Abstract
Preadmission administration of antibiotics to patients with suspected meningococcal infection has decreased the likelihood of obtaining an isolate and has stimulated development of rapid and reliable non-culture-based diagnostic methods. The sensitivity of the conventional test card latex agglutination test (TCLAT) for detection of capsular polysaccharide has been reported to be suboptimal. In the United Kingdom meningococcal DNA detection by PCR has become readily available and is now used as a first-line investigation. Recently, the performance of latex antigen detection has been markedly improved by ultrasound enhancement. Three tests for laboratory confirmation of meningococcal infection, (i) PCR assays, (ii) TCLAT, and (iii) ultrasound-enhanced latex agglutination test (USELAT), were compared in a retrospective study of 125 specimens (serum, plasma, and cerebrospinal fluid specimens) from 90 patients in whom meningococcal disease was suspected on clinical grounds. Samples were from patients with (i) culture-confirmed meningococcal disease, (ii) culture-negative but PCR-confirmed meningococcal disease, and (iii) clinically suspected but non-laboratory-confirmed meningococcal disease. USELAT was found to be nearly five times more sensitive than TCLAT. Serogroup characterization was obtained by both PCR and USELAT for 44 samples; all results were concordant and agreed with the serogroups determined for the isolates when the serogroups were available. For 12 samples negative by USELAT, the serogroup was determined by PCR; however, for 12 other specimens for which PCR had failed to indicate the serogroup, USELAT gave a result. USELAT is a rapid, low-cost method which can confirm a diagnosis, identify serogroups, and guide appropriate management of meningococcal disease contacts. A complementary non-culture-based confirmation strategy of USELAT for local use supported by a centralized PCR assay service for detection of meningococci would give the benefits of timely information and improved epidemiological data.
Growth of Neisseria meningitidis from a normally sterile site has long been the definitive test for the diagnosis of meningococcal disease, although microscopy of Gram-stained films of cerebrospinal fluid (CSF) can demonstrate the presence of diplococci and strongly supports the diagnosis. The recent trend in the United Kingdom to preadmission administration of antibiotics to patients with suspected cases of meningococcal disease coupled with a greater circumspection about obtaining CSF from patients in whom the clinical picture is typical has decreased the likelihood of culture confirmation (6). Rapid, non-culture-based methods for determination of the causative meningococcal serogroup have been developed to improve epidemiological information and to guide contact management by chemoprophylaxis and vaccination. PCR-based DNA detection (9, 21, 23, 24, 25, 26) represents an improvement in non-culture-based serogroup confirmation over that currently available through capsular polysaccharide detection by the conventional test card latex agglutination test (TCLAT).
The Public Health Laboratory Service (PHLS) Meningococcal Reference Unit (MRU) for England and Wales is screening more than 10,000 samples per annum using a sensitive and specific PCR assay (ctrA assay) to detect meningococcal DNA (20). The ctrA-reactive specimens are further tested by the siaD serogroup B and C PCR assays (5). The siaD assay is inherently less sensitive than the ctrA assay, with the result that only 65 to 70% of ctrA assay-positive samples provide a serogroup result (20).
Commercial TCLATs can be used by nonspecialist laboratories for the rapid detection of serogroup-specific meningococcal antigen in CSF or serum with minimal preprocessing and modest expenditure, but poor sensitivity has been reported (12). Recently, improved performance of antigen detection has been described by performing assays in an ultrasonic standing wave. Latex particles suspended in the wave are subjected to physical forces that promote formation of aggregates (8) by increasing particle-particle contact. Enhanced agglutination between antibody-coated microparticles in the presence of antigen occurs, giving substantial increases in sensitivity of antigen detection compared to that of TCLAT (14, 16, 17, 27). Application of the ultrasound-enhanced latex agglutination test (USELAT) for detection of capsular polysaccharide antigen from N. meningitidis serogroup A, B, C, Y, and W135 strains has demonstrated sensitivity enhancements with clinical samples without nonspecific reactivity (2, 18).
The aim of this study was to compare PCR and latex agglutination tests (LATs) for the non-culture-based diagnosis of meningococcal infection and also to determine whether USELAT testing of clinical samples could be a practical first-line investigation in general microbiology laboratories.
(This work has been presented in part at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 24 to 27 September 1998, and the 11th Pathogenic Neisseria Conference, Nice, France, November 1998.)
MATERIALS AND METHODS
Patients and samples.
One hundred twenty-five specimens from 90 patients were studied (Table 1). All patients had clinical features consistent with meningococcal infection (meningococcal meningitis and/or septicemia) as the most probable diagnosis. Samples had been submitted to the MRU specifically for testing by PCR assays for the detection of meningococci. Specimens were retrieved following storage at −80°C for examination by TCLAT and USELAT. Primary culture and other investigations for the detection of bacterial pathogens were performed at the laboratories that submitted the specimens. Apart from one patient in whom Streptococcus pneumoniae was subsequently identified as the causative organism, no etiology other than meningococci was definitively identified. Samples negative by the meningococcus-specific PCR assays and latex tests were not investigated further. The study samples were selected to include (i) PCR-positive specimens from patients from whom meningococci had been cultured from normally sterile sites, (ii) specimens which were positive by PCR but which were culture negative, (iii) specimens which were positive by culture but which were PCR negative, and (iv) PCR- and culture-negative samples.
TABLE 1.
Samples used in the study
| N. meningitidis culture result | No. of samples (no. of patients)
|
|
|---|---|---|
| Serum or plasma | CSFa | |
| Positive | 37b (21c) | 5 (5) |
| Negative | 76d (55e) | 7 (7) |
| Total | 113 (78) | 12 (12) |
CSF samples were from 12 patients, 9 of whom also had one or more serum or plasma samples tested.
Includes one sample that was positive for meningococcal serogroup Y by culture and three samples that were positive for meningococcal serogroup 29E by culture.
One patient each with culture-proven meningococcal serogroup Y and serogroup 29E infection.
One sample was culture positive for S. pneumoniae.
One patient had invasive S. pneumoniae infection.
Isolate confirmation and characterization.
Isolates of N. meningitidis are received at the PHLS MRU from nearly all culture-positive patients identified by clinical microbiology laboratories throughout England and Wales. Strains are confirmed to be meningococci and are characterized with a panel of antisera (1, 10, 13, 28) to define the serogroup, serotype, and serosubtype.
Molecular detection.
Clinical samples for DNA-based non-culture-based diagnosis of meningococcal infection are submitted to MRU and are stored at 4°C prior to testing. Template DNA is extracted from 100 μl of the clinical specimen (CSF, serum, or plasma) with a commercial kit (DNAzol: Gibco BRL, Paisley, Scotland) to produce 50 μl (final volume) in sterile water (sterile injectible), of which 2 μl is used in each PCR assay.
The ctrA and siaD PCR assays used are similar to those published previously (5, 20) but are modified for use in the automated TaqMan PCR system (Perkin-Elmer Applied Biosystems, Warrington, United Kingdom). The closed-tube format allows direct detection of the PCR-amplified product without additional processing (3) and minimizes the risk of contamination. The current ctrA TaqMan primers detect meningococcal DNA from the sialic acid-containing meningococcal serogroups, namely, B, C, Y, and W135, which covers 98 to 99% of invasive meningococcal strains in the United Kingdom, where serogroup A infection is rare.
Latex agglutination reagents.
Commercially available latex agglutination reagents (Wellcogen kit; Murex Diagnostics, Dartford, United Kingdom) for the detection of meningococcal polysaccharide were used in the TCLAT and USELAT formats. The N. meningitidis ACYW135 reagent detects four serogroups (serogroups A, C, Y, and W135). The N. meningitidis B-Escherichia coli K1 monoclonal antibody latex reagent exploits the cross-reactivity of E. coli K1 and N. meningitidis serogroup B polysaccharide and, as such, is not strictly specific.
Ultrasonic equipment.
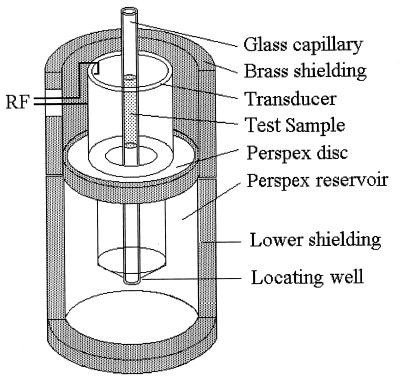
The ultrasound apparatus has been described in detail elsewhere (15, 16). Essentially, a tubular ultrasonic transducer (PCA4; Morgan Matroc, Wrexham, United Kingdom) with a fundamental thickness resonance frequency of 1.5 MHz was mounted as shown schematically in Fig. 1. The transducer was driven at 4.5 MHz by the 20 Vp-p (volts peak-peak) output of an RF amplifier (model 240L; ENI, Rochester, N.Y.). The amplifier input came from a Hewlett-Packard 33120A frequency synthesizer.
FIG. 1.
Section through the ultrasonic chamber showing the tubular transducer (dimensions: height, inside diameter, 20 mm; 31.5 mm; and wall thickness, 1.9 mm) and reservoir which was filled with distilled water up to a level about 1 mm from the transducer’s upper edge. The test sample is shown within the glass capillary, which is held on the transducer axis by a central hole in the perspex lid (not shown) and the lower locating well. The transducer was driven in radial resonance by connecting the output from an RF voltage generator to the electrodes of the inner and outer walls of the transducer.
Conventional agglutination and ultrasonic test procedure.
Specimens (serum, plasma, or CSF specimens) were prepared for processing, in accordance with Wellcogen kit instructions, which included centrifugation and subsequent filtration to remove protein (29) with 0.2 μm-pore-size Millex-GS (Sigma Chemical Co., Poole, United Kingdom) membrane filters when the sample volume permitted. Each patient specimen was tested with N. meningitidis B-E. coli K1 and N. meningitidis ACYW135 test latexes by using the appropriate negative controls supplied with the kits. The manufacturer’s instructions were followed for performance and interpretation of TCLAT.
In the USELATs, 25 μl of test sample was mixed on a nonadsorbing surface with 25 μl of diluted test or control latex suspension prediluted 1 in 8 in phosphate-buffered saline (pH 7.4). With a syringe, the reaction droplet was immediately drawn into a 2-mm (internal diameter) glass capillary (Fisher Scientific, Loughborough, United Kingdom) which was positioned on the transducer axis and sonicated for 60 s in the axial high-acoustic-pressure region of the sound field (15). On expulsion from the capillary onto a test card, the droplet was stirred four times to break up nonspecific aggregates formed as a result of ultrasonic concentration. Droplets were loaded into microslides (Camlab, Cambridge, United Kingdom) with a 200-μm-path-length cross section by capillarity and were examined for agglutination by microscopy (×10 objective). Samples were scored as positive when particle agglutination clearly exceeded any slight residual particle aggregation in control samples. The investigators who performed the LATs were unaware of PCR or culture results.
RESULTS
Detection of meningococci.
Table 2 demonstrates the benefit of non-culture-based detection by comparing the number of samples positive by the PCR screening assay (ctrA assay) and USELAT for culture-confirmed and culture-unconfirmed samples. The total numbers positive by the ctrA PCR assay and USELAT were 77 (62%) and 61 (49%), respectively, whereas 42 (34%) samples were positive by culture. Forty-five ctrA PCR assay-positive and 32 USELAT-positive samples were found among 83 samples from culture-negative patients.
TABLE 2.
Detection of N. meningitidis in samples by ctrA PCR assay and USELAT for patients with culture-confirmed and non-culture-confirmed cases of clinical meningococcal infection
| Culture result | No. of samples
|
||||
|---|---|---|---|---|---|
| PCR positive
|
PCR negative
|
Total | |||
| USELAT negative | USELAT positive | USELAT positive | USELAT negative | ||
| Positive | 5 | 27 | 2 | 8 | 42 |
| Negative | 16 | 29 | 3 | 35 | 83 |
| Total | 21 | 56 | 5 | 43 | 125 |
Thirty-five specimens from patients with clinically suspected cases of meningococcal infection were culture negative and negative or nonreactive by both PCR and USELAT (Table 2). Eight samples were from patients with meningococcal infections that were confirmed by culture but negative for meningococcal polysaccharide and DNA; three of the samples were from a single patient with culture-proven serogroup 29E meningococcal infection. Neither PCR assays nor USELAT identifies serogroup 29E.
Twenty-seven (64%) samples from patients with culture-proven cases of meningococcal infection were positive by both USELAT and PCR. There were no discrepancies in the serogroup results obtained. The value and complementarity of the two non-culture-based methods were demonstrated by the results for culture-negative specimens, for which either or both USELAT and the PCR assay were positive (Table 2). Within the culture-unconfirmed subset of samples, 29 samples were positive by both PCR and USELAT. In addition 16 PCR-positive samples were USELAT negative and 3 PCR-negative specimens were USELAT positive (Table 2).
Serogrouping of N. meningitidis infection by PCR and USELAT.
There were 56 siaD PCR-positive samples (32 serogroup B-positive samples and 24 serogroup C-positive samples). USELAT indicated the serogroup in 44 (79%) specimens for which the siaD PCR assay result was positive (23 N. meningitidis B-E. coli K1-positive samples and 21 ACYW135-positive samples). There was complete concordance between the USELAT and PCR serogroup identifications for 44 samples (Table 3). When considering all results, USELAT and PCR were in agreement for 99 (79.2%) of the 125 samples overall.
TABLE 3.
Comparison of MRU meningococcal PCR assays with USELAT
| PCR and result | No. of samples with the indicated result by the following assay
|
|||
|---|---|---|---|---|
| PCR |
N. meningitidis USELAT
|
|||
| B-E. coli KI positive | ACYW135 positive | USELAT negative | ||
| siaD positive for serotype B | 32 | 23 | 0 | 9 |
| siaD positive for serotype C | 24 | 0 | 21 | 3 |
| ctrA positive (siaD negative) | 21 | 7 | 5a | 9 |
| PCR negative (ctrA negative) | 48 | 1 | 4 | 43b |
Includes one sample positive for meningococcal serogroup Y by culture.
Includes three samples positive for meningococcal serogroup 29E by culture and one sample positive for S. pneumoniae by culture.
In total, 31 and 30 samples were characterized as meningococcal serogroup B and serogroup ACYW135, respectively, by USELAT (Table 3). Twelve specimens in which the serogroup was not determined by the siaD PCR assay were positive by the ctrA PCR assay when USELAT indicated the causative serogroup involved (5 ACYW135-positive specimens and 7 B-E. coli K1-positive specimens).
Four samples were ACYW135 positive and one was N. meningitidis B-E. coli K1 positive by USELAT when the ctrA PCR assay was nonreactive. Three of these PCR-negative samples were culture negative. One of the three PCR- and culture-negative samples was plasma from a patient in whom meningococcal infection had been confirmed by the ctrA PCR assay with CSF. The other two samples were from a single patient in whom there was no further laboratory evidence of meningococcal disease.
Twenty-one samples (nine infected with serogroup B, three infected with serogroup C, and nine infected with an undetermined serogroup) were PCR (ctrA and/or siaD PCR)-positive and USELAT negative, and five of these samples were from patients with culture-proven cases of meningococcal disease (Table 3).
Comparison of ultrasound-enhanced detection with the standard test card method.
Overall, 61 (49%) specimens were positive by USELAT, whereas 13 (10%) were positive by TCLAT. Table 4 shows that 84% (27 of 32) of the total culture-positive and PCR-positive samples (Table 2) were positive by USELAT, whereas 22% (7 of 32) were positive by the commercial test card procedure. No discrepant serogroup results were found by either latex agglutination method. There were no TCLAT-positive results among the PCR-negative samples, whereas USELAT was positive for five PCR-negative samples.
TABLE 4.
Detection of N. meningitidis by USELAT, TCLAT, ctrA PCR assay, and culture
| PCR result | Culture result | No. of samples | No. of samples USELAT positive | No. of samples TCLAT positive |
|---|---|---|---|---|
| Positive | Positive | 32 | 27 | 7 |
| Negative | 45 | 29 | 6 | |
| Negative | Positive | 10a | 2 | 0 |
| Negative | 38b | 3 | 0 | |
| Total tested by LAT | 125 | 61 | 13 |
Includes three samples taken from one patient from whom serogroup 29E meningococci were isolated.
Includes one serum sample from a patient with meningococcal meningitis confirmed by PCR (ctrA PCR assay positive) with CSF and includes one sample from a patient with invasive S. pneumoniae infection.
Sensitivity of meningococcal detection.
By using the clinical diagnosis of meningococcal disease as a standard, the relative sensitivities of culture, USELAT, TCLAT, the ctrA PCR assay, and the siaD PCR assay were 28% (26 of 90), 50% (45 of 90), 10% (9 of 90), 67% (60 of 90), and 48% (43 of 90), respectively. Results for 26 patients with culture-confirmed meningococcal disease and for whom the specimen from the site that was culture positive was available for PCR and LAT gave USELAT, TCLAT, ctrA PCR assay, and siaD PCR assay sensitivities of 76% (20 of 26), 19% (5 of 26), 88% (23 of 26), and 69% (18 of 26), respectively. There were no discrepancies in the serogroups identified in 15 patients who were positive by culture, the siaD PCR assay, and USELAT.
DISCUSSION
Rapid laboratory confirmation of meningococcal infection can help ensure the most appropriate patient care and contact management to prevent secondary cases of infection. Nonculture-based methods, specifically, PCR assays, have greatly improved the rate of confirmation of infection in the United Kingdom and have resulted in closer approximation of the number of patients notified by clinicians to the office of National Statistics (United Kingdom) on the basis of clinical diagnosis and the total number of laboratory-proven cases of infection (7, 20). Increased confidence in the available epidemiological information is especially important prior to the anticipated introduction of serogroup C conjugate meningococcal vaccines to the immunization schedule in the United Kingdom within the next 2 years (11) so that the effect of the new vaccine can be accurately monitored.
The comparison of USELAT and a PCR test for the detection of meningococci demonstrated that there was good concordance between the results of the two assays. Overall, the ctrA PCR screening assay yielded 13% more positive results than USELAT. It is probably more appropriate, however, to compare USELAT with the siaD PCR since by determining the serogroup they provide equivalent epidemiological information.
The siaD PCR assay detected 4% fewer positive results; moreover, the results for small numbers of specimens could be available more rapidly by USELAT than by the PCR. A single USELAT result would be possible within 10 min following boiling and filtration, which take about 20 min; each additional test would take another 8 to 10 min. This compares with about 2.5 h for a single PCR assay. PCR assays are, however, more efficient with large numbers of samples; results from 80 assays can be available within 5 h, which includes the time required for specimen processing.
When both USELAT and the siaD PCR assay were positive, there were no discrepancies in the meningococcal serogroups identified. USELAT provided serogroup characterization in instances in which the PCR assays could only confirm meningococcal infection and in five (4%) specimens identified meningococcal antigen when the ctrA PCR screening assay had been negative. The 12 specimens positive by USELAT (7 N. meningitidis B-E. coli K1-positive specimens and 5 ACYW135-positive specimens and negative by siaD PCR are balanced by the 12 USELAT-negative samples positive by the siaD PCR assay (9 serogroup B-infected specimens and three serogroup C-infected specimens).
In this study of 90 patients with clinical meningococcal disease, the most sensitive test for meningococcal detection was the ctrA PCR assay. USELAT was more sensitive than the siaD PCR assay for serogroup confirmation, and both assays were superior to standard LAT (with Murex reagents) and culture. The relative positions of the sensitivities of the USELAT, TCLAT, and the two PCR assays remained the same when the sensitivities were determined for the subset of samples from patients with culture-confirmed cases of infection. Previous studies of USELAT (2) and meningococcal PCR (5) estimate 100% specificity. A single sample from a patient with an S. pneumoniae infection and three samples from one patient infected with meningococcal serogroup 29E were nonreactive by both USELAT and PCR.
The likelihood of serogroup determination by USELAT or the siaD PCR will be affected by the amount of target present. PCR techniques detect unique N. meningitidis gene sequences present as single copies on each genome, giving a lower limit of detection (250 organisms/ml of fluid tested). In contrast, USELAT detects extracellular capsular polysaccharide, which is produced in excess and which blebs off the organism into the surrounding medium. LAT was performed with 25 μl of a 1-in-4-diluted plasma or serum sample, representing approximately 6 μl of the original specimen, while the 2 μl of the DNA extract tested by PCR was from 4 μl of plasma or serum from the original sample. The volume of the specimen from which target for the respective tests was obtained was therefore equivalent.
One of the five USELAT (ACYW135)- and ctrA PCR-positive samples was found to be a culture-proven serogroup Y infection not detected by the siaD PCR assay used. It is possible that any of the other four samples could have been from patients with serogroup Y or W135 infection, but it is most unlikely that any were from patients with serogroup A infections, since only two cases of infection with these globally important strains have been identified in the United Kingdom over the last 3 years. An alternative explanation is that the samples contained small numbers of genome copies that were detectable by the ctrA PCR assay but not by the siaD PCR assay because the currently available assays exhibit different sensitivities (20).
The sensitivities of the PCR assays may be improved by better DNA extraction and by redesigning the TaqMan assay to include probes for the less common serogroups that cause infection in England and Wales. The current TaqMan ctrA PCR assay detects serogroups B, C, W135, and Y. Serogroups A, X, 29E, and Z are not detected by the current TaqMan ctrA PCR assay but can be detected by a ctrA PCR and enzyme-linked immunosorbent assay (20). The siaD primers described previously (5) were redesigned for the TaqMan assay for increased sensitivity. Serogroup Y- and W135-specific (siaD) PCR assays have recently been developed in a PCR and enzyme-linked immunosorbent assay format (4) and will, in future, be used with specimens that are positive by the ctrA PCR assay but that are nonreactive by the serogroup B- or C-specific siaD PCR assay.
Because of developments in the molecular characterization of strains by the sequencing of alleles of housekeeping or outer membrane protein-encoding genes, such assays are now able to furnish more information than can be provided by any serologically based method (22). The facility of translating sequencing techniques into a non-culture-based diagnostic format is being explored. Data generated in this way could provide important additional information for the management of clusters of infections and outbreaks.
All the LAT investigations were initially performed 2 months after the PCR assays, demonstrating that polysaccharide can persist in stored specimens. In performing the tests independently at the two study sites (Cardiff and Manchester), the technique was found to be portable between laboratories. Undoubted advantages of USELAT over PCR are the lower costs in terms of labor, staff training, equipment, and reagents. A compact voltage-generating device with the essential characteristics of the test equipment described in Materials and Methods has recently been developed (Electro-Medical Supplies, Wantage, United Kingdom). The performance of the device is under evaluation at six hospital locations in the British Isles. If trials are successful a device could become commercially available for less than $4,000. The possibility of using the ultrasound equipment for other latex agglutination-based assays (14, 16) would make any investment even more cost-effective. To develop and maintain confidence on the part of both operators and end users as to the validity of the test results, it would be necessary to develop an external quality assurance scheme (perhaps comprising a panel of negative material and stringently quantified meningococcal polysaccharide samples weighted to include a high proportion of samples containing amounts close to detection thresholds).
Ultrasound assays with individual serogrouping latex reagents for serogroups A, C, Y, and W135 (currently commercially available from Sanofi and bioMerieux) are under evaluation to see if it is possible to provide specific information on the serogroup. Performance of the test with the serogroup-specific latex agglutination reagent after demonstration of reactivity with the pooled reagent would be efficient and cost-effective. Knowledge of the local serogroup incidence would dictate the sequence in which such specific assays were performed. A DNA-based detection strategy would require five separate PCR assays (meningococcal DNA detection, followed by detection of DNAs of serogroups B, C, Y, and W135), and the cost would be considerably greater than those of the LATs.
Although the increased overall sensitivity of the ctrA PCR assay over that of USELAT was clearly shown in this study, the latter would be an extremely useful method of case confirmation in those countries where rapid PCR diagnosis is unavailable. USELAT could notably improve the epidemiological data from areas of the world where routine culture is often impracticable.
USELAT could be used as a first-line investigation in local microbiology laboratories (19) for rapid non-culture-based diagnosis with relevant clinical samples collected as soon after admission as possible. Potentially helpful additional information can be obtained from PCR-based testing schemes, and this will increase over the next few years. When meningococcal PCR assays are available, as is currently the case at reference units in the United Kingdom and the Republic of Ireland, early specimens from all patients with suspected meningococcal infection should be sent for PCR, regardless of any other techniques that are available locally.
ACKNOWLEDGMENTS
The Meningitis Research Foundation, Bristol, United Kingdom, and the United Kingdom Biotechnology and Biological Sciences Research Council (BBSRC) Analytical Biotechnology Initiative have provided support for this study.
REFERENCES
- 1.Abdillahi H, Poolman J T. Neisseria meningitidis group B serosubtyping using monoclonal antibodies in whole cell ELISA. Microb Pathogen. 1988;4:27–32. doi: 10.1016/0882-4010(88)90045-9. [DOI] [PubMed] [Google Scholar]
- 2.Barnes R A, Jenkins P, Coakley W T. Preliminary clinical evaluation of meningococcal disease and bacterial meningitis by ultrasonic enhancement. Arch Dis Child. 1998;78:58–60. doi: 10.1136/adc.78.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassler H A, Flood S J A, Livak K J, Marmaro J, Knorr R, Batt C A. The use of a fluorogenic probe in a PCR-based assay for the detection of Listeria monocytogenes. Appl Environ Microbiol. 1995;61:3724–3728. doi: 10.1128/aem.61.10.3724-3728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borrow R, Claus H, Chaudhry U, Guiver M, Kaczmarski E B, Frosch M, Fox A J. siaD PCR ELISA for the confirmation and identification of serogroup Y and W135 meningococcal infections. FEMS Microbiol Lett. 1998;159:209–214. doi: 10.1111/j.1574-6968.1998.tb12862.x. [DOI] [PubMed] [Google Scholar]
- 5.Borrow R, Claus H, Guiver M, Smart L, Jones D M, Kaczmarski E B, Frosch M, Fox A J. Non-culture diagnosis and serogroup determination of meningococcal B and C infection by a sialyltransferase (siaD) PCR ELISA. Epidemiol Infect. 1997;118:111–117. doi: 10.1017/s0950268896007261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cartwright K, Reilly S, White D, Stuart J. Early treatment with parenteral penicillin in meningococcal disease. Br Med J. 1992;305:484. doi: 10.1136/bmj.305.6846.143. . (Letter.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Communicable Disease Surveillance Centre. Enhanced surveillance of meningococcal disease in five English regions: first quarter 1998. CDR Weekly. 1998;8:183. , 186. [PubMed] [Google Scholar]
- 8.Coakley W T. Ultrasonic separations in analytical biotechnology. Trends Biotechnol. 1997;15:506–511. doi: 10.1016/s0167-7799(97)01122-0. [DOI] [PubMed] [Google Scholar]
- 9.Davison E, Borrow R, Guiver M, Kaczmarski E B, Fox A J. The adaptation of the IS1106 PCR to a PCR ELISA format for the diagnosis of meningococcal infection. Serodiagn Immunother Infect Dis. 1996;8:51–56. [Google Scholar]
- 10.Eldridge J, Sutcliffe E M, Abbott J D. Serological grouping of meningococci and detection of antigen in cerebrospinal fluid by coagglutination. Med Lab Sci. 1978;35:63–66. [PubMed] [Google Scholar]
- 11.Fairley C K, Begg N, Borrow R, Fox A J, Jones D M, Cartwright K A V. Conjugate meningococcal serogroup A and C vaccine: reactogenicity and immunogenicity in UK infants. J Infect Dis. 1996;174:1360–1363. doi: 10.1093/infdis/174.6.1360. [DOI] [PubMed] [Google Scholar]
- 12.Finlay F O, Witherow H, Rudd P T. Latex agglutination testing in bacterial meningitis. Arch Dis Child. 1995;73:160–161. doi: 10.1136/adc.73.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frasch C E, Zollinger W D, Poolman J T. Serotype antigens of Neisseria meningitidis and a proposed scheme for designation of serotypes. Rev Infect Dis. 1985;7:504–509. doi: 10.1093/clinids/7.4.504. [DOI] [PubMed] [Google Scholar]
- 14.Grundy M A, Barnes R A, Coakley W T. Highly sensitive detection of fungal antigens by ultrasound-enhanced latex agglutination. J Med Vet Mycol. 1995;33:201–203. doi: 10.1080/02681219580000411. [DOI] [PubMed] [Google Scholar]
- 15.Grundy M A, Bolek W E, Coakley W T, Benes E. Rapid agglutination testing in an ultrasonic standing wave. J Immunol Methods. 1993;165:47–57. doi: 10.1016/0022-1759(93)90105-g. [DOI] [PubMed] [Google Scholar]
- 16.Grundy M A, Moore K, Coakley W T. Increased sensitivity of diagnostic latex agglutination tests in an ultrasonic standing wave field. J Immunol Methods. 1994;176:169–177. doi: 10.1016/0022-1759(94)90311-5. [DOI] [PubMed] [Google Scholar]
- 17.Gualano M P, Grundy M A, Coakley W T, Parry S H, Stickler D J. Ultrasound-enhanced latex agglutination for the detection of bacterial antigens in urine. Br J Biomed Sci. 1995;52:178–183. [PubMed] [Google Scholar]
- 18.Jenkins P, Barnes R A, Coakley W T. Detection of meningitis antigens in buffer and body fluids by ultrasound-enhanced particle agglutination. J Immunol Methods. 1997;205:191–200. doi: 10.1016/s0022-1759(97)00076-8. [DOI] [PubMed] [Google Scholar]
- 19.Kaczmarski E B, Cartwright K A V. Control of meningococcal disease—guidance for microbiologists. Comm Dis Rep Rev. 1995;5:R156. [PubMed] [Google Scholar]
- 20.Kaczmarski E B, Ragunathan P L, Marsh J, Gray S J, Guiver M. Creating a national service for the diagnosis of meningococcal disease by polymerase chain reaction. Comm Dis Public Health. 1998;1:54–56. [PubMed] [Google Scholar]
- 21.Kristiansen B, Ask E, Jenkins A, Fermer C, Radström P, Skold O. Rapid diagnosis of meningococcal meningitis by polymerase chain reaction. Lancet. 1991;337:1568–1579. doi: 10.1016/0140-6736(91)93262-8. [DOI] [PubMed] [Google Scholar]
- 22.Maiden M C J, Bygraves J A, Feil E, Morelli G, Russell J E, Urwin R, Zhang Q, Zhou J, Zurth K, Caugant D A, Feavers I M, Achtman M, Spratt B G. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci USA. 1998;95:3140–3145. doi: 10.1073/pnas.95.6.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLaughlin G L, Howe D K, Biggs D R, Smith A R, Ludwinski P, Fox B C, Tripathy D N, Frasch C E, Wenger J D, Casey R B. Amplification of rDNA loci to detect and type N. meningitidis and other eubacteria. Mol Cell Probes. 1993;7:7–17. doi: 10.1006/mcpr.1993.1002. [DOI] [PubMed] [Google Scholar]
- 24.Newcombe J, Cartwright K A V, Palmer W H, McFadden J. PCR of peripheral blood for diagnosis of meningococcal disease. J Clin Microbiol. 1996;34:1637–1640. doi: 10.1128/jcm.34.7.1637-1640.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ni H, Knight A I, Cartwright K A V, Palmer W H, McFadden J. Polymerase chain reaction for diagnosis of meningococcal meningitis. Lancet. 1992;340:1432–1434. doi: 10.1016/0140-6736(92)92622-m. [DOI] [PubMed] [Google Scholar]
- 26.Saunders N B, Zollinger W D, Rao V B. A rapid and sensitive PCR strategy employed for amplification and sequencing of porA from a single colony-forming unit of Neisseria meningitidis. Gene. 1993;137:153–162. doi: 10.1016/0378-1119(93)90001-j. [DOI] [PubMed] [Google Scholar]
- 27.Thomas N E, Coakley W T. Measurement of antigen concentration by an ultrasound-enhanced latex immunoagglutination assay. Ultrasound Med Biol. 1996;22:1277–1284. doi: 10.1016/s0301-5629(96)00148-2. [DOI] [PubMed] [Google Scholar]
- 28.Wedege E, Hoiby E A, Rosenqvist E, Froholm L O. Serotyping and serosubtyping of Neisseria meningitidis isolates by coagglutination, dot-blotting and ELISA. J Med Microbiol. 1990;31:195–201. doi: 10.1099/00222615-31-3-195. [DOI] [PubMed] [Google Scholar]
- 29.Weinberg G A, Storch G A. Preparation of urine samples for use in commercial latex agglutination tests for bacterial antigens. J Clin Microbiol. 1985;21:899–901. doi: 10.1128/jcm.21.6.899-901.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]