Abstract
We had a 72-year-old man with advanced gastric cancer, poorly differentiated adenocarcinoma, receiving chemotherapy with S-1 (tegafur, gimeracil, and oteracil potassium) plus oxaliplatin. Ascites developed despite remission of gastric cancer and metastasis. Given no malignant cells in ascites, leg edema, renal impairment, hypoalbuminemia, and massive proteinuria, we diagnosed as nephrotic syndrome with microscopic hematuria. Renal biopsy showed membranoproliferative glomerulonephritis with no deposition of immunoglobulins and complements. Of note, electronic microscopy found organized deposits with microtubular structures in the glomerular capillary lumens and subendothelial spaces. The liquid chromatography-tandem mass spectrometry method detected fibrinogen alpha chain, beta chain, gamma chain, and fibronectin, and we eventually diagnosed cryofibrinogen-associated glomerulonephritis. Cryofibrinogen was not detected in plasma. He was expired at 5 months following renal biopsy due to the progression of refractory nephrotic syndrome. In addition to the detailed assessment of specifically organized deposits, the analysis using liquid chromatography-tandem mass spectrometry method is useful to diagnose cryofibrinogen-associated glomerulonephritis. We should consider cryofibrinogen-associated glomerulonephritis as a differential diagnosis when the patients with malignancy showed abnormal urinalysis and renal impairment, though it is a rare disease.
Keywords: Nephrotic syndrome, Membranoproliferative glomerulonephritis, Organized deposit, Mass spectrometry, LC–MS/MS
Introduction
Cryofibrinogen is one of the cryoprotein, which precipitates in the plasma rested at a cold place for over 72 h and dissolves again rested at room temperature. Cryofibrinogen is not detected in the serum but is detected in the plasma, and it is distinguished from cryoglobulin [1]. Major symptoms of cryofibrinogenemia are purpura, Raynaud’s syndrome, livedo reticularis, skin ulcer, and skin necrosis, all of which are triggered by cold irritation. Thromboembolic events and end-organ dysfunction are fatal comorbidities [2].
There is a scarcity of studies reporting cryofibrinogen-associated glomerulonephritis, showing a sign of membranoproliferative glomerulonephritis (MPGN) in the optical microscope, negative immunoglobulins and complements in the immunofluorescence method, and unique tubular-structured organized deposits in the electronic microscope [3].
We experienced a patient who suffered nephrotic syndrome accompanying renal impairment and microscopic hematuria during chemotherapy for advanced gastric cancer, which was eventually diagnosed as cryofibrinogen-associated glomerulonephritis by renal biopsy and liquid chromatography-tandem mass spectrometry (LC–MS/MS) method.
Case report
Before admission
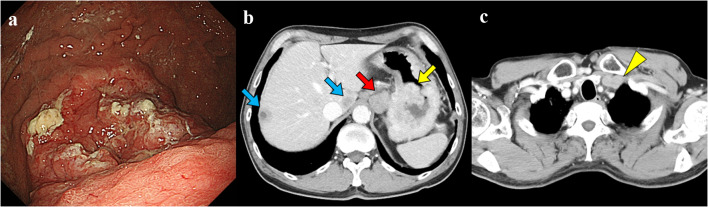
One year ago, a 72-year-old man was diagnosed with advanced gastric cancer, poorly differentiated adenocarcinoma, with multiple hepatic and abdominal lymph node metastasis (Fig. 1a,b) as well as deep venous thrombosis at the left subclavian vein (Fig. 1c), which was treated by anticoagulant therapy with 15 mg per day of the oral factor Xa inhibitor edoxaban. At the time of diagnosis of gastric cancer, serum albumin was 3.3 g/dL and serum creatinine was 0.91 mg/dL, and urinalysis showed proteinuria (dipstick test 2 +) and hematuria (dipstick test 1 +). He had a smoking history with 40 cigarettes per day for 30 years until the age of 53.
Fig. 1.
Imaging findings at the time of gastric cancer diagnosis. a Endoscopic findings of the stomach. A deeply ulcerated tumor with ulcer mounds on the upper gastric body. b Abdominal contrast-enhanced computed tomography findings. The yellow arrow indicates primary tumor of gastric cancer. The red arrow indicates abdominal lymph node metastasis. The blue arrows indicate liver metastasis. c Chest contrast-enhanced computed tomography findings. The yellow arrowhead indicates the thrombosis in his left subclavian and brachiocephalic vein
Seven cycles of chemotherapy using S-1 (tegafur, gimeracil, and oteracil potassium) plus oxaliplatin reduced the sizes of gastric cancer and metastasis. However, ascites developed 5 months later. Five cools of weekly paclitaxel monotherapy as a secondary therapy further improved the disease, whereas the ascites worsened. Peritoneal dissemination was denied by the cytology of ascites.
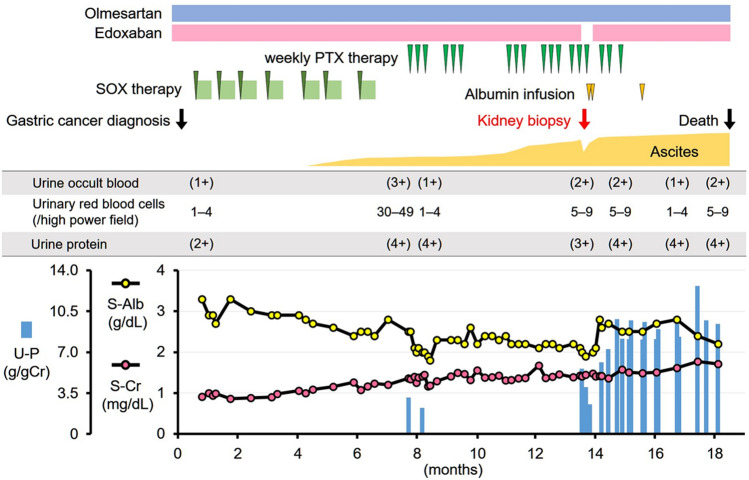
Given the worsening renal function (1.41 mg/dL of serum creatinine), hypoalbuminemia (2.0 g/dL of serum albumin), proteinuria (5.4 g/gCre), and bilateral leg edema, the nephrotic syndrome was suspected as a cause of worsening ascites. He was admitted to our institute to receive further investigation (Fig. 2).
Fig. 2.
Clinical course. SOX S-1 (tegafur, gimeracil, and oteracil potassium) plus oxaliplatin, PTX paclitaxel, U-P urine protein, S-Alb serum albumin, S-Cr serum creatinine
On admission
Body height was 157 cm and body weight was 67.3 kg. He had a weight gain of 10 kg in the last 6 months. Blood pressure was 164/88 mmHg. His abdomen was distended and had a fluid wave. Pitting edema was observed in bilateral legs. He had no skin lesion.
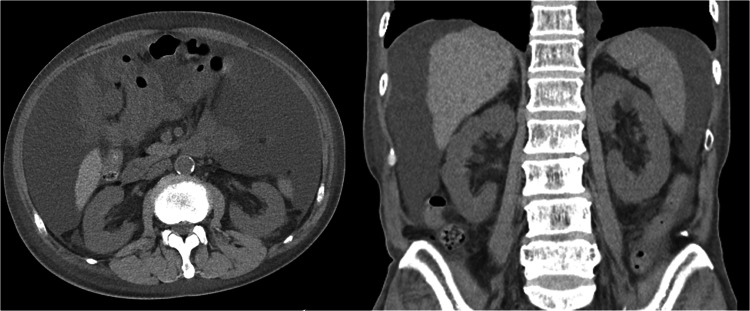
Laboratory data are shown in Table 1. Given the proteinuria, microscopic hematuria, hypoalbuminemia, and dyslipidemia, he was diagnosed with nephrotic syndrome. Computed tomography showed massive ascites without renal atrophy (Fig. 3). Deep venous thrombosis observed in the left subclavian vein disappeared following 6-month edoxaban therapy.
Table 1.
Laboratory data on kidney biopsy
| Laboratory test | Result | Reference ranges |
|---|---|---|
| Urinalysis | ||
| Urine-specific gravity | 1.014 | |
| Urine protein | (3 +) | |
| Urine occult blood | (2 +) | |
| Urine sedimentation | ||
| Red blood cells, (/high power field) | 5–9 | |
| White blood cells, (/high power field) | 1–4 | |
| Fatty casts, (/low power field) | 1 | |
| Urine chemistry and immunological test | ||
| Urine protein, g/g of creatinine | 5.4 | |
| Urine immunofixation electrophoresis | undetectable | |
| Complete blood cell counts | ||
| White blood cells, (/µL) | 4690 | 3300–8600 |
| Red blood cells,(× 104/µL) | 238 | 435–555 |
| Hemoglobin, (g/dL) | 7.3 | 13.7–16.8 |
| Platelets,(× 104/µL) | 21.5 | 15.8–34.8 |
| Serum chemistry | ||
| Total protein, (g/dL) | 4.3 | 6.7–8.3 |
| Albumin, (g/dL) | 2.0 | 4.1–5.1 |
| Aspartate aminotransferase, (IU/L) | 46 | 12–31 |
| Alanine aminotransferase, (IU/L) | 35 | 8–40 |
| Lactate dehydrogenase, (IU/L) | 269 | 110–210 |
| Blood urea nitrogen, (mg/dL) | 29 | 8–22 |
| Creatinine, (mg/dL) | 1.41 | 0.60–1.10 |
| Total cholesterol, (mg/dL) | 297 | 150–220 |
| Low density lipoprotein cholesterol, (mg/dL) | 180 | 65–163 |
| Triglyceride, (mg/dL) | 250 | 30–150 |
| Sodium, (mEq/L) | 141 | 138–146 |
| Potassium, (mEq/L) | 4.3 | 3.6–4.9 |
| Chloride, (mEq/L) | 112 | 99–109 |
| Calcium, (mg/dL) | 7.6 | 8.8–10.1 |
| Serum immunological test | ||
| Hemoglobin A1c, (%) | 5.5 | 4.6–6.2 |
| C-reactive protein, (mg/dL) | 0.21 | 0.00–0.14 |
| Immunoglobulin G, (mg/dL) | 402 | 861–1747 |
| Immunoglobulin A, (mg/dL) | 299 | 93–393 |
| Immunoglobulin M, (mg/dL) | 100 | 33–183 |
| Complement 3, (mg/dL) | 112.7 | 73.0–138.0 |
| Complement 4, (mg/dL) | 24.4 | 11.0–31.0 |
| 50% hemolytic complement activity, (U/mL) | 47 | 30–46 |
| Cryoglobulin | negative | |
| Serum immunofixation electrophoresis | undetectable | |
| Antinuclear antibody | negative | |
| Myeloperoxidase-ANCA | negative | |
| Proteinase 3-ANCA | negative | |
| Anti-glomerular basement membrane antibody | negative | |
| Anti-cardiolipin-β2-glycoprotein I complex antibody | negative | |
| Anti-cardiolipin immunoglobulin G, (U/mL) | negative | |
| Hepatitis B surface antigen | negative | |
| Hepatitis C virus antibody | negative | |
| Coagulation and fibrinolysis examination | ||
| Prothrombin time, INR | 0.90 | 0.85–1.15 |
| Activated partial thromboplastin time, sec | 29.3 | 26.5–41.8 |
| Fibrinogen, (mg/dL) | 324 | 200–400 |
| Fibrinogen/fibrin degradation products, (µg/mL) | 38.8 | < 5.0 |
| D-dimer, (µg/mL) | 13.1 | ≤ 1.0 |
| Tumor marker | ||
| Carcinoembryonic antigen, (ng/mL) | 3.2 | ≤ 3.4 |
| Carbohydrate antigen 19–9, (U/mL) | 23 | ≤ 37 |
| Carbohydrate antigen 125, (U/mL) | 885 | ≤ 35 |
ANCA anti-neutrophil cytoplasmatic antibody, INR international normalized ratio
Fig. 3.
Computed tomography findings at the time of kidney biopsy (horizontal view [left] and coronal view [right]). Bilateral kidneys were not atrophied. Massive ascites was observed
Renal biopsy
We performed a renal biopsy to further investigate the pathology of nephrotic syndrome and construct a therapeutic strategy.
A renal biopsy obtained 43 renal corpuscles under light microscopy. Although mild interstitial fibrosis and tubular atrophy were shown, the main lesion was the glomerular lesion (Fig. 4a). Of them, three glomeruli showed global sclerosis, and others had lobular accentuation of the glomerular capillary tufts with marked mesangial expansion (Fig. 4b). The capillary walls showed expansion of subendothelial spaces and double contours without spike formation (Fig. 4c). The infiltration of foam cells (CD68 positive macrophages) was found in glomerular capillaries (Fig. 4d). Any glomeruli had no crescent formations. The glomerular lesion was membranoproliferative pattern with nodular formation.
Fig. 4.
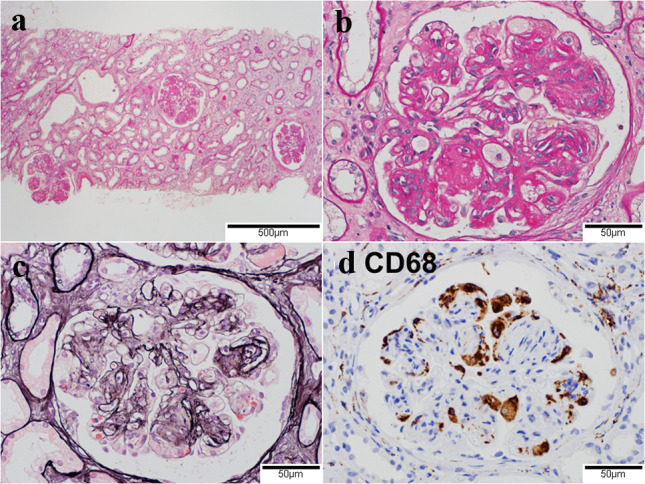
Light microscopic findings in the glomerulus. a Nodular lesions in glomeruli at low magnification. Mild infiltration of inflammatory cells in the renal interstitium and tubular atrophy. (Periodic acid-Schiff stain, original magnification ×40). b Lobular accentuation of the glomerular capillary tufts with marked mesangial expansion and capsular adhesion. Infiltration of foam cells in glomerular capillaries. (Periodic acid-Schiff stain, original magnification ×200). c Expansion of subendothelial spaces and double contours in the glomerular capillary walls. (Periodic acid-methenamin-silver stain, original magnification ×200). d CD68 positive infiltrating cells in the glomerular capillaries. (Immunoenzyme stain, original magnification ×200)
The immunofluorescence staining found no deposition of immunoglobulins (Fig. 5a, b) and complements (Fig. 5c). However, fibrinogen was positive in segmental glomerular capillaries (Fig. 5d).
Fig. 5.
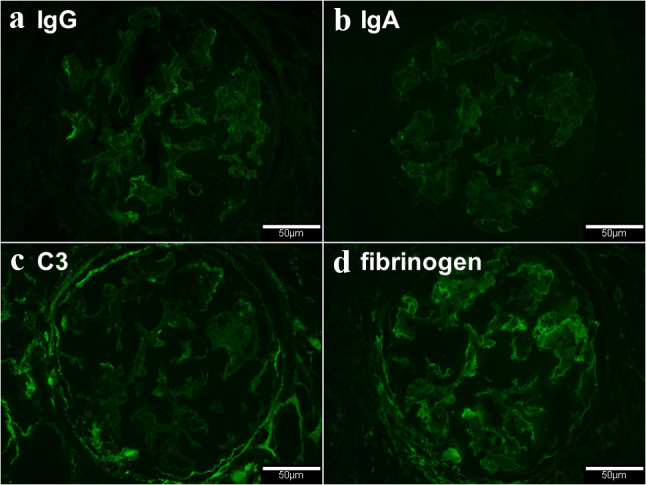
Immunofluorescence findings of the glomerulus. a Immunoglobulin G negative (direct immunofluorescence, original magnification ×200). b Immunoglobulin A negative (direct immunofluorescence, original magnification ×200). c Complement 3 negative (direct immunofluorescence, original magnification ×200). d Segmental deposition of fibrinogen in the glomerular capillaries (direct immunofluorescence, original magnification ×200)
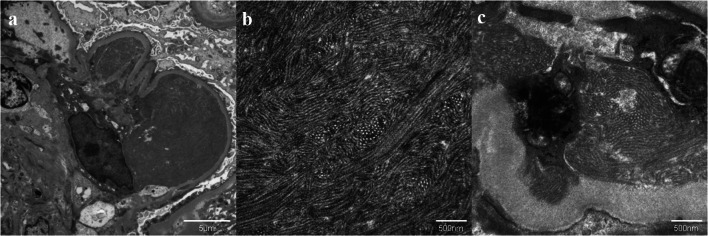
The electron microscopy showed organized deposits characterized by randomly arranged tubular structure in the glomerular capillary lumens (Fig. 6a, b) and subendothelial spaces (Fig. 6c). These microtubules were 40–50 nm in diameter and had no layered structure. Besides, the electron micrograph revealed increased mesangial matrix and diffuse foot process effacement without subepithelial electron-dense deposits. We diagnosed him MPGN-like lesion given these findings.
Fig. 6.
Electron microscopic findings in the glomerulus. a Large amounts of organized deposits in the subendothelial spaces (original magnification ×3000). b Higher magnified image of these deposits. Randomly arranged microtubular structures with 40–50 nm diameter. (original magnification ×20,000). c Similar microtubular structures in another subendothelial space. (Original magnification ×20,000)
Diagnostic strategy
We considered cryoglobulinemic glomerulonephritis and immunotactoid glomerulopathy given the organized deposits with unique microtubular structures. However, we denied these diseases given no cryoglobulinemia or M-protein in blood and urine. The immunofluorescent staining showed no deposit of immunoglobulin in the glomerulus.
We considered idiopathic nodular glomerulosclerosis given the finding of nodular lesion in the glomerulus and previous history of heavy smoking. We also considered chemotherapy-induced thrombotic microangiopathy. However, these diseases could not explain the uniquely organized deposits in the glomerulus.
LC–MS/MS assessment
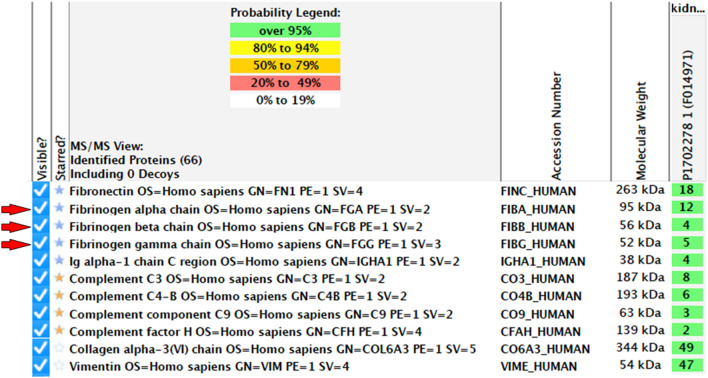
We further assessed the etiology of deposited microtubules for the definitive diagnosis. Following the laser microdissection, we performed the LC–MS/MS procedure to determine the deposited protein, demonstrating the fibrinogen alpha chain, beta chain, and gamma chain, in addition to fibronectin (Fig. 7). Glomerular deposits were stained red by Masson’s trichrome staining, and the deposits showed positive in immunostaining for fibrinogen (Fig. 8). We finally reached the definitive diagnosis of cryofibrinogen-associated glomerulonephritis.
Fig. 7.
Liquid chromatography-tandem mass spectrometry (LC–MS/MS) findings. LC–MS/MS using the Scaffold database identified fibrinogen alpha chain, beta chain and gamma chain (red arrows) in addition to fibronectin
Fig. 8.
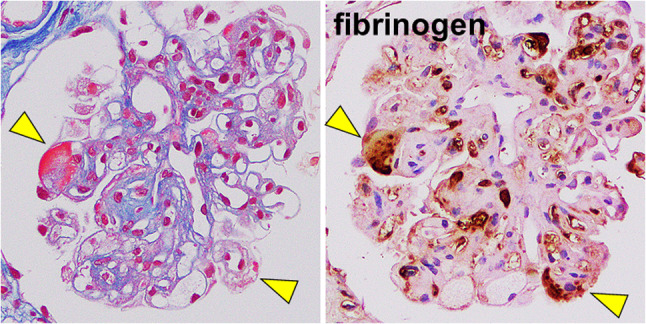
Serial sections of Masson’s trichrome stain and immunostaining for fibrinogen. Serial sections of Masson’s trichrome stain and immunostaining for fibrinogen showed that red color of deposition in glomeruli in Masson staining was identified by positive for fibrinogen, indicating fibrinogen deposition in subendothelial areas in glomeruli
We could not detect cryofibrinogen in the plasma obtained at the time of renal biopsy, although we cannot exclude the possibility of false-negative result.
Clinical course (Fig. 2)
We considered that the nephrotic syndrome was associated with gastric cancer, and continued the chemotherapy, which was eventually terminated 1 month after the renal biopsy due to his worsening performance status. The nephrotic syndrome was refractory to the continued anticoagulation therapy. He was expired 5 months after the renal biopsy due to chemotherapy-induced side effects, malnutrition, and the progression of metastasis, followed by the fatal cerebral bleeding.
Discussion
Nephrotic syndrome with cancer
It is well known that the nephrotic syndrome is often accompanied by malignancy classically as membranous nephropathy due to solid tumors or minimal change disease due to Hodgkin lymphoma [4]. Various medications used for chemotherapy also induce nephrotic syndrome [5]. Renal biopsy is required for the definite diagnosis in patients with malignancy and nephrotic syndrome.
Among the medications utilized in this patient, oxaliplatin is reported to be associated with thrombotic microangiopathy or hemolytic uremic syndrome [6]. However, the patient had no hemolysis or thrombocytopenia. We did not use anti-vascular endothelial growth factor agents such as bevacizumab.
Cryofibrinogen and cancer
Approximately 40–70% of all cryofibrinogenemia develops secondary to other diseases, which are stratified in bacterial and viral infections, autoimmune diseases, lymphoproliferative disorders, and solid tumors [7–9]. Approximately 4–17% of the secondary cryofibrinogenemia is caused by malignancy, most of which are hematological malignancy [7–9], except for one report of colorectal cancer [7]. Detailed mechanism of the development of cryofibrinogenemia in patients with malignancy remains unknown.
Cryofibrinogen and thrombotic complications
Dominant comorbidities of cryofibrinogenemia are skin lesions and thrombotic events. Approximately 5–26% of cryofibrinogenemia accompanies arterial or venous thrombosis [8–10]. His atypical left subclavian vein thrombosis might have association with cryofibrinogen.
Cryofibrinogen-associated glomerulonephritis
Approximately 10% of patients with cryofibrinogenemia had some renal disease [7, 11]. However, its detailed pathological assessments remain unreported [3, 12–15]. Sethi and colleagues reported two cases as cryofibrinogen-associated glomerulonephritis [3].
Clinically, patients with cryofibrinogen-associated glomerulonephritis show a variety of proteinuria, hematuria, and renal impairment (Table 2). Cryofibrinogen-associated glomerulonephritis does not always accompany skin lesions, as our patient.
Table 2.
Literature review of clinical characteristics of the cryofibrinogen-associated glomerulonephritis patients
| Reference | Age | Sex | Co-morbidities | Proteinuria | Hematuria | Serum creatinine | Skin lesions | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Nash JW, et al. [12] | 41 | F |
Type 1 diabetes mellitus Hypothyroidism Deep venous thrombosis |
NR | NR | NR (Renal failure) | (+) | Plasmapheresis | NR |
| Singh A, et al. [13] | 66 | M | Hypothyroidism |
(4+) > 7 g/day |
8–10/HPF | 1.4 mg/dL |
Erythema Ulcers |
PSL Warfarin |
NR |
| Sethi S, et al. [3] | 66 | M |
MGUS COPD Congestive heart failure |
NR | NR | NR (AKI) |
Purpuric rash Ulcers |
PSL Aspirin |
Dialysis dependent |
| Sethi S, et al. [3] | 70 | M |
Hepatitis B virus carrier Prostate cancer (post-surgery) |
(3+) 8 g/day |
(2+) | 2.2 mg/dL | None |
PSL CY |
Improvement in kidney function (serum creatinine level decreased from 3.60 to 1.70 mg/dL after 6 months of treatment) |
| Sudo M, et al. [14] | 60 | M | Accelerated hypertension |
(4+) 7.6 g/day |
(3+) 30–49 /HPF |
3.53 mg/dL | None |
mPSL Heparin |
Unknown due to transfer to another hospital (Serum creatinine 4.14 mg/dl) |
| Ibuki E, et al. [15] | 78 | M |
Hepatitis B virus carrier Hepatitis C virus carrier Gastric cancer (post-surgery) Nontuberculous mycobacterial infection |
(4+) 4.5 g/day |
(3+) | 3.51 mg/dL | None | None |
Dialysis dependent Sudden death |
| Our case | 72 | M |
Advanced gastric cancer Deep venous thrombosis |
(3+) 5.4 g/gCr |
(2+) 5–9 /HPF |
1.41 mg/dL | None | DOAC (Edoxaban) | Died of cerebral hemorrhage |
F female, M male, MGUS monoclonal gammopathy of undetermined significance, COPD chronic obstructive pulmonary disease, NR not reported, AKI acute kidney injury, PSL prednisone, CY cyclophosphamide, mPSL methylprednisolone, DOAC direct oral anticoagulant
Pathologically, cryofibrinogen-associated glomerulonephritis shows MPGN with no immunoglobulin deposition, at which fibrinogen is stained at the segmental capillary wall (Table 3). An ultrastructural study shows organized deposits of large-bore with multilayered tubular structures and fine fibrillary structures. In our patient, organized deposits were small tubular-like and had relatively thinner diameter. In the electronic microscope, settled protein in plasma shows the tubular structure, which is similar to those observed in the glomerulus [3, 15], although we could not assess it. In plasma, the settlement of fibrinogen might be facilitated when fibronectin connects fibrin and fibrinogen [16]. Concentrated plasma cryofibrinogen might deposit in the glomerulus and cause nephritis.
Table 3.
Literature review of histopathological findings of cryofibrinogen-associated glomerulonephritis
| Reference | Age | Sex | Histopathological findings | Identified proteins by LC–MS/MS | ||
|---|---|---|---|---|---|---|
| LM | IF | EM (features of organized deposits) | ||||
| Nash JW, et al. [12] | 41 | F | Eosinophilic nodular deposits within glomerular capillaries | Not reported | aggregates of fibrillary material arranged in a parallel array 20 nm in diameter | Not reported |
| Singh A, et al. [13] | 66 | M | MPGN |
IgG(+), IgM(+) C3( +) |
Not identified | Not reported |
| Sethi S, et al. [3] | 66 | M | MPGN |
IgG (−), IgA(−), IgM(−) C3 (+, weakly) Fibrinogen (+, segmental) |
Randomly arranged large fibrils with tubular structures a large central bore; some have double or triple layering 158 nm (121–211 nm) in diameter |
Fibrinogen α, β and γ chain alpha2-macroglobulin haptoglobin C3 Filamin Apolipoprotein B-100 |
| Sethi S, et al. [3] | 70 | M | MPGN |
IgG (−), IgA(−), IgM(−) C3 (−) Fibrinogen (+, segmental) |
Randomly arranged large fibrils with tubular structures a large central bore; some have double or triple layering 158 nm (121–211 nm) in diameter |
Not reported |
| Sudo M, et al. [14] | 60 | M | MPGN |
IgG (−), IgA(−), IgM(−) C3 (+) Fibrinogen (+) |
Randomly arranged tubular structures 60 nm in diameter |
Fibrinogen α, β and γ chain Fibronectin |
| Ibuki E, et al. [15] | 78 | M | MPGN with crescent formation |
IgG (−), IgA(−), IgM(+) C3 (+) Fibrinogen (+, weakly) |
Randomly arranged large fibrils with large central bores and double layer structures 185 nm (150–220 nm) in diameter |
Fibrinogen α, β and γ chain Fibronectin Filamin-A C3 |
| Our case | 72 | M | MPGN |
IgG (−), IgA(−), IgM(−) C3 (−) Fibrinogen (+, segmental) |
Randomly arranged microtubular structures 40–50 nm in diameter |
Fibrinogen α, β and γ chain Fibronectin |
LM light microscopy, IF immunofluorescence microscopy, EM electron microscopy, LC–MS/MS liquid chromatography-tandem mass spectrometry, MPGN membranoproliferative glomerulonephritis, Ig immunoglobulin, C3 complement 3
Of note, we observed endocapillary infiltration of foam cells (CD68 positive macrophages), which is similarly observed in cryoglobulinemic glomerulonephritis. This might represent immunoreaction to the deposited cryoprotein.
The existence of immunoglobulin-negative organized deposits is quite important in the pathological diagnosis of cryofibrinogen-associated GN, at which the types of protein are determined by the LC–MS/MS method. In our patient, we found fibrinogen alpha chain, beta chain, and gamma chain, in addition to fibronectin using the LC–MS/MS method.
Given the existence of atypical deep venous thrombosis before any therapeutic interventions, the gastric cancer itself might be the dominant cause of cryofibrinogenemia. Despite the remission of gastric cancer during chemotherapy, nephrotic syndrome rather worsened. Chemotherapy, such as oxaliplatin [6], might have induced cryofibrinogen-associated glomerulonephritis accompanying interstitial fibrosis and tubular atrophy.
Treatment of cryofibrinogen-associated glomerulonephritis
There is no established therapy for the cryofibrinogenemia, including cryofibrinogen-associated glomerulonephritis (Table 2). Specific therapies for the underlying diseases are recommended for the secondary cryofibrinogenemia. Steroid, immunosuppressant, anti-coagulation, and plasma exchange are attempted empirically [2].
In this patient, we could not continue chemotherapy due to his worsening physical status. Continued anti-coagulation therapy using edoxaban improved subclavian vein thrombosis, whereas nephrotic syndrome remained progressed. Detailed association between direct oral anti-coagulants and cryofibrinogen-associated glomerulonephritis remains the future concern. Further studies are warranted to establish the therapeutic strategy for cryofibrinogen-associated glomerulonephritis, given the increasing diagnosis of cryofibrinogen-associated glomerulonephritis owing to the spread of LC–MS/MS methodology.
Conclusion
We had a patient with progressive gastric cancer, whose cryofibrinogen-associated glomerulonephritis progressed against continued anticoagulation therapy, despite the remission of malignancy due to chemotherapy.
In addition to the detailed assessment of specifically organized deposits, the analysis using LC–MS/MS method is useful to diagnose cryofibrinogen-associated glomerulonephritis. We should consider cryofibrinogen-associated glomerulonephritis as a differential diagnosis when the patients with malignancy showed abnormal urinalysis and renal impairment, though it is a rare disease.
Declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
All procedures performed in this case were in accordance with the ethical standards of the 1964 Helsinki declaration.
Informed consent
Informed consent was obtained from the patient for the publication of this case report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Korst DR, Kratochvil CH. Cryofibrinogen in a case of lung neoplasm associated with thrombophlebitis migrans. Blood. 1955;10:945–953. doi: 10.1182/blood.V10.9.945.945. [DOI] [PubMed] [Google Scholar]
- 2.Moiseev S, Luqmani R, Novikov P, Shevtsova T. Cryofibrinogenaemia-a neglected disease. Rheumatology. 2017;56:1445–1451. doi: 10.1093/rheumatology/kew379. [DOI] [PubMed] [Google Scholar]
- 3.Sethi S, Yachoui R, Murray DL, Radhakrishnan J, Alexander MP. Cryofibrinogen-associated glomerulonephritis. Am J Kidney Dis. 2017;69:302–308. doi: 10.1053/j.ajkd.2016.08.031. [DOI] [PubMed] [Google Scholar]
- 4.Cambier JF, Ronco P. Onco-nephrology: glomerular diseases with cancer. Clin J Am Soc Nephrol. 2012;7:1701–1712. doi: 10.2215/CJN.03770412. [DOI] [PubMed] [Google Scholar]
- 5.Jhaveri KD, Shah HH, Calderon K, Campenot ES, Radhakrishnan J. Glomerular diseases seen with cancer and chemotherapy: a narrative review. Kidney Int. 2013;84:34–44. doi: 10.1038/ki.2012.484. [DOI] [PubMed] [Google Scholar]
- 6.Dahabreh I, Tsoutsos G, Tseligas D, Janinis D. Hemolytic uremic syndrome following the infusion of oxaliplatin: case report. BMC Clin Pharmacol. 2006;6:5. doi: 10.1186/1472-6904-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saadoun D, Elalamy I, Ghillani-Dalbin P, Sene D, Delluc A, Cacoub P. Cryofibrinogenemia: new insights into clinical and pathogenic features. Am J Med. 2009;122:1128–1135. doi: 10.1016/j.amjmed.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 8.Blain H, Cacoub P, Musset L, Costedoat-Chalumeau N, Silberstein C, Chosidow O, et al. Cryofibrinogenaemia: a study of 49 patients. Clin Exp Immunol. 2000;120:253–260. doi: 10.1046/j.1365-2249.2000.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soyfoo MS, Goubella A, Cogan E, Wautrecht JC, Ocmant A, Stordeur P. Clinical significance of cryofibrinogenemia: possible pathophysiological link with Raynaud’s phenomenon. J Rheumatol. 2012;39:119–124. doi: 10.3899/jrheum.110793. [DOI] [PubMed] [Google Scholar]
- 10.Belizna C, Loufrani L, Subra JF, Godin M, Jolly P, Vitecocq O, et al. A 5-year prospective follow-up study in essential cryofibrinogenemia patients. Autoimmun Rev. 2011;10:559–562. doi: 10.1016/j.autrev.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Terrier B, Izzedine H, Musset L, Ghillani P, Deray G, Saadoun D, et al. Prevalence and clinical significance of cryofibrinogenaemia in patients with renal disorders. Nephrol Dial Transplant. 2011;26:3577–3581. doi: 10.1093/ndt/gfr079. [DOI] [PubMed] [Google Scholar]
- 12.Nash JW, Ross P, Jr, Neil Crowson A, Taylor J, Morales JE, Yunger TM, et al. The histopathologic spectrum of cryofibrinogenemia in four anatomic sites. Skin, lung, muscle, and kidney. Am J clin pathol. 2003;119:114–122. doi: 10.1309/KB5ARGWVL1R2BPBN. [DOI] [PubMed] [Google Scholar]
- 13.Singh A, Gaber LW. Nephrotic syndrome and chronic renal insufficiency associated with essential cryofibrinogenemia. Nephrol Dial Transplant. 2007;22:1772–1775. doi: 10.1093/ndt/gfm045. [DOI] [PubMed] [Google Scholar]
- 14.Sudo M, Sakamaki Y, Hosojima M, Yamamoto S, Ito Y, Imai N, et al. Cryofibrinogen-associated glomerulonephritis diagnosed by mass spectrometry and immunoelectron microscopy. Hum Pathol Case Rep. 2019;15:83–87. doi: 10.1016/j.ehpc.2018.12.002. [DOI] [Google Scholar]
- 15.Ibuki E, Shiraishi A, Sofue T, Kushida Y, Kadota K, Honda K, et al. Characteristic electron-microscopic features of cryofibrinogen-associated glomerulonephritis: a case report. BMC Nephrol. 2020;21:27. doi: 10.1186/s12882-020-1696-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stathakis NE, Karamanolis D, Koukoulis G, Tsianos E. Characterization of cryofibrinogen isolated from patients plasma. Haemostasis. 1981;10:195–202. doi: 10.1159/000214404. [DOI] [PubMed] [Google Scholar]