Abstract
Abstract
Baculoviruses are insect pathogens widely used as biotechnological tools in different fields of life sciences and technologies. The particular biology of these entities (biosafety viruses 1; large circular double-stranded DNA genomes, infective per se; generally of narrow host range on insect larvae; many of the latter being pests in agriculture) and the availability of molecular-biology procedures (e.g., genetic engineering to edit their genomes) and cellular resources (availability of cell lines that grow under in vitro culture conditions) have enabled the application of baculoviruses as active ingredients in pest control, as systems for the expression of recombinant proteins (Baculovirus Expression Vector Systems—BEVS) and as viral vectors for gene delivery in mammals or to display antigenic proteins (Baculoviruses applied on mammals—BacMam). Accordingly, BEVS and BacMam technologies have been introduced in academia because of their availability as commercial systems and ease of use and have also reached the human pharmaceutical industry, as incomparable tools in the development of biological products such as diagnostic kits, vaccines, protein therapies, and—though still in the conceptual stage involving animal models—gene therapies. Among all the baculovirus species, the Autographa californica multiple nucleopolyhedrovirus has been the most highly exploited in the above utilities for the human-biotechnology field. This review highlights the main achievements (in their different stages of development) of the use of BEVS and BacMam technologies for the generation of products for infectious and noninfectious human diseases.
Key points
• Baculoviruses can assist as biotechnological tools in human health problems.
• Vaccines and diagnosis reagents produced in the baculovirus platform are described.
• The use of recombinant baculovirus for gene therapy–based treatment is reviewed.
Keywords: Baculovirus, AcMNPV, BEVS, BacMam, Human diseases
Introduction
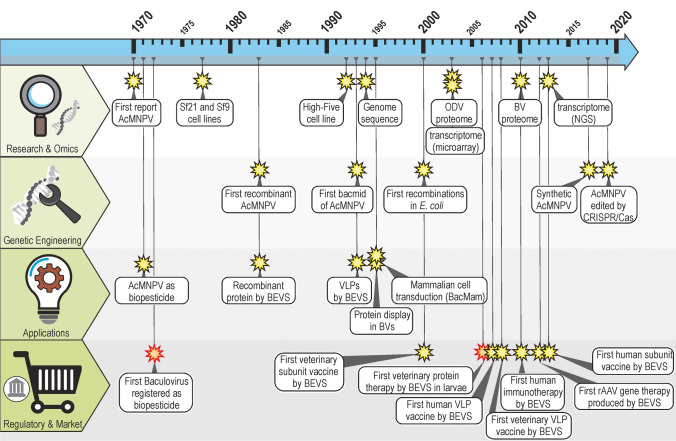
Technologies in the life sciences have grown exponentially since the birth of genetic engineering in the 1970s, a central discipline within biotechnology. The possibility of intervening and modifying the double-stranded DNA molecules of organisms and their associated mobilomes (viruses, plasmids, transposons) has made possible an expansion of the general knowledge about living matter but has also facilitated the emergence of numerous goods and services that have improved the quality of human life. For example, baculoviruses, despite being insect parasites, have become highly useful tools for the development of beneficial products in human health. This review will describe the main technologies related to baculoviruses and human health, the progress in this field, and the opportunities and prospects arising from that work. The recurring challenges of emerging human pathogens that rapidly spread locally or internationally, such as influenza viruses, arboviruses, and coronaviruses, plus the old problems that have plagued humanity for centuries (e.g., cancer and coronary and genetic diseases), constitute a fertile field where baculoviruses and their associated technologies can provide benefits.
Survey methodology
Extensive literature research was conducted using the following electronic databases: PubMed; Science Direct; Google Scholar; and Scopus. The keyword combinations such as baculovirus and molecular biology, baculovirus and viral cycle, baculovirus and expression vector, baculovirus and diagnosis, baculovirus and vaccine, baculovirus and virus like particles, baculovirus and display, emerging and viral diseases, baculovirus and influenza virus, baculovirus and arbovirus, baculovirus and Alphavirus, baculovirus and Flavivirus, baculovirus and dengue virus, baculovirus and zika virus, baculovirus and yellow fever virus, baculovirus and West Nile virus, baculovirus and japanese encephalitis virus, baculovirus and chikungunya virus, baculovirus and coronavirus, baculovirus and SARS-CoV, baculovirus and MERS-CoV, baculovirus and SARS-CoV-2, baculovirus and gene therapy, baculovirus and adeno-associated viruses, baculovirus and BacMam, BacMam and gene therapy, BacMam and cancer, BacMam and vascular diseases, BacMam and tissue engineering, or BacMam and regenerative medicine were utilized to build literature review. All articles were exhaustively studied to be employed as references in the present work.
Original papers, mainly from the last decade, were selected where the BEVS platform was used for the diagnosis, treatment, and prevention of diseases that affected humanity in recent years. A similar criterion was applied for the conceptual use of baculoviruses as gene therapy vectors, selecting those studies that carried out tests on animals. Also, some older papers are considered when their inclusion is important for the topic development. All the articles were selected based on their scientific importance and publication year.
Baculoviruses
The planet’s virome is a space rich in a diversity of parasitic entities of organisms, which abundance and variety offer promising opportunities for biotechnological applications (Paez-Espino et al. 2016). Many viruses that do not infect humans or other mammals thus become tempting instruments for the development of associated technologies—for example, the production of recombinant proteins or the generation of viral vectors adapted to the delivery of specific sequences to target cells and tissues, among other possible applications (Mateu 2011). In this regard, baculoviruses stand out as a prominent group of viral entities with a major role in biotechnology.
Baculoviruses in nature
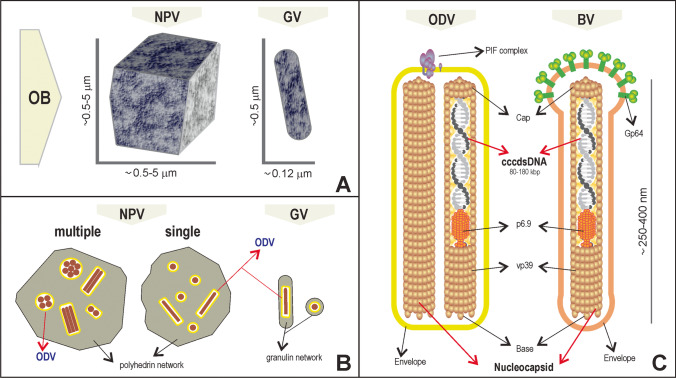
Baculoviruses are double-stranded DNA viruses that infect insects (larval stage) of the orders Lepidoptera, Hymenoptera, and Diptera (Fig. 1). The viral genomes are made up of a large circular molecule (about 80–180 kbp) that is packed in two different structures (Fig. 1C): budded viruses (BVs) or occlusion-derived viruses (ODVs). In both types of virions the viral DNA is associated with proteins conforming to a bacilliform structure known as a nucleocapsid (Nc) which in turn is enveloped by a lipid membrane that is different between BVs and ODVs (Rohrmann 2019). Essential proteins for supporting entry into susceptible cells are located in these membranes. Thus, the F and GP64 proteins are present in the BV envelopes (Westenberg et al. 2007), whereas the “per-os–infectivity factors” (the PIF complex), responsible for penetrating the midgut-epithelium cells of insects, occur in the ODV membranes (Wang et al. 2019b). We need to note that ODVs are, in turn, immersed in a protein crystal—mainly up made of polyhedrin (polh) or granulin depending on the species—denominated occlusion body (OB) that give them great stability in the environment (Fig. 1A). Hence, the name of nucleopolyhedrosis or granulosis that the diseases from these viruses also receive from the microscopically observable shape of their OBs in infected cells. Moreover, certain species of nucleopolyhedrosis carry several Ncs in their ODVs, while others carry only one unit, causing the former species to be referred to as “multiple nucleopolyhedroviruses” and the latter as “single nucleopolyhedroviruses.” Granuloviruses usually carry a single Nc per ODV (Fig. 2B) (Rohrmann 2019).
Fig. 1.
Baculovirus virions. Illustrations describing the main characteristics of baculovirus morphologies. A Representation of typical shapes and dimensions of the occlusion bodies (OBs) of nucleopolyhedroviruses (NPV) and granuloviruses (GVs). B Representation of cross-sections on the OBs, revealing the occlusion-derived viruses (ODVs) present in multiple nucleopolyhedroviruses (MNPVs), single nucleopolyhedroviruses (SNPVs), and GVs. The latter illustrate two different cuts for each particle. C Comparative illustration between ODVs and budded viruses (BVs). The nucleocapsids are the same, but the envelopes are different (that of ODV is derived from the nuclear membrane of the infected cell, whereas that of BV is derived from the plasma membrane). Ncs are polar structures manifesting a different composition and differing forms in the extreme ends and are composed of mainly the VP39 protein. The covalently closed circular double-stranded DNA (cccdsDNA) is associated with a basic protein called p6.9. The per-os–infectivity (PIF) complex is composed of various proteins and is present in only the ODV envelope. The fusogenic GP64 protein is present only in the BV envelope of Group I-alphabaculoviruses (e.g., in AcMNPV), while in the remaining clades this role is performed by the F protein
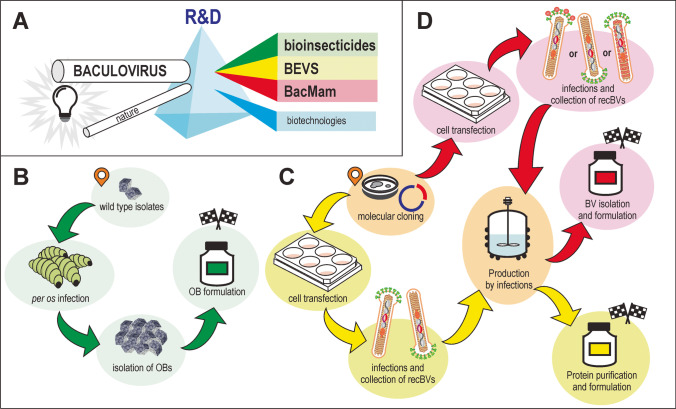
Fig. 2.
Applications of baculoviruses. Illustrations indicating the main uses of baculoviruses. A Because of the research and development carried out on the biology of baculoviruses, 3 main applications have been generated: bioinsecticides, baculovirus expression vector systems (BEVS), and baculoviruses applied to mammals (BacMam). B Main procedures for the generation of bioinsecticide products based on occlusion bodies (OBs). C Main procedures for the generation of protein products based on recombinant budded viruses (recBVs) infecting insect cells and subsequent downstream processes. D. Main procedures for the generation of virion products based on recBVs infecting insect cells and subsequent downstream processes. Only 3 alternatives of recBVs are illustrated: virions that display proteins in the envelope; virions that carry transgenes for the expression in mammal cells; virions that display proteins in the nucleocapsid
The viral cycle begins when susceptible larvae consume OBs. In the midgut, the crystals dissolve through the action of alkaline pH and proteases, and the ODVs are released. These viral forms, once in intestinal cells, will initiate the primary infection, which will then spread to different tissues of the insect through the production of BVs (secondary infection) generally ending in the death of the larva (Saxena et al. 2018).
The family Baculoviridae are composed of numerous species classified into 4 genera (Jehle et al. 2006): Alphabaculovirus (lepidopteran-specific nucleopolyhedroviruses); Betabaculovirus (lepidopteran-specific granuloviruses); Deltabaculovirus (dipteran-specific nucleopolyhedroviruses); and Gammabaculovirus (hymenopteran-specific nucleopolyhedroviruses). The genomes contain about 90–180 protein-encoding genes, of which 38 (known as core genes) are shared by all members (Miele et al. 2011; Garavaglia et al. 2012; Javed et al. 2017). Genes are expressed differentially throughout a viral cycle, either mediated by the host's transcription machinery (early stages) or by the virus-encoded RNA polymerase (late stages). The variable gene content generates a great intra- and interspecies—indeed, pangenomic—diversity (Garavaglia et al. 2012), with many auxiliary genes having been described that, for example, favor viral dissemination (Ishimwe et al. 2015) or produce notable changes in the physiologic state of the larvae (Gasque et al. 2019). The baculoviral prototype species is Autographa californica multiple nucleopolyhedrovirus (AcMNPV; of the genus Alphabaculovirus), from whose properties most nonagricultural biotechnological applications are derived.
Biosecurity aspects for humans and the environment
Baculoviruses are abundant in nature and play fundamental ecologic roles in the population dimension of the insects they infect (Cory and Hails 1997). Because most of these pathogens have a very narrow host range, they depend on particular species of invertebrates for their natural sustenance (Rohrmann 2019). Baculoviruses do not infect vertebrates and although BVs have been found to be able to transduce cells from animals that are not their natural hosts—including mammals (Airenne et al. 2011; Ono et al. 2018; Parsza et al. 2020)—viral DNA is unable to replicate and support progeny generation (Tija et al. 1983; Kost and Condreay 2002; Parsza et al. 2020). In fact, cell lines from mammals transduced with baculoviruses revealed that some viral early genes (e.g., PE38 and IE-01) evidenced a limited expression while the late viral genes (dependent on viral RNA polymerase) remained silent (Shin et al. 2020).
As to immunologic aspects, baculoviruses generate a low response in mammals, but do not evoke systemic antiviral reactions (Gronowski et al. 1999; Abe et al. 2003; Bocca et al. 2013), rather an induction of innate immunity that produces a type I interferon–mediated by Toll-like receptor–dependent and Toll-like receptor–independent pathways (Abe et al. 2005, 2009). Other transcriptomic analyses found similar evidence, including that baculoviruses slightly induce genes associated with Toll-like receptors, cytokine signaling, and complement (Shin et al. 2020).
The BV forms of baculoviruses handled under laboratory conditions are not infective to natural hosts—they would be infective only by injection into larvae—and are completely safe for human operators, as previously mentioned. In view of such considerations, these virions are generally regarded as entities that can be manipulated in facilities with biosafety level 1, as indicated in the guidelines for working with viral vectors of research-and-development laboratories from prestigious universities and institutions worldwide. As has been demonstrated through their uses in agriculture, baculoviruses do not infect nontarget organisms and do not produce adverse effects on plants or humans and other animals (Kost and Condreay 2002).
Baculoviruses in biotechnology
The initial application of baculoviruses (Fig. 2) was the use as active ingredients for bioinsecticide products for the control of agricultural and forestry pests (Haase et al. 2015; Lacey et al. 2015). Currently recognized by the International Committee on Taxonomy of Viruses are 84 species—55 alphabaculoviruses, 26 betabaculoviruses, 1 deltabaculovirus, and 2 gammabaculoviruses (Harrison et al. 2018), most being pathogenic for specific arthropods that are harmful to crops. For this reason, numerous products have been developed and placed on the market, thus participating as one of the alternatives for integrated pest management since the second half of the twentieth century. This development has also enabled the regulatory agencies of many countries to certify the safety of these viruses for humans and other nontarget organisms. We need to emphasize that for this application in the agricultural sector wild-type baculoviral OBs are used and that their production is carried out mainly through infections in larvae reared in insectaries (Haase et al. 2015).
Subsequently, other uses emerged for members of Baculoviridae owing to (i) the facilities offered by genetic engineering through viral-DNA manipulation (Luckow et al. 1993), (ii) the natural condition that the viral genome is infective per se in host cells (Rohrmann 2019), and (iii) the availability of more than 320 insect cell lines capable of multiplying baculoviruses under in vitro conditions (Lynn and Harrison 2016). Unlike the circumstance in the agricultural sector, most of these new applications derive from a single alphabaculovirus, AcMNPV. In particular, the identification of two main groups of applications for engineered baculoviruses is possible: (a) systems for the expression of recombinant proteins within eukaryotic contexts and (b) the use of recombinant virions in mammals for different goals. The former are generally called Baculovirus Expression Vector Systems or simply BEVS (O’Reilly et al. 1994; Possee et al. 2020) and comprise platforms composed of vectors for molecular cloning that enable modifications of the AcMNPV genome (many being based on bacmids) and susceptible insect cell lines plus their culture media or a whole insect as a biofactory (Martínez-Solís et al. 2019). The latter group contains engineered baculoviruses applied to mammals and is recognized as BacMam technologies (Airenne et al. 2013; Mansouri and Berger 2018; Ono et al. 2018). Unlike BEVS, which are a means for the generation of products (e.g., recombinant proteins, virus-like particles), in the BacMam technology, the virions are usually the final product for, among other applications, immunogenic or gene therapy uses (as protein-display systems or as gene vectors). These developments have led to different regulatory considerations because in some instances baculoviruses can be only a contaminant, while in others they are the main ingredient of the products. In contrast to the use of this technology in the production of bioinsecticides (where OBs of many viral species and production systems are used in larvae), genome-engineered BVs of AcMNPV multiplied on the Sf9 or Sf21 insect cell lines, among others, are generally the bases of the BEVS and BacMam technologies (Kwang et al. 2016; Possee et al. 2020).
Baculoviruses as tools for the control and diagnosis of emerging human infectious diseases
The BEVS is a simpler, safer, faster, and easier-to-scale-up method to produce recombinant proteins at a lower cost than traditional systems based on mammalian cell lines. In recent years, this platform has been widely used in academia and industry to produce viral structural proteins (VSPs) for the development of vaccines and therapeutic and diagnostic assays to respond quickly in the event of epidemiologic emergencies (Kumar et al. 2018).
The AcMNPV is the most studied and implemented baculovirus for biotechnological purposes in the world (Premanand et al. 2018). Several commercially susceptible cell lines are available such as those mentioned above derived from the ovary of Spodoptera frugiperda larva (Sf21, Sf9, expresSF +) or Trichoplusia ni larva (BT1-Tn5B1-4, marketed as High Five™) (Martínez-Solís et al. 2019). As a low-cost alternative, the use of whole insect larvae or pupae as an ersatz bioreactor is possible. This technology enables a higher yield than insect cell lines in only a few weeks and at a lower cost. Among the lepidopteran species, the most exploited are S. frugiperda, Rachiplusia nu, and T. ni. In addition, species like Bombyx mori (silkworm) are widely exploited in Asian countries as small biofactories. This species and its derived cell line Bm5, however, are susceptible to another nucleopolyhedrovirus belonging to the family Baculoviridae, the Bombyx mori nucleopolyhedrovirus (Targovnik et al. 2016).
BEVS offers a eukaryotic environment providing adequate posttranslational modification, but the insects are not capable of producing human-type N-glycoproteins (Fabre et al. 2020). Since 1983, when the first protein was expressed by means of this system, different strategies have been implemented in order to improve the technology, upon considering both the host and the viral vector (Martínez-Solís et al. 2019). For instance, the N-glycosylation profile has been improved by the development of novel insect cell lines and viral vectors capable of producing human glycosyltransferase (Palmberger et al. 2013; Maghodia et al. 2021 ). In addition, the system provides strong promoters such as polh and p10 (derived from the polyhedrin and p10 genes, respectively) to produce recombinant proteins. Moreover, new chimeric promoters (polh-pSeL, polhpB2, pB2p10) and other elements have been incorporated into the viral vector to increase the yield and stability of the protein to be expressed (López-Vidal et al. 2013; Martínez-Solís et al. 2019).
In recombinant-protein production, the use of BEVS has two main methodological steps. First, the recombinant virus must be produced, and next the host has to be infected to achieve the final bioproduct. The whole process takes between 4 and 8 weeks (Cox 2012; Cox et al. 2015; Targovnik et al. 2016). Various approaches have been developed for the generation of recombinant baculovirus (rec-baculovirus) including homologous recombination (i.e., BaculoGold™ system, BD Bioscience; BacPAK™ system, Clontech), site-specific transposition (i.e., Bac-to-Bac™ system, Thermo Fisher; Multibac™ system, Geneva Biotech) or combined technologies (i.e., flashBAC™ system, Oxford Expression Technologies) (Martínez-Solís et al. 2019). Furthermore, the systems commercially available have enabled a simultaneous expression of two proteins, as in the example of the Bac-to-Bac™ system (Thermo Fisher), or a multiprotein complex with novel systems such as MultiBac™ (Geneva Biotech). Of course, this degree of expression can be expanded by using internal–ribosome-entry-site sequences or 2A peptides (or by customizing commercial vectors through the addition of new transcriptional units). Moreover, as mentioned before, the system is completely secure from biohazard and represents no form of risk to operators because the baculovirus cannot replicate in mammalian cells. For all of the above considerations, BEVS is a system with great biotechnological value for recombinant-protein production.
As we have emphasized, BEVS is widely used to produce VSPs (Mazalovska and Kouokam 2020). When one or more of these VSPs are coexpressed, they can self-assemble forming nonreplicative and nonpathogenic particles known as virus-like particles (VLPs). VLPs are one of the most promising tools in vaccine development. Various VLP-based vaccines that are currently at a clinical status have been manufactured through the use of BEVS, as has been extensively reviewed by Kumar et al. (2018). In this regard, vaccines against infectious diseases caused by Ebola virus, Enterovirus, Parvovirus, Norwalk virus, Polyomavirus, Papillomavirus, Simian virus 40, Rotavirus, Human immunodeficiency virus, and Respiratory syncytial virus are now being studied (Kumar et al. 2018). These vaccines represent a safer choice than established technologies, like inactivated or attenuated viruses, because those immunogens successfully mimic the virion morphology without the presence of any nucleic acid and, at the same time, are more likely to induce stronger cellular and humoral responses than single-protein vaccines. Within the context of emerging viral infections such as influenza, arboviral, or coronavirus-related diseases, VLPs represent an even more relevant technology in view of their faster development times and greater flexibility than with traditional approaches for endemic diseases like the influenza vaccine manufactured in eggs (Maranga et al. 2002; López-Macías 2012). For the development of these strategies, baculovirus technology is a powerful ally because of the ability to assemble viral particles with high protein yields, with safer and simpler handling, and in the absence of adventitious agents or egg-related contaminants.
In addition, and likewise, for vaccine purposes, the baculovirus itself can be used as an antigen-presenting vehicle by displaying immunogenic peptides or recombinant proteins on its surface (Tsai et al. 2020). This technology (being able to be included in BacMam approaches for vaccine uses) is known as baculovirus display and has the ability to mount a strong immune response. The antigen presentation on the BV surface is achieved mainly by the fusion of heterologous proteins with GP64, the major surface glycoprotein of group-1 alphabaculovirus BVs (cf. the BV, Fig. 1C) (Kost et al. 2005; Xu et al. 2011). Moreover, baculoviruses can be used as DNA vectors to express the antigen in the tissue where the vaccine is injected, as is the circumstance with the new generation of recombinant adenovirus-based vaccines.
In summary, baculovirus technology has been successfully implemented (i) to express VSPs for use as vaccine candidates, with those immunogens exhibiting strong humoral and cellular responses, (ii) to develop diagnostic tests, and (iii) to produce VLPs with excellent results and comparative advantages versus established systems—including administration routes and the potential use as a delivery vector. All of these advantages indicate the widespread use of this technology as a tool for studying and developing strategies for future emerging virus threats and pandemics. In the next section, we will describe the use of baculovirus in the fight against the main emerging viral diseases, where most of the research work has been carried out in recent years (i.e., influenza viruses, arboviruses, and coronaviruses). The use of the platform for the diagnosis and prevention of other clinically significant viral diseases, however, are comprehensively reviewed by Kumar et al. (2018).
Influenza vaccines and diagnostic-reagent production
Influenza—caused by the virus of the same name—is a highly contagious respiratory disease spread worldwide that exhibits high rates of morbidity and mortality. Among the four types of influenza viruses, A and B are responsible for the greatest number of seasonal infectious, producing around 5 million cases of virus-mediated severe flu and around 650,000 fatal cases per year all over the world (Harding and Heaton 2018; Basak et al. 2020). Furthermore, influenza A has caused four pandemics since 1918 (Harding and Heaton 2018), one being produced by the H1N1 influenza A in 2009 (Shim et al. 2019).
Influenza is a segmented negative-strand RNA virus belonging to the Orthomyxoviridae family. The virus envelope is formed by a lipid bilayer and three transmembrane surface proteins encoded by the viral genome: hemagglutinin (HA), neuraminidase (NA), and the proton-channel protein M2 (Veit and Thaa 2011). These components together with the viral-matrix protein 1 (M1) contribute to the virus assembly (Hilsch et al. 2014). Among the VSPs, the viral glycoprotein, HA and NA, are the most antigenic surface species involved in the viral pathogenesis—i.e., attachment and release, respectively (Kash et al. 2004). At least 18 different HA subtypes exist (H1–H18) along with 11 NA subtypes (N1–N11) (Kosik and Yewdell 2019).
Serious influenza infection is prevented through a vaccination that reduces the impact of the disease (Fauci 2006). Because HA is the only influenza component responsible for the induction of neutralizing and protective antibodies in infected individuals, hemagglutinin is usually the principal component in vaccine production (Ting-Hui-Lin et al. 2019). Notwithstanding, antibodies directed against NA reduce the viral replication and the severity of the disease by blocking the viral receptor; therefore, NA can also be an effective ingredient for influenza vaccines (Deng et al. 2012). As both the antigens HA and NA are subject to selected point mutation, the formulation of the influenza vaccines must be annually updated in the fight against the seasonal flu (Fauci 2006). Often, new influenza subtypes with new antigenic properties can arise because of the reassortment of viral genome components among different virus strains. This trait shuffling may be a cause of pandemic outbreaks (Harding and Heaton 2018).
The conventional production platforms for the inactivated influenza virus vaccines are based on embryonated eggs and, in consequence, do not offer a rapid response in the circumstance of a pandemic. The production requires around 6 months after the circulating strain be detected (Ting-Hui-Lin et al. 2019). This use of eggs works well, although the approach has many other drawbacks. For example, the whole-virus manipulation requires specialized containment laboratories, thus complicating the scaling-up in a pandemic outbreak. In addition, an adaptation of the viral strain to multiplication in eggs is also usually necessary, a time-consuming process that is not always successful. Furthermore, the production requires the use of antibiotics and noxious chemicals for the final inactivation of the virus. and this type of vaccine is even not recommended for sufferers of egg-protein allergy. To overcome these limitations, different alternative systems to produce the influenza vaccines in a more rapid and cost-effective way are being explored (Harding and Heaton 2018; Ting-Hui-Lin et al. 2019). Among these approaches, the recombinant subunit protein–based vaccines have been extensively explored, with the associated manufacturing process not requiring specialized facilities for virus manipulation. The production involves the generation of the viral-envelope antigen via recombinant-DNA technology and further utilization of the purified antigen as the active ingredient. Consequently, BEVS emerged as a suitable alternative flexible platform for the fast production of the viral antigen by the infection of insect cells with rec-baculoviruses containing the HA gene (He et al. 2009). Within this context, in 2013 the Food and Drug Administration (FDA) in the USA licensed the first recombinant HA-based trivalent influenza-virus vaccine (a cocktail of three strains) named FluBlok® developed by the Sanofi Pasteur-Protein Science Corporation and produced in infected expresSF + insect cells. This cell line was established to grow in serum-free medium at high densities in suspension cultures that are scalable in simple stirred tank reactors (Buckland et al. 2014). The three HA variants are expressed by coinfection with three independent rec-baculoviruses encoding different human hemagglutinins (Meghrous et al. 2009). Standardized upstream and downstream processes have been developed to rapidly produce new HA variants and the entire procedure requires only 8 weeks (Cox et al. 2015). This vaccine has been demonstrated to be safe, immunogenic, and effective after several clinical trials were conducted (King et al. 2009); and in 2017, the trivalent vaccine was replaced by a quadrivalent version.
Moreover, with an aim at reducing the production costs and improving the antigen-expression levels, living insect larvae were also implemented as biofactories. Thus, the expression of the HA ectodomain—fused with the retention signal KDEL, the (Lys-Asp-Glu-Leu) endoplasmic-reticulum protein—in T. ni larvae infected with rec-baculoviruses was four-fold higher than that obtained with Sf21 insect cells (Gomez-Casado et al. 2011). In addition, the HA ectodomain fused with the viral signal peptide of GP64 was more highly expressed in the hemolymph of Spodoptera litura larvae (Hsieh et al. 2018). This signal peptide facilitated the necessary posttranslational modifications along with the secretion of the recombinant protein (Targovnik et al. 2019). The HA concentration in the hemolymph was around 100-fold higher than in Sf21 cell-culture medium (Hsieh et al. 2018). In all these reports, the larva-derived HA induced protective immunity in vaccinated mice.
Recently, BEVS has also been used as an antigen-expression platform for the development of diagnostic tests for influenza. Shim et al. (2019) developed a process to produce in insect cells the HA ectodomain as a suitable antigen to develop an enzyme-linked immunosorbent assay (ELISA) for detecting anti-HA antibody in serum samples derived from infected and vaccinated individuals (Shim et al. 2019).
Furthermore, vaccines based on NA have also been proposed to prevent viral spread (Faletti et al. 2014) such as FluNhance™ (Sanofi Pasteur-Protein Science Corporation), which is an influenza-subunit vaccine based on a recombinant expression of the NA antigen in insect cells. This product is currently undergoing clinical trial (phase II challenge) and would be used as a booster of the already licensed vaccine to thus induce a broader and more protective immunity. Individuals vaccinated with FluNhance™ in combination with the conventional influenza virus vaccine have developed milder illnesses (Johansson et al. 2002). We must also mention that NA has likewise been successfully expressed in insect larvae. In fact, the production of the same amount of NA that is generated in 153 mL of cell suspensions was possible with only six larvae of R. nu (Faletti et al. 2014).
In addition, BEVS were used to develop an influenza-VLP vaccine by coexpressing HA, NA, and M1 proteins in insect cells (Bright et al. 2007), thus representing a significant improvement in immunogenicity with respect to the recombinant-subunit vaccine. These VLPs can simulate the virus structure and induce protective immunity against the infection owing to the production of high anti-HA– and anti-NA–antibody titers (Bright et al. 2007; He et al. 2009). In this regard, different eukaryotic cells have been explored for the production of VLP vaccines against influenza (Deng et al. 2012). Nevertheless, the BEVS resulted in the best approach for that purpose because they provided an efficient platform to express multiple recombinant proteins simultaneously (Lai et al. 2019). Several reports demonstrated the effectiveness of VLPs produced in BEVS to confer protection against the influenza virus when those prototypes were administered via either the intramuscular or intranasal immunization routes in mice challenged with a lethal dose of the virus (Bright et al. 2007; Hahn et al. 2013; Smith et al. 2013; Ren et al. 2018; Lai et al. 2019). Within this context, Novavax manufactures a VLP vaccine for different influenza A and B strains through the coexpression of only the three structural proteins (HA, NA, and M1) in Sf9 insect cells infected with three different rec-baculoviruses (Hahn et al. 2013). The Novavax influenza VLP vaccine has proved both safe and immunogenic in phase I and phase II clinical trials (Deng et al. 2012; Hahn et al. 2013). The production, formulation, and inspection require only 2 months, after which time the product-release test needs 3 weeks more (Hahn et al. 2013). Recently, Lai et al. (2019) developed an improved method of manufacturing H7N9 VLP vaccine by coexpressing the H7, N9, and M1 proteins in High Five™ insect cells and an adequate level of dissolved oxygen (150 mmHg) at a high multiplicity of infection (Lai et al. 2019).
An alternative strategy has been established to produce vaccines based on nanoparticles. Thus, the production of NanoFlu® (a quadrivalent nanoparticle vaccine) involving the expression of HA through the use of BEVS has been developed by Novavax. Once the four HA variants are simultaneously expressed in Sf9 cells, the proteins are assembled into a nanoparticle during the purification. The vaccine is finally formulated with the patented saponin-based adjuvant Matrix M™ (Novavax) that improves the activation of innate immune cells and antigen presentation (Khalaj-Hedayati et al. 2020). The NanoFlu® vaccine is currently in phase III clinical trials (Shinde et al. 2021).
Unlike the variable HA proteins, the conserved M2 ectodomain has been studied as a presumed universal antigenic target for developing vaccines that produce cross-protective immunity. Kim et al. (2013) developed a VLP-based vaccine candidate by Sf9-cell coinfection with a rec-baculovirus expressing five tandem repeats of the heterologous M2 ectodomain (M2e5x) from different influenza A strains and a baculovirus expressing the M1 matrix protein. To improve the incorporation of M2 into the VLPs, M2e5X was fused to the HA transmembrane domain (Kim et al. 2013). This vaccine induces lower neutralizing antibodies than the VLP based on HA expression, but the M2e5X’s VLPs improved the effectiveness of the traditional attenuated influenza-virus–based vaccine and stimulated cross protection when tested in mice (Kim et al. 2013; Lee et al. 2019).
The simultaneous coexpression of multiple proteins to achieve influenza vaccines involves the coordinate synchronous cell infection with several rec-baculoviruses. This strategy may be inefficient on large scale, or else would require baculovirus shuttle vectors that accept more than one heterologous gene so as to result in larger DNAs that are unstable (Sequeira et al. 2018; Martínez-Solís et al. 2019). To circumvent this obstacle, several reports indicated that recombinant insect cells would provide a promising platform to manufacture functional influenza VLPs (Matsuda et al. 2020). For instance, High Five™ cells stably transformed with HA and M1 efficiently produce VLPs at a yield comparable to those obtained with the baculovirus–insect-cell system (Matsuda et al. 2020). Another report developed a combined approach to express five HAs at the same time to produce a pentavalent HA VLP. The strategy involved High Five™ cells stably transformed with two different HA genes and the subsequent infection with a rec-baculovirus that encoded the other three HAs plus M1. This study demonstrated an efficient and scalable platform to produce multivalent VLPs (Sequeira et al. 2018).
The use of nonreplicative baculoviruses as gene-delivery DNA vectors for the assembly of VLPs in mammalian cells represents another promising strategy for influenza-vaccine development. Regarding this BacMam application, a rec-baculovirus was constructed, carrying influenza HA, NA, and M1 genes under the control of the human cytomegalovirus immediate-early enhancer and promoter. To improve targeted gene delivery, Gwon et al. (2016) incorporated into the baculovirus genome the envelope-glycoprotein–encoding sequence from the human endogenous retrovirus. As a result, mice immunized with the recombinant BVs produced a strong humoral response and neutralizing antibodies after the challenge with a lethal dose of influenza virus (Gwon et al. 2016).
Finally, the antigen-displaying alternative baculoviruses are also being explored as another approach to developing vaccines for influenza. The HA incorporation into the baculovirus surface demonstrated an ability to elicit strong humoral and cell-mediated immunity that protected animals from the lethal challenge of influenza viruses (Prabakaran et al. 2008; Prabakaran and Kwang 2014; Yu et al. 2020). This vaccine can be administrated by the subcutaneous or intranasal route, thus enabling a new alternative in the development of vaccines against mucosal pathogens (Prabakaran and Kwang 2014; Sim et al. 2016). The rec-baculoviruses displaying HA antigens against influenza disease are extensively reviewed by Premanand et al. (2018).
Table 1 provides a summary of the principal influenza vaccines developed through the use of BEVS and BacMam platforms.
Table 1.
Summary of vaccine produced in BEVS and BacMam platform in the campaign against influenza-virus infection
| Baculovirus tool | Host | Protein and Strain | Type of vector | Development stage | References |
|---|---|---|---|---|---|
| Protein or subunit expression | |||||
| Sf9 cells | HA from A/H6N1 | BaculoGold™ (BD Biosciences) | Exploratory | Faletti et al. (2014) | |
| Sf9 cells | HA from A/H1N1, H3N2, and two strain B. Contain saponin-based Matrix M adjuvant | ND | Phase-III clinical trial (Nanoflu™, Novavax) | Shinde et al. (2021) | |
| expresSF + cells | NA (strain ND) | ND |
Clinical trial II (FluNhance ™, Sanofi Pasteur-Protein Science Corporation) |
Deng et al. (2012) | |
| expresSF + cells | HA from A/H1N1, A/H3N2, and two strain B | ND | Approved (FlubloK, Sanofi Pasteur-Protein Science Corporation) | Buckland et al. (2014) | |
| T. ni larvae | HA ectodomain fused with KDEL from A/H1N1 | ND | Preclinical | Gomez-Casado et al. (2011) | |
| S. litura larvae | HA from A/H6N1 |
Bac to Bac™ (Thermo Fisher) |
Preclinical | Hsieh et al. (2014) | |
| R. nu larvae | NA from A/H1N1 | BaculoGold™ (BD Biosciences) | Exploratory | Faletti et al. (2014) | |
| VLP | |||||
| Sf9 cells | HA, NA, M1 from H3N2 and H7N9 |
Bac to Bac™ (Thermo Fisher) |
Preclinical | Bright et al. (2007); Smith et al. (2013); Ren et al. (2018) | |
| Sf9 cells | HA, NA, M1 from H5N1 |
Bac to Bac™ (Thermo Fisher) |
Phase-I/II clinical trial | Khurana et al. (2011) | |
| Sf9 cells | M2e5x (five tandem repeat) from A/H3N2, two A/H1N1, and two A/H5N1 |
Bac to Bac™ (Thermo Fisher) |
Preclinical | Kim et al. (2013) | |
| Sf9 cells | HA, NA, M1 from H7N9 | ND | Phase-I/II clinical trial | Hahn et al. (2013) | |
| Sf9 cells | HA, NA, M1 from A/H1N1, A/H3N2 and two strain B | ND | Phase-IIa clinical trial (Novavax) | Kumar et al. (2018) | |
| High Five™ cells | HA, NA, M1 from A/H7N9 |
Bac to Bac™ (Thermo Fisher) |
Exploratory | Lai et al. (2019) | |
| Display | |||||
| Sf9 cells | HA from A/H7N9 | ND | Preclinical | Prabakaran et al. (2008) | |
| Sf9 cells | HA from A/H1N1 |
Bac to Bac™ (Thermo Fisher) |
Preclinical | Sim et al. (2016) | |
| Gene delivery | |||||
| Sf9 cells | HA, NA, M1 from A/H1N1 |
Bac to Bac™ (Thermo Fisher) |
Preclinical | Gwon et al. (2016) | |
M1 viral-matrix protein 1; HA hemagglutinin; NA neuraminidase; VLP virus-like particles. All preclinical tests were performed in mice; ND no data
Arboviruses vaccines and diagnostic-reagent production
Arboviruses (arthropod-borne viruses) are responsible for a large number of viral diseases that are transmitted to humans through the bites of arthropods (such as mosquitoes and ticks). While certain arboviral infections can be asymptomatic or cause mild febrile illness, others can be more severe causing encephalitis, hemorrhagic fever, joint pain, or even lead to death (Marchi et al. 2018). In fact, arboviral diseases represent 17% of communicable diseases worldwide, affecting millions of people and causing an estimated more than 700,000 deaths annually (Kading et al. 2020; World Health Organization 2020). In the last 50 years, these diseases have spread rapidly, causing epidemics around the world. Vaccination is the most effective method of preventing such infectious diseases, though few commercial vaccines have been approved for humans against certain arboviruses such as the Yellow fever virus (YFV), the Dengue virus (DENV). and the Japanese encephalitis virus (JEV), for which entities the vaccine development involves whole-virus manipulation (Krol et al. 2019). The recombinant-subunit and VLP vaccine approaches offer a quicker and lower-risk alternative to vaccine manufacturing, thus avoiding exposure to biohazardous agents (Cho et al. 2008). Accordingly, several eukaryotic expression platforms such as plant, yeast, mammalian, and insect cells have been exploited to produce recombinant antigens for controlling and preventing an imminent arbovirus outbreak (Martínez et al. 2012; Wilder-Smith et al. 2017; Girard et al. 2020). Since arboviruses replicate efficiently in arthropod cells, BEVS has emerged as a suitable and biosafe technology to produce authentic arbovirus proteins for the manufacture of new vaccines and diagnostic methods in the campaign against arboviruses transmitted by mosquitos, the former proteins mainly from the genera Flavivirus (Family Flaviviridae) and Alphavirus (Family Togaviridae), as summarized in Table 2.
Table 2.
Summary of products manufactured in BEVS and BacMam platform in the campaign against arbovirus infections
| Arbovirus | Product | Baculovirus tool | Structural protein | Type of vector | Host | References |
|---|---|---|---|---|---|---|
| Dengue virus | ||||||
| Vaccine (preclinical stage) | Protein or subunit expression | E ectodomain from DENV-2 | AcRP23-lacZ | Sf9 cells | Staropoli et al. (1997) | |
| Vaccine (preclinical stage) | Protein or subunit expression | Fusion of E ectodomain from DENV-1, DENV-3, and DENV-4 | pVL (stratagene) and AcMNPV genome | Sf9 cells | Rantam et al. (2015) | |
| Vaccine (preclinical stage) | Protein or subunit expression | Consensus E protein |
Bac to Bac™ (Thermo Fisher) |
Sf9 cells | Sun et al. (2017) | |
| Vaccine (preclinical stage) | Protein or subunit expression | E full-length from DENV-2 | pBlueBacIII + linearized AcMNPV (Invitrogen) | High Five™ cells | Kelly et al. (2000) | |
| Vaccine (preclinical stage) | Protein or subunit expression | DomIII from DENV-1 and DENV-2 fused to HFB |
Bac to Bac™ (Thermo Fisher) |
R. nu larvae | Cerezo et al. (2020) | |
| Vaccine (exploratory stage) | VLP | C, prM, E from DENV-2 |
Bac to Bac™ (Thermo Fisher) |
B. mori larvae | Utomo et al. (2019) | |
| Two-step MAC-ELISA | Protein or subunit expression | Tandem repeat of DomIII from four serotype | pAcGP67/pSecG2T (BD Bioscience) AcPak6 DNA (Invitrogen) | Sf9 cells | Niu et al. (2015) | |
| Zika virus | ||||||
| Vaccine (preclinical stage) | VLP | prM-E |
Bac to Bac™ (Thermo Fisher) |
Sf9 cells | Dai et al. (2018) | |
| Vaccine (preclinical stage) | Display | E ectodomain |
Bac to Bac™ (Thermo Fisher) |
Sf9 cells | Luo et al. (2020) | |
| Point-of-care IgG and IgM diagnosis | Protein- or subunit-expression platform | E, NS1 | BaculoGold™ (BD Biosciences) | Sf9 cells | Kim et al. (2018) | |
| Yellow fever virus | ||||||
| Vaccine (preclinical stage) | Protein or subunit expression | E, NS1 | ND | Sf9 cells | Despres et al. (1991) | |
| IgM capture ELISA | Protein or subunit expression | E | pSynXIV/ vSynV1gal | High Five™ cells | Barros et al. (2011) | |
| West Nile virus | ||||||
| Vaccine (preclinical stage) | Protein or subunit expression | E |
pPSC12 (Protein Science Corporation)/ Bsu linearized AcMNPV |
expresSF + cells | Bonafé et al. (2009) | |
| Vaccine (preclinical stage) | VLP | NS1 |
Bac to Bac™ (Thermo Fisher) |
Sf9 cells | Qiao et al. (2004) | |
| Competitive ELISA | Protein or subunit expression | NS1 |
Bac to Bac™ (Thermo Fisher) |
Sf9 cells | Yeh et al. (2012) | |
| IgG indirect ELISA | Protein or subunit expression | E |
Bac to Bac™ (Thermo Fisher) |
T. ni larvae | Alonso-Padilla et al. (2010) | |
| Chikungunya virus | ||||||
| Vaccine (exploratory stage) | Protein or subunit expression | E1, E2 |
Bac to Bac™ (Thermo Fisher) |
Sf21 cells | Metz et al. (2011) | |
| Vaccine (exploratory stage) | VLP | E1, E2 |
Bac to Bac™ (Thermo Fisher) |
Sf21 cells | Metz et al. (2013) | |
| Vaccine (exploratory stage) | VLP | E1, E2 |
Bac to Bac™ (Thermo Fisher) |
Sf9 Basic cells | Wagner et al. (2014) | |
| IgM indirect ELISA and immunochromatographic assay | Protein or subunit expression | C |
Bac to Bac™ (Thermo Fisher) |
Sf9 cells | Cho et al. (2008) | |
| IgG capture ELISA | Protein or subunit expression | E1 |
Bac to Bac™ (Thermo Fisher) |
Sf9 cells | Kumar et al. (2014) | |
E envelope; prM premembrane; C capsid; NS1 nonstructural protein 1; DomIII E protein domain III; HFB hydrophobin; VLP virus-like particles. All preclinical tests were performed in mice; ND no data
Flaviviruses are enveloped, positive-sense, single-stranded RNA viruses whose genomes encode six nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B) essential for viral-RNA replication along with another three structural proteins present in the virion: the capsid (C), the membrane (M), and the envelope (E). In particular, the ectodomain of the E glycoprotein is divided into three structurally distinct domains (DomI, DomII, and DomIII), where DomIII is implemented in viral binding to the host-cell receptor and is the main antigenic component to induce protective immunity against flavivirus infection (Modis et al. 2003). Therefore, the E protein, and especially its DomIII, is a prime candidate antigen for vaccine and specific diagnostic-kit development. The Flaviviridae family contains five species with great impact on public health in recent years: the DENV, the Zika virus (ZIKV), and the YFV, which are transmitted by mosquitoes of Aedes spp.; plus the JEV and the West Nile virus (WNV), which are transmitted by mosquitoes of Culex spp. (Gould et al. 2017).
Nowadays, DENV, the causative agent of dengue fever and dengue hemorrhagic fever, is one of the contagious viral diseases most widely distributed throughout the world. An increasing number of dengue outbreaks have occurred in recent times, resulting in around 400 million reported dengue-infection cases yearly (Pierson and Diamond 2020). Unlike other flaviviruses, the DENV is subdivided into four serotypes (DENV1 to DENV4). Although great efforts have been made to develop vaccines that simultaneously protect against all four variants (VanBlargan et al. 2013), only one vaccine for DENV infection is currently approved and with partial effectiveness depending on the serotype and the individual’s basal immunologic status and age (Hernández-Ávila and Santos-Preciado 2016). Dengvaxia (Sanofi Pasteur) is a live-attenuated tetravalent vaccine based on the yellow fever viral backbone that expresses the precursor membrane (prM) and the E proteins from the four DENV serotypes (VanBlargan et al. 2013). In order to achieve a more effective vaccine, BEVS has currently been used to safely generate dengue antigens (Metz and Pijlman 2011). In this regard, the E ectodomain, comprising the three E domains, was successfully expressed in insect cells through the use of rec-baculoviruses (Delenda et al. 1994; Staropoli et al. 1997). The protein retained its antigenicity and was capable of eliciting neutralizing antibodies that protected mice from lethal virus infection (Eckels et al. 1994). In the second approach, the DENV-2 E was expressed, but fused to the prM translocation signal. As a result, an aggregated E was obtained with a native folding exposing functional epitopes that induced neutralizing antibodies in mice (Kelly et al. 2000). As a low-cost strategy, the production of dengue antigens in whole-insect larvae is possible. Within this context, Cerezo et al. (2020) demonstrated that the DomIII from DENV-1 and DENV-2 fused to hydrophobin (DomIIIHFB) and produced in insect larvae could elicit serotype-specific neutralizing antibodies in mice without cross-reacting against heterologous serotypes and other flaviviruses. Therefore, the DomIIIHFB expression belonging to the four serotypes would be useful for providing reagents for the formulation of a low-cost tetravalent subunit vaccine (Cerezo et al. 2020).
An alternative approach involving the expression of synthetic consensus proteins based on the amino acid sequence belonging to all serotypes has emerged as an excellent alternative to achieve tetravalent vaccine antigens. For instance, a consensus protein of the dengue E ectodomain, has been expressed in Sf9 cells infected with rec-baculoviruses. The antigen was capable of eliciting high titers of specific protective antibodies against all the serotypes. Moreover, the vaccine could activate an antigen-specific T-cell response (Sun et al. 2017). In addition, a fusion of the E ectodomain belonging to three distinct DENV serotypes (1, 3, and 4) was expressed with the ability to induce humoral and cellular responses (Rantam et al. 2015). A recombinant fusion E protein can also be useful as a reagent for efficient immunodiagnostic-kit development with the potential to detect all four serotypes at once. In this scenario, a tandem repeat of DomIII belonging to four serotypes was expressed in Sf9 cells infected with rec-baculoviruses. The protein was successfully used as an antigen for developing an ELISA capture to detect anti-DENV IgM antibodies in sera from patients at early stages of infection (Niu et al. 2015). In addition, the use of infected R. nu larvae to express DomIII from DENV-2 resulted in a low-cost platform to produce immunodiagnostic reagents. The VLP approach has also been employed to produce a dengue vaccine. There, the coexpression of the structural proteins C, prM, and E belonging to DENV-2 was found to lead to VLP formation when expressed in baculovirus-infected silkworm larvae (Utomo et al. 2019). Furthermore, DENV-2 VLPs were also produced by a transient-expression system through transfection of prM and E genes into Sf9 cells (Kuwahara and Konishi 2010).
ZIKV, for its part, is a flavivirus globally disseminated and associated with possible neurologic complications, the coexpression of whose structural proteins (prM and E) in Sf9 insect cells infected with rec-baculovirus also led to an assembly of VLPs . After administration to mice, the ZIKV VLPs induced a protective immunity that elicited neutralizing antibodies, virus-specific IgG titers, and a T-cell immune response (Dai et al. 2018). Recently, a report described for the first time the display of the E protein on the BV surface and the resulting immunogenic and protective effect against ZIKV. To the display, E was fused with the signal peptide and the transmembrane domain from AcMNPV GP64 (Luo et al. 2020). For the purpose of ZIKV diagnosis, the nonstructural proteins NS1 and E from the ZIKV were efficiently produced in insect cells infected with rec-baculoviruses. The purified antigen and monoclonal antibodies were used to develop a point-of-care diagnosis to rapidly detect IgG and IgM antibodies against ZIKV in sera from patients (Kim et al. 2018). The test appears as a novel technology and an alternative compared to traditional methods used in ZIKV diagnosis.
The E and NS1 proteins, or a fusion of both (E-NS1), have also been expressed in insect cells infected with rec-baculoviruses in order to produce a vaccine candidate against yellow fever (YF), a reemerging viral zoonosis caused by the YFV. Of all construction tested, E-NS1 protein has resulted in the most immunogenic construction, whose administration proved to protect mice from a lethal viral challenge (Despres et al. 1991). YF infection is prevented by a highly effective vaccine (YFV-17D), but major outbreaks still occur due to the failure of vaccination campaigns in certain regions, mainly belonging to Africa and Central and South America (Pijlman 2015). Despite the proven efficacy of the vaccine, it is based on attenuated viruses, vaccination is not recommended for persons older than 60 years or younger than 6 years of age, or for allergic and immunocompromised patients. Therefore, BEVS offers an alternative platform to develop immunogens that could be administered regardless of people’s age or health status (Araujo et al. 2020). Furthermore, a YF diagnosis can be performed with the E antigen expressed in High Five™ cells as a reagent to develop an immunoassay to detect specific antibodies in the serum of infected patients (Barros et al. 2011).
Among the flaviviruses transmitted by Culex spp., one of the most widely distributed throughout the world is the WNV, causing a generally asymptomatic infection, but certain cases of infection can lead to severe clinical manifestations and even death (Krol et al. 2019). Several vaccine candidates have been developed against the WNV through the use of BEVS. For example, the soluble ectodomain E protein was expressed in expresSF + cells, while WNV-antigenic VLPs were also produced in Sf9 cells by the coexpression of prM and E VSPs (Qiao et al. 2004; Bonafé et al. 2009). Both vaccine candidates resulted in stable, nontoxic, immunogenic preparations that were protective in model animals inoculated with WNV lethal doses. The diagnosis of WNV is currently carried out by an ELISA test involving the whole virus as a reagent. Nevertheless, the development of a cheaper and safer serological test through the use of the recombinant protein E expressed in T. ni larvae infected with rec-baculoviruses was possible. The antigenic component resulted correctly folded like the native counterpart and was recognized by the sera from infected animals (Alonso-Padilla et al. 2010). In another study, recombinant NS1 produced in rec-baculovirus-infected Sf9 insect cells was used as a reagent to develop a competitive ELISA assay (Yeh et al. 2012).
The JEV is another serious disease-producing flavivirus transmitted by Culex spp. mosquitoes. The infection is spread mainly in Asian countries causing around 50,000 cases with some 15,000 deaths per year (Nerome et al. 2018). Even though a vaccine against JEV has been approved for human use (IXIARO, Valneva), BEVS has been explored as an alternative platform to achieve more cost-effective prototypes. In this regard, the E protein has been successfully expressed in insect cells infected with rec-baculoviruses, where the recombinant protein induced neutralizing antibodies in mice. Moreover, through the expression of the E protein alone or the coexpression of the E with prM in rec-baculovirus-infected Sf9 insect cells, the production of JEV VLPs was possible that were 10 times more abundant than those produced in Chinese-hamster-ovary (CHO) cells (Yamaji and Konishi 2013; Du et al. 2015). In addition, stably transformed insect cells were developed for the constitutive production of JEV VLPs (Yamaji and Konishi 2013). As a low-cost alternative, large amounts of VLPs were furthermore produced in silkworm pupae infected with rec-baculoviruses that subsequently induced protection in mice (Nerome et al. 2018).
Finally, the Chikungunya virus (CHIKV) belonging to Alphavirus genus is the causative agent of chikungunya fever, an infectious disease that has recently captured the scientific community´s attention as an emerging threat to public health (Kading et al. 2020). No vaccine or specific treatment for CHIKV infection is currently available (Kuo et al. 2016). Like the Flavivirus, the Alphavirus contains enveloped positive-sense single-stranded RNA molecule as genome. The mature virion consists of three structural proteins: the Capsid (C); and the two major envelope surface glycoproteins, E1 and E2, that form the spike complex on the virion surface. E1 and E2 are highly immunogenic and are involved in mediating the fusion and interaction with the host receptor during the infection. BEVS were also extensively used in the production of alphavirus antigens to manufacture vaccine candidates and diagnostic tests. Several studies indicated that the structural proteins E1 and E2 expressed through the BEVS platform are correctly processed (Metz et al. 2011, 2013). At this point, an immunoassay to detect specific IgM and IgG antibodies from infected patients has been developed with the structural CHIKV proteins expressed in BEVS as antigens (Cho et al. 2008; Kumar et al. 2014). In addition, a stable Sf9 cell line was developed that expressed the CHIKV E2 as an antigen for the serodiagnosis of CHIK (Chua et al. 2016). A study has found that CHIKV structural proteins expressed in baculovirus-infected Sf9 cells self-assemble into VLPs (Metz et al. 2013; Wagner et al. 2014). To enhance the yield of CHIKV VLPs, a new Sf9 cell line, designated Sf9Basic, was developed with the ability to grow at high pH (Wagner et al. 2014). Moreover, CHIKV VLPs with Matrix M™ as an adjuvant were more immunogenic than a subunit vaccine in animal models and protected against lethal viral doses. Thus, these VLPs constitute promising candidates for vaccines to prevent CHIKV infection (Metz et al. 2013; Wagner et al. 2014).
Coronaviruses vaccines and diagnostic-reagent production
Coronaviruses—enveloped, positive-sense, single-stranded RNA viruses, with large genomes ranging from 26 to 32 kb—are phylogenetically divided into four genera: Alpha- and Beta- (those further subdivided into four lineages A, B, C, and D) plus Gamma- and Deltacoronavirus (Pallesen et al. 2017). To date, the seven known coronaviruses are able to infect humans, of which four (HCoV-OC43, HCov-229E, HCoV-HKU1, and HCov-NL63) circulate endemically in humans and are generally considered harmless causing only mild respiratory diseases. In contrast, the Middle East respiratory syndrome coronavirus (MERS-CoV) isolated in 2012 in Saudi Arabia, the severe acute respiratory syndrome coronavirus (SARS-CoV) isolated in 2003 in south China, and the newly identified SARS-CoV-2 isolated in 2019 in Wuhan, China, produce much more severe clinical outcomes, with pulmonary, cardiovascular, and renal involvement. In 2002, the SARS epidemic produced a total of 916 deaths in 8,098 patients diagnosed in several countries around the globe, while the MERS epidemic caused 858 deaths in 2,254 cases (Song et al. 2019) and the current SARS-CoV-2 pandemic over 4 million deaths in more than 190 million cases worldwide (World Health Organization 2021).
Coronaviruses encode four structural proteins (Zeng et al. 2004): the membrane protein (M), essential for assembly and budding; the envelope protein (E), also involved in assembly; the spike protein (S), responsible for viral entry and membrane fusion; and the nucleocapsid protein (N). During infection, the spike behaves as the main antigenic determinant and target of neutralizing antibodies, with its receptor-binding domain being the region where most sites are concentrated for neutralizing-antibody recognition.
BEVS can be a useful technology for studying coronaviruses and developing diagnostic tests and vaccines, as this platform has already been successfully implemented in the production of commercially available vaccines such as those for influenza (Cox and Hashimoto 2011). Examples of BEVS-mediated immunogens and reagents are summarized in Table 3. Thus, BEVS were used to produce the recombinant S protein derived from the Urbani Strain of SARS-CoV that evoked high titers of neutralizing antibodies in mice thus immunized that strikingly cross-neutralized other strains including Tor2, GD03T13, SZ3, and Palm Civet’s SARS-CoV (He et al. 2006). Dai et al. (2020) also described a universal design for use against all beta coronaviruses (SARS, SARS-CoV-2, and MERS-CoV), a vaccine based on a dimeric form of the S-protein–receptor-binding domain, which immunogen induced a high level of neutralizing antibodies in mice and conferred protection against intranasal challenge with MERS-CoV (Dai et al. 2020). A SARS-CoV-2–subunit vaccine was also developed by Novavax, produced from the full-length spike glycoprotein expressed in Sf9 insect cells, which antigen was demonstrated to have increased thermal stability compared to the wild-type spike protein, an essential variable to consider in view of the known stringent temperature requirements for novel RNA vaccines. When used to immunize mice and baboons in combination with the Matrix-M™ adjuvant, the recombinant antigen elicited high titers of anti-S antibodies and strong multifunctional T- and B-cell responses (Tian et al. 2021). The Novavax vaccine is currently in phase III human trials, and the safety and efficacy of the vaccine have been verified when coadministered with seasonal influenza vaccines (Jhaveri et al. 2020).
Table 3.
Summary of products manufactured in BEVS and BacMam platform in the campaign against coronavirus infections
| Coronaviruses | Product | Baculovirus tool | Structural protein | Vector type | Host | References |
|---|---|---|---|---|---|---|
| MERS-CoV | ||||||
| Vaccine (preclinial stage) | Protein or subunit expression | RBD-dimer |
Bac to Bac™ (Thermo Fisher) |
Sf9 cells | Dai et al. (2020) | |
| Vaccine (preclinial stage) | VLP | M, E, S |
Bac to Bac™ (Thermo Fisher) |
Sf9 cells | Wang et al. (2017b) | |
| Vaccine (preclinial stage) | VLP | RBD |
Bac to Bac™ (Thermo Fisher) |
Sf9 cells | Wang et al. (2017a) | |
| Indirect and IgG-ELISA sandwich | Protein or subunit expression | S | Bac to Bac™(Thermo Fisher) | Sf9 cells | Lee et al. (2018) | |
| SARS-CoV | ||||||
| Vaccine (preclinial stage) | Protein or subunit expression | S |
Bac to Bac™ (Thermo Fisher) |
expresSF + | He et al. (2006) | |
| Vaccine (exploratory stage) | VLP | M, E, S | BaculoGold™ (BD Biosciences) | Sf21 cells | Ho et al. (2004) | |
| Vaccine (preclinial stage) | VLP | M, E, S |
Bac to Bac™ (Thermo Fisher) |
Sf21 cells | Lu et al. (2007) | |
| Vaccine (preclinical stage) | VLP | E, M from Sars-CoV and S from bat-CoV |
Bac to Bac™ (Thermo Fisher) |
Sf21 cells | Bai et al. (2008a) | |
| Vaccine (exploratory stage) | VLP | M, N, E, S |
pAcP102X/pAcVC3 /linearized AcMNPV |
Sf9 cells | Mortola and Roy (2004) | |
| Vaccine (preclinial stag) | VLP | S with influenza M1 |
Bac to Bac™ (Thermo Fisher) |
Sf9 cells | Liu et al. (2011) | |
| Vaccine (preclinial stage) | Display | S |
Bac to Bac™ (Thermo Fisher) |
Sf9 cells | Feng et al. (2006) | |
| Vaccine (preclinical stage) | Gene delivery | N, S |
Bac to Bac™ (Thermo Fisher) |
Sf9 cells | Bai et al. (2008b) | |
| Immunofluorescence assay | Protein or subunit expression | N195 (N) and Sc (S) fusion protein |
Bac to Bac™ (Thermo Fisher) |
Sf9 cells | He et al. (2005) | |
| IgG indirect ELISA | Protein or subunit expression | N | pAc-cHis/linearized AcMNPV | Tn5 cells | Saijo et al. (2005) | |
| SARS-CoV-2 | ||||||
| Vaccine (exploratory stage) | Protein or subunit expression | S | BmNPV bacmid (Qd04)/pFastBac1 | B. mori larvae | Fujita et al. (2020) | |
| Vaccine (phase III clinical trial, Novavax) | VLP technology | S with Matrix M adjuvant | BacVector™ (Millipore) | Sf9 cells | Tian et al. (2021) | |
| Vaccine (exploratory stage) | VLP | M, E, S |
Bac to Bac™ (Thermo Fisher) |
ExpiSf9™ | Mi et al. (2021) | |
| Indirect IgG ELISA | Protein or subunit expression | S complete and RBD |
Bac to Bac™ (Thermo Fisher) |
High Five™ cells | Amanat et al. (2020) | |
| Indirect IgG ELISA | Protein or subunit expression | S |
Bac to Bac™ (Thermo Fisher) |
R. nu larvae | Smith et al. (2021) | |
RBD receptor-binding domain; VLP virus-like particles; M membrane; E envelope; S spike; N nucleoprotein. All preclinical tests were developed in mice, except MERS-CoV VLP from Wang et al. (2017b) that was performed in macaques and Sars-CoV-2 VLP from Tian et al. (2021) was performed in baboons and mice; ND no data
As an alternative to cultured insect cells, whole living insects have also been studied as biofactories for the production of coronavirus recombinant proteins. For instance, the silkworm larvae successfully expressed the SARS-CoV-2 spike protein (Fujita et al. 2020), thus indicating that the system could be a viable vehicle for generating diagnostic kits and therapeutic proteins for coronaviruses. One such kit for the serological testing of COVID-19 based on the expression of the S protein from SARS-CoV-2 in rec-baculovirus–infected R nu larvae has already been produced with excellent results (Smith et al. 2021). Likewise, Escribano et al. (2020) have proposed the use of pupae from T. ni as a living bioreactor for the large-scale production of recombinant proteins, a technology they named CrisBio®. This approach was successfully used for the production of veterinary-virus vaccines (Escribano et al. 2020) and is now shifting into human zoonotic diseases with a successful proof-of-concept test for avian flu (Sisteré-Oró et al. 2020) and a subsequent grant to develop a COVID-19 vaccine as well.
Baculoviruses were also tested as vectors to mediate the gene expression for bat-coronavirus proteins in mammalian cells under the control of mammalian promoters (a BacMam approach). The immunization of mice with such viruses induced the expression of high titers of antibodies as measured by ELISA assays along with Th1-cell responses as visualized by ELISPOT (Bai et al. 2008b). Another alternative approach is the use of a baculovirus-facilitated surface display of the spike protein, which approach was tested by Feng et al. (2006), who accordingly demonstrated the capability of inducing a serum-neutralizing activity against SARS-CoV (Feng et al. 2006). A baculovirus display of the S protein can also be used to study the physiopathology of SARS-CoV along with the contribution of specific amino acids in triggering immune responses, as proven by Chang et al. (2004), the knowledge of which specificity can aid in targeted-drug design (Chang et al. 2004). The BacMam approach for coronaviruses vaccine development is summarized in Table 3.
The development of serological tests can also be achieved with BEVS, because of the technology’s ease of use and substantial protein yields. BEVS has accordingly been successfully implemented to produce ELISA and/or immunofluorescence assays for SARS-CoV (He et al. 2005; Saijo et al. 2005), MERS (Lee et al. 2018), and SARS-CoV-2 (Amanat et al. 2020).
Many attempts have been made to achieve a VLP vaccine via the baculovirus system since the first SARS outbreak. First Ho et al. (2004) and then Mortola and Roy (2004) and Lu et al. (2007) provided evidence that VLPs composed of the S, M, and E proteins from SARS-CoV form correctly when simultaneously expressed in infected-Sf9 cells (Ho et al. 2004; Mortola and Roy 2004; Lu et al. 2007). What is still not clear, though, is if the incorporation of the N protein is necessary for VLP formation or the latter depends on viral species and the expression system used (Naskalska et al. 2018). VLPs for SARS-CoV-2 have also been successfully produced in ExpiSf9 cells—a nonengineered derivative of the Sf9 insect cell line adapted for the first time to grow at high-density and with a faster duplication rate and adequate morphology (Mi et al. 2021). In addition, hybrid SARS-like VLPs—formed by the S protein from Bat-CoV and the M and E proteins from SARS-CoV—were found to up-regulate the level of costimulatory molecules for optimal activation of T cells along with the secretion of cytokines by immature dendritic cells at much higher levels than obtained with monoligated SARS-CoV VLPs (Bai et al. 2008a), a finding of great utility in view of the need for strong cellular immunity on top of humoral neutralizing responses. Chimeric SARS-CoV VLPs were also successfully produced in insect cells by coexpression of the SARS-CoV S protein combined with the influenza M1 protein (Liu et al. 2011), which immunogens protected mice against infection without the need for adjuvants as opposed to the full-length S protein alone, which did require adjuvants. Chimeric VLPs like these could prove a powerful option since influenza antigens can stimulate the memory of CD4 cells from past infections and accelerate the activation of the antigen-specific B-cells against coronaviruses. Furthermore, VLPs produced in insect cells were also effective against MERS-CoV infection in nonhuman primates and mice, through eliciting high specific antibody titers and Th1 cellular responses (Wang et al. 2017a, b).
The administration route is also an essential aspect to consider against respiratory infections such as coronaviruses because of the need for a mucosal-antibody response to combat viral upper respiratory tract replication. The nasal administration in mice of VLPs produced in insect cells induced production of detectable IgA against the S protein in saliva, pulmonary mucosa, intestines, and the urinary tract; and although serum IgG levels were higher with intraperitoneal administration, the neutralizing activity was higher with intranasal administration (Lu et al. 2010), thus proving the versatility and effectiveness of the baculovirus system for these kinds of strategies.
The study of VLPs usually concentrates on two main topics, the particle assembly and the ability to elicit an immune response, but as indicated by Naskalska et al. (2018), the SARS-Cov-2 VLPs can also be useful as delivery vectors for cells expressing the angiotensin-converting enzyme 2, the specific receptor for SARS-CoV-2 (Naskalska et al. 2018). These kinds of vectors would exhibit a narrow tissue tropism, similar to what was observed with antibody–drug conjugates, thus highlighting one more tool that the baculovirus provides in the fight against coronaviruses and other infections.
Baculoviruses as tools for the treatment of noninfectious human diseases via gene therapy
Baculoviruses represent a flexible tool for producing biologics for therapeutic purposes. Certain recombinant proteins produced in BEVS are being analyzed for the treatment of noninfectious diseases and are in different stages of medical evaluation. Already on the market is a recombinant protein (Provenge®) produced in BEVS for the treatment of prostate cancer developed by Dendreon (Kumar et al. 2018), but in recent years, most of the studies have been directed mainly toward evaluating the use of baculoviruses for gene therapy–based treatment, either by producing adeno-associated viruses (AAVs) or through the BacMam technology.
BEVS associated with gene therapy: baculovirus and AAV
Gene therapy is a medical procedure that bases the treatment of diseases on the use of genetic sequences as active ingredients. Accordingly, different virus species have been engineered to generate viral vectors that enable the possibility of directing the active ingredients to the targeted cells—via approaches involving an in vivo administration to the patient's body or an ex vivo modification of patient cells or tissues similar to a cell therapy followed by reintroduction into the patient—but without the recombinant’s replication or progeny generation in either approach. Among the most widely studied options that have reached the pharmaceutical market are gene therapies mediated by AAVs (Ginn et al. 2018).
AAVs are small (about 20–25 nm) icosahedral nonenveloped single-strand (ss-) DNA viruses (genus: Dependovirus; family: Parvoviridae) whose infections in humans manifest no significant clinical consequences despite being very widespread. In particular, AAVs require helper viruses (e.g., adenoviruses, herpes-simplex viruses) to sustain their generation of progeny. The ssDNA (about 4.7 kb in length) is flanked by inverted terminal repeats (ITRs; of about 145 b, generating hairpin structures) and encode two sets of proteins: those associated with replication (Rep proteins); and the virion structural elements (Cap proteins). The ITRs govern the main processes of the multiplication cycle —i.e., double-stranded (ds-) DNA generation, replication, packaging—and, together with the Rep proteins, are responsible for the possible genomic integration (at a locus located in the long arm of chromosome 19) when helper viruses are not present (Mezzina and Merten 2011). AAV-based viral vectors have been extensively evaluated for gene therapy because of their simplicity, satisfactory immune profile, the occurrence of serotypes with different tissue tropisms, and the ability to transduce nondividing cells, thus ensuring a long-term expression of the transgene—n. b.: the nucleic acid remains episomal in the absence of Rep proteins (Keeler and Flotte 2019). In essence, the recombinant AAVs (rAAV) carry an ssDNA that contains the gene of interest (GOI, which is replacing certain viral genes) flanked by the ITRs. Products such as Glybera® (alipogene tiparvovec), Luxturna® (voretigene neparvovec), or Zolgensma® (onasemnogene abeparvovec) are AAV-based gene therapies to treat lipoprotein-lipase deficiency, inherited retinal diseases, and spinal muscular atrophy, respectively (Ginn et al. 2018; Keeler and Flotte 2019).
The initial procedures for producing rAAVs involved transfecting mammalian-cell lines (mainly human-embryonic-kidney HEK 293 cells) with plasmids that carried the therapeutic sequence and the ITRs, plus other helpers with the AAV genes and those needed from other viruses (generally, adenovirus genes). Although undergoing improvements over time with the purpose of achieving scalable systems (including, for example, the development of mammalian packaging cells), the approach did not enable productions in the yield necessary for therapy in human beings (Galibert and Merten 2011). Those limitations led to studies that evaluated the rAAV production by complementation systems that involved adenoviruses, herpes-simplex viruses, or baculoviruses (Aponte-Ubillus et al. 2018). In the first report where BEVS were employed for this purpose, three rec-baculoviruses were used (rep and cap genes in two independent BVs and the ITR-GOI-ITR construct in the third: the so-called ThreeBac system) along with Sf9 cells (Urabe et al. 2002). Subsequently, different optimizations were performed including the use of only two rec-baculoviruses—i.e., the TwoBac system; one combing both the rep and the cap genes and the other carrying the ITR-GOI-ITR (Wu et al. 2019) plus the later generation of a OneBac system consisting of packaging-Sf9 cells that expressed rep and cap genes when infected with a rec-baculovirus that carried the therapeutic nucleic acid (Mietzsch et al. 2015). Subsequent optimizations of the OneBac platform enabled quite good yields of whole particles with little contaminating DNA (Mietzsch et al. 2015; Joshi et al. 2019). In another study, an alternative OneBac system was developed that involved a rec-baculovirus expressing both rep and cap genes and carrying the ITR-GOI-ITR construct (Wu et al. 2018). What is interesting to us about this work is that these studies enabled the insect larval platform to produce rAAV, thus significantly lowering the overall costs. In another report, the genomic stability of rec-baculoviruses was evaluated in accordance with the genetic-engineering platforms to produce those recombinants, either through transposons or homologous recombination (Aurelién et al. 2021). All these current contributions are enabling the insect-cell–rAAV-production platform to remain a viable alternative for the gene-therapy industry, thus underscoring that approach as a principal milestone after Glybera® was produced by a ThreeBac system.
One of the main problems in rAAV production is the generation of empty capsids or capsids carrying contaminating DNA. For this reason, all the technologies developed for this purpose not only aim at high general yields but also must ensure a definitive production of complete particles free of helper DNA. These remaining aspects of the present state of the art constitute a significant challenge that makes this bioprocess one that still requires critical improvement on any of the production platforms and, of course, leaves open the opportunity for innovations.
BacMam technology associated with gene therapy
In addition to the application of BEVS to produce protein-based drugs for numerous noninfectious human diseases, baculoviruses can also be used as viral gene-delivery vectors for therapeutic or immunogenic purposes. These BacMam applications emerged in the mid-1990s with the report that BVs constructed with the prototype species AcMNPV could transduce, but would not infect, mammalian cells (Hofmann et al. 1995); thus enabling the expression of a given GOI assembled with a typical mammalian-gene syntax (as opposed to the insect syntax used when applying BEVS or a baculovirus display platform). Later ODVs (Fig. 1C) were found to not possess this capability (Mäkelä et al. 2008), with the result that the range of the baculovirus species sharing this characteristic was then expanded by adding studies about the BVs of Bombyx mori nucleopolyhedrovirus (Kenoutis et al. 2006; Liu et al. 2017) and the Anticarsia gemmatalis nucleopolyhedrivirus AgMNPV (Parsza et al. 2020). In particular, these benefits in mammalian cells were reported to be due to the GP64 protein present in the BV envelopes of group 1-alphabaculoviruses (Kataoka et al. 2012; Luz-Madrigal et al. 2013); consequently narrowing down the feasibility of such applications to this specific subgroup of species, for which the viral entry mechanism would be based on clathrin-mediated endocytosis (Fujita et al. 2006; Liu et al. 2014; Hu et al. 2019b). Even variants of lentiviruses containing different GP64 proteins evidenced cell-expanded transduction efficiencies (Sinn et al. 2017).
The reasons that led so many researchers to explore this application of AcMNPV BVs (Ono et al. 2014; Mansouri and Berger 2018) resides mainly in biosafety issues: quite simply stated, the virus does not infect mammals (Kost and Condreay 2002) and furthermore exhibits a natural efficiency in transducing different mammalian cells including stem cells (Airenne et al. 2011). In addition to those essential properties, the virus is easy to manipulate for generating recombinant virions (Luckow et al. 1993; Airenne et al. 2011) and producing those constructs on an industrial scale (He et al. 2005; Kwang et al. 2016); possesses an extensive capacity for carrying exogenous DNA of up to at least 38 kbp, thus enabling the insertion of several GOIs at once (Cheshenko et al. 2001); and is characterized by an excellent immunogenic profile when administered in vivo as a result of the lack of preexisting immunity in mammals (Bocca et al. 2013). Another advantage is the compatibility of the baculovirus system carrying GOIs that will express suicide proteins in mammalian cells (useful in cancer treatments), but not in insect cells (where the viral vector is produced) owing to the use of introns and other typical elements of mammalian genetics (Chen 2019).
Despite these definitive benefits, the usually short-expression time in vivo of the GOI in mammals (around 7–14 days postinoculation) should be considered, which timing limits readministrations (Luo et al. 2013), mainly because of certain generic immunologic responses that baculoviruses trigger—those being more pronounced upon systematic administration, though lesser upon local inoculation (Ono et al. 2014). Among such responses, the innate immune system is a clear example because the administration of BVs has been reported to upregulate the RIG-1–like receptor (Balasundaram et al. 2017) and the Toll-like–receptor–9 signaling pathways (Abe et al. 2009; Boulaire et al. 2009), thus activating type-I interferon (Hervas-Stubbs et al. 2007; Abe et al. 2009) and natural-killer cells (Moriyama et al. 2017). In addition, a few other genes are induced by baculoviruses, such as those encoding complement factors and adhesion molecules, or those involved in cytokine-cytokine receptor interaction ( Shin et al. 2020). Other in vitro studies revealed that the cGAS-STING nucleic-acid–sensing pathway would act as a decisive agent in generating an antiviral state produced by interferon types I and III (Amalfi et al. 2020). In this regard, the unmethylated CpG DNA of baculoviruses would be one of the main causes of the low transgene expression in certain cells triggering the different responses mentioned (Ono et al. 2018). At around the same time, baculovirus-transduced cell transplantation in animal models (associated with ex-vivo–gene-therapy approaches) gave very good results and did not elicit a systemic induction of monocytes and CD8 + T cells as did other viruses (Chuang et al. 2009). Another relevant aspect to be considered is regarding the GP64 viral protein of BVs (Fig. 1C). Although this fusogenic factor is useful and sufficient for the baculoviral Ncs to enter the cells and then, in turn, penetrate the nucleus in an actin-dependent manner (Fujita et al. 2006; Long et al. 2006), that factor—though generally considered polytropic—can manifest differences according to the mammalian cells targeted (Airenne et al. 2011; Kataoka et al. 2012; Luz-Madrigal et al. 2013).
Nevertheless, a lot of effort is being made to overcome the limitations (Aulicino et al. 2020) that other viral vectors used in gene therapy do not escape, where those vectors can generate much greater immune responses as a result of being derived from mammalian pathogens and have other serious disadvantages including the limited duration of the transgenes they carry and the costs of scaled-up production. Thus, several investigations have been reported that have offered improvements in baculoviral vectors for BacMam-type applications. In this, list of developments can be mentioned: (i) the pseudo-typed BVs, which constructs express in insect cells the stomatitis-vesicular-virus glycoprotein (Barsoum et al. 1997; Kolangath et al. 2014) or other factors from eukaryote parasites and viruses (Tamura et al. 2016; Hu et al. 2019a) to increase transduction capability in mammals; (ii) the development of baculoviral-hybrid vectors that achieve a longer duration of the GOI expression by, for example, the presence of the ORI P of the Epstein-Barr virus, either alone (Shan et al. 2006; Suzuki et al. 2009) or combined with the FLP/FRP, ɸC31/attB-P or Cre/Loxp recombinase systems (Lo et al. 2009, 2017; Sung et al. 2013); (iii) the inclusion of the inverted terminal repeats and the Rep gene of the adeno-associated virus (Wang 2008) or the inverted terminal repeats and the transposase gene of the Sleeping-Beauty transposon (Turunen et al. 2014); and (iv) genome modifications to increase the BV yield in insect cells (Graves et al. 2018), among other optimizations. In addition, progress has been made in different formulations to achieve better results after the administration of recBVs in mammals. Thus, binary complexes between rec-baculoviruses and nanoparticles composed of the HIV trans-activator–of–transcription protein and DNA molecules encoding therapeutic genes have been studied (Paul et al. 2011) to combine the benefits of viral horizontal-gene-transfer systems with those based on nonviral complexes. Similar efforts have been made involving galactosylated-polyethylenimine–DNA complexes (Kim et al. 2009), cationic polyamidoamine-dendrimer synthetic nanoparticles (Paul et al. 2013), or nanomagnetic particles (Zhu et al. 2020) in combination with rec-baculoviruses. This last approach, combined with the use of magnetic fields, helps overcome serum inactivation and achieves local expression of the GOI after even administrations that are systemic. Moreover, recombinant BVs that express complement-regulatory proteins on the virion surfaces exhibited a higher complement resistance than nonmodified variants (Kawai et al. 2018), while other studies tested the use of different compounds in baculovirus formulations to inhibit the complement system (Kaikkonen et al. 2010) or to improve transduction by decreasing the endosomal pH (Hu et al. 2019b). Of interest to this field was that baculoviruses expressing the adenovirus receptor have been developed to perform combined gene therapies with both viral vectors (Hong et al. 2017). Furthermore, the downstream processing of vectors during in vivo expansion received attention in order to improve the quality and quantity of BVs recovered from infected insect cells (Kwang et al. 2016; Nasimuzzaman et al. 2018) including the development of different chromatographic methods (Nasimuzzaman et al. 2016; Lothert et al. 2020).
Although no BV-vector-based therapies have yet reached a clinical stage, several biodistribution and efficacy studies in different animal models utilizing recombinant AcMNPVs have been performed in the last 10 years. These approaches were designed mainly for the treatment of cancer, heart and/or vascular diseases, and tissue engineering and/or regenerative medicine and include preclinical assays in mice, rats, rabbits, dogs, and pigs with various types of therapeutic sequences and different BV technologies (Table 4).
Table 4.
Some successful preclinical gene-therapy assays in mammals based on BacMam technology
| Disease | AI (sequence) | Type of vector | Animal model | In vivo/ ex vivo | Dose (PFU) | Reference |
|---|---|---|---|---|---|---|
| Cancer | ||||||
| Hepatoma | Gene encoding Apoptin from the chicken anemia virus |
Bac to Bac™ (Thermo Fisher) Pseudotyped with VSV-G |
Mice |
In vivo (intra-tumor) |
1 × 108–1 × 1010 | Pan et al. (2010) |
| Glioblastoma | Gene encoding thymidine kinase from herpes simplex virus |
Bac to Bac™ (Thermo Fisher) |
Mice | Ex vivo (human MSC-like cells transduced) | MOI 100 | Bak et al. (2011) |
| Prostate and ovarian tumors | Gene encoding an antiangiogenic fusion protein |
Bac to Bac™ (Thermo Fisher) Hybrid system with Sleeping Beauty transposon |
Mice |
In vivo (intra-tumor) |
3 × 109 | Luo et al. (2013) |
| Hepatocellular carcinoma | Gene expressing LncRNA PTENP1 | Hybrid system with Sleeping Beauty transposon | Mice |
In vivo (intra-tumor) |
1 × 109 Bac-SB + 2 × 108 Bac-GOI | Chen et al. (2015) |
| Bladder tumor | Genes encoding CD40 ligand and IL-15 | BacPAK™ (Clontech) | Mice |
In vivo (intra-tumor) |
1 × 108 | Ang et al. (2016) |
| Pituitary tumor | Gene expressing shRNA | BacPAK™ (Clontech) | Mice | In vivo (intra-tumor) | 1 × 108 | Gottardo et al. (2018) |
| Hypopharyngeal carcinoma | Gene encoding sodium iodide symporter (NIS) |
Bac to Bac™ (Thermo Fisher) Hybrid system with AAV |
Mice | Ex vivo (human bone marrow mesenchymal stem cells -BMSCs- transduced) combined with 131 I therapy | MOI 400 | Wang et al. (2019a); Wang et al. (2020) |
| Cardiovascular diseases | ||||||
| Myocardial infarction | Gene encoding angiopoietin-1 | BaculoGold™ (BD Biosciences) and Tat/DNA nanoparticles | Rats | In vivo (peri-infarct regions) | 1 × 1010 | Paul et al. (2011) |
| Atherosclerosis related cardiovascular diseases | Gene encoding vascular endothelial growth factor (VEGF) |
Bac to Bac™ (Thermo Fisher) Evaluation variants Pseudo-typed with VSV-G |
Rabbits | In vivo (skeletal muscle) | 1 × 109 | Heikura et al. (2012) |
| Myocardial infarction | Gene encoding angiopoietin-1 | BaculoGold™ (BD Biosciences) and Tat/DNA nanoparticles | Rats | Ex vivo (modified human adipose tissue derived stem cells -hASCs- transduced) | MOI 100 | Paul et al. (2012) |
| In-stent restenosis | Gene encoding vascular endothelial growth factor (VEGF) | BaculoGold™ (BD Biosciences) with cationic Polyamidoamine dendrimer synthetic nanoparticles (PAMAM) | Dogs | In vivo (baculovirus based gene eluting stent) | 5 × 1012 | Paul et al. (2013) |
| Myocardial infarction | Gene encoding vascular endothelial growth factor (VEGF) |
Bac to Bac™ (Thermo Fisher). Use of Bac-GOI/ORI P (EBV) + Bac-recombinase (FLP) |
Rabbits | Ex vivo (rabbit adipose-derived stem cell -ASC- transduced) |
MOI 150 Bac-GOI/ORI P + MOI 50 Bac-FLP |
Yeh et al. (2014) |
| Limb ischemic disease | Gene encoding vascular endothelial growth factor (VEGF) |
Bac to Bac™ (Thermo Fisher). Use of Bac-GOI/ORI P (EBV) + Bac-recombinase (FLP) |
Mice | Ex vivo (mouse adipose-derived stromal cells -ADSC- transduced) |
MOI 150 Bac-GOI/ORI P + MOI 15 Bac-FLP |
Makarevich et al. (2015) |
| Ischaemia–reperfusion (I/R) injury associated with kidney transplantation | Gene encoding manganese superoxide dismutase (SOD-2) |
flashBAC ULTRA™ DNA (Oxford Expression Technologies Ltd) |
Porcine kidneys | Ex vivo (human kidney 2 -HK-2- cells transduced) | MOI 150 | Hitchman et al. (2017) |
| Peripheral arterial disease | Gene encoding a mutant oxygen-resistant hypoxia-inducible factor 1-alpha (mHIF-1α) |
Bac to Bac™ (Thermo Fisher) |
Rabbits | In vivo (skeletal muscle) | 1 × 109 | Giménez et al. (2020) |
| Tissue engineering/regenerative medicine | ||||||
| Massive segmental bony defects following trauma or tumor resection |
Genes encoding bone morphogenetic protein-2 (BMP2) and vascular endothelial growth factor (VEGF) |
Bac to Bac™ (Thermo Fisher) Use of Bac-GOI/ORI P (EBV) + Bac-recombinase (FLP) |
Rabbits | Ex vivo (rabbit adipose-derived stem cells -ASCs- transduced) |
MOI 100 Bac-GOI/ORI P + MOI 100 Bac-FLP |
Lin et al. (2011); Lin et al. (2014) |
| Massive segmental bony defects following trauma or tumor resection |
Genes encoding bone morphogenetic protein-2 (BMP2) and vascular endothelial growth factor (VEGF) |
Bac to Bac™ (Thermo Fisher) Use of Bac-GOI/ORI P (EBV) + Bac-recombinase (FLP optimized) |
Minipigs | Ex vivo (rabbit adipose-derived stem cells -ASCs- transduced) |
MOI 150 Bac-GOI/ORI P + MOI 100 Bac-FLP |
Lin et al. (2015) |
| Osteoporosis | Gene expressing a miR-214 sponge |
Bac to Bac™ (Thermo Fisher) Use of Bac-GOI/ORI P (EBV) + Bac-recombinase (Cre) |
Rats | Ex vivo (rat adipose-derived stem cells -ASCs- transduced) |
MOI 150 Bac-GOI/ORI P + MOI 100 Bac-Cre |
Li et al. (2016); Li et al. (2017) |
| Degenerative disc disease |
Gene encoding bone morphogenetic protein-7 (BMP-7) |
Bac to Bac™ (Thermo Fisher) |
Rats | Ex vivo (rat bone marrow–derived mesenchymal stem cells -BMDMSC- transduced) | MOI 25 | Liao (2016a) |
| Unstable spine |
Gene encoding bone morphogenetic protein-7 (BMP-7) |
Bac to Bac™ (Thermo Fisher) |
Rabbits | Ex vivo (rat bone marrow–derived mesenchymal stem cells -BMDMSC- transduced) | MOI 25 | Liao (2016b) |
| Rheumatoid arthritis | Gene encoding Coxsackie-adenovirus receptor (CAR) in BV + human adenovirus type 5 (HAdV5)-PUMA (p53 upregulated modulator of apoptosis) |
Bac-N-Blue™ (Thermo Fisher) |
Rats | In vivo (intra-articular) | 1 × 105 BVs + 1 × 109 HAdV5-PUMA | Hong et al. (2017) |
| Peripheral nerve injuries |
Gene encoding CRISPRa system for multiplexed activation of brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF) and nerve growth factor (NGF) genes |
Bac to Bac™ (Thermo Fisher) Use of Bac-GOI/ORI P (EBV) + Bac-recombinase (Cre) |
Rats | Ex vivo (rat adipose-derived stem cells -ASC- transduced) |
MOI 150 Bac-GOI/ORI P + MOI 100 Bac-Cre |
Hsu et al. (2019) |
| Other diseases | ||||||
| Hepatic encephalopathy | Gene encoding the glutamine synthetase |
Bac to Bac™ (Thermo Fisher) |
Rats | In vivo (intramuscular) | 9 × 107 | Torres-Vega et al. (2015) |
AI active ingredient; PFU plaque-forming unit; MOI multiplicity of infection; AAV adeno-associated virus; GOI gene of interest; SB Sleeping Beauty transposase; EBV Epstein-Barr virus; HAdV human adenovirus
We need to mention that certain studies were carried out in nonhuman primates (Balasundaram et al. 2017) or using whole-human-blood samples (Georgopoulos et al. 2009) and cells from human donors (Bak et al. 2011; Paul et al. 2011; Graves et al. 2018; Wang et al. 2019a, 2020). In addition, owing to the excellent transport capability of baculoviruses, new approaches in synthetic biology are being explored in vitro that enable, for example, the incorporation of complex gene circuits that can express a therapeutic gene in target cells and not in others (Lin et al. 2018).
Furthermore, in vivo and ex vivo studies involving animals and human cells have demonstrated the feasibility and safety of a BV-mediated gene therapy, thus creating new possibilities for the future approval of clinical trials and giving us the ability to realistically imagine the clinical approval of the first BV-based gene therapy in the near future. Overall, BVs have been used to treat several noninfectious diseases but mostly are in use for cancer therapy and tissue engineering and/or regenerative medicine.
Concluding remarks and perspectives
Baculoviruses, and especially AcMNPV, have been exploited in diverse biotechnological uses for years, generating products and conceptual tests in different technological fields that include agriculture (the first and most obvious biotech use), but also animal and human health. All these new technological opportunities emerged because of the basic studies carried out on baculoviruses in general, and on AcMNPV in particular—it is chosen as the prototype of this family, which includes more than 80 species and hundreds of isolates (Fig. 3). Although baculoviruses (or at least their effects) have been known since the nineteenth century through the consequences they produced in colonies of B. mori larvae reared for silk production, only by the middle of the twentieth century did the baculoviruses begin to be identified as viral entomopathogens (Rohrmann 2019). In the early 1970s, a baculovirus isolated from Autographa californica larvae began to be extensively characterized (Vail et al. 1971). Thus, owing to the development of the insect-cell lines Sf21, Sf9, and High Five™ that were useful for multiplying viruses under laboratory conditions (Vaughn et al. 1977; Wickham et al. 1992), AcMNPV virions became subjected to multiomics studies that included the genome sequencing (Ayres et al. 1994), the determination of differential gene expression by means of transcriptomic approaches (Yamagishi et al. 2003; Chen et al. 2013), and the identification of the proteomic composition from both BVs (Wang et al. 2010) and ODVs (Braunagel et al. 2003).
Fig. 3.
Milestones in baculovirus research and development. The scheme is a timeline of the last 50 years where some ground-breaking discoveries and developments involving baculoviruses are cited, with a particular focus on AcMNPV, the most widely studied and applied species of Baculoviridae. These achievements are classified according to 4 categories: basic research and applications of omic technologies in AcMNPV, plus the development of cell lines to multiply the vector; the use of genetic-engineering tools on the AcMNPV genome; biotechnological applications of AcMNPV virions; and regulatory approvals of some AcMNPV-based products (or baculovirus based products). Only in the example of the first registered biopesticide (Elcar®) and the first veterinary therapy based on recombinant protein produced in Bombyx mori larvae (Virbagen® Omega) are mentioned products generated using other baculoviral species (Helicoverpa zea NPV and Bombyx mori NPV, respectively; milestones indicated with orange stars). All the references that support these milestones are included in the main text
During this time, the genetic engineering procedures were also applied to the AcMNPV genome to generate engineered virions for both basic and applied research. These modifications were first based on homologous-recombination processes performed in insect-cell culture (Smith et al. 1983), which experiments then enabled the generation of AcMNPV bacmids (Luckow et al. 1993). These Escherichia coli megaplasmids containing the baculoviral genome created other possibilities for genomic modification in bacteria (Bideshi and Federici 2000) that promoted progress in the central aspects of functional genomics by knock-out and complementation approaches. Moreover, in recent times, the most sophisticated and powerful tools of genetic engineering have been used on AcMNPV, including the generation of synthetic genomes (Shang et al. 2017) or the gene-editing procedures mediated by CRISPR/Cas technology (Pazmiño-Ibarra et al. 2019), thus auguring a future replete with new knowledge and improvements in application.
Therefore, the versatility manifested by AcMNPV in tolerating genomic changes and the possibilities for scaling-up in cell culture not only enabled the use of this baculovirus as a biopesticide (Vail et al. 1971), but also favored the development of innovations for the expression of recombinant proteins (Smith et al. 1992) and VLPs (Pearson and Polly 1993) for the generation of protein-display platforms (Boublik et al. 1995), or for use as a virus vector in gene deliveries to mammalian cells (Hofmann et al. 1995). Accordingly, BEVS and BacMam technologies were born in just over 10 years between 1983 and 1995, and during that time, everything was prepared for their transfer to the human- and veterinary-health industries. Although Elcar®, the first baculovirus-based biopesticide registered between 1972 and 1975, did not contain AcMNPV as the active ingredient (Ignoffo 1999), most the other products registered for vertebrates (human and nonhuman) are derived from this species. In this regard, the statement is appropriate that the first 4 commercial products reaching the market derived from the use of BEVS platforms in human health were Cervarix® (a human VLP vaccine against human papillomavirus; GlaxoSmithKline, 2007); Provenge® (a recombinant protein for prostate-cancer immunotherapy; Dendreon, 2010); Flublok® (a human-subunit vaccine against influenza; Protein Sciences Corporation, 2013); and Glybera® (human gene-therapy product for the treatment of lipoprotein-lipase deficiency based on recAAV produced by BEVS) (Kumar and Gong 2018). In addition, 8 products for veterinary use produced in BEVS are already on the market. Among those that can be highlighted are Porcilis Pesti (MSD), the first veterinary subunit vaccine against swine flu, and Circumvent PCV (MSD), the first veterinary VLP vaccine against swine circovirus type 2, both produced in insect cells. In addition, Virbagen® Omega (Virbac) was the first veterinary protein therapy produced in insect larva for the treatment of feline viral diseases.
In the coming years, owing to the development of more extensive knowledge about baculoviruses, which new perspective will enable the development of the next generation of vectors for producing better-engineered virions that would be more useful for the aforementioned applications and for other innovations, we could expect that these technologies will be well valued by and appropriate for more human- and veterinary-health companies. Furthermore—and in view of the approvals received in recent years by various human-gene-therapy products based on viral vectors (Ginn et al. 2018) and the new generation of vaccines that use virions (Rawat et al. 2021)—we would expect that in this third decade of the twenty-first century, clinical trials in humans may begin to promote BacMam technologies and bring those procedures to the pharmaceutical market (Aulicino et al. 2020). The usefulness of the application of baculoviruses in human and animal health is already indisputable. The joint efforts between the baculovirus-genome engineering and improvement in the production and the downstream processes will enable baculovirus and/or insect-cell systems to be more productive and less expensive. Moreover, a greater number of registered products will favor a higher level of knowledge in regulatory agencies. All these propitious conditions in combination will enhance the use of baculoviral systems and derived technologies in the pharmaceutical industry.
Acknowledgements
We dedicate this review to the memory of Dr. Pablo Daniel Ghiringhelli, a prominent Latin American baculovirologist.
Author contribution
AMT, MVM, and MNB were involved in the conceptualization and supervision; AMT, JAS, GJM, and MNB were involved in literature review and writing; AMT and MNB editing the article; IS, FUCW, MVN, and MVM revised the article. All authors read and approved the manuscript.
Funding
This work was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica de Argentina (PICT2015-2061; PICT 2018–4321; PICT 2015–1992, Consejo Nacional de Investigaciones Científicas y Técnicas de Argentina (CONICET, PIP 00224/15), Universidad de Buenos Aires (UBACyT 2016-20020150100145BA), and Universidad Nacional de Quilmes (PPROF-2020). AMT, MVM, and MNB are career researchers of CONICET. JAS, GJM, IS, and MVN are research fellows of CONICET.
Declarations
Ethics statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abe T, Takahashi H, Hamazaki H, Miyano-Kurosaki N, Matsuura Y, Takaku H. Baculovirus induces an innate immune response and confers protection from lethal influenza virus infection in mice. J Immunol. 2003;171:1133–1139. doi: 10.4049/jimmunol.171.3.1133. [DOI] [PubMed] [Google Scholar]
- Abe T, Hemmi H, Miyamoto H, Moriishi K, Tamura S, Takaku H, Akira S, Matsuura Y. Involvement of the toll-like receptor 9 signaling pathway in the induction of innate immunity by baculovirus. J Virol. 2005;79:2847–2858. doi: 10.1128/jvi.79.5.2847-2858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe T, Kaname Y, Wen X, Tani H, Moriishi K, Uematsu S, Takeuchi O, Ishii KJ, Kawai T, Akira S, Matsuura Y. Baculovirus induces type i interferon production through toll-like receptor-dependent and -independent pathways in a cell-type-specific manner. J Virol. 2009;83:7629–7640. doi: 10.1128/jvi.00679-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airenne KJ, Makkonen KE, Mähönen AJ, Ylä-Herttuala S. Baculoviruses mediate efficient gene expression in a wide range of vertebrate cells. Methods Mol Biol. 2011;737:279–301. doi: 10.1007/978-1-61779-095-9_12. [DOI] [PubMed] [Google Scholar]
- Airenne KJ, Hu YC, Kost TA, Smith RH, Kotin RM, Ono C, Matsuura Y, Wang S, Ylä-Herttuala S. Baculovirus: an insect-derived vector for diverse gene transfer applications. Mol Ther. 2013;21:739–749. doi: 10.1038/mt.2012.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Padilla J, Jiménez de Oya N, Blázquez AB, Loza-Rubio E, Escribano JM, Saiz JC, Escribano-Romero E. Evaluation of an enzyme-linked immunosorbent assay for detection of West Nile virus infection based on a recombinant envelope protein produced in Trichoplusia ni larvae. J Virol Methods. 2010;166:37–41. doi: 10.1016/j.jviromet.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Amalfi S, Molina GN, Bevacqua RJ, López MG, Taboga O, Alfonso V. Baculovirus transduction in mammalian cells is affected by the production of type I and III interferons, which is mediated mainly by the cGAS-STING pathway. J Virol. 2020;94:e01555–e1620. doi: 10.1128/jvi.01555-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanat F, Stadlbauer D, Strohmeier S, Nguyen THO, Chromikova V, McMahon M, Jiang K, Arunkumar GA, Jurczyszak D, Polanco J, Bermudez-Gonzalez M, Kleiner G, Aydillo T, Miorin L, Fierer DS, Lugo LA, Kojic EM, Stoever J, Liu STH, Cunningham-Rundles C, Felgner PL, Moran T, García-Sastre A, Caplivski D, Cheng AC, Kedzierska K, Vapalahti O, Hepojoki JM, Simon V, Krammer F. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26:1033–1036. doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang WX, Zhao Y, Kwang T, Wu C, Chen C, Toh HC, Mahendran R, Esuvaranathan K, Wang S. Local immune stimulation by intravesical instillation of baculovirus to enable bladder cancer therapy. Sci Rep. 2016;6:1–12. doi: 10.1038/srep27455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aponte-Ubillus JJ, Barajas D, Peltier J, Bardliving C, Shamlou P, Gold D. Molecular design for recombinant adeno-associated virus (rAAV) vector production. Appl Microbiol Biotechnol. 2018;102:1045–1054. doi: 10.1007/s00253-017-8670-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo SC, Pereira LR, Alves RPS, Andreata-Santos R, Kanno AI, Ferreira LCS, Gonçalves VM. Anti-flavivirus vaccines: review of the present situation and perspectives of subunit vaccines produced in escherichia coli. Vaccines. 2020;8:1–30. doi: 10.3390/vaccines8030492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulicino F, Capin J, Berger I. Synthetic virus-derived nanosystems (Svns) for delivery and precision docking of large multifunctional DNA circuitry in mammalian cells. Pharmaceutics. 2020;12:1–20. doi: 10.3390/pharmaceutics12080759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurelién J, Brun L, Jimenez Gil P, Menard L, Boucelha M, Broucque F, Roblin A, Vandenberghe LH, Adjali O, Robin C, François A, Penaud-Budloo M, Ayuso E. Homologous recombination offers advantages over transposition-based systems to generate recombinant baculovirus for adeno-associated viral vector production. Biotechnol J. 2021;16:e2000014. doi: 10.1002/biot.202000014. [DOI] [PubMed] [Google Scholar]
- Ayres M, Howard C, Kuzio J, Lopez-Ferber M, Possee R. The complete DNA sequence of Autographa californica nuclear polyhedrosis virus. Virology. 1994;202:586–605. doi: 10.1006/viro.1994.1380. [DOI] [PubMed] [Google Scholar]
- Bai B, Hu Q, Hu H, Zhou P, Shi Z, Meng J, Lu B, Huang Y, Mao P, Wang H. Virus-like particles of SARS-like coronavirus formed by membrane proteins from different origins demonstrate stimulating activity in human dendritic cells. PLoS ONE. 2008;3:e2685. doi: 10.1371/journal.pone.0002685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai B, Lu X, Meng J, Hu Q, Mao P, Lu B, Chen Z, Yuan Z, Wang H. Vaccination of mice with recombinant baculovirus expressing spike or nucleocapsid protein of SARS-like coronavirus generates humoral and cellular immune responses. Mol Immunol. 2008;45:868–875. doi: 10.1016/j.molimm.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak XY, Lam DH, Yang J, Ye K, Wei ELX, Lim SK, Wang S. Human embryonic stem cell-derived mesenchymal stem cells as cellular delivery vehicles for prodrug gene therapy of glioblastoma. Hum Gene Ther. 2011;22:1365–1377. doi: 10.1089/hum.2010.212. [DOI] [PubMed] [Google Scholar]
- Balasundaram G, Kwang TW, Wang S. cDNA microarray assays to evaluate immune responses following intracranial injection of baculoviral vectors in non-human primates. J Neurochem. 2017;140:320–333. doi: 10.1111/jnc.13884. [DOI] [PubMed] [Google Scholar]
- Barros MCES, Galasso TGCM, Chaib AJM, Degallier N, Nagata T, Ribeiro BM. Yellow fever virus envelope protein expressed in insect cells is capable of syncytium formation in lepidopteran cells and could be used for immunodetection of YFV in human sera. Virol J. 2011;8:2–11. doi: 10.1186/1743-422X-8-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsoum J, Brown R, McKee M, Boyce FM. Efficient transduction of mammalian cells by a recombinant baculovirus having the vesicular stomatitis virus G glycoprotein. Hum Gene Ther. 1997;8:2011–2018. doi: 10.1089/hum.1997.8.17-2011. [DOI] [PubMed] [Google Scholar]
- Basak S, Kang HJ, Lee SH, Chu KB, Moon EK, Quan FS. Influenza vaccine efficacy induced by orally administered recombinant baculoviruses. PLoS ONE. 2020;15:1–13. doi: 10.1371/journal.pone.0233520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bideshi DK, Federici BA. The Trichoplusia ni granulovirus helicase in unable to support replication of Autographa californica multicapsid nucleopolyhedrovirus in cells and larvae of T. ni. J Gen Virol. 2000;81:1593–1599. doi: 10.1099/0022-1317-81-6-1593. [DOI] [PubMed] [Google Scholar]
- Bocca AL, do Espirito Santos Barros MC, Martins GKM, de Araújo ACO, Souza MJÔ, Ribeiro AM, Figueiredo F, Ribeiro BM. Immunological effects of Anticarsia gemmatalis multiple nucleopolyhedrovirus (AgMNPV) by stimulation of mice in vivo and in vitro. Virus Res. 2013;176:119–127. doi: 10.1016/j.virusres.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Bonafé N, Rininger JA, Chubet RG, Foellmer HG, Fader S, Anderson JF, Bushmich SL, Anthony K, Ledizet M, Fikrig E, Koski RA, Kaplan P. A recombinant West Nile virus envelope protein vaccine candidate produced in Spodoptera frugiperda expresSF+ cells. Vaccine. 2009;27:213–222. doi: 10.1016/j.vaccine.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boublik Y, Di Bonito PD, Jones IM. Eukaryotic virus display: Engineering the major surface glycoprotein of the Autographa Californica nuclear polyhedrosis virus (acnpv) for the presentation of foreign proteins on the virus surface. Bio/technology. 1995;13:1079–1084. doi: 10.1038/nbt1095-1079. [DOI] [PubMed] [Google Scholar]
- Boulaire J, Zhao Y, Wang S. Gene expression profiling to define host response to baculoviral transduction in the brain. J Neurochem. 2009;109:1203–1214. doi: 10.1111/j.1471-4159.2009.06015.x. [DOI] [PubMed] [Google Scholar]
- Braunagel SC, Russell WK, Rosas-Acosta G, Russell DH, Summers MD. Determination of the protein composition of the occlusion-derived virus of Autographa californica nucleopolyhedrovirus. Proc Natl Acad Sci U S A. 2003;100:9797–9802. doi: 10.1073/pnas.1733972100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright RA, Carter DM, Daniluk S, Toapanta FR, Ahmad A, Gavrilov V, Massare M, Pushko P, Mytle N, Rowe T, Smith G, Ross TM. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine. 2007;25:3871–3878. doi: 10.1016/j.vaccine.2007.01.106. [DOI] [PubMed] [Google Scholar]
- Buckland B, Boulanger R, Fino M, Srivastava I, Holtz K, Khramtsov N, McPherson C, Meghrous J, Kubera P, Cox MMJ. Technology transfer and scale-up of the Flublok® recombinant hemagglutinin (HA) influenza vaccine manufacturing process. Vaccine. 2014;32:5496–5502. doi: 10.1016/j.vaccine.2014.07.074. [DOI] [PubMed] [Google Scholar]
- Cerezo J, Targovnik AM, Smith ME, González Maglio D, Luppo VC, Morales MA, Miranda MV, Rodríguez Talou J. Simple production of hydrophobin-fused domain III of dengue envelope protein and induction of neutralizing antibodies against the homotypic serotype of dengue virus. Biotechnol Lett. 2020;42:419–428. doi: 10.1007/s10529-019-02767-2. [DOI] [PubMed] [Google Scholar]
- Chang Y-J, Liu CY-Y, Chiang B-L, Chao Y-C, Chen C-C. Induction of IL-8 release in lung cells via activator protein-1 by recombinant baculovirus displaying severe acute respiratory syndrome-coronavirus spike proteins: identification of two functional regions. J Immunol. 2004;173:7602–7614. doi: 10.4049/jimmunol.173.12.7602. [DOI] [PubMed] [Google Scholar]
- Chen H. Production of viral vectors with suicide genes by utilizing the intron-splicing mechanism of insect cells. Methods Mol Biol. 2019;1895:97–109. doi: 10.1007/978-1-4939-8922-5_8. [DOI] [PubMed] [Google Scholar]
- Chen Y-R, Zhong S, Fei Z, Hashimoto Y, Xiang JZ, Zhang S, Blissard GW. The Transcriptome of the Baculovirus Autographa californica Multiple Nucleopolyhedrovirus in Trichoplusia ni Cells. J Virol. 2013;87:6391–6405. doi: 10.1128/jvi.00194-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CL, Tseng YW, Wu JC, Chen GY, Lin KC, Hwang SM, Hu YC. Suppression of hepatocellular carcinoma by baculovirus-mediated expression of long non-coding RNA PTENP1 and MicroRNA regulation. Biomaterials. 2015;44:71–81. doi: 10.1016/j.biomaterials.2014.12.023. [DOI] [PubMed] [Google Scholar]
- Cheshenko N, Krougliak N, Eisensmith RC, Krougliak VA. A novel system for the production of fully deleted adenovirus vectors that does not require helper adenovirus. Gene Ther. 2001;8:846–854. doi: 10.1038/sj.gt.3301459. [DOI] [PubMed] [Google Scholar]
- Cho B, Kim J, Cho JE, Jeon BY, Park S. Expression of the capsid protein of Chikungunya virus in a baculovirus for serodiagnosis of Chikungunya disease. J Virol Methods. 2008;154:154–159. doi: 10.1016/j.jviromet.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Chua CL, Sam IC, Chan YF. Expression and purification of E2 glycoprotein from insect cells (Sf9) for use in serology. Methods Mol Biol. 2016;1426:51–61. doi: 10.1007/978-1-4939-3618-2_5. [DOI] [PubMed] [Google Scholar]
- Chuang CK, Wong TH, Hwang SM, Chang YH, Chen GY, Chiu YC, Huang SF, Hu YC. Baculovirus transduction of mesenchymal stem cells: In vitro responses and in vivo immune responses after cell transplantation. Mol Ther. 2009;17:889–896. doi: 10.1038/mt.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory JS, Hails RS. The ecology and biosafety of baculoviruses. Curr Opin Biotechnol. 1997;8:323–327. doi: 10.1016/S0958-1669(97)80011-0. [DOI] [PubMed] [Google Scholar]
- Cox MMJ. Recombinant protein vaccines produced in insect cells. Vaccine. 2012;30:1759–1766. doi: 10.1016/j.vaccine.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MMJ, Hashimoto Y. A fast track influenza virus vaccine produced in insect cells. J Invertebr Pathol. 2011;107:S31–S41. doi: 10.1016/j.jip.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Cox MMJ, Izikson R, Post P, Dunkle L. Safety, efficacy, and immunogenicity of Flublok in the prevention of seasonal influenza in adults. Ther Adv Vaccines. 2015;3:97–108. doi: 10.1177/2051013615595595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S, Zhang T, Zhang Y, Wang H, Deng F. Zika virus baculovirus-expressed virus-like particles induce neutralizing antibodies in mice. Virol Sin. 2018;33:213–226. doi: 10.1007/s12250-018-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L, Zheng T, Xu K, Han Y, Xu L, Huang E, An Y, Cheng Y, Li S, Liu M, Yang M, Li Y, Cheng H, Yuan Y, Zhang W, Ke C, Wong G, Qi J, Qin C, Yan J, Gao GF. A universal design of Betacoronavirus vaccines against COVID-19, MERS, and SARS. Cell. 2020;182:722–733.e11. doi: 10.1016/j.cell.2020.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delenda C, Frenkiel M, Deuvel V. Protective efficacy in mice of a secreted form of recombinant dengue-2 virus envelope protein produced in baculovirus infected insect cells. Arch Virol. 1994;139:197–207. doi: 10.1007/BF01309465. [DOI] [PubMed] [Google Scholar]
- Deng MP, Hu ZH, Wang HL, Deng F. Developments of subunit and VLP vaccines against influenza a virus. Virol Sin. 2012;27:145–153. doi: 10.1007/s12250-012-3241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres P, Dietrich J, Girard M, Bouloy M. Recombinant baculoviruses expressing yellow fever virus E and NS1 proteins elicit protective immunity in mice. J Gen Virol. 1991;72:2811–2816. doi: 10.1099/0022-1317-72-11-2811. [DOI] [PubMed] [Google Scholar]
- Du R, Yin F, Wang M, Hu Z, Wang H, Deng F. Glycoprotein E of the Japanese encephalitis virus forms virus-like particles and induces syncytia when expressed by a baculovirus. J Gen Virol. 2015;96:1006–1014. doi: 10.1099/vir.0.000052. [DOI] [PubMed] [Google Scholar]
- Eckels K, Dubois D, Summers P, Schlesinger J, Shelly M, Cohen S, Zhang Y, Lai C, Kurane I, Rothman A, Hasty S, Howard B. Immunization of monkeys with baculovirus-dengue type-4 recombinants containing envelope and nonstructural proteins: evidence of priming and partial protection. Am J Trop Med Hyg. 1994;50:472–478. doi: 10.4269/ajtmh.1994.50.472. [DOI] [PubMed] [Google Scholar]
- Escribano JM, Cid M, Reytor E, Alvarado C, Nuñez MC, Martínez-Pulgarín S, Dalton RM. Chrysalises as natural production units for recombinant subunit vaccines. J Biotechnol X. 2020;6:100019. doi: 10.1016/j.btecx.2020.100019. [DOI] [PubMed] [Google Scholar]
- Fabre M, Arrías P, Massón T, Pidre M, Romanowski V. Baculovirus-derived vectors for immunization and therapeutic applications. In: Ennaji M, editor. Emerging and Reemerging Viral Pathogens. United States: Academic Press; 2020. pp. 197–224. [Google Scholar]
- Faletti L, Urtasun N, Targovnik A, Arregui M, Levin G, Maroniche G, Wolman F, Cascone O, Miranda M. Expression of recombinant Influenza A H1N1 neuraminidase in Rachiplusia nu larvae. Curr Topic Virol. 2014;12:65–75. [Google Scholar]
- Fauci AS. Pandemic influenza threat and preparedness. Emerg Infect Dis. 2006;12:73–77. doi: 10.3201/eid1201.050983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q, Liu Y, Qu X, Deng H, Ding M, Lau TLT, Yu ACH, Chen J. Baculovirus surface display of SARS coronavirus (SARS-CoV) spike protein and immunogenicity of the displayed protein in mice models. DNA Cell Biol. 2006;25:668–673. doi: 10.1089/dna.2006.25.668. [DOI] [PubMed] [Google Scholar]
- Fujita R, Matsuyama T, Yamagishi J, Sahara K, Asano S, Bando H. Expression of Autographa californica multiple nucleopolyhedrovirus genes in mammalian cells and upregulation of the host β-actin gene. J Virol. 2006;80:2390–2395. doi: 10.1128/jvi.80.5.2390-2395.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita R, Hino M, Ebihara T, Nagasato T, Masuda A, Lee JM, Fujii T, Mon H, Kakino K, Nagai R, Tanaka M, Tonooka Y, Moriyama T, Kusakabe T. Efficient production of recombinant SARS-CoV-2 spike protein using the baculovirus-silkworm system. Biochem Biophys Res Commun. 2020;529:257–262. doi: 10.1016/j.bbrc.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galibert L, Merten OW. Latest developments in the large-scale production of adeno-associated virus vectors in insect cells toward the treatment of neuromuscular diseases. J Invertebr Pathol. 2011;107:S80–S93. doi: 10.1016/j.jip.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Garavaglia MJ, Miele SAB, Iserte JA, Belaich MN, Ghiringhelli PD. The ac53, ac78, ac101, and ac103 genes are newly discovered core genes in the family Baculoviridae. J Virol. 2012;86:12069–12079. doi: 10.1128/jvi.01873-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasque SN, van Oers MM, Ros VI. Where the baculoviruses lead, the caterpillars follow: baculovirus-induced alterations in caterpillar behaviour. Curr Opin Insect Sci. 2019;33:30–36. doi: 10.1016/j.cois.2019.02.008. [DOI] [PubMed] [Google Scholar]
- Georgopoulos LJ, Elgue G, Sanchez J, Dussupt V, Magotti P, Lambris JD, Tötterman TH, Maitland NJ, Nilsson B. Preclinical evaluation of innate immunity to baculovirus gene therapy vectors in whole human blood. Mol Immunol. 2009;46:2911–2917. doi: 10.1016/j.molimm.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giménez CS, Castillo MG, Simonin JA, Núñez Pedrozo CN, Pascuali N, del Rosario Bauzá M, Locatelli P, López AE, Belaich MN, Mendiz AO, Crottogini AJ, Cuniberti LA, Olea FD. Effect of intramuscular baculovirus encoding mutant hypoxia-inducible factor 1-alpha on neovasculogenesis and ischemic muscle protection in rabbits with peripheral arterial disease. Cytotherapy. 2020;22:563–572. doi: 10.1016/j.jcyt.2020.06.010. [DOI] [PubMed] [Google Scholar]
- Ginn SL, Amaya AK, Alexander IE, Edelstein M, Abedi MR. Gene therapy clinical trials worldwide to 2017: an update. J Gene Med. 2018;20:1–16. doi: 10.1002/jgm.3015. [DOI] [PubMed] [Google Scholar]
- Girard M, Nelson CB, Picot V, Gubler DJ. Arboviruses: A global public health threat. Vaccine. 2020;38:3989–3994. doi: 10.1016/j.vaccine.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Casado E, Gomez-Sebastian S, Núñez MC, Lasa-Covarrubias R, Martínez-Pulgarín S, Escribano JM. Insect larvae biofactories as a platform for influenza vaccine production. Protein Expr Purif. 2011;79:35–43. doi: 10.1016/j.pep.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Gottardo MF, Pidre ML, Zuccato C, Asad AS, Imsen M, Jaita G, Candolfi M, Romanowski V, Seilicovich A. Baculovirus-based gene silencing of Humanin for the treatment of pituitary tumors. Apoptosis. 2018;23:143–151. doi: 10.1007/s10495-018-1444-0. [DOI] [PubMed] [Google Scholar]
- Gould E, Pettersson J, Higgs S, Charrel R, de Lamballerie X. Emerging arboviruses: Why today? One Heal. 2017;4:1–13. doi: 10.1016/j.onehlt.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves LP, Aksular M, Alakeely RA, Buck DR, Chambers AC, Murguia-Meca F, Plata-Muñoz JJ, Hughes S, Johnson PRV, Possee RD, King LA. Improved baculovirus vectors for transduction and gene expression in human pancreatic islet cells. Viruses. 2018;10:1–17. doi: 10.3390/v10100574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronowski AM, Hilbert DM, Sheehan KCF, Garotta G, Schreiber RD. Baculovirus stimulates antiviral effects in mammalian cells. J Virol. 1999;73:9944–9951. doi: 10.1128/jvi.73.12.9944-9951.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwon YD, Kim S, Cho Y, Heo Y, Cho H, Park K, Lee HJ, Choi J, Poo H, Kim YB. Immunogenicity of virus like particle forming baculoviral DNA vaccine against pandemic influenza H1N1. PLoS ONE. 2016;11:1–17. doi: 10.1371/journal.pone.0154824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase S, Sciocco-Cap A, Romanowski V. Baculovirus insecticides in Latin America: historical overview, current status and future perspectives. Viruses. 2015;7:2230–2267. doi: 10.3390/v7052230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn BTJ, Courbron D, Hamer M, Masoud M, Wong J, Taylor K, Hatch J, Sowers M, Shane E, Nathan M, Jiang HUA, Wei Z, Higgins J, Roh K, Burd J, Chinchilla-olszar D, Malou-williams M, Baskind DP, Smith GE. Rapid manufacture and release of GMP batch of avian influenza A (H7N9) virus like particles vaccine made using recombinant baculovirus-sf9 insect cell culture technology. Bioprocess J. 2013;12:4–17. doi: 10.12665/J122.Hahn. [DOI] [Google Scholar]
- Harding AT, Heaton NS. Efforts to improve the seasonal influenza vaccine. Vaccines. 2018;6:19. doi: 10.3390/vaccines6020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RL, Herniou EA, Jehle JA, Theilmann DA, Burand JP, Becnel JJ, Krell PJ, van Oers MM, Mowery JD, Bauchan GR. ICTV virus taxonomy profile: Baculoviridae. J Gen Virol. 2018;99:1185–1186. doi: 10.1099/jgv.0.001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Manopo I, Lu L, Leung BP, Chng HH, Ling AE, Chee LL, Chan SW, Ooi EE, Sin YL, Ang B, Kwang J. Novel immunofluorescence assay using recombinant nucleocapsid-spike fusion protein as antigen to detect antibodies against severe acute respiratory syndrome coronavirus. Clin Diagn Lab Immunol. 2005;12:321–328. doi: 10.1128/CDLI.12.2.321-328.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Li J, Heck S, Lustigman S, Jiang S. Antigenic and immunogenic characterization of recombinant baculovirus-expressed severe acute respiratory syndrome coronavirus spike protein: implication for vaccine design. J Virol. 2006;80:5757–5767. doi: 10.1128/jvi.00083-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Madhan S, Kwang J. Baculovirus vector as a delivery vehicle for influenza vaccines. Expert Rev Vaccines. 2009;8:455–467. doi: 10.1586/erv.09.2. [DOI] [PubMed] [Google Scholar]
- Heikura T, Nieminen T, Roschier M, Karvinen H, Kaikkonen M, Mähönen A, Laitinen O, Lesch H, Airenne K, Rissanen T, Ylä-Herttuala S. Pro-opiomelanocortin gene delivery suppresses the growth of established Lewis lung carcinoma through a melanocortin-1 receptor-independent pathway. J Gene Med. 2012;14:44–53. doi: 10.1002/jgm. [DOI] [PubMed] [Google Scholar]
- Hernández-Ávila M, Santos-Preciado JI. Análisis de la evidencia sobre eficacia y seguridad de la vacuna de dengue CYD-TDV y su potencial registro e implementación en el Programa de Vacunación Universal de México. Salud Publica Mex. 2016;58:71–83. doi: 10.21149/spm.v58i1.7670. [DOI] [PubMed] [Google Scholar]
- Hervas-Stubbs S, Rueda P, Lopez L, Leclerc C. Insect Baculoviruses strongly potentiate adaptive immune responses by inducing type I IFN. J Immunol. 2007;178:2361–2369. doi: 10.4049/jimmunol.178.4.2361. [DOI] [PubMed] [Google Scholar]
- Hilsch M, Goldenbogen B, Sieben C, Höfer CT, Rabe JP, Klipp E, Herrmann A, Chiantia S. Influenza a matrix protein m1 multimerizes upon binding to lipid membranes. Biophys J. 2014;107:912–923. doi: 10.1016/j.bpj.2014.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchman E, Hitchman RB, King LA. BacMam Delivery of a Protective Gene to Reduce Renal Ischemia-Reperfusion Injury. Hum Gene Ther. 2017;28:747–756. doi: 10.1089/hum.2016.100. [DOI] [PubMed] [Google Scholar]
- Ho Y, Lin PH, Liu CYY, Lee SP, Chao YC. Assembly of human severe acute respiratory syndrome coronavirus-like particles. Biochem Biophys Res Commun. 2004;318:833–838. doi: 10.1016/j.bbrc.2004.04.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann C, Sandig V, Jennings G, Rudolph M, Schlag P, Strauss M. Efficient gene transfer into human hepatocytes by baculovirus vectors. Proc Natl Acad Sci U S A. 1995;92:10099–10103. doi: 10.1073/pnas.92.22.10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SS, Marotte H, Courbon G, Firestein GS, Boulanger P, Miossec P. PUMA gene delivery to synoviocytes reduces inflammation and degeneration of arthritic joints. Nat Commun. 2017;8:1–11. doi: 10.1038/s41467-017-00142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh SC, Tsai WY, Nerurkar VR, Wang WK. Characterization of the ectodomain of the envelope protein of dengue virus type 4: expression, membrane association, secretion and particle formation in the absence of precursor membrane protein. PLoS ONE. 2014;9:8–10. doi: 10.1371/journal.pone.0100641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh MS, He JL, Wu TY, Juang RH. A secretary bi-cistronic baculovirus expression system with improved production of the HA1 protein of H6 influenza virus in insect cells and Spodoptera litura larvae. J Immunol Methods. 2018;459:81–89. doi: 10.1016/j.jim.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu MN, Liao HT, Truong VA, Huang KL, Yu FJ, Chen HH, Nguyen TKN, Makarevich P, Parfyonova Y, Hu YC. CRISPR-based activation of endogenous neurotrophic genes in adipose stem cell sheets to stimulate peripheral nerve regeneration. Theranostics. 2019;9:6099–6111. doi: 10.7150/thno.36790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Li Y, Deng F, Hu Z, Wang H, Wang M. Improving Baculovirus transduction of mammalian cells by incorporation of Thogotovirus glycoproteins. Virol Sin. 2019;34:454–466. doi: 10.1007/s12250-019-00133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Li Y, Ning Y-J, Deng F, Vlak JM, Hu Z, Wang H, Wang M. The major hurdle for effective baculovirus transduction into mammalian cells is passing early endosomes. J Virol. 2019;93:e00709–e719. doi: 10.1128/JVI.00709-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignoffo C. The first viral pesticide: past, present, and future. J iIdustrial Microbiol Biotechnol. 1999;22:407–417. doi: 10.1038/sj.jim.2900654. [DOI] [Google Scholar]
- Ishimwe E, Hodgson JJ, Clem RJ, Passarelli AL. Reaching the melting point: degradative enzymes and protease inhibitors involved in baculovirus infection and dissemination. Virology. 2015;479–480:637–649. doi: 10.1016/j.virol.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed MA, Biswas S, Willis LG, Harris S, Pritchard C, van Oers MM, Donly BC, Erlandson MA, Hegedus DD, Theilmann DA. Autographa californica multiple nucleopolyhedrovirus AC83 is a Per Os infectivity factor (PIF) protein required for occlusion-derived virus (ODV) and budded virus nucleocapsid assembly as well as assembly of the PIF complex in ODV envelopes. J Virol. 2017;91:e02115–e2116. doi: 10.1128/jvi.02115-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehle JA, Blissard GW, Bonning BC, Cory JS, Herniou EA, Rohrmann GF, Theilmann DA, Thiem SM, Vlak JM. On the classification and nomenclature of baculoviruses: a proposal for revision. Arch Virol. 2006;151:1257–1266. doi: 10.1007/s00705-006-0763-6. [DOI] [PubMed] [Google Scholar]
- Jhaveri R, Infectious P, Lurie RH. The next set of COVID-19 vaccines: leveraging new development platforms to increase access for more people around the world. Clin Ther. 2020;43:702–710. doi: 10.1016/j.clinthera.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson BE, Pokorny BA, Tiso VA. Supplementation of conventional trivalent influenza vaccine with purified viral N1 and N2 neuraminidases induces a balanced immune response without antigenic competition. Vaccine. 2002;20:1670–1674. doi: 10.1016/S0264-410X(01)00490-X. [DOI] [PubMed] [Google Scholar]
- Joshi PRH, Cervera L, Ahmed I, Kondratov O, Zolotukhin S, Schrag J, Chahal PS, Kamen AA. Achieving high-yield production of functional AAV5 gene delivery vectors via fedbatch in an insect cell-one baculovirus system. Mol Ther - Methods Clin Dev. 2019;13:279–289. doi: 10.1016/j.omtm.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kading RC, Brault AC, Beckham JD. Global perspectives on arbovirus outbreaks: a 2020 snapshot. Trop Med Infect Dis. 2020;5:142. doi: 10.3390/tropicalmed5030142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaikkonen MU, Maatta AI, Ylä-Herttuala S, Airenne KJ. Screening of complement inhibitors: shielded baculoviruses increase the safety and efficacy of gene delivery. Mol Ther. 2010;18:987–992. doi: 10.1038/mt.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash JC, Basler CF, García-Sastre A, Carter V, Billharz R, Swayne DE, Przygodzki RM, Taubenberger JK, Katze MG, Tumpey TM. Global host immune response: pathogenesis and transcriptional profiling of type A influenza viruses expressing the hemagglutinin and neuraminidase genes from the 1918 pandemic virus. J Virol. 2004;78:9499–9511. doi: 10.1128/jvi.78.17.9499-9511.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka C, Kaname Y, Taguwa S, Abe T, Fukuhara T, Tani H, Moriishi K, Matsuura Y. Baculovirus GP64-mediated entry into mammalian cells. J Virol. 2012;86:2610–2620. doi: 10.1128/jvi.06704-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai Y, Kawabata C, Sakaguchi M, Tamura T. Protection of baculovirus vectors expressing complement regulatory proteins against serum complement attack. Biol Pharm Bull. 2018;41:1600–1605. doi: 10.1248/bpb.b18-00451. [DOI] [PubMed] [Google Scholar]
- Keeler AM, Flotte TR. Recombinant adeno-associated virus gene therapy in light of Luxturna (and Zolgensma and Glybera): where are we, and how did we get here? Annu Rev Virol. 2019;6:601–621. doi: 10.1146/annurev-virology-092818-015530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly EP, Greene JJ, King AD, Innis BL. Purified dengue 2 virus envelope glycoprotein aggregates produced by baculovirus are immunogenic in mice. Vaccine. 2000;18:2549–2559. doi: 10.1016/S0264-410X(00)00032-3. [DOI] [PubMed] [Google Scholar]
- Kenoutis C, Efrose RC, Swevers L, Lavdas AA, Gaitanou M, Matsas R, Iatrou K. Baculovirus-mediated gene delivery into mammalian cells does not alter their transcriptional and differentiating potential but is accompanied by early viral gene expression. J Virol. 2006;80:4135–4146. doi: 10.1128/jvi.80.8.4135-4146.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalaj-Hedayati A, Chua CLL, Smooker P, Lee KW. Nanoparticles in influenza subunit vaccine development: immunogenicity enhancement. Influenza Other Respi Viruses. 2020;14:92–101. doi: 10.1111/irv.12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana S, Wu J, Verma N, Verma S, Raghunandan R, Manischewitz J, King LR, Kpamegan E, Pincus S, Smith G, Glenn G, Golding H. H5N1 virus-like particle vaccine elicits cross-reactive neutralizing antibodies that preferentially bind to the oligomeric form of influenza virus hemagglutinin in humans. J Virol. 2011;85:10945–10954. doi: 10.1128/jvi.05406-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Choi JY, Jiang HL, Arote R, Jere D, Cho MH, Je YH, Cho CS. Hybrid of baculovirus and galactosylated PEI for efficient gene carrier. Virology. 2009;387:89–97. doi: 10.1016/j.virol.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Kim MC, Song JM, Eunju O, Kwon YM, Lee YJ, Compans RW, Kang SM. Virus-like particles containing multiple M2 extracellular domains confer improved cross-protection against various subtypes of influenza virus. Mol Ther. 2013;21:485–492. doi: 10.1038/mt.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Lee J, Kim YE, Chong CK, Pinchemel Y, Reisdörfer F, Coelho JB, Dias RF, Bae PK, Gusmão ZPM, Ahn HJ, Nam HW. Development of a rapid diagnostic test kit to detect IgG/IgM antibody against zika virus using monoclonal antibodies to the envelope and non-structural protein 1 of the virus. Korean J Parasitol. 2018;56:61–70. doi: 10.3347/kjp.2018.56.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JC, Cox MM, Reisinger K, Hedrick J, Graham I, Patriarca P. Evaluation of the safety, reactogenicity and immunogenicity of FluBlok® trivalent recombinant baculovirus-expressed hemagglutinin influenza vaccine administered intramuscularly to healthy children aged 6–59 months. Vaccine. 2009;27:6589–6594. doi: 10.1016/j.vaccine.2009.08.032. [DOI] [PubMed] [Google Scholar]
- Kolangath SM, Basagoudanavar SH, Hosamani M, Saravanan P, Tamil Selvan RP. Baculovirus mediated transduction: analysis of vesicular stomatitis virus glycoprotein pseudotyping. VirusDisease. 2014;25:441–446. doi: 10.1007/s13337-014-0229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik I, Yewdell JW. Influenza hemagglutinin and neuraminidase: Yin–yang proteins coevolving to thwart immunity. Viruses. 2019;11:346. doi: 10.3390/v11040346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost TA, Condreay JP. Innovations—Biotechnology: Baculovirus vectors as gene transfer vectors for mammalian cells: Biosafety considerations. Appl Biosaf. 2002;7:167–169. doi: 10.1177/153567600200700312. [DOI] [Google Scholar]
- Kost TA, Condreay JP, Jarvis DL. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat Biotechnol. 2005;23:567–575. doi: 10.1038/nbt1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol E, Brzuska G, Szewczyk B. Production and biomedical application of flavivirus-like particles. Trends Biotechnol. 2019;37:1202–1216. doi: 10.1016/j.tibtech.2019.03.013. [DOI] [PubMed] [Google Scholar]
- Kumar P, Pok KY, Tan LK, Angela C, Leo YS, Ng LC. Development and evaluation of baculovirus-expressed Chikungunya virus E1 envelope proteins for serodiagnosis of Chikungunya infection. J Virol Methods. 2014;206:67–75. doi: 10.1016/j.jviromet.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Kumar N, Pandey N, Halder A. Preventive, diagnostic and therapeutic application of baculovirus expression vector system. In: Kumar D, Gong C, editors. Trends in insect molecular biology and biotechnology. Cham: Springer; 2018. pp. 163–191. [Google Scholar]
- Kuo SC, Teng CY, Ho YJ, Chen YJ, Wu TY. Using bicistronic baculovirus expression vector system to screen the compounds that interfere with the infection of Chikungunya virus. Methods Mol Biol. 2016;1426:263–272. doi: 10.1007/978-1-4939-3618-2_24. [DOI] [PubMed] [Google Scholar]
- Kuwahara M, Konishi E. Evaluation of extracellular subviral particles of dengue virus type 2 and Japanese encephalitis virus produced by Spodoptera frugiperda cells for use as vaccine and diagnostic antigens. Clin Vaccine Immunol. 2010;17:1560–1566. doi: 10.1128/CVI.00087-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwang TW, Zeng X, Wang S. Manufacturing of AcMNPV baculovirus vectors to enable gene therapy trials. Mol Ther - Methods Clin Dev. 2016;3:15050. doi: 10.1038/mtm.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey LA, Grzywacz D, Shapiro-Ilan DI, Frutos R, Brownbridge M, Goettel MS. Insect pathogens as biological control agents: back to the future. J Invertebr Pathol. 2015;132:1–41. doi: 10.1016/j.jip.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Lai CC, Cheng YC, Chen PW, Lin TH, Tzeng TT, Lu CC, Lee MS, Hu AYC. Process development for pandemic influenza VLP vaccine production using a baculovirus expression system. J Biol Eng. 2019;13:1–9. doi: 10.1186/s13036-019-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Ko HL, Lee EY, Park HJ, Kim YS, Kim YS, Cho NH, Park MS, Lee SM, Kim J, Kim H, Seong BL, Nam JH. Development of a diagnostic system for detection of specific antibodies and antigens against Middle East respiratory syndrome coronavirus. Microbiol Immunol. 2018;62:574–584. doi: 10.1111/1348-0421.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YT, Kim KH, Ko EJ, Kim MC, Lee YN, Hwang HS, Lee Y, Jung YJ, Kim YJ, Santos J, Perez DR, Kang SM. Enhancing the cross protective efficacy of live attenuated influenza virus vaccine by supplemented vaccination with M2 ectodomain virus-like particles. Virology. 2019;529:111–121. doi: 10.1016/j.virol.2019.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li KC, Chang YH, Yeh CL, Hu YC. Healing of osteoporotic bone defects by baculovirus-engineered bone marrow-derived MSCs expressing MicroRNA sponges. Biomaterials. 2016;74:155–166. doi: 10.1016/j.biomaterials.2015.09.046. [DOI] [PubMed] [Google Scholar]
- Li KC, Chang YH, Hsu MN, Lo SC, Li WH, Hu YC. Baculovirus-mediated miR-214 knockdown shifts osteoporotic ASCs differentiation and improves osteoporotic bone defects repair. Sci Rep. 2017;7:1–13. doi: 10.1038/s41598-017-16547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao JC. Cell therapy using bone marrow-derived stem cell overexpressing BMP-7 for degenerative discs in a rat tail disc model. Int J Mol Sci. 2016;17:1–13. doi: 10.3390/ijms17020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao JC. Bone marrow mesenchymal stem cells expressing baculovirus-engineered bone morphogenetic protein-7 enhance rabbit posterolateral fusion. Int J Mol Sci. 2016;17:1073. doi: 10.3390/ijms17071073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Lin KJ, Kao CY, Chen MC, Lo WH, Yen TC, Chang YH, Hu YC. The role of adipose-derived stem cells engineered with the persistently expressing hybrid baculovirus in the healing of massive bone defects. Biomaterials. 2011;32:6505–6514. doi: 10.1016/j.biomaterials.2011.05.059. [DOI] [PubMed] [Google Scholar]
- Lin CY, Chang YH, Sung LY, Chen CL, Lin SY, Li KC, Yen TC, Lin KJ, Hu YC. Long-term tracking of segmental bone healing mediated by genetically engineered adipose-derived stem cells: focuses on bone remodeling and potential side effects. Tissue Eng - Part A. 2014;20:1392–1402. doi: 10.1089/ten.tea.2013.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Wang YH, Li KC, Sung LY, Yeh CL, Lin KJ, Yen TC, Chang YH, Hu YC. Healing of massive segmental femoral bone defects in minipigs by allogenic ASCs engineered with FLPo/Frt-based baculovirus vectors. Biomaterials. 2015;50:98–106. doi: 10.1016/j.biomaterials.2015.01.052. [DOI] [PubMed] [Google Scholar]
- Lin MW, Tseng YW, Shen CC, Hsu MN, Hwu JR, Chang CW, Yeh CJ, Chou MY, Wu JC, Hu YC. Synthetic switch-based baculovirus for transgene expression control and selective killing of hepatocellular carcinoma cells. Nucleic Acids Res. 2018;46:e93. doi: 10.1093/nar/gky447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YV, Massare MJ, Barnard DL, Kort T, Nathan M, Wang L, Smith G. Chimeric severe acute respiratory syndrome coronavirus (SARS-CoV) S glycoprotein and influenza matrix 1 efficiently form virus-like particles (VLPs) that protect mice against challenge with SARS-CoV. Vaccine. 2011;29:6606–6613. doi: 10.1016/j.vaccine.2011.06.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, JooLei KY, Wang P. Visualization of intracellular pathways of engineered baculovirus in mammalian cells. Virus Res. 2014;181:81–91. doi: 10.1016/j.virusres.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Li Y, Hu X, Yi Y, Zhang Z. Gene delivery and gene expression in vertebrate using baculovirus Bombyx mori nucleopolyhedrovirus vector. Oncotarget. 2017;8:106017–106025. doi: 10.18632/oncotarget.22522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo WH, Hwang SM, Chuang CK, Chen CY, Hu YC. Development of a hybrid baculoviral vector for sustained transgene expression. Mol Ther. 2009;17:658–666. doi: 10.1038/mt.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo SC, Li KC, Chang YH, Hsu MN, Sung LY, Vu TA, Hu YC. Enhanced critical-size calvarial bone healing by ASCs engineered with Cre/loxP-based hybrid baculovirus. Biomaterials. 2017;124:1–11. doi: 10.1016/j.biomaterials.2017.01.033. [DOI] [PubMed] [Google Scholar]
- Long G, Pan X, Kormelink R, Vlak JM. Functional entry of baculovirus into insect and mammalian cells is dependent on clathrin-mediated endocytosis. J Virol. 2006;80:8830–8833. doi: 10.1128/jvi.00880-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Macías C. Virus-like particle (VLP)-based vaccines for pandemic influenza: Performance of a VLP vaccine during the 2009 influenza pandemic. Hum Vaccines Immunother. 2012;8:402–405. doi: 10.4161/hv.8.3.18757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Vidal J, Gómez-Sebastián S, Sánchez-Ramos I, Escribano JM. Characterization of a Trichoplusia ni hexamerin-derived promoter in the AcMNPV baculovirus vector. J Biotechnol. 2013;165:201–208. doi: 10.1016/j.jbiotec.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Lothert K, Sprick G, Beyer F, Lauria G, Czermak P, Wolff MW. Membrane-based steric exclusion chromatography for the purification of a recombinant baculovirus and its application for cell therapy. J Virol Methods. 2020;275:113756. doi: 10.1016/j.jviromet.2019.113756. [DOI] [PubMed] [Google Scholar]
- Lu X, Chen Y, Bai B, Hu H, Tao L, Yang J, Chen J, Chen Z, Hu Z, Wang H. Immune responses against severe acute respiratory syndrome coronavirus induced by virus-like particles in mice. Immunology. 2007;122:496–502. doi: 10.1111/j.1365-2567.2007.02676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Huang Y, Huang L, Li B, Zheng Z, Chen Z, Chen J, Hu Q, Wang H. Effect of mucosal and systemic immunization with virus-like particles of severe acute respiratory syndrome coronavirus in mice. Immunology. 2010;130:254–261. doi: 10.1111/j.1365-2567.2010.03231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow VA, Lee SC, Barry GF, Olins PO. Efficient generation of infectious recombinant baculoviruses by site-specific transposon-mediated insertion of foreign genes into a baculovirus genome propagated in Escherichia coli. J Virol. 1993;67:4566–4579. doi: 10.1128/jvi.67.8.4566-4579.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W-Y, Lin S-Y, Lo K-W, Lu C-H, Hung C-L, Chen C-Y, Chang C-C, Hu Y-C. Adaptive immune responses elicited by baculovirus and impacts on subsequent transgene expression in vivo. J Virol. 2013;87:4965–4973. doi: 10.1128/jvi.03510-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D, Miao Y, Ke X, Tan Z, Hu C, Li P, Wang T, Zhang Y, Sun J, Liu Y, Wang H, Zheng Z. Baculovirus surface display of zika virus envelope protein protects against virus challenge in mouse model. Virol Sin. 2020;35:637–650. doi: 10.1007/s12250-020-00238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luz-Madrigal A, Asanov A, Camacho-Zarco AR, Sampieri A, Vaca L. A Cholesterol recognition amino acid consensus domain in GP64 fusion protein facilitates anchoring of Baculovirus to mammalian cells. J Virol. 2013;87:11894–11907. doi: 10.1128/jvi.01356-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn DE, Harrison RL. Available lepidopteran insect cell lines. Methods Mol Biol. 2016;1350:119–142. doi: 10.1007/978-1-4939-3043-2_6. [DOI] [PubMed] [Google Scholar]
- Maghodia AB, Geisler C, Jarvis DL. A new Bacmid for customized protein glycosylation pathway engineering in the baculovirus-insect cell system. ACS Chem Biol. 2021 doi: 10.1021/acschembio.0c00974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarevich PI, Boldyreva MA, Gluhanyuk EV, Efimenko AY, Dergilev KV, Shevchenko EK, Sharonov GV, Gallinger JO, Rodina PA, Sarkisyan SS, Hu YC, Parfyonova YV. Enhanced angiogenesis in ischemic skeletal muscle after transplantation of cell sheets from baculovirus-transduced adipose-derived stromal cells expressing vegf165. Stem Cell Res Ther. 2015;6:1–20. doi: 10.1186/s13287-015-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä AR, Tuusa JE, Volkman LE, Oker-Blom C. Occlusion-derived baculovirus: Interaction with human cells and evaluation of the envelope protein P74 as a surface display platform. J Biotechnol. 2008;135:145–156. doi: 10.1016/j.jbiotec.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Mansouri M, Berger P. Baculovirus for gene delivery to mammalian cells: Past, present and future. Plasmid. 2018;98:1–7. doi: 10.1016/j.plasmid.2018.05.002. [DOI] [PubMed] [Google Scholar]
- Maranga L, Cruz PE, Aunins JG, Carrondo MJT. Production of core and virus-like particles with baculovirus infected insect cells. Adv Biochem Eng Biotechnol. 2002;74:183–206. doi: 10.1007/3-540-45736-4_9. [DOI] [PubMed] [Google Scholar]
- Marchi S, Trombetta C, Montomoli E. Emerging and re-emerging arboviral diseases as a global health problem. In: Majumder MAA, Kabir R, Rahman S, editors. Public Health-Emerging and Re-emerging Issues. London: Intech; 2018. pp. 25–46. [Google Scholar]
- Martínez CA, Giulietti AM, Rodríguez Talou J. Research advances in plant-made flavivirus antigens. Biotechnol Adv. 2012;30:1493–1505. doi: 10.1016/j.biotechadv.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Martínez-Solís M, Herrero S, Targovnik AM. Engineering of the baculovirus expression system for optimized protein production. Appl Microbiol Biotechnol. 2019;103:113–123. doi: 10.1007/s00253-018-9474-7. [DOI] [PubMed] [Google Scholar]
- Mateu MG. Virus engineering: Functionalization and stabilization. Protein Eng Des Sel. 2011;24:53–63. doi: 10.1093/protein/gzq069. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Tanijima T, Hirose A, Masumi-Koizumi K, Katsuda T, Yamaji H. Production of influenza virus-like particles using recombinant insect cells. Biochem Eng J. 2020;163:107757. doi: 10.1016/j.bej.2020.107757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazalovska M, Kouokam JC. Progress in the production of virus-like particles for vaccination against hepatitis E virus. Viruses. 2020;12:1–16. doi: 10.3390/v12080826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meghrous J, Mahmoud W, Jacob D, Chubet R, Cox M, Kamen AA. Development of a simple and high-yielding fed-batch process for the production of influenza vaccines. Vaccine. 2009;28:309–316. doi: 10.1016/j.vaccine.2009.10.048. [DOI] [PubMed] [Google Scholar]
- Metz SW, Pijlman GP. Arbovirus vaccines; opportunities for the baculovirus-insect cell expression system. J Invertebr Pathol. 2011;107:S16–S30. doi: 10.1016/j.jip.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Metz SW, Geertsema C, Martina BE, Andrade P, Heldens JG, Van Oers MM, Vlak JM, Pijlman GP. Functional processing and secretion of Chikungunya virus E1 and E2 glycoproteins in insect cells. Virol J. 2011;8:353. doi: 10.1186/1743-422X-8-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz SW, Martina BE, van den Doel P, Geertsema C, Osterhaus AD, Vlak JM, Pijlman GP. Chikungunya virus-like particles are more immunogenic in a lethal AG129 mouse model compared to glycoprotein E1 or E2 subunits. Vaccine. 2013;31:6092–6096. doi: 10.1016/j.vaccine.2013.09.045. [DOI] [PubMed] [Google Scholar]
- Mezzina M, Merten O. Adeno-associated viruses Mauro Mezzina and Otto-Wilhelm Merten Abstract. Methods Mol Biol. 2011;737:211–234. doi: 10.1007/978-1-61779-095-9_9. [DOI] [PubMed] [Google Scholar]
- Mi Y, Xie T, Zhu B, Tan J, Li X, Luo Y, Li F, Niu H, Han J, Lv W, Wang J. Production of sars-cov-2 virus-like particles in insect cells. Vaccines. 2021;9:1–8. doi: 10.3390/vaccines9060554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miele SAB, Garavaglia MJ, Belaich MN, Ghiringhelli PD. Baculovirus: molecular insights on their diversity and conservation. Int J Evol Biol. 2011;2011:1–15. doi: 10.4061/2011/379424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietzsch M, Casteleyn V, Weger S, Zolotukhin S, Heilbronn R. OneBac 2.0: Sf9 cell lines for production of AAV5 vectors with enhanced infectivity and minimal encapsidation of foreign DNA. Hum Gene Ther. 2015;26:688–697. doi: 10.1089/hum.2015.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modis Y., Ogata S., Clements D., Harrison S. C. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proceedings of the National Academy of Sciences. 2003;100(12):6986–6991. doi: 10.1073/pnas.0832193100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama T, Suzuki T, Chang MO, Kitajima M, Takaku H. Baculovirus directly activates murine NK cells via TLR9. Cancer Gene Ther. 2017;24:175–179. doi: 10.1038/cgt.2017.2. [DOI] [PubMed] [Google Scholar]
- Mortola E, Roy P. Efficient assembly and release of SARS coronavirus-like particles by a heterologous expression system. FEBS Lett. 2004;576:174–178. doi: 10.1016/j.febslet.2004.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasimuzzaman M, Lynn D, van der Loo JC, Malik P. Purification of baculovirus vectors using heparin affinity chromatography. Mol Ther - Methods Clin Dev. 2016;3:16071. doi: 10.1038/mtm.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasimuzzaman M, Van Der Loo JCM, Malik P. Production and purification of baculovirus for gene therapy application. J vis Exp. 2018;2018:1–6. doi: 10.3791/57019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naskalska A, Dabrowska A, Nowak P, Szczepanski A, Jasik K, Milewska A, Ochman M, Zeglen S, Rajfur Z, Pyrc K. Novel coronavirus-like particles targeting cells lining the respiratory tract. PLoS ONE. 2018;13:1–21. doi: 10.1371/journal.pone.0203489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerome K, Yamaguchi R, Fuke N, Izzati UZ, Maegawa K, Sugita S, Kawasaki K, Kuroda K, Nerome R. Development of a Japanese encephalitis virus genotype V virus-like particle vaccine in silkworms. J Gen Virol. 2018;99:897–907. doi: 10.1099/jgv.0.001081. [DOI] [PubMed] [Google Scholar]
- Niu G, Pang Z, Guan C, Qi J, Li D. Dengue virus envelope domain III protein based on a tetravalent antigen secreted from insect cells: Potential use for serological diagnosis. Virus Res. 2015;201:73–78. doi: 10.1016/j.virusres.2015.02.008. [DOI] [PubMed] [Google Scholar]
- O′Reilly D, Miller L, Luckow V. Baculovirus expression vector: a laboratory manual. United State: Oxford University Press; 1994. [Google Scholar]
- Ono C, Ninomiya A, Yamamoto S, Abe T, Wen X, Fukuhara T, Sasai M, Yamamoto M, Saitoh T, Satoh T, Kawai T, Ishii KJ, Akira S, Okamoto T, Matsuura Y. Innate immune response induced by baculovirus attenuates transgene expression in mammalian cells. J Virol. 2014;88:2157–2167. doi: 10.1128/jvi.03055-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono C, Okamoto T, Abe T, Matsuura Y. Baculovirus as a tool for gene delivery and gene therapy. Viruses. 2018;10:510. doi: 10.3390/v10090510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez-Espino D, Eloe-Fadrosh EA, Pavlopoulos GA, Thomas AD, Huntemann M, Mikhailova N, Rubin E, Ivanova NN, Kyrpides NC. Uncovering Earth’s virome. Nature. 2016;536:425–430. doi: 10.1038/nature19094. [DOI] [PubMed] [Google Scholar]
- Pallesen J, Wang N, Corbett KS, Wrapp D, Kirchdoerfer RN, Turner HL, Cottrell CA, Becker MM, Wang L, Shi W, Kong WP, Andres EL, Kettenbach AN, Denison MR, Chappell JD, Graham BS, Ward AB, McLellan JS. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc Natl Acad Sci U S A. 2017;114:E7348–E7357. doi: 10.1073/pnas.1707304114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmberger D, Klausberger M, Berger I, Grabherr R. MultiBac turns sweet. Bioengineered. 2013;4:78–83. doi: 10.4161/bioe.22327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Fang L, Fan H, Luo R, Zhao Q, Chen H, Xiao S. Antitumor effects of a recombinant pseudotype baculovirus expressing Apoptin in vitro and in vivo. Int J Cancer. 2010;126:2741–2751. doi: 10.1002/ijc.24959. [DOI] [PubMed] [Google Scholar]
- Parsza CN, Gómez DLM, Simonin JA, Belaich MN, Ghiringhelli PD. Evaluation of the nucleopolyhedrovirus of anticarsia gemmatalis as a vector for gene therapy in mammals. Curr Gene Ther. 2020;21:177–189. doi: 10.2174/1566523220999201217155945. [DOI] [PubMed] [Google Scholar]
- Paul A, Binsalamah ZM, Khan AA, Abbasia S, Elias CB, Shum-Tim D, Prakash S. A nanobiohybrid complex of recombinant baculovirus and Tat/DNA nanoparticles for delivery of Ang-1 transgene in myocardial infarction therapy. Biomaterials. 2011;32:8304–8318. doi: 10.1016/j.biomaterials.2011.07.042. [DOI] [PubMed] [Google Scholar]
- Paul A, Nayan M, Khan AA, Shum-Tim D, Prakash S. Angiopoietin-1-expressing adipose stem cells genetically modified with baculovirus nanocomplex: investigation in rat heart with acute infarction. Int J Nanomedicine. 2012;7:663–682. doi: 10.2147/IJN.S26882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A, Elias CB, Shum-Tim D, Prakash S. Bioactive baculovirus nanohybrids for stent based rapid vascular re-endothelialization. Sci Rep. 2013;3:1–9. doi: 10.1038/srep02366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazmiño-Ibarra V, Mengual-Martí A, Targovnik AM, Herrero S. Improvement of baculovirus as protein expression vector and as biopesticide by CRISPR/Cas9 editing. Biotechnol Bioeng. 2019;116:2823–2833. doi: 10.1002/bit.27139. [DOI] [PubMed] [Google Scholar]
- Pearson L, Polly R. Genetically engineered multi-component virus-like particles as veterinary vaccines. Immunol Cell Biol. 1993;71:381–389. doi: 10.1038/icb.1993.44. [DOI] [PubMed] [Google Scholar]
- Pierson TC, Diamond MS. The continued threat of emerging flaviviruses. Nat Microbiol. 2020;5:796–812. doi: 10.1038/s41564-020-0714-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijlman GP. Enveloped virus-like particles as vaccines against pathogenic arboviruses. Biotechnol J. 2015;10:659–670. doi: 10.1002/biot.201400427. [DOI] [PubMed] [Google Scholar]
- Possee RD, Chambers AC, Graves LP, Aksular M, King LA. Recent developments in the use of baculovirus expression vectors. Curr Issues Mol Biol. 2020;34:215–230. doi: 10.21775/CIMB.034.215. [DOI] [PubMed] [Google Scholar]
- Prabakaran M, Kwang J. Recombinant baculovirus displayed vaccine. Bioengineered. 2014;5:45–48. doi: 10.4161/bioe.26001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabakaran M, Velumani S, He F, Karuppannan AK, Geng GY, Yin LK, Kwang J. Protective immunity against influenza H5N1 virus challenge in mice by intranasal co-administration of baculovirus surface-displayed HA and recombinant CTB as an adjuvant. Virology. 2008;380:412–420. doi: 10.1016/j.virol.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Premanand B, Wee PZ, Prabakaran M. Baculovirus surface display of immunogenic proteins for vaccine development. Viruses. 2018;10:298. doi: 10.3390/v10060298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao M, Ashok M, Bernard KA, Palacios G, Zhou ZH, Lipkin WI, Liang TJ. Induction of sterilizing immunity against west nile virus (WNV), by immunization with WNV-like particles produced in insect cells. J Infect Dis. 2004;190:2104–2108. doi: 10.1086/425933. [DOI] [PubMed] [Google Scholar]
- Rantam FA, Purwati SS, Susilowati H, Sudiana K, Hendrianto E, Soetjipto Analysis of recombinant, multivalent dengue virus containing envelope (E) proteins from serotypes-1, -3 and -4 and expressed in baculovirus. Trials Vaccinol. 2015;4:e75–e79. doi: 10.1016/j.trivac.2013.10.001. [DOI] [Google Scholar]
- Rawat K, Kumari P, Saha L. COVID-19 vaccine: a recent update in pipeline vaccines, their design and development strategies. Eur J Pharmacol. 2021;892:173751. doi: 10.1016/j.ejphar.2020.173751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z, Zhao Y, Liu J, Ji X, Meng L, Wang T, Sun W, Zhang K, Sang X, Yu Z, Li Y, Feng N, Wang H, Yang S, Yang Z, Ma Y, Gao Y, Xia X. Intramuscular and intranasal immunization with an H7N9 influenza virus-like particle vaccine protects mice against lethal influenza virus challenge. Int Immunopharmacol. 2018;58:109–116. doi: 10.1016/j.intimp.2017.12.020. [DOI] [PubMed] [Google Scholar]
- Rohrmann GF (2019) Baculovirus molecular biology, 4th edn. National Center for Biotechnology Information (US), Bethesda [PubMed]
- Saijo M, Ogino T, Taguchi F, Fukushi S, Mizutani T, Notomi T, Kanda H, Minekawa H, Matsuyama S, Long HT, Hanh NTH, Kurane I, Tashiro M, Morikawa S. Recombinant nucleocapsid protein-based IgG enzyme-linked immunosorbent assay for the serological diagnosis of SARS. J Virol Methods. 2005;125:181–186. doi: 10.1016/j.jviromet.2005.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena A, Byram P, Singh S, Chakraborty J, Murhammer D, Giri L. A structured review of baculovirus infection process: integration of mathematical models and biomolecular information on cell-virus interaction. J Gen Virol. 2018;99:1151–1171. doi: 10.1099/jgv.0.001108. [DOI] [PubMed] [Google Scholar]
- Sequeira DP, Correia R, Carrondo MJT, Roldão A, Teixeira AP, Alves PM. Combining stable insect cell lines with baculovirus-mediated expression for multi-HA influenza VLP production. Vaccine. 2018;36:3112–3123. doi: 10.1016/j.vaccine.2017.02.043. [DOI] [PubMed] [Google Scholar]
- Shan L, Wang L, Yin J, Zhong P, Zhong J. An OriP/EBNA-1-based baculovirus vector with prolonged and enhanced transgene expression. J Gene Med. 2006;8:1400–1406. doi: 10.1002/jgm.978. [DOI] [PubMed] [Google Scholar]
- Shang Y, Wang M, Xiao G, Wang X, Hou D, Pan K, Liu S, Li J, Wang J, Arif BM, Vlak JM, Chen X, Wang H, Deng F, Hu Z. Construction and rescue of a functional synthetic baculovirus. ACS Synth Biol. 2017;6:1393–1402. doi: 10.1021/acssynbio.7b00028. [DOI] [PubMed] [Google Scholar]
- Shim DH, Kim MJ, Cha HR, Park ES, Kim AR, Park JH, Park HC, Song D, Lee JM. Development of a ha1-specific enzyme-linked immunosorbent assay against pandemic influenza virus a h1n1. Clin Exp Vaccine Res. 2019;8:70–76. doi: 10.7774/cevr.2019.8.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HY, Choi H, Kim N, Park N, Kim H, Kim J, Kim YB. Unraveling the genome-wide impact of recombinant baculovirus infection in mammalian cells for gene delivery. Genes (basel) 2020;11:1–12. doi: 10.3390/genes11111306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde Vivek, Cho Iksung, Plested Joyce S, Agrawal Sapeckshita, Fiske Jamie, Cai Rongman, Zhou Haixia, Pham Xuan, Zhu Mingzhu, Cloney-Clark Shane, Wang Nan, Zhou Bin, Lewis Maggie, Price-Abbott Patty, Patel Nita, Massare Michael J, Smith Gale, Keech Cheryl, Fries Louis, Glenn Gregory M. Comparison of the safety and immunogenicity of a novel Matrix-M-adjuvanted nanoparticle influenza vaccine with a quadrivalent seasonal influenza vaccine in older adults: a phase 3 randomised controlled trial. The Lancet Infectious Diseases. 2021 doi: 10.1016/S1473-3099(21)00192-4. [DOI] [PubMed] [Google Scholar]
- Sim SH, Kim JY, Seong BL, Nguyen HH, Chang J. Baculovirus displaying hemagglutinin elicits broad cross-protection against influenza in mice. PLoS ONE. 2016;11:1–17. doi: 10.1371/journal.pone.0152485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinn PL, Hwang BY, Li N, Ortiz JLS, Shirazi E, Parekh KR, Cooney AL, Schaffer DV, McCray PB. Novel GP64 envelope variants for improved delivery to human airway epithelial cells. Gene Ther. 2017;24:674–679. doi: 10.1038/gt.2017.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisteré-Oró M, Martínez-Pulgarín S, Solanes D, Veljkovic V, López-Serrano S, Córdoba L, Cordón I, Escribano JM, Darji A. Conserved HA-peptides expressed along with flagellin in Trichoplusia ni larvae protects chicken against intranasal H7N1 HPAIV challenge. Vaccine. 2020;38:416–422. doi: 10.1016/j.vaccine.2019.11.006. [DOI] [PubMed] [Google Scholar]
- Smith GE, Fraser MJ, Summers MD. Molecular engineering of the Autographa californica nuclear polyhedrosis virus genome: deletion mutations within the polyhedrin gene. J Virol. 1983;46:584–593. doi: 10.1128/jvi.46.2.584-593.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GE, Summers MD, Fraser MJ. Production of human beta interferon in insect cells infected with a baculovirus expression vector. 1983. Biotechnology. 1992;24:434–443. doi: 10.1128/mcb.3.12.2156-2165.1983. [DOI] [PubMed] [Google Scholar]
- Smith GE, Flyer DC, Raghunandan R, Liu Y, Wei Z, Wu Y, Kpamegan E, Courbron D, Fries LF, Glenn GM. Development of influenza H7N9 virus like particle (VLP) vaccine: Homologous A/Anhui/1/2013 (H7N9) protection and heterologous A/chicken/Jalisco/CPA1/2012 (H7N3) cross-protection in vaccinated mice challenged with H7N9 virus. Vaccine. 2013;31:4305–4313. doi: 10.1016/j.vaccine.2013.07.043. [DOI] [PubMed] [Google Scholar]
- Smith I, Juan G, Callum M, Sabljic AV, Marfia JI, Bombicino SS, Trabucchi A, Iacono RF, Birenbaum JM, Vazquez SC, Minoia JM, Cascone O, López MG, Taboga O, Targovnik AM, Wolman FJ, Fingermann M, Alonso LG, Valdez SN, Miranda MV. Rapid and cost - effective process based on insect larvae for scale - up production of SARS - COV - 2 spike protein for serological COVID - 19 testing. Biotechnol Bioeng. 2021;118:4129–4137. doi: 10.1002/bit.27889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Xu Y, Bao L, Zhang L, Yu P, Qu Y, Zhu H, Zhao W, Han Y, Qin C. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11:59. doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staropoli I, Frenkiel MP, Mégret F, Deubel V. Affinity-purified dengue-2 virus envelope glycoprotein induces neutralizing antibodies and protective immunity in mice. Vaccine. 1997;15:1946–1954. doi: 10.1016/S0264-410X(97)00128-X. [DOI] [PubMed] [Google Scholar]
- Sun J, Li M, Wang Y, Hao P, Jin X. Elaboration of tetravalent antibody responses against dengue viruses using a subunit vaccine comprised of a single consensus dengue envelope sequence. Vaccine. 2017;35:6308–6320. doi: 10.1016/j.vaccine.2017.09.063. [DOI] [PubMed] [Google Scholar]
- Sung LY, Chen CL, Lin SY, Hwang SM, Lu CH, Li KC, Lan AS, Hu YC. Enhanced and prolonged baculovirus-mediated expression by incorporating recombinase system and in cis elements: a comparative study. Nucleic Acids Res. 2013;41:e139. doi: 10.1093/nar/gkt442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Matsumoto N, Suzuki T, Chang MO, Takaku H. Stable replication of the EBNA1/OriP-mediated baculovirus vector and its application to anti-HCV gene therapy. Virol J. 2009;6:1–8. doi: 10.1186/1743-422X-6-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T, Kawabata C, Matsushita M, Sakaguchi M, Yoshida S. Malaria sporozoite protein expression enhances baculovirus-mediated gene transfer to hepatocytes. J Gene Med. 2016;18:75–85. doi: 10.1002/jgm.2879. [DOI] [PubMed] [Google Scholar]
- Targovnik A, Arregui MB, Bracco LF, Urtasun N, Baieli MF, Segura MM, Simonella MA, Fogar M, Wolman FJ, Cascone O, Miranda MV. Insect Larvae: A New Platform to Produce Commercial Recombinant Proteins. Curr Pharm Biotechnol. 2016;17(431):438. doi: 10.2174/138920101705160303163947. [DOI] [PubMed] [Google Scholar]
- Targovnik AM, Ferrari A, Mc Callum GJ, Arregui MB, Smith I, Bracco LF, Alfonso V, López MG, Martínez-Solís M, Herrero S, Miranda MV. Highly efficient production of rabies virus glycoprotein G ectodomain in Sf9 insect cells. 3 Biotech. 2019;9:1–11. doi: 10.1007/s13205-019-1920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian JH, Patel N, Haupt R, Zhou H, Weston S, Hammond H, Logue J, Portnoff AD, Norton J, Guebre-Xabier M, Zhou B, Jacobson K, Maciejewski S, Khatoon R, Wisniewska M, Moffitt W, Kluepfel-Stahl S, Ekechukwu B, Papin J, Boddapati S, Jason Wong C, Piedra PA, Frieman MB, Massare MJ, Fries L, Bengtsson KL, Stertman L, Ellingsworth L, Glenn G, Smith G. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat Commun. 2021;12:372. doi: 10.1038/s41467-020-20653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tija S, zu Altenschildesche G, Doerfler W. Autographa californica nuclear polyhedrosis virus (AcNPV) DNA does not persist in mass cultures of mammalian cells. Virology Virology. 1983;125:107–117. doi: 10.1016/0042-6822(83)90067-3. [DOI] [PubMed] [Google Scholar]
- Ting-Hui-Lin CMY, Lin CY, Yeh YQ, Jeng US, Wu WG, Lee MS. Improving immunogenicity of influenza virus H7N9 recombinant hemagglutinin for vaccine development. Vaccine. 2019;37:1897–1903. doi: 10.1016/j.vaccine.2018.09.034. [DOI] [PubMed] [Google Scholar]
- Torres-Vega MA, Vargas-Jerónimo RY, Montiel-Martínez AG, Munõz-Fuentes RM, Zamorano-Carrillo A, Pastor AR, Palomares LA. Delivery of glutamine synthetase gene by baculovirus vectors: a proof of concept for the treatment of acute hyperammonemia. Gene Ther. 2015;22:58–64. doi: 10.1038/gt.2014.89. [DOI] [PubMed] [Google Scholar]
- Tsai CH, Wei SC, Lo HR, Chao YC. Baculovirus as versatile vectors for protein display and biotechnological applications. Curr Issues Mol Biol. 2020;34:231–255. doi: 10.21775/CIMB.034.231. [DOI] [PubMed] [Google Scholar]
- Turunen T, Laakkonen J, Alasaarela L, Airenne K, Ylä-Herttuala S. Sleeping Beauty–baculovirus hybrid vectors for long-term gene expression in the eye. J Gene Med. 2014;16:40–53. doi: 10.1002/jgm.2756. [DOI] [PubMed] [Google Scholar]
- Urabe M, Ding C, Kotin RM. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum Gene Ther. 2002;13:1935–1943. doi: 10.1089/10430340260355347. [DOI] [PubMed] [Google Scholar]
- US Department of Health, Human Services (2017) Encouraging vaccine innovation: promoting the development of vaccines that minimize the burden of infectious diseases in the 21 st century. Report to Congress
- Utomo DIS, Hirono I, Kato T, Park EY. Formation of virus-like particles of the dengue virus serotype 2 expressed in silkworm larvae. Mol Biotechnol. 2019;61:852–859. doi: 10.1007/s12033-019-00210-5. [DOI] [PubMed] [Google Scholar]
- Vail PV, Sutter G, Jay DL, Gough D. Reciprocal infectivity of nuclear polyhedrosis viruses of the cabbage looper and alfalfa looper. J Invertebr Pathol. 1971;17:383–388. doi: 10.1016/0022-2011(71)90013-9. [DOI] [Google Scholar]
- VanBlargan LA, Mukherjee S, Dowd KA, Durbin AP, Whitehead SS, Pierson TC. The type-specific neutralizing antibody response elicited by a dengue vaccine candidate is focused on two amino acids of the envelope protein. PLoS Pathog. 2013;9:1–14. doi: 10.1371/journal.ppat.1003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn JL, Goodwin RH, Tompkins GJ, McCawley P. The establishment of two cell lines from the insect Spodoptera frugiperda (lepidoptera; noctuidae) In Vitro. 1977;13:213–217. doi: 10.1007/BF02615077. [DOI] [PubMed] [Google Scholar]
- Veit M, Thaa B. Association of influenza virus proteins with membrane rafts. Adv Virol. 2011;2011:1–14. doi: 10.1155/2011/370606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JM, Pajerowski JD, Daniels CL, McHugh PM, Flynn JA, Balliet JW, Casimiro DR, Subramanian S. Enhanced production of Chikungunya virus-like particles using a high-pH adapted Spodoptera frugiperda insect cell line. PLoS ONE. 2014;9:1–14. doi: 10.1371/journal.pone.0094401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. Hybrid baculovirus - Adeno-associated virus vectors for prolonged transgene expression in human neural cells. J Neurovirol. 2008;14:563–568. doi: 10.1080/13550280802290606. [DOI] [PubMed] [Google Scholar]
- Wang R, Deng F, Hou D, Zhao Y, Guo L, Wang H, Hu Z. Proteomics of the Autographa californica nucleopolyhedrovirus budded virions. J Virol. 2010;84:7233–7242. doi: 10.1128/jvi.00040-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Zheng X, Gai W, Wong G, Wang H, Jin H, Feng N, Zhao Y, Zhang W, Li N, Zhao G, Li J, Yan J, Gao Y, Hu G, Yang S, Xia X. Novel chimeric virus-like particles vaccine displaying MERS-CoV receptor-binding domain induce specific humoral and cellular immune response in mice. Antiviral Res. 2017;140:55–61. doi: 10.1016/j.antiviral.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Zheng X, Gai W, Zhao Y, Wang H, Wang H, Feng N, Chi H, Qiu B, Li N, Wang T, Gao Y, Yang S, Xia X. MERS-CoV virus-like particles produced in insect cells induce specific humoural and cellular immunity in rhesus macaques. Oncotarget. 2017;8:12686–12694. doi: 10.18632/oncotarget.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhu L, Chen X, Huang R, Wang S, Dong P. Human bone marrow mesenchymal stem cells functionalized by hybrid baculovirus-adeno-associated viral vectors for targeting hypopharyngeal carcinoma. Stem Cells Dev. 2019;28:543–553. doi: 10.1089/scd.2018.0252. [DOI] [PubMed] [Google Scholar]
- Wang J, Kong D, Zhu L, Wang S, Sun X. Human bone marrow mesenchymal stem cells modified hybrid baculovirus-adeno-associated viral vectors targeting 131i therapy of hypopharyngeal carcinoma. Hum Gene Ther. 2020;31:1300–1311. doi: 10.1089/hum.2020.081. [DOI] [PubMed] [Google Scholar]
- Wang X, Shang Y, Chen C, Liu S, Chang M, Zhang N, Hu H, Zhang F, Zhang T, Wang Z, Liu X, Lin Z, Deng F, Wang H, Zou Z, Vlak JM, Wang M, Hu Z. Baculovirus Per Os Infectivity Factor Complex: Components and Assembly. J Virol. 2019;93:e02053–18. doi: 10.1128/JVI.02053-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenberg M, Uijtdewilligen P, Vlak JM. Baculovirus envelope fusion proteins F and GP64 exploit distinct receptors to gain entry into cultured insect cells. J Gen Virol. 2007;88:3302–3306. doi: 10.1099/vir.0.83240-0. [DOI] [PubMed] [Google Scholar]
- Wickham TJ, Davis T, Granados RR, Shuler ML, Wood HA. Screening of insect cell lines for the production of recombinant proteins and infectious virus in the baculovirus expression system. Biotechnol Prog. 1992;8:391–396. doi: 10.1021/bp00017a003. [DOI] [PubMed] [Google Scholar]
- Wilder-Smith A, Gubler DJ, Weaver SC, Monath TP, Heymann DL, Scott TW. Epidemic arboviral diseases: priorities for research and public health. Lancet Infect Dis. 2017;17:e101–e106. doi: 10.1016/S1473-3099(16)30518-7. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2020) Vector-borne diseases. https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases. Accessed 13 Sept 2021
- World Health Organization (2021) WHO coronavirus (COVID-19) Dashboard. https://covid19.who.int/. Accessed 13 Sept 2021
- Wu Y, Jiang L, Geng H, Yang T, Han Z, He X, Lin K, Xu F. A recombinant baculovirus efficiently generates recombinant adeno-associated virus vectors in cultured insect cells and larvae. Mol Ther - Methods Clin Dev. 2018;10:38–47. doi: 10.1016/j.omtm.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Mei T, Jiang L, Han Z, Dong R, Yang T, Xu F. Development of versatile and flexible sf9 packaging cell line-dependent onebac system for large-scale recombinant adeno-associated virus production. Hum Gene Ther Methods. 2019;30:172–183. doi: 10.1089/hgtb.2019.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XG, Wang ZS, Zhang Q, Li ZC, Zhao HN, Li W, Tong DW, Liu HJ. Baculovirus surface display of E envelope glycoprotein of Japanese encephalitis virus and its immunogenicity of the displayed proteins in mouse and swine models. Vaccine. 2011;29:636–643. doi: 10.1016/j.vaccine.2010.11.045. [DOI] [PubMed] [Google Scholar]
- Yamagishi J, Isobe R, Takebuchi T, Bando H. DNA microarrays of baculovirus genomes: differential expression of viral genes in two susceptible insect cell lines. Arch Virol. 2003;148:587–597. doi: 10.1007/s00705-002-0922-3. [DOI] [PubMed] [Google Scholar]
- Yamaji H, Konishi E. Production of Japanese encephalitis virus-like particles in insect cells. Bioengineered. 2013;4:37–41. doi: 10.4161/bioe.24514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh JY, Chung KM, Song J. Differentiation of West Nile virus-infected animals from vaccinated animals by competitive ELISA using monoclonal antibodies against non-structural protein 1. Vector-Borne Zoonotic Dis. 2012;12:380–387. doi: 10.1089/vbz.2011.0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh TS, Dean Fang YH, Lu CH, Chiu SC, Yeh CL, Yen TC, Parfyonova Y, Hu YC. Baculovirus-transduced, VEGF-expressing adipose-derived stem cell sheet for the treatment of myocardium infarction. Biomaterials. 2014;35:174–184. doi: 10.1016/j.biomaterials.2013.09.080. [DOI] [PubMed] [Google Scholar]
- Yu L, Pan J, Cao G, Jiang M, Zhang Y, Zhu M, Liang Z, Zhang X, Hu X, Xue R, Gong C. AIV polyantigen epitope expressed by recombinant baculovirus induces a systemic immune response in chicken and mouse models. Virol J. 2020;17:1–13. doi: 10.1186/s12985-020-01388-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng R, Ruan HQ, Jiang XS, Zhou H, Shi L, Zhang L, Sheng QH, Tu Q, Xia QC, Wu JR. Proteomic analysis of SARS associated coronavirus using two-dimensional liquid chromatography mass spectrometry and one-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by mass spectroemtric analysis. J Proteome Res. 2004;3:549–555. doi: 10.1021/pr034111j. [DOI] [PubMed] [Google Scholar]
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/nejmoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]