Key Points
Question
Is using a transanal drainage tube a reliable approach for anastomotic leakage prevention after laparoscopic low anterior resection for rectal cancer?
Findings
In this randomized clinical trial involving 560 patients, there was no difference in the anastomotic leakage rate between patients with (6.4%) and without (6.8%) transanal drainage tubes.
Meaning
A transanal drainage tube may not confer any benefit in anastomotic leakage prevention for patients who have undergone laparoscopic low anterior resection for mid-low rectal cancer without preoperative radiotherapy.
This randomized clinical trial investigates the use of a transanal drainage tube for the prevention of anastomotic leakage after laparoscopic low anterior resection in patients with rectal cancer.
Abstract
Importance
Preventing anastomotic leakage (AL) is crucial for colorectal surgery. Some studies have suggested a positive role of transanal drainage tubes (TDTs) in AL prevention after low anterior resection, but this finding is controversial.
Objective
To assess the effect of TDTs in AL prevention after laparoscopic low anterior resection for rectal cancer.
Design, Setting, and Participants
This multicenter randomized clinical trial with parallel groups (TDT vs non-TDT) was performed from February 26, 2016, to September 30, 2020. Participants included patients from 7 different hospitals in China who were undergoing laparoscopic low anterior resection with the double-stapling technique for mid-low rectal cancer; 576 patients were initially enrolled in this study, and 16 were later excluded. Ultimately, 560 patients were randomly divided between the TDT and non-TDT groups.
Interventions
A silicone tube was inserted through the anus, and the tip of the tube was placed approximately 5 cm above the anastomosis under laparoscopy at the conclusion of surgery. The tube was fixed with a skin suture and connected to a drainage bag. The TDT was scheduled for removal 3 to 7 days after surgery.
Main Outcomes and Measures
The primary end point was the postoperative AL rate within 30 days.
Results
In total, 576 patients were initially enrolled in this study; 16 of these patients were excluded. Ultimately, 560 patients were randomly divided between the TDT group (n = 280; median age, 61.5 years [IQR, 54.0-68.8 years]; 177 men [63.2%]) and the non-TDT group (n = 280; median age, 62.0 years [IQR, 52.0-69.0 years]; 169 men [60.4%]). Intention-to-treat analysis showed no significant difference between the TDT and non-TDT groups in AL rates (18 [6.4%] vs 19 [6.8%]; relative risk, 0.947; 95% CI, 0.508-1.766; P = .87) or AL grades (grade B, 14 [5.0%] and grade C, 4 [1.4%] vs grade B, 11 [3.9%] and grade C, 8 [2.9%]; P = .43). In the stratified analysis based on diverting stomas, there was no significant difference in the AL rate between the groups, regardless of whether a diverting stoma was present (without stoma, 12 [5.8%] vs 15 [7.9%], P = .41; and with stoma, 6 [8.3%] vs 4 [4.5%], P = .50). Anal pain was the most common complaint from patients in the TDT group (130 of 280, 46.4%). Accidental early TDT removal occurred in 20 patients (7.1%), and no bleeding or iatrogenic colonic perforations were detected.
Conclusions and Relevance
The results from this randomized clinical trial indicated that TDTs may not confer any benefit for AL prevention in patients who undergo laparoscopic low anterior resection for mid-low rectal cancer without preoperative radiotherapy.
Trial Registration
ClinicalTrials.gov Identifier: NCT02686567
Introduction
Anastomotic leakage (AL) is one of the most serious complications after low anterior resection (LAR). It has an incidence of 1% to 30%1,2 and is associated with increased medical costs, lengthened hospital stays, increased postoperative morbidity, patient mortality, and an increased rate of recurrence.3 Creation of a diverting stoma is still one of the most common methods used for AL prevention in clinical practice, even though it is argued that diverting stomas cannot reduce the incidence of AL.4,5,6 Despite the clinical benefit of reducing morbidity from AL or symptomatic AL, diverting stomas remain a large source of morbidity for patients, with increased postoperative stay, readmission, dehydration, etc.7,8 In addition, approximately 6% to 40% of stomas fail to reverse for various reasons.9,10
In the last decade, it has been reported that transanal drainage tubes (TDTs) are valuable and safe for preventing AL after LAR.11,12,13,14,15,16,17,18,19,20,21 Application of a TDT is supposed to be beneficial for endoluminal pressure reduction as well as fecal diversion, resulting in a protective effect on anastomotic healing.21,22 Several meta-analytical studies have reported that the TDT is effective in AL prevention.23,24,25,26,27,28 Consequently, the application of TDTs after LAR has progressively increased in recent years, especially in Asia. However, the majority of previous studies have the limitation of retrospective observation, a small sample size, or use of a nonrandomized control group. Moreover, inconsistent results have also been reported, failing to prove the effectiveness of the implementation.14,29,30,31,32,33 In addition, iatrogenic colonic perforations have been reported in some studies.19,31 Thus, due to the lack of a high level of evidence, the role of TDTs in AL prevention after LAR is still unclear and controversial.34
Here, we report a multicenter randomized clinical trial study with the null hypothesis that the AL rate in patients with TDT would be the same as that in patients without TDT within 30 days after laparoscopic LAR. The primary objective of this trial was to evaluate the effect of TDTs on AL incidence following laparoscopic LAR. Secondary objectives included the grades of AL, anal postoperative pain score, and TDT-related adverse events.
Methods
This randomized, multicenter, open-label clinical trial was conducted in 7 hospitals in China from February 26, 2016, to September 30, 2020. The study conformed to the ethical guidelines of the 1975 Declaration of Helsinki (sixth revision, 2008) and was approved by the ethics committee of the Army Medical Center, the Army Medical University. Written informed consent was obtained from all participants. Participants’ race or ethnicity was not collected in this study. There are no published research reports showing that race or ethnicity is related to AL. Therefore, we concluded that this would not lead to selection bias. Each participating hospital also obtained local institutional review board approval. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Inclusion and Exclusion Criteria
Patients who underwent laparoscopic LAR with the double-stapling technique (DST) for mid-low rectal cancer were considered for inclusion. Patients who had undergone preoperative radiotherapy were excluded. Patients who underwent other types of surgeries, including the Hartmann procedure, abdominoperineal resection, intersphincteric resection, etc, were excluded intraoperatively. All preoperative procedures complied with the guidelines for the diagnosis and treatment of colorectal cancer. Details of the eligibility criteria are given in the Box.
Box. Inclusion and Exclusion Criteria.
Inclusion criteria
Age from 18 to 80 y
Primary rectal adenocarcinoma
Tumor location ≤10 cm from anal verge
ASA I, II, or III
Laparoscopic LAR plus DST
Exclusion criteria
Emergency operation
Patients with preoperative radiotherapy
Patients with IBD, FAP, recurrent rectal cancer, or synchronous cancer
Other types of surgeries for rectal cancer
Trial Design and Procedure
Versions 1 through 4 of the trial protocol are available in Supplement 1, Supplement 2, Supplement 3, and Supplement 4, respectively. All procedures were performed by experienced surgeons (A.Z., B.L., B.F., C.L., F.L., J.Z., L.B., M.W., F.G., W.T., and Z.P.) who had performed at least 300 laparoscopic LARs before the study. Preliminary training on the standard operating procedure was administered to all the surgeons and their teams before the initiation of the current study.
Patients were randomly assigned to 2 groups with a 1:1 allocation ratio (TDT and non-TDT groups) after the laparoscopic LAR and DST procedure was chosen during the operation. Simple randomization was obtained through computer-generated random number sequence allocation by the quality control committee. Allocation concealment was performed to ensure that all the intraoperative decisions made by the surgeon were not interfered with by the grouping. After completion of the anastomosis and further diverting stoma construction if necessary, the surgeon was notified to implement the intervention based on the randomization results by the circulating nurse.
Interventions including nutrition screening, nutritional support, and preoperative mechanical bowel preparation combined with oral antibiotics were conducted systematically. All operative procedures fully complied with the guidelines for the diagnosis and treatment of colorectal cancer and the technique of total mesorectal excision. The preservation of the left colonic artery was assessed by the surgeon according to their own experiences and assessment of the patient’s conditions. When the anastomosis was accomplished, the decision regarding diverting stoma construction was made by the surgeon based on an assessment of the risk factors for AL.35,36,37,38,39 Pelvic drainage was used in all cases in this study.
Intervention
A 28F silicone tube (Sumitomo Bakelite Co) was inserted through the anus, and the tip of the tube was placed approximately 5 cm above the anastomosis under laparoscopy at the end of the surgery in patients from the TDT group. The tube was fixed with a skin suture and connected to a drainage bag. The TDT was to be removed at the surgeon’s discretion when the discharge of feces or flatus was clearly and repeatedly observed or when surgeons confirmed the absence of signs of AL, usually at 3 to 7 days after surgery; early removal was allowed if the patient experienced intolerable pain. All patients received the same postoperative protocol.
Definition
Symptomatic AL was defined when the following symptoms were noticed13,19,40,41: discharge of feces, pus, or gas from the pelvic drainage tube or from the vagina or peritonitis or septicemia with pelvic abscess, with or without abdominal pain, fever, leukocytosis, increased procalcitonin, or C-reactive protein. All clinically suspicious symptoms were confirmed by digital rectal examination, computed tomography scan, or surgery when necessary. Asymptomatic AL (also called radiographic AL) was identified when an AL was detected on the computed tomography scan but was not accompanied by any relevant clinical symptoms. The severity grading of AL was defined according to the International Study Group of Rectal Cancer.40 In the present study, AL was referred to as symptomatic AL (grades B and C); asymptomatic AL (grade A) was not considered because no active therapeutic intervention was required. The anastomotic level was defined as the distance from the anal verge and was measured intraoperatively.
End Points
The primary end point was AL within 30 days after surgery. The secondary end points were the AL grade, anal postoperative pain score, and TDT-related adverse events, such as bleeding and iatrogenic colonic perforations.
Statistical Analysis
Based on the synthesis of information from multiple published studies with large sample sizes,21,42,43,44,45,46,47 we assumed that the AL rate was reduced from 10.5% to the expected 4% due to the TDT intervention. With 80% power, a 5% significance level, an expected crossover rate of 1%, and dropout rate of 10%, we needed to recruit 560 patients overall. Sample size estimation was performed using PASS, version 11 (NCSS, LLC).
Analyses of the primary and secondary end points were based on intention-to-treat principles. Per-protocol analysis was restricted to participants who fulfilled the protocol in terms of eligibility, interventions, and outcome assessment. A χ2 test or Fisher exact test was used for the comparison of categorical variables, as appropriate. Continuous variables were expressed as the median and IQR and were analyzed using a Mann-Whitney U test if not normally distributed or were expressed as the mean (SD) and analyzed using t test if normally distributed. The comparison of the AL rate in the 2 groups was performed using a χ2 test and was presented as the relative risk with a 95% CI. Post hoc subgroup analysis was performed according to the presence or absence of a diverting stoma. All analyses were 2-sided, and P < .05 was considered statistically significant. Statistical analysis was performed using SPSS, version 25.0 (IBM Corp).
Results
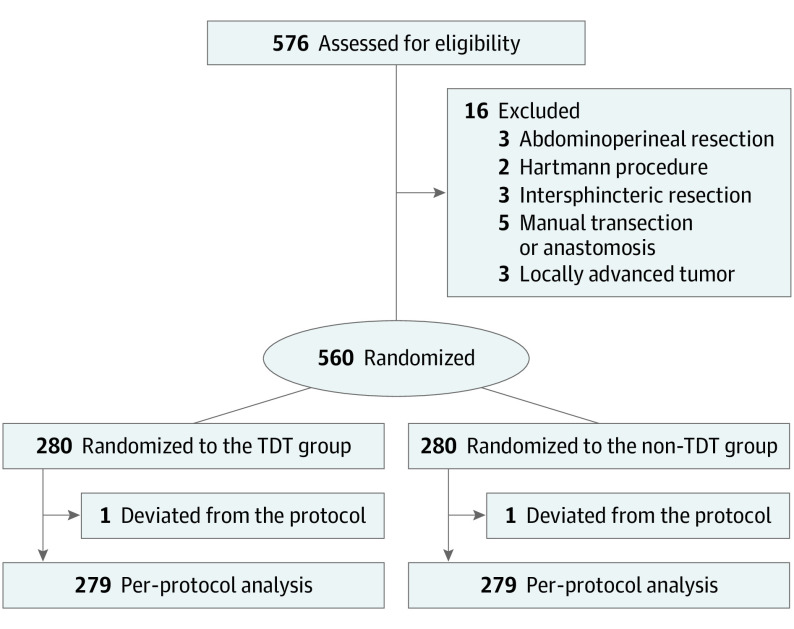
A total of 576 consecutive patients were initially enrolled in this study, and 16 patients were excluded due to changes in surgical procedures, which were inconsistent with LAR plus DST. Finally, 560 patients were randomized into the TDT group (n = 280; median age, 61.5 years [IQR, 54.0-68.8 years]; 177 men [63.2%] and 103 women [36.8%]) and the non-TDT group (n = 280; median age, 62.0 years [IQR, 52.0-69.0 years]; 169 men [60.4%] and 111 women [39.6%]). One case of deviation from the allocated intervention occurred in each group (Figure).
Figure. Study Flow Diagram.
TDT indicates transanal drainage tube.
Intention-to-Treat Analysis
For the intention-to-treat analysis, the median age of the patients was 62.0 years (IQR, 53.0-69.0 years), and there were 346 men (61.8%) and 214 women (38.2%). The total diverting stoma rate was 161 of 560 (28.8%). A body mass index greater than or equal to 25 (calculated as weight in kilograms divided by height in meters squared), a number of stapler firings greater than 1, blood loss of at least 50 mL, and an anastomotic level less than 5 cm are independent risk factors for AL.35,37,48,49,50 Therefore, related continuous variables were transformed into categorical variables. The details of the demographic and surgical characteristics of the patients are presented in Table 1.
Table 1. Demographic and Surgical Characteristics of the Patients.
| Characteristics | No. (%) | |
|---|---|---|
| TDT (n = 280) | Non-TDT (n = 280) | |
| Age, median, (IQR), y | 61.5 (54.0-68.8) | 62.0 (52.0-69.0) |
| Sex | ||
| Male | 177 (63.2) | 169 (60.4) |
| Female | 103 (36.8) | 111 (39.6) |
| BMI | ||
| Median (IQR) | 23.1 (20.8-24.6) | 22.8 (20.7-25.2) |
| ≥25 | 61 (21.8) | 80 (28.6) |
| <25 | 219 (78.2) | 200 (71.4) |
| Diabetes | 26 (9.3) | 29 (10.4) |
| Neoadjuvant chemotherapy | 12 (4.3) | 16 (5.7) |
| Tumor distance from anal verge, cm | ||
| Median (IQR) | 7.0 (6.0-8.0) | 7.0 (5.6-9.0) |
| Mean (SD) | 7.1 (1.9) | 7.2 (2.1) |
| Operation time, median (IQR), min | 180.0 (130.0-220.0) | 175.0 (130.0-230.0) |
| Blood loss, mL | ||
| Median (IQR) | 50.0 (40.0-100.0) | 50.0 (26.3-100.0) |
| Mean (SD) | 67.3 (62.4) | 69.4 (109.4) |
| ≥50 mL | 208 (74.3) | 187 (66.8) |
| <50 mL | 72 (25.7) | 93 (33.2) |
| Anastomotic level, cm | ||
| Median (IQR) | 4.0 (3.0-5.0) | 4.0 (3.0-5.5) |
| ≥5 cm | 121 (43.2) | 130 (46.4) |
| <5 cm | 159 (56.8) | 150 (53.6) |
| No. of stapler firings | ||
| 1 | 62 (22.1) | 47 (16.8) |
| >1 | 218 (77.9) | 233 (83.2) |
| DS | ||
| Absent | 208 (74.3) | 191 (68.2) |
| Present | 72 (25.7) | 89 (31.8) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); DS, diverting stoma; TDT, transanal drainage tube.
Primary End Point
Anastomotic leakage was diagnosed in 37 of 560 patients, for a total AL rate of 6.6%, including 25 patients with grade B and 12 patients with grade C. The AL rate was 6.4% (n = 18) in the TDT group and 6.8% (n = 19) in the non-TDT group, and no significant difference was shown between the groups (relative risk, 0.947; 95% CI, 0.508-1.766, P = .87) (Table 2).
Table 2. Details of Anastomotic Leakage.
| End point | No. (%) | RR (95% CI) | P value | |
|---|---|---|---|---|
| TDT (n = 280) | Non-TDT (n = 280) | |||
| Anastomotic leakage | 18 (6.4) | 19 (6.8) | 0.95 (0.51-1.77) | .87 |
| Grade B | 14 (5.0) | 11 (3.9) | NA | .43 |
| Grade C | 4 (1.4) | 8 (2.9) | ||
Abbreviations: NA, not available; RR, relative risk; TDT, transanal drainage tube.
To further investigate the role of TDT in AL prevention, a stratified analysis was conducted regarding the diverting stoma (Table 3). For patients without a diverting stoma, no significant difference in the AL rate was observed between the TDT group (12 of 208 [5.8%]) and the non-TDT group (15 of 191 [7.9%]; P = .41). A similar result was observed in patients with a diverting stoma (6 of 72 [8.3%] vs 4 of 89 [4.5%]; P = .50). The details of the baseline demographic and surgical characteristics are presented in eTable 1 in Supplement 5.
Table 3. Anastomotic Leakage in Each Subgroup Based on the Presence or Absence of a Diverting Stoma.
| Leakage | With a diverting stoma | Without a diverting stoma | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group, No. (%) | P value | Group, No. (%) | P value | ||||||||
| TDT (n = 72) | Non-TDT (n = 89) | TDT (n = 208) | Non-TDT (n = 191) | ||||||||
| Present | 6 (8.3) | 4 (4.5) | .50 | 12 (5.8) | 15 (7.9) | .41 | |||||
| Grade B | 6 (8.3) | 4 (4.5) | NA | 8 (3.8) | 7 (3.7) | .42 | |||||
| Grade C | 0 | 0 | 4 (1.9) | 8 (4.2) | |||||||
Abbreviations: NA, not applicable; TDT, transanal drainage tube.
Secondary End Points
As reported in some studies,14,31 the application of a TDT might lessen the severity of complications or the reoperation rate in grade-C AL. Therefore, the grades of AL were compared, but no significant difference was found between the TDT and non-TDT groups (grade B, 14 [5.0%] and grade C, 4 [1.4%] vs grade B, 11 [3.9%] and grade C, 8 [2.9%]; P = .43), regardless of whether there was a diverting stoma. Eight patients (2.9%) were diagnosed with grade-C AL in the non-TDT group, which was twice the rate of the TDT group. However, the difference was not statistically significant, whether the analysis was stratified or not. The numerical rating scale was used to assess the anal postoperative pain score of patients in the TDT group. Nearly half of the patients with TDTs (130 of 280; 46.4%) complained of anal pain after surgery. Among them, 8 patients (2.9%) did not describe the details of the numerical rating scale score, 111 patients (39.6%) reported a score of less than 4 points, and 11 patients (3.9%) reported a score of 4 to 7 points. No patient reported a numerical rating scale score greater than 7 points.
In the TDT group, the tubes were retained in the anal canal for a mean (SD) period of 4.2 (1.6) days (median, 4 days; IQR, 3-5 days) after surgery. Premature TDT removal within 2 days occurred in 20 patients (7.1%) as follows: 3 patients complained of intolerable pain or discomfort, 5 patients had TDTs that fell out accidentally, 5 patients had TDTs that were identified as showing loss of function with fecal outflow from the anus along the tubes, and 7 patients had early discharge from the hospital. No bleeding or iatrogenic colonic perforation related to the TDT intervention was observed. The results obtained from the per-protocol analysis were highly consistent with those from the intention-to-treat analysis (eTables 2-5 in Supplement 5).
Discussion
The present study assessed the AL rate and the grades of AL as secondary outcomes within a prospective, multicenter, randomized clinical trial comparing TDT and non-TDT insertion after stapled LAR for rectal cancer. For the primary outcome, the AL rate was 6.8% in the non-TDT group and 6.4% in the TDT group, which was not a significant difference. Our results indicated that the application of a TDT may not affect the AL rate, which is consistent with some previous studies.14,29,30,31,32 Furthermore, a TDT may not decrease the severity of AL.
The actual AL rate in the control group was lower than that used in the sample size calculation, that is, 6.8% instead of 10.5%. The main reason for this difference was the inconsistency of the definition of AL. We used the AL grading scale, which was not available in those previous studies, and our study focused on patients with symptomatic AL only (grades B and C). Another potential reason was the heterogeneity of the study populations. For example, patients who underwent laparoscopic LAR and DST were included, whereas patients who underwent preoperative radiotherapy were excluded from our study.
In the past decade, a positive role of TDTs in AL prevention was proposed by a series of studies.24,27,51,52 However, this conclusion remained in question. Cong and colleagues33 reported that the AL rate in the TDT group was unexpectedly higher than that in the non-TDT group (15.1% vs 4.9%; P = .008). In another retrospective study conducted by Shinji,53 based on the fact that TDTs were used frequently (11 of 18; 61%) in patients who eventually developed AL, the theory that TDTs prevented AL through postoperative rectal decompression was suspect. Zhao et al32 hypothesized that the TDT would reduce anastomotic complications but found that the AL rate was not significantly different between the 2 groups (2.5% vs 7.8%, P = .16). In a study using propensity score matching, Yang et al14 reported no significant difference in the overall AL rate between the 2 groups (9.8% vs 11.8%; P = .65). A similar result was observed by Lee et al31 in their retrospective study, both before and after propensity score matching (5.8% vs 10.7%, P = .65 and 5.8% vs 9.1%, P = .28, respectively). Owing to the inherent limitations of the retrospective methodology, relatively small sample size, and nonconcurrent controls, the level of evidence for the application of TDTs was very low. The earliest randomized clinical trial study was reported in 2011.21 However, there were subsequent concerns regarding patient selection bias in that study, including the factors of tumor location (≤15 cm from anal verge) and type of anastomosis (straight and J-pouch).21
In our current study, all participants underwent laparoscopic LAR, as the laparoscopic surgical approach for rectal cancer is very common and popular in China. To avoid relative heterogeneity, open LARs were excluded. Procedures of transection and anastomosis differing from the DST (eg, transanal transection or intersphincteric resection with a handsewn coloanal anastomosis) were also excluded, as it was reported that the AL rate was different between the DST and other procedures.54 It has been proven that a lower anastomosis level (<5 cm) was an independent risk factor for AL,37,48 and the AL was higher in patients with mid-low rectal cancer who underwent LAR.41,55,56 Thus, only patients whose tumor location was within 10 cm from the anal verge were enrolled in our study. Upper rectal cancers were excluded, as the perioperative characteristics of these patients might be distinct from those of our study concerning the occurrence of anastomosis leaks.
Patients who underwent preoperative radiotherapy were excluded from the current study based on several considerations. First, it was reported that the tissue edema and friability induced by radiotherapy were essentially different from normal tissues without radiotherapy.57,58 Second, the chronic presacral sinus was closely related to neoadjuvant radiotherapy and led to nonhealing of the leakage for at least 1 year.59,60 These 2 factors indicated that preoperative radiotherapy is an important risk factor for anastomosis healing. However, the diversification and individualization of radiotherapy fractionation and the interval between preoperative radiotherapy and surgery might confuse the interpretation of the results.61
Another issue complicating the interpretation of the role of TDTs was the management of patients with diverting stomas; such patients were excluded from some previous studies11,14,15,19,20,21,31,32 but included in others.12,13,16 Although whether a diverting stoma can reduce AL after LAR is still controversial,62,63 it is currently the most popular option to prevent AL. Diverting stomas were included in our study to comprehensively evaluate the role of TDTs in AL prevention for patients with low and high risks of AL. Foreknowledge of TDT application may influence the surgeon’s decision regarding diverting stoma construction. The relationship among TDTs, diverting stomas, and AL is similar to that of exposure, confounder, and outcome.64 Therefore, diverting stomas can be identified as a confounder for studying the role of TDTs in AL prevention. To control confounding, a randomized grouping and allocation concealment was adopted, and a stratified analysis was also performed. In patients without a diverting stoma, the AL rate of the TDT group was close to that of the non-TDT group, with no significant difference. Interestingly, however, 8 patients in the latter group were diagnosed with grade-C AL—twice as many as in the former—although this difference was not statistically significant. This finding may indicate the potential efficacy of TDTs in decreasing grade-C AL if a diverting stoma has not been implemented, but further research with larger populations is needed. In patients with a diverting stoma, the AL rate in the TDT group was even higher than that in the non-TDT group (8.3% vs 4.5%), but the difference was not significant. All these revealed that a TDT may not reduce the AL rate in patients with or without a diverting stoma.
Although a few studies reported that the resting pressure or intraluminal pressure was related to AL,19,21,22 the cause-and-effect relationship remains unsettled. Theoretically, high intraluminal pressure can burst the anastomosis. At the same time, bursting pressure is usually applied in animal leakage models.65 However, there is no evidence that the AL of a patient is caused by increased intraluminal pressure. Therefore, in our opinion, the decrease in intraluminal pressure caused by the TDT is not associated with the occurrence of AL.
Iatrogenic colonic perforations were reported in earlier studies,19,31 and it was reasoned that the TDT was inserted too deeply from the anastomosis. Iatrogenic colonic perforations were not observed in the current study because all cases were diagnosed as mid-low rectal cancer, and the TDT was inserted 3 to 5 cm above the anastomosis. Meanwhile, there were no adverse events, such as bleeding, caused by the TDT in the patients. However, approximately half of the patients complained of anal pain after surgery, and some of them felt that the pain was intolerable. Unexpected early falling out of the TDT was another kind of adverse event that might make a patient nervous.
Limitations
This study had several limitations. First, it may not be possible to extrapolate the results of the current study to the entire population of patients with rectal cancer who undergo laparoscopic LAR plus DST, as patients who had undergone preoperative radiotherapy were excluded from this study. Second, the current study was focused on laparoscopic LARs for mid-low rectal cancers and did not include open surgery.
Conclusions
In conclusion, this prospective randomized clinical trial did not irrefutably demonstrate the usefulness of TDTs in preventing AL after laparoscopic LAR for mid-low rectal cancer without preoperative radiotherapy. This outcome suggests that a TDT might not confer any benefit for AL prevention. Meanwhile, TDTs also caused anal pain and a few mild adverse events. Therefore, we cannot currently recommend using the TDT after laparoscopic LAR.
Trial protocol, original
Trial protocol version 2
Trial protocol version 3
Trial protocol version 4
eTable 1. Demographic and Surgical Characteristics of the Patients in Each Subgroup
eTable 2. Demographic and Surgical Characteristics of the Patients (Per-Protocol Analysis)
eTable 3. The Details of Anastomotic Leakage (Per-Protocol Analysis)
eTable 4. Anastomotic Leakage in Each Subgroup Based on the Presence or Absence of a Diverting Stoma (Per-Protocol Analysis)
eTable 5. Demographic and Surgical Characteristics of the Patients in Each Subgroup (Per-Protocol Analysis)
Data Sharing Statement
Footnotes
Abbreviations: ASA, American Society of Anesthesiologists; DST, double-stapling technique; FAP, familial adenomatous polyposis; IBD, inflammatory bowel disease; LAR, low anterior resection.
References
- 1.Spinelli A, Anania G, Arezzo A, et al. Italian multi-society modified Delphi consensus on the definition and management of anastomotic leakage in colorectal surgery. Updates Surg. 2020;72(3):781-792. doi: 10.1007/s13304-020-00837-z [DOI] [PubMed] [Google Scholar]
- 2.Caulfield H, Hyman NH. Anastomotic leak after low anterior resection: a spectrum of clinical entities. JAMA Surg. 2013;148(2):177-182. doi: 10.1001/jamasurgery.2013.413 [DOI] [PubMed] [Google Scholar]
- 3.Salvans S, Mayol X, Alonso S, et al. Postoperative peritoneal infection enhances migration and invasion capacities of tumor cells in vitro: an insight into the association between anastomotic leak and recurrence after surgery for colorectal cancer. Ann Surg. 2014;260(5):939-943. doi: 10.1097/SLA.0000000000000958 [DOI] [PubMed] [Google Scholar]
- 4.Wu Y, Zheng H, Guo T, Keranmu A, Liu F, Xu Y. Temporary diverting stoma improves recovery of anastomotic leakage after anterior resection for rectal cancer. Sci Rep. 2017;7(1):15930. doi: 10.1038/s41598-017-16311-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman WC Jr., Subramanian M, Jayarajan S, et al. First, do no harm: rethinking routine diversion in sphincter-preserving rectal cancer resection. J Am Coll Surg. 2019;228(4):547-556.e8. doi: 10.1016/j.jamcollsurg.2018.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snijders HS, van Leersum NJ, Henneman D, et al. Optimal treatment strategy in rectal cancer surgery: should we be cowboys or chickens? Ann Surg Oncol. 2015;22(11):3582-3589. doi: 10.1245/s10434-015-4385-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phatak UR, Kao LS, You YN, et al. Impact of ileostomy-related complications on the multidisciplinary treatment of rectal cancer. Ann Surg Oncol. 2014;21(2):507-512. doi: 10.1245/s10434-013-3287-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kye BH, Kim HJ, Kim JG, Cho HM. The nutritional impact of diverting stoma-related complications in elderly rectal cancer patients. Int J Colorectal Dis. 2013;28(10):1393-1400. doi: 10.1007/s00384-013-1699-4 [DOI] [PubMed] [Google Scholar]
- 9.Pan HD, Peng YF, Wang L, et al. Risk factors for nonclosure of a temporary defunctioning ileostomy following anterior resection of rectal cancer. Dis Colon Rectum. 2016;59(2):94-100. doi: 10.1097/DCR.0000000000000520 [DOI] [PubMed] [Google Scholar]
- 10.Kim MJ, Kim YS, Park SC, et al. Risk factors for permanent stoma after rectal cancer surgery with temporary ileostomy. Surgery. 2016;159(3):721-727. doi: 10.1016/j.surg.2015.09.011 [DOI] [PubMed] [Google Scholar]
- 11.Kawada K, Takahashi R, Hida K, Sakai Y. Impact of transanal drainage tube on anastomotic leakage after laparoscopic low anterior resection. Int J Colorectal Dis. 2018;33(3):337-340. doi: 10.1007/s00384-017-2952-z [DOI] [PubMed] [Google Scholar]
- 12.Ito T, Obama K, Sato T, et al. Usefulness of transanal tube placement for prevention of anastomotic leakage following laparoscopic low anterior resection. Asian J Endosc Surg. 2017;10(1):17-22. doi: 10.1111/ases.12310 [DOI] [PubMed] [Google Scholar]
- 13.Goto S, Hida K, Kawada K, et al. Multicenter analysis of transanal tube placement for prevention of anastomotic leak after low anterior resection. J Surg Oncol. 2017;116(8):989-995. doi: 10.1002/jso.24760 [DOI] [PubMed] [Google Scholar]
- 14.Yang CS, Choi GS, Park JS, et al. Rectal tube drainage reduces major anastomotic leakage after minimally invasive rectal cancer surgery. Colorectal Dis. 2016;18(12):O445-O452. doi: 10.1111/codi.13506 [DOI] [PubMed] [Google Scholar]
- 15.Matsuda M, Tsuruta M, Hasegawa H, et al. Transanal drainage tube placement to prevent anastomotic leakage following colorectal cancer surgery with double stapling reconstruction. Surg Today. 2016;46(5):613-620. doi: 10.1007/s00595-015-1230-3 [DOI] [PubMed] [Google Scholar]
- 16.Brandl A, Czipin S, Mittermair R, Weiss S, Pratschke J, Kafka-Ritsch R. Transanal drainage tube reduces rate and severity of anastomotic leakage in patients with colorectal anastomosis: a case controlled study. Ann Med Surg (Lond). 2016;6:12-16. doi: 10.1016/j.amsu.2016.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim MK, Won DY, Lee JK, Kang WK, Kim JG, Oh ST. Comparative study between transanal tube and loop ileostomy in low anterior resection for mid rectal cancer: a retrospective single center trial. Ann Surg Treat Res. 2015;88(5):260-268. doi: 10.4174/astr.2015.88.5.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hidaka E, Ishida F, Mukai S, et al. Efficacy of transanal tube for prevention of anastomotic leakage following laparoscopic low anterior resection for rectal cancers: a retrospective cohort study in a single institution. Surg Endosc. 2015;29(4):863-867. doi: 10.1007/s00464-014-3740-2 [DOI] [PubMed] [Google Scholar]
- 19.Nishigori H, Ito M, Nishizawa Y, et al. Effectiveness of a transanal tube for the prevention of anastomotic leakage after rectal cancer surgery. World J Surg. 2014;38(7):1843-1851. doi: 10.1007/s00268-013-2428-4 [DOI] [PubMed] [Google Scholar]
- 20.Adamova Z. Transanal tube as a means of prevention of anastomotic leakage after rectal cancer surgery. Viszeralmedizin. 2014;30(6):422-426. doi: 10.1159/000369569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao L, Zhang WB, Jiang PC, et al. Can transanal tube placement after anterior resection for rectal carcinoma reduce anastomotic leakage rate? a single-institution prospective randomized study. World J Surg. 2011;35(6):1367-1377. doi: 10.1007/s00268-011-1053-3 [DOI] [PubMed] [Google Scholar]
- 22.Nishigori H, Ito M, Nishizawa Y. A novel transanal tube designed to prevent anastomotic leakage after rectal cancer surgery: the WING DRAIN. Surg Today. 2017;47(4):513-520. doi: 10.1007/s00595-016-1392-7 [DOI] [PubMed] [Google Scholar]
- 23.Wang FG, Yan WM, Yan M, Song MM. Comparison of anastomotic leakage rate and reoperation rate between transanal tube placement and defunctioning stoma after anterior resection: a network meta-analysis of clinical data. Eur J Surg Oncol. 2019;45(8):1301-1309. doi: 10.1016/j.ejso.2019.01.182 [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Cai HK, Tang YH. An updated meta-analysis of transanal drainage tube for prevention of anastomotic leak in anterior resection for rectal cancer. Surg Oncol. 2018;27(3):333-340. doi: 10.1016/j.suronc.2018.05.018 [DOI] [PubMed] [Google Scholar]
- 25.Zhao WT, Li NN, He D, Feng JY. Transanal tube for the prevention of anastomotic leakage after rectal cancer surgery: a systematic review and meta-analysis. World J Surg. 2017;41(1):267-276. doi: 10.1007/s00268-016-3758-9 [DOI] [PubMed] [Google Scholar]
- 26.Yang Y, Shu Y, Su F, Xia L, Duan B, Wu X. Prophylactic transanal decompression tube versus non-prophylactic transanal decompression tube for anastomotic leakage prevention in low anterior resection for rectal cancer: a meta-analysis. Surg Endosc. 2017;31(4):1513-1523. doi: 10.1007/s00464-016-5193-2 [DOI] [PubMed] [Google Scholar]
- 27.Shigeta K, Okabayashi K, Baba H, et al. A meta-analysis of the use of a transanal drainage tube to prevent anastomotic leakage after anterior resection by double-stapling technique for rectal cancer. Surg Endosc. 2016;30(2):543-550. doi: 10.1007/s00464-015-4237-3 [DOI] [PubMed] [Google Scholar]
- 28.Ha GW, Kim HJ, Lee MR. Transanal tube placement for prevention of anastomotic leakage following low anterior resection for rectal cancer: a systematic review and meta-analysis. Ann Surg Treat Res. 2015;89(6):313-318. doi: 10.4174/astr.2015.89.6.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamura K, Matsuda K, Horiuchi T, et al. Laparoscopic anterior resection with or without transanal tube for rectal cancer patients - A multicenter randomized controlled trial. Am J Surg. 2021;222(3):606-612. doi: 10.1016/j.amjsurg.2020.12.054 [DOI] [PubMed] [Google Scholar]
- 30.Challine A, Cazelles A, Frontali A, Maggiori L, Panis Y. Does a transanal drainage tube reduce anastomotic leakage? A matched cohort study in 144 patients undergoing laparoscopic sphincter-saving surgery for rectal cancer. Tech Coloproctol. 2020;24(10):1047-1053. doi: 10.1007/s10151-020-02265-y [DOI] [PubMed] [Google Scholar]
- 31.Lee SY, Kim CH, Kim YJ, Kim HR. Impact of anal decompression on anastomotic leakage after low anterior resection for rectal cancer: a propensity score matching analysis. Langenbecks Arch Surg. 2015;400(7):791-796. doi: 10.1007/s00423-015-1336-5 [DOI] [PubMed] [Google Scholar]
- 32.Zhao WT, Hu FL, Li YY, Li HJ, Luo WM, Sun F. Use of a transanal drainage tube for prevention of anastomotic leakage and bleeding after anterior resection for rectal cancer. World J Surg. 2013;37(1):227-232. doi: 10.1007/s00268-012-1812-9 [DOI] [PubMed] [Google Scholar]
- 33.Cong ZJ, Fu CG, Wang HT, Liu LJ, Zhang W, Wang H. Influencing factors of symptomatic anastomotic leakage after anterior resection of the rectum for cancer. World J Surg. 2009;33(6):1292-1297. doi: 10.1007/s00268-009-0008-4 [DOI] [PubMed] [Google Scholar]
- 34.Wu Z, Wang Q, Li Z, Ji J. Use of transanal drainage tube to prevent anastomotic leakage: intangible differences between the East and the West. Br J Surg. 2021;108(3):e121-e122. doi: 10.1093/bjs/znaa144 [DOI] [PubMed] [Google Scholar]
- 35.Sciuto A, Merola G, De Palma GD, et al. Predictive factors for anastomotic leakage after laparoscopic colorectal surgery. World J Gastroenterol. 2018;24(21):2247-2260. doi: 10.3748/wjg.v24.i21.2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang W, Lou Z, Liu Q, et al. Multicenter analysis of risk factors for anastomotic leakage after middle and low rectal cancer resection without diverting stoma: a retrospective study of 319 consecutive patients. Int J Colorectal Dis. 2017;32(10):1431-1437. doi: 10.1007/s00384-017-2875-8 [DOI] [PubMed] [Google Scholar]
- 37.Qu H, Liu Y, Bi DS. Clinical risk factors for anastomotic leakage after laparoscopic anterior resection for rectal cancer: a systematic review and meta-analysis. Surg Endosc. 2015;29(12):3608-3617. doi: 10.1007/s00464-015-4117-x [DOI] [PubMed] [Google Scholar]
- 38.Ito M, Sugito M, Kobayashi A, Nishizawa Y, Tsunoda Y, Saito N. Relationship between multiple numbers of stapler firings during rectal division and anastomotic leakage after laparoscopic rectal resection. Int J Colorectal Dis. 2008;23(7):703-707. doi: 10.1007/s00384-008-0470-8 [DOI] [PubMed] [Google Scholar]
- 39.Kawada K, Sakai Y. Preoperative, intraoperative and postoperative risk factors for anastomotic leakage after laparoscopic low anterior resection with double stapling technique anastomosis. World J Gastroenterol. 2016;22(25):5718-5727. doi: 10.3748/wjg.v22.i25.5718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahbari NN, Weitz J, Hohenberger W, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010;147(3):339-351. doi: 10.1016/j.surg.2009.10.012 [DOI] [PubMed] [Google Scholar]
- 41.Trencheva K, Morrissey KP, Wells M, et al. Identifying important predictors for anastomotic leak after colon and rectal resection: prospective study on 616 patients. Ann Surg. 2013;257(1):108-113. doi: 10.1097/SLA.0b013e318262a6cd [DOI] [PubMed] [Google Scholar]
- 42.Park JS, Choi GS, Kim SH, et al. Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: the Korean laparoscopic colorectal surgery study group. Ann Surg. 2013;257(4):665-671. doi: 10.1097/SLA.0b013e31827b8ed9 [DOI] [PubMed] [Google Scholar]
- 43.Kang CY, Halabi WJ, Chaudhry OO, et al. Risk factors for anastomotic leakage after anterior resection for rectal cancer. JAMA Surg. 2013;148(1):65-71. doi: 10.1001/2013.jamasurg.2 [DOI] [PubMed] [Google Scholar]
- 44.Snijders HS, Wouters MW, van Leersum NJ, et al. Meta-analysis of the risk for anastomotic leakage, the postoperative mortality caused by leakage in relation to the overall postoperative mortality. Eur J Surg Oncol. 2012;38(11):1013-1019. doi: 10.1016/j.ejso.2012.07.111 [DOI] [PubMed] [Google Scholar]
- 45.Paun BC, Cassie S, MacLean AR, Dixon E, Buie WD. Postoperative complications following surgery for rectal cancer. Ann Surg. 2010;251(5):807-818. doi: 10.1097/SLA.0b013e3181dae4ed [DOI] [PubMed] [Google Scholar]
- 46.den Dulk M, Marijnen CA, Collette L, et al. Multicentre analysis of oncological and survival outcomes following anastomotic leakage after rectal cancer surgery. Br J Surg. 2009;96(9):1066-1075. doi: 10.1002/bjs.6694 [DOI] [PubMed] [Google Scholar]
- 47.Kawada K, Hasegawa S, Hida K, et al. Risk factors for anastomotic leakage after laparoscopic low anterior resection with DST anastomosis. Surg Endosc. 2014;28(10):2988-2995. doi: 10.1007/s00464-014-3564-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu X, Cheng Y. A clinical parameters-based model predicts anastomotic leakage after a laparoscopic total mesorectal excision: a large study with data from China. Medicine (Baltimore). 2015;94(26):e1003. doi: 10.1097/MD.0000000000001003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balciscueta Z, Uribe N, Caubet L, et al. Impact of the number of stapler firings on anastomotic leakage in laparoscopic rectal surgery: a systematic review and meta-analysis. Tech Coloproctol. 2020;24(9):919-925. doi: 10.1007/s10151-020-02240-7 [DOI] [PubMed] [Google Scholar]
- 50.Fukada M, Matsuhashi N, Takahashi T, et al. Risk and early predictive factors of anastomotic leakage in laparoscopic low anterior resection for rectal cancer. World J Surg Oncol. 2019;17(1):178. doi: 10.1186/s12957-019-1716-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cho SH, Lee IK, Lee YS, Kim MK. The usefulness of transanal tube for reducing anastomotic leak in mid rectal cancer: compared to diverting stoma. Ann Surg Treat Res. 2021;100(2):100-108. doi: 10.4174/astr.2021.100.2.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Z, Liang J, Chen J, Mei S, Liu Q. Effectiveness of a transanal drainage tube for the prevention of anastomotic leakage after laparoscopic low anterior resection for rectal cancer. Asian Pac J Cancer Prev. 2020;21(5):1441-1444. doi: 10.31557/APJCP.2020.21.5.1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shinji S, Ueda Y, Yamada T, et al. Male sex and history of ischemic heart disease are major risk factors for anastomotic leakage after laparoscopic anterior resection in patients with rectal cancer. BMC Gastroenterol. 2018;18(1):117. doi: 10.1186/s12876-018-0846-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cong JC, Chen CS, Ma MX, Xia ZX, Liu DS, Zhang FY. Laparoscopic intersphincteric resection for low rectal cancer: comparison of stapled and manual coloanal anastomosis. Colorectal Dis. 2014;16(5):353-358. doi: 10.1111/codi.12573 [DOI] [PubMed] [Google Scholar]
- 55.van der Pas MH, Haglind E, Cuesta MA, et al. ; COlorectal cancer Laparoscopic or Open Resection II (COLOR II) Study Group . Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14(3):210-218. doi: 10.1016/S1470-2045(13)70016-0 [DOI] [PubMed] [Google Scholar]
- 56.Kim CW, Baek SJ, Hur H, Min BS, Baik SH, Kim NK. Anastomotic leakage after low anterior resection for rectal cancer is different between minimally invasive surgery and open surgery. Ann Surg. 2016;263(1):130-137. doi: 10.1097/SLA.0000000000001157 [DOI] [PubMed] [Google Scholar]
- 57.Qin Q, Ma T, Deng Y, et al. Impact of preoperative radiotherapy on anastomotic leakage and stenosis after rectal cancer resection: post hoc analysis of a randomized controlled trial. Dis Colon Rectum. 2016;59(10):934-942. doi: 10.1097/DCR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 58.Kuzu MA, Köksoy C, Akyol FH, Uzal D, Kale T, Demirpence E. Effects of preoperative fractionated irradiation on left colonic anastomoses in the rat. Dis Colon Rectum. 1998;41(3):370-376. doi: 10.1007/BF02237494 [DOI] [PubMed] [Google Scholar]
- 59.Borstlap WAA, Westerduin E, Aukema TS, Bemelman WA, Tanis PJ; Dutch Snapshot Research Group . Anastomotic leakage and chronic presacral sinus formation after low anterior resection: results from a large cross-sectional study. Ann Surg. 2017;266(5):870-877. doi: 10.1097/SLA.0000000000002429 [DOI] [PubMed] [Google Scholar]
- 60.Sloothaak DA, Buskens CJ, Bemelman WA, Tanis PJ. Treatment of chronic presacral sinus after low anterior resection. Colorectal Dis. 2013;15(6):727-732. doi: 10.1111/codi.12094 [DOI] [PubMed] [Google Scholar]
- 61.Erlandsson J, Holm T, Pettersson D, et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017;18(3):336-346. doi: 10.1016/S1470-2045(17)30086-4 [DOI] [PubMed] [Google Scholar]
- 62.Wong NY, Eu KW. A defunctioning ileostomy does not prevent clinical anastomotic leak after a low anterior resection: a prospective, comparative study. Dis Colon Rectum. 2005;48(11):2076-2079. doi: 10.1007/s10350-005-0146-1 [DOI] [PubMed] [Google Scholar]
- 63.Wang L, Hirano Y, Ishii T, et al. Diverting stoma versus no diversion in laparoscopic low anterior resection: a single-center retrospective study in Japan. In Vivo. 2019;33(6):2125-2131. doi: 10.21873/invivo.11713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kyriacou DN, Lewis RJ. Confounding by indication in clinical research. JAMA. 2016;316(17):1818-1819. doi: 10.1001/jama.2016.16435 [DOI] [PubMed] [Google Scholar]
- 65.Bosmans JW, Jongen AC, Boonen BT, et al. Comparison of three different application routes of butyrate to improve colonic anastomotic strength in rats. Int J Colorectal Dis. 2017;32(3):305-313. doi: 10.1007/s00384-016-2718-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol, original
Trial protocol version 2
Trial protocol version 3
Trial protocol version 4
eTable 1. Demographic and Surgical Characteristics of the Patients in Each Subgroup
eTable 2. Demographic and Surgical Characteristics of the Patients (Per-Protocol Analysis)
eTable 3. The Details of Anastomotic Leakage (Per-Protocol Analysis)
eTable 4. Anastomotic Leakage in Each Subgroup Based on the Presence or Absence of a Diverting Stoma (Per-Protocol Analysis)
eTable 5. Demographic and Surgical Characteristics of the Patients in Each Subgroup (Per-Protocol Analysis)
Data Sharing Statement