Abstract
Background
Epstein‐Barr virus (EBV) is detected in a variety of B‐cell lymphomas (BCLs) and B‐cell lymphoproliferative disorders (B‐LPDs). Immunodeficiency has been considered to play a key role in the pathogenesis of these diseases. In addition, immune escape of tumor cells may also contribute to the development of EBV+ BCLs and B‐LPDs. The PD‐1/PD‐L1 pathway is particularly important for immune escape of tumor cells that contribute to development of lymphoma through suppression of cytotoxic T‐cell function. We now consider PD‐L1 immunohistochemistry (IHC) a very useful method for predicting whether tumor cells of lymphoid malignancies are characterized by the immune escape mechanism.
Methods
We reviewed articles of EBV+ BCLs and B‐LPDs from the perspective of immune escape and immunodeficiency, particularly focusing on PD‐L1 IHC.
Results
Based on PD‐L1 IHC, we consider that EBV+ BCL and B‐LPD can be classified into three types: “immunodeficiency”, “immune escape”, and “immunodeficiency + immune escape” type. The immunodeficiency type includes EBV+ diffuse large BCL (DLBCL) of the elderly, EBV+ sporadic Burkitt lymphoma, EBV+ mucocutaneous ulcer, and methotrexate (MTX)‐associated B‐LPD. The immune escape type includes EBV+ classic Hodgkin lymphoma (CHL) and EBV+ DLBCL of the young. The immunodeficiency + immune escape type includes CHL type MTX‐associated LPD and a minor subset of EBV+ DLBCL of the elderly.
Conclusions
Recently, good results have been reported for immune check‐point inhibitors in treating lymphoma. Lymphomas and LPDs characterized by immune escape are regarded as good candidates for PD1/PD‐L1 blockade therapy. Therefore, from both the clinical and pathological perspective, we suggest that lymphoma diagnosis should be made considering immune escape and immunodeficiency.
Keywords: EBV‐positive lymphoma, immune escape, immunodeficiency, lymphoproliferative disorder, PD‐L1
We now consider PD‐L1 immunohistochemistry (IHC) a very useful method for predicting whether tumor cells of lymphoid malignancies are characterized by the immune escape mechanism. Based on PD‐L1 IHC, we consider that EBV + BCL and B‐LPD can be classified into three types: “immunodeficiency,” “immune escape,” and “immunodeficiency + immune escape” type.
1. INTRODUCTION
According to the current WHO classification, Epstein–Barr virus (EBV) is detected in a variety of B‐cell lymphomas (BCLs) and lymphoproliferative disorders (B‐LPDs). 1 Immunodeficiency has been considered as a key factor for the development of EBV+ BCLs and B‐LPDs. 2 , 3 Main causes of immunodeficiency are aging, infection of human immunodeficiency virus, and immunosuppressants used for patients after organ transplantation or with autoimmune disorders. 2 , 3
Immune escape of tumor cells may also play a key role in the pathogenesis of EBV+ BCLs and B‐LPDs. Particularly, the PD‐1/PD‐L1 axis is important for immune escape of tumor cells which contribute to the development of lymphomas via suppression of cytotoxic T‐cell function. 4 , 5 , 6 Some previous studies reported that PD‐L1 expression on tumor cells predicts the worse outcomes for malignant lymphomas. 7 , 8 , 9 , 10 , 11 These worse outcomes are considered to be caused by the immune escape mechanism of the lymphoma cells. Thus, we now consider PD‐L1 IHC a very useful method for predicting whether tumor cells of lymphoid malignancies are characterized by the immune escape mechanism; that is, lymphomas or LPDs expressing PD‐L1 on neoplastic cells are characterized by the immune escape mechanism.
Here, we review EBV+ BCLs and B‐LPDs from the perspective of immune escape and immunodeficiency. The EBV+ BCLs and B‐LPDs we describe in this article include EBV+ diffuse large BCL, not otherwise specified (DLBCL‐NOS), EBV+ sporadic Burkitt lymphoma (sBL), EBV+ classic Hodgkin lymphoma (CHL), EBV+ mucocutaneous ulcer (EBVMCU), methotrexate‐associated B‐LPD (MTX B‐LPD), and CHL type MTX‐associated LPD.
2. IMMUNE ESCAPE AND IMMUNODEFICENCY OF EBV+ BCLS AND B‐LPDS
Immune escape is a key factor of lymphomagenesis. Cancer cells have a variety of mechanisms to escape from immunosurveillance. 12 , 13 For example, they recruit regulatory T cells (Tregs) and myeloid‐derived suppressor cells (MDSCs). These two are major types of immunosuppressive regulatory immune cells. Activated Tregs attenuate the function of tumor‐specific T lymphocytes by secreting IL‐10 and TGF‐β, which are immunosuppressive cytokines, and by expressing PD‐1, CTLA‐4, and PD‐L1. MDSCs are a heterogeneous group of immature myeloid cells and myeloid progenitor cells. MDSCs possess strong immunosuppressive activities regulating the functions of other types of immune cells, such as macrophages, T lymphocytes, dendritic cells, macrophages, and natural killer (NK) cells. Another mechanism is the production of immunosuppressive cytokines, including VEGF, TGF‐β, galectin, or IDO, by cancer cells. They also escape from immunosurveillance by expressing immune checkpoints, for example, PD‐L1 and CTLA‐4. Overall, the expression of PD‐L1 is considered to be the dominant mechanism.
PD‐L1 expression on cancer cells is caused by two general mechanisms, known as innate and adaptive immune resistance. 14 , 15 The former mechanism causes constitutive expression of PD‐L1 via aberrant signaling pathways or genetic alterations of PD‐L1. For example, the aberrant signaling pathways which induce PD‐L1 expression are driven by PTEN losses in glioblastoma, EGFR mutations in lung carcinoma, and BRAF V600E mutations in malignant melanoma. 16 , 17 , 18 , 19 In addition, genetic alterations in PD‐L1, which induce PD‐L1 expression have been reported. Alteration of the 9p24.1/PD‐L1/PD‐L2 has been reported in malignant lymphoma and gastric adenocarcinoma. 20 , 21 Another important genetic alteration in PD‐L1 is 3’‐UTR disruption. Kataoka et al. 22 revealed that PD‐L1 overexpression is caused by PD‐L1 3’‐UTR disruption in multiple cancers. Another mechanism that causes PD‐L1 overexpression is gene fusion between CIITA and upstream of PD‐L1, which is commonly detected in primary mediastinal large B‐cell lymphoma. 23 The CIITA–PD‐L1 fusion places PD‐L1 under the transcriptional control of the CIITA promoter and drives PD‐L1 overexpression. Among the EBV+ BCLs and B‐LPDs, almost all EBV+ CHLs and a subset of EBV+ DLBCLs have alterations in PD‐L1 and PD‐L2. 21 , 24 Another mechanism that induces PD‐L1 expression on tumor cells is adaptive immune resistance. The overexpression of PD‐L1 is driven by interactions with cytokines, particularly IFN‐γ produced by immune cells in the tumor microenvironment (TME). 25 IFN‐γ is secreted by activated CD8+ T cells, activated Th1‐type CD4+ T cells, and NK cells. The cancer cells show adaptive responses against IFN‐γ, resulting in PD‐L1 induction and avoiding attacks by inflammatory immune microenvironment.
The immunodeficiency of EBV+ BCLs and B‐LPDs reviewed in this article is caused by aging or MTX use for autoimmune diseases. Immunosenescence is a gradual decline in immune functions brought on by the aging process. Aging promotes an imbalance between inflammatory and inflammatory‐neutralizing processes. This imbalance cause the low‐grade chronic pro‐inflammatory state, 26 which induces free radicals that cause DNA damage, resulting in carcinogenesis, including lymphomagenesis. 27 Immunosenescence also causes a decrease in the number of total T cells, including cytotoxic CD8+ and helper CD4+ cells, and a gradual decline in the T‐cell antigenic repertoire. 28 , 29 Thus, the number of CD8+ EBV‐specific T cells is reduced. MTX is a cytoreductive folate analog drug commonly used to treat autoimmune diseases, particularly rheumatoid arthritis (RA). 30 Multiple mechanisms are considered to be affected in the treatment of RA. 31 For example, MTX reduces cell proliferation and increases T‐cell apoptosis. Another mechanism is the reduction of the cytokine production induced by T‐cell activation. Therefore, the immunodeficient status of patients due to aging and MTX use suppresses the function of CD8+ EBV‐specific T cells that regulate EBV+ cell proliferation. 32 This immune dysregulation in the surveillance for EBV is presumed to result in the development of EBV+ BCL and B‐LPD.
3. DISEASE OVERVIEW: EBV+ BCLs and B‐LPDs
3.1. EBV+ DLBCL‐NOS
EBV+ DLBCL‐NOS was first described as age‐related EBV‐associated LPD with no evidence of underlying immunodeficiency in 2003. 1 In the previous version of WHO classification, this disease was documented as EBV+ DLBCL of the elderly and is now changed to EBV+ DLBCL‐NOS in the 2017 WHO classification. The age restriction has been deleted because the disease may arise in a wide age range. 33 However, the majority of EBV+ DLBCL‐NOS occur in individuals older than 50, and the percentage of the EBV+ group for all DLBCL‐NOS cases increases with age, peaking in the 80s. 34 , 35 , 36 , 37 , 38 Therefore, immunosenescence is suggested to play an important role in the development of EBV+ DLBCL of the elderly.
In a Japanese cohort, Kiyasu et al. 7 recently reported that 16% (14/90) of patients with EBV+ DLBCL‐NOS expressed PD‐L1. In addition, Takahara et al. 9 recently reported that 11% (6/57) of patients with EBV+ DLBCL‐NOS present with positive staining for PD‐L1 (Figure 1A–C). These two cohorts consisted mainly of old patients. Kataoka et al. 24 reported that 19% (5/27) of EBV+ DLBCL‐NOS cases involved PD‐L1/PD‐L2 somatic alterations. The frequency of PD‐L1/PD‐L2 aberrations in EBV+ DLBCL‐NOS was much higher than the frequency in EBV− DLBCL‐NOS. This frequency was comparable to the PD‐L1‐positive rate in EBV+ DLBCL‐NOS, suggesting that the PD‐L1 overexpression in this disease is mainly due to PD‐L1‐involving genetic alterations. They also found that structural variations (SVs) in PD‐L2 were found exclusively in BCLs, whereas SVs involving PD‐L1 were detected in a variety of solid cancers, T/NK‐cell lymphomas, and BCLs. These results imply that immune escape may be another factor of lymphomagenesis in a minor subset of EBV+ DLBCL of the elderly. Takahara et al. 9 further highlighted that patients with PD‐L1+ EBV+ DLBCL‐NOS were characterized by a poorer prognosis than PD‐L1− cases. Based on this finding, we suggest that, along with immunosenescence, immune escape of tumor cells plays a key role in the lymphomagenesis of PD‐L1+ cases and contributes to their poorer prognosis. On the other hand, compared to immune escape, immunosenescence deeply affect the pathogenesis of PD‐L1− cases, which account for the majority of EBV+ DLBCL of the elderly.
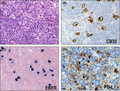
FIGURE 1.
Histological and immunohistochemical features of EBV‐positive diffuse large B‐cell lymphoma (DLBCL) of the elderly. (A) Histologically, a polymorphic pattern is observed in this case (HE ×400). (B) The tumor cells show positive staining for EBER (×400). (C) PD‐L1 expression was detected in 11% of EBV+ DLBCL of the elderly (×400)
The frequency of PD‐L1 positivity was significantly higher in EBV+ LBCL of the young (≤45 years) than EBV+ DLBCL of the elderly. Nicolae et al. 33 revealed that 77% (24/31) of EBV+ LBCL of the young cases expressed PD‐L1. They also revealed that all of the young patients present with nodal lesions, which comprise three morphological patterns: T‐cell/histiocyte‐rich large B‐cell lymphoma (THRBCL)‐like, gray zone lymphoma (GZL), and DLBCL‐NOS. Among the three, the THRBCL‐like pattern presented the highest frequency of PD‐L1 positivity (95%, 20/21). Furthermore, they highlighted that EBV+ LBCL of the young had significantly better overall survival (OS) than EBV+ DLBCL of the elderly (5‐year OS probability: 89.0% and 24.4%, respectively). In particular, young patients with the THRBCL‐like pattern had a superior OS curve compared to the other two patterns. Based on their findings, we speculate that immune escape of neoplastic cells promoted by PD‐L1 expression play a key role in the development of EBV+ LBCL of the young, whereas immunosenescence plays a far lesser role in the pathogenesis because young patients have potent immune responses. The good prognosis of young patients can be explained by the dependence of tumor cells on immune escape. In immune potent patients, when the lymphoma cells are eliminated by chemotherapy, there are no more factors that can lead to lymphomagenesis.
We consider that EBV+ DLBCL of the elderly and EBV+ DLBCL of the young should be regarded as different pathogenic types. The former is categorized as immunosenescent type, and the latter as immune escape type. A minor subset of EBV+ DLBCL of the elderly cases express PD‐L1, and they are considered to be categorized as immunosenescent + immune escape type.
3.2. EBV+ CHL
CHL is a malignant tumor derived from B cell and is composed of Hodgkin and Reed–Sternberg (HRS) cells on a background containing extensive non‐neoplastic immune cells. 1 , 39 Based on the characteristics of infiltrating immune cells and morphological findings, CHL is divided into four histological subtypes: nodular sclerosis (NS), mixed cellularity (MC), lymphocyte‐rich (LR), and lymphocyte‐depleted (LD). 1 The frequencies of EBV positivity on HRS cells varies among the subtypes, and are higher in MC type (75%) and LD type (75%) and lower in NS type (10%–25%) and LR type (variable). 1 , 39 Overall, HRS cells are EBV‐positive in about 40% of CHL cases. 40
CHL is now considered as a representative immune escape‐type lymphoma. Green et al. 41 identified PD‐L1 and PD‐L2 as key targets of 9p24.1 copy gain in cell lines of CHL and HRS cells captured by laser microdissection. They also reported, using quantitative immunohistochemical methods, that PD‐L1 expression level correlated with PD‐L1 copy number. Besides PD‐L1 and PD‐L2, JAK2 is also included in the broader 9p24.1 amplification region. JAK2 copy gain enhances JAK2 expression and JAK/STAT signaling, which leads to the expression of PD‐L1. They also revealed that EBV infection induces the expression of PD‐L1 in CHLs. This increased expression of PD‐L1 is mediated by LMP1 through the JAK/STAT and AP‐1 pathways. Roemer et al. 21 performed FISH in 108 samples from CHL cases to evaluate the alterations of PD‐L1 and PD‐L2. They found that 99% (107/108) of CHLs had PD‐L1 and PD‐L2 aberrations, including polysomy (n = 5), copy gain (n = 61), and amplification (n = 39). They also identified that PD‐L1 expression was associated with relative genetic aberrations. In this series of CHL cases, the EBV+ and EBV− CHLs had similar distribution of genetic alterations. However, HRS cells in EBV+ CHLs had a higher percentage and stronger intensity of PD‐L1‐positive staining, which indicates PD‐L1 expression is further induced by EBV infection. Sakakibara et al. 42 also confirmed in their series that all of the EBV+ CHLs, including one NS type and nine MC type, stained positive for PD‐L1 in IHC (Figure 2A–D). Therefore, immune escape is suggested to be strongly associated with the pathogenesis of EBV+ CHL.
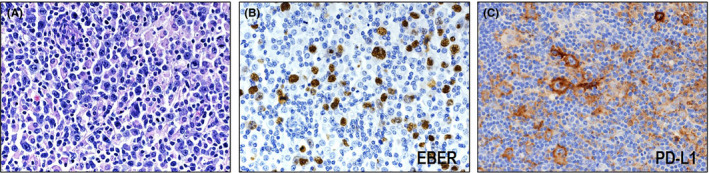
FIGURE 2.
Histological and immunohistochemical features of EBV‐positive mixed cellularity classic Hodgkin lymphoma (CHL). (A) Hodgkin and Reed–Sternberg (HRS) cells proliferate on a background containing extensive non‐neoplastic immune cells (HE ×400). (B) The HRS cells are CD30‐positive (×400) and (C) EBER‐positive (×400). (D) All of the EBV+ CHLs show positive staining for PD‐L1 (×400)
The TME of CHL, including both EBV+ and EBV− cases, has been thoroughly analyzed and its contributions to the immune escape mechanism of CHL highlighted. Previous studies have highlighted that CD4+ T cells are abundant in the TME of CHL. 43 , 44 , 45 , 46 Aoki et al. 45 recently reported a single‐cell transcriptome analysis identifying a novel subset of T cells in the TME of CHL, the LAG3+ T‐cell population. LAG3+ T cells have Treg‐like features and contribute to the immune escape phenotype. Carey et al. 44 found that the majority of PD‐L1+ cells in the TME of CHL are tumor‐associated macrophages (TAMs). These PD‐L1+ TAMs regionally colocalize with HRS cells, suggesting that the elevated expression level of PD‐L1 on TAMs is in response to cytokine production, particularly IFN‐γ, by HRS cells. Consequently, the total amount of PD‐L1 in the vicinity of the HRS cells is increased, and the immune escape mechanism of CHL may be enhanced.
3.3. EBV+ sBL
BL is a B‐cell neoplasm with highly aggressive behavior. BL is classified into three clinical variants: endemic, sporadic, and immunodeficiency‐associated. 1 Endemic BL (eBL) occurs in equatorial Africa and Papua New Guinea, which are also endemic for malaria. 47 Sporadic BL (sBL) is seen worldwide, and immunodeficiency‐associated BL most commonly occurs in HIV patients. 48 , 49 EBV is detected in >95% of eBL, 20%–30% of sBL, and 25%–40% of immunodeficiency‐associated BL. 48 , 50 In Western Europe and North America, sBL occurs mainly in children and young. sBL accounts for 30%–50% of childhood lymphomas in these counties. 51
Satou et al. 50 previously reported that 22% (33/150) of sporadic BL cases in Japan were infected with EBV. Comparing the clinicopathological characteristics of EBV+ and EBV− BL, they found that EBV+ BL had a significantly higher age distribution. The difference in age distribution can be explained by the fact that the most of pediatric cases were EBV−. They speculated that immunodeficiency plays an important role in the development of EBV+ sBL. In old patients with EBV+ sBL, they suggested that the immunodeficiency was caused by aging. Therefore, EBV+ sBL arising in old patients may have a characteristic of age‐related LPD. In contrast, the immunodeficient condition of young patients with EBV+ sBL is caused by an unknown mechanism.
Chen et al. 52 assessed the PD‐L1 expression in a series of EBV+ lymphomas by IHC, but none of the seven cases of EBV+ BL was positive for PD‐L1 on tumor cells. In our cohort of EBV+ sBL, five cases tested for PD‐L1 expression were negative for PD‐L1 (unpublished data). These results suggest that immune escape play a lesser role in the pathogenesis of EBV+ sBL, whereas immunodeficiency has a key function in its development.
3.4. EBVMCU
Dojcinov et al. first reported EBVMCU in 2010, and it was newly categorized as a provisional entity in the 2017 WHO classification. 1 , 53 This disease presents as sharply circumscribed, isolated lesions confined to the oral mucosa, skin, and gastrointestinal tract. There is no evidence of systemic involvement. 53 EBVMCU occurs in individuals of varying immunodeficient status (older age, immunosuppressant drug treatment, treated lymphoma, HIV infection, and primary immunodeficiencies) and is now considered as a specific type of immunodeficiency‐associated LPD. 53 , 54 , 55 , 56 , 57 , 58 , 59 Patients with EBVMCU typically have an indolent, self‐limited course with spontaneous remission (SR) or complete remission (CR) after reduced immunosuppression. Thus, to distinguish EBVMCU from EBV+ DLBCL is important because clinical approach can be different. Recently, Ikeda et al. 54 performed a clinical and pathological study of 34 Japanese patients with EBVMCU; causes of immunosuppression in these 34 patients were MTX use (n = 30), hydroxycarbamide use (n = 1), and aging (n = 3). Therefore, in the Japanese cohort, a majority of the EBVMCUs may have arisen as a subtype of EBV+ MTX B‐LPD, which is described below. EBVMCU was histologically classified into four subtypes: polymorphous; large cell‐rich; CHL‐like; and mucosa‐associated lymphoid tissue lymphoma‐like. The occurrence of IGH rearrangement was not significantly different between EBVMCU and EBV+ DLBCL, or EBVMCU and EBV− DLBCL. Thus, they concluded that EBVMCU was difficult to distinguish from EBV+ DLBCL based on histological and genetical findings, and clinical information is necessary for the accurate diagnosis of EBVMCU.
Daroontum and Satou et al. previously assessed PD‐L1 expression in 25 EBVMCU cases. 55 , 56 None of them presented positive staining for PD‐L1, except for one case (Figure 3A–D). Therefore, immune evasion mechanism may not associate with most EBVMCU cases. This result is expected because EBVMCU is considered as a specific type of immunodeficiency‐associated LPD, and immunodeficiency due to various conditions is regarded as the main cause of the pathogenesis of this disease.
FIGURE 3.
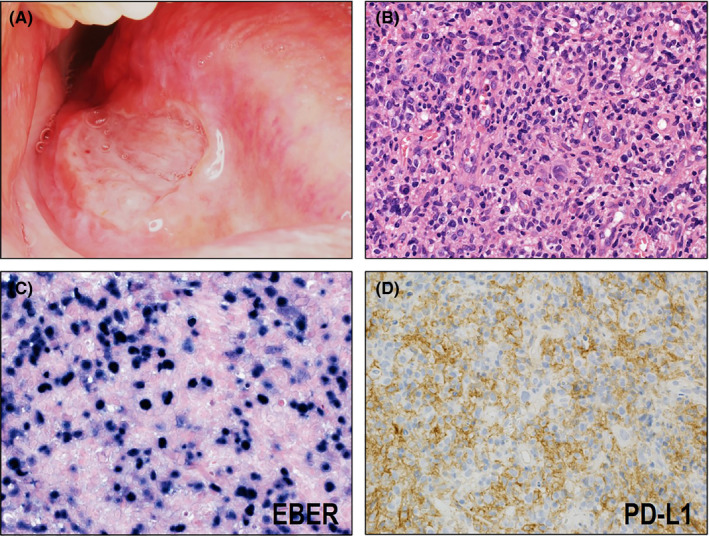
Macroscopic, histological, and immunohistochemical features of EBV‐positive mucocutaneous ulcer (EBVMCU). (A) EBVMCU macroscopically shows a sharply circumscribed mucosal ulcer. (B) Histologically, EBVMCU shows infiltration of intermediate to large atypical lymphoid cells with polymorphous background (HE ×400). (C) EBER is detected in the large atypical lymphoid cells (×400). (D) Most of the cases are negative for PD‐L1 on tumor cells (×400)
3.5. MTX B‐LPD and CHL type MTX‐associated LPD
MTX is an antirheumatic drug widely used to treat autoimmune diseases, particularly rheumatoid arthritis (RA). 30 Although MTX has an excellent inhibitory effect against articular destruction, it increases the risk of LPD. 60 According to the 2017WHO classification, MTX‐associated LPD is categorized as “other immunodeficiency‐associated LPDs.” 1 The iatrogenic immunodeficient status due to MTX use contributes to the development of various types of LPD, including reactive lymphoid hyperplasia, low‐grade BCL, polymorphic B‐LPD, DLBCL, peripheral T‐cell lymphoma, and CHL. 61 , 62 , 63 Among these, DLBCL type occurs most frequently, followed by CHL and polymorphic B‐LPD. Each of these types includes both EBV+ and EBV− cases.
MTX‐associated LPD is unique in that they may present with spontaneous regression after the MTX cessation. 61 , 62 , 63 This phenomenon supports the theory that the disease is caused as a result of immunosuppression induced by MTX. In particular, EBV+ cases have a higher frequency of SR than EBV− cases. 61 , 62 On the other hand, CHL type MTX‐associated LPD is characterized by a lower frequency of SR after MTX cessation. 64 Gion et al. 64 reported that most patients with CHL type could not be cured by MTX cessation alone and required additional chemotherapy. They also revealed that CHL type had an inferior progression‐free survival curve compared to DLBCL type. As many studies of MTX‐associated LPD have been performed, clinicopathological aspects of the disease have been gradually highlighted.
The mechanism underlying the differences in clinical behavior among the types of MTX‐associated LPDs remain to be elucidated. Kohno et al. 65 recently reported interesting findings that may account for the clinical characteristics of CHL type MTX‐associated LPD. They performed a PD‐L1 immunohistochemical assessment in a series of MTX‐associated LPDs, including CHL type (n = 9), DLBCL type (n = 15), and polymorphic B‐LPD type (n = 21). Notably, PD‐L1 expression on neoplastic cells was found exclusively in CHL type (89%, 8/9) (Figure 4A–D). Fluorescence in situ analysis was performed to assess the PD‐L1 copy number status for two cases of CHL type, detecting PD‐L1 gene amplification in one of them. These results imply that, in addition to immunodeficiency due to MTX use, immune escape mechanism contribute to the development of CHL type MTX‐associated LPD. This pathogenic model may account for the lower frequency of SR and frequent demand for additional chemotherapy. Namely, when the immune system of patients recovers after MTX cessation, the antitumor responses of cytotoxic T cells may be regained. This recovery of the antitumor response leads to SR of the lesions. However, the immune escape mechanism of tumor cells in CHL type still remain after MTX cessation, and they evade the patient's immune surveillance. Therefore, the frequency of SR is low in CHL type and additional chemotherapy is required to cure the disease.
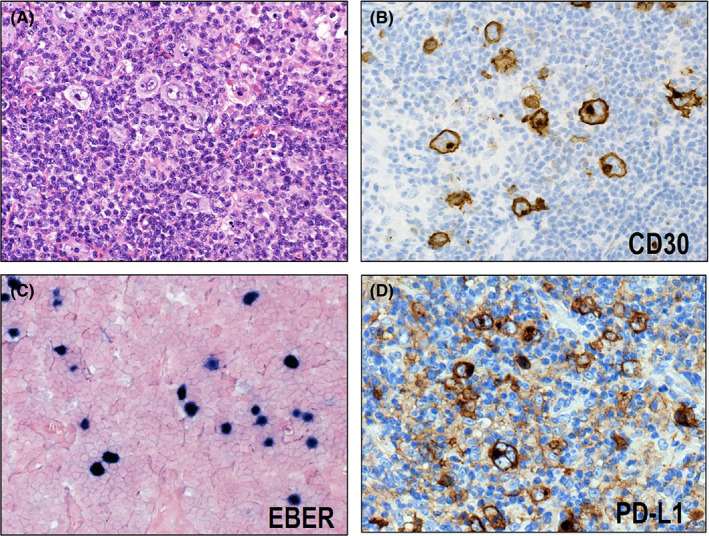
FIGURE 4.
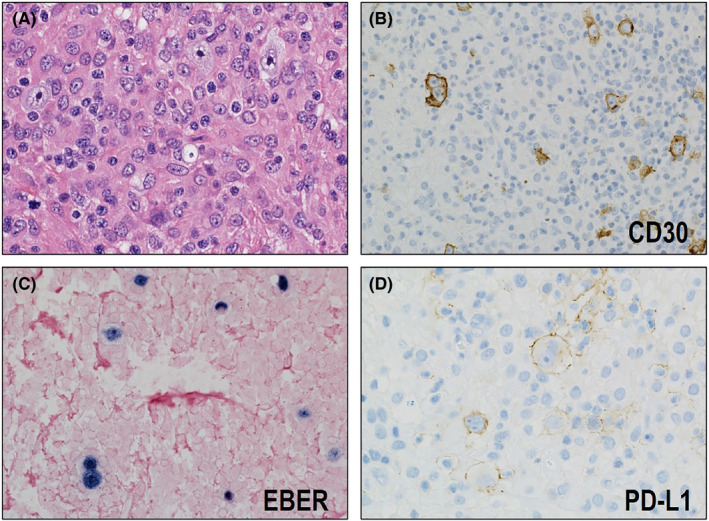
Histological and immunohistochemical features of classic Hodgkin lymphoma (CHL)‐type methotrexate (MTX)‐associated lymphoproliferative disorder (LPD). (A) Hodgkin and Reed–Sternberg (HRS) cells proliferate on a background containing extensive non‐neoplastic immune cells (HE ×400). (B) The HRS cells are CD30‐positive (×400) and (C) EBER‐positive (×400). (D) Most of the CHL‐type MTX‐associated LPDs stain positive for PD‐L1 (×400)
4. CONCLUSIONS
We reviewed EBV+ BCL and B‐LPDs from the perspective of immune escape and immunodeficiency. We used PD‐L1 IHC as an indicator of whether tumor cells harbor an immune escape mechanism, and classified EBV+ BCL and B‐LPD into three types: immunodeficiency, immune escape, and immunodeficiency + immune escape. The immunodeficiency type is PD‐L1‐negative and considered to be due to immunodeficiency caused by aging or MTX use. The immune escape type is PD‐L1‐positive and less associated with immunodeficiency. The immunodeficiency + immune escape type is PD‐L1‐positive and considered to be associated with immunodeficiency.
Recently, good results of immune checkpoint inhibitors in treating lymphoma have been reported. 66 , 67 , 68 , 69 Lymphomas and LPDs characterized by immune escape of the tumor cells are regarded as good candidates for PD1/PD‐L1 blockade therapy. For example, CHL, a representative immune escape‐type lymphoma, shows a good response to PD‐L1 blockade. 66 , 70 , 71 Therefore, from both the clinical and pathological perspective, we suggest that lymphoma diagnosis should be made considering immune escape and immunodeficiency.
CONFLICT OF INTERESTS
The authors declare no competing financial interests.
ACKNOWLEDGMENTS
This work was supported, in part, by grants from the Grants‐in‐Aid for Scientific Research (grant number: 20K16204).
Satou A, Nakamura S. EBV‐positive B‐cell lymphomas and lymphoproliferative disorders: Review from the perspective of immune escape and immunodeficiency. Cancer Med. 2021;10:6777–6785. 10.1002/cam4.4198
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during this study.
REFERENCES
- 1. Swerdlow S, Campo E, Harris N, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. International Agency for Research on Cancer; 2017. [Google Scholar]
- 2. Natkunam Y, Goodlad JR, Chadburn A, et al. EBV‐Positive B‐Cell Proliferations of Varied Malignant Potential: 2015 SH/EAHP workshop report‐part 1. Am J Clin Pathol. 2017;147:129‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Jong D, Roemer MG, Chan JK, et al. B‐cell and classical Hodgkin lymphomas associated with immunodeficiency: 2015 SH/EAHP workshop report‐part 2. Am J Clin Pathol. 2017;147:153‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ilcus C, Bagacean C, Tempescul A, et al. Immune checkpoint blockade: the role of PD‐1‐PD‐L axis in lymphoid malignancies. OncoTargets Ther. 2017;10:2349‐2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD‐1 and its ligands in tolerance and immunity. Ann Rev Immunol. 2008;26:677‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pedoeem A, Azoulay‐Alfaguter I, Strazza M, Silverman GJ, Mor A. Programmed death‐1 pathway in cancer and autoimmunity. Clin Immunolo. 2014;153:145‐152. [DOI] [PubMed] [Google Scholar]
- 7. Kiyasu J, Miyoshi H, Hirata A, et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B‐cell lymphoma. Blood. 2015;126:2193‐2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miyoshi H, Kiyasu J, Kato T, et al. PD‐L1 expression on neoplastic or stromal cells is respectively a poor or good prognostic factor for adult T‐cell leukemia/lymphoma. Blood. 2016;128:1374‐1381. [DOI] [PubMed] [Google Scholar]
- 9. Takahara T, Satou A, Ishikawa E, et al. Clinicopathological analysis of neoplastic PD‐L1‐positive EBV(+) diffuse large B cell lymphoma, not otherwise specified, in a Japanese cohort. Virchows Arch. 2021;478:541‐552. [DOI] [PubMed] [Google Scholar]
- 10. Ishikawa E, Nakamura M, Shimada K, et al. Prognostic impact of PD‐L1 expression in primary gastric and intestinal diffuse large B‐cell lymphoma. J Gastroenterol. 2020;55:39‐50. [DOI] [PubMed] [Google Scholar]
- 11. Suzuki Y, Kohno K, Matsue K, et al. PD‐L1 (SP142) expression in neoplastic cells predicts a poor prognosis for patients with intravascular large B‐cell lymphoma treated with rituximab‐based multi‐agent chemotherapy. Cancer Med. 2020;9:4768‐4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565‐1570. [DOI] [PubMed] [Google Scholar]
- 13. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69‐74. [DOI] [PubMed] [Google Scholar]
- 14. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism‐driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parsa AT, Waldron JS, Panner A, et al. Loss of tumor suppressor PTEN function increases B7–H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84‐88. [DOI] [PubMed] [Google Scholar]
- 17. Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF‐MAPK signaling pathway is essential for cancer‐immune evasion in human melanoma cells. J Exp Med. 2006;203:1651‐1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marzec M, Zhang Q, Goradia A, et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD‐L1, B7–H1). Proc Natl Acad Sci U S A. 2008;105:20852‐20857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Akbay EA, Koyama S, Carretero J, et al. Activation of the PD‐1 pathway contributes to immune escape in EGFR‐driven lung tumors. Cancer Discov. 2013;3:1355‐1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gulley ML. Genomic assays for Epstein‐Barr virus‐positive gastric adenocarcinoma. Exp Mol Med. 2015;47:e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roemer MG, Advani RH, Ligon AH, et al. PD‐L1 and PD‐L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol. 2016;34:2690‐2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kataoka K, Shiraishi Y, Takeda Y, et al. Aberrant PD‐L1 expression through 3'‐UTR disruption in multiple cancers. Nature. 2016;534:402‐406. [DOI] [PubMed] [Google Scholar]
- 23. Steidl C, Shah SP, Woolcock BW, et al. MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nature. 2011;471:377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kataoka K, Miyoshi H, Sakata S, et al. Frequent structural variations involving programmed death ligands in Epstein‐Barr virus‐associated lymphomas. Leukemia. 2019;33:1687‐1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7–h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Franceschi C, Capri M, Monti D, et al. Inflammaging and anti‐inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92‐105. [DOI] [PubMed] [Google Scholar]
- 27. Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276‐285. [DOI] [PubMed] [Google Scholar]
- 28. Dojcinov SD, Fend F, Quintanilla‐Martinez L. EBV‐Positive Lymphoproliferations of B‐ T‐ and NK‐Cell Derivation in Non‐Immunocompromised Hosts. Pathogens. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ok CY, Papathomas TG, Medeiros LJ, Young KH. EBV‐positive diffuse large B‐cell lymphoma of the elderly. Blood. 2013;122:328‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rath T, Rubbert A. Drug combinations with methotrexate to treat rheumatoid arthritis. Clin Exp Rheumatol. 2010;28:S52‐S57. [PubMed] [Google Scholar]
- 31. Wessels JA, Huizinga TW, Guchelaar HJ. Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatol. 2008;47:249‐255. [DOI] [PubMed] [Google Scholar]
- 32. Thorley‐Lawson DA, Gross A. Persistence of the Epstein‐Barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350:1328‐1337. [DOI] [PubMed] [Google Scholar]
- 33. Nicolae A, Pittaluga S, Abdullah S, et al. EBV‐positive large B‐cell lymphomas in young patients: a nodal lymphoma with evidence for a tolerogenic immune environment. Blood. 2015;126:863‐872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oyama T, Ichimura K, Suzuki R, et al. Senile EBV+ B‐cell lymphoproliferative disorders: a clinicopathologic study of 22 patients. Am J Surg Pathol. 2003;27:16‐26. [DOI] [PubMed] [Google Scholar]
- 35. Oyama T, Yamamoto K, Asano N, et al. Age‐related EBV‐associated B‐cell lymphoproliferative disorders constitute a distinct clinicopathologic group: a study of 96 patients. Clin Cancer Res. 2007;13:5124‐5132. [DOI] [PubMed] [Google Scholar]
- 36. Shimoyama Y, Yamamoto K, Asano N, Oyama T, Kinoshita T, Nakamura S. Age‐related Epstein‐Barr virus‐associated B‐cell lymphoproliferative disorders: special references to lymphomas surrounding this newly recognized clinicopathologic disease. Cancer Sci. 2008;99:1085‐1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shimoyama Y, Asano N, Kojima M, et al. Age‐related EBV‐associated B‐cell lymphoproliferative disorders: diagnostic approach to a newly recognized clinicopathological entity. Pathol Int. 2009;59:835‐843. [DOI] [PubMed] [Google Scholar]
- 38. Dojcinov SD, Venkataraman G, Pittaluga S, et al. Age‐related EBV‐associated lymphoproliferative disorders in the Western population: a spectrum of reactive lymphoid hyperplasia and lymphoma. Blood. 2011;117:4726‐4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Connors JM, Cozen W, Steidl C, et al. Hodgkin lymphoma. Nat Rev Dis Primers. 2020;6:61. [DOI] [PubMed] [Google Scholar]
- 40. Piris MA, Medeiros LJ, Chang KC. Hodgkin lymphoma: a review of pathological features and recent advances in pathogenesis. Pathology. 2020;52:154‐165. [DOI] [PubMed] [Google Scholar]
- 41. Green MR, Rodig S, Juszczynski P, et al. Constitutive AP‐1 activity and EBV infection induce PD‐L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res. 2012;18:1611‐1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sakakibara A, Kohno K, Eladl AE, et al. Immunohistochemical assessment of the diagnostic utility of PD‐L1: a preliminary analysis of anti‐PD‐L1 antibody (SP142) for lymphoproliferative diseases with tumour and non‐malignant Hodgkin‐Reed‐Sternberg (HRS)‐like cells. Histopathology. 2018;72:1156‐1163. [DOI] [PubMed] [Google Scholar]
- 43. Greaves P, Clear A, Owen A, et al. Defining characteristics of classical Hodgkin lymphoma microenvironment T‐helper cells. Blood. 2013;122:2856‐2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Carey CD, Gusenleitner D, Lipschitz M, et al. Topological analysis reveals a PD‐L1‐associated microenvironmental niche for Reed‐Sternberg cells in Hodgkin lymphoma. Blood. 2017;130:2420‐2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Aoki T, Chong LC, Takata K, et al. Single‐cell transcriptome analysis reveals disease‐defining T‐cell subsets in the tumor microenvironment of classic Hodgkin lymphoma. Cancer Discov. 2020;10:406‐421. [DOI] [PubMed] [Google Scholar]
- 46. Cader FZ, Schackmann RCJ, Hu X, et al. Mass cytometry of Hodgkin lymphoma reveals a CD4(+) regulatory T‐cell‐rich and exhausted T‐effector microenvironment. Blood. 2018;132:825‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Johnston WT, Mutalima N, Sun D, et al. Relationship between Plasmodium falciparum malaria prevalence, genetic diversity and endemic Burkitt lymphoma in Malawi. Sci Rep. 2014;4:3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lim ST, Karim R, Nathwani BN, Tulpule A, Espina B, Levine AM. AIDS‐related Burkitt's lymphoma versus diffuse large‐cell lymphoma in the pre‐highly active antiretroviral therapy (HAART) and HAART eras: significant differences in survival with standard chemotherapy. J Clin Oncol. 2005;23:4430‐4438. [DOI] [PubMed] [Google Scholar]
- 49. Raphael M, Gentilhomme O, Tulliez M, Byron PA, Diebold J. Histopathologic features of high‐grade non‐Hodgkin's lymphomas in acquired immunodeficiency syndrome. The French Study Group of pathology for human immunodeficiency virus‐associated tumors. Arch Pathol Lab Med. 1991;115:15‐20. [PubMed] [Google Scholar]
- 50. Satou A, Asano N, Nakazawa A, et al. Epstein‐Barr virus (EBV)‐positive sporadic burkitt lymphoma: an age‐related lymphoproliferative disorder? Am J Surg Pathol. 2015;39:227‐235. [DOI] [PubMed] [Google Scholar]
- 51. Mbulaiteye SM, Anderson WF, Ferlay J, et al. Pediatric, elderly, and emerging adult‐onset peaks in Burkitt's lymphoma incidence diagnosed in four continents, excluding Africa. Am J Hematol. 2012;87:573‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen BJ, Chapuy B, Ouyang J, et al. PD‐L1 expression is characteristic of a subset of aggressive B‐cell lymphomas and virus‐associated malignancies. Clin Cancer Res. 2013;19:3462‐3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dojcinov SD, Venkataraman G, Raffeld M, Pittaluga S, Jaffe ES. EBV positive mucocutaneous ulcer–a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol. 2010;34:405‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ikeda T, Gion Y, Sakamoto M, et al. Clinicopathological analysis of 34 Japanese patients with EBV‐positive mucocutaneous ulcer. Modern Pathol. 2020;33:2437‐2448. [DOI] [PubMed] [Google Scholar]
- 55. Satou A, Banno S, Hanamura I, et al. EBV‐positive mucocutaneous ulcer arising in rheumatoid arthritis patients treated with methotrexate: single center series of nine cases. Pathol Int. 2019;69:21‐28. [DOI] [PubMed] [Google Scholar]
- 56. Daroontum T, Kohno K, Eladl AE, et al. Comparison of Epstein‐Barr virus‐positive mucocutaneous ulcer associated with treated lymphoma or methotrexate in Japan. Histopathology. 2018;72:1115‐1127. [DOI] [PubMed] [Google Scholar]
- 57. Hart M, Thakral B, Yohe S, et al. EBV‐positive mucocutaneous ulcer in organ transplant recipients: a localized indolent posttransplant lymphoproliferative disorder. Am J Surg Pathol. 2014;38:1522‐1529. [DOI] [PubMed] [Google Scholar]
- 58. Bunn B, van Heerden W. EBV‐positive mucocutaneous ulcer of the oral cavity associated with HIV/AIDS. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:725‐732. [DOI] [PubMed] [Google Scholar]
- 59. Kleinman S, Jhaveri D, Caimi P, et al. A rare presentation of EBV(+) mucocutaneous ulcer that led to a diagnosis of hypogammaglobulinemia. J Allergy Clin Immunol Pract. 2014;2:810‐812. [DOI] [PubMed] [Google Scholar]
- 60. Bernatsky S, Clarke AE, Suissa S. Hematologic malignant neoplasms after drug exposure in rheumatoid arthritis. Arch Int Med. 2008;168:378‐381. [DOI] [PubMed] [Google Scholar]
- 61. Kurita D, Miyoshi H, Ichikawa A, et al. Methotrexate‐associated lymphoproliferative disorders in patients with rheumatoid arthritis: clinicopathologic features and prognostic factors. Am J Surg Pathol. 2019;43:869‐884. [DOI] [PubMed] [Google Scholar]
- 62. Ichikawa A, Arakawa F, Kiyasu J, et al. Methotrexate/iatrogenic lymphoproliferative disorders in rheumatoid arthritis: histology, Epstein‐Barr virus, and clonality are important predictors of disease progression and regression. Eur J Haematol. 2013;91:20‐28. [DOI] [PubMed] [Google Scholar]
- 63. Satou A, Tabata T, Miyoshi H, et al. Methotrexate‐associated lymphoproliferative disorders of T‐cell phenotype: clinicopathological analysis of 28 cases. Mod Pathol. 2019;32:1135‐1146. [DOI] [PubMed] [Google Scholar]
- 64. Gion Y, Iwaki N, Takata K, et al. Clinicopathological analysis of methotrexate‐associated lymphoproliferative disorders: comparison of diffuse large B‐cell lymphoma and classical Hodgkin lymphoma types. Cancer Sci. 2017;108:1271‐1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kohno K, Suzuki Y, Elsayed AA, et al. Immunohistochemical assessment of the diagnostic utility of PD‐L1 (Clone SP142) for methotrexate‐associated lymphoproliferative disorders with an emphasis of neoplastic PD‐L1 (Clone SP142)‐positive classic Hodgkin lymphoma type. Am J Clin Pathol. 2020;153:571‐582. [DOI] [PubMed] [Google Scholar]
- 66. Ansell SM, Lesokhin AM, Borrello I, et al. PD‐1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372:311‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kwong YL, Chan TSY, Tan D, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T‐cell lymphoma failing l‐asparaginase. Blood. 2017;129:2437‐2442. [DOI] [PubMed] [Google Scholar]
- 68. Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol. 2016;34:2698‐2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nayak L, Iwamoto FM, LaCasce A, et al. PD‐1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood. 2017;129:3071‐3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem‐cell transplantation and brentuximab vedotin: a multicentre, multicohort, single‐arm phase 2 trial. Lancet Oncol. 2016;17:1283‐1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Roemer MGM, Redd RA, Cader FZ, et al. Major histocompatibility complex class II and programmed death ligand 1 expression predict outcome after programmed death 1 blockade in classic Hodgkin lymphoma. J Clin Oncol. 2018;36:942‐950. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during this study.


