Abstract
Background
Novel oral anticoagulants and warfarin are widely used for stroke prevention in patients with atrial fibrillation. The anticoagulation status of patients receiving warfarin or rivaroxaban has been studied. In this study, we aimed to evaluate the effect of dabigatran and warfarin on preventing thrombin generation (TG).
Methods
This retrospective study enrolled 237 nonvalvular atrial fibrillation (NVAF) subjects treated with 110 mg dabigatran etexilate twice daily and 224 NVAF patients received adjusted-dose warfarin (international normalized ratio [INR] of 2 to 3)). Coagulation assays, prothrombin fragment 1 + 2 (F1+2), calibrated automated thrombogram, and thrombin–antithrombin complex (TAT) were detected at the steady state.
Results
Activated partial thromboplastin time (APTT), antithrombin III activity, fibrinogen, and lag time showed no difference between the two groups. Compared to the dabigatran group, prothrombin time and INR values were higher in the warfarin group (all P < .001). Thrombin time, endogenous thrombin potential, peak TG (Cmax), F1+2, and TAT were lower in the warfarin group. The inhibition of TG was still stronger in the warfarin group when the patients were divided into subgroups.
Conclusion
Conventional coagulation assays are suboptimal for assessing the coagulation status of dabigatran. TG could be used as supplementary assays to evaluate the anticoagulation effect of oral anticoagulants. Our results suggest that warfarin may inhibit TG more aggressively than dabigatran in patients regardless of age and kidney function.
Keywords: atrial fibrillation, prothrombin fragment 1 + 2, thrombin generation, thrombin-antithrombin complex
Introduction
Atrial fibrillation (AF) is a major reason for stroke in the world. 1 The incidence of stroke in patients with AF is five times over that of patients without AF. 2 Oral anticoagulants, such as warfarin and dabigatran etexilate, could reduce the occurrence of stroke in AF patients. Warfarin targets not only procoagulants including factor VII, factor IX, factor X, and prothrombin but also anticoagulants including protein C and protein S. Warfarin is cumbersome to use due to the need for frequent monitoring and multiple interactions with food and drugs. However, despite being under frequent monitoring, many patients treated with warfarin still have an unstable international normalized ratio (INR). 3 Dabigatran etexilate is a prodrug that can be hydrolyzed to dabigatran quickly. Dabigatran, a direct thrombin inhibitor, could overcome the limitations of vitamin K antagonists. 4 Coagulation assays are not commonly measured in patients treated with dabigatran etexilate because their pharmacodynamics and pharmacokinetics could be predicted.
Thrombin is a key coagulation factor in the clotting process. It could regulate cell signaling, platelet activation, and blood coagulation cascade. Patients with enhanced TG are vulnerable to thrombotic events, whereas patients with reduced TG are vulnerable to bleeding. The anticoagulant mechanism of warfarin is different from that of dabigatran. Activated partial thromboplastin time (APTT), thrombin time (TT), and prothrombin time (PT) are usually prolonged depending on the plasma dabigatran concentrations. 5 PT and APTT are often prolonged in patients receiving warfarin.
The Randomized Evaluation of Long-Term anticoagulation Therapy (RE-LY) study showed that dabigatran given at a dose of 110 mg have a similar effect with warfarin on preventing stroke and systemic embolism and associates with lower rates of major hemorrhage. 6 In addition, the results of studies in Chinese nonvalvular atrial fibrillation (NVAF) patients are similar to that of global RE-LY study. 7 However, only a few studies reported the difference in the effect of dabigatran and warfarin on calibrated automated thrombogram (CAT), prothrombin fragment 1 + 2 (F1+2), and thrombin-antithrombin complex (TAT). In this study, we aimed to compare the therapeutic efficacy between dabigatran versus warfarin in patients with NVAF.
Methods
Agents
STA®-PTT, STA®-Antithrombin III (ATIII), STA®-Thrombin, STA®-fibrinogen, STA® Néoplastine® CI reagent, Owren-Koller buffer, CaCl2, Platelet-poor plasma (PPP) regent, thrombin calibrator, and FluCa were purchased from Stago (Asnières, France). F1+2 ELISA kit was from Dade Behring (Marburg, Germany). TAT ELISA kit was from Assaypro (MO, United States).
Study Samples
This is a retrospective study. A total of 461 patients diagnosed as NVAF were recruited from January 2016 to December 2020 in First Affiliated Hospital of Soochow University. Patients were informed of the purpose of our study before signing a consent. The ethics committee approved the current study at First Affiliated Hospital of Soochow University. Patients with creatinine clearance (CCr) values of ≥20 ml/min were enrolled in the study. The treatment of dabigatran or warfarin was discussed by patients and physicians. Patients treated with amiodarone or verapamil were excluded. Blood samples were drawn from the antecubital vein using a 21-gauge needle and collected into sodium citrate anticoagulant tubes before the next procedure.
Data Collection
The characteristics of the NVAF patients were collected from the medical records. CHADS2 and CHA2DS2-VASc scores were calculated.
Coagulation Assays
Blood samples were loaded in the Compact Max (Diagnostic Stago). Results of coagulation assays depending on different reagents and machines, so the same batches of reagents were applied. 8 The detection of APTT, TT, and PT was described in detail in our previous study. 9 For detecting ATIII and fibrinogen, 5 μL plasma samples were automatically diluted with 95 μL Owren-Koller buffer. STA®-ATIII thrombin (100 μL) and STA®-ATIII substract (100 μL) were added to detect ATIII values. STA®-fibrinogen (50 μL) mixed with diluted plasma to measure plasma fibrinogen. PT had a local reference range of 10.8 to 13.5 s, INR .8 to 1.5, APTT 23 to 37 s, TT 14 to 21 s, ATIII 70 to 125% and fibrinogen 2 to 5 g/L.
Thrombin Generation Assay
PPP reagent (20 μL) and calibrator (20 μL) were added to the wells of a plate according to the protocols. Blood samples (80 μL) and normal samples (80 μL) containing 10 nM thrombodulin (TM) were thawed at 37°C for 5 min and pipetted into each well. The plate was loaded into the machine and incubated at 37°C for 10 min. The experiment was started after adding FluCa (20 μL) to each well. Results were displayed by a Fluoroskan Ascent fluorometer (Thermo Fisher Scientific, Waltham, MA). The following parameters were showed in the software: lag time, peak TG (Cmax), and endogenous thrombin potential (ETP).
Prothrombin Fragment 1 + 2 Assay
F1+2 was detected as another marker of TG. F1+2 was measured following the manufacturer's instructions. The intra-assay coefficient of variation (CV) was between 3.6% and 5.5% and the inter-CV was between 4.4 and 11.2%.
TAT Assay
TAT is also a marker of TG. Plasma TAT complex levels were measured using a commercially available ELISA kit in accordance with the manufacturer's instructions. The average intra-assay CV was 4.7% and the inter-CV was 9.9%.
Statistical Analysis
Statistical analysis was performed with Stata 14.0. Kolmogorov–Smirnov test was used to test distribution characters of data. All the data were distributed normally and expressed as mean ± standard deviation (SD). An independent sample t-test was used for continuous variables. Categorical variables were reported as frequencies and percentages. To analyze these kinds of variables, the Chi-squared test was applied. P < .05 indicated a statistical difference.
Results
General Characteristics of the Patients
There was a total of 461 patients available for potential analysis, of which 237 were treated with dabigatran etexilate 110 mg twice daily and 224 were treated adjusted-dose warfarin. Between the two groups, there was no significant difference in sex, age, body mass index (BMI), and CHADS2. Age, BMI, and CHADS2 were 69.8 ± 7.2, 24.4 ± 3.1, and 2.18 ± 0.7 in the dabigatran group and 71.3 ± 10.8, 24.7 ± 4.0, and 2.32 ± 1.1 in the warfarin group, respectively (Table 1).
Table 1.
General Characteristics of the Patients.
| Characteristics | Dabigatran | Warfarin | p Value |
|---|---|---|---|
| Age, years (mean ± SD) | 69.8 ± 7.2 | 71.3 ± 10.8 | .079 |
| Male, n (%) | 124 (52.3) | 136 (60.7) | .075 |
| Body mass index | 24.4 ± 3.1 | 24.7 ± 4.0 | .367 |
| Congestive heart failure, n (%) | 75 (31.6) | 59 (26.3) | .220 |
| Hypertension, n (%) | 180 (75.9) | 187 (83.0) | .066 |
| Diabetes mellitus, n (%) | 62 (26.3) | 74 (33.0) | .125 |
| History of cerebral infarction, n (%) | 28 (11.8) | 42 (18.8) | .051 |
| History of smoking, n (%) | 54 (22.8) | 83 (37.1) | <.001 |
| History of drinking, n (%) | 41 (17.3) | 35 (15.6) | .707 |
| CHADS2 (mean ± SD)a | 2.18 ± .7 | 2.32 ± 1.1 | .102 |
| CHA2DS2-VASc (mean ± SD)a | 3.4 ± 1.2 | 3.8 ± 1.3 | <.001 |
| CHADS2 score, n (%) | .401 | ||
| ≤1 | 116 (48.9) | 100 (44.6) | |
| >1 | 121 (51.1) | 124 (55.4) | |
| Creatinine, nmol/L (mean ± SD) | 91.3 ± 20.1 | 95.4 ± 19.7 | .028 |
| CCr, ml/min | 67.6 ± 17.2 | 64.2 ± 15.3 | .026 |
| CCr, n (%) | .302 | ||
| >50 | 175 (73.8) | 155 (69.2) | |
| ≤50 | 62 (26.2) | 69 (30.8) |
Abbreviations: CCr, creatinine clearance; SD, standard deviation.
Comparison of the Coagulation Status Between Dabigatran and Warfarin
In our center, coagulation assays include PT, INR, APTT, TT, ATIII, and fibrinogen. Coagulation assays and TG could reflect the coagulation status of patients. F1+2 and TAT are also markers of TG. Figure 1 and Figure 2 show coagulation status in both groups. The results showed that 60.9% of PT values, 71.7% of APTT levels, and 82.6% of TT values were elevated above the upper reference limit in the dabigatran group. The equivalents in the warfarin group were 100%, 83.3%, and 11.1% respectively. PT and INR were prolonged in the warfarin group than in the dabigatran group (26.3 ± 4.3 s vs 14.5 ± 2.3 s, 2.4 ± 0.4 vs 1.2 ± 0.2, both P < .001). While TT increased obviously in the dabigatran group (118.7 ± 48.9 s). Although APTT was prolonged in the dabigatran group and warfarin group, there was no significant difference (P = .556). In addition, there was no statistical difference in ATIII, fibrinogen, and lag time. The ETP, Cmax, F1+2 and TAT were significantly higher in the dabigatran group than in the warfarin-treated patients (P < .001, P < .001, P < .001 and P = .001, respectively). The results indicated that the inhibition of TG was stronger in the warfarin group.
Figure 1.
The values of PT, INR, TT, APTT, fibrinogen, and ATIII were compared between dabigatran and warfarin. The values are presented as mean ± SD. Abbreviations: APTT, activated partial thromboplastin time; ATIII, antithrombin III; INR, international normalized ratio; PT, prothrombin time; SD, standard deviation; TT, thrombin time.
Figure 2.
The values of lag time, ETP, peak TG (Cmax), prothrombin fragment 1 + 2 (F1+2), and TAT were compared between dabigatran and warfarin. The values are presented as mean ± SD. Abbreviations: ETP, endogenous thrombin potential; SD, standard deviation; TAT, thrombin–antithrombin complex; TG, thrombin generation.
Comparisons of TG, F1+2, and TAT Values Between Dabigatran and Warfarin According to the Age, Renal Function, and CHADS2 Score
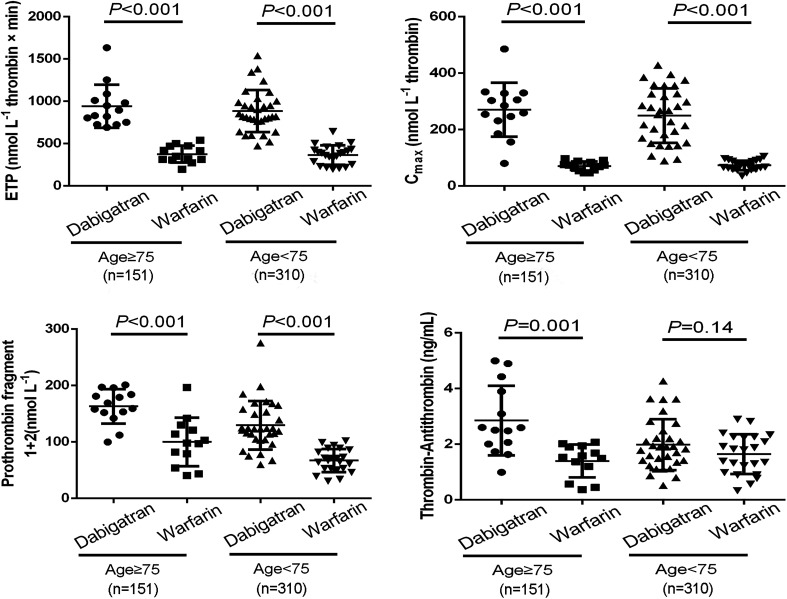
To understand whether the effect of dabigatran or warfarin on TG, F1+2, and TAT values depend on the age, renal function, and CHADS2 score, we divided the data into subgroups. Compared to the dabigatran group, ETP, Cmax, F1+2, and TAT decreased significantly regardless of the age in the warfarin group (Figure 3). As shown in Figure 4, ETP, Cmax, F1+2, and TAT were lower in the warfarin group than in the dabigatran group in patients with a preserved kidney function and those with an impaired kidney function.
Figure 3.
Comparisons of ETP, peak TG (Cmax), fragment 1 + 2 (F1+2) and TAT between dabigatran and warfarin according to age. The values are presented as mean ± SD. Abbreviations: ETP, endogenous thrombin potential; SD, standard deviation; TAT, thrombin–antithrombin complex; TG, thrombin generation.
Figure 4.
Comparisons of ETP, peak TG (Cmax), fragment 1 + 2 (F1+2), and TAT between dabigatran and warfarin according to renal function. The values are presented as mean ± SD. Abbreviations: ETP, endogenous thrombin potential; SD, standard deviation; TAT, thrombin–antithrombin complex; TG, thrombin generation.
Discussion
In this study, TG and conventional coagulation assays were used to assess coagulation status in the NVAF patients. Coagulation assays could only provide assistance for assessing the anticoagulant effect of dabigatran. TG assays showed that warfarin may inhibit TG more aggressively than dabigatran in patients regardless of age and kidney function.
Tajiri and his colleagues investigated the effect of rivaroxaban and warfarin on coagulation status in the NVAF patients. 10 PT and APTT values increased depending on plasma warfarin and rivaroxaban concentrations. PT mainly reflects factor VII activity. In fact, factor VII seems not to play an important role in the warfarin's antithrombotic effect, because venous thrombosis still can be formed in patients with congenital factor VII deficiency from a case report. 11 Blood coagulation cascade is composed of three phases: initiation, amplification, and propagation. The phase of amplification is important for thrombosis. It could infer that factor VII deficiency impairs the initiation of coagulation but not the amplification of coagulation. Tajiri reported PT prolonged with plasma rivaroxaban concentrations. The relationship between plasma rivaroxaban concentrations and APTT is poor. 12 Rivaroxaban might prefer to inhibit the extrinsic pathway. Rivaroxaban and warfarin prevent thrombosis through different mechanisms. The concentration-dependent prolongation of PT and APTT by dabigatran is observed with APTT being the more sensitive test. 5 It would be more interesting to disclose the effect of dabigatran on coagulation status compared with warfarin.
Our previous study demonstrated the effect of dabigatran on coagulation status in humans and rabbits. 9 Coagulation assays combined with TG could reflect coagulation status in humans. Nowadays, few studies showed the coagulation status in NVAF patients receiving dabigatran etexilate or warfarin. Considering the present situation, this retrospective study aimed to compare the therapeutic efficacy between the two groups. Our results showed that no significant difference in sex, age, BMI, and CHADS2 was observed in patients receiving dabigatran etexilate or warfarin. APTT, PT, and TT values prolonged in the dabigatran group. In addition to TT values, PT, and APTT values increased in the warfarin group. There was no significant difference in APTT, fibrinogen, and ATIII levels between the two groups. It is well known that PT and INR could reflect the anticoagulation effect of warfarin. The anticoagulation activity of dabigatran does not need to be monitored in routine clinical practice. However, it is necessary to assess the anticoagulant effect in patients with bleeding or in urgent situations. Although coagulation assays could not predict the anticoagulant effect of dabigatran accurately, but these assays could provide assistance for assessing the anticoagulant effect of dabigatran. A normal PT and APTT exclude supratherapeutic levels in dabigatran-treated patients. It needs our caution when APTT exceeds two times the upper limit of normal. A normal TT could almost rule out the presence of dabigatran. 13 Conventional coagulation assays are suboptimal for assessing the coagulation status of Dabigatran. To detect the anticoagulation effect, CAT, F1+2, and TAT are used as supplementary assays. CAT assay results showed that ETP and Cmax were inhibited more aggressively in the warfarin group. These results could be confirmed by TAT and F1+2 assays. Moreover, these TG markers were still significantly higher in the dabigatran group by dividing into subgroups according to the age, renal function, or CHADS2 score.
In our study, TG was assessed by the CAT method in human plasma. The final concentrations of tissue factor and phospholipids were 5 pM and 4 μM. The concentrations of tissue factor (TF) and phospholipid were used according to the manufacturer's instructions and previous studies. 14 In this condition, TG could be induced completely. Compared with the dabigatran group, CAT assay results showed that ETP and Cmax were inhibited more aggressively in the warfarin group. The results might suggest that patients treated with 110 mg dabigatran twice daily insufficiently suppressing TG. Wagenvoord 15 reported that the low concentrations of dabigatran could paradoxically increase TG due to an enhancement in the activity of the α2-macroglobulin-thrombin (α2MT) complex. The CAT assay uses an algorithm to subtract α2MT activity from the total amidolytic activity. However, the transient enhancement of α2MT induced by dabigatran could not be subtracted, leading to a false increase in TG. But further study showed that α2M did not participate in the enhancement of TG. 16
F1+2 and TAT are other markers of TG and are not affected by α2MT. F1+2 is cleaved when prothrombin is activated and converted to thrombin. 17 TAT forms following the neutralization of thrombin by ATIII and reflects in vivo TG. 18 Compared with the warfarin group, F1+2 and TAT values were significantly higher in the dabigatran group, despite the similar APTT values in the two groups. In anticoagulants-treated patients, prolonged PT or APTT were associated with increased hemorrhagic events. 19 However, there is no evidence disclosing the relationship between an over inhibition of TG and hemorrhagic events in patients treated with anticoagulants. Previous studies demonstrated that lower F1+2 and TAT were associated with a greater blood loss after operation.20,21 These studies may indicate that excessive inhibition of TG leaded to an enhancement risk of bleeding events in patients treated with anticoagulation therapy. In the RE-LY study, dabigatran given at a dose of 110 mg has a similar effect with warfarin on preventing stroke and systemic embolism and was associated with lower rates of major hemorrhage. 6 Recently, a retrospective study in Asian patients showed that dabigatran was associated with a significantly lower stroke/systemic embolism risk and lower major bleeding risk than warfarin. 22 Taken together, our results suggested that an over inhibition of TG may not be necessary for the prevention of thromboembolism, but it would increase the incidence of hemorrhagic events. It is useful to understand the meaning of TG in clinical practice. Further researches are needed to confirm the relationship between TG and hemorrhagic and thrombotic events in NVAF patients treated with anticoagulants.
The present study still has some limitations. Firstly, we collected plasma in patients at trough concentrations of dabigatran. It is difficult to accurately measure when the patients take their medication. This study did not evaluate the relationship between peak concentrations of dabigatran and TG. Secondly, TG might be a marker for predicting major bleeding risk. In the future, clinical trials should be required to found the relationship between TG and thrombosis in the two groups. Finally, this study included a subgroup of patients without indication for dabigatran dose reduction as this remains a confounding factor to the results obtained by the authors.
Acknowledgments
This work was supported by National Natural Science Foundation of China (Grant No. 81770327), Suzhou “Promoting Health through Science and Education” Youth Science and Technology Project (KJXW2019004, KJXW2020001).
Footnotes
Declaration of Conflicting Interests: The authors declare that they have no competing interests.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Natural Science Foundation of China (grant number 81770327) and Suzhou Municipal Science and Technology Bureau (grant number KJXW2019004, KJXW2020001).
ORCID iD: Chi Zhang https://orcid.org/0000-0001-7698-6601
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the european association for cardio-thoracic surgery (EACTS). Eur Heart J. 2021;42(5):373-498. [DOI] [PubMed] [Google Scholar]
- 2.Cheung CC, Nattel S, Macle L, Andrade JG. Atrial fibrillation management in 2021: an updated comparison of the current CCS/CHRS, ESC, and AHA/ACC/HRS guidelines. Can J Cardiol. 2021;(21):313-315. [Google Scholar]
- 3.Sridharan K, Al Banna R, Qader AM, Husain A. Evaluation of inter-patient variability in the pharmacodynamic indices of warfarin. Expert Rev Cardiovasc Ther. 2020;18(11):835-840. [DOI] [PubMed] [Google Scholar]
- 4.Zhu W, Ye Z, Chen S, et al. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients. Stroke: STROKEAHA120031007. 2021.
- 5.Douxfils J, Ageno W, Samama CM, et al. Laboratory testing in patients treated with direct oral anticoagulants: a practical guide for clinicians. J Thromb Haemost. 2018;16(2):209-219. [DOI] [PubMed] [Google Scholar]
- 6.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151. [DOI] [PubMed] [Google Scholar]
- 7.Gao X, Yang YM, Zhu J, Dai Y, Tan HQ, Investigators R-LC. [Dabigatran versus warfarin for the prevention of stroke in Chinese patients with nonvalvular atrial fibrillation: Chinese subpopulation analysis of RE-LY]. Zhonghua Xin Xue Guan Bing Za Zhi. 2016;44(11):929-934. [DOI] [PubMed] [Google Scholar]
- 8.Chin PK, Patterson DM, Zhang M, et al. Coagulation assays and plasma fibrinogen concentrations in real-world patients with atrial fibrillation treated with dabigatran. Br J Clin Pharmacol. 2014;78(3):630-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang C, Zhang P, Li H, et al. The effect of dabigatran on thrombin generation and coagulation assays in rabbit and human plasma. Thromb Res. 2018;165:38-43. [DOI] [PubMed] [Google Scholar]
- 10.Tajiri K, Sato A, Harunari T, Shimojo N, Yamaguchi I, Aonuma K. Impact of rivaroxaban compared with warfarin on the coagulation status in Japanese patients with non-valvular atrial fibrillation: a preliminary analysis of the prothrombin fragment 1 + 2 levels. J Cardiol. 2015;65(3):191-196. [DOI] [PubMed] [Google Scholar]
- 11.Elkhateb IT, Mousa A, Mohye Eldeen R, Soliman Y. Accidentally discovered high INR in pregnancy unmasks an inherited factor VII (FVII) deficiency that is paradoxically associated with thrombotic tendency. BMJ Case Rep. 2021;14(2):e237781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gosselin RC, Adcock D, Hawes EM, Francart SJ, Grant RP, Moll S. Evaluating the use of commercial drug-specific calibrators for determining PT and APTT reagent sensitivity to dabigatran and rivaroxaban. Thromb Haemost. 2015;113(1):77-84. [DOI] [PubMed] [Google Scholar]
- 13.Steffel J, Collins R, Antz M, et al. 2021 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace. 2021. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samama MM, Le Flem L, Guinet C, Gerotziafas G, Depasse F. Three different patterns of calibrated automated thrombogram obtained with six different anticoagulants. J Thromb Haemost. 2007;5(12):2554-2556. [DOI] [PubMed] [Google Scholar]
- 15.Wagenvoord RJ, Deinum J, Elg M, Hemker HC. The paradoxical stimulation by a reversible thrombin inhibitor of thrombin generation in plasma measured with thrombinography is caused by alpha-macroglobulin-thrombin. J Thromb Haemost. 2010;8(6):1281-1289. [DOI] [PubMed] [Google Scholar]
- 16.Wagenvoord RJ, Hemker C. The action mechanism of direct thrombin inhibitors during coagulation. J Thromb Haemost. 2011;9(suppl.S2):573. [Google Scholar]
- 17.Hagii J, Tomita H, Metoki N, et al. Effect of rivaroxaban on prothrombin fragment 1 + 2 compared with warfarin in patients with acute cardioembolic stroke: insight from its serial measurement. Thromb Res. 2016;148:9-14. [DOI] [PubMed] [Google Scholar]
- 18.Lauridsen SV, Hvas CL, Sandgaard E, et al. Thromboelastometry shows early hypercoagulation in patients with spontaneous subarachnoid hemorrhage. World Neurosurg. 2019;130:e140-e149. [DOI] [PubMed] [Google Scholar]
- 19.Inoue H, Okumura K, Atarashi H, et al. Target international normalized ratio values for preventing thromboembolic and hemorrhagic events in Japanese patients with non-valvular atrial fibrillation: results of the J-RHYTHM registry. Circ J. 2013;77(9):2264-2270. [DOI] [PubMed] [Google Scholar]
- 20.Tagawa ST, Dorff TB, Rochanda L, et al. Subclinical haemostatic activation and current surgeon volume predict bleeding with open radical retropubic prostatectomy. BJU Int. 2008;102(9):1086-1091. [DOI] [PubMed] [Google Scholar]
- 21.Karkouti K, McCluskey SA, Syed S, Pazaratz C, Poonawala H, Crowther MA. The influence of perioperative coagulation status on postoperative blood loss in complex cardiac surgery: a prospective observational study. Anesth Analg. 2010;110(6):1533-1540. [DOI] [PubMed] [Google Scholar]
- 22.Bang OY, On YK, Lee MY, et al. The risk of stroke/systemic embolism and major bleeding in Asian patients with non-valvular atrial fibrillation treated with non-vitamin K oral anticoagulants compared to warfarin: results from a real-world data analysis. PLoS One. 2020;15(11):e0242922. [DOI] [PMC free article] [PubMed] [Google Scholar]