Abstract
Background:
Although several injection-based treatments have been proposed to address knee osteoarthritis (OA), it is often difficult to understand the clinical relevance of the obtained results. The psychometric measures of minimal clinically important difference (MCID) and Patient Acceptable Symptom State (PASS) were developed to better interpret study findings.
Purpose:
To establish the MCID and the PASS for the International Knee Documentation Committee (IKDC) Subjective score and the Knee injury and Osteoarthritis Outcome Score (KOOS) in patients treated with intra-articular platelet-rich plasma (PRP) injections for knee OA.
Study Design:
Case series; Level of evidence, 4.
Methods:
This study included 215 patients with knee OA (68% men, 32% women; age, 53.2 ± 11.3 years; body mass index, 26.8 ± 4.3 kg/m2) who underwent intra-articular PRP injections. Patients were assessed through the IKDC Subjective score and KOOS subscales, and the MCID and the PASS for both measures were independently calculated at 6 and 12 months post-injection. The MCID was calculated using the value equal to half of the standard deviation of the overall cohort improvement. The PASS was assessed using a 2-point scale (satisfied or not satisfied), with threshold values being detected through a receiver operating characteristic curve analysis and the Youden index to maximize the sensitivity and the specificity of the threshold values.
Results:
All scores improved significantly from baseline to 6 months and baseline to 12 months (P < .001 for all scores). All scores were stable from 6 to 12 months except for the KOOS Quality of Life subscale, which improved further (P = .033). For the IKDC, the MCID values were 8.6 and 8.5 points and the PASS scores were 59.7 and 62.1 at 6 and 12 months, respectively. Overall, the MCID and the PASS for all KOOS subscales remained constant at the 2 follow-up points. The percentage of patients who achieved the MCID and the PASS was higher than 85% at both 6 and 12 months post-injection.
Conclusion:
This study provided the MCID and PASS thresholds for the IKDC and KOOS scores in patients with knee OA treated with PRP injections. These psychometric measures may allow a better interpretation of the clinical relevance of injection-based treatment outcomes for knee OA.
Keywords: minimal clinically important difference, Patient Acceptable Symptom State, knee, osteoarthritis, injection, treatment
Knee osteoarthritis (OA) is a degenerative joint disease causing the loss of articular cartilage, with concomitant structural and functional changes in the other joint tissues. 27 Its incidence increases with age, affecting more than 10% of people older than 60 years and leading to a large societal and economic burden. 17,24 The treatment options for knee OA range from conservative to surgical methods, with intra-articular injections representing an important solution to provide clinical improvement and to possibly delay more invasive operative treatments. 13,18,31,35 Several products have been used as injection-based treatments, including corticosteroids, hyaluronic acid, and new and promising biologic products such as platelet-rich plasma (PRP) and mesenchymal stromal cells. 5,14,20 To date, no consensus has been reached about the use of such treatments because of the heterogeneity in methodologies used and obtained results in different studies. 2 Also, even when focusing on the most commonly applied patient-reported outcome measures (PROMs), such as the International Knee Documentation Committee (IKDC) Subjective score and the Knee injury and Osteoarthritis Outcome Score (KOOS), it is often difficult to understand the clinical relevance of the findings. 38
In fact, a statistically significant improvement on a PROM does not always reflect a clinically meaningful change for the patient, thus not providing robust evidence of treatment efficacy as a base for guidelines in clinical practice. 5,9 To better determine the clinical relevance of a treatment beyond statistical significance, the magnitude of improvement and satisfaction should achieve a threshold value to be perceived by the patient as significant. 16 For this reason, the psychometric measures of minimal clinically important difference (MCID) and Patient Acceptable Symptom State (PASS) have been developed to assist in interpreting PROM scores. The MCID is defined as the smallest difference in a specific PROM score that patients perceive as beneficial, referring to the amount of absolute change in a PROM score that relates to a clinical improvement, while the PASS defines a level of symptoms that discriminate between feeling well and feeling unwell. 21,23 These 2 psychometric measures for the IKDC and the KOOS have been reported in patients undergoing orthopaedic surgical procedures (eg, microfracture or meniscal allograft transplantation 6,7,25 ); however, their threshold when assessing the clinical relevance of injection-based treatments for knee OA is yet to be defined.
The aim of this study was to establish the MCID and PASS threshold values for the IKDC Subjective and KOOS scores in patients treated with intra-articular injections by investigating a large cohort of patients receiving PRP for knee OA.
Methods
Study Design and Patient Selection
The present study was a review of prospectively collected PROMs (IKDC Subjective score and KOOS subscale scores) from a database of patients for the study of knee OA treated with intra-articular PRP injections between March 2009 and March 2019. Institutional review board approval was obtained for the study protocol, and informed consent was obtained at the time of patient enrollment.
PRP treatment was indicated for unilateral symptomatic knee OA with history of chronic pain (at least 6 months) or swelling; early OA findings at imaging evaluation with signs of cartilage degeneration (Kellgren-Lawrence [K-L] grade = 0, detected on magnetic resonance imaging) or OA (K-L grade = 1-4); age between 18 and 80 years; no major axial deviation (varus >5°, valgus >5° for mechanical alignment); no focal chondral or osteochondral lesions; absence of any concomitant knee lesion causing pain or swelling (i.e., ligamentous or meniscal injury); and absence of hematological or cardiovascular diseases, infections, and immunosuppression. 10 PRP procedures consisted of 1 or 3 (1-week interval) intra-articular injections of 5-mL PRP (based on the institutional protocol available at the time of patient recruitment), which was frozen and activated with calcium gluconate, with a platelet concentration of 4 to 5 times higher than baseline whole blood values, including both PRP with and without leukocytes.
Patients were assessed through the IKDC Subjective score and KOOS subscales independently calculated and reported at the baseline and at 6 and 12 months after the injection. Baseline variables, including age, sex, body mass index (BMI), and K-L grade, were collected from all patients to investigate their influence on clinical significance. The K-L grade was determined by an orthopaedic surgeon (L.A.). From a total of 389 patients available in the database at the time of the study analysis, 215 were included based on the presence of the specific data requested for quantification of the MCID and the PASS, including the anchor question and all needed scores at both the 6- and 12-month follow-up.
Consisting of 18 questions, the IKDC Subjective score was designed as an evaluative measure to detect improvement or deterioration in symptoms (including pain, stiffness, swelling, locking/catching, and giving way), function, and sports activity experienced by patients with a variety of knee conditions. A score of 100 (maximum) indicates the absence of symptoms and limitations in performing daily activities. 19 The KOOS is a knee-specific instrument, developed to assess the patients’ opinions about their knee and associated problems. It holds 42 items in 5 separately scored subscales: Pain, Symptoms, Activities of Daily Living (ADL), Function in Sport and Recreation (Sport/Rec), and knee-related Quality of Life (QOL). 34 The items are scored individually from 0 (extreme knee problems) to 100 (no knee problems).
MCID and PASS Quantification
The MCID was calculated using the distribution method derived from the value equal to half of the standard deviation of the overall cohort improvement at 6 and 12 months. 7,21,25,28 Patients were classified as achieving the MCID if it was achieved in ≥1 of the included outcome measures, as previously described. 7 The PASS was calculated through use of an anchor-based method by asking the patients whether their current state was satisfactory or not through the following question: “Taking into account all the activities of daily life, the level of pain and the functional impairment, are you satisfied with your knee health status?” as previously described. 7,12,21,22,25 The PASS was assessed using a 2-point scale (satisfied or not satisfied), and threshold values were detected through a receiver operating characteristic (ROC) curve analysis and the Youden index in such a way as to maximize the sensitivity and the specificity of the threshold values. The area under the curve (AUC) of the ROC analysis was used to evaluate the ability of the identified PASS threshold values to differentiate between satisfied and unsatisfied patients. For this study, predictive models with AUC values >0.7 were considered acceptable, and those with values >0.8 were considered excellent. 28 As done for the MCID, patients were classified as achieving the PASS if it was achieved in ≥1 of the included outcome measures, as previously defined. 7
Statistical Analysis
All continuous data were expressed in terms of mean ± SD, and categorical variables were expressed as proportions or percentages. The Shapiro-Wilk test was performed to test the normality of continuous variables. The analysis of variance (ANOVA) test was performed to assess between-group differences of continuous and normally distributed and homoscedastic data; when required, the Mann-Whitney test was used otherwise. The Spearman rank correlation was used to assess correlations between scores and continuous data (age and BMI). The Pearson chi-square exact test was performed to investigate relationships between grouping variables (sex, K-L grade, MCID achievement, and PASS achievement). The MCID and PASS were calculated as previously described. A logistic regression analysis was performed to determine whether age, sex, BMI, and baseline PROM values influenced MCID and PASS achievement. For all tests, P < .05 was considered significant. All statistical analyses were performed using SPSS Version 19.0 (IBM Corp).
Results
Characteristics and Outcomes
This analysis included 215 patients from a database of patients with knee OA treated with intra-articular PRP injections (182 received 3 PRP injections with a 1-week interval, 33 a single injection) and evaluated at baseline and 6- and 12-month follow-up. Patients were selected based on the availability of the necessary data to calculate the MCID and the PASS. Among these patients, 146 were men (68%) and 69 women (32%), with a mean age of 53.2 ± 11.3 years and a mean BMI of 26.8 ± 4.3 kg/m2. Further baseline characteristics of the evaluated patients are reported in Table 1.
Table 1.
Baseline Patient Characteristics of the Included Patients Treated With Intra-articular PRP Injections a
| Baseline Characteristic | Value |
|---|---|
| Patients (male/female), n | 215 (146/69) |
| Age, y | 53.2 ± 11.3 [51.6-54.7] |
| BMI, kg/m2 | 26.8 ± 4.3 [26.2-27.3] |
| K-L grade, % | |
| Grade 0 | 4 |
| Grade 1 | 21 |
| Grade 2 | 36 |
| Grade 3 | 29 |
| Grade 4 | 10 |
| IKDC Subjective score | 49.9 ± 15.9 [47.8-52.1] |
| KOOS Pain | 67.6 ± 17.1 [65.3-69.9] |
| KOOS Symptoms | 65.8 ± 17.5 [63.4-68.1] |
| KOOS ADL | 74.4 ± 17.2 [72.1-76.7] |
| KOOS Sport/Rec | 44.9 ± 23.7 [41.7-48.1] |
| KOOS QOL | 37.2 ± 18.5 [34.7-39.7] |
a Values are presented as mean ± SD [95% CI] unless otherwise indicated. ADL, Activities of Daily Living; BMI, body mass index; IKDC, International Knee Documentation Committee; K-L, Kellgren-Lawrence; KOOS, Knee injury and Osteoarthritis Outcome Score; PRP, platelet-rich plasma; QOL, Quality of Life; Sport/Rec, Sport and Recreation.
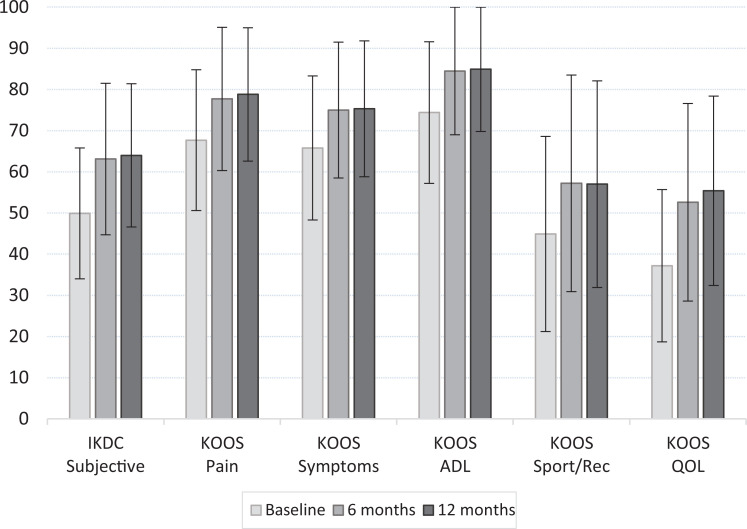
The IKDC Subjective score and KOOS subscales all improved significantly from baseline to 6-month and baseline to 12-month follow-up (all P < .001), as reported in Figure 1 (more details in Appendix Table A1). All scores were stable from 6 to 12 months except for the KOOS QOL subscale, which improved further during that time (P = .033).
Figure 1.
Trends in patient-reported outcome measures during the study period. Data are shown as mean ± SD. All scores improved from the baseline to 6-month and 12-month follow-up (all P < .001). The KOOS QOL further improved from 6 months to 12 months (P = .033). ADL, Activities of Daily Living; IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; QOL, Quality of Life.
Psychometric Analysis
The MCID and PASS values for the IKDC Subjective score and KOOS subscales are reported in Tables 2 and 3. For the IKDC, the MCID values were 8.6 and 8.5 points and the PASS scores were 59.7 and 62.1 at 6 and 12 months, respectively. Overall, the MCID and PASS values for all KOOS subscales remained constant at the 2 follow-up points. The percentage of patients who achieved the MCID and the PASS was higher than 85% at both 6 and 12 months.
Table 2.
MCID Values for the IKDC Subjective Score and KOOS Subscales at 6 and 12 Months After PRP Injection Treatment a
| 6 Months | 12 Months | |||
|---|---|---|---|---|
| Outcome Measure | MCID | Range | MCID | Range |
| IKDC Subjective | 8.6 | (7.3-11.2) | 8.5 | (6.9-10.7) |
| KOOS Pain | 9.3 | (7.7-11.9) | 9.1 | (7.5-11.5) |
| KOOS Symptoms | 8.4 | (7.0-10.7) | 8.2 | (6.6-10.1) |
| KOOS ADL | 9 | (7.1-11) | 9.2 | (7.3-11.2) |
| KOOS Sport/Rec | 12.5 | (10.5-16.1) | 11.6 | (9.8-15.1) |
| KOOS QOL | 10.3 | (8.7-13.3) | 10.3 | (8.5-13.3) |
a Differences in MCID values from 6- to 12-month follow-up were nonsignificant for all outcome measures. ADL, Activities of Daily Living; IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; MCID, minimal clinically important difference; PRP, platelet-rich plasma; QOL, Quality of Life; Sport/Rec, Sport and Recreation.
Table 3.
PASS Values for the IKDC Subjective Score and KOOS Subscales at 6 and 12 Months After PRP Injection Treatment a
| 6 Months | 12 Months | |||
|---|---|---|---|---|
| Outcome Measure | PASS Cutoff Score | Sensitivity; Specificity [Youden] | PASS Cutoff Score | Sensitivity; Specificity [Youden] |
| IKDC Subjective | 59.7 | 0.712; 0.780 [0.491] | 62.1 | 0.710; 0.830 [0.540] |
| KOOS Pain | 73.6 | 0.744; 0.610 [0.354] | 76.4 | 0.710; 0.660 [0.370] |
| KOOS Symptoms | 71.2 | 0.731; 0.576 [0.307] | 73.2 | 0.704; 0.623 [0.326] |
| KOOS ADL | 84.5 | 0.712; 0.627 [0.339] | 84.5 | 0.710; 0.660 [0.370] |
| KOOS Sport/Rec | 47.5 | 0.724; 0.627 [0.351] | 42.5 | 0.790; 0.509 [0.300] |
| KOOS QOL | 47.0 | 0.718; 0.712 [0.430] | 43.9 | 0.772; 0.585 [0.357] |
a ADL, Activities of Daily Living; IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; PASS, Patient Acceptable Symptom State; PRP, platelet-rich plasma; QOL, Quality of Life; Sport/Rec, Sport and Recreation.
The likelihood of achieving the MCID remained constant from 6 to 12 months; in fact, the percentage of patients who achieved MCID was 89.8% at 6 months and 85.6% at 12 months. Some factors were found to influence the achievement of MCID thresholds: age and sex. Older patients were more likely to reach the MCID for the KOOS ADL at 12 months (P = .01). Female patients were more likely to reach the MCID for the KOOS ADL at 6 and 12 months (P = .033 and P = .01, respectively) and the KOOS Pain at 12 months (P = .04). BMI and K-L grade did not significantly influence the achievement of any MCID threshold. The multivariate logistic regression analysis results did not confirm the influence of the aforementioned factors.
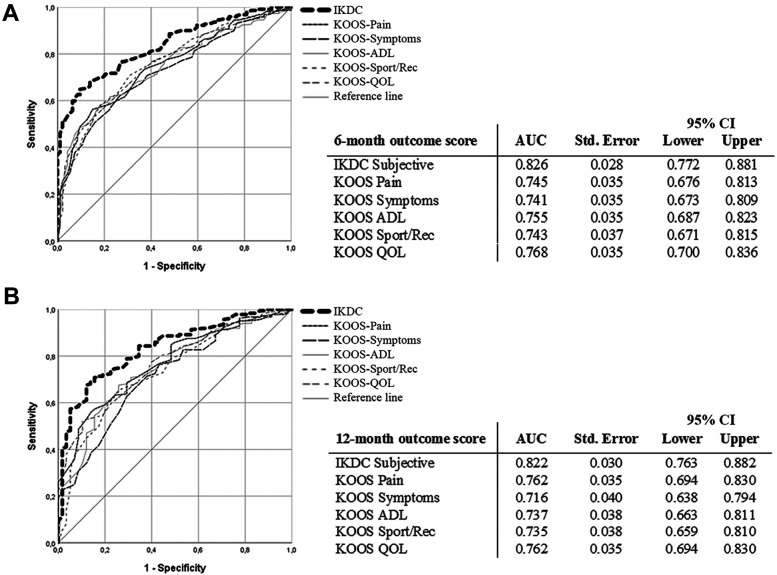
Regarding the PASS, 156 patients (72.6%) were satisfied and 59 (27.4%) not satisfied at 6 months postinjection, while 162 patients (75.3%) were satisfied and 53 (24.7%) not satisfied at 12 months. There were statistically significant differences between satisfied and unsatisfied patients at both follow-ups, both in the IKDC Subjective score (P < .001) and all KOOS subscales (all P < .001). The PASS values at 6- and 12-month follow-up based on the ROC analysis are reported in Table 3 and Figure 2. The percentage of patients who achieved a PASS value was 87% at 6 months and 86% at 12 months. Age, sex, BMI, and K-L grade were not significantly related to the odds of achieving the PASS for any of the evaluated PROMs, as confirmed by the multivariate logistic regression analysis results.
Figure 2.
The ROC curve analysis for the IKDC Subjective score and KOOS subscales for Patient Acceptable Symptom State threshold scores at (A) 6 months and (B) 12 months. The AUC of the ROC analysis for the IKDC Subjective score was >0.8 at both follow-ups and thus were considered excellent. The AUCs of the ROC analysis for all KOOS subscales were >0.7 and thus were considered acceptable. AUC, area under the curve; IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; ROC, receiver operating characteristic.
Discussion
The main contribution of this study was the definition of both MCID and PASS thresholds for the IKDC Subjective score and the KOOS subscales at 6 and 12 months for patients affected by knee OA and treated with intra-articular PRP injections. These results should be analyzed in a more patient-oriented way. Considering the findings of clinical trials on these treatments and the present study being the first to evaluate the MCID and PASS for knee injections, it sets reference values for future studies. This could give more reliable information for the creation of guidelines, allowing better interpretation of the results of clinical trials and helping practicians to counsel patients on the potential of injection treatments to provide clinically meaningful results for knee OA.
To overcome the limitations of PROMs and to better understand the real clinical improvement offered by different treatment options, several different tools have been introduced to help researchers and physicians in evaluating patients. In this context, the Osteoarthritis Research Society International (OARSI) proposed a simplified set of responder criteria for a better interpretation of the results of clinical trials. These criteria categorize an individual’s response to treatment based on definitions of responders and nonresponders. 11 Another applicable method is the pain trajectories evaluation, which aims, through a repetitive assessment of the patient’s pain level, to better characterize and evaluate different profiles of pain progression rather than with less reliable 1-time evaluations. 32,37 Similarly, the MCID and the PASS are important tools for documenting the magnitude of patient improvement and well-being after treatment, allowing a more patient-focused outcome than statistics can provide.
Various methods have been employed to determine MCID and PASS values, with the 2 general approaches most commonly used being distribution-based and anchor-based methods. 21 Distribution-based methods are purely statistical and do not need clinically based questionnaires or questions, but do not consider patient perspectives. 21 Conversely, the anchor-based methods rely on identifying a question that asks directly about subjective clinical change, linking this response with changes in an outcome score, but they are susceptible to a number of well-documented recall biases. 8 These biases might lead to variability in results. For example, a systematic review of estimates of the MCID and the PASS in patients who underwent total knee and hip replacement underlined significant heterogeneity in the calculation methods and in the obtained thresholds. 26 While more studies are needed to understand the most suitable approach, both methods are currently applied.
In the current study, MCID and PASS values were calculated for the IKDC Subjective score and KOOS subscales in patients with knee OA who underwent PRP injection treatment. It is important to underline that the MCID and the PASS are specific to a determined PROM, and results may also vary among different treatment populations. Previous studies have established the thresholds for the MCID and the PASS for the IKDC and KOOS subscales after several diseases or surgical procedures of the knee, ranging from cartilage repair procedures to total knee replacement. 7,26,29,30 Interestingly, the MCID and PASS values reported for these treatments and patient populations differ from those found in the current study, where patients with OA obtained lower MCID thresholds and higher PASS values. This is likely because of the different activity levels and expectations that could influence the patient’s perspective on the obtained results. The heterogeneity of these psychometric measures, according to treatment and population chosen, further underlines the importance of investigating their values within the specific condition studied. Accordingly, it is paramount to use specific values of these psychometric measures in patients with knee OA treated with injection-based therapies, instead of those calculated previously from other patient populations and other procedures. In this way, it will be possible to reduce the risk of underestimating or overestimating the real clinical efficacy of this type of treatment for knee OA. The MCID and the PASS for the IKDC Subjective score and KOOS subscales for injection-based therapies for knee OA had not previously been provided in the literature. The KOOS is one of the most used scores for knee OA, and although more commonly indicated for cartilage lesions, the IKDC score is also often used for knee OA, especially when investigating younger and active populations. 1 Thus, the MCID and PASS of both scores may prove useful to further understand response to treatment in this field.
Injection-based treatments are characterized by disputed findings and difficulties in proving superiority over placebo as well as clear superiority of one product over another, leading to controversial recommendations for injection therapies from different sources. 3,33,36 Accordingly, while treatments such as hyaluronic acid or PRP have been widely used in clinical practice, and positive findings have been reported in several studies, they are not yet recommended by many international societies. 2,4,15 These controversial findings on injection-based treatments for knee OA and the consequent absence of a consensus about their use could be partially due to the limited ability to define exactly the clinical benefit of these treatments, and the definition of threshold values for psychometric measures may help in shedding some light in this direction. 2,9
A strength of this study was the use of ROC analyses with the AUC and Youden values to determine PASS thresholds, allowing identification of reliable values both for the IKDC and the KOOS subscales. In particular, the AUC of the ROC analysis for the IKDC Subjective score was >0.8 at both 6-month and 12-month follow-ups and thus was considered excellent and highly reliable. The AUC of the ROC analysis for all KOOS subscales was >0.7 at both follow-ups, providing acceptable values.
The MCID and PASS are complementary, being focused on different aspects of patient perception, and add to the results quantification obtained through PROMs. The MCID defines whether a patient perceives what he or she thinks is a clinically important difference. Still, this improvement may not be enough to be considered an acceptable state. On the other hand, some patients could define the status reached at the follow-up as acceptable, although without being able to perceive a meaningful treatment-related improvement. Thus, while looking at different perspectives, both measures offer the possibility to delineate treatment responders, which is useful information for researchers, physicians, and patients as well. In this way, they can indicate more realistic expectations on the probability of reaching a perceived benefit from the injection treatment, rather than relying on more impersonal results based on the statistical analysis of mean scores.
This study, while providing these important specific psychometric measures for this patient population, presents some limitations that should be considered in the interpretation of the results. The included patients were treated in a clinical research facility. Thus, the population cannot be considered real practice, and patient expectations and the placebo effect could alter the results. Placebo is an important component of all treatments, especially when interpreting the results of fashionable products like orthobiologics. A recent literature meta-analysis demonstrated long-lasting results in terms of pain and function for saline injections, even higher than what was considered the MCID. 33 Further studies should explore how much of the perceived MCID can be ascribed to the placebo effect and how much to the real benefit offered by the injections. This applies also to different injection-based treatments, which should be investigated with specific analyses to understand possible differences in terms of perceived benefit. However, until further investigations establish differences in perceivable benefit among different injection-based options, the values found in this study could be useful for analyzing the response to these treatments in patients affected by knee OA.
Among other aspects worth further investigation, our relatively small sample size might have hindered the detection of significant factors that could predict the achievement of our MCID and PASS thresholds. In this study, sex and age seemed to influence the MCID, although the multivariate analysis results did not confirm this finding. Thus, larger series of patients should be evaluated to further explore this issue and help identify factors affecting the values of the MCID and the PASS. Another limitation of the study was the higher percentage of male patients compared with female patients, higher than in the general population of patients with knee OA. Also, the study population had only a few advanced OA cases. Some further limitations might be also because of the psychometric measurement methodology itself. In fact, each method presents its own advantages and pitfalls, and a consistent superiority of one method over the others has yet to be demonstrated. Future studies should investigate the most suitable approach optimizing these psychometric thresholds to better understand the patient perception of treatment results after knee OA injections.
Conclusion
This study provides the MCID and PASS thresholds for the IKDC Subjective score and the KOOS subscales in patients with knee OA treated with PRP injections. At 6 and 12 months postinjection, the IKDC Subjective MCID values were 8.6 and 8.5 points and the IKDC PASS scores were 59.7 and 62.1, respectively. Values remained stable at the 2 follow-ups for both the IKDC and the KOOS subscales. The predictive models of the PASS were found to be excellent for the IKDC and acceptable for KOOS values. These psychometric measures may allow researchers to better determine the clinical relevance of the outcome of injection-based treatments for knee OA.
Acknowledgment
The authors thank Elettra Pignotti for providing support with statistical analysis of the data.
APPENDIX
Table A1.
Outcome Scores at Baseline and at 6 and 12 Months After PRP Injection a
| Outcome Measure | Preoperative | 6 Months | 12 Months | P Value |
|---|---|---|---|---|
| IKDC Subjective | 49.9 ± 15.9 | 63.1 ± 18.4 | 64 ± 17.4 | <.0005 |
| KOOS Pain | 67.6 ± 17.1 | 77.7 ± 17.4 | 78.8 ± 16.2 | <.0005 |
| KOOS Symptoms | 65.8 ± 17.5 | 75 ± 16.5 | 75.3 ± 16.5 | <.0005 |
| KOOS ADL | 74.4 ± 17.2 | 84.5 ± 16.3 | 84.9 ± 15.8 | <.0005 |
| KOOS Sport/Rec | 44.9 ± 23.7 | 57.2 ± 26.3 | 57 ± 25.1 | <.0005 |
| KOOS QOL | 37.2 ± 18.5 | 52.6 ± 24 | 55.4 ± 23 | <.0005 |
a Values are presented as mean ± SD. ADL, Activities of Daily Living; IKDC, International Knee Documentation Committee score; KOOS, Knee injury and Osteoarthritis Outcome Score; PRP, platelet-rich-plasma; QOL, Quality of Life; Sport/Rec, Sport and Recreation.
Footnotes
Final revision submitted April 9, 2021; accepted May 4, 2021.
One or more of the authors has declared the following potential conflict of interest or source of funding: S.Z. has received institutional support from Fidia Farmaceutici, Cartiheal, IGEA Clinical Biophysics, Biomet, and Kensey Nash; grant support from I+; and royalties from Springer. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from IRCCS Istituto Ortopedico Rizzoli (protocol No. 0016512).
References
- 1. Altamura SA, Di Martino A, Andriolo L, et al. Platelet-rich plasma for sport-active patients with knee osteoarthritis: limited return to sport. Biomed Res Int. 2020;2020:8243865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the nonsurgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578–1589. [DOI] [PubMed] [Google Scholar]
- 3. Bowman S, Awad ME, Hamrick MW, Hunter M, Fulzele S. Recent advances in hyaluronic acid based therapy for osteoarthritis. Clin Transl Med. 2018;7(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bruyere O, Honvo G, Veronese N, et al. An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Semin Arthritis Rheum. 2019;49(3):337–350. [DOI] [PubMed] [Google Scholar]
- 5. Cavallo C, Boffa A, Andriolo L, et al. Bone marrow concentrate injections for the treatment of osteoarthritis: evidence from preclinical findings to the clinical application. Int Orthop. 2021;45(2):525–538. doi: 10.1007/s00264-020-04703-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chahal J, Van Thiel GS, Mather RC III, et al. The patient acceptable symptomatic state for the modified Harris Hip Score and Hip Outcome Score among patients undergoing surgical treatment for femoroacetabular impingement. Am J Sports Med. 2015;43(8):1844–1849. [DOI] [PubMed] [Google Scholar]
- 7. Chahla J, Kunze KN, Tauro T, et al. Defining the minimal clinically important difference and patient acceptable symptom state for microfracture of the knee: a psychometric analysis at short-term follow-up. Am J Sports Med. 2020;48(4):876–883. [DOI] [PubMed] [Google Scholar]
- 8. Crosby RD, Kolotkin RL, Williams GR. Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol. 2003;56(5):395–407. [DOI] [PubMed] [Google Scholar]
- 9. Dhawan A, Brand JC, Provencher MT, Rossi MJ, Lubowitz JH. Research pearls: the significance of statistics and perils of pooling. Arthroscopy. 2017;33(6):1099–1101. [DOI] [PubMed] [Google Scholar]
- 10. Di Martino A, Di Matteo B, Papio T, et al. Platelet-rich plasma versus hyaluronic acid injections for the treatment of knee osteoarthritis: results at 5 years of a double-blind, randomized controlled trial. Am J Sports Med. 2019;47(2):347–354. [DOI] [PubMed] [Google Scholar]
- 11. Dougados M, Leclaire P, van der Heijde D, Bloch DA, Bellamy N, Altman RD. Response criteria for clinical trials on osteoarthritis of the knee and hip: a report of the Osteoarthritis Research Society International Standing Committee for Clinical Trials response criteria initiative. Osteoarthritis Cartilage. 2000;8(6):395–403. [DOI] [PubMed] [Google Scholar]
- 12. Dwyer T, Zochowski T, Ogilvie-Harris D, Theodoropoulos J, Whelan D, Chahal J. Determining the patient acceptable symptomatic state for patients undergoing arthroscopic partial meniscectomy in the knee. Am J Sports Med. 2020;48(4):847–852. [DOI] [PubMed] [Google Scholar]
- 13. Filardo G, Kon E, Longo UG, et al. Non-surgical treatments for the management of early osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1775–1785. [DOI] [PubMed] [Google Scholar]
- 14. Filardo G, Perdisa F, Roffi A, Marcacci M, Kon E. Stem cells in articular cartilage regeneration. J Orthop Surg Res. 2016;11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Filardo G, Previtali D, Napoli F, Candrian C, Zaffagnini S, Grassi A. PRP injections for the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Cartilage. Published June 19, 2020. doi:10.1177/1947603520931170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harris JD, Brand JC, Cote MP, Faucett SC, Dhawan A. Research pearls: the significance of statistics and perils of pooling: part I, clinical versus statistical significance. Arthroscopy. 2017;33(6):1102–1112. [DOI] [PubMed] [Google Scholar]
- 17. Hsu H, Siwiec RM. Knee Osteoarthritis. StatPearls Publishing; 2021. Updated July 2, 2021. https://www.ncbi.nlm.nih.gov/books/NBK507884/ [PubMed]
- 18. Indelli PF, Giuntoli M. Early osteoarthritis of the knee: from conservative to surgical management. Ann Transl Med. 2018;6(20):398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2001;29(5):600–613. [DOI] [PubMed] [Google Scholar]
- 20. Jones IA, Togashi R, Wilson ML, Heckmann N, Vangsness CT, Jr. Intra-articular treatment options for knee osteoarthritis. Nat Rev Rheumatol. 2019;15(2):77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Katz NP, Paillard FC, Ekman E. Determining the clinical importance of treatment benefits for interventions for painful orthopedic conditions. J Orthop Surg Res. 2015;10:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim DM, Kim TH, Kholinne E, et al. Minimal clinically important difference, substantial clinical benefit, and patient acceptable symptomatic state after arthroscopic rotator cuff repair. Am J Sports Med. 2020;48(11):2650–2659. [DOI] [PubMed] [Google Scholar]
- 23. Kvien TK, Heiberg T, Hagen KB. Minimal clinically important improvement/difference (MCII/MCID) and patient acceptable symptom state (PASS): what do these concepts mean? Ann Rheum Dis. 2007;66(suppl_3):iii40–iii41. doi:10.1136/ard.2007.079798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu JN, Gowd AK, Redondo ML, et al. Establishing clinically significant outcomes after meniscal allograft transplantation. Orthop J Sports Med. 2019;7(1):2325967118818462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. MacKay C, Clements N, Wong R, Davis AM. A systematic review of estimates of the minimal clinically important difference and patient acceptable symptom state of the Western Ontario and McMaster Universities Osteoarthritis Index in patients who underwent total hip and total knee replacement. Osteoarthritis Cartilage. 2019;27(10):1408–1419. [DOI] [PubMed] [Google Scholar]
- 27. Mobasheri A, Batt M. An update on the pathophysiology of osteoarthritis. Ann Phys Rehabil Med. 2016;59(5-6):333–339. [DOI] [PubMed] [Google Scholar]
- 28. Nwachukwu BU, Fields K, Chang B, Nawabi DH, Kelly BT, Ranawat AS. Preoperative outcome scores are predictive of achieving the minimal clinically important difference after arthroscopic treatment of femoroacetabular impingement. Am J Sports Med. 2017;45(3):612–619. [DOI] [PubMed] [Google Scholar]
- 29. Ogura T, Ackermann J, Barbieri Mestriner A, Merkely G, Gomoll AH. Minimal clinically important differences and substantial clinical benefit in patient-reported outcome measures after autologous chondrocyte implantation. Cartilage. 2020;11(4):412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ogura T, Ackermann J, Mestriner AB, Merkely G, Gomoll AH. The minimal clinically important difference and substantial clinical benefit in the patient-reported outcome measures of patients undergoing osteochondral allograft transplantation in the Knee. Cartilage. 2021;12(1):42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ong KL, Runa M, Lau E, Altman R. Is intra-articular injection of Synvisc associated with a delay to knee arthroplasty in patients with knee osteoarthritis? Cartilage. 2019;10(4):423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Previtali D, Andriolo L, Di Laura Frattura G, et al. Pain trajectories in knee osteoarthritis: a systematic review and best evidence synthesis on pain predictors. J Clin Med. 2020;9(9):2828. doi:10.3390/jcm9092828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Previtali D, Merli G, Di Laura Frattura G, Candrian C, Zaffagnini S, Filardo G. The long-lasting effects of “placebo injections” in knee osteoarthritis: a meta-analysis. Cartilage. Published March 18, 2020. doi:10.1177/1947603520906597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sanchez M, Jorquera C, Sanchez P, et al. Platelet-rich plasma injections delay the need for knee arthroplasty: a retrospective study and survival analysis. Int Orthop. 2021;45(2):401–410. [DOI] [PubMed] [Google Scholar]
- 36. Santilli V, Paoloni M, Mangone M, Alviti F, Bernetti A. Hyaluronic acid in the management of osteoarthritis: injection therapies innovations. Clin Cases Miner Bone Metab. 2016;13(2):131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stone AA, Schwartz JE, Broderick JE, Shiffman SS. Variability of momentary pain predicts recall of weekly pain: a consequence of the peak (or salience) Memory Heuristic. Pers Soc Psychol Bull. 2005;31(10):1340–1346. [DOI] [PubMed] [Google Scholar]
- 38. Wang D, Jones MH, Khair MM, Miniaci A. Patient-reported outcome measures for the knee. J Knee Surg. 2010;23(3):137–151. [DOI] [PubMed] [Google Scholar]