Introduction
The opioid crisis continues to be a major public health concern in the United States (U.S.). Globally, 58 million people used opioids in 2018 accounting for the majority of disability-adjusted life years lost [41]. Compared to other regions in the world, pharmaceutical opioids are drivers of the opioid epidemic in North America [41]. In the same year in the U.S., 9.9 million people, representing 3.6% of the population, reported opioid misuse in the past year [40].
Addressing the opioid epidemic in the U.S. has focused heavily on reducing opioid prescribing with a notable effect: the national opioid prescribing rate was 51.4 prescriptions per 100 persons in 2018, the lowest in 13 years [5]. Even so, drug overdoses accounted for 67,367 overdose deaths in 2018 with 69.5% of these deaths involving opioids (n=46,802) [38]. Opioid-related adverse events are on the rise among patients without documented opioid prescriptions [25] along with increased use of illicit opioids like heroin or fentanyl [8].
Following publication of Centers for Disease Control (CDC) Prescribing Opioids for Chronic Pain in 2016 [13], some have raised concern about unintended consequences of aggressive or involuntary tapering of patients on chronic high-dose opioid therapy [24]. CDC Guideline calls for a collaborative approach between clinicians and patients to determine appropriate tapering plans. However, some may integrate elements of the CDC Guideline and reduce opioid prescribing in ways that are not sensitive to patient needs or preferences. In fact, some healthcare professionals have identified multilevel barriers to deprescribing (i.e. dose reduction and discontinuation) [26] while others have suggested opioid “stewardship” toolkits to reduce opioid harms especially in high prescribing settings (e.g. emergency departments) [33].
Few studies have examined unintended effects of abrupt discontinuation on patient outcomes. Patients on chronic opioid therapy in one primary care clinic who discontinued opioid prescriptions had nearly a three-fold increase in overdose risk [17]. Elevated overdose mortality risk remained high for 3 years following discontinuation of prescription opioids in a Massachusetts cohort [27]. Mark et al. found that many long-term opioid users are discontinued quickly and without prior dose reduction, a pattern that is associated with increased risk of adverse events [29]. This finding focused on the relationship between timing of discontinuation and the risk of an adverse opioid-related event among patients on chronic opioid therapy but did not examine the interaction between dose reduction and discontinuation, and did not assess opioid-related deaths or suicide as outcomes.
The aim of this study was to evaluate the association between dose reduction and risk of suicide, opioid overdose, and other opioid-related adverse events among patients with high-dose chronic opioid therapy.
Methods
Data sources
We assembled a dataset of Oregon Medicaid beneficiaries’ claims linked with Prescription Drug Monitoring Program (PDMP) pharmacy data and death certificate data from 2014 to 2017. The linked dataset included Medicaid beneficiaries with any eligibility in the four-year period and at least one opioid prescription or an opioid-related diagnosis (e.g. poisoning, dependence, adverse event). Analysts in the Oregon Public Health Division linked and de-identified the datasets [19]. All activities were approved by the Oregon Health and Science University and Oregon Public Health Division Institutional Review Boards.
Cohort Development and Exposures
Within this linked dataset we first identified patients with chronic opioid therapy (COT) [18]. We identified opioids in PDMP data using the First Databank national drug code (NDC) file’s opioid therapeutic class [32]. COT was defined as 84 or more consecutive days with an opioid available (excluding buprenorphine). We used the prescription fill date and days’ supply variable to estimate opioid availability for each day of the study period, then estimated each patient’s average daily morphine milligram equivalent dose (MME) on every day of their consecutive use period using drug strength, MME conversion factor [37], fill dates, and days’ supply. Next, we applied a 28-day rolling average to smooth out day-to-day variation in MME due to overlapping medication fills. This calculation rolled forward, so for each day we estimated the average daily dose of the 28 preceding days. Patients with at least 84 consecutive days (3 months) of opioid availability with an average daily MME of 50 or greater on each of those days were retained. We selected each patient’s first episode of high-dose COT.
We retained only patients whose COT episode ended in 2014 or 2015 to allow sufficient follow-up. We considered the first day that the average daily dose dropped below 50 MME following at least 84 consecutive days as the end of COT and the index date with which we tracked subsequent dose changes. While dose may vary over time, we only attempted to identify dose trajectories after the average daily dose dropped below 50 MME.
Following this index date, we categorized patients into four mutually exclusive groups (Figure 1). We first identified patients who discontinued opioid prescriptions in the year following high-dose COT, defined as 56 consecutive days without any opioid availability. Discontinuations were identified using the exact daily dosage because any immediate and prolonged stoppage would appear gradual using a rolling average approach. Among this group, we further categorized patients as having either an abrupt discontinuation or a dose reduction prior to discontinuation. The dose change calculation compared two 28-day average MME values: the day immediately preceding the exact discontinuation date and day 29 days prior. Abrupt discontinuation was defined as a dose increase, stable dose, or reduction <50% in the four weeks before discontinuation. A dose reduction prior to discontinuation was defined as a reduction in average daily MME of ≥50% in the four weeks preceding discontinuation.
Figure 1:
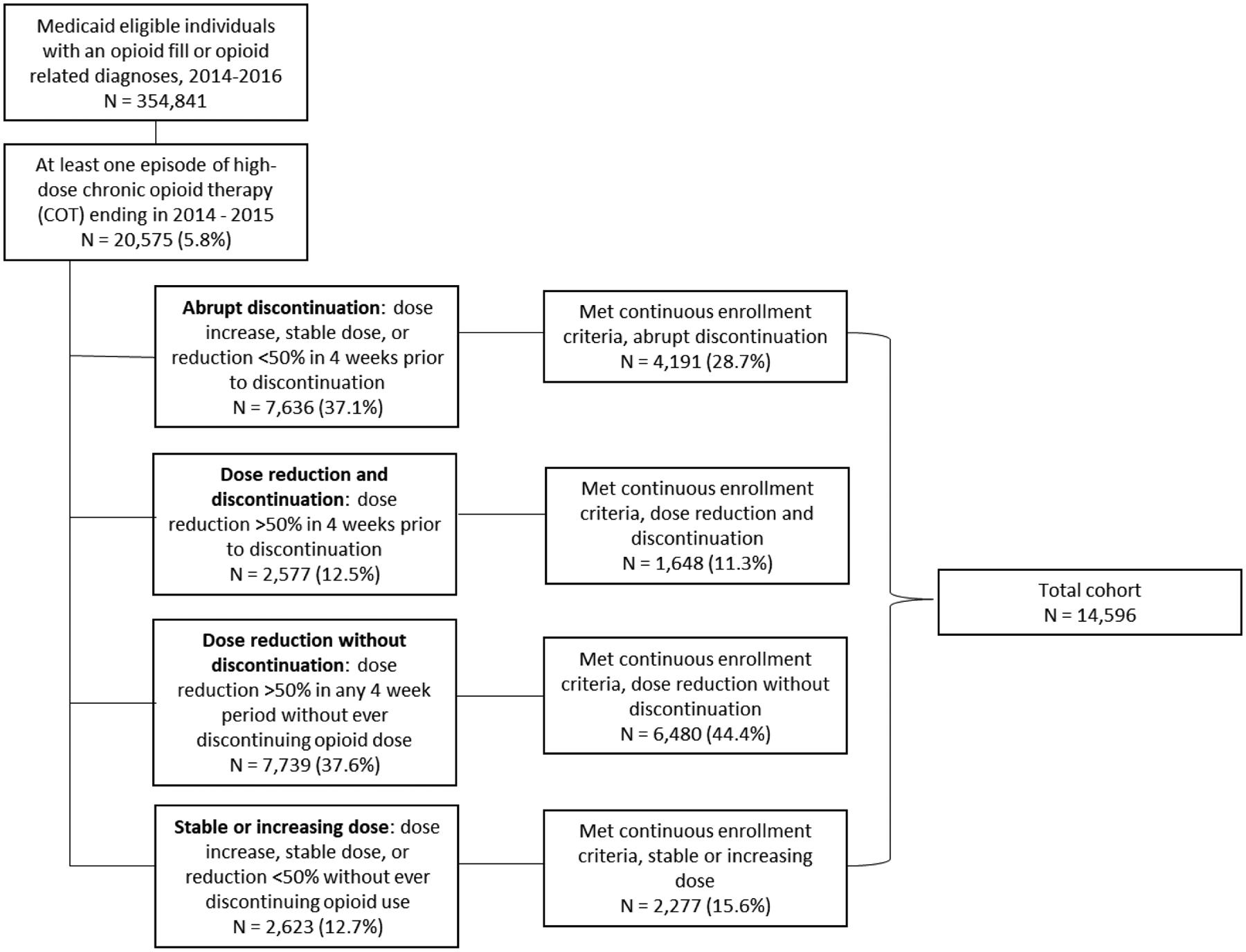
Cohort development of Medicaid patients with abrupt discontinuation, dose reduction and discontinuation, dose reduction without discontinuation, or stable or increasing dose after the end of high-dose chronic opioid therapy
Next, we identified patients who did not discontinue opioid prescriptions in the year after the end of their COT episode. These patients may have had days without opioids totaling less than 56 consecutive days. We categorized COT patients who did not discontinue opioid prescriptions as either having a dose reduction without discontinuation or a stable or increasing dose in the year after the end of their COT episode. A dose reduction without discontinuation was defined as a ≥50% reduction in average daily MME in any 4-week period after the end of the COT period, but without ever discontinuing opioid prescriptions for 56 or more consecutive days. A stable or increasing dose without discontinuation was defined as a dose increase, stable dose, or reduction <50% in the year after the end of the COT episode without ever discontinuing opioid prescriptions for 56 or more consecutive days. Deceased patients with a discontinuation date on or after their date of death were placed in this last group.
Outcomes
Outcomes were assessed in a 12-month period starting 28 days prior to discontinuation for those who discontinued (abrupt discontinuation or dose reduction prior to discontinuation) and following the start of the dose reduction for those with a dose reduction who did not discontinue (Figure 2). For patients with a stable or increasing dose, the 12-month follow-up started at end of their COT episode (i.e. the first day they fell below 50 MME per day). We measured outcomes using death certificate and Medicaid encounter data. To ensure complete capture of non-fatal suicide, overdose, and other related adverse events from claims data, patients were required to have continuous Medicaid enrollment throughout their follow-up period. Patients who died during the follow-up were included until their date of death.
Figure 2:
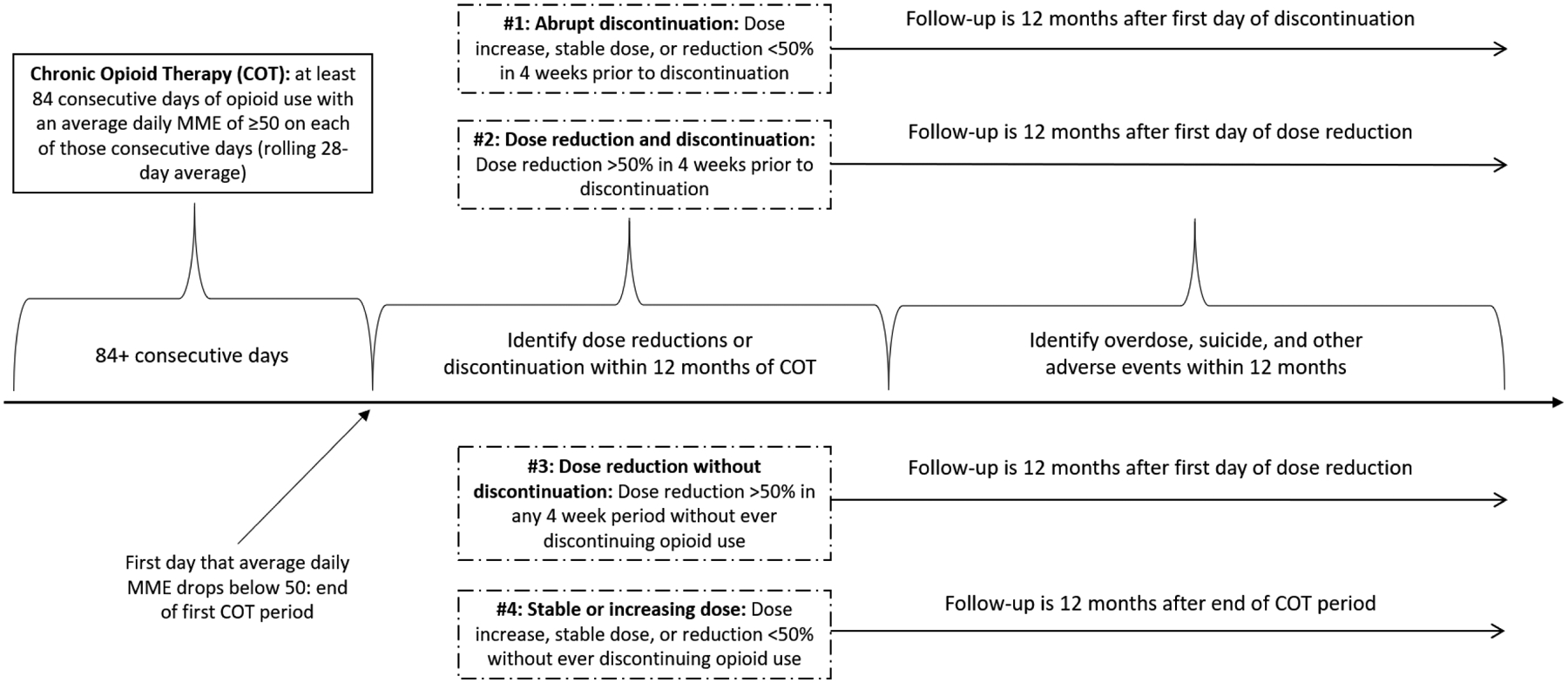
Timeline, from Chronic Opioid Therapy to Dose Trajectory Group to Follow-up Period
We characterized the occurrence of three potential opioid-related events in the follow up period: suicide, opioid overdose, and other opioid-related adverse events. We identified both fatal [11] and non-fatal [21] suicides and opioid overdose events using ICD9 and ICD10 diagnostic codes. Other opioid-related adverse events included a diagnosis indicative of adverse effects, opioid abuse, opioid dependence, and opioid use, unspecified as identified by ICD9 and ICD10 diagnostic codes in any setting (Table A, Appendix A) [22].
Because opioid discontinuation can be implemented for the purpose of clinically indicated buprenorphine induction, we also identified patients who filled at least one buprenorphine prescription during the 12-month follow-up period.
Analyses and Covariable Adjustment
We tabulated the number of patients with each event type according to dose change group and used chi-square tests to identify statistically significant differences. We used Cox proportional hazard models to evaluate the differences in time-to-event risk of any opioid-related event (fatal or non-fatal suicide, fatal or non-fatal overdose, other adverse events) between the dose change groups. The proportional hazards assumption was tested and met based on the graphed Schoenfeld residuals for predictors and covariables. We adjusted tied data using the Efron approximation. We also modeled the odds of filling a buprenorphine prescription after COT using logistic regression. All models were adjusted patient demographics, baseline COT characteristics, and comorbidities.
Demographics included age, race/ethnicity, and rural/urban status. Patient zip code was used to define rural or urban residence according to the Oregon Office of Rural Health [20]. We characterized baseline COT for patients filling opioid and benzodiazepine prescriptions during their COT episode. Specifically, we computed the average MME per day during COT, identified those with concurrent benzodiazepine use, and having multiple providers (4 or more prescribers or 4 or more pharmacies in any six-month period during the COT episode). These variables have been associated with increased risk of poorer opioid related outcomes [2; 12; 36].
We used diagnostic codes appearing in Medicaid claims in the 3 months prior to the end of the index COT episode to characterize any drug abuse, depression, alcohol abuse [14] or chronic pain [30] comorbidities (Table B, Appendix A).
P values were considered significant at p<0.05. Data management and analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, North Carolina).
Results
The linked dataset contained 354,841 patients with Medicaid eligibility and an opioid prescription or opioid-related diagnoses between 2014 and 2017. Of these, 20,575 (5.8%) patients had at least one episode of high-dose COT that ended in 2014 or 2015 (Figure 1).
Of the 14,596 high-dose COT patients that met continuous enrollment criteria, 4,191 (28.7%) abruptly discontinued opioid prescriptions in the year after high-dose COT, 1,648 (11.3%) reduced opioid dose prior to discontinuing, 6,480 (44.4%) had a dose reduction but never discontinued, and 2,277 (15.6%) had a stable or increasing dose in the year following the COT episode (Figure 1).
Patients with an abrupt discontinuation had the highest daily average MME during the COT episode (mean 146.08, SD 735.53) while patients with a stable or increasing dose had the lowest average MME (mean 103.46, SD 76.49) (Table 1). Patients who discontinued opioid prescriptions (abruptly or with a preceding dose reduction) were younger and less likely to be female than patients who did not discontinue opioid prescriptions (with or without a dose reduction). Patients with a dose reduction and discontinuation were more likely to have a multiple prescriber or pharmacy episode during COT or diagnoses of chronic pain, depression, alcohol abuse, or any drug abuse in the 3-months prior to the end of the COT than patients in other groups (Table 1).
Table 1:
Patient characteristics according to opioid dose trajectory in 12 months after the end of high-dose chronic opioid therapy (COT)
| Total | Abrupt discontinuation | Dose reduction and discontinuation | Dose reduction without discontinuation | Stable or increasing dose | |
|---|---|---|---|---|---|
| N (%) | 14,596 | 4,191 (28.7%) | 1,648 (11.3%) | 6,480 (44.4%) | 2,277 (15.6%) |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Average MME during chronic opioid therapy | 129.85 (403.29) | 146.08 (735.53) | 142.13 (114.96) | 125.51 (103.08) | 103.46 (76.49) |
| N (%) | N (%) | N (%) | N (%) | ||
| Top 5 drug types in COT (n, % of prescriptions) | 259,678 (100.0%) | 72,590 (28.0%) | 33,920 (13.1%) | 113,347 (43.6%) | 39,821 (15.3%) |
| Oxycodone HCL | 85,714 (33.0%) | 25,809 (35.5%) | 13,791 (40.7%) | 34,896 (30.8%) | 11,218 (28.2) |
| Hydrocodone / Acetaminophen | 47,039 (18.1%) | 11,092 (15.3%) | 4,299 (12.7%) | 22,328 (19.7%) | 9,320 (23.4%) |
| Morphine Sulfate | 41,705 (16.1%) | 11,602 (16.0%) | 5,032 (14.8%) | 18,761 (16.6%) | 6,310 (15.9%) |
| Methadone HCL | 26,860 (10.3%) | 8,230 (11.3%) | 3,417 (10.1%) | 11,542 (10.2%) | 3,671 (9.2%) |
| Oxycodone HCL / Acetaminophen | 26,213 (10.1%) | 6,598 (9.1%) | 3,128 (9.2%) | 11,671 (10.3%) | 4,816 (12.1%) |
| N (%) | N (%) | N (%) | N (%) | ||
| Benzodiazepine filled | 6,447 (44.2%) | 1,870 (44.6%) | 764 (46.4%) | 2,856 (44.1%) | 957 (42.0%) |
| Multiple prescriber episode during chronic opioid therapy | 3,000 (20.6%) | 889 (21.2%) | 542 (32.9%) | 1,166 (18.0%) | 403 (17.7%) |
| Multiple pharmacy episode during chronic opioid therapy | 1,433 (9.8%) | 463 (11.1%) | 272 (16.5%) | 556 (8.6%) | 142 (6.2%) |
| N (%) | N (%) | N (%) | N (%) | ||
| Female | 8,766 (60.0%) | 2,372 (56.6%) | 917 (55.6%) | 4,081 (63.0%) | 1,396 (61.3%) |
| Rural | 7,113 (48.7%) | 2,038 (48.6%) | 804 (48.8%) | 3,213 (49.6%) | 1,058 (46.5%) |
| Age | |||||
| 0–29 | 459 (3.1%) | 179 (4.3%) | 89 (5.4%) | 140 (2.2%) | 51 (2.2%) |
| 30–39 | 1,828 (12.5%) | 619 (14.8%) | 306 (18.6%) | 676 (10.4%) | 227 (10.0%) |
| 40–49 | 2,803 (19.2%) | 895 (21.4%) | 371 (22.5%) | 1,154 (17.8%) | 383 (16.8%) |
| 50–59 | 4,865 (33.3%) | 1,308 (31.2%) | 533 (32.3%) | 2,224 (34.3%) | 800 (35.1%) |
| 60+ | 4,641 (31.8%) | 1,190 (28.4%) | 349 (21.2%) | 2,286 (35.3%) | 816 (35.8%) |
| Race | |||||
| White | 11,285 (77.3%) | 3,242 (77.4%) | 1,215 (73.7%) | 5,089 (78.5%) | 1,739 (76.4%) |
| Black | 441 (3.0%) | 90 (2.1% | 39 (2.4%) | 207 (3.2%) | 105 (4.6%) |
| Other* | 693 (4.7%) | 180 (4.3%) | 98 (6.0%) | 304 (4.7%) | 111 (4.9%) |
| Unknown* | 2,177 (14.9%) | 679 (16.2%) | 296 (18.0%) | 880 (13.6%) | 322 (14.1%) |
| Comorbidities | |||||
| Alcohol abuse | 205 (1.4%) | 76 (1.8%) | 45 (2.7%) | 69 (1.1%) | 15 (0.7%) |
| Depression | 994 (6.8%) | 290 (6.9%) | 151 (9.2%) | 417 (6.4%) | 136 (6.0%) |
| Drug abuse | 774 (5.3%) | 301 (7.2%) | 160 (9.7%) | 229 (3.5%) | 84 (3.7%) |
| Chronic pain | 6,119 (41.9%) | 1,792 (41.8%) | 887 (53.8%) | 2,483 (38.3%) | 957 (42.0%) |
HCL: hydrochloride
Unknown race includes missing, refused, and unknown. Other race includes all other racial categories.
Overall, 625 (4.3%) patients had an opioid-related event (Table 2). Patients who discontinued opioid prescriptions (with or without a dose reduction) were more likely to have a suicide event (1.0% and 1.4% vs. 0.3% and 0.2%) or experience some other opioid-related harm (3.8% and 4.6% vs. 2.7% and 1.9%) than those who did not discontinue (p<0.0001). Patients with a stable or increasing dose were most likely to experience an opioid overdose (1.7%, p=0.0002) (Table 2).
Table 2:
Number and percent of people with a fatal or non-fatal suicide event, fatal or non-fatal opioid overdose, opioid-related adverse event, or buprenorphine fill in 12 months after discontinuation or dose reduction according to opioid dose trajectory*
| Total | Abrupt discontinuation | Dose reduction and discontinuation | Dose reduction without discontinuation | Stable or increasing dose | p-value | |
|---|---|---|---|---|---|---|
| N (%) | 14,596 | 4,191 (28.7%) | 1,648 (11.3%) | 6,480 (44.4%) | 2,277 (15.6%) | |
| Median days between end of COT and start of follow-up | 8 | 29 | 23 | 30 | 0 | |
| Any opioid-related event** | 625 (4.3%) | 222 (5.3%) | 101 (6.1%) | 220 (3.4%) | 82 (3.6%) | <0.0001 |
| Suicide | 88 (0.6%) | 40 (1.0%) | 23 (1.4%) | 20 (0.3%) | 5 (0.2%) | <0.0001 |
| Fatal | 31 (35.2%) | 23 (57.5%) | 3 (13.0%) | 5 (25.0%) | 0 (0.0%) | <0.0001 |
| Non-fatal | 57 (64.8%) | 17 (42.5%) | 20 (87.0%) | 15 (75.0%) | 5 (100.0%) | |
| Opioid Overdose | 156 (1.1%) | 54 (1.3%) | 15 (0.9%) | 47 (0.7%) | 40 (1.7%) | 0.0002 |
| Fatal | 42 (26.9%) | 11 (20.4%) | 2 (13.3%) | 2 (4.3%) | 27 (67.5%) | <0.0001 |
| Non-fatal | 114 (73.1%) | 43 (79.6%) | 13 (86.7%) | 45 (95.7%) | 13 (32.5%) | |
| Heroin | 14 (9.0%) | 8 (14.8%) | 1 (6.7%) | 2 (4.3%) | 3 (7.5%) | 0.0622 |
| Rx Opioid | 142 (91.0%) | 46 (85.2%) | 14 (93.3%) | 45 (95.7%) | 37 (92.5%) | |
| Other Opioid-related Harms | 462 (3.2%) | 160 (3.8%) | 76 (4.6%) | 182 (2.7%) | 44 (1.9%) | <0.0001 |
| Adverse Effects | 32 (6.9%) | 9 (5.6%) | 3 (3.9%) | 16 (8.8%) | 4 (9.1%) | 0.9117 |
| Opioid Abuse | 60 (13.0%) | 33 (20.6%) | 11 (14.5%) | 12 (6.6%) | 4 (9.1%) | <0.0001 |
| Opioid Dependence | 361 (78.1%) | 177 (73.1%) | 12 (6.6%) | 149 (81.9%) | 36 (81.8%) | <0.0001 |
| Opioid Use, Unspecified | 9 (1.9%) | 1 (0.6%) | 4 (9.1%) | 5 (2.8%) | 0 (0.0%) | 0.0173 |
| Buprenorphine Filled | 327 (2.2%) | 192 (4.6%) | 83 (5.0%) | 46 (0.7%) | 6 (0.3%) | <0.0001 |
Some patients had more than one outcome event, so are counted once in each event type category. For example, an individual with a non-fatal overdose and an opioid abuse indicator would be counted twice, once in each category. Where a patient experienced multiple events of the same type (e.g. multiple non-fatal Rx Opioid overdoses) they are counted once; if multiple events within a category differed, the more severe event was counted (e.g. an individual with a prescription overdose and heroin overdose would be counted once in the heroin category).
Any opioid-related event flags whether a patient had any of the events regardless of the count.
Among patients with an opioid overdose, those who abruptly discontinued opioid prescriptions were more likely than patients in all other groups to overdose on heroin vs prescription opioids (n=8, 14.8%, p=0.0622) while patients with a stable or increasing dose were more likely to experience a fatal overdose (n=27, 67.5%, p<0.0001). Among patients with a suicide event, those with an abrupt discontinuation were more likely to experience a fatal event (n=23, 57.5%, p<0.0001) than patients in other dose change groups (Table 2).
Patients with an abrupt discontinuation (n=192, 4.6%) or dose reduction prior to discontinuation (n=83, 5.0%) were more likely to have filled at least one buprenorphine prescription during the follow-up period than patients in other dose change groups (p<0.0001) (Table 2).
Discontinuation significantly increased risk of suicide compared to those with stable or increasing dose (abrupt, adjusted hazard ratio (aHR) 3.63; 95% CI 1.42–9.25 or with dose reduction, aHR 4.47, 95% CI 1.68–11.88), while discontinuation or dose reduction reduced the risk of overdose compared to those with a stable or increasing dose (aHR 0.36 – 0.62, 95% CI 0.20 – 0.94) (Table 3).
Table 3:
Adjusted hazard ratios of opioid related events and adjusted odds ratios for buprenorphine fills in 12 months after discontinuation or dose reduction, according to opioid dose trajectory
| Risk of any event | Risk of suicide | Risk of overdose | Risk of Adverse events | Buprenorphine Filled | |
|---|---|---|---|---|---|
| aHR (95% CI)* | aHR (95% CI)* | aHR (95% CI)* | aHR (95% CI)* | aOR (95% CI)* | |
| Stable or increasing dose | Reference | Reference | Reference | Reference | Reference |
| Abrupt discontinuation | 1.22 (0.94–1.58) | 3.63 (1.42–9.25) | 0.62 (0.40–0.94) | 1.61 (1.15–2.26) | 15.07 (7.28–38.38) |
| Dose reduction and discontinuation | 1.13 (0.84–1.53) | 4.47 (1.68–11.88) | 0.36 (0.20–0.66) | 1.54 (1.05–2.25) | 14.83 (7.00–38.33) |
| Dose reduction without discontinuation | 0.94 (0.73–1.21) | 1.29 (0.48–3.45) | 0.41 (0.27–0.62) | 1.45 (1.04–2.02) | 2.47 (1.14–6.46) |
aHR: adjusted hazard ratio; OR: odds ratio, CI: confidence interval
Adjusted for age, gender, race, rurality, comorbidities, MME dose, filled benzodiazepines, chronic pain
Those with an abrupt discontinuation (aOR 15.07, 95% CI 7.28–38.38), dose reduction and discontinuation (aOR 14.83, 95% CI 7.00–38.33), or dose reduction without discontinuation (aOR 2.47, 95% CI 1.14–6.46) all had higher odds of filling a buprenorphine prescription relative to those with a stable or increasing dose (Table 3). Full models with adjusted covariates and survival curves can be found in Table 1, Appendix B.
Discussion
In this cohort of Medicaid beneficiaries with an episode of chronic opioid therapy (COT), discontinuation of opioid prescriptions (abrupt or with dose reduction) increased the patient’s risk of suicide or opioid related adverse events in the following year. Patients with an abrupt discontinuation were more likely to overdose on heroin (vs. prescription opioids) or experience a fatal suicide than those in all other dose change groups. Patients on a stable or increasing dose were more likely to experience an overdose event. Patients who discontinued opioid prescriptions had higher odds of filling a buprenorphine prescription.
This study broadens our understanding of the potential risks of opioid discontinuation among individuals using high-dose COT. Similar to Mark et al [29], we find that individuals with an abrupt discontinuation face increased risk for some harms, but we also find increased risk of overdose among those with a stable or increasing dose. Our findings extend these observations to a cohort of individuals with COT at a lower average dose (50 versus 120 MME daily) and highlight specific risks.
There has recently been a shift in deaths from prescription opioids to heroin and synthetic opioid overdose deaths [34]. Prescription opioid use or misuse is common among individuals who initiate heroin [31]; one study suggests that restrictive policies on prescription opioids, sometimes leading to opioid discontinuation, may precipitate heroin use [25]. Moreover, the combination of prescription opioid use prior to injection drug use is associated with increased overdose risk [1]. Consistent with these observations, we find that heroin-related overdose was more common among individuals who abruptly discontinued opioid prescriptions. We also found that fatal overdose was more common among individuals with a stable or increasing dose, though individuals in this group were also more likely to overdose on prescription opioids than heroin.
Growing evidence suggests the use of sublingual buprenorphine may confer analgesic effects in patients with chronic non-cancer pain. In addition, opioid-dependent patients with chronic pain may benefit from reversal of opioid-induced hyperalgesia and improvement in opioid tolerance [6; 7; 10]. In this study, patients who discontinued opioid prescriptions or reduced their dose without ever discontinuing had higher odds of filling a buprenorphine prescription than those who had a stable or increasing dose, suggesting that some patients may be transitioned to buprenorphine. However, the degree to which people were switched to buprenorphine for opioid use disorder, chronic pain management, or both is unclear.
This study raises several important issues. Discontinuation after COT may increase risks of heroin overdose, suicide, or other adverse events, so discontinuation should be carefully considered in partnership with the patient. We found that opioid harms were most common among those with a dose reduction prior to discontinuation, so even when dose reduction is the best identified clinical course, clinical caution is merited [28]. Certain conditions may favor rapid tapering or discontinuation [3], yet in general, the CDC guideline recommends slow dose reductions over a longer time period for patients with COT [4]. We found that stable or increasing dose of opioids carries a notable risk of fatal overdose, and emphasize the importance of patient education and co-prescriptions for naloxone [9]. Lastly, after end of COT, patients had characteristics similar to patients with opioid use disorder (OUD) [15; 23; 39] as well as a high proportion of health services utilization for OUD (opioid misuse and opioid dependence) after opioid discontinuation or reduction [43]. Wei et al, found that 50% of patients on chronic opioid therapy developed OUD within a year [42]; therefore, tapering decisions should account for presence or risk of OUD in patients on COT to reduce any potential harms.
This study has several limitations. First, the observational design does not permit us to disentangle issues related to temporality and causation; we do not know why prescriptions were interrupted. Patients who were incarcerated or hospitalized may have erroneously appeared to discontinue use. The length of COT may be underestimated and we cannot ascertain any differences in the length of COT across the four cohorts because we were limited by the start of our study period [35].
The patient cohort is comprised entirely of Oregon Medicaid members, so may not represent national patterns [16]. We also could not determine clinical indication for COT, thus, we could not evaluate the appropriateness of any opioid use, nor could we determine the appropriateness of the identified dose reductions or discontinuations. As with all studies using claims data, we may underestimate risk if there was not a billed medical visit as a result of an overdose, suicide attempt, or other adverse event. To mitigate this limitation we applied a continuous enrollment criterion and used PDMP data instead of pharmacy claims to ensure that we captured all opioid prescriptions including those paid with cash, and we used vital statistics data to identify fatal events that may not have been identified in claims.
In summary, discontinuation of prescription opioid dose after a period of high-dose long-term use is associated with an increased risk of suicide or other opioid-related harms, while a stable or increasing opioid dose is associated with an increased risk of opioid overdose. Our study suggests that patients on COT require careful risk assessment and supportive interventions when considering opioid discontinuation or continuation at a high dose.
Supplementary Material
Acknowledgements
The authors wish to thank Kun Zhang at the CDC and Christi Hildebran at Comagine Health for their contributions of ongoing support, guidance, and insight. The authors also wish to thank Josh Van Otterloo and the Oregon Health Authority Injury and Violence Prevention Program for their technical help, partnership, and support. This work was made possible by funding from the Centers for Disease Control and Prevention, CDC U01CE002786. Dr. Korthuis time was further supported from grants from the National Institutes of Health, National Institute on Drug Abuse (UG3DA044831, UG1DA015815). Dr. Korthuis serves as principal investigator for NIH-funded grants that receive donated study medications from Indivior (buprenorphine) and Alkermes (extended-release naltrexone). The authors have no conflicts of interest to disclose.
References
- [1].Al-Tayyib AA, Koester S, & Riggs P (2017). Prescription opioids prior to injection drug use: Comparisons and public health implications. Addict Behav, 65, 224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Altekruse SF, Cosgrove CM, Altekruse WC, Jenkins RA, & Blanco C (2020). Socioeconomic risk factors for fatal opioid overdoses in the United States: Findings from the Mortality Disparities in American Communities Study (MDAC). PLoS One, 15(1), e0227966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Berna C, Kulich RJ, & Rathmell JP (2015). Tapering long-term opioid therapy in chronic noncancer pain: evidence and recommendations for everyday practice. Mayo Clin Proc, 90(6), 828–842. doi: 10.1016/j.mayocp.2015.04.003 [DOI] [PubMed] [Google Scholar]
- [4].Centers for Disease, C., & Prevention. (2015). Common elements in guidelines for prescribing opioids for chronic pain. Atlanta: US Department of Health and Human Services, CDC. [Google Scholar]
- [5].Centers for Disease Control and Prevention. (2020). U.S. Opioid Prescribing Rate Maps Retrieved May 9, 2019, from https://www.cdc.gov/drugoverdose/maps/rxrate-maps.html
- [6].Chen KY, Chen L, & Mao J (2014). Buprenorphine–naloxone therapy in pain management. Anesthesiology, 120(5), 1262–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chou R, Ballantyne J, & Lembke A (2019). Rethinking Opioid Dose Tapering, Prescription Opioid Dependence, and Indications for Buprenorphine. Ann Intern Med. doi: 10.7326/M19-1488 [DOI] [PubMed] [Google Scholar]
- [8].Cicero TJ, Ellis MS, & Kasper ZA (2017). Increased use of heroin as an initiating opioid of abuse. Addict Behav, 74, 63–66. doi: 10.1016/j.addbeh.2017.05.030 [DOI] [PubMed] [Google Scholar]
- [9].Coffin PO, Behar E, Rowe C, Santos G-M, Coffa D, Bald M, & Vittinghoff E (2016). Nonrandomized intervention study of naloxone coprescription for primary care patients receiving long-term opioid therapy for pain. Ann Intern Med, 165(4), 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cote J, M. L (2014). Sublingual Buprenorphine as an Analgesic in Chronic Pain: A Systematic Review. Pain Med, 15(7), 1171–1178. [DOI] [PubMed] [Google Scholar]
- [11].Curtin SC, Warner M, & Hedegaard H (2016). Increase in Suicide in the United States, 1999–2014 (Vol. No. 241): US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. [Google Scholar]
- [12].Dilokthornsakul P, Moore G, Campbell JD, Lodge R, Traugott C, Zerzan J, … Page RL 2nd. (2016). Risk Factors of Prescription Opioid Overdose Among Colorado Medicaid Beneficiaries. J Pain, 17(4), 436–443. doi: 10.1016/j.jpain.2015.12.006 [DOI] [PubMed] [Google Scholar]
- [13].Dowell D, Haegerich TM, & Chou R (2016). CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. JAMA, 315(15), 1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Elixhauser A, Steiner CA, Harris D, & Coffey R (1998). Comorbidity measures for use with administrative data. Med Care(36), 8–27. [DOI] [PubMed] [Google Scholar]
- [15].Esposito DB, Cepeda MS, Lyons JG, Yin R, & Lanes S (2019). Medical record-based ascertainment of behaviors suggestive of opioid misuse, diversion, abuse, and/or addiction among individuals showing evidence of doctor/pharmacy shopping. J Pain Res, 12, 2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fenton JJ, A. A, Xing G, Hang L, Altan AE, Tancredi DJ, Jerant A, Magnan E (2019). Trends and Rapidity of Dose Tapering Among Patients Prescribed Long-term Opioid Therapy, 2008–2017. JAMA Netw Open. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gerlach LB, S. J, Kim HM, Maust DT. (2019). Discontinuation of Chronic Benzodiazepine Use Among Adults in the United States. J Gen Intern Med, 34(9), 1833–1840. doi: 10.1007/s11606-019-05098-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hallvik SE, Johnston K, Geddes J, Leichtling G, Korthuis PT, & Hartung DM (2020). Identifying opioid dose reductions and discontinuation among patients with chronic opioid therapy. Pharmacoepidemiol Drug Saf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hartung D, Ahmed SM, Middleton L, Van Otterloo J, Zhang K, Keast S, Kim H, Johnston K, Deyo RA. (2017). Using prescription monitoring program data to characterize out-of-pocket payments for opioid prescriptions in a state Medicaid program. Pharmacoepidemiol Drug Saf, 26(9), 1053–1060. doi: 10.1002/pds.4254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Health, O. O. o. R. (2019). About Rural and Frontier Data. Retrieved June 30 2019, 2019, from https://www.ohsu.edu/oregon-office-of-rural-health/about-rural-and-frontier-data
- [21].Hedegaard H, Schoenbaum M, Claassen C, Crosby AE, Holland K, & Proescholdbell S (2018). Issues in Developing a Surveillance Case Definition for Nonfatal Suicide Attempt and Intentional Self-harm Using International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) Coded Data National Health Statistics Reports (Vol. 108): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. [PubMed] [Google Scholar]
- [22].Heslin KC, Owens PL, Karaca Z, Barrett ML, Moore BJ, & Elixhauser A (2017). Trends in opioid-related inpatient stays shifted after the US transitioned to ICD-10-CM diagnosis coding in 2015. Med Care, 55(11), 918–923. [DOI] [PubMed] [Google Scholar]
- [23].Koons AL, Greenberg MR, Cannon RD, & Beauchamp GA (2018). Women and the experience of pain and opioid use disorder: A literature-based commentary. Clinical Therapeutics, 40(2), 190–196. [DOI] [PubMed] [Google Scholar]
- [24].Kroenke K, Alford DP, Argoff C, Canlas B, Covington E, Frank JW, … Kertesz SG (2019). Challenges with Implementing the Centers for Disease Control and Prevention Opioid Guideline: A Consensus Panel Report. Pain Med. [DOI] [PubMed] [Google Scholar]
- [25].Kuo YF, Raji MA, Liaw V, Baillargeon J, & Goodwin JS (2018). Opioid prescriptions in older medicare beneficiaries after the 2014 federal rescheduling of hydrocodone products. J Am Geriatr Soc, 66(5), 945–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Langford AV, Gnjidic D, Lin C-WC, Bero L, Penm J, Blyth FM, & Schneider CR (2020). Challenges of opioid deprescribing and factors to be considered in the development of opioid deprescribing guidelines: a qualitative analysis. BMJ Qual Saf. [DOI] [PubMed] [Google Scholar]
- [27].Larochelle MR, Bertstein R, Bernson D, Land T, Stopka TJ, Rose AJ, … Walley AY (2019). Touchpoints - Opportunities to predict and prevent opioid overdose: A cohort study. Drug Alcohol Depend, 3(204). doi: 10.1016/j.drugalcdep.2019.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Maani CV, DeSocio PA, Jansen RK, Merrell JD, McGhee LL, Young A, … Serio-Melvin ML (2011). Use of ultra rapid opioid detoxification in the treatment of US military burn casualties. J Trauma Acute Care Surg, 71(1), S114–S119. [DOI] [PubMed] [Google Scholar]
- [29].Mark TL, & Parish W (2019). Opioid medication discontinuation and risk of adverse opioid-related health care events. J Subst Abuse Treat. doi: 10.1016/j.jsat.2019.05.001 [DOI] [PubMed] [Google Scholar]
- [30].Mayhew M, DeBar LL, Deyo RA, Kerns RD, Goulet JL, Brandt CA, & Von Korff M (2019). Development and Assessment of a Crosswalk Between ICD-9-CM and ICD-10-CM to Identify Patients with Common Pain Conditions. J Pain, 20(12), 1429–1445. doi: 10.1016/j.jpain.2019.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mojtabai R (2018). National trends in long‐term use of prescription opioids. Pharmacoepidemiol Drug Saf, 27(5), 526–534. [DOI] [PubMed] [Google Scholar]
- [32].National Center for Injury Prevention and Control. (2018, 2019). CDC compilation of benzodiazepines, muscle relaxants, stimulants, zolpidem, and opioid analgesics with oral morphine milligram equivalent conversion factors, 2018 version, Atlanta, GA: Centers for Disease Control and Prevention. Retrieved August 2020, from https://www.cdc.gov/drugoverdose/resources/data.html [Google Scholar]
- [33].Nelson LS, Mazer-Amirshahi M, & Perrone J (2020). Opioid Deprescribing in Emergency Medicine—A Tool in an Expanding Toolkit. JAMA Network Open, 3(3), e201129–e201129. [DOI] [PubMed] [Google Scholar]
- [34].NIHCM, T. N. I. f. H. C. M. (2019, 2019). The Evolution of the Opioid Crisis: 2000–2017. Retrieved August 29, 2019, from https://www.nihcm.org/categories/the-evolution-of-the-opioid-crisis-2000-2017
- [35].Oliva EM, Bowe T, Manhapra A, Kertesz S, Hah JM, Henderson P, … Gordon AJ (2020). Associations between stopping prescriptions for opioids, length of opioid treatment, and overdose or suicide deaths in US veterans: observational evaluation. BMJ, 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Park TW, Lin LA, Hosanagar A, Kogowski A, Paige K, & Bohnert AS (2016). Understanding risk factors for opioid overdose in clinical populations to inform treatment and policy. Journal of Addiction Medicine, 10(6), 369–381. [DOI] [PubMed] [Google Scholar]
- [37].Prevention, C. f. D. C. a. (2019). Data Resources: Analyzing Prescription Data and Morphine Milligram Equivalents (MME). from https://www.cdc.gov/drugoverdose/resources/data.html
- [38].Scholl L, Seth P, Kariisa M, Wilson N, & Baldwin G (2019). Drug and Opioid-Involved Overdose Deaths — United States, 2013–2017. MMWR Morb Mortal Wkly Rep, 67(51–52), 1419–1427. doi: 10.15585/mmwr.mm6751521e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sullivan MD (2018). Depression effects on long-term prescription opioid use, abuse, and addiction. Clin J Pain, 34(9), 878–884. [DOI] [PubMed] [Google Scholar]
- [40].The Substance Abuse and Mental Health Services Administration. (2019). Key Substance Use and Mental Health Indicators in the United States: Results from the 2018 National Survey on Drug Use and Health. from https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHNationalFindingsReport2018/NSDUHNationalFindingsReport2018.pdf
- [41].United Nations Office on Drug and Crime. (2020). World Drug Report 2020 from https://wdr.unodc.org/wdr2020/field/WDR20_BOOKLET_4.pdf
- [42].Wei YJ, Chen C, Fillingim R, Schmidt SO, & Winterstein AG (2019). Trends in prescription opioid use and dose trajectories before opioid use disorder or overdose in US adults from 2006 to 2016: A cross-sectional study. PLoS Medicine, 16(11), e1002941. doi: 10.1371/journal.pmed.1002941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Williams AR, Samples H, Crystal S, & Olfson M (2020). Acute care, prescription opioid use, and overdose following discontinuation of long-term buprenorphine treatment for opioid use disorder. American Journal of Psychiatry, 177(2), 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


