Abstract
Introduction
We evaluated the arginine‐depleting enzyme pegargiminase (ADI‐PEG20; ADI) with pemetrexed (Pem) and cisplatin (Cis) (ADIPemCis) in ASS1‐deficient non‐squamous non‐small cell lung cancer (NSCLC) via a phase 1 dose‐expansion trial with exploratory biomarker analysis.
Methods
Sixty‐seven chemonaïve patients with advanced non‐squamous NSCLC were screened, enrolling 21 ASS1‐deficient subjects from March 2015 to July 2017 onto weekly pegargiminase (36 mg/m2) with Pem (500 mg/m2) and Cis (75 mg/m2), every 3 weeks (four cycles maximum), with maintenance Pem or pegargiminase. Safety, pharmacodynamics, immunogenicity, and efficacy were determined; molecular biomarkers were annotated by next‐generation sequencing and PD‐L1 immunohistochemistry.
Results
ADIPemCis was well‐tolerated. Plasma arginine and citrulline were differentially modulated; pegargiminase antibodies plateaued by week 10. The disease control rate was 85.7% (n = 18/21; 95% CI 63.7%–97%), with a partial response rate of 47.6% (n = 10/21; 95% CI 25.7%–70.2%). The median progression‐free and overall survivals were 4.2 (95% CI 2.9–4.8) and 7.2 (95% CI 5.1–18.4) months, respectively. Two PD‐L1‐expressing (≥1%) patients are alive following subsequent pembrolizumab immunotherapy (9.5%). Tumoral ASS1 deficiency enriched for p53 (64.7%) mutations, and numerically worse median overall survival as compared to ASS1‐proficient disease (10.2 months; n = 29). There was no apparent increase in KRAS mutations (35.3%) and PD‐L1 (<1%) expression (55.6%). Re‐expression of tumoral ASS1 was detected in one patient at progression (n = 1/3).
Conclusions
ADIPemCis was safe and highly active in patients with ASS1‐deficient non‐squamous NSCLC, however, survival was poor overall. ASS1 loss was co‐associated with p53 mutations. Therapies incorporating pegargiminase merit further evaluation in ASS1‐deficient and treatment‐refractory NSCLC.
Keywords: ADIPemCis, arginine, arginine deiminase, ASS1, KRAS, non‐squamous NSCLC, p53, PD‐L1
We evaluated the arginine‐depleting enzyme pegargiminase (ADI‐PEG20; ADI) with pemetrexed (Pem) and cisplatin (Cis) (ADIPemCis) in ASS1‐deficient non‐squamous non‐small cell lung cancer (NSCLC) via a phase 1 dose‐expansion trial with exploratory biomarker analysis. ADIPemCis was safe and highly active in patients with ASS1‐deficient non‐squamous NSCLC, however, survival was poor overall. ASS1 loss was co‐associated with p53 mutations providing a developmental pathway for further testing pegargiminase in ASS1‐deficient and treatment‐refractory NSCLC.

1. INTRODUCTION
Globally, lung cancer is the leading cause of cancer‐related mortality, accounting for almost one in five deaths. 1 Around 85% of lung cancer patients have non‐small cell lung carcinoma (NSCLC), comprising mostly of adenocarcinoma (40%), squamous carcinoma (30%), and large‐cell undifferentiated carcinoma (10%–15%). 2 Standard of care for patients with first‐line metastatic non‐squamous NSCLC––in the absence of an oncogenic driver such as an EGFR mutation or ALK translocation––is immune checkpoint blockade either alone or in combination with platinum‐based chemotherapy (pemetrexed or paclitaxel‐bevacizumab). While the 5‐year survival has improved from less than 5% a decade ago to over 25% in high (≥50%) PD‐L1‐expressing NSCLC, prognosis for many patients remains poor, emphasizing the need for more options that exploit novel biological pathways. 3
Arginine is a versatile amino acid involved in the biosynthesis of proteins and regulation of numerous cellular processes, including proliferation by modulating polyamine and nucleotide synthesis, hormone synthesis, cell signaling, and vasodilation via nitric oxide. 4 Arginine also has a crucial role in immune system regulation. 5 Normal cells synthesize arginine de novo from citrulline and aspartate via ATP in the urea cycle, however this is dysregulated in arginine auxotrophic cancers––exemplified by loss of the tumor suppressor argininosuccinate synthetase 1 (ASS1)––rendering arginine essential for tumor growth (“arginine auxotrophy”) and thereby leveraging arginine deprivation as an attractive therapeutic strategy. 6 , 7 Moreover, arginine deprivation with pegylated arginine deiminase (ADI‐PEG20; ADI; pegargiminase) disrupts thymidine pools and potentiates antifolate cytotoxicity in preclinical ASS1‐deficient tumor models. 8
In addition to its role as a predictive biomarker for arginine deprivation therapy, ASS1 deficiency is increasingly recognized as a prognostic biomarker for poor survival outcomes in several cancer types, including mesothelioma, sarcomas, bladder, ovarian, and breast cancer by driving increased tumor cell proliferation, invasiveness, and metastasis. 8 , 9 , 10 , 11 , 12 , 13 , 14 Moreover, based on data from The Cancer Genome Atlas (TCGA), ASS1 loss confers significantly reduced 5‐year survival rates (25% “low” vs. 44% “high” ASS1 expression; p = 0.007; n = 500) for all stages of lung adenocarcinoma, including patients in adjuvant and metastatic treatment settings. 15 In particular, low ASS1 combined with high citrin (mitochondrial aspartate transporter) expression conferred poor survival outcomes in both adenocarcinoma and squamous cell lung cancer consistent with the redirection of aspartate for enhanced pyrimidine synthesis in urea cycle‐deficient cancers. 16 Although preclinically ASS1 loss is linked to chemorefractoriness, specific data on the impact of systemic therapy in ASS1 low and high subsets in NSCLC are not currently available.
In the phase I dose‐escalation TRAP study 17 we showed that ADI‐PEG20 with pemetrexed (Pem) and cisplatin (Cis) chemotherapy (ADIPemCis) was tolerable and produced a 100% disease control rate (DCR) and an objective response rate of 78% in nine patients with ASS1‐deficient thoracic cancers (non‐squamous NSCLC = 4; mesothelioma = 5) (Beddowes et al., 2017). However, while the median overall survival––prior to the approval of immune checkpoint therapy––was promising at 13.9 months, the small patient thoracic cohort precluded any firm survival estimates. Recently, we have reported on the safety and activity of ADIPemCis in dose‐expansion cohorts in patients with high‐grade glioma (n = 10), malignant pleural mesothelioma (n = 31), and a placebo‐controlled phase 3 trial of ADIPemCis is now underway in non‐epithelioid mesothelioma based on a robust survival signal. 17 , 18
Therefore, we tested the hypothesis that targeting essential arginine would benefit patients with non‐squamous NSCLC characterized by loss of the ASS1 tumor suppressor. We treated a dose‐expansion cohort of 21 patients with ASS1‐deficient non‐squamous non‐small cell lung cancer at the recommended phase 2 dose (RP2D) of ADI‐PEG 20 (36 mg/m2) in combination with standard doses of pemetrexed and cisplatin. The main aims of this phase 1 dose‐expansion study were to define further the safety, preliminary activity, and survival estimates of the ADIPemCis triplet in patients with non‐squamous NSCLC. Additionally, we sought to translate the correlative molecular biomarkers of ASS1 deficiency in non‐squamous NSCLC by next‐generation sequencing, characterize the levels of PD‐L1 expression immunohistochemically, and explore the treatment resistance.
2. PATIENTS AND METHODS
2.1. Patient eligibility
Patients were aged 18 years or over, with histologically proven argininosuccinate synthetase 1 (ASS1)‐deficient stage IIIB or IV non‐squamous NSCLC (v. Beddowes et al. for methods). Patients had evaluable disease by Response Evaluation Criteria in Solid Tumors criteria (RECIST) 1.1. All patients were chemotherapy naïve. Subjects with EGFR or ALK mutation must have had an EGFR tyrosine kinase inhibitor (TKI) or ALK inhibitor, and must have progressed or been shown to be intolerant of this therapy prior to enrolling into the study. Additional criteria included Eastern Cooperative Oncology Group performance status 0 or 1, adequate hematologic, renal and hepatic function, and a minimum of 12‐week life expectancy. Exclusion criteria included symptomatic brain or spinal cord metastases, recent major surgery, significant concomitant illness, allergy to platinum salts, or pegylated agents. The protocol amendment for the dose‐expansion cohort specified the enrollment of up to 30 patients with non‐squamous NSCLC at the RP2D and required written informed signed consent.
2.2. Study design and treatment
This was a dose‐expansion cohort phase I study in up to 30 patients with non‐squamous NSCLC testing the recommended phase II dose (RP2D) of 36 mg/m2 weekly intramuscular (IM) ADI‐PEG 20 plus 75 mg/m2 cisplatin and 500 mg/m2 pemetrexed given every 3 weeks, and derived from the prior dose‐escalation TRAP study. 19 The initial dose of ADI‐PEG20 was given 48 h prior to the first dose of intravenous chemotherapy. Standard premedication to reduce pemetrexed toxicity was given, namely dexamethasone, daily folic acid (400 µg), and IM hydroxycobalamin (1000 µg) every 9 weeks, starting at least 7 days prior to first dose. Patients received a maximum of four cycles (12 weeks) after which patients who had stable disease or partial response were eligible to continue maintenance single‐agent ADI‐PEG20 or pemetrexed until disease progression or withdrawal. Blood samples were taken at baseline, prior to each cycle of ADIPemCis and on progression of disease or study withdrawal. Baseline tumor biopsies were mandatory and were optional at disease progression.
2.3. Study endpoints
The data lock was performed on the 18 June 2020. The primary endpoints of the dose‐expansion phase were to assess the safety and tolerability of ADIPemCis using National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03; and to estimate the preliminary efficacy or response rate (RR) using RECIST 1.1, by computed tomography (CT) every 6 weeks during ADIPemCis therapy and then every 8 weeks during pegargiminase or pemetrexed maintenance therapy. Secondary endpoints were to calculate the median progression‐free survival (PFS), median overall survival (OS), pharmacodynamics, and immunogenicity. For the translational endpoints, next‐generation sequencing was performed on baseline tumor DNA examining the different somatic variants detected using Ion AmpliSeq Cancer Hotspot Panel v2 (Thermo Fisher Scientific). PD‐L1 expression was assessed using the 22C3 immunohistochemistry (IHC) assay and reported according to the standard criteria (>50%; 1%–49%; <1% expression). Rebiopsied tumor tissue at relapse was assessed for ASS1 IHC using the monoclonal antibody 195‐21‐1 from Polaris Pharmaceuticals, Inc.
2.4. Statistical analyses
No formal sample size calculation was made for the dose‐expansion TRAP study in patients with non‐squamous NSCLC, which aimed to recruit up to 30 patients as per protocol. AEs were collated, and response rates, PFS, and OS were graphed using Prism 8 Version 8.4.3. This trial is registered with clinicaltrials.gov, number. 33
3. RESULTS
3.1. Patient demographics
Patient enrollment into the dose‐expansion study began in March 2015 and was completed in July 2017. Sixty‐seven patients with non‐squamous NSCLC were screened, and 21 patients with ASS1‐deficient tumors were enrolled for the treatment with ADIPemCis as summarized in Figure 1. None of the patients with ASS1‐deficient disease had EGFR mutations or ALK rearrangements. The majority had stage IV lung adenocarcinoma (LUAC) which included patients with initial presentations of superior vena cava obstruction (n = 1), pericardial tamponade (n = 1), and multiple brain metastases (n = 1) requiring stabilization prior to study entry (n = 18/21; 85.7%). All subjects were included for the safety, while 19 were evaluable by RECIST 1.1 (Table 1). Patients with ASS1‐proficient non‐squamous NSCLC who received first‐line platinum and pemetrexed chemotherapy were followed up for survival only.
FIGURE 1.
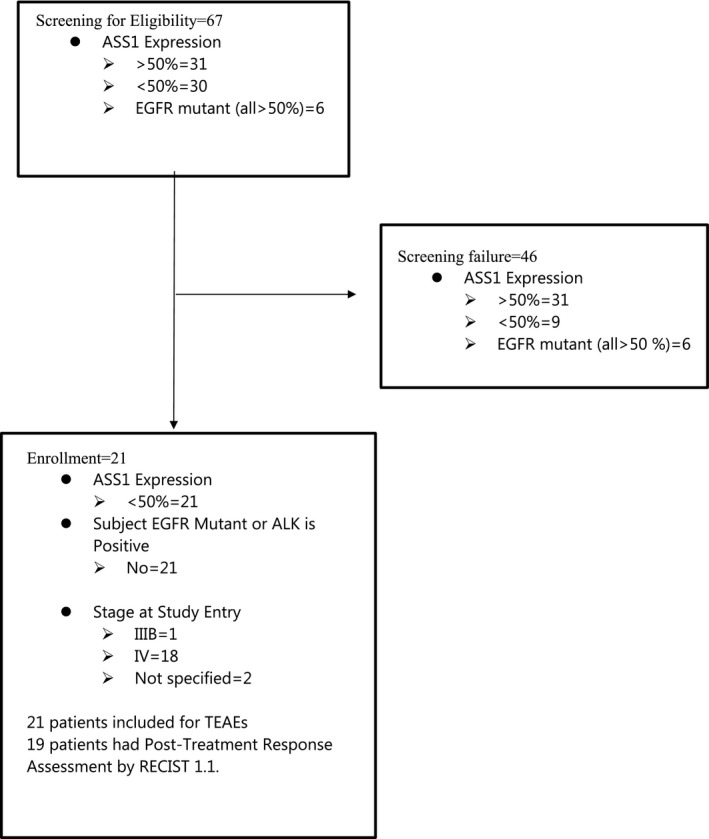
CONSORT diagram
TABLE 1.
Demographics of patients receiving ADIPemCis
| Characteristic | Number of patients (n = 21) |
|---|---|
| Age, years | |
| Median | 60 |
| Range | 39–78 |
| Gender | |
| Male | 13 |
| Female | 8 |
| ECOG performance status | |
| 0 | 6 |
| 1 | 15 |
| Prior therapy | |
| Surgery | 5 |
| External beam radiotherapy | 3 |
| Disease stage a | |
| IIIB | 1 |
| IV | 18 |
| Unknown | 2 |
| Histology | |
| Adenocarcinoma | 19 |
| Large cell carcinoma | 1 |
| Pleomorphic (Giant cell) carcinoma | 1 |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
AJCC eighth edition TNM staging for lung cancer.
3.2. Safety
Consistent with the prior dose‐escalation study, ADIPemCis treatment was well‐tolerated. Treatment‐emergent adverse events were reported in all 21 patients: the majority were related to cisplatin and pemetrexed (81%; n = 17/21), and the rest to pegargiminase (52.4%; n = 11/21). Grade 1/2 adverse events occurred in 36.4% (n = 8/21) and were mostly due to nausea, anorexia, and fatigue, while Grade 3 was reported in 47.6% (n = 10/21) patients; adverse events of Grade 4 occurred in 4.8% (n = 1/21) and grade 5 in 9.5% (n = 2/21) patients, the latter due to disease progression (Table 2; Table S1).
TABLE 2.
Overall adverse events
| Overall AE a summary | Number of subject (n = 21) | % |
|---|---|---|
| Total number of adverse event (AE) | 197 | |
| Number of subject reporting at least one AE | 21 | 100.0 |
| AE by severity | ||
| Grade 1 | 3 | 14.3 |
| Grade 2 | 5 | 23.8 |
| Grade 3 | 10 | 47.6 |
| Grade 4 | 1 | 4.8 |
| Grade 5 (peritoneal PD; CNS PD) | 2 | 9.5 |
| Number of subject reporting at least one SAE | 12 | 57.1 |
| AE related to ADI‐PEG 20 | 11 | 52.4 |
| AE related to cisplatin | 17 | 81.0 |
| AE related to pemetrexed | 17 | 81.0 |
Abbreviations: CNS, central nervous system; PD, progressive disease.
AE is defined as any untoward medical occurrence in a subject administered with ADIPemCis and that does not necessarily have a causal relationship with the treatment.
3.3. Pharmacodynamics
Pegargiminase decreased plasma arginine with a reciprocal increase in plasma citrulline levels in patients and is shown in Figure 2A. Plasma levels of the amino acids remained differentially altered compared with pre‐treatment levels, however, recovery to pre‐treatment levels was evident with the fewer number of on‐treatment patients by 16 weeks. There was a fourfold increase in the titer of ADI‐PEG20 antibodies by 16 weeks consistent with previous studies and is shown in Figure 2B.
FIGURE 2.
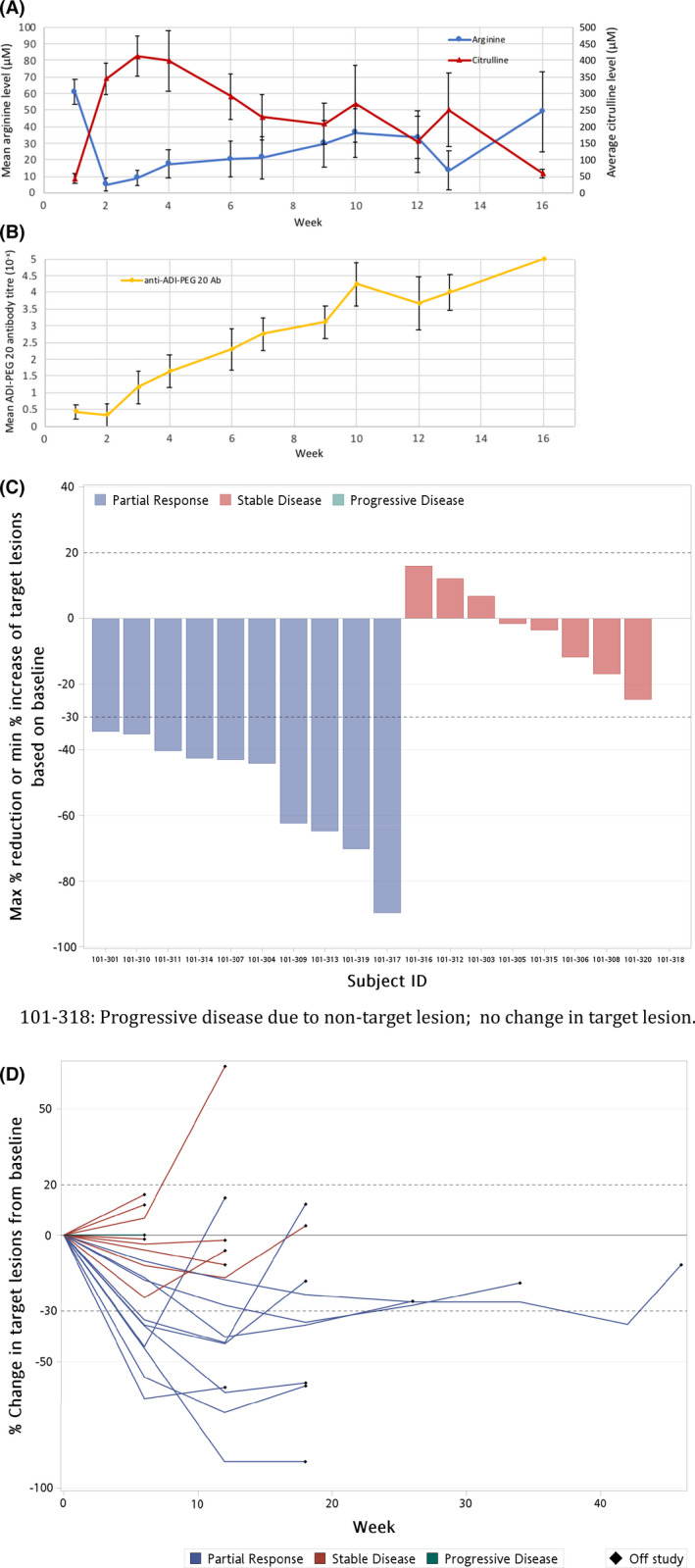
Pharmacodynamics and response. (A) Pharmacodynamics of arginine and citrulline in patients treated with ADIPemCis. Serum [arginine] and [citrulline] are shown by week of treatment (means ± SEM). (B) Serum levels of anti‐ADI‐PEG 20 antibodies in all patients by week of ADIPemCis (Mean ± SEM); Ab, Antibody. (C) Waterfall plot of response by RECIST 1.1. to ADIPemCis. (D) Spider plots showing response duration to ADIPemCis
3.4. Efficacy
ADIPemCis treatment induced a disease control rate of 85.7% (n = 18/21; 95% CI 63.7%–97%), and a partial response rate of 47.6% (n = 10/21, 95% CI 25.7%–70.2%; or n = 10/18 or 55.6% of evaluable patients), in a cohort of patients with non‐squamous NSCLC enriched by ASS1 loss, summarized in Figure 2C,D. The median PFS and OS were 4.2 (95% CI 2.9–4.8) and 7.2 (95% CI 5.1–18.4) months, respectively, and are shown in Figure 3.
FIGURE 3.
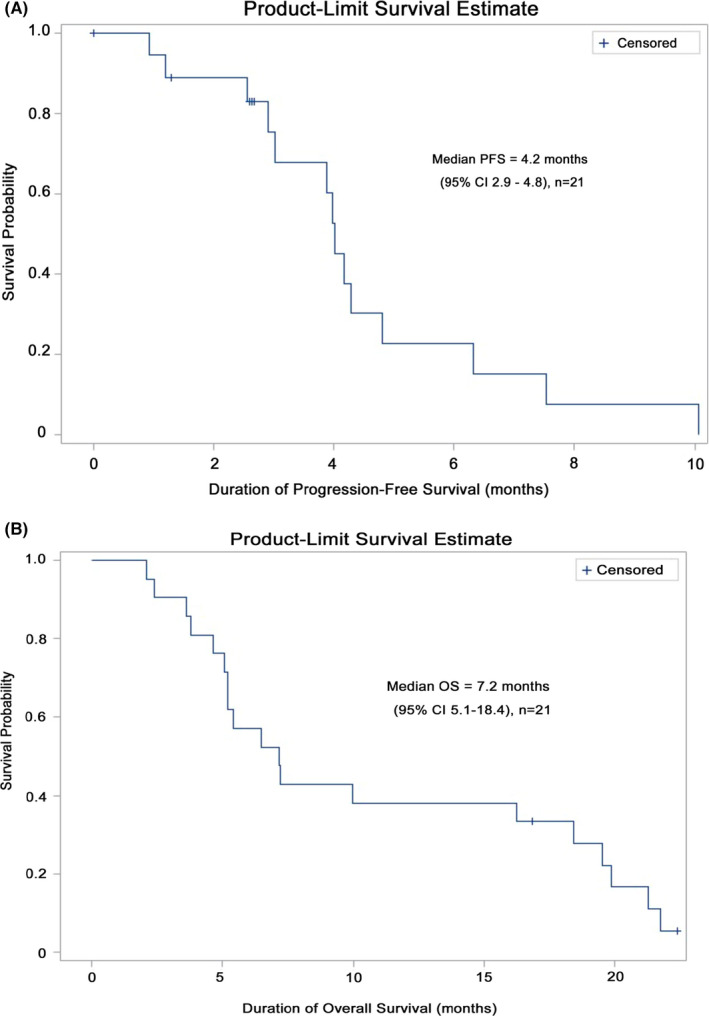
Survival outcomes for ASS1‐deficient patients (A) Progression‐free survival. (B) Kaplan–Meier survival estimates
3.5. Molecular biomarkers
To understand the disconnect between the high partial response rate and the unexpectedly short median PFS and OS, NGS was performed on the baseline tumor biopsies. This revealed a wide spectrum of p53 mutations in 64.7% of patients and KRAS mutations in 35.3% of patients (G12C, G13C, G12D, and Q61H) in ASS1‐deficient non‐squamous NSCLC. Additional mutations were identified including in HRAS, CDKN2A, BRAF (V600E), ATM, RB1, and APC (Table 3). Overall, the expression of PD‐L1 ≥1% was reported in 44.4% of patients (n = 8/18), while 55.6% of patients (n = 10/18) were PD‐L1 negative by the 22C3 IHC assay. Patients with tumoral PD‐L1 ≥1% expression and disease progression were eligible for second‐line pembrolizumab; two patients are in remission at 40 and 47 months from treatment with ADIPemCis following 2 years of immunotherapy (9.5%). Finally, to study drug resistance, three patients receiving maintenance ADI‐PEG20 consented to a rebiopsy revealing ASS1 re‐expression in one patient (n = 1/3; 33%).
TABLE 3.
Individual patient and tumor characteristics
| Subject | Age | Gender | Diagnosis | Disease presentation | PD‐L1 expression a | NGS somatic variants b | ||
|---|---|---|---|---|---|---|---|---|
| TP53 | KRAS | Other | ||||||
| 101‐301 | 64 | M | ADC | Lung, LN | <1% | WT | G12C | |
| 101‐302 | 54 | M | ADC | LN, Adrenal | >50% | R158L | WT | |
| 101‐303 | 51 | F | ADC | Lung | N/A | N/A | N/A | |
| 101‐304 | 60 | M | ADC | Lung | 3% | G245V | G12C | |
| 101‐305 | 45 | M | Large cell | Lung, LN, Peritoneal | <1% | G334V | WT | |
| 101‐306 | 58 | M | ADC | Lung, LN | N/A | WT | WT | |
| 101‐307 | 56 | F | ADC | Lung, LN, Bone | <1% | WT | G12D | |
| 101‐308 | 68 | M | ADC | LN, Bone, Muscle | <1% | R248Q | WT | ATM |
| 101‐309 | 63 | M | ADC | Lung, LN | <1% | N/A | N/A | |
| 101‐310 | 63 | F | ADC | LN, Bone | 5%–10% | G199V | WT | BRAF (V600E) |
| 101‐311 | 39 | M | ADC | Lung, LN, Liver | <1% | N/A | N/A | |
| 101‐312 | 62 | M | ADC | Lung, Adrenal, Bone | <1% | WT | Q61H | |
| 101‐313 | 60 | F | ADC | Lung, LN, Pleural, Pericardial Bone | 80%–90% | E285V | G13C | |
| 101‐314 | 50 | M | ADC | Lung, LN, Liver, Pleural | <1% | WT | WT | |
| 101‐315 | 65 | M | Giant cell | Lung, LN, Adrenal | 100% | Y126D | WT | |
| 101‐316 | 60 | F | ADC | Lung, LN, Adrenal | <1% | WT | G12D | APC |
| 101‐317 | 59 | F | ADC | Lung, LN, Liver, Bone | <1% | G244R | WT | RB1; ERBB2 |
| 101‐318 | 53 | M | ADC | Lung | 10%–20% | G245V | WT | CDKN2A; HRAS |
| 101‐319 | 70 | M | ADC | Lung, LN | 60%–70% | R248L | WT | |
| 101‐320 | 78 | M | ADC | Lung, LN | 60%–70% | R249T | WT | JAK3 P132T |
| 103‐301 | 61 | F | ADC | N/A | N/A | N/A | N/A | |
Abbreviations: ADC, adenocarcinoma; F, female; M, male; N/A, not available; NGS, next‐generation sequencing; PD‐L1, programmed cell death‐ligand 1.
PD‐L1 expression assessed using the 22C3 immunohistochemistry assay.
Somatic variants detected using Ion AmpliSeqTM Cancer Hotspot Panel v2 analysis of tumor DNA.
3.6. ASS1‐positive group
Retrospectively, we determined the median OS of patients with ASS1‐proficient non‐squamous NSCLC screened as part of this study (n = 35). Following exclusion of six ASS1‐positive patients with EGFR mutations, who all received first‐line therapy with a tyrosine kinase inhibitor (TKI: gefitinib, erlotinib, or afatinib), the median OS for patients receiving first‐line platinum (cisplatin or carboplatin) and pemetrexed chemotherapy was 10.2 months (n = 29; 95% CI 5.9–17.3) and is depicted in Figure 4. This included two patients with ALK rearrangements who were eligible for second‐line crizotinib. Overall, three patients are alive from the ASS1‐proficient group including a patient with ALK‐positive disease on crizotinib (10.3%).
FIGURE 4.
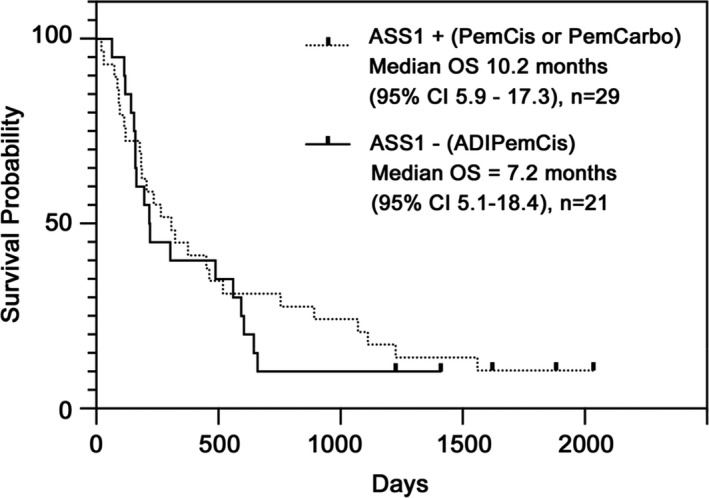
Survival outcomes for ASS1‐proficient patients Kaplan–Meier survival estimates for ASS1‐proficient patients screened as part of this study (n = 29) and treated with standard of care cisplatin and pemetrexed chemotherapy and compared to ASS1‐deficient patients receiving ADIPemCis (n = 21)
4. DISCUSSION
This expansion study of ADIPemCis at the RP2D confirmed good safety and a high rate of disease control and partial response (~50%) in patients with ASS1‐deficient non‐squamous NSCLC consistent with the dose‐escalation study. 19 However, the encouraging response rate did not translate into a prolonged median OS (7.2 months) when contrasted with ASS1‐proficient disease (10.2 months) or compared with ASS1‐agnostic historical controls (10.4–12.6 months) treated with platinum and pemetrexed alone. 20 Notably, p53 and KRAS mutations were co‐associated with ASS1‐deficient non‐squamous NSCLC contributing to the poor clinical outcomes; PD‐L1 expression was commonly absent (<1% staining by the 22C3 assay). In contrast, EGFR‐mutant and ALK rearranged non‐squamous NSCLC tumors were all ASS1‐expressors for which TKI therapies have revolutionized patient management.
Since completing the ADIPemCis dose‐expansion cohort, chemoimmunotherapy with PD1 and PD‐L1 inhibitors has overtaken platinum‐doublet chemotherapy reaching a median OS of 22 months for pembrolizumab with platinum–pemetrexed (vs. 10.7 months for platinum and pemetrexed) in the KEYNOTE‐189 study, and 19.2 months for atezolizumab with bevacizumab, carboplatin, and paclitaxel (vs. 14.7 months for bevacizumab, carboplatin, and paclitaxel) in the IMpower150 study. 21 , 22 Further optimization of chemoimmunotherapy especially in PD‐L1‐negative expressors is a priority area, as benefits are modest compared with disease exhibiting high (≥50%) PD‐L1 expression, where single‐agent PD1i/PDL1i immunotherapy is an approved standard of care. 23 , 24 Two patients treated with ADIPemCis with ≥1% tumoral PDL1 expression remain alive and in remission, post‐pembrolizumab immunotherapy. Both harbored mutations in either JAK3 or CDKN2A which are known to upregulate cancer immune responses in NSCLC. 25 , 26
PD‐L1 <1% expression was reported in 55.6% of patients enrolled on ADIPemCis, a prevalence that is significantly higher than that reported across key studies of pembrolizumab in NSCLC (33%), but consistent with real‐world data (48%–56.4%). 27 , 28 , 29 Recent work suggests that arginine deprivation may potentiate immune checkpoint inhibition and turn “cold” tumors “hot” by increasing the PD‐L1 expression and T‐cell infiltration. 30 , 31 Moreover, urea cycle dysregulated cancers are differentially sensitive to immune checkpoint therapies and further clinical testing will be needed to validate these preclinical data. 32 Specifically, a phase 1 study of pembrolizumab and pegargiminase has completed accrual and, while patients with NSCLC were not enrolled, this immunometabolic approach was tolerable with partial responses in 24% of subjects. 33
The 41% higher prevalence of p53 mutations in our study compared to that expected for patients with LUAC (i.e., 64.7% vs. 46%) is intriguing. 34 Miyamoto et al. showed that wild‐type p53 activates ASS1 in response to genotoxic and nutrient stress driving arginine biosynthesis in line with its known homeostatic functions as a key tumor suppressor pathway. 35 , 36 Specifically, transfection of wild‐type p53 in p53‐deficient (NSCLC) or p53‐mutant (astrocytoma) cancer cell lines was sufficient to restore ASS1 expression. Moreover, the p53‐mediated regulation of ASS1 was cell type‐specific, consistent with the diverse roles of arginine in cellular homeostasis. Thus, our study links various p53 mutations to deregulation of ASS1 in clinical samples for the first time, and also provides an explanation for the poor overall survival seen in the non‐squamous NSCLC patient cohort. Multiple studies have identified p53 mutations as critical in lung carcinogenesis and more recently as a key biomarker of adverse outcomes in NSCLC. 37 , 38 , 39 , 40 Further analysis of the relationship between p53 and ASS1 is warranted, particularly in squamous NSCLC where p53 mutations occur with the highest frequency (81%). Preclinically, ADI‐PEG20 combined with a taxane potentiates gemcitabine cytotoxicity, and is an approach that may have utility in patients with squamous NSCLC. 41 Interestingly, our NSCLC cohort data validate studies showing high rates of ASS1 loss in non‐epithelioid mesothelioma, where p53 mutations have been identified to the exclusion of epithelioid mesothelioma, and underlie similar poor outcomes to platinum–pemetrexed chemotherapy. 42 , 43
Mutations in KRAS were the second most common finding by NGS in our patient cohort with a modestly higher prevalence compared to patients with unselected LUAC reported in the recent published AACR Project GENIE, namely 35.3% versus 26.7%. 44 However, KRAS mutations have been described with a frequency of 33% in the TCGA dataset for LUAC thus a molecular link with ASS1 requires additional scrutiny. 45 Two patients in our study achieved PRs on ADIPemCis despite co‐mutations of p53 and KRAS mutations which portend a poor survival with disease refractory to chemotherapy and radiotherapy. 46 , 47 None of the patients displayed STK11/LKB1 mutations which, linked to KRAS mutations, yield a median OS of 6.4 months versus 16 months for KRAS mutations alone. 48 Interestingly, KEAP1 mutations, which were not represented in the NGS panel, are frequently co‐mutated with LKB1 and similarly herald a poor prognosis characterized by glutamine addiction and sensitivity to glutaminase inhibition in KRAS‐mutant LUAC. 49 Site‐specific differences have also been reported in the median OS for patients with KRAS mutations and LUAC: 3.7 months for bone metastases versus 9.7 months without bone involvement. 50 Importantly, the recent development of K12C‐targeted drugs will leverage novel opportunities for patients with KRAS co‐mutated tumors. 51 Preclinically, we have identified enhanced disease control of an ASS1‐deficient and K12D KRAS‐mutant NSCLC cell line sensitive to ADI‐PEG20 and PD1 blockade. 52
As noted earlier, several studies reinforce that ASS1 deficiency is a poor prognostic biomarker in a variety of solid malignancies. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 16 In the present study, the median OS for ASS1‐deficient compared to ASS1‐proficent patients was numerically shorter by 3 months (i.e., 7.2 vs. 10.2 months) but, based on our small patient population, was not significant. While our study implicate p53, and, in part, KRAS mutations, in the overall poor prognosis, the rapid emergence of resistance to pegargiminase requires greater scrutiny. Thus, several mechanisms are apparent, namely, tumoral re‐expression of ASS1, stromal support via infiltrating macrophages identified in the earlier mesothelioma TRAP dose‐expansion cohort, and potentially drug‐dependent resistance, evidenced by an increasing titer of pegargiminase antibodies and a concomitant rise in arginine levels by week 16. 17 While the pharmacodynamic response was less durable––possibly due to low numbers of patients sampled by week 16––compared with the dose‐escalation ADIPemCis study, it was nonetheless more robust than that reported for doublet chemotherapy with ADI‐PEG20 and docetaxel. 19 , 53 Lastly, preclinical studies in NSCLC also support autophagy as a key modulator of KRAS‐dependent growth warranting further clinical study. 54
In summary, ADIPemCis is safe and active in an expansion cohort of patients with ASS1‐deficient non‐squamous NSCLC. Our data reveal that tumoral ASS1 deficiency enriches for a poor prognosis group of patients characterized by frequent p53 mutations, in which arginine deprivation may provide additional therapeutic benefit warranting further investigation with rationally selected agents.
ETHICAL CONSIDERATIONS
The clinical protocol (ClinicalTrials.gov identifier NCT02029690) was approved by Leeds East Research Ethics Committee (14/YH/0090) and was sponsored by Polaris Pharmaceuticals, Inc.
CONFLICT OF INTEREST
PW Szlosarek received support from the Higher Education Funding Council for England (HEFCE) and research grant support from Polaris Group. Ms Shiu, Ms Kuo, Dr Bomalaski, Dr Johnson, and Dr Feng are paid employees of Polaris Pharmaceuticals. The remaining authors did not report any relevant conflict of interest.
Supporting information
Table S1
ACKNOWLEDGMENTS
The authors are very grateful to all the patients and families who took part in the ADIPemCis (TRAP) dose‐expansion non‐squamous NSCLC cohort study. The authors thank all the staff at the Barts Experimental Cancer Medicine Center (ECMC), Dr Simon Pacey (ECMC, Cambridge), Brendan O’Sullivan (Molecular Pathology, University Hospitals Birmingham NHS Foundation Trust), Suzanne Jordan (Clinical Pathology, Barts), Amy Roe (Cytogenetics and Molecular Haematology, Barts), and Jim Thomson and Bor‐Wen Wu (Polaris Pharmaceuticals, Inc). This work was funded and sponsored by Polaris Pharmaceuticals, Inc., and supported by Barts (Queen Mary University of London) Experimental Cancer Medicine Centre.
Szlosarek PW, Wimalasingham AG, Phillips MM, et al. Phase 1, pharmacogenomic, dose‐expansion study of pegargiminase plus pemetrexed and cisplatin in patients with ASS1‐deficient non‐squamous non‐small cell lung cancer. Cancer Med. 2021;10:6642–6652. 10.1002/cam4.4196
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Inamura K. Lung cancer: understanding its molecular pathology and the 2015 WHO classification. Front Oncol. 2017;7:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non‐small cell lung cancer: a review. JAMA. 2019;322(8):764‐774. [DOI] [PubMed] [Google Scholar]
- 4. Husson A, Brasse‐Lagnel C, Fairand A, Renouf S, Lavoinne A. Argininosuccinate synthetase from the urea cycle to the citrulline‐NO cycle. Eur J Biochem. 2003;270(9):1887‐1899. [DOI] [PubMed] [Google Scholar]
- 5. Kim SH, Roszik J, Grimm EA, Ekmekcioglu S. Impact of l‐arginine metabolism on immune response and anticancer immunotherapy. Front Oncol. 2018;8:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Delage B, Fennell DA, Nicholson L, et al. Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int J Cancer. 2010;126(12):2762‐2772. [DOI] [PubMed] [Google Scholar]
- 7. Keshet R, Szlosarek P, Carracedo A, Erez A. Rewiring urea cycle metabolism in cancer to support anabolism. Nat Rev Cancer. 2018;18(10):634‐645. [DOI] [PubMed] [Google Scholar]
- 8. Allen MD, Luong P, Hudson C, et al. Prognostic and therapeutic impact of argininosuccinate synthetase 1 control in bladder cancer as monitored longitudinally by PET imaging. Cancer Res. 2014;74(3):896‐907. [DOI] [PubMed] [Google Scholar]
- 9. Phillips MM, Sheaff MT, Szlosarek PW. Targeting arginine‐dependent cancers with arginine‐degrading enzymes: opportunities and challenges. Cancer Res Treat. 2013;45(4):251‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Szlosarek PW, Steele JP, Nolan L, et al. Arginine deprivation with pegylated arginine deiminase in patients with argininosuccinate synthetase 1‐deficient malignant pleural mesothelioma: a randomized clinical trial. JAMA Oncol. 2017;3(1):58‐66. [DOI] [PubMed] [Google Scholar]
- 11. Huang HY, Wu WR, Wang YH, et al. ASS1 as a novel tumor suppressor gene in myxofibrosarcomas: aberrant loss via epigenetic DNA methylation confers aggressive phenotypes, negative prognostic impact, and therapeutic relevance. Clin Cancer Res. 2013;19(11):2861‐2872. [DOI] [PubMed] [Google Scholar]
- 12. Qiu F, Chen Y‐R, Liu X, et al. Arginine starvation impairs mitochondrial respiratory function in ASS1‐deficient breast cancer cells. Sci Signal. 2014;7(319):ra31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kobayashi E, Masuda M, Nakayama R, et al. Reduced argininosuccinate synthetase is a predictive biomarker for the development of pulmonary metastasis in patients with osteosarcoma. Mol Cancer Ther. 2010;9(3):535‐544. [DOI] [PubMed] [Google Scholar]
- 14. Nicholson LJ, Smith PR, Hiller L, et al. Epigenetic silencing of argininosuccinate synthetase confers resistance to platinum‐induced cell death but collateral sensitivity to arginine auxotrophy in ovarian cancer. Int J Cancer. 2009;125(6):1454‐1463. [DOI] [PubMed] [Google Scholar]
- 15. Chang JT, Lee YM, Huang RS. The impact of the Cancer Genome Atlas on lung cancer. Transl Res. 2015;166(6):568‐585. 10.1016/j.trsl.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rabinovich S, Adler L, Yizhak K, et al. Diversion of aspartate in ASS1‐deficient tumours fosters de novo pyrimidine synthesis. Nature. 2015;527(7578):379‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Szlosarek PW, Phillips MM, Pavlyk I, et al. Expansion phase 1 study of pegargiminase plus pemetrexed and cisplatin in patients with argininosuccinate synthetase 1‐deficient mesothelioma: safety, efficacy, and resistance mechanisms. JTO Clin Res Rep. 2020;1(4):1‐11. 10.1016/j.jtocrr.2020.100093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hall PE, Lewis R, Syed N, et al. A phase I study of pegylated arginine deiminase (pegargiminase), cisplatin, and pemetrexed in argininosuccinate synthetase 1‐deficient recurrent high‐grade glioma. Clin Cancer Res. 2019;25(9):2708‐2716. [DOI] [PubMed] [Google Scholar]
- 19. Beddowes E, Spicer J, Chan PY, et al. Phase 1 dose‐escalation study of pegylated arginine deiminase, cisplatin, and pemetrexed in patients with argininosuccinate synthetase 1‐deficient thoracic cancers. J Clin Oncol. 2017;35(16):1778‐1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy‐naive patients with advanced‐stage non‐small‐cell lung cancer. J Clin Oncol. 2008;26(21):3543‐3551. [DOI] [PubMed] [Google Scholar]
- 21. Gandhi L, Rodríguez‐Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med. 2018;378(22):2078‐2092. [DOI] [PubMed] [Google Scholar]
- 22. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first‐line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288‐2301. [DOI] [PubMed] [Google Scholar]
- 23. Reck M, Rodríguez‐Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med. 2016;375(19):1823‐1833. [DOI] [PubMed] [Google Scholar]
- 24. Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for first‐line treatment of PD‐L1‐selected patients with NSCLC. N Engl J Med. 2020;383(14):1328‐1339. [DOI] [PubMed] [Google Scholar]
- 25. Van Allen EM, Golay HG, Liu Y, et al. Long‐term benefit of PD‐L1 blockade in lung cancer associated with JAK3 activation. Cancer Immunol Res. 2015;3(8):855‐863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Choi M, Kadara H, Zhang J, et al. Mutation profiles in early‐stage lung squamous cell carcinoma with clinical follow‐up and correlation with markers of immune function. Ann Oncol. 2017;28(1):83‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aggarwal C, Rodriguez Abreu D, Felip E, et al. Prevalence of PD‐L1 expression in patients with non‐small cell lung cancer screened for enrollment in KEYNOTE‐001, ‐010, and ‐024. Ann Oncol. 2016;27:359‐78.26658890 [Google Scholar]
- 28. Dietel M, Savelov N, Salanova R, et al. Real‐world prevalence of programmed death ligand 1 expression in locally advanced or metastatic non‐small‐cell lung cancer: the global, multicenter EXPRESS study. Lung Cancer. 2019;134:174‐179. [DOI] [PubMed] [Google Scholar]
- 29. Gelatti ACZ, Cordeiro de Lima VC, Freitas H, et al. Real‐world prevalence of PD‐L1 expression among tumor samples from patients with non‐small‐cell lung cancer. Clin Lung Cancer. 2020;21(6):e511‐e515. [DOI] [PubMed] [Google Scholar]
- 30. Brin E, Wu K, Lu HT, He Y, Dai Z, He W. PEGylated arginine deiminase can modulate tumor immune microenvironment by affecting immune checkpoint expression, decreasing regulatory T cell accumulation and inducing tumor T cell infiltration. Oncotarget. 2017;8(35):58948‐58963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duan Q, Zhang H, Zheng J, Zhang L. Turning cold into hot: firing up the tumor microenvironment. Trends Cancer. 2020;6(7):605‐618. [DOI] [PubMed] [Google Scholar]
- 32. Lee JS, Adler L, Karathia H, et al. Urea cycle dysregulation generates clinically relevant genomic and biochemical signatures. Cell. 2018;174(6):1559‐70 e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chang KY, Chiang NJ, Wu SY, et al. Phase 1b study of pegylated arginine deiminase (ADI‐PEG 20) plus pembrolizumab in advanced solid cancers. Oncoimmunology. 2021;10(1). 10.1080/2162402X.2021.1943253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gibbons DL, Byers LA, Kurie JM. Smoking, p53 mutation, and lung cancer. Mol Cancer Res. 2014;12(1):3‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miyamoto T, Lo PHY, Saichi N, et al. Argininosuccinate synthase 1 is an intrinsic Akt repressor transactivated by p53. Sci Adv. 2017;3(5):e1603204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Berkers CR, Maddocks OD, Cheung EC, Mor I, Vousden KH. Metabolic regulation by p53 family members. Cell Metab. 2013;18(5):617‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Robles AI, Linke SP, Harris CC. The p53 network in lung carcinogenesis. Oncogene. 2002;21(45):6898‐6907. [DOI] [PubMed] [Google Scholar]
- 38. Donehower LA, Soussi T, Korkut A, et al. Integrated analysis of TP53 gene and pathway alterations in the cancer genome atlas. Cell Rep. 2019;28(5):1370‐1384.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Skoulidis F, Heymach JV. Co‐occurring genomic alterations in non‐small‐cell lung cancer biology and therapy. Nat Rev Cancer. 2019;19(9):495‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aggarwal C, Davis CW, Mick R, et al. Influence of TP53 mutation on survival in patients with advanced EGFR‐mutant non‐small‐cell lung cancer. JCO Precis Oncol. 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Prudner BC, Rathore R, Robinson AM, et al. Arginine starvation and docetaxel induce c‐Myc‐driven hENT1 surface expression to overcome gemcitabine resistance in ASS1‐negative tumors. Clin Cancer Res. 2019;25(16):5122‐5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bueno R, Stawiski EW, Goldstein LD, et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet. 2016;48(4):407‐416. [DOI] [PubMed] [Google Scholar]
- 43. Markowitz P, Patel M, Groisberg R, et al. Genomic characterization of malignant pleural mesothelioma and associated clinical outcomes. Cancer Treat Res Commun. 2020;25:100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Consortium APG . AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov. 2017;7(8):818‐831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cancer Genome Atlas Research N . Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shepherd FA, Lacas B, Le Teuff G, et al. Pooled analysis of the prognostic and predictive effects of TP53 comutation status combined with KRAS or EGFR mutation in early‐stage resected non‐small‐cell lung cancer in four trials of adjuvant chemotherapy. J Clin Oncol. 2017;35(18):2018‐2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ruiz‐Cordero R, Ma J, Khanna A, et al. Simplified molecular classification of lung adenocarcinomas based on EGFR, KRAS, and TP53 mutations. BMC Cancer. 2020;20(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Skoulidis F, Goldberg ME, Greenawalt DM, et al. STK11/LKB1 mutations and PD‐1 inhibitor resistance in KRAS‐mutant lung adenocarcinoma. Cancer Discov. 2018;8(7):822‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Galan‐Cobo A, Sitthideatphaiboon P, Qu X, et al. LKB1 and KEAP1/NRF2 pathways cooperatively promote metabolic reprogramming with enhanced glutamine dependence in KRAS‐mutant Lung adenocarcinoma. Cancer Res. 2019;79(13):3251‐3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lohinai Z, Klikovits T, Moldvay J, et al. KRAS‐mutation incidence and prognostic value are metastatic site‐specific in lung adenocarcinoma: poor prognosis in patients with KRAS mutation and bone metastasis. Sci Rep. 2017;7:39721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ghimessy A, Radeczky P, Laszlo V, et al. Current therapy of KRAS‐mutant lung cancer. Cancer Metastasis Rev. 2020;39(4):1159‐1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pavlyk I, Foster J, Dexter K, et al. Pegylated arginine deiminase sensitizes ASS1‐negative and KRAS mutant non‐small cell lung cancer to PD‐1 blockade immunotherapy. Cancer Res. 2020;80:nr 2217. 10.1158/1538-7445.AM2020-2217 [DOI] [Google Scholar]
- 53. Tomlinson BK, Thomson JA, Bomalaski JS, et al. Phase I trial of arginine deprivation therapy with ADI‐PEG 20 plus docetaxel in patients with advanced malignant solid tumors. Clin Cancer Res. 2015;21(11):2480‐2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Poillet‐Perez L, Xie X, Zhan LE, et al. Autophagy maintains tumour growth through circulating arginine. Nature. 2018;563(7732):569‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


