Abstract
Objective: Birth weight, an important indicator of fetal nutrition and degree of development, may affect the risk of subsequent leukemia. At present, little is known about the effect of birth weight on acute myeloid leukemia (AML) and whether there is a dose-dependent relationship of birth weight with acute lymphoid leukemia (ALL) and AML. To address these questions, the present work aimed to systematically investigate the relationship between birth weight and the risk of subsequent leukemia based on the current epidemiological studies
Methods: Relevant studies were systematically retrieved from electronic databases PubMed, Embase, and Cochrane Library, from inception to May 15th, 2021. Finally, 28 studies (including 21 case-control studies and 7 cohort studies) were included for the final meta-analysis. Results in cohort studies were performed by risk ratios (RRs), while those in case-control studies by odds ratios (ORs), and all results were assessed by adopting the random-effect model. Besides, a dose-dependent analysis was conducted based on the cohort studies.
Results: Compared with the population with normal birth weight (NBW), the population with high birth weight (HBW) might have an increased risk of leukemia (OR 1.33, 95%CI 1.20–1.49; I2 0%). Meanwhile, low birth weight (LBW) was associated with a decreased risk of ALL, as evidenced from the pooled analysis of case-control studies (OR 0.83, 95% CI 0.75–0.92; I2 23.3%). However, relative to NBW population, the HBW population might have an increased risk of ALL (OR 1.28, 95% CI 1.20–1.35; I2 7%). There was no obvious evidence supporting the relationship between LBW and the risk of AML from the pooled analysis of case-control studies (OR, 1.11 95% CI 0.87–1.42; I2 31.7%).
Conclusions: Overall, in children and young adults, HBW population may be associated with the risks of subsequent leukemia and AML relative to NBW population, but the supporting dose-dependent evidence is lacking. In addition, compared with NBW population, there is stronger evidence supporting a significantly increased risk of subsequent ALL in HBW population, and a decreased risk in LBW population in a dose-dependent manner. More prospective studies with large samples are warranted in the future to validate and complement these findings.
Keywords: leukemia, acute leukemia, meta-analysis, high birth weight, low birth weight
Introduction
Leukemia is a malignant clonal disease of hematopoietic stem cells (HSCs). It is characterized by the uncontrolled proliferation and development of leukocytes in the bone marrow and peripheral blood, which in turn invade the internal organs, such as the liver and spleen (1–3). Leukemia, a common hematologic tumor, remains the most common cancer in children. In general, leukemia can be classified into acute and chronic subtypes according to the disease progression degree, of them, acute leukemia is the most common in clinical work. Acute leukemia encompasses acute lymphoid leukemia (ALL) and acute myeloid leukemia (AML). Typically, ALL has been reported to account for approximately 80% of all the diagnosed leukemia cases in children aged 0–19 years, while AML accounts for 15–20% (4). Some perinatal features (such as gestational age, gender, and birth order) and maternal features (like age) are associated with the risk of childhood leukemia. However, the associations of other features with leukemia, especially with the leukemia subtypes, remain to be further elucidated. Currently, the pathogenesis of leukemia is unclear. Typically, exposure early in life may lead to dramatic health consequences, including the risk of cancer in the childhood and throughout the life of an individual (5).
Birth weight, an important indicator of fetal nutrition and degree of development, may affect the risk of subsequent leukemia. Although several previous studies have suggested that high birth weight (HBW) increases the risk of subsequent ALL, the results are inconsistent (6–9). Nevertheless, little is known about the effect of birth weight on AML and whether there is a dose-dependent relationship between birth weight and ALL/AML. To address these questions, this study aimed to systematically investigate the relationship between birth weight and the risk of subsequent leukemia based on existing epidemiological studies.
Methods
This meta-analysis was conducted according to the guidance of Meta-analyses of Observational Studies in Epidemiology (MOOSE) (10). Two investigators (Che and Long) systematically searched the electronic databases PubMed, Embase, and Cochrane Library, from inception to May 15th, 2021, without language restriction. Two groups of medical subject terms (MeSH), including “birth weight” and “leukemia,” were used for study search. Meanwhile, Boolean operator “OR” was used within groups, whereas “AND” was used between groups. To identify more relevant studies, the library entries were retrieved manually. Besides, previous meta-analyses were also reviewed if applicable. The detailed search flow is displayed in Appendix 1 (Supplementary Material).
Study Selection
Inclusion criteria were determined following the PICOS standards: (1) The study population did not have any family history of cancers, exposure to radiation and chemicals, or congenital disease (such as Down's syndrome), with birth weight being the interested exposure. (2) Different birth weight levels were compared. (3) One birth weight group served as the control or reference group. (4) The study outcomes reported the incidence of leukemia. (5) The study types were restricted to case-controlled, cohort studies, or randomized controlled trials (RCTs). (6) The related odds ratios (ORs), risk ratios (RRs), or hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were available or might be calculated. Meanwhile, the exclusion criteria were as follows: (1) The study population had a family history of cancers, exposure to radiation and chemicals, or congenital disease like Down's syndrome, and birth weight was not the interested exposure. (2) The studies did not report the risk of leukemia or acute leukemia by comparing different birth weight levels. (3) Cross-sectional studies, pooled studies, case reports or series, conference abstracts should be excluded. (4) Related ORs, RRs, or HRs were not obtained or converted.
Data Extraction and Quality Assessment
An unified list was used to extract the following baseline data from the included studies, including first author, publication year, country, sample size, date of birth or diagnosis, BMI categories, types of leukemia, ascertainment of leukemia, and maximum adjusted variables. The maximum variables adjusted for ORs, RRs, or HRs were extracted. Any disagreements were solved by the opinion from a third investigator. The quality of included studies was evaluated by using the Newcastle-Ottawa Scale (NOS) items, with the total score of 9 stars (11). Studies with a score ≥6 stars were considered as high-quality studies, otherwise, they were the low-quality studies.
Statistical Analysis
Referring to most of the included studies, this study defined birth weight ≤2,500 g, 2,500–4,000 g, ≥4,000 g as “low birth weight (LBW),” “normal birth weight (NBW),” and “HBW,” respectively. When one study reported different LBWs or HBWs (such as ≤ 2,000 g, 2,000–2,500, 4,000–4,500 g, ≥4,500 g), it was analyzed separately. The primary endpoint of our study was to qualitatively analyze the relationship between birth weight and leukemia/acute leukemia (like LBW vs. NBW, HBW vs. NBW). Generally speaking, HRs can be roughly considered to be equal to RRs in cohort studies (12). Therefore, results of all the cohort studies were performed by RRs, whereas those of case-control studies by ORs. Statistical heterogeneity was evaluated using I2 statistics, with the I2 values of 25, 50, and 75% indicating low, moderate, and high inconsistency, respectively (13). If there were high heterogeneities between studies, subgroup and sensitivity analyses were performed to explore the possible sources of heterogeneity between groups, and meta-regression analysis was further carried out in the case of enough included studies (n > 10). This study used a random-effects model to more conservatively estimate the pooled RRs and ORs, since more robust results were obtained after aggregating with this model. In addition, the risk of potential publication bias was assessed by funnel plots as well as Begg's and Egger's tests (14, 15). Trim and fill analyses were performed if necessary.
The secondary endpoint of this study was to quantitatively assess the effect of birth weight on leukemia. For this purpose, a dose-response meta-analysis was performed on the included cohort studies. To maximally include the available cohort studies, the robust error meta-regression method described by Xu and Doi (16) was utilized to establish a potential dose-response relationship between birth weight and leukemia. In this “one-stage” framework approach, each of the included studies was considered as a cluster across the whole population, so long as the study included at least two categories.
In this paper, the restricted cubic spline was utilized to fit the potential non-linear trend with 3 knots, and non-linear p-values were calculated by testing the second spline coefficients to zero. The non-linear model was adopted when the non-linear p < 0.05; otherwise, the linear model was used. In general, the lowest-dose category should be used as a reference in the included studies; however, when the non-lowest-dose studies were used as reference, they were converted via an Excel macro file produced by Hamling et al. (17) based on the Greenland and Longnecker's (18) theory. The corresponding authors were contacted when the number of cases in a particular category was missing. Also, when the open intervals were studied, the amplitude was assumed to be the same as the adjacent category or 1.2-fold of the node (19). All statistical analyses were performed by Stata 12.0E.
Results
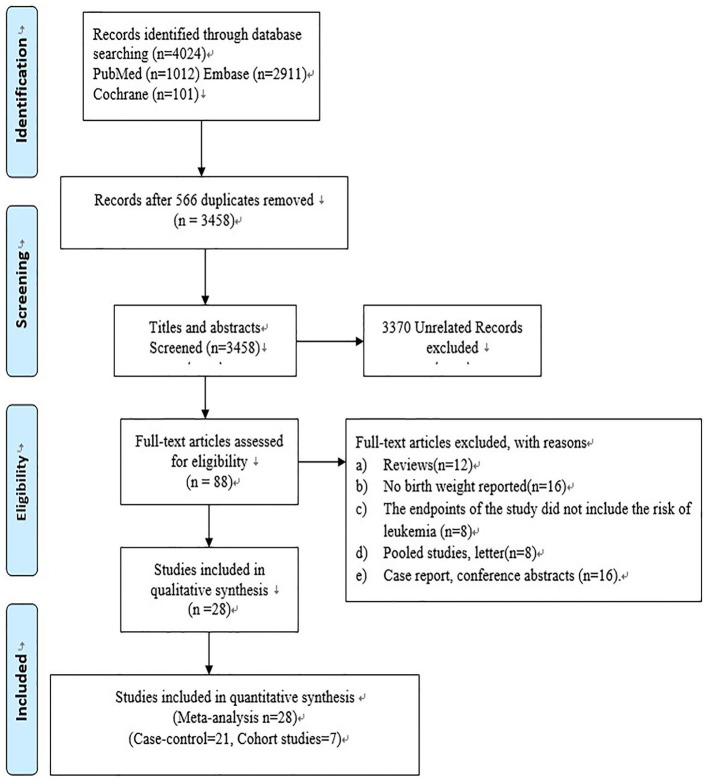
At first, a total of 4,024 studies were included. After removing 566 duplicated studies, 3,458 studies were retained. Then, the titles and abstracts of these 3,458 studies were read, and 3,370 unrelated studies were further excluded; as a result, only 88 studies were left for full-text review. Finally, only 28 studies (including 21 case-control and 7 cohort studies) were included, as shown in Figure 1. The specific reasons of exclusion were listed as follows: (1) reviews (n = 12); (2) no available information of birth weight (n = 16); (3) the study endpoints did not include the risk of leukemia (n = 8); (4) pooled studies, letters (n = 8); and (5) case reports, conference abstracts (n = 16).
Figure 1.
Flow chart of search results.
In the 28 studies, participants were children and young adults <29 years of age. Among the 21 case-control studies (6–9, 20–36), 21 involving altogether 111,643 participants (both cases and controls) reported the association between birth weight and the risk of ALL; 14 including 82,566 participants (both cases and controls) mentioned the relationship between birth weight and the risk of AML; while 7 containing 43,501 participants (both cases and controls) reported the association between birth weight and the risk of total leukemia. Among the 7 cohort studies (37–43), 5 recruiting altogether 4,807,631 participants mentioned the relationship between birth weight and the risk of ALL; 3 including 4,143,450 participants reported the association between birth weight and the risk of AML; and 4 containing 2,261,005 participants stated the relationship between birth weight and the risk of total leukemia. More details about the baseline characteristics are displayed in Table 1. In addition, all the included studies had a score ≥6 stars and were considered as high-quality studies, as shown in Supplementary Table 1.
Table 1.
The detailed baseline information of 28 observational studies.
| Author, year | Country | Sample size | Birth or diagnosed period | Birth weight categories (g) | Types of leukemia | Ascertainment of leukemia | Maximum adjusted variables |
|---|---|---|---|---|---|---|---|
| Cohort studies | |||||||
| Stacy et al. (37) | USA | 1,877,078 | 2003–2015 | <2,000; 2,000–2,499; 3,000–3,499; 3,500–3,999; 4,000–4,499; ≥4,500 | Total | International classification of childhood cancer | Maternal age, race, sex at birth, gestational age |
| Paltiel et al. (38) | Israel | 88,829 | 1964–1976 | ≤2,999; 3,000–3,499; 3,500–3,999; ≥4,000 | Total, ALL, AML | ICD-9 | Maternal origin, mother age, father age, gender, socioeconomic status |
| Heck et al. (39) | China | 2,079,037 | 2004–2014 | <2,500 g; 2,500–3,999 g; ≥4,000 | ALL, AML | ICD-9 ICD-O-3 |
Mother's age, father's age, family income, urbanization level of residence at birth |
| Lee et al. (40) | Singapore | 229,248 | 1992–1999 | 2,500–3,500; >3,500 | Total, ALL | ICCC | Gender, gestational age, birth order, maternal age |
| Murray et al. (41) | UK | 434,933 | 1971–1986 | <3,500 g; ≥3,500 | ALL | Medical records | Maternal age, birth order, Down's syndrome, gestational age, gender, social class |
| Spracklen et al. (42) | USA | 65,850 | 1993–1998 | <2,721; 2,721–3,624; 3,624–4,490; ≥4,490 | Total | Medical records | Age, race, education, normalized socioeconomic status, BMI, smoking status, alcohol use |
| Westergaard et al. (43) | Denmark | 1,975,584 | 1968–1992 | <2,510; 2,510–3,009; 3,010–3,509; 3,510–4,009; 4,010–4,509; ≥4,510 | ALL, AML | ICO | Gender, gestational age, maternal age, birth order calendar period |
| Case-control | |||||||
| Jiménez-Hernández et al. (20) | Mexico | Case 1,455 Control 1,455 |
2010–2015 | <2,500; 2,500–3,499; 3,500–4,000; >4,000 | ALL, AML | Medical Records | Child's sex, overcrowding index, birth order, mother's age at the time of pregnancy |
| Barahmani et al. (24) | USA | Case 575 Control 11,379 |
1995–2003 | ≤2,500; 2,500–3,999; ≥4,000 | ALL | Medical records | Infant gender, maternal age |
| Dorak et al. (28) | UK | Case 732 Control 3,723 |
1968–1992 | ≤2,500; 2,500–2,999; 3,000–3,499; 3,500–3,999; ≥4,000 | ALL | Medical records | None |
| Hjalgrim et al. (29) | Denmark | Case 2,204 Control 10,745 |
1984–1999 | <1,500; 1,500–1,999; 2,000–2,499; 2,500–2,999; 3,000–3,499; 3,500–3,999; 4,000–4,499; ≥4,500 | ALL, AML | FAB classification M0–M7 |
None |
| Koifman et al. (6) | Brazil | Case 201 Control 440 |
1999–2005 | <2,500; 2,500–2,999; 3,000–3,499; 3,500–3,999; ≥4,000 | Total, ALL, AML | Medical records | Gender, income, maternal age, hormone intake, pesticide exposure during pregnancy |
| Groves et al. (25) | USA | Case 401 Control 1,592 |
1995–2002 | ≤2,500; 2,500–3,999; ≥4,000 | ALL | Medical records | None |
| Ma et al. (7) | USA | Case 366 Control 460 |
1995–2002 | ≤2,500; 2,500–3,999; ≥4,000 | ALL, AML | Medical records | Household income, maternal education |
| Oksuzyan et al. (9) | USA | Case 5,788 Control 5,788 |
1988–2008 | <2,500; 2,500–3,000; 3,000–3,500; 3,500–4,000; 4,000–4,500; ≥4,500 | Total, ALL, AML | Medical records | Gestational age, birth order, mother's age, father's education, child's race, and payment source for delivery |
| Ou et al. (30) | USA | Case 1,842 Control 1,986 |
1989–1993 | ≤3,000; 3,001–3,500; 3,501–4,000; >4,000 | ALL | Medical records | Socioeconomic status, maternal age, race |
| Podvin et al. (31) | USA | Case 595 Control 5,950 |
1981–2002 | ≤2,500; 2,500–3,999; ≥4,000 | Total, ALL, AML | ICD-O-3 | Maternal age |
| Reynolds et al. (32) | USA | Case 1,728 Control 2,802 |
1988–1997 | ≤2,500; 2,500–3,999; ≥4,000 | ALL, AML | Medical records | Gestational age |
| Smith et al. (34) | UK | Case 3,651 Control 6,337 |
1991–1996 | ≤2,500; 2,500–3,999; ≥4,000 | Total, ALL, AML | Medical records | study region, sex and age |
| Sprehe et al. (35) | USA | Case 2,254 Control 11,734 |
1995–2003 | ≤2,500; 2,500–3,999; ≥4,000 | Total, ALL, AML | Medical records | Birth year, gestational age, maternal age |
| Yeazel et al. (36) | USA | Case 3,711 Control 816 |
1982–1989 | ≤2,797; 2,798–3,291; 3,292–3,547; 3,548–3,859; >3,859 | ALL, AML | Medical records | Maternal age, birth order, gestational age, gender |
| Roman et al. (26) | UK | Case 128 Control 286 |
1962–1992 | <2,500; 2,500–3,500; >3,500 | Total, ALL, AML | Medical records | Gender, age, study region |
| Schüz et al. (21) | Germ total | Case 755 Control 2,057 |
1992–1994 | ≤2,500; 2,500–3,999; ≥4,000 | ALL, AML | Medical records | Maternal age, degree of urbanization, and socioeconomic status |
| McLaughlin et al. (23) | USA | Case 148 Control 9,667 |
1985–2001 | <2,500; 2,500–2,999; 3,000–3,499; 3,500–3,999; 4,000–4,499; ≥4,500 | ALL, AML | ICD-O-3 | Birth year, gender, race, and ethnicity, maternal age |
| Jourdan-Da Silva et al. (22) | USA | Case 473 Control 567 |
1995–1998 | <2,500; 2,500–2,999; 3,000–3,499; 3,500–3,999; ≥4,000 | ALL, AML | Medical records | Gender, age at diagnosis, region of residence at diagnosis |
| Cnattingius et al. (27) | Sweden | Case 610 Control 3,061 |
1973–1989 | <1,500; 1,500–1,999; 2,000–2,499; 2,500–2,999; 3,000–3,499; 3,500–3,999; 4,000–4,499; ≥4,500 | ALL | ICD-7 | Gestational age |
| Okcu et al. (8) | USA | Case 104 Control 245 |
1995 | 2,500–3,999; ≥4,000 | Total, ALL | Medical records | Year of birth, gender, gestational age, maternal age, Oksuzyan tobacco use, parity, race/ethnicity |
| Savitz et al. (33) | USA | Case 68 Control 208 |
1976–1983 | ≤2,500; 2,500–3,999; ≥4,000 | ALL | Medical records | None |
ICD-9, International Classification of Diseases, Ninth Revision codes; ICD-O-3, International Classification of Diseases for Oncology, Version 3; ICD-7, International Classification of Diseases Seventh Revision; ICO, International Classification of Diseases for Oncology. FAB classification M0–M7, French American, and British classification system; ICCC, International Childhood Cancer Classification.
Meta-Analysis
Total Leukemia
As presented in Figure 2, no obvious evidence was found between LBW and the risk of total leukemia from the pooled analysis of enrolled case-control studies (OR 0.90, 95% CI 0.75–1.07; I2 27.6%). Compared with the NBW population, the HBW population might have an increased risk of leukemia (OR 1.33, 95% CI 1.20–1.49; I2 0%). The potential publication bias was evaluated by the funnel plot, as shown in Supplementary Figure 1A. Visually, the funnel plot was symmetrical, and both Begger's (p = 1.00) and Egger's (p = 0.881) tests did not reveal any evidence of publication bias. Moreover, sensitivity analysis was conducted by removing one study each time, and the pooled results showed little change, as shown in Supplementary Figure 2.
Figure 2.
The odds ratios for low birth weight, high birth weight, and total leukemia.
Compared with the NBW population, no obvious evidence was detected between LBW/HBW and the risk of leukemia from the pooled analysis of 4 cohort studies (RR 0.94, 95% CI 0.68–1.30; I2 0% for LBW; RR 1.27, 95% CI 0.89–1.83; I2 31% for HBW), respectively, as presented in Supplementary Figure 3A. Due to the limited number of available studies, publication bias test and sensitivity analysis were not conducted further.
According to Supplementary Figure 4A, the dose-response analysis from 4 cohort studies showed that the risk of leukemia did not increase with the increase in birth weight.
ALL
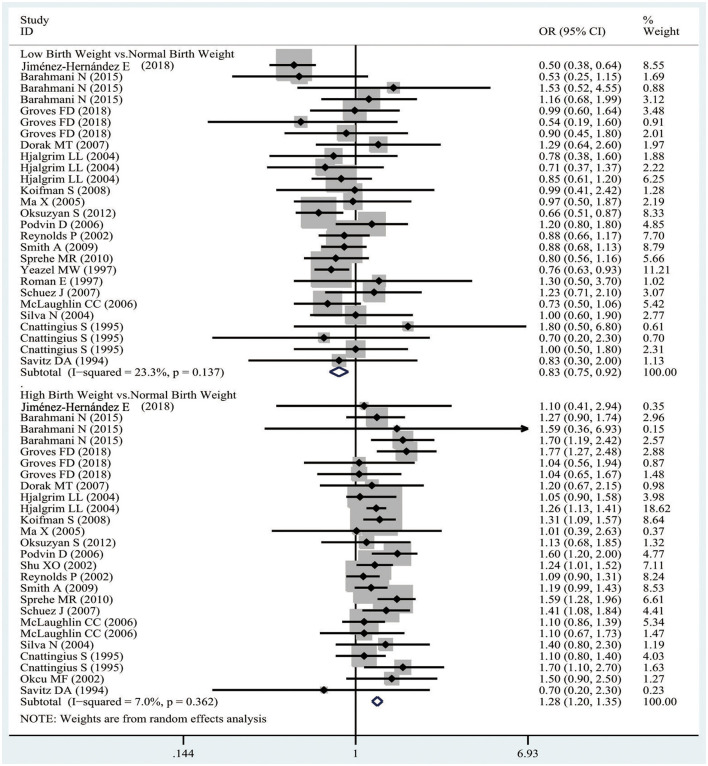
It was demonstrated from Figure 3 that, there was obvious evidence supporting that LBW was related to a decreased risk of ALL from the pooled analysis of case-control studies (OR 0.83, 95%CI 0.75–0.92; I2 23.3%). However, compared with the NBW population, the HBW population might have an increased risk of ALL (OR 1.28, 95%CI 1.20–1.35; I2 7%). In addition, the publication bias was evaluated by the funnel plot, as presented in Supplementary Figure 1B. Visually, the funnel plot was asymmetrical, and both Begger's (p = 0.203) and Egger's (p = 0.256) tests revealed no obvious evidence of publication bias. Also, sensitivity analysis was conducted by removing one study each time, and the pooled results showed slight change, as shown in Supplementary Figure 5.
Figure 3.
The odds ratios for low birth weight, high birth weight, and ALL.
Similar results were obtained the pooled analysis of cohort studies, suggesting that the LBW population had a decreased risk of ALL compared with the NBW population (RR 0.66, 95% CI 0.47–0.93; I2 0%), and the HBW population had an increased risk of ALL (RR 1.49, 95% CI 1.16–1.91; I2 0%), as observed from Supplementary Figure 3B. Due to the limited number of available studies, the publication bias test and sensitivity analysis were not conducted further.
As observed from Supplementary Figure 4B, the dose-response analysis of 5 cohort studies showed that the risk of ALL significantly increased when the birth weight increased from 1,750 to 5,000 g.
AML
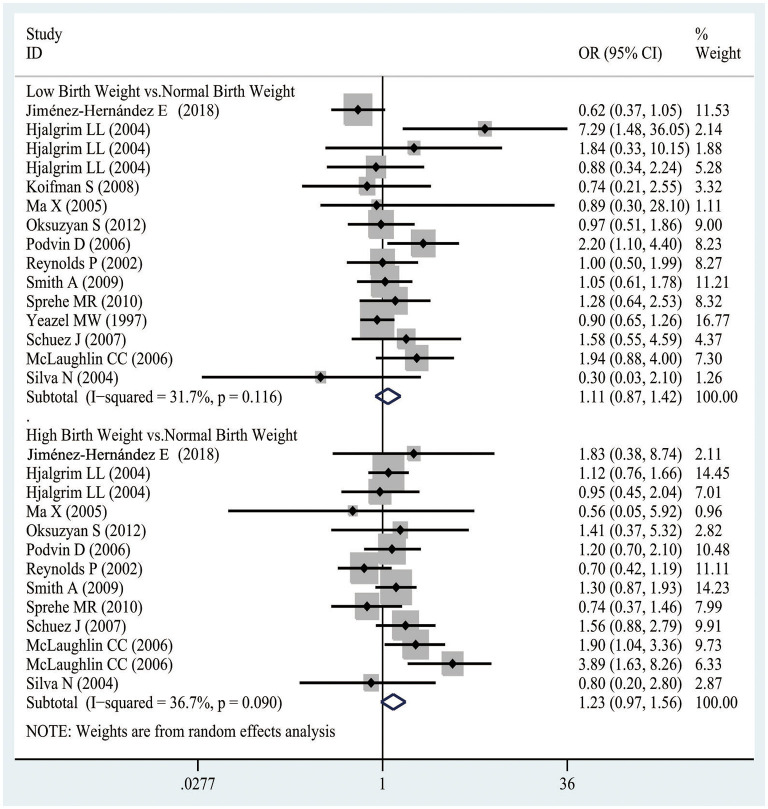
In Figure 4, there was no obvious evidence supporting the relationship between LBW and the risk of AML, as evidenced from the pooled analysis of case-control studies (OR, 1.11 95% CI 0.87–1.42; I2 31.7%). Similarly, compared with the NBW population, the HBW population was not associated with an increased risk of AML (OR 1.23, 95% CI 0.97–1.56; I2 36.7%). Besides, the publication bias was assessed by the funnel plot, as displayed in Supplementary Figure 1C. Visually, the funnel plot was asymmetrical, meanwhile, Begger's (p = 0.650) and Egger's (p = 0.434) tests did not indicate any evidence of publication bias. Also, sensitivity analysis was conducted by removing one study each time, and the pooled results showed little change, as shown in Supplementary Figure 6.
Figure 4.
The odds ratios for low birth weight, high birth weight, and AML.
Results from the pooled analysis of cohort studies demonstrated that the HBW population had an increased risk of AML compared with the NBW population (RR 1.88, 95% CI 1.10–3.22; I2 0%), as observed from Supplementary Figure 1. Due to the limited number of available studies, the publication test and sensitivity analysis were not conducted further.
However, the dose-response analysis of 3 cohort studies suggested that the risk of AML did not significantly increase with the increase in birth weight, as shown in Supplementary Figure 4C.
Subgroup Analysis and Meta-Regression
A moderate heterogeneity was found in the association between birth weight and AML, therefore, subgroup analysis and meta-regression were performed based on the features below, including child age, sample size, publication year, country, and study quality (Table 2). According to subgroup analysis, child age and study quality might be the potential sources of heterogeneity between LBW and AML, whereas sample size, child age, publication year, country, and study quality might be the potential sources of heterogeneity between HBW and AML. However, no potential source of heterogeneity was detected through meta-regression.
Table 2.
Subgroup analysis for birth weight and AML.
| Items | Low birth weight | High birth weight | ||||||
|---|---|---|---|---|---|---|---|---|
| n | OR, 95% CI; I2 | p a | p b | n | OR, 95% CI; I2 | p a | p b | |
| Sample size (cases) | 0.414 | 0.577 | ||||||
| ≥500 | 9 | 1.1 (0.84–1.44); 38.5% | 0.093 | 8 | 1.1 (0.91–1.33); 0% | 0.532 | ||
| <500 | 4 | 1.12 (0.53–2.37); 20.8% | 0.285 | 3 | 1.86 (0.93–3.74); 45.9% | 0.136 | ||
| Children age | 0.537 | 0.632 | ||||||
| 10+ years | 9 | 1.08 (0.79–1.49); 41.5% | 0.072 | 8 | 1.22 (0.99–1.50); 0% | 0.961 | ||
| ≤10 years | 4 | 1.25 (0.82–1.85); 0% | 0.498 | 3 | 1.36 (0.64–2.87); 81.8% | 0.001 | ||
| Published year | 0.996 | 0.460 | ||||||
| ≥2010 | 3 | 0.87 (0.57–1.34); 31.7% | 0.231 | 3 | 0.94 (0.53–1.66); 0% | 0.469 | ||
| <2010 | 10 | 1.22 (0.91–1.64); 29.9% | 0.153 | 8 | 1.28 (0.97–1.67); 45.6% | 0.056 | ||
| Country | 0.642 | 0.944 | ||||||
| America | 10 | 1.04 (0.79–1.38); 33.3% | 0.141 | 8 | 1.25 (0.82–1.91); 54.4% | 0.025 | ||
| Europe | 3 | 1.40 (0.8–2.45); 33.4% | 0.198 | 3 | 1.23 (0.97–1.56); 0% | 0.707 | ||
| Study quality | 0.185 | 0.272 | ||||||
| 8–9 | 8 | 1.41 (0.97–2.05); 23.8% | 0.224 | 7 | 1.38 (1.08–1.77); 28.1% | 0.195 | ||
| 6–7 | 5 | 0.89 (0.71–1.12); 0% | 0.541 | 4 | 0.8 (0.54–1.17); 0% | 0.559 | ||
Pa for heterogeneity within each subgroup. Pb for heterogeneity between subgroups with meta-regression analysis.
Discussion
This meta-analysis suggested that LBW might not increase the risk of total leukemia, ALL, or AML compared with NBW. In contrast, the risk of ALL significantly decreased in the LBW population. In the HBW population, HBW might increase the risk of total leukemia and AML, but there was no dose-response relationship. In addition, compared with the NBW population, the HBW population had an increased risk of ALL, and similar results were obtained from the dose-response analysis of birth weight from 1,750 to 5,000 g.
Birth weight is determined by the nutritional, metabolic, and endocrine differences in the intrauterine environment, and is necessarily closely related to maternal prenatal health and nutritional status. The study by Wiemels et al. showed that chromosomal translocation in acute leukemia, a genetic event, might begin in utero (44). In addition, birth weight has been reported to be associated with multiple growth factors in the intrauterine environments, like insulin-like growth factor-1 (IGF-1), sex steroid hormones, and insulin-like growth factor II (IGF-1I) (45, 46). In utero, growth factors are related to the increased total number of stem cells, which may increase the risks of transformation into tumor cells and leukemia (47). Moreover, IGF-1 is an important embryonic growth factor that increases the stem cell pools in humans and animals. Further, several other hormones and growth factors related to birth weight and stem cell size, including IGF binding protein-3, estriol, and testosterone, can significantly increase the stem cell pool (45).
Birth weight and the risk of subsequent leukemia are often the consequences of multiple reproductive factors including gestational age, race, diet, and micronutrients. As reported in the study by Barahmani et al., the offspring of Hispanic whites with large gestational age were associated with a 50% increased risk of ALL compared to the non-Hispanic whites (24). Besides, a study from Denver indicates that animal foods are rich in iron, while plant foods may hinder iron absorption, and the increased iron absorption may increase the risk of ALL in children (48). Meanwhile, another meta-analysis shows that an increased maternal iron intake by 10 mg per day is associated with an increased offspring birth weight by 15.1 g (49).
Although some existing biological evidence suggests that a larger birth size may indicate that more cells are at risk of carcinogenesis (50, 51), the exact mechanism remains unclear. LBW may reflect a poor intrauterine environment or impaired fetal nutrition. In addition, it may also be associated with certain complications during the maternal pregnancy, such as intrauterine growth restriction (IUGR), preeclampsia, or preterm delivery. However, there is still no well-understood mechanism to explain this negative association of LBW with ALL.
Noteworthily, the present study has the following strengths. First, this was the first dose-response analysis that quantitatively assessed the association between birth weight and the risk of leukemia. Meanwhile, all the available studies were included for qualitative analysis, which verified and supplemented the dose-response analysis. Second, there was low inter-heterogeneity between the studies, which enabled the homogeneity between studies. Third, all the extracted ORs or RRs were adjusted by maximum variables, and the random-effect model was adopted to improve the stability of the pooled results.
Inevitably, certain limitations should also be noted in this study. First, most of the included studies were originated from America, Europe, and Asia, while there were relatively few studies from other regions, and the impact on research remained unclear. Meanwhile, the number of cohort studies was limited, which made it impossible for further analysis. Second, this meta-analysis suggested that HBW was associated with a high risk of leukemia, but whether there is a causal relationship between the two remains to be further investigated. Besides, the impacts of other potential perinatal variables (including full-term or preterm birth, birth order, breastfeeding, or feeding) and maternal characteristics (like the presence of maternal diseases and complications such as diabetes, eclampsia, maternal age, and weight) on our results remained to be further confirmed. Although all of the extracted ORs or RRs were adjusted by the maximum variables, the effects of residual confounding variables were still unknown. Third, the dose-response analyses on birth weight were conducted within the range of 1,750–5,000 g, and the results beyond this range were still unknown. Last but not least, all the populations included in the study were children and young adults (<29 years), and the effect of birth weight on the risk of leukemia beyond this range is still unknown.
Conclusions
Overall, in children and young adults, HBW may be related to an increased risk of subsequent leukemia and AML in HBW population compared with the NBW population, but support from dose-dependent evidence is lacking. In addition, stronger evidence supports a significantly increased risk of subsequent ALL in the HBW population compared with the NBW population, whereas a decreased risk in the LBW population, and this association is also found in the dose-response. More prospective studies with large samples are still warranted in the future to validate and complement these findings.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Author Contributions
HC participated in the data collection, data review, relevant data extraction, data analysis, statistical analysis, and the writing of the manuscript. DL, QS, and YL participated in checking data extraction as well as in the data analysis, statistical analysis, and the writing of the manuscript. LW participated in checking data extraction as well as in the statistical analysis and the writing of the manuscript. All authors saw and approved the final version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2021.722471/full#supplementary-material
The plots of the funnel for birth weight and Total leukemia (A), ALL (B), and AML (C).
The sensitivity analyses for low birth weight (A), high birth weight (B), and Total leukemia.
The RRs of birth weight and total leukemia (A), AML (B), and ALL (C).
The dose-response of birth weight and total leukemia (A), AML (B), and ALL (C).
The sensitivity analyses for low birth weight (A), high birth weight (B), and ALL.
The sensitivity analyses for low birth weight (A), high birth weight (B), and AML.
Quality assessment of the 28 observational studies.
References
- 1.Kampen KR. The discovery and early understanding of leukemia. Leuk. Res. (2012) 36:6–13. 10.1016/j.leukres.2011.09.028 [DOI] [PubMed] [Google Scholar]
- 2.Jakobsen NA, Vyas P. From genomics to targeted treatment in haematological malignancies: a focus on acute myeloid leukaemia. Clin. Med. (2018) 18:47. 10.7861/clinmedicine.18-2-s47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Licht JD, Sternberg DW. The molecular pathology of acute myeloid leukemia. Hematology. (2005) 2005:137–42. 10.1182/asheducation-2005.1.137 [DOI] [PubMed] [Google Scholar]
- 4.Marcotte EL, Spector LG, Mendes-de-Almeida DP, Nelson HH. The prenatal origin of childhood leukemia: potential applications for epidemiology and newborn screening. Front Pediatr. (2021) 9:639479. 10.3389/fped.2021.639479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baird J, Jacob C, Barker M, Fall CHD, Hanson M, Harvey NC, et al. Developmental origins of health and disease: a life course approach to the prevention of non-communicable diseases. Healthcare. (2017) 5:14. 10.3390/healthcare5010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koifman S, Pombo-de-Oliveira MS, Brazilian Collaborative Study Group of Infant Acute Leukemia . High birth weight as an important risk factor for infant leukemia. Br J Cancer. (2008) 98:664–7. 10.1038/sj.bjc.6604202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma X, Metayer C, Does MB, Buffler PA. Maternal pregnancy loss, birth characteristics, and childhood leukemia (United States). Cancer Causes Control. (2005) 16:1075–83. 10.1007/s10552-005-0356-9 [DOI] [PubMed] [Google Scholar]
- 8.Okcu MF, Goodman KJ, Carozza SE, Weiss NS, Burau KD, Bleyer WA, et al. Birth weight, ethnicity, and occurrence of cancer in children: a population-based, incident case-control study in the State of Texas, USA. Cancer Causes Control. (2002) 13:595–602. 10.1023/A:1019555912243 [DOI] [PubMed] [Google Scholar]
- 9.Oksuzyan S, Crespi CM, Cockburn M, Mezei G, Kheifets L. Birth weight and other perinatal characteristics and childhood leukemia in California. Cancer Epidemiol. (2012) 36:e359–65. 10.1016/j.canep.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 11.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. (1998) 280:1690–1. 10.1001/jama.280.19.1690 [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 15.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu C, Doi SAR. The robust error meta-regression method for dose-response meta-analysis. Int J Evidence Based Healthc. (2018) 16:138–44. 10.1097/XEB.0000000000000132 [DOI] [PubMed] [Google Scholar]
- 17.Hamling J, Lee P, Weitkunat R, Ambuhl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Statist Med. (2008) 27:954–70. 10.1002/sim.3013 [DOI] [PubMed] [Google Scholar]
- 18.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. (1992) 135:1301–9. 10.1093/oxfordjournals.aje.a116237 [DOI] [PubMed] [Google Scholar]
- 19.Cheng W, Zhang Z, Cheng W, Yang C, Diao L, Liu W. Associations of leisure-time physical activity with cardiovascular mortality: a systematic review and meta-analysis of 44 prospective cohort studies. Eur J Prevent Cardiol. (2018) 25:1864–72. 10.1177/2047487318795194 [DOI] [PubMed] [Google Scholar]
- 20.Jiménez-Hernández E, Fajardo-Gutiérrez A, Núñez-Enriquez JC, Martín-Trejo JA, Espinoza-Hernández LE, Flores-Lujano J, et al. A greater birthweight increases the risk of acute leukemias in Mexican children-experience from the Mexican Interinstitutional Group for the Identification of the Causes of Childhood Leukemia (MIGICCL). Cancer Med. (2018) 7:1528–36. 10.1002/cam4.1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schüz J, Forman MR. Birthweight by gestational age and childhood cancer. Cancer Causes Control. (2007) 18:655–63. 10.1007/s10552-007-9011-y [DOI] [PubMed] [Google Scholar]
- 22.Jourdan-Da Silva N, Perel Y, Méchinaud F, Plouvier E, Gandemer V, Lutz P, et al. Infectious diseases in the first year of life, perinatal characteristics and childhood acute leukaemia. Br J Cancer. (2004) 90:139–45. 10.1038/sj.bjc.6601384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLaughlin CC, Baptiste MS, Schymura MJ, Nasca PC, Zdeb MS. Birth weight, maternal weight and childhood leukaemia. Br J Cancer. (2006) 94:1738–44. 10.1038/sj.bjc.6603173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barahmani N, Dorak MT, Forman MR, Sprehe MR, Scheurer ME, Bondy ML, et al. Evaluating the role of birth weight and gestational age on acute lymphoblastic leukemia risk among those of Hispanic ethnicity. Pediatr Hematol Oncol. (2015) 32:382–9. [PubMed] [Google Scholar]
- 25.Groves FD, Watkins BT, Roberts DJ, Tucker TC, Shen T, Flood TJ. Birth weight and risk of childhood acute lymphoblastic leukemia in Arizona, Illinois, and Kentucky. South Med J. (2018) 111:579–84. 10.14423/SMJ.0000000000000873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roman E, Ansell P, Bull D. Leukaemia and non-Hodgkin's lymphoma in children and young adults: are prenatal and neonatal factors important determinants of disease? Br J Cancer. (1997) 76:406–15. 10.1038/bjc.1997.399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cnattingius S, Zack MM, Ekbom A, Gunnarskog J, Kreuger A, Linet M, et al. Prenatal and neonatal risk factors for childhood lymphatic leukemia. J Natl Cancer Inst. (1995) 87:908–14. 10.1093/jnci/87.12.908 [DOI] [PubMed] [Google Scholar]
- 28.Dorak MT, Pearce MS, Hammal DM, McNally RJ, Parker L. Examination of gender effect in birth weight and miscarriage associations with childhood cancer (United Kingdom). Cancer Causes Control. (2007) 18:219–28. 10.1007/s10552-006-0093-8 [DOI] [PubMed] [Google Scholar]
- 29.Hjalgrim LL, Rostgaard K, Hjalgrim H, Westergaard T, Thomassen H, Forestier E, et al. Birth weight and risk for childhood leukemia in Denmark, Sweden, Norway, and Iceland. J Natl Cancer Inst. (2004) 96:1549–56. 10.1093/jnci/djh287 [DOI] [PubMed] [Google Scholar]
- 30.Ou SX, Han D, Severson RK, Chen Z, Neglia JP, Reaman GH, et al. Birth characteristics, maternal reproductive history, hormone use during pregnancy, and risk of childhood acute lymphocytic leukemia by immunophenotype (United States). Cancer Causes Control. (2002) 13:15–25. 10.1023/A:1013986809917 [DOI] [PubMed] [Google Scholar]
- 31.Podvin D, Kuehn CM, Mueller BA, Williams M. Maternal and birth characteristics in relation to childhood leukaemia. Paediatr Perinat Epidemiol. (2006) 20:312–22. 10.1111/j.1365-3016.2006.00731.x [DOI] [PubMed] [Google Scholar]
- 32.Reynolds P, Von Behren J, Elkin EP. Birth characteristics and leukemia in young children. Am J Epidemiol. (2002) 155:603–13. 10.1093/aje/155.7.603 [DOI] [PubMed] [Google Scholar]
- 33.Savitz DA, Ananth CV. Birth characteristics of childhood cancer cases, controls, and their siblings. Pediatr Hematol Oncol. (1994) 11:587–99. 10.3109/08880019409141806 [DOI] [PubMed] [Google Scholar]
- 34.Smith A, Lightfoot T, Simpson J, Roman E, UKCCS investigators. Birth weight, sex and childhood cancer: a report from the United Kingdom Childhood Cancer Study. Cancer Epidemiol. (2009) 33:363–7. 10.1016/j.canep.2009.10.012 [DOI] [PubMed] [Google Scholar]
- 35.Sprehe MR, Barahmani N, Cao Y, Wang T, Forman MR, Bondy M, et al. Comparison of birth weight corrected for gestational age and birth weight alone in prediction of development of childhood leukemia and central nervous system tumors. Pediatr Blood Cancer. (2010) 54:242–9. 10.1002/pbc.22308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeazel MW, Ross JA, Buckley JD, Woods WG, Ruccione K, Robison LL. High birth weight and risk of specific childhood cancers: a report from the Children's Cancer Group. J Pediatr. (1997) 131:671–7. 10.1016/S0022-3476(97)70091-X [DOI] [PubMed] [Google Scholar]
- 37.Stacy SL, Buchanich JM, Ma ZQ, Mair C, Robertson L, Sharma RK, et al. Maternal obesity, birth size, and risk of childhood cancer development. Am J Epidemiol. (2019) 188:1503–11. 10.1093/aje/kwz118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paltiel O, Harlap S, Deutsch L, Knaanie A, Massalha S, Tiram E, et al. Birth weight and other risk factors for acute leukemia in the Jerusalem Perinatal Study cohort. Cancer Epidemiol Biomarkers Prev. (2004) 13:1057–64. [PubMed] [Google Scholar]
- 39.Heck JE, Lee PC, Wu CK, Tsai HY, Ritz B, Arah OA, et al. Gestational risk factors and childhood cancers: a cohort study in Taiwan. Int J Cancer. (2020) 147:1343–53. 10.1002/ijc.32905 [DOI] [PubMed] [Google Scholar]
- 40.Lee J, Chia KS, Cheung KH, Chia SE, Lee HP. Birthweight and the risk of early childhood cancer among Chinese in Singapore. Int J Cancer. (2004) 110:465–7. 10.1002/ijc.20159 [DOI] [PubMed] [Google Scholar]
- 41.Murray L, McCarron P, Bailie K, Middleton R, Davey Smith G, Dempsey S, et al. Association of early life factors and acute lymphoblastic leukaemia in childhood: historical cohort study. Br J Cancer. (2002) 86:356–61. 10.1038/sj.bjc.6600012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spracklen CN, Wallace RB, Sealy-Jefferson S, Robinson JG, Freudenheim JL, Wellons MF, et al. Birth weight and subsequent risk of cancer. Cancer Epidemiol. (2014) 38:538–43. 10.1016/j.canep.2014.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Westergaard T, Andersen PK, Pedersen JB, Olsen JH, Frisch M, Sørensen HT, et al. Birth characteristics, sibling patterns, and acute leukemia risk in childhood: a population-based cohort study. J Natl Cancer Inst. (1997) 89:939–47. 10.1093/jnci/89.13.939 [DOI] [PubMed] [Google Scholar]
- 44.Wiemels JL, Xiao Z, Buffler PA, Maia AT, Ma X, Dicks BM, et al. In utero origin of t(8;21) AML1-ETO translocations in childhood acute myeloid leukemia. Blood. (2002) 99:3801–5. 10.1182/blood.V99.10.3801 [DOI] [PubMed] [Google Scholar]
- 45.Baik I, Devito WJ, Ballen K, Becker PS, Okulicz W, Liu Q, et al. Association of fetal hormone levels with stem cell potential: evidence for early life roots of human cancer. Cancer Res. (2005) 65:358–63. [PubMed] [Google Scholar]
- 46.Michels KB, Xue F. Role of birthweight in the etiology of breast cancer. Int J Cancer. (2006) 119:2007–25. 10.1002/ijc.22004 [DOI] [PubMed] [Google Scholar]
- 47.Trichopoulos D, Lipworth L. Is cancer causation simpler than we thought, but more intractable? Epidemiology. (1995) 6:347–9. 10.1097/00001648-199507000-00003 [DOI] [PubMed] [Google Scholar]
- 48.Peters JM, Preston-Martin S, London SJ, Bowman JD, Buckley JD, Thomas DC. Processed meats and risk of childhood leukemia (California, USA). Cancer Causes Control. (1994) 5:195–202. 10.1007/BF01830266 [DOI] [PubMed] [Google Scholar]
- 49.Haider BA, Olofin I, Wang M, Spiegelman D, Ezzati M, Fawzi WW, et al. Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. (2013) 346:f3443. 10.1136/bmj.f3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCormack VA, dos Santos Silva I, Koupil I, Leon DA, Lithell HO. Birth characteristics and adult cancer incidence: Swedish cohort of over 11,000 men and women. Int J Cancer. (2005) 115:611–7. 10.1002/ijc.20915 [DOI] [PubMed] [Google Scholar]
- 51.Ruder EH, Dorgan JF, Kranz S, Kris-Etherton PM, Hartman TJ. Examining breast cancer growth and lifestyle risk factors: early life, childhood, and adolescence. Clin Breast Cancer. (2008) 8:334–42. 10.3816/CBC.2008.n.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The plots of the funnel for birth weight and Total leukemia (A), ALL (B), and AML (C).
The sensitivity analyses for low birth weight (A), high birth weight (B), and Total leukemia.
The RRs of birth weight and total leukemia (A), AML (B), and ALL (C).
The dose-response of birth weight and total leukemia (A), AML (B), and ALL (C).
The sensitivity analyses for low birth weight (A), high birth weight (B), and ALL.
The sensitivity analyses for low birth weight (A), high birth weight (B), and AML.
Quality assessment of the 28 observational studies.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.