Abstract
Purpose
Relapse and treatment adherence to paliperidone palmitate once-monthly (PP1M) and three-monthly (PP3M) formulations in patients with schizophrenia were evaluated and compared using health claims data.
Patients and Methods
Data (June 2015─June 2018) obtained from the MarketScan® Multi-State Medicaid Database were retrospectively analyzed. Patients aged ≥18 years with ≥1 claim for schizophrenia diagnosis prior to and/or at index date (i.e., date of first PP3M prescription record for PP3M patients and same month/year as the matched PP3M patients for PP1M patients) and continuous enrollment in the insurance plan for ≥12 months prior to index date (baseline) were included. PP1M cohort included patients who received ≥4 PP1M doses. PP3M patients were matched with PP1M patients (1:3) using propensity score matching and prevalent new user design. Outcome measures were relapse rate, time to relapse, proportion of days covered (PDC), and level of treatment adherence defined by PDC in five levels. Time to relapse was compared by Kaplan–Meier survival curves and log-rank test with the hazard ratio calculated using Cox proportion hazards model; PDC by t-test, and relapse rate and PDC categories by chi-square test.
Results
A total of 1564 patients (428 PP3M and 1136 PP1M) were included. Relapse rate was lower in PP3M cohort (10.5%) compared with PP1M cohort (15.7%). Incidence rate of relapse was 8.98/100 person-years (PY) in PP3M cohort and 13.81/100 PY in PP1M cohort. After a mean (SD) follow-up of 456.1 (240.28) days in PP3M cohort and 465.4 (237.95) days in PP1M cohort, PP3M patients had a significantly lower relapse risk (hazard ratio: 0.65, 95% CI: 0.47, 0.90) than PP1M patients. Treatment adherence was significantly (p<0.0001) higher in PP3M versus PP1M cohort.
Conclusion
Risk of relapse was significantly lower, and treatment adherence was significantly higher in PP3M cohort compared with PP1M cohort. Higher treatment adherence was associated with lower relapse rate.
Keywords: health claims, paliperidone palmitate, relapse rate, schizophrenia, three-monthly, treatment adherence
Introduction
Schizophrenia is a chronic condition characterized by hallucinations, delusions, and impaired cognition and perception. Estimates of schizophrenia prevalence in the United States Medicaid population range from 2.16% to 4.01%.1 Most patients with schizophrenia experience frequent symptomatic exacerbations and relapse, triggered most often by treatment non-adherence or treatment discontinuation.2,3 Illness relapse is associated with an increased risk of serious, negative outcomes, such as increased overall mortality, violence, damaged relationships, increased stigmatization, disruptions in employment and education, decreased brain volume, treatment resistance, worsening of baseline level of functioning, and increased individual and societal economic burdens.3–7 Treatment with antipsychotic medications, compared to no treatment, in patients with schizophrenia - a cohort known to suffer from excess mortality compared to the general population - is also associated with a reduced risk of death.3,8 Relapse prevention is therefore the main goal of schizophrenia management.9
Nonadherence to antipsychotic therapy is a prominent driver of healthcare resource utilization (HRU) among patients with schizophrenia. Approximately 40%–60% of patients with schizophrenia are partially or totally nonadherent to antipsychotic therapy;10 with some estimates being as high as 89%.11 Indeed, even medication gaps of 1 to 10 continuous days is associated with a two-fold increase in risk of hospitalization.12 This increase in risk adds on to the annual economic burden of schizophrenia in the United States, which was estimated to be USD 155.7 billion in 2013, with a direct healthcare cost of USD 38 billion. Moreover, societal cost per patient may be as high as USD 95,000 per year according to a 2015 estimate.13
Long acting injectable antipsychotics (LAIs) eliminate the need for daily medication dosing14 and reduce the risk of relapse and hospitalization compared to oral antipsychotics.15–21 Paliperidone palmitate (PP) is a second-generation antipsychotic available in once-monthly (PP1M) and three-monthly (PP3M) dosing formulations. PP3M initiation may occur after a minimum of four months (i.e., 13 weeks) of continuous treatment with PP1M, with the last two doses of PP1M recommended to be the same dosage strength.22 PP3M has shown efficacy versus placebo23 and non-inferiority versus PP1M in reducing the risk of schizophrenia relapse.24 It can be hypothesized that a LAI such as PP3M, which has a longer dosing interval compared to monthly LAIs, may offer a greater level of treatment adherence and thereby reduce relapse rates. This hypothesis, however, would be difficult to test in clinical trials since adherence in trials is protocol-driven and is likely to be higher than that observed in routine clinical practice. Additionally, in a clinical trial, a disproportionate adherence level between treatment arms may introduce bias in estimation of outcome measures; hence, it is reasonable to compare treatment adherence levels in real-world settings. Therefore, we conducted the present study to compare rates of treatment adherence and relapse after initiation of PP3M treatment versus continued PP1M treatment (i.e., patients who do not switch to PP3M treatment) in Medicaid patients with schizophrenia using health claims data generated in routine clinical practice.
Methods
Data Sources
Data were obtained from MarketScan® Multi-State Medicaid Database (MDCD), a US health claims database. The MDCD contains enrollment information (eg, demographics, period of enrollment, plan type), inpatient and outpatient services records, medical and pharmacy claims data, and financial information of more than 10 million Medicaid enrollees each year from approximately 10 states.
Use of the MDCD was reviewed by the New England Institutional Review Board and determined to be exempt from review board approval, as this study does not involve human subjects research. All data were de-identified and fully complied with the US Health Insurance Portability and Accountability Act of 1996 regulations.
International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM), National Drug Code, Current Procedural Terminology, and The Healthcare Common Procedure Coding System codes were used to retrieve data related to diagnoses, medication records, and procedures (Supplementary Table 1).
Study Design and Sample Selection
This retrospective cohort study was conducted from 01 June 2015 to 30 June 2018. For the PP3M cohort, date of the first PP3M prescription record was assigned as the index date. Patients aged ≥18 years who had at least one claim for schizophrenia diagnosis (Supplementary Table 1), anytime up to the index visit, and a continuous enrollment in the insurance plan for at least 12 months prior to the index date (ie, baseline period) and 12 months after the index date (ie, follow-up period) were included in the study. Only patients with Medicaid drug coverage included in the study to exclude patients with both Medicare and Medicaid (dual) coverage. To minimize the effect of potential confounding, the following were excluded: patients with a claim for bipolar, dementia or autism diagnosis prior to the index date; patients treated with clozapine during the baseline period, using clozapine treatment as a means of indirectly identifying patients with treatment-resistant schizophrenia; patients who had not received ≥4 lead-in treatment with PP1M prior to the index date; patients treated with a concomitant antipsychotic medication on the index date. Patients were followed up from the index date until they left the insurance plan (ignoring breaks of <30 days), end of the study (cut-off date of data received before analysis), or two years after the index date, whichever occurred first.
Study Cohorts and Matching
The PP3M cohort was selected from patients who received PP3M after ≥4 lead-in doses of PP1M at an equivalent dosage strength (Supplementary Table 2) to the index PP3M dose. The first PP1M dose after the lead-in doses was considered the index date for the matched PP1M cohort. The PP3M patients were matched with PP1M patients at a 1:3 ratio using propensity score matching. Matching was conducted stratifying by lead-in PP1M injection counts, PP1M dose strength, the index month (i.e., these 3 factors were exact match). For each stratum, propensity score was calculated using factors: age categories, gender, baseline depression, Charlson Comorbidity Index Score, Elixhauser Comorbidity Index Score. Two matching approaches were applied for cohort selection. The prevalent new user design (PNUD) approach is presented as the primary analysis; the concurrent control approach was a sensitivity analysis.
In the PNUD approach, a PP1M patient in the control cohort was identified to have the same strength and the number of lead-in doses of PP1M as the matched PP3M patient in the case cohort and in addition, had the index date in the same month as that of the matched case; those PNUD control PP1M patients may have switched to PP3M later. This design avoids potential bias introduced due to the use of future information on exposure to determine cohort membership. For the sensitivity analysis (concurrent control group), PP1M patients whose index date was after June 2015 and who had never switched to PP3M were included.
Demographics, Clinical Characteristics and Outcome Measures
Demographics and clinical characteristics including age at the index date, age group (18–24, 25–34, 35–44, 45–54, 55–64, ≥65 years), sex, number of lead-in PP1M doses, strength of paliperidone doses received, diagnosis of major depressive disorder, and substance abuse (Supplementary Table 1) during the baseline period, Elixhauser comorbidity score (calculated using the diagnosis codes during the 12-month baseline period), and Charlson comorbidity index (CCI, calculated using the diagnosis codes during the 12-month baseline period) were determined.
Outcome measures were relapse rate, time to relapse, and level of treatment adherence. Relapse was determined to occur if a patient had a claims record for any of the following: hospitalization with schizophrenia, suicidal behavior, suicide attempt, injury with undetermined intent, suicidal ideation, homicidal ideation, exacerbation of schizophrenia, clozapine use, violent behavior, hostility, and aggressive behavior (Supplementary Table 1). The time to relapse was considered as the day of first occurrence of any of the events in these relapse criteria during the follow-up period.
Adherence was measured in terms of the proportion of days covered (PDC), defined as the number of days of the study period covered by a PP3M or PP1M prescription and lack of any other antipsychotics divided by the total number of days in follow-up period. Level of treatment adherence in terms of PDC was categorized as 0–20%, 20–40%, 40–60%, 60–80%, and 80–100%.
Statistical Analysis
Patient characteristics and outcome measures for each cohort were summarized using descriptive statistics. Time to relapse was compared by Kaplan–Meier survival curves and log-rank test with the hazard ratio calculated using Cox proportion hazards model; PDC by t-test, and relapse rate and PDC categories by chi-square test.
Results
PNUD Approach
Patient Characteristics
In the PNUD control approach, 1136 patients who had ≥4 lead-in PP1M dose and were non-bipolar were included in the PP1M cohort and 428 patients in the PP3M cohort. After propensity score matching, the PP1M and PP3M cohorts were balanced for baseline characteristics, except for the age category (absolute standardized difference = 0.119). A greater proportion of patients were in the age group of 25–34 years in the PP1M cohort (n=408, 35.9%) and PP3M (n=139, 32.5%) as compared to the other age groups. A total of 942 patients (82.9%) in the PP1M cohort and 347 patients (81.1%) in the PP3M cohort were on higher strength (Level 3 and 4) PP doses (Table 1; Supplementary Table 3).
Table 1.
Demographic and Baseline Characteristics After Matching the PP3M and PP1M Cohorts (“≥4 Lead-in PP1M Dose and Non-Bipolar” Analysis Set) for PNUD and Concurrent Control Approaches
| PNUD Approach | Concurrent Control Approach | |||
|---|---|---|---|---|
| PP1M (n=1136) | PP3M (n=428) | PP1M (n=1525) | PP3M (n=518) | |
| Gender, n (%) | ADS=0.020 | ASD=0.057 | ||
| Women | 311 (27.4) | 121 (28.3) | 422 (27.7) | 152 (29.3) |
| Age categories, n (%) | ASD=0.119 | ASD=0.040 | ||
| 18–24 | 106 (9.3) | 50 (11.7) | 184 (12.1) | 59 (11.4) |
| 25–34 | 408 (35.9) | 139 (32.5) | 505 (33.1) | 169 (32.6) |
| 35–44 | 282 (24.8) | 99 (23.1) | 342 (22.4) | 119 (23) |
| 45–54 | 195 (17.2) | 83 (19.4) | 300 (19.7) | 102 (19.7) |
| 55–64 | 144 (12.7) | 56 (13.1) | 186 (12.2) | 65 (12.5) |
| ≥65 | 1 (0.1) | 1 (0.2) | 8 (0.5) | 4 (0.8) |
| Paliperidone dosea, n (%) | ASD=0.051 | ASD=0.045 | ||
| Level 1b | 4 (0.4) | 2 (0.5) | 13 (0.9) | 3 (0.6) |
| Level 2c | 190 (16.7) | 79 (18.5) | 273 (17.9) | 99 (19.1) |
| Level 3d | 472 (41.5) | 176 (41.1) | 629 (41.2) | 213 (41.1) |
| Level 4e | 470 (41.4) | 171 (40) | 610 (40) | 203 (39.2) |
| Depression diagnosis at baseline, n (%) | ASD=0.022 | ASD=0.012 | ||
| Yes | 213 (18.8) | 84 (19.6) | 267 (17.5) | 93 (18) |
| Charlson Comorbidity Score | ASD=0.039 | ASD=0.056 | ||
| Mean (SD) | 0.6 (1.24) | 0.5 (1.09) | 0.6 (1.31) | 0.5 (1.07) |
| Elixhauser Score | ASD=0.050 | ASD=0.093 | ||
| Mean (SD) | 2.7 (1.97) | 2.6 (1.70) | 2.8 (2.01) | 2.6 (1.74) |
Notes: a156 mg of paliperidone palmitate = 100 mg eq (the dosing scheme for US is different from rest of the world); bPP1M=78 mg; PP3M=273 mg; cPP1M=117 mg; PP3M=410 mg; dPP1M=156 mg; PP3M=546 mg; ePP1M=234 mg; PP3M=819 mg.
Abbreviations: ASD, absolute standardized difference; PNUD, prevalent new user design; PP1M, Paliperidone palmitate once-monthly; PP3M, Paliperidone palmitate 3-monthly.
Relapse
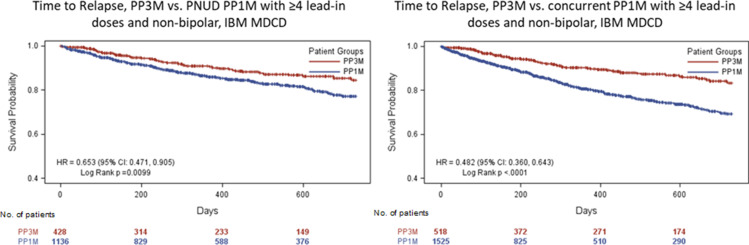
Overall, 179 patients (15.7%) in the PP1M cohort and 45 patients (10.5%) in the PP3M cohort had relapse, and schizophrenia-related hospitalization was the most common relapse criterion (PP1M: 10.7%; PP3M: 5.6%, Table 2). Incidence rate of relapse was 13.81 per-100-person years (PY) in the PP1M cohort and 8.98 per-100-PY in the PP3M cohort (Table 3). After a mean (SD) follow-up of 465.4 (237.9) days in the PP1M cohort and 456.1 (240.2) days in the PP3M cohort (Table 3), risk of relapse was 35% higher in the PP1M cohort (hazard ratio [HR]: 0.65; 95% CI: 0.47─0.90) (Figure 1).
Table 2.
Relapse Rate of Schizophrenia and Relapse Criteria in the PP3M and PP1M Cohorts (“≥4 Lead-in PP1M Dose and Non-Bipolar” Analysis Set) for PNUD and Concurrent Control Approaches
| PNUD Approach | Concurrent Control Approach | |||
|---|---|---|---|---|
| PP1M (n=1136) | PP3M (n=428) | PP1M (n=1525) | PP3M (n=518) | |
| Non-relapse, n (%) | 957 (84.2) | 383 (89.5) | 1269 (83.2) | 462 (89.2) |
| Relapse, n (%) | 179 (15.7) | 45 (10.5) | 256 (16.8) | 56 (10.8) |
| Relapse Criteria, n (%) | ||||
| Schizophrenia- related hospitalization | 121 (10.7) | 24 (5.6) | 161 (10.6) | 31 (6.0) |
| Suicidal ideation | 38 (3.3) | 11 (2.6) | 54 (3.5) | 13 (2.5) |
| Homicidal ideation | 8 (0.7) | 5 (1.2) | 11 (0.7) | 6 (1.2) |
| Suicidal ideation, homicidal ideation | 1 (0.1) | 2 (0.5) | 8 (0.5) | 2 (0.4) |
| Aggressive/violent behavior | 1 (0.1) | - | - | - |
| Suicide attempt | 2 (0.2) | 2 (0.5) | 4 (0.3) | 3 (0.6) |
| Clozapine use | 6 (0.5) | 1 (0.2) | 8 (0.5) | 1 (0.2) |
| Aggressive/violent behavior, hostility | 3 (0.2) | |||
| Suicidal ideation, Suicide attempt | 1 (0.1) | - | 3 (0.2) | - |
| Suicidal ideation, aggressive/violent behavior, hostility | 1 (0.1) | - | ||
Abbreviations: PNUD, prevalent new user design; PP1M, Paliperidone palmitate once-monthly; PP3M, Paliperidone palmitate 3-monthly.
Table 3.
Relapse Incidence Rate and Follow-Up Duration of the PP3M and PP1M Cohorts (“≥4 Lead-in PP1M Dose and Non-Bipolar” Analysis Set) for PNUD and Concurrent Approaches
| PNUD Approach | PP1M | PP3M |
|---|---|---|
| Incidence Rate | ||
| N | 1136 | 428 |
| Person years | 1297 | 501 |
| Event | 179 | 45 |
| Incidence rate | 13.81 | 8.98 |
| Follow-up duration (days), Mean (SD) | 465.4 (237.95) | 456.1 (240.28) |
| Concurrent control approach | ||
| N | 1525 | 518 |
| Person years | 1267 | 591 |
| Event | 256 | 56 |
| Incidence rate | 20.2 | 9.47 |
| Follow-up duration (days), Mean (SD) | 355.9 (258.52) | 447.4 (243.65) |
Abbreviations: PP1M, Paliperidone palmitate once-monthly; PP3M, Paliperidone palmitate 3-monthly; PNUD, prevalent new user design.
Figure 1.
Time to relapse (“≥4 lead-in PP1M dose and non-bipolar” analysis set) for PNUD and Concurrent control approaches.
Abbreviations: PP1M, Paliperidone palmitate once-monthly; PP3M, Paliperidone palmitate 3-monthly; PNUD, prevalent new user design No. of patients.
Treatment Adherence
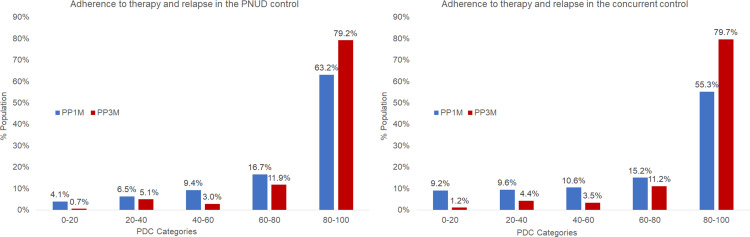
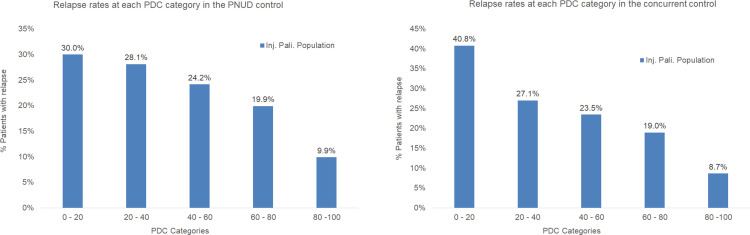
PP3M patients had a significantly higher PDC than PP1M patients with mean (SD) 87% (19%) vs 78% (24%) (p<0.0001) (Table 4). Patients were categorized into five groups based on the PDC on therapy (0–20%, 20–40%, 40–60%, 60–80%, and 80–100%). Level of adherence was significantly (p<0.0001) higher in the PP3M cohort as compared with the PP1M cohort (Figure 2). As both PP3M and PP1M cohorts showed similar pattern of association of relapse and adherence, the results combining the two cohorts were presented in Figure 3, which shows that relapse rate was highest among patients in the PDC category 0–20% and lowest among patients in the PDC category 80–100%.
Table 4.
Proportion Days Covered (PDC) of the PP3M and PP1M Cohorts (“≥4 Lead-in PP1M Dose and Non-Bipolar” Analysis Set) for PNUD and Concurrent Approaches
| PP1M | PP3M | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Approach | N | Mean | SD | Min | Max | N | Mean | SD | Min | Max | p-value |
| PNUD | 1136 | 78% | 24% | 4% | 107% | 428 | 87% | 19% | 13% | 108% | <0.0001 |
| Concurrent | 1525 | 73% | 30% | 4% | 116% | 518 | 87% | 20% | 13% | 109% | <0.0001 |
Abbreviations: PP1M, Paliperidone palmitate once-monthly; PP3M, Paliperidone palmitate 3-monthly; PNUD, prevalent new user design.
Figure 2.
Adherence to therapy in patients of the PNUD and the concurrent control approaches (≥4 lead-in PP1M dose and non-bipolar’ analysis set).
Abbreviations: PDC, proportion of days covered; PNUD, prevalent new user design; PP1M, Paliperidone palmitate once-monthly; PP3M, Paliperidone palmitate 3-monthly.
Figure 3.
Relapse by adherence categories in patients of the PNUD and the concurrent control approaches (≥4 lead-in PP1M dose and non-bipolar’ analysis set).
Abbreviations: PDC, proportion of days covered; PNUD, prevalent new user design; PP1M, Paliperidone palmitate once-monthly; PP3M, Paliperidone palmitate 3-monthly.
A similar trend was observed in the level of adherence in all patients and patients without bipolar disorder wherein, patients of the PP3M cohort showed a significantly higher (p<0.0001) level of adherence as compared with patients of the PP1M cohort (Supplementary Tables 4–6, Supplementary Figures 1–2).
Sensitivity Analyses: Concurrent Control Approach
Patient Characteristics
In the concurrent control group, 1525 patients who had ≥4 lead-in PP1M doses and were non-bipolar were included in the PP1M cohort and 518 patients in the PP3M cohort. A total of 1239 patients (81.2%) from the PP1M cohort and 416 patients (80.3%) from the PP3M cohort were on higher strength (Level 3 and 4) PP doses (Table 1). Most of the patients were men (PP1M: 72.3%, PP3M: 70.7%). A greater proportion of patients were in the 25 to 34-year age group (PP1M: 33.1%; PP3M: 32.6%). Most of the patients did not have depression at baseline (PP1M: 82.5%, PP3M: 82%). After matching propensity scores, baseline characteristics of PP1M and PP3M patients were balanced (Table 1).
Relapse
Overall, 256 (16.8%) of PP1M patients and 56 (10.8%) of PP3M patients relapsed (Table 2). In the PP1M cohort, the incidence rate of relapse was 20.20 per-100-PY, and in the PP3M cohort, the incidence rate was 9.47-per-100-PY (Table 3). Of the various relapse criteria, schizophrenia-related hospitalization (PP1M: 10.6%, PP3M: 6.0%) was the most common, followed by suicidal ideation (PP1M: 3.5%, PP3M: 2.5%) (Table 2). After a mean (SD) follow-up of 355.9 (258.5) days in the PP1M cohort and 447.4 (243.6) days in the PP3M cohort (Table 3), PP3M patients had a significantly lower risk of relapse compared to patients on PP1M (HR: 0.48, 95% CI: 0.36, 0.64) (Figure 1).
Treatment Adherence
PP3M patients had a significantly higher PDC than PP1M patients with mean (SD) 87% (20%) vs 73% (30%) (p<0.0001) (Table 4). A significant (p<0.0001) difference was observed in the adherence to therapy across each PDC category and between PP1M and PP3M cohorts. Similar to the results of PNUD, relapse rate was associated with PDC category as displayed in Figure 3, relapse rate was highest among patients in the PDC category 0–20% and lowest among patients in the PDC category 80–100%.
The results of the PNUD and concurrent control approaches were consistent.
Discussion
Medicaid is the largest payer in the United States for mental health services, including services for schizophrenia.25,26 Health claims data obtained from Medicaid are commonly analyzed in real-world mental-health research. With progressing functional impairment, patients with schizophrenia are more likely to fall into the Medicaid pool, resulting in an over-representation in the Medicaid population compared to the commercially insured population. As the IBM MDCD database collects administrative data from ten states that have varying sociodemographic composition, whose patients were already fully deidentified by IBM and had no contact with the authors, data analyzed in this study are likely to be representative. We retrospectively compared the impact of PP1M and PP3M therapies on treatment adherence and relapse in patients with schizophrenia using health claims data of Medicaid beneficiaries. The current study demonstrates that adherence to therapy was higher in PP3M patients than PP1M patients, and the results are consistent with the known inverse association between higher treatment adherence and lower relapse risk.27 Although PP1M is itself effective in reducing the risk of relapse, results of the present study reiterate that treatment with PP3M, with its longer dosing interval, appears to result in a lower risk of relapse compared to continued treatment with PP1M.
Nonadherence to treatment is a robust predictor of relapse, hospitalizations and poorer long-term outcome. Adherence to treatment is associated with a lower risk of relapse among patients with schizophrenia.28 Less frequent dosing regimens have shown improvement in treatment adherence among patients with schizophrenia and other psychiatric disorders.29 We observed that treatment adherence was higher in the PP3M cohort than in the PP1M cohort and that the relapse rate was lowest among patients in the PDC category 80–100%. Higher treatment nonadherence or lower persistence observed in the PP1M group as compared to PP3M may have corresponded with higher relapse rate in PP1M cohort. A retrospective database analysis of commercially insured patients with schizophrenia who transitioned from PP1M to PP3M showed that the proportion of patients with PDC ≥80% significantly increased (p = 0.007) from 65.1% to 78.9% after transitioning.30 Other studies have also demonstrated improved adherence after transitioning from PP1M to PP3M;31,32 however, those studies did not investigate the effect of adherence on relapse. Previous studies have shown that patients who received PP3M demonstrated improved relapse rates as compared to placebo (incidence of relapse: 8.8% [PP3M] vs 29.0% [placebo]) and PP1M (incidence of relapse: 8.1% [PP3M] vs 9.2% [PP1M]),23,33 thus implying that a reduction in dose frequency was associated with improved adherence, which subsequently reduces the risk of relapse in patients with schizophrenia. Our results from sensitivity analysis of historic and PNUD control groups further substantiated those findings. Of note however, some cases (in both groups) may also have discontinued treatment due to tolerability issues, but that there is no evidence to support that PP1M would be less tolerable than PP3M.
Improved adherence and relapse rate with LAIs may further reduce disease burden, since relapse is the most resource-exhaustive health state of schizophrenia; however, evidence is not conclusive. For example, although Emond et al observed improved treatment adherence with transition from PP1M to PP3M in a commercially insured population with schizophrenia, there was no significant difference in HRU pre- vs post-transition.30 However, transition was associated with a reduced burden of comorbidities such psychoses, diabetes without chronic complication, drug abuse, and substance-related and addictive disorder.30 Similar results were noted in Medicaid beneficiaries where transitioning from PP1M to PP3M was associated with no significant change in HRU and healthcare costs.31 In US veterans, transition was associated with decreases in HRU and costs.34 Therefore, to assess the long-term cost-effectiveness of PP3M, it is important to estimate the effect of improved adherence and relapse rate on HRU and healthcare cost. Furthermore, it is important to note that an improved adherence and decreased relapse rate reduces mortality among patients with schizophrenia.3,35 Cullen et al35 further reported that an annual antipsychotic continuity of greater than 90% was associated with significantly reduced risk of mortality (HR 0.75, 95% CI 0.57–0.99).
Most patients with schizophrenia show signs of cognitive impairment such as deficits in executive functioning, attention, new learning, and decision-making abilities.36 Cognitive deficits in schizophrenia are associated with increased disability, decreased functional connectivity in brain, impaired neuroplasticity, and decreased physiological activity in specific areas of the brain.37 Cognitive deficits in schizophrenia may worsen with increasing age and progression of the disease.36 Additionally, patients with schizophrenia often show gray matter deficits that present as impaired socio-occupational functioning and difficulty in learning.38 Poor response to treatment or lack of treatment due to poor adherence, have been shown to aggravate gray matter volume deficit in patients with schizophrenia.4
Given that schizophrenia is a chronic psychiatric disorder with high heterogeneity in the magnitude of effect, the generalizability of these results must be carefully considered. Furthermore, this study is subject to limitations inherent to retrospective health claims analyses, such as unidentified confounders, coding errors, and reporting bias. Diagnoses were identified using billing codes, and not clinically validated; hence, the possibility of misidentification cannot be ruled out. Since IBM MDCD database does not contain information on clinical assessment such as positive and negative syndrome scale scores, relapse criteria were based only on claims data; hence, relapse rates may be underestimated for all cohorts. The patient severity could also be low owing to exclusion of clozapine and associated treatment-resistant disease due to antipsychotic polytherapy and could be considered as potential confounders. While we used propensity score matching based on obvious confounders to compensate for selection bias, the possibility of residual confounding cannot be excluded. The Medicaid population has an over-representation of patients with mental health diagnoses; therefore, these results may not be generalizable to commercially insured populations or other groups that are socio-economically better-off than the Medicaid pool. The inclusion criterion on follow-up period in this study was >12 months; long-term follow-up data were not available.
In conclusion, the risk of relapse was significantly lower in Medicaid beneficiaries with schizophrenia after they switched to PP3M compared to the risk with continued treatment with PP1M. Treatment adherence was significantly higher with PP3M than with PP1M. An increased level of adherence was associated with a decreased relapse rate, as patients with higher proportion of days covered by their LAI medication showed lower relapse rates. Relapse is a major contributor to schizophrenia burden. Therefore, future studies evaluating PP3M-associated improvement in relapse should attempt to elucidate the association between improved adherence and reduction in relapse rate along with their impact on resource utilization.
Acknowledgments
The authors acknowledge Leo J. Philip, P. Pandey, G. Virya and Shweta Pitre (all from SIRO Clinpharm Pvt. Ltd.) for providing writing assistance and Ellen Baum, PhD (Janssen Global Services, LLC) for additional editorial support.
Funding Statement
This study was sponsored by Janssen Research & Development, LLC.
Abbreviations
HR, Hazard Ratio; HRU, Healthcare Resource Utilization; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; ICD-10-CM, International Classification of Diseases, Tenth Revision, Clinical Modification; LAI, Long-acting Injectable Antipsychotics; MDCD, MarketScan® Multi-State Medicaid Database; PNUD, Prevalent New User Design; PP, Paliperidone Palmitate; PP1M, Paliperidone Palmitate Once-monthly; PP3M, Paliperidone Palmitate Three-monthly; PDC, Proportion of Days Covered; PY, Person-years; SD, Standard Deviation.
Data Sharing Statement
The data used in this study are stored with the MDCD and can be accessed by approaching MDCD.
Ethics Approval and Informed Consent
Use of the MDCD was reviewed by the New England Institutional Review Board and determined to be exempt from review board approval, as this study does not involve human subjects research. All data were de-identified and fully complied with the US Health Insurance Portability and Accountability Act of 1996 regulations.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All the authors were employees of Janssen and its subsidiaries during the study and may own stock or stock options. The authors report no other conflicts of interest in this work.
References
- 1.Lafeuille MH, Patel C, Pilon D, et al. Prevalence, Incidence and Economic Burden of Schizophrenia Among Medicaid Beneficiaries. 32nd Annual Psych Congress; 2019; San Diego, California. [DOI] [PubMed] [Google Scholar]
- 2.Tiihonen J, Tanskanen A, Taipale H. 20-Year Nationwide Follow-Up Study on Discontinuation of Antipsychotic Treatment in First-Episode Schizophrenia. Am J Psychiatry. 2018;175(8):765–773. doi: 10.1176/appi.ajp.2018.17091001 [DOI] [PubMed] [Google Scholar]
- 3.Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274–280. doi: 10.1016/j.schres.2017.12.010 [DOI] [PubMed] [Google Scholar]
- 4.Andreasen NC, Liu D, Ziebell S, Vora A, Ho BC. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. Am J Psychiatry. 2013;170(6):609–615. doi: 10.1176/appi.ajp.2013.12050674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emsley R, Chiliza B, Asmal L. The evidence for illness progression after relapse in schizophrenia. Schizophr Res. 2013;148(1–3):117–121. doi: 10.1016/j.schres.2013.05.016 [DOI] [PubMed] [Google Scholar]
- 6.Herings RM, Erkens JA. Increased suicide attempt rate among patients interrupting use of atypical antipsychotics. Pharmacoepidemiol Drug Saf. 2003;12(5):423–424. doi: 10.1002/pds.837 [DOI] [PubMed] [Google Scholar]
- 7.Takeuchi H, Siu C, Remington G, et al. Does relapse contribute to treatment resistance? Antipsychotic response in first- vs. second-episode schizophrenia. Neuropsychopharmacology. 2019;44(6):1036–1042. doi: 10.1038/s41386-018-0278-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laursen TM, Nordentoft M, Mortensen PB. Excess early mortality in schizophrenia. Annu Rev Clin Psychol. 2014;10:425–448. doi: 10.1146/annurev-clinpsy-032813-153657 [DOI] [PubMed] [Google Scholar]
- 9.Chen EY-H, Hui CL-M, Dunn EL-W, et al. A prospective 3-year longitudinal study of cognitive predictors of relapse in first-episode schizophrenic patients. Schizophr Res. 2005;77(1):99–104. doi: 10.1016/j.schres.2005.02.020 [DOI] [PubMed] [Google Scholar]
- 10.Wu EQ, Shi L, Birnbaum H, Hudson T, Kessler R. Annual prevalence of diagnosed schizophrenia in the USA: a claims data analysis approach. Psychol Med. 2006;36(11):1535–1540. doi: 10.1017/S0033291706008191 [DOI] [PubMed] [Google Scholar]
- 11.Kishimoto T, Hagi K, Nitta M, et al. Effectiveness of Long-Acting Injectable vs Oral Antipsychotics in Patients With Schizophrenia: a Meta-analysis of Prospective and Retrospective Cohort Studies. Schizophr Bull. 2018;44(3):603–619. doi: 10.1093/schbul/sbx090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiden PJ, Kozma C, Grogg A, Locklear J. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatric Services. 2004;55(8):886–891. doi: 10.1176/appi.ps.55.8.886 [DOI] [PubMed] [Google Scholar]
- 13.Jin H, Mosweu I. The societal cost of schizophrenia: a systematic review. Pharmacoeconomics. 2017;35(1):25–42. doi: 10.1007/s40273-016-0444-6 [DOI] [PubMed] [Google Scholar]
- 14.Leucht C, Heres S, Kane JM, Kissling W, Davis JM, Leucht S. Oral versus depot antipsychotic drugs for schizophrenia–a critical systematic review and meta-analysis of randomised long-term trials. Schizophr Res. 2011;127(1–3):83–92. doi: 10.1016/j.schres.2010.11.020 [DOI] [PubMed] [Google Scholar]
- 15.Baser O, Xie L, Pesa J, Durkin M. Healthcare utilization and costs of Veterans Health Administration patients with schizophrenia treated with paliperidone palmitate long-acting injection or oral atypical antipsychotics. J Med Econ. 2015;18(5):357–365. doi: 10.3111/13696998.2014.1001514 [DOI] [PubMed] [Google Scholar]
- 16.Kishimoto T, Nitta M, Borenstein M, Kane JM, Correll CU. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957–965. doi: 10.4088/JCP.13r08440 [DOI] [PubMed] [Google Scholar]
- 17.Lafeuille MH, Grittner AM, Fortier J, et al. Comparison of rehospitalization rates and associated costs among patients with schizophrenia receiving paliperidone palmitate or oral antipsychotics. Am J Health Syst Pharm. 2015;72(5):378–389. doi: 10.2146/ajhp140219 [DOI] [PubMed] [Google Scholar]
- 18.Lafeuille MH, Laliberte-Auger F, Lefebvre P, Frois C, Fastenau J, Duh MS. Impact of atypical long-acting injectable versus oral antipsychotics on rehospitalization rates and emergency room visits among relapsed schizophrenia patients: a retrospective database analysis. BMC Psychiatry. 2013;13:221. doi: 10.1186/1471-244X-13-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcus SC, Zummo J, Pettit AR. Antipsychotic Adherence and Rehospitalization in Schizophrenia Patients Receiving Oral Versus Long-Acting Injectable Antipsychotics Following Hospital Discharge. J Manag Care Spec Pharm. 2015;21(9):754–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Offord S, Wong B, Mirski D, Baker RA, Lin J. Healthcare resource usage of schizophrenia patients initiating long-acting injectable antipsychotics vs oral. J Med Econ. 2013;16(2):231–239. doi: 10.3111/13696998.2012.751025 [DOI] [PubMed] [Google Scholar]
- 21.Tiihonen J, Haukka J, Taylor M, Haddad PM, Patel MX, Korhonen P. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168(6):603–609. doi: 10.1176/appi.ajp.2011.10081224 [DOI] [PubMed] [Google Scholar]
- 22.Gopal S, Vermeulen A, Nandy P, et al. Practical guidance for dosing and switching from paliperidone palmitate 1 monthly to 3 monthly formulation in schizophrenia. Curr Med Res Opin. 2015;31(11):2043–2054. doi: 10.1185/03007995.2015.1085849 [DOI] [PubMed] [Google Scholar]
- 23.Berwaerts J, Liu Y, Gopal S, et al. Efficacy and safety of the 3-month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2015;72(8):830–839. doi: 10.1001/jamapsychiatry.2015.0241 [DOI] [PubMed] [Google Scholar]
- 24.Savitz AJ, Xu H, Gopal S, et al. Efficacy and safety of paliperidone palmitate 3-month formulation for patients with schizophrenia: a randomized, multicenter, double-blind, noninferiority study. Int J Neuropsychopharmacol. 2016;19(7):pyw018. doi: 10.1093/ijnp/pyw018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown JD, Barrett A, Ireys H, Caffery E, Hourihan K. Evidence-based practices for Medicaid beneficiaries with schizophrenia and bipolar disorder; 2012. Available from: http://aspe.hhs.gov/daltcp/reports/2012/ebpsbd.pdf. Accessed September9, 2021.
- 26.Kronick RG, Bella M, Gilmer TP. Faces of Medicaid III: refining the Portrait of People with Multiple Chronic Conditions. 2009; Available from: https://www.chcs.org/media/Faces_of_Medicaid_III.pdf. Accessed September9, 2021.
- 27.Emsley R, Kilian S. Efficacy and safety profile of paliperidone palmitate injections in the management of patients with schizophrenia: an evidence-based review. Neuropsychiatr Dis Treat. 2018;14:205–223. doi: 10.2147/NDT.S139633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higashi K, Medic G, Littlewood KJ, Diez T, Granstrom O, De Hert M. Medication adherence in schizophrenia: factors influencing adherence and consequences of nonadherence, a systematic literature review. Ther Adv Psychopharmacol. 2013;3(4):200–218. doi: 10.1177/2045125312474019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medic G, Higashi K, Littlewood KJ, Diez T, Granstrom O, Kahn RS. Dosing frequency and adherence in chronic psychiatric disease: systematic review and meta-analysis. Neuropsychiatr Dis Treat. 2013;9:119–131. doi: 10.2147/NDT.S39303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emond B, El Khoury AC, Patel C, et al. Real-world outcomes post-transition to once-every-3-months paliperidone palmitate in patients with schizophrenia within US commercial plans. Curr Med Res Opin. 2019;35(3):407–416. doi: 10.1080/03007995.2018.1560220 [DOI] [PubMed] [Google Scholar]
- 31.Emond B, Joshi K, El Khoury AC, et al. Adherence, healthcare resource utilization, and costs in Medicaid beneficiaries with schizophrenia transitioning from once-monthly to once-every-3-months paliperidone palmitate. Pharmacoeconomics-Open. 2019;3(2):177–188. doi: 10.1007/s41669-018-0089-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joshi K, Lafeuille M-H, Brown B, et al. Baseline characteristics and treatment patterns of patients with schizophrenia initiated on once-every-three-months paliperidone palmitate in a real-world setting. Curr Med Res Opin. 2017;33(10):1763–1772. doi: 10.1080/03007995.2017.1359516 [DOI] [PubMed] [Google Scholar]
- 33.Savitz AJ, Xu H, Gopal S, et al. Efficacy and Safety of Paliperidone Palmitate 3-Month Formulation for Patients with Schizophrenia: a Randomized, Multicenter, Double-Blind, Noninferiority Study. Int J Neuropsychopharmacol. 2016;19(7):pyw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DerSarkissian M, Lefebvre P, Joshi K, et al. Health care resource utilization and costs associated with transitioning to 3-month paliperidone palmitate among US veterans. Clin Ther. 2018;40(9):1496–1508. doi: 10.1016/j.clinthera.2018.07.011 [DOI] [PubMed] [Google Scholar]
- 35.Cullen BA, McGinty EE, Zhang Y, et al. Guideline-concordant antipsychotic use and mortality in schizophrenia. Schizophr Bull. 2013;39(5):1159–1168. doi: 10.1093/schbul/sbs097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohs RC. Cognition in Schizophrenia: natural History, Assessment, and Clinical Importance. Neuropsychopharmacology. 1999;21(2):S203–S210. doi: 10.1016/S0893-133X(99)00120-7 [DOI] [Google Scholar]
- 37.Tripathi A, Kar SK, Shukla R. Cognitive Deficits in Schizophrenia: understanding the Biological Correlates and Remediation Strategies. Clin Psychopharmacol Neurosci. 2018;16(1):7–17. doi: 10.9758/cpn.2018.16.1.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rasser PE, Schall U, Todd J, et al. Gray matter deficits, mismatch negativity, and outcomes in schizophrenia. Schizophr Bull. 2011;37(1):131–140. doi: 10.1093/schbul/sbp060 [DOI] [PMC free article] [PubMed] [Google Scholar]