Abstract
Background and Aims
Matrix metalloproteinases [MMPs] play an important role in extracellular matrix regulation during cell growth and wound healing. Increased expression of MMP-12 [human macrophage elastase] has been reported in inflammatory bowel disease [IBD] which is characterised by the loss of epithelial tight junction [TJ] barrier function and an excessive inflammatory response. The aim of this study was to investigate the role of MMP-12 in intestinal TJ barrier function and inflammation.
Methods
Wild type [WT] and MMP-12-/- mice were subjected to experimental acute or chronic dextran sodium sulphate [DSS] colitis. The mouse colonic permeability was measured in vivo by recycling perfusion of the entire colon and ex vivo by Ussing chamber studies.
Results
DSS administration increased colonic permeability through modulation of TJ proteins and also increased MMP-12 expression in the colonic mucosa of WT mice. The acute as well as chronic DSS-induced increase in colonic TJ permeability and the severity of DSS colitis was found to be markedly attenuated in MMP-12-/- mice. The resistance of MMP-12-/- mice to DSS colitis was characterised by reduced macrophage infiltration and transmigration, and reduced basement membrane laminin degradation. Further in vitro and in vivo studies show that macrophage transmigration across the epithelial layer is MMP-12 dependent and the epithelial TJ barrier is compromised during macrophage transmigration.
Conclusions: Together, these data demonstrate that MMP-12 mediated degradation of basement membrane laminin, macrophage transmigration, and associated loss of intestinal TJ barrier are key pathogenic factors for intestinal inflammation.
Keywords: Matrix metalloproteinase, tight junction, macrophage
1. Introduction
The intestinal epithelium separates the luminal environment from the intestinal mucosa and underlying host tissue. In an intact epithelial lining, the apical intercellular tight junctions [TJ] are primary determinants of intestinal permeability. The TJs form a selective barrier allowing transport of water, ions, and nutrients while restricting toxins and pathogenic organisms. Increased intestinal permeability caused by defects in intestinal epithelial TJ barrier is an important reason for the development of intestinal inflammation.1–4 Disruption of the TJ barrier is thought to allow increased antigenic penetration, causing amplified, unresolved inflammation in inflammatory bowel diseases [IBD] including Crohn’s disease [CD] and ulcerative colitis [UC], coeliac disease, and ischaemia-reperfusion injury.3,5 Thus, the function of the intestinal TJ barrier is critical for prevention and resolution of intestinal inflammatory diseases.1,6 The understanding of the physiological regulation of the intestinal TJ barrier and its dysregulation under pathological conditions has been continuously evolving.
Matrix metalloproteinases [MMPs] are Zn2+ dependent endopeptidases that degrade and regulate the extracellular matrix [ECM].7,8 Based on substrate specificity and homology, the MMPs are classified into major subgroups such as collagenases [MMP-1, -8, -13, -18], gelatinases [MMP -2, -9], stromelysins [MMP -3, -7, -10, -11, -19], elastase [MMP -12], and membrane type [MMP -1, -5].7,9 The various domains of MMPs include the catalytic domain that has a Zn2+ binding site, a prodomain which maintains the latency through interaction with the catalytic domain, and the hemopexin-like C-terminal domain that defines the substrate specificity.9 Secreted as zymogens, proteolytic cleavage of the cysteine residue in the prodomain activates MMPs. Due to their ability to degrade ECM, MMPs play an important role in tissue remodelling and wound healing. However, tissue damage and sustained inflammatory response can be caused by dysregulated MMP expression.7,10,11 Beside ECM degradation and remodelling, MMPs are involved in the cleavage of surface receptors, cytokine activation, cell migration and differentiation, and degradation of junctional proteins.12–14
MMP-12 [human macrophage elastase] was initially discovered as a metalloproteinase produced by human alveolar macrophages.15 MMP-12 is capable of degrading several ECM components including elastin, type IV collagen, laminin, and fibronectin.16 Since macrophages from MMP-12 knockout mice are unable to degrade ECM and penetrate reconstituted basement membrane,17 MMP-12 is considered to have a role in macrophage migration. MMP-12 is associated with several inflammatory conditions including actinic damage,18 cutaneous granulomas,19 psoriasis,20 atherosclerosis,21 aneurysm,22 and especially chronic obstructive pulmonary disease [COPD].23 The ability of MMP-12 to induce an inflammatory response, as well as its role in tissue remodelling, makes it a potential target in inflammatory conditions.24
Among intestinal inflammatory conditions, MMP-12 levels were found to be increased multi-fold in UC and CD tissue,25,26 and in T cell mediated model of gut injury.27 In CD, MMP-12 mRNA and proteins were highly expressed in inflamed mucosa and decreased in patients who responded to anti-tumournecrosis factor alpha [TNF-α] infliximab antibody treatment. MMP-12 downregulation was also accompanied by a simultaneous improvement of the histological score, suggesting an important role for MMP-12 in inflammation and wound healing.28 Furthermore, mucosal damage induced by trinitrobenzenesulphonic acid [TNBS] administration was found to be prevented in MMP-12 knockout [MMP-12-/-] mice.25 The role of MMP-12 in intestinal epithelial permeability, and particularly its function in intestinal inflammation, is not entirely clear. In the present study, we investigated the role of MMP-12 in intestinal epithelial TJ permeability using Caco-2 cell culture and a chemically induced acute and chronic dextran sodium sulphate [DSS] colitis model which morphologically and symptomatically reproduce epithelial damage seen in human ulcerative colitis,29–32 in the context of its function in macrophage migration and ECM degradation. Our results show that the degradation of basement membrane laminin, macrophage infiltration and transmigration across the colonic epithelium, loss of intestinal TJ barrier due to macrophage transmigration, and severity of experimental colitis are substantially attenuated in absence of MMP-12.
2. Materials and Methods
2.1. Experimental animals and induction of colitis
Studies were approved by the Institutional Animal Care and Use Committee. The generation of MMP-12 knockout [MMP-12−/−] [Jackson laboratory, stock: 004855] has been described previously.17 In the acute colitis model, male and female C57BL/6J WT or MMP-12-/- mice, 9 weeks old, received 3% dextran sodium sulphate [DSS] [molecular mass, 36 000–50 000 daltons; MP Biomedicals, Santa Ana, CA] in autoclaved drinking water for 7 days. In the chronic DSS colitis model, mice received three cycles of 5 days of 2% DSS in drinking water followed by 5 days of plain water. The body weights of mice were monitored daily, and the disease activity index and histological grading of colitis lesions were carried out, as described previously.33 The DSS colitis data represent a minimum of three independent experiments with n ≥ 3. The trinitrobenzenesulphonic acid [TNBS] murine colitis model was established using standard protocol.33
2.2. Determination ofmouse colonic permeability in vivo and measurement of transepithelial electrical resistance [TER]
The colonic permeability in DSS colitis in an in vivo mouse model system was established using a recycling colonic perfusion method.34 After 7 days of DSS treatment, mice were anaesthetised using isofluorane and colon was isolated following mid-abdominal incision. The colon was cannulated at the proximal and distal ends via a rectal opening with a 0.88-mm diameter plastic tube. An external recirculating pump was used to recirculate the perfusate of Krebs-phosphate saline buffer for a 2-h perfusion period at a constant flow rate [0.75 mL/min]. The body temperature of the mouse was maintained at 370C with a temperature-controlled warming blanket. The colonic permeability was assessed by measuring the luminal-to-serosal flux rate of paracellular probe, fluorescein isothiocyanate-labelled dextran [molecular weight, 10 000 g/mol]. The water absorption was determined by using a non-absorbable marker sodium ferrocyanide or by measuring the difference between the initial and final volume of the perfusate. For measurement of transepithelial resistance, colonic tissues were harvested immediately after euthanasia, cut longitudinally, and placed on 0.03-cm2-aperture Ussing chambers [Physiologic Instruments, CA].35 Transepithelial electrical resistance [TER, Ω·cm2] was calculated from the spontaneous potential difference and short-circuit current. The paracellular permeability was assessed by mucosal-to-serosal fluxes of [3H]-inulin and [14C]-urea, as described by us previously.36 For wound healing assay,37 the Caco-2 monolayer was wounded with a 100-μl pipette tip and the wounds were imaged at time 0, 4, 24, and 48 h. The percent wound healing was calculated from the area resurfaced and the original area of wound, for individual wounds [as represented in Supplementary Figure 1, available as Supplementary data at ECCO-JCC online]. During the wound healing, the Caco-2 cells were incubated with normal media, human recombinant MMP-12 [catalytic domain] [Enzo Life Sciences, BML-SE138], and MMP-12 inhibitor MMP408 [Sigma, 444291].
2.3. Gel electrophoresis and western blotting
The lysates of colonic mucosa were prepared and processed for SDS-PAGE as described previously.38 Equal amounts of protein were loaded in individual wells on the SDS-PAGE gel. After protein transfer to membrane, the membranes were probed using anti-occludin [Invitrogen, 71–1500], claudin-1, -2, -3 [Invitrogen, 71–7800, 32–5600, and 34–1700], myosin light chain [MLC] [Cell Signaling, 3672], phospho-MLC [Cell Signaling, 3671], MMP-12 [Proteintech, 22989-1-AP], laminin [L9393, Sigma], and β-actin [Santa Cruz Biotechnology, sc-1615] antibodies. Human colonic biopsy samples for MMP-12 western blotting and immunofluorescence were obtained under the Institutional Review Board approved protocol [study #10–481].
2.4. Confocal immunofluorescence and immunohistochemistry
Immunohistochemistry for MMP-12, macrophage marker CD68 and F4/80, and laminin on colonic tissue was performed by standard methods. The colon cryosections were fixed in acetone and permeabilised with 0.1% Triton X-100 in PBS at room temperature for 5 min. The sections were then blocked in normal serum and labelled with primary antibodies in blocking solution overnight at 4°C [MMP-12, Proteintech, 22989-1-AP; F4/80, biogems, 02922-20; CD68, abcam, ab955; Laminin α1, R & D systems, MAB4656]. After PBS washes, the sections were incubated in Alexa Fluor-488 or Cy-3-conjugated secondary antibodies [Invitrogen]. ProLong Gold antifade reagent [Invitrogen, CA] containing DAPI as a nuclear stain was used to mount the sections on glass slides. The slides were examined using a confocal fluorescence microscope Leica SP8. Images were processed with LAS X software [Leica Microsystems].
2.5. Flow cytometry
Flow cytometric analysis was performed to assess changes in macrophage population in WT and MMP12 -/- mice colon upon DSS treatment, by modification of previously described protocol.39–41 The mice colons were harvested and digested with Collagenase D [Roche], DNAse-I [Roche] and Dispase-II [Roche] at concentrations of 0.05 g, 0.05 g and 0.3 g/ 100 ml. Cells obtained from the digestion step were subjected to a 80‐40% Percoll [Sigma] gradient centrifugation to enrich the leukocyte population. From the enrichment step, approximately 1 X 106 cells from each sample were stained with Fixable Viability Dye eFluorTM 780 [eBioscienceTM, Invitrogen, Carlsbad, CA] in FACS buffer [Ca2+/Mg2+ free DPBS with 2% FBS and 5 mM EDTA] for 10 min at 4oC. Followed by washing, the cells were suspended in 100 μ l FACS buffer with 0.125–1 μ g [1:100 dilution] fluorophore conjugated monoclonal antibodies to stain surface markers for 60 min at 4 oC. Macrophages were identified as CD45- FITC+, MHC-II-PE Cy7+, CD64-BV421+, CX3CR1-BV-605+, and Ly6C-BV786 -/hi cells. The macrophage population was further assessed for CD14-PE staining and CD14hi cells were demarcated as pro-inflammatory M1 macrophage subtype.41–43 Following the staining step, cells were washed with FACS buffer, fixed with 2% paraformaldehyde and analysed using a BD-LSRFortessaTM [BD- Biosciences, San Jose, CA] flow cytometer. Flow cytometry data were analysed using FlowJo version 10 software [FlowJo LLC, Ashland, OR]. Gating used for analysis of the flow cytometry data for the macrophage subtyping was performed using appropriate fluorescence minus one [FMO] controls. A representation of the flow cytometry gating strategy used to identify the macrophage population and its subtypes is illustrated in Supplementary Figure 2, available as Supplementary data at ECCO-JCC online. All monoclonal fluorescent dye-tagged antibodies were purchased from BioLegend [San Diego, CA].
2.6. Macrophage transmigration across the Caco-2 cell monolayer
The Caco-2 cells obtained from the American Type Culture Collection were maintained at 37°C in DMEM culture medium supplemented with 10% fetal bovine serum. The U937 cells [ATCC] were maintained in RPMI1640 Medium with 10% fetal bovine serum. Caco-2 cells were grown in an upside-down fashion [apical side facing the base of the transwell] on laminin-rich [60% laminin] matrigel [Corning, 354230] covered 8.0- μ m pore size transwell inserts [Corning, 3422], for 15-days or till the monolayers were confluent. U937 macrophages were transfected with On-Target Smartpool MMP-12 or non- target control siRNA [Dharmacon]. Then 24 h after transfection, macrophages were treated with Phorbol 12-myristate 13-acetate [PMA, Sigma, P8139, 100 ng/ml] for another 48 h and equal number of U937 macrophages were added to the apical chamber [basal side of Caco-2 cells] in the presence of N-Formyl-Met-Leu-Phe [fMLP, Sigma, F3506, 10 μ m] in the basal chamber [apical side of Caco-2 cells] for indicated time intervals. The macrophages transmigrated to the apical side of Caco-2 cells were counted using a Neubauer chamber and presented as percent transmigration. Macrophages were stained with a fluorescent dye [PKH26, Sigma] to aid in identification and counting. In simultaneous experiments, activated macrophages were incubated with MMP-12 inhibitor, MMP408 [Sigma, 444291, 10 μ g/ml].
2.7. Macrophage transmigration in mouse intestine
The macrophage transmigration in mouse intestine was studied using the intestinal loop method, described by Flemming et al.,44 with modifications. In brief, mice were injected with mouse recombinant MMP-12 (R & D systems, 3467-MPB-020, intraperitoneally [i.p.], 2 μ g/mouse) with or without LPS [Sigma, L4391, i.p., 5 mg/kg], for 48 h. An intestinal loop was isolated following isofluorane anaesthesia and mid-line abdominal incision. The 4-cm loop was flushed with warm HBSS, ligated, injected with 400 ml of 1 μ M fMLP, and inserted back into the abdominal cavity. After 1 h, the loop was excised, the contents were collected, and filtered through a 35- μ m cell strainer. The contents were centrifuged [400 g, 5 min, 40C], re-suspended in PBS, and stained for F4/80 and CD68. Following counterstaining in DAPI, the cells were visualised on a Neubauer chamber under fluorescence microscope and counted. Immediately following the intestinal loop procedure, the corresponding intestinal tissue was mounted on an Ussing chamber to study TJ permeability.
2.8. Statistical analysis
Data are reported as mean ± standard error of the mean [SE]. Whenever needed, data were analysed by using an analysis of variance [ANOVA] for repeated measures [SigmaStat, Systat Software, San Jose, CA]. A Tukey’s test was used for post-hoc analysis between treatments following ANOVA [p < 0.05].
3. Results
3.1. MMP-12 expression in ulcerative colitis and experimental colitis
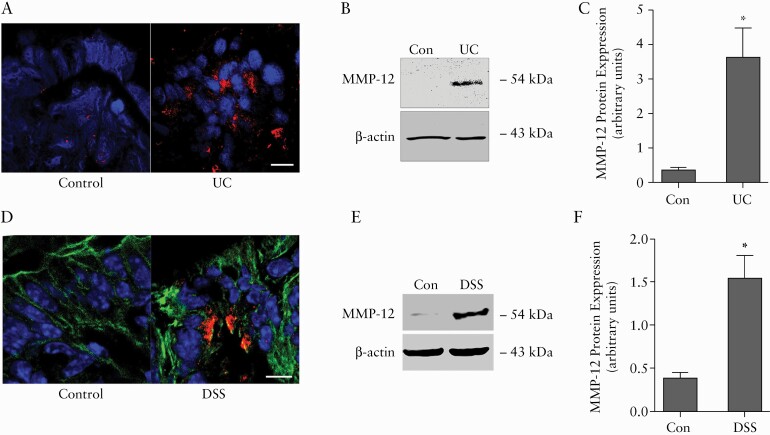
To understand the role of MMP-12 in intestinal inflammation, we first examined the immunolocalisation and protein levels of MMP-12 in intestinal inflammation. In confocal immunofluorescence examination, sparse staining for MMP-12 was observed in the colonic epithelium of healthy control patients. However, in ulcerative colitis [UC], the colonic mucosa showed strong staining for MMP-12, particularly under the colonic surface epithelium [Figure 1A]. In western blot analysis, the total protein level of MMP-12 in colonic tissue was found to be markedly increased in UC patients [Figure 1B and C]. Similarly, in experimental acute DSS colitis, increased MMP-12 immunostaining was detected in the lamina propria and under the surface epithelium [Figure 1D]. Increased levels of MMP-12 in DSS colitis were also confirmed by western blot [Figure 1E and F]. Increased expression of MMP-12 has been reported previously in another experimental model of TNBS colitis.25 Thus MMP-12 expression is increased during intestinal inflammation, indicating a potential role of MMP-12 in the inflammatory response.
Figure 1.
MMP-12 immunolocalisation and protein expression in intestinal inflammation. A: In confocal immunofluorescence, MMP-12 protein [red] was detected within the colonic mucosa in ulcerative colitis [UC]. Nuclei are stained in blue. White bar: 15 μ m. B: In western blot analysis, MMP-12 protein levels in the colon were significantly elevated in UC. β -actin is shown as a loading control. Representation of more than three blots in each group. C: Densitometry analysis for MMP-12 protein levels. D: In confocal immunofluorescence, MMP-12 protein [red] was detected under the colonic epithelial layer in DSS colitis. Actin is stained in green and nuclei are stained in blue. White bar: 15 μ m. E: In western blot analysis, MMP-12 protein levels in the colon were significantly elevated in DSS colitis. β -actin is shown as a loading control. Representation of three blots in each group. F: Densitometry analysis for MMP-12 protein levels. *p < 0.01. DSS, dextran sodium sulphate.
3.2. Severity of DSS colitis is reduced in MMP-12-/- mice
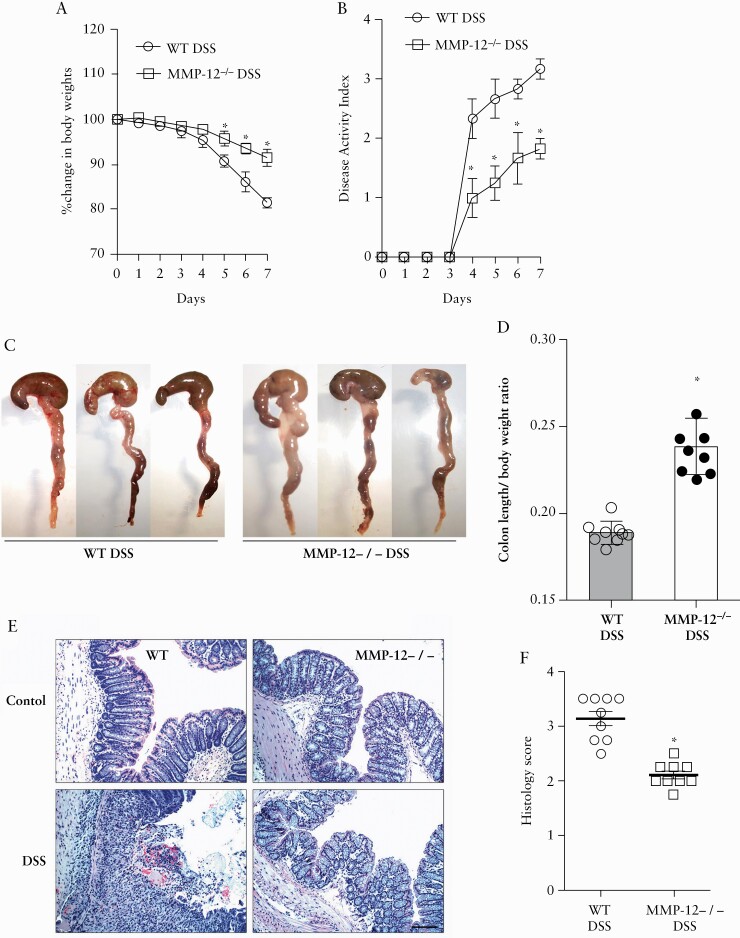
We next sought to determine the role of MMP-12 during intestinal inflammation. Using MMP-12-/- mice, we observed that the severity of experimental colitis after 7 days of oral administration of 3% DSS was significantly lower in MMP-12-/- mice as compared with the WT mice. Following DSS administration, the loss of body weight and the disease activity index33 were significantly attenuated in MMP-12-/- mice compared with WT mice [Figure 2A and B]. In gross examination, WT DSS colons were shortened and dilated with bloody contents [Figure 2C]. The ratio of colon length to body weight was significantly higher in MMP-12-/- DSS mouse colon, compared with WT mice, respectively [p < 0.001] [Figure 2D]. Likewise, DSS MMP-12-/- mice showed much less severe histopathological changes of colonic inflammation, including epithelial erosion, neutrophilic and mononuclear infiltration, loss of crypts, and oedema in the muscularis layer of the colon, when compared with WT DSS mice [histological score: 3.13 ± 0.13 and 2.11 ± 0.07 for WT and MMP-12-/- mice, respectively, on a scale of 0–4, p < 0.001] [Figure 2D and E]. We also assessed the severity of inflammation in DSS colitis by quantifying select cytokines in DSS colitis tissues. The quantitative polymerase chain reaction [qPCR] studies on colonic mucosa showed that the mRNA expression of pro-inflammatory IFNγ, IL-1β, and TNF-α were markedly increased in WT DSS colitis [Figure 3]. The mRNA expression of IFNγ, IL-1β, and TNF-α was found to be significantly lower in MMP12-/- DSS colitis compared with WT DSS colitis. Overall, the severity of DSS colitis was significantly reduced in MMP-12-/- mice when compared with WT mice.
Figure 2.
Severity of DSS colitis is attenuated in MMP-12-/- mice. A: The percent reduction in the body weight during DSS colitis was attenuated in MMP-12-/- mice compared with WT mice. B: The disease activity index during DSS colitis was found to be lower in MMP-12-/- mice compared with WT mice. C: Gross images of DSS colitis revealing attenuated changes of colon shortening and hyperaemia in MMP-12-/- mice, compared with WT mice. D: The colon length/body weight ratio was calculated based on starting body weights of mice. This ratio was significantly lower in the WT DSS group compared with the MMP-12-/- DSS group. E: The histological examination showed loss of colonic surface and crypt epithelium, haemorrhages, and diffuse inflammatory cell infiltration in WT DSS colon. The MMP-12-/- mice showed mild inflammation compared with WT DSS colon. H & E stain, black bar = 25 μ m. F: The histological score of DSS colitis was significantly lower in MMP-12-/- mice compared with WT mice. *p < 0.01 versus WT DSS colitis. DSS, dextran sodium sulphate; WT, wild-type; H & E, haematoxylin and eosin.
Figure 3.
Inflammatory cytokines in MMP-12-/- DSS colitis. The qPCR analysis of DSS colitis tissue showed decreased mRNA expression of [A] IFN-γ, [B] TNF-α, [C] IL-1β, and [D] IL-10 in MMP-12-/- DSS colon versus WT DSS colon. The mRNA expression of respective cytokines was normalised to mRNA expression of GAPDH. *p < 0.01 versus other groups, one-way analysis of variance [ANOVA] [Dunnett’s multiple comparisons test]. DSS, dextran sodium sulphate; WT, wild-type; qPCR, quantitative polymerase chain reaction.
3.3. DSS-induced epithelial permeability is attenuated in MMP12-/- mice
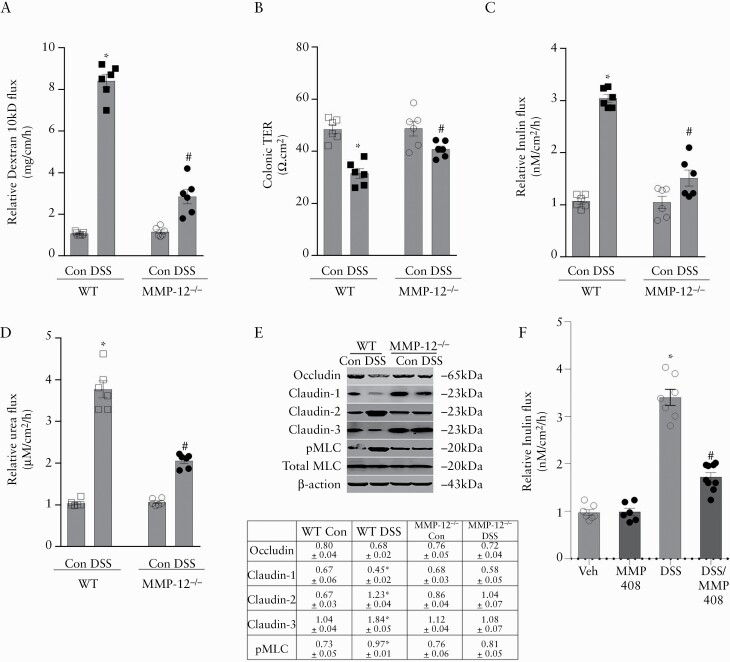
Clinical as well as experimental colitis is marked by increase in intestinal paracellular permeability and epithelial TJ barrier disruption.3,5 Given the protective phenotype of MMP-12-/- mice in DSS colitis, we sought to determine if MMP-12 is involved in intestinal barrier function and intestinal inflammation. The mouse colonic paracellular permeability in DSS colitis was assessed by an in vivo method of whole-length colonic recycling perfusion with Texas red-labelled dextran [10 kDa] as a macromolecular marker. We found that the baseline colonic dextran flux in MMP-12-/- mice was not significantly different from wild type [WT] mice. Administration of DSS caused a substantial increase in dextran flux in the colon of WT DSS-treated mice. This DSS-induced increase in dextran flux was found to be significantly attenuated in MMP-12-/- mouse colon [Figure 4A]. Additionally, in ex vivo studies with Ussing chambers, DSS-induced decrease in colonic transepithelial resistance [TER] was significantly attenuated in MMP-12-/- mice compared with WT mice [Figure 4B]. The colonic flux of paracellular markers inulin and urea was also significantly lower in MMP-12-/- DSS mice compared with WT DSS mice [Figure 4C and D]. We next investigated select TJ proteins whose protein expression is commonly altered in DSS colitis mucosa [Figure 4E]. DSS-induced reduction in TJ barrier forming occludin, claudin-1, and claudin-3 was found to be largely inhibited in MMP-12-/- DSS colitis. Also, DSS-induced increase in pore-forming TJ protein claudin-2 was found to be prevented in MMP-12-/- colitis. Intestinal TJ permeability is also regulated by myosin light chain kinase [MLCK] via rearrangement of the perijunctional actin-myosin ring. MLCK phosphorylates the myosin regulatory light chain [MLC], resulting in contraction of perijunctional actomyosin and increased TJ permeability.45–47 DSS induced marked phosphorylation of MLC [pMLC] in WT mice but not in MMP-12-/- mice. These results indicate overall preservation of colonic TJ barrier in MMP-12-/- DSS mice compared with WT DSS mice. Thus, MMP-12 expression was increased in DSS colitis and DSS-induced increase in colonic TJ permeability was found to be significantly attenuated in MMP-12-/- mice, suggesting an important role of MMP-12 in DSS-induced increase in colonic TJ permeability during intestinal inflammation. In addition, pharmacological inhibition of MMP-12 did not have any effect on the baseline TJ permeability but significantly attenuated DSS-induced increase in mouse colonic TJ permeability [Figure 4F].
Figure 4.
Role of MMP-12 in colonic TJ barrier. A: In in-vivo colonic recycling perfusion, DSS colitis caused a multifold increase in Texas red-labelled dextran [10 kDa] flux in the colon of WT but not MMP-12-/- mice. B: In Ussing chamber studies, DSS-induced drop in the electrical resistance [TER] of colonic tissue was attenuated in MMP-12-/- mice. DSS-induced increase in colonic inulin [C] and urea [D] flux was attenuated in MMP-12-/- mice. * , #p < 0.01 versus WT control and each other, one-way ANOVA [Tukey’s multiple-comparison test]. E: Effect of DSS colitis on various TJ proteins. DSS-induced decrease in occludin, claudin-1, claudin-3, and pMLC was prevented in MMP-12-/- mice. The expression of pore-forming claudin-2 was increased in WT DSS but not MMP-12-/- DSS mice. Representation of three blots in each group. Densitometry for blots is shown in the table. Arbitrary values: occludin, claudin-1, -2, and -3 represent respective TJ protein expression normalised to β -actin. The pMLC values represent pMLC expression normalised to total MLC; *p < 0.05 versus WT control, one-way ANOVA [Dunnett’s multiple comparisons test]. F: MMP-12 inhibition with MMP408 attenuated DSS-induced increase in mouse colonic inulin flux. MMP408 [5 mg/kg] was administered orally in 0.5% methylcellulose solution for 3 days before studying the colonic permeability. #p < 0.01 versus other groups, one-way ANOVA [Dunnett’s multiple comparisons test]. DSS, dextran sodium sulphate; WT, wild-type; TJ, tight junction; ANOVA, analysis of variance; pMLC, phosphorylated myosin light chain.
3.4. Reduced epithelial macrophage infiltration in MMP-12-/- DSS colitis
Macrophages play a central role in intestinal inflammation during DSS colitis48,49 and MMP-12 secreted by macrophages has been shown to have a role in macrophage migration.17 We next examined macrophage infiltration in DSS colitis in WT and MMP-12-/- mice. In confocal immunofluorescence examination, negligible staining for macrophage marker F4/80 was seen in control WT and MMP-12-/- mouse colon. In WT DSS colitis, significantly increased F4/80 staining was seen in the colonic mucosa. The F4/80-staining cells were present in the lamina propria, near the apical membrane of surface epithelium, and in the lumen of crypts [Figure 5A]. Also, F4/80-positive cells were present commonly within the paracellular space in between adjacent colonic epithelial cells in WT DSS colitis [Figure 5A].This increase in F4/80 staining in WT DSS colitis was not observed in MMP-12-/- mouse DSS colon [Figure 5A and B]. Another macrophage marker, CD68 staining, showed exactly similar increased staining within the surface and crypt epithelium and lamina propria of WT DSS colon but was negligible in MMP12-/- DSS colon [not shown].
Figure 5.
Colonic macrophage infiltration in MMP-12-/- mice. A: In confocal immunofluorescence examination, negligible staining for macrophage marker F4/80 [green] was seen in control WT and MMP-12-/- mice colon. In WT DSS colitis, significantly increased F4/80 staining was seen in the lamina propria, close to the apical membrane of surface epithelium [arrows], and in the lumen of crypts [arrowheads]. The F4/80-positive cells were also present within the paracellular space in between adjacent colonic epithelial cells in WT DSS colitis [lower two panels, arrows]. The MMP-12-/- DSS colon showed negligible presence of F4/80-positive cells. Actin is stained in red and nuclei are stained in blue. White bar: 25 μ m. B: Quantification of F4/80 staining intensity. * ,#p < 0.01 versus WT control and each other, one-way ANOVA [Tukey’s multiple comparison test. C and D: Flow cytometry for relative abundance of CD45+, MHC-II+, CD64+, CX3CR1+ and Ly6C-/hi macrophages [C], and CD14hi M1 macrophage subtype [D] in the colonic lamina propria of control and DSS-treated WT and MMP12 -/- mice. Data are expressed as mean ± SE, n = 3–5, a, b, and c, *p < 0.01 versus each other in one-way ANOVA followed by Dunnett’s multiple comparison test. DSS, dextran sodium sulphate; WT, wild-type; ANOVA, analysis of variance; SE, standard error of the mean.
We further quantified macrophage abundance in the control and DSS-administered WT and MMP-12-/- mouse colons. In flow cytometric analysis, DSS colitis markedly increased the abundance of macrophages in the colonic lamina propria in WT mice. The macrophage number in the colonic lamina propria of MMP-12-/- DSS mice was, however, significantly lower compared with WT DSS mice [Figure 5C]. Further sorting of pro-inflammatory M1 macrophages revealed that the DSS-induced increase in the mouse colonic M1 population was significantly prevented in MMP-12-/- mice compared with WT mice [Figure 5D]. Thus this data confirmed that the macrophage infiltration in and through the colonic mucosal epithelium in DSS colitis is mediated by MMP-12.
3.5. Laminin degradation in DSS colitis is prevented in MMP-12-/- mice
Various substrates of MMP-12 include extracellular matrix proteins elastin, type IV collagen, laminin, and fibronectin vitronectin, proteoglycan, chondroitin suphfate, and myelin basic protein.15,16,50 Laminins, as heterotrimeric glycoproteins, are major components of the basement membrane present underneath the epithelial cells, separating them from the lamina propria.51 Genome-wide association studies have identified laminin gene LAMB1 as a susceptibility locus for ulcerative colitis,52 and deficiency of epithelial basement membrane laminin in ulcerative colitis has been documented.53
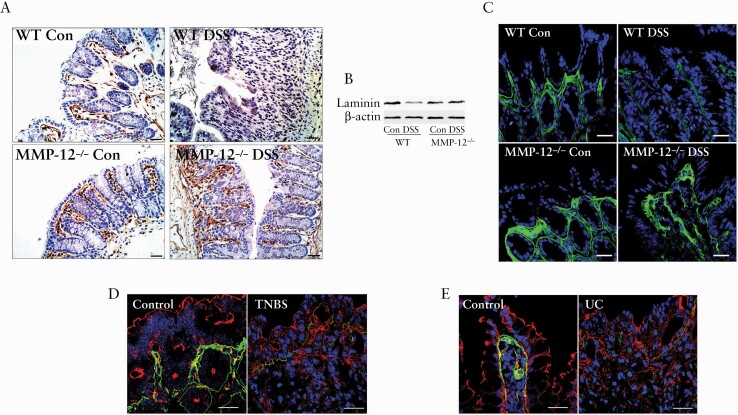
We next examined the possibility of laminin being a relevant substrate of MMP-12 in DSS colitis. In immunohistochemical examination, laminin was stained at the basement membrane below the epithelial lining of colon in WT and MMP-12-/- control mice [Figure 6A]. In WT DSS colitis, laminin staining at the basement membrane was found to have completely disappeared. In contrast, MMP-12-/- DSS mice colon showed strong staining for laminin in the basement membranes [Figure 6A]. In western blotting, laminin protein expression was significantly reduced in WT DSS colon and not MMP-12-/- DSS colon, compared with respective control colons [Figure 6B]. Laminins are heterotrimeric glycoproteins composed of α, β, and γ chains with each chain having multiple subunits. Laminin α1 and α5 are the common subunits expressed in intestine.54 In confocal immunofluorescence, immunolocalisation of laminin α1 in the basement membrane was found to be markedly lost in WT DSS colitis but not in MMP-12-/- DSS colitis [Figure 6C]. At least two commercial lamininα5 antibodies did not stain laminin in the basement membrane in mouse colon. Laminin α1 was also found to be degraded in TNBS mouse colitis [Figure 6D] as well as in ulcerative colitis [Figure 6E]. Over all, these data indicate that laminin degradation is a consistent feature of intestinal inflammation, and genetic inhibition of MMP-12 prevents the loss of laminin during murine DSS colitis.
Figure 6.
Immunolocalisation of laminin in intestinal inflammation. A: In immunohistochemical examination, polyclonal laminin antibody detected the basement membrane [brown] below the epithelial lining of control colon in WT and MMP-12-/- mice. The laminin staining at the basement membrane was found to be completely disappeared in WT DSS colon but not in MMP-12-/- DSS mice colon. Nuclei are counterstained with haematoxylin. Black bar = 30 μ m. B: In western blotting, laminin protein expression was significantly reduced in WT DSS colon and not in MMP-12-/- DSS colon. Representation of three blots in each group. C: The laminin α1-specific staining [green] in the basement membrane was found to be markedly lost in WT DSS colitis but not in MMP-12-/- DSS colitis. The laminin α1 expression [green] was found to be lost in murine TNBS colitis [D] and in UC [E], compared with respective controls. Actin is stained in red and nuclei are stained in blue. White bar: 50 μ m. Representation of several microscopic areas, from three or more samples in each group. DSS, dextran sodium sulphate; WT, wild-type; TNBS, trinitrobenzene sulphonic acid; UC, ulcerative colitis.
3.6. Chronic DSS colitis is attenuated in MMP-12-/- mice
Considering the chronic nature of IBD, we also examined the role of MMP-12 in the chronic DSS colitis model. Consistent with the findings in the acute DSS colitis model, the severity of chronic DSS colitis was found to be attenuated in MMP-12-/- mice, compared with WT mice. MMP-12-/- mice showed reduced loss of body weight during chronic DSS colitis [Figure 7A]. Compared with WT mice, the colonic TER was higher and paracellular inulin flux was lower in MMP-12-/- mice, indicating preservation of TJ barrier during chronic DSS colitis [Figure 7B and C]. The histological changes of complete colonic mucosal obliteration, loss of crypts, and mucosal and submucosal mononuclear infiltration were less commonly seen in MMP-12-/- mice compared with WT mice [Figure 7D and E, histological score of 3.02 ± 0.17 and 2.05 ± 0.18 in WT and MMP-12-/- mice, respectively, p < 0.03]. In WT chronic DSS colitis, significantly increased F4/80 staining was seen in the lamina propria, within the apical membrane of surface epithelium, and in the lumen of crypts [Figure 7F]. Also, laminin degradation seen in WT chronic DSS colon was found to be substantially prevented in MMP-12-/- chronic DSS colon [Figure 7G]. Thus the clinical and histological severity, loss of TJ barrier, macrophage infiltration within the surface epithelium and colonic mucosa, and laminin degradation during chronic DSS colitis was found to be attenuated in the absence of MMP12.
Figure 7.
Chronic DSS colitis in MMP-12-/- mice. A: The percent reduction in body weight during chronic DSS colitis was attenuated in MMP-12-/- mice compared with WT mice. In Ussing chamber studies following chronic DSS colitis, colonic TER was higher [B] and inulin flux was lower [C] in MMP-12-/- mice compared with WT mice. *p < 0.01 versus WT. D: Following chronic DSS colitis, MMP-12-/- mice showed attenuated inflammation in colon compared with WT colon. H & E stain, black bar = 25 μ m. E: The histological score of chronic DSS colitis was significantly lower in MMP-12-/- mice compared with WT mice. *p < 0.01 versus WT chronic DSS colitis. F: In confocal immunofluorescence examination, MMP-12-/- chronic DSS colon showed negligible staining for macrophage marker F4/80 [green], compared with WT chronic DSS colon. Actin is stained in red and nuclei are stained in blue. White bar: 25 μ m. G: The laminin α1-specific staining [green] in the basement membrane was found to be markedly lost in WT chronic DSS colitis but not in MMP-12-/- chronic DSS colitis. Actin is stained in red and nuclei are stained in blue. White bar: 25 μ m. Representation of several microscopic areas, from three or more samples in each group. DSS, dextran sodium sulphate; WT, wild-type; TER, transepithelial electrical resistance; H & E, haematoxylin and eosin.
3.7. Macrophage transmigration across the intestinal epithelium is MMP-12 dependent
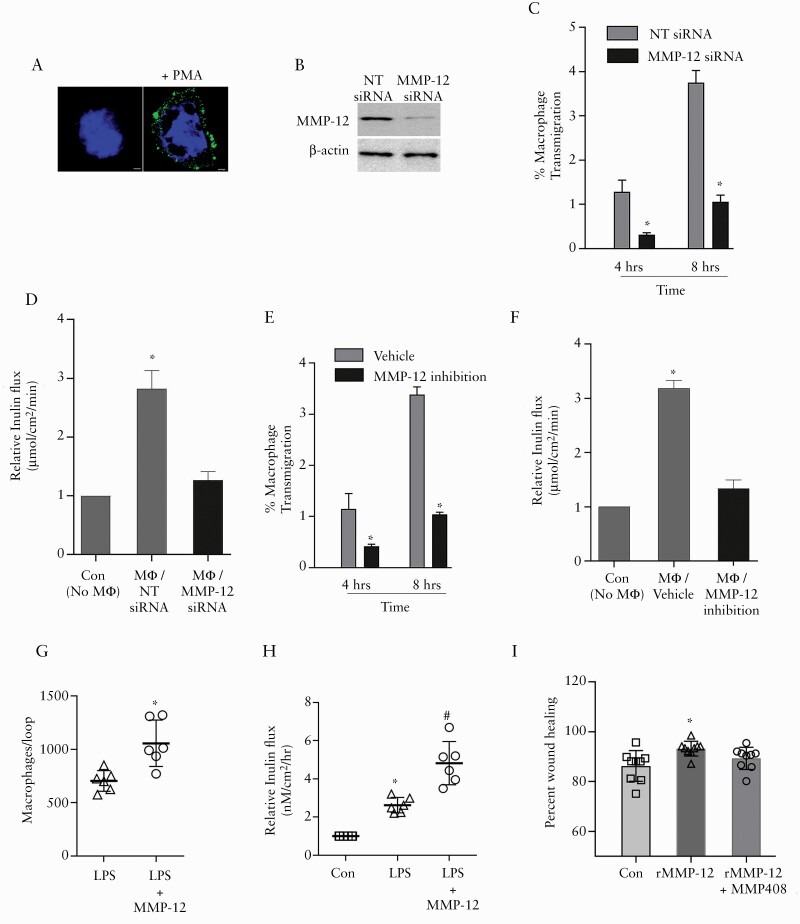
The above studies in mouse colon showed reduced basement membrane laminin degradation and macrophage infiltration in absence of MMP-12, suggesting a role of MMP-12 in macrophage migration. We hypothesise that during inflammation, MMP-12 enables macrophages to degrade basement membrane and transmigrate through TJs, resulting in increased TJ permeability and exaggerated inflammatory response. To further examine this hypothesis, we investigated the role of MMP-12 in macrophage transmigration in an in-vitro human intestinal Caco-2 cell and U937 macrophage co-culture model. To mimic in vivo conditions, U937 macrophage cell transmigration was studied across Caco-2 cells which were grown on laminin-rich matrigel covered transwell inserts in a basolateral to apical fashion, as detailed in the Methods section. In transmigration assay, U937 macrophages were activated with phorbol myristate acetate [PMA] to produce MMP-12, before addition on the basolateral side of Caco-2 cell monolayers [Figure 8A]. To study the role of MMP-12 in macrophage transmigration, U937 macrophages were transfected with MMP-12 or non-target [NT] siRNA. The efficacy of MMP-12 knockdown was confirmed by western blot [Figure 8B]. Measured numbers of U937 macrophages were added on the basolateral side of Caco-2 cells in the transwells. A chemo-attractive gradient was provided in a basolateral to apical direction by adding N-Formyl-Methionyl-Leucyl-Phenylalanine [fMLP] on the apical side of Caco-2 cell monolayers. Compared with NT siRNA-transfected macrophages, MMP-12 siRNA-transfected U937 macrophages showed significantly less transmigration across Caco-2 cells at different time points [Figure 8C]. The paracellular flux measured across Caco-2 cells at the end of transmigration studies showed significant increase in inulin flux in Caco-2 cells incubated with NT siRNA-transfected U937 macrophages compared with Caco-2 cells without incubation with U937 macrophages [Figure 8D]. The inulin flux in Caco-2 cells incubated with MMP-12 siRNA-transfected U937 macrophages was found to be significantly lower compared with the Caco-2 cells incubated with NT siRNA-transfected U937 macrophages. Under a similar experimental set-up, specific MMP-12 inhibitor MMP408 reduced the macrophage transmigration and the associated increase in paracellular inulin permeability [Figure 8E and F].
Figure 8.
A: PMA-activated U937 macrophages showed abundant expression of MMP-12 [green]. Nuclei: blue. B: The U937 macrophages transfected with MMP-12 siRNA showed marked reduction in MMP-12 expression, compared with non-target [NT] siRNA-transfected U937 cells. C: MMP-12 siRNA transfection reduced fMLP-induced, basolateral to apical transmigration of PMA-activated U937 macrophages across Caco-2 cells at time points shown. *p < 0.01 versus respective NT siRNA group. D: The Caco-2 paracellular inulin flux at the end of transmigration studies was significantly higher in Caco-2 cells incubated with NT siRNA-transfected U937 macrophages compared with Caco-2 cells incubated with MMP-12 siRNA-transfected U937 macrophages. * p < 0.01 versus all other groups, one-way ANOVA [Tukey’s multiple comparison test]. Under similar experimental set up as in [C], specific MMP-12 inhibitor MMP408 reduced the U937 macrophage transmigration [E, *p < 0.01 versus respective vehicle group] and associated increase in paracellular inulin flux [F, *p < 0.01 versus all other groups, one-way ANOVA, Tukey’s multiple comparison test]. The luminal fMLP chemoattractant gradient-induced macrophage transmigration [G] and associated inulin flux [H] in the intestinal loop were higher in mice injected with LPS and MMP-12, compared with mice injected with LPS alone. *,#p < 0.01 versus control and other groups. I: Wound healing assay revealed significantly higher percent wound healing after 48 h in Caco-2 cells incubated with recombinant MMP-12. The data represent area healed based on original wound area [as shown in Supplementary Figure 1], calculated using imageJ program. #p < 0.01 versus other groups, one-way ANOVA [Dunnett’s multiple comparisons test]. ANOVA, analysis of variance.
The role of MMP-12 in macrophage transmigration was further examined in vivo by an intestinal loop method in mice. In this method, originally described by Flemming et al.,44 a chemoattractant gradient is established within an intestinal loop to study trafficking of leukocytes across the intestinal epithelium into the lumen. To study macrophage transmigration, the mice were injected with LPS with or without recombinant mouse MMP-12, 48 h before the intestinal loop procedure. We found that the luminal fMLP chemoattractant gradient-induced macrophage transmigration in the intestinal loop was higher in mice injected with LPS and MMP-12, compared with mice injected with LPS alone [Figure 8G]. Immediately following the intestinal loop procedure, the tissues were mounted on an Ussing chamber to study TJ permeability. The paracellular flux of inulin was found to be significantly higher in mice injected with LPS and MMP-12, compared with mice injected with LPS alone [Figure 8H]. These in vitro and in vivo results demonstrate that the macrophage transmigration across the intestinal epithelium is MMP-12 dependent and intestinal epithelial TJ permeability is increased during macrophage transmigration. We also examined the role of MMP-12 in wound healing, for which wounded Caco-2 cell monolayers were incubated with the human recombinant MMP-12 catalytic domain alone or in presence of MMP-12 inhibitor. These results indicated that MMP-12 promotes wound healing in Caco-2 cells [Figure 8I and Supplementary Figure 1]. Consistent with the previous reports,55 these results indicate the potentially physiological role of MMP-12 during early wound healing.
4. Discussion
Accumulating evidence from IBD patient samples and animal and mathematical models of IBD, as well as IBD susceptible risk loci identification, have all suggested that macrophages are a major celltype contributing to IBD pathogenesis.56–67 In fact, inflammatory macrophages accumulate in the intestinal mucosa of individuals with CD and UC.57,60 Moreover, the current therapeutic targets used in IBD, such as anti-TNFα, -IL-12p40, and -IL-23p19, are cytokines largely produced by macrophages.68 Nevertheless, the complete understanding of how inflammatory macrophages affect the intestinal microenvironment remains unknown. Here, we report on the role of MMP-12 and macrophages in mouse colonic TJ permeability and DSS-induced colitis. MMP-12 is considered as a potentially inflammatory mediator based on its increased expression in IBD tissue25 and the animal models of colitis27; however, the function of MMP-12 during intestinal inflammation is unclear. We show how macrophage transmigration across the intestinal epithelium is MMP-12 dependent and how intestinal epithelial TJ permeability is increased during macrophage transmigration.
Macrophages play a central role in intestinal homeostasis and inflammation. During intestinal homeostasis, macrophages have an anti-inflammatory role where they function to clear apoptotic cells and pathogens without initiating an inflammatory response. These anti-inflammatory macrophages also maintain intestinal homeostasis through the production of immunoregulatory cytokines that act on regulatory T cells, stem cell renewal, and peristalsis.56 During inflammation, there is an accumulation of inflammatory macrophages in intestinal tissue, which in the presence of improper differentiation and processing, may produce excessive pro-inflammatory cytokines such as TNF-α and IL-1 β.56,69 In states of chronic intestinal inflammation such as seen in UC and CD, inflammatory macrophages are believed to contribute to intestinal inflammation through pro-inflammatory cytokine production and subsequent activation of T cells and various other effector cells.56 Similar to previous reports,70,71 we found activated macrophages abundantly present in the WT DSS-treated colonic mucosa. The abundance of activated macrophages as quantified by flow cytometry, and the production of pro-inflammatory cytokines as measured by RT-PCR, were markedly diminished in MMP-12-/- DSS colonic mucosa. These data point out the ability of MMP-12 to enable macrophage migration and infiltration into the colonic mucosa, providing a new functional mechanism on how macrophages contribute to intestinal inflammation by disrupting the intestinal mucosal integrity.
The presence of macrophages within the colonic epithelial paracellular space and luminal side of crypts was of particular interest to us. We hypothesised that such macrophage transmigration would require degradation of subepithelial basement membrane. The investigation into the relevant MMP-12 target of the basement membrane component, indeed, revealed marked loss of laminin in colonic inflammation. The loss of laminin was evident in murine WT DSS colitis and TNBS colitis, and also in UC specimens. Laminin has been previously shown to be targeted by MMP-12,16 and the laminin gene LAMB1 has been identified as a susceptibility locus for ulcerative colitis in genome-wide association studies.52 Moreover, loss of basement membrane in human explant cultures was reported in a model of T cell injury.72 In another study, transgenic mice overexpressing LMα1 and LMα5 showed attenuated early-stage inflammation but enhanced tumour formation in the DSS-AOM colon carcinogenesis model, supporting the role of basement membrane in intestinal homeostasis and stability during early stages of inflammation.54 Beyond the previous in vitro data,72 we now provide in vivo evidence of laminin degradation in IBD as well as in experimental colitis. The effect of the loss of basement membrane on the disease process can be multifaceted. The role of basement membrane in the integrity of intestinal mucosa during inflammation53 and preservation of the basement membrane in MMP-12-/- DSS colon, in our study, are comparable to the MMP-12 dependent loss of extracellular matrix in the bronchial wall in COPD73 and internal elastic lamina in atherosclerotic plaque.74 Also, MMP-12 and MMP-3 downregulation was found to be accompanied by a simultaneous improvement of the histological score in CD patients.28 Beside their role in structural integrity, ECM fragments, including those from laminin, can act as chemoattractants and have various immune and inflammatory effects.75
In agreement with others and our previous reports,4,76,77 DSS administration significantly increased colonic TJ paracellular permeability in WT mice. However, the acute or chronic DSS-induced increase in colonic TJ permeability for various-sized probes was significantly attenuated in MMP-12-/- mice. Also, the severity of DSS colitis, as assessed by loss of body weight, clinical disease activity scores, pro-inflammatory cytokine expression, and colonic histological scores of inflammation was significantly diminished in MMP-12-/- DSS mice compared with WT DSS mice. To our knowledge, this is a first report of acute or chronic DSS colitis in MMP-12 knockout mice. The analysis of select TJ proteins indicated overall preservation of TJ composition during the DSS-induced colitis in MMP-12-/- mice. Thus, reduced increase in the colonic TJ permeability was associated with the reduced severity of DSS colitis in MMP-12-/- mice, compared with WT mice. Beside the genetic deletion of MMP-12, pharmacological inhibition of MMP-12 also attenuated DSS-induced increase in mouse colonic TJ permeability, supporting the role of MMP-12 in increasing intestinal TJ permeability during inflammation. The beneficial effect of MMP-12 in in-vitro wound healing possibly indicates the physiological role of MMP-12 during wound healing, in the absence of inflammation. In later stage of inflammation, however, inhibition of MMP-12 attenuates the loss of TJ barrier. Moreover, morphological evidence of wound healing does not directly correspond to the recovery of TJ barrier,78 and the role of MMP-12 in wound healing needs to be studied further in in-vivo studies.
To further confirm MMP-12-mediated macrophage transmigration and possible increase in TJ permeability during this process as seen in mouse colon, we used a relevant in vitro model where macrophages were placed on the basal side of intestinal epithelial Caco-2 cells grown on laminin-rich basement membrane. In the presence of an apical chemotactic gradient, macrophages transmigrated to the apical side of Caco-2 cells. The macrophage transmigration was however significantly reduced when MMP-12 was inhibited in macrophages, genetically or pharmacologically. Also, importantly, the epithelial TJ permeability was found to be increased during the macrophage transmigration. Transmigration of macrophages across Caco-2 monolayers has been reported previously in response to chemoattractants adenosine and uridine 5’-triphosphate.79 Also, in mouse intestine, macrophages have been shown to transmigrate in early response to luminal presence of Salmonella spp.80 Though this mechanism would capture pathogen before trespassing into the intestinal epithelium, it would also lead to the loss of cells that are critical for intestinal immune homeostasis,80,81 potentially making it an aberrant immune response. Though exact mechanism is not known, our study clearly indicates that macrophage transmigration is associated with the loss of basement membrane laminin and intestinal TJ barrier. Transmigrating macrophages may mechanically cause TJ barrier disruption during the transmigration, or it may be the result of signalling events initiated due to the presence of activated macrophages in the proximity of epithelial cells in the basolateral domain. Consistent with the numerous reports on the critical role of macrophages in intestinal homeostasis, excessive macrophage activation and infiltration may contribute to intestinal damage, as suggested by the reduced severity of DSS colitis and reduced presence of activated macrophages in the colonic mucosa of MMP-12-/- mice in our study.
In summary, we have provided support for inflammatory macrophages being a primary driver of intestinal inflammation and we demonstrate how macrophages, through MMP-12, disrupt the colonic basement membrane and TJ barrier. Macrophages are considered a major cell type driving IBD pathogenesis and, with current therapeutics targeting the key inflammatory cytokines produced by macrophages, our work demonstrates an additional mediator of intestinal inflammation. Understanding the complete arsenal of inflammatory macrophages allows us to understand the numerous ways in which macrophages cause intestinal damage, as well as to identify new interventions against intestinal inflammation.
Funding
This research work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases grants DK100562 [PN], DK114024 [PN], and DK-106072 [TM]. MN is supported by Crohn’s & Colitis Foundation Award 694583. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Conflict of Interest
No conflicts of interest, financial or otherwise, are declared by the authors.
Author Contributions
MN, TM, and PN: concept and design of research; MN, ASG, KS, ES, and PN performed experiments; MN, ASG, KS, EFC, and PN analysed data; MN, ASG, EFC, KS, AG, SS, TM, and PN interpreted results of experiments; MN, KS, EFC, and PN prepared figures and drafted the manuscript; MN, TM, AG, SS, EFC, and PN edited and revised manuscript. All authors approved the final version of the manuscript.
Supplementary Material
Acknowledgements
The authors thank the Imaging and Animal Facility cores at the Penn State College of Medicine for their excellent technical assistance.
References
- 1. Arnott ID, Kingstone K, Ghosh S. Abnormal intestinal permeability predicts relapse in inactive Crohn disease. Scand J Gastroenterol 2000;35:1163–9. [DOI] [PubMed] [Google Scholar]
- 2. Arrieta MC, Madsen K, Doyle J, Meddings J. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut 2009;58:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 2009;9:799–809. [DOI] [PubMed] [Google Scholar]
- 4. Nighot P, Young K, Nighot M, et al. Chloride channel ClC-2 is a key factor in the development of DSS-induced murine colitis. Inflamm Bowel Dis 2013;19:2867–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ma T, Nighot P, Al-sadi R. Tight junctions and the intestinal barrier. In: Said H, editor. Physiology of the Gastrointestinal Tract. 6th edn. Cambridge, MA: Academic Press; 2018: 587–640. [Google Scholar]
- 6. Wyatt J, Vogelsang H, Hübl W, Waldhöer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn’s disease. Lancet 1993;341:1437–9. [DOI] [PubMed] [Google Scholar]
- 7. Ravi A, Garg P, Sitaraman SV. Matrix metalloproteinases in inflammatory bowel disease: boon or a bane? Inflamm Bowel Dis 2007;13:97–107. [DOI] [PubMed] [Google Scholar]
- 8. Medina C, Radomski MW. Role of matrix metalloproteinases in intestinal inflammation. J Pharmacol Exp Ther 2006;318:933–8. [DOI] [PubMed] [Google Scholar]
- 9. Naito Y, Yoshikawa T. Role of matrix metalloproteinases in inflammatory bowel disease. Mol Aspects Med 2005;26:379–90. [DOI] [PubMed] [Google Scholar]
- 10. Garg P, Vijay-Kumar M, Wang L, Gewirtz AT, Merlin D, Sitaraman SV. Matrix metalloproteinase-9-mediated tissue injury overrides the protective effect of matrix metalloproteinase-2 during colitis. Am J Physiol Gastrointest Liver Physiol 2009;296:G175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Medina C, Videla S, Radomski A, et al. Increased activity and expression of matrix metalloproteinase-9 in a rat model of distal colitis. Am J Physiol Gastrointest Liver Physiol 2003;284:G116–22. [DOI] [PubMed] [Google Scholar]
- 12. Corry DB, Kiss A, Song LZ, et al. Overlapping and independent contributions of MMP2 and MMP9 to lung allergic inflammatory cell egression through decreased CC chemokines. FASEB J 2004;18:995–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Medina C, Santana A, Paz MC, et al. Matrix metalloproteinase-9 modulates intestinal injury in rats with transmural colitis. J Leukoc Biol 2006;79:954–62. [DOI] [PubMed] [Google Scholar]
- 14. Van den Steen PE, Proost P, Wuyts A, Van Damme J, Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood 2000;96:2673–81. [PubMed] [Google Scholar]
- 15. Shapiro SD, Kobayashi DK, Ley TJ. Cloning and characterization of a unique elastolytic metalloproteinase produced by human alveolar macrophages. J Biol Chem 1993;268:23824–9. [PubMed] [Google Scholar]
- 16. Gronski TJ Jr, Martin RL, Kobayashi DK, et al. Hydrolysis of a broad spectrum of extracellular matrix proteins by human macrophage elastase. J Biol Chem 1997;272:12189–94. [DOI] [PubMed] [Google Scholar]
- 17. Shipley JM, Wesselschmidt RL, Kobayashi DK, Ley TJ, Shapiro SD. Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc Natl Acad Sci U S A 1996;93:3942–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saarialho-Kere U, Kerkelä E, Jeskanen L, et al. Accumulation of matrilysin [MMP-7] and macrophage metalloelastase [MMP-12] in actinic damage. J Invest Dermatol 1999;113:664–72. [DOI] [PubMed] [Google Scholar]
- 19. Vaalamo M, Kariniemi AL, Shapiro SD, Saarialho-Kere U. Enhanced expression of human metalloelastase [MMP-12] in cutaneous granulomas and macrophage migration. J Invest Dermatol 1999;112:499–505. [DOI] [PubMed] [Google Scholar]
- 20. Suomela S, Kariniemi AL, Snellman E, Saarialho-Kere U. Metalloelastase [MMP-12] and 92-kDa gelatinase [MMP-9] as well as their inhibitors, TIMP-1 and -3, are expressed in psoriatic lesions. Exp Dermatol 2001;10:175–83. [DOI] [PubMed] [Google Scholar]
- 21. Matsumoto S, Kobayashi T, Katoh M, et al. Expression and localization of matrix metalloproteinase-12 in the aorta of cholesterol-fed rabbits: relationship to lesion development. Am J Pathol 1998;153:109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Curci JA, Liao S, Huffman MD, Shapiro SD, Thompson RW. Expression and localization of macrophage elastase [matrix metalloproteinase-12] in abdominal aortic aneurysms. J Clin Invest 1998;102:1900–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Belvisi MG, Bottomley KM. The role of matrix metalloproteinases [MMPs] in the pathophysiology of chronic obstructive pulmonary disease [COPD]: a therapeutic role for inhibitors of MMPs? Inflamm Res 2003;52:95–100. [DOI] [PubMed] [Google Scholar]
- 24. Nénan S, Boichot E, Lagente V, Bertrand CP. Macrophage elastase [MMP-12]: a pro-inflammatory mediator? Mem Inst Oswaldo Cruz 2005;100[Suppl 1]:167–72. [DOI] [PubMed] [Google Scholar]
- 25. Pender SL, Li CK, Di Sabatino A, Sabatino AD, MacDonald TT, Buckley MG. Role of macrophage metalloelastase in gut inflammation. Ann N Y Acad Sci 2006;1072:386–8. [DOI] [PubMed] [Google Scholar]
- 26. Vaalamo M, Karjalainen-Lindsberg ML, Puolakkainen P, Kere J, Saarialho-Kere U. Distinct expression profiles of stromelysin-2 [MMP-10], collagenase-3 [MMP-13], macrophage metalloelastase [MMP-12], and tissue inhibitor of metalloproteinases-3 [TIMP-3] in intestinal ulcerations. Am J Pathol 1998;152:1005–14. [PMC free article] [PubMed] [Google Scholar]
- 27. Salmela MT, MacDonald TT, Black D, et al. Upregulation of matrix metalloproteinases in a model of T cell mediated tissue injury in the gut: analysis by gene array and in situ hybridisation. Gut 2002;51:540–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Di Sabatino A, Saarialho-Kere U, Buckley MG, et al. Stromelysin-1 and macrophage metalloelastase expression in the intestinal mucosa of Crohn’s disease patients treated with infliximab. Eur J Gastroenterol Hepatol 2009;21:1049–55. [DOI] [PubMed] [Google Scholar]
- 29. Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium [DSS]-induced colitis in mice. Curr Protoc Immunol 2014;104:15.25.1–15.25.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Randhawa PK, Singh K, Singh N, Jaggi AS. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J Physiol Pharmacol 2014;18:279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jurjus AR, Khoury NN, Reimund JM. Animal models of inflammatory bowel disease. J Pharmacol Toxicol Methods 2004;50:81–92. [DOI] [PubMed] [Google Scholar]
- 32. Gaudio E, Taddei G, Vetuschi A, et al. Dextran sulfate sodium [DSS] colitis in rats: clinical, structural, and ultrastructural aspects. Dig Dis Sci 1999;44:1458–75. [DOI] [PubMed] [Google Scholar]
- 33. Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc 2007;2:541–6. [DOI] [PubMed] [Google Scholar]
- 34. Ye D, Guo S, Al-Sadi R, Ma TY. MicroRNA regulation of intestinal epithelial tight junction permeability. Gastroenterology 2011;141:1323–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nighot PK, Blikslager AT. ClC-2 regulates mucosal barrier function associated with structural changes to the villus and epithelial tight junction. Am J Physiol Gastrointest Liver Physiol 2010;299:G449–56. [DOI] [PubMed] [Google Scholar]
- 36. Nighot PK, Moeser A, Ali RA, Blikslager AT, Koci MD. Astrovirus infection induces sodium malabsorption and redistributes sodium hydrogen exchanger expression. Virology 2010;401:146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fernando EH, Gordon MH, Beck PL, MacNaughton WK. Inhibition of intestinal epithelial wound healing through protease-activated receptor-2 activation in Caco2 cells. J Pharmacol Exp Ther 2018;367:382–92. [DOI] [PubMed] [Google Scholar]
- 38. Nighot PK, Hu CA, Ma TY. Autophagy enhances intestinal epithelial tight junction barrier function by targeting claudin-2 protein degradation. J Biol Chem 2015;290:7234–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol 2008;Chapter 14:Unit 14 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weigmann B, Tubbe I, Seidel D, Nicolaev A, Becker C, Neurath MF. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat Protoc 2007;2:2307–11. [DOI] [PubMed] [Google Scholar]
- 41. Curato C, Bernshtein B, Aychek T, Jung S. In vivo analysis of intestinal mononuclear phagocytes. Methods Mol Biol 2016;1423:255–68. [DOI] [PubMed] [Google Scholar]
- 42. Isidro RA, Appleyard CB. Colonic macrophage polarization in homeostasis, inflammation, and cancer. Am J Physiol Gastrointest Liver Physiol 2016;311:G59–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Geem D, Medina-Contreras O, Kim W, Huang CS, Denning TL. Isolation and characterization of dendritic cells and macrophages from the mouse intestine. J Vis Exp 2012.. PMID: 22644046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Flemming S, Luissint AC, Nusrat A, Parkos CA. Analysis of leukocyte transepithelial migration using an in vivo murine colonic loop model. JCI Insight 2018;3:e99722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shen L, Black ED, Witkowski ED, et al. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J Cell Sci 2006;119:2095–106. [DOI] [PubMed] [Google Scholar]
- 46. Turner JR, Rill BK, Carlson SL, et al. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol 1997;273:C1378–85. [DOI] [PubMed] [Google Scholar]
- 47. Yuhan R, Koutsouris A, Savkovic SD, Hecht G. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology 1997;113:1873–82. [DOI] [PubMed] [Google Scholar]
- 48. Smith P, Mangan NE, Walsh CM, et al. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J Immunol 2007;178:4557–66. [DOI] [PubMed] [Google Scholar]
- 49. Qualls JE, Kaplan AM, van Rooijen N, Cohen DA. Suppression of experimental colitis by intestinal mononuclear phagocytes. J Leukoc Biol 2006;80:802–15. [DOI] [PubMed] [Google Scholar]
- 50. Chandler S, Cossins J, Lury J, Wells G. Macrophage metalloelastase degrades matrix and myelin proteins and processes a tumour necrosis factor-alpha fusion protein. Biochem Biophys Res Commun 1996;228:421–9. [DOI] [PubMed] [Google Scholar]
- 51. Simon-Assmann P, Lefebvre O, Bellissent-Waydelich A, Olsen J, Orian-Rousseau V, De Arcangelis A. The laminins: role in intestinal morphogenesis and differentiation. Ann N Y Acad Sci 1998;859:46–64. [DOI] [PubMed] [Google Scholar]
- 52. Barrett JC, Lee JC, Lees CW, et al. ; UK IBD Genetics Consortium; Wellcome Trust Case Control Consortium 2 . Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat Genet 2009;41:1330–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schmehl K, Florian S, Jacobasch G, Salomon A, Körber J. Deficiency of epithelial basement membrane laminin in ulcerative colitis affected human colonic mucosa. Int J Colorectal Dis 2000;15:39–48. [DOI] [PubMed] [Google Scholar]
- 54. Spenlé C, Hussenet T, Lacroute J, et al. Dysregulation of laminins in intestinal inflammation. Pathol Biol [Paris] 2012;60:41–7. [DOI] [PubMed] [Google Scholar]
- 55. Wolf M, Maltseva I, Clay SM, Pan P, Gajjala A, Chan MF. Effects of MMP12 on cell motility and inflammation during corneal epithelial repair. Exp Eye Res 2017;160:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Na YR, Stakenborg M, Seok SH, Matteoli G. Macrophages in intestinal inflammation and resolution: a potential therapeutic target in IBD. Nat Rev Gastroenterol Hepatol 2019;16:531–43. [DOI] [PubMed] [Google Scholar]
- 57. Bernardo D, Marin AC, Fernández-Tomé S, et al. Human intestinal pro-inflammatory CD11chighCCR2+CX3CR1+ macrophages, but not their tolerogenic CD11c-CCR2-CX3CR1- counterparts, are expanded in inflammatory bowel disease. Mucosal Immunol 2018;11:1114–26. [DOI] [PubMed] [Google Scholar]
- 58. Bain CC, Scott CL, Uronen-Hansson H, et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol 2013;6:498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zigmond E, Varol C, Farache J, et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity 2012;37:1076–90. [DOI] [PubMed] [Google Scholar]
- 60. Kamada N, Hisamatsu T, Okamoto S, et al. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest 2008;118:2269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zigmond E, Bernshtein B, Friedlander G, et al. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity 2014;40:720–33. [DOI] [PubMed] [Google Scholar]
- 62. Wendelsdorf K, Bassaganya-Riera J, Hontecillas R, Eubank S. Model of colonic inflammation: immune modulatory mechanisms in inflammatory bowel disease. J Theor Biol 2010;264:1225–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dahan S, Rabinowitz KM, Martin AP, Berin MC, Unkeless JC, Mayer L. Notch-1 signaling regulates intestinal epithelial barrier function, through interaction with CD4+ T cells, in mice and humans. Gastroenterology 2011;140:550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zheng X, Tsuchiya K, Okamoto R, et al. Suppression of hath1 gene expression directly regulated by hes1 via notch signaling is associated with goblet cell depletion in ulcerative colitis. Inflamm Bowel Dis 2011;17:2251–60. [DOI] [PubMed] [Google Scholar]
- 65. Wehkamp J, Salzman NH, Porter E, et al. Reduced Paneth cell alpha-defensins in ileal Crohn’s disease. Proc Natl Acad Sci U S A 2005;102:18129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gersemann M, Becker S, Kübler I, et al. Differences in goblet cell differentiation between Crohn’s disease and ulcerative colitis. Differentiation 2009;77:84–94. [DOI] [PubMed] [Google Scholar]
- 67. Okamoto R, Tsuchiya K, Nemoto Y, et al. Requirement of Notch activation during regeneration of the intestinal epithelia. Am J Physiol Gastrointest Liver Physiol 2009;296:G23–35. [DOI] [PubMed] [Google Scholar]
- 68. Neurath MF. Current and emerging therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol 2017;14:269–78. [DOI] [PubMed] [Google Scholar]
- 69. Grainger JR, Konkel JE, Zangerle-Murray T, Shaw TN. Macrophages in gastrointestinal homeostasis and inflammation. Pflugers Arch 2017;469:527–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang SW, Bai YF, Weng YY, et al. Cinobufacini ameliorates dextran sulfate sodium-induced colitis in mice through inhibiting M1 macrophage polarization. J Pharmacol Exp Ther 2019;368:391–400. [DOI] [PubMed] [Google Scholar]
- 71. Mai CT, Wu MM, Wang CL, Su ZR, Cheng YY, Zhang XJ. Palmatine attenuated dextran sulfate sodium [DSS]-induced colitis via promoting mitophagy-mediated NLRP3 inflammasome inactivation. Mol Immunol 2019;105:76–85. [DOI] [PubMed] [Google Scholar]
- 72. Pender SL, Tickle SP, Docherty AJ, Howie D, Wathen NC, MacDonald TT. A major role for matrix metalloproteinases in T cell injury in the gut. J Immunol 1997;158:1582–90. [PubMed] [Google Scholar]
- 73. Li W, Li J, Wu Y, et al. A selective matrix metalloprotease 12 inhibitor for potential treatment of chronic obstructive pulmonary disease [COPD]: discovery of [S]-2-[8-[methoxycarbonylamino]dibenzo[b,d]furan-3-sulfonamido]-3-methylbutanoic acid [MMP408]. J Med Chem 2009;52:1799–802. [DOI] [PubMed] [Google Scholar]
- 74. Yamada S, Wang KY, Tanimoto A, et al. Matrix metalloproteinase 12 accelerates the initiation of atherosclerosis and stimulates the progression of fatty streaks to fibrous plaques in transgenic rabbits. Am J Pathol 2008;172:1419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Adair-Kirk TL, Senior RM. Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol 2008;40:1101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Klepsch V, Gerner RR, Klepsch S, et al. Nuclear orphan receptor NR2F6 as a safeguard against experimental murine colitis. Gut 2018;67:1434–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nighot P, Al-Sadi R, Rawat M, Guo S, Watterson DM, Ma T. Matrix metalloproteinase 9-induced increase in intestinal epithelial tight junction permeability contributes to the severity of experimental DSS colitis. Am J Physiol Gastrointest Liver Physiol 2015;309:G988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nighot PK, Moeser AJ, Ryan KA, Ghashghaei T, Blikslager AT. ClC-2 is required for rapid restoration of epithelial tight junctions in ischemic-injured murine jejunum. Exp Cell Res 2009;315:110–8. [DOI] [PubMed] [Google Scholar]
- 79. Langlois C, Gendron FP. Promoting MPhi transepithelial migration by stimulating the epithelial cell P2Y[2] receptor. Eur J Immunol 2009;39:2895–905. [DOI] [PubMed] [Google Scholar]
- 80. Man AL, Gicheva N, Regoli M, et al. CX3CR1+ cell-mediated salmonella exclusion protects the intestinal mucosa during the initial stage of infection. J Immunol 2017;198:335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Regoli M, Bertelli E, Gulisano M, Nicoletti C. The multifaceted personality of intestinal CX3CR1+ macrophages. Trends Immunol 2017;38:879–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.