Abstract
There is limited evidence about the prognostic utility of right ventricular dysfunction (RVD) in patients with coronavirus disease 2019 (COVID‐19). We assessed the association between RVD and mortality in COVID‐19 patients. We searched electronic databases from inception to February 15, 2021. RVD was defined based on the following echocardiographic variables: tricuspid annular plane systolic excursion (TAPSE), tricuspid S′ peak systolic velocity, fractional area change (FAC), and right ventricular free wall longitudinal strain (RVFWLS). All meta‐analyses were performed using a random‐effects model. Nineteen cohort studies involving 2307 patients were included. The mean age ranged from 59 to 72 years and 65% of patients were male. TAPSE (mean difference [MD], −3.13 mm; 95% confidence interval [CI], −4.08–−2.19), tricuspid S′ peak systolic velocity (MD, −0.88 cm/s; 95% CI, −1.68 to −0.08), FAC (MD, −3.47%; 95% CI, −6.21 to −0.72), and RVFWLS (MD, −5.83%; 95% CI, −7.47–−4.20) were significantly lower in nonsurvivors compared to survivors. Each 1 mm decrease in TAPSE (adjusted hazard ratio [aHR], 1.22; 95% CI, 1.08–1.37), 1% decrease in FAC (aHR, 1.09; 95% CI, 1.04–1.14), and 1% increase in RVFWLS (aHR, 1.33; 95% CI, 1.19–1.48) were independently associated with higher mortality. RVD was significantly associated with higher mortality using unadjusted risk ratio (2.05; 95% CI, 1.27–3.31), unadjusted hazard ratio (3.37; 95% CI, 1.72–6.62), and adjusted hazard ratio (aHR, 2.75; 95% CI, 1.52–4.96). Our study shows that echocardiographic parameters of RVD were associated with an increased risk of mortality in COVID‐19 patients.
Keywords: coronavirus disease 2019, mortality, right ventricular dysfunction, systematic review
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), continues to be a public health problem across the globe. 1 Although the lungs seem to be the main target of SARS‐CoV‐2 infection, there are a variety of cardiovascular presentations provoked directly or indirectly by SARS‐CoV‐2, including acute myocardial infarction, heart failure, arrhythmias, and myocarditis/pericarditis. 2 The right ventricular compromise is increasingly recognized as an important complication in COVID‐19 patients. 3 However, there is conflicted data about its prognostic utility in this population. Therefore, we performed a systematic review and meta‐analysis to assess the association between right ventricular dysfunction (RVD) and mortality in COVID‐19 patients.
2. METHODS
This review was reported according to the preferred reporting items for systematic reviews and meta‐analyses statement. 4
2.1. Search strategy
We searched in the following databases: PubMed, Embase, Scopus, and Web of Science. The search was conducted from inception to February 15, 2021. The complete search strategy is available in Table S1. There were no restrictions on publication date or language. Furthermore, we performed a manual search of reference lists of included studies and relevant reviews to identify additional studies.
2.2. Eligibility criteria
The inclusion criteria were the following: (i) Cohort studies that included adult patients (≥18 years old) diagnosed with COVID‐19 by reverse transcription‐polymerase chain reaction and (ii) Studies that assessed the association between RVD assessed by echocardiography and mortality. We excluded commentaries, conference abstracts, systematic reviews, and narrative reviews.
2.3. Study selection
We downloaded all articles from electronic search to EndNote X8 software and duplicate records were removed. Titles and abstracts were independently screened by two review authors (Carlos Diaz‐Arocutipa and Jose Saucedo‐Chinchay) to identify relevant articles. Additionally, the same review authors (Carlos Diaz‐Arocutipa and Jose Saucedo‐Chinchay) independently examined the full‐text of each article and registered reasons for the exclusion. Any disagreement on title/abstract and full‐text selection was resolved by consensus.
2.4. Echocardiographic parameters
We assess any of the following echocardiographic parameters: tricuspid annular plane systolic excursion (TAPSE), tricuspid S′ peak systolic velocity, fractional area change (FAC), and right ventricular free wall longitudinal strain (RVFWLS). In addition, if available, the investigator‐defined RVD was also evaluated.
2.5. Data extraction
The information from each study was independently extracted by two review authors (Carlos Diaz‐Arocutipa and Jose Saucedo‐Chinchay) using a standardized data extraction form that was previously piloted. Any disagreement was resolved by consensus. We extracted the following data: first author name, year of publication, country, study design, sample size, population, age, sex, comorbidities, timing of echocardiographic evaluation, echocardiographic parameters of right ventricular function, and mortality.
2.6. Risk of bias assessment
The Newcastle‐Ottawa scale (NOS) was used to assess the risk of bias of cohort studies. 5 Each study was classified into the following groups: low risk of bias (8–9 points), moderate risk of bias (5–7 points), and high risk of bias (0–4 points). The risk of bias was independently evaluated by two review authors (Carlos Diaz‐Arocutipa and Jose Saucedo‐Chinchay) and any disagreement was resolved by consensus.
2.7. Statistical analyses
We performed all meta‐analyses using a random‐effects model. The between‐study variance was estimated using the Paule‐Mandel estimator. 6 We pooled unadjusted and adjusted effect measures for binary variables and mean differences (MD) with their 95% confidence interval (95% CI) for continuous variables. In the case of studies that have only reported median and interquartile range, the mean and SD were estimated using the method published by Wan et al. 7 Heterogeneity among studies was evaluated using the chi‐squared test (threshold p < .10) and I2 statistic. Heterogeneity was defined as low if I2 < 30%, moderate if I2 = 30%–60%, and high if I2 > 60%. Publications bias was assessed by visual inspection of funnel plots. Statistical tests for funnel plot asymmetry were not performed since at least 10 studies per outcome are required. Subgroup analyses were performed according to the type of population (consecutive vs. nonconsecutive) and proportion of mechanically ventilated patients (<50% vs. ≥50%). All meta‐analyses were conducted using the meta package from R 3.6.3 (www.r-project.org). A two‐tailed p < .05 was considered statistically significant.
3. RESULTS
3.1. Study selection
Our electronic search retrieved 807 articles. After the removal of duplicates, 334 articles were screened by title/abstract, and of those, 253 were excluded. After a full‐text assessment of 81 remaining articles, 62 were excluded due to no full‐text (n=2), other population (n=5), conference abstract (n=5), commentary (n=8), other exposure (n=8), and other outcome (n=9). Finally, 19 articles were selected (Supplemental Figure 1).8‐26
3.2. Study characteristics
The main characteristics of the included studies were summarized in Table 1. A total of 2307 patients were included. The mean age ranged from 59 to 72 years and 65% of patients were male. Most studies were from Italy (n = 5) and United Kingdom (n = 4). The most common comorbidities were hypertension (51%), diabetes (25%), obesity (22%), and coronary artery disease (14%). The median number of days on which echocardiographic evaluation was performed, ranged from 1 to 8 days after hospital admission. RVFWLS was analyzed using the GE EchoPAC in four studies and the Philips QLAB 13 in two studies. The length of follow‐up varied across studies although was mostly at 30 days and in‐hospital. Three studies8,12,14 included only patients hospitalized in the intensive care unit (ICU), while the remaining studies included ICU and non‐ICU patients. The proportion of patients who required mechanical ventilation varied between 4% and 98%. Overall, the mortality ranged from 9% to 44% across studies. Information about the adjusted effect estimates and the included variables in the multivariate analysis are shown in Table S2. The echocardiographic parameters evaluated and the definitions of mortality and RVD can be found in Table S3.
TABLE 1.
Characteristics of included studies
| Study | Country | Sample size | Population | Timing of evaluation a | Age a | Male | Comorbidities | Mechanical ventilation | Mortality |
|---|---|---|---|---|---|---|---|---|---|
| Bagate, 2021 8 | France | 67 | Consecutive ICU patients with COVID‐19 | 1 (1–3) days after ICU admission | 61 (50–70) | 82% | Hypertension (54%), diabetes (36%), HF (10%), AF (9%) | 98% | 39% |
| Bursi, 2020 9 | Italy | 49 | Hospitalized patients with COVID‐19 who underwent TTE | 8 (4–15) days since symptoms onset | 65.7 ± 12.6 | 63% | Hypertension (49%), diabetes (18%), HF (6%), CAD (22%), COPD (12%), AF (8%) | 22% | 33% |
| Chen, 2020 10 | USA | 143 | Consecutive hospitalized patients with COVID‐19 | NR | 67 ± 16 | 62% | Hypertension (69%), diabetes (38%), CAD (30%), CKD (19%) | 35% | 28% |
| Crook, 2021 11 | UK | 27 | Hospitalized patients with COVID‐19 who required a TTE | NR | 64.5 (37–80) | 73% | Hypertension (43%), diabetes (20%), HF (7%), CAD (3%), stroke (7%) | 67% | 26% |
| D'Alto, 2020 12 | Italy | 94 | Hospitalized patients with ARDS and COVID‐19 | 3 (range 1–7 days) after admission | 63.6 ± 12.7 | 74% | Hypertension (67%), diabetes (17%), CAD (18%), obesity (33%) | 39% | 26% |
| D'Andrea, 2020 13 | Italy | 89 | Consecutive hospitalized patients with COVID‐19 | NR | 64.6 (range: 20–88) | 60% | NR | 9% | 18% |
| Gonzalez, 2020 14 | Spain | 52 | COVID‐19 Patients admitted to the ICU | 6 (2–10) days after ICU admission | 59.3 ± 13.5 | 69% | Hypertension (40%), diabetes (29%), CAD (2%), AF (4%), CKD (2%) | 88% | 15% |
| Kim, 2020 15 | USA | 268 | Consecutive hospitalized patients who underwent TTE | 6 (1–15) days after admission | 65.1 ± 14.1 | 62% | Hypertension (65%), diabetes (39%), CAD (20%), obesity (32%), COPD (7%) | 54% | NR |
| Lassen, 2020 16 | Denmark | 214 | Consecutive hospitalized patients with COVID‐19 | 4 (2–8) days after admission | 68.9 ± 13.5 | 55% | Hypertension (57%), diabetes (25%), hyperlipidemia (40%), HF (10%), CAD (16%), COPD (15%) | NR | 12% |
| Li 1, 2020 17 | China | 157 | Consecutive hospitalized patients with COVID‐19 | NR | 62.3 ± 13.1 | 50% | Hypertension (45%), diabetes (15%), obesity (15%), COPD (6%), CAD (17%), CKD (2%) | 24% | 15% |
| Li 2, 2020 18 | China | 120 | Consecutive hospitalized patients with COVID‐19 | 7 (3–10) days after admission | 61 ± 14 | 48% | Hypertension (40%), diabetes (12%), obesity (18%), COPD (5%), CAD (9%), CKD (14%) | 12% | 15% |
| Mahmoud, 2020 19 | UK | 74 | Hospitalized patients with COVID‐19 who underwent TTE | 5 (3–10) days after admission | 59 ± 13 | 78% | Hypertension (42%), diabetes (36%), CKD (11%), stroke (7%), CAD (9%) | 82% | 38% |
| Moody, 2020 20 | UK | 164 | Hospitalized patients with COVID‐19 who underwent TTE | 3 (2–5) days | 61 ± 13 | 78% | Hypertension (41%), diabetes (32%), CKD (12%), stroke (7%), CAD (13%) | 73% | 40% |
| Pagnesi, 2020 21 | Italy | 200 | Consecutive hospitalized non‐ICU patients with COVID‐19 | NR | 62 (55–74) | 65% | Hypertension (42%), diabetes (18%), CAD (7%), HF (3%), dyslipidemia (22%), COPD (5%) | 4% | 9% |
| Rath, 2020 22 | Germany | 123 | Consecutive hospitalized patients with COVID‐19 | Within 24 h after admission | 68 ± 15 | 63% | Hypertension (70%), diabetes (24%), dyslipidemia (37%), obesity (19%), CKD (11%), CAD (23%) | 40% | 13% |
| Rothschild, 2020 23 | Israel | 100 | Consecutive hospitalized patients with COVID‐19 who underwent speckle‐tracking TTE | Within 24 h after admission | 64.3 ± 20.7 | 64% | NR | 19% | 23% |
| Stockenhuber, 2020 24 | UK | 34 | Consecutive hospitalized patients with COVID‐19 who underwent TTE | NR | 72 ± 2.6 | 79% | Hypertension (53%), diabetes (35%), CKD (32%), CAD (9%), stroke (9%) | 32% | 44% |
| Szekely, 2020 25 | Israel | 200 | Consecutive hospitalized patients with COVID‐19 | Within 24 h after admission | 64.2 ± 19.2 | 60% | Hypertension (54%), diabetes (28%), CAD (15%), HF (11%), stroke (10%), COPD (6%), obesity (24%) | 21% | 14% |
| Xie, 2020 26 | China | 132 | Consecutive hospitalized patients with COVID‐19 | 7 (3–11) days after admission | 61 ± 13 | 51% | Hypertension (44%), diabetes (11%), obesity (15%), COPD (4%), CAD (14%), CKD (1%) | 24% | 14% |
Abbreviations: CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; COVID‐19, coronavirus disease 2019; HF, heart failure; ICU, intensive care unit; NR, not reported; TTE, transthoracic echocardiography; USA, United States of America; UK, United Kingdom.
Data are mean ± SD or median (interquartile range).
3.3. Risk of bias assessment
According to the NOS assessment, 10 studies had a moderate risk of bias and nine studies had a low risk of bias (Table S4). None of the studies was scored as high risk of bias.
3.4. Tricuspid annular plane systolic excursion
TAPSE was significantly lower (MD, −3.13 mm; 95% CI, −4.08 to −2.19; I2 = 63%) in nonsurvivors compared to survivors (Figure 1A). Each 1 mm decrease in TAPSE was significantly associated with a higher mortality using both unadjusted hazard ratio (uHR, 1.36; 95% CI, 1.15–1.62; I2 = 94%) and adjusted hazard ratio (aHR, 1.22; 95% CI, 1.08–1.37; I2 = 65%) (Figure 2A and Figure 3A). Funnel plots did not show asymmetry (Figure S2).
FIGURE 1.
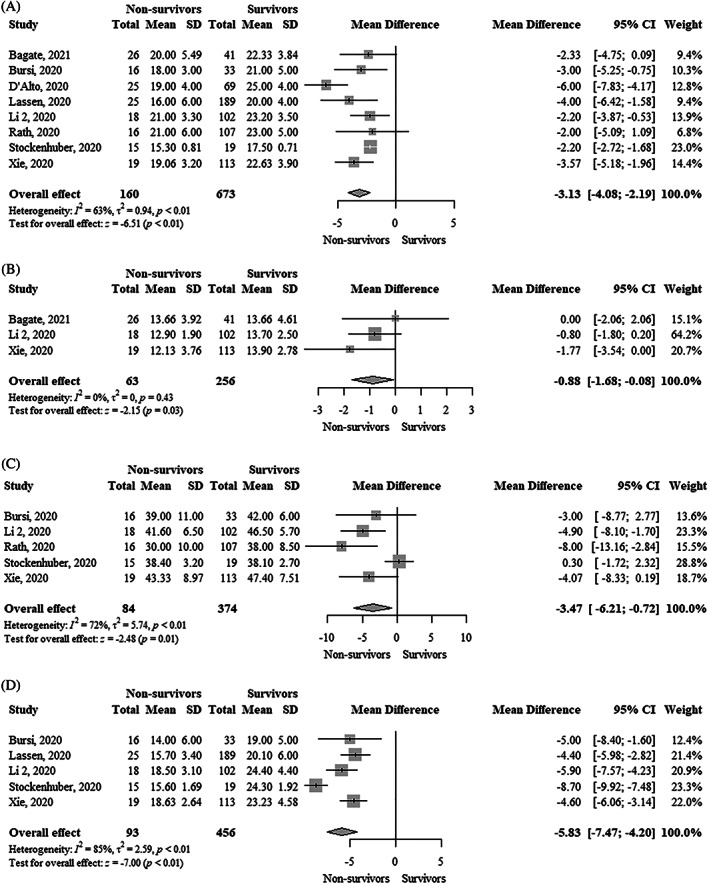
Forest plot showing the comparison of (A) Tricuspid annular plane systolic excursion (mm), (B) Tricuspid S′ peak systolic velocity (cm/s), (C) Fractional area change (%), and (D) Right ventricular free wall longitudinal strain (%) between survivors and nonsurvivors with COVID‐19. COVID‐19, coronavirus disease 2019
FIGURE 2.
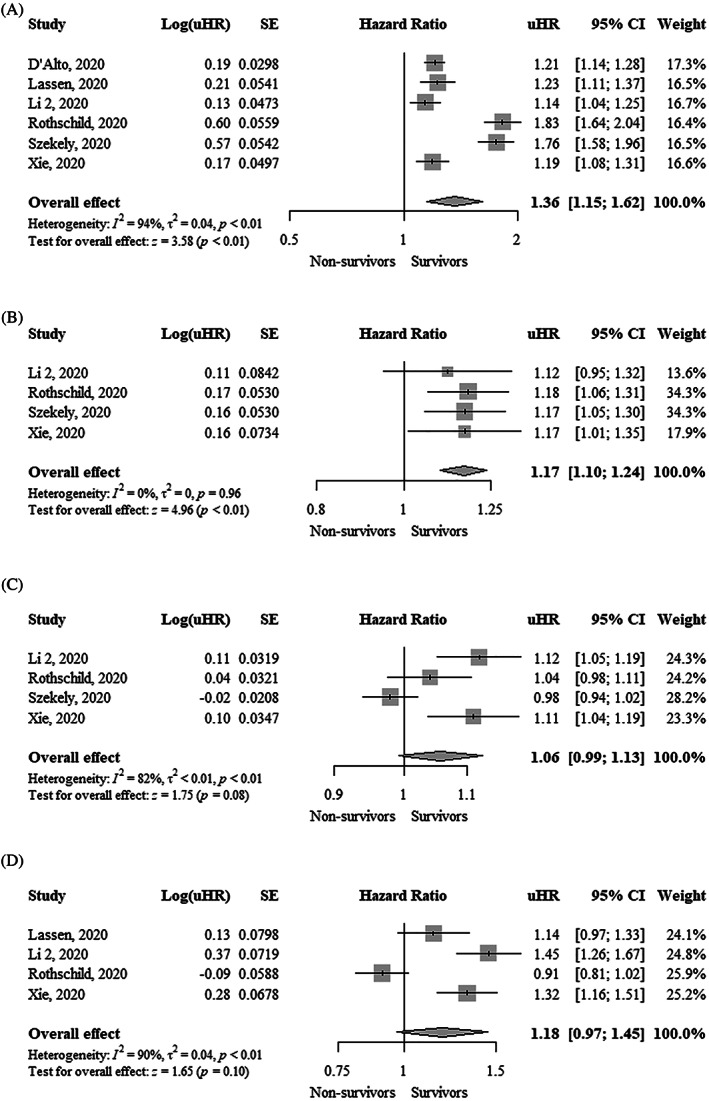
Forest plot showing the association between one unit change in (A) Tricuspid annular plane systolic excursion (mm), (B) Tricuspid S′ peak systolic velocity (cm/s), (C) Fractional area change (%), and (D) Right ventricular free wall longitudinal strain (%) and mortality in COVID‐19 patients (unadjusted estimates). CI, confidence interval; COVID‐19, coronavirus disease 2019; uHR, unadjusted hazard ratio
FIGURE 3.
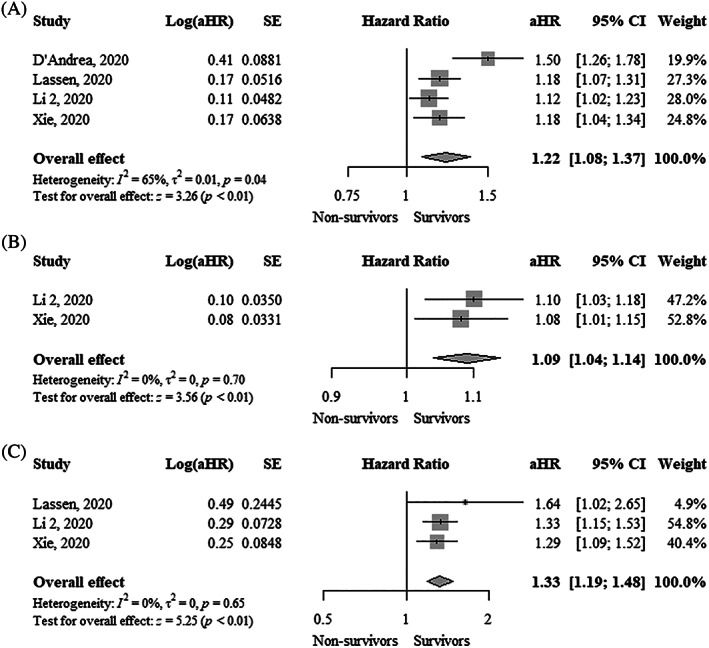
Forest plot showing the association between one unit change in (A) Tricuspid annular plane systolic excursion (mm), (B) Fractional area change (%), and (C) Right ventricular free wall longitudinal strain (%) and mortality in COVID‐19 patients (adjusted estimates). aHR, adjusted hazard ratio; CI, confidence interval; COVID‐19, coronavirus disease 2019
3.5. Tricuspid S′ peak systolic velocity
Tricuspid S′ peak systolic velocity was significantly lower (MD, −0.88 cm/s; 95% CI, −1.68 to −0.08; I2 = 0%) in nonsurvivors compared to survivors (Figure 1B). Funnel plot did not show asymmetry (Figure S3). Each 1 cm/s decrease in tricuspid S′ peak systolic velocity was significantly associated with higher mortality (uHR, 1.17; 95% CI, 1.10–1.24; I2 = 0%) (Figure 2B). Funnel plot showed asymmetry (Figure S3).
3.6. Fractional area change
FAC was significantly lower (MD, −3.47%; 95% CI, −6.21 to −0.72; I2 = 72%) in nonsurvivors compared to survivors (Figure 1C). FAC was not significantly associated with higher mortality using an uHR (1.06; 95% CI, 0.99–1.13; I2 = 82%) (Figure 2C). In contrast, the adjusted hazard ratio (aHR, 1.09; 95% CI, 1.04–1.14; I2 = 0%) showed that each 1% decrease in FAC was significantly associated with higher mortality (Figure 3B). Funnel plots did not show asymmetry (Figure S4).
3.7. Right ventricular free wall longitudinal strain
RVFWLS was significantly lower (MD, −5.83%; 95% CI, −7.47 to −4.20; I2 = 85%) in survivors compared to nonsurvivors (Figure 1D). RVFWLS was not significantly associated with higher mortality using aHR (1.18; 95% CI, 0.97–1.45; I2 = 90%) (Figure 2D). In contrast, the adjusted hazard ratio (aHR, 1.33; 95% CI, 1.19–1.48; I2 = 0%) showed that each 1% increase of RVFWLS was significantly associated with higher mortality (Figure 3C). Funnel plots did not show asymmetry (Figure S5).
3.8. Right ventricular dysfunction
Overall, 22% of patients had RVD and the absolute risk of mortality in patients with RVD was 40% in eight studies. RVD was significantly associated with higher mortality using unadjusted risk ratio (uRR, 2.05; 95% CI, 1.27–3.31; I2 = 64%), uHR (3.37; 95% CI, 1.72–6.62; I2 = 46%), and adjusted hazard ratio (aHR, 2.75; 95% CI, 1.52–4.96; I2 = 45%) (Figure 4). Funnel plots did not show asymmetry (Figure S6).
FIGURE 4.
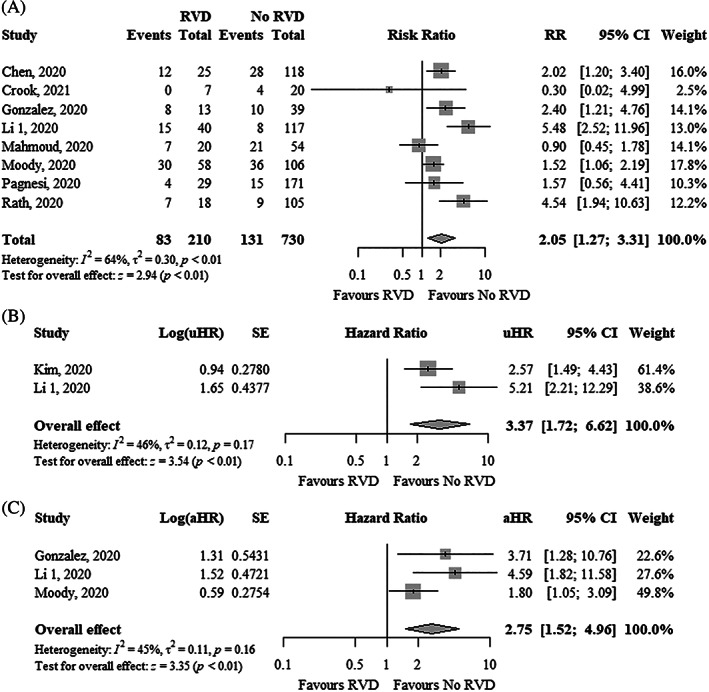
Forest plot showing the (A) RR, (B) uHR, and (C) aHR between right ventricular dysfunction and mortality in COVID‐19 patients. aHR, adjusted hazard ratio; CI, confidence interval; COVID‐19, coronavirus disease 2019; RR, risk ratio; uHR, unadjusted hazard ratio
3.9. Subgroup analyses
Subgroup analyses by type of population (consecutive vs. nonconsecutive) and proportion of mechanical ventilation (<50% vs. ≥50%) showed that RVD was significantly associated with an increased risk of mortality only in studies with consecutive samples (Figure S7) and studies with <50% of mechanically ventilated patients (Figure S8), respectively.
4. DISCUSSION
The current study demonstrates that abnormalities in the echocardiographic markers of RVD, namely, TAPSE, tricuspid S′ peak systolic velocity, FAC, and RVFWLS, were significantly associated with poor survival in patients with COVID‐19. Specifically, each 1 mm decrease in TAPSE, each 1% decrease of FAC, and each 1% increase of RVFWLS were independently associated with higher mortality. Likewise, RVD was significantly associated with higher mortality using unadjusted and adjusted outcome measures. The risk of bias was low or moderate across the studies.
Although it is well known that COVID‐19 patients can develop left and right ventricular abnormalities,27,28 several reports indicate that RVD occurs more frequently than left ventricular dysfunction in these patients.19,29,30 Furthermore, SARS‐CoV‐2 can cause pulmonary damage at different levels (vasculature, parenchyma, and interstitium) leading to patients requiring mechanical ventilation. These unique pathophysiological alterations place the right ventricle at high risk for failure in COVID‐19 patients.
RVD is a known prognostic factor in several cardiovascular disorders such as heart failure, pulmonary hypertension, and valvular heart disease.31 However, its role in systemic viral infections such as SARS‐CoV‐2 is less known. 3 Recently, there are several single‐center studies with small sample sizes that have evaluated the impact of RVD on the prognosis of patients with COVID‐19. The largest published study was a multicenter registry that included 268 hospitalized patients with COVID‐19 who underwent transthoracic echocardiography.15 This study found that right ventricular dilatation and dysfunction independently increased the risk of mortality. In addition, the adverse right ventricular remodeling (dilatation or dysfunction) provided incremental risk stratification beyond clinical‐ and biomarker‐based assessments. Interestingly, RVD occurred less frequently than dilatation and was present mainly in cases with greater dilatation, suggesting that probably dilatation is the initial response to the myocardial insult that ultimately results in RVD. We found that RVD was independently associated with an almost threefold increase in mortality in COVID‐19 patients. Overall, our finding highlights the possible prognostic value of the right ventricular function assessment that can potentially improve the risk stratification of these patients.
How RVD impacts clinical outcomes in COVID‐19 patients remains incompletely understood. RVD may contribute to the rapid hemodynamic deterioration of these patients by affecting the left ventricular forward stroke volume.31 Another possible explanation is that the presence of myocardial damage will provide the pathological substrate for malignant ventricular arrhythmias, predisposing to sudden cardiac death. However, although we found that RVD increases the risk of death in COVID‐19 patients, it is also possible the option of a common cause for both problems.
In the context of COVID‐19, the two main pathophysiological mechanisms that may explain RVD include increased afterload and impaired contractility.32 Although both mechanisms can coexist, the first mechanism seems to predominate because the right ventricle is highly sensitive to changes in afterload.
There are some clinical conditions associated with SARS‐CoV‐2 infection that can lead to increased afterload such as pulmonary thromboembolism, acute respiratory distress syndrome, and mechanical ventilation in intubated patients.32 Many studies have shown an elevated incidence of pulmonary thromboembolism in COVID‐19 patients.33 Likewise, several reports are showing that these patients exhibit marked alterations of the coagulation pathways as evidence by increased levels of D‐dimer, fibrinogen, prothrombin time, and activated partial thromboplastin time.34 A recent autopsy study of patients who died of COVID‐19 showed a higher incidence of deep vein thrombosis, pulmonary embolism, and microthrombi formation within the small to the mid‐sized pulmonary vasculature.35 This prothrombotic state can be explained by the interaction of several pathophysiological mechanisms. The initial response provoked by the SARS‐CoV‐2 infection is characterized by the release of pro‐inflammatory cytokines such as IL‐1β, IL‐6, and TNF‐α which, in turn, induces the activation of pro‐coagulant factors and platelets, impairs fibrinolysis, and promotes the endothelial damage.36 In addition, endothelial cells are an important viral target by expressing the ACE2 receptor. Reduced ACE2 expression may lead to an imbalance of the renin‐angiotensin‐aldosterone system favoring the pro‐coagulant and vasoconstrictor effects mediated by angiotensin II.36
Acute respiratory distress syndrome is a major cause of death in COVID‐19 patients with an incidence of up to 68% in hospitalized patients.37 RVD is an independent predictor of mortality in ARDS patients and its development is attributed to the increased pulmonary vascular resistance, which is mediated by vascular remodeling, right ventricular–pulmonary arterial uncoupling, and thrombosis/vasoconstriction of the pulmonary vasculature.38
Mechanical ventilation, especially when high levels of positive end‐expiratory pressure are required, reduces venous return and increases pulmonary vascular resistance.39 Considering that up to 33% of hospitalized patients with COVID‐19 are mechanically ventilated,40 the potential damage that this invasive therapy may cause to the right ventricle should be considered in all cases. Thus, the management of this equipment should be adequately optimized in order to reduce the risk of RVD.
Currently, it is recognized that several cardiac cells such as cardiomyocytes, pericytes, and fibroblasts can also express the ACE2 receptor and are therefore susceptible to infection by SARS‐CoV‐2.36 Many cases of acute myocarditis in patients with COVID‐19 have been described in the literature and it is considered a cause of right and/or left heart failure.41 Interestingly, there are published autopsy reports that have shown lymphocytic infiltrates in the right ventricular myocardium of these patients.35 Overall, it appears that direct viral damage, exacerbated systemic inflammatory response, and other pathogenic mechanisms such as epicardial and microvascular coronary artery disease secondary to thrombosis also contribute to the development of ventricular dysfunction in COVID‐19 patients.27
The assessment of right ventricular systolic function has historically been a difficult task given its complex geometric shape. Although echocardiography has some limitations, it remains the most commonly used imaging tool worldwide.42 TAPSE, tricuspid S′ peak systolic velocity, and FAC are the most frequently used echocardiographic parameters to evaluate right ventricular systolic function in daily clinical practice.42,43 Of note, these parameters can assess both longitudinal (e.g., TAPSE and tricuspid S′ peak systolic velocity) and radial function (e.g., FAC) of the right ventricle.44 More recent techniques such as speckle‐tracking echocardiography are increasingly used to estimate the global and regional systolic function.43 This technique allows evaluating the longitudinal myocardial deformation of the right ventricle, particularly of the free wall (e.g., RVFWLS). Among these four echocardiographic parameters, our results indicate that TAPSE, FAC, and RVFWLS were significantly associated with mortality in COVID‐19 patients in adjusted models. Overall, these findings suggest that both one‐ and two‐dimensional parameters have a prognostic value. Right ventricular ejection fraction by three‐dimensional echocardiography is another parameter that evaluates the global right ventricular systolic function. Unfortunately, none of the included studies in our study used this measure, although it seems to be more accurate and with better reproducibility compared to other parameters. Nevertheless, the assessment of right ventricular function by three‐dimensional echocardiography needs a very good acoustic. This parameter is quite difficult to obtain in ICU patients, particularly when ventilated and with often a poor acoustic window.
Our study has some limitations. First, heterogeneity was high in several pooled estimates. Possible reasons for heterogeneity include sample size, the definition of RVD, type of population, time of follow‐up, and type of echocardiogram analysts. Second, data on the prevalence of myocarditis and acute pulmonary thromboembolism was not reported in included studies, limiting the applicability of the proposed pathophysiological hypothesis. Third, since almost half of the included studies were conducted in only two countries (Italy and the United Kingdom), our results are not necessarily generalizable to other regions with different mortality rates. Fourth, the time from hospital admission to echocardiogram performance varied among studies with a median ranging from 1 day to 8 days and being performed in a quarter of the studies after approximately 1 week. This variation in echocardiogram time could influence the likelihood of developing RVD as patients could worsen during hospitalization, potentially introducing selection bias. Fifth, TAPSE and tricuspid S′ peak systolic velocity only reflects basal right ventricular function. In clinical conditions with regional dysfunction such as pulmonary thromboembolism, which has been reported in many COVID‐19 patients, both measures may underestimate global right ventricular function. Therefore, the prevalence of RVD may be even higher in the COVID‐19 setting. Finally, since most of the included studies did not report adjusted effects, there is an increased risk of bias in their pooled estimates. Although we also reported meta‐analyses of adjusted estimates of available studies, there is still a possibility of residual confounding. Thus, our results should be interpreted with caution.
5. CONCLUSION
Our review shows that RVD was associated with an increased risk of mortality in COVID‐19 patients. Among echocardiographic parameters, TAPSE, FAC, and RVFWLS were independently associated with higher mortality. Although our results need to be confirmed by prospective studies with larger sample sizes, they suggest that echocardiographic assessment of right ventricular systolic function may add prognostic information during the risk stratification of COVID‐19 patients.
CONFLICT OF INTEREST
None of the authors reported any conflicts of interest.
AUTHOR CONTRIBUTIONS
Carlos Diaz‐Arocutipa and Jose Saucedo‐Chinchay involved in concept/design. Carlos Diaz‐Arocutipa and Jose Saucedo‐Chinchay involved in data acquisition. Carlos Diaz‐Arocutipa, Jose Saucedo‐Chinchay, and Edgar Argulian involved in data analysis/interpretation. Carlos Diaz‐Arocutipa drafted the article. Jose Saucedo‐Chinchay and Edgar Argulian critically revised the article. Carlos Diaz‐Arocutipa, Jose Saucedo‐Chinchay, and Edgar Argulian approved the article.
Supporting information
Appendix S1: Supporting Information
Diaz‐Arocutipa C, Saucedo‐Chinchay J, Argulian E. Association between right ventricular dysfunction and mortality in COVID‐19 patients: A systematic review and meta‐analysis. Clin Cardiol. 2021;44(10):1360‐1370. doi: 10.1002/clc.23719
Contributor Information
Carlos Diaz‐Arocutipa, Email: cdiazar@usil.edu.pe.
Jose Saucedo‐Chinchay, Email: joseluissaucedo.ch@gmail.com.
Edgar Argulian, Email: edgar.argulian@mountsinai.org.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.WHO Coronavirus Disease (COVID‐19) Dashboard. https://covid19.who.int/ 2021.
- 2. Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID‐19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17(9):543‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lan Y, Liu W, Zhou Y. Right ventricular damage in COVID‐19: association between myocardial injury and COVID‐19. Front Cardiovasc Med. 2021;8:606318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wells G., Shea B., O'Connell D., Peterson J., Welch V., Losos M. and Tugwell P.. The Newcastle‐Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta‐analysis, 2021, http://www.ohri.ca/programs/clinical_epidemiology/oxfordasp.
- 6. Langan D, Higgins JPT, Simmonds M. Comparative performance of heterogeneity variance estimators in meta‐analysis: a review of simulation studies. Res Synth Methods. 2017;8(2):181‐198. [DOI] [PubMed] [Google Scholar]
- 7. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bagate F, Masi P, d'Humières T, et al. Advanced echocardiographic phenotyping of critically ill patients with coronavirus‐19 sepsis: a prospective cohort study. J Intensive Care Med. 2021;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bursi F, Santangelo G, Sansalone D, et al. Prognostic utility of quantitative offline 2D‐echocardiography in hospitalized patients with COVID‐19 disease. Echocardiography. 2020;37(12):2029‐2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen LQ, Burdowski J, Marfatia R, et al. Reduced cardiac function is associated with cardiac injury and mortality risk in hospitalized COVID‐19 patients. Clin Cardiol. 2020;43(12):1547‐1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crook RL, Williams H, Green M, et al. Prospective multicentre cohort study of transthoracic echocardiography provision in the south west of the UKduring the first wave of SARS‐CoV‐2 pandemic. Open Heart. 2021;8(1):e001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. D'Alto M, Marra AM, Severino S, et al. Right ventricular‐arterial uncoupling independently predicts survival in COVID‐19 ARDS. Crit Care. 2020;24(1):670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. D'Andrea A, Scarafile R, Riegler L, et al. Right ventricular function and pulmonary pressures as independent predictors of survival in patients with COVID‐19 pneumonia. JACC Cardiovasc Imaging. 2020;13(11):2467‐2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gonzalez‐Fernandez O, Ponz de Antonio I, Rosillo Rodriguez SO, Ruiz Cantador J, Figueira Iglesias JC, Lopez‐Sendon Hentschel JL. D‐dimer and right ventricular abnormalities as prognostic factors in critically ill COVID‐19 patients. Rev Esp Cardiol. 2020;73(11):966‐968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim J, Volodarskiy A, Sultana R, et al. Prognostic utility of right ventricular remodeling over conventional risk stratification in patients with COVID‐19. J Am Coll Cardiol. 2020;76(17):1965‐1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lassen MCH, Skaarup KG, Lind JN, et al. Echocardiographic abnormalities and predictors of mortality in hospitalized COVID‐19 patients: the ECHOVID‐19 study. ESC Heart Fail. 2020;7(6):4189‐4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li Y, Li H, Li M, Zhang L, Xie M. The prevalence, risk factors and outcome of cardiac dysfunction in hospitalized patients with COVID‐19. Intensive Care Med. 2020;46(11):2096‐2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li Y, Li H, Zhu S, et al. Prognostic value of right ventricular longitudinal strain in patients with COVID‐19. JACC Cardiovasc Imaging. 2020;13(11):2287‐2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mahmoud‐Elsayed HM, Moody WE, Bradlow WM, et al. Echocardiographic findings in patients with COVID‐19 pneumonia. Can J Cardiol. 2020;36(8):1203‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moody WE, Mahmoud‐Elsayed HM, Senior J, et al. Impact of right ventricular dysfunction on mortality in patients hospitalized with COVID‐19. CJC Open. 2021;3(1):91‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pagnesi M, Baldetti L, Beneduce A, et al. Pulmonary hypertension and right ventricular involvement in hospitalised patients with COVID‐19. Heart. 2020;106(17):1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rath D, Petersen‐Uribe Á, Avdiu A, et al. Impaired cardiac function is associated with mortality in patients with acute COVID‐19 infection. Clin Res Cardiol. 2020;109(12):1491‐1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rothschild E, Baruch G, Szekely Y, et al. The predictive role of left and right ventricular speckle‐tracking echocardiography in COVID‐19. JACC Cardiovasc Imaging. 2020;13(11):2471‐2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stockenhuber A, Vrettos A, Androschuck V, et al. A pilot study on right ventricular longitudinal strain as a predictor of outcome in COVID‐19 patients with evidence of cardiac involvement. Echocardiography. 2021;38:222–229. [DOI] [PubMed] [Google Scholar]
- 25. Szekely Y, Lichter Y, Hochstadt A, et al. The predictive role of combined cardiac and lung ultrasound in Coronavirus Disease. J Am Soc Echocardiogr. 2021;34:642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xie Y, Wang L, Li M, et al. Biventricular longitudinal strain predict mortality in COVID‐19 patients. Front Cardiovasc Med. 2020;7:632434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bieber S, Kraechan A, Hellmuth JC, et al. Left and right ventricular dysfunction in patients with COVID‐19‐associated myocardial injury. Infection. 2021;49(3):491‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Soulat‐Dufour L, Fauvel C, Weizman O, et al. Prognostic value of right ventricular dilatation in patients with COVID‐19: a multicentre study. Eur Heart J Cardiovasc Imaging. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Silverio A, Di Maio M, Scudiero F, et al. Clinical conditions and echocardiographic parameters associated with mortality in COVID‐19. Eur J Clin Invest. 2021;e13638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Szekely Y, Lichter Y, Taieb P, et al. Spectrum of cardiac manifestations in COVID‐19: a systematic echocardiographic study. Circulation. 2020;142(4):342‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sanz J, Sánchez‐Quintana D, Bossone E, Bogaard HJ, Naeije R. Anatomy, function, and dysfunction of the right ventricle: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2019;73(12):1463‐1482. [DOI] [PubMed] [Google Scholar]
- 32. Park JF, Banerjee S, Umar S. In the eye of the storm: the right ventricle in COVID‐19. Pulm Circ. 2020;10(3):2045894020936660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Malas MB, Naazie IN, Elsayed N Mathlouthi A Marmor R Clary B. Thromboembolism risk of COVID‐19 is high and associated with a higher risk of mortality: a systematic review and meta‐analysis. EClinicalMedicine. 2020;29:100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Luo HC, You CY, Lu SW, Fu YQ. Characteristics of coagulation alteration in patients with COVID‐19. Ann Hematol. 2021;100(1):45‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID‐19: a prospective cohort study. Ann Intern Med. 2020;173(4):268‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bonnemain J, Ltaief Z, Liaudet L. The right ventricle in COVID‐19. J Clin Med. 2021;10(12):2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tzotzos SJ, Fischer B, Fischer H Zeitlinger M. Incidence of ARDS and outcomes in hospitalized patients with COVID‐19: a global literature survey. Crit Care. 2020;24(1):516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zochios V, Parhar K, Tunnicliffe W, Roscoe A Gao F. The right ventricle in ARDS. Chest. 2017;152(1):181‐193. [DOI] [PubMed] [Google Scholar]
- 39. Disselkamp M, Adkins D, Pandey S, Coz Yataco AO. Physiologic approach to mechanical ventilation in right ventricular failure. Ann Am Thorac Soc. 2018;15(3):383‐389. [DOI] [PubMed] [Google Scholar]
- 40. Wunsch H. Mechanical ventilation in COVID‐19: interpreting the current epidemiology. Am J Respir Crit Care Med. 2020;202(1):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Castiello T Georgiopoulos G Finocchiaro G, et al. COVID‐19 and myocarditis: a systematic review and overview of current challenges. Heart Fail Rev. 2021;1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schneider M, Aschauer S, Mascherbauer J, et al. Echocardiographic assessment of right ventricular function: current clinical practice. Int J Cardiovasc Imaging. 2019;35(1):49‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1‐39.e14. [DOI] [PubMed] [Google Scholar]
- 44. Zaidi A, Knight DS, Augustine DX, et al. Echocardiographic assessment of the right heart in adults: a practical guideline from the British Society of Echocardiography. Echo Res Pract. 2020;7(1):G19‐g41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


