Abstract
Understanding the magnitude and causes of isotopic fractionation between organisms and their dietary resources is crucial for gaining knowledge on stable isotope ecology. However, little is known regarding the diet-tissue fractionation values of marine ciliates, which play a critical role in the reconstruction of microbial food webs. In the present study, we conducted experiments on two benthic (Pseudokeronopsis pararubra and Protocruzia labiata) and two pelagic (Strombidium sulcatum and Uronemella filificum) marine ciliates, where they were fed with isotopically constant foods (Chaetoceros calcitrans and Isochrysis galbana) under laboratory culture conditions to determine their carbon and nitrogen isotopic fractionation values (Δ13C and Δ15N). The stable isotope values (δ13C and δ15N) of ciliates for all experiments rapidly increased after the initial feeding, with half-lives ranging from 6.1 to 23.0h for δ13C and from 3.1 to 24.9h for δ15N. The Δ13C and Δ15N for all ciliates represented significantly positive enrichments, with overall mean fractionations of 0.6±0.2 and 1.2±0.4, respectively. Irrespective of the dietary type, both Δ13C and Δ15N were very similar for the same ciliate species. These results suggest that Δ13C and Δ15N for marine ciliates are similar to those found in common marine organisms with very little food-dependent variation. Overall, quantifying the specific isotopic fractionation of marine ciliates is expected to provide fundamental information on the trophic transfer of carbon, nitrogen, and energy flow through the microbial pathway in marine ecosystems.
Keywords: isotopic fractionation, Δ13C, Δ15N, protozoa, stable isotopes, turnover rates, feeding experiment
Introduction
Stable isotope ratios of carbon and nitrogen have been used to identify trophic pathways of organic matter and trophic relationships within animal communities, and the isotopic ratios of an organism’s tissues reflect those of its dietary sources (Fry and Sherr, 1984; Peterson and Fry, 1987; Michener and Schell, 1994; Layman et al., 2012). Typical enrichment of heavier isotopes in animal tissues occurs during the course of physiological metabolism, in the range of ≤1‰ for carbon (DeNiro and Epstein, 1978; Fry and Sherr, 1984) and 2–4‰ for nitrogen (Vander Zanden and Rasmussen, 2001; Post, 2002). The smaller fractionation of δ13C makes it useful for identifying the origins of dietary sources for organisms with distinctly different δ13C signatures among different organic matter sources, whereas δ15N is better suited for estimating their trophic levels (Peterson and Fry, 1987). However, estimating the contribution of dietary sources to the consumers’ production using stable isotopes can cause uncertainties owing to a variety of biotic and abiotic factors, there being too many sources of organic matter, isotopic discrimination between consumers and resources, and physiological conditions (Boecklen et al., 2011). Above all, estimates of trophic discrimination between consumers and resources (expressed by Δ13C for carbon and Δ15N for nitrogen) are considerably variable, which may result in various errors in the determination of resource contribution and trophic level (McCutchan et al., 2003). The application of actual trophic discrimination from stable isotope ratios will improve the accuracy of estimates of the contribution of dietary sources to the trophic level of consumers within diverse natural ecosystems (Post, 2002; McCutchan et al., 2003). Thus, understanding the magnitude and causes of isotopic discrimination between consumers and resources is crucial for understanding trophic ecology using stable isotope tracers.
Ciliates are one of the major functional groups in marine ecosystems (Pierce and Turner, 1992; Löder et al., 2011; Xu et al., 2018). They play important roles as intermediators in the pathways of material and energy flows from pico- and nanoplanktonic producers to higher trophic levels in marine ecosystems through the microbial food web (Montagnes et al., 2010; Zingel et al., 2019). Microzooplankton, including ciliates, is known to consume 60–75% of primary production and most of the bacterial secondary production in the oceans (Sherr and Sherr, 1994; Calbet and Landry, 2004). Several studies have focused on the importance of ciliates as a food source for mesozooplankton to establish the relevance of the trophic link in oceanic biogeochemical cycles on a global scale (Fessenden and Cowles, 1994; Calbet and Saiz, 2005). Given the magnitude of carbon flux from ciliates in marine food webs for carbon budget estimates, a comprehensive understanding of the phytoplankton-ciliate-mesozooplankton trophic pathways is required. However, the strength and variability of trophic linkages are difficult to quantify in complex natural systems, thus rendering the comparison of ecosystems unviable (Gutiérrez-Rodríguez et al., 2014). To determine the trophic pathways of ciliates within complex microbial food chains, tracking the dietary utilization of ciliates in food webs and demonstrating their nutritional contribution to higher trophic levels should be considered in a rigorous trophic analysis. Recently, stable isotope analysis has been applied to resolve trophic connections between phytoplankton, heterotrophic protists, and mesozooplankton, quantifying the energy flows of carbon and nitrogen through complex microbial food webs (Gutiérrez-Rodríguez et al., 2014; Décima et al., 2017). The isotopic discrimination values of specific organisms are necessary to determine the dietary sources that contribute to their nutrition and trophic levels in the food web (Post, 2002). Although isotopic values can provide quantitative and qualitative information regarding the transfer of material and energy in marine food webs, little is known about the diet-tissue fractionation values of marine ciliates that can be used as a critical value to reconstruct dietary sources and food web structures.
In the present study, we conducted feeding experiments on benthic and pelagic marine ciliates in laboratory cultures to determine the carbon and nitrogen isotopic fractionation values between diet and consumers. Such feeding experiments are indispensable for isolating and collecting sufficient amounts of ciliate samples for isotope analyses. During the experiments, the ciliates were fed with an isotopically constant food source over a period of time to precisely estimate isotopic fractionation. Furthermore, we also investigated whether the different types of diets for ciliates would influence their carbon and nitrogen isotopic fractionation through the isotope analysis of the ciliate populations raised on each type of diet.
Materials and Methods
Cultures and Feeding Experiments
Two benthic (Pseudokeronopsis pararubra and Protocruzia labiata) and two pelagic (Strombidium sulcatum and Uronemella filificum) ciliates were obtained from the marine ciliate resource bank of Korea, Gangneung-Wonju National University, Gangneung, Korea (Figure 1). All ciliate organisms were cultured in f/2 seawater media at 20°C under a 14:10 light:dark (L:D) cycle with cool-white fluorescent lamps (irradiance 60μmol photons m2 s−1). The stock cultures were acclimated in cell culture flasks containing 300ml fresh f/2-Si seawater media and wheat grains (Triticum aestivum L.) as food to achieve initial cell concentrations.
Figure 1.
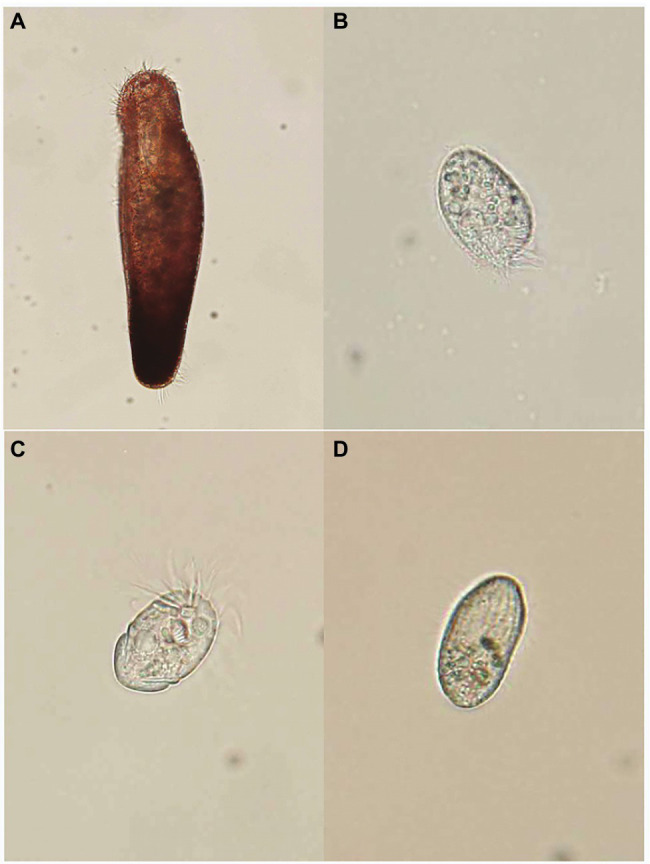
Specimens of four ciliates: (A) Pseudokeronopsis pararubra, (B) Protocruzia labiata, (C) Strombidium sulcatum, and (D) Uronemella filificum.
Feeding experiments lasted 8days in 500ml transparent polycarbonate Nalgene bottles, which were considered sufficient for reaching isotope equilibrium. The experimental conditions were maintained in all the bottles and checked every 12h (temperature, average 15.3±0.6°C; salinity, average 25.0±0.3; dissolved oxygen, average 5.3±0.4mgL−1). Living microalgae for feeding experiments were supplied every day (average concentration 10×103±480 cells mL−1) to the ciliate bottles. The diatom and haptophyte species, Chaetoceros calcitrans and Isochrysis galbana, respectively, which are dietary items of ciliates, were pure-cultured in rotating cell culture flasks in a low-temperature incubator until a uniform exponential growth phase. Controls of the four ciliates cultured with wheat grains were also maintained under the same experimental conditions. All treatment and control cultures were performed in duplicates and incubated for the experiments. The 3–5 ciliate samples from each bottle were collected at 0, 1, 4, 8, 12, 24, 48, 96, and 192h for analyses of cell abundance and carbon and nitrogen stable isotopes. The settled detrital materials were removed every day by careful pipetting to prevent the proliferation of bacterial populations and particle sedimentation.
At each collection, for the carbon and nitrogen stable isotope analyses, 20–40ml subsamples (presented from a preliminary experiment) from the treatment and control culture bottles were pre-filtered through 40μm Nitex sieves to remove any microalgae-derived particles, and then, the remaining ciliates were filtered onto pre-combusted (450°C, 4h) Whatman GF/F filters. Two diatom samples were obtained by filtering the culture water (approximately 10ml) through pre-combusted Whatman GF/F filters. All filter samples were oven-dried at 50°C for 48h, wrapped in aluminum foil, and stored at −40°C until isotope analysis.
Carbon and Nitrogen Stable Isotope Analyses
To measure the stable isotope ratios, the filter samples were sealed in a tin disk. The sealed samples were analyzed using an elemental analyzer (Vario MICRO Cube, Elementar, Hanau, Germany) coupled to a continuous-flow isotope-ratio mass spectrometer (CF-IRMS; Isoprime 100; Isoprime Ltd., Cheadle Hulme, United Kingdom). The samples were oxidized at a high temperature (1,150°C) in the elemental analyzer, and the resultant CO2 and N2 gases were transferred into the CF-IRMS using helium as a carrier gas. The stable isotope ratios are expressed as δ notation relative to Pee Dee Belemnite for carbon and atmospheric air for nitrogen, using the equation: δ (‰)=[(Rsample/Rstandard)−1]×103, where R is the 13C/12C or 15N/14N ratio. The international standard reference materials, sucrose (ANU C12H22O11, δ13C=−10.47±0.13‰, NIST, Gaithersburg, MD, United States), and ammonium sulfate [(NH4)2SO4, δ15N=−1.8±0.1‰, NIST] were used for calibrating carbon and nitrogen, respectively, after analyzing every 10 unknown samples. The analytical precisions, based on repeated analyses of acetanilide (Thermo Scientific) as an internal standard, were within 0.07‰ for δ13C and 0.11‰ for δ15N.
Carbon and Nitrogen Isotope Fractionations and Turnover Times
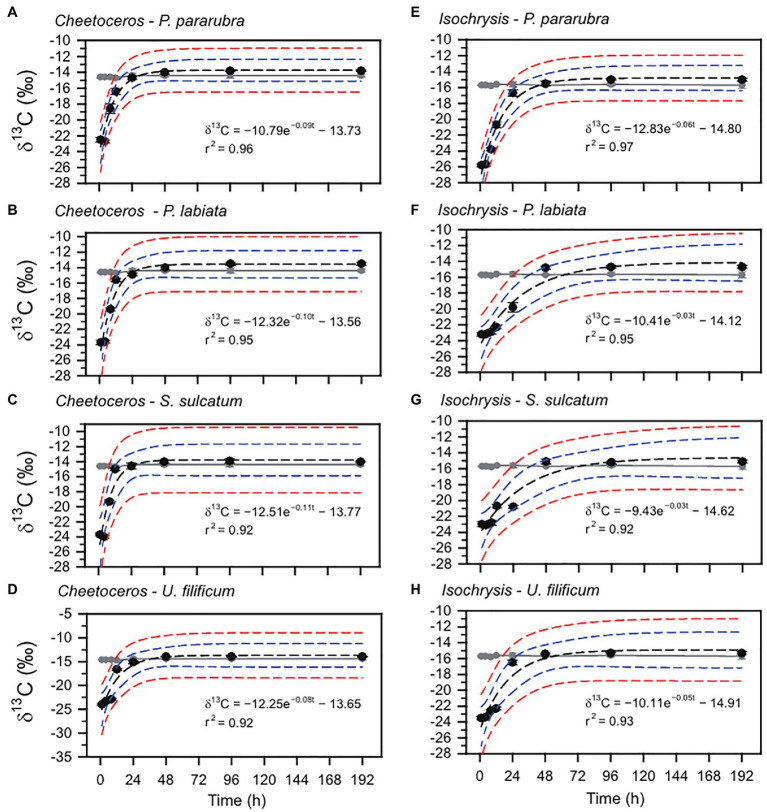
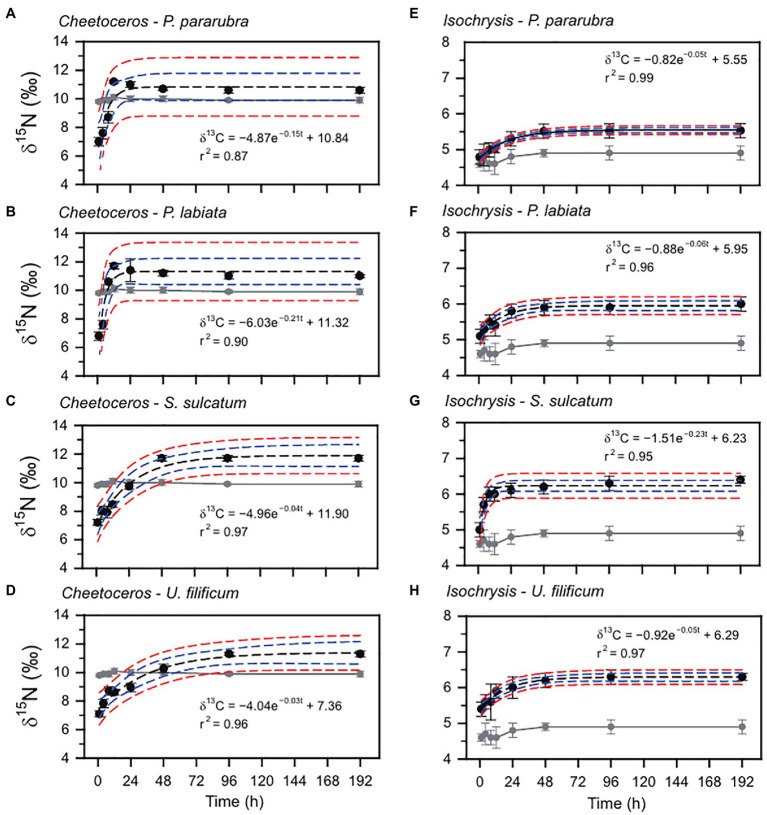
The trophic enrichment factors (TEFs, Δ) were calculated using the equation (Fry, 2006): Δ=δc−δf, where δc and δf refer to the carbon and nitrogen isotope ratios of the consumer species and food source at isotopic equilibrium, respectively. The equilibrated times were estimated for each feeding experiment by fitting the exponential decay model to the time of the asymptotic isotope values of consumers on the food source. To compare the variability in isotope fractionation between consumers and diets and among consumers, the standard deviations from the isotope mean values after equilibration were calculated.
To estimate the turnover time of carbon and nitrogen in the algal species, C. calcitrans and I. galbana, we fitted the isotope data to an exponential rise to a maximum equation, y=a+bect, using SigmaPlot for Windows (version 14.0; Bosley et al., 2002). Here, y represents the δ13C or δ15N values of the two diets at the time, a and b are the regression coefficients (parameters) that provide the best fit between the equation and the data, c is the turnover rate of carbon or nitrogen in the two diets, and t is the time (h) of the diet switch. We calculated the half-life of carbon and nitrogen in the tissue using the equation half-life=ln(0.5)/c (Hobson and Clark, 1992).
Statistical Analysis
All data were examined for normality using the Shapiro–Wilk test and homogeneity of variance with the Levene’s test prior to statistical analysis. Significant differences in the δ13C and δ15N values of microalgal diets and ciliates among culturing times during the feeding experiments, and the Δ13C and Δ15N values among culturing ciliates were analyzed using a one-way ANOVA. Subsequently, a Tukey’s multiple comparison post-hoc test was used to evaluate differences among variables. The significance level among treatments was α=0.05. All statistical analyses were performed using IBM SPSS Statistics software (version 23.0, IBM, Armonk, NJ, United States).
Results
Carbon and Nitrogen Stable Isotope Ratios and Turnover Times
The isotopic ratios of the two dietary microalgae (C. calcitrans and I. galbana) remained constant throughout the culture period (Table 1). There were no significant differences in the δ13C and δ15N values of both C. calcitrans and I. galbana at various sampling times, showing overall mean values of −14.5±0.3‰ and 9.9±0.2‰ (ANOVA, F8,30=1.04, p=0.435 for δ13C and F8,30=0.89, p=0.539 for δ15N) and−15.7±0.2‰ and 4.7±0.2‰ (ANOVA, F8,28=0.33, p=0.947 for δ13C and F8,28=1.39, p=0.260 for δ15N), respectively.
Table 1.
Mean δ13C and δ15N values of food sources (Chaetoceros calcitrans and Isochrysis galbana) for marine ciliates during the entire experiment.
| Time(h) | Chaetoceros calcitrans | Isochrysis galbana | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| δ13C | δ15N | δ13C | δ15N | |||||||
| n | Mean | SD | Mean | SD | n | Mean | SD | Mean | SD | |
| 0 | 5 | −14.3 | 0.3 | 9.9 | 0.2 | 5 | −15.6 | 0.2 | 4.6 | 0.2 |
| 1 | 3 | −14.6 | 0.1 | 9.8 | 0.1 | 3 | −15.7 | 0.1 | 4.6 | 0.1 |
| 4 | 3 | −14.6 | 0.2 | 9.9 | 0.1 | 3 | −15.7 | 0.3 | 4.7 | 0.3 |
| 8 | 3 | −14.6 | 0.2 | 9.9 | 0.2 | 3 | −15.8 | 0.1 | 4.6 | 0.2 |
| 12 | 4 | −14.7 | 0.2 | 10.1 | 0.2 | 3 | −15.6 | 0.1 | 4.6 | 0.3 |
| 24 | 4 | −14.4 | 0.3 | 10.0 | 0.2 | 3 | −15.6 | 0.3 | 4.8 | 0.2 |
| 48 | 3 | −14.4 | 0.2 | 10.0 | 0.2 | 3 | −15.7 | 0.1 | 4.9 | 0.1 |
| 96 | 3 | −14.4 | 0.3 | 9.9 | 0.1 | 3 | −15.6 | 0.2 | 4.9 | 0.2 |
| 192 | 3 | −14.4 | 0.2 | 9.9 | 0.2 | 3 | −15.7 | 0.4 | 4.9 | 0.2 |
| Overall | −14.5 | 0.3 | 9.9 | 0.2 | −15.7 | 0.2 | 4.7 | 0.2 | ||
| F-value | 1.04 | 0.89 | 0.33 | 1.39 | ||||||
| Significance (p) | 0.435 | 0.539 | 0.947 | 0.260 | ||||||
One-way ANOVA of δ13C and δ15N values among time factors for each food source. Data represent means±1 SD. Significance at p<0.05.
The changes in the ciliate δ13C and δ15N values obtained from the feeding experiments are summarized in Table 2. The initial mean δ13C and δ15N values of the four ciliates were significantly different (ANOVA, F3,31=41.95, p<0.001 and F3,31=33.07, p<0.001, respectively), especially as shown by the differences between benthic (−26.2±0.2‰ and 6.2±0.2‰) and pelagic (−25.5±0.2‰ and 6.7±0.1‰) species. For all experiments, the δ13C and δ15N values of ciliates rapidly increased after the first feeding (Tukey post-hoc test, p<0.01 for all cases), which remained constant and in equilibrium with their dietary items over the next 96h (Tukey post-hoc test, p>0.05 for all cases; Figures 2, 3). Half-lives of ciliates estimated by turnover rates varied considerably, ranging from 6.1h (S. sulcatum fed with I. galbana) to 23.0h (S. sulcatum fed with C. calcitrans) for δ13C and from 3.1h (S. sulcatum fed with I. galbana) to 24.9h (U. filificum fed with C. calcitrans) for δ15N (Table 3). At equilibrium times of 96h and 192h, the differences in δ13C and δ15N values for both C. calcitrans (ANOVA, F3,23=11.41, p<0.001 and F3,31=51.84, p<0.001, respectively) and I. galbana (ANOVA, F3,23=14.50, p<0.001 and F3,31=33.07, p<0.001, respectively) were significant among the ciliates. For both the C. calcitrans and I. galbana experiments, the asymptotic δ13C and δ15N values were the highest for P. labiata (−13.5±0.2‰ and−14.7±0.2‰) and S. sulcatum (11.7±0.2‰ and 6.4±0.1‰) and the lowest for U. filificum (−14.0±0.2‰ and−15.3±0.1‰) and P. pararubra (10.6±0.2‰ and 5.5±0.1‰), respectively.
Table 2.
Mean δ13C and δ15N values (‰) of marine ciliates (Pseudokeronopsis pararubra, Protocruzia labiata, Strombidium sulcatum, and Uronemella filificum) fed with Chaetoceros calcitrans and Isochrysis galbana during the duration of the entire experiment.
| Time (h) | Pseudokeronopsis pararubra | Protocruzia labiata | Strombidium sulcatum | Uronemella filificum | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| δ13C | δ15N | δ13C | δ15N | δ13C | δ15N | δ13C | δ15N | |||||||||||||
| Chaetoceros calcitrans | ||||||||||||||||||||
| n | Mean | SD | Mean | SD | n | Mean | SD | Mean | SD | n | Mean | SD | Mean | SD | n | Mean | SD | Mean | SD | |
| 0 | 4 | −26.3a | 0.2 | 6.3a | 0.2 | 4 | −26.2a | 0.2 | 6.1a | 0.2 | 4 | 25.6a | 0.2 | 6.7a | 0.1 | 4 | −25.5a | 0.2 | 6.5a | 0.2 |
| 1 | 5 | −22.5b | 0.3 | 7.0b | 0.3 | 5 | −23.7b | 0.3 | 6.8b | 0.3 | 5 | −23.7b | 0.2 | 7.2b | 0.2 | 5 | −24.0b | 0.2 | 7.1b | 0.2 |
| 4 | 5 | −22.7b | 0.2 | 7.6b | 0.4 | 5 | −23.6b | 0.2 | 7.6c | 0.3 | 5 | −24.0b | 0.2 | 8.0c | 0.3 | 5 | −23.3c | 0.3 | 7.8c | 0.3 |
| 8 | 5 | −18.6c | 0.6 | 8.7c | 0.4 | 5 | −19.4c | 0.2 | 10.6d | 0.2 | 5 | −19.3c | 0.2 | 7.9c | 0.4 | 5 | −22.9d | 0.2 | 8.7d | 0.3 |
| 12 | 5 | −16.4d | 0.4 | 11.2e | 0.1 | 5 | −15.6d | 0.2 | 11.7f | 0.2 | 5 | −15.0d | 0.2 | 8.5d | 0.2 | 5 | −16.6e | 0.2 | 8.6d | 0.2 |
| 24 | 5 | −14.7e | 0.2 | 11.0e | 0.2 | 5 | −14.9e | 0.2 | 11.4ef | 0.8 | 5 | −14.6d | 0.4 | 9.7e | 0.2 | 5 | −15.1f | 0.3 | 8.9e | 0.3 |
| 48 | 3 | −14.0f | 0.2 | 10.7d | 0.1 | 3 | −14.0f | 0.3 | 11.2e | 0.2 | 3 | −14.0e | 0.2 | 11.7f | 0.2 | 3 | −13.9g | 0.1 | 10.3f | 0.3 |
| 96 | 3 | −13.8f | 0.1 | 10.6d | 0.2 | 3 | −13.5g | 0.2 | 11.0e | 0.2 | 3 | −13.9e | 0.3 | 11.7f | 0.2 | 3 | −13.9g | 0.1 | 11.3g | 0.1 |
| 192 | 3 | −13.8f | 0.1 | 10.6d | 0.2 | 3 | −13.5g | 0.2 | 11.0e | 0.1 | 3 | −14.0e | 0.1 | 11.7f | 0.2 | 3 | −13.9g | 0.1 | 11.3g | 0.2 |
| Isochrysis galbana | ||||||||||||||||||||
| 0 | 4 | −26.2a | 0.2 | 6.4d | 0.2 | 4 | −26.2a | 0.2 | 6.0c | 0.1 | 4 | −25.5a | 0.1 | 6.8e | 0.1 | 4 | −25.4a | 0.2 | 6.6e | 0.2 |
| 1 | 5 | −25.8b | 0.2 | 4.8a | 0.2 | 5 | −23.2b | 0.2 | 5.1a | 0.2 | 5 | −23.0b | 0.3 | 5.0a | 0.2 | 5 | −23.5b | 0.2 | 5.4a | 0.2 |
| 4 | 5 | −25.7b | 0.2 | 4.8a | 0.3 | 5 | −23.2b | 0.2 | 5.3ab | 0.2 | 5 | −23.1b | 0.3 | 5.7b | 0.2 | 5 | −23.4b | 0.2 | 5.6a | 0.2 |
| 8 | 5 | −23.8c | 0.2 | 5.0ab | 0.2 | 5 | −22.9b | 0.3 | 5.5 b | 0.2 | 5 | −22.8b | 0.3 | 6.0bc | 0.2 | 5 | −22.6c | 0.2 | 5.6ab | 0.5 |
| 12 | 5 | −20.7d | 0.4 | 5.0ab | 0.1 | 5 | −22.3c | 0.2 | 5.4b | 0.3 | 5 | −20.7c | 0.2 | 6.0bc | 0.2 | 5 | −22.3c | 0.2 | 5.9b | 0.2 |
| 24 | 5 | −16.7e | 0.4 | 5.3bc | 0.2 | 5 | −19.8d | 0.6 | 5.8c | 0.2 | 5 | −20.8c | 0.2 | 6.1bc | 0.2 | 5 | −16.5d | 0.3 | 6.0b | 0.3 |
| 48 | 3 | −15.5f | 0.3 | 5.5c | 0.2 | 3 | −14.8e | 0.2 | 5.9c | 0.2 | 3 | −15.1d | 0.3 | 6.2c | 0.2 | 3 | −15.4e | 0.1 | 6.2c | 0.2 |
| 96 | 3 | −15.0g | 0.1 | 5.5c | 0.2 | 3 | −14.7e | 0.2 | 5.9c | 0.2 | 3 | −15.2d | 0.2 | 6.3c | 0.2 | 3 | −15.3e | 0.1 | 6.3c | 0.2 |
| 192 | 3 | −15.0g | 0.2 | 5.5c | 0.2 | 3 | −14.7e | 0.2 | 6.0c | 0.2 | 3 | −15.1d | 0.2 | 6.4cd | 0.1 | 3 | −15.3e | 0.2 | 6.3cd | 0.1 |
Data represent means±1 SD. Means followed by the same superscript letter in each row are not significantly different (p<0.01, Tukey’s post-hoc test).
Figure 2.
Carbon stable isotope variation and exponential model for P. pararubra, Protocruzia labiate, Strombidium sulcatum, and Uronemella filificum fed with Chaetoceros calcitrans (Chaeto) and Isochrysis galbana (Isochrysis). Black and gray circles are the mean of carbon stable isotope values (δ13C) for ciliates and foods, respectively. Vertical lines are ± SD. Black and blue dashed lines indicate the fitting curve and 95% confidence, respectively.
Figure 3.
Nitrogen stable isotope variation and exponential model for P. pararubra, Protocruzia labiate, Strombidium sulcatum, and Uronemella filificum fed with Chaetoceros calcitrans (Chaeto) and Isochrysis galbana (Isochrysis). Black and gray circles are the mean of nitrogen stable isotope values (δ15N) for ciliates and foods, respectively. Vertical lines are ± SD. Black and blue dashed lines indicate fitting curve and 95% confidence, respectively.
Table 3.
Turnover rate and half-life (h) of carbon and nitrogen elements in marine ciliates (Pseudokeronopsis pararubra, Protocruzia labiata, Strombidium sulcatum, and Uronemella filificum) fed with Chaetoceros calcitrans and Isochrysis galbana calculated using the exponential equation (y=a+bect) and regressions of carbon and nitrogen stable isotope data vs. time since diet switch.
| Isotope | Diet | Species name | Turnover rate | Half-life (h) |
|---|---|---|---|---|
| δ13C | Chaetoceros calcitrans | Pseudokeronopsis pararubra | −0.09 | 7.4 |
| Protocruzia labiata | −0.10 | 6.9 | ||
| Strombidium sulcatum | −0.11 | 6.1 | ||
| Uronemella filificum | −0.08 | 9.2 | ||
| Isochrysis galbana | Pseudokeronopsis pararubra | −0.06 | 11.3 | |
| Protocruzia labiata | −0.03 | 22.7 | ||
| Strombidium sulcatum | −0.03 | 23.0 | ||
| Uronemella filificum | −0.05 | 14.6 | ||
| δ15N | Chaetoceros calcitrans | Pseudokeronopsis pararubra | −0.15 | 4.6 |
| Protocruzia labiata | −0.21 | 3.4 | ||
| Strombidium sulcatum | −0.04 | 18.2 | ||
| Uronemella filificum | −0.03 | 24.9 | ||
| Isochrysis galbana | Pseudokeronopsis pararubra | −0.05 | 14.7 | |
| Protocruzia labiata | −0.06 | 11.4 | ||
| Strombidium sulcatum | −0.23 | 3.1 | ||
| Uronemella filificum | −0.05 | 13.3 |
Carbon and Nitrogen Stable Isotope Fractionations
The δ13C and δ15N fractionations (Δ13C and Δ15N) for all experiments were significantly positive enrichments, with overall mean fractionations of all ciliates being 0.6±0.2 and 1.2±0.4, respectively (Table 4). There were significant differences in the Δ13C and Δ15N calculated at the initial and equilibrium (96–192h) isotope values among the ciliates (ANOVA, F3,47=26.28, p<0.001 and F3,47=78.67, p<0.001, respectively), which were relatively small. The Δ13C of benthic ciliates (0.64, P. pararubra fed with I. galbana to 1.00, P. labiata fed with C. calcitrans) was higher than that of pelagic ciliates (0.40, S. sulcatum fed with I. galbana to 0.57, S. sulcatum fed C. calcitrans), whereas the Δ15N of benthic ciliates (0.68, P. pararubra fed with C. calcitrans to 1.18, P. labiata fed with I. galbana) was relatively lower than that of pelagic ciliates (1.38, U. filificum fed C. calcitrans to 1.73, S. sulcatum fed C. calcitrans). Regardless of the dietary type, both the carbon and nitrogen isotopic fractionations of all ciliates were very similar for the same species.
Table 4.
Trophic enrichment factors (TEFs, Δ13C, and Δ15N) of marine ciliates (Pseudokeronopsis pararubra, Protocruzia labiata, Strombidium sulcatum, and Uronemella filificum) fed with Chaetoceros calcitrans and Isochrysis galbana calculated from the equilibrium equation (Fry, 2006).
| Species name | Final value at equilibrium | TEFs | |||
|---|---|---|---|---|---|
| n | Mean | SD | |||
| δ13C | Chaetoceros calcitrans (food) | 31 | −14.5 | 0.3 | |
| Pseudokeronopsis pararubra | 6 | −13.8 | 0.1 | 0.69 | |
| Protocruzia labiata | 6 | −13.5 | 0.2 | 1.00 | |
| Strombidium sulcatum | 6 | −13.9 | 0.1 | 0.57 | |
| Uronemella filificum | 6 | −14.0 | 0.2 | 0.51 | |
| Isochrysis galbana (food) | 29 | −15.7 | 0.2 | ||
| Pseudokeronopsis pararubra | 6 | −15.0 | 0.2 | 0.64 | |
| Protocruzia labiata | 6 | −14.7 | 0.2 | 0.95 | |
| Strombidium sulcatum | 6 | −15.2 | 0.2 | 0.51 | |
| Uronemella filificum | 6 | −15.3 | 0.1 | 0.40 | |
| δ15N | Chaetoceros calcitrans (food) | 31 | 9.9 | 0.2 | |
| Pseudokeronopsis pararubra | 6 | 10.6 | 0.2 | 0.68 | |
| Protocruzia labiata | 6 | 11.0 | 0.1 | 1.06 | |
| Strombidium sulcatum | 6 | 11.3 | 0.1 | 1.38 | |
| Uronemella filificum | 6 | 11.7 | 0.2 | 1.73 | |
| Isochrysis galbana (food) | 29 | 4.7 | 0.2 | ||
| Pseudokeronopsis pararubra | 6 | 5.5 | 0.1 | 0.76 | |
| Protocruzia labiata | 6 | 5.9 | 0.1 | 1.18 | |
| Strombidium sulcatum | 6 | 6.3 | 0.1 | 1.59 | |
| Uronemella filificum | 6 | 6.3 | 0.1 | 1.55 | |
Discussion
In general, the stable isotope approach is a well-known key tool for understanding the trophic relationship between an organism and its diet. In addition, it is used to trace the pathways of organic matter within the food web, based on the common assumption of a stepwise trophic enrichment of δ13C by ≤1‰ (DeNiro and Epstein, 1978; Fry and Sherr, 1984) and δ15N by 2–4‰ (Vander Zanden and Rasmussen, 2001; Post, 2002). However, these common enrichment factors may not always apply to all individual species, because of differences in physiological status, major biochemical components (i.e., lipids, proteins, and carbohydrates), and assimilated diet during metabolic processes (DeNiro and Epstein, 1978; Focken and Becker, 1998). Furthermore, food type and quality can influence the trophic enrichment factor and isotopic turnover rates of consumer species (Remy et al., 2017). Our results showed that despite the significant differences in Δ13C and Δ15N, benthic and pelagic ciliates feeding on different microalgae had similar patterns of isotopic fractionation (i.e., TEF and isotopic turnover rate) under controlled laboratory conditions to those previously reported for other aquatic organisms (McCutchan et al., 2003; Dubois et al., 2007). Overall, the present study provides experimental quantitative data on species-specific TEFs and dietary source variations in the trophic fractionation process.
Ecological information on the influence of resource parameters on TEFs and fractionation processes of carbon and nitrogen stable isotopes in marine ciliates appears to be very uncommon compared to other aquatic organisms. In the present study, the Δ13C (0.4 to 1.0) and Δ15N (0.7 to 1.7) ciliates were found to be slightly enriched relative to their diets (both C. calcitrans and I. galbana), which was consistent with previously reported trophic fractionation ranges of aquatic consumers (Vander Zanden and Rasmussen, 2001; McCutchan et al., 2003). Similarly, previous laboratory studies on consumer TEFs have reported a general pattern showing little change in δ13C and relatively high enrichment in δ15N between prey and predators (Fry and Sherr, 1984; Minagawa and Wada, 1984). For both carbon and nitrogen, Δ13C and Δ15N were generally positive in most studies (McCutchan et al., 2003; Caut et al., 2009). However, several studies have shown that there are considerable variations in carbon and nitrogen TEFs among species due to species-specific mechanisms, the respective feeding ecology, and the associated environmental conditions (Lesage et al., 2002; Yokoyama et al., 2005; Heethoff and Scheu, 2016; Jenkins et al., 2018). Our study showed significant differences in the TEFs between benthic and pelagic ciliates, as reported by some studies on natural ecosystems, which reported that there was a clear discrimination in δ13C signatures between these two groups because of the difference in carbon fixation by benthic and pelagic primary producers (Fry and Sherr, 1984; France, 1995; Kang et al., 2015). Similarly, the TEFs of the marine amphipods Ampithoe valida and Parhyale hawaiensis, fed on fresh and detrital algae in laboratory experiments, showed considerable variation per species, ranging from −1.5 to −0.4 and−1.3 to −0.3 for Δ13C, and from −0.7 to −0.1 and 2.2 to 2.7 for Δ15N, respectively (Macko et al., 1982). Significant differences in Δ13C and Δ15N between herbivores (−0.4±1.1‰ and 2.5±2.5‰, respectively) and carnivores (0.9±1.0‰ and 3.2±0.4‰, respectively) were observed in laboratory and field experiments, owing to the effect of assimilate and metabolic factors by feeding type (Vander Zanden and Rasmussen, 2001). Water temperature may affect turnover rates and TEFs of carbon and nitrogen in juvenile winter flounder, which is related to differences in physiological processes (Bosley et al., 2002). Overall, despite the species-specific fractionation factors between the ciliates, their Δ13C and Δ15N did not vary with differences in species and diets under identical experimental conditions and were within the previously reported ranges.
The Δ13C and Δ15N values of animals are generally known to be influenced by different diets (McCutchan et al., 2003). Above all, herbivorous species showed highly variable Δ15N compared to carnivorous species based on laboratory and field estimations (Vander Zanden and Rasmussen, 2001). This tendency may be related to different food qualities, in which the protein content of different diets can affect the TEFs for consumers (McCutchan et al., 2003). Specifically, Δ15N may differ with the C/N ratios of diets, resulting from the degree of palatability, the consequent assimilation efficiency, and the physiological process of internal element recycling (Adams and Sterner, 2000; Remy et al., 2017). However, several studies have reported that Δ13C may not vary with the type and quality of diets, in contrast to the food-dependent variation in TEFs (Vanderklift and Ponsard, 2003; Mancinelli, 2012). Our study also showed no significant differences in either Δ13C or Δ15N between the two groups of ciliates fed with two different microalgal species. However, a significant difference was observed in the C/N ratios between the two diets in a laboratory experiment (unpublished data; Student’s t-test, p<0.05; 6.2±0.4 for C. calcitrans and 5.6±0.3 for I. galbana). Collectively, these results suggest that the differences in taxonomic class and quality of the two food sources for ciliates as herbivores may not result in highly variable TEFs.
Knowledge of the isotopic turnover rates of consumer tissues over time is fundamental to understanding dietary changes and quantifying the relative importance of dietary items in aquatic ecosystems (Tieszen et al., 1983; Bosley et al., 2002). As the dietary items of organisms in natural ecosystems change over time, it is necessary to retain information on the amount of time required for the organism’s isotopic ratio to equilibrate with the isotopic ratio of the last ingested food. Such information obtained through controlled laboratory experiments will improve the ecological applications of stable isotope tracers to infer the feeding strategy or history of animals (Gannes et al., 1997; Herzka et al., 2001). In the present study, the turnover time for both benthic and pelagic ciliates to equilibrate with the isotopic ratios of the two microalgae as their dietary items was 96h. Despite the substantial variability in half-lives among ciliates (6.1–23.0h for δ13C and 3.1–24.9h for δ15N), the turnover periods were relatively very fast in comparison with other animals, showing a wide range from several days to 1year (Bosley, 1998). Given that the turnover rate of the amphipod Gammarus for δ13C (half-life 12.5 d and 51.6 d fed with animal and litter, respectively) was fast because of the rapid assimilation of food sources (Remy et al., 2017), our results may be unique to the physiological responses of ciliates. Several studies have demonstrated that the turnover rates of organisms are very specific to the taxon or species level (Fry and Arnold, 1982; Tieszen et al., 1983; Yokoyama et al., 2005). Hobson and Clark (1992) found that the turnover rates of Japanese quail may vary according to the life stage and the particular tissue and body compartment, owing to differences in the level of metabolic activity.
The turnover rate of an organism can be significantly influenced by the speed of growth, likely reflecting the rapid shift of the isotopic ratios of consumer tissues to the existing diet (Bosley et al., 2002). By comparing rapidly and slowly growing individuals, Fry and Arnold (1982) demonstrated that turnover rates of post-larval brown shrimp can be directly related to growth. In fact, the rapid change in the carbon and nitrogen isotopic ratios of the four ciliates may be closely associated with their fast growth and metabolism; similarly, high growth rates are observed in microalgal species (Banse, 1982). Therefore, our results suggest that the rapid assimilation of microalgae, together with the exponential growth of ciliates, may contribute to their fast turnover rates.
Conclusion
The results of this study, involving controlled feeding experiments with microalgal diets, indicate that the TEFs of carbon and nitrogen for marine ciliates are similar to those found for common marine organisms with very little food-dependent variation. Although stable isotope analysis is a useful tool for assessing the trophic role of an organism in food webs, isotopic fractionation is generally species-specific in animals, and information on the TEFs of carbon and nitrogen for marine ciliates is still lacking. In this respect, the knowledge of species-specific TEFs needs to be based on precise estimation of the trophic level of an organism and the contribution of potential food sources to nutrition, by mixing models. Furthermore, considering the trophic importance of marine ciliates with respect to carbon flow, our study, which quantified their specific isotopic fractionation, validates the importance of stable isotope ecology in marine microbial food webs. Overall, these results are expected to provide fundamental information on the trophic transfer of carbon, nitrogen, and energy flow through microbial pathways in marine ecosystems.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material; further inquiries can be directed to the corresponding author.
Author Contributions
JYP and HJP designed the experiments and wrote the main manuscript text. J-HJ and HGP provided all the ciliate and microalgae samples. JHK performed the statistical analyses. C-KK revised the manuscript. All authors analyzed the data and reviewed the manuscript.
Funding
This research was supported by the National Research Foundation of Korea (NRF-2018R1C1B6004501), Ministry of Science and ICT, and Long-term change of structure and function in marine ecosystems of Korea project funded by the Ministry of Oceans and Fisheries, Korea.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Dong Young Kim (Ph.D. student) at Gwangju Institute of Science and Technology for performing the stable isotope analyses.
References
- Adams T. S., Sterner R. W. (2000). The effect of dietary nitrogen content on trophic level 15N enrichment. Limnol. Oceanogr. 45, 601–607. doi: 10.4319/lo.2000.45.3.0601 [DOI] [Google Scholar]
- Banse K. (1982). Cell volumes, maximal growth rates of unicellular algae and ciliates, and the role of ciliates in the marine pelagial. Limnol. Oceanogr. 27, 1059–1071. doi: 10.4319/lo.1982.27.6.1059 [DOI] [Google Scholar]
- Boecklen W. J., Yarnes C. T., Cook B. A., James A. C. (2011). On the use of stable isotopes in trophic ecology. Annu. Rev. Ecol. Evol. Syst. 42, 411–440. doi: 10.1146/annurev-ecolsys-102209-144726 [DOI] [Google Scholar]
- Bosley K. L. (1998). Tissue Turnover of Carbon and Nitrogen in Juvenile Fish, Estimated with Stable-Isotope Ratios, and the Effects of Preservatives and Acidification on the Stable Isotope Ratios of Marine Animals. MS thesis, NJ, Rutgers University. [Google Scholar]
- Bosley K. L., Witting D. A., Chambers R. C., Wainright S. C. (2002). Estimating turnover rates of carbon and nitrogen in recently metamorphosed winter flounder Pseudopleuronectes americanus with stable isotopes. Mar. Ecol. Prog. Ser. 236, 233–240. doi: 10.3354/meps236233 [DOI] [Google Scholar]
- Calbet A., Landry M. R. (2004). Phytoplankton growth, microzooplankton grazing, and carbon cycling in marine systems. Limnol. Oceanogr. 49, 51–57. doi: 10.4319/lo.2004.49.1.0051 [DOI] [Google Scholar]
- Calbet A., Saiz E. (2005). The ciliate-copepod link in marine ecosystems. Aquat. Microb. Ecol. 38, 157–167. doi: 10.3354/ame038157 [DOI] [Google Scholar]
- Caut S., Angulo E., Courchamp F. (2009). Variation in discrimination factors (Δ15N and Δ13C): the effect of diet isotopic values and applications for diet reconstruction. J. Appl. Ecol. 46, 443–453. doi: 10.1111/j.1365-2664.2009.01620.x [DOI] [Google Scholar]
- Décima M., Landry M. R., Bradley C. J., Fogel M. L. (2017). Alanine δ15N trophic fractionation in heterotrophic protists. Limnol. Oceanogr. 62, 2308–2322. doi: 10.1002/lno.10567 [DOI] [Google Scholar]
- DeNiro M. J., Epstein S. (1978). Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta 42, 495–506. doi: 10.1016/0016-7037(78)90199-0 [DOI] [Google Scholar]
- Dubois S., Jean-Louis B., Bertrand B., Lefebvre S. (2007). Isotope trophic-step fractionation of suspension-feeding species: implications for food partitioning in coastal ecosystems. J. Exp. Mar. Biol. Ecol. 351, 121–128. doi: 10.1016/j.jembe.2007.06.020 [DOI] [Google Scholar]
- Fessenden L., Cowles T. J. (1994). Copepod predation on phagotrophic ciliates in Oregon coastal waters. Mar. Ecol. Prog. Ser. 107, 103–111. doi: 10.3354/meps107103 [DOI] [Google Scholar]
- Focken U., Becker K. (1998). Metabolic fractionation of stable carbon isotopes: implications of different proximate compositions for studies of the aquatic food webs using δ13C data. Oecologia 115, 337–343. doi: 10.1007/s004420050525, PMID: [DOI] [PubMed] [Google Scholar]
- France R. L. (1995). Carbon-13 enrichment in benthic compared to planktonic algae: foodweb implications. Mar. Ecol. Progr. Ser. 124, 307–312. doi: 10.3354/meps124307 [DOI] [Google Scholar]
- Fry B. (2006). Stable Isotope Ecology. New York: Springer. [Google Scholar]
- Fry B., Arnold C. (1982). Rapid 13C/12C turnover during growth of brown shrimp (Penaeus aztecus). Oecologia 54, 200–204. doi: 10.1007/BF00378393, PMID: [DOI] [PubMed] [Google Scholar]
- Fry B., Sherr E. (1984). δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems. Contrib. Mar. Sci. 27, 49–63. [Google Scholar]
- Gannes L. Z., O’Brien D. M., Martinez del Rio C. (1997). Stable isotopes in animal ecology: assumptions, caveats, and a call for more laboratory experiments. Ecology 78, 1271–1276. doi: 10.1890/0012-9658(1997)078[1271:SIIAEA]2.0.CO;2 [DOI] [Google Scholar]
- Gutiérrez-Rodríguez A., Décima M., Popp B. N., Landry M. R. (2014). Isotopic invisibility of protozoan trophic steps in marine food webs. Limnol. Oceanogr. 59, 1590–1598. doi: 10.4319/lo.2014.59.5.1590 [DOI] [Google Scholar]
- Heethoff M., Scheu S. (2016). Reliability of isotopic fractionation (Δ15N, Δ13C) for the delimitation of trophic levels of oribatid mites: diet strongly affects Δ13C but not Δ15N. Soil Biol. Biochem. 101, 124–129. doi: 10.1016/j.soilbio.2016.07.013 [DOI] [Google Scholar]
- Herzka S. Z., Holt S. A., Holt G. J. (2001). Documenting the settlement history of individual fish larvae using stable isotope ratios: model development and validation. J. Exp. Mar. Biol. Ecol. 265, 49–74. doi: 10.1016/S0022-0981(01)00324-0 [DOI] [Google Scholar]
- Hobson K. A., Clark R. G. (1992). Assessing avian diets using stable isotopes II: factors influencing diet-tissue fractionation. Condor 94, 189–197. doi: 10.2307/1368808 [DOI] [Google Scholar]
- Jenkins W., Demopoulos A. W. J., Sikkel P. C. (2018). Host feeding ecology and trophic position significantly influence isotopic discrimination between a generalist ectoparasite and its hosts: implications for parasite-host trophic studies. Food Webs 16:e00092. doi: 10.1016/j.fooweb.2018.e00092 [DOI] [Google Scholar]
- Kang C. K., Park H. J., Choy E. J., Choi K. S., Hwang K., Kim J. B. (2015). Linking intertidal and subtidal food webs: consumer-mediated transport of intertidal benthic microalgal carbon. PLoS One 10:e0139802. doi: 10.1371/journal.pone.0139802, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layman C. A., Araujo M. S., Boucek R., Hammerschlag-Peyer C. M., Harrison E., Jud Z. R., et al. (2012). Applying stable isotopes to examine food-web structure: an overview of analytical tools. Biol. Rev. 87, 545–562. doi: 10.1111/j.1469-185X.2011.00208.x, PMID: [DOI] [PubMed] [Google Scholar]
- Lesage V., Hammill M. O., Kovacs K. M. (2002). Diet-tissue fractionation of stable carbon and nitrogen isotopes in phocid seals. Mar. Mamm. Sci. 18, 182–193. doi: 10.1111/j.1748-7692.2002.tb01027.x [DOI] [Google Scholar]
- Löder M. G., Meunier C., Wiltshire K. H., Boersma M., Aberle N. (2011). The role of ciliates, heterotrophic dinoflagellates and copepods in structuring spring plankton communities at Helgoland roads, North Sea. Mar. Biol. 158, 1551–1580. doi: 10.1007/s00227-011-1670-2 [DOI] [Google Scholar]
- Macko S. A., Lee W. Y., Parker P. L. (1982). Nitrogen and carbon isotope fractionation by two species of marine amphipods: laboratory and field studies. J. Exp. Mar. Biol. Ecol. 63, 145–149. doi: 10.1016/0022-0981(82)90028-4 [DOI] [Google Scholar]
- Mancinelli G. (2012). On the trophic ecology of Gammaridea (Crustacea: Amphipoda) in coastal waters: a European-scale analysis of stable isotopes data. Estuar. Coast. Shelf Sci. 114, 130–139. doi: 10.1016/j.ecss.2011.12.003 [DOI] [Google Scholar]
- McCutchan J. H., Lewis W. M., Jr., Kendall C., McGrath C. C. (2003). Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102, 378–390. doi: 10.1034/j.1600-0706.2003.12098.x [DOI] [Google Scholar]
- Michener R. H., Schell D. M. (1994). “Stable isotope ratios as tracers in marine aquatic food webs,” in Stable Isotopes in Ecology and Environmental Science. eds. Lajtha K., Michener R. H. (United States: Blackwell Scientific Publications; ), 138–157. [Google Scholar]
- Minagawa M., Wada E. (1984). Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochim. Cosmochim. Acta 48, 1135–1140. doi: 10.1016/0016-7037(84)90204-7 [DOI] [Google Scholar]
- Montagnes D. J. S., Dower J. F., Figueiredo G. M. (2010). The protozooplankton-ichthyoplankton trophic link: An overlooked aspect of aquatic food web. J. Eukaryot. Microbiol. 57, 223–228. doi: 10.1111/j.1550-7408.2010.00476.x, PMID: [DOI] [PubMed] [Google Scholar]
- Peterson B. J., Fry B. (1987). Stable isotopes in ecosystem studies. Annu. Rev. Ecol. Syst. 18, 293–320. doi: 10.1146/annurev.es.18.110187.001453 [DOI] [Google Scholar]
- Pierce R. W., Turner J. T. (1992). Ecology of planktonic ciliates in marine food webs. Rev. Aquat. Sci. 6, 139–181. [Google Scholar]
- Post D. M. (2002). Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83, 703–718. doi: 10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2 [DOI] [Google Scholar]
- Remy F., Darchambeau F., Melchior A., Lepoint G. (2017). Impact of food type on respiration, fractionation and turnover of carbon and nitrogen stable isotopes in the marine amphipod Gammarus aequicauda (Martynov, 1931). J. Exp. Mar. Biol. Ecol. 486, 358–367. doi: 10.1016/j.jembe.2016.10.031 [DOI] [Google Scholar]
- Sherr E. B., Sherr B. F. (1994). Bacterivory and herbivory: key roles of phagotrophic protists in pelagic food web. Micro. Ecol. 28, 223–235. doi: 10.1007/BF00166812 [DOI] [PubMed] [Google Scholar]
- Tieszen L. L., Boutton T. W., Tesdahl K. G., Slade N. A. (1983). Fractionation and turnover of stable carbon isotopes in animal tissues: implications for δ13C analysis of diet. Oecologia 57, 32–37. doi: 10.1007/BF00379558, PMID: [DOI] [PubMed] [Google Scholar]
- Vander Zanden M. J., Rasmussen J. B. (2001). Variation in δ13C and δ15N trophic fractionation: implications for aquatic food web studies. Limnol. Oceanogr. 46, 2061–2066. doi: 10.4319/lo.2001.46.8.2061 [DOI] [Google Scholar]
- Vanderklift M. A., Ponsard S. (2003). Sources of variation in consumer-diet delta N-15 enrichment: a meta-analysis. Oecologia 136, 169–182. doi: 10.1007/s00442-003-1270-z, PMID: [DOI] [PubMed] [Google Scholar]
- Xu G., Yang E. J., Jiang Y., Cho K. H., Jung J., Lee Y., et al. (2018). Can pelagic ciliates indicate vertical variation in the water quality status of western Arctic pelagic ecosystems? Mar. Pollut. Bull. 133, 182–190. doi: 10.1016/j.marpolbul.2018.05.017, PMID: [DOI] [PubMed] [Google Scholar]
- Yokoyama H., Tamaki A., Harada K., Shimoda K., Koyama K., Ishihi Y. (2005). Variability of diet-tissue isotopic fractionation in estuarine macrobenthos. Mar. Ecol. Prog. Ser. 296, 115–128. doi: 10.3354/meps296115 [DOI] [Google Scholar]
- Zingel P., Agasild H., Karus K., Buholce L., Nõges T. (2019). Importance of ciliates as food for fish larvae in a shallow sea bay and a large shallow lake. Eur. J. Protistol. 67, 59–70. doi: 10.1016/j.ejop.2018.10.004, PMID: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material; further inquiries can be directed to the corresponding author.