Abstract
Purpose
Although acne vulgaris (AV) is a common disease and can persist into adulthood, there are few large-scale epidemiological studies on the prevalence of acne vulgaris in adults. The aim of our study was to characterise the epidemiology and comorbidity of acne vulgaris in working adults in Germany.
Patients and Methods
Within the framework of a cross-sectional study, a total of 161,269 employees underwent dermatological whole-body examinations in more than 500 German companies between 2001 and 2016. Point prevalence rates for acne vulgaris and further skin diseases and their 95% confidence intervals were calculated and differences between participants with and without acne vulgaris were tested with chi-squared tests.
Results
Mean age was 43.2 years ± 10.9, 55.5% were male. In total, n = 5311 people (3.3%) with acne vulgaris were identified. Prevalence decreased by age. Controlling for age and gender, acne was significantly associated with folliculitis (OR = 1.91; CI: 1.76–2.07), contact dermatitis (OR = 1.74; CI: 1.08–2.81), rosacea (OR = 1.74; CI: 1.40–2.15), pyoderma (OR = 1.58; 1.22–2.06), seborrheic dermatitis (OR = 1.47; CI: 1.27–1.71), hand eczema (OR = 1.34; CI: 1.00–1.76), verruca vulgaris plantaris (OR = 1.29; CI: 1.09–1.51), tinea pedis (OR = 1.27; CI: 1.10–1.47), spider veins (OR = 1.26; CI: 1.16–1.38) and telangiectasia (OR = 1.15; CI: 1.02–1.30).
Conclusion
These data underline the importance of acne vulgaris in the adult population. Further studies to better understand the pathophysiology of AV and its comorbidity in different phases of adulthood would be desirable to develop appropriate guidelines and therapy concepts.
Keywords: acne vulgaris, comorbidities, epidemiology, inflammation
Introduction
Acne vulgaris (AV) is a chronic inflammatory skin disease arising from the pilosebaceous unit1 with a prevalence of up to 85% of all young people aged 12–24 years.2 15–20% of cases are classified as moderate to severe.3 AV can also persist into adulthood. The published prevalence of AV in adulthood varies between 3 and 43% depending on the study.4,5
Acne lesions are divided into inflammatory and non-inflammatory lesions. Inflammatory lesions include papules, pustules and nodules, while non-inflammatory lesions include comedones. The skin symptoms are particularly rich in sebaceous glands, such as the face, neck, chest, shoulder or back.6 Health-related quality of life in acne is particularly impaired by the visibility of lesions and consecutive psychosocial strain.7,8
Three factors play an important role in the pathogenesis of AV: seborrhea, abnormal follicular keratinisation and dysbiosis, which is associated on the one hand with the predominance of phylotype IA1 of Cutibacterium acnes and on the other hand by overpresentation of Proteobacteria and Firmicutes.9,10 Interestingly, a study by Dreno et al showed that Staphylococci also seem to be involved in the pathogenesis of acne when they colonised acne lesions such as comedones, papules or pustules more frequently than non-lesional skin.10 Perifollicular hyperkeratosis initially leads to the formation of microcomedones. Aggravated by the frequently increased sebum production during puberty, a microenvironment is formed which favours colonisation with Propionibacterium acnes.1,11 In addition, the inflammatory reaction results in the blocking of receptors such as histamine receptor, hormonal dihydrotestosterone (DHT) receptor, neuromodulatory receptors and corticotropin-releasing hormone (CRH) receptor.12,13 Recent studies show that different dietary substances can also lead to increased sebum production by activating specific receptors, such as peroxisome proliferator-activated receptors stimulated by free fatty acids and cholesterol, insulin-like growth factor (IGF)-1 receptor stimulated by sugar and leptin receptor activated by fat.14,15 Leptin, a hormone secreted by adipocytes, not only regulates body weight, but also activates pro-inflammatory enzymes and cytokines such as Interleukin-6/8, thus providing a link between fat metabolism and inflammatory response in other tissues.16 Some studies suggest that the prevalence of acne correlates with the glycemic index.17,18 It has been shown that a carbohydrate-rich diet correlates with the severity of the disease and that diet and lifestyle play an important role in the pathogenesis of the disease.
Publications have associated AV with a variety of comorbidities including depression and anxiety,19 upper respiratory tract infections, increased upper gastrointestinal diseases20 and increased risk of endometriosis.21 In addition, AV is frequently associated with syndromes such as polycystic ovary syndrome22 or PASH (Pyoderma gangrenosum, acne and suppurative hidradenitis) syndrome.23
The prevalence of AV also seems to vary according to skin type and ethnic group. The few comparative studies report that people with fair skin suffer more frequently from acne than people with black skin.24–26
It has been shown several times that AV is more common in men during adolescence,4 but more common in women in adulthood.27
The impact of socio-economic factors on the prevalence of AV remains unclear. In 1986, Cunliffe et al found a markedly higher unemployment rate in people with AV compared to the control group.28 Among 896 employees of a German city, no difference in prevalence was observed between the individual social classes in the study by Schäfer et al in 2001.4 However, a high socioeconomic status was identified as a risk factor for the development of AV in the 0–17 age group in Bettoli’s study in 2014.20
Although AV is a common disease and can persist into adulthood, there are few large-scale population-based epidemiological studies on the prevalence of AV in adults. The aim of our study was to characterise the epidemiology and comorbidity of AV in working adults in Germany.
Materials and Methods
People and Centres
Within the framework of a cross-sectional study, a total of 161,269 employees underwent dermatological whole-body examinations as part of voluntary company-based skin screenings in more than 500 German companies between 2001 and 2016. All employees were invited to participate regardless of age, sex or social status.
The participating companies belonged to different branches of industry and commerce and varied in size (150–65,000 employees). Most of the companies belonged to the following fields: car industry, insurance companies, banking, energy companies, chemical industry and printing houses. Smaller companies with less than 200 employees included banks and postal services. The screenings were carried out on behalf of the companies or company health insurers by HSH GmbH, which has been active in the field of secondary prevention in the dermatological, phlebological and cardiovascular field since 1989.
A positive ethics vote has been obtained from the responsible ethics committee for the conduct of the study. Only patients aged 16 years and older with AD who had signed an informed consent form were included.
Assessments
To reduce selection bias, the screenings were conducted within the working hours. For each participant a standardised whole-body examination was carried out by experienced and trained dermatologists. The average duration of the examination was 15 minutes. The diagnoses were based on the clinical examination exclusively. AV was considered prevalent if at least IGA (Investigator Global Assessment) grade 2 was present, what is a mild form with less than half of the face infested with a few open or closed comedones, papules and pustules. In addition to the documentation of acne, all other dermatological findings were also documented in a standardised PC-based case report form by an assisting person.
Statistics
Statistical analyses were conducted using SPSS 23.0 (IBM, Armonk, NY) for Windows. Point prevalence rates for AV and further skin diseases and their 95% confidence intervals (95% CI) were calculated and differences between participants with and without acne were tested with chi-squared tests. Logistic regression analysis was applied to test, whether further skin diseases are associated with AV when controlling for age and gender. Bonferroni correction of p-values was applied to account for inflation of nominal α-level due to multiple significance testing in the logistic regression analysis.
Results
People and Centres
In this study the mean age of N = 161,269 was 43.2 years ± 10.9, 55.5% were male. The largest proportion of the population had skin phototype II with 101,455 people (65.5%) followed by skin phototype III with 37,695 people (24.3%), skin phototype I with 13,696 people (8.8%) and skin phototype IV to VI with 2149 people (1.4%). With 32.7% (n = 52,700 people) the age group between 40 and 49 years was most frequently represented. The following distribution was found for other age groups: age group 30–39 years n = 41,332 (25.6%), age group 50–59 years n = 37,419 (23.2%), age group 16–29 years n = 19,661 (12.2%) and age group 60–70 years n = 10,157 (6.3%).
In total, N = 5311 people (3.3%) with AV were identified. The mean age in this group was 33.9 years, 55.9% (n = 2970) were male.
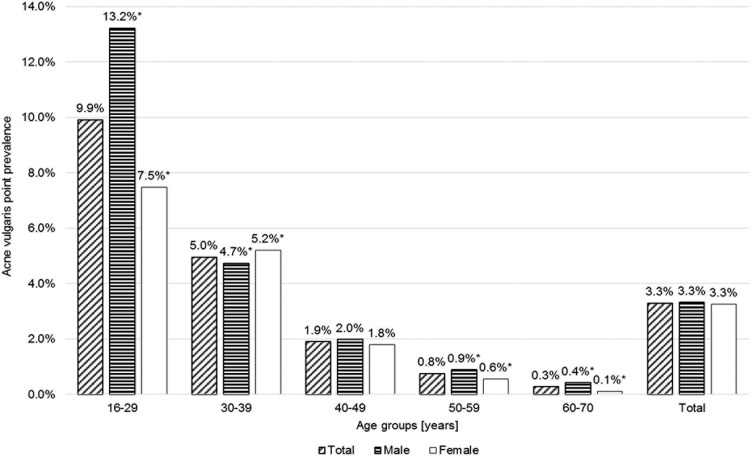
Prevalence decreased by age (Figure 1). There were no statistically significant gender differences in the prevalence of acne. However, when classified by age group, men were significantly more affected in people under 30 years (13.2% vs 7.5%), but AV was significantly more prevalent in women at the age of 30 to 39 (5.2% vs 4.7%).
Figure 1.
Prevalence of acne vulgaris by age group and gender. * significant difference between men and women, p ≤ 0.05.
Prevalence of Acne and Skin Phototype
The prevalence of acne was highest in people with phototype I (n = 511; 3.6%) and phototype IV (n = 80; 3.6%). Individuals with phototype III (n = 1141; 2.9%) had the lowest prevalence, followed by phototype II (n = 3556; 3.4%).
Cutaneous Comorbidities in People with Acne
In groupwise comparisons, the following skin diseases showed a significantly higher prevalence in people with acne compared to people without acne (Table 1): folliculitis, seborrheic dermatitis, plantar verruca vulgaris and pyodermia. A significantly lower prevalence was found for exsiccation dermatitis, onychomycosis, psoriasis and vascular lesions of the skin.
Table 1.
Frequency of Skin Conditions in People with Acne Vulgaris (AV) in Total and by Gender, N = 161,269; 95% Confidence Intervals Permit Comparison with People Showing No Signs of Acne
| Total with AV (n = 5311) | Male with AV (n = 2970) | Female with AV (n = 2341) | Total without AV (n = 155,958) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | |
| Inflammatory skin diseases | ||||||||||||
| Atopic dermatitis | 82 | 1.5 | (1.23–1.92) | 45 | 1.5 | (1.11–2.03) | 37 | 1.6 | (1.11–2.18) | 2,173 | 1.4 | (1.34–1.45) |
| Contact dermatitis | 19 | 0.4 | (0.22–0.56) | 9 | 0.3 | (0.14–0.58) | 10 | 0.4 | (0.20–0.79) | 330 | 0.2 | (0.19–0.24) |
| Exsiccation dermatosis | 31 | 0.6 | (0.40–0.83) | 16 | 0.5 | (0.31–0.87) | 15 | 0.6 | (0.36–1.06) | 1,526 | 1.0 | (0.93–1.03) |
| Hand eczema | 51 | 1.0 | (0.71–1.26) | 38 | 1.3 | (0.91–1.76) | 13 | 0.6 | (0.30–0.95) | 1,218 | 0.8 | (0.74–0.83) |
| Intertriginous dermatitis | 37 | 0.7 | (0.49–0.96) | 18 | 0.6 | (0.36–0.96) | 19 | 0.8 | (0.49–1.27) | 1,008 | 0.7 | (0.61–0.69) |
| Psoriasis | 66 | 1.2 | (0.96–1.58) | 43 | 1.5 | (1.05–1.95) | 23 | 1.0 | (0.62–1.47) | 3,209 | 2.1 | (1.99–2.13) |
| Rosacea | 94 | 1.8 | (1.43–2.17) | 61 | 2.1 | (1.57–2.64) | 33 | 1.4 | (0.97–1.98) | 3,292 | 2.1 | (2.04–2.18) |
| Seborrheic dermatitis | 204 | 3.8 | (3.33–4.41) | 165 | 5.6 | (4.74–6.47) | 39 | 1.7 | (1.18–2.28) | 4,962 | 3.2 | (3.09–3.27) |
| Viral diseases of the skin | ||||||||||||
| Verruca vulgaris feet | 167 | 3.1 | (2.69–3.66) | 84 | 2.8 | (2.26–3.50) | 83 | 3.6 | (2.82–4.40) | 3,613 | 2.3 | (2.24–2.39) |
| Verruca vulgaris hands | 26 | 0.5 | (0.32–0.72) | 21 | 0.7 | (0.44–1.08) | 5 | 0.2 | (0.07–0.50) | 820 | 0.5 | (0.49–0.56) |
| Fungal diseases of the skin | ||||||||||||
| Onychomycosis | 222 | 4.2 | (3.65–4.77) | 170 | 5.7 | (4.90–6.65) | 52 | 2.2 | (1.66–2.91) | 9,773 | 6.3 | (6.14–6.39) |
| Pityriasis versicolor | 37 | 0.7 | (0.49–0.96) | 25 | 0.8 | (0.54–1.24) | 12 | 0.5 | (0.26–0.90) | 1,549 | 1.0 | (0.94–1.04) |
| Tinea corporis | 17 | 0.3 | (0.19–0.51) | 14 | 0.5 | (0.26–0.79) | 3 | 0.1 | (0.03–0.37) | 676 | 0.4 | (0.40–0.47) |
| Tinea pedis | 223 | 4.2 | (3.67–4.79) | 180 | 6.1 | (5.21–7.01) | 43 | 1.8 | (1.33–2.47) | 7,115 | 4.6 | (4.46–4.67) |
| Bacterial diseases of the skin | ||||||||||||
| Folliculitis | 865 | 16.3 | (15.22–17.41) | 577 | 19.4 | (17.87–21.08) | 288 | 12.3 | (10.92–13.81) | 11,819 | 7.6 | (7.44–7.72) |
| Pyoderma | 70 | 1.3 | (1.03–1.67) | 51 | 1.7 | (1.28–2.26) | 19 | 0.8 | (0.49–1.27) | 778 | 0.5 | (0.46–0.54) |
| Vascular lesions of the skin | ||||||||||||
| Haemangioma | 1521 | 28.6 | (27.22–30.11) | 886 | 29.8 | (27.90–31.86) | 635 | 27.1 | (25.06–29.32) | 65,954 | 42.3 | (41.97–42.61) |
| Naevus flammeus | 220 | 4.1 | (3.61–4.73) | 101 | 3.4 | (2.77–4.13) | 119 | 5.1 | (4.21–6.08) | 7,765 | 5.0 | (4.87–5.09) |
| Spider veins | 752 | 14.2 | (13.17–15.21) | 203 | 6.8 | (5.93–7.84) | 549 | 23.5 | (21.53–25.50) | 30,086 | 19.3 | (19.07–19.51) |
| Telangiectasia | 298 | 5.6 | (4.99–6.29) | 136 | 4.6 | (3.84–5.42) | 162 | 6.9 | (5.90–8.07) | 12,198 | 7.8 | (7.68–7.96) |
Abbreviations: AV, acne vulgaris; CI, confidence interval.
Seborrheic dermatitis, onychomycosis, tinea pedis, folliculitis and pyodermia affected significantly more often men than women. Vascular lesions of the skin were more frequent in women (Table 1).
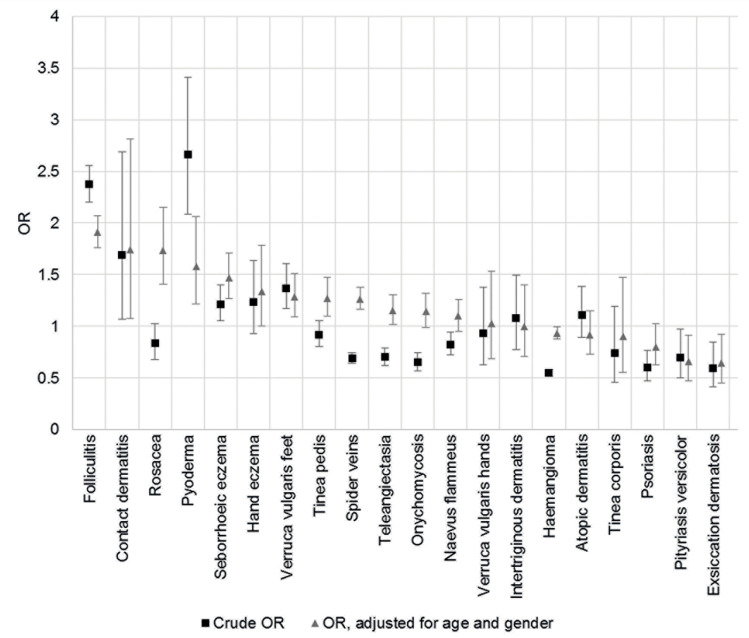
Logistic regression showed that the probability of having acne vulgaris was lower with increasing age (OR = 0.91; CI: 0.91–0.91) and in women (OR = 0.81; CI: 0.76–0.85). Controlling for age and gender, acne was a significantly associated with: folliculitis (OR = 1.91; CI: 1.76–2.07), contact dermatitis (OR = 1.74; CI: 1.08–2.81), rosacea (OR = 1.74; CI: 1.40–2.15), pyoderma (OR = 1.58; CI: 1.22–2.06), seborrheic dermatitis (OR = 1.47; CI: 1.27–1.71), hand eczema (OR = 1.34; CI: 1.00–1.76), verruca vulgaris plantaris (OR = 1.29; CI: 1.09–1.51), tinea pedis (OR = 1.27; CI: 1.10–1.47), spider veins (OR = 1.26; CI: 1.16–1.38) and telangiectasia (OR = 1.15; CI: 1.02–1.30) (Table 2). Less commonly associated with AV were the following diseases: exsiccation dermatosis (OR = 0.64; CI: 0.45–0.92), pityriasis versicolor (OR = 0.66; CI: 0.47–0.92) and haemangioma (OR = 0.93; CI: 0.88–1.00). After Bonferroni correction, folliculitis, pyoderma, rosacea, seborrheic dermatitis, spider veins and tinea pedis remained statistically significant. Figure 2 shows crude OR and OR controlled for age and gender. Significant difference between the crude OR and OR controlled for age and sex shows up only for co-morbidity rosacea. Without control for age and sex, rosacea is not more frequent, but is significantly more often associated with AV in terms of age and gender.
Table 2.
Odds Ratio of the Occurrence of Different Skin Conditions Among People with Acne Vulgaris, Logistic Regression Analysis Controlling for Age and Gender, N = 161,269
| Skin Disease | OR | 95% CI of OR | p ≤ |
|---|---|---|---|
| Atopic dermatitis | 0.92 | 0.73–1.15 | 0.464 |
| Contact dermatitis | 1.74 | 1.08–2.81 | 0.024 |
| Exsiccation dermatosis | 0.64 | 0.45–0.92 | 0.017 |
| Folliculitis | 1.91 | 1.76–2.07 | 0.001* |
| Haemangioma | 0.93 | 0.88–1.00 | 0.040 |
| Hand eczema | 1.34 | 1.00–1.79 | 0.049 |
| Intertriginous dermatitis | 1.00 | 0.71–1.40 | 0.986 |
| Naevus flammeus | 1.10 | 0.95–1.26 | 0.201 |
| Onychomycosis | 1.14 | 0.99–1.32 | 0.068 |
| Pityriasis versicolor | 0.66 | 0.47–0.92 | 0.013 |
| Psoriasis | 0.80 | 0.62–1.03 | 0.078 |
| Pyoderma | 1.58 | 1.22–2.06 | 0.001* |
| Rosacea | 1.74 | 1.40–2.15 | 0.001* |
| Seborrheic dermatitis | 1.47 | 1.27–1.71 | 0.000* |
| Spider veins | 1.26 | 1.16–1.38 | 0.001* |
| Telangiectasia | 1.15 | 1.02–1.30 | 0.025 |
| Tinea corporis | 0.90 | 0.55–1.48 | 0.682 |
| Tinea pedis | 1.27 | 1.10–1.47 | 0.001* |
| Verruca vulgaris (feet) | 1.28 | 1.09–1.51 | 0.003 |
| Verruca vulgaris (hands) | 1.03 | 0.69–1.53 | 0.897 |
Notes: *Remains significant at nominal α ≤ 0.05 level after Bonferroni correction for 22 significance tests.
Abbreviations: CI, confidence interval; OR, odds ratio.
Figure 2.
Odds ratios (crude and adjusted for age and gender) and their 95% confidence intervals to develop further skin diseases when acne vulgaris is present.
Abbreviation: OR, odds ratio.
Discussion
The aim of this study was to determine the prevalence of acne vulgaris (AV) and common cutaneous comorbidity in the adult working population in Germany. In the cohort of 161,269 employees, the total prevalence of AV was 3.3%. These data correspond with the published data where the prevalence of each degree of AV was described for people aged over 25 years between 3% for men and 12% for women.4,29 In consensus with previous publications, we also found gender differences in the prevalence of AV being significantly more prevalent in adolescent men and in women aged 30–39. While AV in adolescents is mainly due to the physiologically increased production of sebum and follicular hyperkeratinisation by pubertal maturation, pathological hormonal fluctuations or hypersensitivity to normal androgen level were described in adults.30–32 In women, however, the use of cosmetic products, diets and comorbidities such as polycystic ovary syndrome also play an important role.33 Premenstrual flare of AV has been described with up to 78% of cases.34,35 In women, the influence of cycle-dependent hormone level fluctuations on the flares seems to be significant.
In our study, AV prevalence was highest in people with skin type I and IV with 3.6%. However, due to less frequent occurrences, people with skin types V and VI after Fitzpatrick were also grouped under skin type IV. The ethnic origin seems to be related to the frequency of AV. Several studies have shown that AV is more common in people of Caucasian descent than in black subjects.24,36 Further studies on larger patient groups with different ethnic origins would be desirable.
We were able to show that people with AV suffer more frequently from pustular and/or infectious skin diseases such as folliculitis or pyoderma. One reason could be the cutaneous dysbiosis due to increased androgen-induced sebum production. Moreover, hormonal imbalances, psychosocial stressors and genetic factors may also contribute to the association between these conditions.
Interestingly, our study showed gender-specific differences in comorbidities related to the skin. Men with AV were more frequently affected by tinea pedis. One explanation might be that men were more likely to engage in physically strenuous activities associated with increased exposure to dirt, wearing protective footwear and sweating. Gender differences in personal hygiene could be another reason.
One limitation of this study is the focus on the working population. However, though data on the prevalence of AV in the unemployed population group are missing, a strong “healthy worker effect” is not expected in this condition. Nevertheless, it has been shown that socio-economic differences exist in the prevalence of AV and that unemployed people suffer more frequently from AV.28 This may have led to an underestimation of prevalence in our study.
Another limitation is the voluntary nature of the study. People rejecting skin examinations might potentially show altered frequencies of skin affections. AV is associated with a severe reduction in quality of life and social isolation.37 It is possible that especially people with a severe AV did not dare to participate in the screenings. Even if the current data cannot be directly transferred to the overall general population, they show robust results due to the size of the cohort of 161,269 people and the standardised kind of whole-body examinations by trained dermatologists.
Conclusion
Overall, the current data underline the importance of AV in the adult population. Furthermore, the associations of AV with other inflammatory and infectious skin diseases show the need for adapted counselling and skin care and treatment for these people. Further studies to better understand the pathophysiology of AV and its comorbidity in different phases of adulthood would be desirable to develop appropriate guidelines and therapy concepts.
Acknowledgments
The authors thank the Scientific Communication Team of the IVDP, in particular Merle Twesten and Mario Gehoff, for copy editing.
Funding Statement
There is no funding to report.
Compliance with Ethical Standards
All procedures performed in studies involving human participants were in accordance with the standards of the ethics committee of the Medical Association of Hamburg and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The UKE clinical ethics approved this study. Informed consent was obtained from all individual participants included in the study.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Moradi Tuchayi S, Makrantonaki E, Ganceviciene R, Dessinioti C, Feldman SR, Zouboulis CC. Acne vulgaris. Nat Rev Dis Primers. 2015;1:15029. doi: 10.1038/nrdp.2015.29 [DOI] [PubMed] [Google Scholar]
- 2.White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2 Pt 3):S34–7. doi: 10.1016/s0190-9622(98)70442-6 [DOI] [PubMed] [Google Scholar]
- 3.Law MPM, Chuh AAT, Lee A, Molinari N. Acne prevalence and beyond: acne disability and its predictive factors among Chinese late adolescents in Hong Kong. Clin Exp Dermatol. 2010;35(1):16–21. doi: 10.1111/j.1365-2230.2009.03340.x [DOI] [PubMed] [Google Scholar]
- 4.Cunliffe WJ, Gould DJ. Prevalence of facial acne vulgaris in late adolescence and in adults. Br Med J. 1979;1(6171):1109–1110. doi: 10.1136/bmj.1.6171.1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schäfer T, Nienhaus A, Vieluf D, Berger J, Ring J. Epidemiology of acne in the general population: the risk of smoking. Br J Dermatol. 2001;145(1):100–104. doi: 10.1046/j.1365-2133.2001.04290.x [DOI] [PubMed] [Google Scholar]
- 6.Williams HC, Dellavalle RP, Garner S. Acne vulgaris. Lancet. 2012;379(9813):361–372. doi: 10.1016/S0140-6736(11)60321-8 [DOI] [PubMed] [Google Scholar]
- 7.Dunn LK, O’Neill JL, Feldman SR. Acne in adolescents: quality of life, self-esteem, mood, and psychological disorders. Dermatol Online J. 2011;17(1):1. [PubMed] [Google Scholar]
- 8.Vilar GN, LAd S, Sobral Filho JF. Quality of life, self-esteem and psychosocial factors in adolescents with acne vulgaris. An Bras Dermatol. 2015;90(5):622–629. doi: 10.1590/abd1806-4841.201533726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Neill AM, Gallo RL. Host-microbiome interactions and recent progress into understanding the biology of acne vulgaris. Microbiome. 2018;6(1):177. doi: 10.1186/s40168-018-0558-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dreno B, Martin R, Moyal D, Henley JB, Khammari A, Seité S. Skin microbiome and acne vulgaris: staphylococcus, a new actor in acne. Exp Dermatol. 2017;26(9):798–803. doi: 10.1111/exd.13296 [DOI] [PubMed] [Google Scholar]
- 11.Dréno B. What is new in the pathophysiology of acne, an overview. J Eur Acad Dermatol Venereol. 2017;31(Suppl 5):8–12. doi: 10.1111/jdv.14374 [DOI] [PubMed] [Google Scholar]
- 12.Pelle E, McCarthy J, Seltmann H, et al. Identification of histamine receptors and reduction of squalene levels by an antihistamine in sebocytes. J Invest Dermatol. 2008;128(5):1280–1285. doi: 10.1038/sj.jid.5701160 [DOI] [PubMed] [Google Scholar]
- 13.Zouboulis CC. Sebaceous gland receptors. Dermatoendocrinol. 2009;1(2):77–80. doi: 10.4161/derm.1.2.7804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trivedi NR, Cong Z, Nelson AM, et al. Peroxisome proliferator-activated receptors increase human sebum production. J Invest Dermatol. 2006;126(9):2002–2009. doi: 10.1038/sj.jid.5700336 [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Li W-H, Anthonavage M, Eisinger M. Melanocortin-5 receptor: a marker of human sebocyte differentiation. Peptides. 2006;27(2):413–420. doi: 10.1016/j.peptides.2005.05.030 [DOI] [PubMed] [Google Scholar]
- 16.Törőcsik D, Kovács D, Camera E, et al. Leptin promotes a proinflammatory lipid profile and induces inflammatory pathways in human SZ95 sebocytes. Br J Dermatol. 2014;171(6):1326–1335. doi: 10.1111/bjd.13229 [DOI] [PubMed] [Google Scholar]
- 17.Burris J, Rietkerk W, Woolf K. Relationships of self-reported dietary factors and perceived acne severity in a cohort of New York young adults. J Acad Nutr Diet. 2014;114(3):384–392. doi: 10.1016/j.jand.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 18.Mahmood SN, Bowe WP. Diet and acne update: carbohydrates emerge as the main culprit. J Drugs Dermatol. 2014;13(4):428–435. [PubMed] [Google Scholar]
- 19.Kouotou EA, Adegbidi H, Bene Belembe R, et al. Acné au Cameroun: qualité de vie et comorbidités psychiatriques [Acne in Cameroon: quality of life and psychiatric comorbidities]. Ann Dermatol Venereol. 2016;143(10):601–606. doi: 10.1016/j.annder.2016.05.011 [DOI] [PubMed] [Google Scholar]
- 20.Bettoli V, Zauli S. The epidemiology and comorbidities of severe acne in children aged 0-17 years. Br J Dermatol. 2014;170(5):1013–1014. doi: 10.1111/bjd.13043 [DOI] [PubMed] [Google Scholar]
- 21.Xie J, Kvaskoff M, Li Y, et al. Severe teenage acne and risk of endometriosis. Hum Reprod. 2014;29(11):2592–2599. doi: 10.1093/humrep/deu207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenfield RL. The diagnosis of polycystic ovary syndrome in adolescents. Pediatrics. 2015;136(6):1154–1165. doi: 10.1542/peds.2015-1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonbol H, Duchatelet S, Miskinyte S, Bonsang B, Hovnanian A, Misery L. PASH syndrome (pyoderma gangrenosum, acne and hidradenitis suppurativa): a disease with genetic heterogeneity. Br J Dermatol. 2018;178(1):e17–e18. doi: 10.1111/bjd.15740 [DOI] [PubMed] [Google Scholar]
- 24.Cheng CE, Irwin B, Mauriello D, Liang L, Pappert A, Kimball AB. Self-reported acne severity, treatment, and belief patterns across multiple racial and ethnic groups in adolescent students. Pediatr Dermatol. 2010;27(5):446–452. doi: 10.1111/j.1525-1470.2010.01286.x [DOI] [PubMed] [Google Scholar]
- 25.El-Essawi D, Musial JL, Hammad A, Lim HW. A survey of skin disease and skin-related issues in Arab Americans. J Am Acad Dermatol. 2007;56(6):933–938. doi: 10.1016/j.jaad.2007.01.031 [DOI] [PubMed] [Google Scholar]
- 26.Freyre EA, Rebaza RM, Sami DA, Lozada CP. The prevalence of facial acne in Peruvian adolescents and its relation to their ethnicity. J Adolesc Health. 1998;22(6):480–484. doi: 10.1016/s1054-139x(97)00277-2 [DOI] [PubMed] [Google Scholar]
- 27.Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41(4):577–580. [PubMed] [Google Scholar]
- 28.Cunliffe WJ. Acne and unemployment. Br J Dermatol. 1986;115(3):386. doi: 10.1111/j.1365-2133.1986.tb05757.x [DOI] [PubMed] [Google Scholar]
- 29.Goulden V, Clark SM, Cunliffe WJ. Post-adolescent acne: a review of clinical features. Br J Dermatol. 1997;136(1):66–70. doi: 10.1111/j.1365-2133.1997.tb08748.x [DOI] [PubMed] [Google Scholar]
- 30.Addor FAS, Schalka S. Acne in adult women: epidemiological, diagnostic and therapeutic aspects. An Bras Dermatol. 2010;85(6):789–795. doi: 10.1590/s0365-05962010000600003 [DOI] [PubMed] [Google Scholar]
- 31.Elsaie ML. Hormonal treatment of acne vulgaris: an update. Clin Cosmet Investig Dermatol. 2016;9:241–248. doi: 10.2147/CCID.S114830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seirafi H, Farnaghi F, Vasheghani-Farahani A, et al. Assessment of androgens in women with adult-onset acne. Int J Dermatol. 2007;46(11):1188–1191. doi: 10.1111/j.1365-4632.2007.03411.x [DOI] [PubMed] [Google Scholar]
- 33.Shrestha S. Correlation of hormonal profile and lipid levels with female adult acne in a Tertiary Care Center of Nepal. J Nepal Health Res Counc. 2018;16(2):222–227. doi: 10.33314/jnhrc.v16i2.1178 [DOI] [PubMed] [Google Scholar]
- 34.Poli F, Dreno B, Verschoore M. An epidemiological study of acne in female adults: results of a survey conducted in France. J Eur Acad Dermatol Venereol. 2001;15(6):541–545. doi: 10.1046/j.1468-3083.2001.00357.x [DOI] [PubMed] [Google Scholar]
- 35.Stoll S, Shalita AR, Webster GF, Kaplan R, Danesh S, Penstein A. The effect of the menstrual cycle on acne. J Am Acad Dermatol. 2001;45(6):957–960. doi: 10.1067/mjd.2001.117382 [DOI] [PubMed] [Google Scholar]
- 36.Hazen H. Personal observations upon skin diseases in the American Negro. J Cutaneous Dis. 1914;32:704–706. [Google Scholar]
- 37.Dreno B, Bagatin E, Blume-Peytavi U, Rocha M, Gollnick H. Female type of adult acne: physiological and psychological considerations and management. J Dtsch Dermatol Ges. 2018;16(10):1185–1194. doi: 10.1111/ddg.13664 [DOI] [PubMed] [Google Scholar]