Abstract
Coronavirus disease 2019 (COVID-19) is a systemic disease that can be life-threatening involving immune and inflammatory responses, and that can result in potentially lethal complications, including venous thrombo-embolism (VTE). Forming an integrative approach to thrombo-prophylaxis and coagulation treatment for COVID-19 patients ensues. We aim at reviewing the literature for anticoagulation in the setting of COVID-19 infection to provide a summary on anticoagulation for this patient population. COVID-19 infection is associated with a state of continuous inflammation, which results in macrophage activation syndrome and an increased rate of thrombosis. Risk assessment models to predict the risk of thrombosis in critically ill patients have not yet been validated. Currently published guidelines suggest the use of prophylactic intensity over intermediate intensity or therapeutic intensity anticoagulant for patients with critical illness or acute illness related to COVID-19 infection. Critically ill COVID-19 patients who are diagnosed with acute VTE are considered to have a provoking factor, and, therefore, treatment duration should be at least 3 months. Patients with proximal deep venous thrombosis or pulmonary embolism should receive parenteral over oral anticoagulants with low-molecular-weight heparin or fondaparinux preferred over unfractionated heparin. In patients with impending hemodynamic compromise due to PE, and who are not at increased risk for bleeding, reperfusion may be necessary. Internists should remain updated on new emerging evidence regarding anticoagulation for COVID-19 patients. Awaiting these findings, we invite internists to perform individualized decisions that are unique for every patient and to base them on clinical judgment for risk assessment.
Keywords: deep venous thrombosis, pulmonary embolism, COVID-19, anticoagulants, direct oral anticoagulants, heparins
Introduction
According to the World Health Organization, coronavirus disease 2019 (COVID-19) has become a Public Health Emergency of International Concern with more than 32 million individuals affected worldwide. 1 COVID-19 disease involves various systems, including the cardiovascular, gastrointestinal, respiratory, nervous, hematopoietic, and immune systems. It is, therefore, a systemic disease that can be life-threatening involving immune and inflammatory responses, manifested by endothelial cell dysfunction, complement activation, and a hypercoagulable state.2-6 This can result in potentially lethal complications even in young healthy individuals, including disseminated intravascular coagulopathy, myocarditis, and venous thrombo-embolism.4,7-9
Understanding the hematologic findings of patients with severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) virus infection is essential to promote their care and improve outcomes. Hematologic findings include lymphopenia, thrombocytopenia/thrombocytosis, and elevated D-dimer. Hypercoagulability is a frequent finding in COVID-19 disease especially among hospitalized patients where it can predict worsening outcomes and mortality. More so, the incidence of venous thrombo-embolism (VTE) and pulmonary embolism (PE) is greater in this patient population when compared to other patients with an acute illness.10-12 In the setting of the magnitude and hematologic consequences of this pandemic on 1 hand, and the absence of clear consensus regarding anticoagulation (AC) management on the other, forming an integrative approach to thrombo-prophylaxis and coagulation treatment for COVID-19 disease ensues.
In this paper, we aim at reviewing the literature for AC in the setting of COVID-19 disease to provide a summary of AC for this patient population.
Background and Significance
PE in the setting of COVID-19 pneumonia ranks the second-highest among the causes of death in patients with SARS-COV-2 virus infection after pneumonia itself. According to 80 autopsy results published in a large series by Edler et al incidence was 21% and 11% for massive and symptomatic peripheral PE, respectively, and 19% of cases had a deep venous thrombosis (DVT) without PE. Moreover, a comparison of autopsies from 7 patients who died from COVID-19 infection to 7 others who died from H1N1 influenza infection showed a 9-fold increased prevalence of alveolar capillaries microthrombi (P < .001). 13 Moreover, Cui et al 14 showed a 25% incidence of symptomatic VTE detected by CT pulmonary angiography and/or ultrasonography among patients with severe SARS-COV-2 virus infection requiring an intensive care unit (ICU) admission, 40% of whom died. This parallels published data from a Dutch cohort of184 COVID-19 ICU patients in which the incidence of symptomatic VTE detected by CT pulmonary angiography was high reaching 49% with the majority involving segmental and subsegmental pulmonary arteries. 15 A lower incidence, 21%, was reported from an Italian cohort of 388 patients. 27.6% were treated in ICU and 6.6% on the general wards. 16 Interestingly, 38% of patients who died at home had VTE, which also indicates that the risk for VTE is not only dependent on hospitalization. 17 These findings imply that COVID-19 disease imposes a hypercoagulable state on patients, thus carrying a higher incidence of VTE than other types of severe pneumonia.
COVID-19-associated hypercoagulable state stems from the patient's inflammatory response to the SARS-CoV-2 and innate immune activation. “Physiological immunothrombosis” refers to the intricate relationship between hemostasis and the immune system where the 2 systems complement each other and provide host defense and limit the dissemination of pathogenic organisms. Dysregulation of physiological immuno-thrombosis can result in excessive formation of immunologically mediated thrombi that predominantly affect the microvasculature. It is proposed to be an important pathological mechanism in patients with COVID-19, whereby innate immune cell activation and endothelial dysfunction contribute to the observed prothrombotic state. 18 Moreover, it has been recently shown that COVID-19 pathogenesis is associated with coagulopathy that differs from sepsis-associated disseminated intravascular coagulation (DIC). Unlike sepsis-induced coagulopathy and DIC, which are manifestations of systemic coagulopathy, the newly introduced entity “Pulmonary Intravascular Coagulopathy” is a manifestation of a local coagulation disorder in the lung. It is a kind of immune thrombosis that is distinct from the classical DIC and with pathological findings showing lungs that are edematous with patchy hemorrhage with diffuse alveolar damage and extensive fibrin thrombi in distended small vessels and capillaries. 19 Similar to the acute phase of other viral infections, adaptive immunity initially results in increased fibrin deposition. COVID-19 disease is associated with a state of continuous inflammation, which results in macrophage activation syndrome (MAS) and cytokine release syndrome. Cytokine release is a major predisposing factor to acute respiratory distress syndrome (ARDS) and multiorgan failure, and MAS results in a pro-inflammatory pathway that results in an increased rate of thrombosis.8,11,20,21 While the exact mechanism through which COVID-19 disease carries a hypercoagulable prothrombotic state remains to be better delineated, activation of the above coagulative cascade can favor arterial and venous thrombosis and can predispose to DIC. 12
With the increased incidence of DVT and VTE in SARS-COV-2 infected patients on the one hand and the morbidity and mortality of the associated hypercoagulable state on the other, the need for prophylactic and therapeutic AC ensues. Development of such strategies should aim at the management of “endotheliits,” or endothelial inflammation, inhibition of serine proteases expressed by the virus, and inhibition of the complement and the intrinsic pathways of blood coagulation in hemostasis. 11 An integrative approach to AC for the physicians consists of thrombo-prophylaxis, therapeutic AC, and thrombolysis.
Thrombo-Prophylaxis for COVID-19 Disease
There is no clear consensus regarding the most appropriate choice of anticoagulant for thrombo-prophylaxis in SARS-COV-2 infected patients. Several drugs are used, including low-molecular-weight heparin (LMWH), unfractionated heparin (UFH), vitamin K antagonists, or direct oral anticoagulants (DOACs). In addition to their anticoagulant properties, heparins have an anti-inflammatory effect. 22 There are not yet clear guidelines regarding the best agent and selection is based on the availability of the anticoagulant, familiarity of the medical team and patient, in addition to patient-related factors, namely creatinine clearance, history of heparin-induced thrombocytopenia, risk of gastrointestinal bleed, and medical conditions that can compromise gastrointestinal tract absorption. 23
Despite the increased incidence of DVT and VTE in SARS-COV-2 infected patients, the optimal thrombo-prophylaxis dose remains unclear. A retrospective study by Artifoni et al 24 showed a 22.5% incidence of VTE and a 10% incidence of PE among noncritically ill SARS-COV-2 infected patients despite thrombo-prophylaxis. As a result, they recommended that hospitalized patients with COVID-19 disease generally receive a higher therapeutic dose of LMWH of 1 mg/kg twice daily than the usual recommended prophylaxis dose of 0.5 mg/kg daily. Cassini et al recommended the use of prophylactic dose anticoagulant in the acute setting of COVID-19 disease in patients who are hospitalized. 25 On the other hand, Flumignan et al conducted a Cochrane Database Systematic review that included 7 retrospective nonrandomized studies including hospitalized SARS-COV-2 infected patients. They concluded that there is insufficient evidence to determine the risks and benefits of prophylactic anticoagulants for this patient population. There are 22 ongoing studies that aim at evaluating more than 15,000 participants in this setting, which indicates that more evidence is expected to be published.11,21
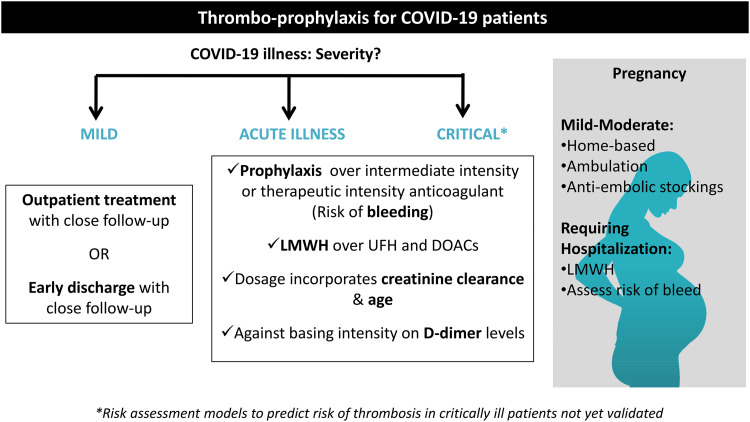
For patients with mild COVID-19 disease, outpatient treatment or early discharge may be considered, with close follow-up. 26 The American Society of Hematology (ASH) suggests the use of prophylactic intensity over intermediate intensity or therapeutic intensity anticoagulant for patients with critical illness related to SARS-COV-2 viral infection and who do not have suspected or confirmed DVT/PE. As for patients with acute illness related to COVID-19 disease who do not satisfy critical illness and who do not have suspected or confirmed DVT/PE, the ASH also suggests the use of prophylaxis intensity over intermediate intensity or therapeutic intensity anticoagulant. 23 This suggestion was based on the results of 3 international clinical trials, namely the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia, Accelerating COVID-19 Therapeutic Interventions and Vaccines-4, and Antithrombotic Therapy to Ameliorate Complications of COVID-19.27,28 The common primary objective of these trials was to evaluate for the benefit of full intensity anticoagulant compared to prophylactic dose anticoagulant in patients with moderately-critically ill COVID-19 disease. Critical illness related to the SARS-COV-2 virus was defined as a life-threatening condition requiring advanced clinical care and support through admission to an ICU or coronary care unit (CCU). On the other hand, patients with acute illness were those requiring hospitalization without advanced clinical care and support and, thus, not requiring admission to ICU or CCU. 29
The ASH recommendations acknowledge that these results were based on a planned interim analysis and that physicians should remain updated on further results that may arise and should weigh the benefit versus harms of AC when doing so. 23 Individualized decision that is unique for every patient should be made based on risk assessment. While there are risk assessment models to predict the risk of thrombosis in critically ill patients, they have not yet been validated for COVID-19 disease.30-33 Patients with COVID-19 disease generally have elevated levels of D-dimer, C-reactive protein, and erythrocyte sedimentation rate. Whether these parameters should be used to risk-stratify these patients for thrombosis or whether they should affect a decision on thrombo-prophylaxis remains unclear. 12 Interestingly, while D-dimer is not included in the ASH guidelines and was considered as useful to be monitored according to the International Society on Thrombosis and Hemostasis (ISTH), the AC panel recommends against monitoring D-dimer serial levels and against increasing the dose intensity of AC depending on these levels.23,34
Physicians should assess for the risk of thrombosis and the risk of bleed when making their decision regarding the intensity of AC in moderately or critically ill patients. ASH acknowledges that physicians can choose to give higher-intensity anticoagulants for patients who are at high risk of thrombosis and with low risk of bleeding.12,23 To date, there is no strong evidence supporting the use of therapeutic or intermediate intensity AC in thrombo-prophylaxis for COVID-19 disease. There were 5 observational studies that included patients with SARS-COV-2 infection who were critically ill. These studies reported the correlation between therapeutic AC and mortality, between intermediate intensity AC and risk of PE, between intermediate intensity AC and risk of DVT, or between therapeutic intensity AC and the risk of DVT or PE, respectively.35-39 None of the studies, however, reported the relationship between therapeutic or intermediate intensity AC and complications, such as multiorgan failure or intracranial hemorrhage. Therapeutic AC reduced all-cause mortality with an odds ratio of 0.73 (95% confidence interval [CI], 0.33 - 1.76), which translated to 52 less deaths per 1000 patients. 36 It also resulted in a decrease in the risk of VTE with an OR of 0.87 [95% CI, 0.45 - 1.67], translating to a small reduction in the number of affected cases by 15 patients per 1000.34,35 Intermediate intensity AC was associated with a decrease in the risk of PE with an OR of 0.09 [95% CI, 0.02 - 0.57], which translated to 88 less cases of PE per 1000 patients. 39 It was associated with a decrease in the risk of DVT with an OR of 0.35 [95% CI, 0.06 - 2.02], which translated to 66 less cases of DVT per 1000 patients. 38 Therefore, there was only a small absolute risk reduction for DVT and PE for patients receiving intermediate or therapeutic intensity AC with a low reduction in mortality and incidence as compared to prophylaxis dose anticoagulant.
Not only there is no strong evidence regarding the use of therapeutic intensity AC in thrombo-prophylaxis for COVID-19 disease, but also it can be associated with an increased risk of bleeding. Pesavento et al 40 showed that it may increase the risk for major bleed with an adjusted hazard ratio (HR) of 3.89 [95% CI, 1.90 - 7.97] in a cohort of 324 SARS-COV-2 infected patients, 84 of whom were provided with therapeutic dose as thrombo-prophylaxis. This did not support the use of therapeutic AC in noncritically ill patients. Furthermore, a matched case-control study showed an increased incidence of upper and lower gastrointestinal bleed. 41
While the above-mentioned ASH guidelines described recommendations regarding thrombo-prophylaxis, other published guidelines addressed broader clinical questions. While they do recommend prophylactic intensity anticoagulants for acutely ill and critically ill patients infected with the SARS-COV-2 virus, the 2020 CHEST COVID-19 guidelines recommend LMWH over the use of UFH, which requires a twice-daily dose, to limit the exposure of house staff to infected patients. It is worth noting that ASH does acknowledge the importance of attempting at limiting this exposure but does not specify the recommendation of an anticoagulant over another. 42 In addition, they recommend caution from the use of DOACs due to concern regarding drug–drug interaction with other treatments given to SARS-COV-2 infected patients and the potential increased risk of bleeding in case of rapid clinical worsening during the course of the hospitalization. 23
The AC forum interim clinical guidance recommends prophylactic anticoagulants while adjusting the dosage based on creatinine clearance and age. Unlike ASH guidelines, however, the AC forum recommended that patients with critical illness should receive intermediate intensity anticoagulant rather than prophylaxis dose. Their recommendation applies to patients who do not have DIC or who have DIC yet without evidence of bleeding. This recommendation was based on both, expert opinion and extrapolation from published data from studies on AC for critically ill patients in influenza pneumonia, trauma, and bariatric surgeries.43-45 ASH guidelines, on the other hand, do not include DIC as a predictor for clinical outcomes of COVID-19 disease that remains to be better described. ASH also did not address the role of thrombo-prophylaxis following discharge from the hospital nor the role of screening duplex ultrasound in COVID-19 patients who do not have symptoms suggestive of thrombosis. 23
The ISTH-Scientific and Standardization Committee (SSC) interim recommendations suggest that SARS-COV-2 infected patients with acute or critical illness should receive a prophylaxis dose of LMWH or UFH while considering intermediate-dose LMWH for patients who are considered by the physicians to be at an elevated risk for DVT or PE. Moreover, they suggest against the use of therapeutic dose AC until there is more evidence from currently ongoing randomized controlled trials. Interestingly, they recommend considering additional methods, including mechanical intermittent pneumatic compression, with pharmacologic anticoagulants for critically ill patients. This is contrary to critically ill patients who do not have SARS-COV-2 infection, where pharmacologic prophylaxis alone is recommended rather than a combined multimodal approach.23,46,47 Figure 1 shows the approach to thrombo-prophylaxis in COVID-19 disease, and Table 1 compares consensus recommendations by 3 main societies/panels.
Figure 1.
Thrombo-prophylaxis for coronavirus disease 2019 (COVID-19) patients.
Table 1.
Comparison of Consensus Recommendations by 3 Main Societies/Panels.
| American Society of Hematology (ASH) | AC panel | International Society on Thrombosis and Hemostasis (ISTH) | |
|---|---|---|---|
| D-dimer |
|
|
|
| DIC |
|
|
|
| AC |
|
|
|
|
|
||
| Other Comments |
|
N/A |
|
Abbreviations: DIC, disseminated intravascular coagulation; DVT/PE, deep venous thrombosis/pulmonary embolism; LMWH, low-molecular-weight heparin; UFH, unfractionated heparin; SARS-COV-2, severe acute respiratory syndrome coronavirus-2; AC, anticoagulation; COVID-19, coronavirus disease 2019.
Treatment of Documented VTE in COVID-19 Disease
Management of SARS-COV-2 infected patients with acute PE has not yet been described in the outpatient setting, but the approach to these patients can follow existing guidelines for inpatients. 26 Critically ill patients with COVID-19 disease who are diagnosed with acute DVT or VTE are considered to have a provoking factor, and, therefore, treatment duration should be at least 3 months. Patients with proximal DVT or PE should receive parenteral over oral anticoagulants, with LMWH or fondaparinux preferred over UFH. The decision to use UFH should include the risk of house staff exposure to patients with COVID-19 disease as it requires twice-daily dosing. In patients with an elevated risk for bleeding, including those with compromised renal function, UFH is preferred over LMWH or fondaparinux.23,26
SARS-COV-2 infected patients whose PE is complicated by cardiopulmonary complications can have an elevated jugular venous pressure, worsening arterial blood gas, signs of shock, and/or right ventricular strain. Thrombolysis may increase the risk of bleeding, including intracranial hemorrhage and diffuse alveolar hemorrhage. While the ASH guidelines do not address the role of thrombolysis in COVID-19 patients diagnosed with PE, the 2020 CHEST COVID-19 panel recommends against the use of thrombolysis for patients who are diagnosed with PE. However, in patients with impending hemodynamic compromise due to PE, and who are not at increased risk for bleeding, reperfusion may be necessary.23,26 In addition, when a cardiopulmonary arrest is suspected to be the result of a massive PE in the setting of COVID-19 disease, thrombolysis can be considered. However, prior to its use, physicians should attempt at ruling out the possibility of preexisting pulmonary hypertension or severe ARDS as the underlying etiology of right ventricular strain, more than the SARS-COV-2 infection itself, before initiating thrombolysis empirically.11,26
While DOACs are the standard treatments for VTE and atrial fibrillation, their use in hospitalized patients with COVID-19 disease is not straightforward. ISTH did not mention recommendations regarding the use of DOACs in COVID-19 disease, and DOACs were not a preferable option in 7 recently published guidelines on AC in this patient population.48,49 As such, LMWH and UFH are preferable over DOACs in patients with SARS-COV-2 infection. DOACs interact with cytochrome P450 (CYP)-based metabolic pathways. A variety of drugs can modify the DOAC pharmacodynamic and pharmacokinetic profile thus resulting in modification of their action, either enhancement or reduction, and exposing patients to a risk of uncontrolled bleeding or thrombosis, respectively. These drugs include antivirals, antimicrobials, antihypertensives, bronchodilators, dexamethasone, and immunosuppressive agents. 49 Of an Italian cohort of 1039 patients hospitalized with COVID-19 pneumonia and who received antiviral treatment, 32 were on DOACs 12 of whom remained on DOACs drug during the course of hospitalization. In all of these 12 patients, a marked increase in the DOACs serum level was noted as compared to prehospitalization. 50 Moreover, it is established that IL-6 can downregulate the CYP3A4 enzyme, which suggests that the patient's immune response, characterized by a rise in IL-6, can alter this enzyme. Therefore, blocking IL-6 by tocilizumab or sarilumab, which is the focus of current research, can alter this enzyme, which can, in turn, alter DOACs levels.51,52 In patients who were already on DOACs prior to admission, DOACs should be stopped and switched to LMWH or UFH until antiviral or anti-IL6 treatment is completed. Interestingly, since the effect of dexamethasone on CYP3A4 lasts for around a week, DOACs can be restarted again after discharge with a minimum of 1 week after stopping dexamethasone. 49
Special Populations
Pregnancy induces a hypercoagulable state with up to a 6-fold increased risk of VTE. As such, SARS-COV-2 infection poses a challenge in pregnancy with up to 10% ICU admissions and risk for morbidity and mortality. 52 While there are no clear guidelines for AC in pregnant SARS-COV-2 infected patients, the ISTH conducted a structured literature search for recommendations on AC for this patient population. For pregnant women with mild to moderate COVID-19 disease, mobilization, and management as an outpatient can be done. The use of antiembolic stockings at home may also be encouraged. Pregnant women with COVID-19 disease requiring hospitalization carry an 18-fold increased risk for VTE after discharge, especially when older than 35 years, in the third trimester of pregnancy, and when admitted for 3 days or longer. 53 The Royal College of Obstetricians and Gynecologists group recommends LMWH unless there is a specific contraindication, particularly an increased risk of bleeding.53-56
Hospitalized pregnant women should receive LMWH as prophylaxis for 14 days after which the need for AC should be reassessed according to the risk–benefit balance, which depends on the severity of COVID-19 disease and comorbidities. For women with severe symptoms related to COVID-19 disease and/or with obstetrics complications, prophylactic LMWH should be continued during pregnancy, and up to 6 weeks postpartum. This is of particular importance during the third trimester as there is a greater risk of VTE. For women in which VTE is documented, AC should be continued until 6 weeks postpartum for a minimum of a total duration of 3 months since initiation.57,58
Interestingly, there has been recent concern regarding a possible increased risk for thrombosis following vaccination for the SARS-COV-2 virus. The first to note this was the Austrian National Competent Authority, which reported to the European Medicines Agency (EMA) thrombosis in 4 immunized people, and suspended the use of a batch of the AstraZeneca COVID vaccine. 59 This was followed by several other countries, including Denmark, who suspended its use as well for similar cases. 60 This concern was later transformed to attention to cerebral venous sinus thrombosis, which is a rare condition occurring in around 15 cases per million people and affecting younger adults, women more than men, especially pregnant women or those on hormonal contraception. 61 Other clotting conditions were reported following vaccination, namely arterial thrombosis and splanchnic vein thrombosis. The EMA compared these clinical pictures with heparin-induced thrombocytopenia (HIT) and confirmed their similarity. Patients who were included in these series had antibodies against platelet factor 4 (PF4), as seen in HIT. As a result, a new entity referred to as “Vaccine Induced Immune Thrombotic Thrombocytopenia” was introduced. Potential suggested therapeutic options include high-dose intravenous immunoglobulins and nonheparin anticoagulants.62-64 In addition to the AstraZeneca vaccine, the use of the Johnson & Johnson (Janssen) COVID-19 vaccine was also associated with thrombosis in 6 reported cases to date and to our knowledge. 65 There are no clear guidelines regarding the role of thromboprophylaxis following COVID-19 vaccination, and there is still much that is not known about thrombosis linked to vaccination. It remains, however, the case that the benefits of COVID-19 vaccination outweigh the risks. People should seek vaccination and medical advice at the same time.
Conclusion and Take-Home Messages
Hypercoagulability is a frequent finding in hospitalized patients with COVID-19 disease and can predict worsening outcomes and mortality. More so, the incidence of VTE and PE is greater in this patient population when compared to other patients with an acute illness. While there are risk assessment models to predict the risk of thrombosis in critically ill patients, they have not yet been validated for SARS-COV-2 infection. Table 2 shows a summary of our take-home messages. Currently published guidelines suggest the use of prophylactic intensity over intermediate intensity or therapeutic intensity anticoagulant for patients with critical illness or acute illness related to COVID-19 disease and who do not have suspected or confirmed DVT/PE. Critically ill patients with COVID-19 disease who are diagnosed with acute VTE are considered to have a provoking factor, and, therefore, treatment duration should be at least 3 months. Patients with proximal DVT or PE should receive parenteral over oral anticoagulants with LMWH or fondaparinux preferred over UFH. Physicians should remain updated on new emerging evidence regarding AC for COVID-19 disease, particularly with the currently ongoing studies that are evaluating more than 15,000 participants. Awaiting these findings, we invite physicians to perform individualized decisions that are unique for every patient and to base them on clinical judgment for risk assessment.
Table 2.
Take-Home Messages.
|
Abbreviations: VTE, venous thrombo-embolism; DVT/PE, deep venous thrombosis/pulmonary embolism; LMWH, low-molecular-weight heparin; UFH, unfractionated heparin; SARS-COV-2, severe acute respiratory syndrome coronavirus-2; AC, anticoagulation; COVID-19, coronavirus disease 2019.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Firas Kreidieh https://orcid.org/0000-0003-2751-5154
References
- 1.Boulos MNK, Geraghty EM. Geographical tracking and mapping of coronavirus disease COVID-19/severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) epidemic and associated events around the world: how 21st century GIS technologies are supporting the global fight against outbreaks and epidemics. 2020:1-12.
- 2.World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected—interim guidance, March 13, 2020. Accessed July 26, 2020 at: https://www.who.int/publications/i/item/clinical-management-of-covid-19
- 3.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;95(7):834-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maglakelidze N, Manto KM, Craig TJ. A review: does complement or the contact system have a role in protection or pathogenesis of COVID-19? Pulmonary Therapy. 2020;6(2):169.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(23):2950-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75(18):2352-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet. 2020;395(10229):1033-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M, Ling M. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan. China: A Retrospective Case Series Study. 2020.
- 10.Gerotziafas GT, Catalano M, Colgan MP, et al. Guidance for the management of patients with vascular disease or cardiovascular risk factors and COVID-19: position paper from VAS-European independent foundation in angiology/vascular medicine. Thromb Haemostasis. 2020;120(12):1597-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanff TC, Mohareb AM, Giri J, et al. Thrombosis in COVID-19. Am J Hematol. 2020;95(12):1578-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreidieh F, Temraz S. SARS-CoV-2 infected patient: from a hematologist's perspective. Mediterr J Hematol Infect Dis. 2020;12(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383:120-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui S, Chen S, Li X, et al. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klok FA, Kruip MJ, Van der Meer NJ, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edler C, Schröder AS, Aepfelbacher M, et al. Correction to: dying with SARS-CoV-2 infection—an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Leg Med. 2020;134(4):1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loo J, Spittle DA, Newnham M. COVID-19, immunothrombosis and venous thromboembolism: biological mechanisms. Thorax. 2021;76(4):412-420. [DOI] [PubMed] [Google Scholar]
- 19.Belen-Apak FB, Sarıalioğlu F. Pulmonary intravascular coagulation in COVID-19: possible pathogenesis and recommendations on anticoagulant/thrombolytic therapy. J Thromb Thrombolysis. 2020;50(2):278-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chousterman BG, Swirski FK, Weber GF. Cytokine storm and sepsis disease pathogenesis. In Seminars in Immunopathology. Vol. 39(5). Springer Berlin Heidelberg; 2017. [DOI] [PubMed] [Google Scholar]
- 21.England JT, Abdulla A, Biggs CM, et al. Weathering the COVID-19 storm: lessons from hematologic cytokine syndromes. Blood Rev. 2021;45:100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flumignan RLG, de Sá Tinôco JD, Pascoal PIF, et al. Prophylactic anticoagulants for people hospitalised with COVID-19. Cochrane Database Syst Rev. 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuker A, Tseng EK, Nieuwlaat R, et al. American Society of hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021;5(3):872-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Artifoni M, Danic G, Gautier G, et al. Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: incidence and role of D-dimer as predictive factors. J Thromb Thrombolysis. 2020;50:211-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fontana P, Casini A, Robert-Ebadi H, et al. Venous thromboembolism in COVID-19: systematic review of reported risks and current guidelines. Swiss Med Wkly. 2020;150(w20301):1–9. [DOI] [PubMed] [Google Scholar]
- 26.Moores LK, Tritschler T, Brosnahan S, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest. 2020;158(3):1143-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.https://clinicaltrials.gov/ct2/show/NCT04505774, accessed April 4, 2021.
- 28.https://clinicaltrials.gov/ct2/show/NCT04372589, accessed April 4, 2021.
- 29.https://www.nih.gov/news-events/news-releases/nih-activ-trial-blood-thinners-pausesenrollment-critically-ill-covid-19-patients, accessed April 4, 2021.
- 30.Barbar S, Noventa F, Rossetto V, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua prediction score. J Thromb Haemost. 2010;8(11):2450-2457. [DOI] [PubMed] [Google Scholar]
- 31.Spyropoulos AC, Anderson FA, Jr, FitzGerald G, et al. IMPROVE Investigators. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011;140(3):706-714. [DOI] [PubMed] [Google Scholar]
- 32.Decousus H, Tapson VF, Bergmann J-F, et al. IMPROVE Investigators. Factors at admission associated with bleeding risk in medical patients: findings from the IMPROVE investigators. Chest. 2011;139(1):69-79. [DOI] [PubMed] [Google Scholar]
- 33.Darzi AJ, Repp AB, Spencer FA, et al. Risk-assessment models for VTE and bleeding in hospitalized medical patients: an overview of systematic reviews. Blood Adv. 2020;4(19):4929-4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Llitjos JF, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18(7):1743-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferguson J, Volk S, Vondracek T, Flanigan J, Chernaik A. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: a single health system study. J Clin Pharmacol. 2020;60(11):1411-1415. [DOI] [PubMed] [Google Scholar]
- 37.Fraisse M, Logre E, Pajot O, Mentec H, Plantefève G, Contou D. Thrombotic and hemorrhagic events in critically ill COVID-19 patients: a French monocenter retrospective study. Crit Care. 2020;24(1):275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trigonis RA, Holt DB, Yuan R, et al. Incidence of venous thromboembolism in critically ill coronavirus disease 2019 patients receiving prophylactic anticoagulation. Crit Care Med. 2020;48(9):e805-e808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taccone FS, Gevenois PA, Peluso L, et al. Higher intensity thromboprophylaxis regimens and pulmonary embolism in critically ill coronavirus disease 2019 patients. Crit Care Med. 2020;48(11):e1087-e1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pesavento R, Ceccato D, Pasquetto G, et al. The hazard of (sub)therapeutic doses of anticoagulants in non-critically ill patients with COVID-19: the Padua province experience. J Thromb Haemost. 2020;18(10):2629-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin TA, Wan DW, Hajifathalian K, et al. Gastrointestinal bleeding in patients with coronavirus disease 2019: a matched case-control study. Am J Gastroenterol. 2020;115(10):1609-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Hajizadeh N, Moore EE, et al. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. 2020;18(7):1752-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Obi AT, Tignanelli CJ, Jacobs BN, et al. Empirical systemic anticoagulation is associated with decreased venous thromboembolism in critically ill influenza A H1N1 acute respiratory distress syndrome patients [published correction appears in J VascSurg venous LymphatDisord. 2019;7(4):621]. J VascSurg Venous LymphatDisord. 2019;7(3):317-324. [DOI] [PubMed] [Google Scholar]
- 44.Walker CK, Sandmann EA, Horyna TJ, Gales MA. Increased enoxaparin dosing for venous thromboembolism prophylaxis in general trauma patients. Ann Pharmacother. 2017;51(4):323-331. [DOI] [PubMed] [Google Scholar]
- 45.Ikesaka R, Delluc A, Le Gal G, Carrier M. Efficacy and safety of weight-adjusted heparin prophylaxis for the prevention of acute venous thromboembolism among obese patients undergoing bariatric surgery: a systematic review and meta-analysis. Thromb Res. 2014;133(4):682-687. [DOI] [PubMed] [Google Scholar]
- 46.Schünemann HJ, Cushman M, Burnett AE, et al. American Society of hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arabi YM, Al-Hameed F, Burns KEA, et al. Adjunctive intermittent pneumatic compression for venous thromboprophylaxis. N Engl J Med. 2019;380(14):1305-1315. [DOI] [PubMed] [Google Scholar]
- 48.Flaczyk A, Rosovsky RP, Reed CT, et al. Comparison of published guidelines for management of coagulopathy and thrombosis in critically ill patients with COVID 19: implications for clinical practice and future investigations. Crit Care. 2020;24:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schutgens RE. DOAC in COVID-19: yes or no? HemaSphere. 2021;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Testa S, Prandoni P, Paoletti O, et al. Direct oral anticoagulant plasma levels’ striking increase in severe COVID-19 respiratory syndrome patients treated with antiviral agents: the Cremona experience. J Thromb Haemost. 2020;18:1320-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jover R, Bort R, Gómez-Lechón MJ, Castell JV. Down-regulation of human CYP3A4 by the inflammatory signal interleukin 6: molecular mechanism and transcription factors involved. FASEB J. 2002;16(13):1-29. [DOI] [PubMed] [Google Scholar]
- 52.O’Hare R. Imperial College London. Arthritis Drug Effective in Treating Sickest Covid-19 Patients, 2020. Available at: https://www.imperial.ac.uk/news/209033/arthritis-drug-effective-treating-sickest-covid-19/. Accessed November 30, 2020.
- 53.Kadir RA, Kobayashi T, Iba T, et al. COVID-19 coagulopathy in pregnancy: critical review, preliminary recommendations, and ISTH registry—communication from the ISTH SSC for Women's Health. J Thromb Haemost. 2020;18(11):3086-3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu J, Yuan E, Lee L. Gestational age-specific reference intervals for routine haemostatic assays during normal pregnancy. Clin Chim Acta. 2012;413:258-261. [DOI] [PubMed] [Google Scholar]
- 55.Abdul Sultan A, West J, Tata LJ, Fleming KM, Nelson-Piercy C, Grainge MJ. Risk of first venous thromboembolism in pregnant women in hospital: population based cohort study from England. Br Med J. 2013;347:f6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Greer IA, Nelson-Piercy C. Low-molecular-weight heparins for thromboprophylaxis and treatment of venous thromboembolism in pregnancy: a systematic review of safety and efficacy. Blood. 2005;106:401-407. [DOI] [PubMed] [Google Scholar]
- 57.Wiegers H, Middeldorp S. Contemporary best practice in the management of pulmonary embolism during pregnancy. Ther Adv Respir Dis. 2020;14:1-20. 10.1177/1753466620914222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lou-Mercadé AC, Gavín O, Oros D, et al. Prevention of thrombosis in pregnant women with suspected SARS-CoV-2 infection: clinical management algorithm. Ultrasound Obstet Gynecol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.EMA. Covid-19 vaccine AstraZeneca: PRAC preliminary view suggests no specific issue with batch used in Austria, 10 March 2021. https://www.ema.europa.eu/en/news/covid-19-vaccine-astrazeneca-prac-preliminary-view-suggests-no-specific-issue-batch-used-austria
- 60.AstraZeneca vaccine: timeline of what's happened since European countries suspended use of Covid jab. ITV News 2021 Apr 7. https://www.itv.com/news/2021-04-07/astrazeneca-vaccinetimeline-of-whats-happened-since-european-countries-suspended-use-of-covid-jab
- 61.Medicherla CB, Pauley RA, de Havenon A, Yaghi S, Ishida K, Torres JL. Cerebral venous sinus thrombosis in the COVID-19 pandemic. J Neuroophthalmol. 2020;40:457-462. doi: 10.1097/WNO.0000000000001122. pmid: 33186264. [DOI] [PubMed] [Google Scholar]
- 62.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021. doi: 10.1056/NEJMoa2104840. pmid: 33835769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021. doi: 10.1056/NEJMoa2104882. pmid: 33835768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hunter PR. Thrombosis after COVID-19 vaccination. Br Med J. 2021;373:n958. [DOI] [PubMed] [Google Scholar]
- 65.Centre for Disease Control. DC Joint CDC and FDA Statement on Johnson & Johnson COVID-19 vaccine. https://www.cdc.gov/media/releases/2021/s0413-JJ-vaccine.htm