Abstract
An internet questionnaire survey for investigating empirical antibiotic usage and bacterial superinfections in patients with coronavirus disease-2019 (COVID-19) in Japan was conducted among the chief physicians of respiratory disease departments of 715 Japanese Respiratory Society-certified hospitals using Google Forms between January 28, 2021 and February 28, 2021. Responses to the questionnaire survey were obtained from 198 of 715 hospitals (27.6%). The survey revealed that the complication incidences of community-acquired pneumonia; hospital-acquired pneumonia, including ventilator-associated pneumonia; and sepsis were 2.86, 5.59, and 0.99%, respectively, among patients with moderate/severe and critical COVID-19. Bacterial co-infection and secondary infection rarely affected patients with COVID-19 in Japan, and the isolated pathogens were not specific to these patients. Moreover, the anti-inflammatory effects of macrolides for COVID-19 were not observed in several studies. These results might be useful in clinical practice for COVID-19.
Keywords: COVID-19, Antibiotic stewardship, Community-acquired pneumonia, Hospital-acquired pneumonia, Sepsis
1. Introduction
The worldwide spread of the coronavirus disease-2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2, has resulted in more than four million deaths as of July 2021 [1]. Bacterial infections complicate viral airway infections, and those secondary to severe influenza virus infections are fatal. Therefore, it is crucial to focus on addressing secondary infection or co-infection in patients with COVID-19. Moreover, it is essential to investigate their epidemiology in patients with COVID-19 to practice antibiotic stewardship and reduce unnecessary antibiotic prescriptions. Several studies have reported low incidence rates [2,3]. However, nationwide large-scale data are limited. Therefore, we conducted a nationwide internet questionnaire surveillance study in Japan to clarify empiric antibiotic usage and incidences of bacterial pneumonia and sepsis, complicating COVID-19.
2. Materials and methods
An internet questionnaire survey was conducted among the chief physicians of the respiratory disease departments of 715 Japanese Respiratory Society-certified hospitals using Google Forms between January 28, 2021 and February 28, 2021. The questionnaire included the total number of COVID-19 cases diagnosed, based on reverse transcription-polymerase chain reaction testing in each hospital from the beginning of the COVID-19 pandemic to January 27, 2021. The questionnaire included COVID-19 severity according to the National Institute of Health treatment guidelines [4], use of empirical antibiotic treatment, type and duration of empirical antibiotic treatment, and complications of community-acquired pneumonia (CAP), hospital-acquired pneumonia (HAP) excluding ventilator-associated pneumonia (VAP), and sepsis. The contents of the questionnaires related to this study are summarized in supporting information. Informed written consent was not required because the study utilized anonymized patient data and was approved by the Japanese Respiratory Society.
3. Results
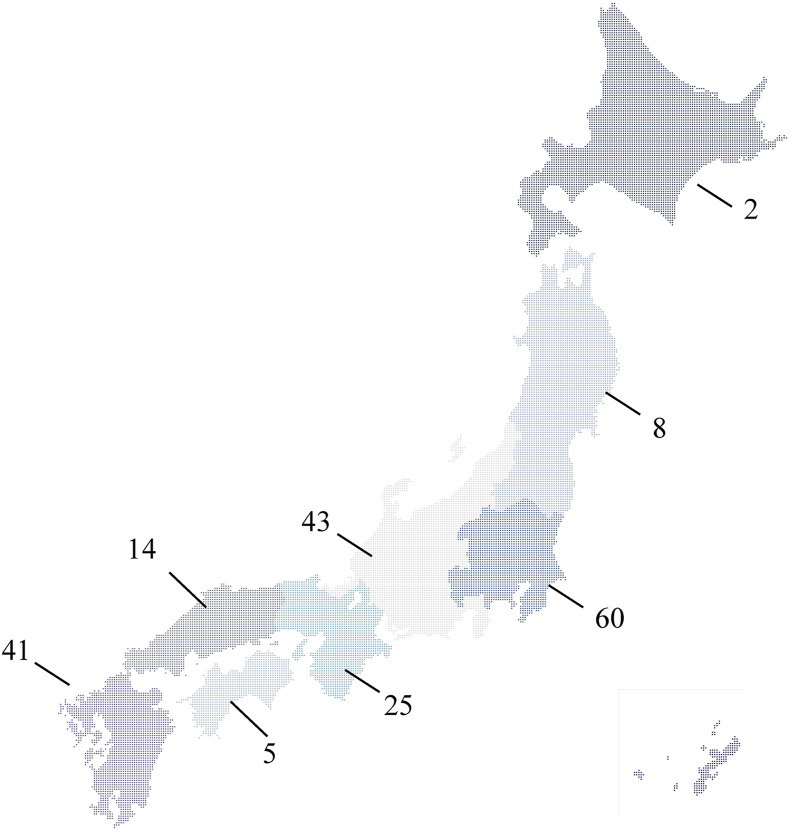
Responses to the questionnaire survey were obtained from 198 of 715 hospitals (27.6%). Of these facilities, approximately half were tertiary medical centers and 40 (20.2%) were university hospitals. The hospitals were distributed throughout Japan, with 2 in Hokkaido; 8 in Tohoku; 60 in Kanto, including Tokyo; 43 in Chubu-Hokuriku-Koshinetsu; 25 in Kinki; 14 in Chugoku; 5 in Shikoku; and 41 in Kyusyu (Fig. 1 ). A total of 10,047 patients with moderate/severe COVID-19 and 1664 patients with critical COVID-19 in all facilities participated in the survey.
Fig. 1.
Number of facilities that participated in this survey in each region of Japan.
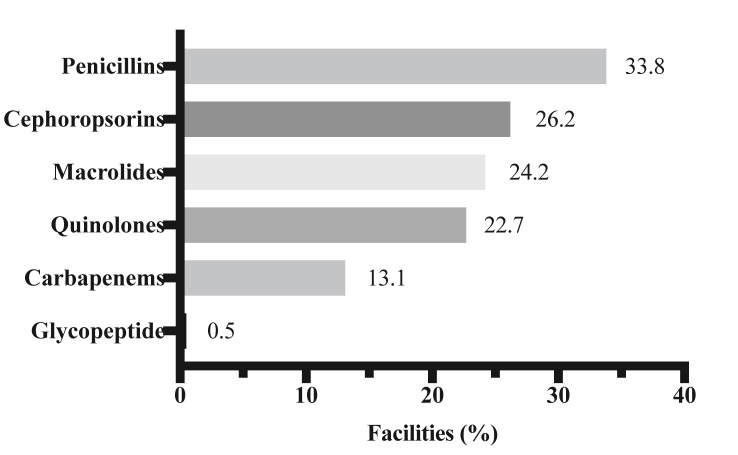
Answers to the question “Do you use empirical antibiotics treatment when patients with COVID-19 are admitted to your hospital?” were as follows: “Yes” (13%, n = 26), “Yes, if patients have severe pneumonia” (38%, n = 75), and “No” (49%, n = 97). The mean duration (±standard deviation) of empirical antibiotic treatment was 6.39 days (±2.92). The most frequently administered antibiotic was penicillin, followed by cephalosporins, macrolides, quinolones, and carbapenems (Fig. 2 ). In terms of complications, there were 336 cases of CAP, 403 cases of HAP excluding VAP, 252 cases of VAP, and 116 cases of sepsis (bacteremia and candidemia cases). The isolated or detected pathogens in pneumonia and sepsis in patients with COVID-19 are summarized in Table 1, Table 2 .
Fig. 2.
Types of antibiotics used for empirical therapy for patients with COVID-19 who were admitted in the facilities that participated in this survey. Multiple answers were allowed in this question.
Table 1.
Causative pathogens in bacterial pneumonia complicating COVID-19.
| Causative pathogens | Number of cases |
|---|---|
| Staphylococcus aureus | 33 |
| Streptococcus pneumoniae | 29 |
| Pseudomonas aeruginosa | 25 |
| Klebsiella pneumoniae | 23 |
| Haemophilus influenzae | 17 |
| Escherichia coli | 5 |
| Legionella spp. | 2 |
| Stenotrophomonas maltophilia | 3 |
| Others | 11 |
| Total | 148 |
Table 2.
Isolated pathogens in sepsis complicating COVID-19.
| Isolated pathogens | Number of cases |
|---|---|
| Staphylococcus aureus | 28 |
| Candida species | 9 |
| Pseudomonas aeruginosa | 7 |
| Escherichia coli | 7 |
| Staphylococcus epidermidis | 4 |
| Klebsiella pneumoniae | 2 |
| Others | 15 |
| Total | 72 |
4. Discussion
This report investigated empirical antibiotic usage and frequency of bacterial superinfections in patients with COVID-19 in Japan. Empirical usage rate was as high as 51% of the facilities, including for the answer option “if patients were in a severe condition due to COVID-19.” Antibiotic usage rate was not as high as those in previous studies by Rawson et al. [2] and Kabara et al. [3], in which the rates of antibiotic usage were 72% and 69%, respectively. Since the present study was conducted a couple months after these previous studies, the physicians may have already been aware that the co-infection rate was not as high as expected in the early phase of the COVID-19 pandemic. Macrolides were the third most frequently used antibiotic in our survey, and their anti-inflammatory effects have been utilized for COVID-19 treatment worldwide. However, azithromycin failed to improve survival rate or clinical outcomes in hospitalized patients with COVID-19 [5] and did not alleviate symptoms in outpatients with COVID-19 [6]. Therefore, empirical macrolide administrations should be avoided unless pneumonia due to atypical pathogens, such as Mycoplasma pneumoniae and Legionella spp., is highly suspected. Moreover, Petit et al. reported that reducing empiric antibiotic therapy including administration period did not affect the outcome of COVID-19 in a single-center study [7].
The complication rate of CAP was only 2.86% in patients with moderate/severe and critical COVID-19 (336/11,714). This did not differ from the result of a previous meta-analysis, which reported a complication rate of 3.5% (95% confidence interval [CI] 0.4–6.7%) [8]. Nosocomial pneumonia, including HAP and VAP, was observed in 5.59% (655/11,711), and sepsis was observed in 0.99% (116/11,711). The isolated bacterial pathogens in our survey were identical to those of previous studies on patients with COVID-19, which were common pathogens in CAP and HAP [9,10]. The secondary infection rate of these two complications was 6.58% (771/11,711), but overlapping cases were possibly included. Our data indicated a lower complication rate compared to 14.3% (95% CI, 9.6–18.9%), obtained from a previous meta-analysis [8]. The pathogens isolated from patients with sepsis in our survey were the typical pathogens of catheter-related bloodstream infections.
Our study had several limitations. First, the available data were limited to the nature of the questionnaire-based study, and the accuracy of data is lower compared to observational studies. Second, since the response rate from the survey was relatively low (27.6%) and biased toward referral centers, the results of this study might not be generalized. Third, the diagnosis of pneumonia was based on the judgment of each physician, rather than the defined diagnostic criteria. Fourth, our survey data were obtained from an internet questionnaire survey. Therefore, direct comparisons with previous observational studies are not applicable.
In conclusion, bacterial co-infection and secondary infection were rarely observed in patients with COVID-19 in this survey, and the isolated pathogens were not specific to patients with COVID-19. The results obtained from this survey might be useful in clinical practice for COVID-19. Further large-scale observational studies are required to confirm our result.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
H·K received honoraria from MSD Co., Ltd., Astellas Pharma Inc., Pfizer Inc., Asahi Kasei Pharma Corporation, Shionogi Co., Ltd., and Sumitomo Dainippon Pharma Co., Ltd. The other authors have no conflict of interest.
Acknowledgments
We appreciate the Japanese Respiratory Society-certified hospitals that participated in this survey.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resinv.2021.09.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Johns Hopkins University and Medicine. Coronavirus resource center.
- 2.Rawson T.M., Moore L.S.P., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M., et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71(9):2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karaba S.M., Jones G., Helsel T., Smith L.L., Avery R., Dzintars K., et al. Prevalence of co-infection at the time of hospital admission in COVID-19 patients, A multicenter study. Open Forum Infect Dis. 2021;8(1):ofaa578. doi: 10.1093/ofid/ofaa578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NIH. COVID-19 treatment guidelines, https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/.
- 5.RECOVERY Collaborative Group Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10274):605–612. doi: 10.1016/S0140-6736(21)00149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oldenburg C.E., Pinsky B.A., Brogdon J., Chen C., Ruder K., Zhong L., et al. Effect of oral azithromycin vs placebo on COVID-19 symptoms in outpatients with SARS-CoV-2 infection: a randomized clinical trial. JAMA. 2021 doi: 10.1001/jama.2021.11517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pettit N.N., Nguyen C.T., Lew A.K., Bhagat P.H., Nelson A., Olson G., et al. Reducing the use of empiric antibiotic therapy in COVID-19 on hospital admission. BMC Infect Dis. 2021;21(1):516. doi: 10.1186/s12879-021-06219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langford B.J., So M., Raybardhan S., Leung V., Westwood D., MacFadden D.R., et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metlay J.P., Waterer G.W. Treatment of community-acquired pneumonia during the coronavirus Disease 2019 (COVID-19) pandemic. Ann Intern Med. 2020;173(4):304–305. doi: 10.7326/M20-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Senok A., Alfaresi M., Khansaheb H., Nassar R., Hachim M., Al Suwaidi H., et al. Coinfections in patients hospitalized with COVID-19: a descriptive study from the United Arab Emirates. Infect Drug Resist. 2021;14:2289–2296. doi: 10.2147/IDR.S314029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.