Abstract
Background
The reported incidence of venous thromboembolism (VTE) in COVID-19 patients varies widely depending on patient populations sampled and has been predominately studied in hospitalized patients. The goal of this study was to assess the evolving burden of COVID-19 and the timing of associated VTE events in a systems-wide cohort.
Methods
COVID-19 PCR positive hospitalized and non-hospitalized patients ≥18 years of age tested between 1/1/2020 through 12/31/2020 were retrospectively analyzed using electronic medical records from multiple states across the Mayo Clinic enterprise.
Radiology reports within 90 days before and after confirmed COVID-19 diagnosis were examined for VTE outcomes using validated Natural Language Processing (NLP) algorithms.
Results
A 29-fold increased rate of VTE compared to the pre-COVID-19 period was noted during the first week following the first positive COVID-19 test (RR: 29.39; 95% CI 21.77-40.03). The rate of VTE steadily decreased and returned to baseline by the 6th week. Among 366 VTE events, most occurred during (n = 243, 66.3%) or after (n = 111, 30.3%) initial hospitalization. Only 11 VTE events were identified in patients who did not require hospitalization (3.0% of total VTE events). VTE and mortality increased with advancing age with a pronounced increased each decade in older patients.
Conclusion
We observed a profoundly increased risk of VTE within the first week after positive testing for COVID-19 that returned to baseline levels after 6 weeks. VTE events occurred almost exclusively in patients who were hospitalized, with the majority of VTE events identified within the first days of hospitalization.
Keywords: SARS-CoV-2, COVID-19, Venous thromboembolism, Deep vein thrombosis, Pulmonary embolism
1. Introduction
Severe acute respiratory syndrome (SARS) Coronavirus 2 (SARS-CoV-2), also known as COVID-19, has spread around the world causing significant morbidity and mortality [1] and has created significant challenges for health care systems and staff [2], [3]. COVID-19 daily mortality rates have exceeded those from cardiovascular disease and cancer [4] with a unique and alarming association with thrombosis [5]. Hematologic derangements that constitute a form of coagulopathy often manifest in the form of venous thromboembolism (VTE) [6], [7], [8]. Numerous reports from various countries have demonstrated a high incidence of VTE in COVID-19 patients [9], [10], [11], however, there is a lot of heterogeneity reported in the risk of VTE [12]. Furthermore, the precise timing of VTE events as they relate to the diagnosis of COVID-19 has not been well studied. Much of the published data shows a high incidence of VTE in critically ill hospitalized patients, the group with the most severe presentations and most comorbidities. While managing VTE risk in hospitalized patients is essential, an exclusive focus on this setting limits the scope of the exploration of the relationship between COVID-19 and thrombosis.
The combination of referral bias of sicker individuals to tertiary referral centers, screening regimens to assess for asymptomatic VTE, examination of only hospitalized or intensive care patients, and nonstandard VTE definitions (including fistula thrombosis and superficial vein thrombosis) has led to some very high reported rates of venous thrombosis that may not be reflective of the population of patients more broadly who acquire COVID-19. VTE estimates in hospitalized patients range from 4.5-85% with pooled estimates from observational studies in meta-analyses estimating VTE incidence between 17 and 26% with noted concerns of heterogeneity and bias [13], [14]. One meta-analysis of pulmonary emboli in hospitalized COVID-19 patients found the incidence to be much lower than others had reported (3.0%) when examining studies larger than 400 patients [15] and importantly, some studies have not demonstrated an overall increased thrombotic risk in COIVD-19 patients compared to other hospitalized patients [16].
In this study, we evaluate VTE outcomes in COVID-19 infections across all patients (inpatients and outpatients) in the Mayo Clinic enterprise representing geographically diverse locations and different care settings (tertiary referral and regional hospitals). We further sought to evaluate the relative magnitude of risk in relation to baseline VTE rates as well as specifically examining timing of VTE and mortality as it relates to COVID-19 testing and hospitalization.
2. Methods
2.1. Population and setting
An electronic search of a unified data platform of laboratory records was performed from 1/1/2020 through 12/31/2020 to identify all Mayo Clinic enterprise patients at least 18 years or older with a COVID-19 positive PCR test. Mayo Clinic enterprise consists of numerous outpatient and inpatient locations utilizing a common medical record system, including three tertiary referral centers (Rochester Minnesota, Phoenix Arizona, and Jacksonville Florida) and many regional Mayo Clinic Health System sites in Minnesota, Wisconsin, and Iowa. The date of the first positive COVID-19 PCR test recorded (collected date) was used as the initial diagnosis date for the cohort. Prophylactically dosed anticoagulation is recommended for all COVID-19 positive patients admitted to the hospital without contraindications with official enterprise-wide anticoagulation guidelines released in May of 2020 [5]. Patients with contraindications for anticoagulant prophylaxis are recommended to use sequential compression devices when feasible. Over time, patients with COVID-19 were treated with evolving regimens during the study period based on most recent clinical trials and guidance statements [17]. This study was approved by the Mayo Clinic Institutional Review Board in Rochester, MN, and patients without Minnesota research authorization were excluded.
2.2. Data collection
Basic demographic variables including age at positive COVID-19 testing, sex, race, zip code, and death date were extracted from electronic records. Using the cohort of positive COVID-19 patients, electronic medical records were searched to identify all hospital admissions from 1/1/2020 through 1/31/2021. A population of COVID-19 positive hospitalized patients was created by including all admissions that occurred within a 90-day window before and after positive testing. For patients with multiple admissions after the COVID-19 positive test, only initial admission was analyzed. This admission was selected by identifying the numerically smallest difference between the admission date and COVID-19 testing date. All radiology text reports of computed tomography (CT) scans that included the chest and contained IV contrast (including CT angiograms), and all venous Duplex ultrasounds of the upper or lower extremity were compiled within 90 days before and after the COVID-19 testing date. Due to the nature of COVID-19 pneumonia and because it is now an infrequent diagnostic tool for PE, ventilation/perfusion scans were not included.
2.3. Natural language processing
VTE was defined as upper or lower extremity deep vein thrombosis (DVT) (proximal and distal) and pulmonary embolism (PE) and were identified by analyzing the text from radiology reports. The VTE outcomes did not include atypical sites of venous thrombosis (e.g. splanchnic vein thrombosis) or superficial thrombophlebitis. Natural language algorithms (NLP) to identify new/acute, or progressive PE and new/acute, or progressive DVT were created, validated, and applied to the text from the radiology report using the “simple NLP” program [18]. A separate database of imaging reports (not this cohort) was initially used for algorithm development (PE and DVT). Radiology imaging reports from the derivation and testing database were reviewed and interpreted as positive or negative by a physician. Upper extremity DVTs were designated in this category if the NLP algorithm was positive on Duplex ultrasound studies with a special designation for the upper extremities. For the DVT algorithm, 1752 lower extremity Duplex ultrasound reports and 787 upper extremity Duplex ultrasound reports were ultimately reviewed and interpreted to create the testing database. The NLP algorithm for DVT in the lower extremity reports had a 98.2% sensitivity and a 99.6% specificity and for the upper extremity had a 97.8% sensitivity and a 99.2% specificity. The NLP algorithm for PE correctly identified 325 of 327 reports with PE (as defined above; sensitivity of 99.4%) and 672 out of 673 reports without PE (specificity of 99.9%).
2.4. Analysis
The primary outcome of interest was the first incident VTE after the first COVID-19 positive testing. The precise date of thrombus confirmation by imaging was determined by using the date of the first positive PE/DVT on CT/ultrasound (if multiple were positive). All radiology reports within 90 days before and after positive COVID-19 testing were examined. The VTE percentage and mortality rates were stratified and tabulated into age groups (rounded to the nearest decade). A histogram of events with COVID-19 testing date was created and no large spike in VTE diagnoses was observed in the 2 weeks before COVID-19 testing. Therefore, first VTE events occurring in a 90-day window before COVID-19 testing was used to calculate a baseline incident rate of VTE that reflected the inherent risk of the population based on age, gender, and other defined and undefined comorbidities. This baseline incidence rate of VTE was then compared to the incidence rate of VTE in weekly intervals after COVID-19 testing until rates returned to baseline levels. The relative increase in risk for VTE that could be attributed to COVID-19 infection and its sequelae was then determined. Person-time follow-up for each weekly interval was readjusted to account for patients who died during that period. An incidence rate ratio (IRR) was calculated for each comparison with 95% confidence intervals. Based on the increase in the frequency of both VTE and death observed with increasing age, incidence rates and IRR were recalculated after stratifying age at 65 years.
To evaluate the cohort for possible biases associated with loss to follow-up, a subgroup analysis was performed evaluating VTE outcomes and death in a subset of patients residing only in counties contained within the Rochester Epidemiology Project. Patients residing in these counties were identified using the patient-provided mailing address zip code. Residents of these counties with COVID-19 testing represent a local population of Mayo Clinic primary care patients where outcomes of VTE and death would, as a rule, be mostly uniformly captured and any escalation of care from a regional hospital to a tertiary hospital contain complete information.
3. Results
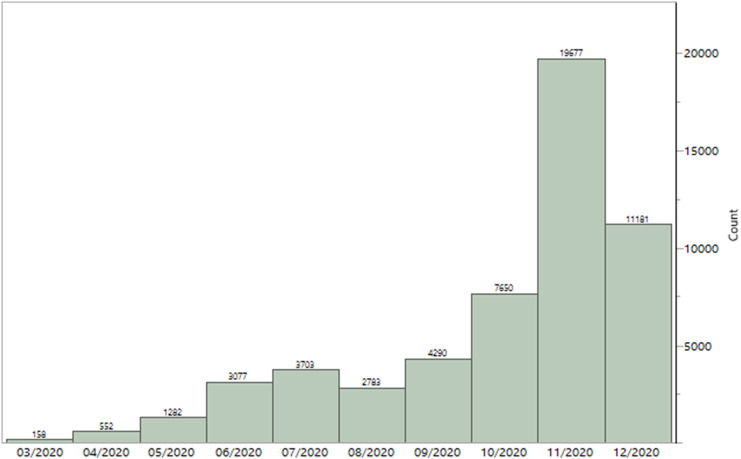
A total of 54,354 patients with COVID-19 positive PCR were identified from March 01, 2020, to December 31, 2020. The age of the cohort ranged from 18 to 101 years with a mean age of 44 ± 18 years with a similar proportion of males and females. Most patients with COVID-19 were Caucasians (82.5%). Consistent with national trends, the number of COVID-19 positive cases demonstrated a bimodal curve with increasing cases from March 2020 to July 2020, with a brief reduction in August before cases again escalated. Cases peaked in November with 19,677 patients, more than doubled compared to October (Fig. 1 ).
Fig. 1.
Monthly number of patients with positive COVID-19 testing.
A total of 426 VTE were identified in the 90 days before and after COVID-19 PCR testing, among which 366 occurred in the 90 days after testing. Among the 366 patients with VTE after COVID-19 testing, 131 (35.8%) were DVT (upper or lower extremity), 192 (52.5%) were PE, and 43 (11.7%) had PE and DVT. A significant increase in the frequency of VTE (within 90 days) and death (within 30 days) was observed with advancing age in COVID-19 patients and was most pronounced after the 6th decade of life (Table 1 ). Data in the subgroup of patients (n = 34,785) from Rochester Epidemiology Project (REP) counties were consistent with the overall findings and trends for both VTE and death.
Table 1.
First venous thromboembolism or death after COVID-19 diagnosis by age group.
| VTE before COVID-19a n (%) |
ALL patients |
REP county patients |
|||||
|---|---|---|---|---|---|---|---|
| Age groupb | N | VTEc n (%) |
Deathd n (%) |
N | VTEc n (%) |
Deathd n (%) |
|
| 60 (0.11) | Total | 54,353 | 366 (0.67) | 429 (0.79) | 34,785 | 181 (0.52) | 261 (0.75) |
| 1 (0.01) | 20 | 9598 | 4 (0.04) | 3 (0.03) | 6415 | 2 (0.03) | 3 (0.05) |
| 1 (0.01) | 30 | 10,039 | 13 (0.13) | 1 (0.01) | 6896 | 1 (0.01) | 1 (0.02) |
| 8 (0.09) | 40 | 9137 | 24 (0.26) | 5 (0.05) | 6131 | 9 (0.15) | 3 (0.05) |
| 12 (0.14) | 50 | 8810 | 30 (0.34) | 8 (0.09) | 5406 | 13 (0.24) | 4 (0.07) |
| 9 (0.11) | 60 | 8516 | 91 (1.07) | 45 (0.53) | 5104 | 43 (0.84) | 24 (0.47) |
| 14 (0.29) | 70 | 4858 | 92 (1.89) | 83 (1.71) | 2808 | 53 (1.89) | 45 (1.60) |
| 8 (0.32) | 80 | 2514 | 75 (2.98) | 151 (6.01) | 1469 | 41 (2.79) | 86 (5.85) |
| 6 (0.75) | 90 | 800 | 34 (4.25) | 108 (13.5) | 505 | 18 (3.56) | 76 (15.1) |
| 1 (1.23) | 100 | 81 | 3 (3.70) | 25 (30.9) | 51 | 1 (1.96) | 19 (37.3) |
| P valuee | <0.001 | <0.001 | <0.001 | <0.001 | |||
Abbreviations: REP = Rochester Epidemiology Project, VTE = venous thromboembolism.
VTE events within 90 days before COVID-19 positive testing.
Age rounded to the nearest decade and grouped.
First VTE event within 90 days after COVID-19 positive testing.
Death within 30 days.
Pearson Chi-squared.
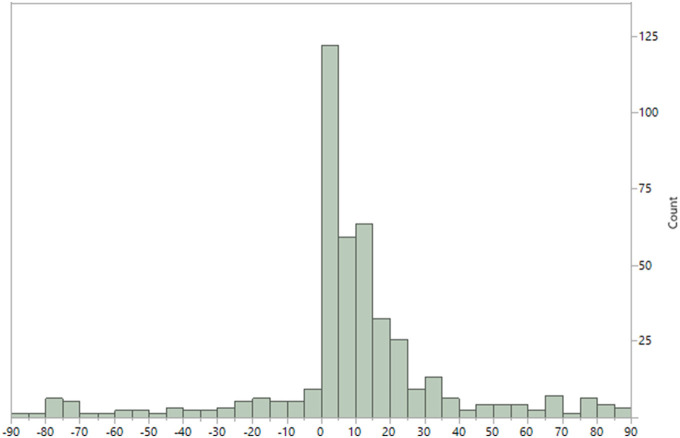
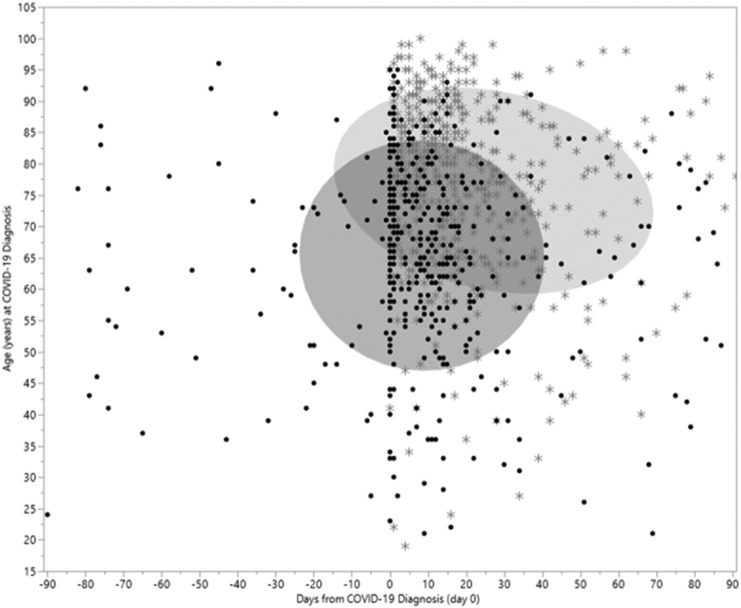
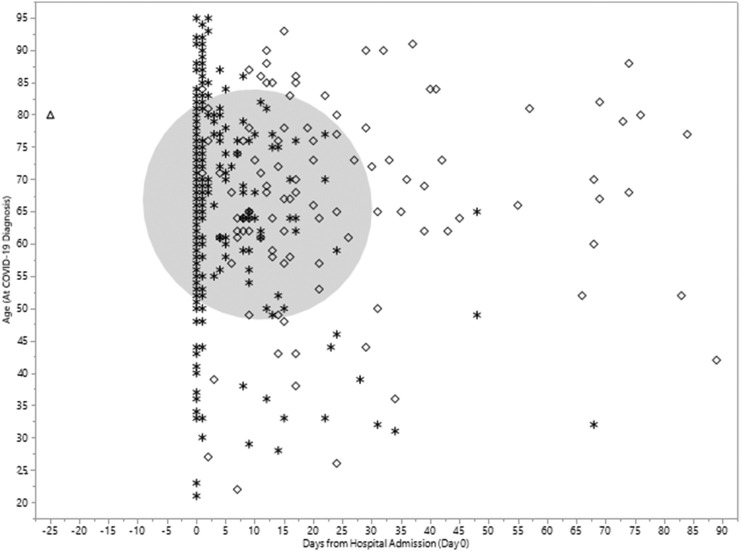
Most VTE events were identified within three weeks after positive testing for COVID-19. The number of VTE events returned to baseline approximately 40 days after the COVID-19 testing (Fig. 2 ). A 29-fold increased rate of VTE (131.7 per 1000 person-years; Table 2 ) was noted during the first week following the COVID-19 testing (RR: 29.39; 95% CI 21.77 to 40.03) compared to the pre-COVID timeframe. The rate progressively decreased until returning to near basal values by the 6th week (7.80 per 1000 person-years). The baseline rate of VTE during the pre-COVID timeframe for all patients was 4.5 (per 1000 person-years) and varied by age. In both age groups, the rate of VTE in the first week after COVID-19 infection was the highest and with each successive week, it continued to decrease. The timeframe of VTE and death in relation to COVID-19 testing date plotted by age (Fig. 3 ) shows the overall pattern of events. Among hospitalized COVID-19 patients, the pattern of VTE during and after hospital admission is demonstrated in Fig. 4 .
Fig. 2.
Histogram of timing of venous thromboembolism diagnosis in relationship to COVID-19 positive PCR (day 0) testing.
Table 2.
Risk of VTE by week after positive COVID-19 testing.
| Time interval | # VTE events | At risk | # Deaths | VTE Rate | IRR | 95% CI |
|---|---|---|---|---|---|---|
| All patients | ||||||
| 90 days before diagnosis | 60 | 54,353 | 4.5 | ref | ||
| Week 1 | 137 | 54,293 | 103 | 131.7 | 29.39 | 21.77, 40.03 |
| Week 2 | 94 | 54,053 | 132 | 90.7 | 20.25 | 14.68, 28.12 |
| Week 3 | 51 | 53,827 | 117 | 49.4 | 11.04 | 7.57, 16.04 |
| Week 4 | 22 | 53,659 | 57 | 21.4 | 4.78 | 2.88, 7.71 |
| Week 5 | 19 | 53,580 | 35 | 18.5 | 4.13 | 2.41, 6.83 |
| Week 6 | 8 | 53,526 | 30 | 7.8 | 1.74 | 0.78, 3.50 |
| Age < 65 | Mean age 40.0, SD 13.85 | |||||
| 90 days before diagnosis | 31 | 46,100 | 2.7 | ref | ||
| Week 1 | 48 | 46,069 | 17 | 54.4 | 19.92 | 12.71, 31.58 |
| Week 2 | 48 | 46,004 | 12 | 54.4 | 19.95 | 12.73, 31.62 |
| Week 3 | 24 | 45,944 | 14 | 27.3 | 9.99 | 5.80, 17.04 |
| Week 4 | 14 | 45,906 | 12 | 15.9 | 5.83 | 3.01, 10.85 |
| Week 5 | 10 | 45,880 | 12 | 11.4 | 4.17 | 1.95, 8.32 |
| Week 6 | 1 | 45,858 | 6 | 1.1 | 0.42 | 0.02, 2.19 |
| Age > 65 | Mean age 74.1, SD 7.42 | |||||
| 90 days before diagnosis | 29 | 8253 | 14.3 | ref | ||
| Week 1 | 89 | 8224 | 86 | 564.7 | 39.60 | 26.27, 61.06 |
| Week 2 | 46 | 8049 | 120 | 298.2 | 20.91 | 13.17, 33.61 |
| Week 3 | 27 | 7883 | 103 | 178.7 | 12.53 | 7.37, 21.25 |
| Week 4 | 8 | 7753 | 45 | 53.8 | 3.78 | 1.62, 8.02 |
| Week 5 | 9 | 7700 | 23 | 61.0 | 4.28 | 1.92, 8.82 |
| Week 6 | 7 | 7668 | 24 | 47.6 | 3.34 | 1.36, 7.36 |
| Week 7 | 1 | 7637 | 7 | 6.8 | 0.48 | 0.02, 2.52 |
VTE rate per 1000 person-years.
Abbreviations: CI = confidence interval, IRR = incidence rate ratio, SD = standard deviation, VTE = venous thromboembolism.
Fig. 3.
Timing of venous thromboembolism and death in relationship to COVID-19 diagnosis. Legend: (dot) VTE event, (star) Death. Ellipse (shades 50% of events).
Fig. 4.
Timing of venous thromboembolism events among hospitalized patients occurring after confirmed positive COVID-19 testing. Legend: (triangle) VTE before admission, (star) VTE during admission, (diamond) VTE after admission. Circle (shades 50% of VTE events).
Hospital admission occurred in a total of 4340 patients (8.0%) within 90 days of COVID-19 testing. Among these patients, 2049 had their first positive PCR test on the day of admission. Eighteen admissions were ongoing at the time of data analysis. The median length of stay (LOS) was 2 days with a range from 0 to 209 (mean 5.0, SD 8.92). The mean LOS peaked in May (10.1 days) and has down trended to approximately 4 days in each month from September through December. Among hospitalized patients, 1 had VTE after COVID-19 testing but before hospital admission, 5.6% (n = 243) had VTE during the admission, and 2.6% (n = 111) had VTE after discharge and within 90 days after COVID-19 testing. For patients not requiring admission (n = 50,013), 0.02% (n = 11) had VTE diagnosed within 90 days after COVID-19 testing. For patients with a VTE occurring while admitted and after COVID-19 testing, the median length of stay was 9 days (mean 14.5 days) with a range from 0 to 119 days. In patients with VTE occurring after initial admission, the median LOS was 1 day (mean 3.5 days) and the median days to VTE post-discharge was 14.5 (IQR 7-28). Of 366 total VTE events, 243 (66.3%) were diagnosed during initial hospitalization whereas 111 occurred after discharge from initial hospitalization. Only 11 patients had VTE (3.0%) but never required hospitalization and 1 patient had VTE prior to admission. Only 11 patients with VTE (3.0%) did not require hospitalization. Among VTE events occurring in the hospital (n = 243), the majority (64%) were identified on the day of hospitalization (n = 116) or the next day (n = 40). Among these 156 VTE events, 113 (72%) were initially identified as PE and 43 as DVT. Most (n = 109) were identified on typical CT angiography of the chest (PE protocol study) rather than as an incidental finding. A total of 51 patients were diagnosed with COVID-19, hospitalized, and found to have VTE on the same day. When excluding VTE events identified during the first 2 days of hospitalization, 87 “hospital-acquired” VTE events occurred of which the pertinent imaging study performed identified DVT in 53 patients and PE in 34. Of the 53 patients with DVTs, 17 (32%) were upper extremity DVTs and 36 were lower extremity DVTs.
To evaluate for changing mortality and VTE rates throughout 2020, possibly due to different strains of the virus or improved medical treatments, we performed a rolling analysis evaluating 30-day outcomes by month of first positive PCR testing. Monthly frequencies of VTE varied between 0.40 and 1.1% and death varied between 0.56 and 2.0% (Sup Fig. 1). The risk of hospitalization, death, and VTE increased with age (Sup Fig. 2). Although hospitalization for COVID-19 decreased (especially in older adults), frequencies of VTE and death remained largely similar since June and did not significantly worsen with the surge in cases in the last quarter of 2020.
Suppl Fig. 1.
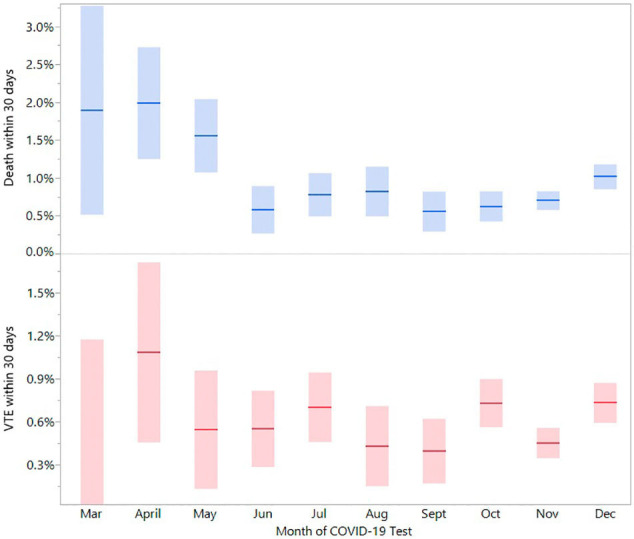
Venous thromboembolism and death in COVID-19 positive patients by month of 2020 (Percent with 95% confidence intervals).
Suppl Fig. 2.
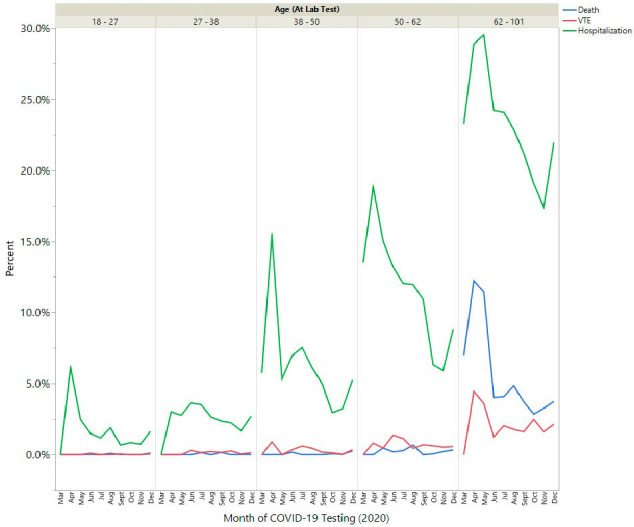
Frequency of hospitalization, death, and VTE within 30 days of positive COVID-19 testing by age group.
4. Discussion
The central findings of our analysis are 1) an overall 29-fold increase in VTE events was noted during the first week after COVID-19 testing 2) VTE incidence and death increased with age with a pronounced increase each decade in older patients; 3) the frequency of VTE was much higher for hospitalized patients compared to non-hospitalized patients (5.6% vs 0.02%); 4) patients 65 years of age or older had a near 40-fold increased VTE rate in the first week; 5) VTE rate decreased to baseline pre-COVID-19 levels 6-7 weeks afterward.
Apart from hospitalized patients, the burden of thromboembolic disease in COVID-19 patients is not well established. For hospitalized patients, the frequency of VTE in this study was similar to some studies [19], [20], [21], [22] but significantly lower than others [23], [24], [25]. Patients not requiring hospital admission had a very low incidence of VTE (0.02%) which should be reassuring to providers and patients. This confirms the prevailing hypothesis that thromboembolic risk in COVID-19 closely mirrors the severity of illness. These patients presumably had low severity of COVID-19 infection, minimal cytokine storm, were more ambulatory, would be less likely to have co-morbidities, and were younger. The results of the current study are consistent with the study of Piazza et al., where 715 COVID-19 patients managed as outpatients did not experience a VTE event. In a study from Denmark using a nationwide population-based registry of COVID-19 patients, the overall 30 day incidence of VTE was 0.4% (9460 patients) [26], which is similar to the 90 day incidence of VTE of 0.67% in this study (54,354 patients). In a research letter, 26,104 adult members of the Kaiser Permanente health plan with COVID-19 reported a 30 day VTE incidence of 0.8% [27].
It is evident from the current study that most VTE events are diagnosed within the first three weeks of COVID-19 testing and with each passing week, the rate decreases. The rate of VTE was highest in the first week after testing, particularly in older patients. The rate then steadily decreases reaching almost pre-COVID-19 baseline rate by the 6th week. By the end of the first two weeks, the rate is reduced by half. This finding has not been reported previously in patients with COVID-19 infection. The baseline, pre-COVID, rate of VTE was expectedly higher than population-based epidemiology studies [28], consistent with data that shows that the population most susceptible to COVID-19 infection has more comorbidities and are “primed” for thromboembolic events. Recent data suggest a role for extended post-hospital prophylaxis in patients for about 4-6 weeks with a prophylactic dose of low molecular weight heparin or a direct oral anticoagulant (DOAC) for vulnerable patients [29]. However, in our study, the rate drops rapidly in the first few weeks following testing for COVID-19, suggesting 4-6 weeks of prophylaxis from time of diagnosis, rather than the time of hospital discharge might be more appropriate. Some institutions have implemented various doses of DVT prophylaxis for hospitalized COVID-19 patients [5] whereas others have instituted therapeutic anticoagulation over standard DVT prophylaxis [30], [31]. We identified that a sizable proportion of diagnosed VTE events (n = 156) occurred either on admission or the next day. We find it extremely unlikely that any hospital anticoagulation strategy would reduce VTE events identified on the first or second day after hospitalization. These events almost certainly were “present on admission” and may not have been imaged promptly or imaging may not have been immediately available in the case of Duplex ultrasound. Standard pharmacologic DVT prophylaxis is not currently recommended in non-hospitalized patients [32] but these results highlight the importance of studies evaluating prophylactic anticoagulation strategies in high risk outpatients.
The frequency of VTE in COVID-19 patients following initial hospitalization in this study was 2.6% which is higher than others have reported [33], [34]. In the study by Ayra et al., 9 patients (0.48%) out of 1877 after hospital discharges were diagnosed with VTE within 42 days. When this VTE rate was compared to hospital discharges in the pre-COVID-19 era in 2019, the rate of VTE was not significantly different between both patient populations (OR 1.6; 95% CI 0.77-3.1; P = 0.2). The higher frequency of VTE in the current study can in part be explained by the longer follow-up (90 days). Also, the subsequent VTE events post initial hospitalization, which may have occurred during possible second hospitalization were also included if they fell within the study period. Data from this study also demonstrate overall shorter hospital length of stays in the last 4 months of 2020, possibly reflecting the increasing ability for some locations to finish remdesivir infusions as an outpatient when appropriate. This reduction in hospital length of stay, at a time we have identified as particularly high risk, would transition more events to the post-discharge setting that might have been inpatient events earlier in the pandemic as reported by other studies. This further reflects that the thrombotic risk in COVID-19 patients is high during acute illness [33] regardless of the need to remain in the hospital. Hence, merely a diagnosis of COVID-19 should not influence the decision for post-discharge extended prophylaxis. That decision needs to be individualized depending upon the patient's co-morbid conditions, additional VTE risk factors, and risk of bleeding, the way it is implemented following hospital discharge for any other medical illness [35].
To our knowledge, this study represents the largest sample of COVID-19 patients evaluated for VTE events and contains a systems-wide cohort of hospitalized and non-hospitalized patients. The precise timing of VTE was known by utilizing a highly accurate NLP algorithm analyzing the imaging reports of each patient. While we would anticipate this would correlate with the timing of onset of the thrombotic event itself, the precise timing of symptoms of the thrombotic event cannot be ascertained from this study. Compared to traditional methods of VTE ascertainment, [International Classification of Disease (ICD) codes] our approach represents a significant improvement, bypassing the well-described inaccuracies known to occur with coding definitions and allows for a much more precise estimate of the timing of thrombosis. In general, upper extremity DVT is less well studied than “traditional” (i.e. lower extremity) DVT and may be even less well captured by ICD codes. Our study demonstrates the significant burden of upper extremity DVT in hospital-acquired events (32%). This finding is likely largely a result of central venous catheters in critically ill patients and is similar to what has been described in non-COVID-19 related hospitalized patients previously [36]. It is possible that this study missed VTE events that were diagnosed on a clinical basis alone due to concern for sending COVID-19 patients for imaging studies.
Unlike health care institutions that received an overwhelming influx of COVID-19 patients early in the pandemic leading unfortunately to rationing of healthcare resources, this was not the case among the institutions included in this study. Furthermore, our previous studies have suggested that COVID-19 patients received at least an equal number of imaging studies compared to non-COVID-19 patients suggesting no hesitancy to order appropriate imaging tests [16]. Another strength of this study was the use of pre and post COVID-19 VTE rates within the same population. The calculated rate ratio is, therefore “self-adjusted” and does not require multivariable adjustment. The calculated rate ratio captures the entirety of VTE risk associated with COVID-19, encompassing the associated sequela from severe illness (hospitalization, organ failure, etc.). An inherent limitation to our analysis is the retrospective nature of this study and possible loss to follow-up. The population selected reflected a cohort of patients who would seek their follow-up within the same interconnected healthcare system. This determination was verified by examining a subset of patients within a specific geographic region that demonstrated a similar outcome ascertainment. Inclusion of only patients with PCR confirmed positive cases is both a strength and limitation, as some symptomatic patients who elected not to seek medical care could have been missed. Since symptoms of COVID-19 mimic many other viral presentations, utilizing only PCR confirmed cases ensures an accurate cohort. Another limitation was the inability to identify atypical site thrombosis, arterial thromboembolic events, and bleeding events. Using our NLP algorithm, we were not able to differentiate proximal from distal lower extremity DVT, which might alter the management. All VTE were identified by imaging in this study, therefore higher utilization of chest imaging for other purposes in hospitalized patients may have inflated the number of incidental pulmonary emboli as compared to the outpatient only population. Anticoagulation strategies (prophylactic or therapeutic) before and after COVID-19 testing could not be assessed in this study. However, the observed VTE rates should be concerning in light of the use of standard of care anticoagulant prophylaxis in hospitalized patients. Among hospitalized patients, the division between ICU and general ward could not be made reliably, and due to the inclusion of community and tertiary care hospitals, the heterogeneity between these patients would have likely significantly varied anyway.
5. Conclusions
In this large observational study, we found that there is a profoundly increased rate of VTE within the first week following positive COVID-19 testing, with the highest rate in patients 65 years and older. The rate then declined and returned to baseline by the 6th week. The number of VTE events identified on initial admission and after COVID-19 PCR confirmation highlight the challenges for prevention in these patients. Further prospective studies are needed to understand the safety and efficacy of increased intensity or individualized anticoagulation strategies to mitigate the risk of VTE in hospitalized patients and the utility of post-hospital discharge prophylaxis.
The following are the supplementary data related to this article.
Authorship
All authors were involved in the conception and design or analysis and interpretation of the data, drafting of the manuscript or revising it critically, and read and approved the final manuscript.
Authorship contributions
Concept and design: DEH, RDM.
Statistical analysis and interpretation: DEH, AKP.
Initial draft: AKP, DEH, RDM.
Critical revision: RC, LJP, EW, RP, AA, PD, MS, WEW.
Final approval: AKP, RDM, RC, RC, LJP, EW, RP, AA, PD, MS, WEW, DEH.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davidson P.M., Padula W.V., Daly J., Jackson D. Moral outrage in COVID19-understandable but not a strategy. J. Clin. Nurs. 2020;29(19–20):3600–3602. doi: 10.1111/jocn.15318. [DOI] [PubMed] [Google Scholar]
- 3.Rajkumar R.P. COVID-19 and mental health: a review of the existing literature. Asian J. Psychiatr. 2020;52 doi: 10.1016/j.ajp.2020.102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woolf S.H., Chapman D.A., Lee J.H. COVID-19 as the leading cause of death in the United States. JAMA. 2021;325(2):123–124. doi: 10.1001/jama.2020.24865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McBane R.D., 2nd, Torres Roldan V.D., Niven A.S., et al. Anticoagulation in COVID-19: a systematic review, meta-analysis, and rapid guidance from Mayo Clinic. Mayo Clin. Proc. 2020;95(11):2467–2486. doi: 10.1016/j.mayocp.2020.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colling M.E., Kanthi Y. COVID-19-associated coagulopathy: an exploration of mechanisms. Vasc. Med. 2020;25(5):471–478. doi: 10.1177/1358863X20932640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaudhary R., Garg J., Houghton D.E., et al. Thrombo-inflammatory biomarkers in COVID-19: systematic review and meta-analysis of 17,052 patients. Mayo Clin. Proc. Innov. Qual. Outcomes. 2021;5(2):388–402. doi: 10.1016/j.mayocpiqo.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leonard-Lorant I., Delabranche X., Severac F., et al. Acute pulmonary embolism in patients with COVID-19 at CT angiography and relationship to d-dimer levels. Radiology. 2020;296(3):E189–E191. doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Llitjos J.F., Leclerc M., Chochois C., et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemost. 2020;18(7):1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lodigiani C., Iapichino G., Carenzo L. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nopp S., Moik F., Jilma B., Pabinger I., Ay C. Risk of venous thromboembolism in patients with COVID‐19: a systematic review and meta‐analysis. Research and Practice in Thrombosis and Haemostasis. 2020;4(7):1178–1191. doi: 10.1002/rth2.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porfidia A., Valeriani E., Pola R., Porreca E., Rutjes A.W.S., Nisio M.D. Venous thromboembolism in patients with COVID-19: systematic review and meta-analysis. Thromb. Res. 2020;196:67–74. doi: 10.1016/j.thromres.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiménez D., García-Sanchez A., Rali P., et al. Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019 a systematic review and meta-analysis. Chest. 2021;159(3):1182–1196. doi: 10.1016/j.chest.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallastegui N., Zhou J.Y., Drygalski A.V., RFW Barnes, Fernandes T.M., Morris T.A. Pulmonary embolism does not have an unusually high incidence among hospitalized COVID19 Patients. Clin. Appl. Thromb. Hemost. 2021;27(1-9) doi: 10.1177/1076029621996471. 107602962199647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaudhary R., Padrnos L., Wysokinska E., et al. Macrovascular thrombotic events in a Mayo Clinic Enterprise-wide sample of hospitalized COVID-19–Positive compared with COVID-19–Negative patients. Mayo Clin. Proc. 2021;96(7):1718–1726. doi: 10.1016/j.mayocp.2021.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Horo J.C., Cerhan J.R., Cahn E.J., Bauer P.R., Temesgen Z., Ebbert J.…Badley A.D. Mayo Clinic Proceedings. Vol. 96, No. 3. 2021. Outcomes of COVID-19 with the Mayo Clinic model of care and research; pp. 601–618. March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swartz J., Koziatek C., Theobald J., Smith S., Iturrate E. Creation of a simple natural language processing tool to support an imaging utilization quality dashboard. Int. J. Med. Inform. 2017;101:93–99. doi: 10.1016/j.ijmedinf.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Piazza G., Campia U., Hurwitz S., et al. Registry of arterial and venous thromboembolic complications in patients with COVID-19. J. Am. Coll. Cardiol. 2020;76(18):2060–2072. doi: 10.1016/j.jacc.2020.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rali P., O'Corragain O., Oresanya L., Yu D., Sheriff O., Weiss R.…Healy M. Incidence of venous thromboembolism in coronavirus disease 2019: an experience from a single large academic center. J. Vasc. Surg. Venous Lymphat. Disord. 2021;9(3):585–591. doi: 10.1016/j.jvsv.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill J.B., Garcia D., Crowther M., et al. Frequency of venous thromboembolism in 6513 patients with COVID-19: a retrospective study. Blood Adv. 2020;4(21):5373–5377. doi: 10.1182/bloodadvances.2020003083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganatra S., Dani S.S., Redd R., et al. Outcomes of COVID-19 in patients with a history of cancer and comorbid cardiovascular disease. J. Natl. Compr. Cancer Netw. 2020;1–10 doi: 10.6004/jnccn.2020.7658. [DOI] [PubMed] [Google Scholar]
- 23.Middeldorp S., Coppens M., van Haaps T.F., et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020;18(8):1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grillet F., Behr J., Calame P., Aubry S., Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected with pulmonary CT angiography. Radiology. 2020;296(3):E186–E188. doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klok F.A., Kruip M., van der Meer N.J.M., et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb. Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dalager-Pedersen Michael, Lund Lars Christian, Mariager Theis, Winther Rannva, Hellfritzsch Maja, Larsen Torben Bjerregaard, Thomsen Reimar Wernich, Johansen Nanna Borup, Søgaard Ole Schmeltz, Nielsen Stig Lønberg, Omland Lars Haukali, Lundbo Lene Fogt, Israelsen Simone Bastrup, Harboe Zitta Barrella, Pottegård Anton, Nielsen Henrik, Bodilsen Jacob. 2021. Venous Thromboembolism and Major Bleeding in Patients With Coronavirus Disease 2019 (COVID-19): A Nationwide, Population-Based Cohort Study, Clinical Infectious Diseases; p. ciab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roubinian N.H., Dusendang J.R., Mark D.G., et al. Incidence of 30-day venous thromboembolism in adults tested for SARS-CoV-2 infection in an integrated health care system in northern California. JAMA Intern. Med. 2021;181(7):997–1000. doi: 10.1001/jamainternmed.2021.0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heit J.A., Spencer F.A., White R.H. The epidemiology of venous thromboembolism. J. Thromb. Thrombolysis. 2016;41(1):3–14. doi: 10.1007/s11239-015-1311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spyropoulos A.C., Levy J.H., Ageno W., et al. Scientific and standardization committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020;18(8):1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lynn L., Reyes J.A., Hawkins K., et al. The effect of anticoagulation on clinical outcomes in novel coronavirus (COVID-19) pneumonia in a U.S. cohort. Thromb. Res. 2021;197:65–68. doi: 10.1016/j.thromres.2020.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuker A., Tseng E.K., Nieuwlaat R., et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021;5(3):872–888. doi: 10.1182/bloodadvances.2020003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emert R., Shah P., Zampella J.G. COVID-19 and hypercoagulability in the outpatient setting. Thromb. Res. 2020;192:122–123. doi: 10.1016/j.thromres.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts L.N., Whyte M.B., Georgiou L., et al. Postdischarge venous thromboembolism following hospital admission with COVID-19. Blood. 2020;136(11):1347–1350. doi: 10.1182/blood.2020008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eswaran H., Jarmul J.A., Shaheen A.W., Meaux D., Long T., Saccoccio D., Moll S. Vascular thromboembolic events following COVID‐19 hospital discharge: incidence and risk factors. Research and Practice in Thrombosis and Haemostasis. 2021;5(2):292–295. doi: 10.1002/rth2.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patell R., Bogue T., Koshy A., et al. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood. 2020;136(11):1342–1346. doi: 10.1182/blood.2020007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winters J.P., Callas P.W., Cushman M., Repp A.B., Zakai N.A. Central venous catheters and upper extremity deep vein thrombosis in medical inpatients: the medical inpatients and thrombosis (MITH) study. J. Thromb. Haemost. 2015;13(12):2155–2160. doi: 10.1111/jth.13131. [DOI] [PubMed] [Google Scholar]