Abstract
Background
There is an unmet need for non-invasive biomarkers for the diagnosis of nonalcoholic steatohepatitis (NASH) in non-specialized settings. We aimed to develop and validate a non-invasive test for diagnosing NASH in individuals with biopsy-proven nonalcoholic fatty liver disease (NAFLD).
Methods
We developed a non-invasive test named the acNASH index that combines serum creatinine and aspartate aminotransferase levels in a derivation cohort of 390 Chinese NAFLD patients admitted to the hepatology center of the First Affiliated Hospital of Wenzhou Medical University (China) between December 2016 and September 2019 and subsequently validated in five external cohorts of different ethnicities of patients with biopsy-confirmed NAFLD (pooled n=1,089).
Findings
The performance of the acNASH index for identifying NASH (defined as NAFLD activity score ≥5 with score of ≥1 for each steatosis, lobular inflammation and ballooning) was good in the derivation cohort with an area under receiver operating characteristics (AUROC) of 0·818 (95%CI 0·777-0·860). A cutoff of acNASH index <4·15 gave a sensitivity (Se) of 91%, a specificity (Sp) of 48% and a negative predictive value (NPV) of 83% for ruling-out NASH, conversely, a cutoff of acNASH >7·73 gave a Sp of 91%, Se of 53% and a positive predictive value (PPV) of 85% for ruling-in NASH. In the pooled validation cohort (n=1,089), the diagnostic performance of the index was also good with AUROC=0·805 (95%CI 0·780-0·830), NPV of 93% for ruling-out NASH and PPV of 73% for ruling-in NASH. Subgroup analyses showed similar performance in patients with diabetes or subjects with normal serum transaminase levels.
Interpretation
The acNASH index shows promising utility as a simple non-invasive biomarker for diagnosing NASH among adults with biopsy-proven NAFLD of different ethnicities from different countries.
Funding
The National Natural Science Foundation of China (82070588), High Level Creative Talents from Department of Public Health in Zhejiang Province (S2032102600032) and Project of New Century 551 Talent Nurturing in Wenzhou.
Keywords: Nonalcoholic steatohepatitis, primary care, screening, metabolic dysfunction-associated fatty liver disease, nonalcoholic fatty liver disease, scoring system
Abbreviations: NASH, nonalcoholic steatohepatitis; NAFLD, nonalcoholic fatty liver disease; AUROC, area under receiver operating characteristics; PPV, positive predictive value; NPV, negative predictive value; T2DM, type 2 diabetes mellitus; ALT, alanine aminotransferase; PERSONS, Prospective Epidemic Research Specifically Of NASH; e-GFR, estimated glomerular filtration rate; SCr, serum creatinine; HBV, chronic viral hepatitis B; HCV, chronic viral hepatitis C; BMI, body mass index; NAS, NAFLD Activity Score; CRN, Clinical Research Network; Se, sensitivity; Sp, specificity; GAA, guanidine-acetic acid; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; AST, aspartate aminotransferase
Research in context.
Evidence before this study
The global prevalence of nonalcoholic steatohepatitis (NASH) is increasing but liver biopsy remains the gold standard for diagnosing NASH. To date, there is no simple noninvasive test for diagnosing NASH. We searched the PubMed database from Jan 1, 2010, to Dec 31, 2020 for research published with the search terms “nonalcoholic steatohepatitis” AND “diagnosis”, without language restrictions.
Added value of this study
Younger age, higher serum aspartate aminotransferase (AST) and lower serum creatinine (SCr) levels were independent predictors of NASH. A simple formula – the acNASH index, was derived from serum AST and SCr levels to identify patients with biopsy-proven NASH. The acNASH index showed better performance compared to existing non-invasive prediction models and had satisfactory discriminatory ability for identifying NASH in each tested external validation cohort.
Implications of all the available evidence
These results may provide a simple method to accurately identify patients with suspected NASH, who might be candidates for potential pharmacological therapy, specialist referral, or further histologic liver evaluation. The acNASH index may also reduce the number of patients with NAFLD who are subjected to an unnecessary liver biopsy.
Alt-text: Unlabelled box
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) affects more than a quarter of the world's adult population, paralleling the escalating incidence of obesity around the global world [1]. Within the spectrum of liver disease in NAFLD, non-alcoholic steatohepatitis (NASH), which is associated with an accelerated fibrosis progression, heralds the liver condition that greatly increases mortality [2]. The mortality rate has been estimated to be 25.6 per 1,000 person-years, doubling the mortality rate of overall NAFLD population where it is 11.77 per 1,000 person-years. NAFLD has become the second-leading indication for liver transplantation in the USA and Europe, although continues to cause considerable health, social and economic burdens in the next few years globally [1,3].
The global prevalence of NASH is as high as 37% amongst patients with type 2 diabetes mellitus (T2DM) [4]. However, a diagnosis of NASH is often not considered even when patients have increased serum alanine aminotransferase (ALT) levels [5]. Also, identifying patients with NASH in primary care or in non-specialist clinics is uncommon in many parts of the world. Even more worrying is the fact that 90% of all individuals with NAFLD have fairly normal serum ALT levels, as shown in the US-National Health and Nutrition Examination Survey III database [6]. Thus, it is crucial to determine what proportion of subjects with normal serum ALT levels or with type 2 diabetes mellitus has NASH, to screen who may at greatest risk of clinical progression and benefit from treatment with the emerging range of new pharmacotherapies, in primary care centers to referral.
Liver biopsy is currently the ‘gold standard’ for diagnosing NASH, however, its low acceptance and unwanted acute complications are well documented [7]. Consequently, there is an urgent need for an accurate and non-invasive test for identifying patients with NASH that could be used in non-specialist settings [8]. Thus, the aim of this study was to develop and validate a simple and reliable non-invasive test to identify patients with NASH in a derivation cohort, then validate the test in five different cohorts of patients of different ethnicities across the globe.
2. Methods
2.1. Study design
The development and validation of the new non-invasive test was reported following the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) as detailed in Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis TRIPOD checklist.
2.2. Derivation cohort
2.2.1. Study and participants
Participants were recruited from the well-characterized Prospective Epidemic Research Specifically Of NASH (PERSONS) cohort [9]. All these participants were admitted to the hepatology center of the First Affiliated Hospital of Wenzhou Medical University because of abnormal liver imaging, abnormal liver function test, and/or abnormal fibrosis tests between December 2016 to September 2019. Individuals interested in participating will be provided an explanation of the study by a hepatologist until they have completely understood the risks and benefits. The protocol of the study was approved by the internal review board for ethics at the First Affiliated Hospital of Wenzhou Medical University (2016-246, 1 December 2016) and the same protocol was registered in the Chinese Clinical Trial Registry (ChiCTR-EOC-17013562). All participants signed a written informed consent to participate in this study.
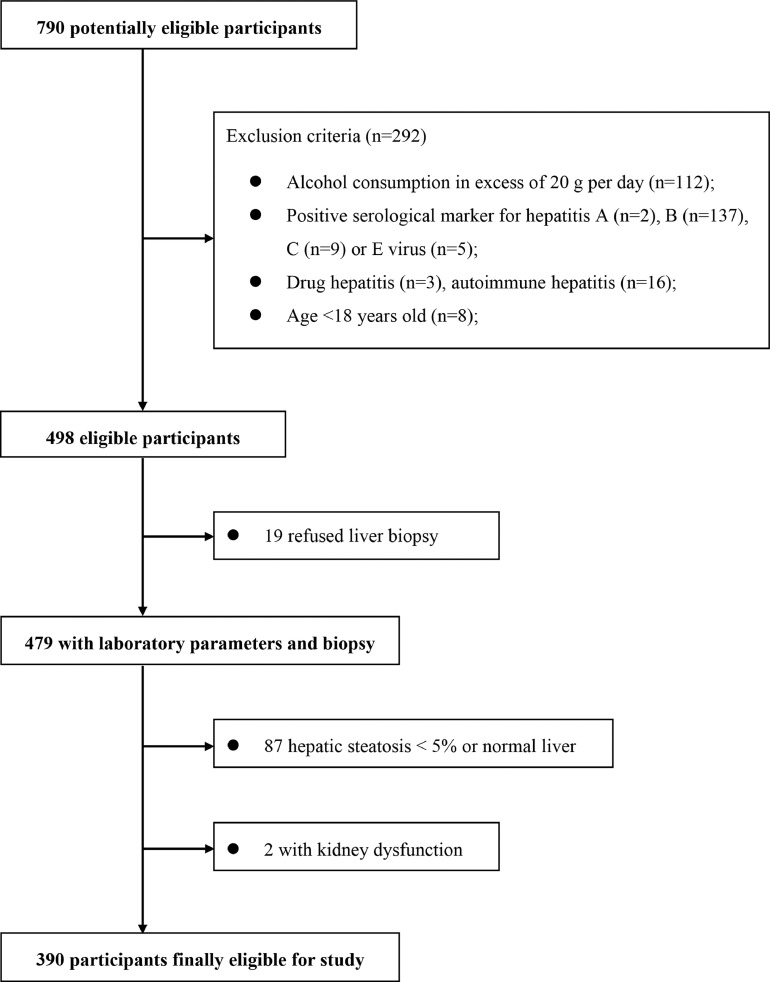
A total of 790 potentially eligible participants who had undergone liver biopsy were consecutively recruited from December 2016 to January 2019 at the First Affiliated Hospital of Wenzhou Medical University. As shown in Figure 1, from the initial cohort of participants with suspected NAFLD (based on imaging methods and/or persistently elevated serum liver enzymes) we excluded 400 patients with: (1) kidney dysfunction (i.e. those with a previous history of kidney disease such as other known causes of chronic or acute kidney diseases, as well as presence of urinary tract infection, overt proteinuria, estimated glomerular filtration rate (e-GFR) <60 ml/min/1.73 m2 or serum creatinine (SCr) levels >133 μmol/L); (2) liver diseases other than NAFLD, such as chronic viral hepatitis B [HBV] or C [HCV] (i.e. defined as positivity for HBV surface antigen, HBV DNA, anti-HCV antibodies and HCV RNA), and autoimmune hepatitis (based on serum autoantibodies or histology); (3) use of potentially hepatotoxic drugs; (4) significant alcohol intake (defined as more than 20 grams of alcohol per day for both sexes); (5) congestive heart failure or significant valvular diseases; (6) active cancers; (7) liver steatosis <5% on liver biopsy; and (8) missing data of relevant clinical variables.
Figure 1.
Flow diagram for the Chinese derivation study.
2.2.2. Patient characteristics
The following variables were recorded for each patient: age, sex, height, weight, body mass index (BMI), waist circumference, hip circumference, body composition analysis (including skeletal muscle, muscle weight and fat free weight), presence of diabetes and hypertension. For each participant, an 8-hour fasting blood sample was taken and then transported within 1-hour to our central laboratory for assessment.
2.2.3. Liver biopsy examination
All the eligible patients underwent ultrasound-guided percutaneous biopsy (16-gauge Hepafix needle). Liver specimens were embedded in paraffin and underwent H&E and Masson's trichrome staining. An experienced liver pathologist (X.D. Wang), who was blinded to participants’ clinical data, reviewed all liver histology specimens. Histological parameters, such as steatosis, ballooning and lobular inflammation, were scored according to the NAFLD Activity Score (NAS) system according to Kleiner et al. [10] NAFLD was diagnosed by observing steatosis grade >5% without other causes of fatty liver, and definite NASH was considered as NAS ≥5 with score of ≥1 for each steatosis, lobular inflammation and ballooning, using the NASH Clinical Research Network (CRN) scoring system.
2.2.4. Outcome
The main outcome was to develop and validate an index combining serum creatinine and aspartate aminotransferase for the diagnostic of definite NASH.
2.2.5. External validation cohorts
We included patients with biopsy-proven NAFLD from five international cohorts: France (Department of Hepato-Gastroenterology, Angers University Hospital, Angers), Turkey (Liver Research Unit, Institute of Gastroenterology, Marmara University, Istanbul), Malaysia (Gastroenterology and Hepatology Unit, Gastrointestinal Endoscopy Unit, Department of Medicine, University of Malaya, Kuala Lumpur), Egypt (Department of Endemic Medicine, Faculty of Medicine, Helwan University, Cairo) and Spain (Hospital Universitario Virgen del Rocío de Sevilla, Instituto de Biomedicina de Sevilla, Biomedical Research Networking Center in Hepatic and Digestive Diseases, Sevilla). The data from these five tertiary Gastroenterology centers were collected to perform the external validation cohort of the acNASH index.
All data from the external validation cohorts were gathered within the framework of clinical studies approved by the local ethics committees and in accordance with the declaration of Helsinki. Patients who participated in the study from each country all gave written consent. Date of enrollment and description for each study are provided in Supplementary Table 1. All liver biopsies were analyzed by experienced liver pathologists, who were blinded to patients’ clinical details. Histological grading for patients from each study were assessed according to the NASH-CRN scoring system [10]. For the external validation cohorts we imposed the same inclusion criteria as for the derivation cohort, as specified above. Potential risks of bias in each external validation cohort were evaluated in Supplementary Table 2.
2.2.6. Statistical analysis
Statistical analyses were performed using SPSS (version R23.0.0.0) and MedCalc (version 18.2.1, MedCalc software bvba). Continuous variables were expressed as mean ± SD and compared using the Student's t-test. Categorical variables were expressed as frequency (%) and compared using the chi-square test or the Fisher's exact test, as appropriate. Correlation was evaluated by the Spearman's rank correlation coefficients. To explore potential biomarkers for the prediction model, univariable regression analysis assessing forty-two variables was performed in the derivation cohort. All continuous variables were calculated to assess the linear relationship with logit p (a probability of NASH occurrence), otherwise, logarithmic transformation for independent variables, was undertaken for logistic multivariable regression analyses. Continuous variables that were not normally distributed, and that it was not possible to normalize by logarithmic transformation, also was not satisfactory using logarithm transformation were eliminated from further analyses. Significant variables from the univariable regression analysis combined with clinical results were then subjected to multivariable regression analysis by forward logistic regression to identify independent factors associated with definite NASH on liver histology (as defined above in the Methods section). A 2-sided P-values <0·05 were considered statistically significant.
A formula that could best predict the presence of NASH was constructed and developed into a risk score. The risk score was then simplified into a prediction index termed the acNASH index. ROC curves were used to assess the diagnostic performance of the acNASH index and compared with other established non-invasive models for predicting NASH. Cut-off values giving the best balance of Se and Sp were identified using the Youden's index (i.e. Se and Sp ≥0·91) in the derivation cohort and applied to the external cohorts. When appraising performance at a given cut-off, Se, Sp, PPV and NPV were analysed. External validation of the acNASH index was performed on each external cohort.
2.2.7. Role of the funding source
The study sponsor had no role in the design, data collection, or implementation of the study, or the analysis or reporting of the results. Ming-Hua Zheng had full access to all the data in the study and accept responsibility to submit for publication.
3. Results
A total of 390 patients with biopsy-proven NAFLD were enrolled in the derivation cohort for constructing the acNASH index, and 1,089 patients with biopsy-proven NAFLD from five independent Gastroenterology centers were enrolled in the external validation cohort. The main clinical, biochemical and liver histology characteristics of patients in the derivation cohort are summarized in Table 3.
Table 3.
Clinical and histological characteristics of patients in the derivation cohort of Chinese patients with biopsy-proven NAFLD.
| Variables | Non-NASH | Definite NASH | P value |
|---|---|---|---|
| (N =200) | (N =190) | ||
| Demographics | |||
| Age (years) | 43·73±11·14 | 38·26±12·22 | <0·001 |
| Sex (male) | 156 (78·0%) | 143 (75·3%) | 0·049 |
| Body measurements | |||
| Height (cm) | 168·36±7·88 | 167·41±8·88 | 0·268 |
| Weight (kg) | 75·09±12·31 | 77·47±14·37 | 0·080 |
| BMI (kg/m2) | 26·40±3·21 | 27·47±3·41 | 0·002 |
| Waist circumference (cm) | 91·12±8·64 | 92·86±8·81 | 0·054 |
| WHR | 0·93±0·06 | 0·92±0·06 | 0·728 |
| Body composition analysis | |||
| Skeletal muscle mass(kg) | 29·48±8·26 | 30·66±6·38 | 0·209 |
| Total muscle mass (kg) | 49·62±13·43 | 50·82±11·03 | 0·441 |
| Fat free mass (kg) | 52·48±14·21 | 53·80±11·67 | 0·421 |
| Fat mass (kg) | 19·69±7·72 | 24·45±6·82 | <0·001 |
| Laboratory parameters | |||
| AST (U/L) | 33·60±19·27 | 60·06±36·78 | <0·001 |
| ALT (U/L) | 51·53±54·15 | 103·36±78·88 | <0·001 |
| AST/ALT ratio | 0·78±0·30 | 0·69±0·30 | 0·003 |
| GGT (U/L) | 60·35±67·11 | 82·41±105·91 | 0·014 |
| Alkaline phosphatase (U/L) | 83·17±25·41 | 87·66±32·80 | 0·063 |
| Albumin (g/L) | 46·10±3·54 | 46·70±4·20 | 0·131 |
| Platelet count (x109/L) | 240·88±58·91 | 248·94±56·86 | 0·170 |
| Hemoglobin (g/L) | 148·26±13·15 | 148·77±14·58 | 0·715 |
| Fasting glucose (mmol/L) | 5·58±1·43 | 5·80±1·65 | 0·162 |
| HbA1c (%) | 6·20±1·50 | 6·03±1·23 | 0·235 |
| HOMA-IR | 4·71±6·56 | 6·35±9·00 | 0·040 |
| BUN (mmol/L) | 4·97±1·26 | 4·59±1·25 | 0·003 |
| Creatinine (μmol/L) | 69·78±13·76 | 64·38±14·24 | <0·001 |
| e-GFR | 112·38±17·47 | 120·26±18·36 | <0·001 |
| INR | 0·97±0·06 | 0·97±0·06 | 0·991 |
| Total bilirubin (μmol/L) | 14·07±6·70 | 14·45±6·54 | 0·564 |
| Total cholesterol (mmol/L) | 4·91±1·18 | 5·30±1·11 | 0·001 |
| Triglyceride (mmol/L) | 2·19±1·29 | 2·37±1·44 | 0·211 |
| Uric acid (μmol/L) | 380·07±88·72 | 414·66±118·84 | 0·001 |
| Alpha-fetal protein (ng/ml) | 3·20±1·58 | 3·06±2·19 | 0·483 |
| Hyaluronic acid (ng/ml) | 50·21±34·20 | 53·45±54·73 | 0·487 |
| P3NP (ng/ml) | 18·58±5·51 | 25·11±30·18 | 0·004 |
| Ⅳ-C (ng/ml) | 18·14±5·34 | 22·68±11·94 | <0·001 |
| Laminin (ng/ml) | 8·77±7·72 | 10·42±10·42 | 0·098 |
| Novel biomarkers related to NAFLD | |||
| CK-18 M30 (U/L) | 175·78±195·09 | 449·64±615·02 | <0·001 |
| Concomitant diseases | |||
| Hypertension (%) | 65 (32·5%) | 61 (32·1%) | 0·934 |
| Type 2 diabetes (%) | 61 (30·5%) | 50 (26·3%) | 0·647 |
| Non-invasive models | |||
| ION | 43·26±58·41 | 68·10±82·17 | <0·001 |
| HAIR | 0·84±0·66 | 1·16±0·60 | <0·001 |
| NICE model | -2·61±2·39 | 0·07±3·61 | <0·001 |
| Histological characteristics | |||
| Steatosis | <0·001 | ||
| 1 | 146 (73·0%) | 10 (5·3%) | |
| 2 | 46 (23·0%) | 48 (25·3%) | |
| 3 | 8 (4·0%) | 132 (69·4%) | |
| Ballooning | <0·001 | ||
| 0 | 37 (18·5%) | 0 | |
| 1 | 127 (63·5%) | 83 (43·7%) | |
| 2 | 36 (18·0%) | 107 (56·3%) | |
| Lobular inflammation | <0·001 | ||
| 0 | 23 (11·5%) | 0 | |
| 1 | 173 (86·5%) | 112 (58·9%) | |
| 2 | 4 (2·0%) | 75 (39·5%) | |
| 3 | 0 | 3 (1·6%) | |
| NAS | 3·21±0·75 | 5·63±0·70 | <0·001 |
| Fibrosis stage | 0·257 | ||
| 0 | 69 (34·5%) | 45 (23·8%) | |
| 1 | 95 (47·5%) | 97 (51·3%) | |
| 2 | 31 (15·5%) | 35 (18·5%) | |
| 3 | 5 (2·5%) | 10 (5·3%) | |
| 4 | 0 | 2 (1·1%) | |
Note: ALT = alanine aminotransferase, AST = aspartate aminotransferase, BMI = body mass index, BUN = blood urea nitrogen, CK-18 M30 = cytokeratine-18 neoepitope M30, e-GFR = estimated glomerular filtration rate, GGT = γ-glutamyl transferase, HbA1c = glycated hemoglobin, HOMA-IR = homeostasis model assessment of insulin resistance, INR = international normalized ratio, Ⅳ-C = type IV collagen, NAS = NAFLD activity score, P3NP = procollagen-3 N-terminal peptide, WHR = Waist to hip ratio
Variables associated with definite NASH were first calculated using univariable regression analysis. All continuous variables were calculated to assess the linear relationship with logit p, otherwise, logarithmic transformation was undertaken for multivariable analyses. Age, sex, BMI, AST, ALT, ALT/AST ratio, creatinine, eGFR, total cholesterol, uric acid, P3NP, type IV collagen were statistically significant, but sex had no clinical significance while HOMA-IR had clinical significance. Subsequent multivariable regression analysis including 9 variables showed that younger age, higher serum AST and lower serum Cr levels were independent predictors of NASH (Supplementary Table 3). The best regression formula determined by the multivariable regression analysis for predicting definite NASH was constructed as follows: risk score = -0·031 * age – 2·352 * Ln SCr (μmol/L) + 1·988 * Ln AST (U/L).
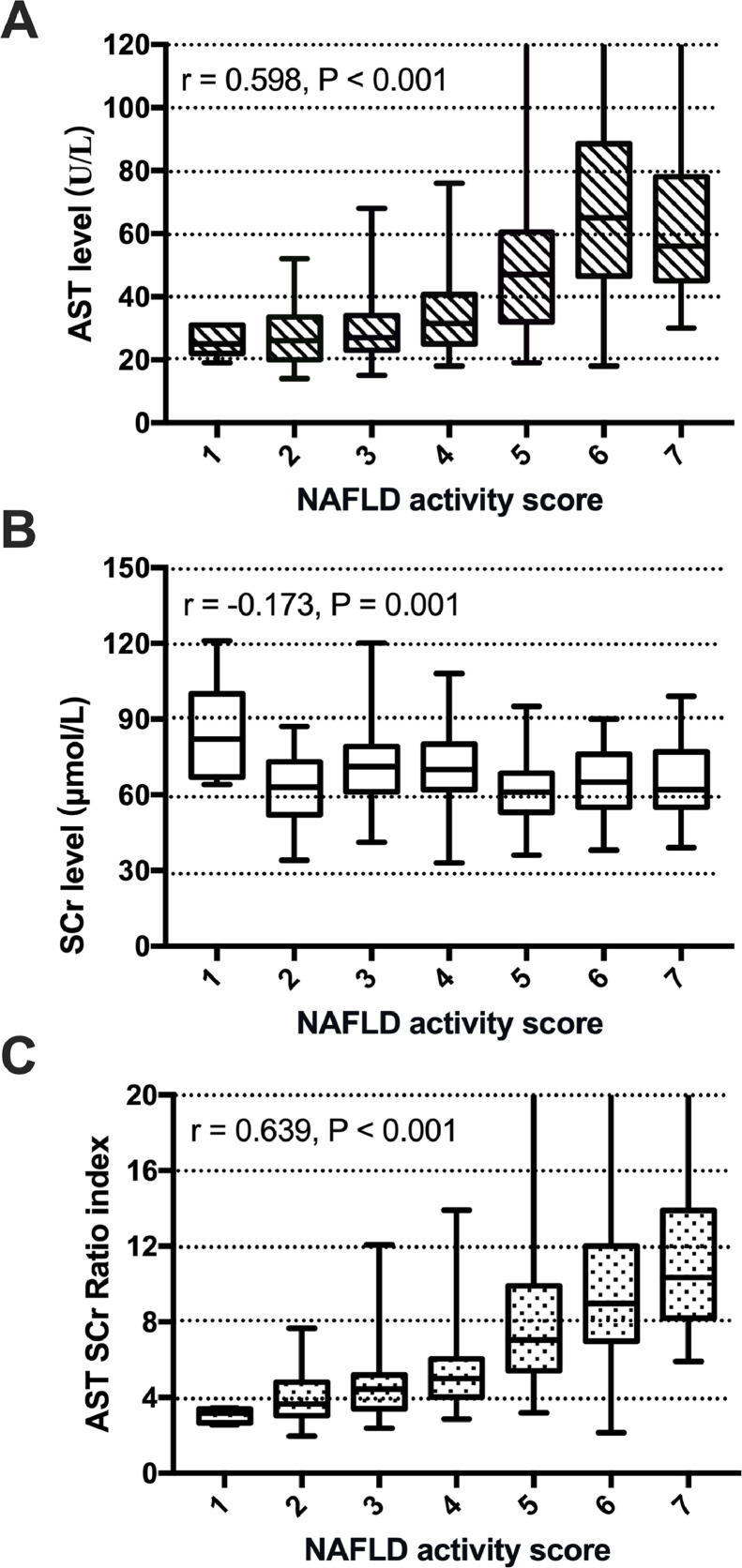
Since age, serum AST and SCr levels were the most important predictors of NASH, further analyses were performed (Figure 2). These analyses showed that there was no significant association between age and NAS score, while an increase in NAS score was significantly associated with a gradual increase in serum AST levels (r =0·598, P <0·001) and a decrease in SCr levels (r =-0·173, P =0·001). Since serum AST was increased and serum creatinine was decreased in patients with NASH, we derived a simple formula with serum AST as the numerator and serum creatinine as the denominator to further increase the separation between patients with NASH and those with simple steatosis using this derived measurement. We refer to this measurement as the AST to creatinine ratio index (acNASH), which was calculated as follows: acNASH = AST (U/L)/SCr (μmol/L) * 10.
Figure 2.
Box plot of serum AST level (A), SCr level (B), and AST-to-SCr ratio index (C) values in relation to the histological NAS. The box represents the interquartile range. The whiskers indicate the highest and lowest values, and the asterisks represent outliers. The line across the box indicates the median value.
As shown in Figure 2C, there was a significant association between acNASH and the NAS score. The correlation coefficient was higher than that of serum AST or SCr levels alone (r =0·639, P <0·001). The AUROC for AST alone, ALT alone, and AST/ALT ratio, which were included in univariable and multivariable regression analyses in the derivation cohort were 0·782, 0·767 and 0·370 (not shown), respectively. All of these AUROCs were lower than the acNASH index. Comparisons of ROC curves between acNASH, AST, ALT and AST/ALT ratio of subgroups in the derivation cohort of Chinese patients with NAFLD were shown in Supplementary Table 12. The AUROC of AST was 0·704 and the AUROC of acNASH was 0·829 in subgroups of patients with normal ALT. The cut-off values of AST for a Se and Sp of ≥0·91 were 26·5 and 52 (not shown), respectively. Using a dual cut-off approach, PPV and NPV in the derivation cohort of AST were 0·83 and 0·82 (not shown). With this, 47% of the patients fell into the indeterminate range or the “grey zone” between the two cut-offs, comparing with the AST, 7% (n=25) (not shown) of the patients were predicted better when using the acNASH.
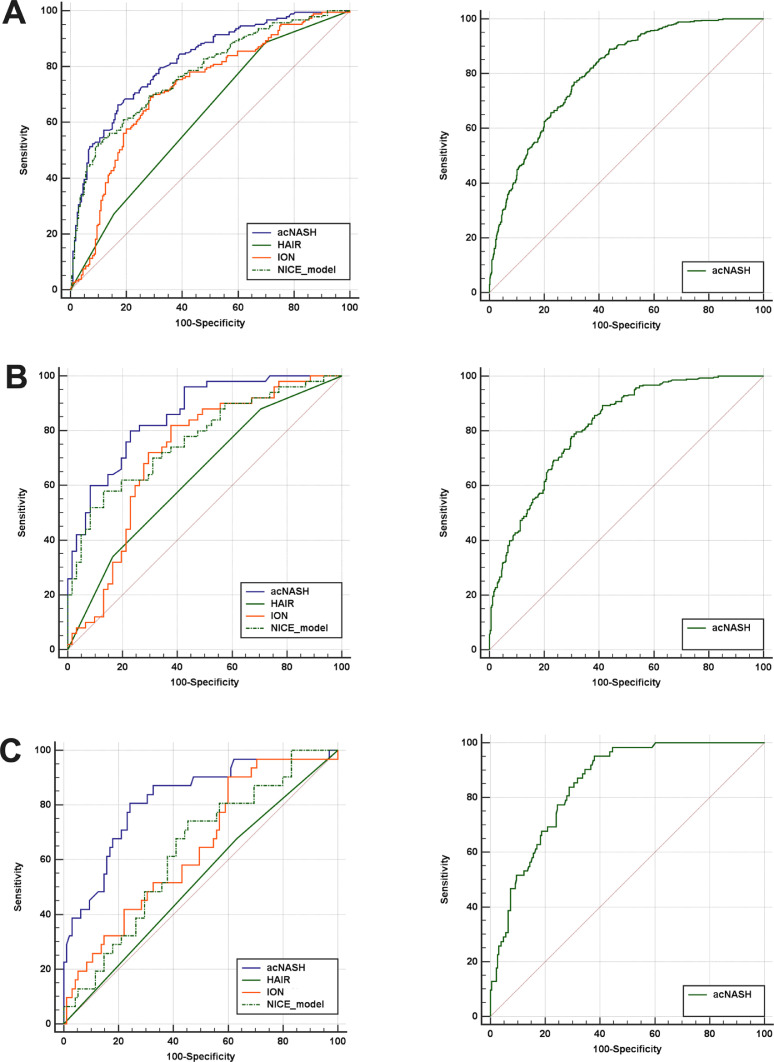
The acNASH index showed better performance compared to other existing non-invasive prediction models (see Supplementary Methods) for identifying definite NASH (Figure 3A). In the derivation cohort the AUROCs for the acNASH, HAIR, ION and NICE models were 0·818, 0·621, 0·720 and 0·776, respectively (Supplementary Table 4).
Figure 3.
ROC curves of the acNASH (the blue lines), HAIR (the green lines), ION (the solid orange lines) and NICE models (the green dotted line) for the prediction of definite NASH on liver histology in the derivation cohort (on the left of the figure) as well as ROC curves in the external validation cohorts (on the right of the figure) (A). ROC curves in patients with T2DM of the derivation cohort as well as ROC curves of the external validation cohorts (B). ROC curves in patients with normal serum ALT levels of the derivation cohort, as well as ROC curves of the external validation cohorts (C).
Patients in the derivation and validation cohorts differed significantly in demographics, adiposity measures, laboratory parameters and liver histology characteristics (Table 1). Nonetheless, with the exception of the Spanish cohort, the acNASH index showed good discriminatory ability for identifying definite NASH in all external validation cohorts, the best performance was observed in the Malaysia cohort with an AUROC of 0·852 (95% CI 0·805-0·898).
Table 1.
Main patient characteristics of the derivation Chinese cohort and external validation cohorts
| Variables | Country | ||||||
|---|---|---|---|---|---|---|---|
| Derivation cohort (N=390) | French cohort (N=448) | Turkish cohort (N=172) | Malaysian cohort (N=270) | Egyptian cohort (N=61) | Spanish cohort (N=138) | P value | |
| Demographics | |||||||
| Age (years) | 41·06±11·98 | 57·45±11·68 | 48·43±11·15 | 52·57±11·36 | 44·79±9·16 | 52·72±10·92 | P<0.05 |
| Sex (male) | 299 (76·7%) | 278 (62·1%) | 91 (52·9%) | 137 (50·7%) | 21 (34·4%) | 54 (39·1%) | P<0.05 |
| Body measurements | |||||||
| Height (cm) | 167·90±8·39 | 167·53±9·60 | 164·5±11·00 | 161·12±8·71 | 165·80±7·29 | 166·70±9·72 | P<0.05 |
| Weight (kg) | 76·25±13·39 | 93·50±19·69 | 90·84±16·51 | 78·64±14·82 | 93·86±13·23 | 89·35±18·12 | P<0.05 |
| BMI (kg/m2) | 26·92±3·35 | 33·26±6·17 | 33·53±4·91 | 30·21±4·64 | 34·07±4·68 | 32·00±5·38 | P<0.05 |
| Waist circumference (cm) | 91·97±8·76 | 113·13±14·74 | 108·33±10·91 | 98·71±10·75 | 108·43±12·25 | 105·13±12·85 | P<0.05 |
| WHR | 0·92±0·06 | 1·03±0·09 | 0·98±0·07 | 0·93±0·07 | NA | NA | / |
| Laboratory parameters | |||||||
| AST (U/L) | 46·49±31·98 | 44·59±32·69 | 51·55±36·12 | 43·65±23·51 | 48·30±40·04 | 50·52±28·49 | P=0.06 |
| ALT (U/L) | 76·78±72·98 | 61·18±39·36 | 79·66±65·81 | 66·55±42·29 | 55·75±34·43 | 76·48±47·40 | P<0.05 |
| Alkaline phosphatase (U/L) | 85·36±29·29 | 77·69±28·78 | 88·92±46·59 | 82·39±30·88 | 108·55±41·03 | 106·19±59·70 | P<0.05 |
| Albumin (g/L) | 46·39±3·88 | 42·73±3·56 | 45·35±3·58 | 42·16±3·76 | 4·15±0·45 | 44·45±4·34 | P<0.05 |
| Platelet count (x109/L) | 244·81±57·98 | 223·16±64·32 | 227·44±64·79 | 271·00±69·28 | 245·95±68·82 | 228·57±61·74 | P<0.05 |
| Fasting glucose (mmol/L) | 5·69±1·54 | 7·00±2·54 | 6·63±2·12 | 6·96±2·59 | NA | 6·94±2·81 | P<0.05 |
| HbA1c (%) | 6·12±1·38 | 6·49±1·21 | 6·25±1·20 | 6·97±2·44 | 6·60±0·85 | 6·59±1·48 | P<0.05 |
| Creatinine (μmol/L) | 67·15±14·24 | 72·01±15·60 | 67·34±15·55 | 73·05±19·16 | 66·85±16·03 | 71·76±14·80 | P<0.05 |
| e-GFR (CKD-EPI) | 110·37±14·46 | 92·68±15·73 | 101·67±14·66 | 92·52±20·80 | 100·11±19·18 | 89·22±18·55 | P<0.05 |
| Total bilirubin (μmol/L) | 14·25±6·62 | 11·19±6·60 | 16·38±14·75 | 11·89±6·41 | 13·02±6·00 | 12·13±7·99 | P<0.05 |
| Total cholesterol (mmol/L) | 5·10±1·16 | 4·94±1·22 | NA | 4·77±1·10 | 5·65±1·22 | 5·09±1·31 | P<0.05 |
| Triglycerides (mmol/L) | 2·28±1·37 | 1·91±1·22 | 2·03±1·06 | 1·71±0·83 | 1·89±0·84 | 1·85±0·97 | P<0.05 |
| Concomitant diseases | |||||||
| Hypertension (%) | 126 (32·3%) | 315 (70·3%) | 77 (44·8%) | 168 (62·2%) | 21 (34·4%) | 50 (36·2%) | P<0.05 |
| Type 2 diabetes (%) | 111 (28·5%) | 231 (51·6%) | 93 (54·1%) | 209 (77·4%) | 15 (24·6%) | 58 (42·0%) | P<0.05 |
| Non-invasive models | |||||||
| ION | 55·85±72·06 | 107·97±239·94 | 61·04±45·67 | / | / | / | / |
| Histological characteristics | |||||||
| Steatosis | P<0.05 | ||||||
| 1 | 156 (40·0%) | 214 (47·8%) | 25 (14·5%) | 80 (29·6%) | 23 (37·7%) | 47 (34·1%) | |
| 2 | 94 (24·1%) | 137 (30·6%) | 62 (36·0%) | 148 (54·8%) | 22 (36·0%) | 54 (39·1%) | |
| 3 | 140 (35·9%) | 97 (21·7%) | 85 (49·4%) | 42 (15·6%) | 16 (26·2%) | 37 (26·8%) | |
| Ballooning | P<0.05 | ||||||
| 0 | 37 (9·5%) | 93 (20·8%) | 6 (3·5%) | 67 (24·8%) | 35 (57·4%) | 63 (45·7%) | |
| 1 | 210 (53·8%) | 215 (48·0%) | 86 (50·0%) | 139 (51·5%) | 19 (31·1%) | 61 (44·2%) | |
| 2 | 143 (36·7%) | 140 (31·3%) | 80 (46·5%) | 64 (23·7%) | 7 (11·5%) | 14 (10·1%) | |
| Lobular inflammation | P<0.05 | ||||||
| 0 | 23 (5·9%) | 70 (15·6%) | 3 (1·7%) | 3 (1·1%) | 32 (52·5%) | 47 (34·1%) | |
| 1 | 285 (73·1%) | 325 (72·5%) | 67 (39·0%) | 168 (62·2%) | 13 (21·3%) | 75 (54·3%) | |
| 2 | 79 (20·3%) | 53 (11·8%) | 69 (40·1%) | 96 (35·6%) | 16 (26·2%) | 14 (10·1%) | |
| 3 | 3 (0·8%) | 0 | 33 (19·2%) | 3 (1·1%) | 0 | 2 (1·4%) | |
| NAS | 4·39±1·41 | 3·80±1·38 | 5·55±1·54 | 4·22±1·31 | 4·77±1·37 | 3·36±1·48 | P<0.05 |
| Fibrosis stage | P<0.05 | ||||||
| 0 | 114 (29·2%) | 47 (10·5%) | NA | 71 (26·3%) | NA | 46 (33·3%) | |
| 1 | 191 (49·0%) | 98 (21·9%) | NA | 115 (42·6%) | NA | 33 (23·9%) | |
| 2 | 65 (16·7%) | 132 (29·5%) | NA | 22 (8·1%) | NA | 25 (18·1%) | |
| 3 | 14 (3·6%) | 135 (30·1%) | NA | 53 (19·6%) | NA | 27 (19·6%) | |
| 4 | 3 (0·8%) | 36 (8·0%) | NA | 9 (3·3%) | NA | 7 (5·1%) | |
Abbreviations: ALT = alanine aminotransferase, AST = aspartate aminotransferase, BMI = body mass index, e-GFR = estimated glomerular filtration rate, GGT = γ-glutamyl transferase, HbA1c = glycated hemoglobin, HOMA-IR = homeostasis model assessment of insulin resistance, NA = not available, NAS = NAFLD activity score, P3NP = procollagen-3 N-terminal peptide, WHR = waist to hip ratio.
Notes: Inter-group difference analysis of continuous variables was evaluated by one-way ANOVA analysis and of categorical variables was evaluated by chi-square test.
In the derivation cohort, the cut-off values for a Se and Sp of ≥0·91 were 4·15 and 7·73, respectively (Table 2). Using a dual cut-off approach, PPV and NPV in the derivation cohort were 0·83 and 0·85. With this, 40% of the patients fell into the indeterminate range or the “grey zone” between the two cut-offs. When applying these cut-offs in the external validation cohorts, the Turkish cohort had the highest PPV of 0·92, whereas the French, Malaysian, Egyptian and Spanish cohorts had a PPV of 0·69, 0·79, 0·82, and 0·45, respectively. The NPV remained high (0·93) in the pooled external validation cohort, in which the Turkish cohort had the lowest NPV (0·75).
Table 2.
Performance of the acNASH for the diagnosis of definite NASH on histology in the derivation cohort and external validation cohorts.
| Cohort(s) | AUROC (95% CI) | N | Prevalence of definite NASH | Diagnostic performance using dual cut-offs (cut-offs from derivation cohort) |
||
|---|---|---|---|---|---|---|
| rule-out zone | grey zone | rule-in zone | ||||
| Derivation cohort | 0·818 (0·777-0·860) | 390 | 190 (48·7%) | acNASH<4·15 | acNASH: 4·15-7·73 | acNASH >7·73 |
| n=115 (29%) | n=158 (40%) | n=119 (30%) | ||||
| Se=0·91 | Sp=0·91 | |||||
| Sp=0·48 | Se=0·53 | |||||
| NPV=0·83 | PPV=0·85 | |||||
| French cohort | 0·807 (0·767-0·848) | 448 | 148 (33·0%) | acNASH<4·15 | acNASH: 4·15-7·73 | acNASH >7·73 |
| n=126 (28%) | n=224 (50%) | n=98 (22%) | ||||
| Se=0·97 | Sp=0·90 | |||||
| Sp=0·40 | Se=0·46 | |||||
| NPV=0·96 | PPV=0·69 | |||||
| Turkish cohort | 0·810 (0·738-0·883) | 172 | 124 (72·1%) | acNASH<4·15 | acNASH: 4·15-7·73 | acNASH >7·73 |
| n=28 (16%) | n=92 (53%) | n=52 (30%) | ||||
| Se=0·94 | Sp=0·92 | |||||
| Sp=0·44 | Se=0·39 | |||||
| NPV=0·75 | PPV=0·92 | |||||
| Malaysian cohort | 0·852 (0·805-0·898) | 270 | 105 (38·9%) | acNASH<4·15 | acNASH: 4·15-7·73 | acNASH >7·73 |
| n=88 (33%) | n=110 (41%) | n=72 (27%) | ||||
| Se=0·93 | Sp=0·91 | |||||
| Sp=0·49 | Se=0·54 | |||||
| NPV=0·92 | PPV=0·79 | |||||
| Egyptian cohort | 0·809 (0·701-0·918) | 61 | 34 (55·7%) | acNASH<4·15 | acNASH: 4·15-7·73 | acNASH >7·73 |
| n=9 (15%) | n=35 (57%) | n=17 (28%) | ||||
| Se=1·00 | Sp=0·89 | |||||
| Sp=0·33 | Se=0·41 | |||||
| NPV=1·00 | PPV=0·82 | |||||
| Spanish cohort | 0·785 (0·704-0·866) | 138 | 35 (25·4%) | acNASH<4·15 | acNASH: 4·15-7·73 | acNASH >7·73 |
| n=31 (22%) | n=58 (42%) | n=49 (36%) | ||||
| Se=1·00 | Sp=0·74 | |||||
| Sp=0·30 | Se=0·63 | |||||
| NPV=1·00 | PPV=0·45 | |||||
| Pooled patient cohorts | 0·805 (0·780-0·830) | 1089 | 446 (41·0%) | acNASH<4·15 | acNASH: 4·15-7·73 | acNASH >7·73 |
| n=282 (26%) | n=519 (48%) | n=288 (26%) | ||||
| Se=0·96 | Sp=0·86 | |||||
| Sp=0·41 | Se=0·47 | |||||
| NPV=0·93 | PPV=0·73 | |||||
Performance associated with a dual cut-off approach is evaluated using the acNASH index when the cut-offs are calculated in the derivation cohort and applied in external validation cohorts. The lower cut-off constitutes a rule-out cut-off and is based on a sensitivity ≥0·91 in the derivation cohort. The higher cut-off constitutes a rule-in cut-off and is based on a specificity ≥0·91 in the derivation cohort. Individuals with an acNASH score between the rule-out and rule-in cut-offs are in the grey zone. In the rule-out group, the sensitivity is provided together with the specificity and negative predictive value to appraise the rule-out performance of the score. In the rule-in group, the specificity is provided together with the sensitivity and positive predictive value to appraise the rule-in performance of the score.
Abbreviations: AUROC: area under the receiver operating curve, NASH: non-alcoholic fatty liver disease, NPV: negative predictive value, PPV: positive predictive value, Se: sensitivity, Sp: specificity.
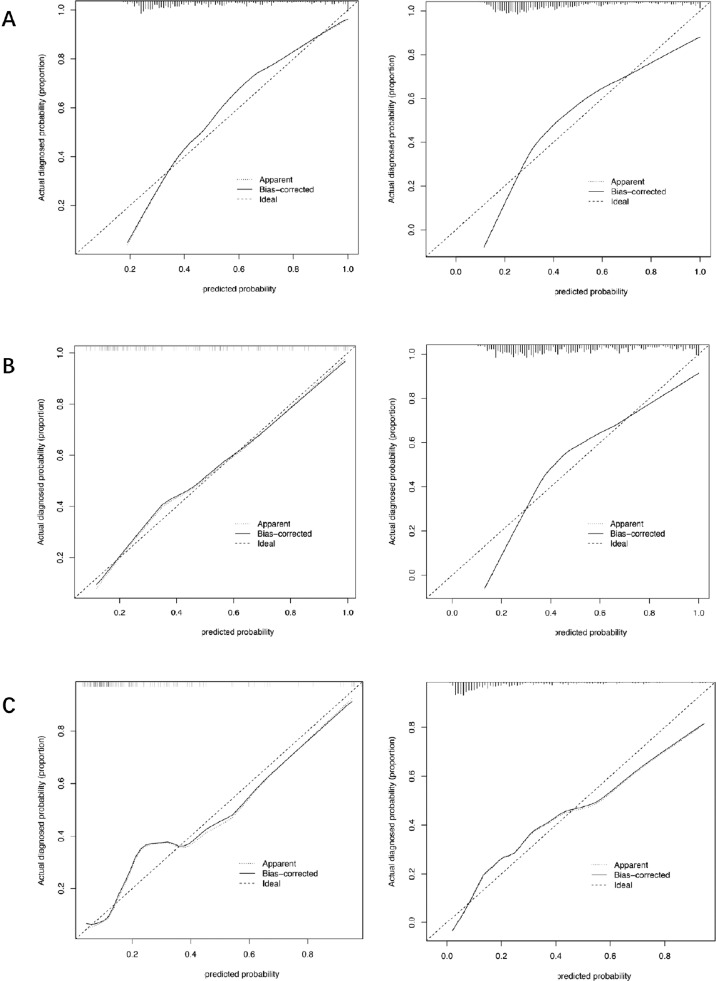
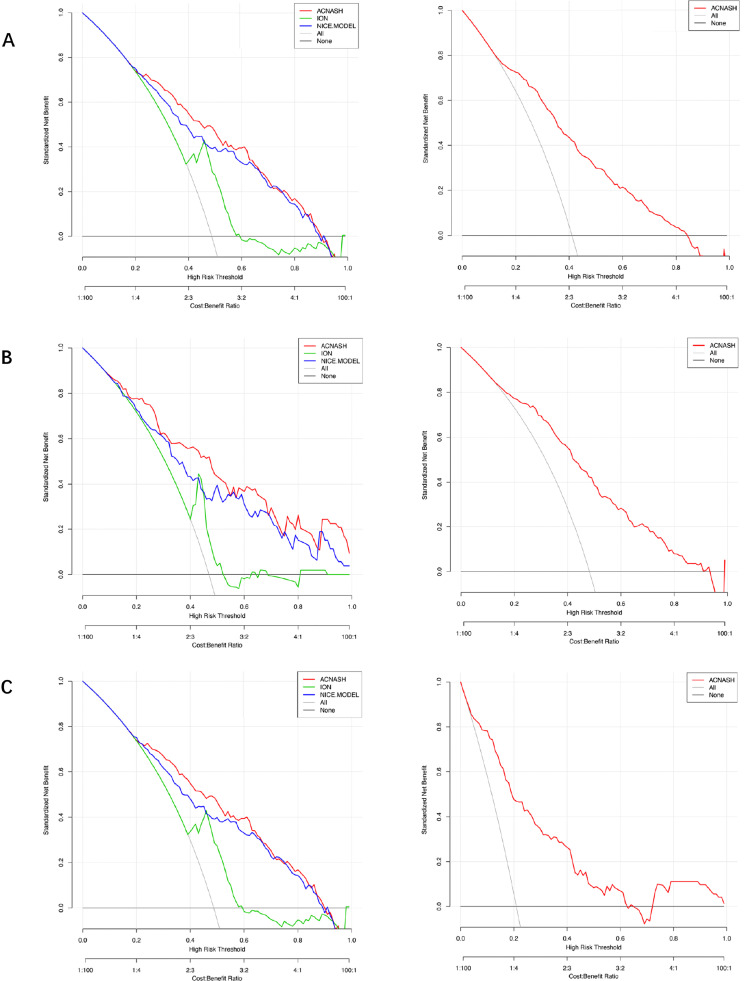
The calibration curves are shown in Figures 4 for both the derivation and validation cohorts, and curves show good agreement. To evaluate clinical utility, decision curve analysis (DCA) was also undertaken. The plots of DCA for the derivation and validation cohorts are shown in Figure 5, indicating the positive net benefit of the established models.
Figure 4.
Calibration curves. The x-axis designates the predicted probability of definite NASH on liver histology in the derivation cohort based on the acNASH index, and the y-axis indicates the actual diagnose of definite NASH. Calibration curves of the acNASH for the prediction of definite NASH on histology in the derivation cohort (on the left of the figure), as well as calibration curves in the external validation cohorts (on the right of the figure) (A). Calibration curves in patients with T2DM of the derivation cohort, as well as calibration curves of the external validation cohorts (B). Calibration curves in patients with normal serum ALT levels of the derivation cohort as well as Calibration curves of the external validation cohorts (C). The solid line represents equality between the predicted and actual values.
Figure 5.
Decision curve analysis (DCA) curves of the acNASH (the red lines), ION (the green lines) and NICE models (the blue line) for the prediction of definite NASH on liver histology in the derivation cohort (on the left of the figure) as well as DCA curves in the external validation cohorts (on the right of the figure) (A). DCA curves in patients with T2DM of the derivation cohort, as well as DCA curves of the external validation cohorts (B). DCA curves in patients with normal serum ALT levels of the derivation cohort, as well as DCA curves of the external validation cohorts (C).
In addition, we tested the diagnostic performance of the acNASH index in patients with either T2DM or normal ALT levels both in derivation cohort and in external cohorts, and performance was not materially different in these important subgroups. Pairwise comparisons of AUROC between the acNASH index and other existing prediction models for NASH in patients with either T2DM or normal ALT levels of the derivation cohort are shown in Supplementary Tables 5 and 7, respectively. The ROC curves of T2DM patients with normal ALT levels were plotted in Figure 3B and 3C, respectively. The diagnostic ability of acNASH for identifying NASH was superior to other established non-invasive methods in both patient subgroups. In the external validation cohorts, similar performances of the acNASH index in terms of AUROC were observed among patients with T2DM and those with normal ALT levels (Supplementary Tables 6 and 8).
4. Discussion
In this study, we suggested that age, serum AST and SCr levels were the strongest predictors of NASH confirmed by liver histology. To further improve the predictive utility of these variables for identifying patients with NASH, we developed a simple score that we refer to as the acNASH index. The acNASH index comprised dividing the AST level by the serum SCr concentration since AST levels were higher and serum SCr levels were lower in patients with NASH than in patients with simple steatosis. Addition of age to these two variables did not improve the diagnostic performance of the acNASH index and, therefore, age was excluded. The diagnostic performance of the acNASH index was tested in a derivation cohort and, subsequently, validated in five external cohorts.
Many biomarkers have been investigated for predicting NASH, i.e. the more rapidly progressive form of NAFLD. These biomarkers range from clinical parameters, biochemical variables, metabolic factors and plasma lipids that can reflect the complex molecular mechanisms underlying the pathogenesis and progression of NASH [11]. Other investigators have proposed various non-invasive approaches to predict NASH, including novel serum markers, algorithms or imaging techniques [12], [13], [14], [15], [16], [17], [18]. However, the large majority of these non-invasive methods lack the potential for widespread implementation in primary care settings, across different ethnic groups and geographical locations. Furthermore, these prediction models do not meet the need for a simple, inexpensive, and accurate method for screening NASH in routine clinical care.
It is well known that AST is a mitochondrial enzyme that is released during hepatocyte damage in patients with chronic liver diseases. Therefore, an increase in serum AST levels may represent mitochondrial damage in NASH, although the exact mechanism of increased AST levels in the pathogenesis of NASH is unclear [19]. However, and consistent with data reported by both Zhou et al [17] and Feldstein et al [12], who have used targeted mass spectrometry-based methods to construct a model for NASH, in our study higher serum AST levels were also found to be a strong risk factor for predicting NASH.
Though some studies have reported the utility of SCr levels as a surrogate marker of the body's skeletal muscle mass in patients with NAFLD [20,21], we found no significant differences in skeletal muscle mass between patients with NASH and non-NASH in our derivation cohort. A potentially more complicated mechanism explaining the changes in SCr levels may involve creatine synthesis. The formation of Cr is a passive process by two steps, involving the conversion of arginine to guanidine-acetic acid (GAA) by L-arginine-glycine amidinotransferase, which is affected by urea cycle [22], and subsequently Cr conversion from GAA by guanidinoacetate N-methyltransferase, which occurs in the liver and is affected by remethylation cycle via S-adenosylmethionine [23]. NAFLD may affect both the urea cycle and remethylation, which may, at least in part, explain why SCr levels are lower in patients with NASH.
Moreover, we explored the utility of kidney function parameters in predicting the presence of NASH, because people with NAFLD are known to have extra-hepatic manifestations, including CKD [24,25]. Calculation of e-GFR by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) study equation [26] was also included into our regression models for assessing the diagnostic performance for NASH, as well as to dispel the underlying influence by ethnicity and sex (Supplementary Table 11). The results show that the regression model using serum AST and SCr levels was simpler and had greater accuracy, compared to that using serum levels of AST and eGFR (as estimated by the CKD-EPI equation) in predicting the presence of NASH.
NASH is commonly asymptomatic and with a prevalence of 59·1% in the biopsied NAFLD patients, patients at high risk of NASH should be referred for consideration of liver biopsy, but there are no accepted well-defined screening recommendations [3,27]. To improve the long-term clinical outcomes of patients with NASH, early diagnosis, timely referral, and effective clinical interventions are necessary especially in the setting of primary care and general non-hepatology practices. Compared with other screening tests, the non-invasive model - acNASH index appears to show more effective discriminatory ability and possesses a satisfactory clinical potential.
In our study, the overall diagnostic performance of the acNASH index was satisfactory, though it varied from country to country. The diagnostic performance of the acNASH index in the Chinese, Malaysian, Turkish and Egyptian cohorts of NAFLD patients was (slightly) better than in French and Spanish cohorts, so highlighting the existence of ethnic differences in the patient characteristics.
The acNASH index had similar diagnostic performance for identifying NASH in patients with established T2DM or patients with normal ALT levels as compared to that in the pooled validation cohorts. Therefore, the acNASH index may be used in non-hepatology settings (e.g. diabetology clinics) and aid clinicians stratify patients with NAFLD for their risk of NASH prior to referral to specialized centers for more specific testing or treatment, especially when more advanced diagnostic methods are not available, and could potentially decrease the cost for healthcare systems [28]. Screening for NASH in the primary setting emerges to be an important unmet need, and the acNASH index provides a clinically useful tool that can be applied directly in clinical practice using routinely available and inexpensive biochemical parameters.
Our study has several important strengths. Firstly, the major advantage of the acNASH index is its simplicity and its very low cost. Secondly, the diagnostic performance of the acNASH index for identifying NASH is good both in the derivation cohort and in most of the external validation cohorts in which AUROCs ranged from 0·785 to 0·852, with PPVs 0·45-0·92 and NPVs of 0·75-0·99, using a dual cut-off approach that was derived from the derivation cohort. Thirdly, we have recruited patients with NAFLD who met eligibility criteria in six Gastroenterology centers across the globe. Ethnic diversity within our study further supports the clinical utility of the acNASH index in patients in primary care across various regions of the world.
That said, we acknowledge that there are also some important limitations to our study. Firstly, SCr levels may be influenced by several conditions, such as skeletal muscle mass, dietary protein intake, kidney diseases, long-term use of medications for hypertension, T2DM or dyslipidemia. This suggests that the aforementioned influencing factors should be always considered prior to the application of the acNASH index. It is also worth highlighting that SCr level is unreliable in severe chronic liver diseases (e.g. decompensated cirrhosis or liver failure), although it should be noted that none of our NAFLD patients were in these categories. Several medications for diabetes and dyslipidemia may affect results based on changes in serum creatinine, in AST/ALT ratio and even in NAFLD/NASH (e.g., GLP-1 agonists and pioglitazone). Information of medication use was not included, though the prevalence of T2DM was similar between patients with non-NASH and those with definite NASH in the derivation cohort, and may be potential confounding factors. The prevalence of established T2DM in the NASH group was very similar to that observed in the non-NASH group. This finding contradicts previous studies showing that patients with T2DM are at higher risk of NASH and more advanced disease and it is possible that there was some undetected selection bias. However, in our cohort, the percentage of patients with less advanced liver disease was high and 75.1% of patients had fibrosis stages 0 – 1. HOMA-IR is not such a good proxy for insulin resistance in patients with long standing T2DM where beta cell function has deteriorated and decreased insulin secretory capacity has resulted in low fasting insulin concentrations. Since approximately 25-30% of our cohort had T2DM, it is plausible that the HOMA-IR is not a good proxy for insulin resistance in our cohort. Due to the variability in ultrasound readings, diagnosis of fatty liver may be subject to error. However, we have excluded from the analysis those who did not have steatosis diagnosed by liver biopsy in order to avoid bias. Finally, our study was based on liver pathology results, which may be subject to potential sampling errors, as well as intra- and inter-observer variability that may complicate the association between liver histology data and blood biomarkers [7],[78].
In conclusion, our newly proposed and validated acNASH index is a simple and inexpensive test that shows promising utility for identifying patients with biopsy-confirmed NASH across different countries and in subjects with varying ethnicities. We consider that acNASH index could be used for identifying those patients in primary care, who merit consideration for referral to liver specialists for more detailed investigation. Further studies are required to corroborate our findings.
Contributors
M-HZ, X-XW and KIZ were involved in study design and data interpretation, verification. X-XW performed data analysis. XXW and KIZ wrote the manuscript. Data collection was done by X-XW, KIZ, JB, W-KC, YY, MR-G, MEK and Z-MH. GT and CDB conducted critical revision and writing of the manuscript. All authors reviewed and commented on the manuscript and approved the final version.
Data sharing statement
The data underlying the results of this study are available upon request because they contain potentially sensitive information. Interested researchers can contact the corresponding author for data access requests via email (zhengmh@wmu.edu.cn).
Declaration of Competing Interest
The authors declare no conflict of interest.
Funding
The National Natural Science Foundation of China (82070588), High Level Creative Talents from Department of Public Health in Zhejiang Province (S2032102600032) and Project of New Century 551 Talent Nurturing in Wenzhou. This work is a part of the PERSONS study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.101145.
Appendix. Supplementary materials
References
- 1.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 2.Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM. Association Between Fibrosis Stage and Outcomes of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology. 2020;158(6):1611–1625. doi: 10.1053/j.gastro.2020.01.043. e12. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology (Baltimore, Md) 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 4.Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. Journal of hepatology. 2019;71(4):793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 5.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology (Baltimore, Md) 2018;67(1):328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 6.Health USDo, Control HSCfD, Statistics PNCfH. National Health and Nutrition Examination Survey III, 1988-1994. Inter-university Consortium for Political and Social Research [distributor]; 1998.
- 7.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology (Baltimore, Md) 2009;49(3):1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 8.Castera L, Friedrich-Rust M, Loomba R. Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156(5):1264–1281. doi: 10.1053/j.gastro.2018.12.036. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou YJ, Gao F, Liu WY, Wong GL, Mahadeva S, Wang XD. Screening for compensated advanced chronic liver disease using refined Baveno VI elastography cutoffs in Asian patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2021;54(4):470–480. doi: 10.1111/apt.16487. [DOI] [PubMed] [Google Scholar]
- 10.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology (Baltimore, Md) 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 11.Byrne CD, Targher G. What’s new in NAFLD pathogenesis, biomarkers and treatment? Nat Rev Gastroenterol Hepatol. 2020;17(2):70–71. doi: 10.1038/s41575-019-0239-2. [DOI] [PubMed] [Google Scholar]
- 12.Anty R, Iannelli A, Patouraux S, Bonnafous S, Lavallard VJ, Senni-Buratti M. A new composite model including metabolic syndrome, alanine aminotransferase and cytokeratin-18 for the diagnosis of non-alcoholic steatohepatitis in morbidly obese patients. Alimentary pharmacology & therapeutics. 2010;32(11-12):1315–1322. doi: 10.1111/j.1365-2036.2010.04480.x. [DOI] [PubMed] [Google Scholar]
- 13.Dixon JB, Bhathal PS, O'Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121(1):91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 14.Feldstein AE, Lopez R, Tamimi TA, Yerian L, Chung YM, Berk M. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Journal of lipid research. 2010;51(10):3046–3054. doi: 10.1194/jlr.M007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poynard T, Ratziu V, Charlotte F, Messous D, Munteanu M, Imbert-Bismut F. Diagnostic value of biochemical markers (NashTest) for the prediction of non alcoholo steato hepatitis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006;6:34. doi: 10.1186/1471-230X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Younes R, Rosso C, Petta S, Cucco M, Marietti M, Caviglia GP. Usefulness of the index of NASH - ION for the diagnosis of steatohepatitis in patients with non-alcoholic fatty liver: An external validation study. Liver Int. 2018;38(4):715–723. doi: 10.1111/liv.13612. [DOI] [PubMed] [Google Scholar]
- 17.Younossi ZM, Jarrar M, Nugent C, Randhawa M, Afendy M, Stepanova M. A novel diagnostic biomarker panel for obesity-related nonalcoholic steatohepatitis (NASH) Obes Surg. 2008;18(11):1430–1437. doi: 10.1007/s11695-008-9506-y. [DOI] [PubMed] [Google Scholar]
- 18.Younossi ZM, Page S, Rafiq N, Birerdinc A, Stepanova M, Hossain N. A biomarker panel for non-alcoholic steatohepatitis (NASH) and NASH-related fibrosis. Obes Surg. 2011;21(4):431–439. doi: 10.1007/s11695-010-0204-1. [DOI] [PubMed] [Google Scholar]
- 19.Rej R. Measurement of aminotransferases: Part 1. Aspartate aminotransferase. Critical reviews in clinical laboratory sciences. 1984;21(2):99–186. doi: 10.3109/10408368409167137. [DOI] [PubMed] [Google Scholar]
- 20.Heymsfield SB, Arteaga C, McManus C, Smith J, Moffitt S. Measurement of muscle mass in humans: validity of the 24-hour urinary creatinine method. Am J Clin Nutr. 1983;37(3):478–494. doi: 10.1093/ajcn/37.3.478. [DOI] [PubMed] [Google Scholar]
- 21.Baxmann AC, Ahmed MS, Marques NC, Menon VB, Pereira AB, Kirsztajn GM. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol. 2008;3(2):348–354. doi: 10.2215/CJN.02870707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Chiara F, Heeboll S, Marrone G, Montoliu C, Hamilton-Dutoit S, Ferrandez A. Urea cycle dysregulation in non-alcoholic fatty liver disease. Journal of hepatology. 2018;69(4):905–915. doi: 10.1016/j.jhep.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 23.Kashani K, Rosner MH, Ostermann M. Creatinine: From physiology to clinical application. Eur J Intern Med. 2020;72:9–14. doi: 10.1016/j.ejim.2019.10.025. [DOI] [PubMed] [Google Scholar]
- 24.Sun DQ, Zheng KI, Xu G, Ma HL, Zhang HY, Pan XY. PNPLA3 rs738409 is associated with renal glomerular and tubular injury in NAFLD patients with persistently normal ALT levels. Liver Int. 2020;40(1):107–119. doi: 10.1111/liv.14251. [DOI] [PubMed] [Google Scholar]
- 25.Targher G, Byrne CD. Non-alcoholic fatty liver disease: an emerging driving force in chronic kidney disease. Nat Rev Nephrol. 2017;13(5):297–310. doi: 10.1038/nrneph.2017.16. [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong VW, Adams LA, de Lédinghen V, Wong GL, Sookoian S. Noninvasive biomarkers in NAFLD and NASH - current progress and future promise. Nat Rev Gastroenterol Hepatol. 2018;15(8):461–478. doi: 10.1038/s41575-018-0014-9. [DOI] [PubMed] [Google Scholar]
- 28.Bazick J, Donithan M, Neuschwander-Tetri BA, Kleiner D, Brunt EM, Wilson L. Clinical Model for NASH and Advanced Fibrosis in Adult Patients With Diabetes and NAFLD: Guidelines for Referral in NAFLD. Diabetes Care. 2015;38(7):1347–1355. doi: 10.2337/dc14-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.