Summary
Background
Despite standard curative-intent treatment with neoadjuvant cisplatin-based chemotherapy, followed by radical surgery in eligible patients, muscle-invasive urothelial carcinoma has a high recurrence rate and no level 1 evidence for adjuvant therapy. We evaluated atezolizumab as adjuvant therapy in the phase 3 IMvigor010 study.
Methods
In this multicentre, open-label, randomised phase 3 trial, patients aged 18 years and older with histologically confirmed muscle-invasive urothelial carcinoma and Eastern Cooperative Oncology Group performance status of 0, 1 or 2 were enrolled within 14 weeks after radical cystectomy or nephroureterectomy with lymph node dissection. Patients had ypT2-4a or ypN+ tumours following neoadjuvant chemotherapy or pT3-4a or pN+ tumours if no neoadjuvant chemotherapy was received. Patients not treated with neoadjuvant chemotherapy must have been ineligible for or declined cisplatin-based adjuvant chemotherapy. No postsurgical radiation or prior adjuvant chemotherapy was allowed. Patients were randomised 1:1 using a permuted block method and interactive voice-web response system to receive atezolizumab 1200 mg given intravenously every 3 weeks or up to 1 year or to observation. Randomisation was stratified by previous neoadjuvant chemotherapy use, number of lymph nodes resected, pathologic nodal status, tumour stage, and PD-L1 expression on tumor-infiltrating immune cells. The primary endpoint was disease-free survival, tested in the intention-to-treat population. This trial is registered with ClinicalTrials.gov, NCT02450331, and is ongoing but not recruiting patients.
Findings
Between October 5, 2015, and July 30, 2018, we enrolled 809 patients, of whom 406 were assigned atezolizumab, and 403 were assigned observation. Median follow-up was 21·9 months (interquartile range: 13·2–29·8). Median disease-free survival with atezolizumab and observation was 19·4 (95% CI: 15·9–24·8) and 16·6 (11·2–24·8) months, respectively; the hazard ratio was 0·89 (95% CI, 0·74–1·08; P=0·24). The most common grade 3 or 4 adverse events were urinary tract infection (occurring in 31 of 390 [8%)] treated patients in the atezolizumab and 20 [5%] of 397 in the observation arm), as well as pyelonephritis (in 12 (3%]) vs 14 (4%]) and anaemia (in 8 [2%] vs 7 [2%]). Serious adverse events occurred in 122 patients (31%) who received atezolizumab and 71 (18%) who underwent observation. Sixty-three patients who received atezolizumab (16%) had a treatment-related grade 3 or 4 adverse event. One treatment-related grade 5 adverse event of acute respiratory distress syndrome was observed in the atezolizumab arm.
Interpretation
IMvigor010—to our knowledge the largest, first-completed phase 3 adjuvant study to evaluate the role of a checkpoint inhibitor in muscle-invasive urothelial carcinoma—did not meet its primary endpoint of improved disease-free survival over observation. Atezolizumab was generally tolerable, with no new safety signals; however, higher frequencies of adverse events leading to discontinuation were reported than in metastatic urothelial carcinoma studies. These data do not support the use of adjuvant checkpoint inhibitor therapy in the setting evaluated in IMvigor010 at this time.
Funding
F. Hoffmann-La Roche Ltd./Genentech, Inc.
Introduction
Muscle-invasive urothelial carcinoma of the bladder or upper urinary tract has a high recurrence risk despite treatment with curative-intent therapy. Locoregional or distal recurrence or metastasis tend to occur within 2 years of radical cystectomy in approximately half of patients.1-3 Radical surgery (cystectomy for bladder tumours or nephroureterectomy for upper tract tumours [≈10% of patients with urothelial cancer]) with lymph node dissection remains the current standard of care. In urothelial carcinoma of the bladder, neoadjuvant cisplatin-based combination chemotherapy has been shown to provide an overall survival benefit in patients eligible for cisplatin compared with surgery alone4-7 and its use is increasing.8 In upper tract urothelial carcinoma, the use of either neoadjuvant or adjuvant chemotherapy have shown clinical benefit in preliminary clinical trials and meta-analyses, but definitive overall survival data from phase 3 trials are pending at this time.7,9 However, up to half of patients are cisplatin ineligible due to renal insufficiency and/or other clinical factors10 or decline treatment and are treated with radical surgery alone.
Patients with high-risk features at the time of surgery have a substantial risk of recurrence and mortality.4,8 However, no standard-of-care adjuvant therapy exists for these high-risk patients. Although cisplatin-based adjuvant chemotherapy is frequently used (if not previously given as neoadjuvant therapy), guideline recommendations vary in levels of consensus.4-6,11 Multiple clinical trials have been conducted in this setting, but either have not completed their planned accrual or have not demonstrated a definitive overall survival benefit for adjuvant therapy.12-15
Since the late 2010s, several immune checkpoint inhibitors have been approved for metastatic urothelial cancer16-22 and non–muscle-invasive bladder cancer.23 Specifically, the anti–programmed death-ligand 1 (PD-L1) immune checkpoint inhibitor atezolizumab is indicated for locally advanced or metastatic urothelial carcinoma in certain types of patients—patients with previously platinum-treated disease, cisplatin-ineligible disease with PD-L1 expression (PD-L1–stained tumour-infiltrating immune cells covering ≥ 5% of the tumour area), and, in the United States, platinum-ineligible disease—among other solid tumour types.24,25 However, these agents have not been investigated as adjuvant therapy in the context of a phase 3 trial. To test the hypothesis that adjuvant atezolizumab monotherapy would reduce, compared with observation, the risk of recurrence or death after radical surgery in patients with high-risk muscle-invasive urothelial carcinoma, we conducted the open-label, multicentre, randomised phase 3 trial IMvigor010. Here we report the primary analysis from this study.
Methods
Study design and participants
In our randomised, open-label IMvigor010 trial, we enrolled patients from 24 countries or regions. Eligible patients were aged ≥ 18 years and had histologically confirmed muscle-invasive urothelial carcinoma of either the bladder or, following a protocol amendment, the upper tract (i.e., urothelial carcinoma of the renal pelvis or ureters). Patients with mixed histology were required to have a dominant urothelial carcinoma pattern. Enrolment of patients with upper tract urothelial carcinoma was capped at 10% of the study population to reflect the real-world distribution of patients. Patients who did not receive prior neoadjuvant chemotherapy (or only received 1 cycle of a platinum-based regimen) must have declined or been ineligible for cisplatin-based adjuvant chemotherapy based on impaired renal function (glomerular filtration rate < 60 mL/min), grade 2 or greater hearing loss, grade 2 or greater peripheral neuropathy, or Eastern Cooperative Oncology Group performance status of 2. Patients were required to have undergone radical surgical resection (cystectomy for patients with bladder cancer or nephroureterectomy for patients with upper tract urothelial carcinoma) with lymph node dissection, to the extent deemed necessary by the treating surgeon, with negative surgical margins ≤ 14 weeks before enrolment (≤ 12 weeks per earlier protocol version). For patients with upper tract disease, excision of bladder cuff was required. Based on pathologic stage per tumour-node-metastasis (TNM) classification (Union for International Cancer Control–American Joint Committee on Cancer 7th edition), the disease must have been M0 and either (1) ypT2-4a or ypN+ (ypT2-4 or ypN+ for upper tract urothelial cancer) in patients treated with prior neoadjuvant chemotherapy or (2) pT3-4a or pN+ (pT3-4 or pN+ for upper tract disease) in patients without prior neoadjuvant chemotherapy. These criteria for patient selection aimed to focus on a population at high risk for recurrence (with an expected 5-year recurrence risk of approximately 50%). The absence of pathologic residual disease and the absence of metastasis—as confirmed by negative postoperative radiologic imaging, an Eastern Cooperative Oncology Group performance status ≤ 2, and adequate hematologic and end-organ function—were required for enrolment. Patients with a history of autoimmune disease, ongoing infection, or significant cardiovascular disease (New York Heart Association Class II or greater) were excluded. Patients with a history of autoimmune-related hypothyroidism or Type 1 diabetes mellitus were eligible provided that they were on a stable dose of thyroid replacement hormone and/or stable dose of insulin. Central evaluation of a surgical resection or lymph node sample for PD-L1–expression testing using the VENTANA SP142 IHC assay (Ventana Medical Systems, Oro Valley, Arizona, USA) was also required. All patients were required to submit a sample at the time of cystectomy or nephroureterectomy (post-neoadjuvant chemotherapy for applicable patients); PD-L1 status was determined from this sample. Adequate hematologic and end-organ function laboratory defined by laboratory test results obtained within 14 days prior to the first study treatment.
In IMvigor010 enrolment was initially restricted to patients whose tumours expressed PD-L1 (IC2/3 status, i.e., PD-L1–expressing tumour-infiltrating immune cells covering ≥ 5% of the tumour area); however, on the basis of IMvigor210 cohort 120 results that demonstrated that clinical activity was not dependent on PD-L1 status, we amended the protocol to expand enrolment to patients regardless of PD-L1 status. Adjuvant chemotherapy or radiation therapy for urothelial carcinoma following surgical resection was not allowed, and for patients with upper tract urothelial carcinoma, post-surgical intravesical chemotherapy or bacille Calmette-Guérin was not allowed based on treatment patterns in clinical practice at the time of study design.
The trial was conducted according to Good Clinical Practice and the Declaration of Helsinki. All patients provided written informed consent. Protocol approval was obtained from independent review boards or ethics committees at each site. For the study protocol, see the Appendix.
Randomisation and masking
Patients were enrolled by the investigators and randomised 1:1 to atezolizumab or observation using a permuted block method and interactive voice-web response system. Randomisation was stratified by number of lymph nodes resected (< 10 vs ≥ 10), nodal status (positive vs negative), tumour stage after surgical resection (≤ pT2 vs pT3/pT4), PD-L1 status (IC0/1 [PD-L1–expressing tumour-infiltrating immune cells covering < 5% of the tumour area)] vs IC2/3), and prior neoadjuvant chemotherapy (yes vs no).
Procedures
Every 3 weeks, patients were either treated with intravenous atezolizumab 1200 mg or observed; this continued for a total of 16 cycles or 1 year, whichever occurred first, or until recurrence (as determined by the investigator based on radiographic evidence with confirmatory biopsy), unacceptable toxicity, withdrawal of consent, or study termination by the sponsor. Disease recurrence was determined by the investigator based on radiographic evidence. Imaging assessments for recurrence were performed at baseline and every 12 weeks in the first year following randomisation; upon completion of the period of treatment or observation, surveillance for tumour recurrence was performed every 12 weeks for years 2 and 3, every 24 weeks for years 4 and 5, and at year 6. In the absence of a disease-free survival event (defined below), surveillance for tumour recurrence was conducted regardless of whether the patient had started a new anticancer therapy, until withdrawal of consent, loss to follow-up, or study termination, whichever occurred first. Disease recurrence assessments followed the same schedule (every 12 weeks) for patients in the observation arm as for those in the atezolizumab arm. Safety assessments were performed every 3 weeks by means of formal clinic visits alternating with telephone calls. Crossover was not permitted as part of the protocol; however, subsequent non-protocol therapies were permitted after disease recurrence and were recorded during study follow-up. Dose reductions of atezolizumab were not permitted. Patients could temporarily suspend study treatment for up to 42 days beyond the last dose if they experienced adverse events that required a dose to be held. Further details are included in the study protocol. Patients with disease recurrence continued to be followed for survival until death, withdrawal of consent, or loss to follow-up. Patients could withdraw consent at any time or if the investigator or sponsor determined that continuing in the study could jeopardise the patient’s safety or that discontinuation was in the best interest of the patient. All adverse events were recorded. Serious adverse events and adverse events of special interests were recorded until 90 days after the last dose of study drug or initiation of another anti-cancer therapy; serious adverse events that were deemed to be related to study drug treatment or study procedure could be reported after the 90 day reporting period. All other adverse events were recorded until 30 days after the last dose of study drug.
Outcomes
The primary efficacy endpoint was investigator-assessed disease-free survival. A disease-free survival event was defined by local (pelvic) or urinary tract recurrence or distant urothelial carcinoma metastasis or death from any cause. Overall survival, defined as the time from randomisation to death from any cause, was a secondary efficacy endpoint. Safety was evaluated per the National Cancer Institute Common Terminology Criteria for Adverse Events, Version 4·0. Additional trial endpoints that the study was not powered to test formally included disease-specific survival, distant metastasis-free survival, and non-urinary tract recurrence-free survival (secondary efficacy endpoints for which data were not yet mature at the time of primary analysis), as well as pharmacokinetic evaluations, patient reported outcomes (per EuroQol 5-dimension, 5-level version), and exploratory biomarkers. Efficacy analyses were performed based on the intention-to-treat population (defined as all randomised patients, whether or not the patient received the assigned treatment), and the safety population was defined as patients who either received at least one dose of atezolizumab or had at least one post-baseline safety assessment (regardless of their assigned treatment).
Statistical analysis
All statistical analyses were done using SAS (version 9.4). IMvigor010 was designed to enrol 800 patients to evaluate the primary endpoint, investigator-assessed disease-free survival (see Supplementary Methods). A definitive analysis of disease-free survival in the intention-to-treat population was planned (to take place after ≈377 events), at which time an interim analysis of overall survival was also planned to occur. During the course of the study, the timing of the disease-free survival analysis was updated in order to ensure a minimum of 12 months of follow-up from last patient enrolled for efficacy and safety. The following assumptions were used. Type I error was controlled for disease-free survival and overall survival at α=0·05 (two-sided), and treatment arms were compared in a hierarchical fashion: if results from the disease-free survival analysis were statistically significant at α=0·05, the analysis of overall survival was to be performed at α=0·05, with interim boundaries for significance calculated based on α=0·05. The trial had at least 80% power for the primary analysis of disease-free survival in the intention-to-treat population, with a hazard ratio for disease recurrence or death of 0·75 (corresponding to median disease-free survival durations of 26·7 months in the atezolizumab arm and 20 months in the observation arm). The median disease-free survival duration for the observation arm was estimated based on the population anticipated to be enrolled (10% T2N0; 55% T3/T4N0; 35% T[any]N+, extrapolated from references 2, 15, and 26. The trial had 80% power for the analysis of overall survival, with a hazard ratio for death of 0·76 (corresponding to median overall survival durations of 44·7 months in the atezolizumab arm and 34 months in the observation arm). Three analyses of overall survival were planned (two interim analyses and one final analysis). Disease-free survival and overall survival were compared between treatment groups using the log-rank test (stratified by nodal status, post-resection tumour stage, and PD-L1 status). Hazard ratios for recurrence or death were estimated using a stratified Cox proportional-hazards model. The proportional hazards assumption for the Cox model was assessed visually. Kaplan-Meier methodology was applied to disease-free survival and overall survival, with 95% CIs constructed by Brookmeyer-Crowley methodology for medians or by Greenwood formula for analyses at annual timepoints.
As a prespecified exploratory analysis, efficacy was examined in subgroups defined by demographic and baseline characteristics. In a post hoc exploratory analysis, the effect of baseline covariates on the prognosis for disease-free survival was evaluated in the observation arm patients with multivariable Cox model; factors were evaluated initially in a univariable model, one at a time at significance level α=0·05, followed by stepwise selection at α=0·05 significance level for factors retained in the final multivariable model.
An independent data monitoring committee reviewed safety and trial conduct every 6 months. The trial is registered with ClinicalTrials.gov, NCT02450331.
Role of the funding source
The trial sponsor, F. Hoffmann-La Roche/Genentech, Inc., provided the study drug, atezolizumab, and support for the trial and collaborated with authors on trial design, data collection, analysis, interpretation and writing of the report. All authors affirm the accuracy and completeness of the data and that trial was conducted per protocol. NND, VD and YS had full access to the raw data, and all authors had access to all the data from the study. The corresponding author had access to all data from the study and the final responsibility to submit for publication. All authors contributed to preparation of the manuscript with editorial assistance provided by professional medical writers funded by the sponsor and gave final approval to submit the manuscript.
Results
Between October 5, 2015, and July 30, 2018, 809 patients from 24 countries or regions were enrolled in 192 centres. As of the clinical cutoff date, November 30, 2019, 406 patients randomised to atezolizumab and 403 randomised to observation were evaluated in the intention-to-treat population (Figure 1). Overall, out of the 406 patients in the atezolizumab arm vs 403 patients in the observation arm, 160 (39%) vs 179 (44%) discontinued treatment vs observation, 39 (10%) vs 50 (12%) withdrew from the study, 7 (2%) vs 9 (2%) were lost to follow-up and 114 (28%) vs 120 (30%) died, respectively. One hundred nineteen patients with IC2/3 tumours were enrolled under a protocol with PD-L1 IC selection criteria, and 690 patients were enrolled following an amendment that removed requirements for PD-L1 IC2/3 status (Table S2). Baseline characteristics were generally well balanced (Table 1), with the exception of a slightly higher frequency of higher age-adjusted Charlson Comorbidity index (≥ 4) in the atezolizumab arm compared with the observation arm (210 patients [53%] out of 400 patients in the atezolizumab arm vs 190 [47%] out of 401 patients in the observation arm). Four hundred sixty-three patients (57%) out of 809 patients were reported to have undergone standard lymph node dissection, 187 patients (23%) out of 809 patients had extended dissection, 65 patients (8%) out of 809 patients had limited dissection, with the remaining 94 patients (12%) of 809 being unreported. Of the 809 patients enrolled, 28 (3.5%) patients were enrolled despite at least one exclusion criterion. Most notably, 3 patients (0.4%) had known autoimmune disease and 2 patients (0.2%) had a history of other malignancies within the last 5 years. Further, 47 (5.8%) patients were enrolled despite not meeting at least one inclusion criterion, 4 patients (0.5%) did not have an eligible histology type or muscle-invasive disease stage and 4 patients (0.5%) had residual disease or metastases at baseline.
Figure 1.
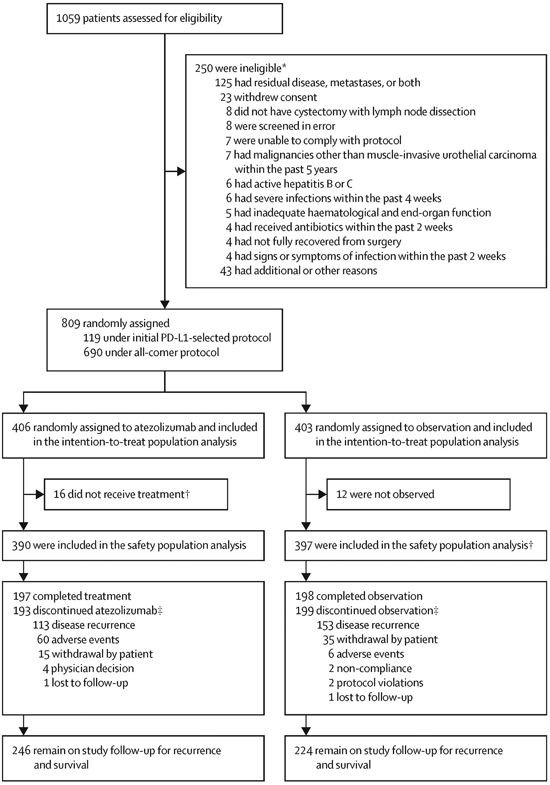
Randomisation and trial populations. *For patients who were screened more than once, reasons for ineligibility refer to the first screening. For patients who were initially ineligible but later re-screened and became eligible, reasons are not included.
Table 1.
Characteristics of the patients at baseline
| Intention-to-treat population | ||
|---|---|---|
| Atezolizumab (n = 406) |
Observation (n = 403) |
|
| Median age (interquartile range), years | 67 (60-72) | 66 (60-73) |
| Race | ||
| White | 320 (79%) | 307 (76%) |
| Asian | 64 (16%) | 68 (17%) |
| Black or African American | 3 (1%) | 3 (1%) |
| American Indian or Alaska Native | 1 (< 1%) | 0 |
| Other/Unknown | 18 (4%) | 25 (6%) |
| Male | 322 (79%) | 316 (78%) |
| Region | ||
| North America | 115 (28%) | 126 (31%) |
| Europe | 227 (56%) | 210 (52%) |
| Asia | 61 (15%) | 64 (16%) |
| Australia | 3 (1%) | 3 (1%) |
| Primary tumour site | ||
| Bladder | 377 (93%) | 378 (94%) |
| Upper tract (ureter, renal pelvis) | 29 (7%) | 25 (6%) |
| Pathologic tumour stage* | ||
| ≤ pT2 | 104 (26%) | 101 (25%) |
| pT3/4 | 302 (74%) | 302 (75%) |
| Pathologic nodal status* | ||
| Positive | 212 (52%) | 208 (52%) |
| Negative | 194 (48%) | 195 (48%) |
| Tumour stage and N0 nodal status† | ||
| pT2N0 | 34 (8%) | 39 (10%) |
| pT3N0 | 124 (31%) | 119 (30%) |
| pT4N0 | 32 (8%) | 33 (8%) |
| No. of lymph nodes resected* | ||
| < 10 | 95 (23%) | 94 (23%) |
| ≥ 10 | 311 (77%) | 309 (77%) |
| Eastern Cooperative Oncology Group performance status | ||
| 0 | 248 (61%) | 259 (64%) |
| 1 | 142 (35%) | 130 (32%) |
| 2 | 16 (4%) | 14 (3%) |
| Age-adjusted Charlson Comorbidity Index‡ | ||
| 0-1 | 55 (14%) | 61 (15%) |
| 2-3 | 135 (34%) | 150 (37%) |
| ≥ 4 | 210 (53%) | 190 (47%) |
| PD-L1 IHC status*,§ | ||
| IC0/1 | 210 (52%) | 207 (51%) |
| IC2/3 | 196 (48%) | 196 (49%) |
| Prior neoadjuvant chemotherapy* | ||
| Yes | 196 (48%) | 189 (47%) |
| No | 210 (52%) | 214 (53%) |
Data are n (%) unless otherwise stated. Percentages may not add to 100% due to rounding.
Per interactive voice/web response system.
Per electronic case report form.
Percentages based on 400 patients in the atezolizumab arm and 401 patients in the observation arm.
Archival and/or fresh pre-treatment formalin-fixed paraffin-embedded tumour tissue from all patients (surgical resection or lymph node dissection) were prospectively tested for PD-L1 status per a central laboratory and used as a stratification factor. A total of 119 patients were enrolled under a protocol using IC2/3 selection; 690 patients were enrolled under an “all-comer” protocol that enrolled 273 patients with IC2/3 tumours (40%) and 417 patients with IC0/1 tumours (60%).
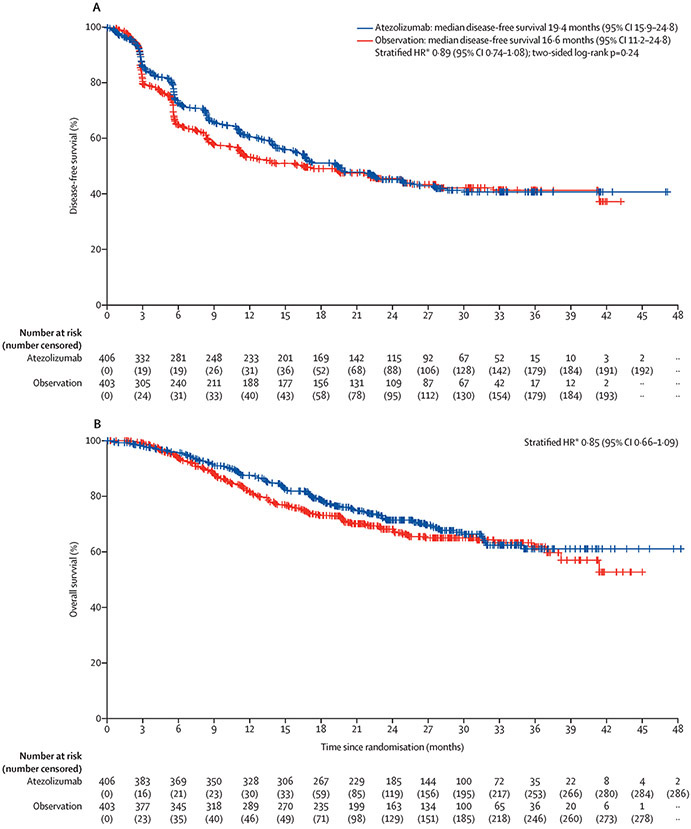
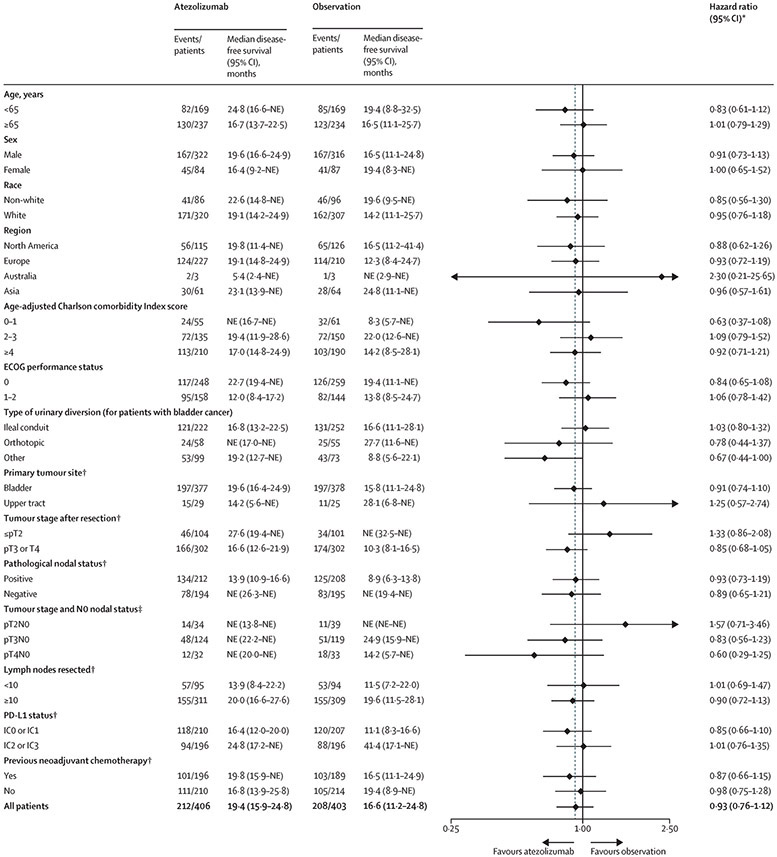
Median follow-up in the intention-to-treat population was 21·9 months (interquartile range [25%,75%], 13·2–29·8). A total of 212 (52%) out of 406 patients in the atezolizumab arm and 208 out of 403 patients (52%) in the observation arm experienced urothelial carcinoma recurrence or died. The stratified hazard ratio for recurrence or death for atezolizumab vs observation was 0·89 (95% CI, 0·74–1·08; P=0·24; Figure 2A). Median disease-free survival was 19·4 months (95% CI, 15·9–24·8) in the atezolizumab arm and 16·6 months (95% CI, 11·2–24·8) in the observation arm (Figure 2A). In prespecified patient subgroups, disease-free survival was similar, with no treatment benefit observed based on PD-L1 status (Figure 3, Figure S1). The majority of recurrences occurred as distant recurrences, with no major difference between the atezolizumab and observation arms (see Table S3 for further detail on recurrence patterns). In an ad-hoc exploratory analysis, univariable and multivariable analyses revealed that lower PD-L1 status, higher tumour stage, and positive nodal status were significant determinants of disease-free survival prognosis for patients in the observation arm (Table S4).
Figure 2.
Kaplan-Meier plots for (A) investigator-assessed disease-free survival and (B) overall survival in the intention-to-treat population. HR, hazard ratio. *Stratified by post-resection tumour stage, nodal status, and programmed death-ligand 1 status. †Two-sided.
Figure 3.
Forest plot analyses of disease-free survival in key subgroups. Median follow-up in the intention-to-treat population was 21·9 months (interquartile range 13·2-29·8); thus, observed median disease-free survival durations longer than 21·9 months may be unstable and subject to change with longer follow-up. Tumor stage after resection refers to pathologic staging at cystectomy or nephroureterectomy. DFS, disease-free survival; HR, hazard ratio; IHC, immunohistochemistry; NE, not estimable; PD-L1, programmed death-ligand 1. *Unstratified analyses. †Per interactive voice/web response system. ‡Per electronic case report form.
At the data cutoff and first interim analysis of overall survival in the intention-to-treat population, 118 (29%) out of 406 patients in the atezolizumab arm and 124 out of 403 patients in the observation arm (31%) had died. The stratified hazard ratio for death for atezolizumab versus observation was 0·85 (95% CI, 0·66–1·09; Figure 2B). Formal testing of overall survival as the secondary endpoint was not permitted based on the hierarchical study design; the median overall survival was not reached. The 12-month overall survival rate was 88% (95% CI, 84–91%) in the atezolizumab arm and 81% (95% CI, 78–85) in the observation arm; the 18-month overall survival rates were 79% (95% CI, 75–83) and 73% (95% CI, 69–78), respectively (Figure 2B).
Subsequent anticancer therapy was administered to 127 out of 406 patients (31%) and 138 out of 403 patients (34%) in the atezolizumab and observation arms, respectively (Table S5); the most common subsequent non-protocol therapies included chemotherapy (108 out of 406 [27%] patients in the atezolizumab arm vs 99 out of 403 patients [25%] in the observation arm), immunotherapy (35 out of 406 patients [9%] in the atezolizumab arm vs 84 out of 403 patients [21%] in the observation arm), and targeted therapy (22 out of 406 patients [5%] vs 7 out of 403 [2%] patients in the atezolizumab and observation arms, respectively).
Of the 390 patients randomised to the atezolizumab arm, 6 patients did not receive treatment, but had post-baseline safety assessments; therefore, they were evaluated in the safety population of the observation arm. The population evaluated for safety included 390 patients in the atezolizumab arm and 397 patients in the observation arm; a total of 197 out of 390 (51%) and 198 out of 397 (50%) patients completed treatment or observation, respectively. Median treatment duration for atezolizumab was 10·3 months (interquartile range, 3·5-10·5). Out of 390 patients, a total of 141 patients (36%) received atezolizumab for ≤ 6 months, 40 (10%) for > 6 to ≤ 9 months, and 204 (52%) for > 9 to ≤ 12 months. Most patients discontinued treatment due to recurrence (113 out of 390 patients [29%] in the atezolizumab arm; 153 out of 397 patients [39%] in the observation arm) or adverse events (60 out of 390 patients [15%] and 6 out of 397 patients [2%] in the atezolizumab and observation arms, respectively). A total of 114 out of 390 patients who received atezolizumab (29%) and 124 out of 397 patients (31%) who underwent observation, had died. Most deaths were due to recurrence (n = 88 out of 114 [77%] in the atezolizumab arm and n = 96 out of 124 [77%] in the observation arm) rather than due to adverse events of any attribution (n = 7 out of 114 [6%] and 8 out of 124 [7%], respectively) other reasons (n = 16 out of 114 [14%] and 16 out of 124 [13%], respectively). Deaths that occurred after the adverse event reporting period that were not treatment related or due to disease recurrence were classified as other. One out of 114 patients (< 1%) in the atezolizumab arm died as a result of a treatment-related adverse event (acute respiratory distress syndrome) (Table 2).
Table 2.
Treatment-related adverse events*
| Atezolizumab (n = 390) | ||||
|---|---|---|---|---|
| Grade 1-2 | Grade 3 | Grade 4 | Grade 5 | |
| All adverse events | 212 (54%) | 58 (15%) | 5 (1%) | 1 (<1%) |
| Pruritus | 73 (19%) | 2 (1%) | 0 | 0 |
| Fatigue | 62 (16%) | 1 (<1%) | 0 | 0 |
| Diarrhoea | 34 (9%) | 3 (1%) | 0 | 0 |
| Rash | 32 (8%) | 1 (<1%) | 0 | 0 |
| Arthralgia | 22 (6%) | 5 (1%) | 0 | 0 |
| Asthenia | 20 (5%) | 3 (1%) | 0 | 0 |
| Pyrexia | 21 (5%) | 2 (1%) | 0 | 0 |
| Infusion-related reaction | 18 (5%) | 2 (1%) | 0 | 0 |
| Alanine aminotransferase increased | 14 (4%) | 4 (1%) | 0 | 0 |
| Aspartate aminotransferase increased | 12 (3%) | 2 (1%) | 0 | 0 |
| Rash maculo-papular | 9 (2%) | 2 (1%) | 0 | 0 |
| Anaemia | 7 (2%) | 2 (1%) | 0 | 0 |
| Pneumonitis | 4 (1%) | 2 (1%) | 0 | 0 |
| Colitis | 1 (<1%) | 4 (1%) | 0 | 0 |
| Lipase increased | 2 (1%) | 2 (1%) | 1 (<1%) | 0 |
| Amylase increased | 3 (1%) | 2 (1%) | 0 | 0 |
| Acute kidney injury | 1 (<1%) | 2 (1%) | 0 | 0 |
| Urinary tract infection | 1 (<1%) | 2 (1%) | 0 | 0 |
| Autoimmune hepatitis | 0 | 2 (1%) | 0 | 0 |
| Immune-mediated enterocolitis | 0 | 2 (1%) | 0 | 0 |
| Systemic immune activation | 0 | 2 (1%) | 0 | 0 |
| Small intestine ulcer | 0 | 0 | 1 (<1%) | 0 |
| Bacterial sepsis | 0 | 0 | 1 (<1%) | 0 |
| Neuroborreliosis | 0 | 0 | 1 (<1%) | 0 |
| Hyperamylasaemia | 0 | 0 | 1 (<1%) | 0 |
| Hyperlipasaemia | 0 | 0 | 1 (<1%) | 0 |
| Acute respiratory distress syndrome | 0 | 0 | 0 | 1 (<1%) |
| Myocardial infarction | 0 | 1 (<1%) | 0 | 0 |
| Adrenal insufficiency | 0 | 1 (<1%) | 0 | 0 |
| Endocrine pancreatic disorder | 0 | 1 (<1%) | 0 | 0 |
| Proctitis | 0 | 1 (<1%) | 0 | 0 |
| Stomatitis | 2 (1%) | 1 (<1%) | 0 | 0 |
| Lithiasis | 0 | 1 (<1%) | 0 | 0 |
| Hepatitis | 0 | 1 (<1%) | 0 | 0 |
| Liver disorder | 0 | 1 (<1%) | 0 | 0 |
| Hypersensitivity | 1 (<1%) | 1 (<1%) | 0 | 0 |
| Hepatic enzyme increased | 0 | 1 (<1%) | 0 | 0 |
| Decreased appetite | 17 (4%) | 1 (<1%) | 0 | 0 |
| Diabetes mellitus | 0 | 1 (<1%) | 0 | 0 |
| Hypokalaemia | 2 (1%) | 1 (<1%) | 0 | 0 |
| Arthritis | 2 (1%) | 1 (<1%) | 0 | 0 |
| Myalgia | 10 (3%) | 1 (<1%) | 0 | 0 |
| Polymyalgia rheumatica | 1 (<1%) | 1 (<1%) | 0 | 0 |
| Headache | 16 (4%) | 1 (<1%) | 0 | 0 |
| Peripheral neuropathy | 3 (1%) | 1 (<1%) | 0 | 0 |
| Autoimmune nephritis | 0 | 1 (<1%) | 0 | 0 |
| Hydronephrosis | 0 | 1 (<1%) | 0 | 0 |
| Nephritis | 2 (1%) | 1 (<1%) | 0 | 0 |
| Renal injury | 0 | 1 (<1%) | 0 | 0 |
| Tubulointerstitial nephritis | 0 | 1 (<1%) | 0 | 0 |
| Pulmonary embolism | 1 (<1%) | 1 (<1%) | 0 | 0 |
| Dermatitis allergic | 0 | 1 (<1%) | 0 | 0 |
| Drug eruption | 0 | 1 (<1%) | 0 | 0 |
| Palmar-plantar erythrodysaesthesia syndrome | 0 | 1 (<1%) | 0 | 0 |
| Rash papular | 1 (<1%) | 1 (<1%) | 0 | 0 |
Data are n (%).
Grade 1-2 treatment-related adverse events in ≥ 10% of patients in either arm, and grade 3, 4 or 5 treatment-related adverse events in all patients are shown.
The majority of adverse events were low grade (Tables 2 and S6-9). Adverse events of any cause occurred in 368 out of 390 patients (94%) in the atezolizumab arm and 313 out of 397 patients (79%) in the observation arm. The most common grade 3 or 4 adverse events observed were urinary tract infection, occurring in 31 of 390 (8%) and 20 (5%) of 397 treated patients in the atezolizumab and observation arms, respectively]), as well as pyelonephritis (in 12 (3%]) and 14 (4%]) and anaemia (in 8 [2%] and 7 [2%]). Serious adverse events occurred in 122 patients (31%) who received atezolizumab and 71 (18%) who underwent observation. Adverse events deemed related to treatment were recorded only for the atezolizumab arm, and those of all grades occurred in 276 (71%) out of 390 atezolizumab-treated patients. The most common treatment-related adverse events of any grade that occurred in more than 30 (8%) out of 390 patients in the atezolizumab arm were pruritus (n = 75, 19%), fatigue (n = 63, 16%), diarrhoea (n = 37, 10%), rash (n = 33, 9%), and hypothyroidism (n = 31, 8%). A total of 63 (16%) out of 390 patients had a grade 3 or 4 treatment-related adverse event. Serious adverse events related to treatment occurred in 41 out of 390 patients (11%) in the atezolizumab arm, with pyrexia being the most frequently occurring treatment-related serious adverse event, observed in 6 out of 390 patients (2%).
A total of 127 out of 390 patients treated with atezolizumab (33%) had an adverse event leading to dose interruption. A total of 61 out of 390 atezolizumab-treated patients (16%) had an adverse event leading to treatment discontinuation (Table S9); the most common events were colitis (in 6 patients [2%]) and pruritus (in 4 patients [1%], out of 390 patients).
Grade 3-4 adverse events of special interest occurred in 37 out of 390 patients (10%) in the atezolizumab arm and 4 out of 397 patients (1%) in the observation arm (Table S8); none of the adverse events of special interest led to patient death. Ten out of 390 patients the atezolizumab arm (3%) and 1 out of 397 patients in the observation arm (< 1%) had an adverse event of special interest requiring systemic corticosteroids.
Discussion
To our knowledge, IMvigor010 was the first randomised phase 3 trial to investigate the utility of a checkpoint inhibitor in high-risk muscle-invasive urothelial carcinoma—to determine whether adjuvant atezolizumab could reduce recurrence following primary therapy, compared with observation alone. The study did not meet its primary endpoint of disease-free survival. No pre-specified subgroups, including those defined by PD-L1 status, primary tumour site, or nodal status showed significant treatment benefit with atezolizumab. Overall survival follow-up is currently immature and ongoing; additional exploratory biomarker and subgroup analyses warrant further study. The safety profile for atezolizumab monotherapy was consistent with that in prior studies in the advanced setting, albeit with higher frequencies of immune-mediated adverse events of special interest and adverse events leading to discontinuation.
To our knowledge, IMvigor010 was the first fully enrolled and completed adjuvant study of an immune checkpoint inhibitor in muscle-invasive urothelial carcinoma and also the first such study of an anti–PD-L1 agent. This large global study was able to enrol patients more quickly (patients enrolled between October 5, 2015, and July 30, 2018) than historic adjuvant studies, many of which were prematurely terminated. IMvigor010 enrolled adequately surgically treated patients; up to 80% of patients had optimal lymph node dissection. However, the enrolled population comprised a higher-risk population than anticipated on the basis of prior adjuvant studies (eg, approximately 85% of study participants were found to be either pT3/T4 or pN+ at surgical resection). In IMvigor010, patients with ypT2-stage who received neoadjuvant chemotherapy (non-responders) were included as a high-risk population, which is a definition beyond that specified by the National Comprehensive Care Network guidelines. The prior receipt of neoadjuvant chemotherapy in the study population (50%) was also higher than expected compared with historical and real-world data (approximately 20%).8,27 The effects of these above characteristics on study outcomes are not clear, although they may have contributed to median disease-free survival in the observation arm being lower than expected.
Immunotherapy is now an established treatment option for advanced urothelial carcinoma—as switch maintenance therapy in patients benefiting from platinum-based chemotherapy, as first-line treatment for certain patients, and for the treatment of patients who progressed on platinum. Phase 1-2 studies of neoadjuvant immunotherapy have also reported high rates of pathologic complete response of approximately 30–45%,28-30 but correlation with survival benefit is yet to be confirmed· One explanation why treatment benefit with atezolizumab was not observed in the adjuvant setting could be related to the absence of tumour burden (eg, micro-metastases)—and thus neoantigen, required to stimulate anti-tumour T-cell activity—although similar findings in melanoma have not been observed. This hypothesis is in line with enhanced benefit suggested here in patients with high tumour burden (pT3/T4 in contrast from patients with ≤ pT2 subgroup) and will be explored further through collective efforts from ongoing studies spanning several checkpoint inhibitors and combinations in the perioperative setting (NCT03244384, NCT02632409, NCT03661320, NCT04209114, NCT03924895, NCT03924856, NCT03732677, NCT03472274).
In our study, PD-L1 expression on tumour-infiltrating immune cells did not appear to influence disease-free survival benefit but instead was a positive prognostic factor for disease-free survival, consistent with studies in metastatic urothelial carcinoma using the VENTANA SP142 IHC assay16,17 or the VENTANA SP263 assay,22 but not the 22C3 assay (which evaluates PD-L1 scoring using a different algorithm and cutoffs).21 Results from our multivariable analyses suggesting prognostic roles for PD-L1 status, as well as higher tumour stage and positive nodal status, are consistent with current knowledge. Consistent with results of studies in triple-negative breast cancer,31 PD-L1 status may be less relevant as a biomarker in early muscle-invasive urothelial carcinoma than in advanced disease—perhaps due to distinct tumour biology in different disease stages or due to PD-L1 expression in the tumour microenvironment being absent following resection or different from that in resected tumour samples. Further investigation of the tumour biology underlying responses to checkpoint inhibitors in the adjuvant setting and of other biomarkers (eg, tumour mutational burden, gene expression profiling) is warranted.
Safety was generally consistent with that observed in previous atezolizumab monotherapy studies, with no new safety signals identified and only 1 treatment-related death, of acute respiratory distress syndrome. Higher frequencies of adverse events of special interest with atezolizumab (mainly grade 1-2) were seen, but those leading to corticosteroid use were lower in IMvigor010 than in prior metastatic studies (eg, 8% in IMvigor130, arm B),17 consistent with results from prior adjuvant studies and potentially reflective of hesitation to use steroids in the adjuvant setting. Compared with patients in the metastatic setting, patients in the adjuvant setting may be more immuno-competent, rendering them more prone to immune-mediated adverse events in general. Adverse events leading to treatment discontinuation also occurred at a relatively higher frequency than in metastatic trials (eg, 6% in IMvigor130, arm B),17 with gastrointestinal and dermatologic adverse events being most common. In IMvigor010, baseline age-adjusted Charlson Comorbidity Index score was ≥ 4 in over half of patients, which reflects a population with higher co-morbidities and may have affected baseline tolerance for management of adverse events and completion of adjuvant therapy. The slight imbalance in age-adjusted Charlson Comorbidity Index score ≥ 4 between arms for this subset may have arisen from this criterion being removed as a randomisation stratification factor during the course of the study when the study was expanded to enrol an all-comer population to allow for inclusion of PD-L1 status as a stratification factor.
Collectively, these tolerability observations point to patients’ and physicians’ lower motivation to tolerate adverse events in an early-stage disease or the greater tendency to pursue other treatment options in the early-stage vs metastatic setting, consistent with observations in other adjuvant studies (eg, as seen with tyrosine kinase inhibitor use in renal cell carcinoma).32 Early treatment discontinuations may have limited the immune response necessary for an effective mechanism of action in this class of drugs that is sometimes associated with delayed onset of action.
IMvigor010 provides needed insights into the perioperative management of urothelial carcinoma as well as into trial design for checkpoint inhibitor studies; however, several points of note, including limitations, merit further consideration. It is the opinion of the authors that as of the clinical data cutoff, the minimum and median follow-up durations were sufficient for performing the primary analysis; disease-free survival results were considered mature, with all patients having completed treatment, and the median disease-free survival durations reached in both arms. However, this primary endpoint may not aptly describe clinical benefit with immunotherapy given the delayed onset of action. Another consideration is that not all patients with disease recurrence had confirmed biopsies (approximately 35% of recurrences were biopsy proven). In IMvigor010, although interim overall survival generally mirrored disease-free survival results, the data are not yet mature and overall survival could also not be formally tested as a secondary endpoint based on the primary disease-free survival findings; mature overall survival data will be reported in due course. Subsequent non-protocol checkpoint inhibitor use, higher in the observation arm, might also affect overall survival findings. Lack of pathologic complete response after neoadjuvant chemotherapy is a known surrogate for future poor outcomes and could also represent an improved measure of treatment efficacy in immunotherapy clinical trials given the mechanism of action of these agents; sequencing studies done prior to and following treatment identified potentially targetable genomic alterations that could help aid future development of customized therapies for non-responding patients.33 Defining optimal adjuvant therapy treatment paradigms for particular populations is an ongoing effort that contemporary prospective data from other studies are helping to address; for instance, two trials have demonstrated a disease-free survival benefit with adjuvant chemotherapy in patients with upper tract urothelial carcinoma,9,34 and one trial has demonstrated positive interim disease-free survival results with adjuvant checkpoint inhibitor in muscle-invasive urothelial carcinoma (NCT02632409), although overall survival data are not yet available. Notwithstanding, there are unmet needs for patients with muscle-invasive urothelial carcinoma who are not eligible for chemotherapy. Future biomarker-driven and/or clinically defined studies evaluating atezolizumab are warranted, as are investigations of checkpoint inhibitor mono- or combination therapy, in other urothelial carcinoma treatment settings and other early cancer types.
Supplementary Material
Research in Context.
Evidence before this study
We searched PubMed and major international congress presentations for articles published in English from March 1, 1991 to April 1, 2015, with MeSH search terms “muscle-invasive bladder cancer” or “muscle-invasive urothelial cancer” combined with “adjuvant” or “programmed cell death 1,” “PD-1,” programmed cell death-ligand 1,” “PD-L1,” “immune checkpoint inhibitor,” or “cancer immunotherapy.” This search identified that surgical resection was the mainstay treatment for muscle-invasive urothelial carcinoma and that neoadjuvant cisplatin-based chemotherapy in eligible patients appeared to improve survival, although a large number of patients tended to be ineligible due to pre-existing comorbidities or other clinical characteristics. Muscle-invasive urothelial carcinoma appeared to carry a high risk of recurrence and mortality, with an unmet need and no level 1 evidence in support of adjuvant therapy after radical cystectomy. Based on approvals for atezolizumab and related in-class agents in locally advanced or metastatic urothelial carcinoma, as well as favourable data for related agents as perioperative therapy in other cancers, investigation of atezolizumab in muscle-invasive urothelial carcinoma was warranted.
Added value of this study
To our knowledge, IMvigor010 is the first reported phase 3 adjuvant checkpoint inhibitor study to fully accrue and be completed in a broad population of patients with muscle-invasive urothelial carcinoma at a high risk of recurrence. Atezolizumab did not prolong disease-free survival in the intent-to-treat population compared with observation. The PD-L1 biomarker did not enrich for disease-free survival benefit favouring atezolizumab. The safety profile of atezolizumab was comparable to that seen with atezolizumab in prior studies in advanced cancer; however, slightly higher frequencies of adverse events of special interest were seen. The frequency of adverse events leading to treatment discontinuation were higher than in studies in advanced cancer and mostly consisted of grade 1 or 2 skin or gastrointestinal toxicities. These data do not support the use of atezolizumab in the setting evaluated in IMvigor010 at this time.
Implications of all the available evidence
Immune checkpoint inhibitors are tolerable and effective in advanced urothelial carcinoma and can be associated with long-term remissions—features that are attractive for the adjuvant setting in patients with high-risk muscle-invasive urothelial carcinoma. To our knowledge, IMvigor010 is the first completed adjuvant phase 3 trial using an anti-PD-L1/PD-1 agent to be reported, and it can provide insights into the underlying biology that may help predict benefit with immunotherapy in patients with urothelial carcinoma. The lack of treatment benefit with atezolizumab seen within the parameters of our study indicates that results cannot be extrapolated to the adjuvant setting and leaves open questions about the role of early immune checkpoint inhibition in the absence of visible disease. The relationships of PD-L1 status and other biomarkers require further evaluation.
Acknowledgments
The study was supported by F. Hoffmann-La Roche Ltd./Genentech, Inc., a member of the Roche Group. We thank the patients who participated in the trial and the clinical site investigators. Medical writing assistance for this manuscript was provided by Ashley J. Pratt, PhD, and Priscilla Hong, PharmD, of Health Interactions, Inc., and funded by F. Hoffmann-La Roche Ltd. Jonathan E Rosenberg acknowledges support from the National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748. Peter H O’Donnell is a National Institutes of Health grant recipient.
Footnotes
Data sharing
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
The investigators in the IMvigor010 study are listed in the Supplementary Appendix (Table S1), available at thelancet.com.
Declaration of interests
All authors report editorial support from F Hoffmann-La Roche. J Bellmunt received institutional research funding from Millennium, Pfizer/EMD Serono, and Sanofi, advisory or consulting fees from Astellas Pharma, AstraZeneca/MedImmune, Bristol-Myers Squibb, Genentech, Merck, Novartis, Pfizer, and Pierre Fabre, honoraria from UpToDate, stock ownership of Rainier, and travel, accommodation, or expenses support from Ipsen, MSD Oncology, and Pfizer. MH received research funding from AstraZeneca, Bayer, Genentech, and Pfizer, honoraria or advisory fees from Astellas Pharma, AstraZeneca, Bayer, Bristol Myers Squibb, Daiichi Sankyo, Genentech, PER, Pfizer, Projects in Knowledge, Research to Practice, and Sanofi/Genzyme, and travel, accommodation, or expenses support from Astellas Pharma, Bayer, Genentech/Roche, and Pfizer. MH also has two pending patents and one licensed patent with Imbio to disclose. JEG received consulting fees or honoraria from Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Janssen-Cilag, Merck, MSD, Pfizer, and Roche. PA received advisory/consulting fees and honoraria from MSD Oncology, Sanofi, and Roche/Genentech. SO received research funding from Ipsen and Sanofi, advisory/consulting fees and honoraria from Astellas, Bayer, Bristol Myers Squibb, Eisai, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, and Sanofi, and travel, accommodations, or expenses support from Bayer, Bristol Myers Squibb, Eisai, Merck Sharp & Dohme, Novartis, and Pfizer. DC received consulting fees from Astellas Pharma, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Ipsen, Janssen Oncology, Lilly, MSD Oncology, Novartis, Pfizer, Pierre Fabre, Roche/Genentech, and Sanofi, research funding support from Janssen Oncology, and travel, accommodation, or expenses support from AstraZeneca Spain, Bristol Myers Squibb, Pfizer, and Roche/Genentech. SD received honoraria from Ferring, MDxHealth Olympus, Pacific Edge, Photocure, QED Therapeutics, , and Spectrum Pharmaceuticals, advisory/consulting fees from Ferring, Photocure, QED Therapeutics, and Taris, and research funding, travel accommodation, or expenses support from Photocure. HN received research funding support from Ono and Chugai, advisory/consulting fees from Astellas Pharma, AstraZeneca, Bayer, Bristol Myers Squibb, Chugai, Janssen, and MSD, and participated in speakers’ bureaus for Astellas Pharma, AstraZeneca, Chugai, and MSD. MM is an employee of Genentech and has stock ownership of Roche. VD is an employee of Genentech and has stock ownership of Roche. SM is an employee of Genentech and has stock ownership of Roche. PG received consulting fees from AstraZeneca, Bayer, Bristol Myers Squibb, Clovis Oncology, Driver, EMD Serono, Exelixis, Foundation Medicine, GlaxoSmithKline, Genentech, Genzyme, Heron Therapeutics, Janssen, Merck, Mirati Therapeutics, Pfizer, Roche, Seattle Genetics and QED Therapeutics, participation in an educational program for Bristol Myers Squibb; and institutional research funding support from AstraZeneca, Bavarian Nordic, Bayer, Bristol Myers Squibb, Clovis Oncology, Debiopharm, Genentech, GlaxoSmithKline, Immunomedics, Kure It Cancer Research, Merck, Mirati Therapeutics, OncoGenex, Pfizer and QED Therapeutics. YS is an employee of Genentech and has stock ownership of Roche. AD received consulting fees from AstraZeneca and Nektar and advisory fees from Genentech, Merck, Seattle Genetics, PACT Pharma, and Janssen. PHO received honoraria from Astellas Pharma, Atheneum Partners, Dedham Group, FirstWord Publication, Genentech/Roche, Health Advances, Janssen, Merck, OncLive, Schlesinger Associates, and Seattle Genetics, advisory fees from Merck, research funding support from Acerta Pharma, AstraZeneca/MedImmune, Boehringer Ingelheim, Bristol Myers Squibb, Genentech/Roche, Janssen, Merck, and Seattle Genetics, travel, accommodations and expenses support from Genentech/Roche, Janssen, Merck, and Seattle Genetics/Astellas, and other support from Janssen, Nektar and NIH. JER has stock ownership of Illumina and Merck, honoraria from Chugai Pharma, advisory fees from Adicet Bio, Astellas Pharma, AstraZeneca, Bayer, BioClin Therapeutics, Boehringer Ingelheim, Bristol Myers Squibb, EMD Serono, GlaxoSmithKline, Inovio Pharmaceuticals, Janssen Oncology, Lilly, Merck, Mirati Therapeutics, Pfizer, Pharmacyclics, QED Therapeutics, Roche/Genentech, Seattle Genetics, research funding support from Astellas Pharma, AstraZeneca, Bayer, Genentech/Roche, Incyte, Novartis, QED Therapeutics, Seattle Genetics, and travel, accommodations, and expenses support from Bristol Myers Squibb and Genentech/Roche. JER also declares an institutional patent. DMG received grants to the institution from Genentech, Merck, Calithera, and Astellas and consulting fees or honoraria from Seattle Genetics/Astellas, Pfizer, Eisai, Merck, and Exelixis. DPP has stock ownership of Bellicum Pharmaceuticals and TYME, received consulting fees from Advanced Accelerator Applications, Amgen, Astellas Pharma, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Clovis Oncology, Exelixis, Incyte, Ipsen, Janssen, Lilly, Pfizer, Pharmacyclics, Roche, Seattle Genetics, and UroGen Pharma, research funding support from Advanced Accelerator Applications, Astellas Pharma, AstraZeneca, Bayer, Bristol Myers Squibb, Clovis Oncology, Endocyte, Genentech, Innocrin Pharma, Lilly, MedImmune, Merck, Novartis, Pfizer, Progenic, Roche, Sanofi, and Seattle Genetics, and personal fees for expert testimony from Celgene and Sanofi. JH-C received research support from Foundation Medicine and Genentech. J Bedke received consulting fees and honoraria from AstraZeneca, Astellas, Bristol Myers Squibb, Eisai, EUSA, Ipsen, Novartis, Roche, Pfizer, Merck, and Janssen. ARK has stock ownership from ECOM Medical, received consulting fees from AstraZeneca, Bayer, Bristol Myers Squibb, EMD Serono, Exelixis, Genentech, Novartis, and Pfizer, and has participated in speakers’ bureaus with Amgen, Astellas Medivation, AstraZeneca, Bristol Myers Squibb, Eisai, EMD Serono, Exelixis, Genentech/Roche, Janssen, Merck, Novartis, Pfizer, and Sanofi, research funding support from Astellas Pharma, AstraZeneca, Bavarian Nordic, Bayer, BeyondSpring Pharmaceuticals, BioClin Therapeutics, Bristol Myers Squibb, Clovis Oncology, Eisai, Epizyme, Exelixis, Genentech/Roche, Immunomedics, Janssen, Macrogenics, and Seattle Genetics, and travel, accommodations, and expenses support from Astellas Medivation, AstraZeneca, Bayer, Eisai, Exelixis, Genentech/Roche, Janssen, Novartis, Pfizer, and Prometheus. YZ has served on an advisory board for Amgen, Roche Diagnostics, Novartis, Janssen, Eisai, Exelixis, Castle Bioscience, Array, Bayer, Pfizer, Clovis, and EMD Serono, received institutional grant or research support for clinical trials from NewLink Genetics, Pfizer, Exelixis, and Eisai, and has served on a data and safety monitoring committee for Janssen Research and Development. MSvdH received research support from Bristol Myers Squibb, AstraZeneca, and Roche and consultancy fees to the institution from Bristol Myers Squibb, Merck/MSD, Roche, AstraZeneca, Seattle Genetics, and Janssen. CNS received honoraria from Astellas Pharma, AstraZeneca, Ipsen, Janssen, Pfizer, Sanofi Genzyme, Bayer, Bristol Myers Squibb, Clovis Oncology, Eisai, Incyte, Merck, MSD, Novartis, Roche, UroToday and Medscape. NND is an employee of Genentech, has stock ownership of Roche, and has received travel, accommodation, and expenses support from Genentech. TP received honoraria and consulting fees from Astellas, AstraZeneca, Bristol Myers Squibb, Eisai, Exelixis, Incyte, Ipsen, Johnson & Johnson, Merck, Merck Serono (EMD Serono), MSD, Novartis, Pfizer, Roche, and Seattle Genetics, research funding support from Astellas, AstraZeneca, Bristol Myers Squibb, Exelixis, Ipsen, Johnson & Johnson, Merck, Merck Serono (EMD Serono), MSD, Novartis, Pfizer, Seattle Genetics, , and Roche, and received travel, accommodation, or expenses support from AstraZeneca, MSD, Ipsen, Pfizer, and Roche.
Contributor Information
Joaquim Bellmunt, Beth Israel Deaconess Medical Center and PSMAR-IMIM Lab, Harvard Medical School, Boston, MA, USA.
Maha Hussain, Robert H Lurie Comprehensive Cancer Center, Northwestern University, Chicago, IL, USA..
Jürgen E Gschwend, Rechts der Isar Hospital, Department of Urology, Technical University of Munich, Germany.
Peter Albers, Heinrich-Heine University Düsseldorf, Medical Faculty, Department of Urology, University Hospital Düsseldorf, Germany.
Stephane Oudard, Georges Pompidou European Hospital, University of Paris, Paris, France.
Daniel Castellano, University Hospital 12 de Octubre, Medical Oncology Department CIBER-ONC, Madrid, Spain.
Siamak Daneshmand, USC Norris Comprehensive Cancer Center, Los Angeles, CA, USA.
Hiroyuki Nishiyama, Department of Urology, Faculty of Medicine University of Tsukuba, Ibaraki, Japan.
Martin Majchrowicz, Genentech, Inc., South San Francisco, CA.
Viraj Degaonkar, Genentech, Inc., South San Francisco, CA.
Yi Shi, Genentech, Inc., South San Francisco, CA.
Sanjeev Mariathasan, Genentech, Inc., South San Francisco, CA.
Petros Grivas, University of Washington, Seattle Cancer Care Alliance and Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Alexandra Drakaki, University of California, Los Angeles, Los Angeles, CA.
Peter H O’Donnell, University of Chicago, Chicago, IL, USA.
Jonathan E Rosenberg, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA; Weill Cornell Medical College, New York, NY, USA.
Daniel M Geynisman, Department of Hematology/Oncology, Fox Chase Cancer Center, Philadelphia, PA, USA.
Daniel P Petrylak, Yale Cancer Center, New Haven, CT, USA.
Jean Hoffman-Censits, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD, USA.
Jens Bedke, Department of Urology, University of Tübingen, Germany.
Arash Rezazadeh Kalebasty, University of California Irvine, CA, USA.
Yousef Zakharia, University of Iowa, Holden Comprehensive Cancer Center, Iowa City, IA, USA.
Michiel S van der Heijden, Netherlands Cancer Institute, Amsterdam, the Netherlands.
Cora N Sternberg, Englander Institute for Precision Medicine, Weill Cornell Medicine, New York-Presbyterian, New York, NY, USA.
Nicole N Davarpanah, Genentech, Inc., South San Francisco, CA.
Thomas Powles, Barts Cancer Institute, Queen Mary University of London, St Bartholomew’s Hospital, London, UK.
References
- 1.Raghavan D, Shipley WU, Garnick MB, et al. Biology and management of bladder cancer. N Engl J Med 1990; 322: 112938. [DOI] [PubMed] [Google Scholar]
- 2.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol 2001; 19: 666–75 [DOI] [PubMed] [Google Scholar]
- 3.Stenzl A, Cowan NC, De Santis M, et al. The updated EAU guidelines on muscle invasive and metastatic bladder cancer. Eur Urol 2009; 55: 815–25. [DOI] [PubMed] [Google Scholar]
- 4.NCCN Clinical Practice Guidelines in Oncology. Bladder Cancer. V6·2020. https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. [Google Scholar]
- 5.AUA/ASCO/ASTRO/SUO MIBC guidelines 2017. https://www.auanet.org/guidelines/bladder-cancer-non-metastatic-muscle-invasive-guideline (accessed June 24, 2020).
- 6.EAU MIBC 2020 guidelines. EAU Guidelines. Presented at the EAU Annual Congress Amsterdam 2020. ISBN 978-94-92671-07-3. https://uroweb.org/guideline/bladder-cancer-muscle-invasive-and-metastatic/ (accessed June 24, 2020).
- 7.Leow JJ, Chong YL, Chang SL, Valderrama BP, Powles T, Bellmunt J. Neoadjuvant and adjuvant chemotherapy for upper tract urothelial carcinoma: a 2020 systematic review and meta-analysis, and future perspectives on systemic therapy. Eur Urol [E-pub]. doi: 10.1016/j.eururo.2020.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Drakaki A, Pantuck A, Mhatre SK, et al. “Real-world” outcomes and prognostic indicators among patients with high-risk muscle-invasive urothelial carcinoma. Urol Oncol 2020;S1078-1439(20)30334-3. [DOI] [PubMed] [Google Scholar]
- 9.Birtle A, Johnson M, Chester J, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomised controlled trial. Lancet 2020; 395: 1268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. J Clin Oncol 2011; 29: 2432–8. [DOI] [PubMed] [Google Scholar]
- 11.Bellmunt J, Orsola A, Leow JJ, et al. Bladder cancer: ESMO practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014; 25(suppl 3): iii40–8—including 2019 online-only update. [DOI] [PubMed] [Google Scholar]
- 12.Cognetti F, Ruggeri EM, Felici A, et al. Adjuvant chemotherapy with cisplatin and gemcitabine versus chemotherapy at relapse in patients with muscle-invasive bladder cancer submitted to radical cystectomy: an Italian, multicenter, randomized phase III trial. Ann Oncol 2012; 23: 695–700. [DOI] [PubMed] [Google Scholar]
- 13.Stadler WM, Lerner SP, Groshen S, et al. Phase III study of molecularly targeted adjuvant therapy in locally advanced urothelial cancer of the bladder based on p53 status. J Clin Oncol 2011; 29: 3443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sternberg CN, Skoneczna I, Kerst JM, et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol 2015; 16: 76–86. [DOI] [PubMed] [Google Scholar]
- 15.Paz-Ares LG, Solsona E, Esteban E, et al. Randomized phase III trial comparing adjuvant paclitaxel/gemcitabine/cisplatin (PGC) to observation in patients with resected invasive bladder cancer: results of the Spanish Oncology Genitourinary Group (SOGUG) 99/01 study. J Clin Oncol 2010; 28(18_suppl) (abstr LBA4518). [Google Scholar]
- 16.Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial [published correction appears in Lancet 2018; 392: 1402]. Lancet 2018; 391: 748–57. [DOI] [PubMed] [Google Scholar]
- 17.Galsky MD, Arija JÁA, Bamias A, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2020; 395: 1547–57. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016; 387: 1909–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balar AV, Castellano D, O'Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017; 18: 1483–92. [DOI] [PubMed] [Google Scholar]
- 20.Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017; 389: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017; 376: 1015–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powles T, Park SH, Voog E, et al. Maintenance avelumab + best supportive care (BSC) versus BSC alone after platinum-based first-line (1L) chemotherapy in advanced urothelial carcinoma (UC): JAVELIN Bladder 100 phase III interim analysis. ASCO20 Virtual Scientific Program; May 29-31, 2020. (abstr LBA1). [Google Scholar]
- 23.Balar AV, Kulkarni GS, Uchio EM, et al. KEYNOTE-057: phase II trial of pembrolizumab for patients with high-risk nonmuscle invasive bladder cancer unresponsive to bacillus Calmette-Guérin. Presented at Genitourinary Cancers Symposium 2019; February 15, 2019; San Francisco, CA: (abstr 350). [Google Scholar]
- 24.TECENTRIQ (atezolizumab) [package insert]. South San Francisco, CA: Genentech, Inc.; 2020. [Google Scholar]
- 25.TECENTRIQ (atezolizumab) [summary of product characteristics]. Grenzach-Wyhlen, Germany: Roche Registration GmbH; 2020. [Google Scholar]
- 26.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003; 349: 859–66. [DOI] [PubMed] [Google Scholar]
- 27.David KA, Milowsky MI, Ritchey J, Carroll PR, Nanus DM. Low incidence of perioperative chemotherapy for stage III bladder cancer 1998 to 2003: a report from the National Cancer Data Base. J Urol 2007; 178: 451–4. [DOI] [PubMed] [Google Scholar]
- 28.Necchi A, Anichini A, Raggi D, et al. Pembrolizumab as Neoadjuvant Therapy Before Radical Cystectomy in Patients With Muscle-Invasive Urothelial Bladder Carcinoma (PURE-01): An Open-Label, Single-Arm, Phase II Study. J Clin Oncol 2018; 36: 3353–3360. [DOI] [PubMed] [Google Scholar]
- 29.Powles T, Kockx M, Rodriguez-Vida A, et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat Med 2019; 25:1706–1714. [DOI] [PubMed] [Google Scholar]
- 30.van Dijk N, Gil-Jimenez A, Silina K, et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial. Nat Med 2020. [E-pub]. doi: 10.1038/s41591-020-1085-z. [DOI] [PubMed] [Google Scholar]
- 31.Schmid P, Cortes J, Pusztai L et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med 2020; 382: 810–21. [DOI] [PubMed] [Google Scholar]
- 32.Ravaud A, Motzer RJ, Pandha HS, et al. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med 2016; 375: 2246–54. [DOI] [PubMed] [Google Scholar]
- 33.Liu D, Abbosh P, Keliher D et al. Mutational patterns in chemotherapy resistant muscle-invasive bladder cancer. Nat Commun 2017; 8:2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo Y, Feng BF, Wei DC, et al. [Prospective controlled observation of effect 725 of adjuvant chemotherapy on survival and prognosis of high-risk upper tract urothelial carcinoma patients underwent radical nephroureterectomy]. Zhonghua Yi Xue Za Zhi 2019; 99: 3158–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.