was 158 cm and her body weight had increased from 60 kg before pregnancy to 66 kg. Her BP and pulse rate were 133/103 mmHg and 98/min, respectively. Although physical examination revealed pretibial pitting edema, she had no symptoms, such as rashes, joint pain, or abdominal pain. Table 1 shows her laboratory test results on admission. Although total bilirubin [T.Bil] was within normal range (0.6 mg/dL), hypoalbuminemia, aggravation of renal function, liver damage (AST 63 IU/L and ALT 84 IU/L), elevated LDH levels (486 IU/L), and decreased platelet count (17.4 × 104 /μL) were detected. Prothrombin time (PT) and activated partial thromboplastin time (APTT) were within normal range. While she was positive for antinuclear antibodies, her complement levels were normal and she was negative for various autoantibodies. The patient tested negative for anti-phospholipid antibodies (aPL). The patient’s thyroid function was well controlled with medication. Twenty-four-hour urinary collection revealed high protein levels (7.5 g/day). We suspected hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome or thrombotic thrombocytopenic purpura (TTP) due to her elevated liver enzyme level and low platelet count. However, there were no positive findings suggesting hemolysis as her haptoglobin concentration was 26 mg/dL and schistocytosis was not detected. Because she did not meet the diagnostic criteria for HELLP syndrome and had no symptoms, we performed a careful follow-up. TTP was later denied because her disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) activity was 90%. Although acute fatty liver of pregnancy (AFLP) is one of the differential diagnoses of liver damage in a pregnant woman, AFLP typically presents with abdominal symptoms and laboratory abnormalities, such as elevation of aminotransferases ranging from 5 to 10 times the upper limit of normal, increased bilirubin levels, and prolonged PT and APTT with decreased antithrombin III levels [7]. However, the patient in this case had no abdominal symptoms; her serum bilirubin levels and coagulation function were within the normal range, and liver enzyme elevation was relatively mild for AFLP. Therefore, we ruled out AFLP. Deep venous thrombosis was not detected on limb vascular echo, and neither hypertensive nor ischemic changes were confirmed by fundus examination. The patient’s BP further increased to 175/128 mmHg after hospitalization. Therefore, methyldopa was added, and her BP decreased to 138/94 mmHg.
Table 1.
Laboratory data on admission
| Blood cell count | Immunology | Endocrinology | ||||||
| WBC | 11,190 | /μL | CRP | 0.95 | mg/dL | TSH | 0.35 | μU/mL |
| Hb | 11.7 | g/dL | IgG | 550 | mg/dL | FT3 | 2.9 | pg/mL |
| Plt | 17.4 × 104 | /μL | IgA | 117 | mg/dL | FT4 | 1.4 | ng/dL |
| IgM | 92 | mg/dL | ||||||
| Blood chemistry | CH50 | 60 | U/mL | Urinalysis | ||||
| TP | 5.1 | g/dL | C3 | 129 | mg/dL | Gravity | 1.016 | |
| Alb | 2.5 | g/dL | C4 | 21 | mg/dL | pH | 5.5 | |
| T-Bil | 0.6 | mg/dL | Antinuclear antibody | Protein | 7.5 | g/day | ||
| AST | 63 | IU/L | Homogenous | 1: 80 | Blood | 1–4 | /HPF | |
| ALT | 84 | IU/L | Speckled | 1: 80 | Ccr (24 h) | 92.1 | mL/min | |
| LDH | 486 | IU/L | Anti-ds DNA antibody | < 10 | U/mL | |||
| ALP | 394 | IU/L | Anti-Smith antibody | (–) | NAG | 35.5 | U/L | |
| γGTP | 70 | IU/L | Anti-β2-glycoprotein I antibody | < 1.2 | U/mL | β2-MG | 169 | μg/L |
| BUN | 11.6 | mg/dL | Anti-cardiolipin antibody | < 8 | U/mL | α1-MG | 23 | mg/L |
| Cr | 0.69 | mg/dL | Lupus anticoagulant | 1.07 | ||||
| UA | 6.0 | mg/dL | ||||||
| eGFR | 78 | ml/min/1.73m2 | Coagulation | |||||
| Na | 139 | mEq/L | PT | 9.8 | sec | |||
| K | 3.6 | mEq/L | PT-INR | 0.8 | ||||
| Cl | 105 | mEq/L | APTT | 27.9 | sec | |||
| Ca | 8.1 | mg/dL | Fibrinogen | 468 | mg/dL | |||
| P | 4.8 | mg/dL | D-dimer | 5.2 | μg/mL | |||
| Glucose | 114 | mg/dL | AT | 88 | % | |||
| HbA1c (NSGP) | 5.3 | % | ADAMTS13 | 90 | % | |||
| Haptoglobin | 26 | mg/dL |
WBC white blood cell, Hb hemoglobin, Plt platelet, TP total protein, Alb albumin, T-Bil total bilirubin, AST aspartate aminotransferase, ALT alanine aminotransferase, LDH lactate dehydrogenase, ALP alkaline phosphatase, γGTP γ-glutamyl transpeptidase, BUN blood urea nitrogen, Cr creatinine, UA uric acid, eGFR estimated glomerular filtrationn rate, CRP C-reactive protein, CH50 complement activity, PT prothrombin time, INR International Normalized Ratio, APTT activated partial thromboplastin time, AT antithrombin, ADAMTS13 a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13, TSH thyroid stimulating hormone, FT3 free thyroxine 3, FT4 free thyroxine 4, Ccr creatinine clearance, NAG N-acetylglutamate, MG microglobulin
We performed a kidney biopsy at 18 weeks of gestation to clarify the underlying renal disease as the renal manifestations were abnormal for HDP. Light microscopic examination showed 20 enlarged glomeruli. The narrowing of capillary lumens associated with endothelial cell swelling (known as endotheliosis) was prominent (Fig. 1a). In addition, three glomeruli showed glomerular epithelial hypercellularity and sclerosing change with the adhesion of the glomerular capillaries to Bowman’s capsule at the tubular outlet, the so-called tip lesion of focal segmental glomerulosclerosis (FSGS) caused by the glomerular epithelial cell injury (Fig. 1b). A double contour of the capillary wall was also observed (Fig. 1c). While slight thickening of the endothelium and partial hyaline degeneration were observed in the small vessels, no fibrinoid necrosis or chronic changes like onion skin lesions were observed. The tubulointerstitial lesions were not injured. Immunofluorescence examination showed level 2 + staining for immunoglobulin G (IgG), immunoglobulin M (IgM), and C4 and level 1 + staining for immunoglobulin A (IgA) and fibrinogen at the capillary walls and mesangial areas (Fig. 1d). In contrast, the samples were negative for C1q and C3. Electron microscopy showed endothelial cell swelling and the disappearance of the endothelial fenestra (Fig. 1e). Although effacement was not evident, slight villous changes of the foot process were observed. Diffuse double contour was present due to mesangial interposition (data not shown). Moreover, electron-dense deposits (EDD) were also detected in the subendothelial lesions (Fig. 1f). Although the presence of EDD and antinuclear antibody positivity suggested immunocomplex deposition, we concluded by mass spectrometry that the EDD was non-specific plasma exudation. Thrombotic microangiopathy (TMA) was provided as the pathological definitive diagnosis of the renal biopsy, with findings coincident with cases of HDP as described later.
Fig. 1.
Renal biopsy findings. a Most of the capillary lumens are narrowed due to endothelial cell swelling (periodic acid-Schiff stain; × 400). b Glomerular epithelial hypercellularity and sclerosing change and adhesion of glomerular capillaries to Bowmans’s capsule are observed (indicated by an asterisk) (periodic acid-Schiff stain; × 400). c The glomerular basement membranes show partial double contours (indicated by arrows) (periodic acid-silver methenamine stain; × 1000). d Immunofluorescence staining showing positive immunoglobulin G (IgG) staining of mesangial and capillary areas (× 400). e Electron microscopic examination showing endothelial cell swelling with disappearance of the fenestra (arrows) and subendothelial edema (asterisk). f Electron microscopic examination showing narrowing of the capillary lumens due to endothelial cell swelling with electron-dense deposits (EDD) (asterisk)
Continuously, her BP was well controlled and the levels of liver enzyme and LDH were decreased (T.Bil 0.4 mg/dL; AST 26 IU/L; ALT 27 IU/L; and LDH 348 IU/L) and platelet count was increased (46.9 × 104 /μL). However, congestive symptoms due to nephrotic syndrome worsened and fetal echo examination revealed asymmetric-type fetal growth restriction. The fetal estimated weight was 185 g, which was -1.8 SD; moreover, the resistance index (RI) of the umbilical artery was 0.9, which was higher than the 90% tile of the RI at 19 weeks of gestation. We had to judge quickly whether the patient could continue her pregnancy because she was nearing 22 weeks of gestation, the period allowed for abortion, when the renal biopsy results were obtained. Although she strongly hoped to continue the pregnancy, we recommended termination because she did not have any specific nephritis or causative TMA diseases that could be treated; for example, lupus nephritis. Moreover, her maternal risk was high and the fetal growth restriction was already significant. Finally, we decided to end her pregnancy at 20 weeks of gestation with the consent of the patient and her family. Her infant weighed 180 g and did not have any congenital deformities. Asymmetrical fetal growth restriction was observed and the patient’s placenta showed ischemic change, which were consistent with HDP.
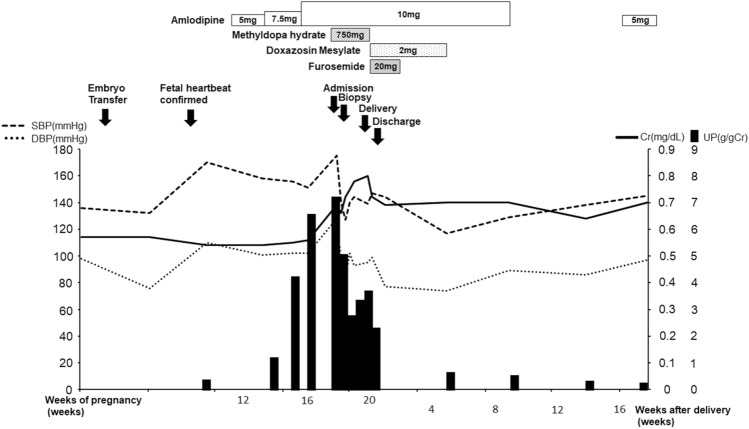
Her clinical course is shown in Fig. 2. At the end of pregnancy, oral administration of furosemide was started; sufficient diuresis was obtained and her leg edema improved rapidly. The levels of LDH, liver enzymes, and platelet were normal 1 month after the delivery (T.Bil 0.6 mg/dL; AST 16 IU/L; ALT 11 IU/L; LDH 182 IU/L; and platelet 28.5 × 104 /μL). Furthermore, renal function improved to her pre-pregnancy values (sCr 0.63 mg/dL) and urinary protein levels decreased and became negative (urinary protein < 150 mg/g Cr) 5 months after the termination. Because her BP also decreased, all antihypertensive drugs were discontinued. However, because her BP increased again, she resumed amlodipine and continued this treatment after 1 year. In addition, we also investigated the causes of secondary hypertension. No abnormal findings of the aorta and renal arteries or adrenal glands were found on computed tomography. Endocrine tests did not show abnormal data suggestive of secondary hypertension. Therefore, secondary hypertension was ruled out.
Fig. 2.
Clinical course of the patient. The solid line indicates serum creatinine (Cr) levels. The black bars indicate the urinary protein (UP) excretion levels. The dashed and dotted lines indicate the systolic blood pressure (SBP) and diastolic blood pressure (DBP), respectively
Discussion
We encountered a case of HDP in which hypertension and proteinuria presented in very early pregnancy. The patient was diagnosed with TMA due to HDP according to the renal biopsy findings and her clinical course. HDP usually develops after 20 weeks of gestation with the appearance or exacerbation of hypertension and proteinuria. Therefore, other glomerular injuries should be suspected if such symptoms appear before 20 weeks of gestation [8]. Previously reported cases of HDP that occurred before 20 weeks of gestation showed renal involvement due to trophoblastic diseases or antiphospholipid syndrome (APS) [2]. We did not initially consider HDP as she did not have trophoblastic disease and APS was unlikely because she did not meet the clinical and laboratory criteria for APS (Sydney criteria) [9]. However, her renal biopsy showed no specific nephritis but did unexpectedly show TMA. Renal TMA is sometimes associated with HDP. Glomerular endothelial cell swelling due to endothelial cell damage and the formation of a double contour of the glomerular capillary walls are observed. Characteristically, the rapid improvement of hypertension and renal abnormalities without specific treatment after the end of pregnancy leads to the diagnosis of HDP. Pregnancy-associated atypical hemolytic uremic syndrome (P-aHUS) is one of the differential diagnoses of TMA. Fakhouri et al. reported that P-aHUS occurred mainly in the postpartum period (79%), and that long-term outcomes were poor; 76% of patients had reached end-stage renal disease or required renal transplantation despite the treatment [10]. Our patient’s TMA was confirmed by a kidney biopsy performed at 18 weeks of gestation, and her renal function recovered promptly after delivery without treatment. As mentioned above, the clinical course of our case was quite different from that of P-aHUS. Therefore, we concluded that the patient did not suffer from P-aHUS.
Seronegative APS (SN-APS) has also been suggested as a differential diagnosis. SN-APS is defined in patients with clinical APS features according to Sydney criteria, but without detectable conventional aPL and obstetric events are more frequent than thrombosis in SN-APS patients [11]. Our case did not meet the laboratory and clinical criteria for SN-APS. In addition, even though miscarriages due to APS are mostly caused in middle to late pregnancy, and prolongation of APTT levels and hypocomplementemia are common in active APS patients [12], she miscarried in the early stage of pregnancy, and the levels of APTT and serum complement were within the normal range. These results indicate that APS could not be conclusively diagnosed in our patient unless miscarriage occurs again, which would indicate pregnancy-related morbidity suggestive of APS. Therefore, it is impossible for us to deny that she was not diagnosed with SN-APS. Moreover, vascular lesions were characterized by occlusion of small vessels associated with fibrous intimal hyperplasia of interlobular arteries and recanalizing thrombi in arteries and arterioles in the chronic phase of APS. However, TMA similar to HDP has been reported in the acute phase of APS [13]. Moreover, the ischemic placental tissue showed no specific findings to confirm the diagnosis. Therefore, it is also difficult to distinguish APS and HDP based on the findings of the kidney and placenta. The screening for presence of non-conventional aPL is uncommon in everyday clinical practice. However, because 68% of obstetric SN-APS patients are positive for non-conventional aPL [14], measuring aPL levels may help confirm the presence of SN-APS.
In the relevant literature, only five cases of HDP have been reported to develop before 20 weeks of gestation without trophoblastic diseases and APS [2–6]. To our knowledge, our case of onset at 9 weeks of gestation is the earliest report. The “Best Practice Guide 2015 for Care and Treatment of Hypertension in Pregnancy” published by the Japan Society for the Study of Hypertension in Pregnancy, listed medical histories of hypertension, chronic kidney disease and diabetes, first pregnancy, multiple pregnancies, older pregnancy, obesity, pregnancy by assisted reproductive technology (ART), thrombotic diathesis, and thyroid dysfunction as risk factors for the development of HDP [9, 15]. All the five above-reported cases [2–6] had at least one risk factor. The risk factors in our patient were advanced age, hypothyroidism, and transfer of a cryopreserved-warmed embryo (ART) and it was likely that she had chronic hypertension. The relative risk (RR) for the development of SPE is 6.5 (95% confidence interval [CI] 4.2–12.5) even in patients with mild hypertension [16]. In addition, Qin et al. [17] reported a significantly increased risk of developing HDP in ART singleton pregnancies to spontaneously conceived singleton pregnancies (RR 1.30 [95%CI 1.04–1.62]), and Sites et al. [18] reported an increased risk for HDP in cases involving the transfer of cryopreserved-warmed embryos as compared to fresh embryo transfer among ART pregnancies conceived using autologous eggs (odds ratio 2.17 [95%CI 1.67–2.82]). Thus, our case had many risk factors and was prone to developing HDP.
Various factors influence HDP onset, with an imbalance between placental growth factor (PlGF) and soluble fms-like tyrosine kinase 1 (sFlt-1) particularly important for its onset. sFlt-1 acts as an antagonist of vascular endothelial growth factor (VEGF) and PlGF. The sFlt-1:PlGF ratio can reportedly be used to predict the development of HDP [19] and pregnancy could be continued by removing sFlt-1 by plasma exchange [20]. However, these factors are rarely used in clinical practice in Japan. In normal pregnancy, placental formation begins at about 7 weeks of gestation and the infiltration of extravillous trophoblast (EVT) into the decidua is shallow until 11 weeks of gestation. At 12–16 weeks of gestation, EVT infiltrates the myometrium and replaces the spiral arteries, allowing maternal blood to flow into the intervillous space [21]. However, insufficient remodeling of the spiral artery causes the placenta to become ischemic with increasing levels of inflammatory cytokines and reactive oxygen species, increasing sFlt-1, and decreasing PlGF in HDP cases. Increased sFlt-1 suppresses the action of VEGF and causes systemic vascular endothelial cell damage [22]. In the kidney, impaired VEGF function results in endothelial cell damage and podocytopathy, leading to the appearance of urinary proteins [23]. In this case, sFlt-1 was predicted to increase because fetal echography had already revealed fetal growth restriction and placental blood flow insufficiency at the time of admission. Therefore, we did not measure sFlt-1 in this case. Levine et al. reported increased sFlt-1 from 5 weeks before the onset of HDP [24]. This suggests that our case might have increased sFlt-1 levels from 4 weeks of gestation, before placental formation. Additionally, there may have been a deficiency in immune tolerance to the fetus. A deficiency of immune tolerance has also been proposed as a cause of HDP. The regulatory T cells play an important role, especially during the implantation stage, while macrophages and natural killer cells play an important role in the subsequent placental formation [25]. Decreased regulatory T cells have been reported in patients with HDP [26]; thus, we speculated that the abnormalities due to immunological incompatibility in our case may have occurred from the very early stages of pregnancy.
In a typical case of HDP, EDD is observed in subendothelial lesions by electron microscopy and IgM and fibrinogen immunostaining, with positivity for IgA, IgG, and complements sometimes is also observed in immunofluorescence examinations. The depositions are reportedly not immune complexes but rather fibrinogen-based exudative substances caused by increased permeability due to endothelial damage [27]. We also confirmed by mass spectrometry in our case that no immunocomplexes were attached. Based on these findings, the renal pathology in our case was consistent with HDP, and she was finally diagnosed with TMA due to HDP.
HDP-induced renal damage is reversible and has a relatively good prognosis. In our case, the renal manifestations tended to improve rapidly from the end of pregnancy. However, the patient’s BP was increased without aggravation of abnormal urinary protein excretion after the discontinuation of antihypertensive drugs 2 months after the end of pregnancy. The patient may have had predisposing factors for hypertension before pregnancy. Additionally, renal injures caused by HDP may have affected her BP after the end of her pregnancy. The pros and cons of a next pregnancy require careful consideration, as the relative risks of developing HDP at the next pregnancy are high for pregnant women with a history of HDP during the first pregnancy (RR 32 [95%CI 17–48]) and it is likely to recur, especially if it develops early in pregnancy [28]. If the patient would like to get pregnant again, we plan to screen for the presence of non-conventional aPL. If she tests positive, we would consider her as an SN-APS patient and initiate antiplatelet therapy.
In conclusion, we report a rare case of severe HDP that developed at 9 weeks of gestation with diffuse endotheliosis and pathology of secondary FSGS. Our case is the earliest report of HDP onset. As the number of pregnant women with similar risk factors for HDP, as in our case, is predicted to increase with the aging of pregnant women in Japan, it is necessary to distinguish not only specific underlying glomerular diseases but also HDP, especially in pregnant women with risk factors for HDP before 20 weeks of gestation.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Makino S, Takeda J, Takeda S, Watanabe K, Matsubara K, Nakamoto O, Ushijima J, Ohkuchi A, Koide K, Mimura K, Morikawa M, Naruse K, Tanaka K, Nohira T, Metoki H, Kawabata I, Takagi K, Yamasaki M, Ichihara A, Kimura T, Saito S, Seki H. New definition and classification of “hypertensive disorders of pregnancy (HDP) Hypertens Res Pregnancy. 2019;7:1–5. doi: 10.14390/jsshp.HRP2019-010. [DOI] [Google Scholar]
- 2.Hazra S, Waugh J, Bosio P. ‘Pure’ pre-eclampsia before 20 weeks of gestation: a unique entity. BJOG. 2003;110:1034–1035. doi: 10.1111/j.1471-0528.2003.02134.x. [DOI] [PubMed] [Google Scholar]
- 3.Imasawa T, Nishiwaki T, Nishimura M, Shikama N, Matsumura R, Nagai M, Soyama A, Koike K, Kitamura H, Joh K. A case of “pure” preeclampsia with nephrotic syndrome before 15 weeks of gestation in a patient whose renal biopsy showed glomerular capillary endotheliosis. Am J Kidney Dis. 2006;48:495–501. doi: 10.1053/j.ajkd.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 4.Stillman IE, Karumanchi SA. The glomerular injury of preeclampsia. J Am Soc Nephrol. 2007;18:2281–2284. doi: 10.1681/ASN.2007020255. [DOI] [PubMed] [Google Scholar]
- 5.Maya ID. Hypertension and proteinuria in a 17-year-old at 19weeks’gestation. Am J Kidney Dis. 2008;51:155–159. doi: 10.1053/j.ajkd.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka M, Tsujimoto Y, Goto K, Kumahara K, Onishi S, Iwanari S, Fumihara D, Miki S, Ikeda M, Sato K, Sato H, Hirose M, Takeoka H. Preeclampsia before 20 weeks of gestation: a case report and review of the literature. CEN Case Rep. 2015;4:55–60. doi: 10.1007/s13730-014-0140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Ghaziani TT, Wolf JL. Acute fatty liver disease of pregnancy: updates in pathogenesis, diagnosis, and management. Am J Gastroenterol. 2017;112:838–846. doi: 10.1038/ajg.2017.54. [DOI] [PubMed] [Google Scholar]
- 8.Paller MS. Hypertension in pregnancy. J Am Soc Nehorol. 1998;9:314–321. doi: 10.1681/ASN.V92314. [DOI] [PubMed] [Google Scholar]
- 9.Pignatelli P, Ettorre E, Menichelli D, Pani A, Violi F, Pastori D. Seronegative antiphospholipid syndrome:refining the value of “non-criteria” antibodies for diagnosis and clinical management. Haematologica. 2020;105:562–572. doi: 10.3324/haematol.2019.221945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fakhouri F, Roumenina L, Provot F, Sallée M, Caillard S, Couzi L, Essig M, Ribes D, Dragon-Durey MA, Bridoux F, Rondeau E, Frémeaux-Bacchi V. Pregnancy-associated hemolytic uremic syndrome revisited in the era of complement gene mutations. J Am Soc Nephrol. 2010;21:859–867. doi: 10.1681/ASN.2009070706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abisror N, Nguyen Y, Marozio L, Valverde EE, Udry S, Pleguezuelo DE, Billoria P, Mayer PK, Urbanski G, Zigon P, Moreuil CD, Hoxha A, Bezanahary H, Carbillion L, Kayem G, Bornes M, Yelnik C, Johanet C, Nicaise RP, Lambert M, Salle V, Latino OJ, Hachulla E, Benedetto C, Bourrienne MC, Benhamou Y, Alijotas RJ, Fain O, Mekinian A. Obstetrical outcome, and treatments in seronegative primary APS: data from European retrospective study. RMD Open. 2020;6:e001340. doi: 10.1136/rmdopen-2020-001340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oku K, Atsumi T, Bohgaki M, Amengual O, Kataoka H, Horita T, Yasuda S, Koike T. Complement activation in patients with primary antiphospholipid syndrome. Ann Rheum Dis. 2009;68:1030–1035. doi: 10.1136/ard.2008.090670. [DOI] [PubMed] [Google Scholar]
- 13.Nochy D, Daugas E, Droz D, Beaufils H, Grunfeld JP, Piette JC, Bariety J, Hill G. The intrarenal vascular lesions associated with primary antiphospholipid syndrome. J Am Soc Nephrol. 1999;10:507–518. doi: 10.1681/ASN.V103507. [DOI] [PubMed] [Google Scholar]
- 14.Mekinian A, Bourrienne MC, Carbillon L, Benbara A, Noemie A, Chollet MS, Tigaizin A, Montestruc F, Fain O, Nicaise RP. Non-conventional antiphospholipid antibodies in patients with clinical obstetrical APS: Precvalence and treatment efficacy in pregnancies. Semin Arthritis Rheum. 2016;46:232–237. doi: 10.1016/j.semarthrit.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Japan Society for the Study of HYPERTENSION IN PREGNANCY. Best Practice Guide 2015 for Care and Treatment of Hypertension in Pregnancy. 1st ed. Medical View; 2015
- 16.Rey E, Couturier A. The prognosis of pregnancy in women with chronic hypertension. Am J Obstet Gynecol. 1994;171:410–416. doi: 10.1016/0002-9378(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 17.Qin J, Liu X, Sheng X, Wang H, Gao D. Assisted reproductive technology and the risk of pregnancy-related complications and adverse pregnancy-related complications and adverse pregnancy outcomes in singleton pregnancies: a meta-analysis of cohort studies. Fertil Steril. 2016;105:73–85. doi: 10.1016/j.fertnstert.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Sites CK, Wilson D, Barsky M, Bernson D, Bernstein IM, Boulet S, Zhang Y. Embryo cryopreservation and preeclampsia risk. Fertil Steril. 2017;108:784–790. doi: 10.1016/j.fertnstert.2017.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennström M, Olovsson M, Brennecke SP, Stepan H, Allegranza D, Dilba P, Schoedl M, Hund M, Verlohren S. Predictive Value of the sFlt-1: PlGF Ratio in Women with Suspected Preeclampsia. N Engl J Med. 2016;374:13–22. doi: 10.1056/NEJMoa1414838. [DOI] [PubMed] [Google Scholar]
- 20.Thadhani R, Hagmann H, Schaarschmidt W, Roth B, Cingoez T, Karumanchi SA, Wenger J, Lucchesi KJ, Tamez H, Lindner T, Fridman A, Thome U, Kribs A, Danner M, Hamacher S, Mallmann P, Stepan H, Benzing T. Removal of soluble fms-like tyrosine kinase-1 by dextran sulfate apheresis in preeclampsia. J Am Soc Nephrol. 2016;27:903–913. doi: 10.1681/ASN.2015020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakashima A, Yamanaka-Tatematsu M, Fujita N, Koizumi K, Shima T, Yoshida T, Nikaido T, Okamoto A, Yoshimori T, Saito S. Impaired autophagy by soluble endoglin, under physiological hypoxia in early pregnant period, is involved in poor placentation in preeclampsia. Autophagy. 2013;9:303–316. doi: 10.4161/auto.22927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seki H. Balance of antiangiogenic and angiogenic factors in the context of the etiology of preeclampsia. Acta Obstet Gynecol Scand. 2014;93:959–964. doi: 10.1111/aogs.12473. [DOI] [PubMed] [Google Scholar]
- 23.Sani HM, Vahed SZ, Ardalan M. Preeclampsia: a close look at renal dysfunction. Biomed Pharmacother. 2019;109:408–416. doi: 10.1016/j.biopha.2018.10.082. [DOI] [PubMed] [Google Scholar]
- 24.Levine RJ, Maynard SE, Qian C, Lim K, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 25.Saito S, Sakai M, Sasaki Y, Nakashima A, Shiozaki A. Inadequate tolerance induction may induce preeclampsia. J Reprod Immunol. 2007;76:30–39. doi: 10.1016/j.jri.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki Y, Kolarz DD, Suzuki D, Sakai M, Ito M, Shima T, Shiozaki A, Rolinski J, Saito S. Proportion of peripheral blood and decidual CD4+ CD25bright regulatory T cells in pre-eclampsia. Clin Exp Immunol. 2007;149:139–145. doi: 10.1111/j.1365-2249.2007.03397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jennette JC, Olson JL, Schwartz MM, Silva FG. Heptinstall’s pathology of the kidney: hypertensive disorders of pregnancy. Netherlands: Wolters Kluwer; 2007. pp. 773–785. [Google Scholar]
- 28.Zhang J, Troendle JF, Levine RJ. Risks of hypertensive disorders in the second pregnancy. Paediatr Perinat Epidemiol. 2001;15:226–231. doi: 10.1046/j.1365-3016.2001.00347.x. [DOI] [PubMed] [Google Scholar]