Abstract
Background and Aims
This study aimed to compare real-world clinical effectiveness and safety of vedolizumab, an α4β7-integrin inhibitor, and anti-tumour necrosis factor-α [anti-TNFα] agents in biologic-naïve ulcerative colitis [UC] and Crohn’s disease [CD] patients.
Methods
This was a 24-month retrospective medical chart study in adult UC and CD patients treated with vedolizumab or anti-TNFα in Canada, Greece and the USA. Inverse probability weighting was used to account for differences between groups. Primary outcomes were cumulative rates of clinical effectiveness [clinical response, clinical remission, mucosal healing] and incidence rates of serious adverse events [SAEs] and serious infections [SIs]. Secondary outcomes included cumulative rates of treatment persistence [patients who did not discontinue index treatment during follow-up] and dose escalation and incidence rates of disease exacerbations and disease-related surgeries. Adjusted analyses were performed using inverse probability weighting.
Results
A total of 1095 patients [604 UC, 491 CD] were included. By 24 months, rates of clinical effectiveness were similar between groups, but incidence rates of SAEs (hazard ratio [HR] = 0.42 [0.28–0.62]) and SIs (HR = 0.40 [0.19–0.85]) were significantly lower in vedolizumab vs anti-TNFα patients. Rates of treatment persistence [p < 0.01] by 24 months were higher in vedolizumab patients with UC. Incidence rates of disease exacerbations were lower in vedolizumab patients with UC (HR = 0.58 [0.45–0.76]). Other outcomes did not significantly differ between groups.
Conclusion
In this real-world setting, first-line biologic therapy in biologic-naïve patients with UC and CD demonstrated that vedolizumab and anti-TNFα treatments were equally effective at controlling disease symptoms, but vedolizumab has a more favourable safety profile.
Keywords: Vedolizumab, biologic-naïve, real-world effectiveness
1. Introduction
Inflammatory bowel disease [IBD] is a collective term for several conditions manifesting through gastrointestinal tract inflammation and include ulcerative colitis [UC] and Crohn’s disease [CD].1 UC and CD are chronic diseases often requiring life-long treatment and frequent hospitalization, resulting in reduced quality of life and substantial healthcare utilization.2 Biologic therapies are a class of drugs used in the treatment of moderate–severe IBD. While anti-tumour necrosis factor-alpha [anti-TNFα] agents were the first biologics approved for IBD treatment, loss of response to treatment and the systemic nature of anti-TNFα treatments have led to a need to identify inhibitors of alternative signalling pathways.3
Vedolizumab is a gut-selective anti-lymphocyte trafficking [GSALT] agent approved for the treatment of moderate–severe UC and CD. It is a humanized monoclonal antibody which selectively antagonizes α4β7 gastrointestinal integrin receptors, resulting in reduced lymphocyte trafficking into intestinal tissue.4 In the GEMINI phase III clinical trials, vedolizumab demonstrated greater efficacy in biologic-naïve patients versus those with non-response to anti-TNFα agents.5,6 Real-world studies of vedolizumab have shown high rates of clinical effectiveness over 44 weeks7 (24/35 [68.6%] of biologic-naïve patients in clinical remission) and similar effectiveness to anti-TNFα treatments [12-month clinical remission: vedolizumab 38%, anti-TNFα 34%] in predominately anti-TNFα-exposed patients.8 In biologic-naïve patients [IBM Explorys database], greater treatment persistence of vedolizumab vs infliximab [77.6% vs 64.6%; p = 0.0005] was observed over 24 months.9 To guide physician decision-making on the most suitable first-line biologic, comparing anti-TNFα to vedolizumab in a real-world setting of biologic-naïve patients is critical.
The primary objective of this study was to compare the real-world clinical effectiveness and safety of vedolizumab and anti-TNFα over 24 months after treatment initiation in biologic-naïve UC and CD patients. Additional objectives over 24 months were to describe treatment patterns associated with first-line biologic use as well as the incidence of IBD-related surgical procedures and disease exacerbations. This analysis also looked at the clinical effectiveness of first-line biologic anti-TNFα compared to second-line anti-TNFα post-vedolizumab discontinuation over 6 months.
2. Materials and methods
2.1. Study design
This was a multi-country [USA, Canada and Greece], multi-centre, retrospective cohort study conducted with an eligibility period for initiating first-line biologic vedolizumab or anti-TNFα treatment between May 20, 2014 and July 31, 2017. Eligibility periods were based on approval dates by the Food and Drug Administration [May, 20 2014], Health Canada [UC: May 19, 2015, CD: May 19, 2016], and the European Medicines Agency [May 22, 2014]. Data abstraction occurred from September 21, 2017 to December 14, 2018. Adult patients [≥18 years] diagnosed with UC or CD, who were biologic-naïve and initiated first-line biologic ‘index treatment’ with either vedolizumab or an anti-TNFα [infliximab, infliximab biosimilars, adalimumab, adalimumab biosimilars, golimumab or certolizumab pegol; USA and Canada and only] during the eligibility periods, and had ≥6 months of follow-up data were included in the study. Patients were identified using hospital medical record databases. This study included patients receiving concomitant non-biologic therapies [aminosalicylates, corticosteroids, immunomodulators]. While data collection included three CD patients receiving ustekinumab, these patients were not included in the final analysis dataset to focus results on the comparison between vedolizumab and anti-TNFα treatments. In Canada, CD patients were also eligible if they had received vedolizumab prior to approval through a compassionate use access programme [n = 9]. For Greece, only patients who initiated vedolizumab were included in the study. Patients were excluded if their index treatment was administered as part of a clinical trial, if they had initiated treatment as combination therapy with two biologic agents, or if they had received prior treatment with biologic agents. In the USA, patients were randomly selected in order to include similar numbers of vedolizumab and anti-TNFα patients and to minimize the potential for selection bias. Random selection was performed after patients were deemed eligible for the study, prior to data abstraction.
The post-index treatment follow-up period was defined as time between index treatment initiation and the earliest date of chart abstraction initiation, death, last contact with the site or 6 months post-treatment discontinuation [Canada only]. Data were also collected at baseline [index treatment initiation] and prior to treatment initiation, as far back as disease diagnosis.
There were 37 sites included in this study across three countries [USA: 15 sites; Canada: 13 sites; Greece: nine sites]; sites comprised university/academic hospitals and private practice centres and were geographically dispersed and of varied sizes. The study received ethics and any other local approvals in line with country-specific requirements.
2.2. Data collection and outcome measures
All data were collected retrospectively from patient medical charts. Patient demographics, and clinical and treatment history were collected and disease severity at baseline was determined using the closest assessment to treatment initiation [within ≤6 months prior to index] using a hierarchical algorithm of standard clinical assessments for severity [Supplementary Table 1]. Data collected post-index treatment initiation included treatment patterns, clinical effectiveness, disease exacerbations [worsening of symptoms attributed to CD or UC], hospitalizations, surgical procedures and safety events. Study outcomes of interest for treatment patterns included treatment persistence, defined as patients who did not discontinue their index treatment for any reason during the study follow-up period, and dose escalation, defined as an increase in index treatment frequency [vedolizumab] or dose and/or treatment frequency [anti-TNFα] for two or more consecutive drug administrations. Due to limited follow-up on the subset of patients on second-line biologic anti-TNFα treatment post-vedolizumab discontinuation, outcomes were assessed up to 6 months after initiating second-line anti-TNFα. Clinical outcomes were assessed, and clinical response, clinical remission and mucosal healing were defined based on a hierarchal algorithm utilizing standard measures of disease activity [definitions below; further detailed in Supplementary Figures 1–10].
2.2.1. Clinical outcome definitions in patients with UC
Clinical response was defined as: Mayo overall score: reduction in score of at least three points and a decrease of at least 30% from the baseline score, with a decrease of at least one point on the rectal bleeding subscale OR an absolute rectal bleeding score of 0 or 1, or if unknown, partial Mayo score: reduction in score of at least two points and a decrease of at least 30% from the baseline score, with a decrease of at least one point on the rectal bleeding subscale OR an absolute rectal bleeding score of 0 or 1 or if unknown, treatment response recorded in the medical chart as ‘complete response’ or ‘partial response’, or if unknown, physician global assessment: decrease of ≥1 point [or improvement by ≥1 category] from baseline.
Clinical remission was defined as Mayo overall score: score ≤2 points, with no individual subscore >1; or in the absence of available subscores patients were classified in remission if the overall score was ≤2, or if unknown, partial Mayo score: score ≤2 points, with no individual subscore >1; or in absence of available subscores patients were classified in remission if the overall score was ≤2, or if unknown, treatment response recorded in the medical chart as ‘in remission’, or if unknown, physician global assessment: score of 0 [normal].
Mucosal healing was defined as endoscopic assessment score = 0 or 1 [i.e. normal or inactive disease or mild disease], or if unknown, one or more endoscopic procedure finding[s] from the case report form (CRF) drop down list indicating inactive disease [no findings/no active disease, no erosion, no ulcers, no inflammation or inflammatory activity, or no pathological findings], Ulcerative Colitis Endoscopic Index of Severity (UCEIS) score = 0 [i.e. normal or inactive disease or mild disease].
2.2.2. Clinical outcome definitions in patients with UC
Clinical response was defined as Crohn’s Disease Activity Index [CDAI]: positive change in category from baseline [CDAI categories include: score <150, score of 151–219, score of 220–450, score >450], or if unknown, Harvey–Bradshaw Index [HBI] Overall: decrease of ≥3 points from baseline, or if unknown, Modified HBI: this has the same cut-offs and definition as HBI Overall, shown above, or if unknown, treatment response recorded in the medical chart as ‘complete response’ or ‘partial response’.
Clinical remission was defined as CDAI score of <150 points, or if unknown, HBI score of ≤4 points, or if unknown, Modified HBI score of ≤4 points, or if unknown, remission status recorded in the medical chart as ‘in remission’.
Mucosal healing was defined as endoscopic assessment score = 0 or 1 [i.e. normal or inactive disease or mild disease], or if unknown, Simple Endoscopic Score for Crohn’s Disease [SES-CD] score of <3 [not derived but based on CRF documentation of score] [SESCD], ‘lack of ulceration’ defined by one or more of the following endoscopic procedure finding[s] from the CRF drop down list: either selection of ‘no ulcers’ or free-text indication of ‘lack of ulceration’, or if unknown, one or more endoscopic procedure finding[s] from the CRF drop down list indicating inactive disease [no findings/no active disease, no erosion, no ulcers, no inflammation or inflammatory activity, or no pathological findings].
2.2.3. Primary non-response [PNR] and secondary loss of response [SLOR] definitions
Patients were classified as having PNR or SLOR if they either discontinued their index treatment due to inability to obtain a response or loss of response, if they had a disease exacerbation, a bowel-related surgical procedure, an IBD-related hospitalization [excluding hospitalizations for treatment infusions/injections or diagnostic/evaluation procedures or tests], or a dose escalation for any reason ≤14 weeks post-index treatment initiation [PNR] and >14 weeks post-index treatment initiation [SLOR].
2.2.4. Safety outcomes
Safety outcomes of interest included serious adverse events [SAEs] and serious infections [SIs] occurring from treatment initiation up to five half-lives post-treatment discontinuation [see Appendix 1, available as Supplementary data at ECCO-JCC online.] in order to cover the period of time of detectable serum drug levels. Adverse event [AEs] or infections were classified as serious if they were life threatening, required hospital admission, resulted in significant disability/incapacity or were recorded as an important medical event.
2.3. Statistical analyses
Results were conducted separately by disease type and compared by vedolizumab vs anti-TNFα. Unadjusted comparisons were performed using t-tests or non-parametric tests for continuous data and chi-square tests for categorical data. p-values were provided from log-rank tests.
Cumulative rates were calculated for patients with ≥1 outcome assessment and using Kaplan–Meier analyses. Cumulative rates were calculated by computing the probability of the occurrence of an event [response, remission, etc.] at a time point and then multiplying by any earlier computed probabilities. Kaplan–Meier figures show the number of patients at risk over time. The number at risk decreases over time from the total number of patients at time zero, as the n at risk only includes patients still on treatment and who have clinical outcomes that can still be assessed. At the last time point, patients still at risk are those who have not reached the outcome [and have data that can be assessed]. Due to variability of real-world clinical assessments, time windows of ±1.5 months were used for 12-, 18- and 24-month outcome assessments. Adjusted disease exacerbations, disease-related surgeries, SAEs and SIs were presented as counts of first occurrences using incidence rates [IRs] expressed per 100 person-years [100 PYs] of exposure and hazard ratios with 95% confidence interval [CI].
To account for differences in baseline characteristics between groups, all adjusted analyses were performed using inverse probability weighting [IPW].10 Baseline covariates included in the model were: age, sex, disease location, disease duration [≤2 years, 2–5 years, 5–10 years, >10 years, unknown], disease-related hospitalizations, disease severity, steroid dependency status, fistula status [for CD only] and a composite biochemical marker (faecal calprotectin [FCP], C-reactive protein [CRP], albumin). See Supplementary Tables 2 and 3 for additional information on the IPW adjustments and Supplementary Figures 11 and 12 for the distribution of propensity scores before and after weighting. IPW weights were not trimmed for this analysis. A p-value below 0.05 was considered statistically significant. All analyses were conducted using SAS version 9.4 software [SAS Institute].
3. Results
3.1. Patient population
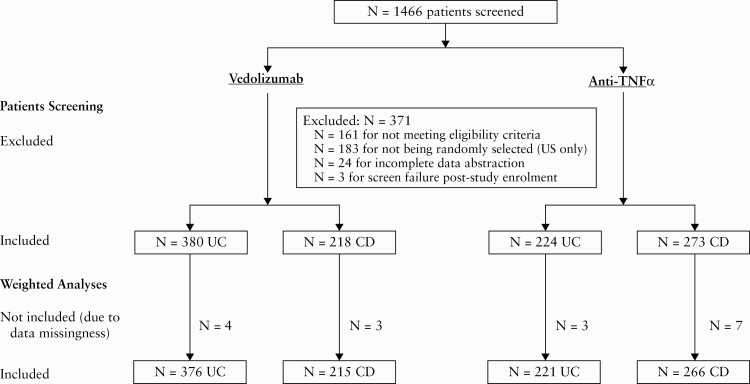
A total of 1466 patients were screened with 1095 patients (598 vedolizumab [380 UC, 218 CD] and 497 anti-TNFα [224 UC, 273 CD]) included in the final dataset. In total, 371 patients were excluded for: not meeting eligibility criteria [161 patients], not being randomly selected [USA only; 183 patients], incomplete data abstraction [24 patients], or were a screen failure post-study enrolment [three patients] [Figure 1].
Figure 1.
Patient screening and analysis flowchart.
3.2. Baseline and clinical characteristics
3.2.1. Ulcerative colitis
3.2.1.1 Unadjusted comparisons
A total of 604 patients with UC were included; 380 [62.9%] were treated with vedolizumab and 224 [37.1%] with an anti-TNFα [62 adalimumab, 24 golimumab, 137 infliximab, one infliximab-abda]. At baseline, 59.5% of the vedolizumab-treated patients and 48.7% of the anti-TNFα-treated patients were male [p = 0.01], had a mean [SD] age of 45.7 [17.4] vs 39.6 [15.7] years [p < 0.01], and included a greater proportion of patients in the longer disease duration categories [p < 0.01]. A larger proportion of anti-TNFα patients had moderate to severe disease [82.7% vs 72.4%; p < 0.01] [Table 1].
Table 1.
Unadjusted baseline demographics and clinical characteristics of biologic-naïve ulcerative colitis and Crohn’s disease patients treated with vedolizumab or anti-TNFα therapy
| Baseline characteristics | Ulcerative colitis | Crohn’s disease | ||||
|---|---|---|---|---|---|---|
| Vedolizumab | Anti-TNFα | p-value | Vedolizumab | Anti-TNFα | p-value | |
| N = 380 | N = 224 | N = 218 | N = 273 | |||
| Demographics | ||||||
| Mean [SD] age, years | 45.7 [17.4] | 39.6 [15.7] | <0.01 | 51.7 [16.8] | 39.7 [14.8] | <0.01 |
| Sex [male], n [%] | 226 [59.5] | 109 [48.7] | 0.01 | 114 [52.3] | 139 [50.9] | 0.76 |
| BMI, n with available data [%] | 227 [59.7] | 129 [57.6] | 0.20 | 135 [61.9] | 178 [65.2] | 0.07 |
| <18.5 [underweight], n [%] | 4 [1.8] | 4 [3.1] | 4 [3.0] | 5 [2.8] | ||
| 18.5–24.9 [normal], n [%] | 93 [41.0] | 60 [46.5] | 46 [34.1] | 68 [38.2] | ||
| 25.0–29.9 [overweight], n [%] | 74 [32.6] | 35 [27.1] | 53 [39.3] | 41 [23.0] | ||
| ≥30 [obese], n [%] | 56 [24.7] | 30 [23.2] | 32 [23.7] | 64 [36.0] | ||
| Smoking status, n with available data [%] | 314 [82.6] | 195 [87.1] | 0.04 | 189 [86.7] | 234 [85.7] | 0.39 |
| Current, n [%] | 23 [7.3] | 15 [7.7] | 32 [16.9] | 35 [15.0] | ||
| Former, n [%] | 95 [30.3] | 40 [20.5] | 39 [20.6] | 65 [27.8] | ||
| Never smoked, n [%] | 196 [62.4] | 140 [71.8] | 118 [62.4] | 134 [57.3] | ||
| Clinical characteristics | ||||||
| Disease duration, n with available data [%] | 320 [84.2] | 181 [80.8] | <0.01 | 176 [80.7] | 220 [80.6] | <0.01 |
| <2 years, n [%] | 86 [26.9] | 90 [49.7] | 50 [28.4] | 111 [50.5] | ||
| 2 to <5 years, n [%] | 72 [22.5] | 39 [21.5] | 32 [18.2] | 30 [13.6] | ||
| ≥5 years, n [%] | 162 [50.6] | 52 [28.7] | 94 [53.4] | 79 [35.9] | ||
| Median [min–max] observation period, [months] | 16.4 [3.0–47.0] | 21.3 [3.5–51.1] | 0.48 | 15.7 [4.2–45.9] | 19.3 [6.0–51.0] | <0.01 |
| Ulcerative colitis disease location at index, n with available data [%] | 339 [89.2] | 196 [87.5] | 0.07 | – | – | |
| Extensive colitis [proximal to hepatic flexure], n [%] | 155 [45.7] | 107 [54.6] | – | – | ||
| Left sided [distal to splenic flexure], n [%] | 160 [47.2] | 72 [36.7] | – | – | ||
| Ulcerative proctitis, n [%] | 24 [7.1] | 17 [8.7] | – | – | ||
| Crohn’s disease location at index, n with available data [%] | – | – | 196 [89.9] | 230 [84.2] | 0.01 | |
| Colonic with/without upper GI disease, n [%] | – | – | 42 [21.4] | 67 [29.1] | ||
| Ileal with/without upper GI disease, n [%] | – | – | 85 [43.4] | 71 [30.9] | ||
| Ileocolonic with/without upper GI disease, n [%] | – | – | 69 [35.2] | 92 [40.0] | ||
| Disease severity at index, n with available data [%] | 323 [85.0] | 190 [84.8] | <0.01 | 180 [82.6] | 220 [80.6] | 0.01 |
| Moderate, n [%] | 178 [55.1] | 86 [45.3] | 84 [46.7] | 93 [42.3] | ||
| Severe, n [%] | 56 [17.3] | 71 [37.4] | 17 [9.4] | 50 [22.7] | ||
| Disease behaviour, n with available data [%] | – | – | 154 [70.6] | 178 [65.2] | 0.64 | |
| Non-stricturing, non-penetrating, with or without perianal disease, n [%] | – | – | 92 [59.7] | 105 [59.0] | ||
| Penetrating, with or without perianal disease, n [%] | – | – | 17 [11.0] | 21 [11.8] | ||
| Stricturing, with or without perianal disease, n [%] | – | – | 45 [29.2] | 52 [29.2] | ||
| Active fistula at index, n with available data [%] | – | – | 189 [86.7] | 239 [87.5] | <0.001 | |
| Active fistula, n [%] | – | – | 8 [4.2] | 40 [16.7] | ||
| Prior non-biologic therapy, n with available data [%] | 371 [97.6] | 218 [97.3] | 0.40 | 176 [80.7] | 238 [87.2] | 0.05 |
| Most common types of prior non-biologic therapy | ||||||
| Prednisone [CS], n [%] | 215 [58.0] | 152 [69.7] | 65 [36.9] | 132 [55.5] | ||
| Mesalazine [5-ASA], n [%] | 316 [85.1] | 177 [81.2] | 78 [44.4] | 100 [42.0] | ||
| Azathioprine [IMM], n [%] | 104 [28.0] | 59 [27.1] | 61 [34.7] | 80 [33.6] | ||
| CS bridging therapya, n with available data [%] | 70 [18.4] | 60 [26.8] | 0.17 | 37 [17.0] | 57 [20.9] | 0.43 |
| n [%] | 18 [25.7] | 20 [33.3] | 10 [27.0] | 18 [31.6] | ||
| Steroid-dependent, n with available data [%] | 380 | 224 | 0.27 | 218 | 273 | 0.63 |
| n [%] | 116 [30.5] | 78 [34.8] | 32 [14.7] | 36 [13.2] | ||
| Composite biochemical marker, n with available data [%] | 286 [75.2] | 172 [76.8] | 0.04 | 158 [72.5] | 194 [71.1] | 0.07 |
| Within normal range, n [%] | 78 [27.3] | 32 [18.6] | 41 [25.9] | 35 [18.0] | ||
| Outside normal range, n [%] | 208 [72.7] | 140 [81.4] | 117 [74.1] | 159 [82.0] | ||
| Prior UC- or CD-related surgeries [since diagnosis], n [%] | 1 [0.3] | 1 [0.5] | 1.00 | 10 [4.6] | 16 [5.9] | 0.53 |
| UC- or CD-related hospitalizations [12 months prior], n [%] | 25 [6.6] | 40 [17.9] | <0.01 | 22 [10.1] | 35 [12.8] | 0.35 |
Abbreviations: ASA = aminosalicylate; BMI = body mass index; CD = Crohn’s disease; CS = corticosteroid; GI = gastrointestinal; IMM = immunomodulator; SD = standard deviation; TNF = tumour necrosis factor; UC = ulcerative colitis.
aPatients were classified as having CS bridging therapy at index treatment initiation if CS started ≤1 month [≤30.5 days] prior to index AND was ongoing at index initiation AND was discontinued within 3 months [≤91 days] following index treatment initiation.
3.2.1.2 Adjusted comparisons
A total of 597 patients were included; 376 [63.0%] were treated vedolizumab and 221[37.0%] with an anti-TNFα agent [62 adalimumab, 24 golimumab, 134 infliximab, one infliximab-abda]. None of the covariates adjusted for in the IPW model were different between cohorts [Supplementary Tables 2 and 4].
3.2.2. Crohn’s disease
3.2.2.1. Unadjusted comparisons
A total of 491 patients with CD were included; 218 [44.4%] were treated with vedolizumab and 273 [55.6%] with an anti-TNFα [144 adalimumab, 120 infliximab, two infliximab-abda, three infliximab-dyyb, four certolizumab pegol]. At baseline, approximately half of CD patients treated with vedolizumab [52.3%] or anti-TNFα [50.9%] were male, had a mean [SD] age of 51.7 [16.8] vs 39.7 [14.8] years [p < 0.01], and included a greater proportion of patients in the longer disease duration categories [p < 0.01]. A substantially larger proportion of anti-TNFα patients had moderate to severe disease [65.0% vs 56.1%; p = 0.01] [Table 1].
3.2.2.2. Adjusted comparisons
A total of 481 patients with CD were included and, of these, 215 [44.7%] initiated treatment with vedolizumab and 266 [55.3%] initiated treatment with an anti-TNFα [142 adalimumab, 115 infliximab, two infliximab-abda, three infliximab-dyyb, four certolizumab pegol]. None of the covariates adjusted for in the IPW model were different between cohorts [Supplementary Tables 3 and 4].
4. Primary outcomes
4.1. Clinical effectiveness [clinical response, clinical remission and mucosal healing]
4.1.1. Ulcerative colitis
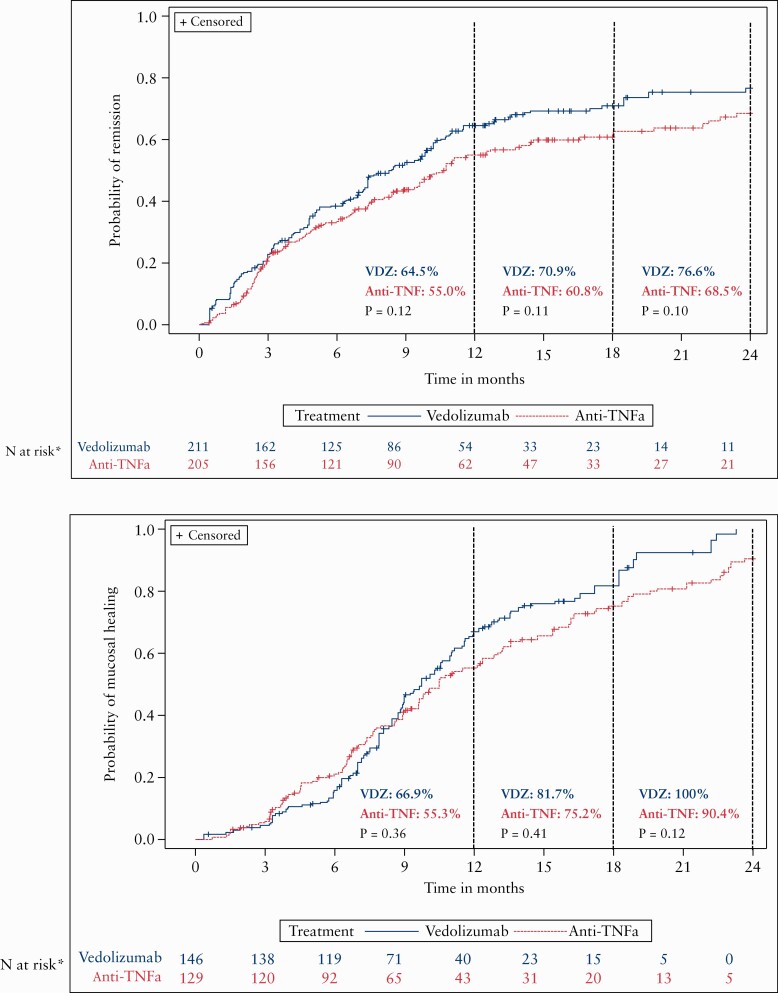
In the adjusted cumulative analyses, there were similar rates of response [vedolizumab: 88.3%; anti-TNFα: 86.2%, p = 0.64], remission [vedolizumab: 65.9%; anti-TNFα: 48.6%; p = 0.09; Figure 2] and mucosal healing [vedolizumab: 86.6%; anti-TNFα: 80.6%; p = 0.66; Figure 2] between cohorts over 24 months. Unadjusted analyses are shown in Supplementary Figure 13. The statistical analysis section in the Methods provides further description of which patients are included in the n at risk.
Figure 2.
Adjusted cumulative rates of clinical remission and mucosal healing in ulcerative colitis patients.*The sum of the patient weights for each group still on treatment and at clinical outcome can still be assessed. p-values are unadjusted log-rank values. Annotated data are the rates at each time point as indicated by the dashed line.
4.1.2. Crohn’s disease
In the adjusted analyses, there were similar rates of response [vedolizumab: 84.0%; anti-TNFα: 72.1%; p = 0.27], remission [vedolizumab: 76.6%; anti-TNFα: 68.5%; p = 0.10; Figure 3] and mucosal healing [vedolizumab: 100%; anti-TNFα: 90.4%; p = 0.12; Figure 3] between cohorts over 24 months. As stated in the statistical analyses section of the Methods, rates are based on the n at risk, and thus rates of 90–100% do not mean that 90–100% of all CD patients reached this outcome. The statistical analysis section in the Methods provides further description of which patients are included in the n at risk.
Figure 3.
Adjusted cumulative rates of clinical remission and mucosal healing in Crohn’s disease patients.*The sum of the patient weights for each group still on treatment and at clinical outcome can still be assessed. Annotated data are the rates at each time point as indicated by the dashed line. p-values are unadjusted log-rank values.
4.1.3. Safety in unadjusted and adjusted populations
4.1.3.1. Inflammatory bowel disease
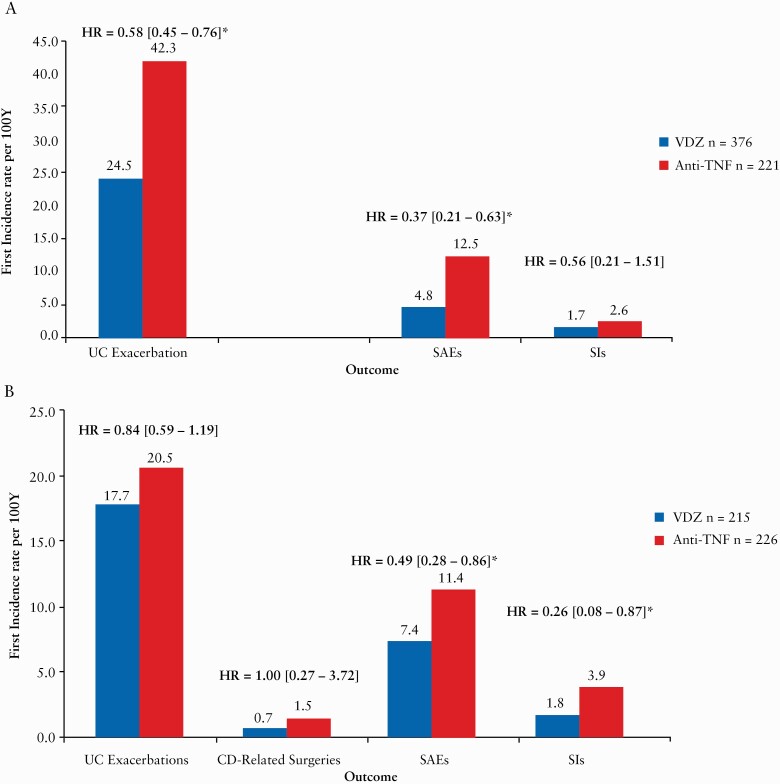
Adjusted results from the combined UC and CD populations showed that vedolizumab patients were less likely to experience SAEs (hazard ratio [HR] = 0.42 [0.28–0.62]) and SIs (HR = 0.40 [0.19–0.85]).
4.1.3.1.1. Ulcerative colitis
Unadjusted results in the UC population showed that overall, SAEs occurred in 6.8% and 19.2% of patients in the vedolizumab and anti-TNFα cohorts, respectively. See Supplementary Tables 5 and 7 for the most frequent SAEs and SIs.
Adjusted results showed that vedolizumab patients were less likely to experience SAEs (HR = 0.37 [0.21–0.63]), but not SIs (HR = 0.56 [0.21–1.51]) [Figure 4A].
Figure 4.
The adjusted first incidence of disease exacerbations, disease-related surgeries, serious adverse events and serious infections of patients with ulcerative colitis [A] and Crohn’s disease [B].Hazard ratios are from adjusted Cox models [number of patients experiencing an AE of interest/time in years patients were at risk, multiplied by 100]. SAE: serious adverse event, SI: serious infection. No UC-related surgeries are shown as the vedolizumab cohort had none.
4.1.3.1.2. Crohn’s disease
Unadjusted results showed that, overall, SAEs occurred in 8.3% of vedolizumab and 19.0% of anti-TNFα patients respectively. See Supplementary Tables 6 and 8 for the most frequent SAEs and SIs.
Adjusted results showed that vedolizumab patients were less likely to experience SAEs (HR = 0.49 [0.28–0.86]) and SIs (HR = 0.26 [0.08–0.87]) [Figure 4B].
5. Secondary outcomes
5.1. Treatment patterns
5.1.1. Ulcerative colitis
Adjusted results showed that the estimated cumulative probability of treatment persistence was significantly higher for vedolizumab compared with anti-TNFα patients [p < 0.01, Figure 5]. The primary reasons for treatment discontinuation were PNR (vedolizumab: 37/376 [9.8%]; anti-TNFα: 38/221 [17.2%]) and SLOR (vedolizumab: 24/376 [6.4%]; anti-TNFα: 21/221 [9.5%]). One vedolizumab [0.3%] and five anti-TNFα [2.3%] patients discontinued due to SAEs (vedolizumab: drug-induced lupus; anti-TNFα: fever/chills, severe shortness of breath, lupus-like reaction, pain [chest pain]). The cumulative probability of dose escalation was significantly less for vedolizumab compared to anti-TNFα patients [p < 0.01]. The primary reasons for dose escalation are shown in Appendix 2, available as Supplementary data at ECCO-JCC online.
Figure 5.
Adjusted cumulative rates of treatment persistence in ulcerative colitis and Crohn’s disease patients.*The sum of the patient weights for each group still on treatment and at clinical outcome can still be assessed.
5.1.2. Crohn’s disease
In adjusted analyses, the probability of treatment persistence was similar between the vedolizumab and anti-TNFα patient cohorts over 24 months [p = 0.25] [Figure 5]. The primary reasons for discontinuation were PNR (vedolizumab: 19/215 [8.8%]; anti-TNFα: 13/266 [4.9%]) and SLOR (vedolizumab: 9/215 [4.2%]; anti-TNFα: 10/266 [3.8%]). Three [1.4%] vedolizumab and seven [2.6%] anti-TNFα patients discontinued treatment due to SAEs [vedolizumab: Bell’s palsy, recurrent respiratory tract infections, carcinoid tumour; anti-TNFα: rash, basal cell carcinoma, allergic reaction, autoimmune hepatitis, pelvic abscess, lupus-like reaction]. The cumulative probability of dose escalation was similar for vedolizumab and anti-TNFα patients [p = 0.18]. The primary reasons for dose escalation are shown in Appendix 2.
5.2. Disease exacerbations and disease-related surgeries
5.2.1. Ulcerative colitis
Vedolizumab patients were significantly less likely to experience a disease exacerbation (HR = 0.58 [0.45–0.76]). Vedolizumab patients had zero colectomies and anti-TNF patients had one, so an HR was not calculated [Figure 4A].
5.2.2. Crohn’s disease
Rates of disease exacerbations (HR = 0.84 [0.59–1.19]) and disease-related surgeries (HR = 1.00 [0.27–3.72]) were not different between cohorts [Figure 4B].
5.3. Treatment persistence, clinical response and clinical remission of first-line and second-line biologic anti-TNFα by 3 and 6 months
Adjusted analyses were not performed due to small sample size.
5.3.1. Ulcerative colitis
The unadjusted rates of treatment persistence, clinical response and clinical remission at 6 months for first-line biologic anti-TNFα patients were similar to second-line biologic anti-TNFα patients [Supplementary Figure 15].
5.3.2. Crohn’s disease
The unadjusted cumulative rates of treatment persistence and clinical response were similar between first-line biologic anti-TNFα patients and second-line biologic anti-TNFα patients at 6 months [Supplementary Figure 14]. The rate of clinical remission was significantly higher in patients on second-line compared to first-line biologic anti-TNFα (Supplementary Figure 16; first-line [n at risk = 136]: 36.2%; second-line [n at risk = 1]: 74.6%, p < 0.01).
6. Discussion
This long-term [24-month], comparative real-world study in UC and CD patients fills an important evidence gap, comparing the effectiveness and safety of vedolizumab and anti-TNFα agents in biologic-naïve patients. Retrospective data from >1000 IBD patients demonstrated that the vedolizumab cohort had similar rates of clinical effectiveness and were significantly less likely to experience SAEs or SIs compared to the anti-TNFα cohort up to 2 years post-treatment initiation. Results support the long-term effectiveness and safety of vedolizumab as a first-line biologic treatment in IBD patients.
Treatment persistence, hypothesized to be related to treatment effectiveness and safety, was similar to a retrospective vedolizumab study with a median follow-up of 17 months [58% persistence; biologic-naïve patients/patients with prior anti-TNFα]11 and to a database study [the IBM Explorys database] by 24 months [77.6%; biologic-naïve patients].9 However, as rates of clinical response and remission were not different between treatment cohorts in the current study, it is possible that treatment persistence in vedolizumab-treated patients with UC has other origins [i.e. safety or other reasons].
Rates of dose escalation are in line with current literature [4–60%12 over 12 months], with lower rates observed for patients on vedolizumab compared to those on anti-TNFα treatments.13 Although research supports dose escalations being used to recapture response following SLOR,14 rates of escalation for this study may have been impacted by the bias that vedolizumab treatment labels did not include escalation instructions [USA or Canada]. In real-world practice, prescribing behaviour can vary across sites, so it is possible the data do not fully align with approved labels.
Effectiveness comparisons between first-line and second-line biologic anti-TNFα treatment cohorts suggest that the effectiveness of anti-TNFα may not be compromised by prior vedolizumab exposure. This finding aligns with literature showing 75% [9/12] of UC patients who failed first-line vedolizumab had improved clinical symptoms 3 months post-second-line anti-TNFα initiation.15 However, sample sizes were small and larger investigations are needed. Previously, the GEMINI trials5,6 [biologic-naïve remission rates, UC: 47%; CD: 49%,] and the VICTORY consortium [database of outcomes for IBD patients from a multi-centre research group]16 [biologic-naïve: 61%] showed remission rates similar to the current study. It is common for patients to be switched between a variety of treatments until clinical symptoms improve,17 so it is important to consider how a given treatment affects subsequent-line clinical effectiveness. Vedolizumab may fit both criteria as a first-line biologic that is clinically effective and does not reduce the effectiveness of second-line treatments. The results need to be confirmed by a larger sample of patients.
Rates of mucosal healing were similar between vedolizumab and anti-TNFα patients. Results at 12 months (vedolizumab: 55.0% [UC]) may be similar to the VICTORY consortium at 12 months [biologic-naïve patients: 51%].18 Results differ from the recent VARSITY trial at week 52 [vedolizumab: 39.7%; adalimumab: 27.7%; p = 0.0005]19 potentially due to real-world study design or the current study not focusing on one specific anti-TNFα. Due to sample size and data availability limitations, it was not possible to assess relationships between mucosal healing and clinical remission.
This study showed that UC patients treated with vedolizumab were less likely to experience exacerbations, which suggests that their disease may be better controlled in the long term. It is also the first study to demonstrate a more favourable tolerability profile of vedolizumab compared to anti-TNFα treatments in a population consisting solely of biologic-naïve IBD patients. Safety results are in line with other studies, including the VICTORY consortium18 and the GEMINI 1 trial,5 which showed low incidence of SAEs (VICTORY: 6%, GEMINI 1 [biologic-naïve patients]: 9%) and SIs (VICTORY: 4%, GEMINI 1 [biologic-naïve patients]: 1%) on vedolizumab.20 Furthermore, systematic reviews showed that vedolizumab treatment is associated with low rates of AEs, SAEs and SIs,21 whilst anti-TNFα treatments are associated with increased SIs and postoperative complications.6,16 These findings may be explained by differences in the mechanisms of action between anti-TNFα and vedolizumab agents. However, as SAEs and SIs were reported in the current study irrespective of the relationship to treatment, incidences may not reflect those directly related to treatments.
This real-world study had several limitations. Due to the retrospective design, data were limited to what was in patient medical charts and data quality varied by site; however, rigorous data quality control was undertaken. There were differences in some of the unadjusted baseline characteristics between the vedolizumab and anti-TNFα cohorts, which is why weighted analyses were performed. Also, individual comparisons of anti-TNFα treatments vs vedolizumab were not performed. For clinical effectiveness, sample sizes were smaller by 24 months and patients may also have achieved outcomes before the event being recorded in the chart, which may affect the Kaplan–Meier estimates. There may be factors not measured [e.g. patient decision, reimbursement] that impacted rates of treatment persistence. The anti-TNFα second-line cohort had a small sample size, short follow-up time and results that were unadjusted, which may decrease the generalizability of the findings. In Greece, only vedolizumab patients were included; not collecting anti-TNFα patients from all sites is a comparative analysis limitation. Nine [4.1%] CD patients treated with vedolizumab in Canada received treatment as part of a compassionate use access programme prior to approval, although it is not expected that any bias affected the study results. Lastly, although the composite biochemical marker was designed based on clinical cut-offs from the literature that may differentiate inactive from active disease, it is not a validated algorithm.
This study also had multiple strengths as this is the first large-scale, long-term study to assess and compare treatment outcomes in biologic-naïve ‘real life’ IBD populations. IPW preserves sample size and adjusted for multiple covariates to balance the baseline characteristics between the treatment cohorts. The utilization of data from medical records allowed for the data capture as occurring in real-world practice.
In conclusion, the results from this real-world study of patients with IBD suggest that first-line vedolizumab and anti-TNFα treatments yield similar rates of clinical effectiveness up to 2 years post-treatment initiation, and that vedolizumab has a more favourable safety profile than anti-TNFα treatments. These real-world data suggest vedolizumab may have a favourable benefit–risk profile as a first-line biologic treatment for patients with IBD.
Supplementary Material
Acknowledgments
We would like to acknowledge the following individuals for their efforts in data collection:
Funding
The study was funded by Takeda Pharmaceuticals Company Ltd.
Conflict of Interest
BB, research support was provided to sites for data collection where AY, UK, GM, MSS, JS, AS, GM, AG, PK, KS, SM, UN, EB, CF, CB and DR received honoraria from Takeda; MB and DS are employees of Evidera, which received funding from Takeda Pharmaceuticals Company Ltd. DD is an employee of and has stock or stock options in Takeda Pharmaceuticals Company Ltd. Grant support: N/A
Canada: Kenneth Atkinson, Columbia Gastroenterology management Inc, New Westminster, BC; Alain Bitton, McGill University Health Centre, Montreal, QC; Bertus Eksteen, Aspen Woods Clinic, Calgary, AB; Astrid Greenup, St. Paul’s Hospital, Vancouver, BC; Jaime Gregor, LHSC – Victoria Hospital, London, ON; Jean-Rene La Chance, CIUSSS de l’EST-de-l’lle’de-Montreal, Montreal, QC; Allen Lim, West Edmonton GI Consultants, Edmonton, AB; Neeraj Narula, HHSC – McMaster University Medical Centre, Hamilton, ON; Rima Petroniene, Barrie GI, Barrie, ON; Richmond Sy, Ottawa Hospital Research Institute, Ottawa, ON; Margaret Walshe, Mount Sinai Hospital, University of Toronto, ON; Petros Zezos, Mount Sinai Hospital, University of Toronto, Toronto, ON.
Greece: Dimitrios Christodoulou, Ioannina University Hospital, Ioánnina; Konstantinos Fasoulas, Theagenio Anticancer Hospital of Thessaloniki, Thessaloniki; Kostantinos Goumas, Erythros Stavros Hospital, Athens; Anastasios Grammatopoulos, Metropolitan Hospital, Athens; Ionnais Koutroubakis, University Hospital of Crete, Heraklion; George Papatheodoridis, Laiko Hospital, Athens; Spiros Potamianos, University of Larissa Hospital, Larissa; Kostantinos Thomopoulos, Patra Rio University Hospital, Rio; Emmanuela Tsoukali, Evangelismos-Ophthalmiatreion Athinon-Polykliniki, Athens; Maria Tzouvala, General Hospital of Nikaia, Nikaia; Basilios Xourgias, Tzaneion General Hospital of Piraeus, Piraeus.
USA: Mazer Ally, United Gastroenterologists, Murrieta, CA; Brian Behm, University of Virgina, Charlottesville, VA; Alexandra Bruss, Medical College of Wisconsin, Milwaukee, WI; Jonathan Chapman, Gastroenterology Associates LLC, Baton Rouge, LA; Philip Ginsburg, Gastroenterology Center of Connecticut, Hamden, CT; William Holderman, Digestive Health Specialists, Tacoma, WA; Ilyas Ikramuddin, Dayton Gastroenterology Inc, Dayton, OH; Manreet Kaur, Baylor University Medical Center, Dallas, TX; Lizbeth Nunez, Medical College of Wisconsin, Milwaukee, WI; Lilani Perera, Aurora Medical Center, Grafton, WI; Timothy Ritter, Texas Digestive Disease Consultants, Southlake, TX; David Sales, Northwest Gastroenterology, Arlington Heights, IL; Michael Schwartz, Medical College of Wisconsin, Milwaukee, WI; Florin Selaru, John Hopkins University, Baltimore, MD; Martin Wolff, Gotham Medical Associates, PLLC, New York City, NY.
We would like to acknowledge the following individuals for their efforts in the management of study activities:
Song Wang, Takeda Pharmaceuticals USA Inc, Cambridge, MA, USA; Neil Brett, Evidera, Montreal, Canada; Chris Colby, Evidera, San Francisco, CA, USA; Hankyul Kim, Evidera, Waltham, MA, USA; Claudia Lopez, Takeda Pharmaceuticals USA Inc, Deerfield, IL, USA; Athanasios Natsios, Takeda Hellas S.A., Athens, Greece; Sumit Saha, Takeda Canada Inc, Ontario, Canada; Christina Kifnidi, Takeda Hellas S.A., Athens, Greece; Shashi Adsul, Takeda Pharmaceuticals International AG, Zurich, Switzerland; Haridarshan Patel, Takeda Pharmaceuticals USA Inc, Deerfield, IL USA.
Author Contributions
ICMJE criteria for authorship were read and met by all authors. All authors agree with the manuscript’s results and conclusions. BB, AY, GJM, MSS, DD, MB and DS designed the study. All authors contributed to data collection. MB, DS and DD analysed the data with data interpretation led by BB, AY and GJM and contributions by all authors. MB, DS and DD wrote the first draft of the manuscript and all authors critically reviewed the manuscript. All authors approved the final version of the manuscript.
Ethics Approval and Consent to Participate
The study was approved by the local ethics committee at each participating site. Patients alive at the time of chart abstraction [99% of patients] signed an informed consent form prior to participation in this study, if there was no waiver of informed consent.
Consent for Publication
Not applicable.
Conference Presentation
European Crohn’s and Colitis Organisation Congress 2018 [Vienna, Austria], 2019 [Copenhagen, Denmark] and 2020 [Vienna, Austria]; United European Gastroenterology Week 2018 [Vienna, Austria] and 2019 [Barcelona, Spain]; American College of Gastroenterology Annual Meeting 2018 [Philadelphia, PA, USA] and 2019 [San Antonia, TX, USA]; Digestive Disease Week 2019 [San Diego, CA, USA] and 2020 [Virtual].
Data Availability Statement
The data underlying this article were provided by Takeda under licence/by permission. Data will be shared on request to the corresponding author with permission of Takeda.
References
- 1. Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007;448:427–34. [DOI] [PubMed] [Google Scholar]
- 2. Kappelman MD, Porter CQ, Galanko JA, et al. Utilization of healthcare resources by U.S. children and adults with inflammatory bowel disease. Inflamm Bowel Dis 2011;17:62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ardizzone S, Bianchi Porro G. Biologic therapy for inflammatory bowel disease. Drugs 2005;65:2253–86. [DOI] [PubMed] [Google Scholar]
- 4. Wyant T, Fedyk E, Abhyankar B. An overview of the mechanism of action of the monoclonal antibody vedolizumab. J Crohns Colitis 2016;10:1437–44. [DOI] [PubMed] [Google Scholar]
- 5. Feagan BG, Rubin DT, Danese S, et al. Efficacy of vedolizumab induction and maintenance therapy in patients with ulcerative colitis, regardless of prior exposure to tumor necrosis factor antagonists. Clin Gastroenterol Hepatol 2017;15:229–39.e5. [DOI] [PubMed] [Google Scholar]
- 6. Sands BE, Sandborn WJ, Van Assche G, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease in patients naïve to or who have failed tumor necrosis factor antagonist therapy. Inflamm Bowel Dis 2017;23:97–106. [DOI] [PubMed] [Google Scholar]
- 7. Kopylov U, Verstockt B, Biedermann L, et al. Effectiveness and safety of vedolizumab in anti-TNF-naïve patients with inflammatory bowel disease – a multicenter retrospective European study. Inflamm Bowel Dis 2018;24:2442–51. [DOI] [PubMed] [Google Scholar]
- 8. Bohm M, Sagi S, Fischer M, et al. Comparative effectiveness of vedolizumab and tumor necrosis factor-antagonist therapy in Crohn’s disease: a multicenter consortium propensity score-matched analysis. Gastroenterology 2018;154:S–82. [Google Scholar]
- 9. Patel H, Latremouille-Viau D, Burne R, et al. Comparison of real-world treatment outcomes with vedolizumab versus infliximab in biologic-naïve patients with inflammatory bowel disease. Crohn’s & Colitis 360 2019;1:otz022. [Google Scholar]
- 10. Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000;11:561–70. [DOI] [PubMed] [Google Scholar]
- 11. Eriksson C, Marsal J, Bergemalm D, et al. ; SWIBREG Vedolizumab Study Group. Long-term effectiveness of vedolizumab in inflammatory bowel disease: a national study based on the Swedish National Quality Registry for Inflammatory Bowel Disease (SWIBREG). Scand J Gastroenterol 2017;52:722–9. [DOI] [PubMed] [Google Scholar]
- 12. Schreiber S, Jang BI, Borzan V, et al. Tu2018 – novel formulation of CT-P13 [infliximab biosimilar] for subcutaneous administration: initial results from a phase I open-label randomized controlled trial in patients with active Crohn’s disease. Gastroenterology 2018;154: S1371. [Google Scholar]
- 13. Ehehalt R, Schubert S, Stein D, et al. Treatment patterns of vedolizumab and anti-TNF-a use among patients with UC and CD in Germany: a multicenter retrospective chart review. In Advances in Inflammatory Bowel Diseases (AIBD) Annual Conference, Orlando, FL, December 8–10, 2016. [Google Scholar]
- 14. Dalal SR, Cohen RD. What to do when biologic agents are not working in inflammatory bowel disease patients. Gastroenterol Hepatol 2015;11:657–65. [PMC free article] [PubMed] [Google Scholar]
- 15. Ritter TE, Fourment C, Okoro TC, et al. Failure of vedolizumab as first-line biologic does not decrease response rates of second-line therapy. Am J Gastroenterol 2018;113:S382–3. [Google Scholar]
- 16. Ford AC, Peyrin-Biroulet L. Opportunistic infections with anti-tumor necrosis factor-α therapy in inflammatory bowel disease: meta-analysis of randomized controlled trials. Am J Gastroenterol 2013;108:1268–76. [DOI] [PubMed] [Google Scholar]
- 17. Brady JE, Stott-Miller M, Mu G, Perera S. Treatment patterns and sequencing in patients with inflammatory bowel disease. Clin Ther 2018;40:1509–21.e5. [DOI] [PubMed] [Google Scholar]
- 18. Narula N, Peerani F, Meserve J, et al. Vedolizumab for ulcerative colitis: treatment outcomes from the VICTORY consortium. Am J Gastroenterol 2018;113:1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sands BE, Peyrin-Biroulet L, Loftus EV Jr, et al. ; VARSITY Study Group. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med 2019;381:1215–26. [DOI] [PubMed] [Google Scholar]
- 20. Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 2017;66:839–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Battat R, Ma C, Jairath V, Khanna R, Feagan BG. Benefit–risk assessment of vedolizumab in the treatment of Crohn’s disease and ulcerative colitis. Drug Saf 2019;42:617–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were provided by Takeda under licence/by permission. Data will be shared on request to the corresponding author with permission of Takeda.